2021 45(1): 139-147
DOI:10.32604/biocell.2021.013277

www.techscience.com/journal/biocell
| BIOCELL 2021 45(1): 139-147 DOI:10.32604/biocell.2021.013277 |  www.techscience.com/journal/biocell |
Reversal of multidrug resistance and antitumor promoting activity of 3-oxo-6β-hydroxy- β-amyrin isolated from Pistacia integerrima
1Department of Chemistry, University of Swabi, Anbar, 23561, Khyber Pakhtunkhwa Pakistan
2Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah, 24211, Saudi Arabia
3State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing, 100029, China
4Chemical Departments, Faculty of Science, Northern Borders University, Arar, 91431, Saudi Arabia
5Department of Pharmacy, Southeast University, Banani-1213, Dhaka, Bangladesh
6Department of Global Medical Science, Wonju College of Medicine, Yonsei University, Seoul, South Korea
7Botany Department, Faculty of Science, Tanta University, Tanta, 31527, Egypt
8Institute of Chemical Sciences, University of Peshawar, Peshawar, 25120, Pakistan
9H.E.J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi, 75270, Pakistan
10University Institute of Diet and Nutritional Sciences, Faculty of Allied Health Sciences, The University of Lahore, Lahore, 54000, Pakistan
11Department of Medical Microbiology and Immunobiology, Faculty of Medicine, University of Szeged, Szeged, 6700, Hungary
12Department of Oto-Rhino-Laryngology and Head-Neck Surgery, Faculty of Medicine, University of Szeged, Szeged, 6700, Hungary
13Department of Pharmacy, Abdul Wali Khan University, Mardan, 23200, Pakistan
14Department of Chemistry, The University of Jordan, Amman, 11942, Jordan
15LCM Laboratory, Faculty of Sciences, Mohamed First University, Oujda, 60000, Morocco
16Institution of Health Science of Tahirah Al Baeti Bulukumba, South Sulawesi, 92352, Indonesia
17Bioinformatics and Medical Informatics Research Center, San Diego State University, San Diego, 92182, USA
*Address correspondence to: Abdur Rauf, mashaljcs@yahoo.com; Saud Bawazeer, ssbawazeer@uqu.edu.sa
Received: 31 July 2020; Accepted: 28 September 2020
Abstract: The bioactive triterpenoid 3-oxo-6-β-hydroxy-β-amyrin (1) has been isolated from multiple plant sources. In this study, chloroform fraction of Pistacia integerrima extract was processed for the isolation of the compound. The compound identity was confirmed by advanced spectroscopy technique. X-ray crystallography was applied for molecular structure confirmation. In addition, compound 1 was screen for its activity on reversal of MDR (multidrug resistance) mediated by P-gp (P-glycoprotein). This was accomplished by using rhodamine123 exclusion on multidrug-resistant human ABCB1 gene transfected mouse T-lymphoma cell line. Outcomes revealed that MDR reversing effect was comparable to verapamil as positive control in vitro. Treatment of TPA-induced tumor promotion with 3-oxo-6β-hydroxy- β-amyrin led to reduction in the applied anti-tumor promotion experiment. The chemo-preventive effect of 3-oxo-6β-hydroxy- β-amyrin was comparable to curcumin as positive control based on the reduction of immediate early tumor antigen expression. Molecular docking by applying Autodock Vina 1 and i-GEMDOCK v 2.1 tools indicated that compound 1 gives good docking results, as determined by their fitness score and specificity. Moreover, results showed that compound 1 isolated from Pistacia integerrima precisely attached to a region where co-crystallized ligand for receptor previously existed. Our findings may explain the use of Pistacia integerrima plant extracts as an anticancer agent in folk medicine.
Keywords: Pistacia integerrima; Anti-tumor properties; X-ray crystallography; POM; Molecular docking
The most important difficulty in chemotherapy and in the cure of cancer are the resistance pattern of cancer cells knowns as multidrug resistance (MDR) (Szabó and Molnar, 2016). The resistance of Cancer cells to anticancer agents is developed through several mechanisms. One of such mechanisms is the overexpression of the ATP-binding cassette (ABC) transporters. These transporters are actually largest family of proteins that are bound to the membrane and bind with ATP (Leonard et al., 2003; Gottesman and Ambudkar, 2001). In energy dependent manner, ABC efflux transporters extrude amphipathic compounds against the concentration gradient. A number of ATP-binding cassette transporters play a physiological role, i.e., protection of liver, kidney and brain (Gottesman and Ambudkar, 2001; Sarkadi et al., 2006; Szakács et al., 2006).
P-glycoprotein is the first known drug efflux encoded with the gene ABCB1. This protein consists of 1,280 amino acids and two transmembrane domains (Szakács et al., 2006). Additionally, in several human cancers the overexpression of this protein occurs and can eject a wide-ranging of drugs, i.e., antibiotics, anticancer, antidepressants, and others (Caraci et al., 2011). So, it decreases drug accumulation in multidrug-resistant cells. Some drugs have been proposed to suppress the activity of ABCB1, e.g., tamoxifen, dexniguldipine, valspodar and tariquidar (Lopez and Martinez-Luis, 2014; Germann et al., 1993). In carcinogenesis studies, assessment of EBV-EA inhibition is employed as preliminary screening model for in-vitro antitumor enhancing potential of different chemo-preventive agents. EBV is a herpes family virus capable of several cancers (such as gastric cancer, nasopharyngeal carcinoma, Hodgkin’s lymphoma, among others). Presence of antibody to the EA of EBV indicates that EBV is active, replicating and capable of cancerous transformation. So, any inhibitor of the EA is considered to have anticancer effect (Kapadia et al., 2000).
MRP1 (multidrug-resistant protein-1) was initially described in doxorubicin-resistant lung cancer cells. Without expression of ABCB1, it displays a multi-drug resistant phenotype (Cole et al., 1992). MRP1 shows overexpression in intestines, blood-brain barrier, & oral mucosa (He et al., 2011); however, among the organ in lung its expressed higher and this may have a protective role in air contamination and toxin enter through inhalation (Sakamoto et al., 2013). The physiological substrates of multidrug-resistant protein-1 are reported to be glutathione conjugates leukotriene C4, bile acid, and folic acid. It showed resistance against, methotrexate, vincristine, and etoposide doxorubicin (Cole and Deeley, 2006). Approximately 31.6% of lung tumors have been identified to have MRP1 expression and showed weak response to cisplatin therapy with paclitaxel, gemcitabine and vinorelbine (Li et al., 2009).
Furthermore, Doyle et al., (1998) first cloned the BCRP (Breast Cancer Resistance Protein) through MCF-7 (drug-resistant breast cancer cell line). Breast cancer resistance protein with a size of 72 kDa is a half transporter member of the ATP-binding cassette transporter subfamily G (ABCG2). Owing to its presence in tissues including liver, placenta, ovary, colon, small intestine, prostate gland, and brain, the expression of BCRP overlaps largely with ABCB1 (Doyle et al., 1998). Similarly, it was noted that overexpression is often linked with the resistance of large number of anticancer compound, i.e., mitoxantrone, antifolates, flavopiridol camptothecins and anthracyclines (Assaraf, 2006; Bihorel et al., 2007; Robey et al., 2007). A number of publications have dealt with the large occurrences of drug efflux mechanism in cancer region. Number of literature described significant relation overexpression of MRP1 or ABCB1 and improper treatment response in leukemia & solid tumors (Larkin et al., 2004; Damiani et al., 2006; Brinkhuis et al., 2002), whereas others literature have also cited a prognostic relationship for BCRP overexpressions (Nampoothiri et al., 2008).
Pistacia integerrima is a well-known member of family Anacardiaceae family, which is commonly known as Kakar sigghi in Eastern Himalayan (Ismail et al., 2011), at an altitude of 2.4 to 3.6 km. This is a medium sized deciduous plant with folkloric health modulating importance for a number of ailments. Previous studies have indicated that this plant has anti-inflammatory, expectorant, blood purifier, gastroprotective, anti-asthmatic, antidiarrheal properties (Uddin et al., 2011; Ahmad et al., 2010). Particularly, the gall of P. integerrima tree has been traditionally used to cure diarrhea, asthma, psoriasis, fever, liver disorders, and oxidative stress, etc. (Uddin et al., 2012a; Uddin et al., 2012b; Ullah et al., 2014). The barks of P. integerrima are also used in the folkloric system for the treatment of cough, asthma, fever, diarrhea, snake bite as well as jaundice (Rahman et al., 2011). Amyrin type of terpenes has been reported for significant anticancer and cytotoxic activity (Wen et al., 2018; Mishra et al., 2016). 3-oxo-6β-hydroxy-β-amyrin is a bioactive amyrin type of triterpenoids which has documented for significant β-secretase, α-glucosidases activity (Bawazeer et al., 2020a). 3-oxo-6β-hydroxy-β-amyrin has also documented for anti-inflammatory, muscle relaxation, gastrointestinal, and anti-pyretic potential (Rauf et al., 2016a; Bawazeer et al., 2020a). In continuation of our phytochemical and pharmacological investigations of P. integerrima extracts, we have isolated the triterpenoid 3-oxo-6 β-hydroxy- β-amyrin (1), also β-sitosterol and stigmasterol from the chloroform fraction of the plant extract. This compound has been identified as 3-oxo-6β-hydroxy- β-amyrin based on 2-D NMR and single X-ray crystallography techniques. Accordingly, the current finding was performed to discover the reversal ability of the isolated compound for multidrug-resistance in mouse lymphoma cells.
All the chemicals as well reagents used in this screening test were commercially grade. Melting points were calculated through Bicote (Bibby Scientific limited, UK) melting point apparatus. We recorded FT-IR spectra as KBr disks, from 400 to 4000 cm–1, on Nicolet 380 FT-IR spectrophotometer (Thermo Scientific, UK), and UV-Vis spectra, 200 to 700 nm, were acquired with the aid of a Hitachi-U-3200 (Japan) instrument with chloroform solutions (1-cm cell). 1H-NMR (500 MHz), 13C-NMR (125 MHz), HMBC (500 MHz), and HSQC (600 MHz) spectra were acquired with a NMR spectrometer (AVANCEIII AV600) equipped with a Cryoprobe in solvent (CDCl3) and employed with TMS (internal standard). Chemical shifts were reported as ppm (parts per million) and expressed as δ units, while the values for J (coupling constant) were expressed in Hz (Hertz). In order to obtain EI-mass spectral data mass spectrometer (JMS-HX-110; JEOL) was employed; EI source 70 eV. Colum chromatography was performed with the help of Merck silica gel-60 having dimensions as 0.063 to 0.200 mm, whereas aluminum TLC-plates in which silica gel was pre-coated with F254 (fluorescence indicator) was supplied by Fluka.
The bark of Pistacia integerrima was procured from village Razagram, KPK, Pakistan (Feb, 2010). Identification and authentication were done by a botanist, Prof. Dr. Abdur Rashid, University of Peshawar (UOP), Pakistan. The voucher specimen (Bot. 20037(PUP) was submitted to herbarium at the Department of Botany, UOP, Pakistan.
After collection, the P. integerrima bark was dried under shade at 25°C followed by grinding to form uniform powdery sample. This powder was then subjected to methanol for extraction purposes (Bawazeer, 2020b). The extraction was repeated three times. Solvents were evaporated from the methanolic extracts, under reduced pressure provided a syrupy liquid (400 g). The syrup liquid substance was sequentially fractioned between butanol-water, chloroform-water, n-hexane-water, and ethyl acetate-water. Afterwards, chloroform (CHCl3) fraction was concentrated by rotary evaporation using anhydrous Na2SO4 and resultant residue obtained weighed 98.6 g. A small quantity (10 gram) of CHCl3 extract was processed through column chromatography (silica gel based) and was initially eluted using n-hexane followed by elution with mixture of n-hexane and ethyl acetate solvent system in an increasing polarity manner. Ten fractions (F-1 to F-10) of the eluted liquid were collected. On TLC profiling, the fraction F-3 (60 mg; eluted with n-hexane-ethyl acetate, 82:18, v/v) resulted colorless crystals (compound 1). Later by decantation process, these crystals were separated from the solution. Finally, compound 1 was identified and characterized as 3-oxo-6β-hydroxy-β-amyrin by different spectroscopic techniques such as 1H-NMR, 13C-NMR, IR, and mass spectral data, the data was compared to literature and found identical (Wang et al., 2005).
In-vitro EVA-EA (Epstein-Barr-Virus early antigen) activation induction assay
Compound 1, 3-oxo-6β-hydroxy- β-amyrin was evaluated in vitro against EBV-EA, activation assay. Concisely, Raji cells (lymphoblastoid cells) derived from Burkitt’s lymphoma carried by EBV genome. These cells were cultured using RPMI-1640 medium provided 10% fetal bovine serum (FBS). Using an indirect immunofluorescence technique, smears from cell suspension were made (Kapadia et al., 2000; Wang et al., 2006). EBV-EA activation was less than 0.1% in experimented subline of Raji cells. For 48 hours, cells were incubated in medium (1 mL) containing butanoic acid (4 mM), TPA (12-O-tetradecanoylphorbol-13-acetate) [32 pM = 20 ng in 2 µL dimethyl sulfoxide (DMSO)] and test compounds (various amount) dissolved in 2 µL of DMSO. By indirect immunofluorescence assay the EBV-EA-inducing cells were stained. A minimum of 500 cells were calculated for each assay, and number of positive (stained cells) were noted. EBV-EA induction (average) of experimented biomolecule (compound-1) was determined in ratio relative to the control experiment (100%), processed with butanoic acid (4 mM) and 12-O-tetradecanoylphorbol-13-acetate (32 pM). Around 35% was calculated the EBV-EA induction. By using trypan blue staining method, viability of treated Raji cells was noted where viability of cells for TPA-positive control was >80%. So, just those compounds showing induction <80% (% of control) of the EBV-active cells (having cell viability >60%) were capable of inhibiting the activation due to promoter substances. Curcumin was used as a standard compound (Wang et al., 2006; Zhang et al., 2013).
The following procedure was applied on mouse lymphoma cells for determination of reversal MDR of compound 1. Medium (McCoy’s 5A) consists of 10% inactivated (by heat) horse serum provided with antibiotics and L-glutamine was employed to grow L5178 and L5178Y MDR cell lines. As mentioned earlier by Rauf et al. (2015b), the L5178 mouse T-cell lymphoma parent cells were stably infected with pHa MDR1/A retrovirus. The L5178Y (MDR1-expressing cell line) was selected by culturing the transfected cells with colchicine containing medium. Then, Cells were distributed using aliquots (0.5 mL) into Eppendorf tubes after being adjusted to a density of 2 × 106 cells per mL and suspended in the McCoy’s 5A medium (serum-free). Final concentrations (4 μg/Ml) of test compounds and positive control (verapamil) were used. Verapamil was used as a positive control as it is a chemosensitizer & Ca+2 channel blocker that inhibits multidrug resistance gene product and therefore reduces MDR1-mediated drug resistance. At room temperature, incubation of samples were carried out for 10 minutes prior to use. Then, added 10 µL of rhodamine-123, (5.20 µM) in samples to employed as an indicator. Cells were further incubated for 20 minutes and washed (two-times), and re-suspended in Phosphate buffered solution (0.5 mL) for examination. With the aid of a flow cytometer i.e., Partec CyFlow (Germany) we measured the fluorescence of cell population, using DMSO as the solvent as well as control. Using this technique, percent FI (Fluorescence Intensity) was measured for treated parental and MDR-cell lines in comparison to untreated cells. On the basis of FI values, fluorescence activity ratio (FAR) was calculated using the following equations (Rauf et al., 2016b).
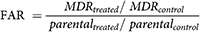
Protein data bank (PDB) was used for the retrieval purposes of crystalline structure of mice P-gp (coded as: 4Q9L) (Sussman et al., 1998). The 3-D structure of P-gp was subjected to an energy minimization process with the aid of a program known as Swiss-PDB viewer (version 4.1.0) (Guex and Peitsch, 1997). For docking studies, Avogardro’s software & Chem sketch was used for the preparation of ligands structures (Hanwell et al., 2012). Similarly, i-GEMDOCK (version 2.1) & Autodock Vina were used for docking purposes (Trott and Olson, 2010; Hsu et al., 2011). For optimization of docking method co-crystallized inhibitor of P-glycoprotein was used (Shityakov and Förster, 2014). Two software namely LIGPLOT plus (version 1.4.5) and discovery studio visualizer was used subsequently for docking analysis (Rauf et al., 2015b; Azam et al., 2013).
Characterization of compound 1
Compound 1, was purified as white crystals. Spectral data employed that the chemical structure of 1 (Fig. 1) has been identified as 3-oxo-6β-hydroxy-β-amyrin. For confirmation, single X-ray crystallography was carried out, which confirmed the structure (Fig. 2).
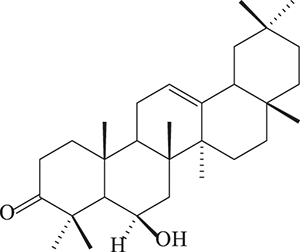
Figure 1: The structure of compound 1 (3-oxo-6β -hydroxy-β-amyrin).
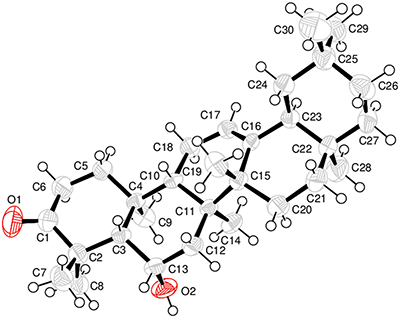
Figure 2: X-ray crystallographic image of compound (1).
Effect in EVA-EA (Epstein-Barr-Virus early antigen) activation induction assay
Tab. 1 presents the results of the EVB-EA activation induction assay. Our findings reveal that compound 1 caused significant antitumor promotion activity at various test concentrations with an IC50 value of 458 µg/mL. However, the standard compound, curcumin was more potent with IC50 of 340 µg/mL. Viability rate of the Raji cells shown by tested compound at a concentration of 1000 (mol ratio/32 pmol TPA) was 60%. Additionally, its effect on MDR mouse lymphoma cell line was also evaluated, results of which are displayed in Tab. 3. These results show that compound 1 exhibits a remarkable effect on MDR-mouse lymphoma cells. Therefore, compound 1 may have a possible chemopreventive effect.
Table 1: Percent Inhibitory Effects on the induction of Epstein-Barr Virus Early Antigen (EBV-EA) by standard compound (curcumin) and isolated compound 1 (3-oxo-6β-hydroxy-β-amyrin)

TPA (32 pmol) = 100%. Negative cont. 0%, Values represent the relative percentage to the positive control, with TPA (32 pmol, 20 ng) representing 100% induction at four different concentrations in terms of molar ratio/32 pmol TPA. Data are expressed as mean ±S.D (n = 3). IC50 values represent the mole ratio of compounds, relative to TPA, required to inhibit 50% of the positive control activated with 32 pmol TPA. Values in parentheses are percentage viability of Raji cells. (In all other experiments, viability was >80%.)
Reversal of MDR in mouse lymphoma cells effects
3-oxo-6-β-Hydroxy-β-amyrin (1) was also assessed for its properties on the reversion of multi-drug resistance mediated by P-gp using rhodamine-123 exclusion study on MDR human ABCB1 gene-transfected mouse T-lymphoma cell line. In vitro results revealed that MDR reversing effect was comparable to verapamil as positive control. The short time experiment exhibited that isolated compound 1 was an active MDR-modulator. Verapamil-a chemosensitizer & Ca2+ channel blocker-acted as a positive control in current experimentation (Tab. 2).
Table 2: Effect of 3-oxo-6β-hydroxy-β-amyrin on reversal of multidrug resistance in MDR mouse lymphoma cells
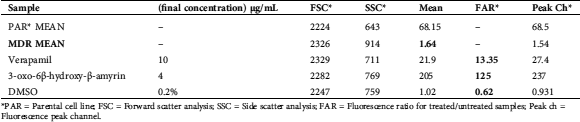
*PAR = Parental cell line; FSC = Forward scatter analysis; SSC = Side scatter analysis; FAR = Fluorescence ratio for treated/untreated samples; Peak ch = Fluorescence peak channel.
Petra/Osiris/Molinspiration (POM) Analyses of compound 1
Presented in Figs. 3 and 4 are the results pertaining to molecular properties predicted for compound 1 such as TPSA, GPCR (G-protein-coupled receptors), ligand and ICM (a protein modeling and design method). Results reveal that compound 1 has limited violation (NV (Number of Violations) = 1) of five rules of Lipinski. In conclusion, compound 1 has potential bioactivity as Nuclear Receptor Ligand and Enzyme Inhibitor (NRL = 0.68 and EI = 0.63 respectively). The bioactivity scores of compound 1 were found to be in accordance with the standard scores of the standard drugs (Tab. 3).
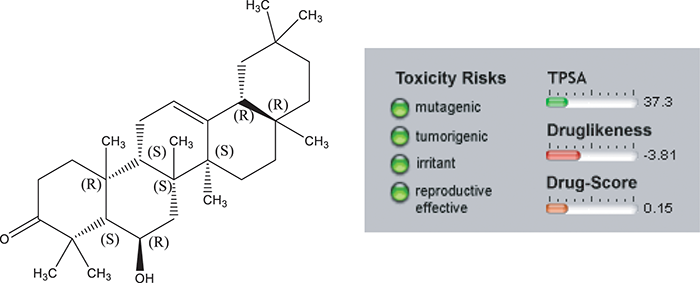
Figure 3: Osiris calculations of drug likeness of compound 1. Toxicity Risks ( : not toxic,
: not toxic,  : slightly toxic,
: slightly toxic,  : highly toxic). Molecular Weight (M.W. < 500 g/mole) is in perfect accordance with Lipinski 5 rules but cLogP > 5.
: highly toxic). Molecular Weight (M.W. < 500 g/mole) is in perfect accordance with Lipinski 5 rules but cLogP > 5.
Our results confirm that free energies of the studied compound are a bit higher than those of Rhodamine123 (Tab. 3). This implies that certain structural features of 3-oxo-6β-hydroxy- β-amyrin may be the reason to its inhibitory properties on P-glycoprotein from mice. Moreover the predicated docked orientation of compound; 3-oxo-6β-hydroxy-β-amyrin shown by sticks red color, the co-crystallized ligand of the receptor is shown by the cyan color while the standard compound Rhodamine 123 is shown by green color in the binding site of P-gp (Fig. 4). Furthermore, as depicted in Fig. 5, docking interactions of 3-oxo-6β-hydroxy-β-amyrin indicate hydrophobic interactions (total: 08) and no hydrogen bonding. The hydrophobic bondings reported come from residues Met68, Leu64, Phe332, Ile336, Gln343, Met945, Tyr949, and Met982. These hydrophobic contacts of 3-oxo-6β-hydroxy- β-amyrin are responsible for its binding capacity to the P-gp receptor.
Table 3: Molinspiration calculations of Bioactivity Scores (BS) of compound 1. [a] Compound 1 has potential bioactivity as Nuclear receptor ligand and enzyme inhibitor (NRL = 0.68 and 0.63 respectively)
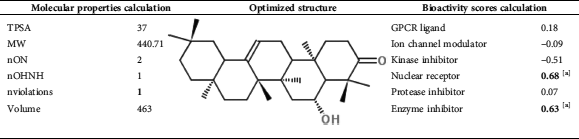
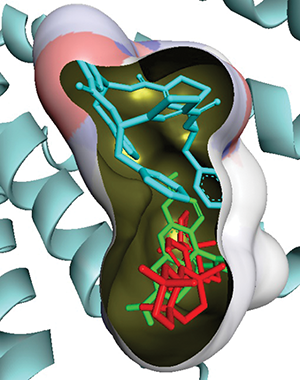
Figure 4: Predicted docked orientation of compound 3-oxo-6β-hydroxy-β-amyrin shown by sticks red color, the co-crystallized ligand of the receptor is shown by the cyan color while the standard compound Rhodamine 123 is shown by green color in the binding site of P-gp.
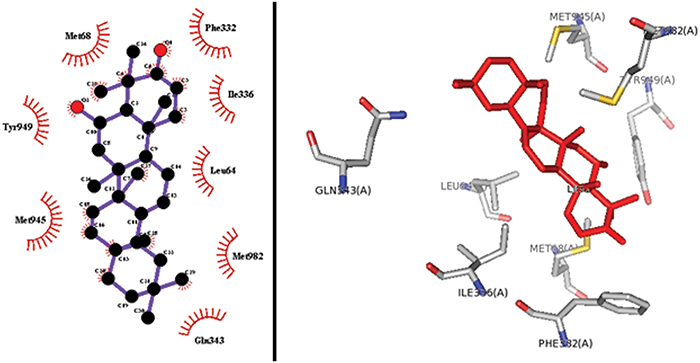
Figure 5: 2-D (left) and 3-D (right) interaction of 3-oxo-6 β-hydroxy- β-amyrin in the binding site of P-glycoprotein.
In-silico screening has been proven to be a vital tool for discovering new inhibitors against receptors. Purposely, we conducted docking studies to recognize the inhibiting potential of 3-oxo-6β-hydroxy- β-amyrin with the P-gp. Results from docking analysis revealed that 3-oxo-6β-hydroxy- β-amyrin gives good docking results on both docking software. As displayed in Figs. 4 and 5, the results showed that compound 1 isolated from Pistacia integerrima precisely attached to a region where co-crystallized ligand for receptor was previously existing. In-silico screening predicts that lesser the free energy (local energy minimization) more effective is the docking score and higher is the activity of compound (Sliwoski et al., 2014).
Cell efflux-pump, including ATP-binding cassette transporters, may be considered as enzyme having varied substrate specificity. Inhibition of ATP-binding cassette transporters may be regarded as a potential opportunity in order to overcome multidrug resistance (MDR). MDR cancer cells may be modulated by administering conventional chemo-therapeutics along with resistance modifiers. For this purpose, numerous natural bioactive biomolecules and synthetic metabolites have been investigated to inhibit the efflux-pump activity (Barath et al., 2006). In this study, FAR (fluorescence activity ratio) value was determined to assess the modulating potential of 3-oxo-6β-hydroxy-β-amyrin on ABCB1 transporter. In flow cytometry, FSC and SSC value enhanced revealing that compound 1 increased the granulation of cytoplasm. Results of FAR value showed that 3-oxo-6β-hydroxy-β-amyrin was an effective MDR-modulator. As shown in Tab. 2, compound 1 exhibited significantly modulated the efflux pump activity (FAR: 125, 4 µg/ml). Similar results were obtained in study conducted on Pistagremic acid (PA), a triterpenoid, present in Pistacia integerrima on reversal of MDR (Rauf et al., 2016c). They concluded that there might be certain important chemical features of Pistagremic acid responsible for its inhibitory potential of P-glycoprotein (P-gp). Likewise, reversal of MDR mediated by P-gp in mouse Lymphoma Cells due to crude extract and two (dihydrokaempferol & naringenin) isolated compounds from Pistacia integerrima has also been experimented by Rauf et al. (2016b). They demonstrated that crude extract, dihydrokaempferol & naringenin were promising modulators of efflux pump activity (FAR = 64.02, 1.58, & 1.79; 4 µg/ml).
The octanol/water partition coefficient, or best known as cLogP, is calculated by the molecular properties mining and quality assurance software package Molinspiration and Osiris (Open Source Independent Review and Interpretation System Background), respectively, as a sum of fragment contributions & correction factors (Husain et al., 2016; Jarrahpour et al., 2010). This procedure can be practically applied to all organic and organometallic based molecules. In addition, the method published by Ertl et al. (2000) is employed in the calculation of the total molecular polar surface area (TPSA). This quantity is simply the sum of fragment contributions; O- and N- centered polar fragments. Furthermore, TPSA characterizes intestinal drug absorption, permeability of Caco-2, bioavailability and penetration of blood-brain barrier.
The rule of five (Ro5) is based on the physicochemical profiles (distribution, absorption, excretion and metabolism) of synthetic drugs, which among other criteria, deals with target and ligand alignment, bioavailability, etc. (Murugan et al., 2015). Natural products do not generally follow the Ro5, due to their intra-molecular hydrogen bonding and easy metabolizibility (Zhang and Wilkinson, 2007).
In summary, findings from this investigation suggest that bark of P. integerrima contain 3-oxo-6β-hydroxy-β-amyrin; which exhibits anticancer activities ranging from cancer chemoprevention to reversal of multidrug resistance of cancer cells. The findings may explain the medicinal use of P. integerrima as an medicinal plant with anticancer effects. However, more detailed research are needed to establish the safety and efficacy of the isolated compound.
Acknowledgement: The authors are highly thankful to the Higher Education commission, Pakistan for funding this research group No. NRPU649.
Availability of Data and Materials: The data such as spectra of compounds 1 associated material used to support the research of this finding are available from corresponding authors upon request.
Funding Statement: This research was funded by Higher Education commission, Pakistan (HEC) (Grant No. NRPU649).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ahmad NS, Waheed A, Farman M, Qayyum A. (2010). Analgesic and anti-inflammatory effects of Pistacia integerrima extracts in mice. Journal of Ethnopharmacology 129: 250–253. DOI 10.1016/j.jep.2010.03.017. [Google Scholar] [CrossRef]
Assaraf YG. (2006). The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resistance Updates 9: 227–246. DOI 10.1016/j.drup.2006.09.001. [Google Scholar] [CrossRef]
Azam F, Abugrain IM, Sanalla MH, Elnaas RF, Rajab IAI. (2013). In silico investigation of the structural requirements for the AMPA receptor antagonism by quinoxaline derivatives. Bioinformation 9: 864–869. DOI 10.6026/97320630009864. [Google Scholar] [CrossRef]
Barath Z, Radics R, Spengler G, Ocsovszki I, Kawase M, Motohashi N, Shirataki Y, Shah A, Molnar J. (2006). Multidrug resistance reversal by 3-formylchromones in human colon cancer and human MDR1 gene-transfected mouse lymphoma cells. In Vivo 20: 645–649. [Google Scholar]
Bawazeer S, Rauf A, Bawazeer S (2020a). Potent in vitro α-Glucosidase and β-secretase inhibition of amyrin type triterpenoid isolated from Datura metel Linnaeus (Angel’s trumpet) fruits. BioMed Research International 2020: 1–5. Article ID 8530165. DOI 10.1155/2020/8530165. [Google Scholar] [CrossRef]
Bawazeer S (2020b). Gastrointestinal motility, muscle relaxation, antipyretic and acute toxicity screening of amyrin type triterpenoid (daturaolone) isolated from Datura metel Linnaeus (Angel’s trumpet) fruits. Frontier in Pharmacology 11: 383. DOI 10.3389/fphar.2020.544794. [Google Scholar] [CrossRef]
Bihorel S, Camenisch G, Lemaire M, Scherrmann JM. (2007). Modulation of the brain distribution of imatinib and its metabolites in mice by valspodar, zosuquidar and elacridar. Pharmaceutical Research 24: 1720–1728. DOI 10.1007/s11095-007-9278-4. [Google Scholar] [CrossRef]
Brinkhuis M, Izquierdo MA, Baak J, Van Diest PJ, Kenemans P, Scheffer GL, Scheper RJ. (2002). Expression of multidrug resistance-associated markers, their relation to quantitative pathologic tumour characteristics and prognosis in advanced ovarian cancer. Analytical Cellular Pathology 24: 17–23. DOI 10.1155/2002/958436. [Google Scholar] [CrossRef]
Caraci F, Crupi R, Drago F, Spina E. (2011). Metabolic drug interactions between antidepressants and anticancer drugs: Focus on selective serotonin reuptake inhibitors and hypericum extract. Current Drug Metabolism 12: 570–577. DOI 10.2174/138920011795713706. [Google Scholar] [CrossRef]
Cole SPC, Deeley RG. (2006). Transport of glutathione and glutathione conjugates by MRP1. Trends in Pharmacological Sciences 27: 438–446. DOI 10.1016/j.tips.2006.06.008. [Google Scholar] [CrossRef]
Cole SPC, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan A, Deeley RG. (1992). Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258: 1650–1654. DOI 10.1126/science.1360704. [Google Scholar] [CrossRef]
Damiani D, Tiribelli M, Calistri E, Geromin A, Chiarvesio A, Michelutti A, Cavallin M, Fanin R. (2006). The prognostic value of P-glycoprotein (ABCB) and breast cancer resistance protein (ABCG2) in adults with de novo acute myeloid leukemia with normal karyotype. Haematologica 91: 825–828. [Google Scholar]
Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. (1998). A multidrug resistance transporter from human MCF-7 breast cancer cells. Proceedings of the National Academy of Sciences 95: 15665–15670. DOI 10.1073/pnas.95.26.15665. [Google Scholar] [CrossRef]
Ertl P, Rohde B, Selzer P. (2000). Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. Journal of Medicinal Chemistry 43: 3714–3717. DOI 10.1021/jm000942e. [Google Scholar] [CrossRef]
Germann UA, Pastan I, Gottesman MM. (1993). P-glycoproteins: Mediators of multidrug resistance. Seminars in Cell Biology 4: 63–76. DOI 10.1006/scel.1993.1008. [Google Scholar] [CrossRef]
Gottesman MM, Ambudkar SV. (2001). Overview: ABC transporters and human disease. Journal of Bioenergetics and Biomembranes 33: 453–458. DOI 10.1023/A:1012866803188. [Google Scholar] [CrossRef]
Guex N, Peitsch MC. (1997). Swiss-Model and the Swiss-Pdb viewer: An environment for comparative protein modeling. Electrophoresis 18: 2714–2723. DOI 10.1002/elps.1150181505. [Google Scholar] [CrossRef]
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. (2012). Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. Journal of Cheminformatics 4: 17. DOI 10.1186/1758-2946-4-17. [Google Scholar] [CrossRef]
He SM, Li R, Kanwar R, Zhou J, SF. (2011). Structural and functional properties of human multidrug resistance protein 1 (MRP1/ABCC1). Current Medicinal Chemistry 18: 439–481. DOI 10.2174/092986711794839197. [Google Scholar] [CrossRef]
Hsu KC, Chen YF, Lin SR. (2011). iGEMDOCK: A graphical environment of enhancing GEMDOCK using pharmacological interactions and post-screening analysis. BMC Bioinformatics 12: S33. DOI 10.1186/1471-2105-12-S1-S33. [Google Scholar] [CrossRef]
Husain A, Ahmad A, Khan SA, Asif M, Bhutani R, Al-Abbasi FA. (2016). Synthesis, molecular properties, toxicity and biological evaluation of some new substituted imidazolidine derivatives in search of potent anti-inflammatory agents. Saudi Pharmaceutical Journal 24: 104–114. DOI 10.1016/j.jsps.2015.02.008. [Google Scholar] [CrossRef]
Ismail M, Muhammad N, Mohani N, Khan MA, Hussain J. (2011). Pharmacognostic and phytochemical investigation of the stem bark of Pistacia integerrima Stew ex Brandis. Journal of Medicinal Plants Research 5: 3891–3895. [Google Scholar]
Jarrahpour A, Motamedifar M, Zarei M, Youssoufi MH, Mimouni M, Chohan ZH, Hadda TB. (2010). Petra, osiris, and molinspiration together as a guide in drug design: Predictions and correlation structure/antibacterial activity relationships of new N-sulfonyl monocyclic β-lactams. Phosphorus, Sulfur, and Silicon 185: 491–497. DOI 10.1080/10426500902953953. [Google Scholar] [CrossRef]
Kapadia GJ, Azuine MA, Takayasu J, Konoshima T, Takasaki M, Nishino H, Tokuda H. (2000). Inhibition of Epstein-Barr Virus early antigen activation promoted by 12-O-tetradecanoylphorbol-13-acetate by the non-steroidal anti-inflammatory drugs. Cancer Letters 161: 221–229. DOI 10.1016/S0304-3835(00)00616-9. [Google Scholar] [CrossRef]
Larkin A, O'Driscoll L, Kennedy S, Purcell R, Moran E, Crown J, Parkinson M, Clynes M. (2004). Investigation of MRP-1 protein and MDR-1 P-glycoprotein expression in invasive breast cancer: A prognostic study. International Journal of Cancer 112: 286–294. DOI 10.1002/ijc.20369. [Google Scholar] [CrossRef]
Leonard GD, Fojo T, Bates SE. (2003). The role of ABC transporters in clinical practice. Oncology 8: 411–424. [Google Scholar]
Li J, Li Z, LI Q, Bao Q, Chen P (2009). Expression of MRP1, BCRP, LRP, and ERCC1 in advanced non–small-cell lung cancer: Correlation with response to chemotherapy and survival. Clinical Lug Cancer 10: 414–421. [Google Scholar]
Lopez D, Martinez-Luis S. (2014). Marine natural products with P-glycoprotein inhibitor properties. Marine Drugs 12: 525–546. DOI 10.3390/md12010525. [Google Scholar] [CrossRef]
Mishra T, Arya RK, Meena S, Joshi P, Pal M, Meena B, Upreti DK, Rana TS, Datta D. (2016). Isolation, characterization and anticancer potential of cytotoxic triterpenes from Betula utilis Bark. PLoS One 11: e0159430. DOI 10.1371/journal.pone.0159430. [Google Scholar] [CrossRef]
Murugan K, Sangeetha S, Ranjitha S, Vimala A, Al-Sohaibani S, Rameshkumar G. (2015). HDACiDB: A database for histone deacetylase inhibitors. Drug Design, Development and Therapy 9: 2257–2264. DOI 10.2147/DDDT.S78276. [Google Scholar] [CrossRef]
Nampoothiri KM, Rubex R, Patel AK, Narayanan SS, Krishna S, Das SM, Pandey A. (2008). Molecular cloning, overexpression and biochemical characterization of hypothetical β-lactamases of Mycobacterium tuberculosis H37Rv. Journal of Applied Microbiology 105: 59–67. DOI 10.1111/j.1365-2672.2007.03721.x. [Google Scholar] [CrossRef]
Rahman SU, Ismail M, Muhammad N, Ali F, Chishti KA, Imran M. (2011). Evaluation of the stem bark of Pistacia integerrima Stew ex Brandis for its antimicrobial and phytotoxic activities. African Journal of Pharmacy and Pharmacology 5: 1170–1174. [Google Scholar]
Rauf A, Maione F, Uddin G, Raza M, Siddiqui BS, Muhammad N, Khan H, Shah SUA, Feo VD, Mascolo N (2016a). Biological evaluation and docking analysis of daturaolone as potential cyclooxygenase inhibitor. Evidence-Based Complementary and Alternative Medicine 2016: 1–7. Article ID 4098686. DOI 10.1155/2016/4098686. [Google Scholar] [CrossRef]
Rauf A, Saleem M, Uddin G, Siddiqui BS, Khan H, Raza M, De Feo V (2015a). Phosphodiesterase-1 inhibitory activity of two flavonoids isolated from Pistacia integerrima J. L. Stewart Galls. Evidence-Based Complementary and Alternative Medicine, 506564. Article ID 50656, DOI 10.1155/2015/506564. [Google Scholar] [CrossRef]
Rauf A, Uddin G, Raza M, Ahmad B, Jehan N, Siddiqui BS, Szabo D (2016b). Reversal of multidrug resistance in mouse lymphoma cells by extracts and flavonoids from Pistacia integerrima. Asian Pacific Journal of Cancer Prevention 17: 51–55. DOI 10.7314/APJCP.2016.17.1.51. [Google Scholar] [CrossRef]
Rauf A, Uddin G, Raza M, Ahmad A, Jehan N, Ahmad B, Nisar M, Molnar J, Csonka A, Szabo D, Khan A (2016c). Reversal of multidrug resistance and computational studies of pistagremic acid isolated from Pistacia integerrima. Asian Pacific Journal of Cancer Prevention 17: 2311–2314. DOI 10.7314/APJCP.2016.17.4.2311. [Google Scholar] [CrossRef]
Rauf A, Uddin G, Siddiqui BS, Molnár J, Csonka Á., Ahmad B, Khan AA (2015b). Rare class of new dimeric naphthoquinones from diospyros lotus have multidrug reversal and antiproliferative effects. Frontiers in Pharmacology 6: 293. DOI 10.3389/fphar.2015.00293. [Google Scholar] [CrossRef]
Robey RW, Polgar O, Deeken J, To KW, Bates SE. (2007). ABCG2: Determining its relevance in clinical drug resistance. Cancer and Metastasis Reviews 26: 39–57. DOI 10.1007/s10555-007-9042-6. [Google Scholar] [CrossRef]
Sakamoto A, Matsumaru T, Yamamura N, Uchida Y, Tachikawa M, Ohtsuki S, Terasaki T. (2013). Quantitative expression of human drug transporter proteins in lung tissues: Analysis of regional, gender, and interindividual differences by liquid chromatography-tandem mass spectrometry. Journal of Pharmaceutical Sciences 102: 3395–3406. DOI 10.1002/jps.23606. [Google Scholar] [CrossRef]
Sarkadi B, Homolya L, Szakács G, Váradi A. (2006). Human multidrug resistance ABCB and ABCG transporters: Participation in a chemoimmunity defense system. Physiological Reviews 86: 1179–1236. DOI 10.1152/physrev.00037.2005. [Google Scholar] [CrossRef]
Shityakov S, Förster C. (2014). In silico structure-based screening of versatile P-glycoprotein inhibitors using polynomial empirical scoring functions. Advances and Applications in Bioinformatics and Chemistry 7: 1–9. DOI 10.2147/AABC.S56046. [Google Scholar] [CrossRef]
Sliwoski G, Kothiwale S, Meiler J, Lowe E. (2014). Computational methods in drug discovery. Pharmacological Reviews 66: 334–395. DOI 10.1124/pr.112.007336. [Google Scholar] [CrossRef]
Sussman JL, Lin D, Jiang J, Manning NO, Prilusky J, Ritter O, Abola EE. (1998). Protein Data Bank (PDBDatabase of three-dimensional structural information of biological macromolecules. Acta Crystallographica Section D: Biological Crystallography 54: 1078–1084. DOI 10.1107/S0907444998009378. [Google Scholar] [CrossRef]
Szabó D, Molnar J. (2016). The role of stereoselectivity of chemosensitizers in the reversal of multidrug resistance of mouse lymphoma cells. Anticancer Research 18: 3039–3044. [Google Scholar]
Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. (2006). Targeting multidrug resistance in cancer. Nature Reviews Drug Discovery 5: 219–234. DOI 10.1038/nrd1984. [Google Scholar] [CrossRef]
Trott O, Olson AJ. (2010). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry 31: 455–461. [Google Scholar]
Uddin G, Rauf A, Arfan M, Waliullah KI, Ali M, Taimur M, Ur-Rehman I, Samiullah (2012a). Pistagremic acid a new leishmanicidal triterpene isolated from Pistacia integerrima Stewart. Journal of Enzyme Inhibition and Medicinal Chemistry 27: 646–848. [Google Scholar]
Uddin G, Rauf A, Al-Othman AM, Collina S, Arfan M, Ali G, Khan I. (2012b). Pistagremic acid, a glucosidase inhibitor from Pistacia integerrima. Fitoterapia 83: 1648–1652. DOI 10.1016/j.fitote.2012.09.017. [Google Scholar] [CrossRef]
Uddin G, Rauf A, Rehman TU, Qaisar M. (2011). Phytochemical screening of Pistacia chinensis var. integerrima. Middle-East Journal of Scientific Research 7: 707–711. [Google Scholar]
Ullah H, Rauf A, Ullah Z, Anwar M, Uddin G, Ayub K. (2014). Density functional theory and phytochemical study of Pistagremic acid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 118: 210–214. DOI 10.1016/j.saa.2013.08.099. [Google Scholar] [CrossRef]
Wang K, Sun H, Wu B, Pan Y. (2005). Two novel olean triterpenoids from celastrus hypoleucus. Helvetica Chimica Acta 88: 990–995. DOI 10.1002/hlca.200590094. [Google Scholar] [CrossRef]
Wang X, Nakagawa-Goto K, Kozuka M, Tokuda H, Nishino H, Lee KH. (2006). Cancer preventive agents. Part 6: Chemopreventive potential of furanocoumarins and related compounds. Pharmaceutical Biology 44: 116–120. DOI 10.1080/13880200600592178. [Google Scholar] [CrossRef]
Wen S, Gu D, Zeng H. (2018). Antitumor effects of beta-amyrin in Hep-G2 liver carcinoma cells are mediated via apoptosis induction, cell cycle disruption and activation of JNK and P38 signalling pathways. JBUON 23: 965–970. [Google Scholar]
Zhang MQ, Wilkinson B. (2007). Drug discovery beyond the rule-of-five. Current Opinion in Biotechnology 18: 478–488. DOI 10.1016/j.copbio.2007.10.005. [Google Scholar] [CrossRef]
Zhang J, Nishimoto Y, Tokuda H, Suzuki N, Yasukawa K, Kitdamrongtham W, Akihisa T. (2013). Cancer chemopreventive effect of bergenin from Peltophorum pterocarpum wood. Chemistry & Biodiversity 10: 1866–1875. DOI 10.1002/cbdv.201300182. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |