2021 45(1): 103-108
DOI:10.32604/biocell.2021.011220

www.techscience.com/journal/biocell
| BIOCELL 2021 45(1): 103-108 DOI:10.32604/biocell.2021.011220 |  www.techscience.com/journal/biocell |
CLEC3A gene three polymorphisms and risk of gastric cancer in Northwestern Chinese population
Department of General Surgery, Tangdu Hospital, Air Force Medical University of People’s Liberation Army, Xi’an, 710038, China
*Address correspondence to: Guoqiang Bao, baogq740905@sina.com
Received: 27 April 2020; Accepted: 19 August 2020
Abstract: This study aimed to evaluate the association between the CLEC3A gene polymorphisms (rs2735401/rs2293776/rs2072665) and the gastric cancer risk in the Northwestern Chinese population. A hospital-based case-control study was conducted on 681 cases and 756 healthy controls. Odds ratio (OR) and 95% confidence intervals (CI) were applied to evaluate the association of the CLEC3A polymorphisms on gastric cancer risk. We found that there was no significant association between the CLEC3A polymorphisms and gastric cancer susceptibility, which was detected in the main analysis or stratification analyses of age, gender, and clinical stages. Our findings verified that the CLEC3A polymorphisms are not associated with gastric cancer susceptibility in the Northwestern Chinese population; other polymorphisms should be investigated to further clarify the susceptibility to gastric cancer.
Keywords: CELC3A; gastric cancer; risk; single nucleotide polymorphisms
Gastric cancer (GC) is one of the most prevalent cancers worldwide and the third major cause of cancer mortality (Bray et al., 2018; Gjyshi et al., 2018). In China, it is the second leading cause of cancer death among both men and women, with approximately 679,100 new cancer cases and 498,000 cancer deaths reported in 2015 (Chen et al., 2016). Although there have been advances in the treatment strategies for gastric cancer, the prognosis of gastric cancer is still poor, the 5-year survival rate is only 20–30% because most cases are diagnosed in an advanced stage (Tahara et al., 2010). GC development is influenced by individual genetic susceptibility, environmental components (Maccormick et al., 2019), and/or dietary habits (Hartgrink et al., 2009). Genetic factors have been found to play an important role in the development of gastric cancer (Baroudi and Benammar-Elgaaied, 2016). To clarify the genetic background of gastric cancer, it is necessary to identify the genetic factors specifically such as single nucleotide polymorphisms (SNP), and the relationship between SNP and gastric cancer has been studied. A study found that the ERCC1, XPG, and mTORC1 Gene may affect the risk of gastric cancer in the Chinese Han population (He et al., 2012; He et al., 2013; He et al., 2018), but no positive association was found between the three LIG3 (DNA ligase III) SNPs and gastric cancer risk in single-locus analysis or combined risk genotypes analysis (Hua et al., 2019).
C-type lectin domain family 3 member A (CLEC3A, originally called CLECSF1) was first described as a cartilage-derived member of the C-type lectin superfamily and according to its domain structure assigned to the tetranectin IX group, together with tetranectin (CLEC3B) and stem cell growth factor (SCGF) with α and β forms (CLEC11A) (Neame et al., 1999). The C-type lectins form a diverse protein family with many different functions across species. Most members are found extracellularly and carry C-type carbohydrate recognition domains (CRD) that, in some cases, specifically recognize or bind proteins, lipids, or carbohydrates in a Ca2+ dependent manner; whereas, those in other proteins only form a structural motif. Human CLEC3A mRNA has been detected in normal breast and breast cancer tissue as well as in two colon cancer cell lines, and CLEC3A associates with cell adhesion (Tsunezumi et al., 2009). Cell adhesion influenced results in tumor cell proliferation and metastasis (Boguslawska et al., 2018). In terms of tumor tissue, CLEC3A expression was markedly higher in breast invasive ductal cancer tissues than normal breast tissues or adjacent normal tissue (Ni et al., 2018). It is suggested that CLEC3A may be related to the development of breast cancer. Additionally, CLEC3A was reported to activate the plasminogen activation via enhancing tissue plasminogen activator (Lau et al., 2018). It was found that the plasminogen activator system was identified as one of the major mechanisms involved in the processes of cell invasion and metastatic spreading (Duffy et al., 2014; Resmini et al., 2017).
The CLEC3A gene coding the CLEC3A protein also resides in the fine-mapped region on chromosome 16q23 (Rezaee et al., 2006). The murine and human CLEC3A gene consists of three exons. The first exon codes for a potential signal peptide with 22 amino acids and the subsequent 16 amino acids. The second exon encodes 27 amino acids and the third a CRD domain of 130 amino acids (Neame et al., 1999). In human colon carcinoma cells, CLEC3A is a membrane-associated substrate for matrix metalloproteinase-7 (MMP-7), and has been speculated that cleavage of CLEC3A by MMP-7 in the tumor microenvironment may affect tumor cell invasion and metastasis by modulating cell adhesion and the plasminogen/plasminogen-activator system (Tsunezumi et al., 2009). In a proteomic analysis of high-density lipoprotein (HDL), CLEC3A was identified as an HDL-associated protein (Rezaee et al., 2006). However, the relationship between CLEC3A polymorphisms and tumors has not been studied.
Therefore, in this study, we analyzed the relationship between the CELC3A gene three polymorphisms (rs2735401 T>G/rs2293776 C>G/rs2072665 T>C) and GC susceptibility in a cohort of GC and healthy controls in the Northwestern Chinese population.
This study was approved by the Ethics Committee of The Air Force Medical University. The procedures were performed according to the approved guidelines and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The signed informed consent was obtained from each participant included in the study.
A total of 681 GC patients and 756 healthy control subjects from Chinese genetically unrelated Northwestern people were enrolled in the study, including subjects from two hospitals in Xijing and Tangdu hospital in Shaanxi province. All patients were diagnosed as having GC based on a histopathological examination. None of the patients had a history of any other tumors. The control subjects were randomly chosen among people living in Shaanxi province, with match age and sex.
Sample collection and genotyping
Peripheral venous blood samples (5 mL) were collected from all subjects in EDTA vacutainers. Genomic DNA was obtained from the peripheral blood lymphocytes of study subjects using the Genomic DNA Extraction Kit (Omega Bio-Tek, Norcross, GA, USA or GoldMag Ltd, Xian, China) according to the manufacturer’s protocol. Agena Mass ARRAYAssay Design 4.0 software was used to design the multiplexed SNP Mass EXTEND assay. The CLEC3A polymorphisms were genotyped on the Agena Mass ARRAY RS1000 platform according to the standard protocol (Applied Biosystems, Foster City, CA, USA). Then, Agena Typer 4.0 software was applied to analyze and manage our data. Quality control was performed with eight negative control and positive control samples in each 384-well plate. In addition, 10% of the samples were randomly selected for a second genotyping for validation of the assay, and the concordance rate was 100%.
We used SPSS version 19.0 software (SPSS, Chicago, IL) and Microsoft Excel (Redmond, WA) to analyze all the related data. A value of p < 0.05 was considered statistically significant. Pearson’s χ2-test was used to detect differences in demographic variables, risk factors distribution, and CLEC3A genotypes distribution between the case and control groups. Frequencies of the variants were estimated using the Hardy–Weinberg equilibrium (HWE) (p-value calculated by the exact test) to compare the expected frequencies of the genotypes in the control groups. PLINK software was used to calculate the odds ratios (OR) and 95% confidence intervals (CI) by unconditional logistic regression analysis with adjustment for age and gender (Jin et al., 2015). Finally, we measured the linkage disequilibrium (LD) between loci, haplotype construction, and the genetic association was calculated by unconditional logistic regression. The Haploview was used to construct haplotype and genetic association at significant polymorphism loci and to estimate the pairwise LD, haplotype software (version 4.2) (Guruvaiah et al., 2014).
Characteristics of the study population
Overall, the selected variables were significantly different between GC patients and controls regarding the distribution of age and gender (p < 0.001 and p < 0.001, respectively). The distributions of age, gender, and clinical stages of the study subjects are summarized in Tab. 1. The mean age was 57.57 ± 10.83 years for GC patients and 52.58 ± 8.71 years for the controls. Males were predominant in both the GC and control groups. The proportion of male subjects was significantly higher in the group with GC (77.4%), whereas the number of female subjects was higher in the control group (35.3%). Most of the patients had stage II disease, followed by stage III, stage I, and stage IV. Tumors less than 5 cm in diameter accounted for more than half of the case group.
Table 1: Characteristics and clinical features of the gastric cancer (GC) cases and the control group
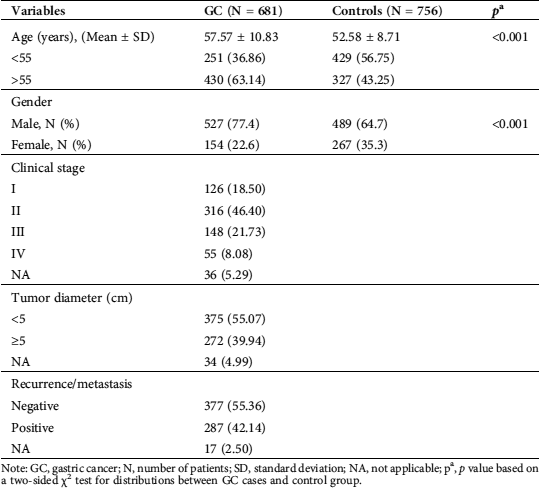
Note: GC, gastric cancer; N, number of patients; SD, standard deviation; NA, not applicable; pa, p value based on a two-sided χ2 test for distributions between GC cases and control group.
Association of the three CLEC3A polymorphisms with the risk of GC
The genotype distributions of the CLEC3A polymorphisms in GC patients and controls are summarized in Tab. 2. Compared with rs2735401 TT homozygote, the frequencies of rs2735401 GT and GG genotypes in GC cases and controls did not show significant difference (p = 0.148 and p = 0.454). The OR after adjustment of risk factors (age and sex) were 1.04 (95%CI = 0.83–1.31, p = 0.145) for GT and 0.83 (95%CI = 0.58–1.21, p = 0.520) for GG genotypes. Individuals with variant genotypes (GT + GG) had a 0.99-fold reduce in risk of GC but had no significant difference from TT genotype (adjusted OR = 0.99, 95%CI = 0.80–1.24, p = 0.96).
Compared with rs2293776 CC homozygote, the frequencies of rs2293776 CG and GG genotypes in GC cases and controls also had no significant difference (p = 0.163 and p = 0.339, respectively). The adjusted OR were 0.98 (95%CI = 0.78–1.25, p = 0.075) for CG group and 0.90 (95%CI = 0.55–1.46, p = 0.863) for GG group. Individuals with variant genotypes (CG + GG) had only a 0.97-fold risk of GC but with no difference compared with CC homozygote carriers (adjusted OR = 0.97, 95%CI = 0.77–1.22, p = 0.79).
Compared with rs2072665 TT homozygote, the frequencies of rs2072665 TC and CC genotypes in GC cases and controls had no significant difference (p = 0.48 and p = 0.37, respectively). The adjusted OR were 1.14 (95%CI = 0.88–1.48, p = 0.33) for TC group and 1.24 (95%CI = 0.91–1.70, p = 0.37) for CC group. Individuals with variant genotypes (TC + CC) had a 1.17-fold risk of GC but with no difference compared with CC homozygote carriers (adjusted OR = 1.17, 95%CI = 0.92–1.50, p = 0.20).
In summary, there was no significant association observed between the CLEC3A polymorphisms and GC susceptibility in any comparison (Tab. 2).
Table 2: Association between CLEC3A rs2735401 T > G, rs2293776 C > G, and rs2072665 T > C polymorphisms and gastric cancer (GC) susceptibility
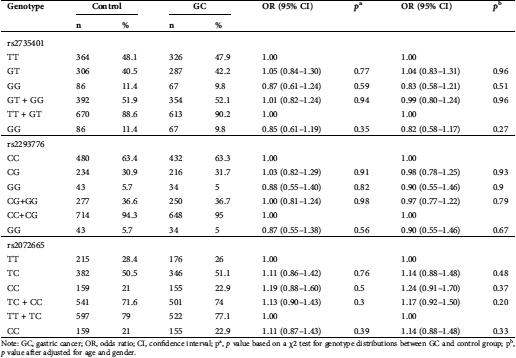
Note: GC, gastric cancer; OR, odds ratio; CI, confidence interval; pa, p value based on a χ2 test for genotype distributions between GC and control group; pb, p value after adjusted for age and gender.
Stratification analysis of the CLEC3A polymorphisms and GC risk
Because age and gender are reported to be the two major risk factors of gastric cancer, and this disease is reported to be more common in men over the age of 55 than in other groups (Billington, 1960; Christie et al., 1997; Silecchia et al., 2005). We further explored the association between the polymorphisms and GC risk in analyses stratified by age, gender, and clinical stages (Tab. 3). We found that regardless of CLEC3A rs2735401 T > G, rs2293776 C > G, or rs2072665 T > C, no significant associations were observed in older than 55 years or in those 55 years or younger. In addition, the three polymorphisms were not significantly associated with GC risk in either females or males. Finally, CG/GG genotypes were not associated with GC risk in patients at Stages I + II or Stages III + IV.
Table 3: Stratification analyses for the association between CLEC3A polymorphisms and gastric cancer (GC) susceptibility
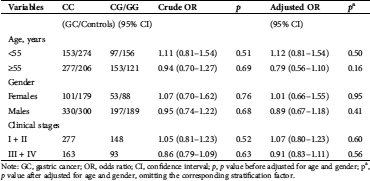
Note: GC, gastric cancer; OR, odds ratio; CI, confidence interval; p, p value before adjusted for age and gender; pa, p value after adjusted for age and gender, omitting the corresponding stratification factor.
In this first hospital-based case-control study, we investigated the association of the CLEC3A polymorphisms with the risk of gastric cancer in 681 patients and 756 healthy controls of Northwestern Chinese origin. We found that the CLEC3A genotypes not significantly increased the risk of gastric cancer in the Northwestern Chinese population. Overall, this study of its kind indicates that the CLEC3A gene polymorphisms may not be associated with GC susceptibility in the Northwestern Chinese population.
The incidence rate of GC ranks fifth, and its mortality remains third among all human cancers in both sexes in 2018 (Bray et al., 2018). The development of gastric cancer represents a complex interaction of infectious agents with environmental and host factors (Rawla and Barsouk, 2019; De Re et al., 2019). As surgical techniques improve and progress is made in traditional radiotherapy, chemotherapy, and the implementation of neoadjuvant therapy, the 5-year survival rate of early gastric cancer can reach >95% (Song et al., 2017). However, the low rate of early diagnosis means that most patients have advanced-stage disease at diagnosis and so the best surgical window is missed, and the 5-year survival rate is low (Wu et al., 2015).
SNPs are the most common type of genetic variation, which makes them excellent biological markers. On the other hand, SNPs, including those that fall within the coding or noncoding regions of genes, may affect the gene transcription and translation, as well as the structure and function of proteins, contributing to changing the host susceptibility to diseases (Liang et al., 2019). At present, studies indicate that gene polymorphisms were closely related to the susceptibility of gastric cancer, including XPG (Wang et al., 2019), MUC4 (Nabatchian et al., 2019), cyclooxygenase-2 (Chen et al., 2019), PSCA (Yan et al., 2019) and so on.
The C-type lectin domain family is classified into 17 subgroups, but the classification criteria are not consistent with regard to function, phylogenesis criteria, or gene structure (Zelensky and Gready, 2005). CLEC3A is a poorly characterized protein belonging to the superfamily of C-type lectins (Lau et al., 2018), and associated with an increased risk of a variety of multiple diseases (Karlsson et al., 2010; Elezagic et al., 2019; Wilson et al., 2016).
Previously, we have found no association of CLEC3A gene expression between cancer and normal tissue samples via immunohistochemical analysis (data not published). Therefore, we ought to explore the role of the CLEC3A polymorphisms via genetic analysis of patient samples. Since there are limited reports illustrating the CLEC3A polymorphisms rs2735401, rs2293776, and rs2072665 in cancer progression while regulating coagulation, we chose to analyze these polymorphisms in GC patient (Lau et al., 2018; Hua et al., 2019). In the present study, we genotyped 681 GC patients and 576 cancer-free controls from two different hospitals to evaluate the association between the CLEC3A gene polymorphisms and GC susceptibility. We found that the CLEC3A gene three polymorphisms were no significant association and GC susceptibility in any comparison. Moreover, although reports showed that age and gender are the two major risk factors of gastric cancer (Lu et al., 2012), our stratified analysis by age and gender did not modify the association between the three polymorphisms and the risk of gastric cancer, similar results were found in clinical stages.
Although our overall results suggest no association, it is important to consider that GC is a multifactorial disease resulting from multiplicative interactions between environmental factors and genetic backgrounds. Thus, a main limitation of this study is the lack of available information on some valuable parameters such as parental exposure, dietary intake, and living environment. Selection bias is another obvious potentially confounding factor, as the study population certainly is not representative of the whole Chinese population.
CLEC3A has been investigated in a few studies, limiting our discussion since we cannot compare our results with other ethnic groups. Thus, to better elucidate the role of the CLEC3A polymorphisms with GC susceptibility, future studies should as many as possible.
Our study represents the first case-control study conducted to date to explore the correlation between the CLEC3A gene polymorphisms and GC risk in the Northwestern Chinese population. We found no such risk, pointing to a need for further validation of this association in other populations. Moreover, further investigations of polymorphisms that might mediate the risk of GC would help gain a better understanding of the pathogenesis and improve prognosis in the face of the increasing incidence of gastric cancer. DECLARATIONS.
Acknowledgement: We would like to thank all the researchers and patients who had participated in this study.
Availability of Data and Materials:The data is available upon the request from the corresponding author.
Funding Statement: This study is supported by National Natural Science Foundation of China (81572916).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding this study.
Baroudi O, Benammar-Elgaaied A. (2016). Involvement of genetic factors and lifestyle on the occurrence of colorectal and gastric cancer. Critical Reviews in Oncology/Hematology 107: 72–81. DOI 10.1016/j.critrevonc.2016.08.014. [Google Scholar] [CrossRef]
Billington BP. (1960). Gastric ulcer: Age, sex, and a curious retrogression. Australasian Annals of Medicine 9: 111–121. DOI 10.1111/imj.1960.9.2.111. [Google Scholar] [CrossRef]
Boguslawska J, Rodzik K, Poplawski P, Kedzierska H, Rybicka B, Sokol E, Tanski Z, Riekielko-Witkowska A. (2018). TGF-β1 targets a microRNA network that regulates cellular adhesion and migration in renal cancer. Cancer Letters 412: 155–169. DOI 10.1016/j.canlet.2017.10.019. [Google Scholar] [CrossRef]
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 68: 394–424. DOI 10.3322/caac.21492. [Google Scholar] [CrossRef]
Chen S, Chen L, Tan Y, Wang J. (2019). Association between rs20417 polymorphism in cyclooxygenase-2 and gastric cancer susceptibility: Evidence from 15 case-control studies. Medicine 98: e15468. DOI 10.1097/MD.0000000000015468. [Google Scholar] [CrossRef]
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. (2016). Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians 66: 115–132. DOI 10.3322/caac.21338. [Google Scholar] [CrossRef]
Christie J, Shepherd NA, Codling BW, Valori RM. (1997). Gastric cancer below the age of 55: Implications for screening patients with uncomplicated dyspepsia. Gut 41: 513–517. DOI 10.1136/gut.41.4.513. [Google Scholar] [CrossRef]
De Re V, Repetto O, De Zorzi M, Casarotto M, Tedeschi M, Giuffrida P, Lenti MV, Magris R, Miolo G, Mazzon C, Zanette G, Alessandrini L, Canzaonieri V, Caggiari L, Zanussi S, Steffan A, Sabatino AD, Cannizzaro R. (2019). Polymorphism in toll-like receptors and Helicobacter pylori motility in autoimmune atrophic gastritis and gastric cancer. Cancers 11: 648. DOI 10.3390/cancers11050648. [Google Scholar] [CrossRef]
Duffy MJ, McGowan PM, Harbeck N, Thomssen C, Schmitt M. (2014). uPA and PAI-1 as biomarkers in breast cancer: Validated for clinical use in level-of- evidence-1 studies. Breast Cancer Research 16: 428. DOI 10.1186/s13058-014-0428-4. [Google Scholar] [CrossRef]
Elezagic D, Morgelin M, Hermes G, Hamprecht A, Sengle G, Lau D, Hollriegl S, Wagener R, Paulsson M, Streichert T, Klatt AR. (2019). Antimicrobial peptides derived from the cartilage-specific C- type Lectin Domain Family 3 Member A (CLEC3A) – potential in the prevention and treatment of septic arthritis. Osteoarthritis and Cartilage 27: 1564–1573. DOI 10.1016/j.joca.2019.06.007. [Google Scholar] [CrossRef]
Gjyshi O, Vashi P, Seewald L, Kohan M, Abboud E, Fowler E, Suppiah R, Halabi H. (2018). Therapeutic and prophylactic gastrectomy in a family with hereditary diffuse gastric cancer secondary to a CDH1 mutation: A case series. World Journal of Surgical Oncology 16: 143. DOI 10.1186/s12957-018-1415-5. [Google Scholar] [CrossRef]
Guruvaiah P, Govatati S, Reddy TV, Lomada D, Deenadayal M, Shivaji S, Bhanoori M. (2014). The VEGF +405 G>C 5' untranslated region polymorphism and risk of PCOS: A study in the South Indian Women. Journal of Assisted Reproduction and Genetics 31: 1383–1389. DOI 10.1007/s10815-014-0310-4. [Google Scholar] [CrossRef]
Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. (2009). Gastric cancer. Lancet 374: 477–490. DOI 10.1016/S0140-6736(09)60617-6. [Google Scholar] [CrossRef]
He J, Qiu LX, Wang MY, Hua RX, Zhang RX, Yu HP, Wang YN, Sun MH, Zhou XY, Yang YJ, Wang JC, Jin L, Wei QY, Li J. (2012). Polymorphisms in the XPG gene and risk of gastric cancer in Chinese populations. Human Genetics 7: 1235–1244. DOI 10.1007/s00439-012-1152-8. [Google Scholar] [CrossRef]
He J, Wang MY, Qiu LX, Zhu ML, Shi TY, Zhou XY, Sun MH, Yang YJ, Wang JC, Jin L, Wang YN, Li J, Yu HP, Wei QY. (2013). Genetic variations of mTORC1 genes and risk of gastric cancer in an Eastern Chinese population. Molecular Carcinogenesis 52: E70–E79. DOI 10.1002/mc.22013. [Google Scholar] [CrossRef]
He J, Zhuo ZJ, Zhang AQ, Zhu JH, Hua RX, Xue WQ, Zhang SD, Zhang JB, Li XZ, Jia WH. (2018). Genetic variants in the nucleotide excision repair pathway genes and gastric cancer susceptibility in a southern Chinese population. Cancer Management and Research 10: 765–774. DOI 10.2147/CMAR.S160080. [Google Scholar] [CrossRef]
Hua RX, Zhuo ZJ, Zhu JH, Zhang SD, Xue WQ, Li XZ, He J, Jia WH. (2019). LIG3 gene polymorphisms and risk of gastric cancer in a Southern Chinese population. Gene 705: 90–94. DOI 10.1016/j.gene.2019.04.072. [Google Scholar] [CrossRef]
Jin T, Wang Y, Li G, Du S, Yang H, Geng T, Hou P, Gong Y. (2015). Analysis of difference of association between polymorphisms in the XRCC5, RPA3 and RTEL1 genes and glioma, astrocytoma and glioblastoma. American Journal of Cancer Research 5: 2294–2300. [Google Scholar]
Karlsson C, Dehne T, Lindahl A, Brittberg M, Pruss A, Sittinger M, Ringe J. (2010). Genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Osteoarthritis and Cartilage 18: 581–592. DOI 10.1016/j.joca.2009.12.002. [Google Scholar] [CrossRef]
Lau D, Elezagic D, Hermes G, Morgelin M, Wohl AP, Koch M, Hartmann U, Hollriegl S, Wagener R, Paulsson M, Streichert T, Klatt AR. (2018). The cartilage- specific lectin C-type lectin domain family 3 member A (CLEC3A) enhances tissue plasminogen activator–mediated plasminogen activation. Journal of Biological Chemistry 293: 203–214. DOI 10.1074/jbc.M117.818930. [Google Scholar] [CrossRef]
Liang P, Zhang W, Wang W, Dai P, Wang Q, Yan W, Wang W, Lei X, Cui D, Yan Z. (2019). PLCE1 polymorphisms and risk of esophageal and gastric cancer in a Northwestern Chinese population. Biomed Research International 2019: 9765191. DOI 10.1155/2019/9765191. [Google Scholar] [CrossRef]
Lu R, Gao X, Chen Y, Ni J, Yu Y, Li S, Guo L. (2012). Association of an NFKB1 intron SNP (rs4648068) with gastric cancer patients in the Han Chinese population. BMC Gastroenterology 12: 87. DOI 10.1186/1471-230X-12-87. [Google Scholar] [CrossRef]
Maccormick TM, Carvalho CES, Bravo Neto GP, Carvalho MDGDC. (2019). Comparative analysis of glutathione transferase genetic polymorphism, Helicobacter pylori and Epstein-Barr virus between the tumor area and the proximal and distal resection margins of gastric cancer. Revista do Celegio Brasileiro de Cirurgioes 46: e2068. DOI 10.1590/0100-6991e-20192068. [Google Scholar] [CrossRef]
Nabatchian F, Rahimi Naiini M, Moradi A, Tabatabaeian H, Hoghoughi N, Azadeh M, Ghaedi K. (2019). miR-581-related single nucleotide polymorphism, rs2641726, located in MUC4 gene, is associated with gastric cancer incidence. Indian Journal of Clinical Biochemistry 34: 347–351. DOI 10.1007/s12291-018-0751-0. [Google Scholar] [CrossRef]
Neame PJ, Tapp H, Grimm DR. (1999). The cartilage-derived, C-type lectin (CLECSF1Structure of the gene and chromosomal location. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression 1446: 193–202. DOI 10.1016/S0167-4781(99)00087-1. [Google Scholar] [CrossRef]
Ni J, Peng Y, Yang FL, Xi X, Huang XW, He C. (2018). Overexpression of CLEC3A promotes tumor progression and poor prognosis in breast invasive ductal cancer. OncoTargets and Therapy 11: 3303–3312. DOI 10.2147/OTT.S161311. [Google Scholar] [CrossRef]
Rawla P, Barsouk A. (2019). Epidemiology of gastric cancer: Global trends, risk factors and prevention. Przeglad Gastroenterologiczny 14: 26–38. [Google Scholar]
Resmini G, Rizzo S, Franchin C, Zanin R, Penzo C, Pegoraro S, Piazza S, Arrigoni G, Sgarra R, Manfioletti G. (2017). HMGA1 regulates the Plasminogen activation system in the secretome of breast cancer cells. Scientific Reports 7: 11768. DOI 10.1038/s41598-017-11409-4. [Google Scholar] [CrossRef]
Rezaee F, Casetta B, Levels JH, Speijer D, Meijers JC. (2006). Proteomic analysis of high-density lipoprotein. Proteomics 6: 721–730. DOI 10.1002/pmic.200500191. [Google Scholar] [CrossRef]
Silecchia G, Greco F, Bacci V, Boru C, Pecchia A, Casella G, Rizzello M, Basso N. (2005). Results after laparoscopic adjustable gastric banding in patients over 55 years of age. Obesity Surgery 15: 351–356. DOI 10.1381/0960892053576622. [Google Scholar] [CrossRef]
Song Z, Wu Y, Yang J, Yang D, Fang X. (2017). Progress in the treatment of advanced gastric cancer. Tumor Biology 39: 1010428317714626. [Google Scholar]
Tahara T, Shibata T, Nakamura M, Yamashita H, Yoshioka D, Okubo M, Yonemura J, Maeda Y, Maruyama N, Kamano T, Kamiya Y, Fujita H, Nakagawa Y, Nagasaka M, Iwata M, Hirata I, Arisawa T. (2010). Association between IL-17A, -17F and MIF polymorphisms predispose to CpG island hyper-methylation in gastric cancer. International Journal of Molecular Medicine 25: 471–477. DOI 10.3892/ijmm_00000367. [Google Scholar] [CrossRef]
Tsunezumi J, Higashi S, Miyazaki K. (2009). Matrilysin (MMP-7) cleaves C-type lectin domain family 3 member A (CLEC3A) on tumor cell surface and modulates its cell adhesion activity. Journal of Cellular Biochemistry 106: 693–702. DOI 10.1002/jcb.22062. [Google Scholar] [CrossRef]
Wang XQ, Terry PD, Li Y, Zhang Y, Kou WJ, Wang MX. (2019). Association of XPG rs2094258 polymorphism with gastric cancer prognosis. World Journal of Gastroenterology 25: 5152–5161. DOI 10.3748/wjg.v25.i34.5152. [Google Scholar] [CrossRef]
Wilson R, Golub SB, Rowley L, Angelucci C, Karpievitch YV, Bateman JF, Fosang AJ. (2016). Novel elements of the chondrocyte stress response identified using an in vitro model of mouse cartilage degradation. Journal of Proteome Research 15: 1033–1050. DOI 10.1021/acs.jproteome.5b01115. [Google Scholar] [CrossRef]
Wu H, Wang W, Tong S, Wu C. (2015). Nucleostemin regulates proliferation and migration of gastric cancer and correlates with its malignancy. International Journal of Clinical and Experimental Medicine 8: 17634–17643. [Google Scholar]
Yan K, Wu K, Lin C, Jie Z. (2019). Impact of PSCA gene polymorphisms in modulating gastric cancer risk in the Chinese population. Bioscience Reports 39: BSR20181025. DOI 10.1042/BSR20181025. [Google Scholar] [CrossRef]
Zelensky AN, Gready JE. (2005). The C-type lectin-like domain superfamily. FEBS Journal 272: 6179– 6217. DOI 10.1111/j.1742-4658.2005.05031.x. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |