2021 45(1): 65-77
DOI:10.32604/biocell.2021.014154

www.techscience.com/journal/biocell
| BIOCELL 2021 45(1): 65-77 DOI:10.32604/biocell.2021.014154 |  www.techscience.com/journal/biocell |
In silico assessment of human health risks caused by cyanotoxins from cyanobacteria
1Department of Biomedical Science and Environmental Biology, College of Life Science, Kaohsiung Medical University, Kaohsiung, 807, Taiwan
2Univ. Lille, Laboratoire LASIRE, UMR CNRS 8516, Equipe Physico-Chimie de l’Environnement, Lille, F-59000, France
3Institute of Marine Biology, National Taiwan Ocean University, Keelung, 202, Taiwan
4Center of Excellence for the Oceans, National Taiwan Ocean University, Keelung, 202, Taiwan
5Center of Excellence for Ocean Engineering, National Taiwan Ocean University, Keelung, 202, Taiwan
6Research Center for Environmental Medicine, KMU-Kaohsiung Medical University, Kaohsiung, 807, Taiwan
7Department of Marine Biotechnology and Resources, National Sun Yat-sen University, Kaohsiung, 804, Taiwan
*Address correspondence to: Hans-Uwe Dahms, hudahms11@gmail.com
Received: 03 September 2020; Accepted: 12 October 2020
Abstract: Harmful algal blooms (HABs) that are formed by cyanobacteria have become a serious issue worldwide in recent years. Cyanobacteria can release a type of secondary metabolites called cyanotoxins into aquatic systems which may indirectly or directly provide health risks to the environment and humans. Cyanotoxins provide some of the most powerful natural poisons including potent neurotoxins, hepatotoxins, cytotoxins, and endotoxins that may result in environmental health risks, and long-term morbidity and mortality to animals and humans. In this research, we used the chemcomputational tool Molinspiration for molecular property predictions, Pred-hERG 4.2 web software for cardiac toxicity prediction, and Pred-Skin 2.0 web software for predicting skin sensitization. We are predicting some toxicological aspects of cyanobacteria here using chemcomputational tools with the hypothesis that cyanotoxins are providing a risk to human health. We are using the tool Pred-hERG 4.2 to predict hERG channel blocking potential and the Pred-skin tool to predict skin sensitization due to cyanotoxins. The potential of anatoxin, ambigol, the microcystin group, and lyngbyatoxin A, lyngbyatoxin B, nodularin-R, and saxitoxin were predicted to cause skin sensitization in the final results (consensus model). Anatoxin-a and lyngbyatoxin were predicted to allow GI absorption and blood–brain barrier penetration. Among the 20 predicted cyanotoxins only aeruginosin 103-A, ambigol A, and ambigol were predicted by Pred-hERG 4.2 according to the applicability domain results as potential cardiotoxins with weak or moderate potency. Lyngbyatoxin shows activity through the GPCR ligand and protease, kinase, and enzyme inhibitor.
Keywords: Cyanotoxins; Predictive model; Molinspiration; Bioactivity score; hERG blocker; Carcinogenicity
Cyanobacteria occur in aquatic environments such as freshwaters (rivers, lakes, ponds, etc.), brackish waters (Hamilton et al., 2014), and the oceans (Schaefer et al., 2020). They are primary producers and play important roles in aquatic ecosystems, converting nitrogen into organic forms that can be used as macronutrients by eukaryotes, and oxygenic photosynthesis (Gademann and Portmann, 2008; Harke et al., 2016; Wood et al., 2007). However, climate change and eutrophication can result in harmful algal blooms (HABs) that are caused by excessive growth of cyanobacteria, and the release of high concentrations of toxic secondary metabolites called cyanotoxins becomes a serious threat. This holds for other organisms as well as for the safety of drinking water, aquatic food sources (Agasild et al., 2019), and public health (Aráoz et al., 2010).
Cyanotoxins provide toxic compounds that may affect the environment, animals, and humans by exposure through, e.g., seafood and aquaculture consumption, water contact during recreation, and drinking water (Meriluoto et al., 2017; Bukaveckas et al., 2018). Cyanotoxins can cause serious public health issues after long-term exposure through contaminated drinking water and recreational contact in fresh- and saltwater systems. Seafood consumption is another concern (Stone and Bress, 2007). Exposure to cyanobacteria can cause health effects in humans. These may include vomiting, flu-like symptoms, rashes, nausea, fever, gastroenteritis, blistered mouth, skin, eye, and ear irritation, visual disturbances, abdominal pain, and systemic effects such as hepatic failure, neurological damage, and death (Brown et al., 2018; Codd et al., 2005; Kubickova et al., 2019). Skin irritation can result from contact with toxic cyanobacteria (Pilotto et al., 2004). Cyanobacteria can also cause allergic reactions with symptoms like hives, conjunctivitis, and asthma (Farrer et al., 2015).
Cyanobacterial toxigenic compounds provide major waterborne health risks globally (Schaefer et al., 2020). Several toxins appear to be confined to specific cyanobacteria, but some cyanobacteria are known to produce a variety of toxic compounds (Haque et al., 2017). Cyanotoxins contain a variety of chemical compounds that differ by their chemical structure. Toxicological endpoints can be separated by their effects on liver toxins, neurotoxins, dermatotoxins, and endotoxins (Brown et al., 2018; Mankiewicz et al., 2003; Haque et al., 2017) (Tab. 1). Different approaches try to classify cyanotoxins, such as their respective biological sources, toxicity, molecular mass, and structural characteristics (Johnson et al., 2011). Reproductive and developmental toxicity, as well as carcinogenicity, are as yet not conclusively connected to cyanotoxins. This holds for marine systems but also for freshwater ones, where cyanotoxins represent an emerging health concern (IJCH Report, 2017).
Table 1: Health risks provided by toxins from cyanobacteria
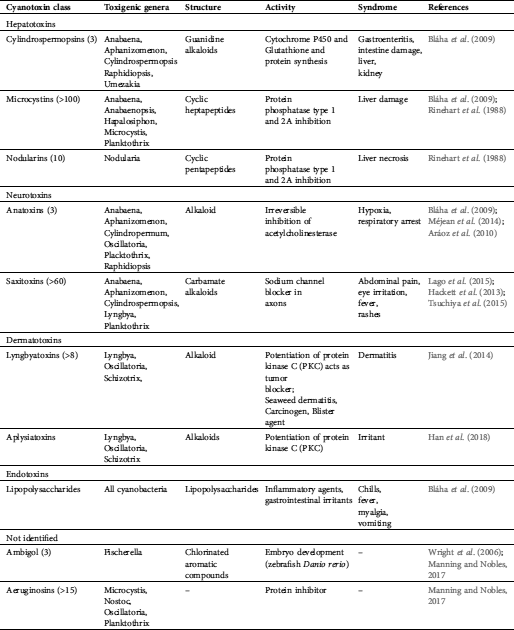
Cyanotoxins can cause alimentary poisoning in humans and animals with different syndromes such as: Diarrheic shellfish poisoning (DSP), amnesic shellfish poisoning (ASP), paralytic shellfish poisoning (PSP), neurotoxic shellfish poisoning (NSP), and azaspiracid shellfish poisoning (ASP) (Bigalke and Rummel, 2005). Several toxic compounds were found in cyanobacteria worldwide from oceanic environments and reservoirs and freshwater lakes worldwide (Huang and Zimba, 2019). Most threatening are neurotoxins affecting the nervous system (e.g., tetrodotoxin and saxitoxin), providing considerable potential as toxins even for military use (Pitschmann and Hon, 2016). For example, saxitoxin (STX) binds to the voltage-gated Na+ channel, subsequently blocking neuronal transmissions (Arnich and Thebault, 2018). It is the most studied and the first known compound to cause paralytic shellfish poisoning (PSP). The aerosol of the unstable saxitoxin degrades per minute at a rate of 17% (Jansson and Åstot, 2015). Saxitoxin is able to overcome the dermal barrier (similar to brevetoxin, anatoxin, and tetrodotoxin). However, it does not reach the efficacy of organophosphate as a chemical warfare agent (CWA) with another example of cyanobacterial neurotoxin, β-N-methylamino-l-alanine (BMAA), which is a cyanobacterial neurotoxin (Kubo et al., 2008). The toxins of several cyanobacterial genera also showed antineoblastic potential in human cell lines and promising results with respect to human adenocarcinomas (Zanchett and Oliveira-Filho, 2013).
We are applying chemoinformatics here as a search for chemical information that transfers chemical data to simulations. In silico approaches in toxicological studies make use of informational techniques that allow the predictions of toxic effects of cyanotoxins. We are using chemcomputational tools here following the hypothesis that cyanotoxins are providing risks to human health.
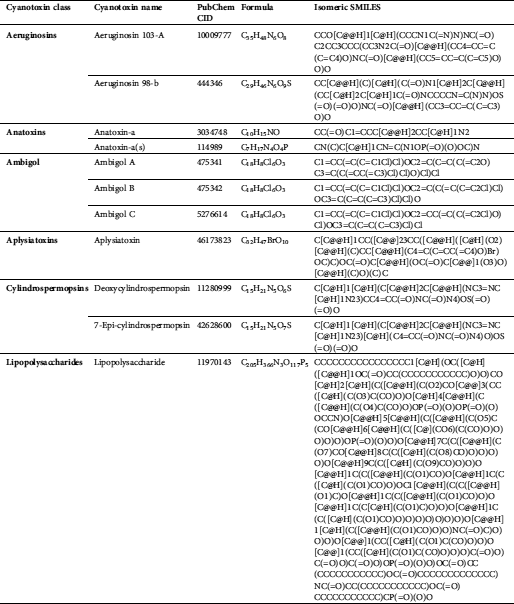
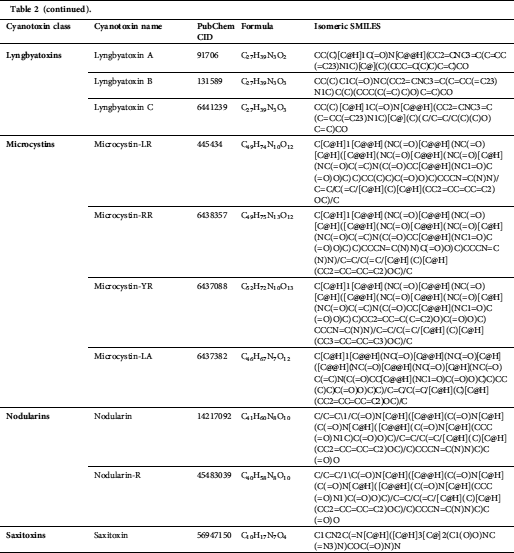
We accessed 21 cyanotoxins through the database of PubChem (https://pubchem.ncbi.nlm.nih.gov/) and a literature report. Isomeric SMILES notations of cyanotoxins collected from PubChem are presented in Tab. 2, and structures sketched by software ChemDraw 18.1 (https://www.perkinelmer.com/au/category/chemdraw) are presented in Fig. 1. Linear kernels like SMILES were used for our toxicity predictions since their similarity functions are computationally more efficient (Frenzel et al., 2017).
We were using Swiss ADME online software (http://www.swissadme.ch) in our toxicity discovery to predict parameters related to “absorption, distribution, metabolism, and excretion” (ADME) such as medicinal chemistry, druglike nature, friendliness of one or multiple small molecules as well as to compute parameters related to their physicochemistry. Swiss ADME enables assessment of ADME parameters of drug candidates and small molecules and provides information that allows early risk assessment in the drug development process. Notably, Swiss ADME provides a platform to assess the drug-likeness of oral bioavailability through Lipinski’s rule of five (Jayaseelan et al., 2012; Lipinski, 2000). Swiss ADME is a more recent and comprehensive site run by the Swiss Institute of Bioinformatics (SIB), which provides bioinformatics services and resources for scientists worldwide. SIB has over 800 scientists and 65 bioinformatic teams from major Swiss research and higher education institutes (Daina et al., 2017).
One form of the Long QT syndrome (LQTS) with a lack of repolarization of the heart after a heartbeat was disturbed, is related to a defect of the hERG protein that affects the K+ channel functioning during cardiac electric excitability. Identification of a hydrophobic area, which represents the putative interface and tight binding region with the interface of the K+ channel, became possible by the screening for mutagenesis of the domain surface. Once this hydrophobic domain of the channel is present, the rate of deactivation is slowed down (Cabral et al., 1998). The first three-dimensional model of a eukaryotic domain called Per-Arnt-Sim (PAS) is structurally similar to a yellow protein functioning as a bacterial light sensor.
In order to confirm or reject the sensitization effect of cyanobacterial compounds evaluated here, we used Pred-Skin 3.0 web online software (http://labmol.com.br/predskin/), available as an online software program (Braga et al., 2017).
The bioactivity potential of cyanotoxins was predicted by calculating the activity score for the ion channel modulator, the GPCR ligand, the nuclear receptor ligand, and the inhibitors of enzymes, proteases, and kinases (Jabeen and Ranganathan, 2019). All the above parameters were tested by applying the drug-likeness score of Molinspiration software (www.molinspiration.com).
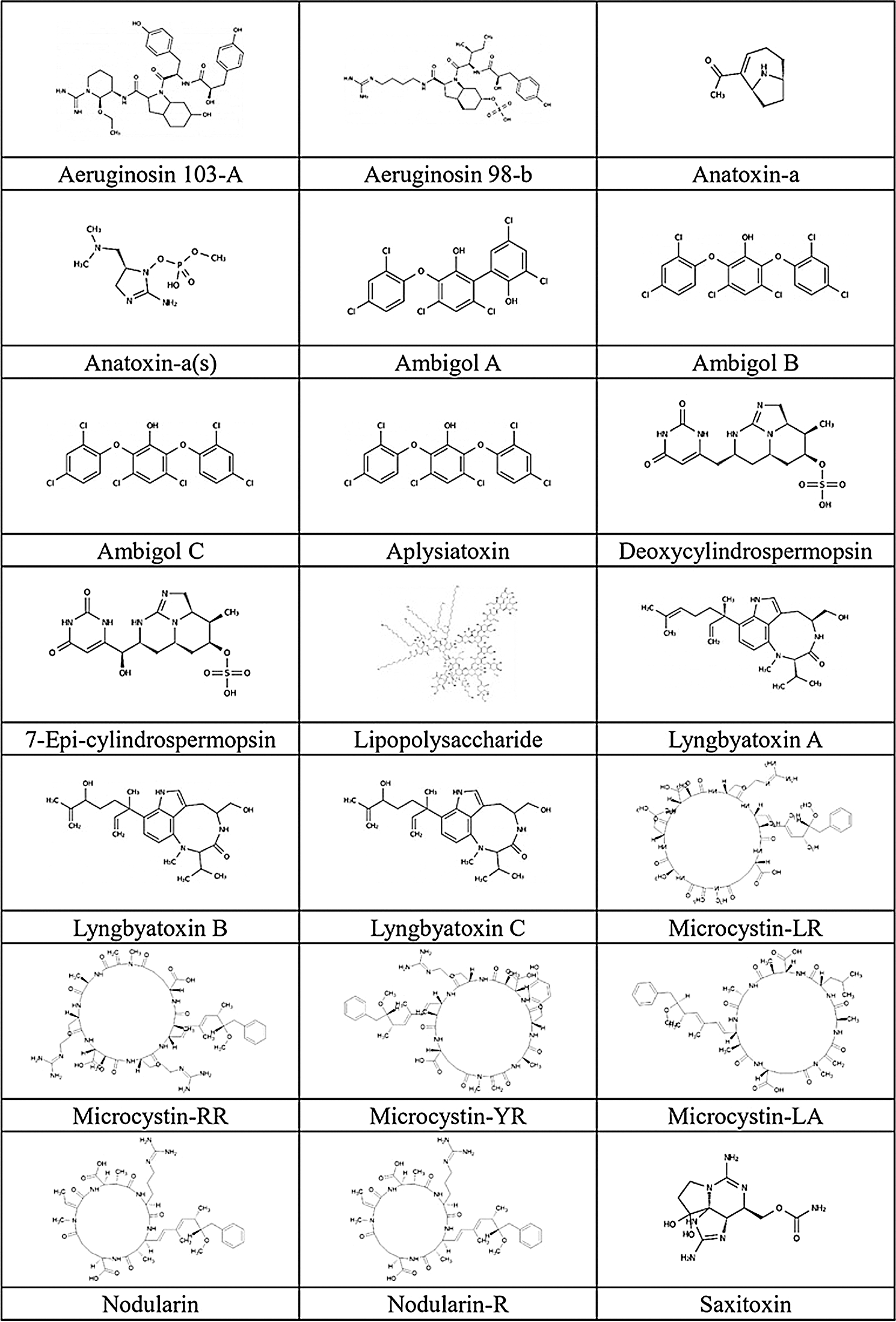
Figure 1: Chemical structure of cyanotoxins.
Table 2: Isomeric SMILES of cyanobacterial toxins
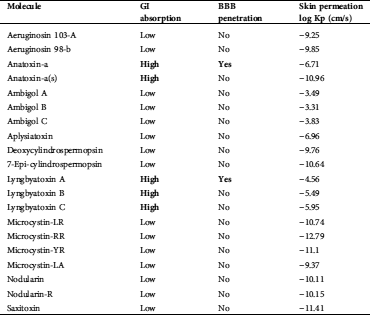
Anatoxin-a and Lyngbyatoxin were predicted to have GI absorption and blood–brain barrier (BBB) penetration (Tab. 3).
Table 3: ADME prediction results of 20 cyanotoxins
Among the 20 predicted cyanotoxins only aeruginosin 103-A, ambigol A, and ambigol B have cardiotoxic potential and weak or moderate potency as predicted by Pred-hERG 4.2 according to the applicability domain results (Tab. 4).
Anatoxin, ambigol, the microcystin group, lyngbyatoxin A, lyngbyatoxin B, nodularin-R, and saxitoxin were predicted to have the potential to cause skin sensitization in the final results (consensus model) (Tab. 5).
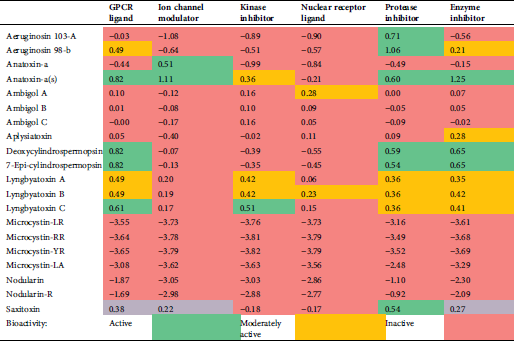
The calculated values of the isolated compound endpoints and the drug-likeness scores are summarized in Tab. 6. Lyngbyatoxin, as an enzyme inhibitor, shows inhibition activity against proteases and kinases through the GPCR ligand and the inhibitors of enzyme, kinase, and protease.
Anatoxin-a and Lyngbyatoxin were predicted to have GI absorption and BBB penetration (Tab. 3). The BBB maintains homeostasis of the Central Nervous System (CNS), which is provided by the permeability BBB (Gao et al., 2017).
The inability of most compounds to cross the BBB is a major limitation to effectively treat diseases in the central nervous system (Alexander et al., 2019). The BBB is a highly evolved microvascular system comprised of brain endothelial cells (ECs) lining the vascular lumen, pericytes in the basal lamina, and associating astrocytic end-feet, microglia, and neurons. This cellular architecture forms functional neurovascular units that regulate molecular trafficking between the blood and the brain (Zhang et al., 2019).
The extent of binding can greatly influence The ADME (adsorption, distribution, metabolism, and excretion) profile that can greatly be affected by toxin binding. The evaluation of biological substance effects very much depends on gastrointestinal absorption (GI absorption). In the evaluation of the biological effects of substances, this is a key criterion (Diukendjieva et al., 2019). Cost-effective and reliable and cost-effective bioavailability studies early in the drug discovery process can lead to an improvement in the success rate for compounds entering clinical trials (Fabini and Danielson, 2017).
Pred-hERG 4.2 server (http://labmol.com.br/predherg) predicts the probability maps where compounds block the K+ ion channel coded by a human Ether-à-go-go-Related Gene (hERG) (Tab. 4). The results show that not all tested marine cyanobacterial toxins are hERG blockers in a multiclass prediction. All tested cyanobacterial compounds are hERG blockers as predicted by the Pred-hERG binary prediction, except anatoxin-A, BMAA, and hypoxanthine.
Anatoxin, ambigol, and the microcystin group, lyngbyatoxin A, lyngbyatoxin B, nodularin-R, and saxitoxin were predicted to cause skin sensitization (Tab. 5). In case a susceptible individual is exposed to a contact allergen, upregulation and clonal expansion of allergen-responsive T-cells occurs (Gilmour et al., 2019).
The computed values of different parameters of the isolated compounds are summarized in Tab. 6. We found that Lyngbyatoxin shows activity through the protease inhibitor, the G-protein-coupled receptor (GPCR ligand), the GPCR ligand, and the kinase inhibitor. In clinical medicine, GPCR ligands became the most successful molecular drug targets. Antagonists, as well as agonists of the GPCR class, were applied in the treatment of every major organ system, including the respiratory, metabolic, urogenital, and cardiovascular systems. Considering their widespread expression and important regulatory and mechanistic characteristics, GPCRs have manifold functions in living beings (Insel et al., 2007; Campbell and Smrcka, 2018). Our study predicted anatoxin-a and lyngbyatoxin to affect GI absorption and BBB penetration. Factors ensuring overall drug absorption include compound solubility and permeability as well as compound dissolution and gastrointestinal conditions, drug- and formulation- related parameters, drug product dissolution, as well as active transport, passive diffusion, and metabolism of toxicants of drugs (Freerks et al., 2019).
Table 4: hERG-predictions of 20 cyanotoxins from Pred-hERG 4.2
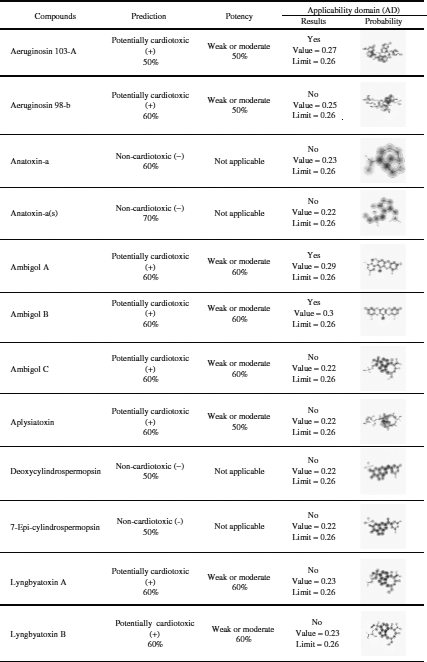
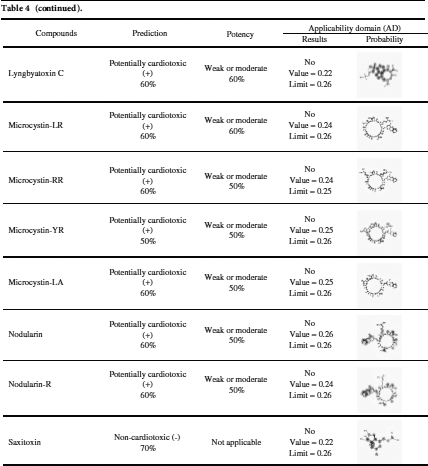
Table 5: Skin sensitization predictions of cyanotoxins
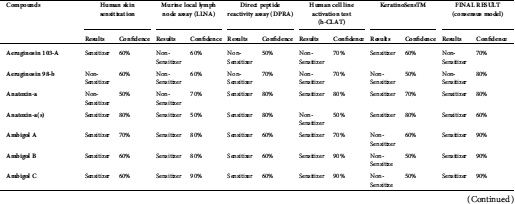
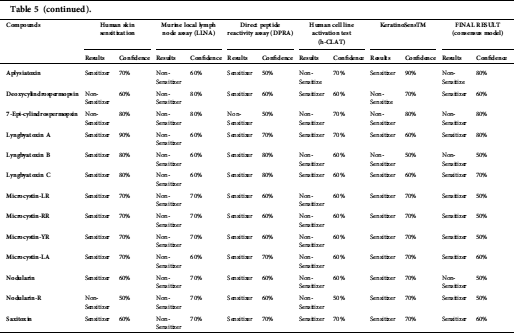
Table 6: Bioactivity potential results as predicted by Molinspiration
Chemcomputation adds to the instrumentation applied in ecotoxicology, such as the prediction of the physiological effects of cyanotoxins. In silico tools add objectivity combines several approaches in repeatable and intelligent ways. Economic demands and fast technological progress are in favor of chemcomputational tools. Cheminformatics allows for higher throughput and constant optimization. Cheminformatic approaches have a higher reproducibility, are less time consuming, and are less expensive. Computational approaches can also prioritize chemicals in order to reduce the amount of costly in vivo and in vitro toxicological screening and provide early alerts for unexplored, newly discovered, or newly developed substances. Through their replacement, they reduce the use of experimental efforts.
A lack of transparency and quality of the training set of experimental data provide some limitations. For example, carcinogenicity prediction can only be applied with non-genotoxic compounds. Neurotoxicity, hepatotoxicity, and developmental toxicity cannot be accurately predicted by in silico methods. These are complex phenomena with multiple endpoints, unlike issues with well-understood mechanisms like mutagenicity, sensitization, and aquatic toxicity – as exemplified in this study by cyanotoxins.
Acknowledgement: We are thankful to Mr. Charli Deepak and Cheng-Han Liu, Mrs. Sravya Kosuru, and Revathi Gurunandam for their assistance in this computational study.
Availability of Data and Materials: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Funding Statement: This research was funded by the Ministry of Science and Technology of Taiwan, R.O.C. to HUD, grant number MOST 107-2621-M-037-001, MOST108-2621-M-037-001, and MOST 109-2621-M-037-001 provided for Tan, Han-Shih. This work was financially supported by the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. An NSYSU/KMU collaboration is acknowledged (108-PO25).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Agasild H, Panksep K, Tonno I, Blank K, Koiv T, Freiberg R, Laugaste R, Jones RI, Noges P, Noges T. (2019). Role of potentially toxic cyanobacteria in crustacean zooplankton diet in a eutrophic lake. Harmful Algae 89: 101688. DOI 10.1016/j.hal.2019.101688. [Google Scholar] [CrossRef]
Alexander A, Agrawal M, Uddin A, Siddique S, Shehata AM, Shaker MA, Rahman SAU, Abdul MIM, Shaker MA. (2019). Recent expansions of novel strategies towards the drugs targeting into the brain. International Journal of Nanomedicine 30: 5895–5909. DOI 10.2147/IJN.S210876. [Google Scholar] [CrossRef]
Aráoz R, Jordi M, de Marsac NT. (2010). Neurotoxic cyanobacterial toxins. Toxicon 56: 813–828. DOI 10.1016/j.toxicon.2009.07.036. [Google Scholar] [CrossRef]
Arnich N, Thebault A. (2018). Dose-response modelling of paralytic shellfish poisoning (PSP) in humans. Toxins 10: 1–20. DOI 10.3390/toxins10040141. [Google Scholar] [CrossRef]
Bigalke H, Rummel A. (2005). Medical aspects of toxin weapons. Toxicology 214: 210–220. DOI 10.1016/j.tox.2005.06.015. [Google Scholar] [CrossRef]
Bláha L, Babica P, Maršálek B. (2009). Toxins produced in cyanobacterial water blooms–toxicity and risks. Interdisciplinary Toxicology 2: 36–41. DOI 10.1016/j.tox.2005.06.015. [Google Scholar] [CrossRef]
Braga RC, Alves VM, Muratov EN, Strickland J, Kleinstreuer N, Trospsha A, Andrade CH. (2017). Pred-Skin: A fast and reliable web application to assess skin sensitization effects of chemicals. Journal of Chemical Information and Modeling 57: 1013–1017. DOI 10.1021/acs.jcim.7b00194. [Google Scholar] [CrossRef]
Brown A, Foss A, Miller MA, Gibson Q. (2018). Detection of cyanotoxins (microcystins/nodularins) in livers from estuarine and coastal bottlenose dolphins (Tursiops truncatus) from Northeast Florida. Harmful Algae 76: 22–34. DOI 10.1016/j.hal.2018.04.011. [Google Scholar] [CrossRef]
Bukaveckas PA, Franklin R, Tassone S, Trache B, Egerton T. (2018). Cyanobacteria and cyanotoxins at the river-estuarine transition. Harmful Algae 76: 11–21. DOI 10.1016/j.hal.2018.04.012. [Google Scholar] [CrossRef]
Buratti FM, Manganelli M, Vichi S, Stefanelli M, Scardala S, Testai E, Funari E. (2017). Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Archives of Toxicology 91: 1049–1130. DOI 10.1007/s00204-016-1913-6. [Google Scholar] [CrossRef]
Cabral JHM, Lee A, Cohen SL, Chait BT, Li M, Mackinnon R. (1998). Crystal structure and functional analysis of the HERG potassium channel N-terminus: A eukaryotic PAS domain. Cell 95: 649–655. DOI 10.1016/S0092-8674(00)81635-9. [Google Scholar] [CrossRef]
Campbell AP, Smrcka AV. (2018). Targeting G protein-coupled receptor signalling by blocking G-proteins. National Reviews Drug Discovery 17: 789–803. DOI 10.1038/nrd.2018.135. [Google Scholar] [CrossRef]
Codd GA, Morrison LF, Metcalf JS. (2005). Cyanobacterial toxins: Risk management for health protection. Toxicology and Applied Pharmacology 203: 264–272. DOI 10.1016/j.taap.2004.02.016. [Google Scholar] [CrossRef]
Daina A, Michielin O, Zoete V. (2017). SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific Reports 7: 42717. DOI 10.1038/srep42717. [Google Scholar] [CrossRef]
Diukendjieva A, Tsakovska I, Alov P, Pencheva T, Pajeva I, Worth AP, Madden JC, Cronin MT. (2019). Advances in the prediction of gastrointestinal absorption: Quantitative Structure-Activity Relationship (QSAR) modelling of PAMPA permeability. Computational Toxicology 10: 51–59. DOI 10.1016/j.comtox.2018.12.008. [Google Scholar] [CrossRef]
Fabini E, Danielson UH. (2017). Monitoring drug–serum protein interactions for early ADME prediction through Surface Plasmon Resonance technology. Journal of Pharmaceutical and Biomedical Analysis 144: 188–194. DOI 10.1016/j.jpba.2017.03.054. [Google Scholar] [CrossRef]
Farrer D, Counter M, Hillwig R, Cude C. (2015). Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins 7: 457–477. DOI 10.3390/toxins7020457. [Google Scholar] [CrossRef]
Freerks L, Soulou EP, Batchelor H, Klein S. (2019). A review of GI conditions critical to oral drug absorption in malnourished children. European Journal of Pharmaceutics and Biopharmaceutics 137: 9–22. DOI 10.1016/j.ejpb.2019.02.001. [Google Scholar] [CrossRef]
Frenzel F, Buhrke T, Wenzel I, Andrack J, Hielscher J, Lampen A. (2017). Use of in silico models for prioritization of heat-induced food contaminants in mutagenicity and carcinogenicity testing. Archives of Toxicology 91: 3157–3174. DOI 10.1007/s00204-016-1924-3. [Google Scholar] [CrossRef]
Gademann K, Portmann C. (2008). Secondary metabolites from cyanobacteria: Complex structures and powerful bioactivities. Current Organic Chemistry 12: 326–341. DOI 10.1002/chin.200842246. [Google Scholar] [CrossRef]
Gao Z, Chen Y, Cai X, Xu R. (2017). Predict drug permeability to blood-brain–barrier from clinical phenotypes: Drug side effects and drug indications. Bioinformatics 33: 901–908. DOI 10.1093/bioinformatics/btw713. [Google Scholar] [CrossRef]
Gilmour N, Kimber I, Williams J, Maxwell G. (2019). Skin sensitization: Uncertainties, challenges, and opportunities for improved risk assessment. Contact Dermatitis 80: 195–200. DOI 10.1111/cod.13167. [Google Scholar] [CrossRef]
Hackett JD, Wisecaver JH, Brosnahan ML, Kulis DM, Anderson DM, Bhattacharya D, Plumely FG, Erdner DL. (2013). Evolution of saxitoxin synthesis in cyanobacteria and dinoflagellates. Molecular Biology and Evolution 30: 70–78. DOI 10.1093/molbev/mss142. [Google Scholar] [CrossRef]
Hamilton DP, Wood SA, Dietrich DR, Puddick J. (2014). Costs of harmful blooms of freshwater cyanobacteria. Cyaobacteria: An Economic Perspective John Wiley & Sons 247–256. DOI 10.1002/9781118402238. [Google Scholar] [CrossRef]
Han BN, Liang TT, Keen LJ, Fan TT, Zhang XD, Xu L, Zhao Q, Wang SP, Lin HW. (2018). Two marine cyanobacterial aplysiatoxins polyketides, neo-debromoaplysiatoxin A and B, with K+ channel inhibition activity. Organic Letters 20: 578–581. DOI 10.1021/acs.orglett.7b03672. [Google Scholar] [CrossRef]
Haque F, Banayan S, Yee J, Chiang YW. (2017). Extraction and applications of cyanotoxins and other cyanobacterial secondary metabolites. Chemosphere 183: 164–175. DOI 10.1016/j.chemosphere.2017.05.106. [Google Scholar] [CrossRef]
Harke MJ, Steffen MM, Gobler CJ, Otten TG, Wilhelm SW, Wood SA, Paerl, HW. (2016). A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium. Microcystis spp. Harmful Algae 54: 4–20. DOI 10.1016/j.hal.2015.12.007. [Google Scholar] [CrossRef]
Huang IS, Zimba PV. (2019). Cyanobacterial bioactive metabolites—A review of their chemistry and biology. Harmful Algae 86: 139–209. DOI 10.1016/j.hal.2019.05.001. [Google Scholar] [CrossRef]
Insel PA, Tang CM, Hahntow I, Michel MC. (2007). Impact of GPCRs in clinical medicine: Monogenic diseases, genetic variants and drug targets. Biochimica et Biophysica Acta (BBA) - Biomembranes 1768: 994–1005. DOI 10.1016/j.bbamem.2006.09.029. [Google Scholar] [CrossRef]
IJCH report. (2017). Human Health Effects of Cyanobacterial Toxins in the Great Lakes Region: A Science and Monitoring Assessment, A report submitted to the International Joint Commission by the Health Professionals Advisory Board February 27. [Google Scholar]
Jabeen A, Ranganathan S. (2019). Applications of machine learning in GPCR bioactive ligand discovery. Current Opinion in Structural Biology 55: 66–76. DOI 10.1016/j.sbi.2019.03.022. [Google Scholar] [CrossRef]
Jansson D, Åstot C. (2015). Analysis of paralytic shellfish toxins, potential chemical threat agents, in food using hydrophilic interaction liquid chromatography–mass spectrometry. Journal of Chromatography A 1417: 41–48. DOI 10.1016/j.chroma.2015.09.029. [Google Scholar] [CrossRef]
Jayaseelan KV, Moreno P, Truszkowski A, Ertl P, Steinbeck C. (2012). Natural product-likeness score revisited: An open-source, open-data implementation. BMC Bioinformatics 13: 471–2105. DOI 10.1186/1471-2105-13-106. [Google Scholar] [CrossRef]
Jiang W, Zhou W, Uchida H, Kikumori M, Irie K, Watanabe R, Suzuki T, Sakamoto B, Kamio M, Nagai H. (2014). A new lyngbyatoxin from the hawaiian cyanobacterium Moorea producens. Marine Drugs 5: 2748–2759. DOI 10.3390/md12052748. [Google Scholar] [CrossRef]
Johnson RC, Kalb SR, Barr JR. (2011). Toxin analysis using mass spectrometry. In: Budowle B, ed. Microbial Forensics. 2nd Edition. Cambridge, Massachusetts: Academic Press, 405–420. [Google Scholar]
Kubickova B, Babica P, Hilscherová K, Sindlerova L. (2019). Effects of cyanobacterial toxins on the human gastrointestinal tract and the mucosal innate immune system. Environmental Sciences Europe 31: 31. DOI 10.20944/preprints201903.0231.v1. [Google Scholar] [CrossRef]
Kubo T, Kato N, Hosoya K, Kaya K. (2008). Effective determination method for a cyanobacterial neurotoxin, β-N-methylamino-L-alanine. Toxicon 51: 1264–1268. DOI 10.1016/j.toxicon.2008.02.015. [Google Scholar] [CrossRef]
Lago J, Rodríguez PL, Blanco L, Vieites JM, Cabado AG. (2015). Tetrodotoxin, an extremely potent marine neurotoxin: Distribution, toxicity, origin and therapeutical uses. Marine Drugs 13: 6384–6406. DOI 10.3390/md13106384. [Google Scholar] [CrossRef]
Lipinski CA. (2000). Drug-like properties and the causes of poor solubility and poor permeability. Journal of Pharmacological and Toxicological Methods 44: 235–249. DOI 10.1016/S1056-8719(00)00107-6. [Google Scholar] [CrossRef]
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. (2001). Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews 23: 3–25. DOI 10.1016/S0169-409X(96)00423-1. [Google Scholar] [CrossRef]
Mankiewicz J, Tarczynska M, Walter Z, Zalewski M. (2003). Natural toxins from cyanobacteria. Acta Biological Cracoviensia. Series Botanica 45: 9–20. DOI 10.1021/bk-1990-0418.ch006. [Google Scholar] [CrossRef]
Manning SR, Nobles DR. (2017). Impact of global warming on water toxicity: Cyanotoxins. Current Opinion in Food Science 18: 14–20. DOI 10.1016/j.cofs.2017.09.013. [Google Scholar] [CrossRef]
Méjean A, Paci G, Gautier V, Ploux O. (2014). Biosynthesis of anatoxin-a and analogues (anatoxins) in cyanobacteria. Toxicon 91: 15–22. DOI 10.1016/j.toxicon.2014.07.016. [Google Scholar] [CrossRef]
Meriluoto J, Blaha L, Bojadzija G, Bormans M, Brient L, Codd GA, Drobac D, Faassen EJ, Fastner J, Hiskia A, Ibelings BW. (2017). Toxic cyanobacteria and cyanotoxins in European waters – recent progress achieved through the CYANOCOST action and challenges for further research. Advances in Oceanography and Limnology 8: 161–178. DOI 10.4081/aiol.2017.6429. [Google Scholar] [CrossRef]
Molinspiration Home Page (2018). [Google Scholar]
Pilotto L, Hobson P, Burch MD, Ranmuthugala G, Attewell R, Weightman W. (2004). Acute skin irritant effects of cyanobacteria (blue green algae) in healthy volunteers. Australian and New Zealand Journal of Public Health 28: 220–224. DOI 10.1111/j.1467-842X.2004.tb00479.x. [Google Scholar] [CrossRef]
Pitschmann V, Hon Z. (2016). Military importance of natural toxins and their analogs. Molecules 21: 556. DOI 10.3390/molecules21050556. [Google Scholar] [CrossRef]
Rinehart KL, Harada K, Namikoshi M, Chen C, Harvis CA. (1988). Nodularin, microcystin and the configuration of ADDA. Journal of the American Chemical Society 110: 8557–8558. DOI 10.1021/ja00233a049. [Google Scholar] [CrossRef]
Schaefer AM, Yrastorza L, Stockley N, Harvey K, Harris N, Grady R, Sullivan J, McFarland M, Reif JS. (2020). Exposure to microcystin among coastal residents during a cyanobacteria bloom in Florida. Harmful Algae 92: 101769. DOI 10.1016/j.hal.2020.101769. [Google Scholar] [CrossRef]
Stone D, Bress W. (2007). Addressing public health risks for cyanobacteria in recreational freshwaters: The Oregon and Vermont framework. Integrated Environmental Assessment and Management 3: 137–143. DOI 10.1002/ieam.5630030112. [Google Scholar] [CrossRef]
Tsuchiya S, Cho Y, Konoki K, Nagasawa K, Oshima Y, Yotsu-Yamashita M. (2015). Synthesis of a tricyclic bisguanidine compound structurally related to saxitoxin and its identification in paralytic shellfish toxin-producing microorganisms. Chemistry A - European Journal 21: 7835–7840. DOI 10.1002/chem.201500064. [Google Scholar] [CrossRef]
Wood SA, Selwood AI, Rueckert A, Holland PT., Milne JR, Smith KF, et al. (2007). First report of homoanatoxin-a and associated dog neurotoxicosis in New Zealand. Toxicon 50: 292–301. DOI 10.1016/j.toxicon.2007.03.025. [Google Scholar] [CrossRef]
Wright AD, Papendorf O, König GM. (2006). Ambigol C and 2,4-dichlorobenzoic acid, natural products produced by the terrestrial cyanobacterium Fischerella ambigua. Journal of Natural Products 3: 456–461. DOI 10.1021/np049640w. [Google Scholar] [CrossRef]
Zanchett G, Oliveira-Filho EC. (2013). Cyanobacteria and cyanotoxins: From impacts on aquatic ecosystems and human health to anticarcinogenic effects. Toxins 5: 1896–1917. DOI 10.3390/toxins5101896. [Google Scholar] [CrossRef]
Zhang Y, Cui G, Wang Y, Gong Y, Wang Y. (2019). SIRT1 activation alleviates brain microvascular endothelial dysfunction in peroxisomal disorders. International Journal of Molecular Medicine 44: 995–1005. DOI 10.3892/ijmm.2019.4250. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |