DOI:10.32604/biocell.2021.014900

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.014900 |  www.techscience.com/journal/biocell |
| Review |
Microfluidic chips for the endothelial biomechanics and mechanobiology of the vascular system
1Beijing Advanced Innovation Centre for Biomedical Engineering, Key Laboratory for Biomechanics and Mechanobiology of Chinese Education Ministry, School of Biological Science and Medical Engineering, Beihang University, Beijing, China, 100083
2School of Engineering Medicine, Beihang University, Beijing, China, 100083
3Artificial Intelligence Key Laboratory of Sichuan Province, School of Automation and Information Engineering, Sichuan University of Science and Engineering, Zigong, China, 643000
4Beijing Research Center of Urban System Engineering, Beijing, China
*Address correspondence to: Xiao Liu, liuxiao@buaa.edu.cn; Xiaoyan Deng, dengxy1953@buaa.edu.cn; Yubo Fan, yubofan@buaa.edu.cn
Received: 06 November 2020; Accepted: 14 January 2021
Abstract: Endothelial cells arranged on the vessel lumen are constantly stimulated by blood flow, blood pressure and pressure-induced cyclic stretch. These stimuli are sensed through mechanical sensory structures and converted into a series of functional responses through mechanotransduction pathways. The process will eventually affect vascular health. Therefore, there has been an urgent need to establish in vitro endothelial biomechanics and mechanobiology of models, which reproduce three-dimensional structure vascular system. In recent years, the rapid development in microfluidic technology makes it possible to replicate the key structural and functionally biomechanical characteristics of vessels. Here, we summarized the progress of microfluidic chips used for the investigation of endothelial biomechanics and mechanobiology of the vascular system. Firstly, we elucidated the contribution of shear stress and circumferential stress, to vascular physiology. Then, we reviewed some applications using microfluidic technology in angiogenesis and vasculogenesis, endothelial permeability and mechanotransduction, as well as the blood-brain barrier under these physical forces. Finally, we discussed the future obstacles in terms of the development and application of microfluidic vascular chips.
Keywords: Endothelial cells; Vascular system; Mechanotransduction; Microfluidic chip
The vascular system, composed of arteries, capillaries, and veins, is essential in maintaining the physiological activities of the human body. Arteries carry oxygenated blood to various organs. This blood contains high-concentration oxygen, abundant nutrients, and immune cells. The veins then transport the deoxygenated blood to the heart, and the two kinds of vessels are connected by capillaries (Aird, 2004; Alitalo et al., 2005; Chiu and Chien, 2011). Despite their unique functions, the inner surface of each vessel is comprised of endothelial cells (ECs) that are directly exposed to the blood. As a result, ECs are constantly affected by hemodynamic forces, including the wall shear stress (WSS) and cyclic circumferential stress (Hahn and Schwartz, 2009; Chatterjee, 2018; Campinho et al., 2020). More detail on the hemodynamics in vasculature was reviewed recently by Secomb (Secomb, 2016). Besides, ECs produce a variety of molecules and hormones that play vital roles in vascular homeostasis, local cellular growth, and inflammatory responses (Baratchi et al., 2017). Based on these physiological features, ECs were sometimes considered to be a dependent organ and used to study vascular physiopathology.
Cells convert mechanical stimulus into electrochemical activity, which is called mechanotransduction. In blood vessels, vascular cells convert cell-generated forces or mechanical stimuli from the extracellular environment into biochemical signals and induce downstream cellular responses in the vascular system (Wang, 2017). Specifically, it has key effects on angiogenesis as well as vascular integrity and remodeling in capillaries. And for arterial and venous ECs, it is crucial to several cellular activities such as regulation of the cytoskeleton structure, cell differentiation and permeability, the expression of cytokines and adhesion molecules, and the communication between cells (Zhou et al., 2014; Gordon et al., 2020).
There were multiple in vitro macro-fluidic systems designed to recapitulate significant characteristics of in vivo flow environment. These systems were mainly modified from parallel-plate flow chamber (Frangos et al., 1985; Wang et al., 2016) and cone plate viscometer (Dewey et al., 1981; Spruell and Baker, 2013). In these platforms, ECs directly exposed to shear stress and molecular signaling and gene expression of ECs under flow shear stress could be analyzed. Compared with conventional in vitro macro-fluidic systems, the fabrication, mechanical control and chemical analysis of the microfluidic systems were much more efficient and economical (Young and Simmons, 2010). For instance, the microfluidic systems with superior microfabrication technology brought achievable models, which improve our understanding of molecules and cells in the vascular system in various mechanical environments (Lee et al., 2018; Skorupska et al., 2018; Castiaux et al., 2019). These microfluidic systems were able to provide precise fluidic control because the micro-channels can be designed with great flexibility and microfluidic flows are laminar (Duncombe et al., 2015). Besides, molecule and cell collection are easier in these microsystems, which brought great convenience for cell collection, cell sorting, and high throughput analyses (Chen et al., 2008; Halldorsson et al., 2015).
Here, we elucidated the contribution of shear stress and stretch to ECs. Cellular responses, including the adaption of the cytoskeleton, the secretion of mechanical responsive molecules, and cell-cell communication, were described. Then, we introduced two categories of recent microfluidic chips mimicking the mechanical environment of vessels: independent vascular systems with only ECs, and organ-on-a-chip models incorporated with ECs, which focused on organ-specific microvascular function. We also provided some microfluidic chips for vascular diseases such as atherosclerosis. Finally, we discussed the limitation and future perspectives of vascular microfluidic chips.
The Hemodynamics and Mechanotransduction of Endothelial Cells in Blood Vessels
The physiological function of ECs could be affected by the blood flow (Fig. 1). It is one thing to know that shear stress can influence ECs through plenty of mechanical responsive protein factors on the cytoskeleton, and quite another to know when ECs are exposed to different mechanical stimulate, specific mechanotransduction will be triggered. Those physical stresses will be transformed into biological signals secreted by ECs. It has been proposed to many mechanotransductions, including ion channels, platelet-endothelial-cell adhesion molecule-1 (PECAM-1), vascular endothelial cadherin (VE-cadherin), and Piezo 1, function in sensing blood flow. This section summarizes the hemodynamic forces in blood vessels and endothelial mechanotransduction pathways, including ECs cytoskeleton, ion channels, junctional proteins, and some membrane proteins.
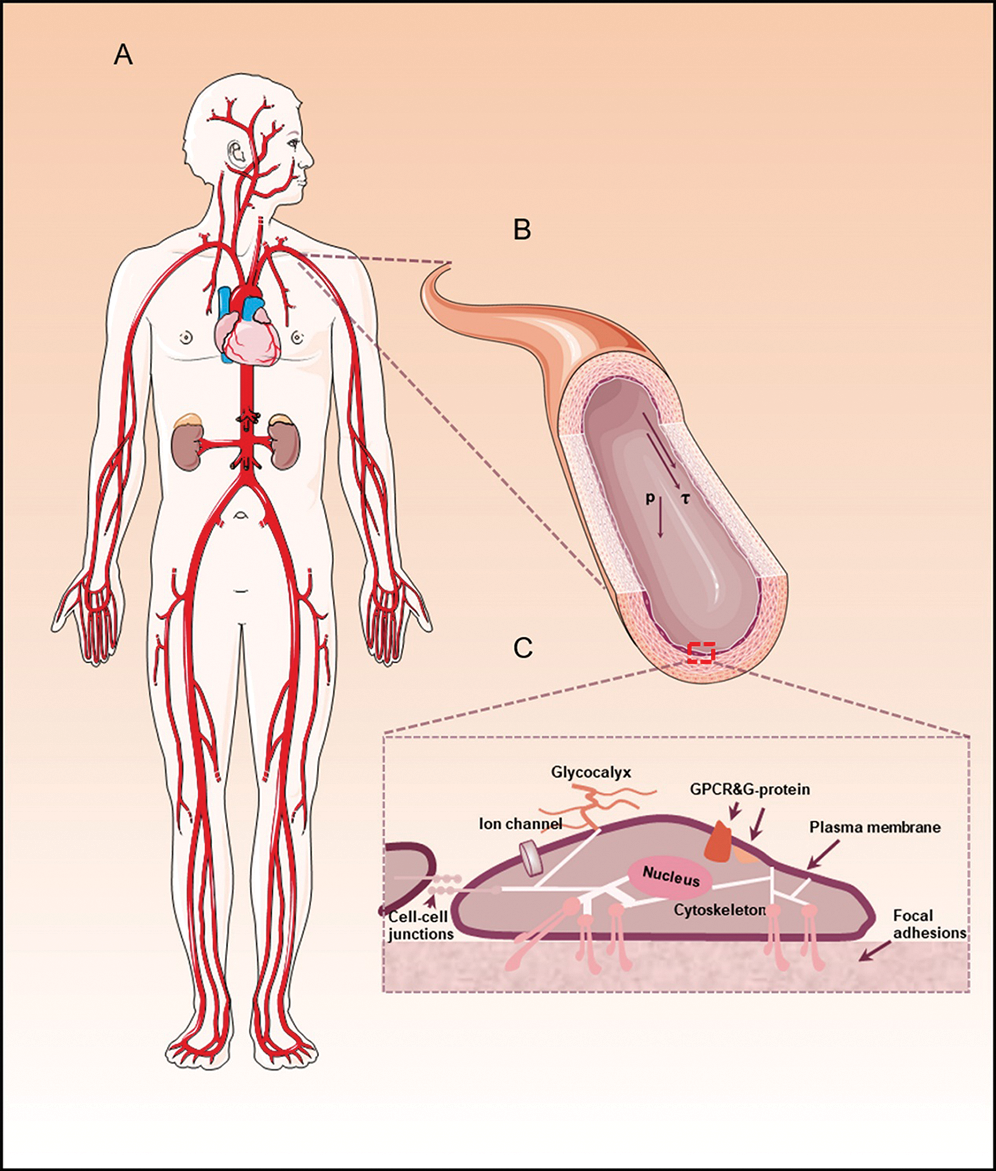
Figure 1: The hemodynamics and mechanotransduction of endothelial cells.
The complex hemodynamic forces acting on endothelial cells in blood vessel
Blood flow and cyclic stretch are typical forms of hemodynamics in vessels, which have two main characteristics. Firstly, they are time-dependent and change with the progress of the cardiac cycle. Secondly, it is also spatially variational because of the complex geometry features of blood vessels such as branching, curvature, taper, and torsion.
Blood flows and their patterns
Patterns of the flows in straight and bifurcating vessels are different. In straight vessels, the flow pattern is laminar, and the WSS on the ECs is largely unidirectional. In contrast, the flow pattern at curvatures of the vessels is usually disturbed and bidirectional. The flow through the branch vessels results in flow separation with transient flow reversals and occasional turbulence (Davies, 2007). In recent years, helical flow patterns in the vascular system induce great attention (Morbiducci et al., 2011; Gallo et al., 2012; de Nisco et al., 2020). The helical flow is characterized by high velocity and high shear stress, which is regarded as a physiological form of blood flow (Liu et al., 2015; Baratchi et al., 2020). This kind of flow is considered to be atheroprotective because it can prevent the accumulation of low-density lipoproteins, enhance blood perfusion and oxygen transport, and reduce the adhesion of blood cells on the arterial wall (Liu et al., 2009; Liu et al., 2010). Interestingly, the helical flow can improve the hemodynamic performance of vascular devices (Liu et al., 2016; Zeller et al., 2016; Zhang et al., 2018).
Cyclic stretch (CS), sometimes called circumferential strain resulted from blood pressure, manifests the expansion and contraction of the vessel wall. Similar to the blood flow, the stretching magnitude is implicated in the systolic and diastolic blood pressure, which are related to the heart cycle frequency (Kaunas et al., 2005). The range of strains varies in different blood vessels. For instance, the arterial strain is 9%–12%, the pulmonary artery strain is 6%–10%, the carotid artery strain is 1%–2%, and the femoral artery strain is 2%–15% (Anwar et al., 2012; Jufri et al., 2015). However, in pathological conditions, such as hypertension, strains can reach as high as 20% (O’Rourke, 1995). Besides, it is also reported that the lag of temporal phase angle exists between the WSS and the CS, this is because of the viscoelastic mechanical properties of the vessels (James and Allen, 2018).
Endothelial mechanotransduction
The ECs can sense physical forces, which interact with the surrounding environments. Then, the stimuli are further transduced into biochemical signals via mechanotransduction pathways. Cytoskeleton can act as key mechanosensory molecules to transduce mechanical stimuli from the endothelium to the nucleus. In addition, multiple mechano-sensors on the membrane of vascular ECs membrane have been reported, such as integrins (Israeli-Rosenberg et al., 2014), ion channels (Delmas and Coste, 2013; Leckband and de Rooij, 2014), junctional proteins (Chen and Tzima, 2009), PECAM-1 (Chen and Tzima, 2009; Conway and Schwartz, 2015; Wang et al., 2015), caveolae (Gilbert et al., 2016; Shihata et al., 2016), membrane lipids and protein(Panciera et al., 2017), glycocalyx (Florian et al., 2003; Zeng et al., 2018; Weinbaum et al., 2020), primary cilia (Luo et al., 2014), etc. Here, we firstly show some recent research of cytoskeleton in response to blood flow. Then, we emphatically discuss the mechanotransduction of ion channels, junctional proteins, and some membrane proteins.
Cytoskeleton in response to blood flow
The cytoskeleton consists of microtubules, actin, and intermediate filaments. These components concatenate different areas of the ECs and play a role in transmitting forces to the basal or lateral areas. These forces come from the apical domain of the cell, which also directly senses shear forces (Huber et al., 2015). The response of ECs to the flow was blocked when actin, microtubules, or intermediate filaments were inhibited (Acharya and Yap, 2016). Furthermore, there was a theory about the cytoskeletal flows: The spatial gradient of motor contractility or cytoskeletal polymerization may induce flow. This theory has been used to study cell division, migration, polarization (Hannezo and Heisenberg, 2019). Besides, the actin polymerization at the leading edge of ECs may be made answerable for the retrograde flow of the actin cytoskeleton. Then, this process will create friction with the extracellular matrix (ECM) and then produced the force to help cell migration (Recho et al., 2013; Barnhart et al., 2015; Bergert et al., 2015). The friction between cells and ECM generates from adhesion receptors, which are the main molecular link between ECs and ECM. The adhesion receptors also act as bidirectional hubs transmitting signals between ECs and the surrounding environment. Interestingly, when exposed to blood flow, actomyosin may reorganize its own network. This process is regulated by the adhesion strength and/or orientation of actin filaments and myosin motors (Allen et al., 2020).
Mechanically activated ion channels
When ion channels sense the stress, the proteins allow the passage of ions selectively. Therefore, it can help maintain the equilibrium of electric potential. Earlier studies have shown that flow can activate ion channels, including the transfer of Na+, Ca2+, K+, Cl− (Barakat et al., 2009), and stretch can activate ion channels, including the transfer of Na+, Ca2+, and K+ (Liu et al., 1998). Of course, they activate immediately and independently when the flow starts (Barakat et al., 2009). To be specific, when the shear force is less than 0.01 Pa, the K+ channels are activated. When the shear force is 0.03 Pa, the Cl− channels are activated (Lieu et al., 2004; Gautam et al., 2006). Meanwhile, the activation of K+ and Cl− channels are also affected by the oscillating flow frequencies below the threshold frequency. In recent years, the Piezo1 channel, a kind of mechanically activated Ca2+ ion channel, was found to be able to sense flow shear stress (Coste et al., 2010; Ranade et al., 2014; Volkers et al., 2015). It is suggested that the Piezo1 channel is regulated by various mechanical stimuli (Nourse and Pathak, 2017). For example, the detection of Piezo1 showed that it changed during exercises because of increased blood flow (Rode et al., 2017; Beech, 2018). Furthermore, Piezo1 may regulate the migration of cells. In a developing vasculature, Piezo1 mediates the migration of ECs through vascular endothelial growth factors (Li et al., 2014). The released Ca2+ in ECs would activate endothelial nitric oxide (NO) synthase, which is necessary for the production of NO. It is a key signaling molecule with a plethora of biological functions (Tejero et al., 2019). In blood vessels, NO produced by ECs would diffuse rapidly to smooth muscle cells. Eventually, it will cause vasodilatation (Maiorana et al., 2003). The NO is considered a hallmark of endothelial dysfunction because NO-mediated vasodilation is induced by blood flow shear stress (Davignon and Ganz, 2004). The formation, transport, and consumption of NO in blood vessels are controlled by the local blood flow pattern, hemoglobin in red blood cells, and NO scavengers in the arterial wall (Qian et al., 2020; Su et al., 2020).
Junctional proteins and some membrane proteins
ECs mainly adhere to the extracellular matrix through focal adhesion of transmembrane. Specifically, the tripeptide alanine-glycine-aspartate is the most characterized integrin-binding ligand. It exists widely in fibronectin, vitronectin, and many other molecules (Ruoslahti, 1996). In addition, focal adhesions involved in cellular migration are dynamic structures. When applied with force, they will reshape and strengthen through actin connections (Geiger et al., 2009). In addition, ECs connect and communicate with neighboring cells through cell connections. For example, the adherent junction such as VE-cadherin is recognized as a mechanosensory. It was first found that VE-cadherin plays a role in the incorporation of vascular endothelial growth factor receptor 2 and PECAM-1 (Tzima et al., 2005). Notably, PECAM-1 is considered a mechanosensor that performs tyrosine phosphorylation under the action of a force from neighboring cells (Li et al., 2005; Collins et al., 2012).
Microfluidic Chips for the Endothelial Biomechanics and Mechanobiology
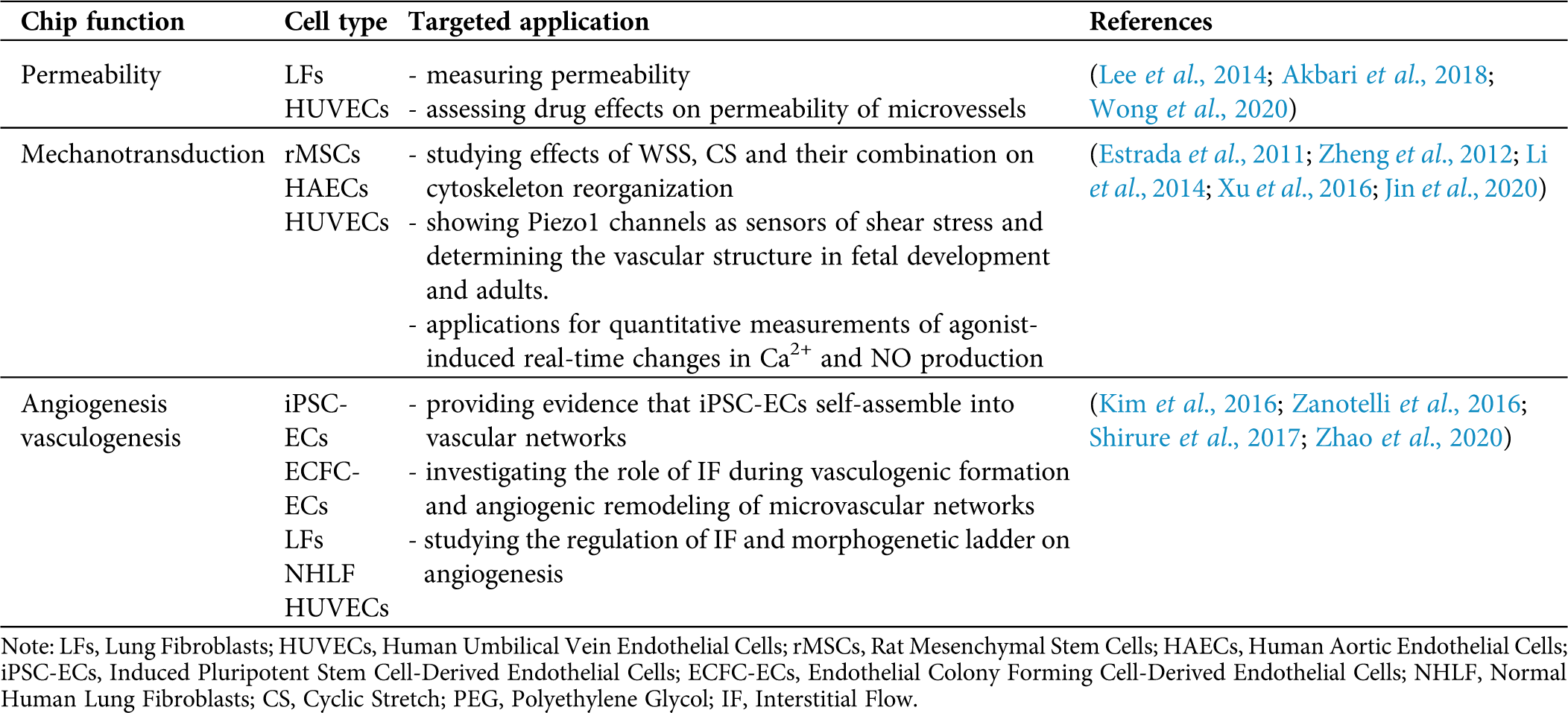
With the increasing development of manufacturing and processing techniques, microfluidic devices can be designed flexibly. The width of the microchannel can range from the nanometer to a few hundred micrometers, and the length can reach several centimeters (Skorupska et al., 2018). Microfluidic devices have many advantages that they can carry out experiments with higher throughput, less reagent consumption, design flexibility and adaptability of experiments under different conditions (Kang et al., 2008). Therefore, microfluidic systems have been made some significant breakthroughs in conventional biological research. They can be applied in multiple experiments, including cell culture, intercellular interaction, cell lysis or cell sorting, and the mimic of different types of tissues (Yum et al., 2014; Zhang et al., 2018; Li et al., 2019). In particular, microfluidic systems have been widely used in biomechanics research (Kurth et al., 2012). Several studies have explored the impact of WSS on cells, and the stimuli can influence cytoskeleton transformation and cell development (Toh and Voldman, 2011; Kurth et al., 2015). Additionally, microfluidic platforms can also apply other forces, such as stretch and compression, on target objects (Dongeun et al., 2010). As a result, microfluidic systems are introduced to mimic the mechanical environments in vessels to conquer the limitation of in vitro vascular system studies. Besides, it can reproduce a pathological environment for cells and be applied in studies of vascular diseases, such as atherosclerosis (AS). A summary of microfluidic chips for the endothelial biomechanics and mechanobiology is provided in Tab. 1.
Table 1: Summary of microfluidic chips for the endothelial biomechanics and mechanobiology
Microfluidic chips for endothelial permeability and mechanotransduction
With the transport barrier formed by blood vessels, cells and nutrients are selectively exchanged to meet the needs of metabolism and homeostasis of surrounding tissues. In addition, whether the ability to regulate vascular permeability is normal or not is fundamental to the judgment of cardiovascular function. Therefore, barrier dysfunction is a hallmark of many cardiovascular diseases (Hahn and Schwartz, 2009; Dongaonkar et al., 2010). Besides, these large amounts of chemical signals were found to affect the degree of permeability (Mehta and Malik, 2006). Blood flow plays a significant role in the guiding of permeability of ECs (Tarbell, 2003). Given the significance of vascular permeability, a variety of microfluidic chips were developed to investigate the selective permeability of the endothelial cells (Shao et al., 2010).
To be specific, Lee et al. designed a novel microfluidic system (Fig. 2A) to form multiple perfusable 3D microvessels with reliable functional barrier properties (Lee et al., 2014). Using this platform, the barrier function measured is similar to the barrier function measured in the in vivo experiment, but the permeability coefficient measured in the in vivo experiment will be relatively low. On the one hand, vessel networks in physiology are bifurcating while previous microfluidic models were limited to the design of the channel, which was often a single channel or two parallel vessel analogs (Bischel et al., 2013; Akbari et al., 2017). Therefore, Akbari et al (Akbari et al., 2018)considered the hemodynamic characteristics of the bifurcated vessels (Fig. 2B). Their model reproduced the state of blood flow at bifurcated vessels and the structure between endothelium and the extracellular matrix (ECM). This model also systematically studied the effect of local hemodynamics on changes in endothelial permeability in the bifurcating vessel structure. They also studied the influence of NO in the guiding of permeability of ECs under shear stress using the device. On the other hand, assessments of barrier function in these microfluidic vascular models were usually limited to fluorescence-based diffusion permeability measurements. To overcome this problem, a recent model established by Wong et al. (2020) measured the permeability of ECs by using an electroactive tracer on a 3D hydrogel-based microfluidic vascular model (Fig. 2C). They measured the permeability of EC under the condition of the cell-sensing mechanical stimulation and after exposure to a permeability mediator.
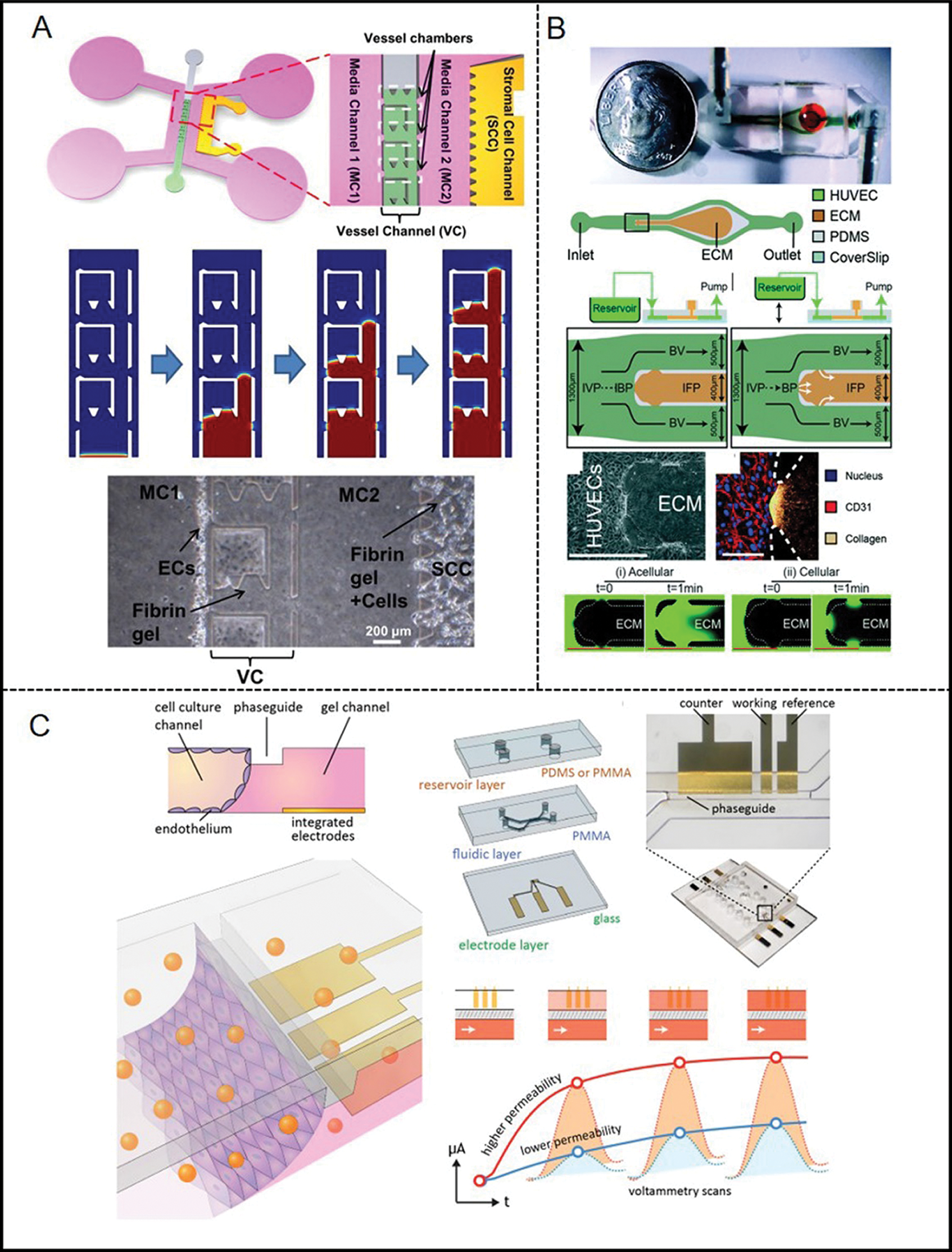
Figure 2: Examples of microfluidic vascular system for endothelial permeability developed by (A) Lee et al. (2014), (B) Akbari et al. (2018), (C) Jin et al. (2020).
In addition to studying endothelial permeability, microfluidic systems have shown tremendous potential in studying the mechanotransduction of ECs. The first related vascular chip, a silicon platform with varying widths of microchannels, was fabricated by Gray et al. (2002) using deep reactive ion etching method. The result showed that the elongation of ECs progressively increased as the channel width decreased. ECs were planted in a 200 mm wide microchannel and formed a monolayer after being sheared by 20 dyne/cm2 shear stress for 16 h. Besides, they considered the magnitude and waveform of shear stress similar to physiological conditions. Although the laminar-steady flow is simple and easy to implement in a chip, but importantly, the comparison between laminar flow and static culture can directly show the effects of shear stress on the cells.
Because the blood flow in the body is pulsatile, other work adds various types of unstable flow to the microfluidic devices. The dynamic effects of pulsatility on cell growth, such as oscillatory and non-reversing pulsatile flows, were tested. Multiple parameters, including flow amplitude, frequency, and reversal degree, could be applied and manually controlled. These applications also showed the flexibility of microfluidic platforms (Vedel et al., 2010; Mohammed et al., 2019). For example, the microfluidic system with pulsatile flow described by Mohammed et al. (2019) can study the mechanobiology of human aortic endothelial cells (Mohammed et al., 2019). To achieve the simplification of microfluidic technologies and minimize their reliance on supporting equipment, they integrate microfluidic channels with miniaturized pumping units and flow sensors. However, the flows mimicked in those chips still have relatively large differences in comparison with the in vivo hemodynamic microenvironment of blood vessels. To deal with the problem, Zheng et al. (2012) developed a microfluidic vascular chip (Fig. 3A). This platform can provide WSS and CS for cultured vascular ECs, which are also the two main mechanical stimulations of the cardiovascular system (Zheng et al., 2012). The chip contains a microchannel layer, an elastic membrane, and a stretch layer. The cells planted on the elastic membrane can be stimulated by WSS and CS simultaneously or independently as the membrane deforms under the action of perfusion. Furthermore, Estrada et al. (2011) fabricated a microfluidic model (Fig. 3B) to culture ECs under physiological flow patterns (Estrada et al., 2011). The system is designed to use a variety of adjustable analog controls, including compliance, resistance, foldable pulse chamber, and control valve, to accurately simulate hemodynamic waveforms.
Given the significance of Piezo1 for ECs, Li et al. (2014) designed microfluidic chambers applying shear stress on ECs. They concluded that the calpain activation will enhance the entry of calcium ions under the action of shear stress. This entry process is regulated by the Piezo1 channel and has a significant impact on endothelial cell organization and alignment. Additionally, Xu et al. (2016) presented detailed procedures of cultured microvessel network development (Fig. 3C). The device possessed long-term perfusion capability and quantitatively measured the agonist-induced changes of Ca2+ in EC. They also measured the production of NO in real time. Although microfluidic chips are now integrated for multiple purposes, one of the most important applications is to monitor in-situ mechanical force trigger signals in real time during vascular mechanical transduction is still a problem to be solved. Recently, the microfluidic vascular chip designed by Jin et al. (2020) is helpful to overcome the challenges (Fig. 3D). The microfluidic vascular chip contains a flexible and stretchable electrochemical sensor, which was constructed using conductive polymer-coated carbon nanotubes. The material has good mechanical compliance and real-time electrochemical performance. Moreover, they also studied the effect of cyclic CS on ECs and detected NO signals and reactive oxygen species under different conditions.
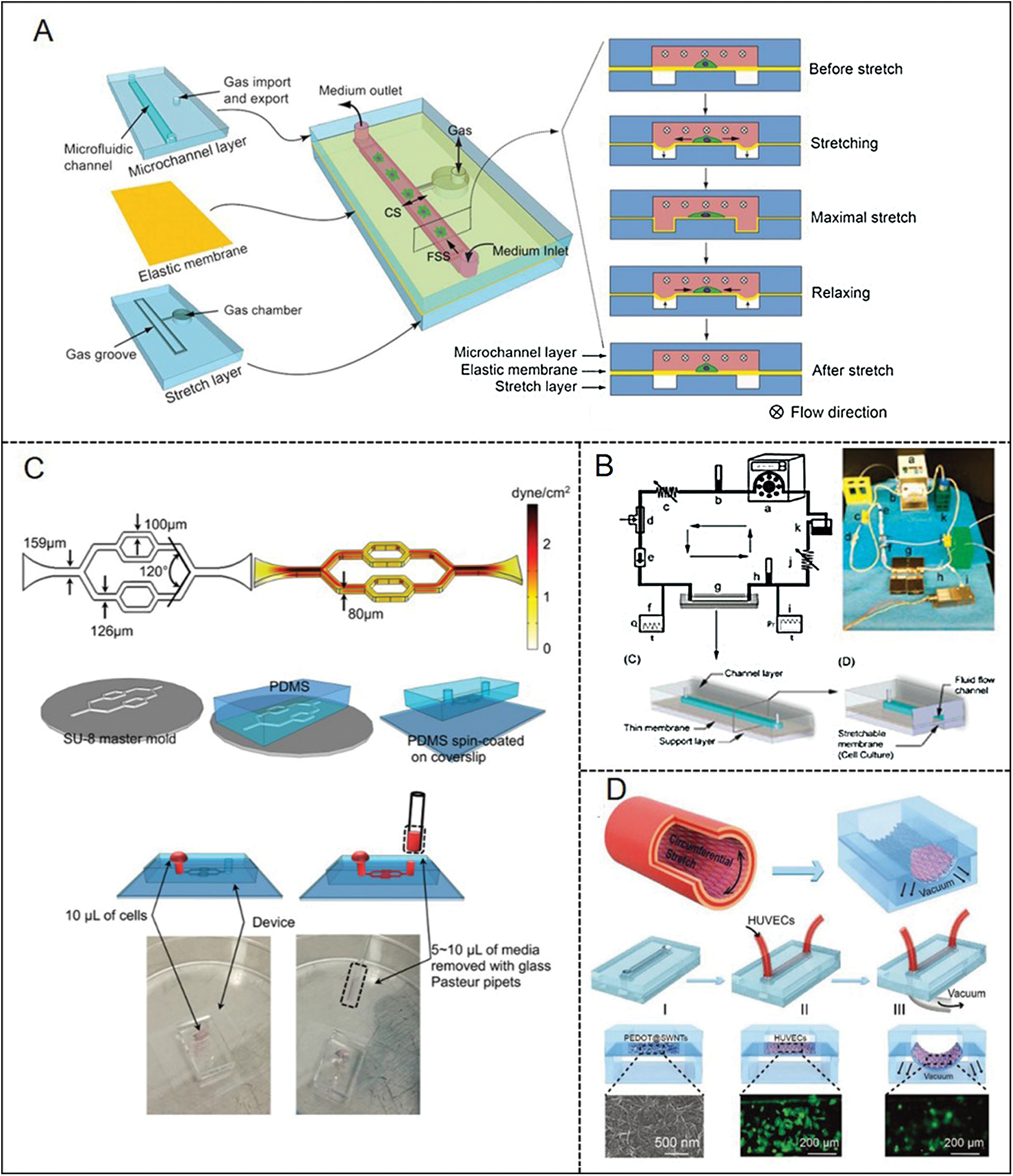
Figure 3: Examples of microfluidic vascular system for endothelial mechanotransduction developed by (A) Zheng et al. (2012), (B) Estrada et al. (2011), (C) Xu et al. (2016), (D) Jin et al. (2020).
Microfluidic chips for angiogenesis and vasculogenesis
Vascular development involves a coordinated process of ECs differentiation, proliferation, and migration. It includes two main processes: Angiogenesis and vasculogenesis. Vasculogenesis is in the early stage of embryonic development. It is a process in which angioblasts differentiated from mesoderm cells gather to form primary capillary plexus (Semenza, 2007). Subsequently, the vascular network is reconstructed during the vascular maturation process, new blood vessels sprout from the existing vascular network and grow to the surrounding tissues. This process is called angiogenesis (Carmeliet, 2000; Feige et al., 2014). Angiogenesis usually occurs during normal embryonic development, but it also occurs during the regeneration of damaged tissues. In addition, it involves the sprouting or division of pre-existing angiogenesis, which is an important natural process in the development and wound repair process, especially in the development of cancer (Adams and Alitalo, 2007; Herbert and Stainier, 2011). In this process, the pivotal molecular mechanism in maintaining vasculature homeostasis is the vascular endothelial growth factor pathway (Olsson et al., 2006). Moreover, both intrinsic molecular pathways and extrinsic stimuli can influence vascular development. Among in vitro models, microfluidic technology also showed excellent performance in reproducing angiogenesis that complex vascular systems were fabricated with fluidic perfusion tightly controlled. The advantages of microfluidic technology also provide a feasible method in studying the effects of hemodynamic forces on cells, cell interactions, and the effects of biochemical/biophysical stimulation on angiogenesis.
In in vitro experiments, a hydrogel was used as a significant component in vascular formation instead of ECM to better reproduce the three-dimensional (3D) in vivo niches of vascularization (Lewis and Gerecht, 2016). Moreover, it becomes possible to recreate the angiogenesis process (Bersini and Moretti, 2015). The microfluidic system well defined the cell patterns, biochemical gradients, and dynamic physical stimulation in microchannels to achieves angiogenesis. The flow channel connecting the microfluidic channel to one or more chambers is usually composed of different 3D matrix microstructures. The materials are selected to emulate the ECM and thereby promote the attachment and sprouting of ECs (Cochrane et al., 2019). For instance, Zanotelli et al. (2016) designed a 3D microvascular model (Fig. 4A) on a tri-channel microfluidic system. The vascular channel is filled with polyethylene glycol hydrogel, and then human-induced pluripotent stem cell-derived endothelial cells are encapsulated in the hydrogel. The hydrogel contained cell-adhesive and MMP-degradable peptides, which could be beneficial for the generation of lumenized vascular networks under flow conditions. Moreover, interstitial flow (IF) is significant for angiogenesis for two reasons. Firstly, Changes in IF can affect pathological and physiological angiogenesis. Second, both IF and morphogen gradients (such as VEGF) may stimulate and guide the growth of new blood vessels (Shirure et al., 2017). Kim et al. (2016) investigated the individual and combined effect of IF and proangiogenic factors (Fig. 4B). As long as IF exists, no matter how to change the flow direction, it will significantly promote the angiogenesis of the microvascular network. Subsequently, Shirure et al. (2017) also presented similar results in terms of the effect of IF on angiogenesis. They developed an in vitro microfluidic platform (Fig. 4C) that can model 3D angiogenesis in the tissue microenvironment. Their results show that under physiological IF (0.1–10 μm/s) conditions, morphogen gradients were eliminated within a few hours. In addition, the results of angiogenesis show that the opposite direction of IF is more conducive to angiogenesis.
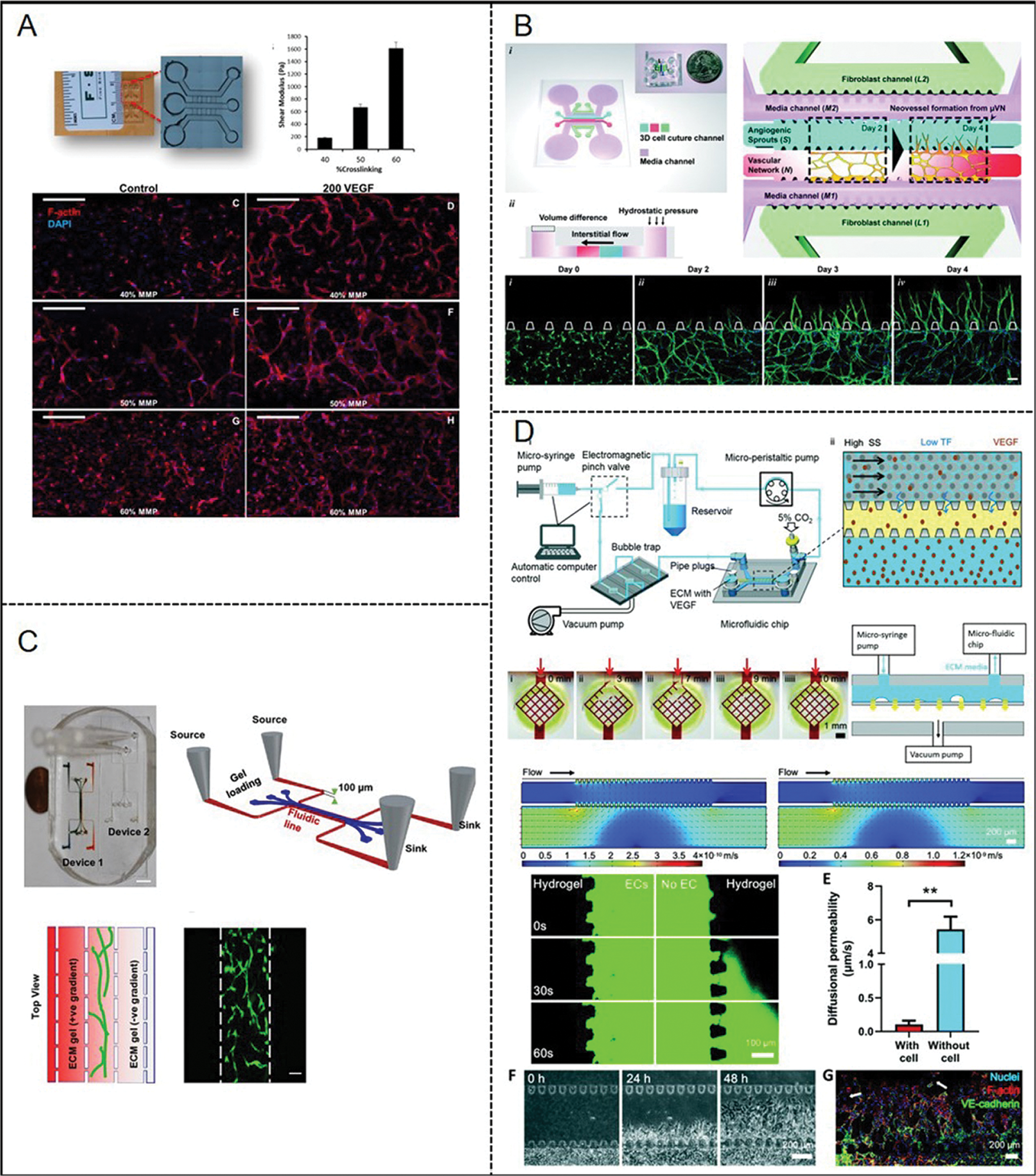
Figure 4: Reported examples of microfluidic vascular systems for angiogenesis and vasculogenesis developed by (A) Zanotelli et al. (2016), (B) Kim et al. (2016), (C) Shirure et al. (2017), (D) Zhao et al. (2020).
Based on microfluidic techniques, our group developed a microfluidic sprouting chip (Fig. 4D) and an automatic system to control the circulatory perfusion system and to simulate the process of neovascularization (Zhao et al., 2020). The microfluidic chip contained an endothelial cell culture channel (having flow shear stress control), a liquid channel (supporting medium and VEGF), and a wide central hydrogel channel (mimicking the ECM) separating two liquid channels. The result showed that high shear stress prevents the initiation of new blood vessel formation and stabilizes the ECs layer. This process is regulated by the mechanical transduction of the heparan sulfate proteoglycan coating on ECs.
Microfluidic chips for endothelial vascularization in blood-brain barrier
The blood-brain barrier (BBB) contains peripheral cells, pericytes, and astrocytes and forms a strictly regulated neurovascular unit (NVU) to control the dynamic balance of the central nervous system to maintain normal brain functions (Arvanitis et al., 2020). The physical barrier has the function of restricting the passage of ions and hydrophilic agents. It is formed by ECs connected by tight junction proteins (such as closed zonule 1) (Wong et al., 2013; Serlin et al., 2015). The microfluidic BBB models replicated realistic dimensions and geometries, so the endothelium can be directly exposed to physiological fluid flow. Using “BBBs-on-chips”, researchers can detect the expression of specific markers, including adhesion proteins and tight junction proteins, to lay the foundation for further understanding of the blood-brain barrier mechanism. Besides, the permeability of the cell barrier could be investigated (van der Helm et al., 2016).
Brown et al. (2015) reported a microfluidic brain vasculature chip (Fig. 5A). The system consists of the vascular and brain chambers and they are closely opposed to each other and separated by a thin polycarbonate (PC) membrane (0.2 μm pores). They cultured primary microvascular endothelial cells, derived from the human brain, on the upper membrane, implanted a gel containing astrocytes under the membrane, and performed blood flow. The amount of ZO-1 was semi-quantified by immunofluorescence. In addition, it was observed that the actin filaments were rearranged along the flow direction, and the percentage of actin filaments was quantified. Their work also confirmed that permeability is related to the active transport of ascorbic acid. Besides, based on the effect of astrocytes in a 3D collagen matrix to modulate brain endothelial barrier function, Sellgren et al. (2015) developed a novel microfluidic device. Their device is composed of two upper and lower polydimethylsiloxane (PDMS) micro-molded channels and a middle nano-porous membrane. The membrane used commercial polyester (PE) and polytetrafluoroethylene (PTFE) nanoporous membranes (pore size 0.4 μm, TClear 3450, Corning, and BGCM00010, Millipore). They incorporated a 3D matrix for supporting astrocytes on the lower membrane and planted ECs on the upper membrane at a perfusion condition. Similar to the foregoing work, Walter et al. (2016) designed a barrier-on-a-chip device (Fig. 5B), which consisted of two PDMS parts with channels. The middle layer is a porous PET membrane with 23 mm thick and 0.45 mm pores. Co-culture of 2 or 3 types of cells (ECs, pericytes, and astrocytes) were available with the flow of culture medium. The whole-cell layer was observed, and the monitoring of transcellular electrical resistance in real time was applied as well. Subsequently, Maoz et al. (2018) considered the interaction of different cells so that the effect of individual cell types or sub-compartments on NVU function would not be missed. They constructed three chips, with a brain chip in the middle and a BBB chip on each side (Fig. 5C). Then, they cultured human brain microvascular endothelial cells on the lower surface of the BBB chip membrane. Besides, in order to simulate the outer wall of brain microvessels, primary brain microvascular pericytes were dispersed in astrocytes and cultured on the upper surface of the membrane. Additionally, it is also a very meaningful work to apply the BBB system to drug development. Ahn et al. (2020) presented a microfluidic microphysiological platform (Fig. 5D) to mimic the key structure and function of the human BBB. Comparing to previous models, it can achieve the reproduction of BBB-specific endothelial characteristics. In addition, they used microfluidic technology to combine HDL mimetic nanoparticles with apolipoprotein A1 (eHNP-A1) to reconstruct the physiological condition of discoidal HDL. Finally, they confirmed that eHNP-A1 entered the brain blood-brain barrier with a relative accumulation of about 3%.
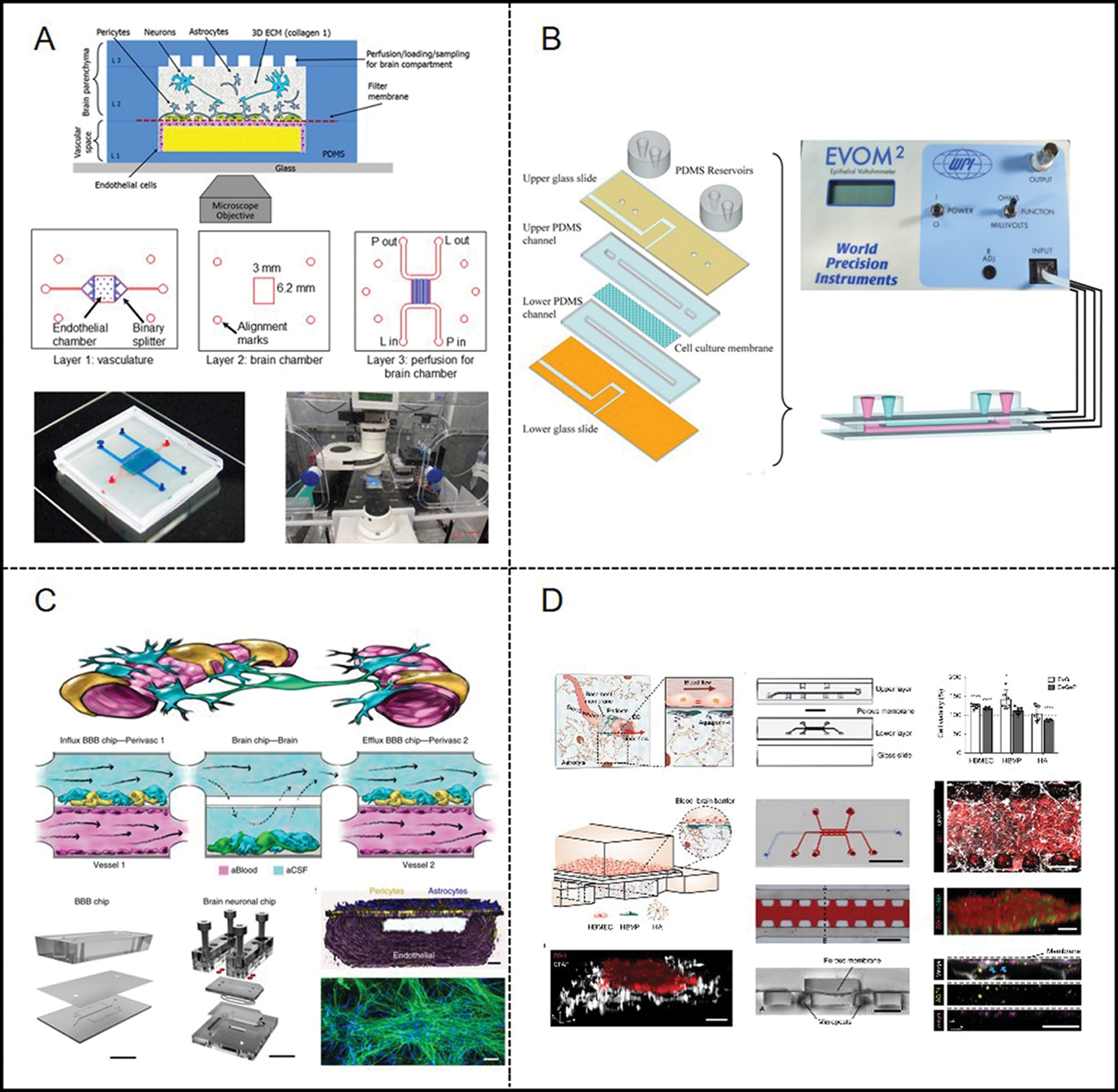
Figure 5: Examples of microfluidic vascular system for blood–brain barrier developed by (A) Brown et al. (2015), (B) Walter et al. (2016), (C) Maoz et al. (2018), (D) Ahn et al. (2020).
Microfluidic chip for atherosclerosis
It is known that AS is a chronic cardiovascular disease that is closely related to the arterial microenvironment. Hemodynamics also affects the process of AS (Zaromitidou et al., 2016). Recently, AS chips containing several cell types and flow patterns have been developed to reproduce the various characteristics of atherosclerotic diseases, including mechanical strain (Zheng et al., 2016), monocyte phenotypes (Foster et al., 2013), disturbed flow (Patibandla et al., 2014), platelet aggregation (Westein et al., 2013), and thrombosis or leukocyte–endothelial interactions at the atherosclerotic plaque (Venugopal Menon et al., 2018). For instance, Zheng et al. (2016) reported a microfluidic model (Fig. 6A) to reconstruct early-stage AS to investigate physiological or AS-prone hemodynamic conditions. In addition, they designed a microfluidic chip that can integrate FSS and CS and study the behavior of ECs under physiological and AS-prone mechanical conditions so as to simulate the mechanical properties of blood vessels. Besides, Venugopal Menon et al. (2018) innovatively introduced a 3D stenosis vessel model to research the effect of hemodynamics on the interaction between leukocytes and endothelium (Fig. 6B). The multilayered microfluidic chip is composed of two orthogonal channels with a cell culture channel (top) and an air channel (bottom), separated by a thin PDMS membrane. It creates an adjustable 3D contraction by pumping air into the bottom channel to simulate narrow plaques of different severity. As mentioned above, helical flows may prevent the occurrence of vascular diseases, such as AS. Therefore, it is of great importance to design experimental models to specifically study helical flows. Jia et al. (2019) developed a microfluidics-based model to fabricate biomimetic helical microfibrous with various complex helical structures. The micro-channels had good perfusability and were able to simulate the swirling blood flow of helical blood vessels. Consequently, the model could reproduce blood function in vitro and be applied for blood-vessel-on-a-chip applications (Jia et al., 2019). Another platform developed by Varma et al. (2020) was able to provide helical flow as well (Fig. 6C). In this platform, grooved surfaces on the channel ceilings created uniform, helical, and chaotic flow. Specific coupling between the spatial profile of flow and human endothelial cell phenotype was characterized in this work (Varma et al., 2020).
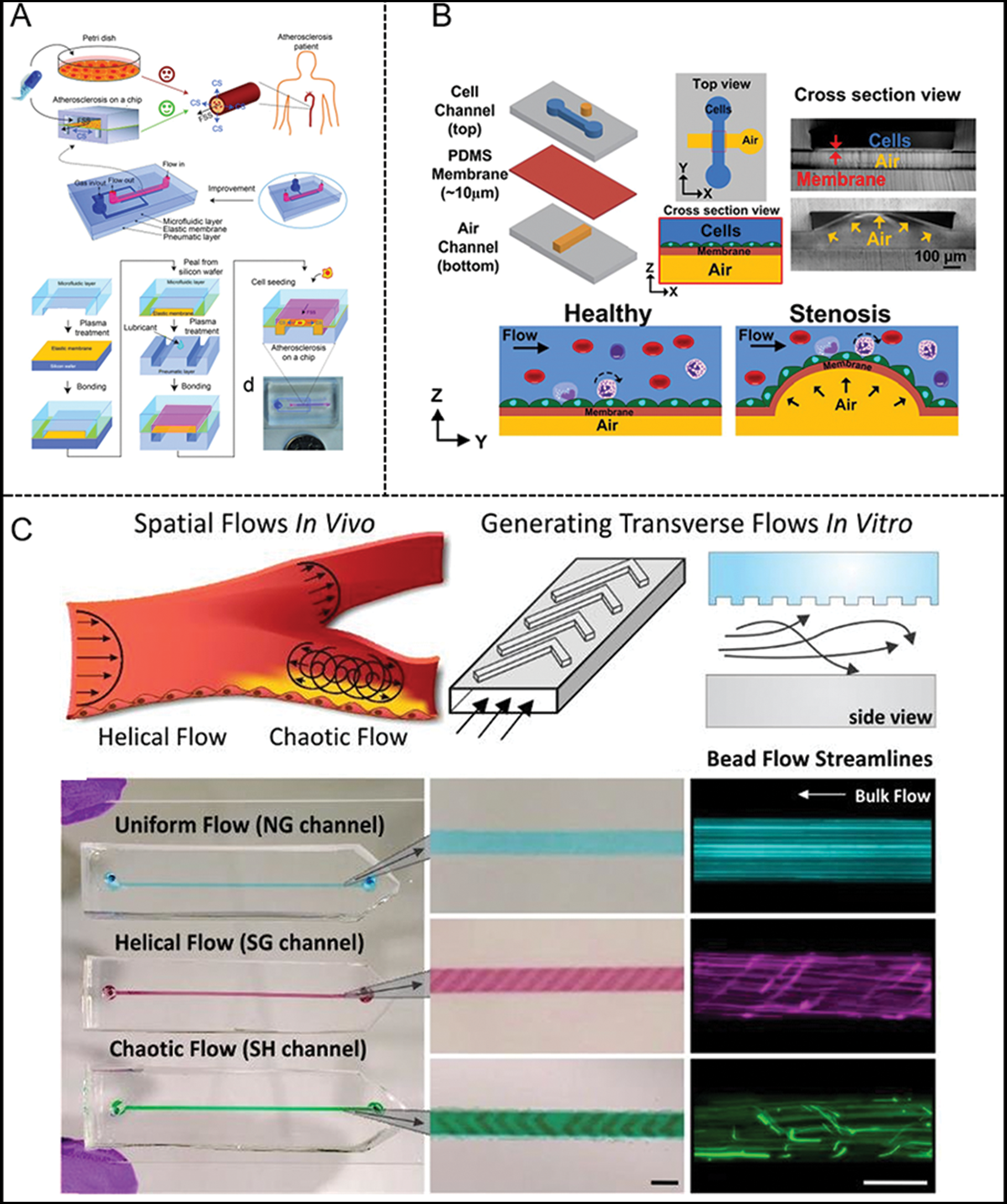
Figure 6: Examples of microfluidic vascular system for atherosclerosis developed by (A) Zheng et al. (2016), (B) Venugopal et al. (2018), (C) Varma et al. (2020).
Conclusions and Future Challenges
We firstly elaborated the contribution of physical forces, such as shear stress, blood pressure, and stretch strain, to ECs in molecular and cellular aspects, including mechanical responsive molecules or proteins involved in cell cytoskeleton and cell-cell communication. Then, we summarized the advanced progress of vascularized micro-tissues using microfluidic technology. These in vitro microfluidic vascularization chips reproduced the mechanical microenvironment of blood vessels of the human body. Overall, the development of organ-on-chip has taken a big step forward, and future technological advances may further accelerate the development of reproducing cell heterogeneity and complex three-dimensional structure models of in vivo tissues on in vitro microfluidic platforms.
However, there are also several challenges in vascular microfluidic systems. Current microfluidic platforms are usually composed of PDMS. The material has its disadvantages in that the absorption of small molecules and cytokines may greatly affect the accuracy of the bioassays. Therefore, more applicable biomaterials are required to develop to modify or replace PDMS. However, the current disease-specific microfluidic chip for drug screen may not fully reproduce the journey of a drug through the human body. It is difficult for vascular chips to reproduce the dynamic structure, the environment, and function changes in the process of organogenesis because they are designed and constructed in a predetermined manner. Furthermore, although the function and applications of the physiological organ-on-a-chip platforms have been accepted, there is still in deficiency of more personalized clinical applications based on microfluidic chips.
Availability of Data and Materials: Data supporting this article are detailed in this manuscript.
Author Contribution: The authors confirm contribution to the paper as follows: Study conception and design: Xiao Liu, Xiaoyan Deng, Yubo Fan. The modification of article: Jing Du, Li Wang. Figure integration: Kexin Li. Draft manuscript preparation: Haoran Su. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This work was supported by the National Natural Science Research Foundation of China (61533016, 11827803, 31971244, 31570947, 11772036, 11421202 and U20A20390), the National Key Research and Development Program of China (2016YFC1102202 and 2016YFC1101101), Beijing Natural Science Foundation (4194079) and the 111 Project (B13003).
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Acharya BR, Yap AS (2016). Cell-cell adhesion and the cytoskeleton. In: Bradshaw RA, Stahl PD (eds.pp. 704–712. Encyclopedia of Cell Biology. Kidlington, Oxford, United Kingdom: Elsevier. [Google Scholar]
Adams RH, Alitalo K (2007). Molecular regulation of angiogenesis and lymphangiogenesis. Nature Reviews Molecular Cell Biology 8: 464–478. DOI 10.1038/nrm2183. [Google Scholar] [CrossRef]
Ahn SI, Sei YJ, Park HJ, Kim J, Ryu Y, Choi JJ, Sung HJ, MacDonald TJ, Levey AI, Kim YT (2020). Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nature Communications 11: a020412. DOI 10.1038/s41467-019-13896-7. [Google Scholar] [CrossRef]
Aird W (2004). Endothelium as an organ system. Critical Care Medicine 32: S271–S279. DOI 10.1097/01.CCM.0000129669.21649.40. [Google Scholar] [CrossRef]
Akbari E, Spychalski GB, Rangharajan KK, Prakash S, Song JW (2018). Flow dynamics control endothelial permeability in a microfluidic vessel bifurcation model. Lab on a Chip 18: 1084–1093. DOI 10.1039/C8LC00130H. [Google Scholar] [CrossRef]
Akbari E, Spychalski GB, Song JW (2017). Microfluidic approaches to the study of angiogenesis and the microcirculation. Microcirculation 24: e12363. DOI 10.1111/micc.12363. [Google Scholar] [CrossRef]
Alitalo K, Tammela T, Petrova T (2005). Lymphangiogenesis in development and human disease. Nature 438: 946–953. DOI 10.1038/nature04480. [Google Scholar] [CrossRef]
Allen GM, Lee KC, Barnhart EL, Tsuchida MA, Wilson CA, Gutierrez E, Groisman A, Theriot JA, Mogilner A (2020). Cell mechanics at the rear act to steer the direction of cell migration. Cell Systems 11: 286–299. e4. DOI 10.1016/j.cels.2020.08.008. [Google Scholar] [CrossRef]
Anwar MA, Shalhoub J, Lim CS, Gohel MS, Davies AH (2012). The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. Journal of Vascular Research 49: 463–478. DOI 10.1159/000339151. [Google Scholar] [CrossRef]
Arvanitis CD, Ferraro GB, Jain RK (2020). The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nature Reviews Cancer 20: 26–41. DOI 10.1038/s41568-019-0205-x. [Google Scholar] [CrossRef]
Barakat AI, Gojova A, Mofrad MRK, Kamm RD (2009). Role of ion channels in cellular mechanotransduction–lessons from the vascular endothelium. Cellular Mechanotransduction. pp. 161–180. Cambridge: Cambridge University Press. [Google Scholar]
Baratchi S, Chen YC, Peter K (2020). Helical flow: A means to identify unstable plaques and a new direction for the design of vascular grafts and stents. Atherosclerosis 300: 34–36. DOI 10.1016/j.atherosclerosis.2020.03.002. [Google Scholar] [CrossRef]
Baratchi S, Khoshmanesh K, Woodman OL, Potocnik S, Peter K, McIntyre P (2017). Molecular sensors of blood flow in endothelial cells. Trends in Molecular Medicine 23: 850–868. DOI 10.1016/j.molmed.2017.07.007. [Google Scholar] [CrossRef]
Barnhart E, Lee KC, Allen GM, Theriot JA, Mogilner A (2015). Balance between cell–substrate adhesion and myosin contraction determines the frequency of motility initiation in fish keratocytes. Proceedings of the National Academy of Sciences of the United States of America 112: 5045–5050. DOI 10.1073/pnas.1417257112. [Google Scholar] [CrossRef]
Beech DJ (2018). Endothelial Piezo1 channels as sensors of exercise. Journal of Physiology 596: 979–984. DOI 10.1113/JP274396. [Google Scholar] [CrossRef]
Bergert M, Erzberger A, Desai RA, Aspalter IM, Oates AC, Charras G, Salbreux G, Paluch EK (2015). Force transmission during adhesion-independent migration. Nature Cell Biology 17: 524–529. DOI 10.1038/ncb3134. [Google Scholar] [CrossRef]
Bersini S, Moretti M (2015). 3D functional and perfusable microvascular networks for organotypic microfluidic models. Journal of Materials Science: Materials in Medicine 26: 1559. DOI 10.1007/s10856-015-5520-5. [Google Scholar] [CrossRef]
Bischel LL, Young EW, Mader BR, Beebe DJ (2013). Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 34: 1471–1477. DOI 10.1016/j.biomaterials.2012.11.005. [Google Scholar] [CrossRef]
Brown JA, Pensabene V, Markov DA, Allwardt V, Neely MD, Shi M, Britt CM, Hoilett OS, Yang Q, Brewer BM (2015). Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics 9: 054124. DOI 10.1063/1.4934713. [Google Scholar] [CrossRef]
Campinho P, Vilfan A, Vermot J (2020). Blood flow forces in shaping the vascular system: A focus on endothelial cell behavior. Frontiers in Physiology 11: 1131. DOI 10.3389/fphys.2020.00552. [Google Scholar] [CrossRef]
Carmeliet P (2000). Mechanisms of angiogenesis and arteriogenesis. Nature Medicine 6: 389–395. DOI 10.1038/74651. [Google Scholar] [CrossRef]
Castiaux AD, Spence DM, Martin RS (2019). Review of 3D cell culture with analysis in microfluidic systems. Analytical Methods 11: 4220–4232. DOI 10.1039/C9AY01328H. [Google Scholar] [CrossRef]
Cochrane A, Albers HJ, Passier R, Mummery CL, van den Berg A, Orlovaa VV, van der Meer AD (2019). Advanced in vitro models of vascular biology: Human induced pluripotent stem cells and organ-on-chip technology. Advanced Drug Delivery Reviews 140: 68–77. DOI 10.1016/j.addr.2018.06.007. [Google Scholar] [CrossRef]
Collins C, Guilluy C, Welch C, O’Brien ET, Hahn K, Superfine R, Burridge K, Tzima E (2012). Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Current Biology 22: 2087–2094. DOI 10.1016/j.cub.2012.08.051. [Google Scholar] [CrossRef]
Conway DE, Schwartz MA (2014). Mechanotransduction of shear stress occurs through changes in VE-cadherin and PECAM-1 tension: Implications for cell migration. Cell Adhesion & Migration 9: 335–339. DOI 10.4161/19336918.2014.968498. [Google Scholar] [CrossRef]
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60. DOI 10.1126/science.1193270. [Google Scholar] [CrossRef]
Chatterjee S (2018). Endothelial mechanotransduction, redox signaling and the regulation of vascular inflammatory pathways. Frontiers in Physiology 9: 314. DOI 10.3389/fphys.2018.00524. [Google Scholar] [CrossRef]
Chen P, Feng X, Du W, Liu BF (2008). Microfluidic chips for cell sorting. Frontiers in Bioscience 13: 2464–2483. DOI 10.2741/2859. [Google Scholar] [CrossRef]
Chen Z, Tzima E (2009). PECAM-1 is necessary for flow-induced vascular remodeling. Arteriosclerosis, Thrombosis, and Vascular Biology 29: 1067–1073. DOI 10.1161/ATVBAHA.109.186692. [Google Scholar] [CrossRef]
Chiu J, Chien S (2011). Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiological Reviews 91: 327–387. DOI 10.1152/physrev.00047.2009. [Google Scholar] [CrossRef]
Davies PF (2007). Endothelial mechanisms of flow-mediated athero-protection and susceptibility. Circulation Research 101: 10–12. DOI 10.1161/CIRCRESAHA.107.156539. [Google Scholar] [CrossRef]
Davignon J, Ganz P (2004). Role of endothelial dysfunction in atherosclerosis. Circulation 109: III27–32. [Google Scholar]
de Nisco G, Hoogendoorn A, Chiastra C, Gallo D, Kok AM, Morbiducci U, Wentzel JJ (2020). The impact of helical flow on coronary atherosclerotic plaque development. Atherosclerosis 300: 39–46. DOI 10.1016/j.atherosclerosis.2020.01.027. [Google Scholar] [CrossRef]
Delmas P, Coste B (2013). Mechano-gated ion channels in sensory systems. Cell 155: 278–284. DOI 10.1016/j.cell.2013.09.026. [Google Scholar] [CrossRef]
Dewey CF, Bussolari SR, Gimbrone MA, Davies PF (1981). The dynamic response of vascular endothelial cells to fluid shear stress. Journal of Biomechanical Engineering 103: 177–185. DOI 10.1115/1.3138276. [Google Scholar] [CrossRef]
Dongaonkar RM, Stewart RH, Geissler HJ, Laine GA (2010). Myocardial microvascular permeability, interstitial oedema, and compromised cardiac function. Cardiovascular Research 87: 331–339. DOI 10.1093/cvr/cvq145. [Google Scholar] [CrossRef]
Dongeun H, Benjamin DM, Akiko M, Martín MZ, Hong YH, Donald EI (2010). Reconstituting organ-level lung functions on a chip. Science 328: 1662–1668. DOI 10.1126/science.1188302. [Google Scholar] [CrossRef]
Duncombe TA, Tentori AM, Herr AE (2015). Microfluidics: Reframing biological enquiry. Nature Reviews Molecular Cell Biology 16: 554–567. DOI 10.1038/nrm4041. [Google Scholar] [CrossRef]
Estrada R, Giridharan GA, Nguyen MD, Roussel TJ, Shakeri M, Parichehreh V, Prabhu SD, Sethu P (2011). Endothelial cell culture model for replication of physiological profiles of pressure, flow, stretch, and shear stress in vitro. Analytical Chemistry 83: 3170–3177. DOI 10.1021/ac2002998. [Google Scholar] [CrossRef]
Feige JJ, Pagès G, Soncin F (2014). Molecular mechanisms of angiogenesis (Chapter 4). pp. 77–97. Paris: Springer. DOI 10.1007/978-2-8178-0466-8. [Google Scholar] [CrossRef]
Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM (2003). Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circulation Research 93: H1145. DOI 10.1161/01.RES.0000101744.47866.D5. [Google Scholar] [CrossRef]
Foster GA, Gower RM, Stanhope KL, Havel PJ, Simon SI, Armstrong EJ (2013). On-chip phenotypic analysis of inflammatory monocytes in atherogenesis and myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America 110: 13944–13949. DOI 10.1073/pnas.1300651110. [Google Scholar] [CrossRef]
Frangos JA, Eskin SG, McIntire LV, Ives CJS (1985). Flow effects on prostacyclin production by cultured human endothelial cells. Science 227: 1477–1479. DOI 10.1126/science.3883488. [Google Scholar] [CrossRef]
Gallo D, Steinman DA, Bijari PB, Morbiducci U (2012). Helical flow in carotid bifurcation as surrogate marker of exposure to disturbed shear. Journal of Biomechanics 45: 2398–2404. DOI 10.1016/j.jbiomech.2012.07.007. [Google Scholar] [CrossRef]
Gautam M, Shen Y, Thirkill TL, Douglas GC, Barakat AI (2006). Flow-activated chloride channels in vascular endothelium. Shear stress sensitivity, desensitization dynamics, and physiological implications. Journal of Biological Chemistry 281: 36492–36500. DOI 10.1074/jbc.M605866200. [Google Scholar] [CrossRef]
Geiger B, Spatz JP, Bershadsky AD (2009). Environmental sensing through focal adhesions. Nature Reviews Molecular Cell Biology 10: 21–33. DOI 10.1038/nrm2593. [Google Scholar] [CrossRef]
Gilbert G, Ducret T, Savineau JP, Marthan R, Quignard JF (2016). Caveolae are involved in mechanotransduction during pulmonary hypertension. American Journal of Physiology-Lung Cellular and Molecular Physiology 310: L1078–L1087. DOI 10.1152/ajplung.00198.2015. [Google Scholar] [CrossRef]
Gordon E, Schimmel L, Frye M (2020). The importance of mechanical forces for in vitro endothelial cell biology. Frontiers in Physiology 11: 1084. DOI 10.3389/fphys.2020.00684. [Google Scholar] [CrossRef]
Gray BL, Lieu DK, Collins SD, Smith RL, Barakat AI (2002). Microchannel platform for the study of endothelial cell shape and function. Biomedical Microdevices 4: 9–16. DOI 10.1023/A:1014211627166. [Google Scholar] [CrossRef]
Hahn C, Schwartz MA (2009). Mechanotransduction in vascular physiology and atherogenesis. Nature Reviews Molecular Cell Biology 10: 53–62. DOI 10.1038/nrm2596. [Google Scholar] [CrossRef]
Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RMT (2015). Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosensors and Bioelectronics 63: 218–231. DOI 10.1016/j.bios.2014.07.029. [Google Scholar] [CrossRef]
Hannezo E, Heisenberg CP (2019). Mechanochemical feedback loops in development and disease. Cell 178: 12–25. DOI 10.1016/j.cell.2019.05.052. [Google Scholar] [CrossRef]
Herbert SP, Stainier DY (2011). Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nature Reviews Molecular Cell Biology 12: 551–564. DOI 10.1038/nrm3176. [Google Scholar] [CrossRef]
Huber F, Boire A, Lopez MP, Koenderink GH (2015). Cytoskeletal crosstalk: When three different personalities team up. Current Opinion in Cell Biology 32: 39–47. DOI 10.1016/j.ceb.2014.10.005. [Google Scholar] [CrossRef]
Israeli-Rosenberg S, Manso AM, Okada H, Ross RS (2014). Integrins and integrin-associated proteins in the cardiac myocyte. Circulation Research 114: 572–586. DOI 10.1161/CIRCRESAHA.114.301275. [Google Scholar] [CrossRef]
James BD, Allen JB (2018). Vascular endothelial cell behavior in complex mechanical microenvironments. ACS Biomaterials Science & Engineering 4: 3818–3842. DOI 10.1021/acsbiomaterials.8b00628. [Google Scholar] [CrossRef]
Jia L, Han F, Yang H, Turnbull G, Wang J, Clarke JV, Shu WM, Guo MY, Li B (2019). Microfluidic fabrication of biomimetic helical hydrogel microfibers for blood-vessel-on-a-chip applications. Advanced Healthcare Materials 8: 1900435. DOI 10.1002/adhm.201900435. [Google Scholar] [CrossRef]
Jin ZH, Liu YL, Fan WT, Huang WH (2019). Integrating flexible electrochemical sensor into microfluidic chip for simulating and monitoring vascular mechanotransduction. Small 16: 1903204. DOI 10.1002/smll.201903204. [Google Scholar] [CrossRef]
Jufri NF, Mohamedali A, Avolio A, Baker MS (2015). Mechanical stretch: Physiological and pathological implications for human vascular endothelial cells. Vascular Cell 7: 8. DOI 10.1186/s13221-015-0033-z. [Google Scholar] [CrossRef]
Kang L, Chung BG, Langer R, Khademhosseini A (2008). Microfluidics for drug discovery and development: From target selection to product lifecycle management. Drug Discovery Today 13: 1–13. DOI 10.1016/j.drudis.2007.10.003. [Google Scholar] [CrossRef]
Kaunas R, Nguyen P, Usami S, Chien S (2005). Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proceedings of the National Academy of Sciences of the United States of America 102: 15895–15900. DOI 10.1073/pnas.0506041102. [Google Scholar] [CrossRef]
Kim S, Chung M, Ahn J, Lee S, Jeon NL (2016). Interstitial flow regulates the angiogenic response and phenotype of endothelial cells in a 3D culture model. Lab on a Chip 16: 4189–4199. DOI 10.1039/C6LC00910G. [Google Scholar] [CrossRef]
Kurth F, Eyer K, Franco-Obregon A, Dittrich PS (2012). A new mechanobiological era: Microfluidic pathways to apply and sense forces at the cellular level. Current Opinion in Chemical Biology 16: 400–408. DOI 10.1016/j.cbpa.2012.03.014. [Google Scholar] [CrossRef]
Kurth F, Franco-Obregon A, Casarosa M, Kuster SK, Wuertz-Kozak K, Dittrich PS (2015). Transient receptor potential vanilloid 2-mediated shear-stress responses in C2C12 myoblasts are regulated by serum and extracellular matrix. FASEB Journal 29: 4726–4737. DOI 10.1096/fj.15-275396. [Google Scholar] [CrossRef]
Leckband DE, de Rooij J (2014). Cadherin adhesion and mechanotransduction. Annual Review of Cell and Developmental Biology 30: 291–315. DOI 10.1146/annurev-cellbio-100913-013212. [Google Scholar] [CrossRef]
Lee H, Kim S, Chung M, Kim JH, Jeon NL (2014). A bioengineered array of 3D microvessels for vascular permeability assay. Microvascular Research 91: 90–98. DOI 10.1016/j.mvr.2013.12.001. [Google Scholar] [CrossRef]
Lee J, Huh HK, Park SH, Lee SJ, Doh J (2018). Endothelial cell monolayer-based microfluidic systems mimicking complex in vivo microenvironments for the study of leukocyte dynamics in inflamed blood vessels. Methods in Cell Biology 146: 23–42. [Google Scholar]
Lewis DM, Gerecht S (2016). Microfluidics and biomaterials to study angiogenesis. Current Opinion in Chemical Engineering 11: 114–122. DOI 10.1016/j.coche.2016.02.005. [Google Scholar] [CrossRef]
Li N, Zhang W, Li Y, Lin JM (2019). Analysis of cellular biomolecules and behaviors using microfluidic chip and fluorescence method. TrAC Trends in Analytical Chemistry 117: 200–214. DOI 10.1016/j.trac.2019.05.029. [Google Scholar] [CrossRef]
Li YSJ, Haga JH, Chien S (2005). Molecular basis of the effects of shear stress on vascular endothelial cells. Journal of Biomechanics 38: 1949–1971. DOI 10.1016/j.jbiomech.2004.09.030. [Google Scholar] [CrossRef]
Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, Mckeown L, Young RS (2014). Piezo1 integration of vascular architecture with physiological force. Nature 515: 279–282. DOI 10.1038/nature13701. [Google Scholar] [CrossRef]
Lieu DK, Pappone PA, Barakat AI (2004). Differential membrane potential and ion current responses to different types of shear stress in vascular endothelial cells. American Journal of Physiology-Cell Physiology 286: C1367–C1375. DOI 10.1152/ajpcell.00243.2003. [Google Scholar] [CrossRef]
Liu X, Hymel LJ, Songu-Mize E (1998). Role of Na+ and Ca2+ in stretch-induced Na+-K+ -ATPase α-subunit regulation in aortic smooth. American Journal of Physiology 274: H83. DOI 10.1152/ajpcell.1998.274.1.C120. [Google Scholar] [CrossRef]
Liu X, Fan Y, Deng X (2010). Effect of spiral flow on the transport of oxygen in the aorta: A numerical study. Annals of Biomedical Engineering 38: 917–926. DOI 10.1007/s10439-009-9878-8. [Google Scholar] [CrossRef]
Liu X, Pu F, Fan Y, Deng X, Li D, Li S (2009). A numerical study on the flow of blood and the transport of LDL in the human aorta: the physiological significance of the helical flow in the aortic arch. American Journal of Physiology-Heart and Circulatory Physiology 297: H163–H170. DOI 10.1152/ajpheart.00266.2009. [Google Scholar] [CrossRef]
Liu X, Wang L, Wang Z, Li Z, Kang H, Fan Y, Sun A, Deng X (2016). Bioinspired helical graft with taper to enhance helical flow. Journal of Biomechanics 49: 3643–3650. DOI 10.1016/j.jbiomech.2016.09.028. [Google Scholar] [CrossRef]
Liu X, Wang M, Zhang N, Fan Z, Fan Y, Deng X (2015). Effects of endothelium, stent design and deployment on the nitric oxide transport in stented artery: A potential role in stent restenosis and thrombosis. Medical & Biological Engineering & Computing 53: 427–439. DOI 10.1007/s11517-015-1250-6. [Google Scholar] [CrossRef]
Luo N, Conwell MD, Chen X, Kettenhofen CI, Westlake CJ, Cantor LB, Wells CD, Weinreb RN, Corson TW, Spandau DF (2014). Primary cilia signaling mediates intraocular pressure sensation. Proceedings of the National Academy of Sciences of the United States of America 111: 12871–12876. DOI 10.1073/pnas.1323292111. [Google Scholar] [CrossRef]
Maiorana DA, O’Driscoll G, Taylor R, Green D (2003). Exercise and the nitric oxide vasodilator system. Sports Medicine 33: 1013–1035. DOI 10.2165/00007256-200333140-00001. [Google Scholar] [CrossRef]
Maoz BM, Herland A, FitzGerald EA, Grevesse T, Vidoudez C, Pacheco AR, Sheehy S, Park TE, Dauth S, Mannix R, Budnik N, Shores K, Cho A, Nawroth JC, Segrè D, Budnik B, Ingber DE, Parker K (2018). A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nature Biotechnology 36: 865–874. DOI 10.1038/nbt.4226. [Google Scholar] [CrossRef]
Mehta D, Malik AB (2006). Signaling mechanisms regulating endothelial permeability. Physiological Reviews 86: 279–367. DOI 10.1152/physrev.00012.2005. [Google Scholar] [CrossRef]
Mohammed M, Thurgood P, Gilliam C, Nguyen N, Pirogova E, Peter K, Khoshmanesh K, Baratchi S (2019). Studying the response of aortic endothelial cells under pulsatile flow using a compact microfluidic system. Analytical Chemistry 91: 12077–12084. DOI 10.1021/acs.analchem.9b03247. [Google Scholar] [CrossRef]
Morbiducci U, Ponzini R, Rizzo G, Cadioli M, Esposito A, Montevecchi FM, Redaelli A (2011). Mechanistic insight into the physiological relevance of helical blood flow in the human aorta: An in vivo study. Biomechanics and Modeling in Mechanobiology 10: 339–355. DOI 10.1007/s10237-010-0238-2. [Google Scholar] [CrossRef]
Nourse JL, Pathak MM (2017). How cells channel their stress: Interplay between Piezo1 and the cytoskeleton. Seminars in Cell & Developmental Biology 71: 3–12. DOI 10.1016/j.semcdb.2017.06.018. [Google Scholar] [CrossRef]
O’Rourke MF (1995). Mechanical principles in arterial disease. Hypertension 26: 2–9. DOI 10.1161/01.HYP.26.1.2. [Google Scholar] [CrossRef]
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L (2006). VEGF receptor signalling-in control of vascular function. Nature Reviews Molecular Cell Biology 7: 359–371. DOI 10.1038/nrm1911. [Google Scholar] [CrossRef]
Panciera T, Azzolin L, Cordenonsi M, Piccolo S (2017). Mechanobiology of YAP and TAZ in physiology and disease. Nature Reviews Molecular Cell Biology 18: 758–770. DOI 10.1038/nrm.2017.87. [Google Scholar] [CrossRef]
Patibandla PK, Rogers AJ, Giridharan GA, Pallero MA, Murphy-Ullrich JE, Sethu P (2014). Hyperglycemic arterial disturbed flow niche as an in vitro model of atherosclerosis. Analytical Chemistry 86: 10948–10954. DOI 10.1021/ac503294p. [Google Scholar] [CrossRef]
Qian S, Ma T, Zhang N, Liu X, Zhao P, Li X, Chen D, Hu L, Chang L, Xu L, Deng X, Fan Y (2020). Spatiotemporal transfer of nitric oxide in patient-specific atherosclerotic carotid artery bifurcations with MRI and computational fluid dynamics modeling. Computers in Biology and Medicine 125: 104015. DOI 10.1016/j.compbiomed.2020.104015. [Google Scholar] [CrossRef]
Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B (2014). Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proceedings of the National Academy of Sciences of the United States of America 111: 10347–10352. DOI 10.1073/pnas.1409233111. [Google Scholar] [CrossRef]
Recho P, Putelat T, Truskinovsky L (2013). Contraction-driven cell motility. Physical Review Letters 111: 629. DOI 10.1103/PhysRevLett.111.108102. [Google Scholar] [CrossRef]
Rode B, Shi J, Endesh N, Drinkhill MJ, Webster PJ, Lotteau SJ, Bailey MA, Yuldasheva NY, Ludlow MJ, Cubbon RM (2017). Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nature Communications 8: 1053. DOI 10.1038/s41467-017-00429-3. [Google Scholar] [CrossRef]
Ruoslahti E (1996). RGD and other recognition sequences for integrins. Annual Review of Cell and Developmental Biology 12: 697–715. DOI 10.1146/annurev.cellbio.12.1.697. [Google Scholar] [CrossRef]
Secomb TW (2016). Hemodynamics. Comprehensive Physiology 6: 975–1003. [Google Scholar]
Sellgren KL, Hawkins BT, Grego S (2015). An optically transparent membrane supports shear stress studies in a three-dimensional microfluidic neurovascular unit model. Biomicrofluidics 9: 687. DOI 10.1063/1.4935594. [Google Scholar] [CrossRef]
Semenza GL (2007). Vasculogenesis, angiogenesis, and arteriogenesis: Mechanisms of blood vessel formation and remodeling. Journal of Cellular Biochemistry 102: 840–847. DOI 10.1002/jcb.21523. [Google Scholar] [CrossRef]
Serlin Y, Shelef I, Knyazer B, Friedman A (2015). Anatomy and physiology of the blood-brain barrier. Seminars in Cell & Developmental Biology 38: 2–6. DOI 10.1016/j.semcdb.2015.01.002. [Google Scholar] [CrossRef]
Shao J, Wu L, Wu J, Zheng Y, Zhao H, Lou X, Jin Q, Zhao J (2010). A microfluidic chip for permeability assays of endothelial monolayer. Biomedical Microdevices 12: 81–88. DOI 10.1007/s10544-009-9362-0. [Google Scholar] [CrossRef]
Shihata WA, Michell DL, Andrews KL, Chin-Dusting JP (2016). Caveolae: A role in endothelial inflammation and mechanotransduction? Frontiers in Physiology 7: R1222. DOI 10.3389/fphys.2016.00628. [Google Scholar] [CrossRef]
Shirure VS, Lezia A, Tao A, Alonzo LF, George SC (2017). Low levels of physiological interstitial flow eliminate morphogen gradients and guide angiogenesis. Angiogenesis 20: 493–504. DOI 10.1007/s10456-017-9559-4. [Google Scholar] [CrossRef]
Skorupska S, Jastrzebska E, Chudy M, Dybko A, Brzozka Z (2018). Microfluidic systems, Cardiac Cell Culture Technologies. pp. 3–21. Cham: Springer. [Google Scholar]
Spruell C, Baker AB (2013). Analysis of a high-throughput cone-and-plate apparatus for the application of defined spatiotemporal flow to cultured cells. Biotechnology and Bioengineering 110: 1782–1793. DOI 10.1002/bit.24823. [Google Scholar] [CrossRef]
Su H, Liu X, Du J, Deng X, Fan Y (2020). The role of hemoglobin in nitric oxide transport in vascular system. Medicine in Novel Technology and Devices 5: 100034. DOI 10.1016/j.medntd.2020.100034. [Google Scholar] [CrossRef]
Tarbell JM (2003). Mass transport in arteries and the localization of atherosclerosis. Annual Review of Biomedical Engineering 5: 79–118. DOI 10.1146/annurev.bioeng.5.040202.121529. [Google Scholar] [CrossRef]
Tejero J, Shiva S, Gladwin MT (2019). Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiological Reviews 99: 311–379. DOI 10.1152/physrev.00036.2017. [Google Scholar] [CrossRef]
Toh YC, Voldman J (2011). Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction. FASEB Journal 25: 1208–1217. DOI 10.1096/fj.10-168971. [Google Scholar] [CrossRef]
Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao GY, DeLisser H, Schwartz MA (2005). A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431. DOI 10.1038/nature03952. [Google Scholar] [CrossRef]
van der Helm MW, van der Meer AD, Eijkel JC, van den Berg A, Segerink LI (2016). Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 4: e1142493. DOI 10.1080/21688370.2016.1142493. [Google Scholar] [CrossRef]
Varma S, Garcia-Cardena G, Voldman J (2020). Unraveling endothelial cell phenotypic regulation by spatial hemodynamic flows with microfluidics. 6: 16. DOI 10.1101/2020.08.28.268599. [Google Scholar] [CrossRef]
Vedel S, Olesen LH, Bruus H (2010). Pulsatile microfluidics as an analytical tool for determining the dynamic characteristics of microfluidic systems. Journal of Micromechanics and Microengineering 20: 035026. DOI 10.1088/0960-1317/20/3/035026. [Google Scholar] [CrossRef]
Venugopal Menon N, Tay HM, Pang KT, Dalan R (2018). A tunable microfluidic 3D stenosis model to study leukocyte-endothelial interactions in atherosclerosis. APL Bioengineering 2: 016103. DOI 10.1063/1.4993762. [Google Scholar] [CrossRef]
Venugopal Menon N, Tay HM, Pang KT, Dalan R Wong SC et al. (2018). A tunable microfluidic 3D stenosis model to study leukocyte-endothelial interactions in atherosclerosis. APL Bioengineering 2: 016103. DOI 10.1063/1.4993762. [Google Scholar] [CrossRef]
Volkers L, Mechioukhi Y, Coste B (2015). Piezo channels: From structure to function. Pflügers Archiv-European Journal of Physiology 467: 95–99. DOI 10.1007/s00424-014-1578-z. [Google Scholar] [CrossRef]
Walter FR, Valkai S, Kincses A, Petneházi A, Czeller T et al. (2016). A versatile lab-on-a-chip tool for modeling biological barriers. Sensors and Actuators B: Chemical 222: 1209–1219. DOI 10.1016/j.snb.2015.07.110. [Google Scholar] [CrossRef]
Wang N (2017). Review of cellular mechanotransduction. Journal of Physics D: Applied Physics 50: 233002. DOI 10.1088/1361-6463/aa6e18. [Google Scholar] [CrossRef]
Wang S, Iring A, Strilic B, Juárez JA, Kaur H, Troidl K, Tonack S, Burbiel JC, Müller CE, Fleming I (2015). P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. Journal of Clinical Investigation 125: 3077–3086. DOI 10.1172/JCI81067. [Google Scholar] [CrossRef]
Wang YX, Xiang C, Liu B, Zhu Y, Luan Y, Liu S, Qin K (2016). A multi-component parallel-plate flow chamber system for studying the effect of exercise-induced wall shear stress on endothelial cells. Biomedical Engineering Online 15: 659–672. [Google Scholar]
Weinbaum S, Cancel LM, Fu BM, Tarbell JM (2020). The glycocalyx and its role in vascular physiology and vascular related diseases. Cardiovascular Engineering and Technology 557: 889. DOI 10.1007/s13239-020-00485-9. [Google Scholar] [CrossRef]
Westein E, van der Meer AD, Kuijpers MJ, Frimat JP, van den Berg A, Heemskerk J (2013). Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proceedings of the National Academy of Sciences of the United States of America 110: 1357–1362. DOI 10.1073/pnas.1209905110. [Google Scholar] [CrossRef]
Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC (2013). The blood-brain barrier: An engineering perspective. Frontiers in Neuroengineering 6: 7. DOI 10.3389/fneng.2013.00007. [Google Scholar] [CrossRef]
Wong JF, Mohan MD, Young EWK, Simmons CA (2020). Integrated electrochemical measurement of endothelial permeability in a 3D hydrogel-based microfluidic vascular model. Biosensors and Bioelectronics 147: 111757. DOI 10.1016/j.bios.2019.111757. [Google Scholar] [CrossRef]
Xu S, Li X, Liu Y, He P (2016). Development and characterization of in vitro microvessel network and quantitative measurements of endothelial [Ca2+]i and nitric oxide production. JoVE (Journal of Visualized Experiments) 111: e54014. [Google Scholar]
Young EWK, Simmons CA (2010). Macro- and microscale fluid flow systems for endothelial cell biology. Lab on a Chip 10: 143–160. DOI 10.1039/B913390A. [Google Scholar] [CrossRef]
Yum K, Hong SG, Healy KE, Lee LP (2014). Physiologically relevant organs on chips. Biotechnology Journal 9: 16–27. DOI 10.1002/biot.201300187. [Google Scholar] [CrossRef]
Zanotelli MR, Ardalani H, Zhang J, Hou Z, Nguyen EH, Swanson S, Nguyen BK, Bolin J, Elwell A, Bischel LL (2016). Stable engineered vascular networks from human induced pluripotent stem cell-derived endothelial cells cultured in synthetic hydrogels. Acta Biomaterialia 35: 32–41. DOI 10.1016/j.actbio.2016.03.001. [Google Scholar] [CrossRef]
Zaromitidou M, Siasos G, Papageorgiou N, Oikonomou E, Tousoulis D (2016). Atherosclerosis and coronary artery disease. Cardiovascular Diseases. pp. 3–24. Academic Press. [Google Scholar]
Zeller T, Gaines PA, Ansel GM, Caro CG (2016). Helical centerline stent improves patency: Two-year results from the randomized mimics trial. Circulation: Cardiovascular Interventions 9: e002930. DOI 10.1161/CIRCINTERVENTIONS.115.002930. [Google Scholar] [CrossRef]
Zeng Y, Zhang XF, Fu BM, Tarbell JM (2018). The role of endothelial surface glycocalyx in mechanosensing and transduction. Molecular, Cellular, and Tissue Engineering of the Vascular System 1097: 1–27. [Google Scholar]
Zhang B, Korolj A, Lai BFL, Radisic M (2018). Advances in organ-on-a-chip engineering. Nature Reviews Materials 3: 257–278. DOI 10.1038/s41578-018-0034-7. [Google Scholar] [CrossRef]
Zhang Q, Gao B, Chang Y (2018). Helical flow component of Left Ventricular Assist Devices (LVADs) outflow improves aortic hemodynamic states. Medical Science Monitor 24: 869–879. DOI 10.12659/MSM.905940. [Google Scholar] [CrossRef]
Zhao P, Liu X, Zhang X, Wang L, Su H, Wang L, He N, Zhang D, Li Z, Kang H, Sun A, Chen Z, Zhou L, Wang M, Zhang Y, Deng X, Fan Y (2020). Flow shear stress controls the initiation of neovascularization via heparan sulfate proteoglycans within biomimic microfluidic model. Lab on a Chip 21: 421–434. [Google Scholar]
Zheng W, Huang R, Jiang B, Zhao Y, Zhang W, Jiang X (2016). An early-stage atherosclerosis research model based on microfluidics. Small 12: 2022–2034. DOI 10.1002/smll.201503241. [Google Scholar] [CrossRef]
Zheng W, Jiang B, Wang D, Zhang W, Wang Z, Wang Z, Jiang X (2012). A microfluidic flow-stretch chip for investigating blood vessel biomechanics. Lab on a Chip 12: 3441–3450. DOI 10.1039/c2lc40173h. [Google Scholar] [CrossRef]
Zhou J, Li YS, Chien S (2014). Shear stress-initiated signaling and its regulation of endothelial function. Arteriosclerosis, Thrombosis, and Vascular Biology 34: 2191–2198. DOI 10.1161/ATVBAHA.114.303422. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |