DOI:10.32604/biocell.2021.015039

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.015039 |  www.techscience.com/journal/biocell |
| Article |
Angelica sinensis polysaccharides ameliorate 5-flourouracil-induced bone marrow stromal cell proliferation inhibition via regulating Wnt/β-catenin signaling
Laboratory of Stem Cell and Tissue Engineering, Department of Histology and Embryology, Chongqing Medical University, Chongqing, 40012, China
*Address correspondence to: Lu Wang, luwang@cqmu.edu.cn
Received: 17 November 2020; Accepted: 11 January 2021
Abstract: Chemotherapy may cause cellular oxidative stress to bone marrow. Oxidative damage of bone marrow hematopoietic microenvironment is closely related to chronic myelosuppression after chemotherapeutic treatment. Angelica sinensis polysaccharides (ASP) are major effective ingredients of traditional Chinese medicine Angelica with multi-target anti-oxidative stress features. In the current study, we investigated the protective roles and mechanisms of ASP on chemotherapy-induced bone marrow stromal cell (BMSC) damage. The human bone marrow stromal cell line HS-5 cells were divided into control group, 5-FU group, 5-FU + ASP group, and 5-FU + LiCl group to investigate the mechanism of ASP to alleviate 5-FU-induced BMSC proliferation inhibition. The results showed that 5-FU inhibits the growth of HS-5 cells in a time and dose-dependent manner; however, ASP partially counteracted the 5-FU-induced decrease in cell viability, whereas Wnt signaling inhibitor Dkk1 antagonized the effect of ASP on HS-5 cells. ASP reversed the decrease in total cytoplasmic β-catenin, p-GSK-3β, and CyclinD1 following 5-FU treatment and modulated nuclear expression of β-catenin, Lef-1, and C-myc proteins. Furthermore, ASP also enhanced the antioxidant capacity of cells and reduced 5-FU-induced oxidative stress, attenuated FoxO1 expression, thus weakened its downstream apoptosis-related proteins and G0/G1 checkpoint-associated p27Kip1 expression to alleviate 5-FU-induced apoptosis and to promote cell cycle progression. All the results above suggest that the protective role of ASP in 5-FU-treated BMSCs proliferation for the chemotherapy may be related to its activating Wnt/β-catenin signaling and keeping homeostasis between β-catenin and FoxO1 under oxidative stress. The study provides a potential therapeutic strategy for alleviating chemotherapeutic damage on BMSCs.
Keywords: Angelica sinensis polysaccharides; 5-Fluorouracil; Wnt/β-catenin signaling pathway; Oxidative stress; Cell proliferation; FoxO1
Myelosuppression is the major side effect of chemotherapy (Chabner and Roberts, 2005; Papac, 2001) that may be caused even by minor doses of chemotherapeutic drugs, leading to hematopoietic dysfunction, hematopoietic reconstitution disorders, and other secondary adverse reactions (Dritschilo and Sherman, 1981; Marsh, 1976). The mechanisms of myelosuppression can be various, including direct cytotoxicity to marrow cells, inhibition of bone marrow stem cell or progenitor cell proliferation, or interference with hematopoietic growth factor and receptor signaling, subsequently affecting the downstream differentiation processes. Chemotherapy-induced cellular damage involved not only hematopoietic stem cells/progenitors but also stromal cells in the hematopoietic microenvironment, which may be the reason for chronic hematopoietic dysfunction (Galotto et al., 1999; Kemp et al., 2010; Li et al., 2004; Nicolay et al., 2016; Oliveira et al., 2014; de Lima Prata et al., 2010). As chemotherapy disrupts the steady-state function of hematopoietic and stromal cell, disruptions over time may cause severe bone marrow toxicity and the failure of cancer treatment. 5-FU, widely used in high-proliferative, tissue-derived cancers, particularly for colorectal cancer and breast cancer, exerts its anti-cancer effects through inhibition of thymidylate synthase (TS) and incorporation of its metabolites into RNA and DNA (Douillard et al., 2000; Longley et al., 2003; de Lima Prata et al., 2010). It was reported that the mechanism of stromal cell proliferation inhibition and apoptosis after 5-FU treatment is oxidative damage (Somaiah et al., 2018; Wang et al., 2015). Our previous findings have confirmed that following oxidative damage of BMSCs 5-FU may alter bioactive substance and cause stress-induced premature senescence (SIPS) of hematopoietic cells (Xiao et al., 2017). However, the specific underlying mechanism of 5-FU-induced BMSC proliferation inhibition remains unclear. Therefore, to explore its related mechanisms to reduce the side effects of chemotherapy drugs and to screen protective drugs during chemotherapy is of clinical guidance significance.
Wnt/β-catenin is an evolutionarily highly conserved signaling pathway that plays a key role in the development and is involved in cell proliferation, differentiation, apoptosis, and localization control (Nusse and Clevers, 2017; Petersen and Reddien, 2009). Particularly, the Wnt pathway involves various signal feedback that maintains the processes of stem cell proliferation, differentiation, and self-renewal (Luis et al., 2011; Richter et al., 2017). The properties of stem cells are conferred by the interaction of stem cells with their local microenvironment. Recent studies have evidenced that the Wnt/β-catenin signaling pathway is closely related to hematopoietic microenvironment affecting hematopoietic microenvironment function extensively, participate in BMSC proliferation, alleviate oxidative stress, and regulate hematopoietic stem cell self-renewal through stroma-dependent manner (Kim et al., 2009; Oh, 2010; Schreck et al., 2014). It is increasingly realized that the microenvironment keeps the threshold of Wnt signaling in stem cells at a physiological range. In the current work, it was clarified herein the roles of Wnt signaling in chemotherapy-induced stromal suppression and the ameliorative effects of ASP.
The Forkhead transcription factors family, including FoxO1 (or Fkhr), FoxO3a (or Fkhrl1), FoxO4 (or Afx), and FoxO6, are critically involved in the regulation of apoptosis, proliferation, and the control of oxidative stress (Eijkelenboom and Burgering, 2013). Stress conditions such as high ROS levels induce FoxO nuclear import and trigger the shifting of β-catenin from TCF/LEF to FoxO-mediated transcription (Behrens et al., 1996; Essers et al., 2005). In the hematopoietic system, activation of FoxO factor is sufficient to activate a variety of proapoptotic genes and to trigger apoptosis. Meanwhile, overexpression of FoxO factors cause a strong inhibition of cell proliferation (Burgering and Medema, 2003; Ma and Wang, 2012). As playing a critical role in proliferation and apoptosis, it has been aware that FoxO factors are closely related to chemotherapy-induced cell damage; nevertheless, studies are needed to clarify the relationship of FoxO factors and Wnt signaling in myelosuppression (Gomes et al., 2008; Greer and Brunet, 2005).
Angelica of Chinese herb is a commonly used medicine to enrich the blood, promote blood circulation and treat menstrual disorders (Dietz et al., 2016; Zhao et al., 2003). Angelica sinensis polysaccharides (ASP) are major effective ingredients of Angelica, with significant bioactivities including anti-oxidation (Lei et al., 2014; Zhuang et al., 2018), anti-tumor (Tsai et al., 2005; Zhang et al., 2016), promoting hematopoiesis (Bradley et al., 1999; Liu et al., 2010a; Wang et al., 2017), and delaying senescence (Lai and Liu, 2015; Mu et al., 2017) effects. ASP shows antioxidant activity by suppressing the production of ROS and regulating several chemical substances associated with oxidative stress (Ai et al., 2013; Wei et al., 2016). Our previous work showed marked antioxidative role of ASP in BMSCs from 5-FU injury in vitro, thus protected hematopoietic cells against SIPS via alleviating oxidative stress, preventing oxidative DNA damage, promoting hematopoietic stimulating factors originated from BMSCs, and enhancing intercellular communication between stromal cells and hematopoietic cells (Xiao et al., 2017). On this basis, we demonstrated herein that ASP alleviated 5-FU-induced stromal cell proliferation inhibition, apoptosis, and oxidative stress damage, and the underlying mechanism may be related to ASP activating Wnt/β-catenin signaling and keeping homeostasis between β-catenin and FoxO1 under oxidative stress.
5-fluorouracil was purchased from Sigma-Aldrich Co., St. Louis, USA. Angelica sinensis polysaccharides are composed of long chains of several monosaccharide units including fucose, galactose, glucose, arabinose, rhamnose and xylose, and all the monosaccharide units are linked via various glycosidic bonds. In the study, ASP were purchased from Ci Yuan Biotechnology Co. Ltd., Shanxi, China. All standards were at least 98% pure, as confirmed by HPLC (Jin et al., 2012; Wei et al., 2016; Zhang et al., 2014; Zhang et al., 2016). LiCl (purity >95%) was purchased from Damao Chemical Reagent Factory, Tianjin, China. Fetal bovine serum (FBS) was purchased from MRC Company, Australia. Dulbecco’s modified Eagle medium high-glucose(H-DMEM) was purchased from Gibco Co., NY, USA. Cell Counting Kit-8 was purchased from Dojindo Laboratories (Japan). EdU Cell Proliferation Assay Kit was purchased from RiboBio Co. Ltd., Guangzhou, China. β-catenin, GSK-3β, p-GSK-3β, Lef-1, Cyclin D1, C-myc, FoxO1, p-FoxO1, p27Kip1, Bim, Bax, Bcl-2, and caspase-3 antibodies were purchased from Cell Signaling Technology, Danvers, USA. Dkk1 was purchased from R&D Systems (USA). Reactive Oxygen Species Assay Kit and Senescence β-Galactosidase Staining Kit were purchased from the Beyotime Institute of Biotechnology, Shanghai, China. Superoxide Dismutase (SOD) assay kit, Malondialdehyde (MDA) assay kit and Catalase (CAT) assay kit, were purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, China.
Cell culture and groups of experiment
Human bone marrow stromal cell line HS-5 was cultured in H-DMEM containing 10% fetal bovine serum and 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were cultured in a humidified atmosphere with 5% CO2 at 37°C. Cells were divided into control group, 5-FU group, 5-FU + ASP group, and 5-FU+ LiCl group. The control group was routinely cultured; 5-FU group was treated with 5-FU on the concentration of 25 μg/mL; 5-FU+ ASP group was pretreated with ASP on the concentration of 100 μg/mL, and 25 μg/mL 5-FU was added after 6 h; 5-FU+ LiCl group was pretreated with LiCl on the concentration of 10 mmol/L, and 25 μg/mL 5-FU was added after 6 h, each group was cultured for 48 h.
Cell viability assay and the screening of drug concentration were performed using the Cell Counting Kit-8. Cells were plated in 96-well plates at a density of 5 × 103 cells per well. The optical density (OD) value at 450 nm was measured using a microplate reader (Massachusetts, USA). The cell viability of HS-5 was calculated according to the formula: Cell viability = [(OD experimental group − OD blank group)/(OD control group − OD blank group)] × 100%. Inhibition rate = [(OD control group − OD experimental group)/(OD control group − OD blank group)] × 100%.
The HS-5 cells were seeded in 96-well plates at a density of 5 × 103 cells per well and treated as described in groups of experiment. After 48 h treatment, cells were exposed to 10 μmol/L EdU solution for 24 h. Cells were washed and fixed in 4% paraformaldehyde at room temperature for 30 min. After washing, cells were permeabilized in PBS containing 0.5% Triton X-100 for 20 min. Then, cells were washed and incubated with 1X Apollo® reaction cocktail for 30 min. Subsequently, cells were stained with Hoechst33342 for 30 min and observed under a fluorescence microscope (Olympus, Japan). Counting 200 cells at random, the proliferation rate of HS-5 cells was defined as the ratio of EdU-positive cells (green cells) to Hoechst33342-positive cells (blue cells).
For cell apoptosis assay, the HS-5 cells were cultured then treated as described in groups of experiment. After 48 h treatment, cells were harvested and centrifuged at 1000 rpm for 5 min. Subsequently, cells were resuspended with 500 µL PBS solution for each tube. Cell apoptosis was detected by flow cytometry. For cell cycle assay, the HS-5 cells were cultured then treated as described in groups of experiment. After 48 h treatment, cells were harvested and fixed with pre-cooled 75% ethanol at 4°C for at least 5 h. After centrifugation, cells were incubated with propidium iodide (PI) and RNase A at 37°C for 30 min in the dark. Cell cycle was detected by the flow cytometry. The apoptosis and cell cycle were analyzed on a FAC-Scan laser flow cytometry (BD Biosciences, New Jersey, USA). The data were processed by Cell Quest software (BD Biosciences, New Jersey, USA).
Sterile glass slides were put into 24-well plates; the HS-5 cells were cultured at a density of 5 × 104 cells per well in 24-well plates then treated as described in groups of experiment. After 48 h treatment, cells were fixed with 4% paraformaldehyde for 30 min at room temperature. After washing, cells were permeabilized in PBS containing 0.5% Triton X-100 for 20 min and then blocked with 10% goat serum for 1 h. Subsequently, cells were incubated with monoclonal antibody β-catenin (1:150) overnight at 4°C. After being washed thrice with PBS solution, cells were incubated with Cy3-labeled goat-anti-rabbit immunofluorescent secondary antibody (1:300) at 37°C for 2 h in the dark. The nuclei were stained with 4’,6-diamidino-2-phenylindole (DIPI) for the last 5 min. The images were observed and acquired under the fluorescence microscope.
The HS-5 cells were treated as described in groups of experiment. After 48 h treatment, cells were incubated with PIPA lysis buffer containing 1% protease inhibitor and phosphatase inhibitor for 30 min on ice, and proteins were isolated after centrifugation. The concentrations of proteins were detected by the BCA Protein Assay Kit (Beyotime, China). The protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, USA). The membranes were blocked with 5% skim milk for 1 h at room temperature and subsequently incubated overnight at 4°C with β-catenin, Cyclin D1, p-GSK-3β, GSK-3β, Lef-1, C-myc, FoxO1, p-FoxO1, p27Kip1, Bim, Bcl-2, Bax, and caspase-3 primary antibodies (1:1000). After washing three times with Tris-Buffered Saline and Tween-20 (TBST), the membranes were incubated with secondary antibodies for 1 h at room temperature. The enhanced chemiluminescence (ECL) kit (Millipore, USA) was used for color development, and Image Lab 5.2.1 software was used for semi-quantitative analysis. The relative expression levels of the target proteins were determined by the ratio of the target protein gray value to the internal reference protein gray value.
Oxidation-associated biological indicators assay
For the detection of intracellular ROS, the HS-5 cells were seeded in 6-well plates at a density of 2 × 105 cells per well then treated as described in groups of experiment. After 48 h treatment, the cells were washed thrice by serum-free medium and then incubated with 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) at 37°C for 20–30 min in the dark. The content of intracellular ROS was observed and acquired under the fluorescence microscope. The average optical density per unit area was analyzed using ImageJ software. For the detection of MDA content and SOD, CAT activity, the HS-5 cells were cultured and treated as described in groups of experiment. After 48 h treatment, cells were harvested, lysed, and centrifuged to collect the supernatant. MDA, SOD, and CAT were measured by the corresponding assay kits according to the manufacturer’s instruction.
For all assays, the experiments were performed at least three times. All the results were analyzed by one-way analysis of variance (ANOVA) with SPSS 20. 0 statistical software. All the data were expressed as mean ± standard deviation (SD). P < 0.05 was considered statistical significance.
5-FU inhibits the growth of HS-5 cells by down-regulating the Wnt/β-catenin signaling pathway
To assess the effect of 5-FU on proliferation, HS-5 cells were treated with 5-FU at different concentrations for 72 h. As shown in Fig. 1A, the inhibition ratio of HS-5 cells increased simultaneously with the increase of 5-FU concentration and the extension of treatment time, suggesting that 5-FU exerted the inhibitory role in HS-5 cell growth in a dose- and time-dependent manner. Treated with 25 μg/mL 5-FU for 48 h, the inhibition ratio of HS-5 cells dropped almost 50%, which meant half of the cells were suppressed to grow (P < 0.05). Thus, 25 μg/mL 5-FU treating for 48 h was selected as an optional condition for the subsequent experiments. Interestingly, in the current study, we found that the inhibitory effect of 5-FU on HS-5 cell growth was relative to the Wnt/β-catenin signaling pathway. Western blot assay demonstrated that 5-FU downregulated the cytoplasmic levels of p-GSK-3β, total β-catenin, and Cyclin D1 in HS-5 cells, followed by nuclear protein expression of β-catenin, Lef-1, and C-myc down-regulation (Fig. 1B). These results hinted that 5-FU promoted the ubiquitination degradation of β-catenin mediated by GSK-3β-complex, inhibited the nuclear translocation of β-catenin, and downregulated the downstream target genes.
Then the activator and antagonist of the Wnt/β-catenin signaling pathway were tested to further illustrate the effect of 5-FU on Wnt/β-catenin signaling. Morphologically, 5, 10, 20 mmol/L of activator LiCl increased the number of HS-5 cells, however, the cellularity in the 40 mmol/L group significantly dropped concomitant with smaller and loosely dispersed shape (Fig. 1C). Also, cell viability was tested by CCK-8 assay. As shown in Fig. 1D, cells treated with 5–20 mmol/L LiCl for 48 h showed different degrees of proliferation, among which the proliferation rate of cells peaked up to 150% of the control at the concentration of 10 mmol/L LiCl. In accord with the result by microscopy, 40 mmol/L LiCl also presented cytotoxicity to HS-5 cells even within 24 h (P < 0.05). Therefore, the pretreatment with 10 mmol/L LiCl was utilized as a positive control in the subsequent experiment. Furthermore, Dkk1, an antagonist for Wnt/β-catenin signaling, was used as a negative control to get more evidence for the Wnt/β-catenin signaling pathway on cell viability. 50 ng/mL Dkk1 treated for 48 h, the cells were dramatically inhibited compared with the control group revealed by the results of CCK-8 (Fig. 1E). All the results above indicate that the effect of 5-FU on inhibition of HS-5 cell growth correlates with the suppression of the Wnt signaling pathway.
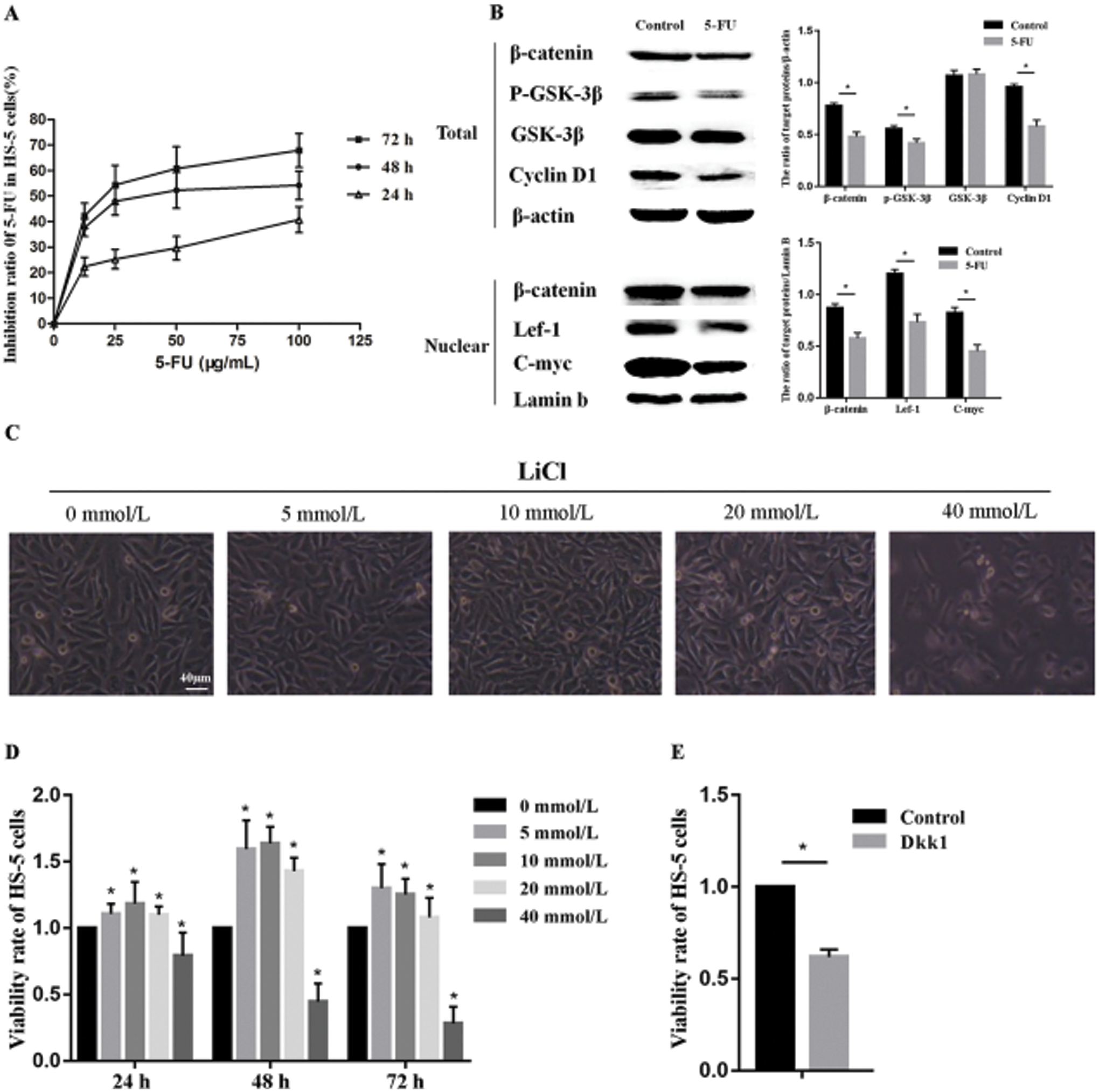
Figure 1: 5-FU inhibits HS-5 cells proliferation by regulating Wnt/β-catenin signaling pathway.
Angelica sinensis polysaccharides antagonize growth inhibition of 5-FU-treated HS-5 cells through the Wnt/β-catenin signaling pathway
Tested by EdU the proportion of proliferating cells in 5-FU group was significantly lower than that of the control group; after pretreatment with ASP and LiCl, the proportion of EdU positive cells increased markedly compared with 5-FU group (Figs. 2A and 2B). The results of CCK-8 showed an obvious reduction after a 48 h-incubation with 5-FU compared with untreated control cells; however, ASP pretreatment partially reversed the reduction. Moreover, the ASP-induced increase in the viability was weakened by Dkk1 (Fig. 2C). Shown by immunofluorescence assay, the cytoplasmic and nuclear expression of β-catenin was decreased obviously after 5-FU treatment, however, ASP and LiCl pretreatment respectively rescued the expression of β-catenin and its nuclear translocation (Fig. 3A). Western blot revealed that ASP and LiCl pretreatment significantly reversed the 5-FU-induced decrease in cytoplasmic expression of total β-catenin, p-GSK-3β, and CyclinD1, meanwhile modulated nuclear expression of β-catenin, Lef-1, and C-myc proteins (Figs. 3B–3I) (P < 0.05). These data hint that ASP may activate Wnt signaling, which may be one mechanism that ASP counteract the inhibiting effect of 5-FU on HS-5 cell growth.
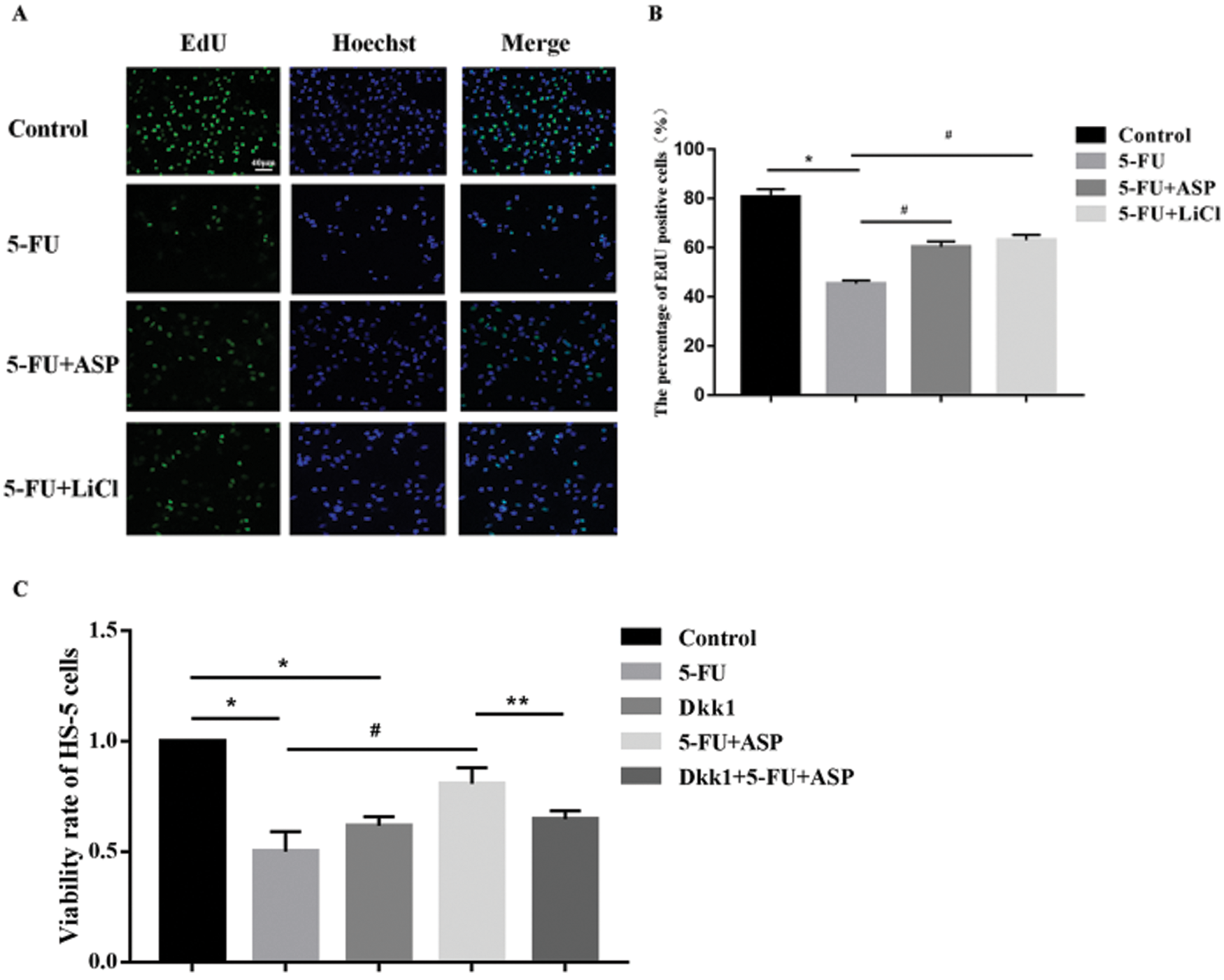
Figure 2: Angelica sinensis Polysaccharides antagonize the growth inhibitory effect of 5-FU on HS-5 cells via up-regulating Wnt/β-catenin signaling.
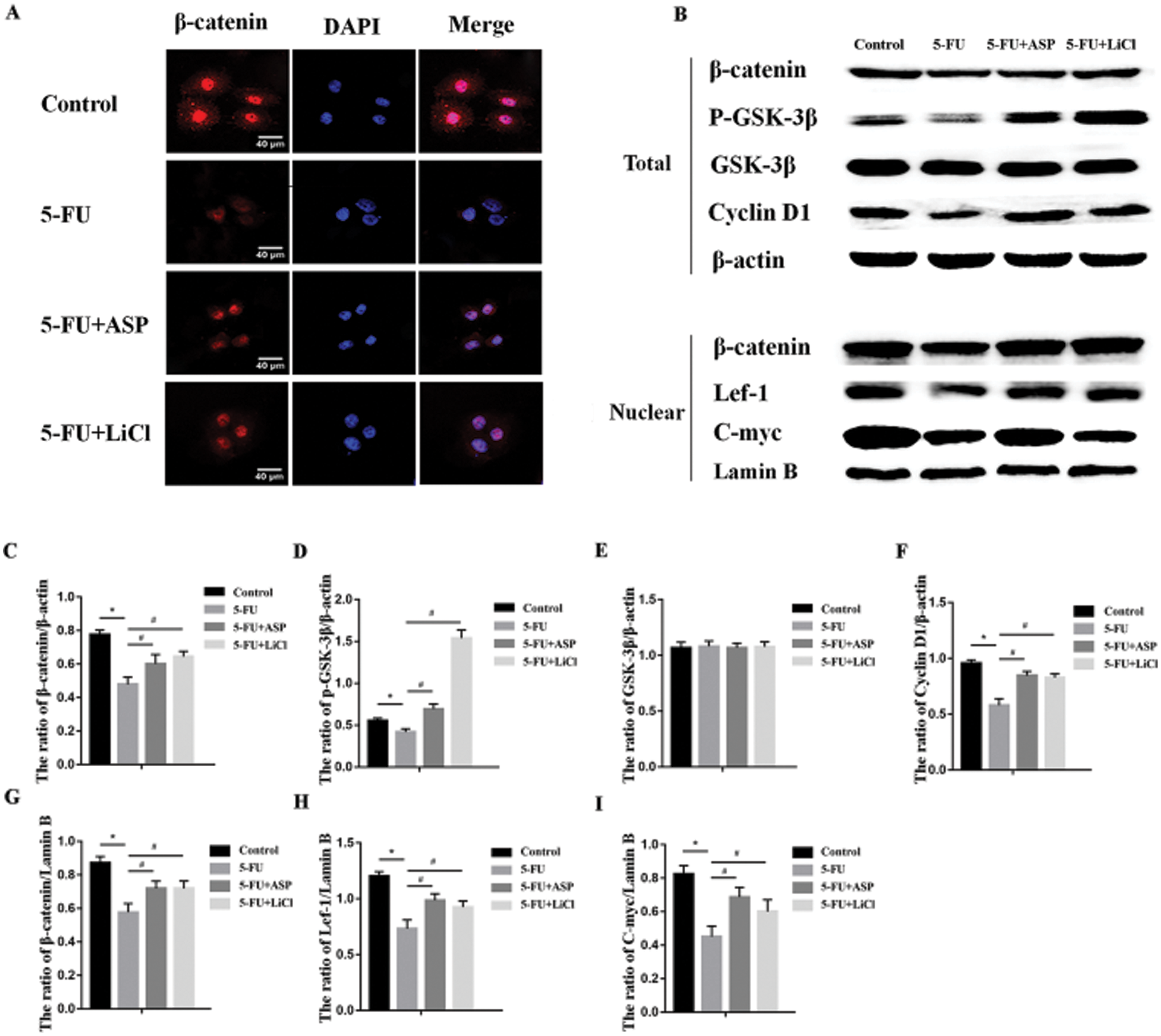
Figure 3: Angelica sinensis Polysaccharides activate Wnt/β-catenin signaling pathway.
Angelica sinensis polysaccharides relieve 5-FU-induced intracellular oxidative stress
To elucidate the mechanism of 5-FU induced damage and ASP mediated protective effect on HS-5 cell growth, we assessed the indexes of oxidative damage. Increases in intracellular ROS and MDA were found in the 5-FU group compared with the control group, whereas single pretreatment of ASP or LiCl reversed the increase dramatically (Figs. 4A and 4C). On the contrary, ASP or LiCl administration protected the antioxidant enzymes, including SOD and CAT in HS-5 cells (Figs. 4D–4E) (P < 0.05). These results demonstrate that 5-FU cause oxidative stress to HS-5 cells, whereas ASP exert a significant anti-oxidative role to alleviate 5-FU-induced oxidative stress, which may be related to the activation of Wnt/β-catenin signaling.
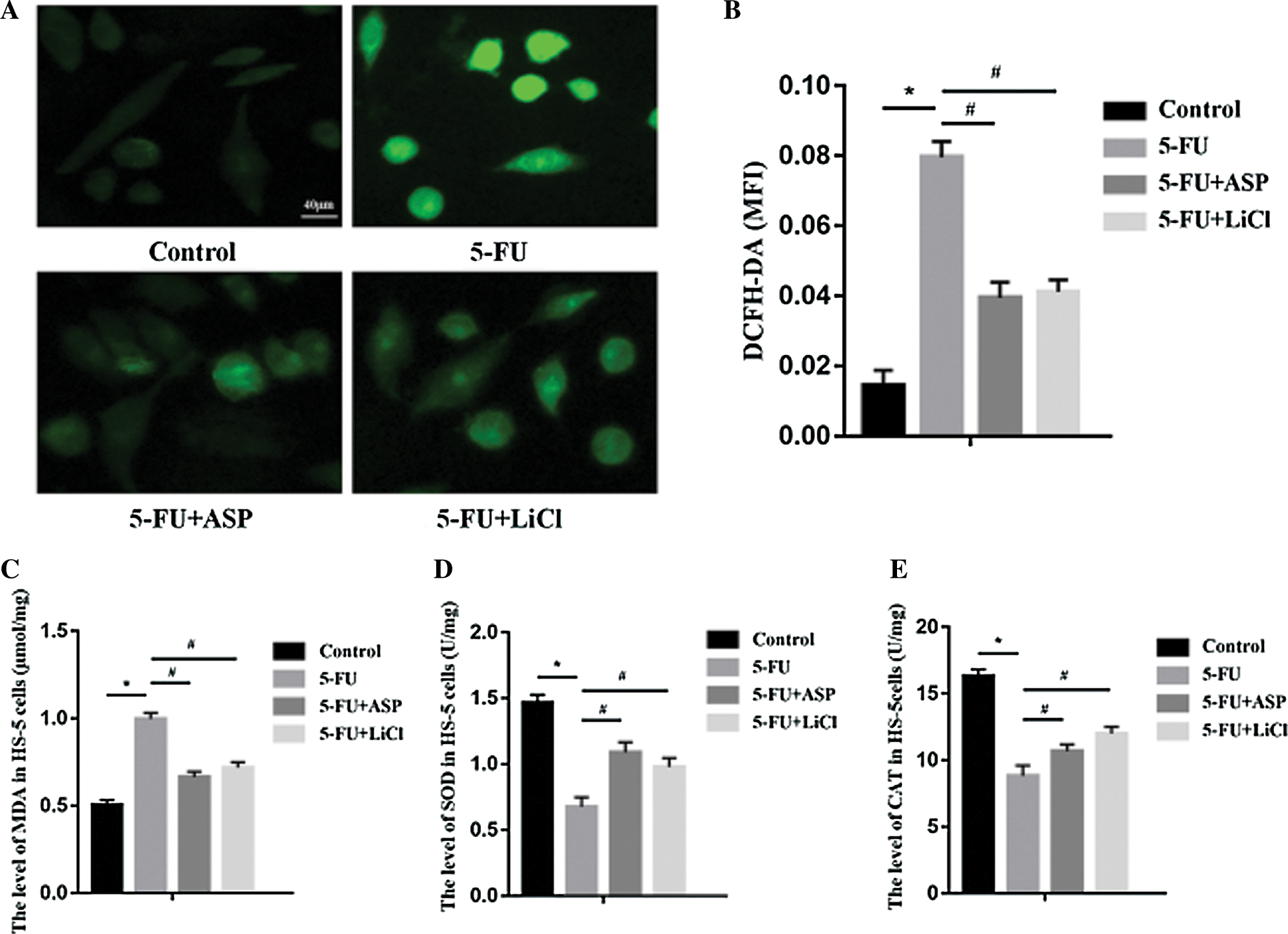
Figure 4: Angelica sinensis Polysaccharides reduce 5-FU-induced intracellular oxidative stress.
Angelica sinensis polysaccharides ameliorate the activation of FoxO1 induced by 5-FU
FoxOs are transcriptional factors closely related to cellular survival and oxidative stress, Notably, activated FoxO1 may impair Wnt signaling via competitive combination with β-catenin in the nucleus. Western blot results demonstrated that compared with the control group FoxO1 expression in the 5-FU group rose concurrently with decreased p-FoxO1 expression. However, ASP or LiCl pretreatment significantly reduced FoxO1 expression via degradation of FoxO1 by an increased p-FoxO1 expression (Fig. 5A). It was inferred antioxidative properties of ASP may play a role in FoxO1 downregulation, which may be another possible mechanism for ASP upregulation of Wnt/β-catenin signaling. FoxOs may orchestrate apoptosis and cell cycle arrest. Here, in the context, the proteins correlating to apoptosis including Bcl-2, Bim, Bax, caspase-3 and cell cycle inhibitor as p27Kip1 were detected via western blot assay. It revealed that ASP or LiCl abrogated 5-FU-induced increase in Bim, Bax caspase-3 and p27Kip1 expression, however enhanced anti-apoptotic protein Bcl-2 expression (Figs. 5B–5F, and 5H) (P < 0.05). The results were in line with the data of flow cytometric analysis that a 2.2-fold increase in the apoptosis rate was found in the 5-FU group compared with the control group, whereas ASP or LiCl significantly decreased apoptotic cell percentage compared with that in 5-FU group (Fig. 5G) (P < 0.05). Meanwhile, ASP or LiCl pretreatment weakened 5-FU induced G0/G1 phase retard concurrent with S and G2/M phase recovery (Fig. 5I). All these data above hinted that the effects of 5-FU on HS-5 growth inhibition may relate to the activation of FoxO1 leading to apoptosis or cycle arrest. The anti-oxidative property of ASP exerts a protective effect against cycle arrest and apoptosis.
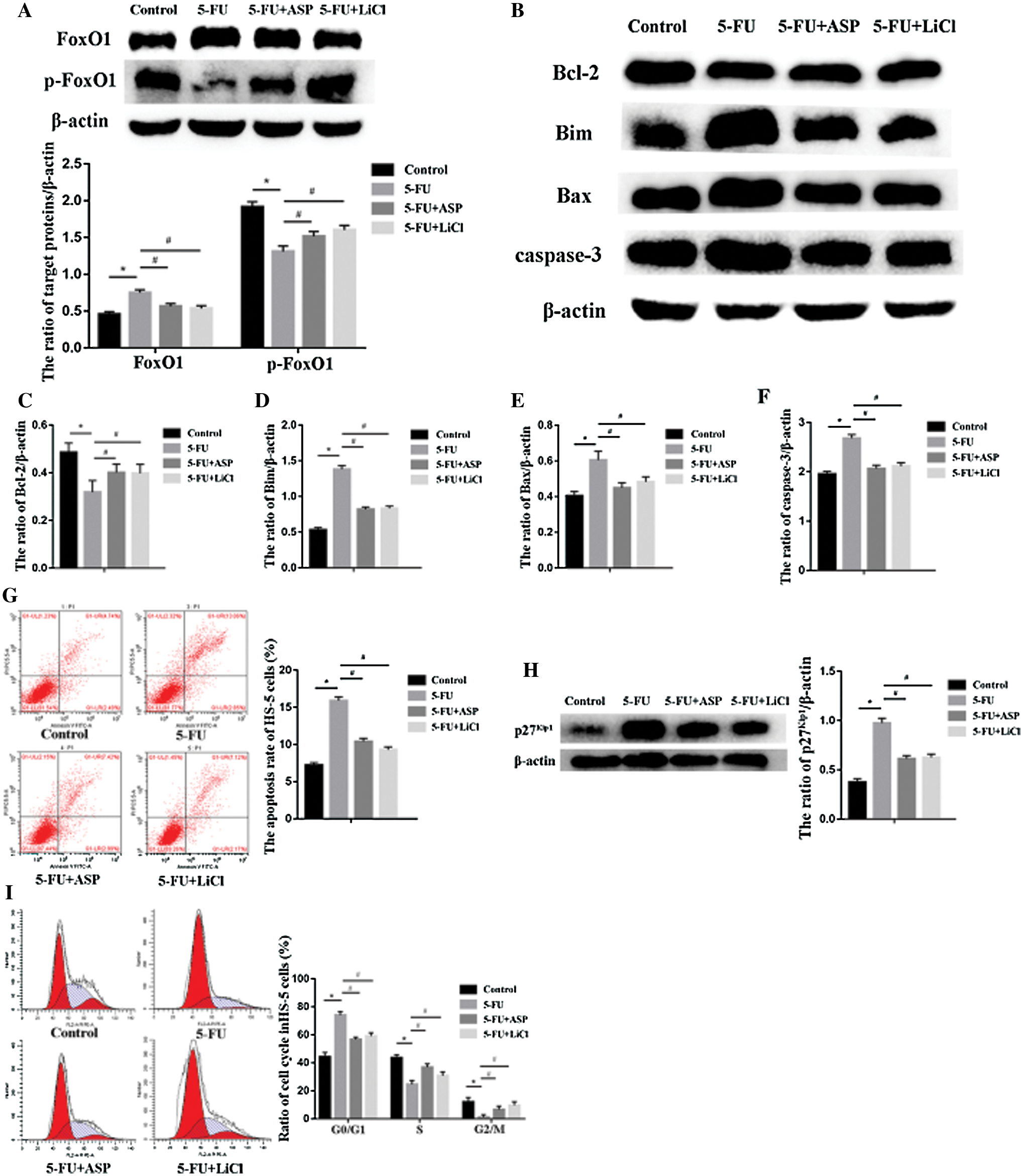
Figure 5: Angelica sinensis Polysaccharides ameliorate 5-FU-induced activation of FoxO1.
Myelosuppression is one of the common side effects of chemotherapy, characterized by depletion of cells within the bone marrow (Ai et al., 2013; Testa et al., 1985). In general, myelosuppression is primarily attributable to the direct cytotoxicity to bone marrow cells, inhibition of bone marrow precursor or progenitor cell proliferation, the reduction in HSC reserves, and impairment in HSC self-renewal. Notably, because of the reduction of HM cellularity in varying degrees, the damaged-hematopoietic microenvironment may result in diminished or delayed hematopoiesis function, immune-related disorders, as well as long-term damage to the bone marrow recovery (Crawford et al., 2004; Kuter, 2015). It has been shown that chemotherapeutic treatment damage the hematopoietic microenvironment in vitro and in vivo (Galotto et al., 1999; Hu et al., 2016; Kemp et al., 2010; Li et al., 2004; Oliveira et al., 2014; de Lima Prata et al., 2010). As chemotherapy disrupts the steady-state function of hematopoietic and stromal cells, disruptions over time may cause severe bone marrow toxicity and the failure of cancer treatment. To ensure this does not occur, finding appropriate agents to promote the recovery process following discontinuation of chemotherapy and to lessen the bone marrow damage has a profound significance.
Since it was first synthesized in 1957, 5-FU has remained one of the most widely used chemotherapeutic agents with broad-spectrum activity against many solid tumors (Wilson et al., 2014). 5-FU exerts its anticancer effects through inhibition of thymidylate synthase (TS) and incorporation of its metabolites into RNA and DNA, leading to cytotoxicity and cell death (Longley et al., 2003). Recent studies have indicated that 5-FU suppressed the proliferation of HSCs and induced the myelosuppression of mice by down-regulating the PI3K-AKT signaling pathway (Wang et al., 2015; Zhang et al., 2019). However, the definite mechanism for 5-FU caused myelosuppression remains unclear. Focused on bone marrow stromal cells, we provided the evidence that 5-FU inhibited stromal cell growth and induced apoptosis, which was related to downregulation of Wnt/β-catenin signaling, also up-regulation of FoxO1 concomitant with an increase of cellular oxidative stress. Furthermore, the current work revealed that anti-oxidative property and role in Wnt signaling regulation might be the key mechanisms of ASP to prevent 5-FU-induced stromal damage.
Stem cells display the defining capacity to self-renew, and their fate is primarily dictated by extrinsic, short-range signals, which typically emanated from the stem cell niche (Losick et al., 2011). The non-hematopoietic cells in the hematopoietic microenvironment have a functional role in regulating hematopoiesis and the signaling pathways that regulate HM may be necessary for the development of functional niches that regulate hematopoietic stem cells and their progenitors (Morrison and Spradling, 2008; Zhang et al., 2003). The Wnt signaling pathway exerts a variety of effects on target cell developmental processes, including cell proliferation, apoptosis, and differentiation. The canonical Wnt pathway affects cellular functions by accumulating β-catenin in the cytoplasm and eventually translocating into the nucleus. Within the nucleus, β-catenin binds to T cell factor (TCF) family/lymphoid enhancer factor (LEF) and regulates cell proliferation through Wnt downstream target genes (Clevers, 2006; Moon et al., 2002; Nusse and Clevers, 2017). It was reported that Wnt/β-catenin signaling regulates HSCs function in a dosage-dependent manner (Fleming et al., 2008; Huang et al., 2012; Malhotra and Kincade, 2009; Mohammed et al., 2016). Various degrees of activation of the pathway may cause different outcomes, leading to either enhanced repopulation capacity or exhaustion of the HSCs. A mild increase in Wnt signaling enhanced HSC function (Famili et al., 2016; Luis et al., 2011). However, a high Wnt level in HSCs eventually leads to stem cell exhaustion and impairment of reconstitution in irradiated recipients (Kirstetter et al., 2006; Ming et al., 2012; Scheller et al., 2006). Most importantly, Wnt signaling regulates HSC reconstruction in a stromal-dependent manner. It was found that when hematopoietic cells were co-cultured with BMSCs supplemented with Wnt3a conditioned medium, the cellularity of Lin−Sca-1+c-kit+ hematopoietic stem cells, was increased, and the hematopoietic transplantation and reconstruction capability were enhanced (Kim et al., 2009; Nemeth et al., 2009). Hence, in the current study, we focused on the Wnt signaling regulation on BMSCs following chemotherapy. It was found that 5-FU induced a decrease in cytoplasmic expression of total β-catenin, p-GSK-3β, and CyclinD1, meanwhile weakened nuclear expression of β-catenin, LEF-1, and C-myc proteins, causing HS-5 cells proliferation inhibition. The results herein are in line with the other data related to the relationship between canonical Wnt signaling and cell proliferation, which has confirmed that Wnt/β-catenin signaling positively stimulates cell growth via cell cycle regulation (Braunschweig et al., 2015; Shtutman et al., 1999).
Reactive oxygen species (ROS) are free radicals and active metabolites of oxygen containing unpaired electrons, which take a significant role in cell signal transduction and regulation (Owusu-Ansah and Banerjee, 2009). Chemical agents, as well as irradiation, can cause persistent ROS production. This accumulation of ROS may lead to excessive oxidative stress and DNA damage such as DSBs (double-strand breaks), which are considered to be the main potential mechanisms causing cellular damage (Meng et al., 2003; Wang et al., 2010). A previous study in our group has demonstrated that 5-FU weakened the antioxidant capacity of HS-5 cells and caused high sensitivity of cells to ROS, thus HS-5 cells underwent DSB which eventually resulted in either apoptosis or senescence (Xiao et al., 2017). Oxidative stress is also related to cell cycle arrest. DSBs initiate DNA damage response through sequential stimulation of ATM, Chk2, and p53 (Sancar et al., 2004). Activation of p53 and its downstream p21 may induce cell cycle arrest. Meanwhile, ROS can activate the p38 MAPK pathway (Ito et al., 2006). Activation of p53 and p38 pathways can converge at p16 and augment of p16 expression may also lead to permanent cell cycle arrest (Beausejour et al., 2003; Iwasa et al., 2003). Interestingly, it is reported that β-catenin may be critical for antagonizing oxidative stress. Exposing β-catenin knockdown mice to chemotherapeutic agent or radiation caused a decreased expression of the hydrogen peroxide (H2O2) detoxifying enzyme catalase and led to the accumulation of ROS and superoxide (O2–) free radicals in cells and an inability to repair DNA damage (Lento et al., 2014). On the opposite, effector molecules generated from oxidative DNA damage may also down-regulate the Wnt pathway by inhibiting transcriptional activity or participating in post-translational modifications to enhance ubiquitination degradation (Lin et al., 2008). The evidence above hints that Wnt signaling is also closely correlated with oxidative stress. Therefore, in the current study, increased oxidative stress may be one of the reasons for the downregulation of Wnt signaling induced by 5-FU treatment. Whereas, a decrease in β-catenin protein accompanying reduction of antioxidase SOD and CAT induced by 5-FU treatment may be another mechanism of cell proliferation inhibition.
Forkhead box O (FOXO) family are transcription factors, which promote cell survival by regulating the cell cycle, apoptosis, and the response to oxidative stress (Eijkelenboom and Burgering, 2013). The accumulation of ROS may interrupt 14-3-3 combine to FoxO via JNK (c-Jun N terminal kinase), permit FoxO entrance into the nucleus, and induce its transcriptional activation (Morrison, 2009; Nakae et al., 2008). FoxO can be phosphorylated by the phosphatidylinositol 3-kinase-Akt pathway (Han et al., 2015; Zhang et al., 2020). It is of note that FoxO-mediated transcription requires the binding of β-catenin. FoxOs can compete with TCF/LEF by directly binding β-catenin, thereby inhibit Wnt/β-catenin downstream signaling (Almeida et al., 2007; Behrens et al., 1996; Hoogeboom et al., 2008; Iyer et al., 2013). It was demonstrated herein, compared with the control group FoxO1 expression in 5-FU treated HS-5 cells rose dramatically concurrent with decreased p-FoxO1 expression. The reason for up-regulation of FoxO1 may be related to 5-FU triggered oxidative stress, whereas FoxO1 up-regulation may be another reason for 5-FU induced decrease in Wnt signaling (Burgering and Medema, 2003; Danciu et al., 2004; Ma and Wang, 2012). FoxO transcription factor family regulates the proteins that are crucial for apoptosis, as well as the proteins involved in the proliferative status of a cell. FoxO factors may regulate antiapoptotic and proapoptotic proteins at multiple levels, finally trigger activation of the effector caspases. Bim promotes apoptosis by inhibition of antiapoptotic Bcl-2 family members or through direct activation of Bax-like molecules. FoxO factors may regulate Bim protein expression to cause cell death due to cytokine deprivation. FoxO factors may also repress transcription of Bcl-XL through the induction of the transcriptional repressor (Dijkers et al., 2000; Stahl et al., 2002; Tang et al., 2002). Caspase-3 is an important effector protease, when it is cleaved, it acts as the final executor during apoptosis. In the current study, it was found that in 5-FU treated HS-5 cells, FoxO1 targeted apoptosis-related proteins to cause an increase in Bim, Bax, and caspase-3, whereas a decrease in Bcl-2. FoxO1 targeted apoptosis to disturb the dynamic balance of the cellularity of HS-5 cells, which may be one of the reasons for cell growth inhibition. Moreover, the cyclin kinase inhibitor p27Kip1, a downstream target of FoxO1, acting as a potent inhibitor of cyclin/CDK complexes in the S-phase of cell cycle progression was also tested (Collado et al., 2000; Kops et al., 2002; Medema et al., 2000; Nakamura et al., 2000). It was found herein that 5-FU increased the expression of p27kip1. In addition, 5-FU simultaneously reduced the expression of Cyclin D1. It is of note that transcriptional repression of D-type cyclins is vital to the FoxO-induced cell-cycle arrest, which is evidenced by transcriptional profiling and mRNA analysis. D-type cyclins are required for phosphorylation and inactivation of the retinoblastoma tumor suppressor protein (pRb), an essential determinant of cell-cycle progression in G1 (Ramaswamy et al., 2002; Schmidt et al., 2002). To sum up, 5-FU-induced HS-5 cell growth inhibition is probably associated with FoxO1 targeted apoptosis or cell cycle arrest.
The traditional Chinese medicine Angelica sinensis, which is commonly used to enrich the blood, promote blood circulation (Wei et al., 2016). The active constituents of Angelica sinensis include polysaccharides, organic acid sand phthalides, among which Angelica sinensis polysaccharides (ASP) are regarded as the main biological activity ingredient responsible for pharmacological effects with multi-target property (Deng et al., 2006). ASP have attracted more and more attention to its beneficial effects, such as hematopoietic effects (Liu et al., 2010b), immunologic enhancement (Yang et al., 2006), anti-tumor activity (Cao et al., 2010; Shang et al., 2003), and anti-radiation damage (Zhao et al., 2012). The antioxidant properties of ASP suppress the production of ROS and protected the endothelial progenitor cells, hepatocytes, myocardial cells, and nerve cells from oxidative damage (Ai et al., 2013; Ji et al., 2014; Zhang et al., 2010). Moreover, the evidence demonstrated that ASP promote cell proliferation, including in total spleen cells, macrophages (Yang et al., 2006), and gastric epithelial cells (Xie et al., 2019). Our previous studies suggested that ASP reduced oxidative stress and oxidative DNA damage, boosted direct cell-cell contact between stromal cells and hematopoietic cells through Cx43 junctions, regulated cytokines, growth factors and chemokines such as CXCL12, SCF, GM-CSF, RANTES and thus provided a homeostatic microenvironment for hematopoietic stem/progenitor cells to regenerate following chemotherapeutic myelosuppression. In the present study, it was further demonstrated that ASP protected HS-5 cells from 5-FU-induced proliferation inhibition and ameliorated cellular oxidative stress via the mechanism of up-regulation of Wnt/β-catenin signaling. Most importantly, it was first evidenced herein that ASP balanced the relationship between FoxO-mediated transcription and Wnt signaling in BMSCs under oxidative stress, which might be promising for the clinical therapeutic use of ASP to myelosuppression.
In conclusion, the present study has reported that ASP protect stromal cells against 5-FU-induced proliferation inhibition and apoptosis via activating the Wnt/β-catenin signaling pathway directly or the indirect effects on Wnt/β-catenin signaling by down-regulation of its antagonizing FoxO1 (Fig. 6), suggesting a broad role for ASP as a potential antioxidant protective agent for chemoradiation therapeutic, preventive agents.
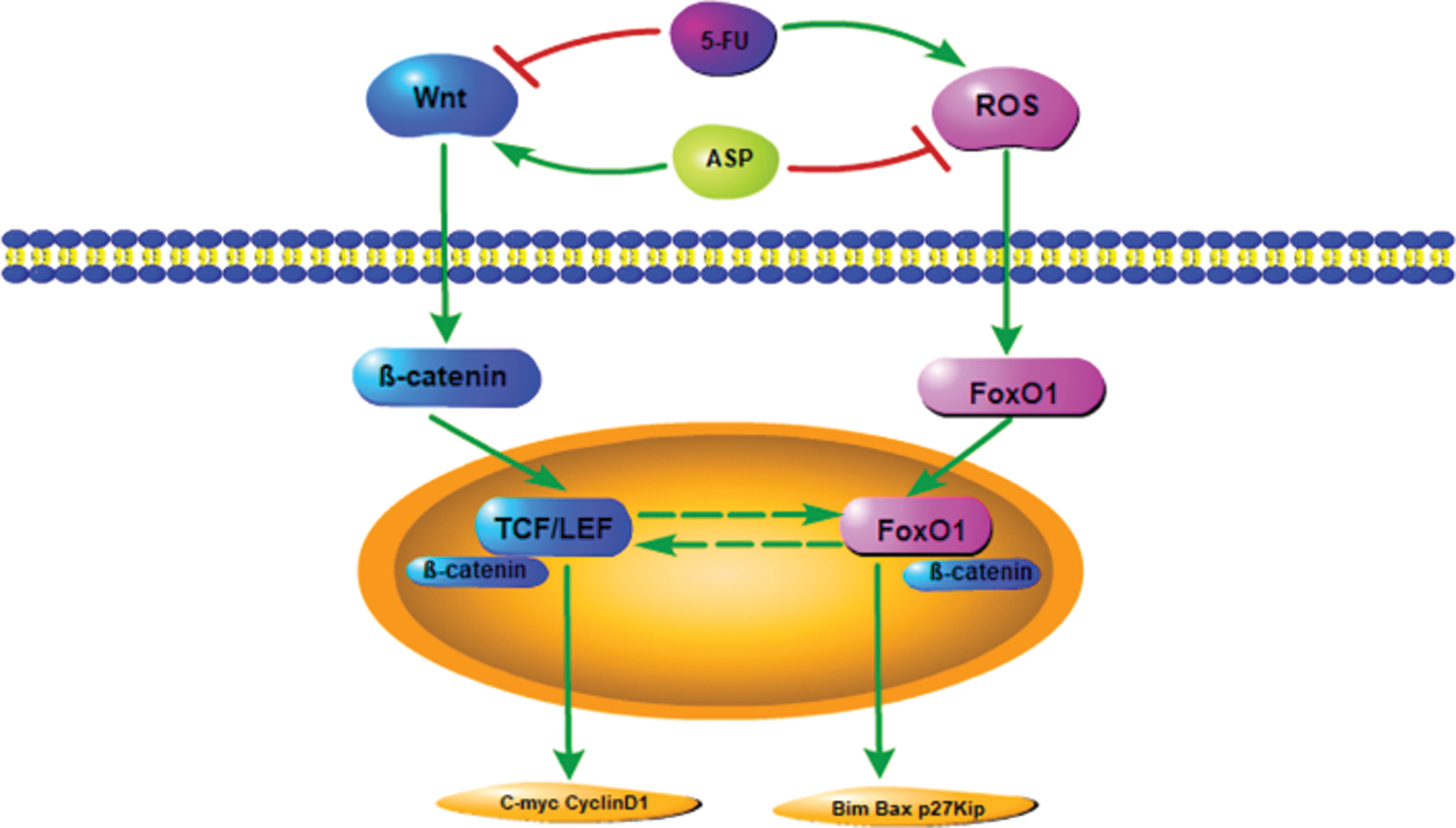
Figure 6: Model of Wnt signaling cascade.
Author Contribution: For research articles, LW conceptualized and designed the experiments; HXZX performed the experiments; RJQ, ZLW and MHX, YX, YPW contributed reagents/materials/analysis tools; HXZX analyzed the data and wrote the paper; LW revised the paper.
Availability of Data and Materials: The datasets used in this study are available from the corresponding author upon reasonable request.
Funding Statement: This work was supported by the National Natural Science Foundation of China (Grant No. 81873103), the Foundation and Frontier Research Project of Chongqing Science and Technology Commission (Grant No. cstc2014jcyjA10001).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ai S, Fan X, Fan L, Sun Q, Liu Y, Tao X, Dai K (2013). Extraction and chemical characterization of Angelica sinensis polysaccharides and its antioxidant activity. Carbohydrate Polymers 94: 731–736. DOI 10.1016/j.carbpol.2013.02.007. [Google Scholar] [CrossRef]
Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC (2007). Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting β-catenin from T cell factor-to forkhead box O-mediated transcription. Journal of Biological Chemistry 282: 27298–27305. DOI 10.1074/jbc.M702811200. [Google Scholar] [CrossRef]
Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J (2003). Reversal of human cellular senescence: Roles of the p53 and p16 pathways. EMBO Journal 22: 4212–4222. DOI 10.1093/emboj/cdg417. [Google Scholar] [CrossRef]
Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W (1996). Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382: 638–642. DOI 10.1038/382638a0. [Google Scholar] [CrossRef]
Bradley RR, Cunniff PJ, Pereira BJ, Jaber BL (1999). Hematopoietic effect of Radix angelicae sinensis in a hemodialysis patient. American Journal of Kidney Diseases 34: 349–354. DOI 10.1016/S0272-6386(99)70367-7. [Google Scholar] [CrossRef]
Braunschweig L, Meyer AK, Wagenfuhr L, Storch A (2015). Oxygen regulates proliferation of neural stem cells through Wnt/β-catenin signalling. Molecular and Cellular Neuroscience 67: 84–92. DOI 10.1016/j.mcn.2015.06.006. [Google Scholar] [CrossRef]
Burgering BM, Medema RH (2003). Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. Journal of Leukocyte Biology 73: 689–701. DOI 10.1189/jlb.1202629. [Google Scholar] [CrossRef]
Cao W, Li XQ, Wang X, Li T, Chen X, Liu SB, Mei QB (2010). Characterizations and anti-tumor activities of three acidic polysaccharides from Angelica sinensis (Oliv.) Diels. International Journal of Biological Macromolecules 46: 115–122. DOI 10.1016/j.ijbiomac.2009.11.005. [Google Scholar] [CrossRef]
Chabner BA, Roberts TG,Jr (2005). Timeline: Chemotherapy and the war on cancer. Nature Reviews Cancer 5: 65–72. DOI 10.1038/nrc1529. [Google Scholar] [CrossRef]
Clevers H (2006). Wnt/β-catenin signaling in development and disease. Cell 127: 469–480. DOI 10.1016/j.cell.2006.10.018. [Google Scholar] [CrossRef]
Collado M, Medema RH, Garcia-Cao I, Dubuisson ML, Barradas M, Glassford J, Rivas C, Burgering BM, Serrano M, Lam EW (2000). Inhibition of the phosphoinositide 3-kinase pathway induces a senescence-like arrest mediated by p27Kip1. Journal of Biological Chemistry 275: 21960–21968. DOI 10.1074/jbc.M000759200. [Google Scholar] [CrossRef]
Crawford J, Dale DC, Lyman GH (2004). Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer 100: 228–237. DOI 10.1002/cncr.11882. [Google Scholar] [CrossRef]
Danciu TE, Gagari E, Adam RM, Damoulis PD, Freeman MR (2004). Mechanical strain delivers anti-apoptotic and proliferative signals to gingival fibroblasts. Journal of Dental Research 83: 596–601. DOI 10.1177/154405910408300803. [Google Scholar] [CrossRef]
Deng S, Chen SN, Yao P, Nikolic D, van Breemen RB, Bolton JL, Fong HHS, Farnsworth NR, Pauli GF (2006). Serotonergic activity-guided phytochemical investigation of the roots of Angelica sinensis. Journal of Natural Products 69: 536–541. DOI 10.1021/np050301s. [Google Scholar] [CrossRef]
Dietz BM, Hajirahimkhan A, Dunlap TL, Bolton JL (2016). Botanicals and their bioactive phytochemicals for women’s health. Pharmacological Reviews 68: 1026–1073. DOI 10.1124/pr.115.010843. [Google Scholar] [CrossRef]
Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ (2000). Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Current Biology 10: 1201–1204. DOI 10.1016/S0960-9822(00)00728-4. [Google Scholar] [CrossRef]
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P (2000). Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 355: 1041–1047. DOI 10.1016/S0140-6736(00)02034-1. [Google Scholar] [CrossRef]
Dritschilo A, Sherman DS (1981). Radiation and chemical injury in the bone marrow. Environmental Health Perspectives 39: 59–64. DOI 10.1289/ehp.813959. [Google Scholar] [CrossRef]
de Lima Prata K, Orellana MD, de Santis GC, Kashima S, Fontes AM, de Cássia Viu Carrara R, Palma PVB, Neder L, Covas DT (2010). Effects of high-dose chemotherapy on bone marrow multipotent mesenchymal stromal cells isolated from lymphoma patients. Experimental Hematology 38: 292–300, e294. DOI 10.1038/nrm3507. [Google Scholar] [CrossRef]
Eijkelenboom A, Burgering BM (2013). FOXOs: Signalling integrators for homeostasis maintenance. Nature Reviews Molecular and Cellular Biology 14: 83–97. DOI 10.1126/science.1109083. [Google Scholar] [CrossRef]
Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC (2005). Functional interaction between β-catenin and FOXO in oxidative stress signaling. Science 308: 1181–1184. DOI 10.1016/j.stemcr.2016.04.009. [Google Scholar] [CrossRef]
Famili F, Brugman MH, Taskesen E, Naber BEA, Fodde R, Staal FJT (2016). High levels of canonical Wnt signaling lead to loss of stemness and increased differentiation in hematopoietic stem cells. Stem Cell Reports 6: 652–659. DOI 10.1016/j.stem.2008.01.003. [Google Scholar] [CrossRef]
Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT (2008). Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2: 274–283. DOI 10.1016/S0301-472X(99)00076-4. [Google Scholar] [CrossRef]
Galotto M, Berisso G, Delfino L, Podesta M, Ottaggio L, Dallorso S, Dufour C, Ferrara GB, Abbondandolo A, Dini G, Bacigalupo A, Cancedda R, Quarto R (1999). Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Experimental Hematology 27: 1460–1466. DOI 10.4161/cc.7.20.6920. [Google Scholar] [CrossRef]
Gomes AR, Brosens JJ, Lam EW (2008). Resist or die: FOXO transcription factors determine the cellular response to chemotherapy. Cell Cycle 7: 3133–3136. DOI 10.1038/sj.onc.1209086. [Google Scholar] [CrossRef]
Greer EL, Brunet A (2005). FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24: 7410–7425. DOI 10.1111/jnc.13199. [Google Scholar] [CrossRef]
Han J, Zhao J, Jiang J, Ma X, Liu X, Wang C, Jiang S, Wan C (2015). Zinc deficiency impairs the renewal of hippocampal neural stem cells in adult rats: Involvement of FoxO3a activation and downstream p27(kip1) expression. Journal of Neurochemistry 134: 879–891. DOI 10.1074/jbc.M706638200. [Google Scholar] [CrossRef]
Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM (2008). Interaction of FOXO with β-catenin inhibits β-catenin/T cell factor activity. Journal of Biological Chemistry 283: 9224–9230. DOI 10.1158/1078-0432.CCR-15-1421. [Google Scholar] [CrossRef]
Hu W, Sung T, Jessen BA, Thibault S, Finkelstein MB, Khan NK, Sacaan AI (2016). Mechanistic investigation of bone marrow suppression associated with Palbociclib and its differentiation from cytotoxic chemotherapies. Clinical Cancer Research 22: 2000–2008. DOI 10.1038/nm.2984. [Google Scholar] [CrossRef]
Huang J, Nguyen-McCarty M, Hexner EO, Danet-Desnoyers G, Klein PS (2012). Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nature Medicine 18: 1778–1785. DOI 10.1038/nm1388. [Google Scholar] [CrossRef]
Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T (2006). Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature Medicine 12: 446–451. DOI 10.1046/j.1365-2443.2003.00620.x. [Google Scholar] [CrossRef]
Iwasa H, Han J, Ishikawa F (2003). Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes to Cells 8: 131–144. DOI 10.1172/JCI68049. [Google Scholar] [CrossRef]
Iyer S, Ambrogini E, Bartell SM, Han L, Roberson PK, de Cabo R, Jilka RL, Weinstein RS, O’Brien CA, Manolagas SC, Almeida M (2013). FOXOs attenuate bone formation by suppressing Wnt signaling. Journal of Clinical Investigation 123: 3409–3419. DOI 10.1016/j.ijbiomac.2014.03.025. [Google Scholar] [CrossRef]
Ji P, Wei Y, Xue W, Hua Y, Zhang M, Sun H, Song Z, Zhang L, Li J, Zhao H, Zhang W (2014). Characterization and antioxidative activities of polysaccharide in Chinese angelica and its processed products. International Journal of Biological Macromolecules 67: 195–200. DOI 10.1016/j.carbpol.2012.04.049. [Google Scholar] [CrossRef]
Jin M, Zhao K, Huang Q, Xu C, Shang P (2012). Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohydrate Polymers 89: 713–722. DOI 10.1007/s00277-009-0896-2. [Google Scholar] [CrossRef]
Kemp K, Morse R, Wexler S, Cox C, Mallam E, Hows J, Donaldson C (2010). Chemotherapy-induced mesenchymal stem cell damage in patients with hematological malignancy. Annals of Hematology 89: 701–713. DOI 10.1002/stem.52. [Google Scholar] [CrossRef]
Kim JA, Kang YJ, Park G, Kim M, Park YO, Kim H, Leem SH, Chu IS, Lee JS, Jho EH, Oh IH (2009). Identification of a stroma-mediated Wnt/β-catenin signal promoting self-renewal of hematopoietic stem cells in the stem cell niche. Stem Cells 27: 1318–1329. DOI 10.1038/ni1381. [Google Scholar] [CrossRef]
Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C (2006). Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nature Immunology 7: 1048–1056. DOI 10.1128/MCB.22.7.2025-2036.2002. [Google Scholar] [CrossRef]
Kops GJPL, Medema RH, Glassford J, Essers MAG, Dijkers PF, Coffer PJ, Lam EWF, Burgering BMT (2002). Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Molecular and Cellular Biology 22: 2025–2036. [Google Scholar]
Kuter DJ (2015). Managing thrombocytopenia associated with cancer chemotherapy. Oncology 29: 282–294. DOI 10.3109/13880209.2015.1027779. [Google Scholar] [CrossRef]
Lai P, Liu Y (2015). Angelica sinensis polysaccharides inhibit endothelial progenitor cell senescence through the reduction of oxidative stress and activation of the Akt/hTERT pathway. Pharmaceutical Biology 53: 1842–1849. DOI 10.4103/1673-5374.128218. [Google Scholar] [CrossRef]
Lei T, Li H, Fang Z, Lin J, Wang S, Xiao L, Yang F, Liu X, Zhang J, Huang Z, Liao W (2014). Polysaccharides from Angelica sinensis alleviate neuronal cell injury caused by oxidative stress. Neural Regeneration Research 9: 260–267. DOI 10.1101/gad.231944.113. [Google Scholar] [CrossRef]
Lento W, Ito T, Zhao C, Harris JR, Huang W, Jiang C, Owzar K, Piryani S, Racioppi L, Chao N, Reya T (2014). Loss of β-catenin triggers oxidative stress and impairs hematopoietic regeneration. Genes & Development 28: 995–1004. DOI 10.1111/j.1365-2141.2004.05200.x. [Google Scholar] [CrossRef]
Li J, Law HK, Lau YL, Chan GC (2004). Differential damage and recovery of human mesenchymal stem cells after exposure to chemotherapeutic agents. British Journal of Haematology 127: 326–334. DOI 10.1210/en.2007-1372. [Google Scholar] [CrossRef]
Lin CL, Wang JY, Ko JY, Surendran K, Huang YT, Kuo YH, Wang FS (2008). Superoxide destabilization of β-catenin augments apoptosis of high-glucose-stressed mesangial cells. Endocrinology 149: 2934–2942. DOI 10.1186/1472-6882-10-79. [Google Scholar] [CrossRef]
Liu C, Li J, Meng FY, Liang SX, Deng R, Li CK, Pong NH, Lau CP, Cheng SW, Ye JY, Chen JL, Yang ST, Yan H, Chen S, Chong BH, Yang M (2010a). Polysaccharides from the root of Angelica sinensis promotes hematopoiesis and thrombopoiesis through the PI3K/AKT pathway. BMC Complementary and Alternative Medicine 10: 79. DOI 10.1016/j.exphem.2010.03.012. [Google Scholar] [CrossRef]
Liu PJ, Hsieh WT, Huang SH, Liao HF, Chiang BH (2010b). Hematopoietic effect of water-soluble polysaccharides from Angelica sinensis on mice with acute blood loss. Experimental Hematology 38: 437–445. DOI 10.1038/nrc1074. [Google Scholar] [CrossRef]
Longley DB, Harkin DP, Johnston PG (2003). 5-fluorouracil: Mechanisms of action and clinical strategies. Nature Reviews Cancer 3: 330–338. DOI 10.1016/j.devcel.2011.06.018. [Google Scholar] [CrossRef]
Losick VP, Morris LX, Fox DT, Spradling A (2011). Drosophila stem cell niches: A decade of discovery suggests a unified view of stem cell regulation. Developmental Cell 21: 159–171. DOI 10.1016/j.stem.2011.07.017. [Google Scholar] [CrossRef]
Luis TC, Naber BA, Roozen PP, Brugman MH, de Haas EF, Ghazvini M, Fibbe WE, van Dongen JJM, Fodde R, Staal FJT (2011). Canonical Wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell 9: 345–356. DOI 10.1042/CBI20120078. [Google Scholar] [CrossRef]
Ma Y, Wang H (2012). PI3K/Akt/FoxO: A novel participant in signal transduction in bone cells under mechanical stimulation. Cell Biology International 36: 923–926. DOI 10.1016/j.stem.2008.12.004. [Google Scholar] [CrossRef]
Malhotra S, Kincade PW (2009). Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell 4: 27–36. [Google Scholar]
Marsh JC (1976). The effects of cancer chemotherapeutic agents on normal hematopoietic precursor cells: A review. Cancer Research 36: 1853–1882. DOI 10.1038/35008115. [Google Scholar] [CrossRef]
Medema RH, Kops GJ, Bos JL, Burgering BM (2000). AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404: 782–787. [Google Scholar]
Meng A, Wang Y, Van Zant G, Zhou D (2003). Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Research 63: 5414–5419. DOI 10.1074/jbc.M112.342089. [Google Scholar] [CrossRef]
Ming M, Wang S, Wu W, Senyuk V, Le Beau MM, Nucifora G, Qian Z (2012). Activation of Wnt/β-catenin protein signaling induces mitochondria-mediated apoptosis in hematopoietic progenitor cells. Journal of Biological Chemistry 287: 22683–22690. DOI 10.1016/j.gendis.2015.12.004. [Google Scholar] [CrossRef]
Mohammed MK, Shao C, Wang J, Wei Q, Wang X, Collier Z, Tang S, Liu H, Zhang F, Huang J, Guo D, Lu M, Liu F, Liu J, Ma C, Shi LL, Athiviraham A, He TC, Lee MJ (2016). Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes & Diseases 3: 11–40. DOI 10.1126/science.1071549. [Google Scholar] [CrossRef]
Moon RT, Bowerman B, Boutros M, Perrimon N (2002). The promise and perils of Wnt signaling through β-catenin. Science 296: 1644–1646. DOI 10.1016/j.tcb.2008.10.003. [Google Scholar] [CrossRef]
Morrison DK (2009). The 14-3-3 proteins: Integrators of diverse signaling cues that impact cell fate and cancer development. Trends in Cell Biology 19: 16–23. DOI 10.1016/j.cell.2008.01.038. [Google Scholar] [CrossRef]
Morrison SJ, Spradling AC (2008). Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 132: 598–611. [Google Scholar]
Mu X, Zhang Y, Li J, Xia J, Chen X, Jing P, Song X, Wang L, Wang Y (2017). Angelica sinensis polysaccharide prevents hematopoietic stem cells senescence in D-galactose-induced aging mouse model. Stem Cells International 2017: 3508907. DOI 10.1016/j.febslet.2007.11.025. [Google Scholar] [CrossRef]
Nakae J, Oki M, Cao Y (2008). The FoxO transcription factors and metabolic regulation. FEBS Letters 582: 54–67. DOI 10.1128/MCB.20.23.8969-8982.2000. [Google Scholar] [CrossRef]
Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR (2000). Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Molecular and Cellular Biology 20: 8969–8982. DOI 10.1002/stem.32. [Google Scholar] [CrossRef]
Nemeth MJ, Mak KK, Yang Y, Bodine DM (2009). β-Catenin expression in the bone marrow microenvironment is required for long-term maintenance of primitive hematopoietic cells. Stem Cells 27: 1109–1119. DOI 10.1038/srep26645. [Google Scholar] [CrossRef]
Nicolay NH, Rühle A, Perez RL, Trinh T, Sisombath S, Weber KJ, Ho AD, Debus J, Saffrich R, Huber PE (2016). Mesenchymal stem cells are sensitive to bleomycin treatment. Scientific Reports 6: 26645. DOI 10.1016/j.cell.2017.05.016. [Google Scholar] [CrossRef]
Nusse R, Clevers H (2017). Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169: 985–999. DOI 10.1517/14712598.2010.504705. [Google Scholar] [CrossRef]
Oh IH (2010). Microenvironmental targeting of Wnt/β-catenin signals for hematopoietic stem cell regulation. Expert Opinion on Biological Therapy 10: 1315–1329. DOI 10.1016/j.toxlet.2013.11.023. [Google Scholar] [CrossRef]
Oliveira MS, Carvalho JL, Campos AC, Gomes DA, de Goes AM, Melo MM (2014). Doxorubicin has in vivo toxicological effects on ex vivo cultured mesenchymal stem cells. Toxicology Letters 224: 380–386. DOI 10.1038/nature08313. [Google Scholar] [CrossRef]
Owusu-Ansah E, Banerjee U (2009). Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461: 537–541. [Google Scholar]
Papac RJ (2001). Origins of cancer therapy. Yale Journal of Biology and Medicine 74: 391–398. DOI 10.1016/j.cell.2009.11.035. [Google Scholar] [CrossRef]
Petersen CP, Reddien PW (2009). Wnt signaling and the polarity of the primary body axis. Cell 139: 1056–1068. DOI 10.1016/j.exphem.2010.01.006. [Google Scholar] [CrossRef]
Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR (2002). A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell 2: 81–91. DOI 10.1016/S1535-6108(02)00086-7. [Google Scholar] [CrossRef]
Richter J, Traver D, Willert K (2017). The role of Wnt signaling in hematopoietic stem cell development. Critical Reviews in Biochemistry and Molecular Biology 52: 414–424. DOI 10.1080/10409238.2017.1325828. [Google Scholar] [CrossRef]
Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S (2004). Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annual Review of Biochemistry 73: 39–85. DOI 10.1146/annurev.biochem.73.011303.073723. [Google Scholar] [CrossRef]
Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A (2006). Hematopoietic stem cell and multilineage defects generated by constitutive β-catenin activation. Nature Immunology 7: 1037–1047. DOI 10.1038/ni1387. [Google Scholar] [CrossRef]
Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJPL, Lam EWF, Burgering BMT, Medema ŔH (2002). Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Molecular and Cellular Biology 22: 7842–7852. DOI 10.1128/MCB.22.22.7842-7852.2002. [Google Scholar] [CrossRef]
Schreck C, Bock F, Grziwok S, Oostendorp RA, Istvanffy R (2014). Regulation of hematopoiesis by activators and inhibitors of Wnt signaling from the niche. Annals of the New York Academy of Sciences 1310: 32–43. DOI 10.1111/nyas.12384. [Google Scholar] [CrossRef]
Shang P, Qian AR, Yang TH, Jia M, Mei QB, Cho CH, Zhao WM, Chen ZN (2003). Experimental study of anti-tumor effects of polysaccharides from Angelica sinensis. World Journal of Gastroenterology 9: 1963–1967. DOI 10.3748/wjg.v9.i9.1963. [Google Scholar] [CrossRef]
Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A (1999). The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proceedings of the National Academy of Sciences of the United States of America 96: 5522–5527. DOI 10.1073/pnas.96.10.5522. [Google Scholar] [CrossRef]
Somaiah C, Kumar A, Sharma R, Sharma A, Anand T, Bhattacharyya J, Das D, Deka Talukdar S, Jaganathan BG (2018). Mesenchymal stem cells show functional defect and decreased anti-cancer effect after exposure to chemotherapeutic drugs. Journal of Biomedical Science 25: 5. DOI 10.1186/s12929-018-0407-7. [Google Scholar] [CrossRef]
Stahl M, Dijkers PF, Kops GJPL, Lens SMA, Coffer PJ, Burgering BMT, Medema RH (2002). The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. Journal of Immunology 168: 5024–5031. DOI 10.4049/jimmunol.168.10.5024. [Google Scholar] [CrossRef]
Tang TT, Dowbenko D, Jackson A, Toney L, Lewin DA, Dent AL, Lasky LA (2002). The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. Journal of Biological Chemistry 277: 14255–14265. DOI 10.1074/jbc.M110901200. [Google Scholar] [CrossRef]
Testa NG, Hendry JH, Molineux G (1985). Long-term bone marrow damage in experimental systems and in patients after radiation or chemotherapy. Anticancer Research 5: 101–110. [Google Scholar]
Tsai NM, Lin SZ, Lee CC, Chen SP, Su HC, Chang WL, Harn HJ (2005). The antitumor effects of Angelica sinensis on malignant brain tumors in vitro and in vivo. Clinical Cancer Research 11: 3475–3484. DOI 10.1158/1078-0432.CCR-04-1827. [Google Scholar] [CrossRef]
Wang K, Wu J, Cheng F, Huang X, Zeng F, Zhang Y (2017). Acidic polysaccharide from Angelica sinensis reverses anemia of chronic disease involving the suppression of inflammatory hepcidin and NF-κB activation. Oxidative Medicine and Cellular Longevity 2017: 7601592. [Google Scholar]
Wang S, Zheng G, Tian S, Zhang Y, Shen L, Pak Y, Shen Y, Qian J (2015). Echinacoside improves hematopoietic function in 5-FU-induced myelosuppression mice. Life Sciences 123: 86–92. DOI 10.1016/j.lfs.2015.01.002. [Google Scholar] [CrossRef]
Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D (2010). Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radical Biology and Medicine 48: 348–356. DOI 10.1016/j.freeradbiomed.2009.11.005. [Google Scholar] [CrossRef]
Wei WL, Zeng R, Gu CM, Qu Y, Huang LF (2016). Angelica sinensis in China—A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. Journal of Ethnopharmacology 190: 116–141. DOI 10.1016/j.jep.2016.05.023. [Google Scholar] [CrossRef]
Wilson PM, Danenberg PV, Johnston PG, Lenz HJ, Ladner RD (2014). Standing the test of time: Targeting thymidylate biosynthesis in cancer therapy. Nature Reviews Clinical Oncology 11: 282–298. DOI 10.1038/nrclinonc.2014.51. [Google Scholar] [CrossRef]
Xiao H, Xiong L, Song X, Jin P, Chen L, Chen X, Yao H, Wang Y, Wang L (2017). Angelica sinensis polysaccharides ameliorate stress-induced premature senescence of hematopoietic cell via protecting bone marrow stromal cells from oxidative injuries caused by 5-fluorouracil. International Journal of Molecular Science 18: 2265. DOI 10.3390/ijms18112265. [Google Scholar] [CrossRef]
Xie X, Liu M, Meng Q (2019). Angelica polysaccharide promotes proliferation and osteoblast differentiation of mesenchymal stem cells by regulation of long non-coding RNA H19: An animal study. Bone & Joint Research 8: 323–332. DOI 10.1302/2046-3758.87.BJR-2018-0223.R2. [Google Scholar] [CrossRef]
Yang T, Jia M, Meng J, Wu H, Mei Q (2006). Immunomodulatory activity of polysaccharide isolated from Angelica sinensis. International Journal of Biological Macromolecules 39: 179–184. DOI 10.1016/j.ijbiomac.2006.02.013. [Google Scholar] [CrossRef]
Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L (2003). Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425: 836–841. DOI 10.1038/nature02041. [Google Scholar] [CrossRef]
Zhang K, Yang Y, Ge H, Wang J, Chen X, Lei X, Zhong J, Zhang C, Xian J, Lu Y, Tan L (2020). Artesunate promotes the proliferation of neural stem/progenitor cells and alleviates ischemia-reperfusion injury through PI3K/Akt/FOXO-3a/p27(kip1) signaling pathway. Sedentary Life and Nutrition 12: 8029–8048. [Google Scholar]
Zhang S, He B, Ge J, Li H, Luo X, Zhang H, Li Y, Zhai C, Liu P, Liu X, Fei X (2010). Extraction, chemical analysis of Angelica sinensis polysaccharides and antioxidant activity of the polysaccharides in ischemia-reperfusion rats. International Journal of Biological Macromolecules 47: 546–550. DOI 10.1016/j.ijbiomac.2010.07.012. [Google Scholar] [CrossRef]
Zhang Y, Cheng Y, Wang N, Zhang Q, Wang K (2014). The action of JAK, SMAD and ERK signal pathways on hepcidin suppression by polysaccharides from Angelica sinensis in rats with iron deficiency anemia. Food & Function 5: 1381–1388. DOI 10.1039/c4fo00006d. [Google Scholar] [CrossRef]
Zhang Y, Ye T, Hong Z, Gong S, Zhou X, Liu H, Qian J, Qu H (2019). Pharmacological and transcriptome profiling analyses of Fufang E’jiao Jiang during chemotherapy-induced myelosuppression in mice. Journal of Ethnopharmacology 238: 111869. DOI 10.1016/j.jep.2019.111869. [Google Scholar] [CrossRef]
Zhang Y, Zhou T, Wang H, Cui Z, Cheng F, Wang KP (2016). Structural characterization and in vitro antitumor activity of an acidic polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydrate Polymers 147: 401–408. DOI 10.1016/j.carbpol.2016.04.002. [Google Scholar] [CrossRef]
Zhao KJ, Dong TT, Tu PF, Song ZH, Lo CK, Tsim KW (2003). Molecular genetic and chemical assessment of radix Angelica (Danggui) in China. Journal of Agricultural and Food Chemistry 51: 2576–2583. DOI 10.1021/jf026178h. [Google Scholar] [CrossRef]
Zhao L, Wang Y, Shen HL, Shen XD, Nie Y, Wang Y, Han T, Yin M, Zhang QY (2012). Structural characterization and radioprotection of bone marrow hematopoiesis of two novel polysaccharides from the root of Angelica sinensis (Oliv.) Diels. Fitoterapia 83: 1712–1720. DOI 10.1016/j.fitote.2012.09.029. [Google Scholar] [CrossRef]
Zhuang C, Wang Y, Zhang Y, Xu N (2018). Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from Angelica sinensis. International Journal of Biological Macromolecules 115: 281–286. DOI 10.1016/j.ijbiomac.2018.04.083. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |