DOI:10.32604/biocell.2021.014581

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.014581 |  www.techscience.com/journal/biocell |
| Article |
Protective effects of Dioscin on TNF-α-induced collagen-induced arthritis rat fibroblast-like synoviocytes involves in regulating the LTB4/BLT pathway
1Department of Physiology, College of Medicine, Nanchang University, Nanchang, 330006, China
2Henan Vocational College of Nursing, Anyan, 455000, China
3Nanchang University Hospital, Nanchang, 330031, China
4Department of Experimental Teaching Center, Nanchang University, Nanchang, 330031, China
*Address correspondence to: Fenfang Hong, hongfenfang@126.com; Shulong Yang, slyang@ncu.edu.cn
Received: 10 October 2020; Accepted: 22 December 2020
Abstract: Background and Objective: LTB4 has been shown to be involved in rheumatoid arthritis (RA) pathogenesis. The effect of Dioscin(Dio) on the LTB4 pathway of RA have not been reported yet. This study aimed at further exploring whether Dioscin’s effects on TNF-α induced collagen-induced arthritis (CIA) rat fibroblast-like synoviocytes (FLS) connected with the LTB4 and its receptor pathway. Materials & Methods: In this experiment, control group, TNF-α group, and different concentrations of Dioscin groups were established. Cell viability was evaluated using MTT assay. The levels of LTB4 in the samples of above groups were measured using ELISA. The mRNA expression levels of LTA4H, BLT1, and BLT2 were detected by quantitative real time PCR, while the expression level of LTA4H proteins were detected using western blot. The distribution of LTA4H was assessed by immunofluorescence assay. Results: the LTB4 level of TNF-α group in sample supernatant was higher than both control group and Dioscin groups with decreased LTB4 levels (p< 0.05). Compared with the control group, the expression of LTA4H was significantly increased in TNF-α group (p < 0.05), whereas LTA4H expressions were significantly decreased in all Dioscin groups when compared to TNF-α group (p < 0.05). The mRNA expressions of BLT1 and BLT2 were markedly higher in TNF-α group than those in control group while Dioscin treatment significantly inhibited the increased expressions of BLT1 and BLT2 induced by TNF-α (p < 0.05). Conclusions: These results firstly demonstrate that the protective effect of Dioscin on TNF-α induced FLS may involve in its reducing LTB4 production by down-regulating LTA4H expression, and may inhibit its downstream pathway by decreasing LTB4 receptors levels. This findings suggest that dioscin produces a potential therapeutic effects for RA via its influencing LTA4H/LTB4/BLT pathway.
Keywords: Rheumatoid arthritis; Dioscin; Fibroblast-like synoviocytes; Leukotriene B4; LTA4 hydrolase; Leukotriene B4 receptor
Rheumatoid arthritis (RA) is a common autoimmune disease, and its primary pathological manifestations include chronic synovial membrane inflammation or proliferation and joint erosion (Tu et al., 2012; Zheng et al., 2019). Abnormal hyperplasia of the synovial membrane is associated with apoptosis and abnormal proliferation of synovial cells, among which fibroblast-like synoviocytes (FLS) are the main cells involved in pannus formation and are actively involved in the progression of RA due to their abnormal apoptosis (Bartok and Firestein, 2010; Bottini and Firestein, 2013). Following stimulation by pathological factors, FLS release various cytokines that cause joint destruction (Friday and Fox, 2016). Proliferative FLS can secrete a large number of inflammatory cytokines, further causing FLS hyperproliferation and continuous inflammatory response. Up to now, the exact pathogenesis of RA has not yet been described, and there is no specific cure for it (Wu et al., 2014; Li et al., 2020).
As metabolic products of eicosanoids, leukotrienes (LTs) are a family of strong proinflammatory lipid mediators, which were discovered in the 1970s (Samuelsson, 1983). cPLA2 releases arachidonic acid (AA) from the cell membrane. AA is oxidized into 5-hydroperoxy-eicosatetraenoic acid, followed by transformation into the unstable epoxide leukotriene A4 (LTA4). LTA4 can be either metabolized into LTB4 (by LTA4 hydrolase (LTA4H)) or into the cysteinyl-leukotriene LTC4 (by LTC4 synthase (LTC4S)), which further degrades into LTD4 and LTE4.
Growing evidence indicates that dysfunction of LT synthesizing enzymes and/or receptors play a pivotal role in RA pathogenesis (Wu et al., 2014). LTB4 is present at high levels in the serum and synovial fluid of RA patients (Elmgreen et al., 1987). As a potent chemotactic mediator, LTB4 may induce most of the RA symptoms through the recruitment of leukocytes by initiating, coordinating, sustaining, and amplifying the inflammatory response (Yousefi et al., 2014). By coupling to BLT1 and BLT2, LTB4 accelerates the neutrophil-dependent increase in microvascular permeability (Yang et al., 2008; Yokomizo, 2015; Guo et al., 2016). Several studies have indicated that the blockade of LTB4 and its high-affinity receptor, BLT1, dramatically inhibits arthritis in an animal model (Zhan et al., 2016; Wei et al., 2017). These data suggested that LTB4 and BLT1 may contribute to the pathogenesis of human RA (Miyabe et al., 2017). In addition, Bi et al. (2017) demonstrated that LTB4 increases the levels of interleukin-32, IFN-γ, chemokines MCP-1, and MIP-1α in synovial cells and facilitates synovial cell apoptosis. Despite such progress so far, few systemic and integrated studies have been employed to explore the cause of abnormal levels of LTB4 or AA metabolites in synovial fluid and serum of patients with active RA (Zhan et al., 2016; Wei et al., 2017). Moreover, LTB4 receptor dysfunction in RA pathogenesis is not fully clear.
Eicosanoids (LTB4, etc.) are responsible for the progressive destruction of bone and cartilage in RA disease, so it is urgent to develop these novel compounds, which target the eicosanoid pathway by repressing the pro-inflammatory eicosanoid production in RA (Hoxha, 2017). Moreover, owing to some adverse effects resulted from long-term drug therapies for RA, it is necessary to seek safe and effective alternatives suitable for long-term chronic use. Ideal therapy of RA always involves mild but simultaneous interventions of multiple targets, which is in line with the philosophy of traditional Chinese medicine (Meng et al., 2015). According to Traditional Chinese Medicine, Dioscin can promote digestion and blood circulation, relax muscles and joints, treat malaria, eliminate phlegm and diuresis, and so on (Wang et al., 2017). Nowadays, it is used as an important synthetic raw material of various steroid hormone drugs. Its functions, such as immunity regulation, anti-inflammation, anti-tumor, and antiplatelet aggregation, have also been revealed (Zhang and Liu, 2010; Guo et al., 2013; Qu et al., 2014). So far, only a few reports have been published about Dioscin treating RA (Chu et al., 2012; Guo et al., 2013; Qu et al., 2014), which mainly focus on inflammatory cytokines, COX pathway, and NF-kB. We previously reviewed the roles of LTB4 in RA pathogenesis, suggesting its potential significance in treating RA (Wu et al., 2014; Zhan et al., 2016). However, Dioscin’s treatment of RA by the LTB4 pathway has not been reported yet. This study aimed to investigate the therapeutic effects and mechanisms of Dioscin against RA in TNF-α induced FLS, trying to provide new experimental evidence and approach for clinical treatment of RA.
The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies (Tveden-Nyborg et al., 2018).
Dioscin monomer was bought from Nanjing Spring & Autumn Biological Engineering Co., Ltd. (Nanjing, China). Tumor necrosis factor (TNF-α) was obtained from Sigma-Aldrich (St. Louis, Missouri, USA). 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT), fluorescein isothiocyanate (FITC) labeled goat anti-rabbit IgG, dimethyl sulfoxide (DMSO), and phosphate-buffered saline (PBS) were obtained from Beijing Zhongshan Biological Technology Co., Ltd. (Beijing, China). Dulbecco’s modified eagle medium (DMEM) culture medium was purchased in Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). Fetal bovine serum (FBS) was bought from Hangzhou Sijiqing Biological Engineering Materials Co., Ltd. (Hangzhou, China). 0.25% trypsin was supplied by Gibco BRL (Grand Island, New York, USA). To prepare the cell frozen stock solution, the allocation ratio of DMSO: FBS: DMEM was equaled to 1:2:7. Antibodies for LTA4H were obtained from Santa Cruz (California, USA). TRIzol was obtained from Takara Bio (Beijing, China). GoScriptTM Reverse Transcription System was obtained from Promega (Wisconsin, USA). Rat LTB4 ELISA assay kit purchased from Beijing Xinpeng Hongye Technology Co., Ltd. (Beijing, China). All other chemicals were of the highest purity commercially available.
Cell culture and drug treatments
Collagen-induced arthritis (CIA) rat fibroblast-like synoviocytes (FLS) were purchased from Shanghai Yansheng Industrial Co., Ltd. (Shanghai, China). The cultivation, resuscitation, and subculture of FLS were done following previous protocols. FLS were cultured in Dulbecco’s modified Eagle’s medium supplemented with 20% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin in a humidified atmosphere under 5% CO2 at 37°C. The cell passage was within 10 passages from when the cell line was purchased. For all experiments, FLS cells were grown to 80–85% confluence, and then, stimulated with 10 ng/mL TNF-α or PBS for 24 h to induce an inflammatory response. Subsequently, different concentrations (0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5 μg/mL) of Dioscin were added to each well for another 24 h, and phosphate-buffered saline (PBS) was used as vehicle control in all experiments.
Cell viability was assessed by a modified MTT assay as described in our previous study (Hong et al., 2015). Cells (1 × 105 cells/mL) were seeded in 96-well plates and incubated for 24 h. Then, the cells were exposed to 10 ng/mL TNF-α or PBS or 10 ng/mL TNF-α together with different concentrations of Dioscin for another 24 h. Thereafter, 10 μL of 5 mg/mL MTT in PBS was added to each well, and cells were incubated for a further 4 h at 37°C. The medium was then replaced by 100 μL of DMSO to dissolve the formed precipitate. The optical density was measured in a microplate reader (Model 680, Bio-Rad, USA) at 490 nm. Viability (%) = (OD of experimental group−OD of blank control group)/(OD of control group−OD of blank control group) × 100%.
Cells (1 × 105 cells/mL) were seeded in 96-well plates and incubated for 24 h. After that, the cells were exposed to 10 ng/mL TNF-α or PBS or 10 ng/mL TNF-α together with different concentrations of Dioscin for another 24 h. Then, 50 μL of the culture medium was assayed according to the LTB4 ELISA kit’s manufacturer’s instructions. The concentration of samples was determined according to the standards.
RNA was extracted from cell samples using TRIzol reagent, and the synthesis of complementary DNA (cDNA) for qPCR analysis was performed using a kit with subsequent melting curve analysis, according to the manufacturer's protocol. The PCR reaction mixture (forward primer, 0.4 μL; reverse primer, 0.4 μL; cDNA, 2 μL; ddH2O, 7 μL; CXR, 0.2 μL; GoTaq qPCR Master Mix, 2×, 10 μL) was prepared using GoTaq®qPCR Master Mix.
The PCR conditions were as follows: 5 min at 95°C, followed by 40 cycles of 15 s at 95°C, 1 min at 60°C. Relative gene expression was determined by the 2−ΔΔCt method (where Ct = threshold cycle) using β-actin as a reference gene. Quantitative real-time PCR primer sequences were shown in Tab. 1.
Table 1: Primer sequences for RT-qPCR

Proteins from cells subjected to every treatment were extracted in a lysis buffer. After lysis on ice for 30 min, cell lysates were then clarified by centrifugation at 12,000 rpm at 4°C for 10 min. Protein concentrations were determined by BCA assay. Immunoblot analysis of protein expressions of LTA4H and GAPDH was performed as described in our previous report. Briefly, 30 μg of protein extracts were separated by 12% SDS–polyacrylamide gels; then, proteins were transferred onto PVDF membranes that were purchased from Millipore (Massachusetts, USA). The membranes were blocked with 5% nonfat milk powder in Tris-buffered saline/0.1% Tween-20 (TBST) for 2 h at room temperature and then incubated overnight with the primary antibody (LTA4H,1:500; GAPTH, 1:2000) at 4°C and anti-rabbit secondary antibody (1:5000) for 2 h at room temperature. After washing thrice, the immunoblots were detected by enhanced chemiluminescence (ECL) detection reagent. The relative band intensity of each protein was normalized for GAPDH.
FLS in 1 × 105 cells/mL were digested and then fully titrated into single cells, inoculated in 6-well plates for 24 h. After discarding the medium, cells were then incubated with 10 ng/mL TNF-α. The complete medium was added to the control group and was discarded after 24 h. The control and TNF-α group were then treated with 0.1% DMSO medium, and the drug groups were treated with different concentrations of Dioscin for 24 h.
After incubation in 0.5% H2O2 followed by normal goat serum to avoid nonspecific immunoreactions, the sections (N = 6, for each group) were incubated with primary antibodies of LTA4H at 4°C overnight. The next day, sections were washed with PBS for 15 min and incubated for 90 min at 37°C with fluorescent secondary antibody. Signals were measured using microscope image-analysis software (Image-Pro Plus, USA).
Values were expressed as
Effects of TNF-α on FLS viability
MTT assay was done to calculate cell viability 24, 48, and 72 h after treating FLS with various concentrations (2.5, 5, 10, and 20 ng/mL) of TNF-α. As shown in Fig. 1, after treatment with TNF-α, FLS viability was increased significantly compared to the control group (p < 0.05). The highest cell survival and growth was observed in FLS exposed to 10 ng/mL TNF-α for 24 h. Thus, this culture condition of FLS was used for further experiments.
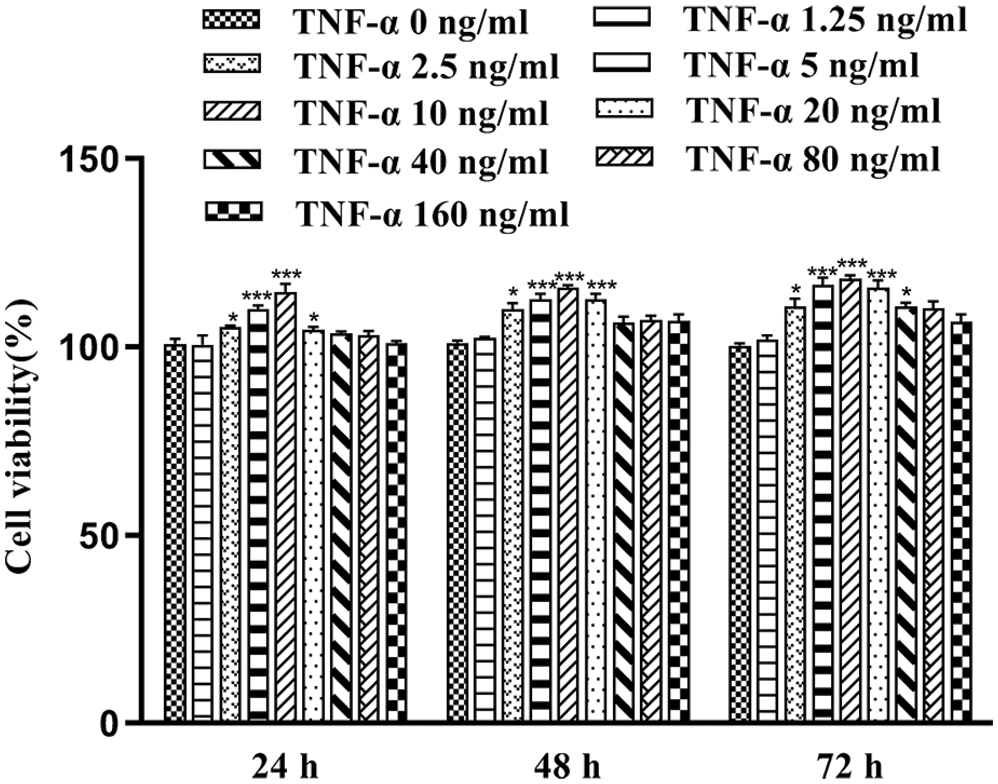
Figure 1: Cell viability of FLS at different incubation times with different concentrations of TNF-α (x̄ ± s, N = 6), *p < 0.05, ***p < 0.01 vs. control group.
Effects of Dioscin on TNF-α induced FLS viability
MTT assay was done to assess the viability of TNF-α induced FLS at 24, 48, and 72 h, after treatment with or without various concentrations of Dioscin. As indicated in Fig. 2, the cell viability of the TNF-α model group was highest. Dioscin at the concentrations of 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5 μg/mL markedly inhibited the survival and growth of FLS induced by TNF-α in a time/dose-dependent manner (p < 0.05), except the 0.5 μg/mL Dioscin group which showed no inhibition on cell viability. The cell viability of TNF-α induced-FLS was 49.46 ± 1.33 after being treated with 3 μg/mL Dioscin for 24 h, which was the closest to 50% cell viability. Based on these results, we selected TNF-α-induced FLS cultured with 1, 2, 3, and 4 μg/mL Dioscin for 24 h to conduct further experiments (Fig. 2).
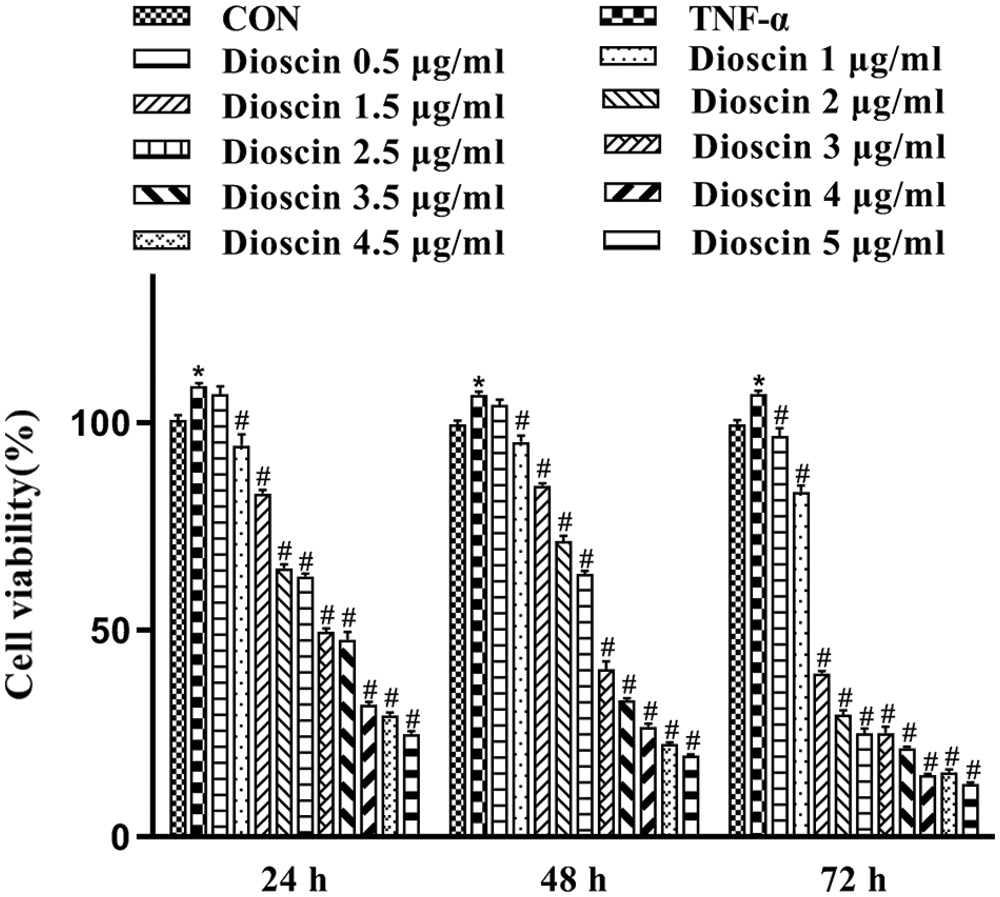
Figure 2: Cell viability of FLS after treatment with different concentrations of Dioscin for different times (x̄ ± s, N = 6).
Effect of Dioscin on the LTB4 concentration in supernatant of TNF-α-induced FLS culture
As shown in Fig. 3, after treatment with 10 ng/mL TNF-α for 24 h, the amount of LTB4 in the cell culture medium significantly increased when compared with the control group (p < 0.05). The TNF-α-induced high LTB4 content was significantly decreased after Dioscin treatment in a dose-dependent manner (p < 0.05).
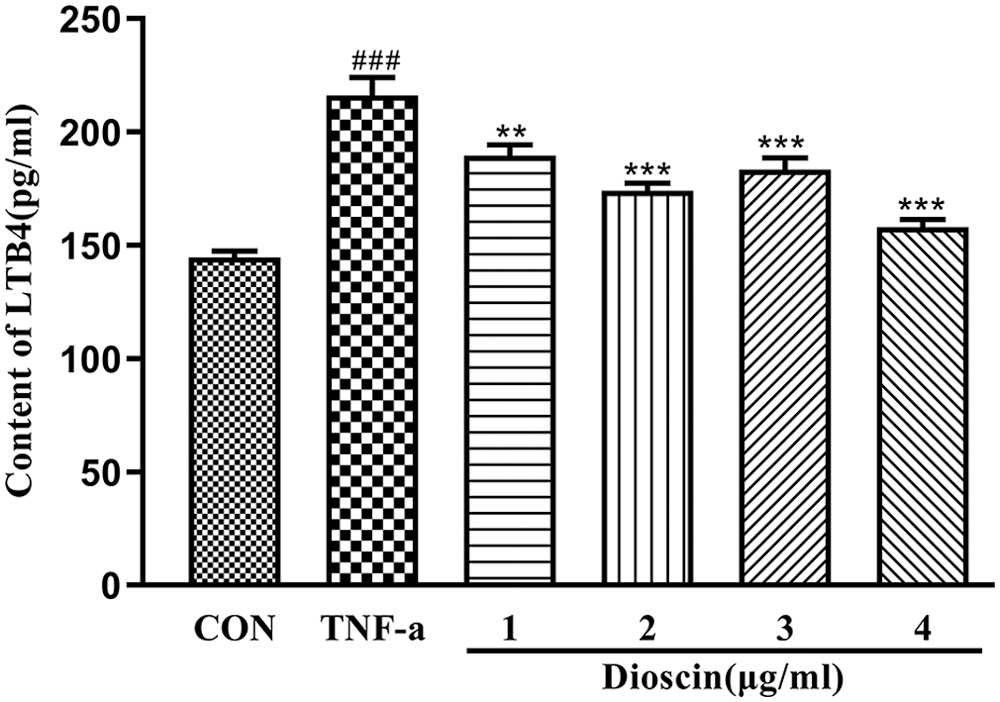
Figure 3: Effect of Dioscin on LTB4 content in TNF-α induced FLS supernatant (x̄ ± s, N = 10).
Effect of Dioscin on the expression of LTA4H in TNF-α-induced FLS
Compared with the control group, the expression levels of LTA4H mRNA and proteins in the TNF-α model group were significantly increased (p < 0.05); treatment with different concentrations of Dioscin significantly reduced the level of LTA4H mRNA and protein expression in TNF-α induced FLS (p < 0.05). At the 3 µg/mL Dioscin concentration, the expression level of LTA4H mRNA was the lowest, while treatment with Dioscin at different concentrations exhibited the inhibitory action in a dose-dependent manner and decreased the expression level of LTA4H protein induced by TNF-α in FLS (Fig. 4).
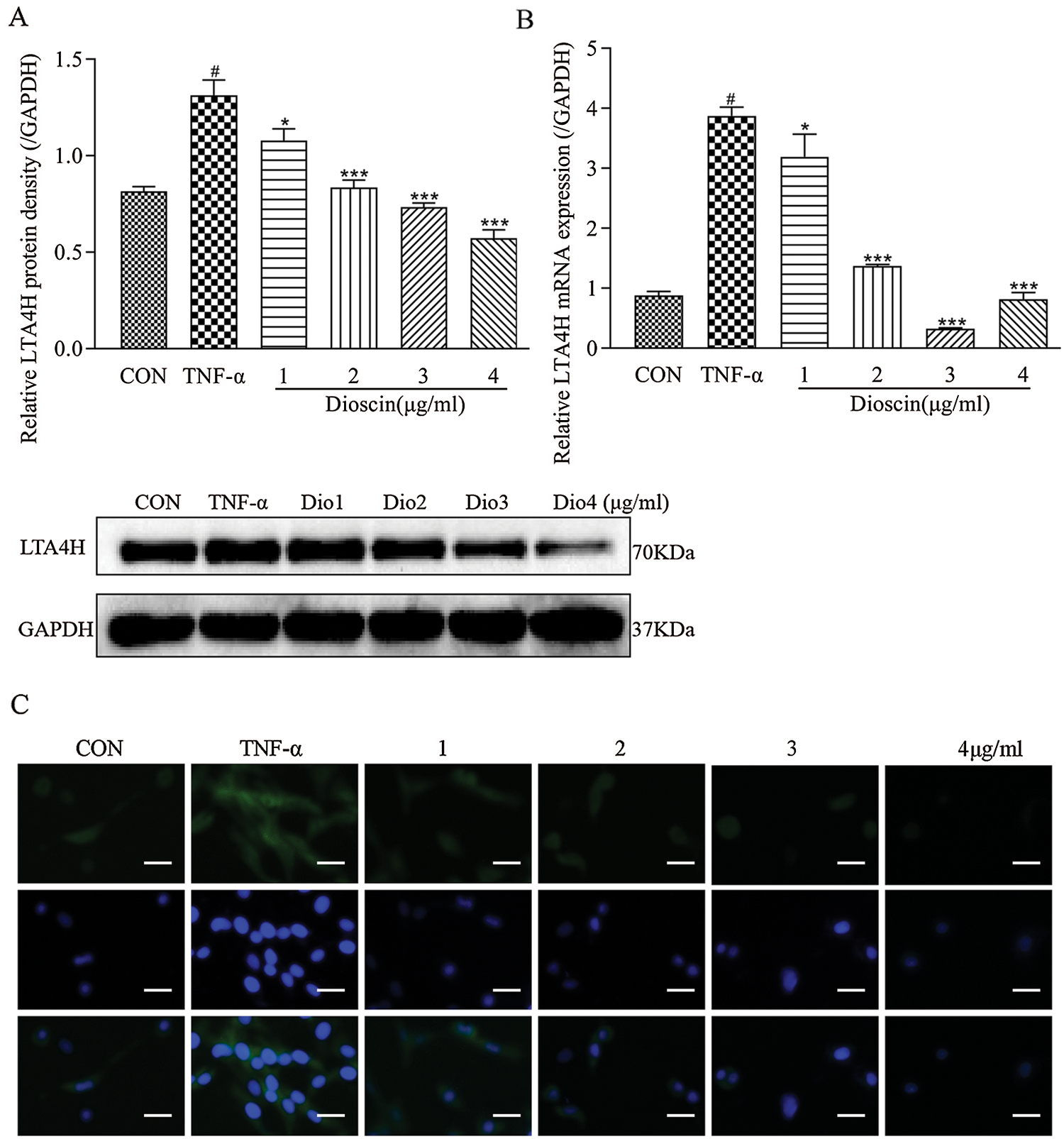
Figure 4: Effect of Dioscin on LTA4H expression in TNF-α induced FLS.
Effect of Dioscin on the expressions of LTB4 receptor in TNF-α-induced FLS
Compared with the control group, the mRNA expressions of BLT1 and BLT2 in the TNF-α group increased markedly, of which the expression level of BLT1 mRNA was six times, and BLT2 was three times higher than those in the control group. Dioscin treatment at 1, 2, and 3 μg/mL reduced the expression levels of BLT1 and BLT2 mRNA at different degrees; however, there were no significant differences between these groups. Treatment with 4 μg/mL Dioscin did not significantly change BLT1 mRNA expression but decreased the expression of BLT2 mRNA significantly compared with the other three groups (Fig. 5).
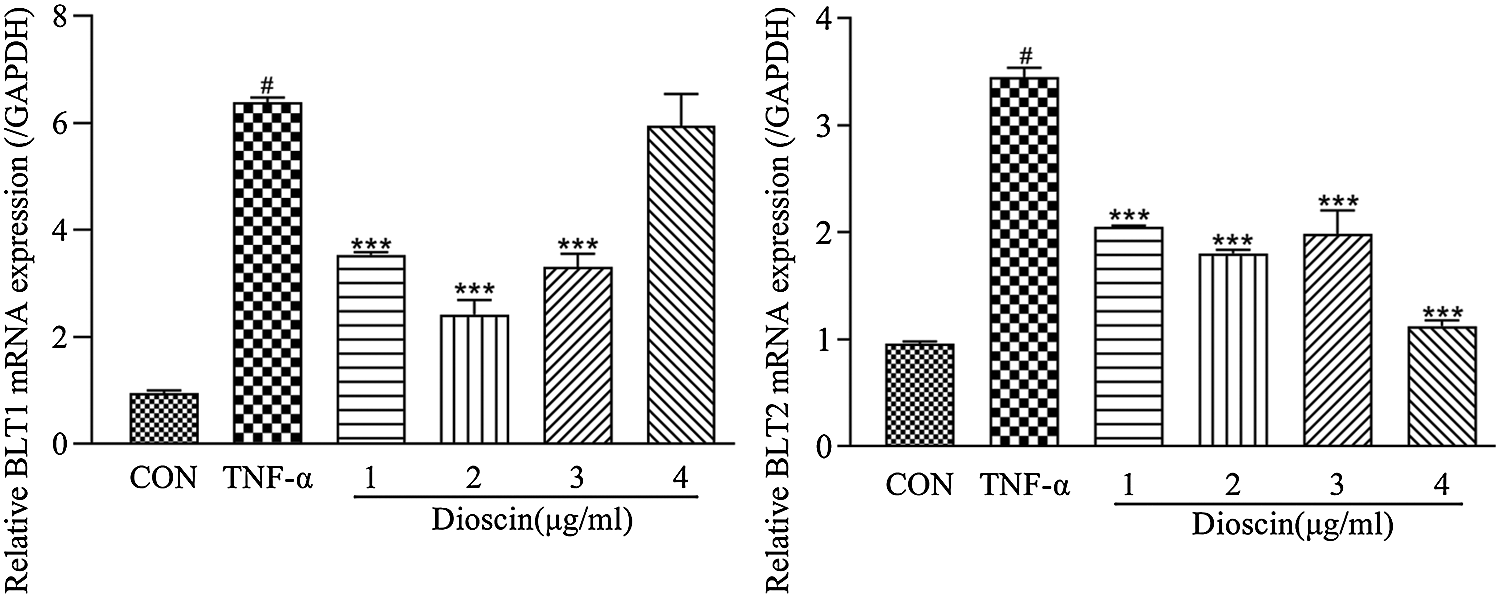
Figure 5: Effect of Dioscin on mRNA expressions of LTB4 receptors BLT1 and BLT2 in TNF-α induced FLS (x̄ ± s, N = 3).
RA is characterized by inflammatory cell infiltration, synovial tissue hyperplasia, bone erosion, and even joint deformity, leading to joint pain and disability, which severely breaks the life quality of RA patients (Wu et al., 2014; Li et al., 2020). FLS plays an important role in the pathogenesis of RA due to its inducing synovial hyperplasia. FLS can also enhance leukocytes’ adhesion and migration into the endothelium and produce high concentrations of CXCL12 to inhibit leukocyte outflow from the joint in RA patients (McGettrick et al., 2009; McGettrick et al., 2010). In the synovium of RA, FLS loses the function of contact-inhibition but exhibits characteristics of tumor-like proliferation, such as proliferation acceleration, apoptosis reduction, and slowing cell aging (Pang et al., 2016). Zou et al. (2017) discovered that FLS is an ideal target to investigate a drug’s therapeutic effects of RA. Previous studies have indicated that the abnormalities of FLS proliferation/apoptosis may be related to inflammatory mediators for the treatment of RA (Crowley et al., 2017; Huang et al., 2017; Sun et al., 2017). TNF-α can induce an excessive activation and proliferation of FLS to produce various kinds of inflammatory factors, leading to synovial hyperplasia and joint damage in RA disease (Pap et al., 2000; Yamanishi and Firestein, 2001). Moreover, exogenous TNF-α has been proved to induce expression and release of endogenous TNF-α, which causes inflammation reactions in vitro, suggesting that the in vitro RA model could be constructed by TNF-α treated FLS (Zhang and Xiao, 2017). In fact, TNF-α induced FLS has been used as an in vitro cellular model of RA to investigate RA pathogenesis and its underlying pharmacological mechanisms (Tian et al., 2010; Zhang and Xiao, 2017). In the present study, 10 ng/mL TNF-α for 24 hFLS proliferation was significantly increased after co-incubating with 10 ng/mL TNF-α for 24 h. The above result is consistent with previous studies (Tian et al., 2010).
As an important steroidal saponin, Dioscin becomes a crucial raw material in steroid hormone drug synthesis (Wang et al., 2017). Although Dioscin has been reported in CIA animal treatments, its underlying mechanisms for RA treatment are still not fully clear (Gao et al., 2012; Guo et al., 2015; Lu, 2017). Using an in vitro RA model, the MTT assay was employed to investigate the appropriate functional concentrations of Dioscin. Our results showed that Dioscin’s concentrations of higher than 1 μg/mL inhibited the proliferation of FLS induced by 10 ng/mL TNF-α treatment for 24 h, in which the inhibition ratio of cell proliferation was close to 50% after treating FLS using 3 μg/mL Dioscin for 24 h, indicating that Dioscin may promote FLS apoptosis in a time/dose-dependent manner. These findings suggest the potential therapeutic function of Dioscin against RA.
Higher LTB4 concentration was observed in the synovia and serum of RA patients (Elmgreen et al., 1987). LTB4 can activate and accumulate many inflammatory factors and immune cells, induce joint swelling and pain, as well as bone destruction, and is directly involved in the occurrence and development of arthritis (Tak and Bresnihan, 2000). Xu et al. (2010) found that the mRNA and protein levels of IL-1β and TNF-α were significantly increased when exogenous LTB4 was added or endogenous LTB4 was produced by lipopolysaccharide stimulation in primary FLS of RA patients, suggesting the involvement of LTB4 in RA pathogenesis. Bi et al. (2017) have also shown that LTB4 exhibited significant toxic effects on synovial cells and promoted apoptosis. Above all, LTB4 plays an important role in the development and progression of RA, and thus, it can be a crucial target for RA therapy. However, up till now, whether Dioscin’s treating RA is related to its effect on LTB4 remains to be investigated. Our results showed, for the first time, that Dioscin inhibited the increase of LTB4 in TNF-α-induced FLS in a dose-dependent manner, indicating that one of the protective effects of Dioscin against RA may be achieved by reducing LTB4 generation. In addition, considering LTA4H is responsible for LTA4 transformation into LTB4, which is a direct functioning enzyme to produce LTB4, we further detected the influence of Dioscin on LTA4H. Our results showed that Dioscin decreased the mRNA and protein expressions of LTA4H in TNF-α-induced FLS at different degrees, firstly suggesting that Dioscin reducing LTB4 generation against RA may be due to its down-regulating LTA4H expression in TNF-α-induced FLS.
It is well known that LTB4 plays a critical role through the two receptors BLT1 and BLT2, among which the affinity of LTB4 with BLT1 is higher than that with BLT2, and it is easy to determine and obtain BLT1, so LTB4 functions via the BLT1 pathway during the treatment of RA and other inflammatory diseases (Yokomizo et al., 2000; Yokomizo, 2015). Chen et al. (2010) have found that FLS produced enough LTB4 to promote arthritis by cross-cell metabolism, and LTB4 levels were increased by 3-fold after TNF-α stimulation of FLS; furthermore, by activating BLT1, high levels of LTB4 regulated the migration and invasion of FLS to promote joint erosion and participate in the occurrence as well as the persistence of RA. For mediating the therapeutic effects against RA, LTB4 functions through the BLT2 pathway, which will be studied later. Mathis et al. (2010) found that arthritis and bone erosion of BLT2-knockout mice were significantly reduced, during which BLT1 expression did not change, indicating the important role of BLT2 in the incidence of RA. At present, there are no reports on the relationships between Dioscin treating RA and LTB4 receptors. Our results, for the first time, discovered that after TNF-α stimulation, the mRNA expression levels of BLT1 and BLT2 of FLS were significantly increased, and Dioscin reversed these alterations in TNF-α-induced FLS. These findings indicate that Dioscin can downregulate the expressions of BLT1 and BLT2, which may block the downstream pathway of LTB4 action and exert its therapeutic effect on TNF-α-induced FLS from CIA rats.
In conclusion, our primary experiments firstly demonstrated that Dioscin produces a protective effect on TNF-α-induced FLS injury via its decreasing LTB4 production by down-regulating the expression of LTA4H, and it may also involve its inhibiting LTB4 downstream pathway by down-regulating the expressions of BLT1 and BLT2. These findings firstly suggest that Dioscin produces a potential therapeutic effect for RA via its influencing LTA4H/LTB4/BLT pathway.
Acknowledgement: Not applicable.
Availability of Data and Materials: The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Author Contribution: Zhiping Wei contributed to acquisition of data; Yajun Liu, Meiwen Yang, MengdiLi, Kexin Li contributed to analysis and interpretation of data; Luxi Zheng, Huiqiong Guo contributed to paper revision in grammars and languages; Fenfang Hong and Shulong Yang are responsible for study conception and design and fund. analysis and interpretation of results, and manuscript preparation.
Ethics Approval: The study was approved by the Ethics Committee of College of Medicine in Nanchang University.
Funding Statement: This work was supported by the National Natural Science Foundation of China (Nos. 82060661, 81660751, 81660151); Jiangxi Provincial Natural Science Foundation of China (No. 20171BAB205085).
Conflicts of Interest: The authors declare that there are no conflicts of interests to report regarding the present study.
Bartok B, Firestein GS (2010). Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunological Reviews 233: 233–255. DOI 10.1111/j.0105-2896.2009.00859.x. [Google Scholar] [CrossRef]
Bi D, Bi D, Zhong M, Zhang H, Jin S, Ma S, Luo H (2017). Effects of leukotriene B4 on interleukin-32, interferon-gamma and chemokines in rats with rheumatoid arthritis. Experimental and Therapeutic Medicine 14: 2925–2930. DOI 10.3892/etm.2017.4845. [Google Scholar] [CrossRef]
Bottini N, Firestein GS (2013). Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted aggressors. Nature Reviews Rheumatology 9: 24–33. DOI 10.1038/nrrheum.2012.190. [Google Scholar] [CrossRef]
Crowley T, O’Neil JD, Adams H, Thomas AM, Filer A, Buckley CD, Clark AR (2017). Priming in response to pro-inflammatory cytokines is a feature of adult synovial but not dermal fibroblasts. Arthritis Research & Therapy 19: 35. DOI 10.1186/s13075-017-1248-6. [Google Scholar] [CrossRef]
Chen M, Lam BK, Luster AD, Zarini S, Murphy RC, Bair AM, Soberman RJ, Lee DM (2010). Joint tissues amplify inflammation and alter their invasive behavior via leukotriene B4 in experimental inflammatory arthritis. Journal of Immunology 185: 5503–5511. DOI 10.4049/jimmunol.1001258. [Google Scholar] [CrossRef]
Chu CM, Zhang HQ, BU P (2012). Experimental study on the therapeutic effect of dioscin on rats with collagen-induced arthritis. Chinese Pharmacological Bulletin 28: 1464–1467. [Google Scholar]
Elmgreen J, Nielsen OH, Ahnfelt-Ronne I (1987). Enhanced capacity for release of leucotriene B4 by neutrophils in rheumatoid arthritis. Annals of the Rheumatic Diseases 46: 501–505. DOI 10.1136/ard.46.7.501. [Google Scholar] [CrossRef]
Friday SC, Fox DA (2016). Phospholipase D enzymes facilitate IL-17- and TNFα-induced expression of proinflammatory genes in rheumatoid arthritis synovial fibroblasts (RASF). Immunology Letters 174: 9–18. DOI 10.1016/j.imlet.2016.04.001. [Google Scholar] [CrossRef]
Gao YX, Liang XJ, Dong WJ, Xiao LJ, Song HR (2012). Effects of total saponin from rhizoma Dioscorea nipponica on NF-κB p65 activity and STAT3 protein expression in joint synovium of CIA rats. Journal of China Medical University 41: 485–489. [Google Scholar]
Guo Y, Liang X, Gao Y, Song H (2015). Effects of diosgenin on VEGF and AP-1 expression in synovial tissues of CIA rats. World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica 17: 1801–1805. [Google Scholar]
Guo Y, Xing E, Song H, Feng G, Liang X, An G, Zhao X, Wang M (2016). Therapeutic effect of dioscin on collagen-induced arthritis through reduction of Th1/Th2. International Immunopharmacology 39: 79–83. DOI 10.1016/j.intimp.2016.06.029. [Google Scholar] [CrossRef]
Guo YC, Gao YX, Song HR (2013). Effects of dioscornin tablet containing serum on NF-kappaB p65, STAT3, and VEGF mRNA expressions in rats’ synovial cell strain RSC-364 induced by IL-17 and TNF-alpha. Zhongguo Zhong Xi Yi Jie He Za Zhi 33: 814–818. [Google Scholar]
Hong FF, Guo FX, Zhou Y, Min QH, Zhang DL, Yang B, Zhou WY, Wu L, Wei ZP, Liu H, Yang SL (2015). Shenfu injection protects human ECV304 cells from hydrogen peroxide via its anti-apoptosis way. Journal of Ethnopharmacology 163: 203–209. DOI 10.1016/j.jep.2015.01.032. [Google Scholar] [CrossRef]
Hoxha M (2017). A systematic review on the role of eicosanoid pathways in rheumatoid arthritis. Advances in Medical Sciences 63: 22–29. DOI 10.1016/j.advms.2017.06.004. [Google Scholar] [CrossRef]
Huang M, Wang L, Zeng S, Qiu Q, Zou Y, Shi M, Xu H, Liang L (2017). Indirubin inhibits the migration, invasion, and activation of fibroblast-like synoviocytes from rheumatoid arthritis patients. Inflammation Research 66: 433–440. DOI 10.1007/s00011-017-1027-5. [Google Scholar] [CrossRef]
Li KX, Zheng LX, Guo HQ, Hong FF, Yang SL (2020). LTB4-induced anti-apoptosis and infiltration of neutrophils in rheumatoid arthritis. Clinical and Experimental Rheumatology 38: 543–551. [Google Scholar]
Lu H (2017). Intervention effect of dioscin on levels expression of OPG/RANKL in serum of rat with rheumatoid arthritis. Guangming Journal of Chinese Medicine 32: 1259–1261. [Google Scholar]
Mathis SP, Jala VR, Lee DM, Haribabu B (2010). Nonredundant roles for leukotriene B4 receptors BLT1 and BLT2 in inflammatory arthritis. Journal of Immunology 185: 3049–3056. DOI 10.4049/jimmunol.1001031. [Google Scholar] [CrossRef]
McGettrick HM, Buckley CD, Filer A, Rainger GE, Nash GB (2010). Stromal cells differentially regulate neutrophil and lymphocyte recruitment through the endothelium. Immunology 131: 357–370. DOI 10.1111/j.1365-2567.2010.03307.x. [Google Scholar] [CrossRef]
McGettrick HM, Smith E, Filer A, Kissane S, Salmon M, Buckley CD, Rainger GE, Nash GB (2009). Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells. European Journal of Immunology 39: 113–125. DOI 10.1002/eji.200838232. [Google Scholar] [CrossRef]
Meng H, Liu Y, Lai L (2015). Diverse ways of perturbing the human arachidonic acid metabolic network to control inflammation. Accounts of Chemical Research 48: 2242–2250. DOI 10.1021/acs.accounts.5b00226. [Google Scholar] [CrossRef]
Miyabe Y, Miyabe C, Luster AD (2017). LTB4 and BLT1 in inflammatory arthritis. Seminars in Immunology 33: 52–57. DOI 10.1016/j.smim.2017.09.009. [Google Scholar] [CrossRef]
Pang X, Zhang Y, Pan J, Zhao Y, Chen Y, Ren X, Ma H, Wei Q, Du B (2016). A photoelectrochemical biosensor for fibroblast-like synoviocyte cell using visible light-activated NCQDs sensitized-ZnO/CH3NH3PbI3 heterojunction. Biosensors and Bioelectronics 77: 330–338. DOI 10.1016/j.bios.2015.09.047. [Google Scholar] [CrossRef]
Pap T, Muller-Ladner U, Gay RE, Gay S (2000). Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Research 2: 361–367. DOI 10.1186/ar113. [Google Scholar] [CrossRef]
Qu X, Zhai Z, Liu X, Li H, Ouyang Z, Wu C, Liu G, Fan Q, Tang T, Qin A, Dai K (2014). Dioscin inhibits osteoclast differentiation and bone resorption though down-regulating the Akt signaling cascades. Biochemical and Biophysical Research Communications 443: 658–665. DOI 10.1016/j.bbrc.2013.12.029. [Google Scholar] [CrossRef]
Samuelsson B (1983). Leukotrienes: Mediators of immediate hypersensitivity reactions and inflammation. Science 220: 568–575. DOI 10.1126/science.6301011. [Google Scholar] [CrossRef]
Sun WX, Liu Y, Zhou W, Li HW, Yang J, Chen ZB (2017). Shikonin inhibits TNF-α production through suppressing PKC-NF-κB-dependent decrease of IL-10 in rheumatoid arthritis-like cell model. Journal of Natural Medicines 71: 349–356. DOI 10.1007/s11418-016-1064-3. [Google Scholar] [CrossRef]
Tak PP, Bresnihan B (2000). The pathogenesis and prevention of joint damage in rheumatoid arthritis: Advances from synovial biopsy and tissue analysis. Arthritis & Rheumatism 43: 2619–2633. [Google Scholar]
Tian J, Gao J, Cen J, Li F, Xie X, Du J, Wang J, Mao N (2010). Effects of resveratrol on proliferation and apoptosis of TNF-α induced rheumatoid arthritis fibrob last-like synoviocytes. China Journal of Chinese Materia Medica 35: 1878–1882. [Google Scholar]
Tu L, Li S, Fu Y, Yao R, Zhang Z, Yang S, Zeng X, Kuang N (2012). The therapeutic effects of daphnetin in collagen-induced arthritis involve its regulation of Th17 cells. International Immunopharmacology 13: 417–423. DOI 10.1016/j.intimp.2012.04.001. [Google Scholar] [CrossRef]
Tveden-Nyborg P, Bergmann TK, Lykkesfeldt J (2018). Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic & Clinical Pharmacology & Toxicology 123: 233–235. DOI 10.1111/bcpt.13059. [Google Scholar] [CrossRef]
Wang SR, Ling S, Zhang QG, Xu JW (2017). Advance on the pharmacological research of dioscin. Chinese Pharmacological Bulletin 33: 161–166. [Google Scholar]
Wei ZP, Hong FF, Yang SL (2017). Research progress on traditional Chinese medicine on the treatment of rheumatoid arthritis. China Journal of Traditional Chinese Medicine and Pharmacy 32: 5477–5481. [Google Scholar]
Wu LM, Ye JF, Xie WH, Li HY, Zhan XQ et al. (2014). Leukotriene B4 in the pathogenesis of rheumatoid arthritis. Chinese Journal of Immunology 30: 689–693. [Google Scholar]
Xu S, Lu H, Lin J, Chen Z, Jiang D (2010). Regulation of TNFα and IL1β in rheumatoid arthritis synovial fibroblasts by leukotriene B4. Rheumatology International 30: 1183–1189. DOI 10.1007/s00296-009-1125-y. [Google Scholar] [CrossRef]
Yamanishi Y, Firestein GS (2001). Pathogenesis of rheumatoid arthritis: The role of synoviocytes. Rheumatic Disease Clinics of North America 27: 355–371. DOI 10.1016/S0889-857X(05)70206-4. [Google Scholar] [CrossRef]
Yang Y, Chen XS, Tian FQ, Shang GL, Wang HW (2008). The effect of juvenile rheumatoid arthritis serum on the LTB4-BLT2 signal pathway of mice dendritic cells. Chinese Journal of Rheumatology 12: 379–381. [Google Scholar]
Yokomizo T (2015). Two distinct leukotriene B4 receptors, BLT1 and BLT2. Journal of Biochemistry 157: 65–71. DOI 10.1093/jb/mvu078. [Google Scholar] [CrossRef]
Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T (2000). A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. Journal of Experimental Medicine 192: 421–432. DOI 10.1084/jem.192.3.421. [Google Scholar] [CrossRef]
Yousefi B, Jadidi-Niaragh F, Azizi G, Hajighasemi F, Mirshafiey A (2014). The role of leukotrienes in immunopathogenesis of rheumatoid arthritis. Modern Rheumatology 24: 225–235. DOI 10.3109/14397595.2013.854056. [Google Scholar] [CrossRef]
Zhan XQ, Xia WH, Wu LM, Ye JF, Li HY, Lin CP, Hong FF, Yang SL (2016). Leukotriene B4 and rheumatoid arthritis treatment. Chinese Journal of Gerontology 36: 1752–1754. [Google Scholar]
Zhang H, Xiao W (2017). TNFR1 and TNFR2 differentially mediate TNF-α-induced inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes. Cell Biology International 41: 415–422. DOI 10.1002/cbin.10735. [Google Scholar] [CrossRef]
Zhang W, Liu B (2010). Advances in research on the pharmacological effects of dioscin. World Journal of Integrated Traditional Chinese and Western Medicine 5: 543–545. [Google Scholar]
Zheng LX, Li KX, Hong FF, Yang SL (2019). Pain and bone damage in rheumatoid arthritis: Role of leukotriene B4. Clinical and Experimental Rheumatology 37: 872–878. [Google Scholar]
Zou Y, Zeng S, Huang M, Qiu Q, Xiao Y, Shi M, Zhan Z, Liang L, Yang X, Xu H (2017). Inhibition of 6-phosphofructo-2-kinase suppresses fibroblast-like synoviocytes-mediated synovial inflammation and joint destruction in rheumatoid arthritis. British Journal of Pharmacology 174: 893–908. DOI 10.1111/bph.13762. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |