DOI:10.32604/biocell.2021.09034

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.09034 |  www.techscience.com/journal/biocell |
| Article |
Ala-Gln improves varicocele-induced testicular injury by increasing HSP70 and antioxidant activity in male rats
1Center for Reproductive Medicine, Cheeloo College of Medicine, Shandong University, Jinan, 250100, China
2Department of Andrology, The Affiliated Hospital of Qingdao University, Qingdao, 266003, China
3Department of Ophthalmology, Qingdao Hospital of Traditional Chinese Medicine (Qingdao Hiser Hospital), Qingdao, 266034, China
4Department of Urology, The Affiliated Hospital of Qingdao University, Qingdao, 266003, China
*Address correspondence to: Xujun Xuan, XuanXuJun@outlook.com
Received: 16 November 2019; Accepted: 12 August 2020
Abstract: This worked aimed to test the hypothesis that L-alanyl-L-glutamine (Ala-Gln) improves the varicocele-induced testicular injury, which causes male infertility. For this purpose, fifty adult male Wistar rats received the varicocele (VC) surgery at the left renal vein. Biomarkers were determined 2, 4, and 8 weeks after VC (n = 10/each detection). Four weeks after VC, rats received Ala-Gln (1.125 g/kg) treatment with and or saline for 1 week (n = 10/each group). Rats in the sham group were also detected for biomarkers at 2, 4, and 8 weeks (n = 10/each detection). VC caused testicular injury detected by hematoxylin–eosin (H&E) staining, immunohistochemistry, and TUNEL assay. HSP70 mRNA was detected quantitative RT-PCR, SOD, and CAT by nitroblue tetrazolium (NBT) method and 8-OHDG by ELISA. The results showed that varicocele induced injury in the testes. The weight of the left testes was lower than that of the right testes in VC-bearing rats (p < 0.01). The relative numbers of sustentacular and spermatogenic cells were decreased after VC (p < 0.01). In sham-4 wk, VC-4wk, VC-5wk and Ala-Gln groups, the apoptosis index was 5.10 ± 1.14, 13.22 ± 3.63, 33.62 ± 3.56 and 22.33 ± 2.61, relative level of HSP70 mRNA 1.00 ± 0.12, 0.53 ± 0.05, 0.51 ± 0.04 and 1.62 ± 0.15 fold, SOD 16.4 ± 0.23, 13.4 ± 0.17, 10.01 ± 1.06 and 19.53 ± 2.26 U/mg protein, CAT 2.16 ± 0.31, 1.07 ± 0.28, and 1.31 ± 0.26 and 3.46 ± 0.71 U/mg, 8-OHDG 5.23 ± 0.67, 6.81 ± 0.78, 7.16 ± 1.22 and 4.14 ± 0.73 pg/μg DNA, respectively (p < 0.01). Our results suggest that Ala-Gln prevented the VC-induced testicular injury. We have firstly reported that Ala-Gln protects against varicocele-induced testicular injuries by up-regulation of HSP70 and antioxidants, SOD and CAT, and down-regulation of oxidant 8-OHDG, resulting in reducing apoptosis in the testis.
Keywords: Varicocele; Ala-Gln; HSP70; SOD; CAT; 8-OHDG
Varicocele (VC) is defined as the abnormal elongation, expansion, and distortion due to poor return flow of slender spermatic vein or venous valve damage (Agarwal et al., 2016; Sadek et al., 2011). Reported by the World Health Organization (WHO), the incidence of VC is about 25–40% in infertile men and may be the main cause of male infertility (Agarwal and Said, 2003; Sigman, 1998). VC enhances oxidative stress resulting in intensive DNA damage in germ cells and spermatozoa (Amin et al., 2018; Khosravanian et al., 2015; Salmani et al., 2020). Heat-shock protein 70 (HSP70) located in the cytoplasm belongs to the HSP family as a protective protein to maintain normal cellular functions (De Maio, 1999; Hassanpour et al., 2017). Due to the ability to bind with pro-apoptotic molecules, HSP70 is known as an anti-apoptotic molecule and protects the cellular DNA content from VCL-induced oxidative stress (Li et al., 1997; Salmani et al., 2020; Shamsi-Gamchi et al., 2018). HSP70 is involved in the regulation of spermatogenesis in different stages with a role oxidative stress in male infertility. The abnormal expression of HSP70 may interfere the male fertility (Erata et al., 2008; Feng et al., 2001; Khosravanian et al., 2015). Men with varicocele are usually with higher levels of seminal oxidants, including malondialdehyde (MDA), nitric oxide (NO), and 8-oxo-2’-deoxyguanosine (8-OHDG), but lower levels of seminal antioxidants, including superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT) (Abd-Elmoaty et al., 2010; Sakamoto et al., 2008).
Glutamine amino acid (Gln) is the most abundant amino acid in the blood and the intracellular free amino acid pool of most tissues (Perriello et al., 1995). Gln induces the expression of HSPs, including HSP70, to protect against endotoxin shock and lipopolysaccharides (LPS)-induced cardiomyocyte damage in rats (Gong and Jing, 2011; Jing et al., 2007; Wischmeyer et al., 2001). L-alanyl-L-glutamine (Ala-Gln) is a dipeptide with the highest stability, solubility, and heat-sterilization. When it intravenously infuses, Ala-Gln promptly hydrolyzed to glutamine and alanine (Albers et al., 1989). In an experimental rat model, the acute Ala-Gln supplementation promotes significant increase of glutamine levels in plasma and tissues (Rogero et al., 2006). In an animal model using Mongolian gerbils, preconditioning with L-Ala-Gln submitted to cerebral ischemia/reperfusion reduces the oxidative stress, nucleus degeneration, and red neuron of and death in the cerebral tissue (Pires et al., 2011). Combining with fish oil containing the ω-3 polyunsaturated fatty acids (PUFAs), the dipeptide Ala-Gln has been used in patients with heart failure (HF) to improve both specific muscle functional parameters and fatigability and overall exercise tolerance (Wu et al., 2015). In addition, the pre-administration of Ala-Gln just before lipopolysaccharide (LPS) in rats effectively protects the lung by enhancing HSP70 expression (Wang et al., 2017). Therefore, Ala-Gln might be a stable HSP70 inducer.
In our study, we have investigated the hypothesis that Ala-Gln protects against the varicocele-induced testicular dysfunction. By administration with Ala-Gln in the VC-bearing rats, we have first found that, in contrast to the effects of varicocele, Ala-Gln increases HSP70 and seminal antioxidants and decreases seminal oxidants, resulting in the reduction of apoptosis in spermatogenic cells and the recovery of testicular injuries.
All experiments were approved by the Animal Experimental Ethics Committee of Shandong University, Jinan, China. Eighty male Wistar rats, at the age of 6.25 ± 0.75 months and weight of 203.20 ± 11.68 g, were provided by Shandong University animal center. Rats were randomly divided into sham (n = 30) and varicocele (VC) (n = 50) groups. All rats fasted for 4 h before the experiments.
Rats were anesthetized with an intraperitoneal (i.p.) injection of 4% chloral hydrate (1 mL/100 g body weight). The left renal vein of the rat in the VP group was carefully separated from the inside of the adrenal vein, the spermatic vein, and the outside the inferior vena cava. A smooth metal rod (0.8 mm diameter) was placed parallel to the left renal vein. The vein and metal rod were ligated with a 3/0 silk, which reduced the vein diameter to approximately half its original diameter. The metal rod was then pulled out. The contents were repositioned, and the skin was carefully sutured layer by layer (Fig. 1A, a). For rats in the sham group, we performed an abdominal median incision. Following exposure, the left renal vein was only separated without ligation. The incision was sutured with 3/0 silk (Fig. 1A, b).
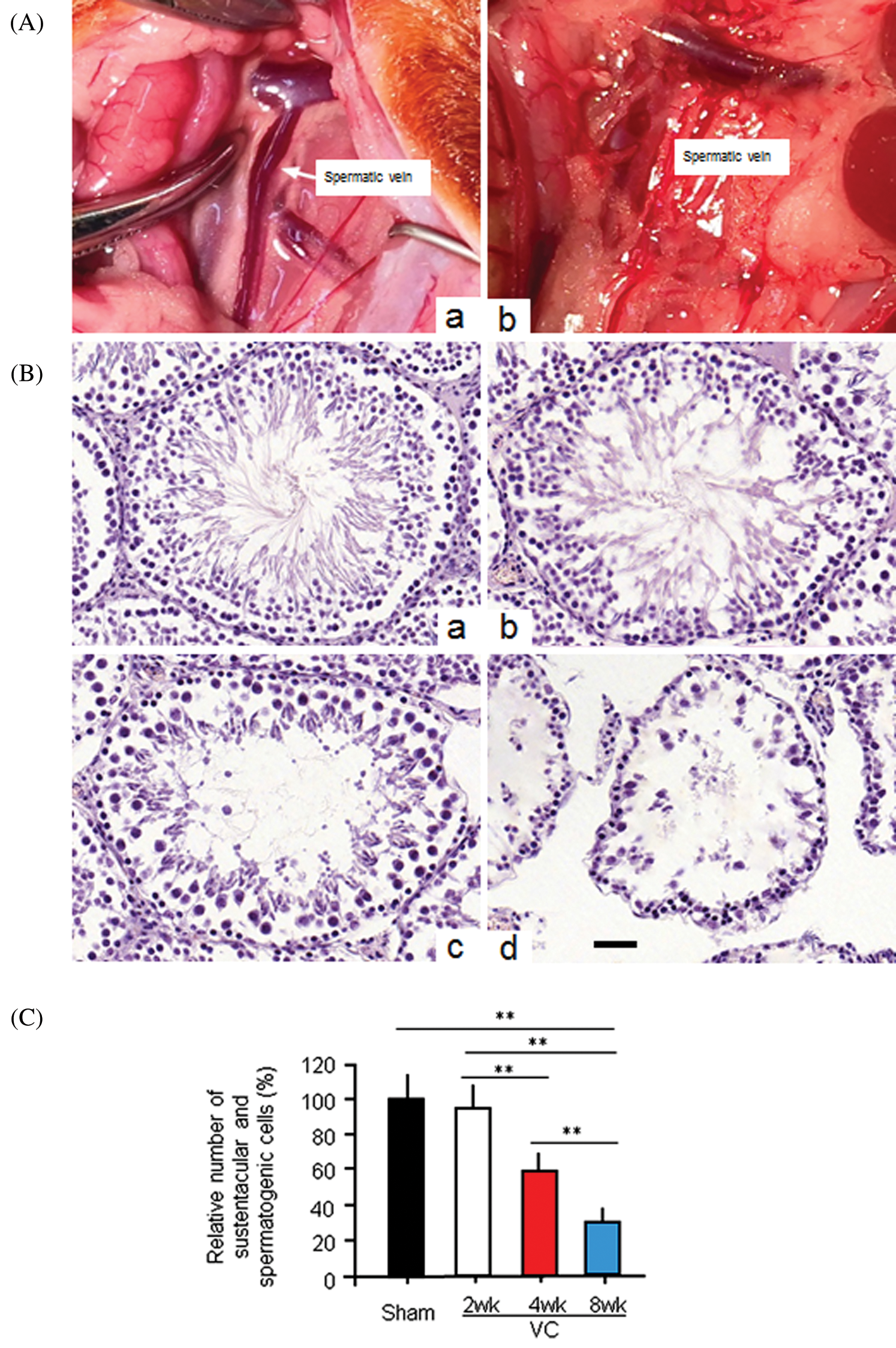
Figure 1: The experimental varicocele induces histopathological changes in rat testes.
To observe the effects of VC, the left and right testes of 10 rats in the VC or the sham group were taken 2, 4, and 8 weeks after surgeries (total 30 rats/each group) for measurement of VC biomarkers. To observe the effect of Ala-Gln on VC-induced testicular injury, 20 VC-bearing rats, 4 weeks after the varicocelectomy, were randomly divided into the Ala-Gln group (n = 10) with a daily i.p. of 1.125 g/kg body weight for one week and the control group (n = 10) with a daily i.p. of the same volume of saline for one week (also named VC-5wk group). The testes in both sides were taken, dissected out from the associated adipose tissue and fascia attached to their surface, and dried on a filter paper after washing with saline. The testes were weighted and then cut in half. One half was fixed in 4% paraformaldehyde for histological examination, and the other half immediately frozen and stored at −80°C for later analysis.
H&E staining, immunohistochemistry and TUNEL assay
After fixation in 4% paraformaldehyde, the half testis was embedded in paraffin and then cut to 5-µm sections. After routinely dewaxing and hydration, sections were stained with hematoxylin and eosin (H&E staining). After the peroxidase block, the section was incubated with the primary antibody, mouse anti-HSP70 monoclonal antibody (1:25) (Santa Cruz Biotech, USA), and then horseradish peroxidase-labeled secondary antibody. The results were observed with an Olympus BX50 light microscope (Olympus, Japan). The staining intensity was assigned a score of 0–3 (0-absent, 1-weak, 2-moderate, and 3-strong). The HSP70-positive staining was defined while score 2 and 3 staining was ≥10% of total cells. Five fields were randomly selected from each section and photographed under the same conditions. The average optical density values of the positive staining were analyzed using CellProfiler, a high-resolution color image reporting system (Matlab, USA). The apoptosis-detection kit (Promega, USA) was used to perform terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay following the manufacturer’s instruction. The nuclei of the TUNEL positive cells stained yellow or dark brown. For calculation of the apoptosis index (AI), 10 fields were randomly selected from each section to count the TUNEL-positive cells.
Quantitative real time RT-PCR (RT-qPCR)
TRIzol reagents (Invitrogen, USA) was used to extract the total RNA from the rat testis tissue. First-strand cDNA was synthesized using a cDNA synthesis kit (Promega, USA) according to the manufacturer's protocol. RT-qPCR was performed with SYBR-Green mix kit (Applied Biosystems, USA), using primers: Hsp70 forward, 5’- ACC GTG CCC GCC TAC-3’ and reverse, 5’- ACA GCG TCC TCT TGG CCC TC-3’, and the internal control, GAPDH, forward, 5’-TGC CAC TCA GAA GAC TGT GG-3’ and reverse, 5’-TTC AGC TCT GGG ATG ACC TT-3’. PCR was performed with 40 cycles of 95°C for 15 s, 60°C for 60 s, and 72°C for 30 s, using the ABI 7900 Real-Time PCR system (Applied Biosystems, USA).
Determination of testicular SOD, CAT and 8-OHDG
The activity of testicular superoxide dismutase (SOD) was assayed using the nitroblue tetrazolium (NBT) method (Sun et al., 1988) and presented as U/mg protein of testicular tissue. The activity of catalase (CAT) was assayed (Aebi, 1984) and presented as U/mg protein of testicular tissue. One unit of CAT equates to the amount of CAT decomposing H2O2 at 1.0 mmol/min. The 8-Hydroxy-2 deoxyguanosine (8-OHDG) level was determined by using the 8-OHDG ELISA kit (Northwest, Canada). Testis DNA was extracted using DNasy Blood and Tissue Kit (Qiagen, USA). The extracted DNA was digested with nuclease P1, and then 8-OHDG was determined following the ELISA protocol. The level of 8-OHDG was presented as pg/μg DNA.
All data were presented as the mean ± standard deviation (SD). Statistical analysis was conducted using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The means were compared using a one-way analysis of variance followed by the Student–Newman–Keuls test for the different groups. ANOVA and post-test were also used. p < 0.05 was considered to indicate a statistically significant difference. All experiments were performed at least three times.
Varicocele induces injury in the testes of VC-model rats
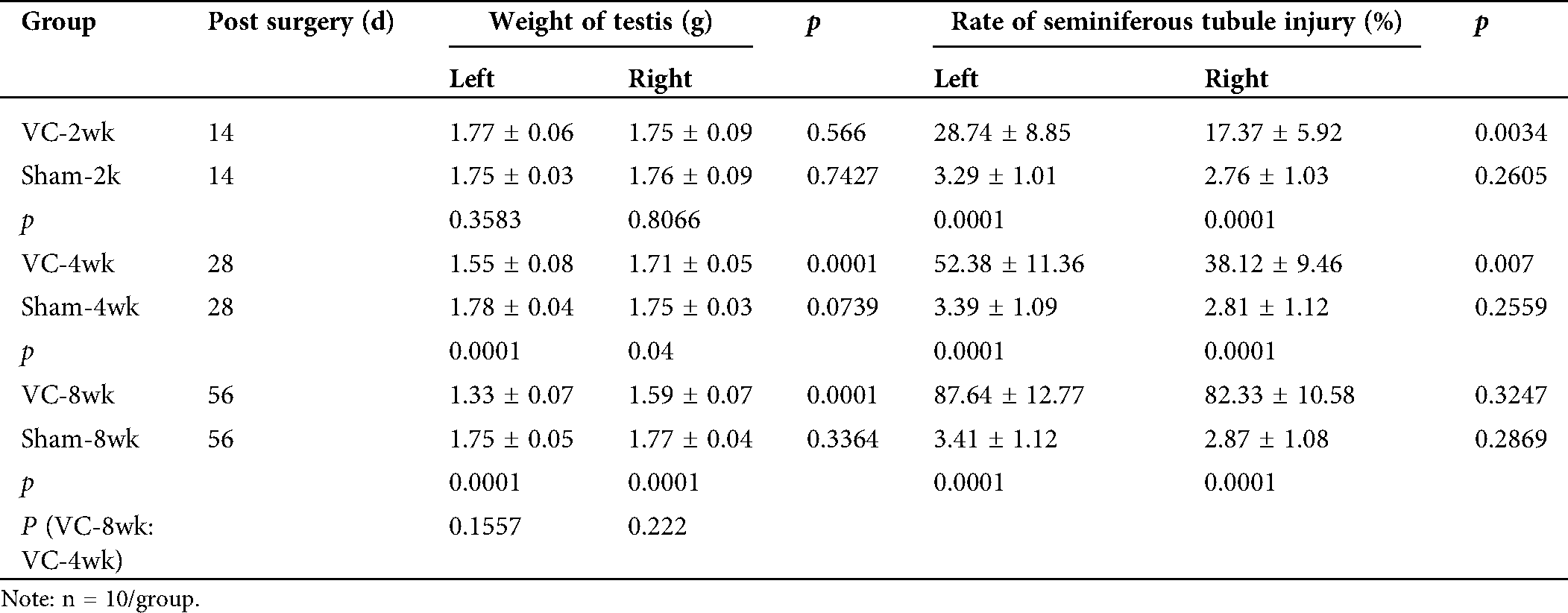
Our primary aim was to test whether Ala-Gln could improve the varicocele (VC) induced testicular injury. Therefore, we successfully established the VC rat model 4 weeks after partial ligation of the left renal vein. The weight of the left testes was significantly lower than that of the right testes in VC-bearing rats (p < 0.01) 4 and 8 weeks after the VC surgery (Tab. 1). The weight of the right testes of rats in the VC group was also significantly lower when compared to that in the sham group (p < 0.05). H&E staining showed the testicular histopathology in shame and VC groups: the cells were counted and normalized to the cell number in the sham group for the relative cell number (%). In the testis of the sham-4wk group, sustentacular and spermatogenic cells were arranged neatly and orderly (100.0 ± 15.3%) (Figs. 1B, 1a, 1C). Two weeks after VC, sustentacular and spermatogenic cells were almost normal (95.4 ± 14.2%) (Figs. 1B, 1b, 1C). Four weeks after VC, the number of seminiferous tubules was decreased, the spermatogenic cells lost (59.3 ± 7.8%), and the spermatogenic process blocked with the interstitial edema (Figs. 1B, 1c, 1C). At the same time, testicular seminiferous tubules were severely atrophied, a large number of spermatogenic cells lost, but sustentacular cells appeared in the vessel wall (32.4 ± 6.3%) (Figs. 1B, 1d, 1C). The bilateral testes obviously displayed the structural impairment, and the pathological changes in left side testis were more serious than that on the right side.
The experimental varicocele increases seminiferous tubule injury
Tab. 1 also showed that the injury rate of the seminiferous tubules in the left testis was 28.74 ± 8.85%, 52.38 ± 11.36%, and 87.64 ± 12.77% in VC-2wk, VC-4wk, and VC-8wk groups, and 3.29 ± 1.01%, 3.39 ± 1.09% and 3.41 ± 1.12% in Sham-2wk, Sham-4wk and Sham-8wk groups (n = 10/each group), respectively, with significant differences (p < 0.01). Two and four weeks after varicocele, the injury rate of the seminiferous tubules in the left testis was higher than that in the right testis (p < 0.01), but no significant difference when comparing the same feature 8 weeks after varicocele (p > 0.05) (Tab. 1). The data suggest that the experimental varicocele increases seminiferous tubule injury.
Table 1: Weight of testis and bilateral seminiferous tubule injury in rats of VC and sham groups
Varicocele decreases the number of HSP70-positive cells
Histological staining showed that the HSP70-positive score of spermatogenic cells was 3 (strong) in the sham-4wk group (n = 10), and 1 (weak) in the VC-4wk group (n = 10), respectively, with significant difference (p < 0.01). The average optical density value of the positive staining was 0.25 ± 0.07 in VC-4wk group (n = 10) and 0.42 ± 0.05 in Sham-4wk group (n = 10), respectively, with significant difference (p < 0.01) (Fig. 2A). The results suggest that HSP70 was widely expressed in rats of the Sham group, and the experimental varicocele reduces the HSP70 expression in spermatogenic cells.
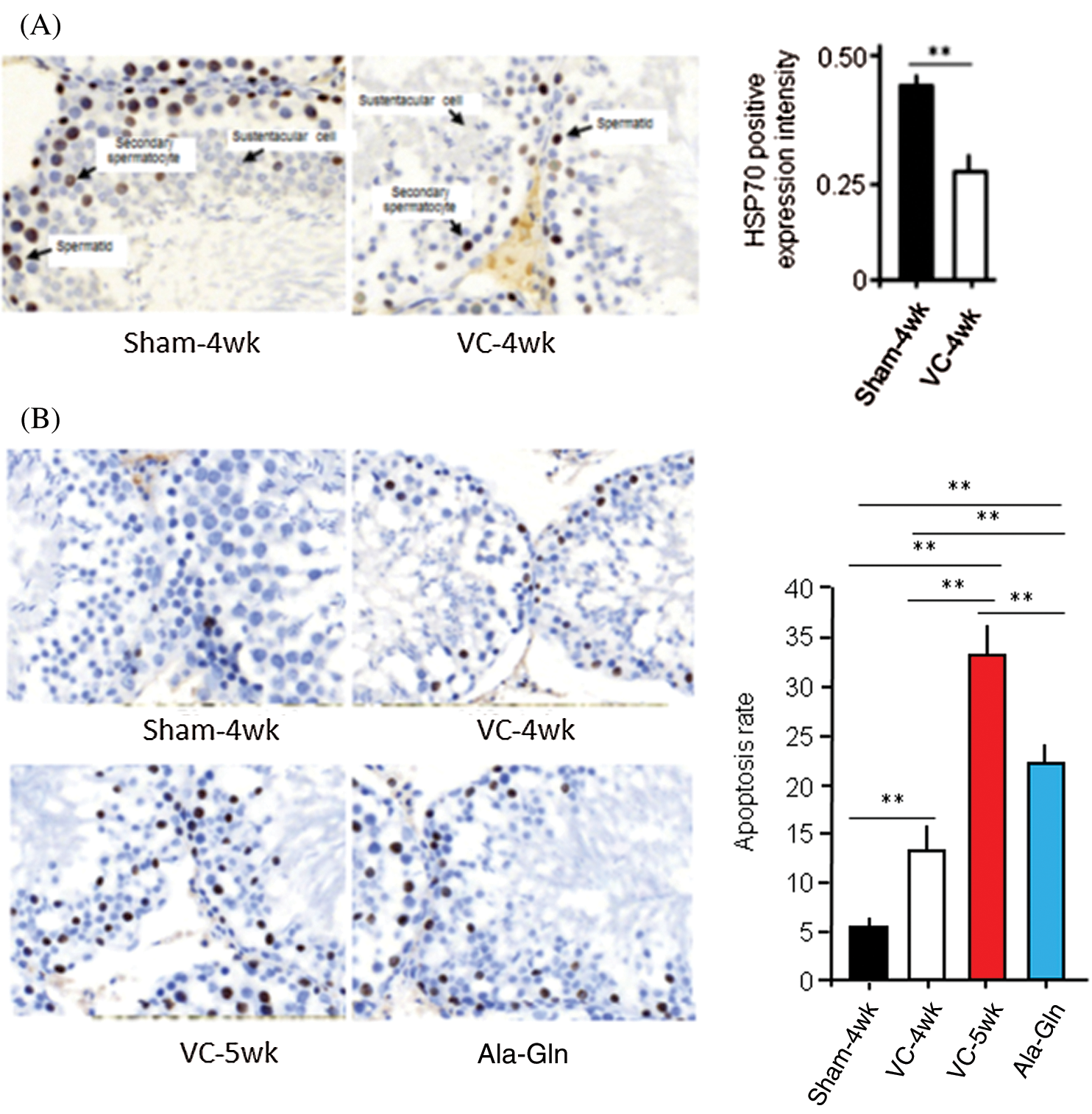
Figure 2: The experimental varicocele and or Ala-Gln treatment affect HSP70 expression and apoptosis.
Varicocele increases apoptosis but Ala-Gln decreases apoptosis in testicular spermatogenic cells
TUNEL was performed, and values of the apoptosis index (AI) were analyzed in rat testes. AI in rats in the sham-4 wk group, VC-4wk group (4 weeks after VC surgery), VC-5wk group (Treatment with saline for 1 week in VC-4wk group), and Ala-Gln group (treatment with Ala-Gln for 1 week in VC-4wk group) was 5.10 ± 1.14, 13.22 ± 3.63, 33.62 ± 3.56 and 22.33 ± 2.61, respectively (n = 10/each group) (ANOVA, p < 0.01) (Fig. 2B). The data suggest that the experimental varicocele increases, but Ala-Gln treatment decreases apoptosis in testicular spermatogenic cells.
VC reduces, but Ala-Gln enhances HSP70 transcription
HSP70 mRNA levels were measured by RT-qPCR in rats with different treatments (n = 10/each group). The relative level of HSP70 mRNA in rats in the sham-4 wk, VC-4wk, VC-5wk, and Ala-Gln groups (n = 10/each group) was 1.00 ± 0.12, 0.53 ± 0.05, 0.51 ± 0.04, and 1.62 ± 0.15 fold, respectively (ANOVA, p < 0.01), suggesting that the experimental varicocele decreases but Ala-Gln treatment increases the HSP70 expression at the transcriptional level (Fig. 3A).
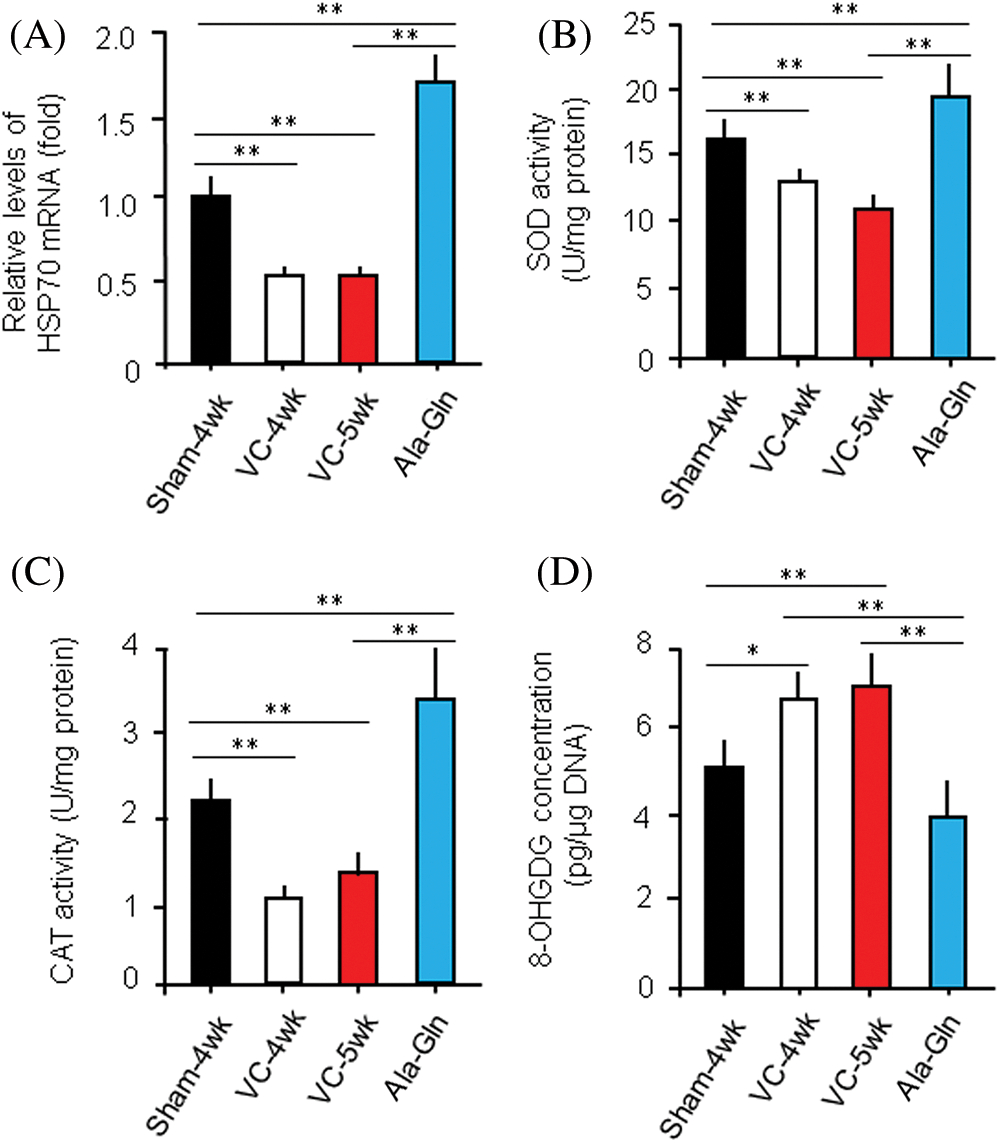
Figure 3: Ala-Gln improves the experimental varicocele (VC)-induced testis injury.
VC reduces, but Ala-Gln enhances the activity of superoxide dismutase (SOD) and catalase (CAT)
The levels of SOD and CAT in the left testes of rats in the sham-4wk, VC-4wk, VC-5wk, and Ala-Gln groups (n = 10/group) were 16.4 ± 0.23 and 2.16 ± 0.31, 13.4 ± 0.17 and 1.07 ± 0.28, 10.01 ± 1.06 and 1.31 ± 0.26, 19.53 ± 2.26 and 3.46 ± 0.71 U/mg protein, respectively (ANOVA, p < 0.01) (Figs. 3B, C), suggesting that the Ala-Gln administration increases the activity of anti-oxidative stress enzymes.
VC increases, but Ala-Gln decreases 8-OHDG concentration in testes
The level of 8-OHDG in the left testes of rats in the sham-4wk, VC-4wk, VC-5wk and Ala-Gln group (n = 10/group) was 5.23 ± 0.67, 6.81 ± 0.78, 7.16 ± 1.22 and 4.14 ± 0.73 pg/μg DNA, respectively (ANOVA, p < 0.01) (Fig. 3D), suggesting that the Ala-Gln administration decreases the concentration of 8-OHDG in testes of rata after VC surgery.
In the present study, we have tested the hypothesis that Ala-Gln enhances the expression of HSP70 and the concentration of antioxidants but reduces the concentration of oxidants and apoptosis in the varicocele (VC)-bearing rats. The VC surgery in rats causes a decrease in the weight of testes and the number of seminiferous tubules and spermatogenic cells, the injury of the bilateral seminiferous tubule, and the block of the spermatogenic process with interstitial edema. Varicocele reduces the HSP70 expression at the transcriptional level in spermatogenic cells and induces apoptosis in testicular spermatogenic cells. Importantly, we have administrated Ala-Gln in the VC-bearing rats, which increases the levels of HSP70 and antioxidants, SOD and CAT, as well as reduces oxidant 8-OHDG and apoptosis in spermatogenic cells. To our knowledge, this study examined, for the first time, the ability of Ala-Gln in the protection against testicular injuries caused by varicocele.
As a major cause of man infertility, varicocele impairs spermatogenesis accompanied by inducing a higher level of oxidative stress (Hendin et al., 1999; Kao et al., 2008). SOD and CAT provide an important defense against the toxicity of the superoxide radical (Chelikani et al., 2004; Kheradmand et al., 2009). The oxidative stress damages the sperm DNA, resulting in male infertility. 8-OHDG is a sensitive marker of the oxidative DNA damage caused by reactive oxygen species (ROS) in sperm and testicular tissue (Sakamoto et al., 2008; Shen et al., 1999; Tugcu et al., 2010). Varicocele causes apoptosis in spermatogenic cells via the mitochondrial signal transduction pathway (Lee et al., 2009; Ning et al., 2017). Hsp70 is an apoptosis inhibitor and is constitutively expressed in spermatogenic cells during spermatogenesis (Dix et al., 1996; Huang et al., 2005). The reduction of HSP70 expression is also a biomarker of apoptosis in varicocele, resulting in male infertility (Purandhar et al., 2014).
It is necessary to find a non-surgical method to improve varicocele-induced testicular injury (Johnson and Sandlow, 2017). Administration of Ala-Gln before torsion/detorsion of the spermatic cord decreases lipid peroxidation during ischemia and protects the testis from oxidative stress by upregulating glutathione (GSH) levels in during reperfusion (Leitao et al., 2011). We have found the effects of Ala-Gln on the treatment of varicocele in a rat model, including the increase in the activity of anti-oxidants SOD and CAT, the decrease in the concentration of oxidant 8-OHDG, the enhancement of anti-apoptotic HSP70 expression, and the inhibition of apoptosis in spermatogenic cells. Our results suggest that Ala-Gln might be valuable for further study in the treatment of varicocele.
We have established a rat varicocele model and administrated Ala-Gln daily for 1 week after varicocele. We have found that Ala-Gln increases the activity of antioxidants SOD and CAT, decreases the concentration of oxidant 8-OHDG, enhances HSP70 expression, and inhibits apoptosis in spermatogenic cells. Therefore, Ala-Gln might be valuable for further study in varicocele treatment.
Availability of Data and Materials Statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abd-Elmoaty MA, Saleh R, Sharma R, Agarwal A. (2010). Increased levels of oxidants and reduced antioxidants in semen of infertile men with varicocele. Fertility and Sterility 94: 1531–1534. DOI 10.1016/j.fertnstert.2009.12.039. [Google Scholar] [CrossRef]
Aebi H. (1984). Catalase in vitro. Methods in Enzymology 105: 121–126. [Google Scholar]
Agarwal A, Said TM. (2003). Role of sperm chromatin abnormalities and DNA damage in male infertility. Human Reproduction Update 9: 331–345. DOI 10.1093/humupd/dmg027. [Google Scholar] [CrossRef]
Agarwal A, Sharma R, Harlev A, Esteves SC. (2016). Effect of varicocele on semen characteristics according to the new 2010 World Health Organization criteria: A systematic review and meta-analysis. Asian Journal of Andrology 18: 163–170. DOI 10.4103/1008-682X.172638. [Google Scholar] [CrossRef]
Albers S, Wernerman J, Stehle P, Vinnars E, Furst P. (1989). Availability of amino acids supplied by constant intravenous infusion of synthetic dipeptides in healthy man. Clinical Science 76: 643–648. DOI 10.1042/cs0760643. [Google Scholar] [CrossRef]
Amin M, Razi M, Sarrafzadeh-Rezaei F, Shalizar Jalali A, Najafi G. (2018). Berberine inhibits experimental varicocele-induced cell cycle arrest via regulating cyclin D1, cdk4 and p21 proteins expression in rat testicles. Andrologia 50: e12984. DOI 10.1111/and.12984. [Google Scholar] [CrossRef]
Chelikani P, Fita I, Loewen PC. (2004). Diversity of structures and properties among catalases. Cellular and Molecular Life Sciences 61: 192–208. DOI 10.1007/s00018-003-3206-5. [Google Scholar] [CrossRef]
De Maio A. (1999). Heat shock proteins: Facts, thoughts, and dreams. Shock 11: 1–12. DOI 10.1097/00024382-199901000-00001. [Google Scholar] [CrossRef]
Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, Poorman-Allen P, Goulding EH, Eddy EM. (1996). Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proceedings of the National Academy of Sciences of the United States of America 93: 3264–3268. DOI 10.1073/pnas.93.8.3264. [Google Scholar] [CrossRef]
Erata GO, Kocak Toker N, Durlanik O, Kadioglu A, Aktan G, Aykac Toker G. (2008). The role of heat shock protein 70 (Hsp 70) in male infertility: Is it a line of defense against sperm DNA fragmentation? Fertility and Sterility 90: 322–327. DOI 10.1016/j.fertnstert.2007.06.021. [Google Scholar] [CrossRef]
Feng HL, Sandlow JI, Sparks AET. (2001). Decreased expression of the heat shock protein hsp70-2 is associated with the pathogenesis of male infertility. Fertility and Sterility 76: 1136–1139. DOI 10.1016/S0015-0282(01)02892-8. [Google Scholar] [CrossRef]
Gong J, Jing L. (2011). Glutamine induces heat shock protein 70 expression via O-GlcNAc modification and subsequent increased expression and transcriptional activity of heat shock factor-1. Minerva Anestesiologica 77: 488–495. [Google Scholar]
Hassanpour H, Bigham Sadegh A, Karimi I, Heidari Khoei H, Karimi A, Edalati Shaarbaf P, Karimi Shayan T. (2017). Comparative expression analysis of HSP70, HSP90, IL-4, TNF, KITLG and KIT-receptor gene between varicocele-induced and non-varicocele testes of dog. International Journal of Fertility & Sterility 11: 148–155. [Google Scholar]
Hendin BN, Kolettis PN, Sharma RK, Thomas Jr. AJ, Agarwal A. (1999). Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. Journal of Urology 161: 1831–1834. DOI 10.1016/S0022-5347(05)68818-0. [Google Scholar] [CrossRef]
Huang SY, Tam MF, Hsu YT, Lin JH, Chen HH, Chuang CK, Chen MY, King YT, Lee WC. (2005). Developmental changes of heat-shock proteins in porcine testis by a proteomic analysis. Theriogenology 64: 1940–1955. DOI 10.1016/j.theriogenology.2005.04.024. [Google Scholar] [CrossRef]
Jing L, Wu Q, Wang F. (2007). Glutamine induces heat-shock protein and protects against Escherichia coli lipopolysaccharide-induced vascular hyporeactivity in rats. Critical Care 11: R34. DOI 10.1186/cc5717. [Google Scholar] [CrossRef]
Johnson D, Sandlow J. (2017). Treatment of varicoceles: Techniques and outcomes. Fertility and Sterility 108: 378–384. DOI 10.1016/j.fertnstert.2017.07.020. [Google Scholar] [CrossRef]
Kao SH, Chao HT, Chen HW, Hwang TI, Liao TL, Wei YH. (2008). Increase of oxidative stress in human sperm with lower motility. Fertility and Sterility 89: 1183–1190. DOI 10.1016/j.fertnstert.2007.05.029. [Google Scholar] [CrossRef]
Kheradmand A, Alirezaei M, Asadian P, Rafiei Alavi E, Joorabi S. (2009). Antioxidant enzyme activity and MDA level in the rat testis following chronic administration of ghrelin. Andrologia 41: 335–340. DOI 10.1111/j.1439-0272.2009.00932.x. [Google Scholar] [CrossRef]
Khosravanian H, Razi M, Farokhi F, Khosravanian N. (2015). Simultaneous administration of dexamethasone and vitamin E reversed experimental varicocele-induced impact in testicular tissue in rats; correlation with Hsp70-2 chaperone expression. International Brazilian Journal of Urology 41: 773–790. DOI 10.1590/S1677-5538.IBJU.2013.0148. [Google Scholar] [CrossRef]
Lee JD, Lee TH, Cheng WH, Jeng SY. (2009). Involved intrinsic apoptotic pathway of testicular tissues in varicocele-induced rats. World Journal of Urology 27: 527–532. DOI 10.1007/s00345-008-0367-8. [Google Scholar] [CrossRef]
Leitao JP, Santos JM, Vasconcelos RC, Garcia JH, Vasconcelos PR, Guimaraes SB. (2011). L-alanyl-glutamine dipeptide pretreatment attenuates ischemia-reperfusion injury in rat testis. Acta Cirurgica Brasileira 26: 21–25. DOI 10.1590/S0102-86502011000700005. [Google Scholar] [CrossRef]
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. (1997). Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489. DOI 10.1016/S0092-8674(00)80434-1. [Google Scholar] [CrossRef]
Ning JZ, Rao T, Cheng F, Yu WM, Ruan Y, Yuan R, Zhu SM, Du Y, Xiao CC. (2017). Effect of varicocelectomy treatment on spermatogenesis and apoptosis via the induction of heat shock protein 70 in varicocele-induced rats. Molecular Medicine Reports 16: 5406–5412. DOI 10.3892/mmr.2017.7239. [Google Scholar] [CrossRef]
Perriello G, Jorde R, Nurjhan N, Stumvoll M, Dailey G, Jenssen T, Bier DM, Gerich JE. (1995). Estimation of glucose-alanine-lactate-glutamine cycles in postabsorptive humans: role of skeletal muscle. American Journal of Physiology 269: C443–C450. DOI 10.1152/ajpcell.1995.269.2.C443. [Google Scholar] [CrossRef]
Pires VL, Souza JR, Guimaraes SB, Silva Filho AR, Garcia JH, Vasconcelos PR. (2011). Preconditioning with L-alanyl-L-glutamine in a Mongolian gerbil model of acute cerebral ischemia/reperfusion injury. Acta Cirurgica Brasileira 26: 14–20. DOI 10.1590/S0102-86502011000700004. [Google Scholar] [CrossRef]
Purandhar K, Jena PK, Prajapati B, Rajput P, Seshadri S. (2014). Understanding the role of heat shock protein isoforms in male fertility, aging and apoptosis. The World Journal of Men's Health 32: 123–132. DOI 10.5534/wjmh.2014.32.3.123. [Google Scholar] [CrossRef]
Rogero MM, Tirapegui J, Pedrosa RG, Castro IA, Pires IS. (2006). Effect of alanyl-glutamine supplementation on plasma and tissue glutamine concentrations in rats submitted to exhaustive exercise. Nutrition 22: 564–571. DOI 10.1016/j.nut.2005.11.002. [Google Scholar] [CrossRef]
Sadek A, Almohamdy AS, Zaki A, Aref M, Ibrahim SM, Mostafa T. (2011). Sperm chromatin condensation in infertile men with varicocele before and after surgical repair. Fertility and Sterility 95: 1705–1708. DOI 10.1016/j.fertnstert.2011.01.008. [Google Scholar] [CrossRef]
Sakamoto Y, Ishikawa T, Kondo Y, Yamaguchi K, Fujisawa M. (2008). The assessment of oxidative stress in infertile patients with varicocele. BJU International 101: 1547–1552. DOI 10.1111/j.1464-410X.2008.07517.x. [Google Scholar] [CrossRef]
Salmani S, Razi M, Sarrafzadeh-Rezaei F, Mahmoudian A. (2020). Testosterone amplifies HSP70-2a, HSP90 and PCNA expression in experimental varicocele condition: Implication for DNA fragmentation. Reproductive Biology 20: 384–395. DOI 10.1016/j.repbio.2020.04.007. [Google Scholar] [CrossRef]
Shamsi-Gamchi N, Razi M, Behfar M. (2018). Testicular torsion and reperfusion: evidences for biochemical and molecular alterations. Cell Stress and Chaperones 23: 429–439. DOI 10.1007/s12192-017-0855-0. [Google Scholar] [CrossRef]
Shen HM, Chia SE, Ong CN. (1999). Evaluation of oxidative DNA damage in human sperm and its association with male infertility. Journal of Andrology 20: 718–723. [Google Scholar]
Sigman MHS. (1998). Male infertility. In: Walsh Pc GR, Pelmutter AD, eds. Campbell’s Urology. 7thedition, Philadelphia: WB Saunders. [Google Scholar]
Sun Y, Oberley LW, Li Y. (1988). A simple method for clinical assay of superoxide dismutase. Clinical Chemistry 34: 497–500. DOI 10.1093/clinchem/34.3.497. [Google Scholar] [CrossRef]
Tugcu V, Gedikbasi A, Mutlu B, Guner E, Uhri M, Andican G, Ozbek E, Tasci AI. (2010). Increased testicular 8-hydroxy-2'-deoxyguanosine (8-OHdG) and inducible nitric oxide synthetase (iNOS) and nuclear factor κB (NF-κB) expressions in experimental rat varicocele. Archivio Italiano di Urologia e Andrologia 82: 148–153. [Google Scholar]
Wang H, Dong Y, Cai Y. (2017). Alanyl-glutamine prophylactically protects against lipopolysaccharide-induced acute lung injury by enhancing the expression of HSP70. Molecular Medicine Reports 16: 2807–2813. DOI 10.3892/mmr.2017.6896. [Google Scholar] [CrossRef]
Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB. (2001). Glutamine induces heat shock protein and protects against endotoxin shock in the rat. Journal of Applied Physiology 90: 2403–2410. DOI 10.1152/jappl.2001.90.6.2403. [Google Scholar] [CrossRef]
Wu C, Kato TS, Ji R, Zizola C, Brunjes DL, Deng Y, Akashi H, Armstrong HF, Kennel PJ, Thomas T, Forman DE, Hall J, Chokshi A, Bartels MN, Mancini D, Seres D, Schulze PC. (2015). Supplementation of l-alanyl-l-glutamine and fish oil improves body composition and quality of life in patients with chronic heart failure. Circulation: Heart Failure 8: 1077–1087. DOI 10.1161/CIRCHEARTFAILURE.115.002073. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |