DOI:10.32604/biocell.2021.010701

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.010701 |  www.techscience.com/journal/biocell |
| Article |
A comparative pattern of lectin-binding in the endometrial glands of the uterus and placenta of healthy buffaloes and bovines at early gestation
1Laboratory of Pathology, Experimental Station Mercedes, Instituto Nacional de Tecnología Agropecuaria, Corrientes, 3470, Argentina
2Laboratory of Theriogenology, School of Veterinary Sciences, Universidad Nacional del Nordeste, Corrientes, W3402BKG, Argentina
3Animal Health Group, Experimental Station Balcarce, Instituto Nacional de Tecnología Agropecuaria, Balcarce, B7620EMA, Argentina
4Laboratory of Histology and Embryology, School of Veterinary Sciences, Universidad Nacional de La Plata, La Plata, 1900, Argentina
*Address correspondence to: Claudio Gustavo Barbeito, barbeito@fcv.unlp.edu.ar
Received: 21 March 2020; Accepted: 02 November 2020
Abstract: Water buffalo (Bubalus bubalis) and domestic cattle (Bos taurus) are closely related species. However, embryo transfer interspecies has been attempted without any success. The failure in hybrid embryo-implantation is associated with the glycocode in the maternal-fetal interface. Glycosylation patterns have been studied in different species of ruminants; however, in B. bubalis, only the binucleated cells (BNC) have been analyzed. This glycocode is essential for a successful embryo-implantation and can be defined by Lectin-Histochemistry (LHC). The aim of this study is to compare the glycosylation pattern of placenta and uterus in water buffaloes and cattle by LHC. Tissue samples of placenta and uterus from pregnant Mediterranean female water buffaloes (Buf1) and Angus cows (Bov1) were analyzed. All animals were euthanized at 98 days of gestation. LHC was carried out using twelve lectins (Con A, LCA, PSA, sWGA, PHA-e, SBA, UEA-1, WGA, RCA-1, PNA, DBA, BSA-1). The intensity of lectin binding was semi-quantitatively scored using a scale of 0 (negative) to 3 (strongly positive). Difference between species was found in trophoblast layer by PSA, SWGA, PNA and BSA-1, in BNC, and in the mononuclear cells by LCA, PSA, PHA-e, DBA, BSA-1, PNA. In utero, differences in the apical cellular membrane and the secretion of glands were identified by DBA and RCA-1, and in the cytoplasm of those glandular epithelial cells by PHA-e, BSA-1, WGA, and SBA. In both species, BNC presented a strong positive reaction with DBA and SBA, a moderate response by LCA, PHA-e, BSA-1 and PNA lectin, and a low reaction by PSA, UEA-1, SWGA, WGA, Con A and RCA-1. The results found in this study suggest that although both species are closely related, glycosylation patterns in the placenta and uterus are different, thus providing a possible reason for embryo transfer not being possible between these species.
Keywords: Lectin-histochemistry; Water buffalo; Placenta; Glycosylation pattern
Water buffalo (Bubalus bubalis) is a species of high importance in beef and milk production due to their better adaption to hot and humid climate than bovines (Bos taurus) and their ability to live and to fatten in marshes and rivers with a poor quality of forage (Konrad et al., 2012). Bovines and water buffaloes have a close phylogenetic relationship but cannot interbreed. There are several differences, such as karyotype (2n:50 in B. bubalis and 2n:60 in B. taurus) or the length of gestation, which is approximately 3–4 weeks longer in buffaloes than in bovines (Schmidt et al., 2006). However, the placenta in both species is synepitheliochorial. This kind of placenta is characterized by the fusion of maternal endometrial cells and fetal trophoblast in the placentome, but remaining large zones of simple apposition in the intercotyledonary areas of the mature placenta (Wooding, 1992; Wooding and Burton, 2008). Macroscopically, both species present similar features (Schmidt et al., 2006) such as to be polycotyledonary, with a similar number of placentomes (92–150 in water buffaloes and 80–140 in cattle), and sharing antigens, which allows that antisera raised against bovine BNC proteins react with buffalo binucleated cells (BNC). As a result of the similarities in the placentation with cattle and the ability to adapt to harsh climates, water buffalo was evaluated as a possible receptor of pure-bred and high-value cattle embryos in wet tropical and subtropical regions, where B. taurus presents difficulties of adaptation. However, all attempts were unsuccessful (Drost et al., 1986). According to Jones and Aplin (2009), glycan expression in the tissue layers may be one of the factors that prevent interbreeding between species because the maternofetal compatibility of the glycocode is essential for the embryonic implantation. The interaction between the trophoblast and endometrial cells depends on a specific glycocode, and the lack of complementarity between them could cause failures in the embryo-implantation in some hybrid species (Jones and Aplin, 2009; Wu et al., 2016). On the other hand, the successful implantation of the hybrid embryo seems to be related to a similar glycosylation pattern between species increasing the chances of implantation whether the implanting embryo has outer trophectoderm cells from the recipient species (Jones and Aplin, 2009). Some hybrids such as the mule, resulting from crossbreeding between the horse (Equus caballus) and the donkey (Equus asinus) have been produced for centuries, but others were created more recently like intergenic chimeras such as between sheep (Ovis aries) and goat (Capra hircus) (Fehilly et al., 1984), or interspecies chimeras in murine species, such as Mus musculus and Mus caroli (Rossant et al., 1983). There are also some hybrids between bovine species, such as Bos indicus (Williams et al., 1990) or Bison bison (Jainudeen and Hafez, 2000) with B. taurus. However, although hybrid embryos between B. taurus and B. bubalis were produced in vitro (Kochhar et al., 2002), neither insemination nor embryo transference resulted in viable pregnancies (Drost et al., 1986; Patil and Totey, 2003).
Lectin-histochemistry (LHC) is mainly used to define the presence of glycoconjugate substances associated with different stages and/or secretion of the cells. Different studies have been performed for the placenta of other species of ruminants, such as bovines, pronghorn, giraffes, and okapis (Jones et al., 2015; Jones et al., 2017). However, in the case of water buffaloes, these studies were partial, and only BNC were analyzed (Carvalho et al., 2006). Therefore, the aim of this study was to compare the glycosylation pattern of placenta and uterus of water buffaloes and bovine at the first trimester of gestation (98 days) by LHC.
Two adult Mediterranean female water buffaloes (B. bubalis) (Buf1) and two Angus cows (B. taurus) (Bov1) from healthy and reproductively normal herds aged between 5 and 7 years old were randomly selected from a group of animals inseminated on the same day.
All animals were confirmed pregnant 30 days after artificial insemination and examined weekly between the 30 to 98 days of gestation (dg) by serial ultrasound scans. At 98 dg, all the animals were sent to a commercial slaughterhouse where both the placenta and uterus were collected. Tissues were analyzed in the post-mortem room, and five samples from cotyledon, intercotyledonary area, and endometrium from every placenta and uterus respectively were taken and stored inside of standard histological cassettes and fixed in 10% formalin solution.
LHC was carried out as previously described (Diaz et al., 2017; Fernández et al., 2014). Briefly, genital tracts were collected, dissected, fixed in 10% buffered, dehydrated through graded alcohol and xylene, and embedded in paraffin wax. Sections (5 µm) were dewaxed and treated with 0.3% hydrogen peroxide in methanol for 30 min at room temperature (to inhibit the endogenous peroxidase) and rinsed four times in 0.01 M phosphate-buffered saline (PBS) (pH 7.2). The sections were then incubated for 1 h at room temperature (RT) with biotinylated lectins. A pattern of twelve lectins (Lectin Kit BK 1000; Vectorlabs, Burlingame, CA, USA) (Tab. 1) were assessed. Lectin concentration was 30 mg/mL in PBS for all lectins except PNA, for which the concentration was 10 mg/mL. The slides were incubated with an avidin-biotin-peroxidase complex (ABC, Vectorlabs, Burlingame, CA, USA) for 45 min at RT. The horseradish peroxidase was activated by incubation for 1–2 min with a diaminobenzidine commercial kit (Dako, Carpinteria, CA, USA). Specimens were rinsed in distilled water, dehydrated with graded ethanol solutions, cleared in xylene, and mounted in Permount® (Fisher Scientific International, Hampton, NH, USA). Negative control samples were similarly processed but without lectin incubation. The intensity of lectin binding was semi-quantitatively scored according to the following scale: 0 (negative), 1 (lightly positive), 2 (moderately positive), and 3 (strongly positive) (Cobo et al., 2004). In addition, a sample of every tissue was stained with hematoxylin-eosin (H&E) for histology assessment.
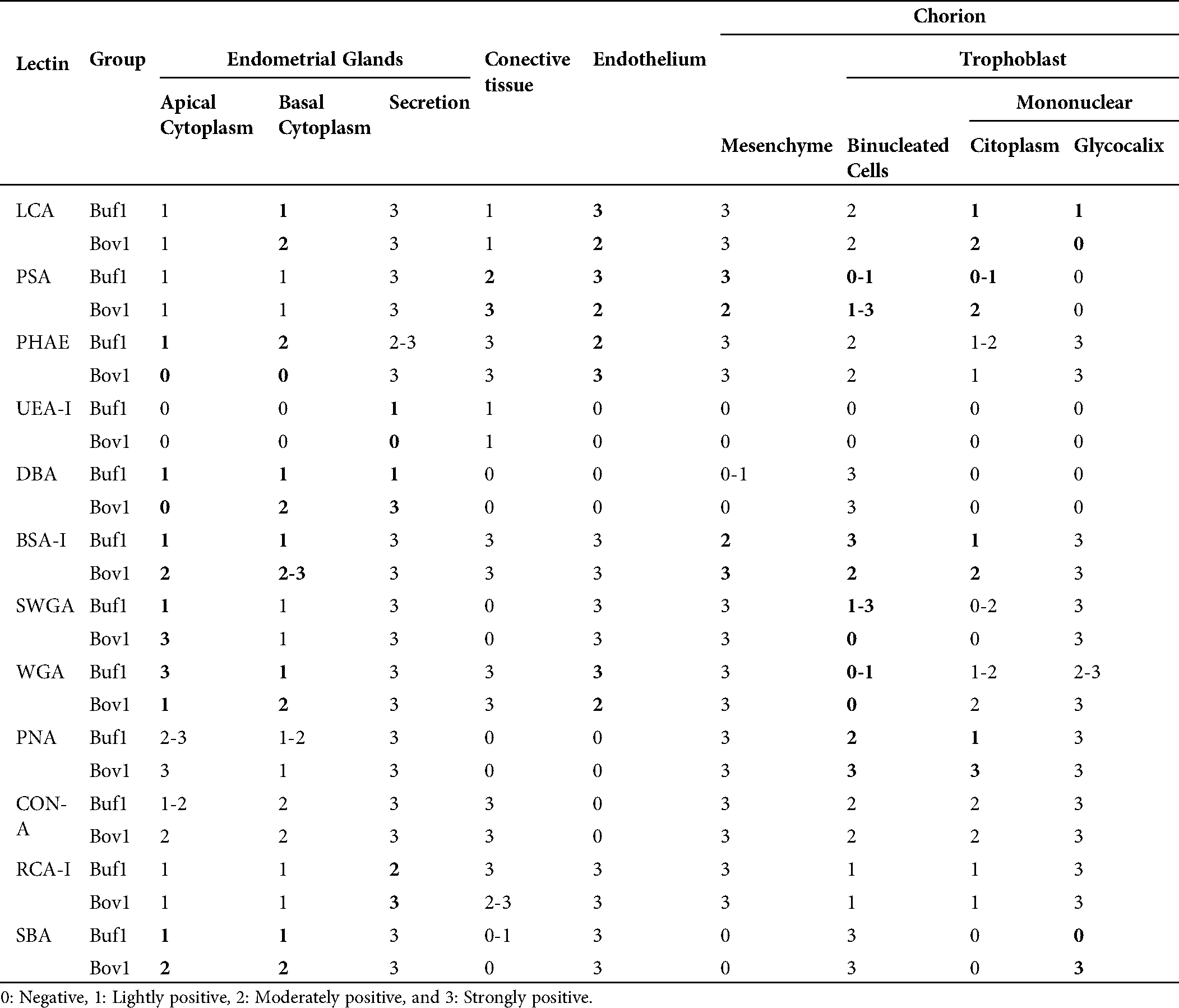
Table 1: Lectins used in this study and their major specificities
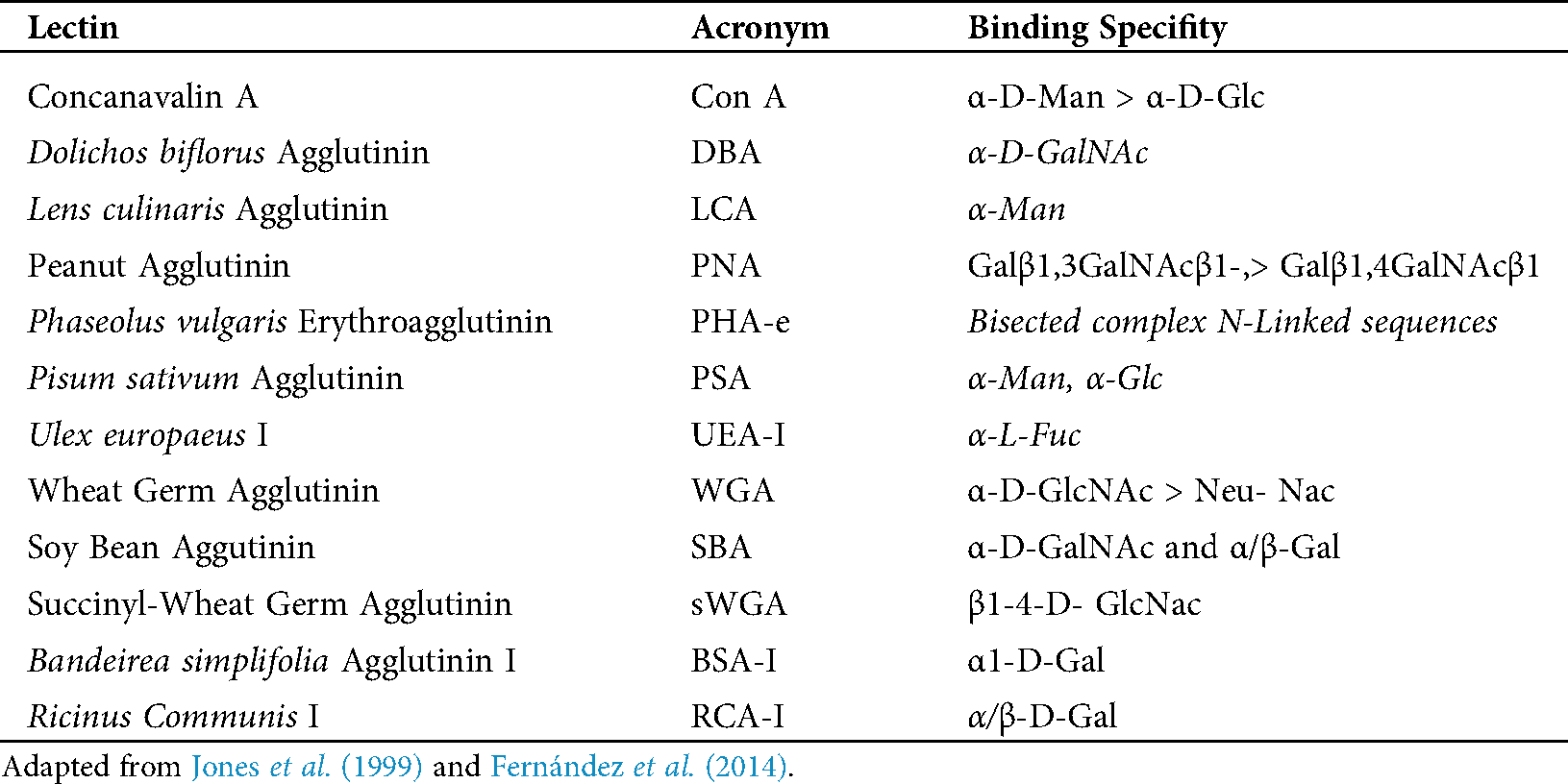
In the chorionic epithelium, the intensity of the lectins’ response showed differences between species. BNC presented differences by PSA, SWGA, PNA and BSA-1 (Fig. 1). The mononuclear cells showed also differences in the cytoplasm but with LCA, PSA, PHA-e, DBA, BSA-1, PNA lectins, and in the glycocalyx of these cells with SBA (Fig. 1).
In the endometrial glandular cells, differences in intensity were also presented in the apical cellular membrane and the glandular secretion by DBA and RCA-1 lectins and in the cellular cytoplasm of the glandular cells by PHA-e, BSA-1, WGA, and SBA lectins (Figs. 2 and 3). The lectin binding pattern for the twelve lectins in the placenta and endometrial glands of buffaloes and cattle are summarized in Tab. 2.
No significative pathological lesions were observed, neither gross nor histologically (HE-staining).
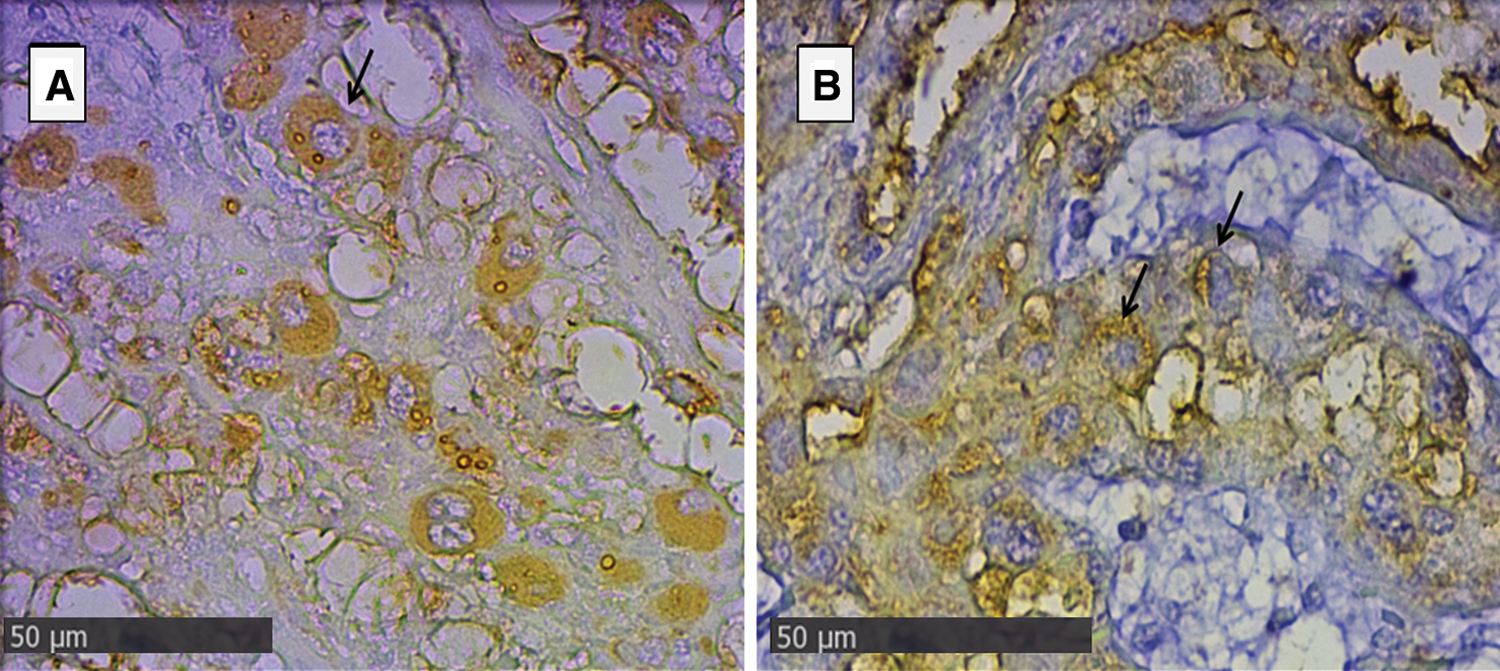
Figure 1: Trophoblast of placenta from groups Bov1 (A) and Bub1 (B).
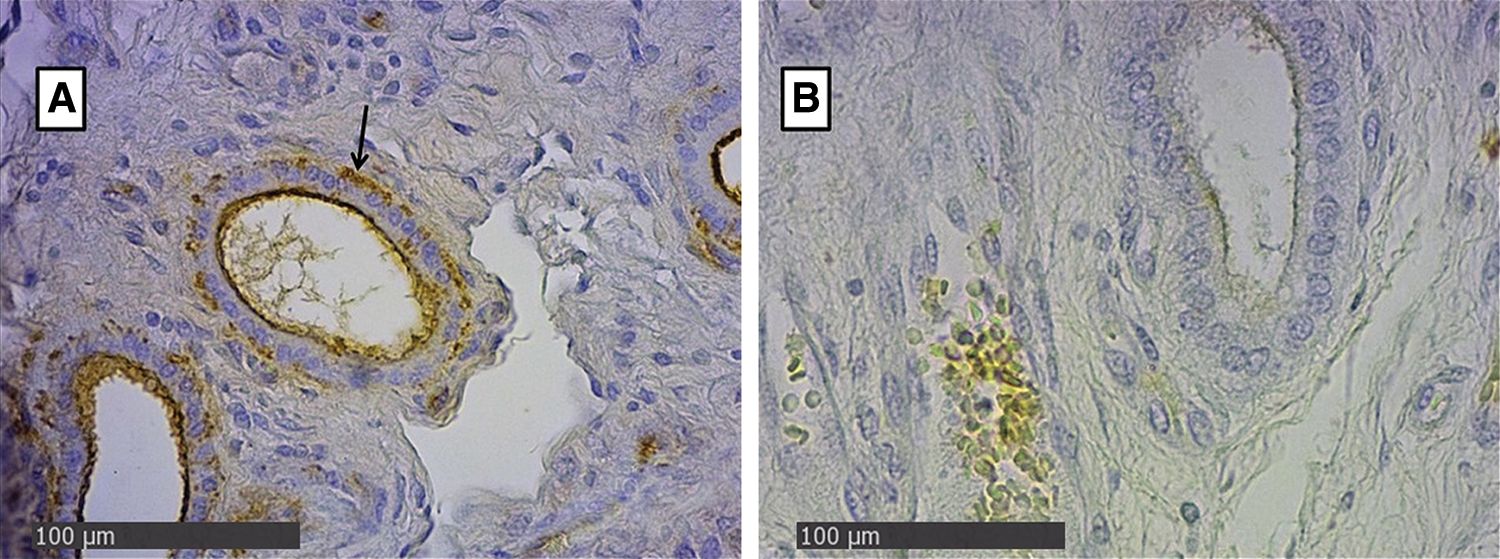
Figure 2: Endometrial glands of groups Bov1 (A) and Buf1 (B).
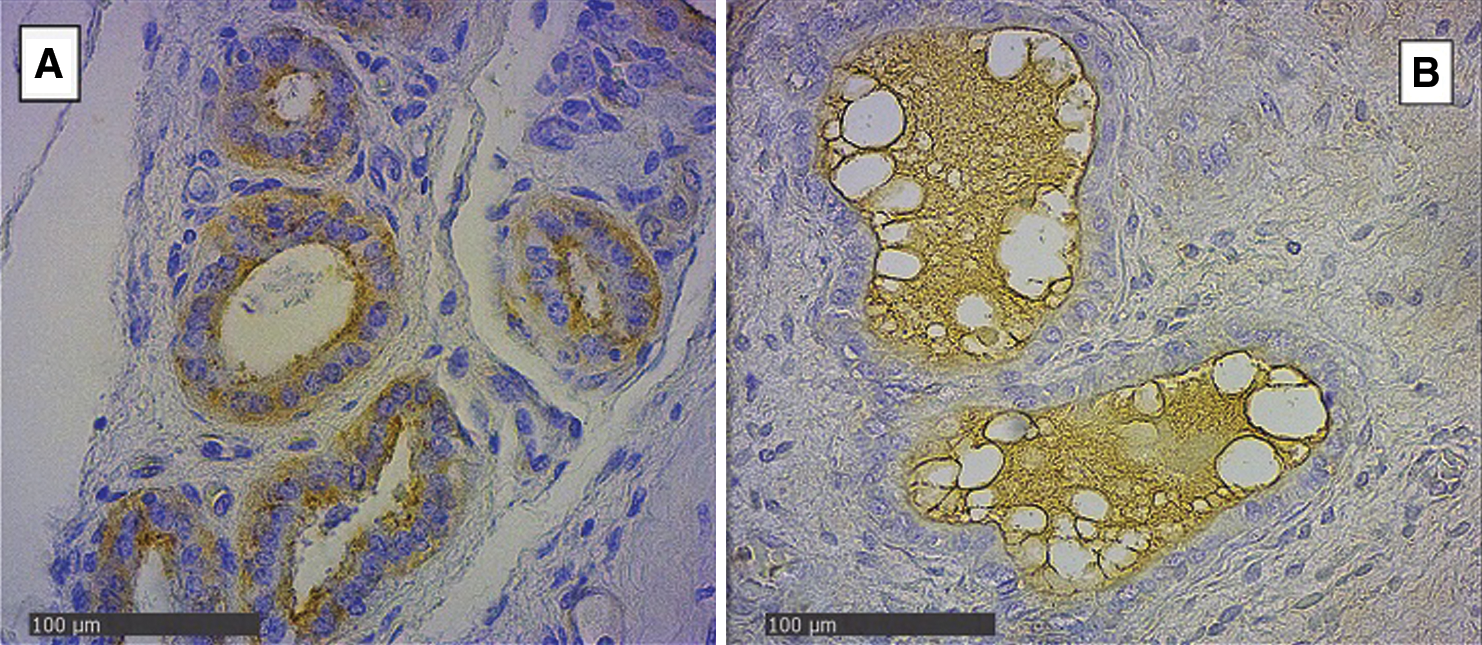
Figure 3: Endometrial glands of groups Bov1 (A) and Buf1 (B). Strongly positive staining in basal and apical cytoplasm of endometrial cells, and B, only secretion of glandular endometrial cells shows a light positive staining. SBA Lectin (400×).
Table 2: Lectin staining of trophoblast and endometrial glands in water buffaloes and cattle
Water buffalo production has exponentially increased in the last decade. This increase has been based on their capacity to adapt to subtropical and tropical climates, where bovine production could be limited (Konrad et al., 2012). Additionally, this species appears to be resistant to some diseases, showing, for example, a lower rate of abortion than bovines to Neosporosis despite being susceptible to the infection (Reichel et al., 2015). However, the origin of this difference is not entirely understood. The differences between species found in the pattern of saccharides during gestation could be an explanation of this feature because of the relationship between microorganisms and receptors in the host cells in the initial infection and the following pathogenesis associated with the infection (Cobo et al., 2004).
The high reactivity of the placenta and endometrial tissues to lectin binding in different species denotes the active function of the glycosides as components for recognition during the union between cells, as in the process of placental remodeling that occurs in the gestation of many species (Fernández et al., 2014; Munson et al., 1989) as well as the cellular recognition by the pathogens and in gestational losses (Cobo et al., 2004; Woudwyk et al., 2015). The LHC technique is used to recognize the distribution and concentration of glycosides as an indirect method to evaluate pathological changes due to pathogen infections in the placenta that could affect the pregnancy (Calkavur et al., 2015; Cobo et al., 2004). Consequently, the definition of a normal pattern is essential to analyze the variations in glycosylation at the maternofetal interface caused by microorganisms, chemicals, or another pathological agent.
The LHC patterns have been analyzed in different species, including dogs (Fernández et al., 2000), domestic cats (Fernández et al., 2014), cattle (Munson et al., 1989), elephants (Jones et al., 2004b), hyenas (Enders et al., 2006), horses (Jones et al., 1999), camelids (Jones et al., 2002), and peccary (Jones et al., 2004a), among others. Although cattle and water buffaloes are close phylogenetically, being both in the Family Bovidae, they have some differences such as in karyotype or the length of gestation (Carvalho et al., 2006). Consequently, the glycosylation patterns in parts of the genital tract or the placenta could not be directly extrapolated from one species to the other as demonstrated in the case of giraffe and okapis, which show several differences suggesting that embryo transfer would not be successful between them (Jones et al., 2015). Previously, in water buffaloes, the pattern of glycosylation had been determined only in BNC (Carvalho et al., 2006). In this study, the presence of glycosides in other parts of the placentome was evaluated and compared with those of cattle, a species which has been well documented following the example of a previous study where patterns of glycosylation in related species were compared (Jones et al., 2015).
In bovines, the glycosylation patterns in the placenta found in this study are similar to those reported by different authors (Jones et al., 2015; Munson et al., 1989), adding confidence to the results. In water buffaloes, like in other species of ruminants such as giraffe, okapi, African buffalo, greater Malayan chevrotain, deer, domestic goat, pronghorn, springbok, and impala, the BNC presented a high concentration of GalNac residues demonstrated as a strong positive reaction with DBA and SBA lectin (Jones et al., 2015; Jones et al., 2017; Klisch et al., 2010; Schmidt et al., 2006). These results were also consistent with those reported previously in water buffaloes (Carvalho et al., 2006). When comparing the positivity with other lectins, BNC in cattle and buffaloes expressed an abundant concentration of D-Man and/or D-Gluc (recognized by LCA), bisected bi-/tri- antennary, complex N-linked sequences (recognized by PHA-e), Galα, 3Gal- and /or Galα1, 4Gal (recognized by BSA-I), Galβ1,3GalNAcβ1 (recognized by PNA), and α-Mannose in non-bisected bi-/tri- antennary, complex N-linked sequences (recognized by PSA), H type 2 antigen [L-Fuc(1,2)Gal1,4GlcNAc1-] (recognized by UEA-1), β1-4-D- GlcNAc (recognized by SWGA), Di-N-acetyl chitobiose, N-acetyl lactosamine and/or some sialyl residues NAc (recognized by WGA), α-D-glucosyl and α-D-mannosyl residues in high mannose, intermediate and small complex N-linked sequences (recognized by Con-A), and β-D-Gal and/or α-D-Gal (recognized by RCA-1).
Although both species present similarities in the patterns of glycosylation, as were observed in other related species of ruminants, some differences were also found. Mononuclear cells expressed different concentrations of α-Man residues (recognized by LCA), non-bisected form of complex N-glycan D-GlcNAc (recognized by PSA), α-D-GalNAc (recognized by BSA-1), β1-4-D- GlcNAc (recognized by SWGA), Galβ1,3GalNAcβ1 (recognized by PNA) in their cytoplasm, as well as for α-Man residues (recognized by LCA) and α-D-GalNAc and α-or β-Gal (recognized by SBA) in their glycocalyx. Furthermore, endometrial glands also presented differences between species. Luminal content of these glands expressed a higher concentration of β-D-Gal; α-D-Gal (recognized by RCA-1) in bovines than in water buffaloes. In glandular cells, the concentration of bisected complex N- Linked sequences (recognized by PHA-e), α1-D Gal (recognized by BSA-1), β1-4-D- GlcNAc (recognized by SWGA), α-D-GlcNAc (recognized by WGA), and α-or β-Gal (recognized by SBA) presented differences between the species with the staining being more intense in the buffaloes for PHA-e, and in the apical cytoplasm for WGA and less intense for BSA-1, and SBA, and in the apical cytoplasm for WGA.
The glycosylation patterns of water buffalo and bovines present some similarities which could be associated with their close phylogenetic relationship. However, the differences found in this study suggests that both species diverged from each other and provides an explanation for the failure of the chimera between these two species.
The successful production of interspecies chimeras seems to be related to a complementary glycoside pattern that regulates the recognition between the trophectoderm of the blastocyst and the endometrium of the maternal uterus, making the implantation of the embryo viable (Jones and Aplin, 2009; Wu et al., 2016). In fact, the chances of successful embryonic implantation are greatly increased whether the implanting embryo has outer trophectoderm cells from the recipient species (Rossant et al., 1982; Rossant et al., 1983). Therefore, the unsuccessful hybrid or embryo transfers between these species (Drost et al., 1986; Patil and Totey, 2003) could be explained by the differences found in this study, in coincidence with those differences found in other species previously reported (Jones and Aplin, 2009).
Although the number of animals used for this study was evaluated to be the minimal necessary respecting the ethical issues and the economic limitations implied on the sacrifice of large animals, the sample size was sufficient to allow conclusions to be drawn from the experiment.
The findings of this study support the hypothesis, which claims that the failure in the embryo implantation between B. bubalis and B. taurus, as well as in other closely related and/or interbreeding species, is related to the difference in the glycosylation pattern in the maternal-fetal interface between these species. Additionally, this is the first study to provide a typical lectin pattern for uterus and placenta of water buffaloes, which can be used in future related studies.
Acknowledgement: The authors thank Dr. Karen Stevenson and Morag Livingstone from Moredun Research Institute, UK, for the English language suggestions.
Availability of Data and Materials: Data supporting in this article is detailed in this manuscript.
Copyright of Figures: All the figures and tables are original, and they have not presented in any place previously.
Ethics Approval: This study was carried out in strict accordance with the Animals (Scientific Procedures) Res N° 16/07 Disp. 174/09 of CICUAE–INTA. The experimental protocol was approved by the Animal Ethics Committee at the National Institute of Agricultural Technology (CICUAE-INTA Mercedes) (Permit No. E01/12). The number of animals was determined according to the 3Rs principles of animal research (Replacement, Reduction, and Refinement). All animals used in this study were handled in strict accordance with good animal practices and the conditions, were fed on natural pasture grass in one paddock, and maintained using standard animal husbandry procedures. Clean water was available ad libitum. Veterinary monitoring was performed daily for any clinical signs, receiving treatment for any secondary disease following standard veterinary practices.
Funding Statement: This study was funded by the UNLP V-273.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Calkavur S, Erdemir G, Onay H, Altun Koroglu O, Yalaz M, Zekioglu O, Aksu G, Ozkinay F, Akercan F, Kultursay N. (2015). Mannose-binding lectin may affect pregnancy outcome. Turkish Journal of Pediatrics 57: 26–33. [Google Scholar]
Carvalho AF, Klisch K, Miglino MA, Pereira FT, Bevilacqua E. (2006). Binucleate trophoblast giant cells in the water buffalo (Bubalus bubalis) placenta. Journal of Morphology 267: 50–56. DOI 10.1002/jmor.10387. [Google Scholar] [CrossRef]
Cobo ER, Campero CM, Gimeno EJ, Barbeito CG. (2004). Lectin binding patterns and immunohistochemical antigen detection in the genitalia of Tritrichomonas foetus-infected heifers. Journal of Comparative Pathology 131: 127–134. DOI 10.1016/j.jcpa.2004.02.003. [Google Scholar] [CrossRef]
Diaz MC, Gonzalez NV, Zanuzzi CN, Najle R, Barbeito CG. (2017). Lectin histochemistry for detecting cadmium-induced changes in the glycosylation pattern of rat placenta. Biotechnic and Histochemistry 92: 36–45. DOI 10.1080/10520295.2016.1185668. [Google Scholar] [CrossRef]
Drost M, Wright JM, Elsden RP. (1986). Intergeneric embryo transfer between water-buffalo and domestic cattle. Theriogenology 25: 13–23. DOI 10.1016/0093-691X(86)90180-9. [Google Scholar] [CrossRef]
Enders AC, Blankenship TN, Conley AJ, Jones CJ. (2006). Structure of the midterm placenta of the spotted hyena, Crocuta crocuta, with emphasis on the diverse hemophagous regions. Cells Tissues Organs 183: 141–155. DOI 10.1159/000095988. [Google Scholar] [CrossRef]
Fehilly CB, Willadsen SM, Tucker EM. (1984). Interspecific chimaerism between sheep and goat. Nature 307: 634–636. DOI 10.1038/307634a0. [Google Scholar] [CrossRef]
Fernández PE, Barbeito CG, Portiansky EL, Gimeno EJ. (2000). Intermediate filament protein expression and sugar moieties in normal canine placenta. Histology and Histopathology 15: 1–6. [Google Scholar]
Fernández PE, Diessler ME, Pachame A, Ortega HH, Gimeno EJ, Portiansky EL, Barbeito CG. (2014). Intermediate filament proteins expression and carbohydrate moieties in trophoblast and decidual cells of mature cat placenta. Reproduction in Domestic Animals 49: 263–269. DOI 10.1111/rda.12265. [Google Scholar] [CrossRef]
Jainudeen MR, Hafez ESE. (2000). Catttle and buffalo. In: Hafez B, Hafez ESE, eds. Reproduction in farm animals. 7th edition, Maryland, USA: Lippincott Williams and Wilkins. [Google Scholar]
Jones CJ, Abd-Elnaeim M, Bevilacqua E, Oliveira LV, Leiser R. (2002). Comparison of uteroplacental glycosylation in the camel (Camelus dromedarius) and alpaca (Lama pacos). Reproduction 123: 115–126. DOI 10.1530/rep.0.1230115. [Google Scholar] [CrossRef]
Jones CJ, Aplin JD. (2009). Glycosylation at the fetomaternal interface: Does the glycocode play a critical role in implantation? Glycoconjugate Journal 26: 359–366. DOI 10.1007/s10719-008-9152-6. [Google Scholar] [CrossRef]
Jones CJ, Santos TC, Abd-Elnaeim M, Dantzer V, Miglino MA (2004a). Placental glycosylation in peccary species and its relation to that of swine and dromedary. Placenta 25: 649–657. DOI 10.1016/j.placenta.2003.12.007. [Google Scholar] [CrossRef]
Jones CJ, Wilsher SA, Wooding FB, Benirschke K, Allen WR. (2015). The binucleate cell of okapi and giraffe placenta shows distinctive glycosylation compared with other ruminants: A lectin histochemical study. Molecular Phylogenetics and Evolution 83: 184–190. DOI 10.1016/j.ympev.2014.12.004. [Google Scholar] [CrossRef]
Jones CJ, Wooding FB, Dantzer V, Leiser R, Stoddart RW. (1999). A lectin binding analysis of glycosylation patterns during development of the equine placenta. Placenta 20: 45–57. DOI 10.1053/plac.1998.0354. [Google Scholar] [CrossRef]
Jones CJ, Wooding FB, Mathias SS, Allen WR (2004b). Fetomaternal glycosylation of early placentation events in the African elephant Loxodonta africana. Placenta 25: 308–320. DOI 10.1016/j.placenta.2003.10.005. [Google Scholar] [CrossRef]
Jones CJP, Silvia WJ, Hamilton CH, Geary TW, Zezeski AL, Wooding FBP. (2017). Glycosylation and immunocytochemistry of binucleate cells in pronghorn (Antilocapra americana, Antilocapridae) show features of both Giraffidae and Bovidae. Placenta 57: 216–222. DOI 10.1016/j.placenta.2017.07.011. [Google Scholar] [CrossRef]
Klisch K, Wooding FB, Jones CJ. (2010). The glycosylation pattern of secretory granules in binucleate trophoblast cells is highly conserved in ruminants. Placenta 31: 11–17. DOI 10.1016/j.placenta.2009.11.001. [Google Scholar] [CrossRef]
Konrad JL, Moore DP, Crudeli G, Caspe SG, Cano DB, Leunda MR, Lischinsky L, Regidor-Cerrillo J, Odeon AC, Ortega-Mora LM, Echaide I, Campero CM. (2012). Experimental inoculation of Neospora caninum in pregnant water buffalo. Veterinary Parasitology 187: 72–78. DOI 10.1016/j.vetpar.2011.12.030. [Google Scholar] [CrossRef]
Kochhar HP, Rao KA, Luciano A, Totey S, Gandolfi F, Basrur P, King W. (2002). In vitro production of cattle-water buffalo (Bos taurus—Bubalus bubalis) hybrid embryos. Zygote 102: 155–162. DOI 10.1017/S0967199402002216. [Google Scholar] [CrossRef]
Munson L, Kao JJ, Schlafer DH. (1989). Characterization of glycoconjugates in the bovine endometrium and chorion by lectin histochemistry. Journals of Reproduction and Fertility 87: 509–517. DOI 10.1530/jrf.0.0870509. [Google Scholar] [CrossRef]
Patil S, Totey S. (2003). Developmental failure of hybrid embryos generated by in vitro fertilization of water buffalo (Bubalus bubalis) oocyte with bovine spermatozoa. Molecular Reproduction and Development 64: 360–368. DOI 10.1002/mrd.10269. [Google Scholar] [CrossRef]
Reichel MP, Mcallister MM, Nasir A, Moore DP. (2015). A review of Neospora caninum in water buffalo (Bubalus bubalis). Veterinary Parasitology 212: 75–79. DOI 10.1016/j.vetpar.2015.08.008. [Google Scholar] [CrossRef]
Rossant J, Mauro VM, Croy BA. (1982). Importance of trophoblast genotype for survival of interspecific murine chimaeras. Development 69: 141–149. [Google Scholar]
Rossant J, Vijh M, Siracusa LD, Chapman VM. (1983). Identification of embryonic cell lineages in histological sections of M. musculus in-equilibrium M. caroli chimaeras. Development 73: 179–191. [Google Scholar]
Schmidt S, Gerber D, Soley JT, Aire TA, Boos A. (2006). Histo-morphology of the uterus and early placenta of the African buffalo (Syncerus caffer) and comparative placentome morphology of the African buffalo and cattle (Bos taurus). Placenta 27: 899–911. DOI 10.1016/j.placenta.2005.09.008. [Google Scholar] [CrossRef]
Williams TJ, Munro RK, Shelton JN. (1990). Production of interspecies chimeric calves by aggregation of Bos indicus and Bos taurus demi-embryos. Reproduction, Fertility and Development 2: 385–394. DOI 10.1071/RD9900385. [Google Scholar] [CrossRef]
Wooding FBP. (1992). The synepitheliochorial placenta of ruminants: Binucleate cell fusions and hormone production. Placenta 13: 101–113. DOI 10.1016/0143-4004(92)90025-O. [Google Scholar] [CrossRef]
Wooding FBP, Burton GJ. (2008). Comparative placentation: Structures, functions and evolution. Berlin: Springer Science and Business Media. [Google Scholar]
Woudwyk MA, Zanuzzi CN, Nishida F, Gimeno EJ, Soto P, Monteavaro CE, Barbeito CG. (2015). Apoptosis and cell proliferation in the mouse model of embryonic death induced by Tritrichomonas foetus infection. Experimental Parasitology 156: 32–36. DOI 10.1016/j.exppara.2015.05.013. [Google Scholar] [CrossRef]
Wu J, Greely HT, Jaenisch R, Nakauchi H, Rossant J, Belmonte JC. (2016). Stem cells and interspecies chimaeras. Nature 540: 51–59. DOI 10.1038/nature20573. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |