DOI:10.32604/biocell.2021.011379

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.011379 |  www.techscience.com/journal/biocell |
| Article |
Different sources of MSCs on pulmonary fibrosis in C57BL/6 mice
1The Center of Lung Cancer Prevention, Hebei Province Chest Hospital, Shijiazhuang, 050041, China
2College of Life Science, Hebei Normal University, Shijiazhuang, 050024, China
*Address correspondence to: Yonghui Yang, yonghui_yang8111@yahoo.com
Received: 04 May 2020; Accepted: 07 October 2020
#These authors contributed equally to this work
Abstract: Since stem cell therapy is the most effective treatment in the field of tissue reparation and reconstitution, the present study aimed to explore the different sources of mesenchymal stem cells (MSCs) on the different effects of pulmonary fibrosis-related cytokines in C57BL/6 mice. For reaching this goal, we isolated MSCs from umbilical cord blood and placenta and used for stem cell therapy in a mouse model of pulmonary fibrosis model. The pulmonary fibrosis model was done by injecting bleomycin into the trachea of C57BL/6 mice. Then we assessed the degree of pulmonary fibrosis in each mouse lung tissue at weeks 1, 2, 3, and 4. In addition, flow cytometry was used to evaluate the frequency of CD73, CD90, CD106, CD34, CD45, CD14 cells at the mononuclear cell level; and western blotting assays revealed the expression of IκB-α. Our results showed that stem cell therapy by placenta-derived MSC had a lower level of CD34, CD45, CD14 cells at the mononuclear cell level, and that improved pulmonary fibrosis at both molecular and pathological levels. In addition, western blotting assays revealed that the expression of IκB-α was down-regulated in MSC-treated animals. In addition, placenta-derived MSC was the most effective in improving pulmonary fibrosis in comparison to other sources. This study suggests that MSC might be a novel therapeutic approach in pulmonary fibrosis due to an enhanced anti-inflammatory effect. Also, MSC modification by gene editing could enhance their therapeutic effect in mouse pulmonary fibrosis.
Keywords: Pulmonary fibrosis; Umbilical cord blood; Umbilical cord placenta; Mesenchymal stem cells; C57BL/6 mice IκB-α
Pulmonary fibrosis is not a single disease; it is a general term for diseases under lung interstitial disease in medical science. Patients with pulmonary fibrosis have typically a low survival period of about 2 to 5 years (King Jr et al., 2009). The main reason for pulmonary interstitial fibrosis is the lung tissue damage causing a series of symptoms; the early stage symptom is diffuse alveolar, and the later stage is excessive proliferation of fibroblasts and the excessive deposition of extracellular matrix, which could cause respiratory failure (Wei and Liu, 2014). The common risk factors for pulmonary fibrosis include environmental, occupational, physical, and chemical, etc. Inflammation of lung injury could be caused by various reasons in the early time and in the late time of pulmonary fibrosis, inflammation is not obvious, and this may be an important cause of failure in the treatment of anti-inflammatory therapy of pulmonary fibrosis. The target of the new anti-fibrosis drug is to interfere, regulate, or even block the process of pulmonary fibrosis development to improve the quality of life of patients and increase lung function. At present, the main treatments of pulmonary fibrosis are anti-inflammatory drugs or immunosuppressive agents, anti-fibrosis drugs, anti-coagulation drugs, lung transplantation, and other measures (Brown and Raghu, 2004). Commonly used drugs include corticosteroids, cyclophosphamide, nitro imidazole sulfur pyrimidine, cyclosporin, mycophenolate mofetil, and colchicine and penicillamine, which can affect the formation of collagen (Selman et al., 2004). A good anti-fibrosis drug needs to meet the following criteria: Reducing the proliferation of lung fibroblast and muscle fibrosis cell and enhancing the apoptosis of lung fibroblast and muscle fibroblast; reducing the synthesis and deposition of the extracellular matrix. So far, there is no effective method to cure the disease.
Recent studies have been focused on stem cell therapy in the field of tissue reparation and reconstitution. Mesenchymal stem cells (MSC) are a kind of cells with highly undifferentiated; they can differentiate into different tissues or structures in different conditions. MSCs have lots of different sources, but their structure and function are roughly the same. There is a common type of bone marrow MSCs, umbilical cord MSCs, umbilical cord blood MSCs (He et al., 2013), among them, bone marrow MSCs also have hematopoietic and immune function (Xu et al., 2007). In recent years, many animal experiments have shown that MSCs can effectively participate in lung tissue repair and immune regulation (Abreu et al., 2011; Gharaee-Kermani et al., 2007; Rojas et al., 2005).
In this study, MSC derived from umbilical cord blood, umbilical cord, and placenta were used to evaluate the pulmonary fibrosis inhibition by using Giemsa staining and flow cytometry (MSC surface markers such as CD73, CD90, CD105 were evaluated). The mouse model of pulmonary fibrosis was established by injecting bleomycin in C57BL/6 mice, and flow cytometry tested the frequency of CD34, CD45, CD14 cell markers at the mononuclear cell level.
Fetal bovine serum was bought from Qingdao Kangyuan Pharmaceutical Co., (China); trypsin was bought from American SHELDON Corporation (USA); Antibodies (i.e., CD73, CD90, CD106, CD34, CD45, CD14) were bought from BD BioSciences (Basel, Switzerland).
The culture and identification of MSCs
The culture and identification of umbilical cord MSCs
Taking 10 cm umbilical cord from the normal healthy newborns and soaking it in the DMEM, then removing it in the ultra-clean bench, using PBS to wash the residual blood, stripping the vascular, and cutting the umbilical cord into pieces, digesting the pieces with 0.1% collagenase II, then according to volume ratio 1:4 mixing the filtrate and PBS, 2500 rpm centrifugation for 10 min, discarding supernatant and re-suspended cells with volume fraction 10% fetal bovine serum DMEN/F12 and then removing it to 25 cm2 breathable culture flask, putting it into the CO2 incubator with 37°C, the volume fraction of CO2 is 5% to culture. A week later, using Giemsa staining to observe the life state of the cells, and flow cytometry to test the cell surface whether there is the existence of surface markers CD73, CD90, CD106, CD34, CD45, CD14.
The culture and identification of umbilical cord blood MSCs
Packing healthy newborn umbilical cord blood whose volume is 100 mL into four centrifuge tube in the ultra-clean bench, then 3500 rpm centrifugation for 10 min, discarding the supernatant, separation liquid separates the cells from the umbilical cord blood, 2300 rpm centrifugation 23 min, putting tunica albuginea into the appropriate amount of saline, 2300 rpm centrifugation for 10 min, discarding the supernatant, finally, putting the cells in the culture medium laying in the CO2 incubator at 37°C, the volume fraction of 5% to culture. A week later, Giemsa staining was used to observe the life state of the cells, and flow cytometry to test the cell surface in terms of the existence of surface markers CD73, CD90, CD106, CD34, CD45, CD14.
The culture and identification of placenta MSCs
Taking the placenta tissue of normal birth under sterile conditions, cutting a few fetal placenta decidual tissue into pieces of 0.25% trypsin digest 10 min, filtering, collecting the cells, and washing it with PBS. Mononuclear cells were obtained by density gradient centrifugation, putting the mononuclear cells into the MSC special culture medium, laying in the 5% volume fraction of CO2 incubator with 37°C, the volume fraction of 5% to culture. A week later, Giemsa staining was used to observe the life state of the cells, and flow cytometry to test the cell surface in terms of the existence of surface markers CD73, CD90, CD106, CD34, CD45, CD14.
C57BL/6 mice model of pulmonary fibrosis
C57BL/6 mice, male, aged 6 to 8 weeks, with an average weight of 19.8 g, were utilized. C57BL/6 mice were treated with a single dose of 5 mg/kg bleomycin to induce pulmonary fibrosis by injecting into the trachea. Bleomycin was dissolved in sterile 0.9% saline and administered in 0.1 mL saline solution per animal. Control animals received 0.1 mL saline alone. Then, the mice were randomly divided into 4 groups, numbers 1–4, the first groups were injected with normal saline, groups 2, 3, 4 were injected with umbilical cord, umbilical cord blood and placenta mesenchymal stem cell suspension. The therapeutic effect of lung tissue reparation and reconstitution in pulmonary fibrosis mice by injection of three kinds of MSC (2 × 106, 4 × 106, 8 × 106) for 12 weeks through vena caudalis.
Each group of rats was respectively intervened by the corresponding measures, and renal histopathological change was observed.
The samples were collected animal euthanasia for 12 weeks of each experimental community by observing the lung tissue of each group to observe the degree of PDGF express every mouse with pulmonary fibrosis. Euthanasia was done by using 7 min exposure to CO2 and followed by cervical dislocation for confirmation of death. There were no requirements for any anesthetic drug. PDGF in renal tissue was detected by the HE-staining technique. Four groups were prepared and stained with hematoxylin-Eosin for assessment of inflammation and histological damage.
Single-cell suspensions from the three kinds of issues were stained with anti-CD73, CD90, CD106, CD34, CD45, CD14 antibody-fluorescein isothiocyanate (FITC) (clone XMG1.2, BD Biosciences) and antibody-PE (clone TC11-18H10, MiltenyiBiotec, Bergisch Gladbach, Germany) antibodies, following the manufacturer’s instructions. Isotype controls were included in all the experiments to adjust the background signal. Flow cytometer analysis was performed in a FACSCalibur instrument and analyzed with Cell Quest Pro software (BD Biosciences).
Lung tissue from the treated and the control group of the mice was frozen. PBS washed the issue and collected them separately. Protein lysate was added to the issue, and the supernatant was collected after centrifugation. Protein concentration was extracted by the BCA method. Then, the SDS-polyacrylamide gel was used to separate the protein of 30 μg per pore, and then separated by gel electrophoresis. After the separation, the protein in the gel was transferred to the PVDF membrane and sealed with the TBS made of 5% skim milk powder. The TBS diluted one PDGF antibody (1:1000) was incubated overnight at 4°C. After washing the membrane, 1H was incubated at room temperature of two 1:1000 (1:2000) diluted with TBS and then washed in the darkroom. Shadow, fixing, pressing, and film scanning. After scanning, the relative expression intensity (Rx) of the target protein was calculated by image quality software. The relative expression intensity (Rx) of the target protein was detected by comparing the gray value (Vx) of the target protein with the gray value of the control protein (Vβ-actin), that is, Rx = Vx/Vβ-actin.
A descriptive analysis of each experiment was performed to compare the incidence and severity of the disease. The impact of the clinical scores for the mice with pulmonary fibrosis and the course of the disease was analyzed by two-way analysis of variance (ANOVA) for repeated measures. Differences between the groups were compared using the Mann–Whitney’s U-test for two independent samples or one-way ANOVA using the Kruskal–Wallis non-parametric test, as required. The analysis was performed using GraphPad Prism software (version 6.0, Graph Pad; CA, USA). p-values below 0.05 were considered statistically significant.
The study was approved by the institutional review board (CWO) of Hebei Chest Hospital Ethics Committee, Shijiazhuang, The People’s Republic of China. All people provided written informed consent
Observation by inverted phase-contrast microscopy
Only spindle-shaped cells were present in the culture. The proportion of the nucleus and cytoplasm was large. The adherent cells’ growth is shown in Figs. 1A–1C. To observe the three kinds of MSCs on morphological characteristics, the cell morphology was observed under an inverted microscope on the 14th day. All the three kings of cells grew adherently and spirally. The third passages of the three kinds of MSCs were used. Because the third generation MSC of placenta mesenchymal has the strongest differentiation ability with more activity. All the following experiments used third-generation placental MSCs.
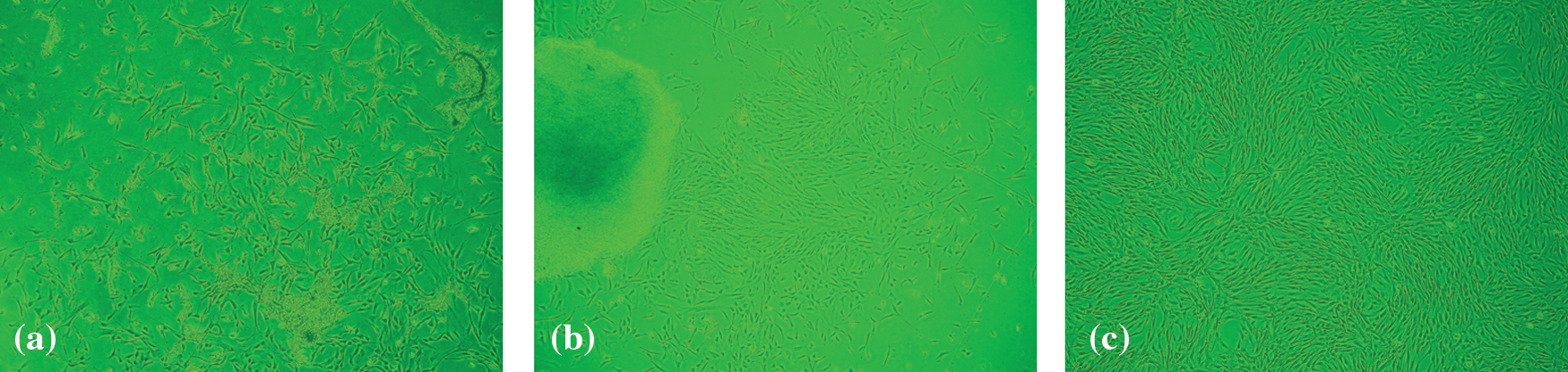
Figure 1: Morphological characteristics and the cell morphology of three sources of mesenchymal stem cells. (a) Umbilical cord blood mesenchymal stem cell. (b) Umbilical cord mesenchymal stem cell. (c) Placenta mesenchymal stem cell
The degrees of PDGF expression in the first group of the mice with pulmonary fibrosis is the most serious, the 2–3 group the degree of pulmonary fibrosis with an obvious difference by HE-staining.
The degrees of PDGF expression in the control group of the mice with pulmonary fibrosis is still the most serious. Compared with the first group, PDGF expression in the degrees of in Figs. 2B and 2C with pulmonary fibrosis is low, placenta mesenchymal stem cell mice pulmonary fibrosis degree is the lowest in Fig. 2D.
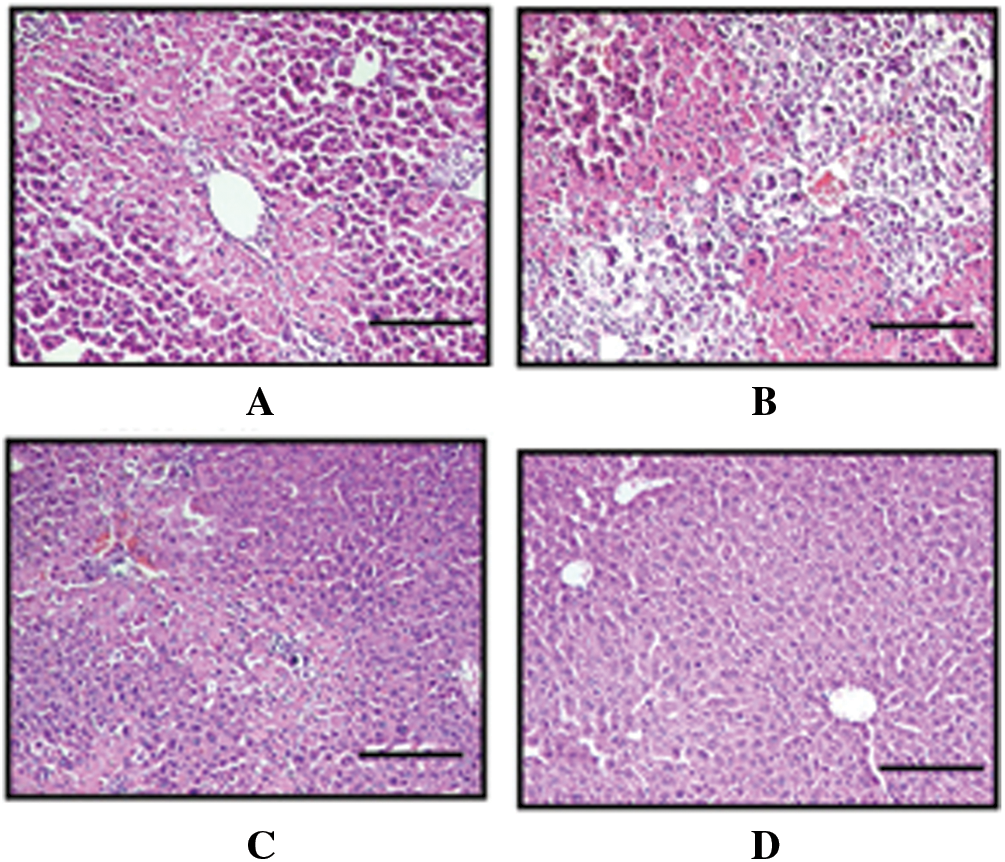
Figure 2: The degrees of PDGF expression in the control and treated mice group. A. Group 1. Phosphate buffer treated B. Group 2. Umbilical cord blood mesenchymal stem cell treated C. Group 3. Umbilical cord mesenchymal stem cell treated D. Group 4. Placenta mesenchymal stem celll stem cell treated
The immunophenotypic analysis showed that the MSCs used in the experiment were negative for the lineage markers CD34 and CD14, strongly positive for the specific surface antigens CD73, CD90, and CD105 (Fig. 3), which are typical of MSCs.
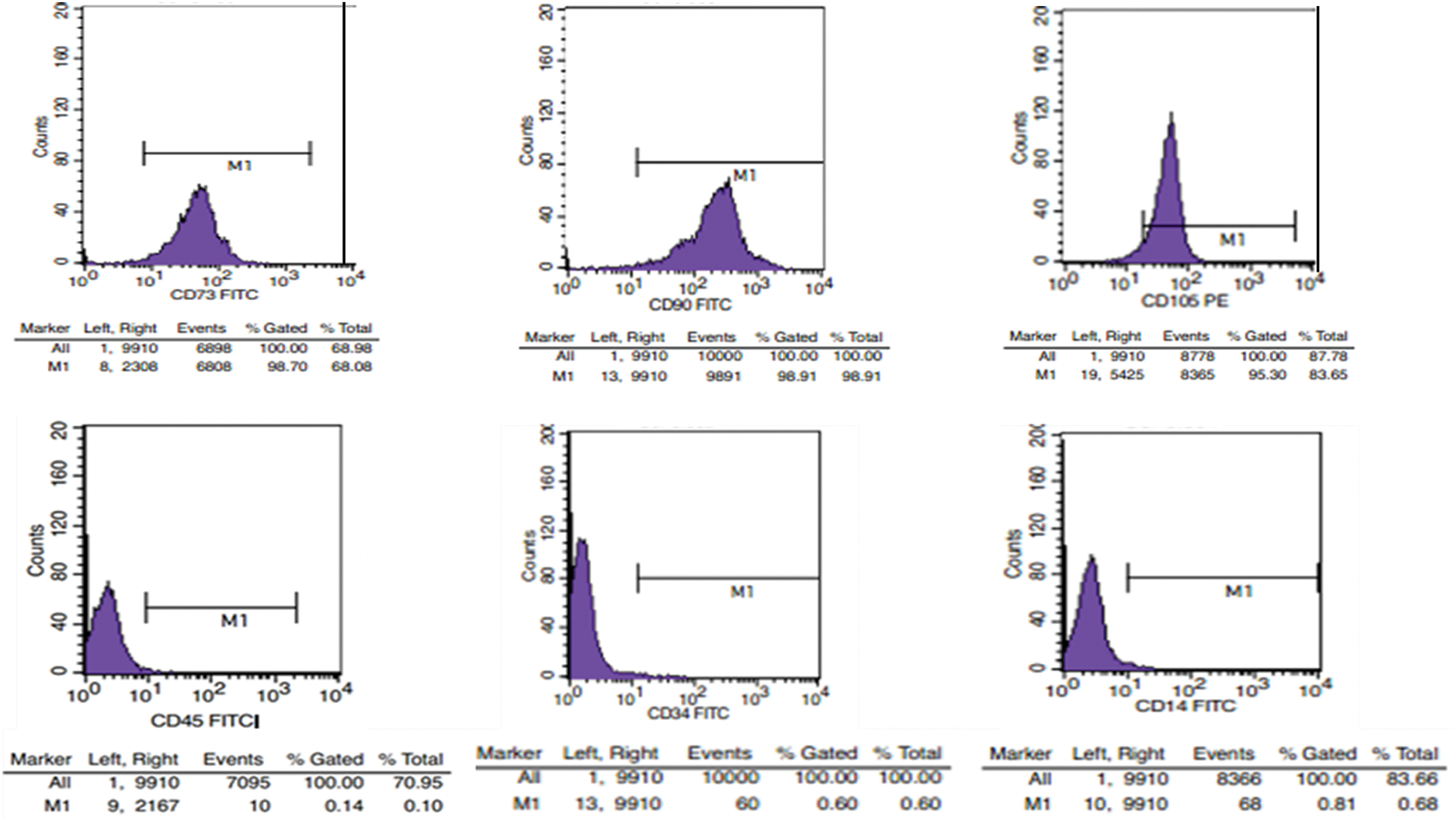
Figure 3: The expression of mesenchymal stem cell surface markers by FCM analysis
The percentage of cell surface markers was analyzed by three independent experiments, as shown in Tab. 1.
Table 1: The percentage of surface markers of mesenchymal stem cell (MSC) from three sources

Different sources of MSCs on pulmonary fibrosis in C57BL/6 mice lung with the expression of IκB-α
The expression of IκB-α can be regulated by several signaling pathways. To investigate how MSC regulates, we analyzed the IκB-α expression. Interestingly, as shown in Fig. 4, Western blotting assays revealed that the expression of IκB-α was down-regulated in different MSC-treated animals, which suggested NF-κB signaling pathway was not activated. As a result, IκB-α downregulated the expression but not the activation of the NF-κB signaling pathway. These data suggest that the reparation activity in pulmonary fibrosis potentially resulted from three kinds of MSCs.
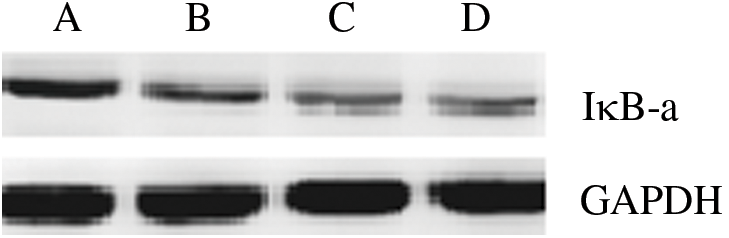
Figure 4: Expression of pulmonary fibrosis -associated IκB-α. IκB-α in the tissue was detected by Western blotting. The relative protein levels of IκB-α was normalized to those of GAPDH. A. Phosphate buffer treated B. Mesenchymal stem cell from umbilical cord blood treated. C. Mesenchymal stem cell from umbilical cord treated. D. Mesenchymal stem cell from placenta treated
In this study, we showed that MSCs come from umbilical cord blood, umbilical cord, and placenta in a mouse model of pulmonary interstitial fibrosis. By observing the degree of pulmonary fibrosis, it was found that the group treated with placenta MSC had lower expression of CD34, CD45, CD14 cells at the mononuclear cell level. Western blotting assays revealed that the expression of IκB-α was down-regulated in different MSC-treated animals. MSC coming from placenta MSCs was significantly more effective in improving pulmonary fibrosis.
It has been suggested that MSC derived from the aborted fetuses, umbilical cord, or discarded test-tube human embryos have high plasticity with low immunogenicity. In this regard, Moodley et al. studied the therapeutic effects of umbilical cord-derived MSC in a bleomycin-induced lung injury model and found that these cells can inhibit lung inflammation and fibrosis by up-regulating anti-inflammatory modulators but downregulating the cytokine expression (Rojas et al., 2005). The systemically administered umbilical cord-derived MSC are present in the injured lung after 2 weeks and may not exactly match with the recipient phenotype to avoid the graft-versus-host reaction (Mudrabettu et al., 2015).
The effect of transplanted placenta-derived MSCs on lung fibrosis was also studied using murine models. Transplantation of allogeneic and xenogeneic placenta-derived MSCs notably reduced bleomycin-induced lung fibrosis by suppressing the infiltration of neutrophils and act as a potential treatment for lung fibrosis (Rojas et al., 2005). Placenta-derived MSCs have immunomodulation properties; therefore, it might be an important agent for lung repair and regeneration like other MSCs (Sueblinvong and Weiss, 2010).
The pathogenesis of interstitial lung disease is not clear and effective treatment is yet to be published. Interstitial lung disease is a disease characterized by diffuse alveolar inflammation and interstitial fibrosis. In recent years, MSCs with the ability of self-renewal and multi-directional differentiation have attracted more and more attention. Under certain conditions, MSCs can differentiate into bone cells, cartilage cells, myocardial cells, nerve cells, and epithelial cells (Rojas et al., 2005). Bone marrow MSCs can increase the number of Treg and reduce the number of T cells (Mudrabettu et al., 2015). MSCs are used in the treatment of severe pulmonary disease, which has become a hot research topic in recent years. Previous studies showed that MSCs can effectively improve acute and chronic lung injury (Sueblinvong and Weiss, 2009, 2010). In the early time, bone marrow MSCs were used to study pulmonary disease, because the source of bone marrow is limited, and, with the growth of the age, the number and proliferation ability of cells decreased significantly. Compared with bone marrow MSCs, umbilical cord blood, umbilical cord, and placenta have a higher potential of proliferation, differentiation, and the ability of immune regulation and control (Chen et al., 2009; Lu et al., 2006; Secco et al., 2008; Tao et al., 2010; Troyer and Weiss, 2008). The experimental results showed that the placenta MSCs have an obvious effect on the treatment of pulmonary fibrosis (Sarugaser et al., 2005).
This study found that MSCs can reduce lung injury induced by bleomycin. MSCs may be a new target for the treatment of interstitial lung disease. MSCs are derived from a mesodermal class of pluripotent stem cells in the development of early, with the potential of self-renewing, high proliferation, and multiple differentiation.
In this study, the experiment was parted into 4 groups to observe the pulmonary fibrosis of mice, and flow cytometry was used to test the frequency of CD73, CD90, CD105, CD45, CD34, and CD14 at the mononuclear cell level of each group of mice lung tissue. By comparing changes of the frequency to determine which MSCs has a more significant effect on the treatment of pulmonary fibrosis. We believe that, with the further development of the study, pulmonary fibrosis will have the potential to be completely cured.
In addition, it has been suggested that activation of NFkB pathways via direct interaction with IkBα resulted in increased expressions of IL-1β, and IL-6, which induce inflammation and lung fibrosis (Kim et al., 2019). Also, previous studies showed that inhibition of the NFkB pathways could significantly inhibit the formation of lung fibrosis. Besides, Jee-Youn Kim et al. showed that activation of NFkB pathways via direct interaction between Hsp27 and IkBα could significantly induce lung fibrosis in radiation-induced lung fibrosis model (Liu et al., 2019).
From our study, because there are not very perfect plans to separate and expand the number of MSCs while studying stem cells derived from the same tissue in different laboratories, there is a big difference in cell phenotype, initiation of heterogeneous cells, differentiation, and proliferation, etc., and also a direct impact on the mesenchymal stem cell application.
To clarify the possible molecular mechanisms of pulmonary fibrosis in response to MSCs treatment, further study showed that IκB-α downregulated the expression promoted through the NF-κB pathway.
In conclusion, our findings show that MSC coming from the placenta might have a better impact on improving pulmonary fibrosis in comparison to another source. In addition, we suggested that this could be due to the downregulation of the IκB-α or NF-κB pathways. In this regard, MSC modification by gene editing could significantly enhance their therapeutic effect in the mouse model of pulmonary fibrosis. However, cell therapy combined with gene therapy needs further study.
Acknowledgement: We thank professors Cheng Zhu and Adam J. Engler for their valuable suggestions on the experiments. We also thank Prof. Yanping Cao for his help in the theoretical analysis. This work was financially supported by the National Natural Science Foundation of China (31001638 & 30870602). This project was supported by the key medical research plan of the Hebei province of China (No .ZD2013063), Tsing-hua University (2009THZ02122), and National Heart, Lung, and Blood Institute Research Grant HL 104402 from the National Institutes of Health of the US Public Health Service.
Author Contributions: JD conceived and designed the experiments, JD, YZ and CY performed the experiments: YZ, JL, SD, YX, LZ, ZW and LJ wrote the manuscript: CY supervised the project: SC.
Availability of Data and Materials: Data supporting this article are details in this manuscript.
Funding Statement: This project was supported by the Key Medical Research Plan of the Hebei Province of China (No. ZD2013063). This project was supported by the National Natural Science Foundation of China (No. 31101638).
Conflicts of Interest: The authors declare no competing financial interests.
Abreu SC, Antunes MA, Pelosi P, Morales MM, Rocco PR. (2011). Mechanisms of cellular therapy in respiratory diseases. Intensive Care Medicine 37: 1421–1431. DOI 10.1007/s00134-011-2268-3. [Google Scholar] [CrossRef]
Brown KK, Raghu G. (2004). Medical treatment for pulmonary fibrosis: Current trends, concepts, and prospects. Clinics in Chest Medicine 25: 759–772. DOI 10.1016/j.ccm.2004.08.003. [Google Scholar] [CrossRef]
Chen MY, Lie PC, Li ZL, Wei X. (2009). Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Experimental Hematology 37: 629–640. DOI 10.1016/j.exphem.2009.02.003. [Google Scholar] [CrossRef]
Gharaee-Kermani M, Gyetko MR, Hu B, Phan SH. (2007). New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: A potential role for stem cells in the lung parenchyma and implications for therapy. Pharmaceutical Research 24: 819–841. DOI 10.1007/s11095-006-9216-x. [Google Scholar] [CrossRef]
He L, Lu Y, Tu J, Al E. (2013). Advances in investigation of mesenchymal stem cells. The Chinese Journal of Dermatovenereology 27: 1061–1065. [Google Scholar]
Kim JY, Jeon S, Yoo YJ, Jin H, Won HY, Yoon K, Hwang ES, Lee YJ, Na Y, Cho J. (2019). The Hsp27-mediated IkBα-NFκB signaling axis promotes radiation-induced lung fibrosis. Clinical Cancer Research 25: 5364–5375. DOI 10.1158/1078-0432.CCR-18-3900. [Google Scholar] [CrossRef]
King TEJr, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, Thomeer M, Valeyre D, du Bois RM. (2009). Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIREA multicentre, randomised, placebo-controlled trial. Lancet 374: 222–228. [Google Scholar]
Liu B, Rong Y, Sun D, Li W, Chen H, Cao B, Wang T. (2019). Costunolide inhibits pulmonary fibrosis via regulating NF-kB and TGF-β1/Smad2/Nrf2-NOX4 signaling pathways. Biochemical and Biophysical Research Communications 510: 329–333. DOI 10.1016/j.bbrc.2019.01.104. [Google Scholar] [CrossRef]
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Han Z-B, Xu ZS, Lu YX, Liu D. (2006). Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 91: 1017–1026. [Google Scholar]
Mudrabettu C, Kumar V, Rakha A, Yadav AK, Ramachandran R, Kanwar DB, Nada R, Minz M, Sakhuja V, Marwaha N. (2015). Safety and efficacy of autologous mesenchymal stromal cells transplantation in patients undergoing living donor kidney transplantation: A pilot study. Nephrology 20: 25–33. DOI 10.1111/nep.12338. [Google Scholar] [CrossRef]
Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. (2005). Bone marrow-derived mesenchymal stem cells in repair of the injured lung. American Journal of Respiratory Cell and Molecular Biology 33: 145–152. DOI 10.1165/rcmb.2004-0330OC. [Google Scholar] [CrossRef]
Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. (2005). Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells 23: 220–229. DOI 10.1634/stemcells.2004-0166. [Google Scholar] [CrossRef]
Secco M, Zucconi E, Vieira NM, Fogaça LL, Cerqueira A, Carvalho MDF, Jazedje T, Okamoto OK, Muotri AR, Zatz M. (2008). Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells 26: 146–150. DOI 10.1634/stemcells.2007-0381. [Google Scholar] [CrossRef]
Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP. (2004). Idiopathic pulmonary fibrosis. Drugs 64: 405–430. DOI 10.2165/00003495-200464040-00005. [Google Scholar] [CrossRef]
Sueblinvong V, Weiss DJ. (2009). Cell therapy approaches for lung diseases: Current status. Current Opinion in Pharmacology 9: 268–273. DOI 10.1016/j.coph.2009.03.002. [Google Scholar] [CrossRef]
Sueblinvong V, Weiss DJ. (2010). Stem cells and cell therapy approaches in lung biology and diseases. Translational Research 156: 188–205. DOI 10.1016/j.trsl.2010.06.007. [Google Scholar] [CrossRef]
Tao R, Han YF, Cai JK. (2010). A safety study of intravenous injection of human umbilical cord mesenchymal stem cells in mice. Junshi Yixue Kexue Yuan Yuankan 34: 293–296. [Google Scholar]
Troyer DL, Weiss ML. (2008). Concise review: Wharton’s Jelly-derived cells are a primitive stromal cell population. Stem Cells 26: 591–599. DOI 10.1634/stemcells.2007-0439. [Google Scholar] [CrossRef]
Wei Y, Liu G. (2014). Advances in the treatment of pulmonary fibrosis. Journal of Clinical Pulmonary Medicine 19: 336–338. [Google Scholar]
Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC, Shi Y. (2007). Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Research 17: 240–248. DOI 10.1038/cr.2007.4. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |