DOI:10.32604/biocell.2021.013842

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.013842 |  www.techscience.com/journal/biocell |
| Article |
Down-regulation of N-methyl-D-aspartate receptor subunits 1 affects neurogenesis of hippocampal neural stem cells
1School of Basic Medical Sciences, Ningxia Medical University, Yinchuan, 750004, China
2Translational Medicine Center, Hong Hui Hospital, Xi’an Jiaotong University College of Medicine, Xi’an, 710054, China
*Address correspondence to: Hao Yang, yanghao.71_99@yahoo.com; Juan Liu, ryuken0518@163.com
Received: 23 August 2020; Accepted: 25 November 2020
Abstract: Schizophrenia is a common and serious mental illness characterized by severe impairments in thinking, emotions, and behaviors. Usually, the cognitive deficits of schizophrenia are closely associated with abnormal neurogenesis due to the hypofunction of certain neural receptors such as N-methyl-D-aspartate receptors (NMDARs), which mediates neurotransmission. However, little is known about the involvement of NMDAR1 in regulating hippocampal neurogenesis in schizophrenia. In the current study, we present evidence suggesting that NMDAR1 regulates hippocampal neurogenesis as lentivirus-mediated shRNA silencing NMDAR1 gene or blocking with MK-801 results in abnormal neurogenesis consistently found in schizophrenia. The important finding was clearly demonstrated by the multiparametric assessments, including morphology, immunofluorescence, western blotting, and flow cytometry. Simultaneously, our results indicated that knockdown and blockade of NMDAR1 significantly attenuated the proliferation of hippocampal neural stem cells (hNSCs) and decreased the differentiation to neurons. More importantly, the blockade of NMDAR1 with MK-801 aggravated the apoptosis of hNSCs. Thus, it is likely that NMDAR1 functions as a new target for the treatment of schizophrenia. Our present study may provide a novel insight for further investigation of the pathogenesis of schizophrenia.
Keywords: Schizophrenia; RNAi; MK-801; NMDAR1; Hippocampal neural stem cells
Schizophrenia is a complex, heterogeneous behavioral and cognitive syndrome that is originated from disruption of brain development caused by genetic or environmental factors, or both (Eltokhi et al., 2020; Owen et al., 2016). Until now, there is no effective curative treatment for this psychotic disorder. Although the exact causes of schizophrenia aren’t fully understood, a growing amount of evidence revealed that schizophrenia pathogenesis is considered to multi-factorial, with likely gene-environment interplays, such as biological specific sets of genes, neurology, psychological and environmental components (Nimgaonkar et al., 2017; Torrey and Yolken, 2019). Hitherto, among several key mechanisms implicated in schizophrenia, abnormal neurogenesis during fetal development may play a central role in the early phase of schizophrenia pathogenesis (Weinberger, 2017). Therefore, there is a very important significance to clarify the pathogenesis of schizophrenia for developing an effective treatment strategy.
MK-801, a non-competitive antagonist of NMDARs, has been widely used to induce a schizophrenia-like phenotype in rodents (Rogoz et al., 2018). NMDARs play diverse roles in synaptic transmission, synaptic plasticity, neuronal development, and neurological diseases (Oshima-Takago and Takago, 2017). Once NMDAR dysfunctions, numerous neural activities responsible for the above-mentioned events will be affected, leading to abnormal neuronal development including the occurrence of schizophrenia (Balu, 2016; Hardingham and Do, 2016). In addition, MK-801 has an influence on the glycolysis process and schizophrenia-related protein expression of different kinds of cells (Cassoli et al., 2016; Brandao-Teles et al., 2017; Guest et al., 2015; Martins-de-Souza et al., 2011), and this effect may be modulated by antipsychotic treatment, implying that MK-801 can induce in vitro neural cells to acquire certain in vivo hippocampal cell biological characteristics in schizophrenia patients. In the present study, we established the schizophrenia-like cell model by means of MK-801.
The glutamatergic hypothesis of schizophrenia states that abnormal expression of glutamatergic systems through NMDARs in the central nervous system (CNS) causes some symptoms of schizophrenia (Zapatero-Solana et al., 2014). NMDAR1 is the key subunit of NMDARs (Ju and Cui, 2016). Recent advances have put forward some direct support for the importance of impaired NMDAR1-mediated glutamatergic pathways in the pathophysiology of schizophrenia (Chen et al., 2020). Significant reduction of the hippocampal volume is a structural hallmark of schizophrenia (Zheng et al., 2019). However, no direct evidence indicates that abnormal hippocampal neurogenesis is likely to contribute to the volume decrease in schizophrenia. Strikingly, the excessive expression of NMDAR1 promotes hippocampal neurogenesis (Kalev-Zylinska et al., 2009). Thus, it is of significance to investigate the relationship of abnormality of NMDAR1 and neurogenesis in schizophrenia.
In the present study, we postulate that the abnormality of NMDAR1 expression may affect hippocampal neurogenesis in schizophrenia. As we expected, knockdown of NMDAR1 by lentivirus-mediated shRNA interference or blockade with MK-801 effectively decreased the proliferation and differentiation of NSCs from the hippocampus and exacerbated apoptosis of NSCs. The present study may lay a foundation for further elucidation of NMDAR1 function on hippocampal neurogenesis in schizophrenia.
DMEM-F12, B27 supplement, fetal bovine serum (FBS) and trypsin were purchased from Thermo Fisher Scientific, Inc., Waltham, MA, USA; Lentivirus carrying the interference NMDAR1 subunits (pLV-EGFP-NMDAR1-shRNA-1, target sequence: CAGTCCCTTTGGCCGATTTAA; pLV-EGFP-shRNA) (pLV-EGFP-NMDAR1-shRNA-2, target sequence: GTGGCTCCACTGACCATTAAC; pLV-EGFP-shRNA) (pLV-EGFP-NMDAR1-shRNA-3, target sequence: GCAGTACCATCCCACTGATAT) were purchased from Cyagen Biosciences, Guangzhou, Guangdong, China; mouse anti-Nestin (ab11306), rabbit anti-SOX2 (ab97959), rabbit anti-NMDAR1 (ab109182), rabbit anti-Ki-67 (ab16667), rabbit anti-NeuN (ab177487) were purchased from Abcam, Cambridge, MA, USA; mouse anti-β-tubulin (ab009) was purchased from liankebio, Hangzhou, Zhejiang, China; HRP-conjugated goat anti-rabbit (zb-2301), HRP-conjugated goat anti-mouse (zb-2305), Rhodamine-conjugated goat anti-mouse lgG (H+L) (zf-0313) and Rhodamine-conjugated goat anti-rabbit lgG (H+L) (zf-0316) were purchased from ZSGB-BIO, Beijing, China; MK-801 was purchased from Sigma-Aldrich, Merck KGaA, Darmstadt, Germany; basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) were purchased from PeproTech, Inc., Rocky Hill, NJ, USA; Annexin V-APC/PI kit was purchased from Nanjing KeyGen Biotech, Jiangsu, China. Cell counting kit-8 was purchased from Wuhan Boster Biological Technology, Hubei, China.
Preparation of NSCs and different treatments
All experiments on animals were conducted in strict accordance with the standards established by the Institutional Animal Care and Use Committee of Ningxia Medical University (license no D2014-014). Primary NSCs were prepared from 1–2-day-old postnatal mice hippocampus. In brief, five postnatal mice were killed by decapitation, and the hippocampi were dissected and enzymatically dissociated into a single-cell suspension. Cells were maintained in the conditional medium at the final concentration of 2% B27, 20 ng/mL bFGF, and 20 ng/mL EGF (PeproTech, USA) and cultured in an incubator with 5% CO2 at 37°C, and the culture medium was changed once every 3 days.
After culture for 5 days, cultured cells were divided into different groups as follows: Control group (treated with DMEM-F12 medium supplemented with 2% B27, 20 ng/mL bFGF, and 20 ng/mL EGF for 96 h), MK-801 group (treated with 200 µM MK-801 for 24 h, and then the medium was switched to DMEM-F12 medium supplemented with 2% B27, 20 ng/mL bFGF and 20 ng/mL EGF for 72 h), MK-801+NMDAR1-shRNA group (pretreated with 200 µM MK-801 for 24 h, followed by removed the medium and switched to DMEM-F12 medium supplemented with 2% B27, 20 ng/mL bFGF and 20 ng/mL EGF adding NMDAR1-shRNA for 72 h).
To determine whether the mock-lentivirus exhibits a cytotoxic influence on cells, we tested cell viability using a modified cell counting kit-8 (CCK-8) assay as described previously (Yang et al., 2020; Dong et al., 2020). In brief, after the treatment of mock-lentivirus for 72 h, the hippocampal NSCs were seeded into a 96-well plate and cultured at 37°C for 24 h. Next, 10 μL of CCK-8 reagent was added to each well, and the cells were incubated at 37°C for an additional 2 h. Finally, the optical density (OD) of the solution was measured at 450 nm to assess cell viability. CCK-8 assay was repeated three times.
The protein extract from cells with different treatments was harvested on ice with lysis buffer (RIPA:PMSF = 100:1) containing protease inhibitor for 30 min. The homogenates of cells were centrifuged at 12000 rpm for 15 min at 4°C. The supernatants were collected for the western blotting according to the protocol described previously. Each protein sample was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were immersed in blocking solution (10% fat-free dried milk in TBST) for 1 h at room temperature (RT). Proteins were probed with rabbit anti-NMDAR1 (1:1000), mouse anti-NeuN (1:1000), and mouse anti-β-tubulin (1:1000) primary antibody overnight at 4°C, respectively. Thereafter, the PVDF membranes were washed with TBST three times for 10 min and incubated with HRP-conjugated goat anti-rabbit antibody (1:5000) and HRP-conjugated goat anti-mouse antibody (1:5000) for 1 h at RT. After thorough washes in TBST, the immunoblots were visualized using enhanced chemiluminescence and imaged using gel systems camera.
Differentiation of hippocampal NSCs
To assess the differentiation ability of NSCs subjected to knockdown of NMDAR1 to neurons, hippocampal NSCs were maintained in DMEM/F12 (Thermo Fisher Scientific, USA) conditioned medium containing 2% FBS (Thermo Fisher Scientific, USA) and 2% B27 (Thermo Fisher Scientific, USA). After 7 days of differentiation, immunocytochemistry staining was conducted as the following experiments and western blot were carried out according to the aforementioned procedures.
NSCs with different treatments in the 24-well plate were fixed with 4% paraformaldehyde solution for 1 h. After washing with D-PBS three times, cells were permeabilized with Triton X-100 in PBS for 10 min, followed by incubation with 8% BSA in D-PBS for 1 h at RT. Thereafter, the cells were incubated overnight at 4°C with the following primary antibodies: mouse anti-Nestin (1:200), rabbit anti-Ki-67 (1:250), rabbit anti-SOX2 (1:200), mouse anti-NeuN (1:200). After the primary antibodies were removed and the samples were extensively washed with D-PBS, the corresponding secondary antibodies Rhodamine-conjugated goat anti-mouse lgG (H+L) (1:200), and Rhodamine-conjugated goat anti-rabbit lgG (H+L) (1:200) were added and incubated at RT for 2 h. The nuclei were counterstained for 10 min with DAPI. Lastly, the percentages of nestin, Ki-67, and NeuN positive cells were observed and counted by fluorescence microscope.
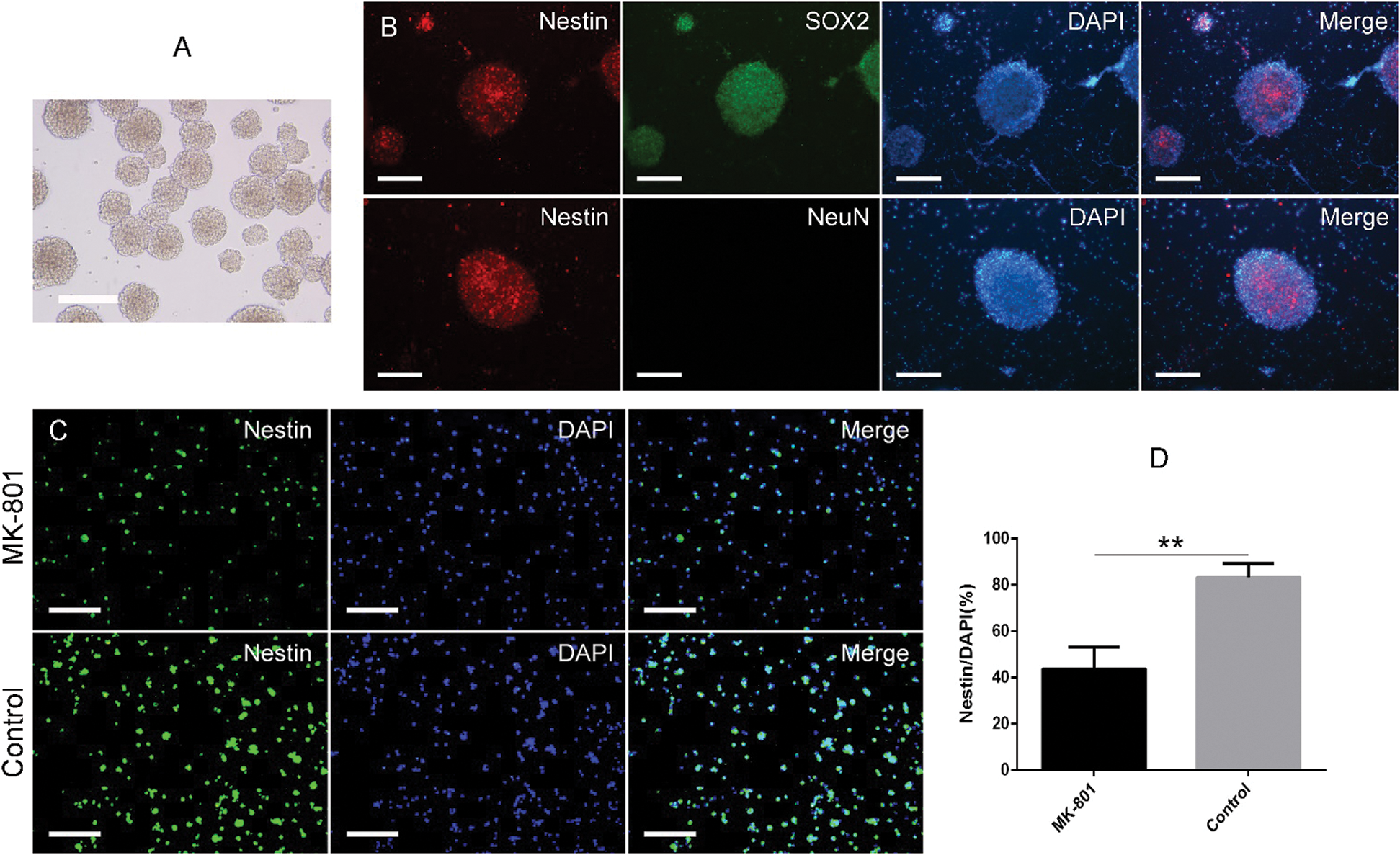
Figure 1: Morphological identification of hippocampal NSCs and effects of MK-801 treatment on nestin expression.
Apoptosis of NSCs was measured by flow cytometry. In brief, NSCs with different treatments were washed with Dulbecco’s Phosphate Buffered Saline (D-PBS) twice and trypsinized with digestion solution containing 0.25% trypsin at 37°C for 15 min, triturated into single-cell suspension, and further filtered through 80-mm nylon mesh prior to analysis. Concomitantly, the cells were resuspended in 500 μL binding buffer and further incubated with 5 μL annexin V-allophycocyanin conjugate and propidium iodide (Annexin V-APC/PI) for flow cytometry analysis. The percentages of apoptosis in hNSCs were determined by flow cytometry assay as described previously (Sun et al., 2019) and analyzed using the FACS express v2.0 software. Annexin V is a sensitive indicator for detecting cells within the population that were undergoing apoptosis. The nucleus of cells in the end stage of apoptosis or necrotic cells is stained by PI.
Statistical analysis was performed by SPSS Statistics Data Editor 17.0 (SPSS, Inc., Chicago, IL, USA). One-way ANOVA was used for intergroup comparisons followed by the S-N-K test and the comparisons of two groups using Independent-Sample t-test. p < 0.05 was considered statistically significant in this study.
Identification of hippocampal NSCs and development of schizophrenia cell model in vitro
To confirm whether the schizophrenia cell model in vitro was successful, we first cultured and identified hippocampal NSCs from the mouse hippocampus based on morphological and phenotypic characteristics. Phase-contrast microscopy showed that hippocampal NSCs exhibited spherical morphology, and the majority of free-floating neurospheres are approximately uniform in size and have good refractivity and stereoscopic vision (Fig. 1A). Further, the identification of hippocampal NSCs was conducted by means of immunofluorescence staining for Nestin, SOX2, and NeuN. As shown in Fig. 1B, almost all neurospheres were positive for Nestin and SOX2 and reached more than 90% positive reactivity. Interestingly, none of them were positive for NeuN, indicating these cells were truly purified NSCs. Twenty-four hours after 200 µM MK-801 treatment, the volume of neurospheres, and percentage of nestin-positive cells remarkably decreased (Fig. 1C). Quantitative analysis revealed that 200 µM MK-801 treatment resulted in an approximately 2-fold reduction in Nestin-positive cells (Fig. 1D), indicating that the MK-801-induced schizophrenia cells model was successful.
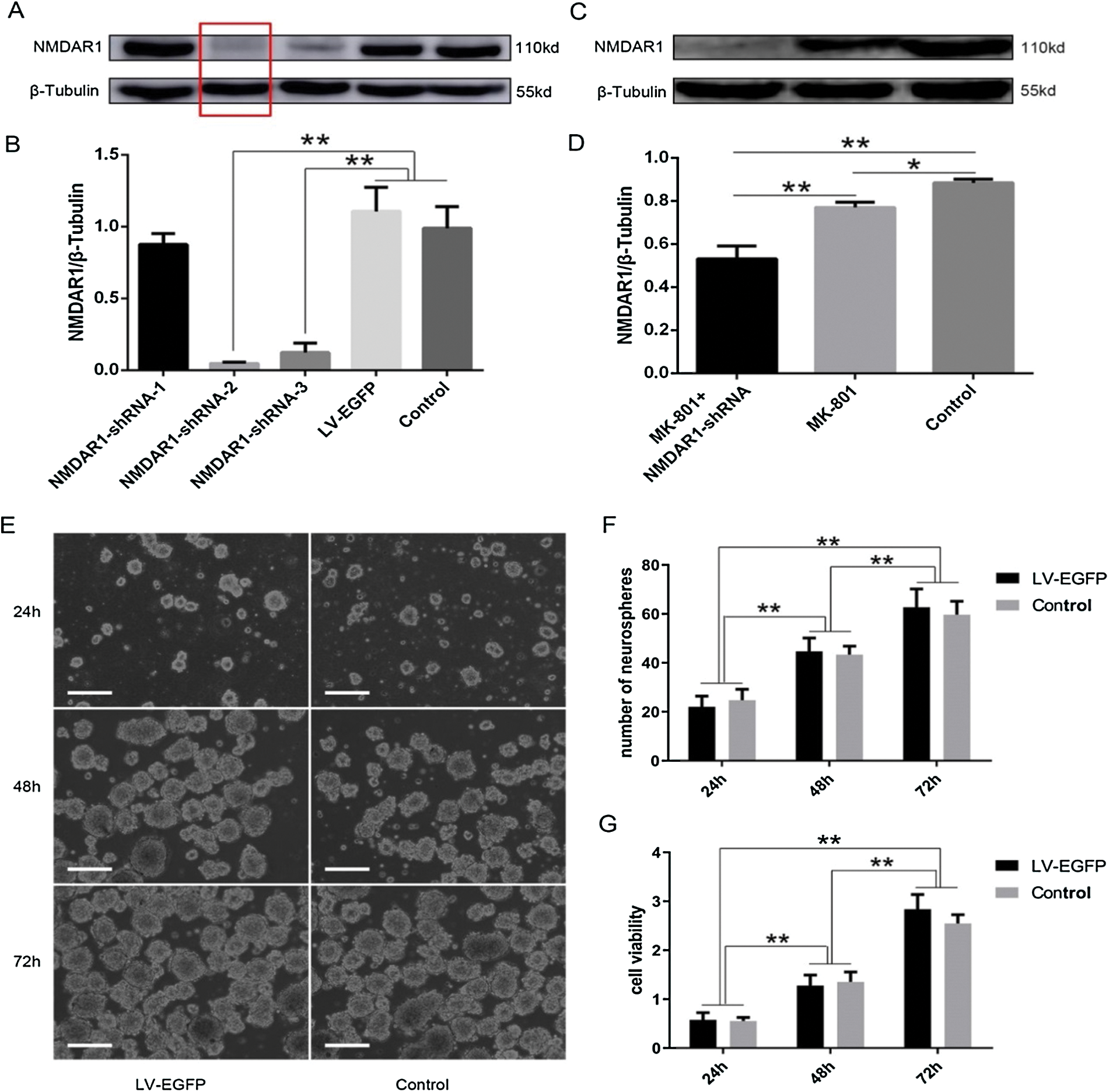
Figure 2: Identification of NMDAR1-pLV-EGFP-shRNA lentiviral vector and transduction of schizophrenia-like hippocampal NSCs.
Construction of NMDAR1-pLV-EGFP-shRNA lentiviral vector and cell transduction
To further validate our hypothesis, the lentiviral vector NMDAR1-pLV-EGFP-shRNA was constructed, identified, and used for cell transfection. After the efficiency of the lentivirus was measured with the method of gradual dilution, the titer level reached 2 × 108–2 × 109 TU/mL was used for cell transfection, followed by examination of the protein expressions of NMDAR1. As shown in Fig. 2, western blot analysis showed that after transduction of the mock lentivirus, no change in NMDAR1 expression was observed between the LV-EGFP and control groups (Fig. 2A), excluding the negative effect of the lentiviral vector on NMDAR1 expression. In reverse, NMDAR1-shRNA transfection resulted in a significant decrease in NMDAR1 expression in the NMDAR1-shRNA-2 and NMDAR1-shRNA-3 groups and was also revealed by the quantitative analysis of band optical density (Fig. 2B). Based on the above results, we selected the NMDAR1-shRNA-2 lentivirus sequence for the following experiments due to its ideal gene silencing. The results showed that after transfection for 72 h in schizophrenia cells model in vitro, NMDAR1 expression was significantly reduced in the MK-801+NR1-shRNA group (Figs. 2C and 2D) as compared with other groups, suggesting the efficiency for NMDAR1 silencing of the lentiviral particles encoding NMDAR1. As shown in Figs. 2E and 2F, the numbers and sizes of neurosphere significantly increased than that of the 48-h group and 24-h group after the 72 h-treatment of mock-lentivirus. However, the numbers and sizes of neurospheres of NSCs have no obvious differences between the mock-lentivirus group and control groups. Similarly, CCK-8 tests showed that there was no significant difference in cell viability of NSCs between the mock-lentivirus groups and the control groups (Fig. 2G). These results suggest that the observed effects mainly attribute to the knockdown of NMDAR1 rather than the cytotoxicity of mock-lentivirus.
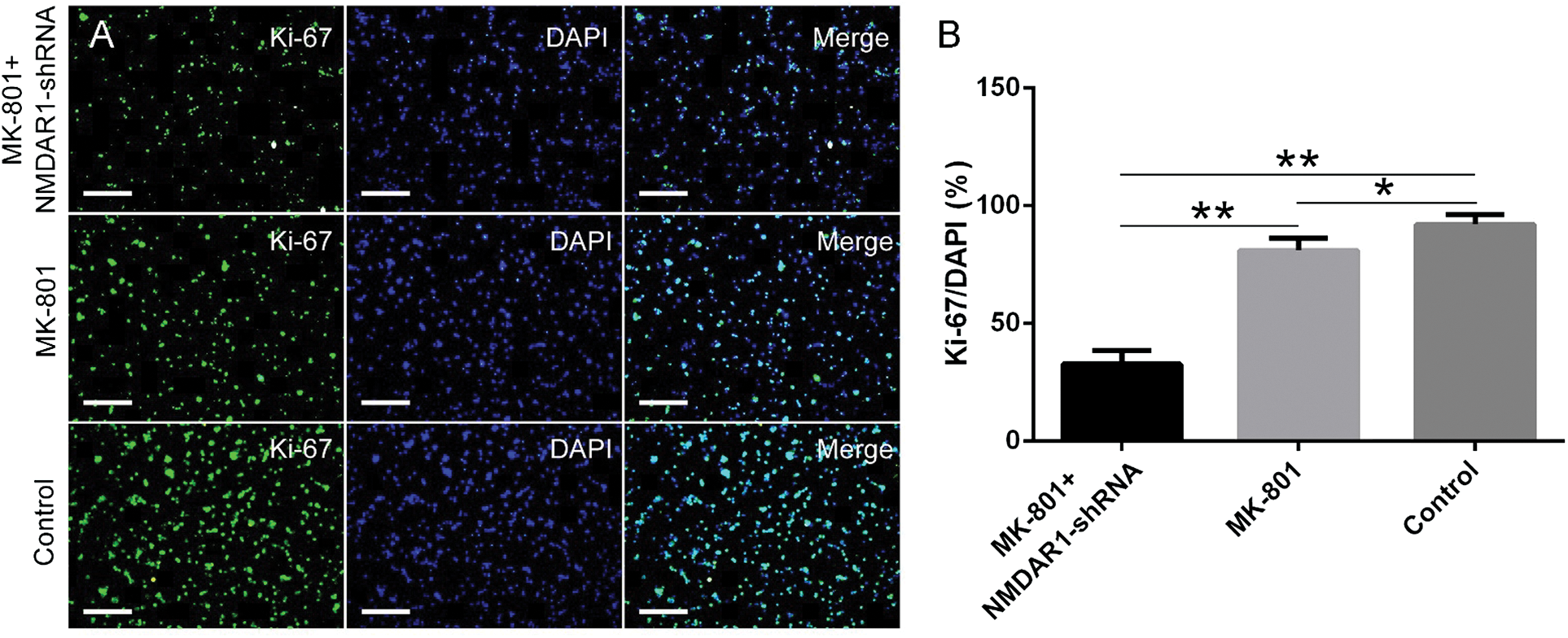
Figure 3: Effects of NMDAR1-shRNA treatment on proliferation of schizophrenia-like hippocampal NSCs.
Knockdown of NMDAR1 inhibits the NSCs proliferation
To examine whether NMDAR1 exerts a possible effect in cell proliferation from the schizophrenia in vitro model, knockdown of NMDAR1 in cells was conducted by NMDAR1-shRNA interference, followed by immunofluorescence staining with the typical proliferation marker Ki-67 was carried out. As shown in Fig. 3A, knockdown of NMDAR1 in combination with 200 µM MK-801 stimulation resulted in a significant decrease in NSCs proliferation as compared with the MK-801 only and control groups, displaying remarkably lower proportion and weaker immunoreactivity of the Ki-67-positive cells in cultures. In comparison, the total number and proportion of Ki-67-positive cells in control groups were larger and higher than that in the other two groups. Consistent with the morphological results, quantitative analysis showed that knockdown of NMDAR1 resulted in a significant decrease in the percentage of Ki-67-positive cells (Fig. 3B), suggesting silencing of NMDAR1 can inhibit NSCs proliferation in the schizophrenia-like cell model.
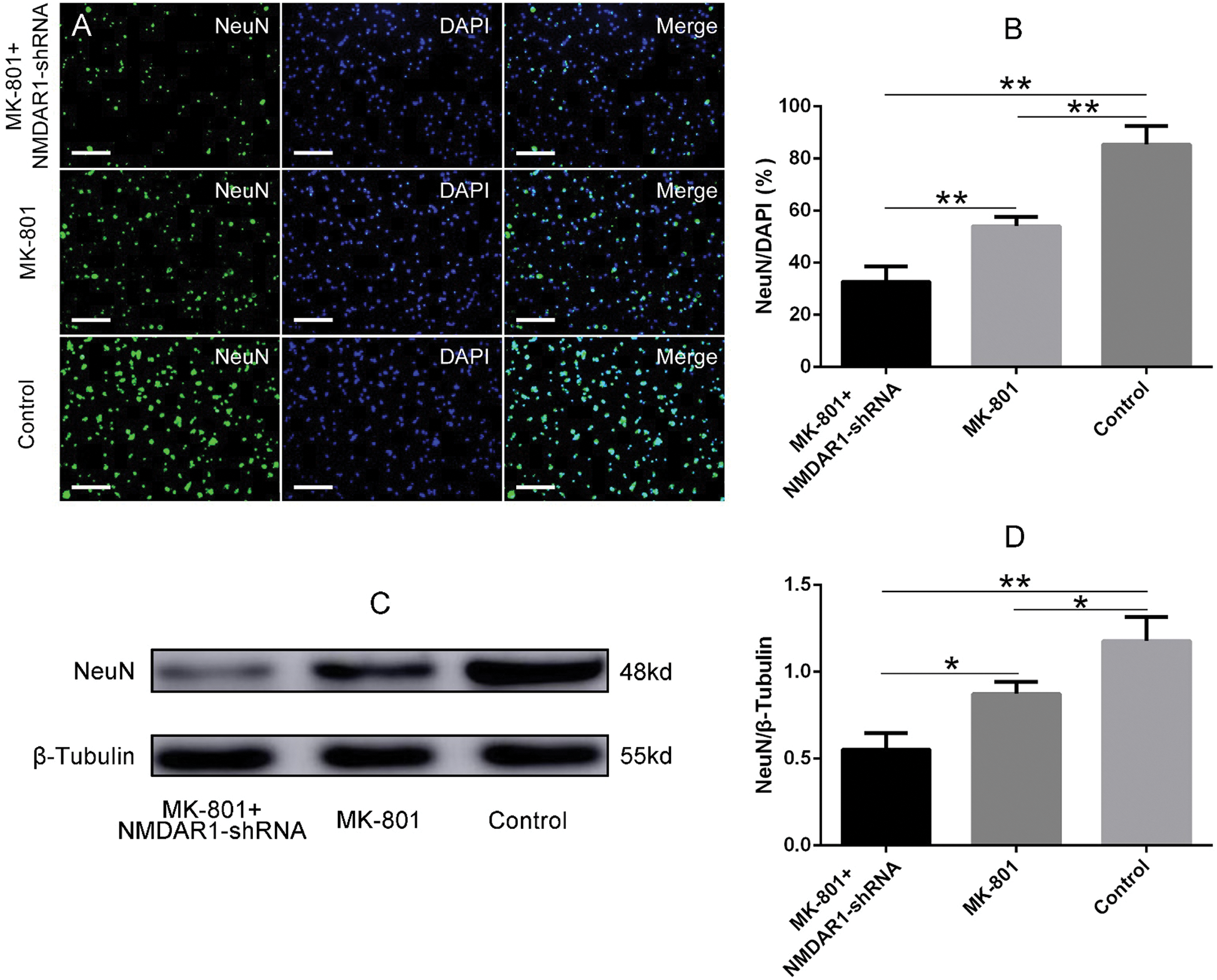
Figure 4: Effects of NMDAR1-shRNA treatment on the differentiation of schizophrenia-like hippocampal NSCs.
Knockdown of NMDAR1 inhibits the differentiation of NSCs to neuronal cells
To investigate whether NMDAR1 is likely to involve in the differentiation of NSCs, Immunofluorescence staining and western blotting test of typical neuronal marker NeuN were performed to assess the NSC differentiation after being treated with the above-mentioned conditions. As shown in Fig. 4A, after 7 days of induction, a large number of cells from in vitro schizophrenia model showed a weaker NeuN immunoreactivity or negative for NeuN, whereas cells in the MK-801 treatment group, the proportion of NeuN positive cells is relatively higher than that in NMDAR1-shRNA+MK-801 group. Reversely, the number and proportion of NeuN positive cells in the normal NSCs group were higher than that in the other two groups. Consistent with the immunofluorescence staining, quantitative analysis showed that knockdown of NMDAR1 significantly inhibits the differentiation of NSCs into neuronal cells, displaying a remarkable decrease in the percentage of NeuN positive cells when compared to MK-801 and control groups (Fig. 4B). Meanwhile, MK-801 alone also resulted in a significant decrease in the percentage of NeuN positive cells (Fig. 4B), implying a critical role of NMDAR1 in neurogenesis. To further substantiate our hypothesis, the protein expression of NeuN in the above treatments was investigated. In line with immunofluorescence results, western blot analysis for NeuN showed a similar result (Fig. 4C) that the weakest blot occurred in the NMDAR1-shRNA+MK-801 group, while the amount of NeuN protein in NSCs treated with MK-801 showed an approximate 1.3-fold decrease (Figs. 4C and 4D), suggesting knockdown of NMDAR1 inhibited the differentiation of schizophrenia-like cells into neurons.
Knockdown of NMDAR1 promotes the apoptosis of NSCs from schizophrenia-like cells model
To characterize both the type and extent of cell death after the knockdown of NMDAR1, the early apoptotic, late apoptotic, and necrotic cells were quantified using AV and PI double staining and flow cytometry. As shown in Fig. 5, the flow cytometry assays showed that knockdown of NMDAR1 significantly reduced the number of normal live cells and elevated the number of late apoptotic and necrotic cells. Likewise, blockade of NMDAR1 by virtue of MK-801 also resulted in significant cell apoptosis as compared with the normal culture (Fig. 5A). Quantitative analysis revealed that knockdown of NMDAR1 significantly promoted the apoptosis of NSCs from the schizophrenia model as compared with the corresponding controls (Fig. 5B), indicating critical roles of NMDAR1 in hippocampal neurogenesis.
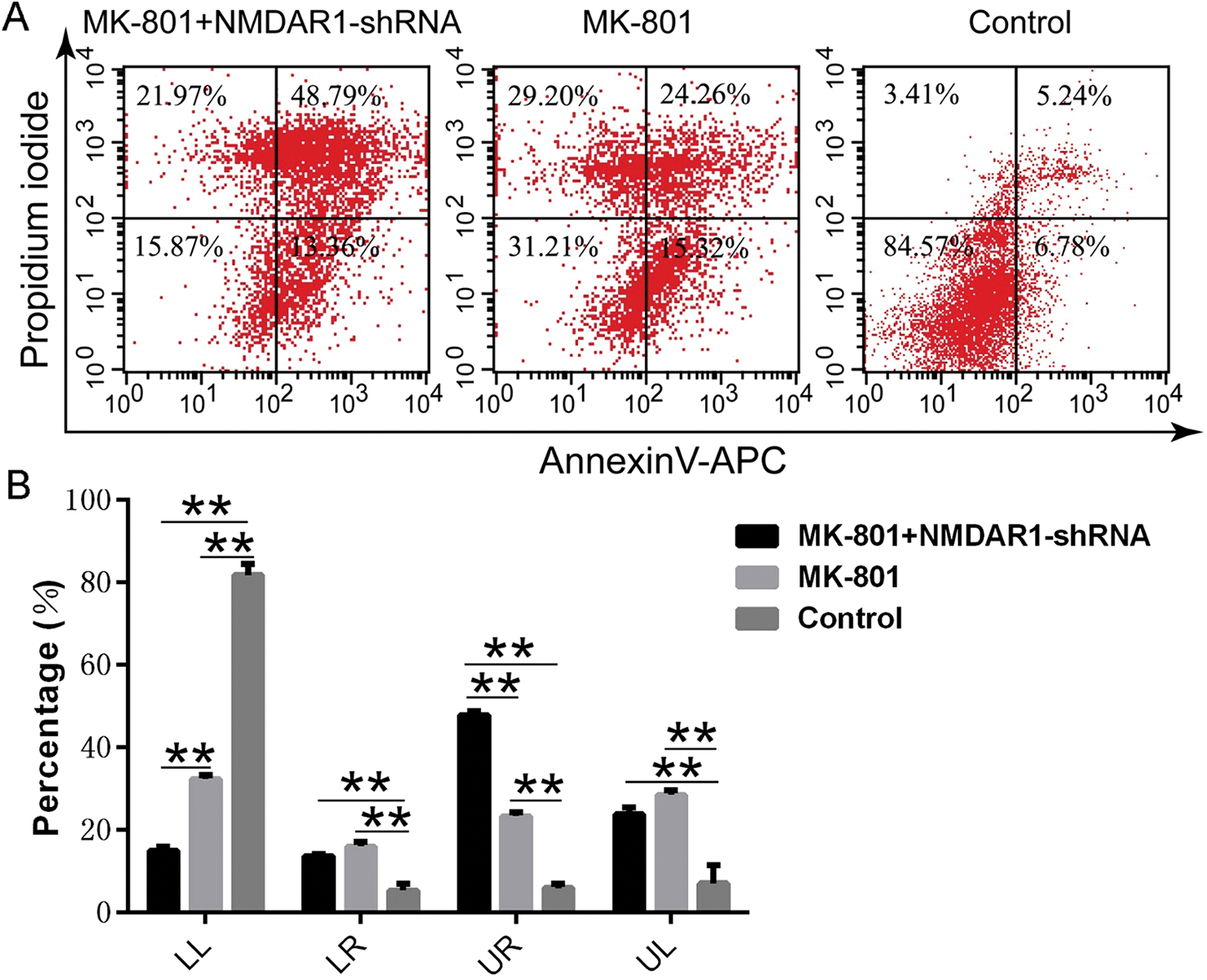
Figure 5: Effects of NMDAR1-shRNA treatment on apoptosis of schizophrenia-like hippocampal NSCs.
Schizophrenia is a group of severe psychoses with a prevalence of approximately 1% among the general population that has a disproportionately negative impact on individuals and society (D’Arcey et al., 2020). However, the specific pathophysiological mechanisms of schizophrenia remain unclear. By far, NMDAR hypofunction is one of the most prevalent models of schizophrenia (Lee and Zhou, 2019). MK-801 is a non-competitive antagonist of NMDAR and is usually used for inducing a schizophrenia-like phenotype in rodents (Rogoz et al., 2018), which leads to different degrees of cognitive impairment (Zhou et al., 2020). MK-801 affects glycolysis in neural cells and this effect may be modulated by antipsychotic treatment (Guest et al., 2015; Brandao-Teles et al., 2017), indicating that MK-801 produces a similar function with the symptom of schizophrenia in vitro levels. Although no animal model of all aspects of schizophrenia can be made accurately, several previous reports and our study have documented that NMDAR hypofunction caused by MK-801 can be used to establish this cell model of schizophrenia (Ding et al., 2018). To clarify whether dysfunction or down-regulation of NMDAR1 affect neurogenesis, resulting in schizophrenia, we established the schizophrenia-like cells model of hNSCs using MK-801. In the present study, MK-801 at a concentration of 200 µM was used, rather than the traditional low dose (in the range of 0.009–0.35 µM) according to MK-801 chirality and NMDAR subunit composition (Traynelis et al., 2010). This is mainly due to the following several points: (1) Pre-experiment results revealed that this dose is relatively optimal (Ding et al., 2018); and (2) The different types of cells possess different biological properties and sensitivity to the drug. Several previous studies demonstrated that in the development of the schizophrenia cell model, various concentrations of MK-801 could be used. For example, the concentration of MK-801 is 50 µM in oligodendrocyte cells (Brandao-Teles et al., 2017; Guest et al., 2015) or 50 mM in oligodendrocyte cells (Cassoli et al., 2016), and 20 or 50 µM MK-801 in astrocytes (Martins-de-Souza et al., 2011). Therefore, we speculated that 200 µM MK-801 used to develop schizophrenia-like cells model in our study may be mainly associated with NSC characteristics (such as strong self-renewal and multipotential differentiation) and sensitivity to MK-801.
Strong evidence implicates that abnormalities of glutamate signaling might account for the negative and cognitive symptoms. NMDAR, one of the most important excitatory amino acid receptor which belongs to the glutamate receptor (Zoodsma et al., 2020), is intimately associated with a variety of physiological processes underlying neural transmission, synaptic plasticity, neuronal development, and cognitive functions (Li et al., 2018). NMDAR1 is the principal subunit of NMDAR assemblies (Ju and Cui, 2016). Several lines of studies showed that the NMDAR1 expression significantly decreased in schizophrenias (Catts et al., 2015; Rodriguez-Munoz et al., 2017; Catts et al., 2016; Areal et al., 2017), implying the intimate associations between the dysfunction of NMDAR1 and schizophrenia. Accordingly, the NMDAR1-knockdown animal model as a representative model was usually utilized to study the role of NMDAR in the pathophysiology of schizophrenia (Ramsey, 2009). In our present study, we firstly used lentivirus-mediated shRNA silencing assay to knockdown NMDAR1 expression to clarify its roles in the development of NSCs. Consistent with the previous reports, the NMDAR1 protein levels significantly decreased in the schizophrenia-like cell model in our study, suggesting that NMDAR1 participates in regulating neurogenesis in schizophrenia. To unravel the potentials of NMDAR1 in schizophrenia pathogenesis, we examined the proliferation, differentiation, and apoptosis of hNSCs in the schizophrenia-like cell model by different assays after knockdown of NMDAR1.
Proliferation, differentiation, survival, and integration of newborn neurons in a neural network are specific manifestations of adult hippocampal neurogenesis (de Miranda et al., 2017). Adult hippocampal neurogenesis is intimately associated with hippocampus-dependent learning and memory and emotional processing (Terranova et al., 2019; Drew and Huckleberry, 2017). Once ventral hippocampal lesions or abnormal neurogenesis, it will implicate the neuropathology of schizophrenia (Peng and Bonaguidi, 2018). Therefore, hippocampal neurogenesis may provide valuable information in identifying schizophrenia. In this study, we present in vitro data emphasizing the potential of NMDAR1 in hippocampal neurogenesis, which is intimately related to schizophrenia. As we expected, the roles of NMDAR1 in regulating neurogenesis in several aspects such as cell proliferation, differentiation, and survival, by in vitro cell model of schizophrenia are revealed. Our further investigation demonstrating the involvement of NMDAR1 in the modulation of neurogenesis provides a molecular mechanism responsible for the pathogenesis of schizophrenia.
The ionotropic glutamate receptor NMDAR1 is permeable to Ca2+ but blocked by Mg2+, which governs key processes, such as cell survival, the release of neurotransmitters (Jimenez-Gonzalez et al., 2019), the initiation of long-term potentiation (LTP), learning and memory formation (Johnson et al., 2019), and induction of synaptic plasticity (Johnson et al., 2019; Mesbahi-Vasey et al., 2017). Once the function of NMDAR is impaired by MK-801, the deleterious cascade event such as blockade of Ca2+ channels, increasing intracellular Ca2+ levels, and final excitotoxicity in cells occurred (Qian et al., 2020). As the disequilibrium of Ca2+ triggers endoplasmic reticulum stress, mitochondrial membrane potential changes, and mitochondrial ATP synthase reversal (Dong et al., 2017). Eventually, the abnormal Ca2+ uptake leads to loss of mitochondrial membrane potential, mitochondrial swelling, and the mitochondrial hyperpermeability transition (Ding et al., 2017). This process aggravates apoptosis of hippocampal NSCs, leading to hippocampal neurogenesis retardation (D'Ascenzo et al., 2006; Ambrosio et al., 1999; Yoon et al., 2003). Our present data showed that the administration of MK-801 decreased the NMDAR1 expression, inhibited NSC proliferation and differentiation, and kindled the apoptosis of hippocampal NSCs. Meanwhile, knockdown of NMDAR1 with lentivirus can progressively strengthen this effect of MK-801 on the regulation of cell proliferation, differentiation, and survival. The underlying mechanisms may be related to the dysfunction of NMDAR1 caused by the abnormal opening of Ca2+ channels, which elicit neurotoxicity and lead to cell death.
Our present study has demonstrated that down-regulation of NMDAR1 progressively enhanced the neurotoxic effects of MK-801 on hNSCs, indicating that knockdown of NMDAR1 has as an inhibitory effect in schizophrenia hippocampal neurogenesis. Conversely, up-regulation of NMDAR1 may reverse this trend and contributes to positive function in schizophrenia hippocampal neurogenesis. This finding provides the basis for us to further understand the mechanism of NMDAR1 in schizophrenia hippocampal neurogenesis, and NMDAR1 may be a new target in the clinical therapy of schizophrenia.
Availability of Data and Materials: The datasets used during the current study are available from the corresponding author on reasonable request.
Author Contribution: The authors confirm contribution to the paper as follows: Study conception and design: (JUAN LIU and HAO YANG); data collection: (YUQING HE and LI GUO); analysis and interpretation of results: (YUQING HE, JUAN DING, HAOWEN LV, QUANRUI MA, CHEN LI); draft manuscript preparation: (YUQING HE, YU SHAO, QIANG LIU and CHUN ZHANG). All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: Animal experimental ethics involved in this experiment was supported by ethics committee of Ningxia medical university, ethical approval code: D2014-014.
Funding Statement: This work was funded by the National Natural Science Foundation of China (Grant No. 82060238) and West China Top Class Discipline Project in Basic Medical Sciences, Ningxia Medical University (Grant No. NXYLXK2017B07).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ambrosio AF, Silva AP, Malva JO, Soares-da-Silva P, Carvalho AP, Carvalho CM. (1999). Carbamazepine inhibits L-type Ca2+ channels in cultured rat hippocampal neurons stimulated with glutamate receptor agonists. Neuropharmacology 38: 1349–1359. DOI 10.1016/S0028-3908(99)00058-1. [Google Scholar] [CrossRef]
Areal LB, Herlinger AL, Pelicao FS, Martins-Silva C, Pires R. (2017). Crack cocaine inhalation induces schizophrenia-like symptoms and molecular alterations in mice prefrontal cortex. Journal of Psychiatric Research 91: 57–63. DOI 10.1016/j.jpsychires.2017.03.005. [Google Scholar] [CrossRef]
Balu DT. (2016). The NMDA receptor and schizophrenia: From pathophysiology to treatment. Advances in Pharmacology 76: 351–382. [Google Scholar]
Brandao-Teles C, Martins-de-Souza D, Guest PC, Cassoli JS. (2017). MK-801-Treated oligodendrocytes as a cellular model to study schizophrenia. Advances in Experimental Medicine and Biology 974: 269–277. [Google Scholar]
Cassoli JS, Iwata K, Steiner J, Guest PC, Turck CW, Nascimento JM, Martins-de-Souza D. (2016). Effect of MK-801 and clozapine on the proteome of cultured human oligodendrocytes. Frontiers in Cellular Neuroscience 10: 52. DOI 10.3389/fncel.2016.00052. [Google Scholar] [CrossRef]
Catts VS, Derminio DS, Hahn CG, Weickert CS. (2015). Postsynaptic density levels of the NMDA receptor NR1 subunit and PSD-95 protein in prefrontal cortex from people with schizophrenia. NPJ Schizophrenia 1: 15037. DOI 10.1038/npjschz.2015.37. [Google Scholar] [CrossRef]
Catts VS, Lai YL, Weickert CS, Weickert TW, Catts SV. (2016). A quantitative review of the postmortem evidence for decreased cortical N-methyl-D-aspartate receptor expression levels in schizophrenia: How can we link molecular abnormalities to mismatch negativity deficits? Biological Psychology 116: 57–67. DOI 10.1016/j.biopsycho.2015.10.013. [Google Scholar] [CrossRef]
Chen K, Yang LN, Lai C, Liu D, Zhu L-Q. (2020). Role of Grina/Nmdara1 in the central nervous system diseases. Current Neuropharmacology 18: 861–867. DOI 10.2174/1570159X18666200303104235. [Google Scholar] [CrossRef]
D’Arcey J, Collaton J, Kozloff N, Voineskos AN, Kidd SA, Foussias G. (2020). The use of text messaging to improve clinical engagement for individuals with psychosis: Systematic review. JMIR Mental Health 7: e16993. DOI 10.2196/16993. [Google Scholar] [CrossRef]
D’Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB, Grassi C. (2006). Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. European Journal of Neuroscience 23: 935–944. DOI 10.1111/j.1460-9568.2006.04628.x. [Google Scholar] [CrossRef]
de Miranda AS, Zhang CJ, Katsumoto A, Teixeira AL. (2017). Hippocampal adult neurogenesis: Does the immune system matter? Journal of the Neurological Sciences 372: 482–495. DOI 10.1016/j.jns.2016.10.052. [Google Scholar] [CrossRef]
Ding J, Shao Y, Zhou HH, Ma QR, Zhang YW, Ding YX, He YQ, Liu J. (2018). Effect of NMDA on proliferation and apoptosis in hippocampal neural stem cells treated with MK-801. Experimental and Therapeutic Medicine 16: 1137–1142. [Google Scholar]
Ding J, Zhou HH, Ma QR, He ZY, Ma JB, Liu YM, Zhang YW, He YQ, Liu J. (2017). Expression of NR1 and apoptosis levels in the hippocampal cells of mice treated with MK-801. Molecular Medicine Reports 16: 8359–8364. DOI 10.3892/mmr.2017.7674. [Google Scholar] [CrossRef]
Dong LG, Lu FF, Zu J, Zhang W, Xu CY, Jin GL, Yang XX, Xiao QH, Cui CC, Xu R, Zhou S, Zhu JN, Shen T, Cui GY. (2020). MiR-133b inhibits MPP+-induced apoptosis in Parkinson’s disease model by inhibiting the ERK1/2 signaling pathway. European Review for Medical and Pharmacological Sciences 24: 11192–11198. [Google Scholar]
Dong Y, Kalueff AV, Song C. (2017). N-methyl-d-aspartate receptor-mediated calcium overload and endoplasmic reticulum stress are involved in interleukin-1β-induced neuronal apoptosis in rat hippocampus. Journal of Neuroimmunology 307: 7–13. DOI 10.1016/j.jneuroim.2017.03.005. [Google Scholar] [CrossRef]
Drew MR, Huckleberry KA. (2017). Modulation of aversive memory by adult hippocampal neurogenesis. Neurotherapeutics 14: 646–661. DOI 10.1007/s13311-017-0528-9. [Google Scholar] [CrossRef]
Eltokhi A, Janmaat IE, Genedi M, Haarman B, Sommer I. (2020). Dysregulation of synaptic pruning as a possible link between intestinal microbiota dysbiosis and neuropsychiatric disorders. Journal of Neuroscience Research 98: 1335–1369. DOI 10.1002/jnr.24616. [Google Scholar] [CrossRef]
Guest PC, Iwata K, Kato TA, Steiner J, Schmitt A, Turck CW, Martins-de-Souza D. (2015). MK-801 treatment affects glycolysis in oligodendrocytes more than in astrocytes and neuronal cells: Insights for schizophrenia. Frontiers in Cellular Neuroscience 9: 180. DOI 10.3389/fncel.2015.00180. [Google Scholar] [CrossRef]
Hardingham GE, Do KQ. (2016). Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nature Reviews Neuroscience 17: 125–134. DOI 10.1038/nrn.2015.19. [Google Scholar] [CrossRef]
Jimenez-Gonzalez V, Ogalla-Garcia E, Garcia-Quintanilla M, Garcia-Quintanilla A. (2019). Deciphering GRINA/Lifeguard1: Nuclear location, Ca2+ homeostasis and vesicle transport. International Journal of Molecular Sciences 20: 4005. DOI 10.3390/ijms20164005. [Google Scholar] [CrossRef]
Johnson LR, Battle AR, Martinac B. (2019). Remembering mechanosensitivity of NMDA receptors. Frontiers in Cellular Neuroscience 13: 533. DOI 10.3389/fncel.2019.00533. [Google Scholar] [CrossRef]
Ju P, Cui D. (2016). The involvement of N-methyl-D-aspartate receptor (NMDAR) subunit NR1 in the pathophysiology of schizophrenia. Acta Biochimica et Biophysica Sinica (Shanghai) 48: 209–219. DOI 10.1093/abbs/gmv135. [Google Scholar] [CrossRef]
Kalev-Zylinska ML, Symes W, Young D, During MJ. (2009). Knockdown and overexpression of NR1 modulates NMDA receptor function. Molecular and Cellular Neuroscience 41: 383–396. DOI 10.1016/j.mcn.2009.04.003. [Google Scholar] [CrossRef]
Lee G, Zhou Y. (2019). NMDAR hypofunction animal models of schizophrenia. Frontiers in Molecular Neuroscience 12: 185. DOI 10.3389/fnmol.2019.00185. [Google Scholar] [CrossRef]
Li Y, Kim R, Cho YS, Song WS, Kim D, Kim K, Roh JD, Chung C, Park H, Yang E, Kim SJ, Ko J, Kim H, Kim MH, Bae YC, Kim E. (2018). Lrfn2-Mutant mice display suppressed synaptic plasticity and inhibitory synapse development and abnormal social communication and startle response. Journal of Neuroscience 38: 5872–5887. DOI 10.1523/JNEUROSCI.3321-17.2018. [Google Scholar] [CrossRef]
Martins-de-Souza D, Lebar M, Turck CW. (2011). Proteome analyses of cultured astrocytes treated with MK-801 and clozapine: Similarities with schizophrenia. European Archives of Psychiatry and Clinical Neuroscience 261: 217–228. DOI 10.1007/s00406-010-0166-2. [Google Scholar] [CrossRef]
Mesbahi-Vasey S, Veras L, Yonkunas M, Johnson JW, Kurnikova MG. (2017). All atom NMDA receptor transmembrane domain model development and simulations in lipid bilayers and water. PLoS One 12: e0177686. DOI 10.1371/journal.pone.0177686. [Google Scholar] [CrossRef]
Nimgaonkar VL, Prasad KM, Chowdari KV, Severance EG, Yolken RH. (2017). The complement system: A gateway to gene–environment interactions in schizophrenia pathogenesis. Molecular Psychiatry 22: 1554–1561. DOI 10.1038/mp.2017.151. [Google Scholar] [CrossRef]
Oshima-Takago T, Takago H. (2017). NMDA receptor-dependent presynaptic inhibition at the calyx of Held synapse of rat pups. Open Biology 7: 170032. DOI 10.1098/rsob.170032. [Google Scholar] [CrossRef]
Owen MJ, Sawa A, Mortensen PB. (2016). Schizophrenia. Lancet 388: 86–97. DOI 10.1016/S0140-6736(15)01121-6. [Google Scholar] [CrossRef]
Peng L, Bonaguidi MA. (2018). Function and dysfunction of adult hippocampal neurogenesis in regeneration and disease. American Journal of Pathology 188: 23–28. DOI 10.1016/j.ajpath.2017.09.004. [Google Scholar] [CrossRef]
Qian N, Lipkin RM, Kaszowska A, Silipo G, Dias EC, Butler PD, Javitt DC. (2020). Computational modeling of excitatory/inhibitory balance impairments in schizophrenia. Schizophrenia Research, DOI 10.1016/j.schres.2020.03.027. [Google Scholar] [CrossRef]
Ramsey AJ. (2009). NR1 knockdown mice as a representative model of the glutamate hypothesis of schizophrenia. Progress in Brain Research 179: 51–58. [Google Scholar]
Rodriguez-Munoz M, Sanchez-Blazquez P, Callado LF, Meana JJ, Garzon-Nino J. (2017). Schizophrenia and depression, two poles of endocannabinoid system deregulation. Translational Psychiatry 7: 1291. DOI 10.1038/s41398-017-0029-y. [Google Scholar] [CrossRef]
Rogoz Z, Wasik A, Lorenc-Koci E. (2018). Combined treatment with aripiprazole and antidepressants reversed some MK-801-induced schizophrenia-like symptoms in mice. Pharmacological Reports 70: 623–630. DOI 10.1016/j.pharep.2018.02.022. [Google Scholar] [CrossRef]
Sun HY, Yang D, Mi J, Yu YQ, Qiu LH. (2019). Histone demethylase Jmjd3 modulates osteoblast apoptosis induced by tumor necrosis factor-alpha through directly targeting RASSF5. Connective Tissue Research 61: 1–9. DOI 10.1080/03008207.2019.1620225. [Google Scholar] [CrossRef]
Terranova JI, Ogawa SK, Kitamura T. (2019). Adult hippocampal neurogenesis for systems consolidation of memory. Behavioural Brain Research 372: 112035. DOI 10.1016/j.bbr.2019.112035. [Google Scholar] [CrossRef]
Torrey EF, Yolken RH. (2019). Schizophrenia as a pseudogenetic disease: A call for more gene-environmental studies. Psychiatry Research 278: 146–150. DOI 10.1016/j.psychres.2019.06.006. [Google Scholar] [CrossRef]
Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. (2010). Glutamate receptor ion channels: Structure, regulation, and function. Pharmacological Reviews 62: 405–496. DOI 10.1124/pr.109.002451. [Google Scholar] [CrossRef]
Weinberger DR. (2017). Future of days past: Neurodevelopment and schizophrenia. Schizophrenia Bulletin 43: 1164–1168. DOI 10.1093/schbul/sbx118. [Google Scholar] [CrossRef]
Yang Z, Lin SD, Zhan F, Liu Y, Zhan YW. (2020). LncRNA GAS5 alleviates rheumatoid arthritis through regulating miR-222-3p/Sirt1 signalling axis. Autoimmunity 17: 1–10. DOI 10.1080/08916934.2020.1846183. [Google Scholar] [CrossRef]
Yoon WJ, Won SJ, Ryu BR, Gwag BJ. (2003). Blockade of ionotropic glutamate receptors produces neuronal apoptosis through the Bax-cytochrome C-caspase pathway: The causative role of Ca2+ deficiency. Journal of Neurochemistry 85: 525–533. DOI 10.1046/j.1471-4159.2003.01724.x. [Google Scholar] [CrossRef]
Zapatero-Solana E, García-Giménez JL, Guerrero-Aspizua S, García M, Toll A, Baselga E, Durán-Moreno M, Markovic J, García-Verdugo JM, Conti CJ, Has C, Larcher F, Pallardó FV, Del Rio M. (2014). Oxidative stress and mitochondrial dysfunction in Kindler syndrome. Orphanet Journal of Rare Diseases 9: 211. DOI 10.1186/s13023-014-0211-8. [Google Scholar] [CrossRef]
Zheng F, Li C, Zhang D, Cui D, Wang Z, Qiu J. (2019). Study on the sub-regions volume of hippocampus and amygdala in schizophrenia. Quantitative Imaging in Medicine and Surgery 9: 1025–1036. DOI 10.21037/qims.2019.05.21. [Google Scholar] [CrossRef]
Zhou X, Cai G, Mao S, Xu D, Xu X, Zhang R, Yao Z. (2020). Modulating NMDA receptors to treat MK-801-induced schizophrenic cognition deficit: Effects of clozapine combining with PQQ treatment and possible mechanisms of action. BMC Psychiatry 20: 106. DOI 10.1186/s12888-020-02509-z. [Google Scholar] [CrossRef]
Zoodsma JD, Chan K, Bhandiwad AA, Golann D, Liu G, Syed S, Napoli A, Burgess HA, Sirotkin HI, Wollmuth LP. (2020). A model to study NMDA receptors in early nervous system development. Journal of Neuroscience 40: 3631–3645. DOI 10.1523/JNEUROSCI.3025-19.2020. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |