DOI:10.32604/biocell.2021.013907

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.013907 |  www.techscience.com/journal/biocell |
| Article |
Antimicrobial and plant growth-promoting activities of bacterial endophytes isolated from Calotropis procera (Ait.) W.T. Aiton
1Plant-Microbe Interactions Lab, Department of Botany, Abdul Wali Khan University Mardan, KP, Pakistan
2Department of Botany, Bacha Khan University Charsadda, Charsadda, 24420, Pakistan
3Department of Food Science and Technology, Abdul Wali Khan University Mardan, Mardan, 23200, Pakistan
4Centre of Biotechnology and Microbiology, University of Peshawar, Peshawar, 25000, Pakistan
5Department of Botany, Islamia College University, Peshawar, 25000, Pakistan
6Department of Applied Biosciences, Kyungpook National University, Daegu, 41566, Korea
*Address correspondence to: In-Jung Lee, ijlee@knu.ac.kr; Muhammad Hamayun, hamayun@awkum.edu.pk
Received: 26 August 2020; Accepted: 22 November 2020
Abstract: Bacterial endophytes are beneficial to their hosts as they can fix nitrogen in the soil and make it available to the host. Endophytic bacteria also secrete plant growth-promoting hormones to support their host plants under normal as well as stress conditions. The current study aimed to isolate endophytic bacteria from different parts of Calotropis procera, i.e., roots, stem and leaves of Calotropis procera (Ait.) W.T. Aiton. Plants were collected from the Lundkhwar, district Mardan. A total of 12 bacterial strains, i.e., six from roots, three from the stem and three from the leaves were isolated. The strains were screened for their growth-promoting activity in rice plants because rice shows a quick and easy response to the bioactive compounds present in the culture filtrate (CF) of the potent endophytic strains. The rice plants were cultivated in pots containing 30 mL of 0.8% w/v water-agar medium. The pots were placed in a growth chamber, operated at 28 ± 0.3°C for 14 h (day); and 25 ± 0.3°C for 10 h (night), at 70% relative-humidity. Among the isolated strains, R1, S1, S3, L1, R5 and R6 showed visible growth promotion in rice plants. The biochemical analysis revealed that the strains were able to produce indole acetic acid (IAA) and flavonoids in higher quantities. Moreover, the strains also produced bioactive compounds that inhibited the growth of Escherichia coli and Aspergillus flavus using the well diffusion method. From the results, it was concluded that these strains can secrete potent compounds that can promote the host plant growth and inhibit the growth of pathogenic microorganisms and, therefore, can be used as bio-fertilizer and bio-control agents.
Keywords: Endophytic bacteria; Growth promotion; Antimicrobial activity; Plant-microbe interaction
Calotropis procera (Ait.) W.T. Aiton belongs to the family Apocynaceae. It is an erect and branched shrub containing milky latex, which is widely used as a medicinal plant in the Indian sub-continent. It has ethnobotanical importance in traditional medicines and is used for the treatment of various diseases (Pattnaik et al., 2017). Besides the ethnobotanical importance, C. procera acts as a reservoir for potent endophytes (Nagda et al., 2017; Rani et al., 2017).
Endophytes are the organisms that establish a mutual yet beneficial relationship with their host plants during long-term evolutionary processes (Ali et al., 2019; Bilal et al., 2018; Gul Jan et al., 2019). Endophytic microbes have the capability to produce a variety of bioactive compounds (Bibi et al., 2018; Hamayun et al., 2017; Ikram et al., 2018). Endophytic microbes live within the plant tissues without harming the host plant (Ismail et al., 2020a; Ismail et al., 2019; Ismail et al., 2018). A variety of endophytic bacterial species are found in the tissues of plants such as Azospirillum, Pseudomonas and Bacillus thatcan be extracted from internal plant parts or from surface-sterilized plant tissues (Phetcharat and Duangpaeng, 2012). The tissues of most plants contain endophytes that secrete secondary metabolites to regulate the plant metabolism, even under stress conditions (Ismail et al., 2020b; Ismail et al., 2020c; Jan et al., 2019). Endophytes can act asbiofertilizers as they can promote the growth of the host species under normal as well as stress conditions (Kang et al., 2019; Khushdil et al., 2019; Mehmood et al., 2019). Endophytes enhance the growth of the host plant by secreting plant hormones, regulating the stomatal opening, enhancing nutrient absorption, and converting the heavy metals from unstable to stable form in the agricultural soil (Muhammad et al., 2019; Nusrat et al., 2019; Qadir et al., 2020). Endophytic bacteria promote the growth of host plants by producing IAA, GA, ACC deaminase, exhibiting phosphate solubilization and siderophore activity, and biologically fix nitrogen fixation (Hamayun et al., 2017; Ikram et al., 2018). Endophytic bacteria are indicated to have an excellent ability to increase plant growth ratios in various ways as they secrete different forms of secondary metabolites in the tissues of that particular plant (Etesami et al., 2014). In recent years, it has been noticed that endophytic bacteria protected the host plants against the nematodes (Mhatre et al., 2019; Su et al., 2017). Moreover, it is observed that bacterial endophytes have an advantageous role in protecting host plants by increasing the phytoremediation process in heavy metals contaminated soils (Zam et al., 2016). Therefore, for the promotion of plant growth, beneficial bacteria may be introduced to the soil to get maximum benefits in terms of high yield. To achieve this aim, endophytic bacteria were isolated from different parts, i.e., roots, stems, leaves of C. procera and screened for plant growth-promoting and antimicrobial activities.
Fresh samples of C. procera were collected from village Lund Khwar (71°59’ E, 34°23’ N with an altitude of 371 m) in district Mardan, Pakistan. The plant samples were collected in polythene bags and safely transferred to the plant–microbe interactions laboratory for further investigation.
Isolation and purification of bacterial endophytes
Plant samples were washed in running tap water to remove dust and debris. The washed plant samples were then cut into parts, i.e., stem, leaves and roots, with the help of sterilized scalpels. The separated plant parts were surface-sterilized by dipping them in 5% sodium hypochlorite solution for 5 min, followed by 70% ethanol for 1 min, and finally rinsed three times in sterile distilled water to remove any traces of sodium hypochlorite and ethanol. From each sterilized plant part, 1 g of tissue was aseptically macerated in 9 mL sterile saline solution using pestle and mortar. About 1 mL of macerated sample was taken and spread ontryptic soy agar and/or nutrient agar plates. The plates were transferred to the incubator, which was then incubated at 37°C for 72 h. The plates were observed for the appearance of bacterial colonies on a daily basis. The plates showed the presence of different bacterial colonies was transferred to the potato agar plates for further purification. Pure colonies were identified by observing the size, color, shape, and growth pattern (Barillot et al., 2013; García-Salamanca et al., 2013).
Indole-3-acetic acid and flavonoids determination
IAA production was examined by a well-established method (Loaces et al., 2011). Briefly, each bacterial suspension (1 × 108 CFU/mL) was inoculated in 10 mL LB broth containing L-tryptophan (100 g/mL) in a 50 mL falcon tube. The broth was incubated at 28°C and 200 rpm for 72 h. Bacterial cells were precipitated by centrifugation (8,000 rpm for 15 min), and the collected supernatant was incubated at room temperature in the dark for 30 min. Pure IAA (Sigma, USA) was used as a standard. The IAA concentration in the culture supernatant was measured at 530 nm with Salkowski’s reagent (12 g/L FeCl3 in 7.9 M H2SO4). Each experiment was repeated three times.
Total flavonoids were estimated by using thealuminum chloride colorimetric method (Saravanan and Parimelazhagan, 2014). In a test tube, 4.3 mL of methanol (80%) was added to 0.5 mL of each culture filtrate. Then, 0.1 mL of aluminum chloride (10%) and 0.1 mL of potassium acetate (10%) was added to the tubes. The samples were incubated for 30 min at room temperature. After incubation, the samples were shaken, and the absorbance was recorded at 510 nm.
Screening for the potent strains
To assess the growth activity of the bacterial isolates, ricewas used as a test plant species. Rice was selected because of the quick response to the plant growth promoters and inhibitors. Initially, seeds of rice were surface-sterilized,as mentioned earlier. The clean seeds were spread in a plate containing sterilized distilled H2O for germination. Germinated seedlings of uniform size (5 per pot) were transplanted inthe pots containing 30 mL of 0.8% w/v water-agar medium. The pots were placed in a growth chamber, operated at 28 ± 0.3°C for 14 h (day) and 25 ± 0.3°C for 10 h (night), at 70% relative-humidity. When the tested rice cultivars reached a two-leaved stage, a 10 μL of lyophilized bacterial filtrate suspension was applied to the tip ofthe apical meristem. The growth attributes of both rice cultivars were analyzed after 10 days of treatment. The experiment was carried in triplicate.
Estimation of rice growth parameters
Root and shoot length was measured manually with the help of a scale. Plant fresh weight was measured directly using an analytical balance, while plant dry weight was estimated after drying the samples in an oven for 48 h at 70°C.
Collection of pathogenic microbes
Escherichia coli and Aspergillus flavus were collected from the laboratory of the Centre of Biotechnology and Microbiology, University of Peshawar.
The antibacterial activity of the extracts was evaluated against the selected pathogenic bacterial strains, i.e., E. coli by agar well-diffusion technique (Zerroug et al., 2018). Each bacterial pathogen (1 × 10–7 CFU/mL) was inoculated into Muller–Hinton agar plates. In each plate, three wells were made using a sterile cork borer. Wells were filled with 100 μL of the endophytic bacterial extract and distilled water as a blank. About 100 μL of standard ampicillin (30 μg/mL) was used as a positive control. Extracts were allowed to diffuse through agar media at room temperature for 2 h, and then the plates were incubated for 24 h at 37°C. The diameters of inhibition zones were recorded. The experiment was repeated three times.
Potato dextrose agar media were used to test the potency of isolated bacterial endophytes from C. procera against the pathogenic A. flavus strain according to a standard protocol (Dellavalle et al., 2011).
All the experiments were performed in triplicate. ANOVA (one-way analysis of variance) was used for the analysis of data and means were compared bya Duncan multiple range test at p < 0.05, using SPSS for Windows 16.0 (SPSS Inc., Chicago, IL, USA). Graphs were constructed by using Graphpad Prism 6.0.
The study was conducted at Plant-microbes interaction laboratory, Department of Botany, Abdul Wali Khan, University, Mardan. Calotropis procera was collected from village Lund Khwar, district Mardan to isolate potent endophytic bacterial strains from different parts of the plants, i.e., leaves, stems and roots. A total of 12 bacterial strains was isolated from various parts of C. procera, i.e., 6 bacterial strains (R1, R2, R3, R4, R5 and R6) were isolated from the roots, 3 strains (L1, L2, L3) from the leaves, and 3 strains (S1, S2 and S3) from the stem.
Among the 12 isolated bacterial strains, the R1 promoted shoot and root length and was considered a potent strain as it increased both shoot and root length of rice plants, while L2 inhibited both shoot and root lengths. The extract of S3 also promoted root and shoot length (Fig. 1).
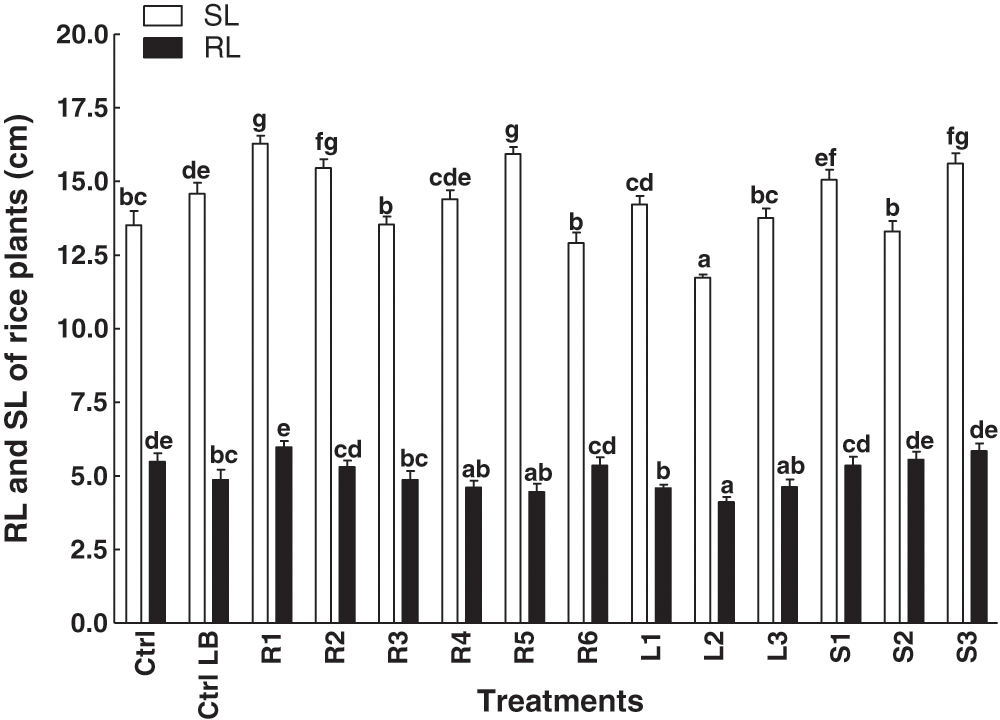
Figure 1: Effect of bacterial endophytes isolated from the various parts of the C. procera on shoot and root length of rice plants. Data are the mean of three replicates with standard error bars. Means that are followed by different letters are significantly different (p = 0.05) from their respective bars. Ctrl: control with distilled water; Ctrl LB: control with the media; R1, R2, R3, R4, R5, R6: endophytes isolated from the roots of the C. procera; L1, L2, L3: endophytes isolated from the leaves of the C. procera; S1, S2, S3: endophytes isolated from the stem of the C. procera.
A total of twelve bacterial extracts was applied to the rice plants. An increase in shoot fresh weight was observed in plants treated with S3 strain isolated from the stem of C. procera. However, a more pronounced effect in terms of weight was recorded in plants treated with the extract from the R1 strain, which was isolated from the root (Fig. 2). Moreover, the other isolated bacterial strains from C. procera didnot show any significant changes in root and shoot weight as compared to the control plants (Fig. 2).
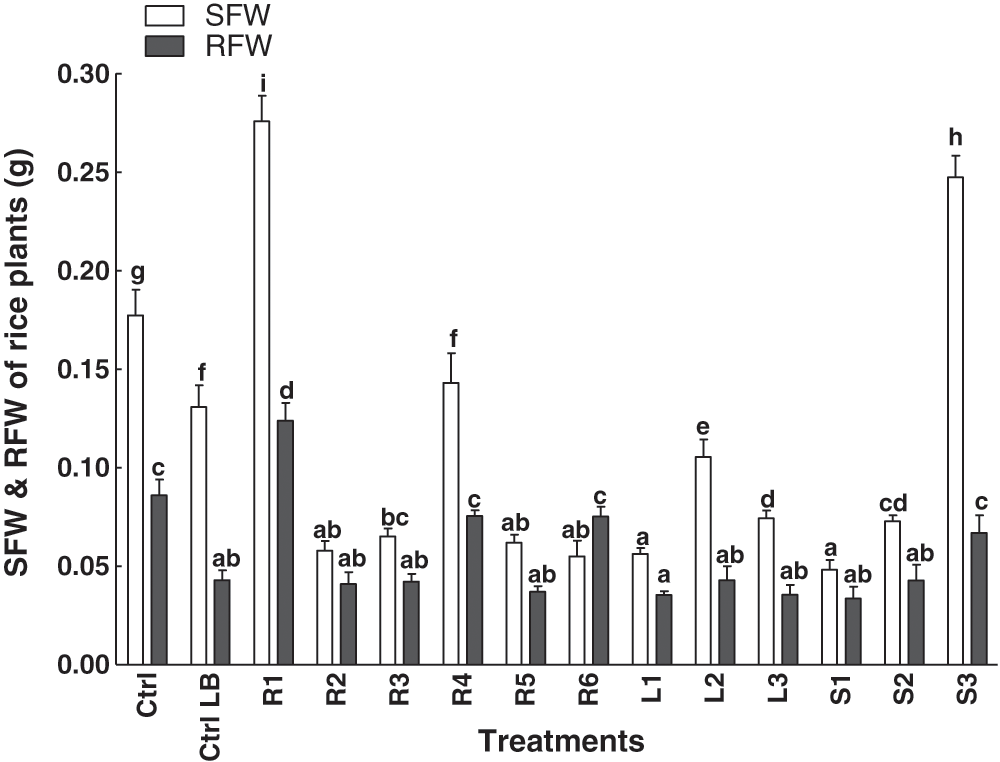
Figure 2: Effect of bacterial endophytes isolated from the various parts of the C. procera on root and shoot fresh weight of rice plant. Data are the mean of three replicates with standard error bars. Means that are followed by different letters are significantly different (p = 0.05) from their respective bars. Ctrl: control with distilled water; Ctrl LB: control with the media; R1, R2, R3, R4, R5, R6: endophytes isolated from the roots of the C. procera; L1, L2, L3:endophytes isolated from the leaves of the C. procera; S1, S2, S3:endophytes isolated from the stem of the C. procera.
The effect of different endophytic bacterial extracts on the dry weight of the rice plant was measured (Fig. 3). Extracts were applied from the embryonic stage to their two-leaf stage. As compared to the controls, the extract of L1 increased the shoots dry weight of the rice plants. However, in the case of root dry weight, the R3 significantly enhanced the root dry weight of rice plants (Fig. 3).
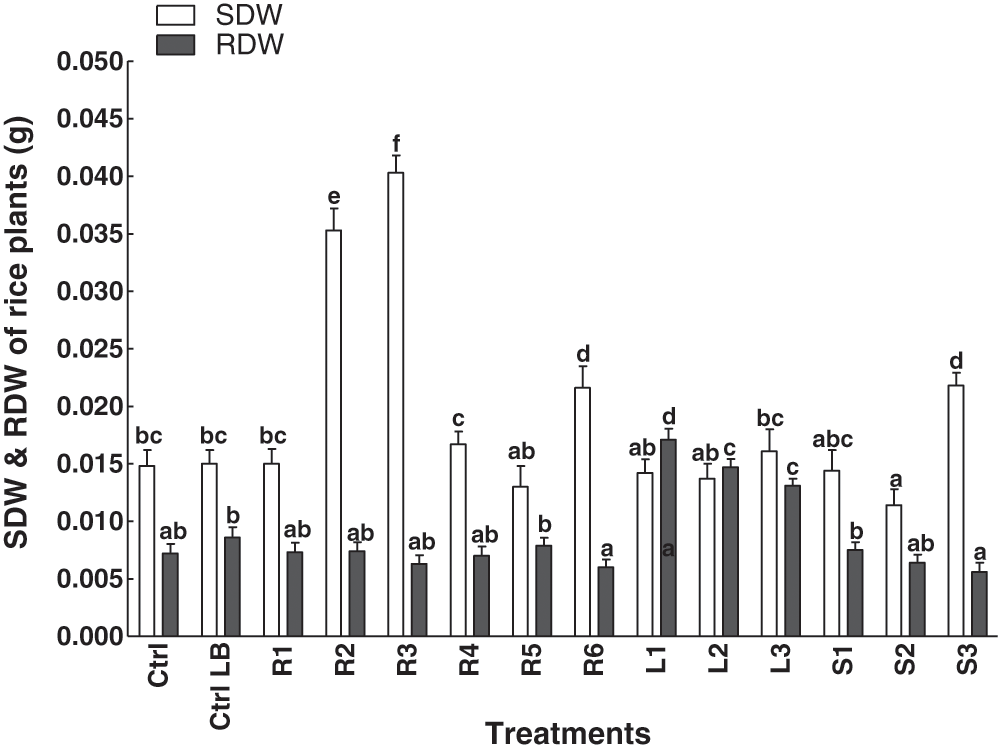
Figure 3: Effect of bacterial endophytes isolated from the various parts of the C. procera on root and shoot dry weight of rice plant. Data are the mean of three replicates with standard error bars. Means that are followed by different letters are significantly different (p = 0.05) from their respective bars. Ctrl: control with distilled water; Ctrl LB: control with the media; R1, R2, R3, R4, R5, R6: endophytes isolated from the roots of the C. procera; L1, L2, L3: endophytes isolated from the leaves of the C. procera; S1, S2, S3: endophytes isolated from the stem of the C. procera.
Indole-3-acetic acid and flavonoid contents
IAA contents of the isolated bacterial strains from various parts of C. procerawere investigated (Fig. 4). The bacterial isolates from roots consisted of the strongest and weakest strains in terms of IAA production. R5 had the highest IAA producing ability, whereas the lowest contents were produced by the isolate R2 (Fig. 4). All the tested strains were capable of producing flavonoids (Fig. 5). The highest flavonoid contents were observed in the R6 treatment, while the lowest flavonoid contents were detected in S3 (Fig. 5).
Antibacterial and antifungal activity
The antibacterial activity was tested by using the isolated strains against E. coli (Tab. 1). The well diffusion method was utilized, and the results were compared with the positive and negative controls. The highest value for inhibition was shown by L1 (8.6 ± 0.7), which was isolated from the leaves. The lowest antibacterial activity was shown by L3 (2.2 ± 0.4), while the effect of L2 (4.3 ± 0.8) was moderate. The isolated endophytes from roots of C. procerashowed variable antibacterial activities, R4 (7.4 ± 0.1) led the antibacterial activity, followed by R3 (5.8 ± 0.7), R1 (5.2 ± 0.6), R6 (4.5 ± 0.4), R5 (4.4 ± 1.1), respectively. The R2 strain isolated from the C. proceraroots showed minimum antibacterial activity (4.0 ± 0.5). The isolated strains from the stem of Calotropis procera also showed variable antibacterial activities (Tab. 1). The strain S2 (6.7 ± 0.8) showed the highest antibacterial activity, followed by S3 (6.2 ± 0.2) and S1 (5.1 ± 0.7). On an overall basis, the highest antibacterial activity was found in the bacterial isolates from the leaves of C. procera in comparison to the isolates from stems and roots (Tab. 1).
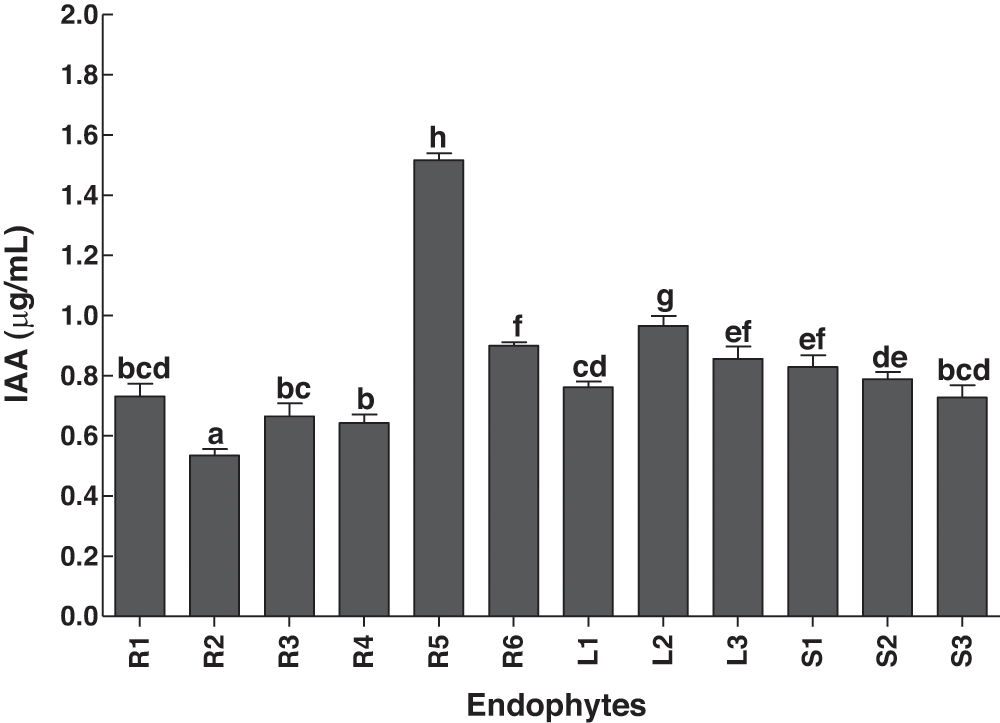
Figure 4: Production of IAA by bacterial endophytes isolated from the various parts of the C. procera. Data are the mean of three replicates with standard error bars. Means that are followed by different letters are significantly different (p = 0.05) from their respective bars. R1, R2, R3, R4, R5, R6: endophytes isolated from the roots of the C. procera; L1, L2, L3: endophytes isolated from the leaves of the C. procera; S1, S2, S3: endophytes isolated from the stem of the C. procera.
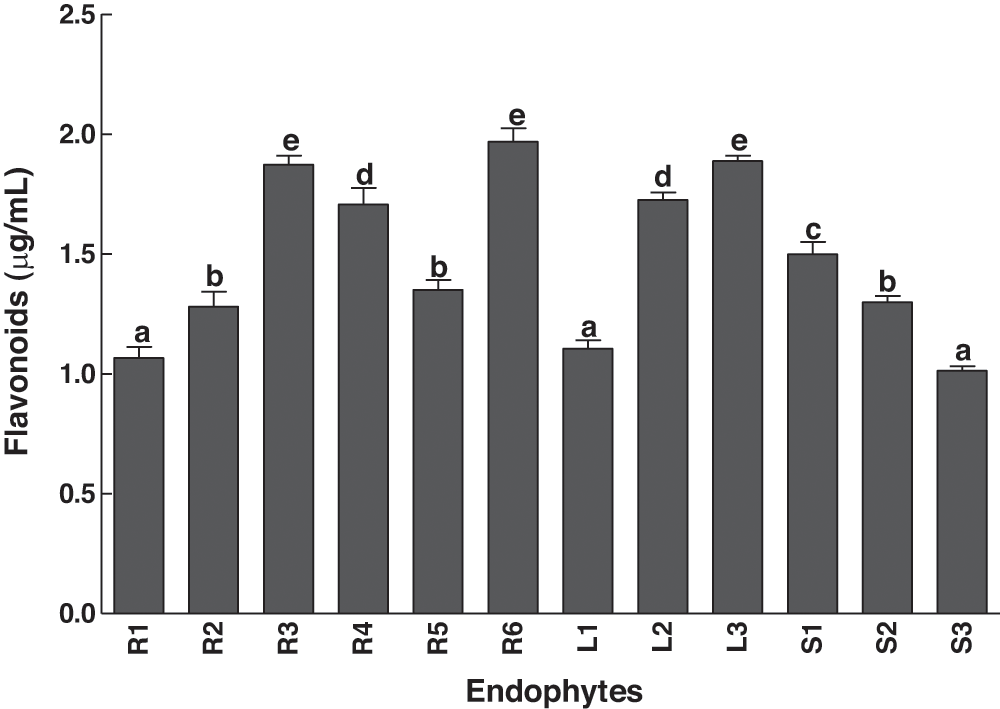
Figure 5: Secretion of total flavonoids by bacterial endophytes isolated from the various parts of the C. procera. Data are the mean of three replicates with standard error bars. Means that are followed by different letters are significantly different (P=0.05) from their respective bars. R1, R2, R3, R4, R5, R6: endophytes isolated from the roots of the C. procera; L1, L2, L3: endophytes isolated from the leaves of the C. procera; S1, S2, S3: endophytes isolated from the stem of the C. procera.
The selected strains were utilized against the pathogenic fungal strain, A. flavus (Tab. 1). Compared with the control the highest antifungal activity was shown by the isolated strain R1 (4.1 ± 0.6), followed by R2 (3.6 ± 0.4), R3 (3.0 ± 0.1), R4 (2.8 ± 0.5), and R5 (2.1 ± 0.1). The lowest antifungal activity was exhibited by R6 (1.8 ± 0.5). Similarly, leaves of C. procera also showed antifungal activities in different concentrations; L3 was recorded as the dominant one (2.6 ± 0.8), followed by L2 (2.2 ± 0.7) and L1 (2.2 ± 0.4). The strains isolated from the stem of C. procera also showed varied antifungal activities (Tab. 1). The highest and most leading strain was S3 (3.2 ± 0.9), followed by S1 (2.4 ± 0.7), and S2 (2.3 ± 0.7) possessed the lowest antifungal activities and was considered as the best source of antifungal agents (Tab. 1).
Endophytes have been studied for decades and reported to have a contribution to growth enhancement and plant health through several metabolic activities (Bilal et al., 2018; Hamayun et al., 2017).
Endophytes can play important beneficiary roles in host plant development under normal and stress conditions. Therefore, the role of endophytes in host plant life is very important that needs to be explored at physiological, biochemical, and molecular levels. In the present study, we have
Table 1: Antibacterial and antifungal properties of bacterial strains isolated from different parts of C. procera
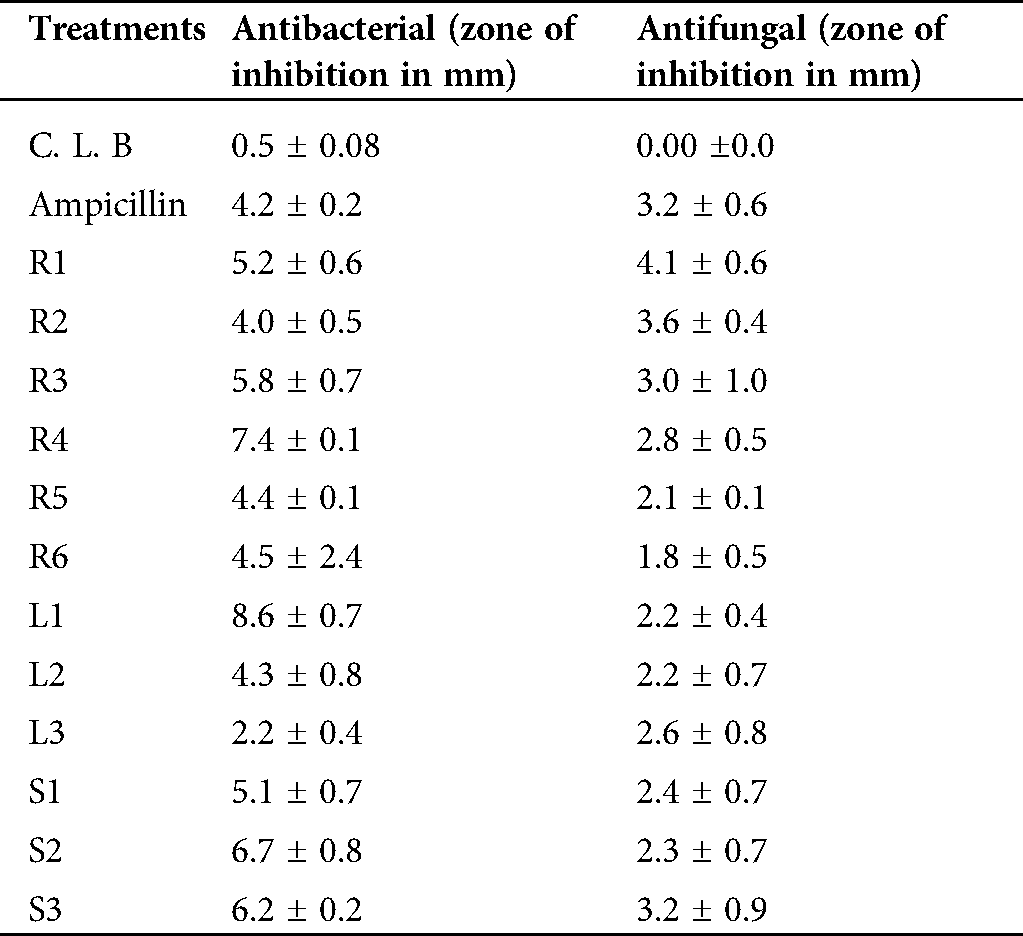
CLB: negative control (media without extract); Ampicillin: positive control (media supplemented with commercial antibiotics); R1, R2, R3, R4, R5, R6: endophytes isolated from roots of C. procera; L1, L2, L3:endophytes isolated from leaves of C. procera; S1, S2, S3: endophytes isolated from stems of C. procera.
isolated 12 bacterial endophytes from the stem, leaves and roots of the C. proceraplant. Out of the 11 isolated bacterial strains, 6 strains (R1, R2, R3, R4, R5 and R6) were isolated from the roots, 3 strains (L1, L2, L3) from the leaves, and 3 strains (S1, S2 and S3) from the stem. The extract of these bacterial endophytes was tested against the growth parameters of the rice plants.
The strain R1 was the potent strain that promoted root and shoot length and weight, which means that the strains can be used as a potent plant growth promoter. On the other hand, the strain L2 inhibited the rice plant growth, which means that the tested strain has the ability to release allelochemicals and can be used as a bio-control agent against weeds. Similar observations have been recorded in previous studies, where plant growth parameters were supported by the endophytes under normal as well as stressful conditions (Ismail et al., 2020c; Muhammad et al., 2019). This shows the importance of endophytes in sustainable agriculture in a continuously changing environment.
Plants contain minute amounts of the IAA, a phytohormone and free acid that helps in the plant growth promotion. Besides plant endogenous IAA, endophytes can also secret IAA to support the host plants under normal as well as stress conditions. In the present study, the isolated strains from the stem, leaves and roots of the C. procera plant secreted different concentrations of IAA. The variability in the release of IAA in different concentrations by different strains might due to the requirements of different precursors. For example, in the presence and absence of precursor tryptophan 66 bacterial strains were able to produce IAA in varying amounts (Phetcharat and Duangpaeng, 2012). Furthermore, the isolated strains from C. procera also produced flavonoids in appreciable quantities, especially the bacterial strain R6. However, the flavonoids contents were different in different bacterial species, which reflects the variability and diversity of endophytes in the same plant species. Our results coincide with the results of (Etesami et al., 2014). In fact, the presence of IAA and flavonoids in the extracts of isolated bacterial species confirms that they could be used as bio-fertilizer and bio-control agents. The endophytic strains thus have the ability to promote plant growth by secreting plant regulators and some other bioactive secondary metabolites, like flavonoids, to resist stresses (Ikram et al., 2018).
There is an increase in the number of infectious diseases, including bacterial infections with various levels of drug resistance. This has resulted in the increasing use of natural products and a search for new antimicrobial drugs. Endophytes are one of the best candidates to explore for new drugs against degenerative diseases. Endophytes certainly contain numerous biologically active compounds, some of which have shown antimicrobial activities against pathogens (Ikram et al., 2019). Similarly, in the current study, the isolated strains from different parts of C. proceraexhibited antimicrobial activity. The extracts of some of the isolated strains showed the highest potency against the pathogenic microbes, while others showed very low activity. Such variability concerning the antimicrobial activity revealed the presence of a diverse group of endophytes in C. procera plants. Moreover, the antimicrobial activity of the extracts from the isolated strains also depends on the solvent system. The antimicrobial compounds in the crude extracts are; therefore, need to be separated bydifferent solvent systems with varying polarity (Khan et al., 2019). Antimicrobial activities of bacterial endophytes isolated from various plant species, such as Aloe vera, P. tenuiflorus, and C. procera has been reported to date (Akinsanya et al., 2015; El-Deeb et al., 2013; Mohamed et al., 2019).
Endophytes are capable of promotingthe plant’s growth and protecting them from several biotic and abiotic environmental stresses. In the current study, we were able to isolate 12 strains of endophytic bacteria from C. procera, but 3 strains, i.e., R1, R4 and L2 can be used as plant growth promoters. Additionally, all the strains were able to show antibacterial activity against E. coli; whereas, the only strain R1 had the highest antifungal activity against A. flavus and can be used as a potent antimicrobial agent.
Ethics Approval: Our study did not involve any human, animal or endangered species.
Consent for Publication: No consent/approval at the national or international level or appropriate permissions and/or licenses for the study was required.
Availability of Data and Material: All the data are included in the manuscript.
Author Contribution: The authors confirm contribution to the paper as follows: Study conception and design: MH, SAK, IJL; data collection: MH, NK, MNK, MQ; analysis and interpretation of results: MH, AH, AI; draft manuscript preparation: MH, AI, SAK; final editing: MH, AH, IJL. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B04035601).
Conflicts of Interest: The authors declare that there is no competing interest of any nature related to this manuscript.
Akinsanya MA, Goh JK, Lim SP, Ting ASY. (2015). Diversity, antimicrobial and antioxidant activities of culturable bacterial endophyte communities in Aloe vera. FEMS Microbiology Letters 362: S15. DOI 10.1093/femsle/fnv184. [Google Scholar] [CrossRef]
Ali S, Khan SA, Hamayun M, Iqbal A, Khan AL, Hussain A, Shah M. (2019). Endophytic fungi from Caralluma acutangula can secrete plant growth promoting enzymes. Fresenius Environmental Bulletin 28: 2688–2696. [Google Scholar]
Barillot CDC, Sarde C-O, Bert V, Tarnaud E, Cochet N. (2013). A standardized method for the sampling of rhizosphere and rhizoplan soil bacteria associated to a herbaceous root system. Annals of Microbiology 63: 471–476. DOI 10.1007/s13213-012-0491-y. [Google Scholar] [CrossRef]
Bibi S, Hussain A, Hamayun M, Rahman H, Iqbal A, Shah M, Irshad M, Qasim M, Islam B. (2018). Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere 211: 653–663. DOI 10.1016/j.chemosphere.2018.07.197. [Google Scholar] [CrossRef]
Bilal L, Asaf S, Hamayun M, Gul H, Iqbal A, Ullah I, Lee I-J, Hussain A. (2018). Plant growth promoting endophytic fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis 76: 117–127. DOI 10.1007/s13199-018-0545-4. [Google Scholar] [CrossRef]
Dellavalle PD, Cabrera A, Alem D, Larrañaga P, Ferreira F, Rizza MD. (2011). Antifungal activity of medicinal plant extracts against phytopathogenic fungus Alternaria spp. Chilean Journal of Agricultural Research 71: 231–239. DOI 10.4067/S0718-58392011000200008. [Google Scholar] [CrossRef]
El-Deeb B, Fayez K, Gherbawy Y. (2013). Isolation and characterization of endophytic bacteria from Plectranthus tenuiflorus medicinal plant in Saudi Arabia desert and their antimicrobial activities. Journal of Plant Interactions 8: 56–64. DOI 10.1080/17429145.2012.680077. [Google Scholar] [CrossRef]
Etesami H, Mirsyed Hosseini H, Alikhani H. (2014). In planta selection of plant growth promoting endophytic bacteria for rice (Oryza sativa L.). Journal of Soil Science and Plant Nutrition 14: 491–503. [Google Scholar]
García-Salamanca A, Molina-Henares MA, Van Dillewijn P, Solano J, Pizarro-Tobías P, Roca A, Duque E, Ramos JL. (2013). Bacterial diversity in the rhizosphere of maize and the surrounding carbonate-rich bulk soil. Microbial Biotechnology 6: 36–44. DOI 10.1111/j.1751-7915.2012.00358.x. [Google Scholar] [CrossRef]
Hamayun M, Hussain A, Khan SA, Kim HY, Khan AL, Waqas M, Irshad M, Iqbal A, Rehman G, Jan S. (2017). Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Frontiers in Microbiology 8: 91. DOI 10.3389/fmicb.2017.00686. [Google Scholar] [CrossRef]
Ikram M, Ali N, Jan G, Hamayun M, Jan FG, Iqbal A. (2019). Novel antimicrobial and antioxidative activity by endophytic Penicillium roqueforti and Trichoderma reesei isolated from Solanum surattense. Acta Physiologiae Plantarum 41: 483. DOI 10.1007/s11738-019-2957-z. [Google Scholar] [CrossRef]
Ikram M, Ali N, Jan G, Jan FG, Rahman IU, Iqbal A, Hamayun M. (2018). IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS One 13: e0208150. DOI 10.1371/journal.pone.0208150. [Google Scholar] [CrossRef]
Ismail I, Hussain A, Mehmood A, Qadir M, Husna H, Iqbal A, Hamayun M, Khan N (2020a). Thermal stress alleviating potential of endophytic fungus Rhizopus oryzae inoculated to sunflower (Helianthus annuus L.) and soybean (Glycine max L.). Pakistan Journal of Botany 52: 1857–1865. DOI 10.30848/PJB2020-5(10). [Google Scholar] [CrossRef]
Ismail I, Hamayun M, Anwar H, Sumera Afzal K, Amjad I, In-Jung L (2020b). An endophytic fungus Aspergillus violaceofuscus can be used as heat stress adaptive tool for Glycine max L. and Helianthus annuus L. Journal of Applied Botany and Food Quality 93: 112–120. [Google Scholar]
Ismail I, Hamayun M, Hussain A, Afzal Khan S, Iqbal A, Lee IJ. (2019). Aspergillus flavus promoted the growth of soybean and sunflower seedlings at elevated temperature. BioMed Research International 2019: 1–13. DOI 10.1155/2019/1295457. [Google Scholar] [CrossRef]
Ismail I, Hamayun M, Hussain A, Iqbal A, Khan SA, Lee IJ (2020c). Aspergillus niger boosted heat stress tolerance in sunflower and soybean via regulating their metabolic and antioxidant system. Journal of Plant Interactions 15: 223–232. DOI 10.1080/17429145.2020.1771444. [Google Scholar] [CrossRef]
Ismail I, Hamayun M, Hussain A, Iqbal A, Khan SA, Lee IJ. (2018). Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. BioMed Research International 2018: 1–11. DOI 10.1155/2018/7696831. [Google Scholar] [CrossRef]
Jan FG, Hamayun M, Hussain A, Iqbal A, Jan G, Khan SA, Khan H, Lee IJ. (2019). A promising growth promoting Meyerozyma caribbica from Solanum xanthocarpum alleviated stress in maize plants. Bioscience Reports 39: 1. DOI 10.1042/BSR20190290. [Google Scholar] [CrossRef]
Gul Jan F, Hamayun M, Hussain A, Jan G, Iqbal A, Khan A, Lee IJ. (2019). An endophytic isolate of the fungus Yarrowia lipolytica produces metabolites that ameliorate the negative impact of salt stress on the physiology of maize. BMC Microbiology 19: 1. DOI 10.1186/s12866-018-1374-6. [Google Scholar] [CrossRef]
Kang SM, Hamayun M, Khan MA, Iqbal A, Lee IJ. (2019). Bacillus subtilis JW1 enhances plant growth and nutrient uptake of Chinese cabbage through gibberellins secretion. Journal of Applied Botany and Food Quality 92: 172–178. [Google Scholar]
Khan W, Subhan S, Shams DF, Afridi SG, Khan AJ, Iqbal A. (2019). In vitro assessment of the antibacterial activity of Datura alba with different solvents. Fresenius Environmental Bulletin 28: 7333–7339. [Google Scholar]
Khushdil F, Jan FG, Jan G, Hamayun M, Iqbal A, Hussain A, Bibi N. (2019). Salt stress alleviation in Pennisetum glaucum through secondary metabolites modulation by Aspergillus terreus. Plant Physiology and Biochemistry 144: 127–134. DOI 10.1016/j.plaphy.2019.09.038. [Google Scholar] [CrossRef]
Loaces I, Ferrando L, Scavino AF. (2011). Dynamics, diversity and function of endophytic siderophore-producing bacteria in rice. Microbial Ecology 61: 606–618. DOI 10.1007/s00248-010-9780-9. [Google Scholar] [CrossRef]
Mehmood A, Hussain A, Irshad M, Hamayun M, Iqbal A, Khan N. (2019). In vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays. Symbiosis 77: 225–235. DOI 10.1007/s13199-018-0583-y. [Google Scholar] [CrossRef]
Mhatre PH, Karthik C, Kadirvelu K, Divya K, Venkatasalam E, Srinivasan S, Ramkumar G, Saranya C, Shanmuganathan R. (2019). Plant growth promoting rhizobacteria (PGPRA potential alternative tool for nematodes bio-control. Biocatalysis and Agricultural Biotechnology 17: 119–128. DOI 10.1016/j.bcab.2018.11.009. [Google Scholar] [CrossRef]
Mohamed NH, Ismail MA, Abdel-Mageed WM, Mohamed Shoreit AA. (2019). Antimicrobial activity of green silver nanoparticles from endophytic fungi isolated from Calotropis procera (Ait) latex. Microbiology 165: 967–975. DOI 10.1099/mic.0.000832. [Google Scholar] [CrossRef]
Muhammad I, Niaz A, Gul J, Amjad I, Muhammad H, Farzana GJ, Anwar H, In-Jung L. (2019). Trichoderma reesei improved the nutrition status of wheat crop under salt stress. Journal of Plant Interactions 14: 590–602. DOI 10.1080/17429145.2019.1684582. [Google Scholar] [CrossRef]
Nagda V, Gajbhiye A, Kumar D. (2017). Isolation and characterization of endophytic fungi from Calotropis procera for their antioxidant activity. Asian Journal of Pharmaceutical and Clinical Research 10: 254. DOI 10.22159/ajpcr.2017.v10i3.16125. [Google Scholar] [CrossRef]
Nusrat B, Gul J, Farzana GJ, Muhammad H, Amjad I, Anwar H, Hazir R, Abdul T, Faiza K. (2019). Cochliobolus sp. acts as a biochemical modulator to alleviate salinity stress in okra plants. Plant Physiology and Biochemistry 139: 459–469. DOI 10.1016/j.plaphy.2019.04.019. [Google Scholar] [CrossRef]
Pattnaik PK, Kar D, Chhatoi H, Shahbazi S, Ghosh G, Kuanar A. (2017). Chemometric profile & antimicrobial activities of leaf extract of Calotropis procera and Calotropis gigantea. Natural Product Research 31: 1954–1957. DOI 10.1080/14786419.2016.1266349. [Google Scholar] [CrossRef]
Phetcharat P, Duangpaeng A. (2012). Screening of endophytic bacteria from organic rice tissue for indole acetic acid production. Procedia Engineering 32: 177–183. DOI 10.1016/j.proeng.2012.01.1254. [Google Scholar] [CrossRef]
Qadir M, Hussain A, Hamayun M, Shah M, Iqbal A, Murad W. (2020). Phytohormones producing rhizobacterium alleviates chromium toxicity in Helianthus annuus L. by reducing chromate uptake and strengthening antioxidant system. Chemosphere 258: 127386. DOI 10.1016/j.chemosphere.2020.127386. [Google Scholar] [CrossRef]
Rani R, Sharma D, Chaturvedi M, Yadav J. (2017). Antibacterial activity of twenty different endophytic fungi isolated from Calotropis procera and time kill assay. Clinical Microbiology: Open Access 06: 280. DOI 10.4172/2327-5073.1000280. [Google Scholar] [CrossRef]
Saravanan S, Parimelazhagan T. (2014). In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Science and Human Wellness 3: 56–64. DOI 10.1016/j.fshw.2014.05.001. [Google Scholar] [CrossRef]
Su L, Shen Z, Ruan Y, Tao C, Chao Y, Li R, Shen Q. (2017). Isolation of antagonistic endophytes from banana roots against Meloidogyne javanica and their effects on soil nematode community. Frontiers in Microbiology 8: 318. DOI 10.3389/fmicb.2017.02070. [Google Scholar] [CrossRef]
Zam S, Syamsuardi AA, Jannah M, Aldi Y, Djamaan A. (2016). Isolation, characterization of endophytic bacteria from Citrus aurantifolia Swingle Leaves and testing of antifungal activity towards Fusarium Oxysporum. Der Pharmachia Lettre 8: 83–89. [Google Scholar]
Zerroug A, Sadrati N, Demirel R, Bakli S, Harzallah D. (2018). Antibacterial activity of endophytic fungus, Penicillium griseofulvum MPR1 isolated from medicinal plant, Mentha pulegium L. African Journal of Microbiology Research 12: 1056–1066. DOI 10.5897/AJMR2018.8887. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |