DOI:10.32604/biocell.2021.015836

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.015836 |  www.techscience.com/journal/biocell |
| Article |
Hellebrigenin induces apoptosis in colorectal cancer Cells through induction of excessive reactive oxygen species
1Guizhou Provincial College-based Key Laboratory for Tumor Prevention and Treatment with Distinctive Medicines, Zunyi Medical University, Zunyi, 563000, China
2Life Sciences Institute, Zunyi Medical University, Zunyi, 563000, China
3Department of Biochemistry and Molecular Biology, Zunyi Medical University, Zunyi, 563000, China
*Address correspondence to: Sanhua Li, zyzmclsh@163.com; Yun Liu, liuyunzmu@126.com
Received: 18 January 2021; Accepted: 20 February 2021
Abstract: Traditional Chinese medicine (TCM) has been increasingly applied in both preventing and treating a variety of cancers in the last decades, attributing to its fewer side effects as compared with chemotherapy drugs. Hellebrigenin, a component of Chanpi from the skin of Bufo bufogargarizans Cantor or Duttaphrynus melanostictus has been reported to have an obvious anti-cancer activity on various cancers. However, the effect and mechanism of hellebrigenin on colorectal cancers were still unknown. Herein, the present study demonstrated hellebrigenin significantly reduced viability and triggered apoptosis via the intrinsic pathway in colorectal cancer cell lines HCT116 and HT29 in vitro and in vivo. Moreover, hellebrigenin led to a reduction of mitochondrial membrane potential. In addition, treatment with hellebrigenin could result in the induction of excessive reactive oxygen species, which led to cell apoptosis. These results indicated that hellebrigenin had anti-cancer potential in the treatment of colorectal cancers.
Keywords: Hellebrigenin; Colorectal cancer; Reactive oxygen species; Apoptosis
The incidence of colorectal cancer (CRC) is ranked the third in terms of morbidity and the fifth in terms of mortality of malignancies in China, where new colorectal cancer cases had reached over 0.8 million and 0.4 million deaths occurred in 2018 (Feng et al., 2019). Chemotherapy is still an important treatment strategy for CRC patients, especially for the ones with metastatic disease. However, there are many side effects such as myelosuppression, gastrointestinal tract reaction, cardiac damage, liver or kidney damage. In addition, the multiple drug resistance of cancer cells limits the application of conventional chemotherapy drugs. It calls for the development of more effective chemotherapy agents.
Traditional Chinese medicine (TCM) has been increasingly applied in preventing and treating a variety of cancers in the last decades because of its characteristics of diverse components, multiple targets, low toxicity, and a few side effects, overall regulation, and synergistic effects (Qi et al., 2015). Skin from Bufo bufo gargarizans Cantor or Duttaphrynus melanostictus, named Chanpi, is one of TCM and is commonly used for bloating, heatstroke coma, vomiting, diarrhea, and cancer.Cinobufacini (Huachansu) is a water-soluble bioactive drug extracted from Chanpi and has been approved for clinical treatment of cancer patients, showing promising therapeutic effects in hepatocellular carcinoma, lung cancer, colorectal and pancreatic cancer (Meng et al., 2009; Qin et al., 2008). Hitherto, the anti-tumor mechanism of Chanpi has not been fully explored because of its complex components.
Bufadienolides are the primary bioactive components of Chanpi, including approximately one hundred monomers, most of which have potent anti-cancer activity and have been drawn attention as novel candidates in cancer therapy (Wang et al., 2011; Zhan et al., 2020). Hellebrigenin is one of the bufadienolides and is an effective constitute of Huachansu injection. It has reported that hellebrigenin could suppress the growth of several cancer cell lines, but few studies demonstrated its effect on colorectal cancer cell lines(Cunha-Filho et al., 2010; Moreno et al., 2013; Wu et al., 2006) and the underlying mechanisms against the growth of colorectal cancer cell lines of hellebrigenin have not yet been disclosed.
In the present study, the effect and mechanism of hellebrigenin on human colorectal cancer cell lines HCT116 and HT29were investigated. We showed that exposure of CRC cell lines to hellebrigenin led to cell death with morphological and biochemical characteristic changes of apoptosis. Further study demonstrated that reactive oxygen species (ROS) were upstream of hellebrigenin-mediated apoptosis, and the apoptosis was triggered through the intrinsic pathway in CRC cells. These findings suggest that hellebrigenin may be a potential chemotherapeutic agent for colorectal cancer treatment.
Human colorectal cancer cell lines HCT116 and HT29 cells were purchased from Genechem Co., Ltd., (Shanghai, China). Hellebrigenin was prepared from water extract of Chanpi and dissolved in DMSO at a stock concentration of 10 mM. Fetal bovine serum was purchased from Gibco (USA). Sulforhodamine B (SRB) was purchased from Sigma (USA). FITC-Annexin V Apoptosis Detection Kit I was purchased from Becton Dickinson Biosciences (USA). Reactive Oxygen Species (ROS) Assay Kit and Mitochondrial Membrane Potential Assay Kit were purchased from Beyotime (China). Antibodies against cleaved caspase-9 (#7237), cleaved caspase-3 (#9664), BAX (#272), BCL-2 (#3498), Cytochrome C (#11940) and VDAC (#4661) were purchased from Cell Signaling Technology (USA). Antibodies against PCNA (EM111201) and β-actin (R1207-1) were obtained from Huabio (China).
The human colorectal cancer cell lines HCT116 and HT29 were cultured in RPMI-1640 medium supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum (FBS) at 37°C in a humidified cell culture incubator containing 5% CO2.
Cells were seeded into 96-well plates at a density of 5 × 103cells per well and cultured for 24 h to allow adherence. Next, cells were incubated with a series of concentrations of hellebrigenin. After incubated for 24, 48 and72 h, cells were performed SRB colorimetry (Skehan et al., 1990). The absorbance at 530 nm of each well was measured by a Spectra Max i3X plate reader (Molecular Devices, USA) and used for calculating cell viability.
Cells were seeded into 6-well plates at a density of 500 cells per well and cultured for 24 h. Next, cells were pretreated with 100 to 400 nM of hellebrigenin for 48 h and then cultured with fresh cell culture medium for 2 weeks. Then, cells were washed with phosphate buffer saline (PBS) followed by fixed with methanol for 1 h and stained with Giemsa solution (Solarbio, Beijing, China). Finally, colonies of cells were counted to evaluate cell proliferation.
Cells were plated into a 6-well plate at a density of 1 × 105cells per well and cultured for 24 h. Then cells were incubated with 100 to 400 nM of hellebrigenin for 48 h. The cells were washed and collected with cold PBS and resuspended in 500 μL binding buffer (0.01 M Hepes-NaOH (pH7.4), 0.14 M NaCl, 2.5 mM CaCl2). Later, cells reacted with 5 μL FITC-Annexin-V and 5 μL PI at room temperature in the dark for 15 min. Finally, the stained samples were analyzed by flow cytometerC6 plus (BD, USA).
Cells were plated into a 6-well plate at a density of 1 × 105cells per well and cultured for 24 h. After incubated with 100 to 400 nM of hellebrigenin for 48 h, cells were stained with Hoechst 33342 (Beyotime, Shanghai, China) for 30 min in the dark. Cells were photographed by fluorescence microscope (Olympus, Tokyo, Japan) in which the bright blue of condensed or fragmented nuclei in apoptotic cells were observed.
Measurement of mitochondrial membrane potential (MMP)
Cells were plated into a 6-well plate at a density of 1 × 105 cells per well and cultured for 24 h, followed by treating with 100 to 400 nM of hellebrigenin for 48 h. Cells were then washed by PBS and cultured in fresh RPMI-1640 medium with 10 μM of 5,5,6,6’-tetrachloro-1,1,3,3’-tetraethyl-imidacarbocyanine (JC-1) for 20 min in the dark. Afterward, cells were washed by JC-1 staining buffer and added fresh RPMI-1640 medium without fetal bovine serum to be photographed by fluorescence microscope.
Cells were plated into a 6-well plate at a density of 1 × 105 cells per well and cultured for 24 h followed by treating with 100 to 400 nM of hellebrigenin for 48 h. Cells then were washed by PBS and incubated in fresh RPMI-1640 medium with 10 μM Dichloro-dihydro-fluoresceindiacetate (DCFH-DA) probe for 30 min in dark. Later, cells washed with ice-cold PBS and added fresh RPMI-1640 medium without fetal bovine serum to be examined by fluorescence microscope.
Extraction of mitochondrial protein
Mitochondria of cells were prepared using Cell Mitochondria Isolation Kit (Beyotime, Shanghai, China). Briefly, cells were collected from culture dishes and suspended in ice-cold PBS. Cells were then precipitated by centrifugation and treated with mitochondrial separation reagent for 15 min in ice. Later, the cell suspension was milled in a glass homogenizer and centrifugated at 600 × g to collect the liquid supernatant. Then, liquid supernatant was centrifugated at 11000 × g to collect precipitant in which mitochondria remained. Finally, the mitochondrial protein was extracted via RIPA lysis buffer.
Xenograft tumor model and compound administration in vivo
HCT116 cells were suspended in PBS at a concentration of 1 × 107 per µL and injected subcutaneously into the right forelimb of male BALB/c nude mouse (N = 10) at 4 weeks of age (Beijing Huahengkang Biological Technology, Beijing, China). Mice were maintained in specific pathogen-free conditions. When the mean volume of the tumors reached approximately 70 mm3, the mice were randomized to either the vehicle control group (N = 5) or hellebrigenin treated group (N = 5). Hellebrigenin was dissolved in normal saline and intraperitoneally injected into mice of the hellebrigenin-treated group at a dose of 2.5 mg/kg for 12 continuous days. Mice of the vehicle control group were treated with the same volume of normal saline and DMSO. Volumes of tumors were measured and calculated every day by the following formula: Volume = 1/2 (length × width2). Then tumor neoplasm was harvested 12 days after compound administration.
Tumor tissues were fixed with formalin, embedded in paraffin, and sectioned. The slices were incubated with primary antibody against PCNA (1:200) overnight at 4°C, and HRP-secondary antibody for 2 h at room temperature and then subjected to 3,3’-diaminobenzidine chromogen development. Samples were photographed by microscope (Olympus, Tokyo, Japan).
Total protein was extracted from cells or tumor tissue by RIPA lysis buffer with proteinase inhibitor PMSF (Beyotime, China) and phosphatase inhibitor (Sigma-Aldrich, USA). Protein samples were quantified, separated by SDS-PAGE in 12% gel, and transferred to PVDF membranes (Millipore, USA). The membranes were blocked with 5% BSA for 2 h at room temperature and probed with primary antibodies at 4°C overnight. Then the membranes were incubated with HRP-conjugated anti-rabbit antibody for 1 h at room temperature. Protein bands were visualized by ChemiDoc Imaging System (Bio-Rad, USA).
All experiments were performed at least three times independently. Statistical analyses were performed using SPSS 17.0 (IBM, Armonk, New York, USA). Statistical significance was determined by ANOVA analysis, and a two-tailed value of p < 0.05 was considered statistically significant.
Hellebrigenin can suppress cell viability and colony formation ability of CRC
To investigate the effect of hellebrigenin on the proliferation of human CRC cells, two human CRC cell lines, HCT116 and HT29, were exposed to various concentration of hellebrigenin (0, 15.62, 31.25, 62.5, 125, 250, and 500 nM) for 24, 48, and 72 h, respectively. The cell viability detected by SRB assay showed that hellebrigenin significantly suppressed the growth of HCT116 and HT29 cells (Figs. 1A and 1B). After treated with hellebrigenin (0, 100, 200, and 400 nM), colonies of CRC cells were much fewer than that of the control group (Figs. 1C and 1D). These results indicated the anti-proliferation effect of hellebrigenin in two CRC cell lines.

Figure 1: Hellebrigenin treatment inhibited proliferation of CRC cells measured via SRB assay in (A) HCT116, (B) HT29.
Hellebrigenin induces apoptosis via intrinsic pathway in CRC cells lines
In order to identify whether the proliferation inhibition by hellebrigenin was associated with apoptosis, we detected apoptotic cells by FITC-Annexin-V and PI staining. Results showed that the proportion of apoptotic cells obviously increased following treatment with 400 nM hellebrigenin for HCT116 and HT29, respectively (Figs. 2A and 2C, p < 0.05). Moreover, changes in nuclear morphometry are the characteristics of cell apoptosis, which could be detected via Hoechst 33342. Brighter blue fluorescence of the condensed cell nuclei was observed in cells treated with 200 and 400 nM hellebrigenin for 48 h than that in the cells of the control group (Fig. 2E, p < 0.05). To further explore whether apoptosis in cells is triggered through the intrinsic pathway, downstream apoptotic proteins BAX, BCL-2, caspase-9, and caspase-3 were detected by western blot. It indicated that BAX, cleaved caspase-3, and cleaved caspase-9 increased while BCL-2 declined in a dose-dependent manner after treated with 100, 200, and 400 nM hellebrigenin (Fig. 2D). Taken together, these results suggested the activation of intrinsic apoptosis by hellebrigenin in CRC cells might contribute to the inhibition of cell growth in CRC cell lines.

Figure 2: Apoptosis induced after cells treated with various doses of hellebrigenin for 48 h by staining with FITC-Annexin-V and PI (A–C).
Hellebrigenin induces apoptosis via the mitochondrial pathway
Mitochondria play an important role in regulating apoptosis, and exhaustion of MMP is a symbolic event in the early stage of intrinsic apoptosis. Subsequently, cytochrome C is released from mitochondria to the cytoplasm, and apoptosis progresses. We examined the change of MMP by fluorescent probe JC-1 with a microscope. MMP significantly declined after treated with 400 nM hellebrigenin for 48 h, evidenced by enhanced green fluorescence representing strengthened permeating of JC-1 monomer in mitochondria (Figs. 3A and 3B, p < 0.05), which indicated that accumulation of damaged mitochondria. Cytochrome C localized in the mitochondrial inner membrane was released from mitochondria to the cytoplasm under the influence of hellebrigenin, especially at 200 and 400 nM, evidenced by results of western blot (Fig. 3C). These results further indicated the apoptosis-inducing function of hellebrigenin in CRC cells. Here, VDAC located at mitochondrial outer membrane was used as an internal reference of the extracted mitochondrial protein.
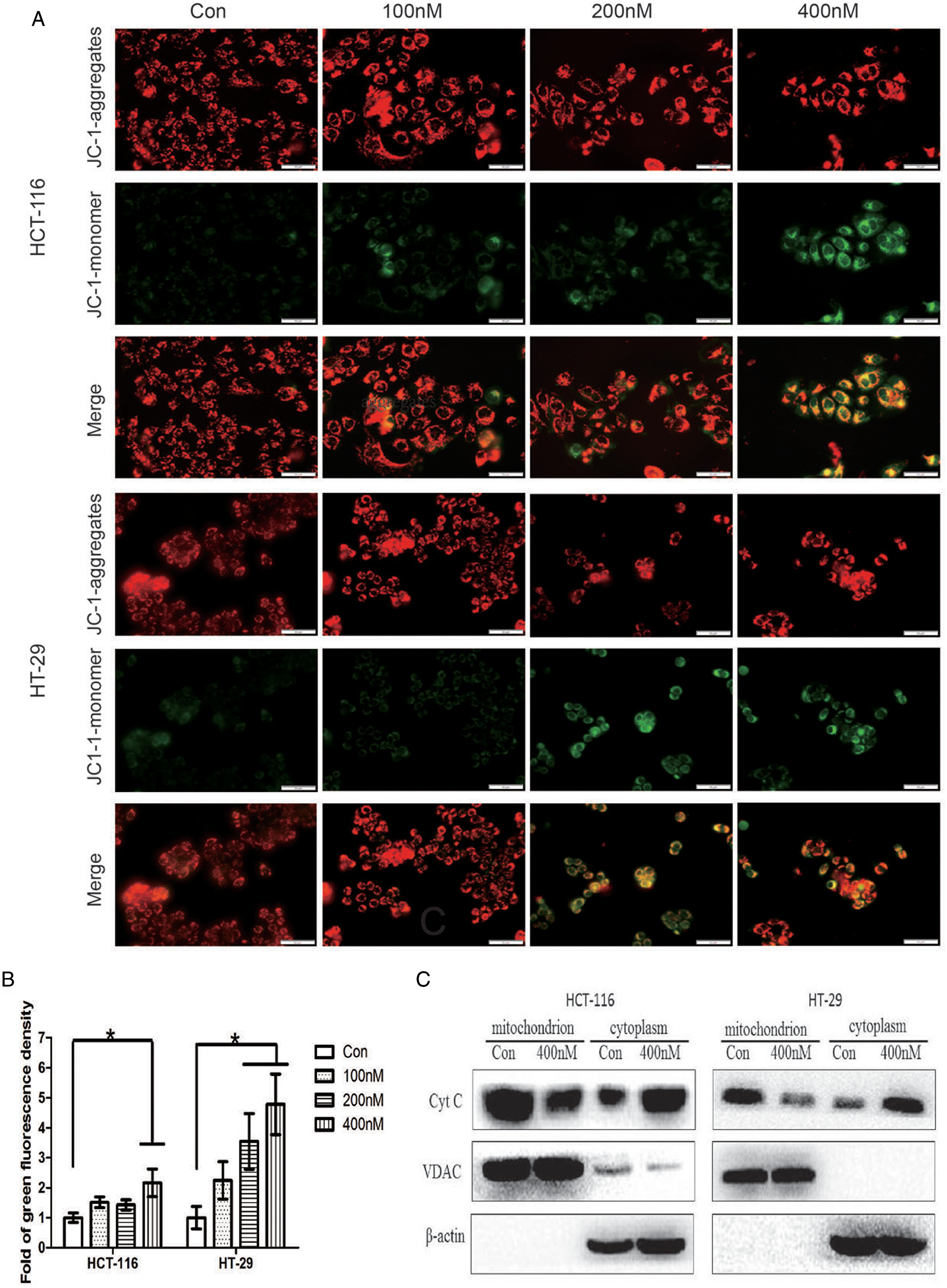
Figure 3: Detection of mitochondrial membrane potential by fluorescent probe JC-1 loading after cells treated with hellebrigenin for 48 h (A and B, the scale bar is 50 μm). Detection of cytochrome C in mitochondrial and cytoplasm by western blot after cells treated with hellebrigenin for 48 h (C). Data of three independent experiments performed one-way ANOVA analysis (*p < 0.05 vs. control).
Hellebrigenin induces accumulation of ROS
An unstable state of ROS is a precipitating factor of apoptosis, and usually, excessive ROS production causes oxidative damage to cells resulting in cell death. In order to find out whether ROS was involved in apoptosis induced by hellebrigenin, we detected the level of ROS in CRC cells by fluorescent probe DCFH-DA. Results showed that the production of ROS elevated, evidenced by green fluorescence increase when cells were treated with 200 and 400 nM hellebrigenin. It revealed that hellebrigenin promoted toxic levels of ROS production to trigger apoptosis in CRC cells (Figs. 4A and 4B).

Figure 4: Detection of ROS by fluorescent probe DCFH-DA loading after cells treated with hellebrigenin for 48 h (A and B, the scale bar is 200 μm). Data of three independent experiments of apoptosis detection performed one-way ANOVA analysis (*p < 0.05 vs. control).
Hellebrigenin suppresses tumor growth in xenograft models
To investigate the antitumor activity of hellebrigenin in vivo, we developed an ectopic transplantation model of tumor cells in nude mice. It showed that hellebrigenin at the chosen dose was able to hinder the increase of tumor volume and weight significantly (Figs. 5A–5C, p < 0.05). Furthermore, hellebrigenin enhanced the expression of BAX, cleaved caspase-9 and cleaved caspase-3, and decreased BCL-2 in three random chosen tumor xenograft models (Fig. 5D). These results revealed that intrinsic apoptosis pathway was also activated by hellebrigenin treatment in vivo. In addition, the protein expression of PCNA in xenograft tumor section was determined via IHC. As the result shown, PCNA decreased in hellebrigenin treated group (Figs. 5E and 5F), which indicated that hellebrigenin might reduce the degree of malignancy of human CRC in vivo.

Figure 5: Anti-proliferation effect of hellebrigenin in CRC xenograft model treated at dose of 2.5 mg/kg/day for 12 days (A) fresh tumor, (B) tumor volume, (C) tumor weight, (D) mitochondrial apoptosis-associated protein BAX, BCL-2, cleaved caspase-3, and cleaved caspase-9 changed in tumor tissue the same as in cell lines, (E) PCNA stained by immunohistochemistry in tumor tissue (the scale bar is 20 μm), (F) relative staining intensity of PCNA (*p < 0.05 vs. control, **p < 0.001 vs. control).
Hellebrigenin is a natural product found in the toad skin secretions and plants of Urginea, including Helleborus and Kalanchoe genera (Tempone et al., 2008). As a compound of bufadienolide, hellebrigenin belongs to the family of cardioactive steroids with high binding affinity and inhibition activity to Na+/K+-ATPase complexes (Moreno et al., 2013). Hellebrigenin also has anti-cancer activity through different molecular mechanisms. For example, it could trigger cell cycle arrest in human glioblastoma U-87 cells at the G2/M phase and promote necrotic cell death through activation of the p38 MAPK signaling pathway (Han et al., 2018). And it showed strong growth inhibition to human leukemia CCRF-CEM cells and multidrug-resistant CEM/ADR5000 cells through binding to tubulin (Abdelfatah et al., 2019). Hellebrigenin induced the G0/G1 arrest and promoted cell early apoptosis and autophagy in both human pancreatic cancer SW1990 and BxPC-3 cells (Wei et al., 2020). It was reported that hellebrigenin induced human hepatocellular carcinoma HepG2 cells DNA damage, mitochondria collapse, cell cycle arrest, and apoptosis (Deng et al., 2014). Hellebrigenin also had anti-proliferative activities against human colon cancel HCT8 cells and HT29 cells (Cunha-Filho et al., 2010; Moreno et al., 2013; Wu et al., 2006). However, the mechanisms of hellebrigenin underlying against colorectal cancer cells are not fully understood yet.
ROS is a set of short-lived molecules with unpaired electrons, including superoxide, hydroxyl, hydrogen peroxide, and hypochlorous acid. Endogenous ROS is byproducts of oxygen consumption and is mainly generated in mitochondria electron transport chain and NADPH oxidases complex (Giorgio et al., 2007; Sabharwal and Schumacker, 2014). The homeostasis of ROS is regulated by a series of antioxidant molecules such as glutathione, peroxiredoxin, thioredoxin, superoxide dismutase, and catalase, which avoid oxidative damage by overproduction of ROS (Nicco and Batteux, 2017). ROS also have long been associated with cancers. Increased levels of ROS have been found in different types of cancer cells compared to their normal counterparts, indicating high metabolism and high antioxidative capacity in the cancer cells. The enhancive level of ROS has shown the ability of activation of pro-survival signaling pathways resulting in function loss of tumor suppressor, adaptation to hypoxia, and generation of oncogenic mutations (Moloney et al., 2017; Sabharwal and Schumacker, 2014). However, toxic levels of ROS production bring oxidative damage to cells resulting in cell cycle arrest, apoptosis, and senescence, which therefore shows anti-tumor potential. Direct ROS-accumulating is an effective anticancer strategy. For instance, doxorubicin can induce the chelation of intracellular iron, leading to the accumulation of hydroxyl radicals and ultimately to cell death (Kotamraju et al., 2002). Cisplatin can directly damage the mitochondrial DNA electron transport chain leading to ETC impairment (Marullo et al., 2013) and also interfere with DNA replication and consequently induce oxidative stress to target cancer cells (Omura and Gynecologic Oncology, 2008)
Although mitochondrion is one of the main ROS generators, increased production of ROS in turn causes damage to the mitochondrial membrane resulting in the release and translocation of cytochrome C from mitochondria to cytosol and then triggering caspase-independent or caspase-dependent apoptosis (Ryter et al., 2007). In our study, hellebrigenin induced excessive production of ROS and then initiated mitochondrial pathway apoptosis evidenced by activation of pro-apoptosis molecules caspase-9, caspase-3, and BAX while inhibition of anti-apoptosis molecule BCL-2. At the same time, mitochondrial transmembrane potential declined, and cytochrome C was translocated from mitochondria to the cytoplasm, indicating the collapse of the mitochondrial membrane. In the tumor-bearing nude mice, hellebrigenin also inhibited ectopic tumor growth significantly through activating the mitochondrial apoptosis pathway. These results suggest hellebrigenin is an intrinsic apoptosis inducer, and its function is involved with excessive ROS production in CRC cells.
Availability of Data and Materials: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Ethics Committee of Zunyi Medical University at 2020-7-30 ([2020]2-148).
Author Contribution: Yun Liu, Sanhua Li, Chunjiao Liu and Qinghong Kong conceptualized and designed the study, aided in acquiring and analyzing data, drafted and critically revised the manuscript. Chunjiao Liu, Gai Huang and Lidan Lu participated in experiments and the data analysis. Fen Pan, Shan Jiang and Lingjie Meng were involved in study design, analyzing and interpreting the data, and critically revised the manuscript. All authors read and approved the final manuscript.
Funding Statement: This work was supported by the Guizhou Provincial Science & Technology Program (QKHZC[2020]4Y154), the Science & Technology Plan of Zunyi (2018 [18]), the Funding of Guizhou Administration of Traditional Chinese Medicine (QZYY-2020-042), the Science and Technology Plan Project of Guizhou (QKHPTRC[2017]5733-059, QKPTRC[2019]-014), Innovation Talent Team of Zunyi (ZSKRC[2019]1), Innovation and Entrepreneurship Project for College Students of Zunyi Medical University (ZYDC2020099), The Science and Technology Plan Project of Guizhou (QKHPTRC[2017]5733–059).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abdelfatah S, Lu X, Schmeda-Hirschmann G, Efferth T (2019). Cytotoxicity and antimitotic activity of Rhinella schneideri and Rhinella marina venoms. Journal of Ethnopharmacology 242: 112049. DOI 10.1016/j.jep.2019.112049. [Google Scholar] [CrossRef]
Cunha-Filho GA, Resck IS, Cavalcanti BC, Pessoa CO, Moraes MO, Ferreira Jé RO, Rodrigues FAR, dos Santos ML (2010). Cytotoxic profile of natural and some modified bufadienolides from toad Rhinella schneideri parotoid gland secretion. Toxicon 56: 339–348. DOI 10.1016/j.toxicon.2010.03.021. [Google Scholar] [CrossRef]
Deng LJ, Hu LP, Peng QL, Yang XL, Bai LL, Yiu A, Li Y, Tian HY, Ye WC, Zhang DM (2014). Hellebrigenin induces cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells through inhibition of Akt. Chemico-Biological Interactions 219: 184–194. DOI 10.1016/j.cbi.2014.06.003. [Google Scholar] [CrossRef]
Feng RM, Zong YN, Cao SM, Xu RH (2019). Current cancer situation in China: Good or bad news from the 2018 Global Cancer Statistics? Cancer Communications 39: 1–12. DOI 10.1186/s40880-018-0346-4. [Google Scholar] [CrossRef]
Giorgio M, Trinei M, Migliaccio E, Pelicci PG (2007). Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nature Reviews Molecular Cell Biology 8: 722–728. DOI 10.1038/nrm2240. [Google Scholar] [CrossRef]
Han L, Yuan B, Shimada R, Hayashi H, Si N, Zhao HY, Bian BL, Tajaki N (2018). Cytocidal effects of arenobufagin and hellebrigenin, two active bufadienolide compounds, against human glioblastoma cell line U-87. International Journal of Oncology 53: 2488–2502. [Google Scholar]
Kotamraju S, Chitambar CR, Kalivendi SV, Joseph J, Kalyanaraman B (2002). Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells: Role of oxidant-induced iron signaling in apoptosis. Journal of Biological Chemistry 277: 17179–17187. DOI 10.1074/jbc.M111604200. [Google Scholar] [CrossRef]
Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, Doetsch PW, Sobol RW (2013). Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One 8: e81162. DOI 10.1371/journal.pone.0081162. [Google Scholar] [CrossRef]
Meng Z, Yang P, Shen Y, Bei W, Zhang Y, Ge Y, Newman RA, Cohen L, Liu L, Thornton B, Chang D Z, Liao Z, Kurzrock R (2009). Pilot study of huachansu in patients with hepatocellular carcinoma, nonsmall-cell lung cancer, or pancreatic cancer. Cancer 115: 5309–5318. DOI 10.1002/cncr.24602. [Google Scholar] [CrossRef]
Moloney JN, Stanicka J, Cotter TG (2017). Subcellular localization of the FLT3-ITD oncogene plays a significant role in the production of NOX- and p22(phox)-derived reactive oxygen species in acute myeloid leukemia. Leukemia Research 52: 34–42. DOI 10.1016/j.leukres.2016.11.006. [Google Scholar] [CrossRef]
Moreno YBL, Katz A, Miklos W, Cimmino A, Tal DM, Ainbinder E, Zehl M, Urban E, Evidente A, Kopp B, Berger W, Feron O, Karlish S, Kiss R (2013). Hellebrin and its aglycone form hellebrigenin display similar in vitro growth inhibitory effects in cancer cells and binding profiles to the alpha subunits of the Na+/K+-ATPase. Molecular Cancer 12: 33. DOI 10.1186/1476-4598-12-33. [Google Scholar] [CrossRef]
Nicco C, Batteux F (2017). ROS modulator molecules with therapeutic potential in cancers treatments. Molecules 23: 84. DOI 10.3390/molecules23010084. [Google Scholar] [CrossRef]
Omura GA, Gynecologic Oncology Group (2008). Progress in gynecologic cancer research: The Gynecologic Oncology Group experience. Seminars in Oncology 35: 507–521. DOI 10.1053/j.seminoncol.2008.07.007. [Google Scholar] [CrossRef]
Qi FH, Zhao L, Zhou AY, Zhang B, Li AY, Wang Z, Han J (2015). The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. BioScience Trends 9: 16–34. DOI 10.5582/bst.2015.01019. [Google Scholar] [CrossRef]
Qin TJ, Zhao XH, Yun J, Zhang LX, Ruan ZP, Pan BR (2008). Efficacy and safety of gemcitabine-oxaliplatin combined with huachansu in patients with advanced gallbladder carcinoma. World Journal of Gastroenterology 14: 5210–5216. DOI 10.3748/wjg.14.5210. [Google Scholar] [CrossRef]
Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AMK (2007). Mechanisms of cell death in oxidative stress. Antioxidants & Redox Signaling 9: 49–89. DOI 10.1089/ars.2007.9.49. [Google Scholar] [CrossRef]
Sabharwal SS, Schumacker PT (2014). Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles' heel? Nature Reviews Cancer 14: 709–721. DOI 10.1038/nrc3803. [Google Scholar] [CrossRef]
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute 82: 1107–1112. DOI 10.1093/jnci/82.13.1107. [Google Scholar] [CrossRef]
Tempone AG, Pimenta DC, Lebrun I, Sartorelli P, Taniwaki NN, de Andrade HF, Antoniazzi MM, Jared CC (2008). Antileishmanial and antitrypanosomal activity of bufadienolides isolated from the toad Rhinella jimi parotoid macrogland secretion. Toxicon 52: 13–21. DOI 10.1016/j.toxicon.2008.05.008. [Google Scholar] [CrossRef]
Wang DL, Qi FH, Tang W, Wang FS (2011). Chemical constituents and bioactivities of the skin of Bufo bufo gargarizans Cantor. Chemistry & Biodiversity 8: 559–567. DOI 10.1002/cbdv.201000283. [Google Scholar] [CrossRef]
Wei X, He J, Gao B, Han L, Mao Y, Zhao H, Si N, Wang H, Yang J, Bian B (2020). Hellebrigenin anti-pancreatic cancer effects based on apoptosis and autophage. PeerJ 8: e9011. DOI 10.7717/peerj.9011. [Google Scholar] [CrossRef]
Wu PL, Hsu YL, Wu TS, Bastow KF, Lee KH (2006). Kalanchosides A-C, new cytotoxic bufadienolides from the aerial parts of Kalanchoe gracilis. Organic Letters 8: 5207–5210. DOI 10.1021/ol061873m. [Google Scholar] [CrossRef]
Zhan X, Wu H, Wu H, Wang R, Luo C, Gao B, Chen Z, Li Q (2020). Metabolites from Bufo gargarizans (Cantor, 1842A review of traditional uses, pharmacological activity, toxicity and quality control. Journal of Ethnopharmacology 246: 112178. DOI 10.1016/j.jep.2019.112178. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |