DOI:10.32604/biocell.2021.014305

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.014305 |  www.techscience.com/journal/biocell |
| Article |
Protective effects of docosahexaenoic acid against non-alcoholic hepatic steatosis through activating of JAK2/STAT3 signaling pathway
1College of Animal Science and Technology, Nanjing Agricultural University, Nanjing, 210095, China
2Nanjing XinRuize Agricultural Biotechnology Co., Ltd., Nanjing, 210095, China
3Jiangsu Key Laboratory for Food Quality and Safety-State Key Laboratory Cultivation Base, Ministry of Science and Technology, Jiangsu Academy of Agricultural Sciences, Nanjing, 210014, China
4College of Pharmacy, Shaanxi University of Chinese Medicine, Xianyang, 712046, China
*Address correspondence to: Huixia Li, lihuixia@njau.edu.cn; Yan Quan, quanyan1@sohu.com
Received: 16 September 2020; Accepted: 24 November 2020
#These authors contributed equally
Abstract: Non-alcoholic fatty liver disease is the most common cause of hepatic dysfunction. In the present study, human normal hepatocyte L02 cells were treated with 50% fetal bovine serum to induce the formation of hepatic steatosis in vitro, and then the cells were treated with docosahexaenoic acid to investigate its protective effect on Non-alcoholic fatty liver disease. Our results showed that 50% of fetal bovine serum significantly induced intracellular lipid accumulation and hepatocyte fatty degeneration within 48 h. The expression level of adipose formation-related genes was significantly up-regulated, such as PPARγ, C/EBPα and SREBP-1; meanwhile, the content of cellular total lipid, total cholesterol and triglycerides were significantly increased after 50% fetal bovine serum treatment. Interestingly, docosahexaenoic acid treatment could inhibit FBS-induced intracellular lipid accumulation in L02 cells and the expression of lipogenic genes. Moreover, docosahexaenoic acid treatment could reduce hepatic steatosis-induced oxidative stress and endoplasmic reticulum stress response, and these responses were shown by the modification of antioxidant enzyme activities and GRP78, CHOP expression. In addition, the results showed that docosahexaenoic acid can activate the JAK2/STAT3 signaling pathway in fatty liver L02 cell; inhibition of JAK2/STAT3 signaling pathway by WP1066 abolished the beneficial effects of docosahexaenoic acid on hepatic steatosis accompanied with the increased expression of lipogenic genes and endoplasmic reticulum stress response. Above all, the present study showed that docosahexaenoic acid can alleviate non-alcoholic hepatic steatosis by activating JAK2/STAT3 signaling pathway.
Keywords: Docosahexaenoic acid; NAFLD; L02 cell; Lipid accumulation; JAK2/STAT3
With the development of the economy and the improvement of life quality, the prevalence of various kinds of “disease of affluence” such as obesity and diabetes has increased rapidly; the non-alcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease. It is estimated that 24–42% of the population in Western countries and 5–42% in Asian countries are affected by (Targher et al., 2016; Zheng et al., 2016). The NAFLD is a common liver disease closely linked with the features of metabolic syndrome; the main characteristic of NAFLD is the accumulation of fat in liver cells in the absence of excessive alcohol intake (Merola et al., 2015; Smith and Adams, 2011). The accumulated-lipid droplets led to a liver more sensitive to inflammatory cytokines, oxidative stress, endoplasmic reticulum (ER), or mitochondrial dysfunction, which further induce the development of nonalcoholic steatohepatitis (NASH) (Lim et al., 2010; Luo et al., 2020). However, there is no validated drug therapy at present. The lifestyle interventions designed to reduce body weight remain the first-line treatments, such as diet and exercise, but several studies demonstrated that lifestyle interventions cannot improve the histological features of NAFLD, but only the metabolic parameters and simple steatosis (Nobili et al., 2008; Vilar-Gomez et al., 2015; Zohrer et al., 2017).
The endoplasmic reticulum (ER) is an important organelle that is responsible for proper and posttranslational modification of proteins, lipid synthesis, and calcium storage. ER stress, which was caused by the accumulation of unfolded protein and calcium depletion, contributed to unfolded protein response (UPR) and the occurrence of diseases. Hepatocytes contain a large amount of ER to synthesize plasma protein, secrete low-density lipoprotein and metabolize xenobiotics. Therefore, excessive ER stress usually caused the dysfunction of ER and liver diseases, such as NAFLD or liver fibrosis (Rutkowski and Kaufman, 2004). GRP78, ATF4, and SREBP-1C, three markers UPR, which are highly expressed under ER stress, might be involved in the formation and development of NAFLD (Lewis and Mohanty, 2010; Zhang et al., 2011; Yamamoto et al., 2010).
Docosahexaenoic acid (DHA) is the major polyunsaturated fatty acids (PUFA) found in marine fish oil, which is the essential fatty acid of mammals. DHA is the main component of the phospholipid of the cell membrane and cannot be synthesized in the body (Horrocks and Yeo, 1999). Previous studies found that PUFA can regulate lipid metabolization in many kinds of animals (Khan et al., 2002; Peyron-Caso et al., 2003). When NAFLD patients were supplemented with PUFA for 6 months, the alanine aminotransferase and triglyceride levels in the liver were significantly decreased, and the symptoms of NAFLD were also relieved (Spadaro et al., 2008). In addition, DHA also improved insulin sensitivity by regulating lipid-related gene expression and ameliorated hepatic triglycerides accumulation in NAFLD mice (Fedor et al., 2012; Sun et al., 2011). Thereby, supplementation of DHA had a potential therapeutic effect on lipogenesis, fatty acid oxidation, and hepatic lipid metabolism (De Castro et al., 2015; Zhang et al., 2013); it can also attenuate fatty liver-caused damage by inhibiting endoplasmic reticulum stress response (Zheng et al., 2016). However, the possible protective mechanisms of DHA on NAFLD are still not clear.
Janus kinase 2 -signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway are involved in many physiological and pathological regulation processes, such as inflammation and apoptosis. Activation of JAK2/STAT3 signaling pathway induced the expression of HMGB1 and inflammatory reaction, which is associated with the NAFLD; rapamycin can inhibit JAK2/STAT3 signaling pathway to reduce HMGB1 expression, which attenuated the liver injury (Zeng et al., 2014). In the present study, we investigate whether DHA could have a protective effect on non-alcoholic hepatic steatosis, and it is unclear whether the beneficial effect of DHA on NAFLD is regulated by JAK2/STAT3 signaling pathway. Our result demonstrated that supplementation of DHA could attenuate lipid droplets-caused oxidative stress and ER stress response in L02 cells through activating the JAK2/STAT3 signaling pathway.
Human normal liver cell line (L02 cell), fetal bovine serum (FBS), Dulbecco minimum essential medium (DMEM), penicillin-streptomycin were purchased from KeyGEN Bio TECH (Nanjing, Jiangsu, China), Docosahexaenoic acid (cat: 6217-54-5) was purchased from Tocris Cookson Ltd (USA), and dissolved in anhydrous alcohol. Real-time quantitative PCR reagents were purchased from Ambion (Austin, TX, USA). Triglycerides (TG) (cat: A110-1), aspartate aminotransferase (AST) (cat: C010-2), lactate dehydrogenase (LDH) (A020-2), total cholesterol (TC) (cat: A111-1), malondialdehyde (MDA) (cat: A003-4), glutathione peroxidase (GSH-Px) (cat: A005) and total superoxide dismutase (SOD) (cat: A001-1-1) kits were purchased from Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). PrimeScriptTM RT Master Mix (cat: RR036A) and SYBR® Premix Ex Taq™ (cat: RR420A) were purchased from TaKaRa Biotechnology. C/EBPα (cat: sc-9314) and PPARγ (cat: sc-1981) antibodies were purchased from Santa Cruz Biotechnology (Beijing, China), a-tubulin (cat: 11H10), P-JAK2 (cat: TYR1007/1008), P-STAT3 (cat: D3A7), and STAT3 (cat: 79D7) antibodies were purchased from Cell Signal, JAK2 (cat: 1001-1015) antibody was purchased from Sigma Aldrich (Shanghai, China), GRP78 (cat: 11587-1-AP), GADPH (cat: 60004-1-lg) and CHOP (cat: 15204-1-AP) antibodies were purchased from Proteintech (Wuhan, China).
Cell culture and DHA treatment
L02 cells were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 IU/mL penicillin, 100 µg/mL streptomycin) in 5% CO2 at 37°C. After the culture reached 80% confluence, cells were seeded in multi-well plates or flasks for 24 h. The cells were then divided into three groups: Control group, FBS-treated groups (24, 48, and 72 h), and DHA-treated group (25 μM). According to previous studies, 50% FBS was able to induce non-alcoholic hepatic steatosis in cell model (Cui et al., 2017; Wu et al., 2010), so the control group cells were cultured in DMEM, and FBS-treated group cells were cultured in 50% FBS medium for 24, 48, and 72 h to induce steatosis. As for the DHA-treated group, the cells first were cultured in 50% FBS medium for 48 h and then cultured in DMEM medium with DHA (25 µM) for 24 h.
The accumulation of lipid droplet in L02 cells were visualized and analyzed by Oil Red O staining (Fu et al., 2016). In brief, the cells are grown in 6-wells plates. After treatment, the cells were washed twice with PBS, and then the cells were fixed with 2 mL of 4% (v/v) formaldehyde for 30 min. Then the cells were stained with 0.5 mL of freshly prepared oil red O solution for 30 min at 37°C. After that, the cells were treated with 70% alcohol and observed under a light microscope.
Determination of the contents of total cholesterol, triglycerides and the activities of aspartate aminotransferase and lactate dehydrogenase
The cells were collected and sonicated on ice. The cell lysates were clarified by centrifugation. Then the total cholesterol (TC), triglyceride (TG), and the enzymatic activities of aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were measured by corresponding assay kit according to the instructions (Nanjing Jiancheng Bioengineering Institute, China).
Measurement of anti-oxidative enzyme activates
Malondialdehyde (MDA), glutathione peroxidase (GSH-Px), and total superoxide dismutase (SOD) activities were measured separately by MDA, GSH-Px, and SOD assay kits (Nanjing Jiancheng Bioengineering Institute, China). The cells were collected and placed in a centrifuge tube for the following experiments. For MDA assay: after adding 500 μL lysis buffer into the cells for 2 min, the cell extraction (100 μL) was mixed with working buffer and incubated at 95°C for 40 min. Then the mixture was centrifuged at 4000 rpm for 10 min, 250 μL of supernatant was added into a 96-well plate, and the absorbance value was measured at 530 nm. MDA content was calculated based on the absorbance value. For GSH-Px assay: After adding the lysis buffer into the tubule, the cell extraction was centrifuged at 3000 rpm for 10 min, and the supernatant was used for measuring GSH-Px. The absorbance value was examined at 412 nm. For SOD assay: the supernatant of cell extraction was added into a 96-well plate and incubated at 37°C for 20 min, then the absorbance value at 450 nm was determined by using Full wavelength marker.
Total RNA extraction and qRT-PCR analysis
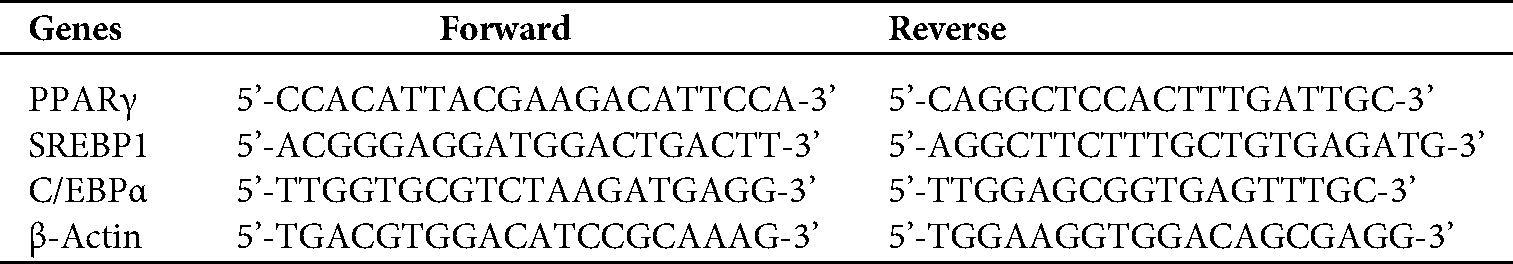
The total RNA was extracted using Trizol reagent, and then reverse transcription was performed using a PrimeScriptTM RT Master Mix. The mRNA expression was quantified using qRT-PCR. The expression levels of all target genes were normalized to those of the endogenous reference gene β-actin using an optimized comparative Ct (2−ΔΔCt) value method, where ΔΔCt = ΔCttarget−ΔCtβ-actin. Primer sequences are listed in Tab. 1.
Table 1: Primer sequences of mRNA
Protein extraction and Western blot analysis
To extract total protein, cells were treated with lysis buffer and centrifuged for 15 min at 4°C. The protein concentration was quantified using the BC protein assay kit. The Western blot procedures were performed as previously described (Chen et al., 2018). The following primary antibodies were used: Goat monoclonal anti-PPARγ, C/EBPα and SREBP-1 (1:500), P-JAK2, JAK2, P-STAT3, STAT3, GRP78, CHOP, GADPH (1:1000) and α-tubulin (1:2500).
All experimental data were expressed as the means ± SEM. Statistical comparisons were made by analysis of variance (ANOVA), followed by Tukey’s multiple comparisons test. Statistical significance was shown as *p < 0.05, **p < 0.01.
Establishment of hepatic steatosis model
Human normal hepatocyte L02 cells were treated with 50% FBS to induce hepatic steatosis, then the cells were stained with Oil Red O to detect the formation of lipid droplets. As shown in Fig. 1, after 50% FBS treatment, there are many small lipid drops were formed in the luminal side of L02 cells (Figs. 1A–1D). Then, the lipid droplets were extracted with isopropyl alcohol, and the absorbance of the extracted solution was measured by a microplate reader at 490 nm. The results showed that intracellular fat content was significantly increased after 50% FBS treatment for 48 and 72 h in L02 liver cells (Fig. 1E).
To further examine the effect of 50% FBS on liver cell steatosis, the intracellular lipogenesis-related indexes TG, TC, and hepatic function-related indexes AST and LDH were examined. The results showed that the contents of TG and TC were significantly increased after 50% FBS treatment compared with the control group in L02 cells (p < 0.01) (Figs. 1F–1G). At the same time, 50% FBS treatment also increased the activity of AST and LDH (Figs. 1H–1I), suggesting that the metabolic function has a defect in 50% FBS-treated L02 liver cells. Above all, the accumulation of lipid droplets and the defect of cell metabolism indicated that the hepatic steatosis model was successfully established after high concentration of FBS treatment.
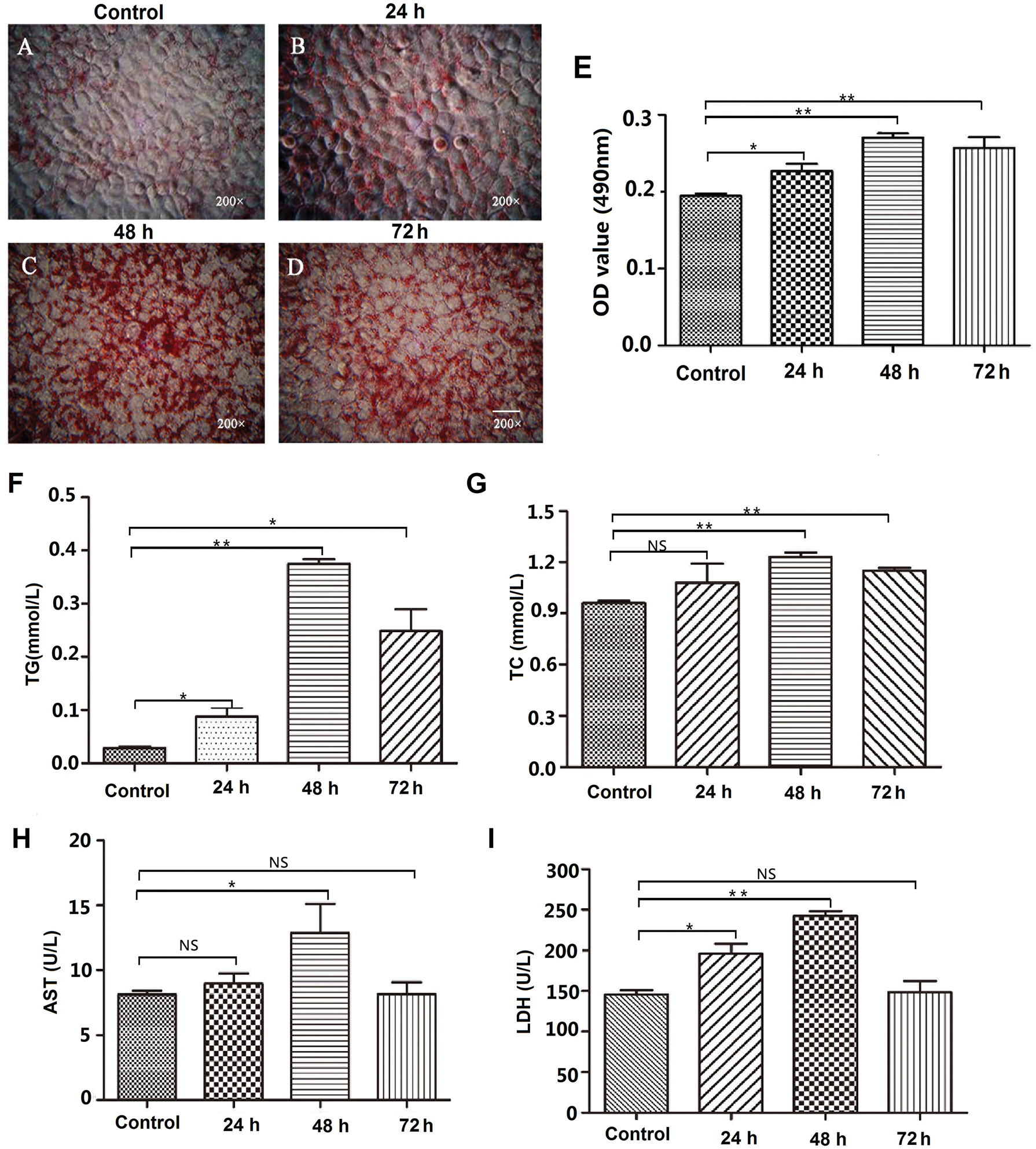
Figure 1: Establishment of the hepatic steatosis model in L02 cells.
Effect of DHA on lipid accumulation in FBS-induced hepatic steatosis
To detect the effect of DHA on non-alcoholic hepatic steatosis, we first examine its effect on lipid droplet formation in L02 cells. Our result showed that the lipid accumulation was significantly inhibited in DHA-treated cells (Figs. 2A–2C); meanwhile, the absorbance of the extracted solution was also significantly decreased after DHA treatment compared with the group of only 50% FBS treatment (Fig. 2D). These results clearly demonstrated that DHA can diminish the accumulation of lipid droplets, which is accompanied by the decrease of TC and TG contents (Figs. 2E, 2F).
To further examine the effect of DHA on hepatic functions, the activities of AST and LDH were measured after DHA treatment. As shown in Figs. 2G, 2H, 50% FBS treatment can significantly increase the activity of AST and LDH compared with the control group. However, the activities of AST and LDH were significantly decreased after DHA treatment (p < 0.05).
To further investigate the mechanism of DHA in lipid droplet formation in NAFLD, the lipogenic genes PPARγ, C/EBPα, and SREBP-1 were examined by qRT-PCR and Western blot. The results showed that the mRNA level and protein expression of PPARγ, C/EBPα, and SREBP-1 were significantly up-regulated in 50% FBS-treated L02 cells; whereas after DHA treatment, it can significantly down-regulated the mRNA level and protein expression of PPARγ, C/EBPα, and SREBP-1 (Figs. 2I–2M).
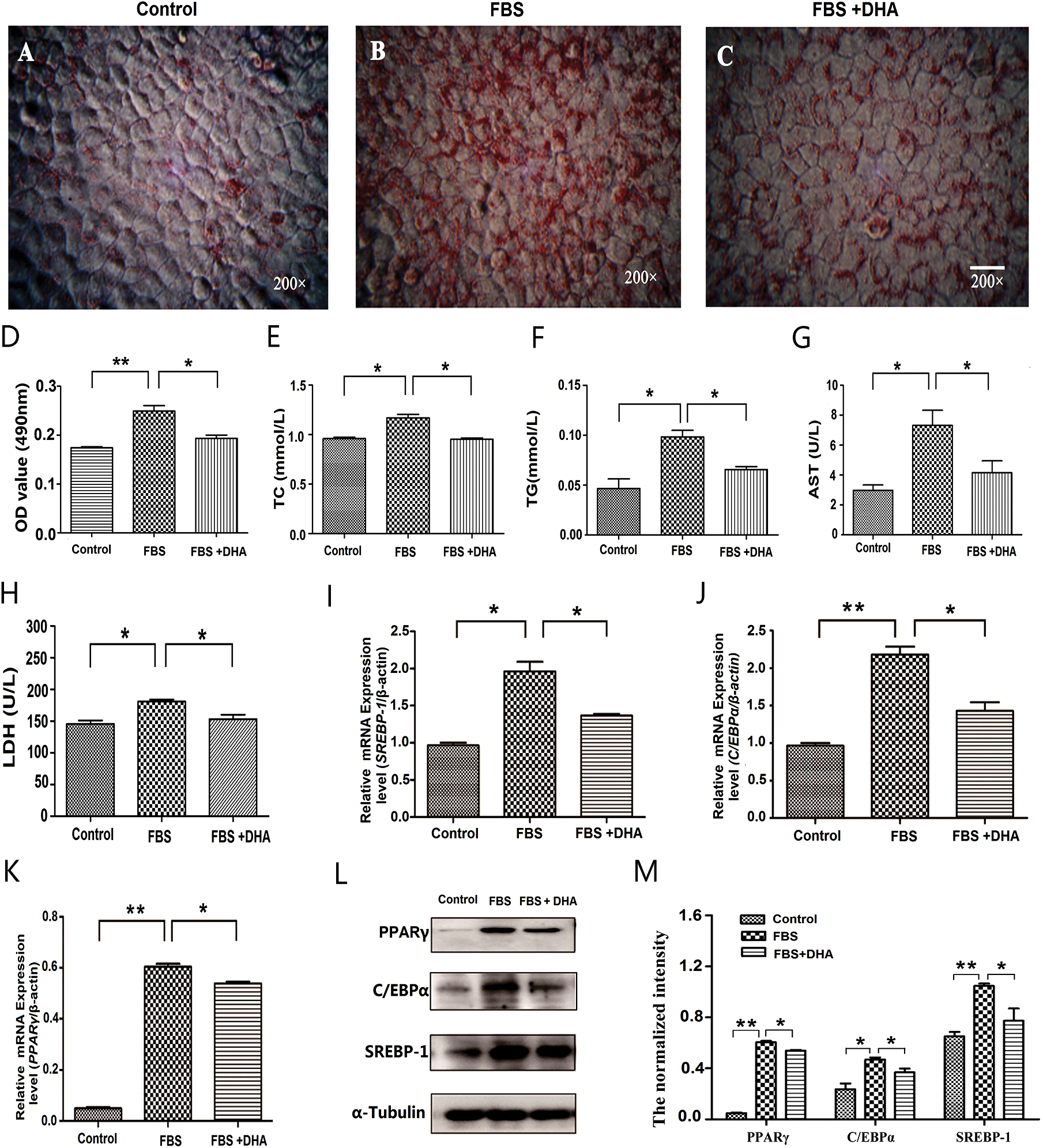
Figure 2: Effect of DHA on lipid accumulation in FBS-induced hepatic steatosis.
Effects of DHA on oxidative stress and ER stress response in FBS-induced hepatic steatosis
To further investigate the effect of DHA on hepatic steatosis, the levels of oxidative stress were analyzed. The cells were incubated with 50% FBS and were cultured with DHA (25 μM) medium for 24 h, then the activity of oxidative stress-related enzymes was measured. Our results showed that the contents of GSH-Px and SOD were significantly reduced in 50% FBS-treated L02 liver cells; whereas after DHA treatment, the contents of SOD and GSH-Px were significantly increased (Figs. 3A, 3B). In contrast, DHA can relieve the activity of MDA in FBS-induced hepatic steatosis (Fig. 3C). These results suggested that DHA can prevent the accumulation of oxidative stress products in fatty liver cells.
Glucose-regulated protein 78 (GRP78) and homologous protein (CHOP) are the protein markers of ER stress-induced unfolded protein response. Thereby, we next examined the effect of DHA on the hepatic steatosis-induced ER stress response. As shown in Figs. 3D, 3E, the protein level of GRP78 and CHOP were significantly increased in 50% FBS-treated L02 liver cells; however, DHA treatment can significantly alleviate FBS-induced ER stress by down-regulating GRP78 and CHOP in L02 cells, indicating that DHA could attenuate the hepatic steatosis-induced ER stress response.
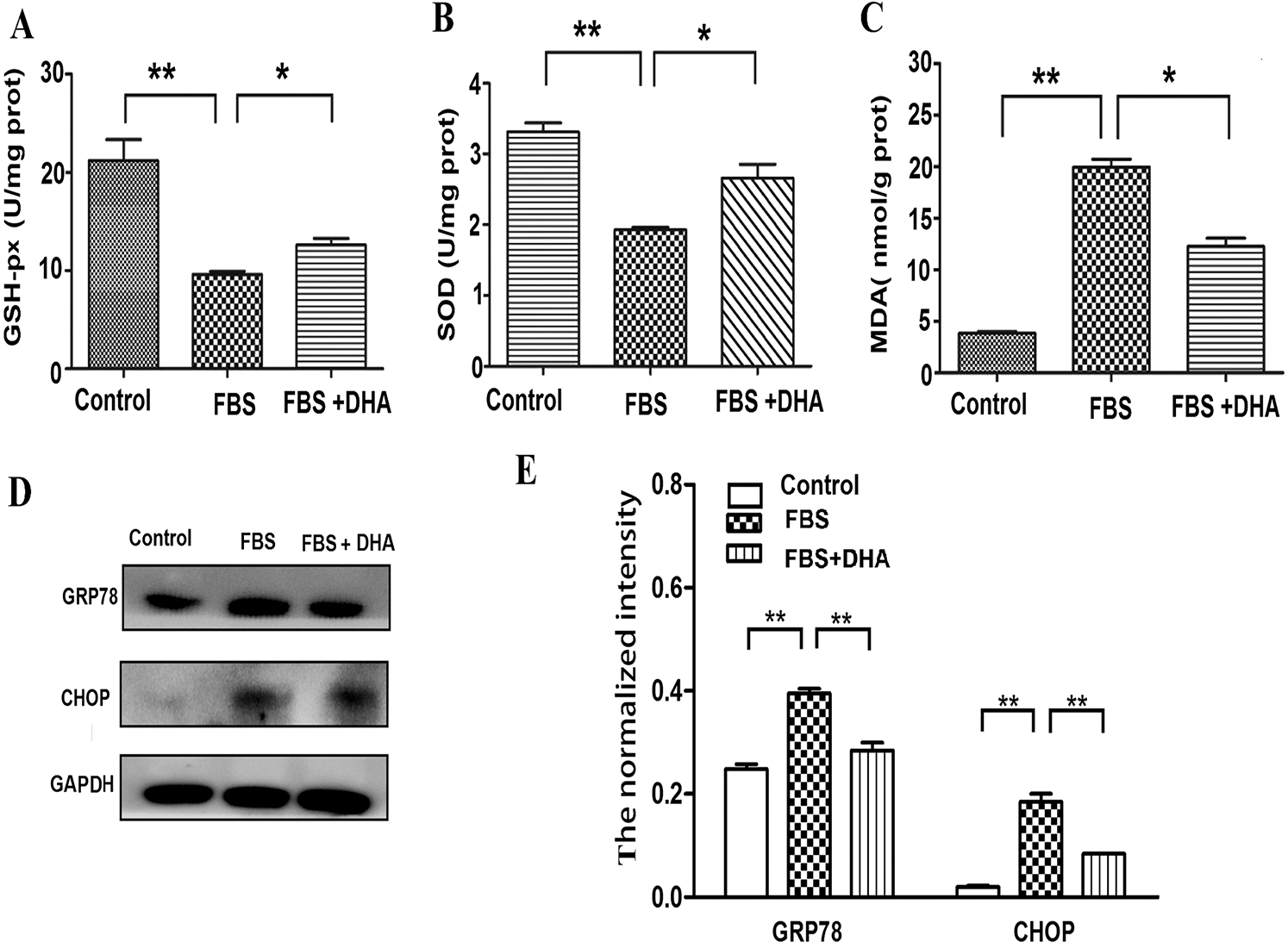
Figure 3: Effects of DHA on oxidative stress and ER-stress response in FBS-induced hepatic steatosis.
DHA attenuate hepatic steatosis through mediating JAK2/STAT3 signaling pathway
To further investigate the protective mechanism of DHA in hepatic steatosis-induced oxidative stress and ER stress, we examined whether the JAK2/STAT3 signaling pathway involved in this process by investigating the expression of JAK2/STAT3 and the phosphorylation of JAK2/STAT3. Our result showed that the phosphorylation of JAK2/STAT3 was significantly decreased in 50% FBS-treated L02 cells compared with the control group. Whereas DHA treatment restored the phosphorylation level of JAK2 and STAT3 (Figs. 4A–4C). These results implied that DHA could attenuate hepatic steatosis-induced injury through activating JAK2/STAT3 signaling pathway.
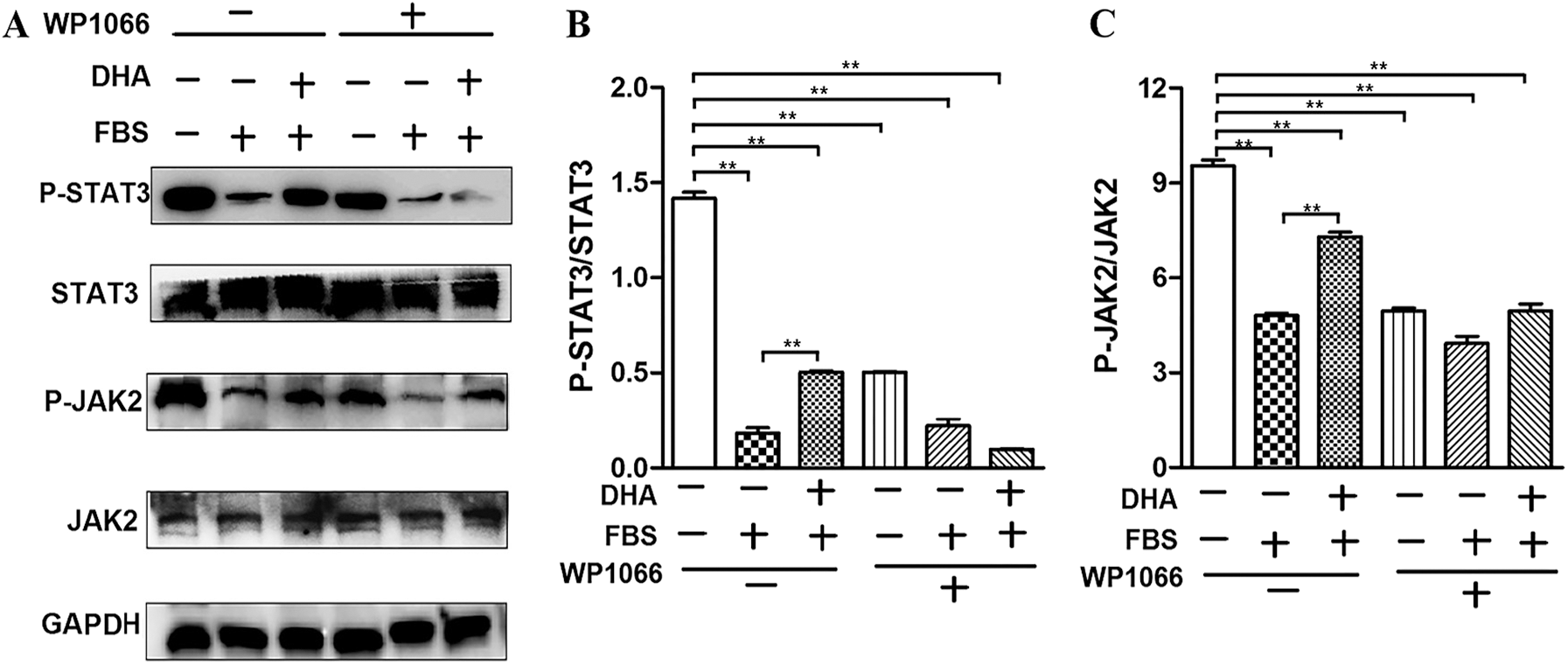
Figure 4: DHA attenuates hepatic steatosis by mediating JAK2/STAT3 signaling pathway.
Inhibition of JAK2/STAT3 signaling pathway reversed the beneficial effect of DHA on hepatic steatosis
WP1066 is considered as a specific inhibitor of the JAK2/STAT3 signaling pathway. As shown in Figs. 4A–4C, WP1066 treatment significantly inhibited the phosphorylation of JAK2 and STAT3, while it also blocked the effect of DHA on the phosphorylation of JAK2 and STAT3, which further demonstrated that DHA could attenuate FBS-induced steatosis hepatocytes via mediating JAK2/STAT3 signaling pathway.
To further confirm the protective role of DHA on the hepatic steatosis-induced injury was associated with JAK2/STAT3 signaling pathway, we treated the cells with WP1066 to determine the effect of DHA on the expression of lipogenic genes and ER stress response. Our results showed that WP1066 treatment can reverse the effect of DHA on PPARγ, C/EBPα, and SREBP-1 expression in FBS-induced hepatic steatosis (Figs. 5A–5E). Meanwhile, WP1066 treatment also blocked the restoration of DHA in FBS-induced ER stress response (Figs. 5F–5G).
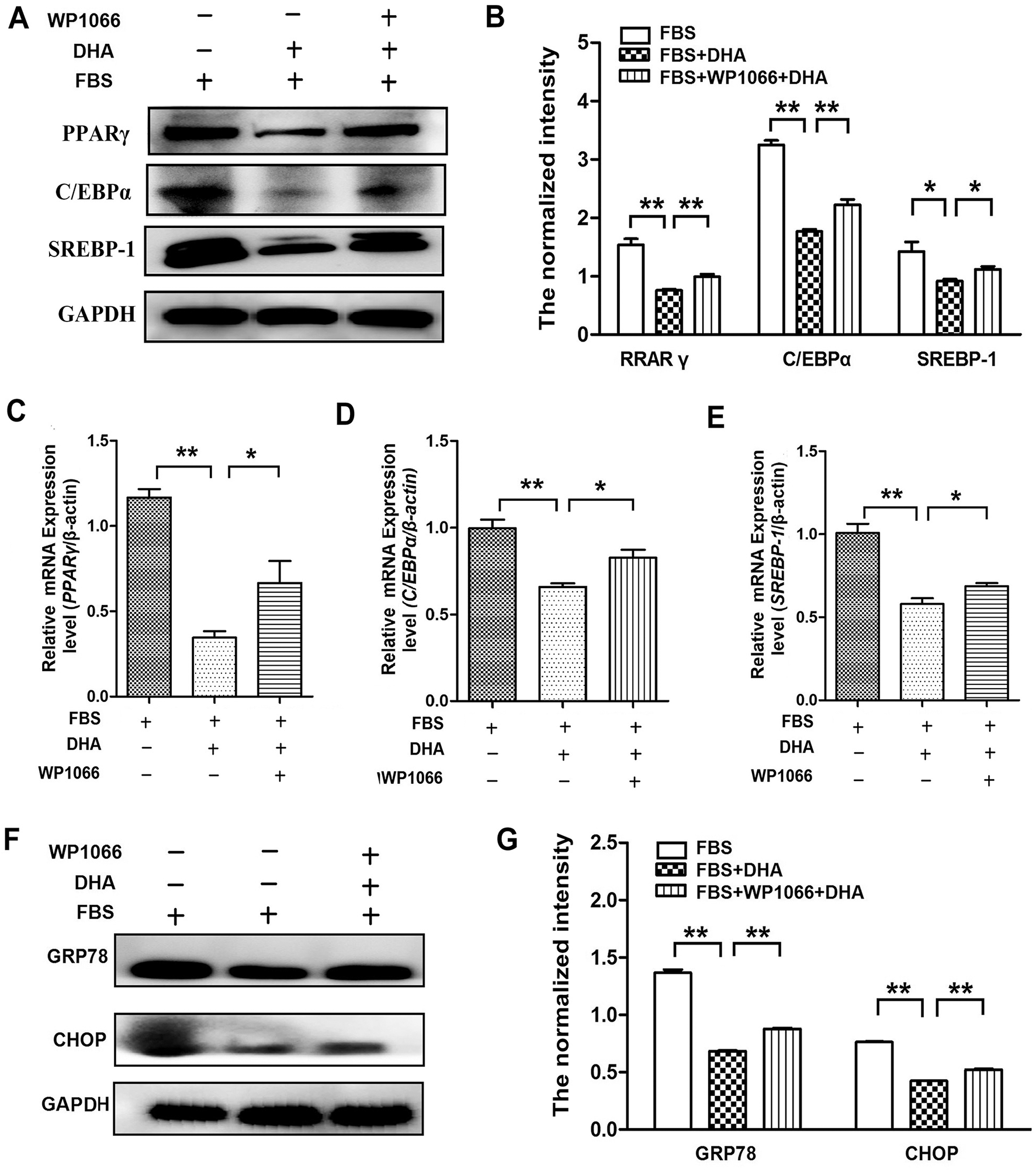
Figure 5: Inhibition of JAK2/STAT3 signaling pathway reversed the beneficial effect of DHA on hepatic steatosis.
In the present work, we treated the L02 cells with 50% FBS to induce hepatic steatosis, which mimics the feature of NAFLD in vitro to study the protective effect of DHA on non-alcoholic hepatic steatosis. The present study provides mechanistic insights into how DHA alleviate the injury of hepatic steatosis in L02 cells. We demonstrated that DHA can reduce the accumulation of lipid droplets in hepatic steatosis and also reduced the hepatic steatosis-induced oxidative stress and ER stress response through activating the JAK2/STAT3 signaling pathway.
The liver is an important organ for maintaining the metabolism of the human body. It plays an important role in the metabolism of various nutrients and drugs and is also the central hub of lipid metabolism in the human body (Hallsworth et al., 2013). Fatty liver has become an important liver disease in the Asia-pacific region, especially the NAFLD has become the major form of chronic liver disease that is characterized by the formation of steatosis, inflammation, and different degree of fibrosis in liver tissue (Ahmed et al., 2010; Fassio et al., 2004). It was well known that excessive accumulation of TC and TG in hepatocytes are the main factors of NAFLD (Wang et al., 2012). Meanwhile, AST and LDH are often used as sensitive and fairly specific biomarkers to evaluate drug-induced hepatocellular injury in preclinical and clinical studies (Schurr and Payne, 2007). Previous studies have shown that supplementation of DHA can inhibit lipogenesis and has a beneficial effect on hepatic lipid metabolism (De Castro et al., 2015; Devarshi et al., 2013). The present study showed that DHA can significantly reduce hepatic lipid accumulation through inhibiting the accumulation of TC and TG and also reducing the activities of AST and LDH. In addition, supplementation of DHA also inhibited the expression of lipogenic genes (such as PPARγ, C/EBP, SREBP-1), suggesting that DHA has anti-hepatic steatosis function in our NAFLD model.
The excessive accumulation of lipid droplets in the liver was associated with the dysfunction of organelles, such as mitochondria and ER. The overloaded lipid increased the synthesis of acetyl-CoA and disturbed the function of the tricarboxylic acid (TCA) cycle during mitochondrial respiration, which increased the reactive oxygen species (ROS) formation. The defect of mitochondrial morphology, electron transport chain, and ATP generation have been shown in NAFLD accompanied by the high level of ROS and inflammation (Sunny et al., 2017; Wang et al., 2020). Therefore, oxidative stress has been considered as one of the major pathogenic mechanisms for the progression of NAFLD (Madan et al., 2006). Different levels of intracellular GSH-Px, SOD, and MDA are important factors to evaluate antioxidant ability. The result showed that the anti-oxidative markers SOD and GSH-Px were significantly increased after DHA treatment, whereas the MDA content was decreased, suggesting that DHA has anti-oxidative functions to relieve the hepatic steatosis-induced oxidative stress.
Under environmental or physiological conditions, when the unfolded protein response (UPR) is not sufficient to maintain normal hepatocellular function, which can induce ER stress and disrupt the ER-dependent liposome homeostasis, thereby stimulating the development of steatosis (Bozaykut et al., 2016). Previous studies showed that the URP was activated in NAFLD patients, accompanied by the increased expression of CHOP and GRP78 (Lee et al., 2017; Zhou et al., 2017). The present study showed that supplementation of DHA significantly alleviated hepatic steatosis-induced ER stress response by down-regulating GRP78 and CHOP. These results indicated that DHA could directly or indirectly target GRP78 and CHOP to inhibit liver lipid accumulation and cell inflammation, which further ameliorate DAFLD development.
JAK-STAT pathway was found in recent years that involved in colonization of the cell increases, differentiation, death depth, and immune regulation and many important biological processes (Zhao et al., 2013). In the high-energy-induced fatty liver rats model, kefir peptides or puerarin can effectively attenuate the symptoms of NAFLD by enhancing the phosphorylation of JAK2 and STAT3, which suggested that the JAK-STAT signaling pathway plays critical roles in NAFLD (Chen et al., 2016). DHA, a natural ligand of PPARγ (Neschen et al., 2007), may activate the JAK2/STAT3 signaling pathway by inhibiting PPARγ (Hwang et al., 2017), thereby alleviating nonalcoholic fatty liver disease. In the present study, we found that the phosphorylation of JAK2 and STAT3 were increased after DHA treatment; meanwhile, WP1066 treatment significantly inhibited the phosphorylation of DHA on JAK2 and STAT3, further suggesting that DHA mediated the JAK2/STAT3 signaling pathway attenuated FBS-induced steatotic hepatocytes. To further demonstrate the effect of DHA on lipid gene expression and endoplasmic reticulum stress via the JAK2/STAT3 pathway. We treated the cells with WP1066, and the results showed that WP1066 treatment could offset the decreased expressions of PPAR, C/EBP, and SREBP-1 in FBS-induced hepatic steatosis induced by DHA. Meanwhile, WP1066 treatment also prevented DHA recovery from FBS induced stress response. The results indicated that DHA attenuated the hepatic steatosis-induced oxidative stress and ER stress through activating the JAK2/STAT3 signaling pathway in the NAFLD.
Our study demonstrated that a high concentration of FBS can cause hepatic steatosis, which can be used to build the in vitro NAFLD model. Meanwhile, DHA could attenuate lipid droplets-caused oxidative stress and ER stress response in L02 cells through activating the JAK2/STAT3 signaling pathway (Fig. 6), which indicated that the JAK2/STAT3 signaling pathway might be an important molecular target of DHA for alleviating NAFLD.
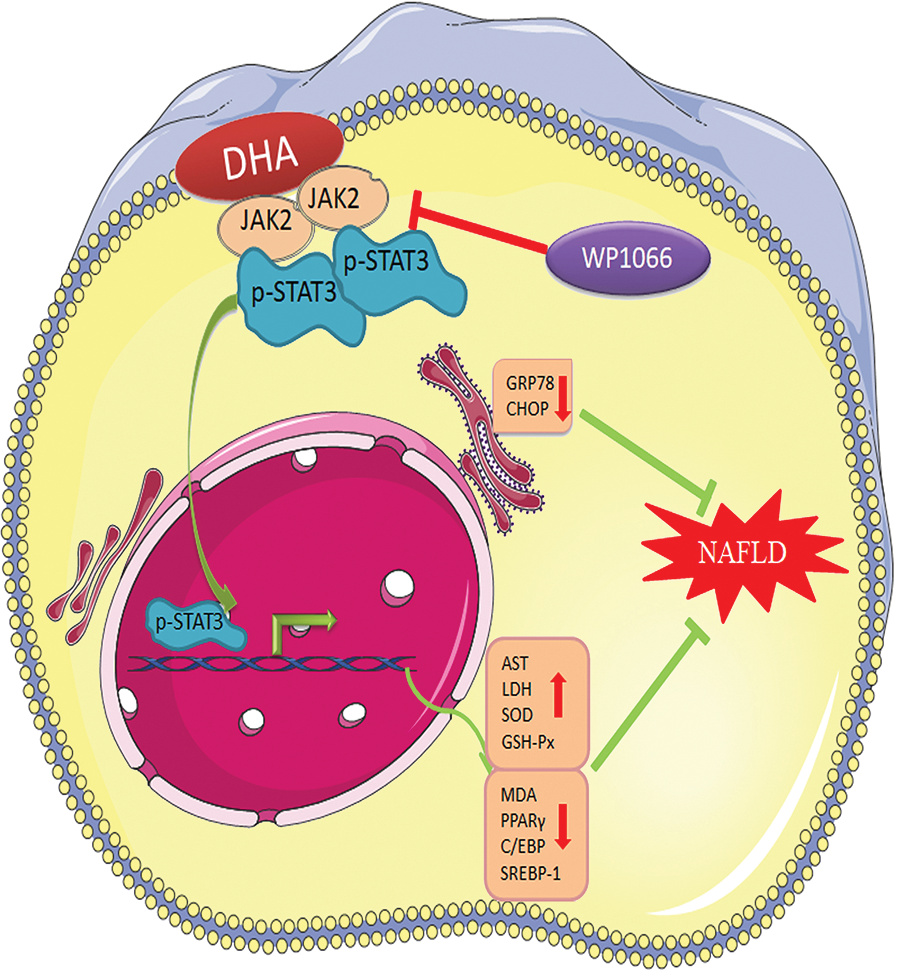
Figure 6: The potential functional mechanism of DHA in non-alcoholic hepatic steatosis.
Ethics Approval: Not applicable.
Availability of Data and Material: All data generated or analyzed during this study are included in this published article.
Author Contribution: YW and YPD conceptualized the study, participated in its design and research. KLC carried out the molecular studies and sample collection. HXL and YQ analyzed data and drafted the manuscript. All authors read and approved the final manuscript for publication.
Funding Statement: This work was supported by the Natural Science Foundation of Jiangsu Province [Grant No. BK20190254], the China Postdoctoral Science Foundation [Grant No. 2019M651755].
Conflicts of Interest: The authors declare that there is no conflict of interests.
Ahmed MH, Abu EO, Byrne CD. (2010). Non-Alcoholic Fatty Liver Disease (NAFLDNew challenge for general practitioners and important burden for health authorities? Primary Care Diabetes 4: 129–137. DOI 10.1016/j.pcd.2010.02.004. [Google Scholar] [CrossRef]
Bozaykut P, Sahin A, Karademir B, Ozer NK. (2016). Endoplasmic reticulum stress related molecular mechanisms in nonalcoholic steatohepatitis. Mechanisms of Ageing and Development 157: 17–29. DOI 10.1016/j.mad.2016.07.001. [Google Scholar] [CrossRef]
Cui MX, Yang LN, Wang XX, Wang L, Li RL, Han W, Wu YJ. (2017). Alleviative effect of ciliary neurotrophic factor analogue on high fat-induced hepatic steatosis is partially independent of the central regulation. Clinical and Experimental Pharmacology and Physiology 44: 395–402. DOI 10.1111/1440-1681.12709. [Google Scholar] [CrossRef]
Chen HL, Tsai TC, Tsai YC, Liao JW, Yen CC, Chen CM. (2016). Kefir peptides prevent high-fructose corn syrup-induced non-alcoholic fatty liver disease in a murine model by modulation of inflammation and the JAK2 signaling pathway. Nutrition & Diabetes 6: e237. DOI 10.1038/nutd.2016.49. [Google Scholar] [CrossRef]
Chen KL, Li L, Yang FX, Li CM, Wang YR, Wang GL. (2018). SIRT7 depletion inhibits cell proliferation, migration, and increases drug sensitivity by activating p38MAPK in breast cancer cells. Journal of Cellular Physiology 233: 6767–6778. DOI 10.1002/jcp.26398. [Google Scholar] [CrossRef]
De Castro GS, Deminice R, Simoes-Ambrosio LM, Calder PC, Jordao AA, Vannucchi H. (2015). Dietary docosahexaenoic acid and eicosapentaenoic acid influence liver triacylglycerol and insulin resistance in rats fed a high-fructose diet. Marine Drugs 13: 1864–1881. DOI 10.3390/md13041864. [Google Scholar] [CrossRef]
Devarshi PP, Jangale NM, Ghule AE, Bodhankar SL, Harsulkar AM. (2013). Beneficial effects of flaxseed oil and fish oil diet are through modulation of different hepatic genes involved in lipid metabolism in streptozotocin-nicotinamide induced diabetic rats. Genes & Nutrition 8: 329–342. DOI 10.1007/s12263-012-0326-2. [Google Scholar] [CrossRef]
Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. (2004). Natural history of nonalcoholic steatohepatitis: A longitudinal study of repeat liver biopsies. Hepatology 40: 820–826. [Google Scholar]
Fedor DM, Adkins Y, Mackey BE, Kelley DS. (2012). Docosahexaenoic acid prevents trans-10, cis-12-conjugated linoleic acid-induced nonalcoholic fatty liver disease in mice by altering expression of hepatic genes regulating fatty acid synthesis and oxidation. Metabolic Syndrome and Related Disorders 10: 175–180. DOI 10.1089/met.2011.0113. [Google Scholar] [CrossRef]
Fu YY, Chen KL, Li HX, Zhou GH (2016). The adipokine Chemerin induces lipolysis and adipogenesis in bovine intramuscular adipocytes. Mol Cell Biochem 418: 39–48. [Google Scholar]
Hallsworth K, Hollingsworth KG, Thoma C, Jakovljevic D, Macgowan GA, Anstee QM, Taylor R, Day CP, Trenell MI. (2013). Cardiac structure and function are altered in adults with non-alcoholic fatty liver disease. Journal of Hepatology 58: 757–762. DOI 10.1016/j.jhep.2012.11.015. [Google Scholar] [CrossRef]
Horrocks LA, Yeo YK. (1999). Health benefits of docosahexaenoic acid (DHA). Pharmacological Research 40: 211–225. DOI 10.1006/phrs.1999.0495. [Google Scholar] [CrossRef]
Hwang JK, Yu HN, Noh EM, Kim JM, Hong OY, Youn HJ, Jung SH, Kwon KB, Kim JS, Lee YR. (2017). DHA blocks TPA-induced cell invasion by inhibiting MMP-9 expression via suppression of the PPAR-gamma/NF-κB pathway in MCF-7 cells. Oncology Letters 13: 243–249. DOI 10.3892/ol.2016.5382. [Google Scholar] [CrossRef]
Khan S, Minihane AM, Talmud PJ, Wright JW, Murphy MC, Williams CM, Griffin BA. (2002). Dietary long-chain n-3 PUFAs increase LPL gene expression in adipose tissue of subjects with an atherogenic lipoprotein phenotype. Journal of Lipid Research 43: 979–985. [Google Scholar]
Lee S, Kim S, Hwang S, Cherrington NJ, Ryu DY. (2017). Dysregulated expression of proteins associated with ER stress, autophagy and apoptosis in tissues from nonalcoholic fatty liver disease. Oncotarget 8: 63370–63381. DOI 10.18632/oncotarget.18812. [Google Scholar] [CrossRef]
Lewis JR, Mohanty SR (2010). Nonalcoholic fatty liver disease: A review and update. Digestive Diseases and Sciences 55: 560–578. [Google Scholar]
Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. (2010). The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nature Reviews Gastroenterology & Hepatology 7: 251–264. DOI 10.1038/nrgastro.2010.41. [Google Scholar] [CrossRef]
Luo X, He Z, Sun X, Gu X, Zhang W, Gao J, Li X, Jia R, Wei J, Yu Y. (2020). DHA protects against hepatic steatosis by activating sirt1 in a high fat diet-induced nonalcoholic fatty liver disease mouse model. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 13: 185–196. DOI 10.2147/DMSO.S232279. [Google Scholar] [CrossRef]
Madan K, Bhardwaj P, Thareja S, Gupta SD, Saraya A. (2006). Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD). Journal of Clinical Gastroenterology 40: 930–935. DOI 10.1097/01.mcg.0000212608.59090.08. [Google Scholar] [CrossRef]
Merola J, Liapakis A, Mulligan DC, Yoo PS. (2015). Non-alcoholic fatty liver disease following liver transplantation: A clinical review. Clinical Transplantation 29: 728–737. DOI 10.1111/ctr.12585. [Google Scholar] [CrossRef]
Neschen S, Morino K, Dong J, Wang-Fischer Y, Cline GW, Romanelli AJ, Rossbacher JC, Moore IK, Regittnig W, Munoz DS, Kim JH, Shulman GI. (2007). n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha–dependent manner. Diabetes 56: 1034–1041. DOI 10.2337/db06-1206. [Google Scholar] [CrossRef]
Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, Piemonte F, Marcellini M, Angulo P. (2008). Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: A randomized, controlled trial. Hepatology 48: 119–128. DOI 10.1002/hep.22336. [Google Scholar] [CrossRef]
Peyron-Caso E, Quignard-Boulange A, Laromiguiere M, Feing-Kwong-Chan S, Veronese A, Ardouin B, Slama G, Rizkalla SW. (2003). Dietary fish oil increases lipid mobilization but does not decrease lipid storage-related enzyme activities in adipose tissue of insulin-resistant, sucrose-fed rats. Journal of Nutrition 133: 2239–2243. DOI 10.1093/jn/133.7.2239. [Google Scholar] [CrossRef]
Rutkowski DT, Kaufman RJ (2004). A trip to the ER: Coping with stress. Trends Cell Biol 14: 20–28. [Google Scholar]
Schurr A, Payne RS. (2007). Lactate, not pyruvate, is neuronal aerobic glycolysis end product: An in vitro electrophysiological study. Neuroscience 147: 613–619. DOI 10.1016/j.neuroscience.2007.05.002. [Google Scholar] [CrossRef]
Smith BW, Adams LA. (2011). Non-alcoholic fatty liver disease. Critical Reviews in Clinical Laboratory Sciences 48: 97–113. DOI 10.3109/10408363.2011.596521. [Google Scholar] [CrossRef]
Spadaro L, Magliocco O, Spampinato D, Piro S, Oliveri C, Alagona C, Papa G, Rabuazzo AM, Purrello F. (2008). Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Digestive and Liver Disease 40: 194–199. DOI 10.1016/j.dld.2007.10.003. [Google Scholar] [CrossRef]
Sun C, Wei ZW, Li Y. (2011). DHA regulates lipogenesis and lipolysis genes in mice adipose and liver. Molecular Biology Reports 38: 731–737. DOI 10.1007/s11033-010-0160-9. [Google Scholar] [CrossRef]
Sunny NE, Bril F, Cusi K. (2017). Mitochondrial adaptation in nonalcoholic fatty liver disease: Novel mechanisms and treatment strategies. Trends in Endocrinology & Metabolism 28: 250–260. DOI 10.1016/j.tem.2016.11.006. [Google Scholar] [CrossRef]
Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. (2016). Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. Journal of Hepatology 65: 589–600. DOI 10.1016/j.jhep.2016.05.013. [Google Scholar] [CrossRef]
Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. (2015). Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149: 367–378.e5. DOI 10.1053/j.gastro.2015.04.005. [Google Scholar] [CrossRef]
Wang J, He W, Tsai PJ, Chen PH, Ye M, Guo J, Su Z. (2020). Mutual interaction between endoplasmic reticulum and mitochondria in nonalcoholic fatty liver disease. Lipids in Health and Disease 19: 187. DOI 10.1186/s12944-020-01210-0. [Google Scholar] [CrossRef]
Wang M, Zhao R, Wang W, Mao X, Yu J. (2012). Lipid regulation effects of Polygoni Multiflori Radix, its processed products and its major substances on steatosis human liver cell line L02. Journal of Ethnopharmacology 139: 287–293. DOI 10.1016/j.jep.2011.11.022. [Google Scholar] [CrossRef]
Wu HR, Chen SH, Lu Y, Li YM. (2010). Comparison of two nonalcoholic hepatocellular steatosis models. Zhonghua Gan Zang Bing Za Zhi 18: 297–299. [Google Scholar]
Yamamoto K, Takahara K, Oyadomari S, Okada T, Sato T, Harada A, Mori K (2010). Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell 21: 2975–2986. [Google Scholar]
Zeng L, Tang WJ, Yin JJ, Zhou BJ. (2014). Signal transductions and nonalcoholic fatty liver: A mini-review. International Journal of Clinical and Experimental Medicine 7: 1624–1631. [Google Scholar]
Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, Hwang SH, Ingham ES, Kieran MW, Weiss RH, Ferrara KW, Hammock BD. (2013). Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proceedings of the National Academy of Sciences of the United States of America 110: 6530–6535. DOI 10.1073/pnas.1304321110. [Google Scholar] [CrossRef]
Zhang K, Wang S, Malhotra J, Hassler JR, Back SH, Wang G, Chang L, Xu W, Miao H, Leonardi R, Chen YE, Jackowski S, Kaufman RJ (2011). The unfolded protein response transducer IRE1alpha prevents ER stress-induced hepatic steatosis. EMBO J 30: 1357–1375. [Google Scholar]
Zhao QH, Wang SG, Liu SX, Li JP, Zhang YX, Sun ZY, Fan QM, Tian JW. (2013). PPARgamma forms a bridge between DNA methylation and histone acetylation at the C/EBPalpha gene promoter to regulate the balance between osteogenesis and adipogenesis of bone marrow stromal cells. FEBS Journal 280: 5801–5814. DOI 10.1111/febs.12500. [Google Scholar] [CrossRef]
Zheng J, Peng C, Ai Y, Wang H, Xiao X, Li J. (2016). Docosahexaenoic acid ameliorates fructose-induced hepatic steatosis involving ER stress response in primary mouse hepatocytes. Nutrients 8: 55. DOI 10.3390/nu8010055. [Google Scholar] [CrossRef]
Zhou X, Han D, Yang X, Wang X, Qiao A. (2017). Glucose regulated protein 78 is potentially an important player in the development of nonalcoholic steatohepatitis. Gene 637: 138–144. DOI 10.1016/j.gene.2017.09.051. [Google Scholar] [CrossRef]
Zohrer E, Alisi A, Jahnel J, Mosca A, Della Corte C, Crudele A, Fauler G, Nobili V. (2017). Efficacy of docosahexaenoic acid–choline–vitamin E in paediatric NASH: A randomized controlled clinical trial. Applied Physiology, Nutrition, and Metabolism 42: 948–954. DOI 10.1139/apnm-2016-0689. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |