DOI:10.32604/biocell.2021.015360

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.015360 |  www.techscience.com/journal/biocell |
| Review |
Oxidative metabolism of photosynthetic species and the exposure to some freshwater and marine biotoxins
1Universidad de Buenos Aires, Facultad de Farmacia y Bioquímica, Fisicoquímica, Buenos Aires, CP 1113, Argentina
2CONICET-Universidad de Buenos Aires. Instituto de Bioquímica y Medicina Molecular (IBIMOL), Buenos Aires, CP 1113, Argentina
*Address correspondence to: Paula Mariela González, paulag@ffyb.uba.ar
Received: 12 December 2020; Accepted: 01 February 2021
Abstract: Environmental climate conditions could lead to an increasing global occurrence of microorganism blooms that synthesize toxins in the aquatic environments. These blooms could result in significantly toxic events. Responses of photosynthetic organisms to adverse environmental conditions implicate reactive oxygen species generation; but, due to the presence of a varied cellular antioxidant defense system and complex signaling networks, this oxidative stress could act as an important factor in the environmental adaptive processes. The objective of this review was to assess how some biotoxins are implicated in the generation of oxidative and nitrosative metabolic changes, not only in biotoxin-producing organisms but also in non-producing organisms. Therefore, toxins may modify the oxidative cellular balance of several other species. Hence, the effect of toxins on the oxidative and nitrosative conditions will be evaluated in freshwater and marine algae and vascular plants. The changing climate conditions could act as agents capable of modifying the community composition leading to alterations in the global health of the habitat, risking the survival of many species with ecological relevance.
Keywords: Blooms; Climate change; Metabolic stress; Photosynthetic organisms; Toxins
Certain species of bacteria, cyanobacteria, dinoflagellates, ciliates, raphidophytes, haptophytes, dinophyceaes, rhodophytes, diatoms and corals synthesize toxic compounds (e.g., biotoxins) (Botana et al., 2019; Vilariño et al., 2018). These molecules can modify the cellular process of other organisms, from plankton to humans. Harmful algae blooms (HAB) are defined as a group of toxic species present both, in freshwater and marine ecosystems. The presence of biotoxins in waters used for consumption, irrigation and recreational purposes represents a public safety concern, implicating also economic risks. Agricultural and aquaculture products are directly affected, altering the consumption of vegetables and animals. Therefore, many toxins are a serious threat to human health linked to poisoning after consumption of contaminated seafood, skin contact with contaminated water, or inhalation (Vilariño et al., 2018).
In the freshwater environment, cyanobacteria species are important producers of different types of cyanotoxins (Singh and Dhar, 2013). The sources and acute toxicities of cyanobacterial toxins, such as cylindrospermopsin (CYN), anatoxin-a (ATX-a), nodularin (NOD), and microcystins (MC), have been described (González-Jartín et al., 2020). On the other hand, marine biotoxins represent a diverse group that includes saxitoxin (STX), domoic acid (DA), ciguatoxin (CTX), yessotoxins (YTX), pectenotoxins (PTXs), brevetoxin (BTX), tetrodotoxin (TTX), okadaic acid (OA), azaspiracid (AZA), palytoxin (PLTX) and many other emerging toxins such as spirolides (SPX) (Otero et al., 2019; Krock et al., 2018; Vilariño et al., 2018). Even more, some of these toxins can be found in both ecosystems, marine and freshwater bodies.
Toxins alter photosynthetic organisms in different ways, such as decreasing the abundance of submerged plants and the diversity of aquatic plant communities (Burkholder et al., 2018). Biotoxins can also contribute to the inhibition of the net photosynthetic rate (Liang and Wang, 2015), alter the growth and development (McElhiney et al., 2001), and reduce the dry weight and the access to nutrients and oxygen (O2) (Yamasaki, 1993). However, not much information has been described in terms of oxidative stress triggering in photosynthetic organisms caused by biotoxins. Recently, changes in oxidative and nitrosative stress associated with HAB have been described in invertebrates (González and Puntarulo, 2016, 2020).
A Special Report from the United Nations’ Intergovernmental Panel on Climate Change (IPCC) in September 2019 was the first IPCC statement to directly link HAB to climate change (Gobler, 2020). Therefore, climate change not only produces direct harm to aquatic organisms but can also modify the distribution and intensity of multiple co-stressors within marine and freshwater ecosystems (Griffith and Gobler, 2020). Also, water pollution and eutrophication contribute to making more intense and frequent the blooms of biotoxin producers. The increment in frequency and magnitude of the HAB phenomena is evident all over the world (Gobler, 2020; Wells et al., 2015). Surprisingly, Krock et al. (2020) reported the presence of a phycotoxin produced by the dinoflagellate Dinophysis sp. in Antarctic waters. Hernando et al. (2018) and Wells et al. (2015) have associated climate change with alterations in the structure and composition of some phytoplanktonic communities, suggesting a prevalence and geographical spread of HAB. Therefore, climate change would lead to an overproduction of biotoxins by many photosynthetic species (Botana et al., 2010).
Widespread information alerts that this increased availability of biotoxins both in freshwater and seawater environments, could have an impact on the trophic chain in terms of alterations in the oxidative balance in many photosynthetic organisms of the aquatic community. It was reported that several biotoxins, such as MC, CYN, STX, and NOD, are found dissolved in freshwater environments (Doucette et al., 2018); thus, the effects on microalgae and vascular plants were studied. On the other hand, NOD and DA can be found dissolved in marine waters due to their hydrophilic nature (Doucette et al., 2018), and their effects were analyzed in marine macro and microalgae. Based on this information, the objective of this review was to assess how these biotoxins are implicated in the generation of oxidative and nitrosative metabolic changes from not only biotoxin-producing organisms but also from non-producing organisms and therefore modifying the oxidative cellular balance of several other species.
Oxidative Stress Generated by HAB
The generation of reactive O2 species (ROS), such as superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH), takes place continuously in living cells, mainly as a byproduct of respiration (Abele and Puntarulo, 2004). They may particularly damage targeting proteins, lipids, and nucleic acids, often leading to cumulative injury (Lushchak, 2011). Harmful effects of ROS generation are due to the increase in their steady-state concentration either by the enhancement of their production rates and/or the decrease of the antioxidant activity.
In freshwater and marine areas of elevated biological productivity, such as phytoplankton blooms, biological production of ROS can be a substantial and dominant ROS source, especially of O2− and H2O2 (Diaz and Plummer, 2018; Marsico et al., 2015; Rusak et al., 2011). Many phytoplankton taxa also produce extracellular ROS under optimal growth conditions in culture. Extracellular ROS production has been quantified in at least 21 eukaryotic phytoplankton species. The majority of these species are capable of triggering HAB (Diaz and Plummer, 2018). Harmful algae species generate much more extracellular ROS than any other phytoplankton taxa. Some biotoxins, like MC, are known to have positive effects as protective elements against reactive species. Therefore, the capacity of some cyanobacteria to produce MC may be a potential selective factor in competing with non-MC-producing strains. This fact was already observed in nitrogen-limited and UV B radiation conditions (Ding et al., 2013). It has been shown that MC prevents oxidative stress (Van de Waal et al., 2011; Zilliges et al., 2011). Recently, Malanga et al. (2019) presented the first in vitro report of the role of MC as an antioxidant for hydrophilic radicals, suggesting that ascorbyl (A•) and •OH radicals could be efficiently quenched by the administration of [D-Leu1] MC-LR.
On the other hand, some raphidophytes species and the dinoflagellate Cochlodinium polykrikoides are known to generate ROS (Kim et al., 1999a; Twiner and Trick, 2000), which can have adverse effects on aquatic organisms. The raphidophyte Chattonella marina generates •OH, and its small and large verruciform protrusions on the cell surface are probably involved in the production of O2− (Kim et al., 1999b; Shimada et al., 1993). Extracellular ROS production by the harmful bloom-forming phytoplankton species C. marina has been implicated in its toxicity (Kim and Oda, 2010). All raphidophytes that produced ROS inhibited the growth of the marine bacterium Vibrio alginolyticus (Oda et al., 1992). The production of small quantities of H2O2 has also been detected in the dinoflagellates Prorocentrum micans and Akashiwo sanguinea (Daugbjerg et al., 2000), and high reactive species concentrations were found in stressed Alexandrium tamarense (Jauzein and Erdner, 2013). The production of ROS by microalgae appears to principally affect fish (Ishimatsu et al., 1996); however, other organisms may also be disturbed. Some fish mortalities may be attributable to the production of brevetoxin-like compounds, to ROS, or a combination of both. Reactive species contribute to pathological changes in the gills of fish, and the brevetoxin-like neurotoxins impair cardiac function (Endo et al., 1992). During plant photosynthesis and metabolism ROS are also generated and are increased in plants exposed to environmental stresses (Dring, 2006).
As it will be shown in this review, the magnitude of the oxidative stress and the specific mechanism of cytotoxicity depend on parameters related to both the photosynthetic species and the factors related to the biotoxin features (Fig. 1). Thus, oxidative stress studies should be faced taking special attention to the organism and the tissue under consideration, as well as the type, concentration, and exposure time to the biotoxin.
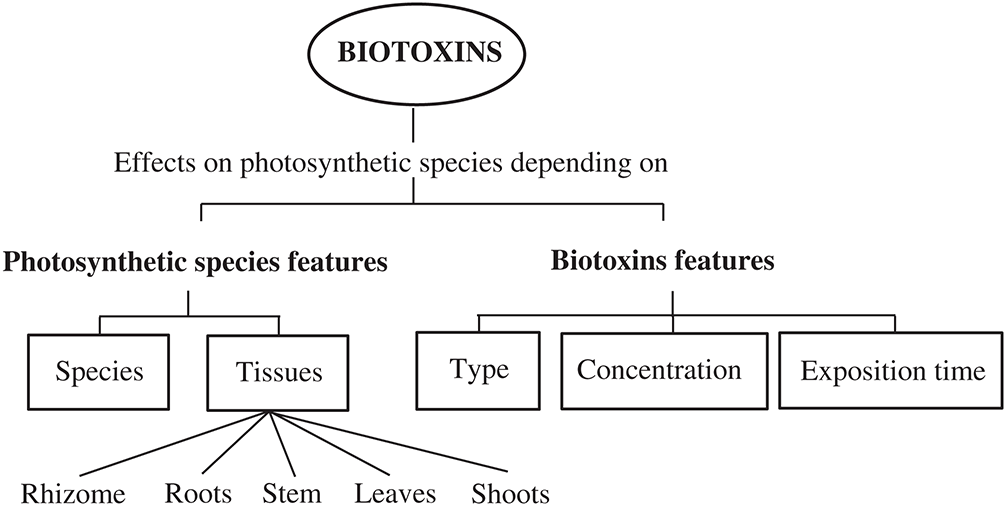
Figure 1: Critical factors that modify the oxidative effects and the mechanisms of cytotoxicity of the biotoxins on photosynthetic organisms.
Oxidative and nitrosative effects of biotoxins on freshwater microalgae and plants
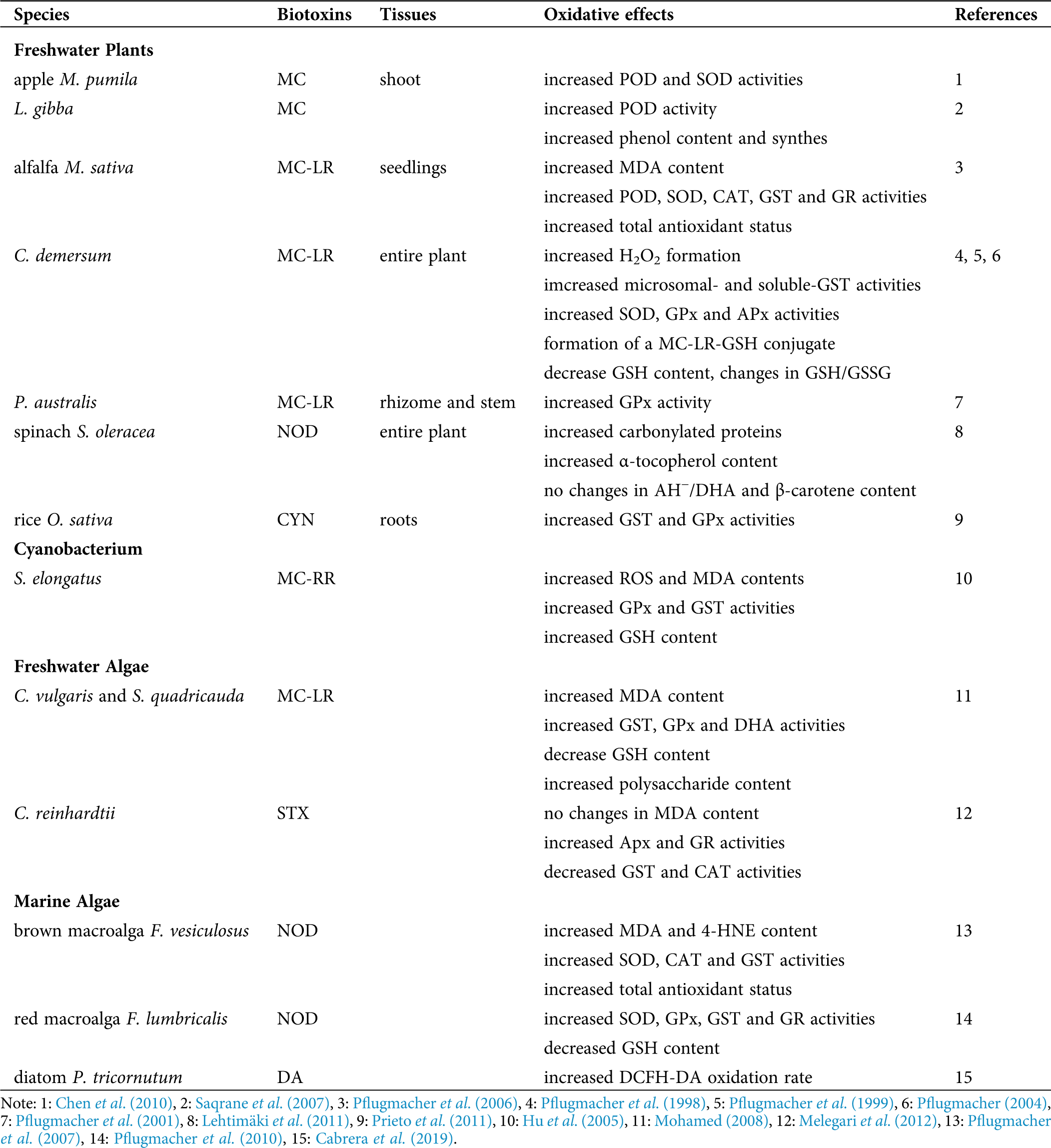
Oxidative damage generated in the lipidic fraction of cells was reported in relation to biotoxin’s exposure in plants and algae. As an indicator of lipid peroxidation, malondialdehyde (MDA) content was shown to be significantly increased in alfalfa Medicago sativa seedlings exposed to MC-LR for 7 days (Pflugmacher et al., 2006). Content of MDA was increased after 3 days of incubation of the green algae Chlorella vulgaris and Scenedesmus quadricauda (commonly associated with toxic cyanobacteria) in an MC-LR concentration-dependent manner, and it was restored to control levels after 14 days of exposure (Mohamed, 2008). The increase in the activities of glutathione S-transferase (GST) and glutathione peroxidase (GPx) and the decrease in the reduced glutathione (GSH) content in both algae species limited the lipid damage restoring the MDA levels. Moreover, antioxidant activities were reestablished to control levels after 3 days of incubation, remaining close to those in control cultures after 14 days of exposure. The changes in these variables correlated with rises in the polysaccharide content, indicating for the first time the involvement of these polysaccharides in protecting the algal cells against MC-induced oxidative stress. Also, the reduction in ROS production correlated only with an increase in the polysaccharide content in toxin-treated cultures, indicating that these polysaccharides were involved in trapping ROS, acting as protective elements. Moreover, the antioxidant activities of these polysaccharides were concentration-dependent. Similarly, Hu et al. (2005) reported elevated ROS and MDA content in the cyanobacterium Synechococcus elongatus exposed to MC-RR. Consequently, an increase in the GSH levels and activities of GPx and GST was triggered in treated cells, compared to control ones.
Melegari et al. (2012) reported a significant decrease in GST and catalase (CAT) activities and increases in the activities of ascorbate peroxidase (APx) and glutathione reductase (GR), as compared to control cultures, with non-significant changes in MDA content in the unicellular algae Chlamydomonas reinhardtii after exposure to STX, probably due to the antioxidant response. Prieto et al. (2011) reported a significant increase in GST and GPx activities in the roots of the rice Oryza sativa after the exposure to CYN produced by the cyanobacteria Aphanizomenon ovalisporum. Pflugmacher et al. (1998) and Pflugmacher et al. (1999) showed that exposure of the entire freshwater plant Ceratophyllum demersum to MC-LR resulted in an elevation of microsomal- and soluble-GST activities and the formation of an MC-LR-GSH conjugate as the first step of the detoxification pathway. Exposure to MC-LR also led to a depletion of the intracellular GSH level, and the change in the value of the GSH/oxidized glutathione (GSSG) ratio was a clear sign of stress in the plant, possibly affecting other metabolic pathways. Elevation of activity of the antioxidant enzymes superoxide dismutase (SOD), GPx, and APx indirectly indicated the triggering of detoxification processes to face the enhanced formation of H2O2 in the plant (Pflugmacher, 2004). Even more, the dehydroascorbate reductase (DHA), one of the connecting enzymes between the GSH-cycle and the ascorbate (AH−)-cycle, was significantly elevated after exposure to MC-LR. This elevation might indicate the need to keep AH− in the reduced state required for the H2O2 detoxification.
Pflugmacher et al. (2001) reported a significant elevation of soluble GST activity in the rhizome and stem parts of Phragmites australis after 24 h of exposure to MC-LR. A significant increase in activities of the antioxidant enzymes peroxidase (POD), SOD, CAT, GST, and GR and in the total antioxidant status in M. sativa seedlings exposed to MC-LR was also observed (Pflugmacher et al., 2006). Chen et al. (2010) reported that in shoot cultures of the apple Malus pumila, MC exposure produced a significant increase in the POD and SOD activities. The POD activity on the free-floating aquatic vascular plant Lemna gibba exposed to MC during 24 h increased by 19% relative to the control, but at higher MC concentration, the activity reminded to control values (Saqrane et al., 2007). An MC dose-dependent increase in phenol content, and the presence of newly synthesized phenolic compounds, were reported in the plant cultures treated with MC, which may be associated with detoxication processes in the plant (Saqrane et al., 2007).
Spinacia oleracea plants exposed to NOD suffered from oxidative stress, as evidenced by a high level of carbonylated proteins, altered levels of enzymatic and non-enzymatic antioxidants (Lehtimäki et al., 2011). The content of APx enzymes was decreased at the thylakoid and stromal cell part, but less drastic changes were detected in the cytoplasmic APx and SOD. Even more, no significant differences in the ratio of AH−/DHA and the content of β-carotene were detected in the treated spinach plants as compared to control ones. However, α-tocopherol content in NOD-treated plants was 1.3-fold higher than in the controls.
Nitric oxide (NO) itself is a rather weak oxidant in comparison with ROS; however, NO can be converted to peroxynitrite (ONOO−) in the presence of O2− (Radi et al., 1991). ONOO− is considered the major cytotoxic agent of reactive nitrogen species (RNS) (Arteel et al., 1999). There are many potential targets of RNS action, including proteins containing transition metal ions either as heme or non-heme complexes, mainly Fe-sulphur centers (Wojtaszek, 2000). Estevez and Puntarulo (2005) reported a significant increase in the NO content at the triggering of the log phase of growth in the Antarctic freshwater algae Chlorella and Chlamydomona sp. This fact suggested that NO could be part of the signaling network operative under specific growing conditions, such as low-temperature stress, and could be an adaptive mechanism to allow optimal cell survival. Even more, mussel hemocytes, cells that represent the key defense element against pathogens, were shown to significantly increase the NO production during a HAB season (González and Puntarulo, 2020). However, the effect of environmental condition changes (e.g., biotoxins released by HAB) on RNS production in photosynthetic organisms under global climate change remains to be studied.
Different oxidative effects were recorded along the years in photosynthetic organisms exposed to biotoxins either in freshwater or marine environments. Tab. 1 summarizes the main features of the information reported in this review.
Table 1: Summary of the main oxidative effects of biotoxins reported in photosynthetic organisms
Oxidative effects of biotoxins on marine algae
The cyclic pentapeptide NOD, produced by the cyanobacteria Nodularia spumigena induced lipid damage in the brown macroalga Fucus vesiculosus (Pflugmacher et al., 2007), and an increase in the concentration of the lipid metabolites MDA and a (4-HNE) was reported. Furthermore, a significant increase in the activities of SOD, CAT and GST, and the total antioxidant status, as compared to controls, was observed. In addition, the red macroalga Furcellaria lumbricalis exposed to NOD increased SOD, GPx, GST and GR activities, and decreased GSH content, as compared to controls (Pflugmacher et al., 2010).
Some diatoms, such as Pseudo-nitzschia australis, produce the toxin DA, but it is not yet clear how DA may affect the oxidative cellular condition on the non-target aquatic organisms, such as the pennate diatom Phaeodactilum tricornutum. The generation of active species, followed by the oxidation rate of 2’,7’ dichlorofluorescein diacetate (DCFH-DA), showed a significant increase in the oxidation rate of the dye in the DA-exposed diatoms in the exponential phase of growth, as compared to controls (Cabrera et al., 2019). By assaying the effect of radical scavengers, it was suggested a similar contribution of several oxidants (H2O2, Fe, and O2−) in the increase of the generation of active species in the diatoms facing the DA exposure.
Diagram in Fig. 2 briefly refers to both, the methods used to assess the oxidative conditions after exposure to biotoxins, and the triggering of the antioxidant system. This critical response of the defense mechanism of photosynthetic organisms might result in the adequate management of the damage produced by biotoxins. The possible contribution of other compounds such as phenols, polysaccharides, and GSH should not be discarded in terms of their participation in the overall protective network triggered against the effect of the biotoxins. It is known that phenolic compounds may suppress or delay the spontaneous autoxidation of organic molecules acting as antioxidants ‘neutralizing’ peroxyl radicals responsible for lipid peroxidation propagation (Foti, 2007). Also, marine polysaccharides could scavenge ROS, regulate the antioxidant system and/or oxidative stress-mediated signaling pathways (Zhong et al., 2019). Nevertheless, lipid damage is detected in many conditions tested, mainly due to the overproduction of dangerous reactive species formation.
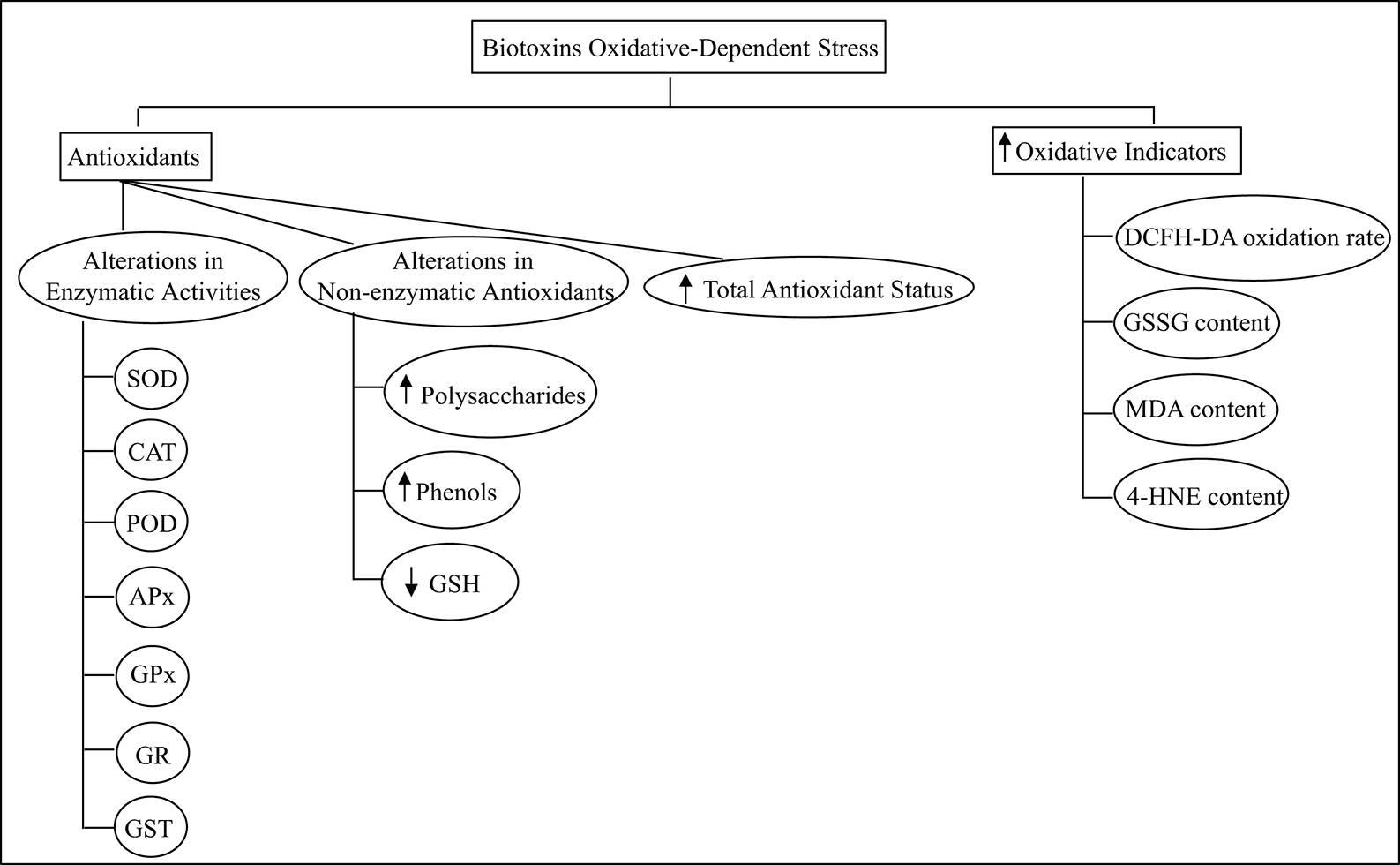
Figure 2: Summary of the oxidative indicators and the antioxidant components of the photosynthetic species defense network assessed to study the biotoxins oxidative-dependent alterations.
Even more, the activities of the enzymes thioredoxins and glutaredoxin are understood as redox controlling enzymes (Meyer et al., 2012). Recent studies by Ventoso et al. (2019) described that the digestive gland of the queen scallop Aequipecten opercularis exposed to DA-producing Pseudo-nitzschia, caused oxidative stress and mitochondrial dysfunction and that the transcriptional response counteracting these effects includes the up-regulation of genes coding for some mitochondrial proteins, proteasome components, and antioxidant enzymes (e.g., thioredoxins and glutaredoxins). Moreover, Chen et al. (2018) reported that blooms of Karenia brevis, which produce brevetoxins, inhibited some forms of NADPH-dependent thioredoxin reductase in the same species.
Alterations of the environmental conditions, such as increases in nitrate (NO3−), phosphate (PO43−) and Fe nutrients, among other factors, are important contributors for the increasing global occurrence of biotoxins blooms (Heisler et al., 2008; Fisher et al., 2006). These blooms would be responsible for releasing biotoxins and increasing ROS in the aquatic environment, where photosynthetic organisms in contact with them will face metabolic changes.
Toxins may reach photosynthetic organisms through different pathways. Aquatic macrophytes and microalgae are naturally into direct contact with these toxins not only in eutrophic water bodies but also seasonally when environmental conditions favor the development of toxic blooms. On the other hand, terrestrial plants are exposed to cyanotoxins via contaminated soils or the irrigation water taken from polluted sources (Chen et al., 2010). The main MC uptake route for P. australis appeared to be the stem and rhizome, from which the toxin is transported into the higher parts of the plant to the leaves (Pflugmacher et al., 2001). The CYN was mainly absorbed by the roots of O. sativa to be further translocated into the leaves (Prieto et al., 2011). In marine organisms, for instance, detectable concentrations of NOD were observed in different tissues of F. vesiculosus, especially in the stem and basal parts (Pflugmacher et al., 2007).
Little information is available about the specific cell targets of biotoxins on photosynthetic organisms. However, it is generally known that the toxic mechanism of MC and NOD is the inhibition of protein phosphatases 1 and 2A in plant cells (Pereira et al., 2011; Peuthert et al., 2008). It is also recognized that CYN may act through the inhibition of GSH and protein synthesis (Froscio et al., 2001; Froscio et al., 2008), but the process may be mediated by cytochrome P-450 generated metabolites (Humpage et al., 2005). Moreover, STX acts as a potent blocker of sodium channels in nerve axons of vertebrates, and its neurotoxicity and potentially lethality were observed in mice and fishes (Falconer, 2008). However, algae are much more resistant to STX than animal cells (Perreault et al., 2011).
The diagram in Fig. 3 summarizes a possible chain of events triggered by global climate change. An alteration in the aquatic community composition through modification in the oxidative and nitrosative metabolism in the organisms could be produced by the enhancement in the magnitude and frequency of HAB. On the other hand, as previously mentioned, some biotoxins (e.g., MC) could show some protective effects from reactive species damage. Thus, they can act as a potential selective factor favoring MC-producing blooms, as compared to non-toxic species development under the same conditions.
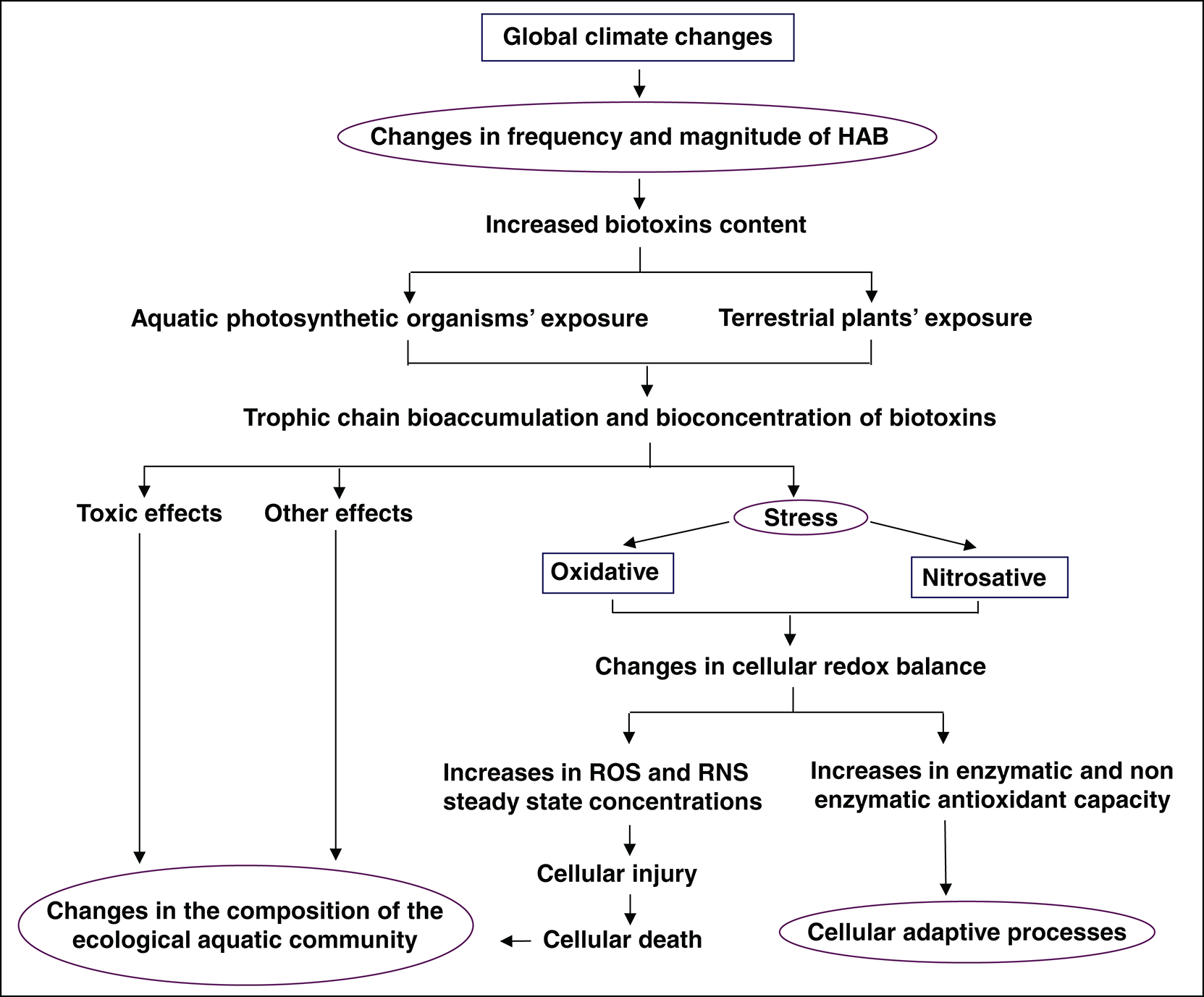
Figure 3: Graphical scheme showing how global climate change may affect the magnitude and frequency of HAB, drastically modifying the aquatic community compositions through responses in the oxidative and nitrosative metabolism.
Based on the actual information available, it seems that the oxidative stress related to the HAB is a critical component of the mechanisms triggered by climate global change. Thus, the antioxidant defense system of photosynthetic organisms is a key feature to manage the damage produced by biotoxins and could be part of the adaptive process. The ability of each species to cope with this hazardous natural condition would allow the adaptation to the new scenario or provoke the organism’s injury/death. The difference, in terms of survival, will be granted by the antioxidant response and the triggering of signaling pathways to express protection mechanisms. It is evident from these data that the biotoxins promote oxidative stress, not only in higher plants but also in algae, independently of their habitat (marine, freshwater, or soil). Therefore, the triggering of oxidative stress seems to be a common occurrence in photosynthetic organisms that respond with similar behaviors. Thus, the metabolic responses may modify the community composition leading to alterations in the global health of each habitat, risking the survival of many species with ecological relevance and economical outbreaks. The available data that explore global change-dependent effects, in terms of oxidative stress due to biotoxin exposure of photosynthetic organisms, especially in marine algae, is scarce up to now. Further studies are required to assess the role of the antioxidant enzymes in the photosynthetic cells exposed to biotoxins in their natural environment.
Future research will need to be focused on clarifying the information on the in situ effects of the changes in the HAB over the antioxidant ability of many photosynthetic communities. Also, the signaling pathways leading to the protection mechanisms observed should be studied. It is important to mention that the nitrosative metabolism implication in this field has not been explored yet. Even though oxidative and nitrosative actions are not the only way biotoxins lead to deleterious alterations on the organisms, their impact on the overall cellular damage is far from being characterized. Clarifying these effects could be a key factor for future advances in this field and could help to design strategies to improve the natural ability of plants and algae to cope with a growing challenge such as global climate change.
Author Contribution: Susana Puntarulo participated in the designing of the original idea and in the manuscript writing. Paula Mariela González contributed to the bibliographic search and in the manuscript writing. Both authors have approved the final version of the manuscript.
Ethics Approval: Not Applicable.
Funding Statement: This work was supported by grants from the University of Buenos Aires (UBACyT, 20020170100199BA) and the National Council for Science and Technology (CONICET, PIP 11220170100539CO). SP and PG are career investigators from CONICET.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abele D, Puntarulo S (2004). Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comparative Biochemistry and Physiology Part A 138: 405–415. DOI 10.1016/j.cbpb.2004.05.013. [Google Scholar] [CrossRef]
Arteel GE, Briviba K, Sies H (1999). Protection against peroxynitrite. FEBS Letters 445: 226–230. DOI 10.1016/S0014-5793(99)00073-3. [Google Scholar] [CrossRef]
Botana LM, Alonso E, Sáinz MJ, Alfonso A, Botana AM, Louzao C, Vilariño N, Vale C, Camiña M, Vieytes MR (2019). Toxins: Neurotoxins. In: Worsfold P, Poole C, Townshend A, Miró M. (eds.) Encyclopedia of Analytical Science (Third Edition). Lugo, Spain: Academic Press, 141–147. DOI 10.1016/B978-0-12-409547-2.14396-3. [Google Scholar] [CrossRef]
Botana LM, Vilariño N, Alfonso A, Vale C, Louzao C, Elliott CT, Campbell K, Botana AM (2010). The problem of toxicity equivalent factors in developing alternative methods to animal bioassays for marine-toxin detection. TrAC Trends in Analytical Chemistry 29: 1316–1325. DOI 10.1016/j.trac.2010.09.004. [Google Scholar] [CrossRef]
Burkholder JAM, Shumway SE, Glibert PM (2018). Food web and ecosystem impacts of harmful algae. In: Shumway SE, Burkholder JAM, Morton SL (eds.Harmful algal blooms, a compendium desk reference. pp. 243–336. NJ: John Wiley & Sons, Ltd. [Google Scholar]
Cabrera J, González PM, Puntarulo S (2019). Oxidative effects of the harmful algal blooms on primary organisms of the food web. BIOCELL 43: 41–50. [Google Scholar]
Chen J, Dai J, Zhang H, Wang C, Zhou G, Han Z, Liu Z (2010). Bioaccumulation of microcystin and its oxidative stress in the apple (Malus pumila). Ecotoxicology 19: 796–803. DOI 10.1007/s10646-009-0456-5. [Google Scholar] [CrossRef]
Chen W, Colon R, Louda JW, Rodriguez del Rey F, Durham M, Rein KS (2018). Brevetoxin (PbTx-2) influences the redox status and NPQ of Karenia brevis by way of thioredoxin reductase. Harmful Algae 71: 29–39. DOI 10.1016/j.hal.2017.11.004. [Google Scholar] [CrossRef]
Daugbjerg N, Hansen G, Larsen J, Moestrup Ø (2000). Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia 39: 302–317. DOI 10.2216/i0031-8884-39-4-302.1. [Google Scholar] [CrossRef]
Diaz JM, Plummer S (2018). Production of extracellular reactive oxygen species by phytoplankton: Past and future directions. Journal of Plankton Research 40: 655–666. DOI 10.1093/plankt/fby043. [Google Scholar] [CrossRef]
Ding Y, Song L, Sedmak B (2013). UVB radiation as a potential selective factor favoring microcystin producing bloom forming cyanobacteria. PLoS One 8: e73919. DOI 10.1371/journal.pone.0073919. [Google Scholar] [CrossRef]
Dring M (2006). Stress resistance and disease resistance in seaweeds: The role of reactive oxygen metabolism. Advances in Botanical Research 43: 175–207. [Google Scholar]
Doucette GJ, Medlin LK, McCarron P, Hess P (2018). Detection and surveillance of harmful algal bloom species and toxins. In: Shumway SE, Burkholder JAM, Morton SL (eds.) Harmful algal blooms, a compendium desk reference. pp. 39–113. NJ: John Wiley & Sons Ltd. [Google Scholar]
Endo M, Onoue Y, Kuroki A (1992). Neurotoxin-induced cardiac disorder and its role in the death of fish exposed to Chattonella marina. Marine Biology 112: 371–376. DOI 10.1007/BF00356281. [Google Scholar] [CrossRef]
Estevez MS, Puntarulo S (2005). Nitric oxide generation upon growth of Antarctic Chlorella sp. cells. Physiologia Plantarum 125: 192–201. DOI 10.1111/j.1399-3054.2005.00561.x. [Google Scholar] [CrossRef]
Falconer IR (2008). Health effects associated with controlled exposures to cyanobacterial toxins. In: Hudnell HK (eds.Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Advances in Experimental Medicine and Biology, vol. 619, pp. 607–612. NY: Springer. [Google Scholar]
Fisher JM, Reese JG, Pellechia PJ, Moeller PL, Ferry JL (2006). Role of Fe(IIIphosphate, dissolved organic matter, and nitrate during the photodegradation of domoic acid in the marine environment. Environmental Science & Technology 40: 2200–2205. DOI 10.1021/es051443b. [Google Scholar] [CrossRef]
Foti MC (2007). Antioxidant properties of phenols. Journal of Pharmacy and Pharmacology 59: 1673–1685. DOI 10.1211/jpp.59.12.0010. [Google Scholar] [CrossRef]
Froscio SM, Humpage AR, Burcham PC, Falconer IR (2001). Cell-free protein synthesis inhibition assay for the cyanobacterial toxin cylindrospermopsin. Environmental Toxicology 16: 408–412. DOI 10.1002/tox.1050. [Google Scholar] [CrossRef]
Froscio SM, Humpage AR, Wickramasinghe W, Shaw G, Falconer IR (2008). Interaction of the cyanobacterial toxin cylindrospermopsin with the eukaryotic protein synthesis system. Toxicon 51: 191–198. DOI 10.1016/j.toxicon.2007.09.001. [Google Scholar] [CrossRef]
Gobler CJ (2020). Climate change and harmful algal blooms: Insights and perspective. Harmful Algae 91: 101731. DOI 10.1016/j.hal.2019.101731. [Google Scholar] [CrossRef]
González PM, Puntarulo S (2016). Seasonality and toxins effects on oxidative/nitrosative metabolism in digestive glands of the bivalve Mytilus edulis platensis. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 200: 79–86. DOI 10.1016/j.cbpa.2016.04.011. [Google Scholar] [CrossRef]
González PM, Puntarulo S (2020). Possible role of seasonality and harmful algal blooms (HAB) on the oxidative and nitrosative metabolisms in hemocytes. Comparative Biochemistry and Physiology Part C 232: 108744. [Google Scholar]
González-Jartín JM, de Castro Alves L, Alfonso A, Piñeiro Y, Yáñez Vilar S, Rodríguez I, González Gomez M, Vargas Osorio Z, Sainz MJ, Vieytes MR, Rivas J, Botana LM (2020). Magnetic nanostructures for marine and freshwater toxins removal. Chemosphere 256: 127019. DOI 10.1016/j.chemosphere.2020.127019. [Google Scholar] [CrossRef]
Griffith AW, Gobler CJ (2020). Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 91: 101590. DOI 10.1016/j.hal.2019.03.008. [Google Scholar] [CrossRef]
Heisler J, Glibert PM, Burkholder JM, Anderson DM, Cochlan W, Dennison WC, Dortch Q, Gobler C, Heil CA, Humphries E, Lewitus A, Magnien R, Marshall HG, Sellner K, Stockwell DA, Stoecker DK, Suddleson M (2008). Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 8: 3–13. DOI 10.1016/j.hal.2008.08.006. [Google Scholar] [CrossRef]
Hernando M, Schloss IR, Almandoz GO, Malanga G, Varela DE, De Troch M (2018). Combined effects of temperature and salinity on fatty acid content and lipid damage in Antarctic phytoplankton. Journal of Experimental Marine Biology and Ecology 503: 120–128. DOI 10.1016/j.jembe.2018.03.004. [Google Scholar] [CrossRef]
Hu ZQ, Liu YD, Li DH, Dauta A (2005). Growth and antioxidant system of the cyanobacterium Synechococcus elongatus in response to microcystin-RR. Hydrobiologia 534: 23–29. DOI 10.1007/s10750-004-1319-y. [Google Scholar] [CrossRef]
Humpage AR, Fontaine F, Froscio S, Burcham P, Falconer IR (2005). Cylindrospermopsin genotoxicity and cytotoxicity: Role of cytochrome P-450 and oxidative stress. Journal of Toxicology and Environmental Health, Part A 68: 739–753. DOI 10.1080/15287390590925465. [Google Scholar] [CrossRef]
Ishimatsu A, Oda T, Yoshida M, Ozaki M (1996). Oxygen radicals are probably involved in the mortality of yellowtail by Chattonella marina. Fisheries Science 62: 836–837. DOI 10.2331/fishsci.62.836. [Google Scholar] [CrossRef]
Jauzein C, Erdner DL (2013). Stress-related responses in Alexandrium tamarense cells exposed to environmental changes. Journal of Eukaryotic Microbiology, 1–13. [Google Scholar]
Kim CS, Lee SG, Lee CK, Kim HG, Jung J (1999a). Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides. Journal of Plankton Research 21: 2105–2115. DOI 10.1093/plankt/21.11.2105. [Google Scholar] [CrossRef]
Kim D, Oda T (2010). Possible factors responsible for the fish killing mechanisms of the red tide phytoplankton, Chattonella marina and Cochlodinium polykrikoides. In: Ishimatsu A, Lie HJ (eds.Coastal Environmental and Ecosystem Issues of the East China Sea, pp. 245–268. Tokyo: TERRAPUB and Nagasaki University. [Google Scholar]
Kim D, Nakamura A, Okamoto T, Komatsu N, Oda T, Ishimatsu A, Muramatsu T (1999b). Toxic potential of the raphidophyte Olisthodiscus luteus: Mediation by reactive oxygen species. Journal of Plankton Research 21: 1017–1027. DOI 10.1093/plankt/21.6.1017. [Google Scholar] [CrossRef]
Krock B, Ferrario ME, Akselman R, Montoya NG (2018). Occurrence of marine biotoxins and shellfish poisoning events and their causative organisms in Argentine marine waters. Oceanography 31: 132–144. DOI 10.5670/oceanog.2018.403. [Google Scholar] [CrossRef]
Krock B, Schloss IR, Trefault N, Tillmann U, Hernando M, Deregibus D, Antoni J, Almandoz GO, Hoppenrath M (2020). Detection of the phycotoxin pectenotoxin‐2 in waters around King George Island. Antarctica Polar Biology 43: 263–277. DOI 10.1007/s00300-020-02628-z. [Google Scholar] [CrossRef]
Lehtimäki N, Shunmugam S, Jokela J, Wahlsten M, Carmel D, Keränen M, Sivonen K, Aro EM, Allahverdiyeva Y, Mulo P (2011). Nodularin uptake and induction of oxidative stress in spinach (Spinachia oleracea). Journal of Plant Physiology 168: 594–600. DOI 10.1016/j.jplph.2010.09.013. [Google Scholar] [CrossRef]
Liang C, Wang W (2015). Response and recovery of rice (Oryza sativa) seedlings to irrigation with microcystin-contaminated water. Environmental Earth Sciences 73: 4573–4580. DOI 10.1007/s12665-014-3746-z. [Google Scholar] [CrossRef]
Lushchak VI (2011). Environmentally induced oxidative stress in aquatic animals. Aquatic Toxicology 101: 13–30. DOI 10.1016/j.aquatox.2010.10.006. [Google Scholar] [CrossRef]
Malanga G, Giannuzzi L, Hernando M (2019). The possible role of microcystin (D-Leu1 MC-LR) as an antioxidant on Microcystis aeruginosa (Cyanophyceae). In vitro and in vivo evidence. Comparative Biochemistry and Physiology Part C 225: 108575. [Google Scholar]
Marsico RM, Schneider RJ, Voelker BM, Zhang T, Diaz JM, Hansel CM, Ushijima S (2015). Spatial and temporal variability of widespread dark production and decay of hydrogen peroxide in freshwater. Aquatic Sciences 77: 523–533. DOI 10.1007/s00027-015-0399-2. [Google Scholar] [CrossRef]
McElhiney J, Lawton LA, Leifert C (2001). Investigations into the inhibitory effects of microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon 39: 1411–1420. DOI 10.1016/S0041-0101(01)00100-3. [Google Scholar] [CrossRef]
Melegari SP, Perreault F, Moukha S, Popovic R, Creppy EE, Matias WG (2012). Induction to oxidative stress by saxitoxin investigated through lipid peroxidation in Neuro 2A cells and Chlamydomonas reinhardtii alga. Chemosphere 89: 38–43. DOI 10.1016/j.chemosphere.2012.04.009. [Google Scholar] [CrossRef]
Meyer Y, Belin C, Delorme-Hinoux V, Reichheld JP (2012). Thioredoxin and glutaredoxin systems in plants: Molecular mechanisms, crosstalks, and functional significance. Antioxidants & Redox Signaling 17: 1124–1160. DOI 10.1089/ars.2011.4327. [Google Scholar] [CrossRef]
Mohamed ZA (2008). Polysaccharides as a protective response against microcystin-induced oxidative stress in Chlorella vulgaris and Scenedesmus quadricauda and their possible significance in the aquatic ecosystem. Ecotoxicology 17: 504–516. DOI 10.1007/s10646-008-0204-2. [Google Scholar] [CrossRef]
Oda T, Ishimatsu A, Shimada M, Takeshita S, Muramatsu T (1992). Oxygen-radical-mediated toxic effects of the red tide flagellate Chattonella marina on Vibrio alginolyticus. Marine Biology 112: 505–509. DOI 10.1007/BF00356297. [Google Scholar] [CrossRef]
Otero P, Miguéns N, Rodríguez I, Botana LM (2019). LC–MS/MS analysis of the emerging toxin pinnatoxin-G and high levels of esterified OA group toxins in Galician commercial mussels. Toxins 11: 394. DOI 10.3390/toxins11070394. [Google Scholar] [CrossRef]
Perreault F, Matias MS, Melegari SP, Creppy EE, Popovic R, Matias WG (2011). Investigation of animal and algal bioassays for reliable saxitoxin ecotoxicity and cytotoxicity risk evaluation. Ecotoxicology and Environmental Safety 74: 1021–1026. DOI 10.1016/j.ecoenv.2011.01.016. [Google Scholar] [CrossRef]
Pflugmacher S (2004). Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquatic Toxicology 70: 169–178. DOI 10.1016/j.aquatox.2004.06.010. [Google Scholar] [CrossRef]
Pflugmacher S, Wiegand C, Oberemm A, Beattie KA, Krause E, Codd GA, Steinberg CEW (1998). Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxication. Biochimica et Biophysica Acta (BBA)-General Subjects 1425: 527–533. DOI 10.1016/S0304-4165(98)00107-X. [Google Scholar] [CrossRef]
Pflugmacher S, Codd GA, Steinberg CEW (1999). Effects of the cyanobacterial toxin microcystin-LR on detoxication enzymes in aquatic plants. Environmental Toxicology 14: 111–115. DOI 10.1002/(SICI)1522-7278(199902)14:1<111::AID-TOX14>3.0.CO;2-3. [Google Scholar] [CrossRef]
Pflugmacher S, Wiegand C, Beattie KA, Krause E, Steinberg CEW, Codd GA (2001). Uptake, effects, and metabolism of cyanobacterial toxins in the emergent reed plant Phragmites australis (cav.) Trin. ex steud. Environmental Toxicology and Chemistry 20: 846–852. DOI 10.1002/etc.5620200421. [Google Scholar] [CrossRef]
Pflugmacher S, Jung K, Lundvall L, Neumann S, Peuthert A (2006). Effects of cyanobacterial toxins and cyanobacterial cell-free crude extract on germination of alfalfa (Medicago sativa) and induction of oxidative stress. Environmental Toxicology and Chemistry 25: 2381–2387. DOI 10.1897/05-615R.1. [Google Scholar] [CrossRef]
Pflugmacher S, Olin M, Kankaanpää H (2007). Nodularin induces oxidative stress in the Baltic Sea brown alga Fucus vesiculosus (Phaeophyceae). Marine Environmental Research 64: 149–159. DOI 10.1016/j.marenvres.2006.12.011. [Google Scholar] [CrossRef]
Pflugmacher S, Olin M, Kankaanpää H (2010). Oxidative stress response in the red alga Furcellaria lumbricalis (Huds.) Lamour. due to exposure and uptake of the cyanobacterial toxin nodularin from Nodularia spumigena. Harmful Algae 10: 49–55. DOI 10.1016/j.hal.2010.06.004. [Google Scholar] [CrossRef]
Pereira SR, Vasconcelos VM, Antunes A (2011). The phosphoprotein phosphatase family of Ser/Thr phosphatases as principal targets of naturally occurring toxins. Critical Reviews in Toxicology 41: 83–110. DOI 10.3109/10408444.2010.515564. [Google Scholar] [CrossRef]
Peuthert A, Lawton L, Pflugmacher S (2008). In vivo influence of cyanobacterial toxins on enzyme activity and gene expression of protein phosphatases in Alfalfa (Medicago sativa). Toxicon 52: 84–90. DOI 10.1016/j.toxicon.2008.04.172. [Google Scholar] [CrossRef]
Prieto A, Campos A, Cameán A, Vasconcelos V (2011). Effects on growth and oxidative stress status of rice plants (Oryza sativa) exposed to two extracts of toxin-producing cyanobacteria (Aphanizomenon ovalisporum and Microcystis aeruginosa). Ecotoxicology and Environmental Safety 74: 1973–1980. DOI 10.1016/j.ecoenv.2011.06.009. [Google Scholar] [CrossRef]
Radi R, Beckman JS, Bush KM, Freeman BA (1991). Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. Journal of Biological Chemistry 266: 4244–4250. DOI 10.1016/S0021-9258(20)64313-7. [Google Scholar] [CrossRef]
Rusak SA, Peake BM, Richard LE, Nodder SD, Cooper WJ (2011). Distributions of hydrogen peroxide and superoxide in seawater east of New Zealand. Marine Chemistry 127: 155–169. DOI 10.1016/j.marchem.2011.08.005. [Google Scholar] [CrossRef]
Saqrane S, hazali El I, Ouahid Y, El Hassni M, El Hadrami I, Bouarab L, del Campo FF, Oudra B, Vasconcelos V (2007). Phytotoxic effects of cyanobacteria extract on the aquatic plant Lemna gibba: Microcystin accumulation, detoxication and oxidative stress induction. Aquatic Toxicology 83: 284–294. DOI 10.1016/j.aquatox.2007.05.004. [Google Scholar] [CrossRef]
Shimada M, Kawamoto S, Nakatsuka Y, Watanabe M (1993). Localization of superoxide anion in the red tide alga Chattonella antiqua. Journal of Histochemistry & Cytochemistry 41: 507–511. DOI 10.1177/41.4.8383714. [Google Scholar] [CrossRef]
Singh NK, Dhar DW (2013). Cyanotoxins, related health hazards on animals and their management: A Review. Indian Journal of Animal Sciences 83: 1111–1127. [Google Scholar]
Twiner MJ, Trick CG (2000). Possible physiological mechanisms for production of hydrogen peroxide by the ichthyotoxic flagellate Heterosigma akashiwo. Journal of Plankton Research 22: 1961–1975. DOI 10.1093/plankt/22.10.1961. [Google Scholar] [CrossRef]
van de Waal DB, Verspagen JM, Finke JF, Vournazou V, Immers AK, Edwin W, Kardinaal A, Tonk L, Becker S, Van Donk E, Visser PM, Huisman J (2011). Reversal in competitive dominance of a toxic versus non-toxic cyanobacterium in response to rising CO2. ISME Journal 5: 1438–1450. DOI 10.1038/ismej.2011.28. [Google Scholar] [CrossRef]
Ventoso P, Pazos AJ, Pérez-Parallé ML, Blanco J, Triviño JC, Sánchez JL (2019). RNA-Seq Transcriptome profiling of the queen scallop (Aequipecten opercularis) digestive gland after exposure to domoic acid-producing Pseudo-nitzschia. Toxins 11: 97. DOI 10.3390/toxins11020097. [Google Scholar] [CrossRef]
Vilariño N, Louzao MC, Abal P, Cagide E, Carrera C, Vieytes MR, Botana LM (2018). Human poisoning from marine toxins: Unknowns for optimal consumer protection. Toxins 10: 324. DOI 10.3390/toxins10080324. [Google Scholar] [CrossRef]
Wells ML, Trainer VL, Smayda TJ, Karlson BS, Trick CG, Kudela RM, Ishikawa A, Bernard S, Wulff A, Anderson DM, Cochlan WP (2015). Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 49: 68–93. DOI 10.1016/j.hal.2015.07.009. [Google Scholar] [CrossRef]
Wojtaszek P (2000). Nitric oxide in plants: To NO or not to NO. Phytochemistry 54: 1–4. DOI 10.1016/S0031-9422(00)00056-X. [Google Scholar] [CrossRef]
Yamasaki S (1993). Probable effects of algal bloom on the growth of Phragmites australis (Cav.) Trin. ex steudel. Journal of Plant Research 106: 113–120. DOI 10.1007/BF02344414. [Google Scholar] [CrossRef]
Zilliges Y, Kehr JC, Meissner S, Ishida K, Mikkat S, Hagemann M, Kaplan A, Börner T, Dittmann E (2011). The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS One 6: e17615. DOI 10.1371/journal.pone.0017615. [Google Scholar] [CrossRef]
Zhong Q, Wei B, Wang S, Ke S, Chen J, Zhang H, Wang H (2019). The antioxidant activity of polysaccharides derived from marine organisms: An overview. Marine Drugs 17: 674. DOI 10.3390/md17120674. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |