DOI:10.32604/biocell.2021.013625

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.013625 |  www.techscience.com/journal/biocell |
| Article |
Immunocytochemical examination of PTEN and Ki-67 for endometrial carcinoma using thin-layer endometrial preparations
1Department of Obstetrics and Gynecology, Beijing Tsinghua Changgung Hospital, Medical Center of Tsinghua University, Beijing, 102218, China
2Department of Gynecology, Beijing Cancer Hospital, Beijing, 100142, China
3Department of Pathology, Beijing Tsinghua Changgung Hospital, Medical Center of Tsinghua University, Beijing, 102218, China
*Address correspondence to: Ke Ma, mka00968@btch.edu.cn
Received: 13 August 2020; Accepted: 21 December 2020
Abstract: The current study is designed to evaluate certain immunocytochemical (ICC) biomarkers to gain a better cytodiagnosis. For this purpose, 85 patients from March 2016 to March 2019 who planned to get a hysteroscopy assay were recruited. Cytological sampling was conducted by scratching the uterus cavity using SAP-1, and the samples were processed as liquid-based smears, using SurePath technology. 36 patients diagnosed with EC or atypical endometrial hyperplasia were recruited in this study. 33 cases were diagnosed with EC, and 3 cases were diagnosed with atypical endometrial hyperplasia, allocated with EC or precancerous lesions group. 26 cases were diagnosed with benign lesions group. Among these cases, 9 cases were diagnosed with endometrial simple hyperplasia, 2 cases were diagnosed with complicated hyperplasia, 5 cases were diagnosed with an irregular proliferation of endometrium and 10 cases were diagnosed with endometrial polyps. There were 23 cases in the healthy group. Staining in thin-layer endometrial preparations by ICC and using H-score or counting the percentage of stained cells. The presentation of PTEN in normal endometrium, benign lesions, and EC/precancerous lesions were different (p < 0.01). Taking the cut-off value of 50 (Youden’s index: 0.698) PTEN expression for the diagnosis of EC/precancerous lesion, the sensitivity and specificity were 83.7% and 86.1%. The presentation of Ki-67 in normal endometrium, benign lesions, and EC/precancerous lesions were different (p < 0.01). Taking the cut-off value of 15% (Youden’s index: 0.76) Ki-67 expression for the diagnosis of EC/precancerous lesion, the sensitivity and specificity were 94.4% and 81.6%. In this study, the use of different cut-off values for Ki-67 and PTEN helped differentiate endometrial lesions. Immunocytochemistry in the ECT detection of PTEN and Ki-67 can improve the diagnostic capabilities of endometrial cancer and precancerous lesions.
Keywords: Immunocytochemical expression; PTEN; Ki-67; Endometrial carcinoma; Thin-layer endometrial preparation
Endometrial carcinoma (EC) is the most common women cancer in the USA. In many developed countries, the incidence rates of EC are higher compared with cervical and ovarian cancers. However, the incidence rates of EC are lower compared with cervical and ovarian cancers (Siegel et al., 2018; Cramer, 2012). In Beijing, uterine corpus cancer has an increasing indication rate due to the introduction of the western lifestyle. Since the prognosis of patients with EC diagnosed at a late stage is very far from ideal, the differential diagnosis of early EC from endometrial hyperplasia is important (Anastasiadis et al., 2000). The abnormal hemorrhage of the uterus is a huge defect the patients often complain about, which implies endometrial sampling (Anastasiadis et al., 2000; Lidor et al., 1986; Montgomery et al., 2004).
EC has been categorized as Type I and Type II cancers. Type I tumors are low-grade EC, which commonly originates in a setting of excessive estrogen and correlates with a favorable prognosis. Type I tumors are generally associated with microsatellite instability and mutations in phosphatase and tensin homolog (PTEN), K-ras, and β-catenin. In comparison, Type II tumors are high-grade undifferentiated or poorly differentiated EC, which have an aggressive biological behavior and hormone-independent nature. Type II tumors often show p53 mutations. In Type I tumors, the transition from healthy endometrial cells to precancerous cells and carcinoma often results from the accumulation of gene abnormalities.
PTEN, with a tumor suppressor on chromosome 10q23, has been found to play a key role in regulating the PTEN-PI3K-AKT pathway. PTEN deficiency has been identified in 30–50% of sporadic EC. Moreover, loss of PTEN has been found to correlate with not only genetic but epigenetic mechanisms. Since a few of these genetic alterations have been discovered in endometrial hyperplasia, these alterations have been regarded as early events in the carcinogenetic process of EC. As a nuclear antigen, Ki-67 has been established as a proliferation marker for cells. In addition, Ki-67 has also been found to be a potential prognostic factor for patients with a variety of solid tumors, including EC. Therefore, it is very necessary to evaluate this gene expression in normal endometrium, precancerous endometrium, and cancerous tissues. Lately, liquid-based cytology has rapidly developed into a promising cell preparation technology, and some studies have shown that they can achieve the same sensitivity as conventional cytological preparations. Several recent studies have reported the diagnostic potential of endometrial liquid-based cytology. Cytology can be used as an alternative diagnostic method (Norimatsu et al., 2006). Recently, we have used Ki-67 and PTEN biomarkers to help us make a more accurate diagnosis.
Our study was aimed to employ immunocytochemical criteria in pre-operative cytological aspirates. Nevertheless, results are from intrauterine cell samples examination prior to hysterectomy directly, which are necessary for validating the reproducibility of the diagnostic criteria derived from endometrial thin-layer endometrial preparations.
The purpose of the current study was to evaluate PTEN and Ki-67 as biomarkers in liquid-based cytology for the detection of endometrial carcinoma cells and precursors.
A total of 85 patients were recruited in the current study, and all of them had received hysteroscopy between March 2016 and March 2019. This study was performed in the Department of Beijing Tsinghua Changgung Hospital (Beijing, China). The Institutional Review Board of the Beijing Tsinghua Changgung Hospital approved this study’s agreement. After provided informed consent, all the patients proceeded sequentially to endometrial cytological sampling, hysteroscopy, and diagnostic and/or therapeutic D&C. Cytology sampling from the uterus cavity was conducted using SAP-1 and then processed to liquid-based smear using SurePath technology before stained by the Papanicolaou method. The correlations between cytological examinations and the D&C histological diagnosis were analyzed. The age of patients ranged from 22 to 75, with a median age of 48 years. 36 cases of endometrial cancer and atypical endometrial hyperplasia were in the experiment, in which 33 cases of endometrial cancer, 3 cases of endometrial atypical hyperplasia, as endometrial cancer or precancerous lesions group. 26 cases were as a benign lesions group with 9 cases of simple endometrial hyperplasia, 2 cases of complex hyperplasia, 5 cases of the irregular proliferation of endometrial, and 10 cases of endometrial polyps. 23 cases were as a healthy group in which 14 cases of proliferative phase, 7 cases of secretory phase, and 2 cases of an atrophic endometrium.
Cytological and histological preparations
Collection of endometrial cytological samples were conducted using SAP-1 device (Saipujiuzhou, Beijing, China; Fig. 1), which is patented and approved to be used in China. The SAP-1 sampler has a size with a diameter of 3 mm and a length of 250 mm, which also has a flexible latex ring with thorns on the side. For avoiding myometrial damage, it is also equipped with a smooth tip. The loop is also covered with a protective sheath. Following release and rotation clockwise and anticlockwise, the loop was used to collect samples at curette edges. The loop then was pulled back into the sheath to avoid cervical contamination. Following removal of the device from the uterine cavity, the specimen attached to the loop was pulled out and put in SurePath (BD Diagnostic, Burlington, NC, USA) vial. Then the vial was shaken vigorously to release specimen cells. Following labeling with patient information, the vial was transferred to the Department of Cytopathology. Centrifugation and suspension were then conducted to achieve mucolysis and hemolysis. Meanwhile, the endometrial cells were separated to achieve blood and mucus. Finally, use AutoCyte PREP automated slide processor (Tri-Path Corporation, USA) to process the vial. Next, stain the slides with routine Papanicolau (Pap) stain.
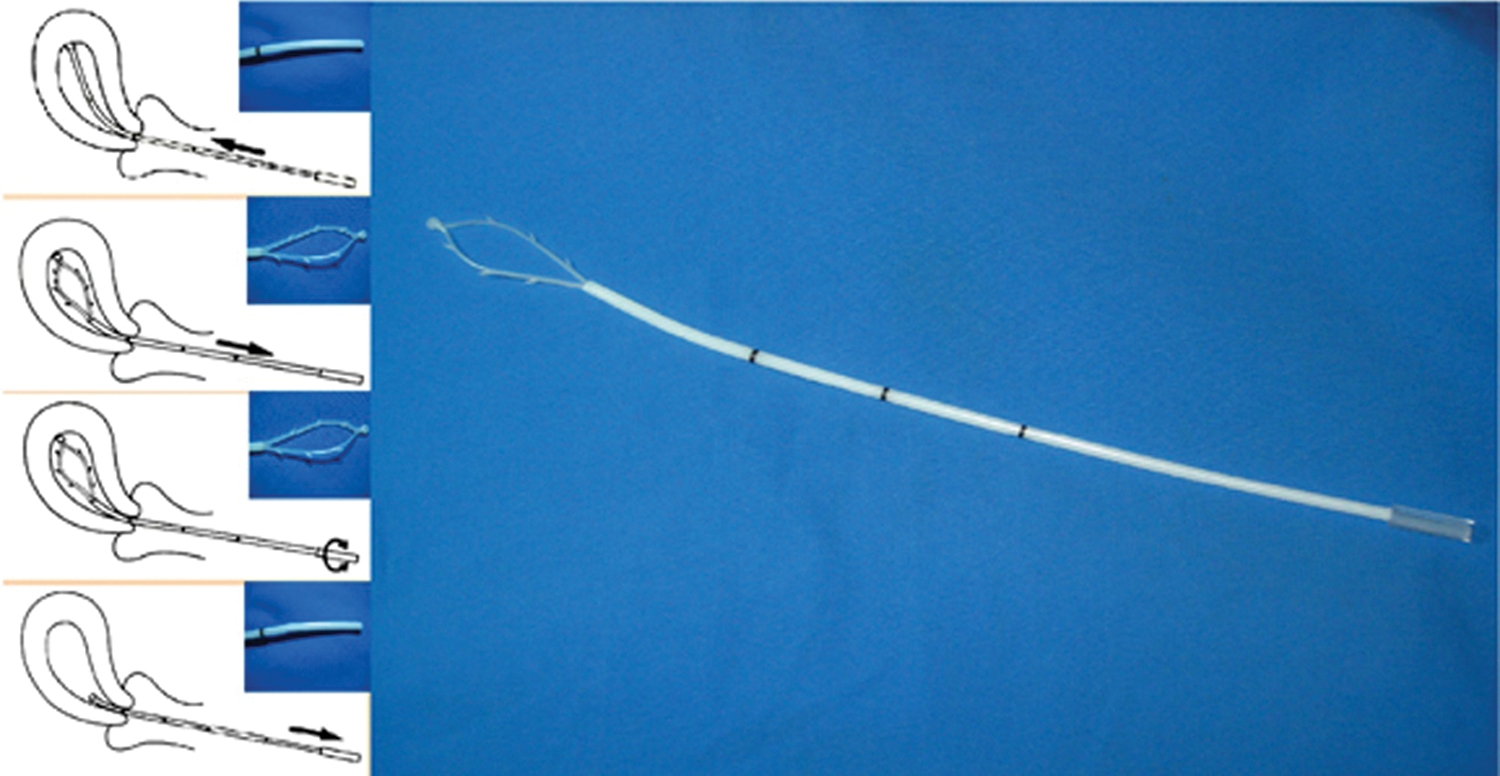
Figure 1: The SAP-1 device.
Following collection of cytological samples, use a 4-mm optic with saline solution distension to conduct hysteroscopy. Collection of histologic samples was conducted via D&C. The endometrial sample was fixed in neutral buffer, then hematoxylin and eosin were used to embed and stain the paraffin.
Two cyto-histo-gynecopathologists made cytological and histological diagnoses blindly. The samples were regarded as unqualified. When the first cytological or histological slide was unqualified, a second one would be used. In the case that both the first and the second sample slides were unqualified, it was taken as “unqualified diagnosis”.
Based on a published diagnostic system, the cytological criteria were used for cellular interpretation (Maksem et al., 2007; Zhao, 2006) The cytological results were classified into 4 groups: normal endometrium, benign endometrial abnormality, atypical endometrial cell, and suspected endometrial carcinoma (Figs. 2A–2D). World Health Organization (WHO) criteria in 2003 were used to make the histological diagnosis (Tavassoli and Devilee, 2003). Analyze the correlation between cytological diagnosis and D&C histological diagnosis.
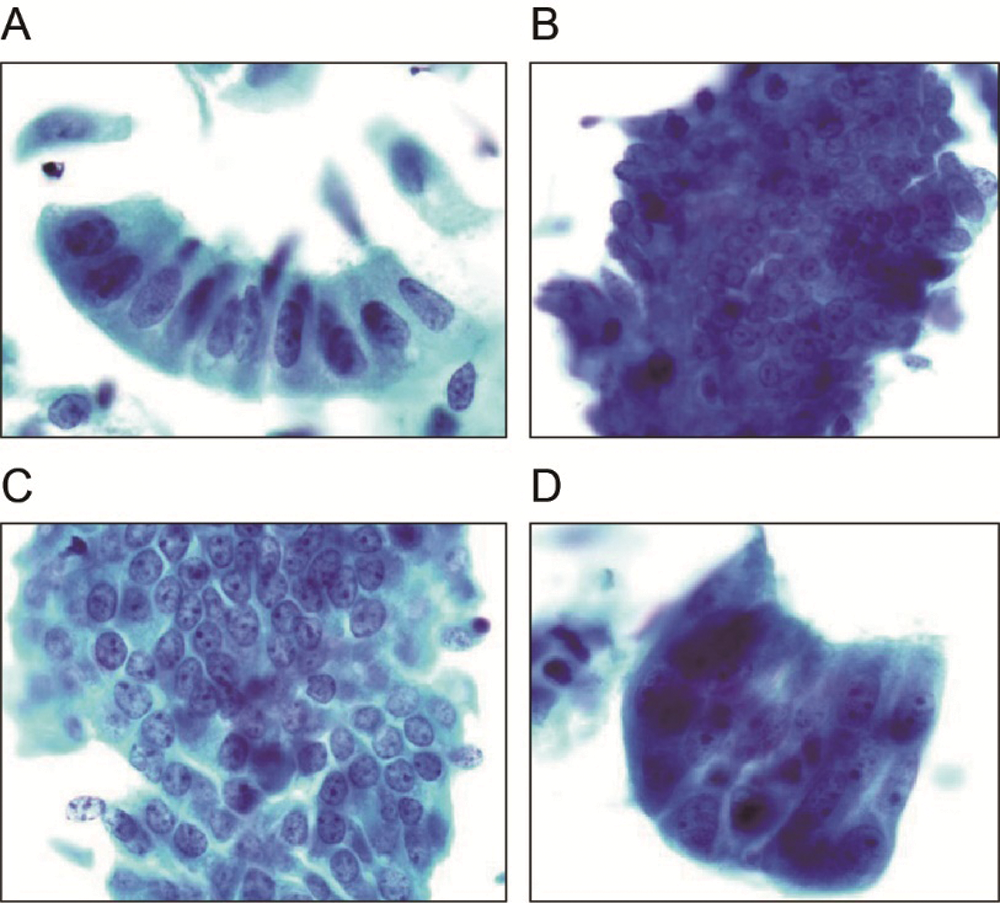
Figure 2: Liquid-based endometrial cytology (Papanicolaou stain; original magnification 100×).
Immunohistochemistry and immunocytochemistry
For immunohistochemistry, the paraffin sections were processed and placed in the distilled water as conventional protocols. Afterward, the paraffin sections were washed three times in PBS before blocking with 3% peroxide-methanol at room temperature. All the following experiments were carried out in a moist chamber: (1) The sections were incubated with EDTA in a microwave oven for 15 min. (2) The specimens were rinsed three times in PBS (3) and then incubated overnight with antibodies against PTEN (6H2.1, Dako, Herndon, VA, USA) and Ki-67 (MIB-1, Zhongshan, Beijing, CN) at dilutions 1:100 and 1:100, respectively. (4) The specimens were rinsed four times with PBS. (5) The ChemMateTM EnVisionTM/HRP (Dako, Herndon, VA, USA) was added, and the specimens were incubated for a half-hour at 37°C. (6) The specimens were rinsed four times with PBS. (7) The color was demonstrated with 3,3-diaminobenzidine (DAB) and was maintained at room temperature in the dark for 3 min. (8) The staining process was ceased by rinsing with distilled water. (9) The specimen was then stained with hematoxylin. (10) The specimens were dehydrated, cleared, and mounted with neutral gums. Positive controls were also stained at the same time.
For immunocytochemistry, immediately in 95% alcohol was used to fix the cytological imprint smears, 50% alcohol, double distilled water successively 5 min. Cytological smears were processed and placed in the distilled water as conventional protocols. Afterward, Rinsing the paraffin sections (3 × 3 min) in PBS and then blocked with 3% peroxide-methanol at room temperature for endogenous peroxidase ablation. Afterward, the paraffin sections were washed three times in PBS before blocking with 3% peroxide-methanol at room temperature. All the following experiments were carried out in a moist chamber: (1) The sections were incubated with EDTA at 60°C for 10 min. (2) The specimens were rinsed three times in PBS, and then (3) incubated overnight in PTEN (6H2.1, Dako, Herndon, VA, USA) and Ki-67 (MIB-1, Zhongshan, Beijing, China) antibodies, at dilutions 1:100 and 1:100, respectively. (4) Rinsing in PBS (4 × 2 min). (5) The ChemMateTM EnVisionTM/HRP (Dako, Herndon, VA, USA) was added, and the specimens were incubated for a half-hour at 37°C. (6) The specimens were rinsed four times with PBS. (7) Coloration with 3,3-diaminobenzidin (DAB), kept at room temperature without light for 1 min. (8) The staining process was ceased by rinsing with distilled water. (9) The specimen was then stained with hematoxylin. (10) The specimens were dehydrated, cleared, and mounted with neutral gums. Positive controls were also stained at the same time. Negative controls were also stained without the primary antibody.
PTEN expression levels were evaluated based on staining intensity (i) and the percentage of positive cells (pi) within the whole tissue section. PTEN staining was evaluated using the H-score = pi (i + 1) × 100, which was calculated by multiplying the pi (0–100%) by the corresponding i (0 = negative, 1 = weak, 2 = moderate, and 3 = strong).
The ki-67 score was the percentage of positively stained nuclei scored. The rate of positively stained nuclei within five high-powered fields (40× magnification) randomly selected across the tumor was calculated. Take the average value for semi-quantitative analysis. Ki-67 and PTEN biomarkers are adjuncts to cytomorphology. The immunocytochemical findings with immunohistochemistry (IHC) are discussed.
Statistical analyses were performed using SPSS (Chicago, IL, USA) 13.0. Spearman’s correlation calculated the associations between biomarker (PTEN and Ki-67) alterations in Immunohistochemistry and Immunocytochemistry. The Kruskal-Wallis test estimated the associations between biomarker (PTEN and Ki-67) alterations and clinicopathological features. The ROC curve was used to evaluate the value of the two indicators in the diagnosis of endometrial carcinoma, and the Chi-square and Fisher’s exact test calculated precancerous lesions. The associations between different ICC scores and clinicopathological features.
The expression of Ki-67 and PTEN in the endometrium
Under light microscopy, Ki-67 was widely expressed in endometrial gland cells and interstitial cells, mainly expressed in the nucleus, and the positive staining was brownish yellow, and the staining was granulated (Fig. 3). The percentage of Ki-67 positive in gland cells was calculated, and the semi-quantitative results of Ki-67 expression in ICC and IHC are shown in Tab. 1. Spearman’s rank correlation test, rs = 0.888, p < 0.001, the Spearman correlation coefficient of Ki-67 expression is positive in ICC and IHC (Fig. 4).
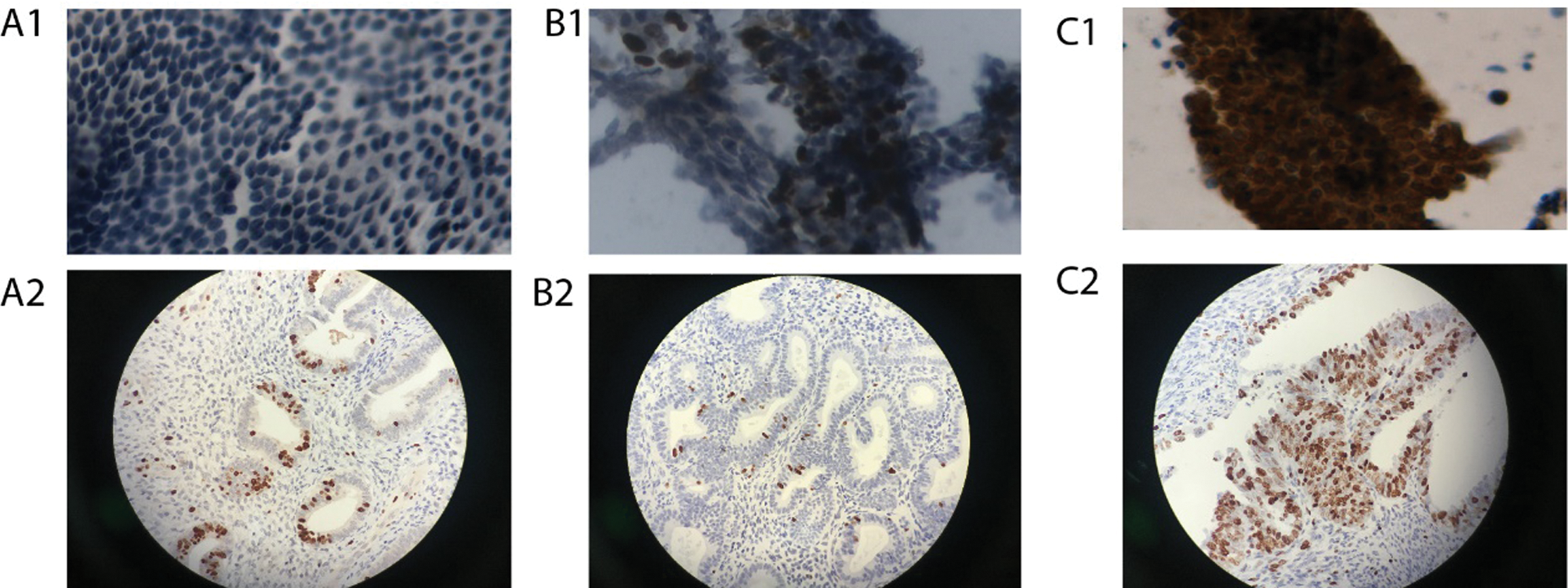
Figure 3: Immunoreactivity for Ki-67 in ICC and IHC (Papanicolaou stain; original magnification 400×).
Table 1: Expression of Ki-67 and PTEN in ICC and IHC
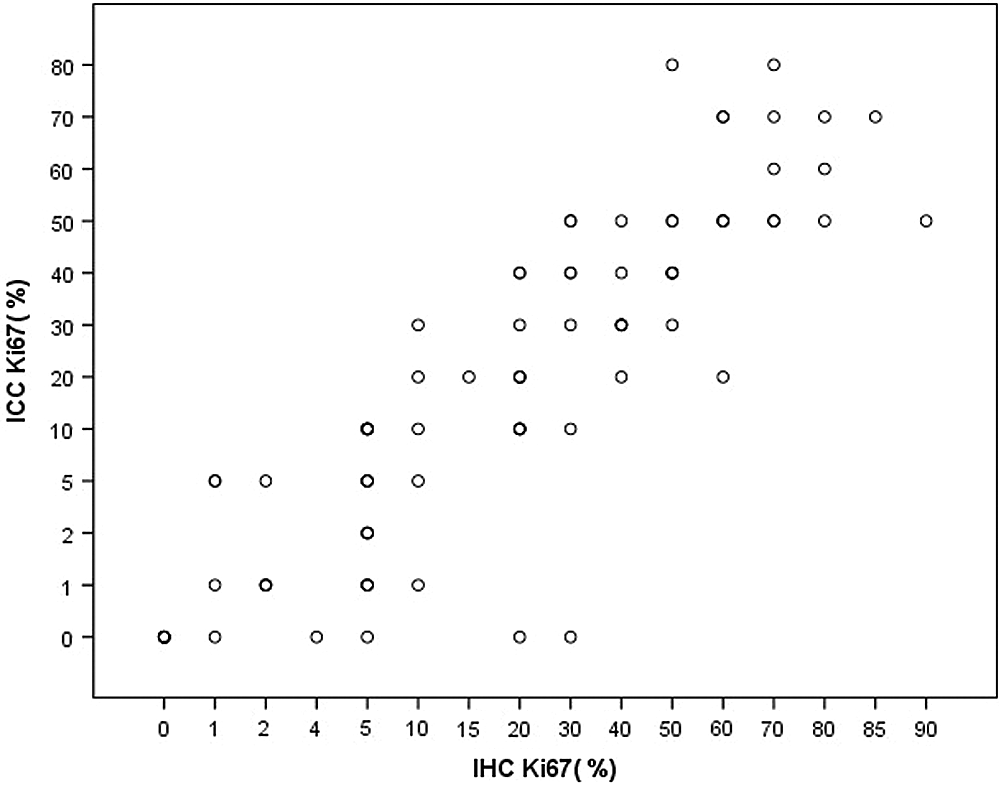
Figure 4: IHC and ICC scatter plot of Ki-67 expression in the endometrium.
The PTEN protein had both a cytoplasmic and nuclear localization. Positive PTEN signals in tumors were heterogeneous with different intensities. PTEN expression levels were evaluated based on staining intensity (i) and the percentage of positive cells (pi) within the whole tissue section. PTEN staining was evaluated using the H-score = pi (i+1) × 100, which was calculated by multiplying the pi (0–100%) by the corresponding i (0 = negative, 1 = weak, 2 = moderate, and 3 = strong) (Fig. 5). The score of PTEN expression in ICC and IHC are shown in Tab. 1. Spearman’s rank correlation test, rs = 0.868, p < 0.001, the Spearman correlation coefficient of PTEN expression is positive in ICC and IHC (Fig. 6).
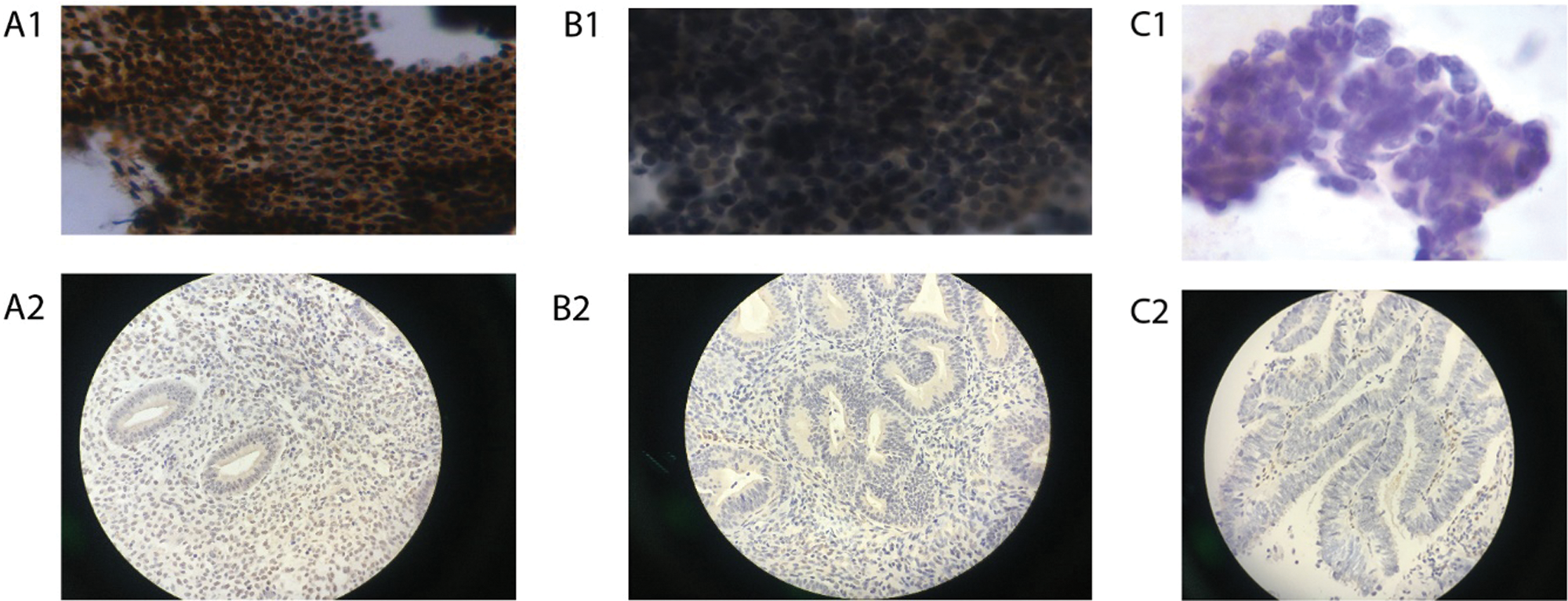
Figure 5: Immunoreactivity for PTEN in ICC and IHC (Papanicolaou stain; original magnification 400×).
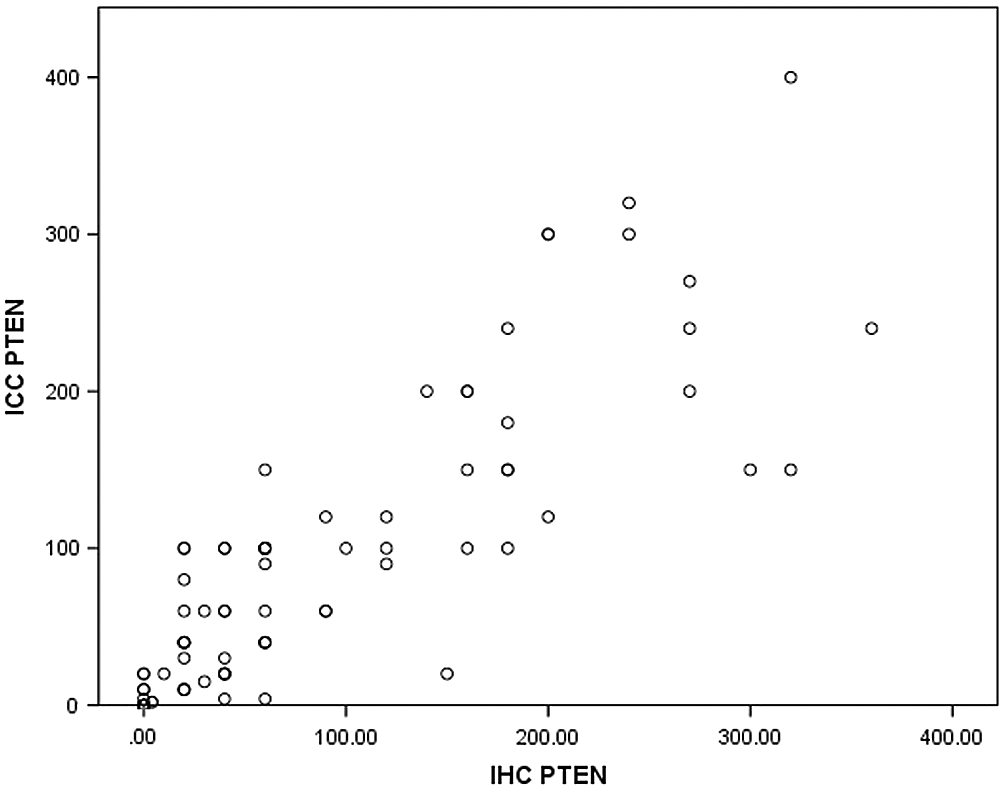
Figure 6: IHC and ICC scatter plot of PTEN expression in the endometrium.
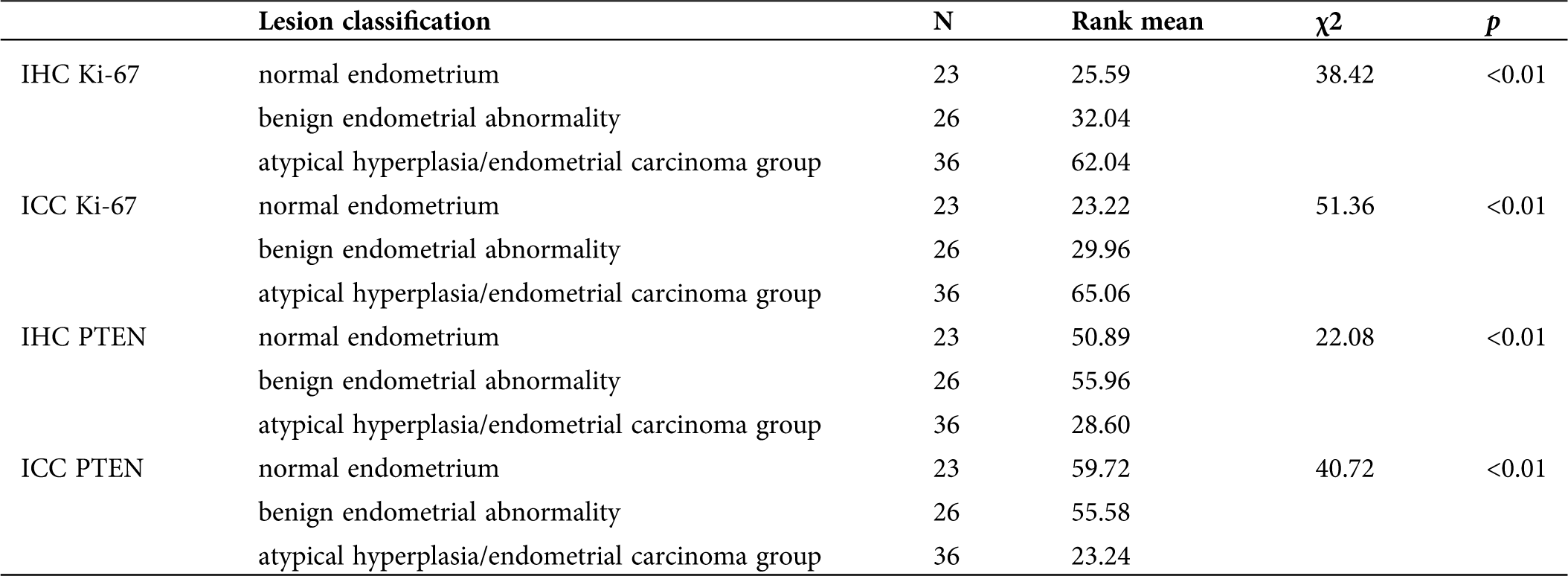
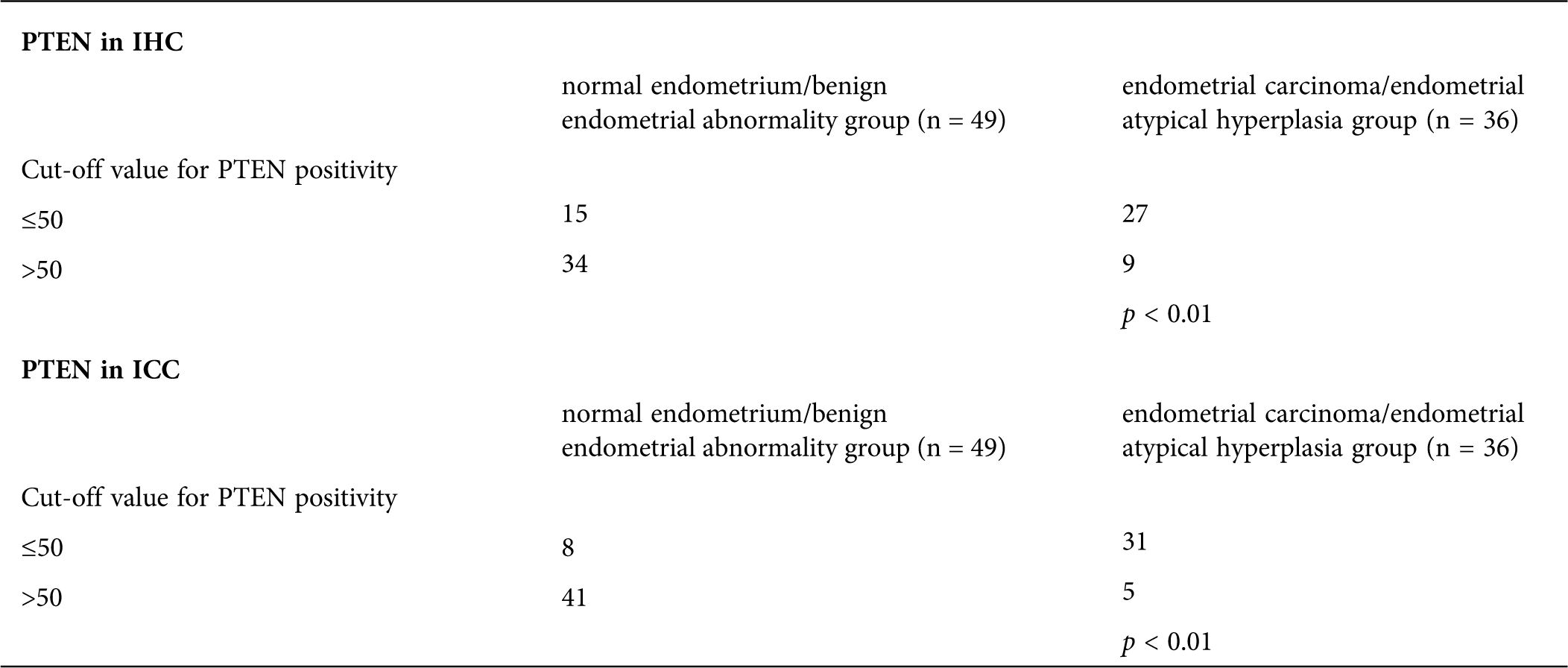
Comparison of Ki-67 and PTEN expression in the normal endometrium group, benign endometrial abnormality group and atypical hyperplasia/endometrial carcinoma group
Whether using IHC or ICC detection methods, Ki-67 and PTEN have significant differences in expression in the normal endometrium group, benign endometrial abnormality group and atypical hyperplasia/endometrial carcinoma group (Tab. 2; Figs. 3 and 5).
Table 2: Expression of Ki-67 and PTEN in each group of ICC and IHC
Determination of the optimal cut-off value for Ki-67 PTEN for the diagnosis of endometrial carcinoma and endometrial atypical hyperplasia
To determine the cut-off value with the best diagnostic efficiency, we performed a ROC curve analysis to determine the optimal cut-off value.
In the Ki-67 group, after the ROC curve analyses (Fig. 7), we determined that 12.5% and 15% were the optimal cut-off value in IHC and ICC (Tab. 3). The sensitivity of Ki-67 in the diagnosis of endometrial carcinoma and endometrial atypical hyperplasia was 94.4% and 94.4% for the cut-off values of 12.5% and 15%. The specificity of the above-mentioned cut-off values was 67.3% and 81.6%.
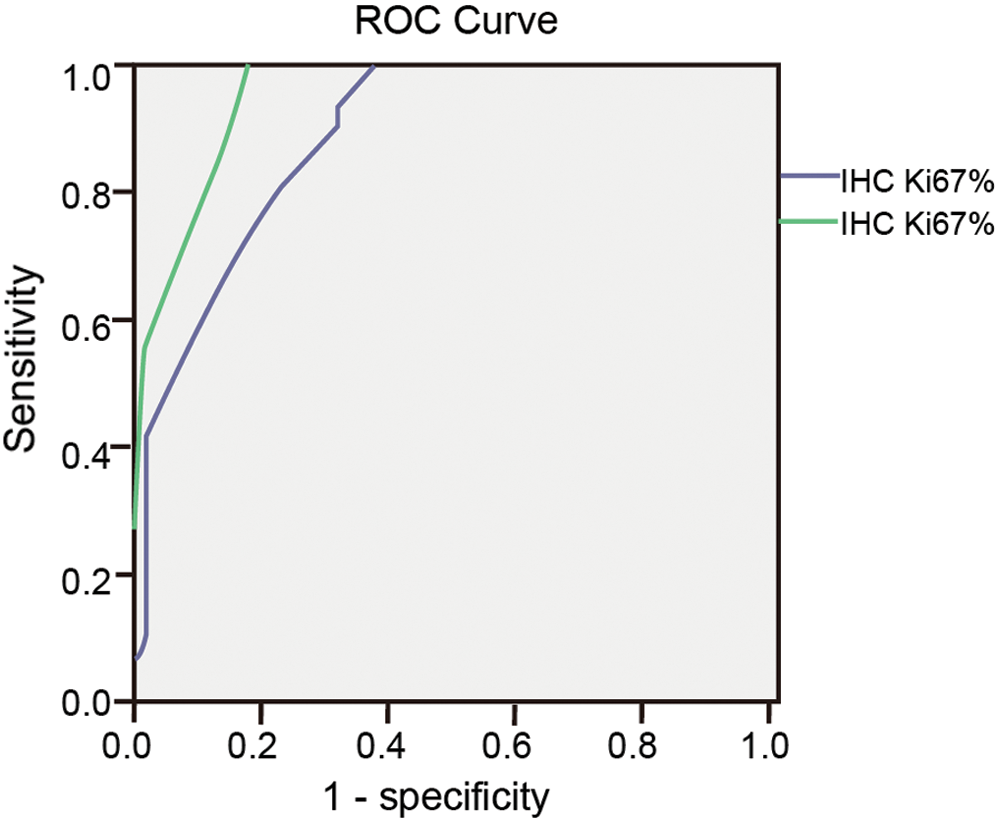
Figure 7: ROC curve of Ki-67 in IHC and ICC.
The Youden indices were 0.617 and 0.76 for the cut-off values (Tab. 3). The area under the ROC curve was 0.889 and 0.950. For Ki-67 at cut-off values 12.5% and 15% in IHC and ICC, the difference between endometrial carcinoma/endometrial atypical hyperplasia group and normal endometrium/benign endometrial abnormality group was statistically significant (p < 0.01 and p < 0.01; Tab. 4).
Table 3: Sensitivity, specificity and Youden’s index of Ki-67% taking different cutoff values in IHC and ICC
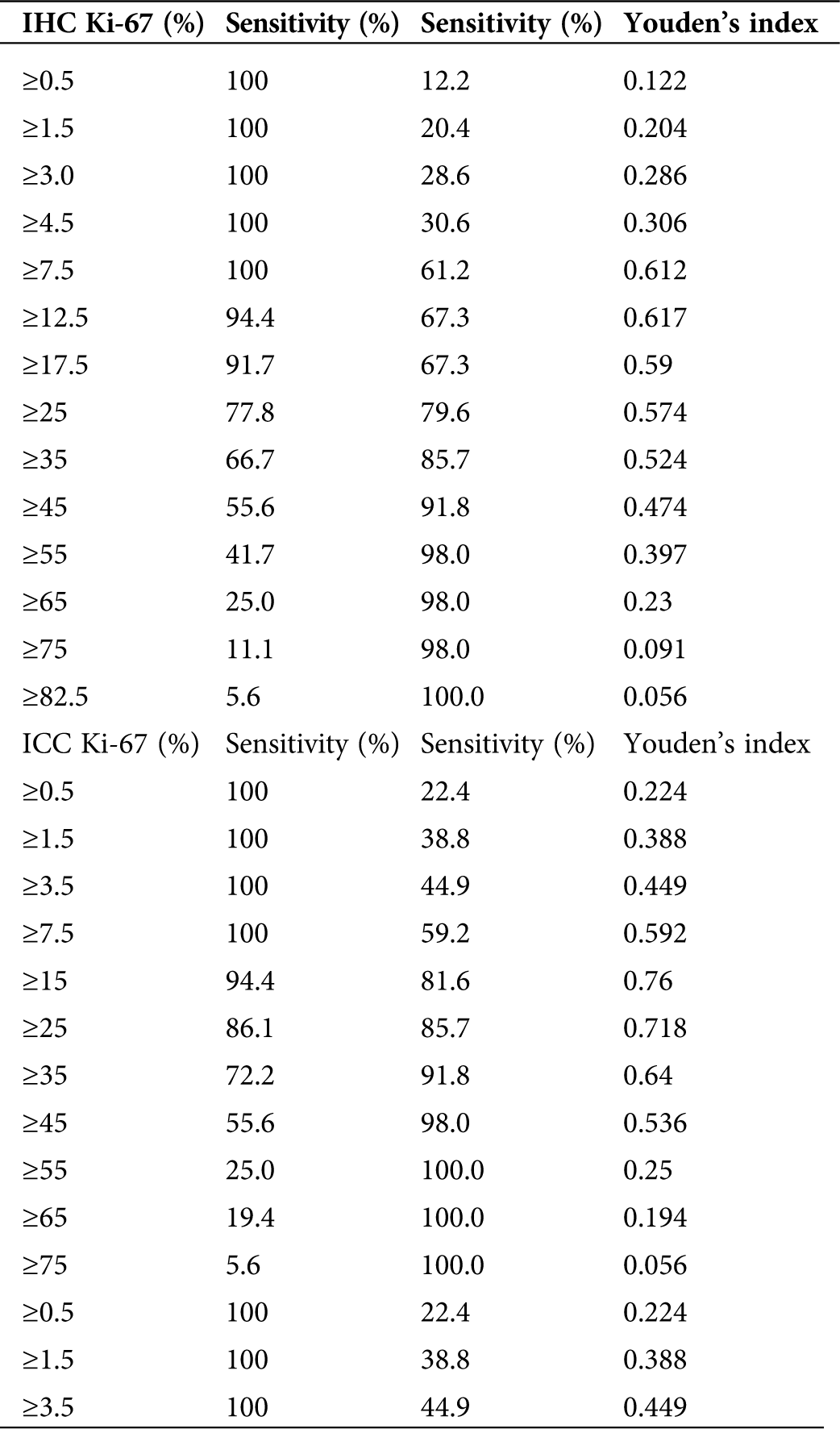
Table 4: The cut-off values for Ki-67 in IHC between endometrial carcinoma/endometrial atypical hyperplasia group and normal endometrium/benign endometrial abnormality group
In the PTEN group, after the ROC curve analyses (Fig. 8), we determined that 50 and 50 were the optimal cut-off value in IHC and ICC (Tab. 5). The sensitivity of PTEN in the diagnosis of endometrial carcinoma and endometrial atypical hyperplasia was 69.4% and 83.7% for the cut-off values of 50 and 50. The specificity of the above-mentioned cut-off values was 75% and 86.1%.
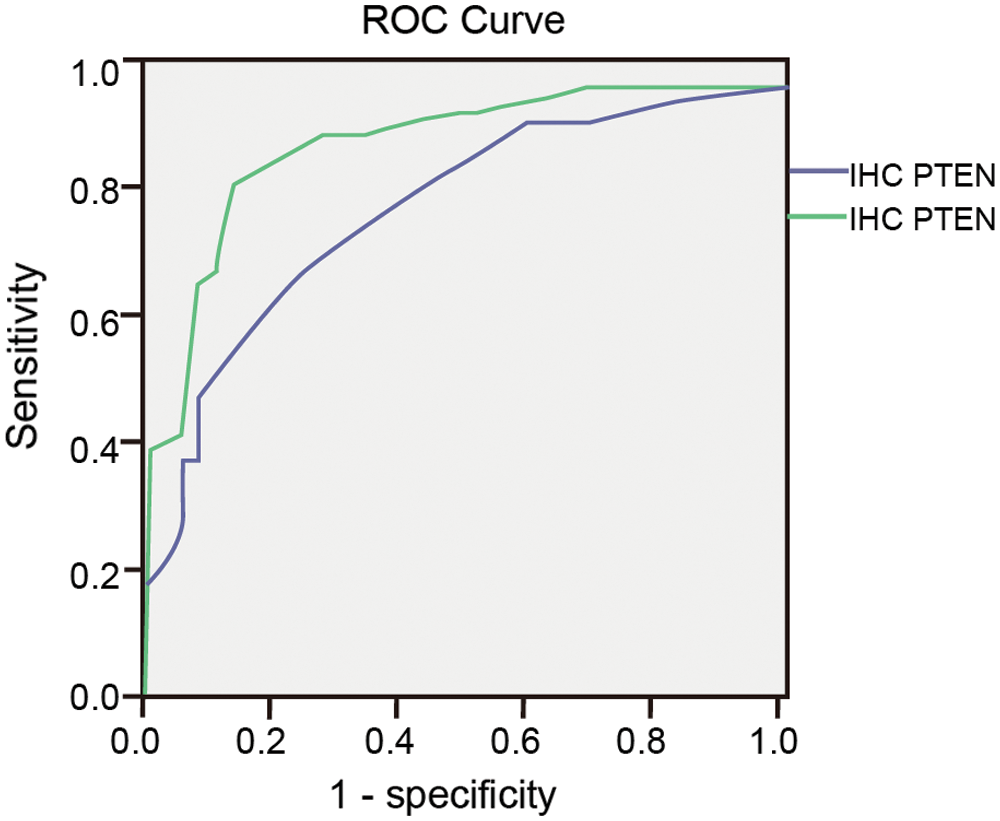
Figure 8: ROC curve of Ki-67 in IHC and ICC.
Table 5: Sensitivity, specificity and Youden’s index of PTEN taking different cut-off values in IHC and ICC
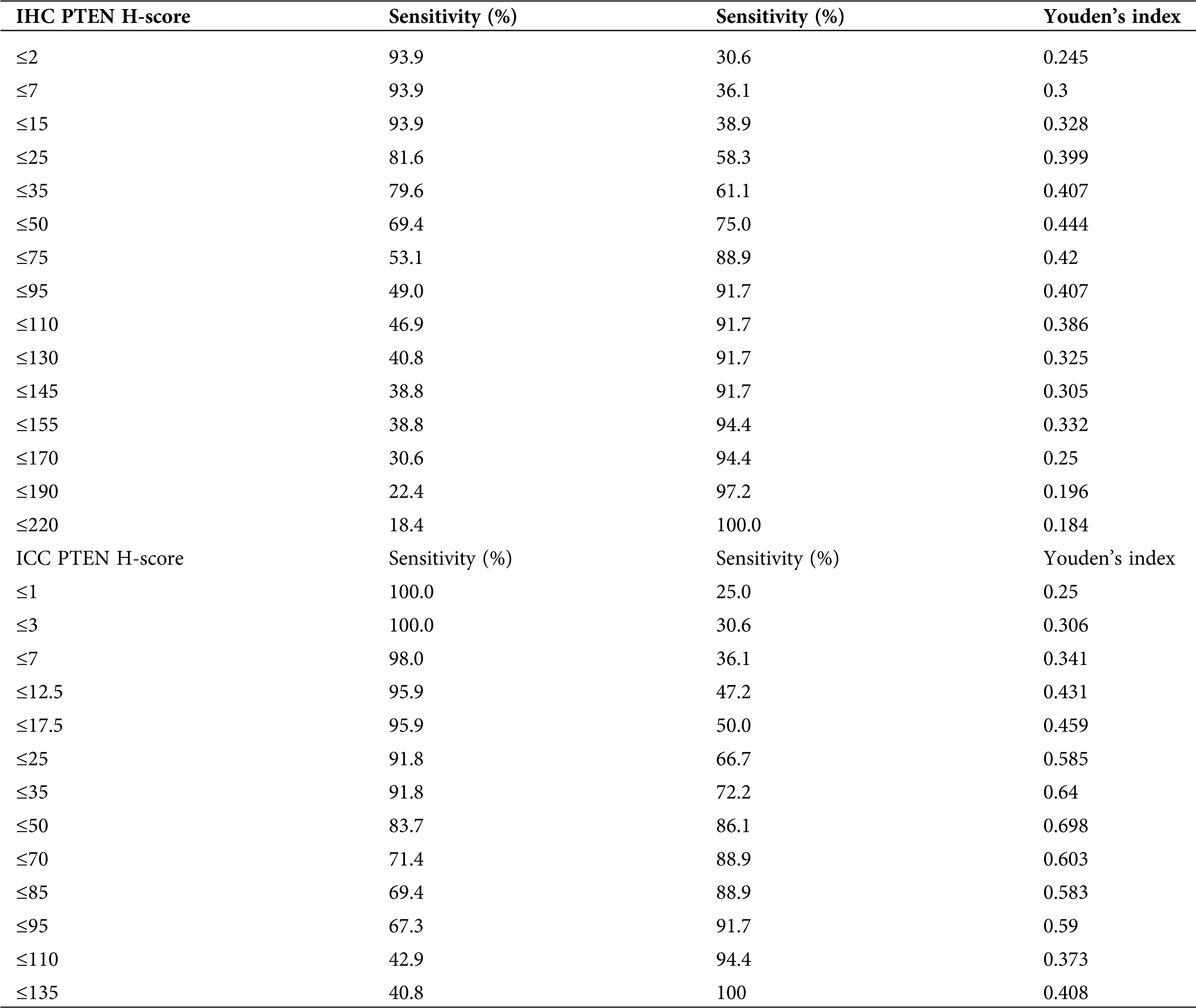
The Youden indices were 0.444 and 0.698 for the cut-off values (Tab. 5). The area under the ROC curve was 0.794 and 0.903.
For PTEN at cut-off values 50 and 50 in IHC and ICC, the difference between the endometrial carcinoma/endometrial atypical hyperplasia group and normal endometrium/benign endometrial abnormality group was statistically significant (p < 0.01 and p < 0.01; Tab. 6)
Table 6: The cut-off values for PTEN in IHC and ICC between endometrial carcinoma/endometrial atypical hyperplasia group and normal endometrium/benign endometrial abnormality group
Endometrial cancer (EC) is a major female reproductive tract malignant tumor in China. In the next few years, this will become an important public health issue. In the United States and Europe, the incidence is increasing, and regular screening of high-risk women will become an important intervention. EC screening is particularly challenging, and there are currently no effective programs to monitor women who are estimated to have a high incidence for this cancer. We have researched endometrial cytology screening for endometrial cancer (Yang et al., 2017; Ma et al., 2016).
Cytological diagnosis of endometrial lesions provides an effective substitution for biopsy, which may help obtain materials to study prognostic and predictive markers and minimize patient discomfort.
Although cervical cancer is more common than EC, its prevalence is gradually reduced by cytology screening. On the contrary, the incidence of EC is on the rise. It is estimated that in developed countries that undergo cervical cancer screening, women who die of EC is about twice those who die of cervical cancer (Siegel et al., 2014). Although cervical cancer screening strategies continue to improve, the use of cytology techniques to screen EC has not been widely accepted by the medical community. Epidemiological data in Japan show that the total mortality rate of EC has dropped from 20 per 100,000 in 1950 to 8 per 100,000 in 1999. This is considered the result of cytological screening and has been widely accepted (Nagase et al., 2004).
LBC, approved for cervical cancer screening by the U.S. Food and Drug Administration in 1996, offers considerable superiorities in improving sample quality. Liquid-based technology has great value in the evaluation of endometrial cells. In this case, the factors that obscure the specimen are more likely to occur and significantly affect the conventional cytological evaluation. Many studies had shown that the diagnostic accuracy of endometrial cytology was improved by liquid-based techniques. Overall, cumulative data on nearly 4000 patients showed a sensitivity ranging from 78% to 100%, specificity from 95% to 100%, PPV from 78 to 100%, and NPV from 96 to 100% (Garcia et al., 2003; Buccoliero et al., 2003; Papaefthimiou et al., 2005; Buccoliero et al., 2007a; Buccoliero et al., 2007b; Kipp et al., 2008; Fambrini et al., 2008; Buccoliero et al., 2008; Remondi et al., 2013; Yang et al., 2013). In our research, atypical hyperplasia or worse were acted as a positive result; the diagnostic accuracy of liquid-based endometrial cytology was 86.1%, sensitivity at 70.3%, specificity at 88.5%, PPV at 48.0%, and NPV at 95.2% (Yang et al., 2017).
Except for giving a morphological diagnosis, LBC can also be used to test molecular changes in residual substances. Based on the literature and our daily experience, at the cellular level, it is challenging to evaluate simple or complex hyperplasia typical or atypical, but we have also demonstrated the possibility of testing LBC molecular techniques, making cytology a unique diagnostic tool. The treatment of uterine hyperplasia is still a clinical dilemma because atypical hyperplasia usually requires a hysterectomy. In our research, we use immunocytochemistry to increase the accuracy of diagnosis to determine which ones are at risk of cancer.
In the present study, we examined our residual material archived from 2016 to 2019 in cytology and histology to assess ki-67 and PTEN express.
Proliferation is essential for the circulating endometrium and is related to the expression of Ki-67, which is positive at all stages of the cell cycle (except G0) and reflect the growth fraction of tumor lesions. Nowadays, the difference in the healthy endometrium/benign endometrial abnormality group and endometrial carcinoma/endometrial atypical hyperplasia group endometrium for different Ki-67 cut-off values proved to be statistically significant.
PTEN is a tumor suppressor gene that is located on chromosome 10q23. PTEN plays an important role in regulating the PTEN-PI3K-Akt pathway. PTEN loss is detected in 30–50% of sporadic endometrial cancers and has been shown to occur through both genetic and epigenetic pathways. Many of these molecular changes seem to accompany early endometrial canceration because they are common in endometrial hyperplasia.
The presentation of Ki-67 and PTEN in normal endometrium, benign lesions, and EC/precancerous lesions were different (p < 0.01). For Ki-67 and PTEN immunoreactivity, the cut-off value of 15 in Ki-67 and the cut-off value of 50 in PTEN were useful in differentiating benign endometrial abnormality group from the endometrial carcinoma/endometrial atypical hyperplasia group. Due to the continuous enrichment of carcinogenic related knowledge, molecular technology will become an effective method to replace unnecessary hysterectomy. In some cases, progesterone therapy can cause regression of the disease, so cytology can be used to track these women.
Apostolou et al. (2014) and Norimatsu et al. (2008) proposed a scoring system by combining the immunocytochemical assessment of KI-67 and P53 using cytological imprints obtained from the removed uterus. The final score is useful for diagnosis. Norimatsu et al. (2008) and Apostolou et al. (2014) have shown that LBC plays an important role in the immunocytochemical expression of PTEN, b-catenin, and p53 in EC samples. In high-risk patients, endometrial cytology screening seems to be an effective option, which is due to the possibility of finding molecular changes.
Endometrial cytology is an efficient method for detecting cancer and precancerous conditions. We proved that it is possible to apply molecular techniques on endometrial, archived LBC.
Generally speaking, the low detection rate of small samples, high diagnostic accuracy, and NPV are basic demands for screening tests. Besides, other elements of screening tests are retained by endometrial LBC, such as a more reasonable cost compared to liquid-based Pap test, remarkable acceptability, and ease of implementation in an office environment. It is estimated that in the next few years, EC can be a serious common health problem, and there will be no reliable means to monitor individuals who develop this cancer and are at increased risk. Combined with lifestyle changes and prevention of risk factors, we believe that regular screening of high-risk women will become the top priority of interventions for the next generation.
The results of this study help to improve the accuracy of cell diagnosis of endometrial disease. The application of immunocytochemistry research results may be extended to the report of endometrial samples, which is a very intricate area in cytology. The results need to be determined under clinical guidance to determine the repeatability of diagnostic criteria.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Author Contribution: The authors confirm contribution to the paper as follows: Study conception and design: Xi Yang, Ke Ma, Rui Chen, Naiyi Zhang, Yiting Meng, Jia Wen, Qinping Liao; data collection: Xi Yang, Ke Ma, Rui Chen, Naiyi Zhang, Yiting Meng, Jia Wen, Qinping Liao; analysis and interpretation of results: Xi Yang, Ke Ma, Rui Chen, Naiyi Zhang, Yiting Meng, Jia Wen, Qinping Liao; draft manuscript preparation: Xi Yang, Ke Ma, Rui Chen, Naiyi Zhang, Yiting Meng, Jia Wen, Qinping Liao. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: This study and all experiments involved are under approval of The Ethical Committee of Beijing Tsinghua Changgung Hospital, with File Code: 17134-0110 in August 31, 2017.
Funding Statement: This work was supported by Beijing Municipal Administration of Hospitals Incubating Program, Code: PX2018039; Beijing Talents Foundation Youth backbone individual program; Beijing Tsinghua Changgung Hospital Fund (Grant No. 120151003)
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Anastasiadis PG, Skaphida PG, Koutlaki NG, Galazios GC, Tsikouras PN, Liberis VA (2000). Descriptive epidemiology of endometrial hyperplasia in patients with abnormal uterine bleeding. European Journal of Gynaecological Oncology 21: 131–134. [Google Scholar]
Apostolou G, Apostolou N, Nikolaidou C, Kavantzas N, Patsouris E, Athanassiadou P (2014). Cytodiagnosis of endometrial carcinoma and hyperplasia on imprint smears with additional immunocytochemistry using Ki-67 and p53 biomarkers. Cytopathology 25: 86–94. DOI 10.1111/cyt.12095. [Google Scholar] [CrossRef]
Buccoliero AM, Caldarella A, Noci I, Borri P, Giachi M, Borrani E, Taddei GL (2003). Thin-layer cytology in endometrial diagnosis. Pathologica 95: 179–184. [Google Scholar]
Buccoliero AM, Castiglione F, Gheri CF, Garbini F, Fambrini M, Bargelli G, Pappalardo S, Scarselli G, Marchionni M, Taddei GL (2007a). Liquid-based endometrial cytology: Its possible value in postmenopausal asymptomatic women. International Journal of Gynecologic Cancer 17: 182–187. DOI 10.1111/j.1525-1438.2006.00757.x. [Google Scholar] [CrossRef]
Buccoliero AM, Fambrini M, Gheri CF, Castiglione F, Garbini F, Barbetti A, Rossi Degl’Innocenti D, Moncini D, Taddei A, Bargelli G, Scarselli G, Marchionni M, Taddei GL (2008). Surveillance for endometrial cancer in women on tamoxifen: The role of liquid-based endometrial cytology-cytohistological correlation in a population of 168 women. Gynecologic and Obstetric Investigation 65: 240–246. DOI 10.1159/000113047. [Google Scholar] [CrossRef]
Buccoliero AM, Gheri CF, Castiglione F, Garbini F, Barbetti A, Fambrini M, Bargelli G, Pappalardo S, Taddei A, Boddi V, Scarselli GF, Marchionni M, Taddei GL (2007b). Liquid-based endometrial cytology: Cyto-histological correlation in a population of 917 women. Cytopathology 18: 241–249. DOI 10.1111/j.1365-2303.2007.00463.x. [Google Scholar] [CrossRef]
Cramer DW (2012). The epidemiology of endometrial and ovarian cancer. Hematology/Oncology Clinics of North America 26: 1–12. DOI 10.1016/j.hoc.2011.10.009. [Google Scholar] [CrossRef]
Fambrini M, Buccoliero AM, Bargelli G, Cioni R, Piciocchi L, Pieralli A, Andersson KL, Scarselli G, Taddei G, Marchionni M (2008). Clinical utility of liquid-based cytology for the characterization and management of endometrial polyps in postmenopausal age. International Journal of Gynecologic Cancer 18: 306–311. DOI 10.1111/j.1525-1438.2007.01019.x. [Google Scholar] [CrossRef]
Garcia F, Barker B, Davis J, Shelton T, Harrigill K, Schalk N, Meyer J, Hatch K (2003). Thin-layer cytology and histopathology in the evaluation of abnormal uterine bleeding. Journal of Reproductive Medicine 48: 882–888. DOI 10.1023/B:JARG.0000006710.64788.99. [Google Scholar] [CrossRef]
Kipp BR, Medeiros F, Campion MB, Distad TJ, Peterson LM, Keeney GL, Halling KC, Clayton AC (2008). Direct uterine sampling with the Tao brush sampler using a liquid-based preparation method for the detection of endometrial cancer and atypical hyperplasia: A feasibility study. Cancer 114: 228–235. DOI 10.1002/cncr.23636. [Google Scholar] [CrossRef]
Lidor A, Ismajovich B, Confino E, David MP (1986). Histopathological findings in 226 women with post-menopausal uterine bleeding. Acta Obstetricia et Gynecologica Scandinavica 65: 41–43. DOI 10.3109/00016348609158227. [Google Scholar] [CrossRef]
Ma K, Yang X, Chen R, Zhao J, Dong Y, Zhang NY, Ma XH, Liao QP (2016). Liquid-based endometrial cytology associated with curettage in the investigation of endometrial carcinoma in postmenopausal women. Taiwanese Journal of Obstetrics and Gynecology 55: 777–781. DOI 10.1016/j.tjog.2015.09.006. [Google Scholar] [CrossRef]
Maksem JA, Meiers I, Robboy SJ (2007). A primer of endometrial cytology with histological correlation. Diagnostic Cytopathology 35: 817–844. DOI 10.1002/dc.20745. [Google Scholar] [CrossRef]
Montgomery BE, Daum GS, Dunton CJ (2004). Endometrial hyperplasia: A review. Obstetrical & Gynecological Survey 59: 368–378. DOI 10.1097/00006254-200405000-00025. [Google Scholar] [CrossRef]
Nagase E, Yasuda M, Kajiwara H, Osamura RY, Yoshitake T, Hirasawa T, Muramatsu T, Miyamoto T, Murakami M, Makino T, Ogawa T (2004). Uterine body cancer mass screening at Tokai University Hospital. Tokai Journal of Experimental and Clinical Medicine 29: 43–48. [Google Scholar]
Norimatsu Y, Miyamoto M, Kobayashi TK, Moriya T, Shimizu K, Yanoh K, Tsukayama C, Miyake Y, Ohno E (2008). Diagnostic utility of phosphatase and tensin homolog, β-catenin, and p53 in endometrial carcinoma by thick-layer endometrial preparations. Cancer 114: 155–164. DOI 10.1002/cncr.23495. [Google Scholar] [CrossRef]
Norimatsu Y, Shimizu K, Kobayashi TK, Moriya T, Tsukayama C, Miyake Y, Ohno E (2006). Cellular features of endometrial hyperplasia and well differentiated adenocarcinoma using the Endocyte sampler: Diagnostic criteria based on the cytoarchitecture of tissue fragments. Cancer 108: 77–85. DOI 10.1002/cncr.21734. [Google Scholar] [CrossRef]
Papaefthimiou M, Symiakaki H, Mentzelopoulou P, Giahnaki AE, Voulgaris Z, Diakomanolis E, Kyroudes A, Karakitsos P (2005). The role of liquid-based cytology associated with curettage in the investigation of endometrial lesions from postmenopausal women. Cytopathology 16: 32–39. DOI 10.1111/j.1365-2303.2004.00224.x. [Google Scholar] [CrossRef]
Remondi C, Sesti F, Bonanno E, Pietropolli A, Piccione E (2013). Diagnostic accuracy of liquid-based endometrial cytology in the evaluation of endometrial pathology in postmenopausal women. Cytopathology 24: 365–371. DOI 10.1111/cyt.12013. [Google Scholar] [CrossRef]
Siegel R, Ma J, Zou Z, Jemal A (2014). Cancer statistics, 2014. CA: A Cancer Journal for Clinicians 64: 9–29. DOI 10.3322/caac.21208. [Google Scholar] [CrossRef]
Siegel RL, Miller KD, Jemal A (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 68: 7–30. DOI 10.3322/caac.21442. [Google Scholar] [CrossRef]
Tavassoli FA, Devilee P (2003). Tumours of the Breast and Female Genital Organs. Pathology and Genetics. World Health Organization Classification of Tumours. Lyon, France: IARC Press. [Google Scholar]
Yang X, Liao QP, Wu C, Zhang NY, Zhao J, Chen R, Zhang N, Ma XH, Gao YN, Gao WL, Zheng H, Zeng Z, Wen J, Dong Y (2013). Screening and sampling of endometrial carcinoma accuracy of the endometrial cytology test for the screening of endometrial cancer. Zhonghua Fu Chan Ke Za Zhi 48: 884–890. [Google Scholar]
Yang X, Ma K, Chen R, Zhao J, Wu C, Zhang N, Ma X, Dong Y, Zhu S, Liao Q (2017). Liquid-based endometrial cytology associated with curettage in the investigation of endometrial carcinoma in a population of 1987 women. Archives of Gynecology and Obstetrics 296: 99–105. DOI 10.1007/s00404-017-4400-2. [Google Scholar] [CrossRef]
Zhao J (2006). The diagnostic system of endometrial cytology. Chinese Journal of Reproductive Health 17: 6–8. DOI 10.3969/j.issn.1671-878X.2006.01.002. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |