DOI:10.32604/biocell.2021.015900

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.015900 |  www.techscience.com/journal/biocell |
| Review |
Biomedical overview of melanin. 1. Updating melanin biology and chemistry, physico-chemical properties, melanoma tumors, and photothermal therapy
1Department of Biology, Faculty of Sciences, Autonomous University of Madrid, Cantoblanco, Madrid, 28049, Spain
2Centro Integrativo de Biología y Química Aplicada, Universidad Bernardo O’Higgins, Santiago, 8370854, Chile
3Universidad de Buenos Aires, Facultad de Ciencias Veterinarias, Cátedra de Histología y Embriología, e Instituto de Investigación y Tecnología en Reproducción Animal, Buenos Aires, C1427CWO, Argentina
*Address correspondence to: Alfonso Blázquez-Castro, alfonso.blazquez@uam.es
Received: 22 January 2021; Accepted: 04 March 2021
Abstract: Melanins (eumelanin, pheomelanin, and allomelanin) represent a very, if not the most, important group of biological pigments. Their biological roles are multiple, from photoprotection to antioxidant activity, heavy metal disposal or the myriad uses of color in organisms across all Phyla. In the first part of this review, eumelanin biology and some chemical aspects will be presented, as well as key physico-chemical features that make this biological pigment so interesting. The principal characteristics of the melanocyte, the melanin-synthesizing cell in mammals, will also be introduced. Transformed melanocytes are the cause of one of the most devastating known cancers: the malignant melanoma. Epidemiology and molecular signaling aspects will be presented next, as well as the principal advances in promising oncotherapies designed and applied for the treatment of melanoma. In particular, on account of the photo-physical properties of melanin, special details will be provided regarding the use of photothermal therapy for melanoma treatment.
Keywords: Antitumor therapies; Biological pigments; Melanin; Melanoma; Photothermal therapy; Skin tumors
The black pigment of the living world is generically called melanin (Greek, µελας, mélas: black), and, chemically, corresponds to aromatic polymers that are widely dispersed in the animal and plant kingdoms. The term melanin was first coined by Berzelius in 1840 to describe black animal pigments. An outstanding feature of melanin is its brown-black color. The pigment occurs at all phyletic levels of biological organization and is considered to be a catechol- or indole-containing macromolecule. Regarding chemical types of melanin (referred as catechol-melanins and indole-melanins) (Nicolaus et al., 1994), it is worth to note the similarity of name of precursors with the known types of neurotransmitters (catechol-amines and indole-amines). For melanin definition and classification, see d’Ischia et al. (2013).
Animal melanin is a light-absorbing indole-polymer derived from the oxidation of tyrosine in melanocytes, and involves eumelanin and pheomelanin (yellow-red, in red hair and feathers), but here the term melanin will refer specifically to eumelanin. Early and present reviews on the occurrence, chemistry, properties, and biosynthesis are available (Swan, 1974; Nicolaus, 1997; d’Ischia et al., 2015; Solano, 2017; Panzella et al., 2018; Huang et al., 2018; D’Alba and Shawkey, 2019; d’Ischia et al., 2019; Cavallini et al., 2020).
Although melanin is the main pigment in the skin of vertebrates, it has been also found in a great variety of organisms such as eubacteria, protozoa, fungi, higher plants, cephalopods, insects, etc. (Nicolaus et al., 1994; Land et al., 2004; d’Ischia et al., 2015; D’Alba and Shawkey, 2019). In plants, melanin corresponds to the catechol-type and is generally named allomelanin. Melanin also occurs in the malignant melanoma, one of the most aggressive human tumors. Neuromelanin is located in the substantia nigra, locus coeruleus, retinal pigmented epithelium, and stria vascularis in the cochlea (Nicolaus, 2005). Neuromelanin depletion damages the function of substantia nigra, and it could be related to human diseases such as parkinsonism, schizophrenia, and deafness (McGuinness et al., 1974). Participation of an altered redox status of melanin in the etiology of melanoma and macular degeneration has been suggested (Sarangarajan and Apte, 2006).
The pigment shows strong adaptive value and has many biological functions. It confers strengthening of plant cell walls, insect cuticles, and bird feathers. Animal melanin is related to skin photoprotection, photoreceptor shielding, thermo-regulation, camouflage and adaptive color responses. Allomelanins are involved in hardening the exterior envelope of spores and seeds (Land et al., 2004).
Likewise, melanins are powerful antioxidant and detoxification agents by removing reactive oxygen species (ROS) and toxic heavy metals. These features have been studied using natural and synthetic melanins, revealing a clear protection against ROS and oxidizing radicals damage (Sarangarajan and Apte, 2006). Trapping of heavy metals, radicals, and harmful chemicals is relevant to body detoxification/protection. The conjugated molecular structure of melanin allows straightforward engagement in redox changes through dynamic equilibrium between quinone and catechol groups.
At present, melanins are of great interest for a broad range of fields, e.g., biomedical research, regenerative medicine (Cavallini et al., 2020), coating, surface, and adhesive new materials (Scognamiglio et al., 2017; d’Ischia, 2018), nanotechnology, and for opto-bioelectronics and photoacoustic devices (Solano, 2017; d’Ischia et al., 2015; d’Ischia et al., 2019). This is due to its striking physicochemical properties: (a) broad-band light absorption spanning the ultraviolet (UV), visible, and near-infrared (NIR) spectrum; (b) paramagnetism; (c) hydration-dependent semi-conductivity; (d) efficient dissipation of the absorbed photon energy as heat; (e) antioxidant properties, both as H-atom donor and as reducing agent; (f) radical-scavenger properties; (g) redox behavior; (h) ion-exchange; (i) high adhesiveness, and (j) metal chelation and binding of drugs and organic compounds. Melanins are also bioavailable, biocompatible and biodegradable, thus representing most promising candidates for biomedical applications.
In addition to melanin involvement in human pigmentation and its disorders (e.g., albinism, vitiligo), melanocytes are the source of the malignant melanoma. Current melanoma treatments are limited mainly to surgery, radiotherapy, and chemotherapy, but for disseminated (metastatic) melanoma there is still no curative therapy. Therefore, new therapeutic modalities based on chemical and physical approaches are necessary. The aim of the first part of this eumelanin review is to describe biological and chemical aspects, physicochemical properties, as well as epidemiology and therapeutic approaches of melanomas, mainly the photothermal therapy. As the literature on melanin is overwhelmingly broad, only the most relevant articles, in our opinion, are mentioned.
Melanin refers to the chemical name of the pigment, whereas melanosome is the specific cellular organelle producing and containing it. Both are produced by melanocytes, a cell type derived from the neuroectoderm layer of the embryo. In mammals, some neural crest cells adopt a melanocytic fate and becomes melanoblasts, which begin to migrate from areas near the neural tube to the developing dermis. Melanoblasts localize in the epidermis, and melanocytes are later found among the skin epithelial cells (keratinocytes) and in the hair follicle, and subsequently they establish a melanocyte stem cell population (see Bejaoui et al., 2020). In melanocytes, melanin is formed within melanosomes (often considered a passive structure), which originate in the Golgi apparatus, and are then transferred to keratinocytes.
Melanosomes within dendritic processes in ramified melanocytes release the melanin content into the intercellular space by exocytosis, and then melanin is internalized in keratinocytes by phagocytosis (D’Alba and Shawkey, 2019). The described epidermal-melanin unit in the skin seems involved in the pathophysiology of melanoma (Jimbow et al., 1991). In basal and suprabasal keratinocytes, melanin granules generally appear with a striking polar distribution, covering the supranuclear region as a hood or cup near the skin surface (Kobayashi et al., 1998; Simpson et al., 2013). Thus melanin provides photoprotection against UV-induced damage of nuclear DNA by shielding nuclei by a melanin “micro-umbrella,” which is easily recognized in Fontana-Masson-stained skin sections (Fig. 1).
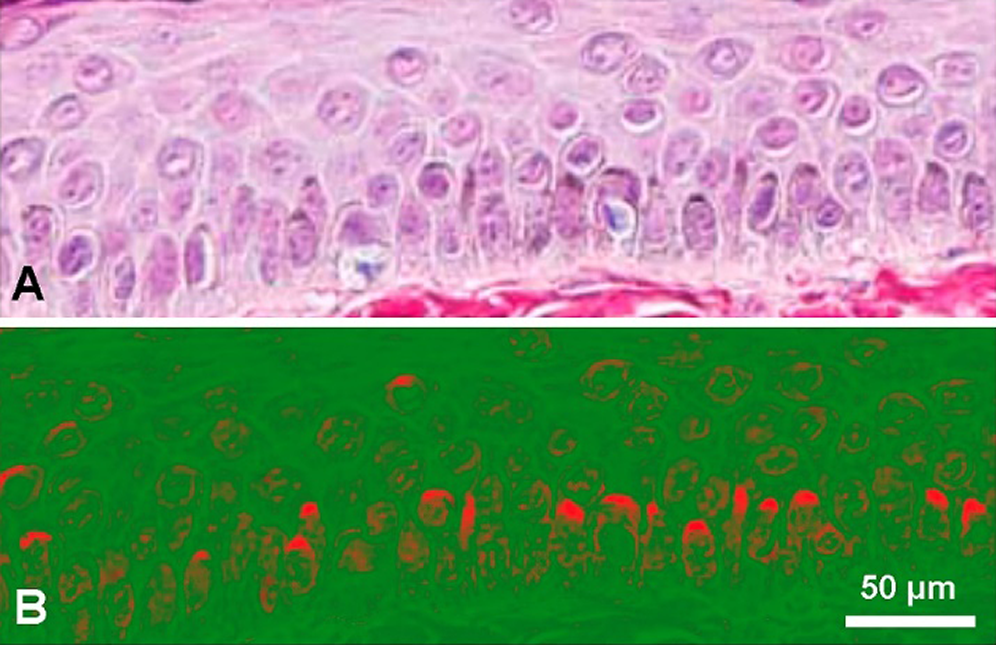
Figure 1: (A) Paraffin section of human skin stained with the Fontana-Masson-picrosirius method, showing keratinocytes with supranuclear melanin-containing micro-umbrellas. Harris hematoxylin counterstaining. Skin surface is at top and a portion of basal membrane is shown at the bottom (Reproduced with permission from Carriel et al. (2011), Journal of Histochemistry and Cytochemistry 59: 270–277). (B) The same picture after processing with ImageJ 1.52v software, using gray gradient converted to false color LUT (red/green). Supranuclear micro-umbrellas are highlighted in red.
Hair is another complex skin structure deeply modified by melanin. Mature hair follicles show a growth cycle consisting of phases of growth (anagen), regression (catagen), and rest (telogen). In the anagen phase, follicle melanocytes synthesize melanin that is then transferred to adjacent specialized keratinocytes, also known as hair progenitor cells. The melanin synthesis is reduced in the catagen phase and is completely absent in the telogen phase. Therefore, the life of follicle melanocytes is linked with the hair growth cycle (Slominski et al., 2005), and both melanogenesis and hair follicle biology are under a complex regulation by transcription factors, melanin-stimulating agents, and several signaling pathways (Bejaoui et al., 2020).
In spite that human hair per se provides a degree of natural sun protection (de Gálvez et al., 2015), skin photoprotection is a relevant function of melanin, and there is evidence that the occurrence of a higher melanin content results in a decreased risk of skin cancer induced by sun exposure. Protection mechanisms involve the absorption of UV photons that might otherwise damage DNA. Solar UV exposure leads to the formation of DNA photoproducts (mainly cyclobutane pyrimidine dimers). UV-induced DNA photoproducts and/or their excision repair products stimulate melanin synthesis (Eller et al., 1996), which in turn may protect epidermal cells against further UV-induced DNA damage. This process promotes the transcription of tyrosinase genes and melanogenesis (Agar and Young, 2005). The protective action may also involve generation of toxic radicals to kill cells that have been exposed to genotoxic doses of light.
Constitutive or facultative pigmentation are viewed as photoprotective, but the function of melanin remains still controversial. In radiotherapy, the treatment with melanin nanoparticles protects against X-ray radiation damage (Na et al., 2019). Melanin also protects cells from different type of radiation including UV, X and gamma rays, and from chemo-, radio- and photo-dynamic therapy. As the presence of melanin in metastatic melanoma attenuates the efficacy of radiotherapy, inhibition of melanogenesis has been suggested as an approach for metastatic melanoma therapy (D’Mello et al., 2016; Brożyna et al., 2016; Sniegocka et al., 2018).
Melanosomes are round or ellipsoid granules, 0.5–1 μm in diameter, belonging to the family of endosome-lysosome organelles. Melanin biosynthesis and melanosome biogenesis are regulated processes in a complex scenario of enzymes, structural scaffolding proteins, metal ions, etc. (Sarangarajan and Apte, 2006). Specific proteins such as MITF and PMEL17 (fibrillary protein of the pre-melanosome matrix) are involved in precursors polymerization (Huang et al., 2018; Sarangarajan and Apte, 2006; Wiriyasermkul et al., 2020).
Melanin biogenesis starts with the oxidation of tyrosine by tyrosinase, which is only active in melanocytes. Tyrosinase is a Cu-binding, integral membrane glycoprotein that initiates melanin synthesis by oxidizing L-tyrosine to DOPA (3,4-dihydroxy-phenylalanine) and DOPA-quinone, which then polymerize spontaneously.
Cu2+-tyrosinase is an essential enzyme for melanin biosynthesis, and it needs neutral pH for activity. In addition to Cu+2-tyrosinase, the tyrosinase-related Zn+2-containing proteins TRP1 and TRP2 (dopachrome tautomerase) are implicated in eumelanin biosynthesis (Sarangarajan and Apte, 2006; Wiriyasermkul et al., 2020). Tyrosinase and TRP2 are transported from the trans-Golgi network to melanosomes in coated vesicles before melanin deposition. Gene expression, enzymatic activity, pH- and ion-homeostasis regulation, and melanosome morphogenesis have been widely reviewed (Nicolaus, 1997; D’Alba and Shawkey, 2019; Sarangarajan and Apte, 2006; Wiriyasermkul et al., 2020; Büngeler et al., 2017).
It is accepted (see D’Alba and Shawkey, 2019) that eumelanosomes are formed through a series of well-defined stages. Stage I begins with an tyrosinase-containing endosome from the Golgi apparatus, showing an incipient protein PMEL17 amyloid-like fibrils, which form a matrix that optimizes melanin polymerization and condensation. Fibrils are completely formed in Stage II, and then the melanosome adopts an ovoid shape. In Stages III and IV, electron-dense melanin is synthesized and progressively deposited on the β-sheet like fibrils until the internal structure of the melanosome is completely obscured at the end of Stage IV. According to Büngeler et al. (2017), morphological aspects of the melanosome formation involves three-steps, and four-levels hierarchical buildup mechanism. Each step increases the size of the melanin particle in the following order: (a) melanin oligomer sheets produce (b) proto-particles (10−9 m), which form onion-like structures and condense into (c) spherical type-A particles (10−8 m) that then aggregate in (d) spherical type-B particles (10−7 m).
Although histopathological sections are currently stained by the hematoxylin-eosin (H&E), method, melanin can be identified in bright-field microscopy of unstained paraffin sections. Due to the anionic character of melanin, it appears additionally stained by the cationic aluminum-hematein complex. Differential diagnosis of melanin in lipofuscinosis, hemosiderosis, hemochromatosis, etc., requires selective histochemical methods. The Fontana-Masson stain (Bancroft and Gamble, 2008; Li et al., 2014) exploits the argentaffin character of melanin, and this stain is applied as a routine diagnostic tool for melanoma and neuroendocrine tumors in paraffin or frozen sections. This allows visualization of melanin as well as argentaffin and chromaffin cells. Lipofuscin granules and fungal (Cladosporium) infections are also revealed in black. Nuclei are commonly counterstained with Harris hematoxylin, safranin, or nuclear fast red. Fontana-Masson picrosirius (Sirius red F3B-picric acid) method has been suggested for the simultaneous staining of melanin and collagen fibers in benign or malignant melanocytic lesions (Carriel et al., 2011).
More selective immunohistochemical staining procedures for melanoma cells are based on HMB45, MART-1 and Sox10 protein antigens (Crawford et al., 2017). The detection of HMB45 indicates that melanogenesis is occurring. The identity of melanocytic cells can be confirmed by their tyrosinase content, which oxidizes L-DOPA to a dark brown, melanin-like pigment. Tyrosinase activity is also histochemically revealed by the fluorescent DOPA-glyoxylic acid reaction (Ichikawa et al., 2009), and a BODIPY-based fluorescent probe (Kim et al., 2011). An elevated expression of tyrosine using a tyramide-Cy3 probe was found in melanoma cells (Angeletti et al., 2004). Metalloproteinases in the stromal matrix of melanomas (possibly related to tumor expansion) have been histochemically detected (Hofmann et al., 2000).
For morphological purposes, dark melanosomes do not interfere with histological studies, but sometimes they can mask results from other histochemical methods such as those based on peroxidase-DAB. Hence, the dark color of melanosomes requires removal, most popular bleaching methods being treatments with KMnO4/oxalic acid (Alexander et al., 1996), or 3–10% H2O2 solutions (Liu et al., 2013). Today, genetic tools and imaging approaches allow the analysis of melanocytic lineage and behavior. For instance, breeding ROSAmT/mG and Tyr::CreERT2 mice generates animals in which melanocytic lineage cells are identified through expression of a green fluorescent protein (Crawford et al., 2017).
Although the detailed structure of eumelanin is poorly known, an overwhelming evidence indicates that it entails an indole backbone with high conjugation degree, thus explaining the strong photon absorption. All natural and synthetic melanins conform to a 3D multi-layer graphite-like aromatic structure, forming amorphous microparticles of different shapes and sizes. Melanin could be now considered a mixture of a pheomelanin core surrounded by a eumelanin shell, with a ratio that determines the final skin and hair color (d’Ischia et al., 2015).
Unfortunately, no melanin sample has yet been fully and unambiguously characterized chemically, and even more simple synthetic melanins appear somewhat heterogeneous (Blois et al., 1964), but X-ray studies indicate that synthetic melanins are essentially similar to “real” eumelanin (Cheng et al., 1994). High molecular weight, poor water solubility, resistance to hydrolysis, and chemical heterogeneity are features that make difficult to analyze the precise chemical structure of eumelanin. However, several models have been suggested, and some of them seem to be plausible.
In the biosynthesis of melanin precursors, the amino acid L-tyrosine is first oxidized to L-DOPA (3,4-dihydroxy-phenylananine), and then to 5,6-dihydroxyindole-2-carboxylic acid (DHICA) and 5,6-dihydroxyindole (DHI). Oxidized and/or decarboxylated forms are indole-5,6-quinone (IQ) (Fig. 2), and indole-5,6-quinone-2-carboxylic acid, respectively. Synthetic melanin-like compounds do not contain protein components, and are formed in vitro by oxidative polymerization of the precursors tyrosine, DOPA, dopamine, DHI, and IQ (Dreyer et al., 2012; Micillo et al., 2016), which occurs spontaneously with time at slight alkaline pH.
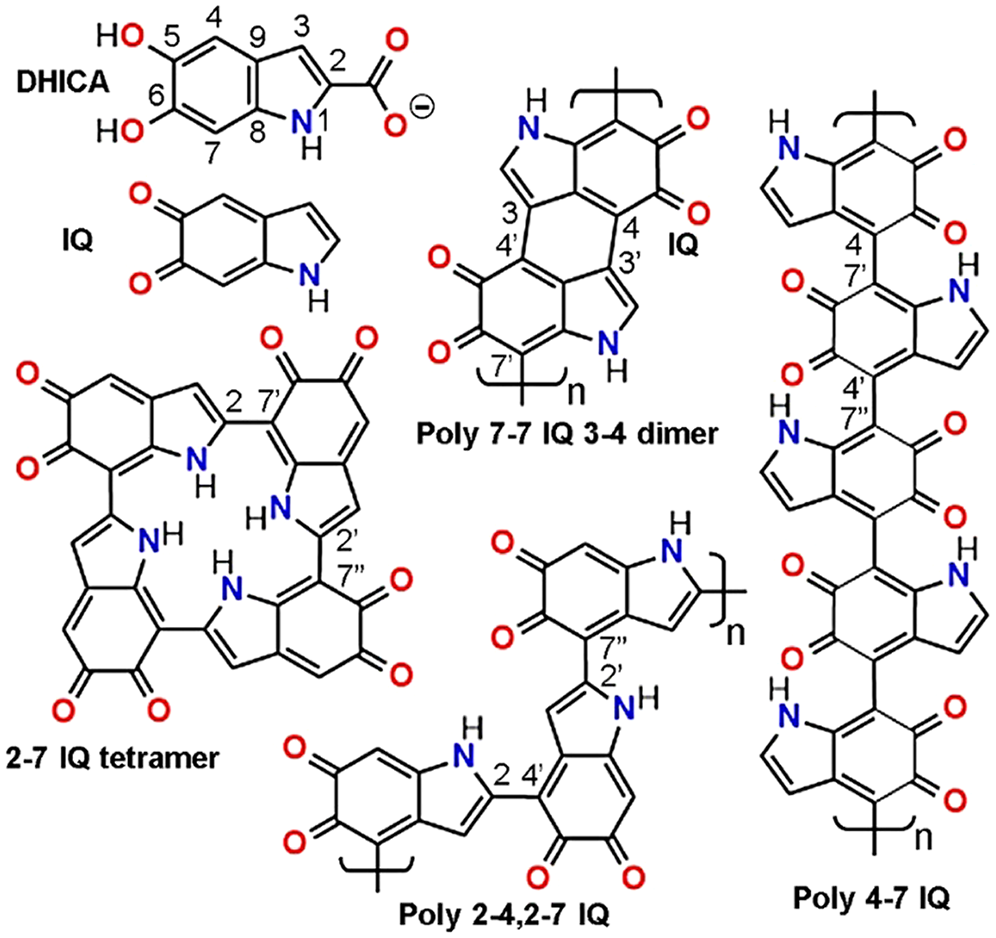
Figure 2: Monomeric melanin precursors: 5,6-dihydroxyindole-2-carboxylic acid (DHICA, with atom numbering), indole-5,6-quinone (IQ), and a gallery of possible IQ-melanin structures. DHICA units can also occur in polymers with free C2 position. Eumelanin polymers are represented according to Liebscher et al. (2013), d’Ischia et al. (2015), and Panzella et al. (2018). Other possible models of eumelanin will be described in the part 2 of this review.
Melanin structures are presented here using the oxidized IQ forms. Simple H-bond aggregates of monomers, and isolated 3,4-dimers of IQ have a very low conjugation degree, and it is unlikely that they can explain the color and characteristic broad-band absorption spectra of melanin. Massive chromophore stacking and π-interactions are known to take place in aromatic compounds, tri- and macrocyclic dyes, either in solution or crystalline state (e.g., thiazine, acridine and phthalocyanine dyes), but they have well-structured spectra and not broad-band absorption (Stockert and Blázquez-Castro, 2017).
On the other hand, cyclic polymerization of IQ results in 2–7 IQ tetramers (Meng and Kaxiras, 2008), and other cyclic oligomers (Chen et al., 2014). It is also not expected that stacking of these structures could account for the broad-band melanin spectra. On the contrary, polymers such as poly 7-7 IQ 3-4 dimer, poly 2-4,2-7 IQ zig-zag chain, and poly 4-7 IQ linear chain (Fig. 2), represent commonly accepted structures of eumelanin (Liebscher et al., 2013; d’Ischia et al., 2015; Panzella et al., 2018). Poly 2-4,2-7 DHI and poly 4-7 IQ only can be formed by using units without 2-carboxylate groups. In the case of 2-4,2-7 zig-zag polymer, a dihedral angle of ~18° occurs between indole rings, allowing π-stacking in an almost planar configuration (Micillo et al., 2016; Panzella et al., 2018).
Due to steric hindrance, the dihedral angle between IQ rings of poly 4–7 IQ is ~40°, which could be considered an impediment for C=C conjugation. However, resonance increases in the first excited state of the stacked IQ rings, because dihedral angles become lower (~20°), allowing greater conjugation. The 3D organization of eumelanin is still poorly known, but there are different models according to the polymeric structure. Stacking of either cyclic tetramers (Chen et al., 2014), linear, or zig-zag chains (Liebscher et al., 2013; d’Ischia et al., 2015), and bundling arrays of linear polymers have been proposed (Micillo et al., 2016; Panzella et al., 2018).
Inspection of molecular orbitals (MOs) from eumelanin structures allows a better understanding of the conjugation changes induced by photoexcitation. A dimeric portion of the poly 4–7 IQ model is shown in Fig. 3. Ground and light-excited molecules with bonding (π) and anti-bonding (π*) electron states (S0 and S1, respectively), result in different MOs, which correspond to the highest-occupied (HOMO), and lowest-unoccupied (LUMO) energy levels, respectively (Stockert and Blázquez-Castro, 2017; Stockert, 2020). In this case, the first excited singlet S1 state (LUMO+0) of the 4–7 IQ dimer has a more extended π-conjugation (Fig. 3C) than that of the ground singlet S0 state (HOMO-0) (Fig. 3B). Thus, the dissipation of electronic energy to the ground state results in massive thermal delivery to the medium. These molecular structural features lead to particular and very interesting physico-chemical properties of eumelanin.
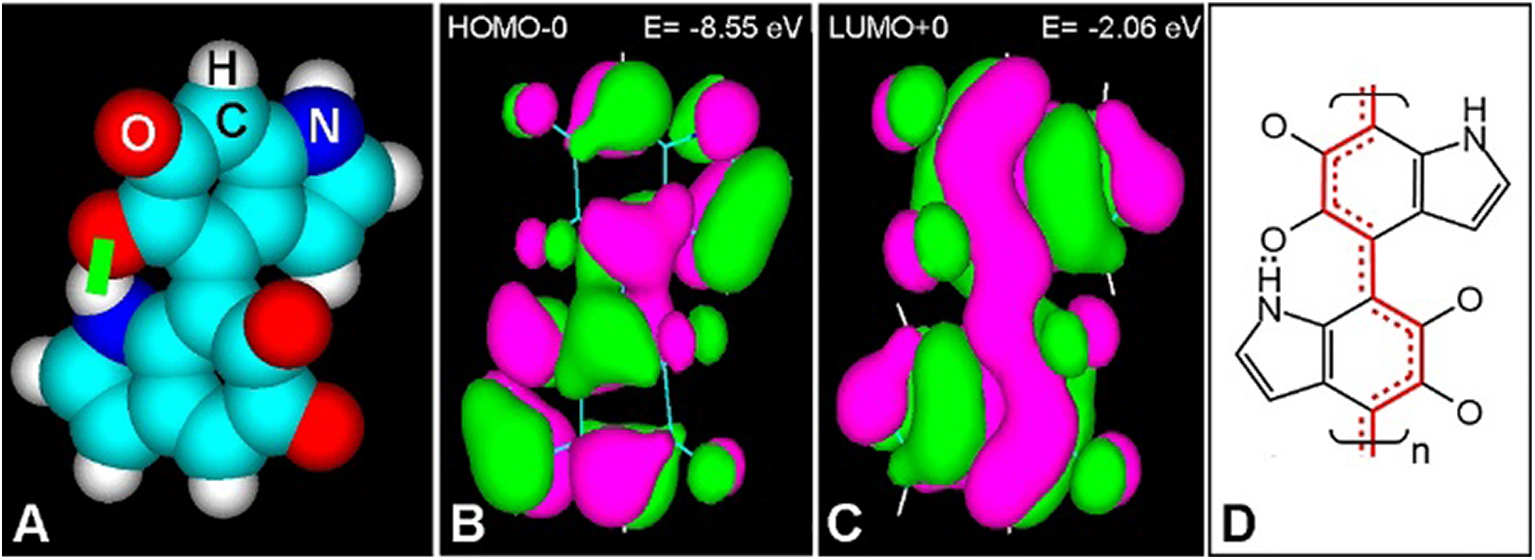
Figure 3: (A) Atomic volume model of the 4-7 IQ dimer with H bond (green bar). (B, C) HOMO-0 (B) and LUMO+0 (C) of the dimer, showing positive (green) and negative (violet) π-orbital lobes with energy (E) values, and orbital contour (1/orbital radius) of 0.02 (HyperChem 7 software, PM3 geometry optimization to 0.1 kcal/Å mol, Goraud shaded isosurface). (D) Resonance pattern of double bonds in the S1 state, according to LUMO+0, showing localized (two lines) and resonant double bonds (red continuous and dashed lines).
The monotonic absorption spectrum and extensive exponential decay of a sample of melanin, with the typical broad-band absorption across the UV-visible-NIR regions, is shown in Fig. 4. Absorption spectra from mammalian, invertebrate (cuttlefish), and synthetic melanins have the same characteristics (Parrish et al., 1983; Plaetzer et al., 2009; Micillo et al., 2016). Some spectra can show small shoulders at ~350–400 and ~550–650 nm, possibly due to a slight heterogeneous composition.
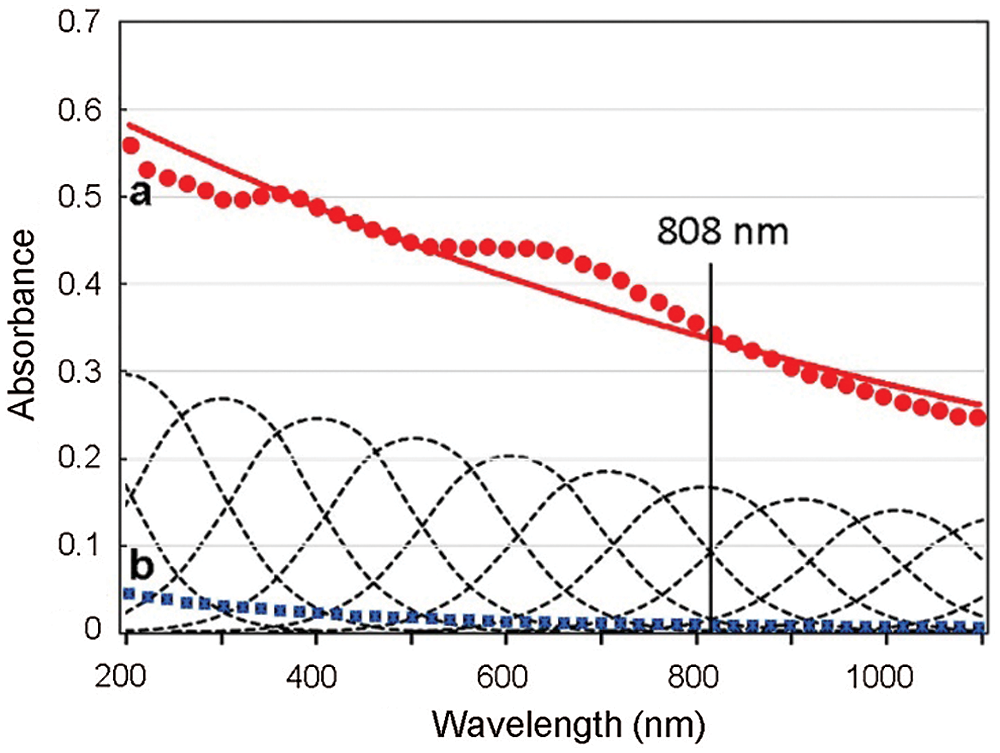
Figure 4: Absorption spectra of sepiomelanin (Sepia officinalis, Freiremar, Spain) (a, red circles, and exponential curve, red line), and China ink (Pelikan black drawing ink, Hannover, Germany) (b, blue squares), both diluted 1:2000 (v/v) in distilled water. Absorption curves of the pigments decrease monotonically from UV to NIR, which is a typical feature of graphitic materials. The Gaussian components of melanin absorption are shown as multiple dashed lines. The 808 nm laser wavelength commonly used for photothermal near infrared (NIR) irradiation is also indicated.
Eumelanin is a stable and amorphous material with rather exotic electrical properties (e.g., semi-conductivity and high density of free spins). Isolated melanosomes and synthetic melanin show a dielectric-semiconductor threshold switch at potentials 2–3 orders of magnitude lower than those reported for inorganic semiconductors (McGuinness et al., 1974). The high density of negative charges on melanin makes it an excellent metal cation trap, and possibly this constitutes one of the biological roles of the pigment, because melanin provides a privileged excretory pathway for heavy metals through keratinocyte desquamation. Melanin shows a strong affinity for metal cations such as Zn2+, Cu2+, Cd2+, Sr2+, Pb2+, Fe3+, Mn3+, La3+, and Gd3+, whereas Na+, K+, Mg2+, and Ca2+ ions bind less strongly (d’Ischia et al., 2015).
Electron paramagnetic resonance (EPR) signal is a specific analytical tool for the detection of melanins, whereas statements based on color and chemical reactivity may be misleading. EPR studies indicate that paramagnetism of synthetic and natural melanin is abolished by cupric and ferric ions and increases by UV irradiation. The observed paramagnetism of melanin is due to the large density of unpaired electrons that may also involve a radical mechanism for melanin biosynthesis (Blois et al., 1964).
These physical data are in agreement with the concept that melanin is a 3D heterogeneous polaron-radical polymer (Olivieri and Nicolaus, 1999), formed by several types of indole units. To explain the function of melanin in both illuminated and non-illuminated areas, participation in energy transduction processes involving strong electron-phonon interactions (fast non-radiative deactivation), ion storage, electromagnetic field sensing, and large density of available energy states have been suggested (Solano, 2017; Wiriyasermkul et al., 2020).
Epidemiological aspects
Malignant melanoma, referred to as “the cancer that rises with the Sun” (Holmes, 2014), is one of the most aggressive human tumors and has the worst prognosis among skin cancers (Lens and Dawes, 2004; Walker and Hayward, 2018), because classical treatments such as radiotherapy and chemotherapy are generally unsuccessful. Melanomas correspond only to 4% of all dermatologic cancers, but are responsible for >80% of deaths provoked by skin cancer. From 1975 to 2013, the incidence of skin melanoma increased 3 times. The incidence of melanoma is continuously increasing, and reached 351,880 cases reported in 2015 (Karimkhani et al., 2017). A number of host characterizations and sun exposure conditions have been identified as risk factors for skin melanomas (Weinstock, 1996; Elwood and Jopson, 1997; Loria and Matos, 2001).
White-skinned populations have the highest incidence of skin melanoma (Alexandrescu et al., 2013; Guy et al., 2015). Black-skinned populations have lower incidences of melanoma (which seems due to the protective effect of melanin against UV radiation), but when tumors do appear they are more aggressive. As it occurs for many cancers, early detection is associated with survival improvement. The high visibility of most skin melanomas makes possible that about 70% are detected before metastatic spreading to lymph nodes or distant sites.
According to epidemiological surveys (Howlader et al., 2016; Karimkhani et al., 2017), there is an increasing and afflicting number of melanoma tumors. In Australia, due to the high solar radiation and the white skin of much of the population, the melanoma incidence in the period 1983–2007 was high, and passed from 23.9 and 25.9 to 58,4 and 41,8 cases/100,000 for men and women, respectively. The 5-years survival is 98% when surgery is done in localized tumors, 62.4% when draining lymph nodes are involved, and 17.9% when there is metastasis in distant organs.
Non-acral cutaneous melanomas can be classified into three main clinic-histopathological categories (Reddy et al., 2017): superficial spreading melanoma (SSM), nodular melanoma (NM), and lentigo malignant melanoma (LMM). The most common type is SSM, which takes place in regions with intermediate sun exposure and has a clear radial growth followed by a vertical growth phase, representing about 65% of all melanomas. NM corresponds to 15% of melanomas and show a rapid vertical growth lacking horizontal growth. LMM represents between 4% and 15% of melanomas, has a slow, radial growth phase before vertical growth, and occurs in sun-damaged regions.
Unfortunately, conventional treatments for metastatic melanoma (radiotherapy, surgery, chemotherapy) have not been much effective. Therefore, novel therapeutic approaches for melanoma are needed to improve the life quality of the patient, and to reduce morbidity, acquired resistance to drugs, and mortality. Adjuvant immune-checkpoint inhibitors and immune-targeted therapies are now promising treatments for advanced melanomas.
Therapeutic approaches
Today the surgical removal of melanomas is the first therapeutic option, because they are considerably refractory to other traditional therapies. In spite of surgical removal, disease recurrence will appear in many of these patients, leading to poor prognosis. Only 14% of metastatic melanomas has a survival over 4 years (Hodis et al., 2012). In most cases, primary tumors can be seen by the naked eye and are easily removed, analyzed for response to the therapy, or used as a source of immune stimulation. Prognostic role of circulating melanoma cells can be detected by PCR for tyrosinase mRNA (Visús et al., 2007). Recently, the interesting concept of circulating tumor DNA (ctDNA) as a “liquid biopsy” for melanoma has been described and applied (Calapre et al., 2017). This ctDNA biomarker is useful to monitor the impact of adjuvant therapy and relapse prediction in advanced melanoma (Tan et al., 2019).
Clinical-genetic risk factors for melanoma are known, as well as a clear causal environmental factor (UV radiation) (Reddy et al., 2017). Advanced and metastatic melanomas are one of the most resistant cancers for chemotherapy, with dacarbazine being for almost 20 years the only drug approved by the US FDA (Jin et al., 2019). The advent of therapeutic approaches based on studies of mutations, gene expression, and signaling has improved the clinical management of metastatic melanoma (Lens and Dawes, 2004). Rapid progress in genomic research has contributed to understand the pathogenesis of melanoma, but the clinical significance of the vast array of genomic alterations is far from being fully characterized.
Melanoma has the highest mutational loading among human tumors (Reddy et al., 2017). Numerous studies on melanoma genetics have been published (Hawryluk and Tsao, 2014; Cancer Genome Atlas Network, 2015; Davis et al., 2018), but significant differences between “driver” mutations—involved in tumor progression and sensitivity to treatments—and abundant neutral “passenger” mutations caused by UV exposure are still unclear. Epidemiological and experimental data on melanoma genesis have shown a causal role for UVA and UVB exposure (Wang et al., 2001).
Driver mutations in melanomas have been recently reviewed (Hawryluk and Tsao, 2014; Reddy et al., 2017), and mainly involve BRAF and RAS genes. BRAF mutations were characterized in human malignancies in 2002, and they occur in about 60% of melanomas. RAF gene from melanoma tumors shows recurrent mutation in exon 15 T1796A of the v-RAF murine sarcoma oncogene homolog B (BRAF), with valine (V) changing into glutamic acid (E) as a result of substitution at this exon (GTG > GAG) in the second site of codon 600 (V600E) of BRAF. This gene encodes for a serine/threonine protein kinase, resulting in a constitutive activation of the mitogen-activated protein kinase (MAPK) signaling (RAS/RAF/MEK/ERK) pathway, involved in proliferation, differentiation, and cell survival (Cancer Genome Atlas Network, 2015; Long et al., 2016).
The BRAF mutation is one of the hallmarks of malignant melanomas, and it results in a ten-fold increase in oncogenic signaling through MEK (Alqathama, 2020). Mutation V600E corresponds to 80% of BRAF-mutant melanomas. Other variant mutations in BRAF are V600K, V600R, V600D, and V600E2. Studies on BRAF mutations related to melanoma pathogenesis, progression and metastasis have been recently reviewed (Alqathama, 2020). It must be noted that about 80% of benign nevi harbor BRAF V600E mutations, and therefore this mutation alone is not sufficient to account for tumorigenesis (Reddy et al., 2017).
The RAS family are relevant regulators in normal cell growth and malignant transformation, involving the MAPK and PI3K pathways. Principal RAS proto-oncogenes are NRAS, HRAS, and KRAS. With 20%, NRAS mutations are the second most common driver mutations in melanoma, and are associated to aggressive tumors and poor patient survival. They occur in melanomas from non-sunlight exposed skin and are related to increased thickness in primary tumors, and high mitotic rates (Hawryluk and Tsao, 2014). As congenital melanocytic nevi also show NRAS mutations, they are insufficient to be the sole drivers for melanoma.
Studies on melanoma genetics have allowed to develop novel and specifically targeted therapeutics. Molecular inhibitors of mutated oncogenic signaling proteins, such as the MAPK pathway components BRAF and mitogen-activated extracellular signal-regulated kinase (MEK), are vemurafenib for BRAF, and imatinib, nilotinib, and dasatinib for tyrosine kinases, showing substantial activity against unresectable melanomas (Larkin et al., 2014; McArthur et al., 2014). Specific inhibitors of mutated BRAF are vemurafenib and dabrafenib, and when used alone or with higher efficacy in combination with downstream MEK inhibitors (e.g., vemurafenib + cobimetinib, dabrafenib + trametinib), they have demonstrated substantial success in patients with unresectable or metastatic BRAF V600-mutant melanoma, leading to their clinic approval (Alqathama, 2020; Hauschild et al., 2012; Robert et al., 2015).
At present, monoclonal antibody immunotherapy and genetically targeted therapy using immune checkpoint inhibitors have demonstrated improved patient response and prolonged survival for advanced melanomas (Jin et al., 2019). Thus, antibody-mediated blockade of immune checkpoints, particularly the anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), anti-programmed cell-death protein 1 (PD-1), and anti–PD ligand-1 (PD-L1), show good anti-tumor effects in metastatic melanoma, with markedly improved clinical response (Achkar and Tarhini, 2017; Schachter et al., 2017). However, numerous patients are still incapable of achieving a clear benefit from immunotherapy. In addition, immune check point inhibitors are expensive and can have considerable toxicity.
Novel multidisciplinary strategies such as adjuvant (McKean and Amaria, 2018), and neoadjuvant treatments for metastatic melanoma are now evaluated in the adjuvant treatment after surgery (Long et al., 2016; Ascierto et al., 2016), and in neoadjuvant protocols to reduce tumor loading previous to chemotherapy and surgery (McKean and Amaria, 2018). Bio-chemotherapeutic regimens combining several agents have also been evaluated for adjuvant protocols, using interleukins, cytokines, hormones, etc. Recently, a great impact in the management of melanoma has been immunotherapeutic approaches that overcome the tumor-mediated immune suppression. This progress is based upon the previous success of using interferon-α for melanoma treatment in the adjuvant setting, as well as interleukin-2 treatment in advanced melanoma (Achkar and Tarhini, 2017).
Immunotherapy by means of vaccination is a recent and promising tool for melanoma treatment. As spontaneous immune recognition of mutations is inefficient, a wider antigenic spectrum (poly-neo-epitopes) is necessary to mobilize immunity against the melanoma cells. Applications of this concept of personalized mutational vaccines have opened a path to personalized immunotherapy for each melanoma patient (Sahin et al., 2017). Effective anti-tumor immunity can be associated to the presence of T cells responsive against tumor neoantigens, a type of highly immunogenic leukocyte antigen-bound peptides arising from tumor-specific mutations.
At present, neoantigens are considered to be the important targets for immunogenic anti-tumor response, but systematic evaluations have only become possible with the recent sequencing of all coding tumor mutations (“mutanome,” the whole pattern of tumor mutations), which can lead to considerable success of immunotherapy. Vaccination with melanoma neoantigens allowed an enhanced tumor control and, when followed by anti-PD-1 (anti-programmed cell death-1) treatment, it resulted in proper tumor regression (Ott et al., 2017).
By combining sequencing analysis and bioinformatics, neoantigens derived from an individual patient’s tumor can be now identified and used to manufacture personalized vaccines. Examples of this new biotechnological immunotherapy have been described (Sahin et al., 2017; Ott et al., 2017). Results reporting the first clinical trials using personalized RNA-based vaccines generated from the mutanome analysis of each individual patient with malignant melanoma were highly positive (Chiocchetti et al., 2017). Synergic approaches using vaccination and checkpoint inhibitor-based immunotherapy are also possible. These approaches as well as other novel designs for melanoma therapy have been recently reviewed (Domingues et al., 2018).
However, there are still drawbacks regarding both conventional and advanced treatments for melanoma. Drawbacks are the toxicity of most of them, low efficiency to remove the primary tumor and to avoid relapses and metastasis, acquired resistance to therapies, and poor survival of patients. Although recent immunological, targeted, and biological therapy seem to show an increasing potential, further efforts are required to find more innovative and improved treatments for melanomas. Thus experimental melanoma models are increasingly used to design and apply new therapeutic strategies.
Malignant melanoma usually produces melanin in large quantities, but there are also amelanotic tumors. Experimental mouse and hamster tumors and cell cultures are used to study the biology of melanoma. Common melanotic cell lines (mouse: B16-F10, Sk-Mel-28, Mel-Ab, Clone M-3 [Cloudman S91]; human: MNT-1, VMM18, A7 [M2A7]; Syrian hamster: RPMI 1846, BHM Ma), and amelanotic cell lines (mouse: B78-H1; human: C32, Hs 695T; Syrian hamster: BHM Ab) are usually employed in oncological research (Sniegocka et al., 2018; Walker and Hayward, 2018).
Novel photochemical and photophysical treatments for melanomas are increasingly applied, and examples are photodynamic therapy (PDT), and photothermal therapy (PTT), respectively. In both cases, the presence of photosensitizers (PSs) is necessary. Photobiomodulation (PBM) generally uses blue LED light to inhibit the growth of melanoma cells in vitro and in vivo by inducing mitochondria-mediated apoptosis (Cook-Moreau et al., 2010; Ohara et al., 2002; Sparsa et al., 2010; Oh et al., 2015), but its mechanistic aspects have not yet been elucidated (Chen et al., 2020). For PBM based on PDT or PTT effects, the presence of endogenous PSs seems to be necessary.
Photodynamic therapy (PDT) is a relatively recent antitumoral treatment based on the selective accumulation of a photosensitizer within tumor cells. When irradiated by a suitable light source it generates ROS, which induce cell death (Stockert et al., 2004; Plaetzer et al., 2009). Although PDT has proven a successful therapeutic modality for numerous malignant tumors, poor results are observed for pigmented melanomas (Kukielczak et al., 1995). The intense light absorption of melanin and its anti-oxidative and radical-scavenger capacity greatly hinders PDT effects. However, indocyanine green (ICG) (Chong et al., 1993; Urbanska et al., 2002; Liggett et al., 2005), and recently a porphycene-sulfonamide (Pan et al., 2021), have been applied for melanoma PDT. It has been claimed by Urbanska et al. (2002) that the successful PDT on pigmented melanomas treated with ICG occurs because the dye has high absorption in the near infrared (NIR) spectral region, whereas melanin practically does not absorb in this region. However, in this case adequate only-NIR irradiation controls were not performed, and so a direct PTT effect of melanin cannot be excluded.
On the other hand, cultured amelanotic melanoma cells and tumors have been applied to investigate mechanistic aspects of PDT response, using Zn- and Al-phthalocyanines to induce cell death (Maduray et al., 2010). Studies on amelanotic melanomas have been carried out with cationic TMPyP porphyrins, showing photo-cleavage of G-quadruplexes that occur in untranslated regions of the mitogenic ras genes and mRNA, causing a decrease of RAS protein with inhibition of the hyperactive and proliferation-stimulating ERK pathway (Rapozzi et al., 2014). Recent studies on PDT applied to melanoma WM35 cells showed higher effects of phthalocyanine compared with porphyrin photosensitizers (Baldea et al., 2015). Likewise, cultured B16-F0 and A375 melanoma cells treated with the new phthalocyanine Pc13 and red light (675 nm) showed necrotic, apoptotic or autophagic cell death mechanisms (Valli et al., 2019; Valli et al., 2020). Unfortunately, in some studies in vitro, it is unclear if cultured melanoma cells are really pigmented or not, and this feature could lead to misleading PDT results.
Anyway, it must be taken into account that behavior and response of cultured cells in vitro can be very different from that of tumors in vivo, the former being highly simplified model systems for tumors in a whole organism, in which the reduced amount of oxygen and the scarce light penetration into tissues are strong limiting factors for an efficient antitumoral phototreatment. In the case of PTT, the presence of photogenerated reactive oxygen species (ROS) is not required for tumor cell damaging, as it occurs using PDT.
PTT is a photophysical therapy based on the photothermal effect, namely, light-to-heat conversion, and photothermal sensitizers have been suggested for possible use in antitumoral therapy (Jori and Spikes, 1990). Efficient photothermal effects require fast conversion of electronic excitation to vibrational excitation, which then decays with heat production (Parrish et al., 1983) inducing denaturation of macromolecules, vaporization, and shock-waves. Organic dyes, nanoparticles, and pigments have been incorporated as NIR-PTT agents. In the first case, cyanines and naphthalocyanines induce damage on amelanotic melanoma cells. ICG has been used for thermotherapy of choroidal melanoma (De Potter and Jamart, 2003). Nanoparticles and delivery strategies are now increasingly applied for PTT (Li et al., 2018).
Regarding pigments, photothermal melanin-based hair removal is widely applied in cosmetics (Haedersdal et al., 2011). In the case of tumors, lethal photothermal effects were induced in murine tumors by treatment with synthetic dopamine-melanin followed by NIR irradiation (Liu et al., 2013; Zheng et al., 2015). Delivery of China ink (carbon black, Color Index: 77266), and melanin from black sesame seeds and cuttlefish were recently used for NIR-PTT of cell cultures and tumors (Blázquez-Castro et al., 2018; Huang et al., 2018). Pulsed PTT of pigmented B16 tumors with broad-band incoherent light (600-800 nm) caused vasculature and melanosome damage, with necrosis of tumor cells (Kostenicha et al., 2000). Human malignant melanomas usually produce melanin in large quantities, and thus melanoma melanin is a specific target chromophore for PTT. The melanin content of the human melanoma cell line IIB-MEL-J (from a metastatic lung nodule) is 4.2 ± 0.3 μg/106 cells and 11.3 ± 0.6 μg/106 cells in exponential and stationary phases, respectively (Bustamante et al., 1991), representing a natural agent for PTT.
As eumelanin can dissipate >99.9% of absorbed UV and visible radiation energy by non-radiative (thermal) decay (Meredith and Riesz, 2004) this endogenous chromophore is just very suitable for direct PTT of melanotic melanoma (Colombo et al., 2019). During continuous PTT irradiation steady heat transfer occurs, favoring volume hyperthermia and denaturation, and the whole illuminated tissue region shows coagulation necrosis. To illustrate this effect, BALB/c mice bearing the experimental melanotic melanoma B16-F10 were irradiated with a continuous wave (cw) 808-nm laser (Colombo et al., 2019). As shown in Fig. 5, severe tissue damage was produced (massive coagulation necrosis, pycnotic nuclei, disrupted tumor cells releasing melanin and cytoplasm fragments). No signs of cell damage were found in the white skin over the irradiated tumor, even when the exciting light had first to traverse this tissue to reach the tumor.
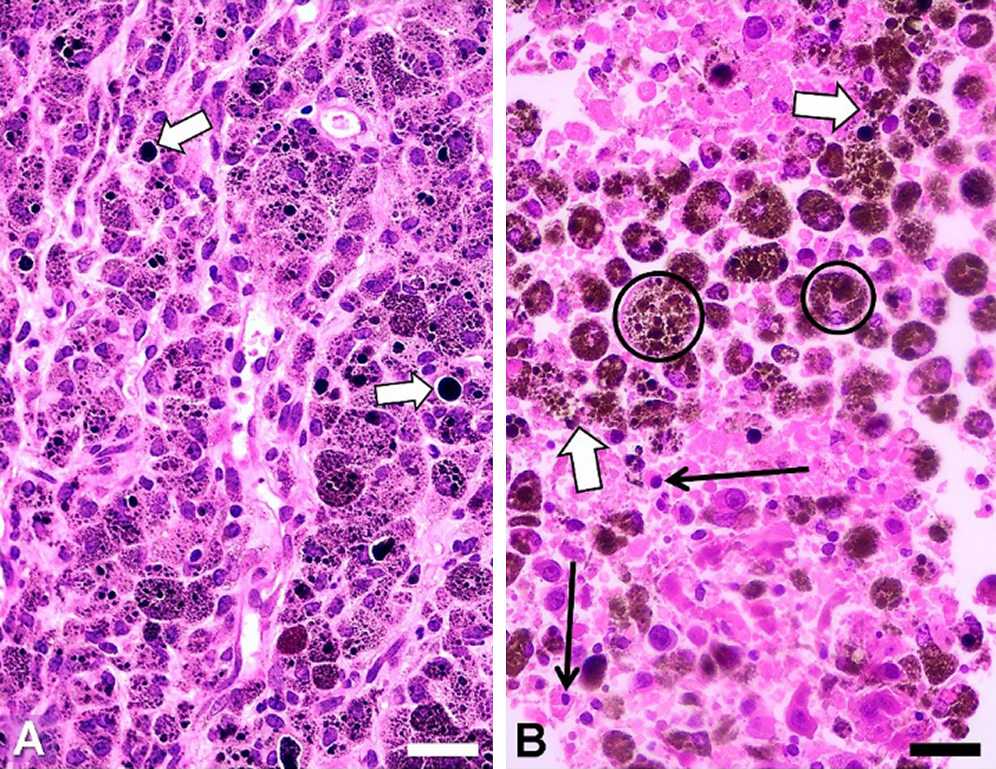
Figure 5: H&E images of paraffin sections from B16-F10 tumors. (A) Non-irradiated tumor, with intracellular melanosomes, and large black extracellular melanin granules (white arrows). (B) Tumor irradiated for 10 min, showing extensive damage: disrupted tumor cells (white arrows), pycnotic nuclei (black arrows), and big brown-black melanophages (encircled). Scale bars: 30 μm. NIR source: portable cw 808 nm laser pointer (GLP-808, Changchun, China; ~200 mW, and ~1.2 mm beam diameter). To reduce light scattering of tissues a drop of glycerol was placed on the depilated skin over the tumor. (Reproduced from Colombo et al. (2019), Biomedical Optic Express 10: 2932–2941).
The presence of a great number of brown-black melanophages (Fig. 5B, encircled) is a relevant feature, because these melanin-targeted cells could be subjected to additional cycles of NIR irradiation. Glycerol can be successfully applied over the tumor to reduce light dispersion caused by keratin (Stockert et al., 2009; Blázquez-Castro et al., 2018). Likewise, as glycerol is a strong protecting agent against cell hyperthermia (Henle and Warters, 1982), application of a glycerol drop on the depilated skin also avoids the non-desired but possible heating and damage of normal tissues.
Therapeutic use of melanin-PTT has several advantages: (a) due to its physicochemical characteristics, melanin appears as the “ideal” photothermal agent, (b) melanin-PTT can be applied in healthcare systems with no access to expensive drugs, (c) damage by NIR irradiation in non-pigmented cells is low or absent, (d) melanin-PTT is independent on the mutation status, only depending on the pigmentation degree, (e) PTT damage of melanoma cells induces the exposition of new antigens and necrotic materials, which could stimulate the immune system (neoantigens), (f) metastatic melanoma cells in-transit toward regional ganglia (Testori et al., 2017) could be destroyed using NIR-PTT of the tumor borders and surgical area during or after surgery, and (g) NIR-excited photo-acoustic waves from melanin can be used for early detection of circulating metastatic cells (O’Brien et al., (2012); Viator et al., 2020). As most melanoma tumor cells are highly pigmented (Wain et al., 2008; Swetter, 2008) (with estimates of amelanotic melanomas being less than 5%), the presence of melanin can allow not only photothermal effects, but also photoacoustic ones. Photoacoustic flow cytometry just allows the detection of circulating melanoma cells, which is based on the production of an ultrasonic signal only when melanin-containing cells are illuminated with a pulsed 532-nm laser beam.
Regarding PTT mechanism, explosive photothermal vaporization (threshold temperature: ~112°C) was early observed in melanosomes in situ from pigmented skin during pulsed ruby laser irradiation (Jacques and McAuliffe, 1991). Likewise, photothermal generation of shock waves and cavitation have been shown to occur by focusing cw laser radiation on aqueous media containing carbon-based pigment particles (Besaga et al., 2014; Padilla-Martinez et al., 2014) or other NIR-absorbing micron-sized particles (Carmona-Sosa et al., 2016). Photothermal decay also generates air micro-bubbles in water suspensions of colloidal carbon particles using cw 808-nm laser radiation (Angelsky et al., 2018).
It is known that absorption and scattering reduce severely light penetration within tissues. On account of deeper tissue penetrance, irradiation with red and NIR light are commonly used for PDT and PTT, respectively. Although in the visible region red light is the most penetrant, NIR irradiation at about 810–820 nm (the biological, diagnostic, and therapeutic window) is the best to reach deep tissues. In PTT of melanomas, the weak absorbance of melanin at 808 nm is compensated by a deep 808-nm penetration. Therefore, irradiation of melanin and other pigments with the 808 nm laser is very adequate because the H2O absorption is negligible, and there are no other tissue chromophores except melanin (Parrish et al., 1983; Plaetzer et al., 2009).
Other melanin-containing experimental models for studying properties and applications of melanin that could be relevant for NIR-induced photothermal effects are available, examples being melanotic insects and amphibian eggs. Macrophages also accumulate selectively within tumor tissues, and this feature has been used to delivery gold nanoparticles phagocytized by macrophages followed by PTT (Yang et al., 2015). Likewise, after phagocytosis of black polymers (e.g., melanins, carbon black), macrophages could be used first to accumulate selectively in non-pigmented tumors, and then followed by NIR-PTT. It is interesting to mention the application of aqueous carbon black suspensions in the preoperative labeling of the human breast carcinoma (Delporte et al., 1994), which could be followed by NIR irradiation to achieve antitumor PTT effects.
In conclusion, it has been shown that melanins represent a highly conserved, and yet highly diverse, family of biological pigments with a complex structure and fascinating properties. They have a definitive role in biological defense against a plethora of agents, acting as photoprotectors, antioxidants, and chelating agents, and they have evolved to fill an enormous niche in biological signaling too, acting as pigments to produce an incredible broad palette of colors with very relevant roles in mating, defense, camouflage and communication.
Melanocytes are fascinating cells involved in defense against external aggressive factors (UV light), but they also help the organism to dispose of internal damaging agents, like heavy metals. Unfortunately, the melanocyte, like any other cell in multicellular organisms, is subject to tumoral initiation and progression. And melanocytes turn out to be one of the toughest cancer cells to be damaged. We have introduced their main biomedical features, relevant mutations, and therapies, both current and prospective, to enhance the success rate in treating melanomas. In our view, in addition to targeted immunotherapy and personalized vaccines, one of the most promising and simple treatments for this disease is melanin-based photothermal therapy. The basics of this technique have been introduced along particular examples of its adequacy. Still, there is a long road ahead both to better understand melanin, melanocytes and their multiple roles in biology, as well as their involvement in the origin and treatment of melanomas.
Acknowledgement: We thank M. M. Blanco, A. G. Casas, L. L. Colombo, O. Gabay, J. Herkovits, D. M. Lombardo, and M. F. Pozzi for valuable collaboration.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: Juan C. Stockert; draft manuscript preparation: Alfonso Blázquez-Castro, Juan C. Stockert; edition and formatting: Alfonso Blázquez-Castro, Juan C. Stockert. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Achkar T, Tarhini AA (2017). The use of immunotherapy in the treatment of melanoma. Journal of Hematology & Oncology 10: 5. DOI 10.1186/s13045-017-0458-3. [Google Scholar] [CrossRef]
Agar N, Young AR (2005). Melanogenesis: a photoprotective response to DNA damage? Mutation Research 571: 121–132. DOI 10.1016/j.mrfmmm.2004.11.016. [Google Scholar] [CrossRef]
Alexander RA, Cree IA, Foss AJ (1996). The immunoalkaline phosphatase technique in immunehistochemistry: the effect of permanganate-oxalate melanin bleaching upon four final reaction products. British Journal of Biomedical Science 53: 170–171. [Google Scholar]
Alexandrescu DT, Maslin B, Kauffman CL, Ichim TE, Dasanu CA (2013). Malignant melanoma in pigmented skin: does the current interventional model fit a different clinical, histologic, and molecular entity? Dermatologic Surgery 39: 1291–1303. DOI 10.1111/dsu.12251. [Google Scholar] [CrossRef]
Alqathama A (2020). BRAF in malignant melanoma progression and metastasis: potentials and challenges. American Journal of Cancer Research 10: 1103–1114. [Google Scholar]
Angeletti C, Khomitch V, Halaban R, Rimm DL (2004). Novel tyramide-based tyrosinase assay for the detection of melanoma cells in cytological preparations. Diagnostic Cytopathology 31: 33–37. DOI 10.1002/dc.20051. [Google Scholar] [CrossRef]
Angelsky OV, Bekshaev AY, Maksimyak PP, Maksimyak AP, Hanson SG (2018). Low-temperature laser-stimulated controllable generation of micro-bubbles in a water suspension of absorptive colloid particles. Optics Express 26: 13995. DOI 10.1364/OE.26.013995. [Google Scholar] [CrossRef]
Ascierto PA, Mc Arthur GA, Dreno B, Atkinson V, Liszkay G, Di Giacomo AM, Mandalà M, Demidov L, Stroyakovskiy D, Thomas L, de la Cruz-Merino L, Dutriaux C, Garbe C, Yan Y, Wongchenko M, Chang I, Hsu JJ, Koralek DO, Rooney I, Ribas A, Larkin J (2016). Cobimetinib combined with vemurafenib in advanced BRAF V600-mutant melanoma (coBRIMupdated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Oncology 17: 1248–1260. DOI 10.1016/S1470-2045(16)30122-X. [Google Scholar] [CrossRef]
Baldea I, Ion RM, Olteanu DE, Nenu I, Tudor D, Filip AG (2015). Photodynamic therapy of melanoma using new, synthetic porphyrins and phthalocyanines as photosensitisers—a comparative study. Clujul Medical 88: 175–180. [Google Scholar]
Bancroft JD, Gamble M (2008). Theory and Practice of Histological Techniques. 6th edition, London: Churchill Livingstone. [Google Scholar]
Bejaoui M, Villareal MO, Isoda H (2020). 3,4,5-Tri-O-caffeoylquinic acid promoted hair pigmentation through β-catenin and its target genes. Frontiers in Cell and Developmental Biology 8: 4216. DOI 10.3389/fcell.2020.00175. [Google Scholar] [CrossRef]
Besaga VR, Maksimyak AP, Maksimyak PP (2014). Laser generation of shock waves in a water suspension with light-absorbing particles. Applied Optics 53: B153–B158. DOI 10.1364/AO.53.00B153. [Google Scholar] [CrossRef]
Blázquez-Castro A, Colombo LL, Vanzulli SI, Stockert JC (2018). NIR pointer laser for in vivo photothermal therapy of murine LM3 tumor using intratumoral China ink as a photothermal agent. Lasers in Medical Sciences 33: 1307–1315. DOI 10.1007/s10103-018-2483-z. [Google Scholar] [CrossRef]
Blois MS, Zahlan AB, Maling JE (1964). Electron spin resonance studies on melanin. Biophysical Journal 4: 471–490. DOI 10.1016/S0006-3495(64)86797-7. [Google Scholar] [CrossRef]
Brożyna AA, Jóźwicki W, Roszkowski K, Filipiak J, Slominski AT (2016). Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget 7: 17844–17853. DOI 10.18632/oncotarget.7528. [Google Scholar] [CrossRef]
Büngeler A, Hämisch B, Strube OI (2017). The supramolecular buildup of eumelanin: structures, mechanisms. controllability International Journal of Molecular Sciences 18: 1901. [Google Scholar]
Bustamante J, Guerra L, Bredeston L, Mordoh J, Boveris A (1991). Melanin content and hydroperoxide metabolism in human melanoma cells. Experimental Cell Research 196: 172–176. DOI 10.1016/0014-4827(91)90247-R. [Google Scholar] [CrossRef]
Calapre L, Warburton L, Millward M, Ziman M, Gray ES (2017). Circulating tumour DNA (ctDNA) as a liquid biopsy for melanoma. Cancer Letters 404: 62e69–69. DOI 10.1016/j.canlet.2017.06.030. [Google Scholar] [CrossRef]
Cancer Genome Atlas Network (2015). Genome classification of cutaneous melanoma. Cell 161: 1681–1696. DOI 10.1016/j.cell.2015.05.044. [Google Scholar] [CrossRef]
Carmona-Sosa V, Alba-Arroyo JE, Quinto-Su PA (2016). Characterization of periodic cavitation in optical tweezers. Applied Optics 55: 1894–1898. DOI 10.1364/AO.55.001894. [Google Scholar] [CrossRef]
Carriel VS, Aneiros-Fernandez J, Arias-Santiago S, Garzón IJ, Alaminos M, Campos A (2011). A novel histochemical method for a simultaneous staining of melanin and collagen fibers. Journal of Histochemistry & Cytochemistry 59: 270–277. DOI 10.1369/0022155410398001. [Google Scholar] [CrossRef]
Cavallini C, Vitiello G, Adinolfi B, Silvestri B, Armanetti P, Manini P, Pezzella A, Marco d’Ischia M, Luciani G, Menichetti L (2020). Melanin and melanin-like hybrid materials in regenerative medicine. Nanomaterials 10: 1518. DOI 10.3390/nano10081518. [Google Scholar] [CrossRef]
Chen CT, Chuang C, Cao J, Ball V, Ruch D, Buehler MJ (2014). Excitonic effects from geometric order and disorder explain broadband optical absorption in eumelanin. Nature Communications 5: 661. DOI 10.1038/ncomms5859. [Google Scholar] [CrossRef]
Chen Z, Li W, Hu X, Liu M (2020). Irradiance plays a significant role in photobiomodulation of B16F10 melanoma cells by increasing reactive oxygen species and inhibiting mitochondrial function. Biomedical Optics Express 11: 27–39. DOI 10.1364/BOE.11.000027. [Google Scholar] [CrossRef]
Cheng JIN, Moss SC, Eisner M, Zschack P (1994). X-ray characterization of melanins—1. Pigment Cell Research 7: 255–262. DOI 10.1111/j.1600-0749.1994.tb00060.x. [Google Scholar] [CrossRef]
Chiocchetti A, Cappellano G, Dianzani U (2017). To each his own: a personalized vaccine for metastatic melanoma. Nature 547: 222–226. DOI 10.1038/nature23003. [Google Scholar] [CrossRef]
Chong LP, Ozler SA, de Queiroz JM, Jr., Liggett PE (1993). Indocyanine green-enhanced diode laser treatment of melanoma in a rabbit model. Retina 3: 251–259. DOI 10.1097/00006982-199313030-00012. [Google Scholar] [CrossRef]
Colombo LL, Vanzulli SI, Blázquez-Castro A, Sanchez Terrero C, Stockert JC (2019). Photothermal effect by 808-nm laser irradiation of melanin: a proof-of-concept study of photothermal therapy using B16-F10 melanotic melanoma growing in BALB/c mice. Biomedical Optics Express 10: 2932–2941. DOI 10.1364/BOE.10.002932. [Google Scholar] [CrossRef]
Cook-Moreau JC, Krausz P, Sturtz FG, Bedane C, Jauberteau-Marchan MO, Ratinaud MH, Bonnetblanc JM (2010). Blue light is phototoxic for B16F10 murine melanoma and bovine endothelial cell lines by direct cytocidal effect. Anticancer Research 30: 143–147. [Google Scholar]
Crawford M, Leclerc V, Dagnino L (2017). A reporter mouse model for in vivo tracing and in vitro molecular studies of melanocytic lineage cells and their diseases. Biology Open 6: 1219–1228. DOI 10.1242/bio.025833. [Google Scholar] [CrossRef]
D’Alba L, Shawkey MD (2019). Melanosomes: biogenesis, properties, and evolution of an ancient organelle. Physiological Reviews 99: 1–19. DOI 10.1152/physrev.00059.2017. [Google Scholar] [CrossRef]
d’Ischia M (2018). Melanin-based functional materials. International Journal of Molecular Sciences 19: 228. DOI 10.3390/ijms19010228. [Google Scholar] [CrossRef]
d’Ischia M, Wakamatsu K, Cicoira F, Di Mauro E, Garcia-Borron JC, Commo S (2015). Melanins and melanogenesis: from pigment cells to human health and technological applications. Pigment Cell & Melanoma Research 28: 520–544. DOI 10.1111/pcmr.12393. [Google Scholar] [CrossRef]
d’Ischia M, Wakamatsu K, NapolitanoA BS, Garcia-Borron JC, Kovacs D, Meredith P, Pezzella A, Picardo M, Tadeusz Sarna T, John D. Simon JD, Ito S (2013). Melanins and melanogenesis: methods, standards. protocols Pigment Cell Melanoma Research 26: 616–633. DOI 10.1111/pcmr.12121. [Google Scholar] [CrossRef]
d’Ischia M, Napolitano A, Pezzella A, Meredith P, Buehler M (2019). Melanin biopolymers: Tailoring chemical complexity for materials design. Angewandte Chemie International Edition. DOI 10.1002/anie.201914276. [Google Scholar] [CrossRef]
D’Mello S, Finlay G, Baguley B, Askarian-Amiri M (2016). Signaling pathways in melanogenesis. International Journal of Molecular Sciences 17: 1144. DOI 10.3390/ijms17071144. [Google Scholar] [CrossRef]
Davis EJ, Johnson DB, Sosman JA, Sunandana Chandra S (2018). Melanoma: what do all the mutations mean? Cancer 124: 3490–3499. DOI 10.1002/cncr.31345. [Google Scholar] [CrossRef]
de Gálvez MV, Aguilera J, Bernabó JL, Sánchez-Roldán C, Herrera-Ceballos E (2015). Human hair as a natural sun protection agent: a quantitative study. Photochemistry and Photobiology 91: 966–970. DOI 10.1111/php.12433. [Google Scholar] [CrossRef]
de Potter P, Jamart J (2003). Adjuvant indocyanine green in transpupillary thermotherapy for choroidal melanoma. Ophthalmology 110: 406–413. DOI 10.1016/S0161-6420(02)01560-9. [Google Scholar] [CrossRef]
Delporte P, Laurent JC, Cambier L (1994). Preoperative marking of non-palpable breast lesions by the stereotaxic tattooing and “harpoon” technique. 670 cases. Journal of Ginecology, Obstetrics and Biology of Reproduction 23: 259–263. [Google Scholar]
Domingues B, Lopes JM, Soares P, Pópulo H (2018). Melanoma treatment in review. ImmunoTargets and Therapy 7: 35–49. DOI 10.2147/ITT.S134842. [Google Scholar] [CrossRef]
Dreyer DR, Miller DJ, Freeman BD, Paul DR, Bielawski CW (2012). Elucidating the structure of poly(dopamine). Langmuir 28: 6428–6435. DOI 10.1021/la204831b. [Google Scholar] [CrossRef]
Eller MS, Ostrom K, Gilchrest BA (1996). DNA damage enhances melanogenesis. Proceedings of the National Academy of Sciences USA 93: 1087–1092. DOI 10.1073/pnas.93.3.1087. [Google Scholar] [CrossRef]
Elwood JM, Jopson J (1997). Melanoma and sun exposure: an overview of published studies. International Journal of Cancer 73: 198–203. DOI 10.1002/(SICI)1097-0215(19971009)73:2<198::AID-IJC6>3.0.CO;2-R. [Google Scholar] [CrossRef]
Guy GPJr., Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC (2015). Centers for disease control and prevention (CDC). Vital signs: melanoma incidence and mortality trends and projections—United States, 1982–2030. Morbility and Mortality Weekly Reports 64: 591–596. [Google Scholar]
Haedersdal M, Beerwerth F, Nash JF (2011). Laser and intense pulsed light hair removal technologies: from professional to home use. British Journal of Dermatology 165: 31–36. DOI 10.1111/j.1365-2133.2011.10736.x. [Google Scholar] [CrossRef]
Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Kaempgen E, Martín-Algarra S, Karaszewska B, Mauch C, Chiarion-Sileni V, Martin AM, Swann S, Haney P, Mirakhur B, Guckert ME, Goodman V, Chapman PB (2012). Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380: 358–365. DOI 10.1016/S0140-6736(12)60868-X. [Google Scholar] [CrossRef]
Hawryluk EB, Tsao H (2014). Melanoma: clinical features and genomic insights. Cold Spring Harbor Perspectives in Medicine 4: a015388. DOI 10.1101/cshperspect.a015388. [Google Scholar] [CrossRef]
Henle KJ, Warters RL (1982). Heat protection by glycerol in vitro. Cancer Research 42: 2171–2176. [Google Scholar]
Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, Dicara D, Ramos AH, Lawrence MS, Cibulskis K, Sivachenko A, Voet D, Saksena G, Stransky N, Onofrio RC, Winckler W, Ardlie K, Wagle N, Wargo J, Chong K, Morton DL, Stemke-Hale K, Chen G, Noble M, Meyerson M, Ladbury JE, Davies MA, Gershenwald JE, Wagner SN, Hoon DSB, Schadendorf D, Lander ES, Gabriel SB, Getz G, Garraway LA, Chin L (2012). A landscape of driver mutations in melanoma. Cell 150: 251–263. DOI 10.1016/j.cell.2012.06.024. [Google Scholar] [CrossRef]
Hofmann U, Westphal J, Van Muijen G, Ruiter D (2000). Matrix metalloproteinases in human melanoma. Journal of Investigative Dermatology 115: 337–344. DOI 10.1046/j.1523-1747.2000.00068.x. [Google Scholar] [CrossRef]
Holmes D (2014). The cancer that rises with the sun. Nature 515: S110–S111. DOI 10.1038/515S110a. [Google Scholar] [CrossRef]
Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF (2016). SEER Cancer Statistics Review, pp. 1975–2013. Bethesda, MD: National Cancer Institute. https://seer.cancer.gov/archive/csr/1975_2013/. [Google Scholar]
Huang L, Liu M, Huang H, Wen Y, Zhang X, Wei Y (2018). Recent advances and progress on melanin-like materials and their biomedical applications. Biomacromolecules 19: 1858–1868. DOI 10.1021/acs.biomac.8b00437. [Google Scholar] [CrossRef]
Ichikawa A, Takagi H, Suda K, Yao T (2009). New methodological approach for the rapid and sensitive detection of melanocytes and melanocytic tumours. Dermatology 219: 195–201. DOI 10.1159/000235571. [Google Scholar] [CrossRef]
Jacques SL, McAuliffe DJ (1991). The melanosome: threshold temperature for explosive vaporization and internal absorption coefficient during pulsed laser irradiation. Photochemistry and Photobiology 53: 769–775. DOI 10.1111/j.1751-1097.1991.tb09891.x. [Google Scholar] [CrossRef]
Jimbow K, Salopek TG, Dixon WT, Searles GE, Yamada K (1991). The epidermal melanin unit in the pathophysiology of malignant melanoma. American Journal of Dermatopathology 13: 179–188. DOI 10.1097/00000372-199104000-00013. [Google Scholar] [CrossRef]
Jin S, Mishra-Kalyani PS, Sridhara R (2019). Unresectable and metastatic melanoma of the skin: Literature review of clinical trials and efficacy endpoints since 2000. Therapeutic Innovation & Regulation Science 54: 59–70. DOI 10.1177/2168479018769286. [Google Scholar] [CrossRef]
Jori G, Spikes JD (1990). Photothermal sensitizers: possible use in tumor therapy. Journal of Photochemistry and Photobiology B: Biology 6: 93–101. DOI 10.1016/1011-1344(90)85078-B. [Google Scholar] [CrossRef]
Karimkhani C, Green AC, Nijsten T, Weinstock MA, Dellavalle RP, Naghavi M, Fitzmaurice C (2017). The global burden of melanoma: results from the Global Burden of Disease Study 2015. British Journal of Dermatology 177: 134–140. DOI 10.1111/bjd.15510. [Google Scholar] [CrossRef]
Kim T, Park J, Park S, Choi Y (2011). Visualization of tyrosinase activity in melanoma cells by a BODIPY-based fluorescent probe. Chemical Communications 47: 12640–12642. DOI 10.1039/c1cc15061h. [Google Scholar] [CrossRef]
Kobayashi N, Nakagawa A, Muramatsu T, Yamashina Y, Shirai T, Hashimoto MW, Ishigaki Y, Ohnishi T, Mori T (1998). Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. Journal of Investigative Dermatology 110: 806–810. DOI 10.1046/j.1523-1747.1998.00178.x. [Google Scholar] [CrossRef]
Kostenicha G, Babushkinab T, Malikb Z, Orenstein A (2000). Photothermic treatment of pigmented B16 melanoma using a broadband pulsed light delivery system. Cancer Letters 157: 161–168. DOI 10.1016/S0304-3835(00)00508-5. [Google Scholar] [CrossRef]
Kukielczak B, Cieszka KA, Matuszak ZT, Subczynski WK (1995). Experimental photodynamic therapy of Bomirski hamster melanoma using merocyanine 540 and visible light. Current Topics in Biophysics 19: 66–70. [Google Scholar]
Land EJ, Ramsden CA, Riley PA (2004). Quinone chemistry and melanogenesis. Methods in Enzymology 378: 88–109. DOI 10.1016/S0076-6879(04)78005-2. [Google Scholar] [CrossRef]
Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, Mandalà M, Demidov L, Stroyakovskiy D, Thomas L, de la Cruz-Merino L, Dutriaux C, Garbe C, Mika A, Sovak MA, Chang I, Choong N, Hack SP, McArthur GA, Ribas A (2014). Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. New England Journal of Medicine 371: 1867–1876. DOI 10.1056/NEJMoa1408868. [Google Scholar] [CrossRef]
Lens MB, Dawes M (2004). Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. British Journal of Dermatology 150: 179–185. DOI 10.1111/j.1365-2133.2004.05708.x. [Google Scholar] [CrossRef]
Li H, Kim J, Hahn HG, Yun J, Jeong HS, Yun HY, Baek KJ, Kwon NS, Min YS, Park KC, Kim DS (2014). KHG26792 inhibits melanin synthesis in Mel-Ab cells and a skin equivalent model. Korean Journal of Physiology and Pharmacology 18: 249–254. DOI 10.4196/kjpp.2014.18.3.249. [Google Scholar] [CrossRef]
Li Z, Yu XF, Chu PK (2018). Recent advances in cell-mediated nanomaterial delivery systems for photothermal therapy. Journal of Materials Chemistry B 6: 1296–1311. DOI 10.1039/C7TB03166A. [Google Scholar] [CrossRef]
Liebscher J, Mrówczyński R, Scheidt HA, Filip C, Hădade ND, Turcu R, Bende A, Beck S (2013). Structure of polydopamine: a never-ending story? Langmuir 29: 10539–10548. DOI 10.1021/la4020288. [Google Scholar] [CrossRef]
Liggett PE, Lavaque AJ, Chaudhry NA, Jablon EP, Quiroz-Mercado H (2005). Preliminary results of combined simultaneous transpupillary thermotherapy and ICG-based photodynamic therapy for choroidal melanoma. Ophthalmic Surgery, Lasers and Imaging Retina 36: 463–470. DOI 10.3928/1542-8877-20051101-06. [Google Scholar] [CrossRef]
Liu Y, Ai K, Liu J, Deng M, He Y, Lu L (2013). Dopamine-melanin colloidal nanospheres: an efficient near infrared photothermal therapeutic agent for in vivo cancer therapy. Advanced Materials 25: 1353–1359. DOI 10.1002/adma.201204683. [Google Scholar] [CrossRef]
Long GV, Weber JS, Infante JR, Kim KB, Daud A, Gonzalez R, Sosman JA, Hamid O, Schuchter L, Cebon J, Kefford RF, Lawrence D, Kudchadkar R, Burris HA, Falchook GS, Algazi A, Lewis K, Puzanov I, Ibrahim N, Sun P, Cunningham E, Kline AS, Del Buono H, McDowell DO, Patel K, Flaherty KT (2016). Overall survival and durable responses in patients with BRAV V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. Journal of Clinical Oncology 34: 871–878. DOI 10.1200/JCO.2015.62.9345. [Google Scholar] [CrossRef]
Loria D, Matos E (2001). Risk factors for cutaneous melanoma: a case-control study in Argentina. International Journal of Dermatology 40: 108–114. DOI 10.1046/j.1365-4362.2001.01132.x. [Google Scholar] [CrossRef]
Maduray K, Karsten A, Odhav B, Nyokong T (2010). The photodynamic therapy effect of aluminum and zinc tetrasulfophthalocyanines on melanoma cancer cells. Proceedings of SPIE-The International Society for Optical Engineering 7376: 73760A. [Google Scholar]
McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, Ribas A, Hogg D, Hamid O, Ascierto PA, Garbe C, Testori A, Maio M, Lorigan P, Lebbé C, Jouary T, Schadendorf D, O’Day SJ, Kirkwood JM, Eggermont AM, Dréno B, Sosman JA, Flaherty KT, Yin M, Caro I, Cheng S, Trunzer K, Hauschild A (2014). Safety and efficacy of vemurafenib in BRAF V600E and BRAF V600K mutation-positive melanoma (BRIM-3extended follow-up of a phase3, randomised, open-label study. Lancet Oncology 15: 323–332. DOI 10.1016/S1470-2045(14)70012-9. [Google Scholar] [CrossRef]
McGuinness JE, Corry PM, Proctor P (1974). Amorphous semiconductor switching in melanins. Science 183: 853–855. DOI 10.1126/science.183.4127.853. [Google Scholar] [CrossRef]
McKean MA, Amaria RN (2018). Multidisciplinary treatment strategies in high-risk resectable melanoma: role of adjuvant and neoadjuvant therapy. Cancer Treatment Review 70: 144–153. DOI 10.1016/j.ctrv.2018.08.011. [Google Scholar] [CrossRef]
Meng S, Kaxiras E (2008). Theoretical models of eumelanin protomolecules and their optical properties. Biophysical Journal 94: 2095–2105. DOI 10.1529/biophysj.107.121087. [Google Scholar] [CrossRef]
Meredith P, Riesz J (2004). Radiative relaxation quantum yields for synthetic eumelanin. Photochemistry and Photobiology 79: 211–216. DOI 10.1562/0031-8655(2004)079<0211:RCRQYF>2.0.CO;2. [Google Scholar] [CrossRef]
Micillo R, Panzella L, Koike K, Monfrecola G, Napolitano A, d’Ischia M (2016). Fifty shades of black and red or how carboxyl groups fine tune eumelanin and pheomelanin properties. International Journal of Molecular Sciences 17: 746. DOI 10.3390/ijms17050746. [Google Scholar] [CrossRef]
Na NTL, Loc SD, Tri NLM, Loan NTB, Son HA, Toan NL (2019). Nanomelanin potentially protects the spleen from radiotherapy-associated damage and enhances immunoactivity in tumor-bearing mice. Materials 12: 1725. DOI 10.3390/ma12172764. [Google Scholar] [CrossRef]
Nicolaus BJR (2005). A critical review of the function of neuromelanin and an attempt to provide a unified theory. Medical Hypotheses 65: 791–796. DOI 10.1016/j.mehy.2005.04.011. [Google Scholar] [CrossRef]
Nicolaus RA (1997). Coloured organic semiconductors: melanin. Rendiconto dell’ Accademia delle Scienze Fisiche e Matematiche, Napoli 64: 325–360. [Google Scholar]
Nicolaus RA, Piatelli M, Fattorusso E (1994). The structure of melanins and melanogenesis. IV. On some natural melanins. Tetrahedron 20: 1163–1172. [Google Scholar]
O’Brien CM, Rood KD, Bhattacharyya K, DeSouza T, Sengupta S, Gupta SK, Mosley JD, Goldschmidt BS, Sharma N, Viator JA (2012). Capture of circulating tumor cells using photoacoustic flowmetry and two phase flow. Journal of Biomedical Optics 17: 061221. DOI 10.1117/1.JBO.17.6.061221. [Google Scholar] [CrossRef]
Oh PS, Na KS, Hwang H, Jeong HS, Lim S, Sohn MH, Jeong HJ (2015). Effect of blue light emitting diodes on melanoma cells: involvement of apoptotic signaling. Journal of Photochemistry and Photobiology B: Biology 142: 197–203. DOI 10.1016/j.jphotobiol.2014.12.006. [Google Scholar] [CrossRef]
Ohara M, Kawashima Y, Katoh O, Watanabe H (2002). Blue light inhibits the growth of B16 melanoma cells. Japanese Journal of Cancer Research 93: 551–558. DOI 10.1111/j.1349-7006.2002.tb01290.x. [Google Scholar] [CrossRef]
Olivieri M, Nicolaus RA (1999). Sulla DHI-melanina. Rendiconto dell’Accademia delle Scienze Fisiche e Matematiche. Napoli 66: 85–96. [Google Scholar]
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ (2017). An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547: 217–221. DOI 10.1038/nature22991. [Google Scholar] [CrossRef]
Padilla-Martinez JP, Berrospe-Rodriguez C, Aguilar G, Ramirez-San-Juan JC, Ramos-Garcia R (2014). Optic cavitation with CW lasers: a review. Physics of Fluids 26: 122007. DOI 10.1063/1.4904718. [Google Scholar] [CrossRef]
Pan Z, Fan J, Xie Q, Zhang X, Zhang W, Ren Q, Li M, Zheng Q, Lu J, Li D (2021). Novel sulfonamide porphyrin TBPoS-2OH used in photodynamic therapy for malignant melanoma. Biomedicine & Pharmacotherapy 133: 111042. DOI 10.1016/j.biopha.2020.111042. [Google Scholar] [CrossRef]
Panzella L, Ebato A, Napolitano A, Koike K (2018). The late stages of melanogenesis: exploring the chemical facets and the application opportunities. International Journal of Molecular Sciences 19: 1753. DOI 10.3390/ijms19061753. [Google Scholar] [CrossRef]
Parrish JA, Anderson RR, Harrist T, Paul B, Murphy GF (1983). Selective thermal effects with pulsed irradiation from lasers: from organ to organelle. Journal of Investigative Dermatology 80: (6 supplement) 75s–80s. [Google Scholar]
Plaetzer K, Krammer B, Berlanda J, Berr F, Kiesslich T (2009). Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Lasers in Medical Sciences 24: 259–268. DOI 10.1007/s10103-008-0539-1. [Google Scholar] [CrossRef]
Rapozzi V, Zorzet S, Zacchigna M, Della Pietra E, Cogoi S, Xodo LE (2014). Anticancer activity of cationic porphyrins in melanoma tumour-bearing mice and mechanistic in vitro studies. Molecular Cancer 13: 75. DOI 10.1186/1476-4598-13-75. [Google Scholar] [CrossRef]
Reddy BY, Miller DM, Tsao H (2017). Somatic driver mutations in melanoma. Cancer 123: 2104–2117. DOI 10.1002/cncr.30593. [Google Scholar] [CrossRef]
Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, Chiarion-Sileni V, Drucis K, Krajsova I, Hauschild A, Lorigan P, Wolter P, Long GV, Flaherty K, Nathan P, Ribas A, Martin AM, Sun P, Crist W, Legos J, Rubin SD, Little SM, Schadendorf D (2015). Improved overall survival in melanoma with combined dabrafenib and trametinib. New England Journal of Medicine 372: 30–39. DOI 10.1056/NEJMoa1412690. [Google Scholar] [CrossRef]
Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Müller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Brück AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Höller C, Utikal J, Huber C, Loquai C, Türeci Ö. (2017). Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547: 222–226. DOI 10.1038/nature23003. [Google Scholar] [CrossRef]
Sarangarajan R, Apte SP (2006). The polymerization of melanin: a poorly understood phenomenon with egregious biological implications. Melanoma Research 16: 3–10. DOI 10.1097/01.cmr.0000195699.35143.df. [Google Scholar] [CrossRef]
Scognamiglio F, Travan A, Turco G, Borgogna M, Marsich E, Pasqua M, Paoletti S, Donati I (2017). Adhesive coatings based on melanin-like nanoparticles for surgical membranes. Colloids and Surfaces B: Biointerfaces. DOI 10.1016/j.colsurfb.2017.04.057. [Google Scholar] [CrossRef]
Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino AS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns P, Blank C, Petrella TM, Hamid O, Zhou H, Ebbinghaus S, Ibrahim N, Robert C (2017). Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390: 1853–1862. DOI 10.1016/S0140-6736(17)31601-X. [Google Scholar] [CrossRef]
Simpson NJ, Wilson JW, Phipps MA, Robles FE, Selim MA, Warren WS (2013). Nonlinear microscopy of eumelanin and pheomelanin with sub-cellular resolution. Journal of Investigative Dermatology 133: 1822–1826. DOI 10.1038/jid.2013.37. [Google Scholar] [CrossRef]
Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ (2005). Hair follicle pigmentation. Journal of Investigative Dermatology 124: 13–21. DOI 10.1111/j.0022-202X.2004.23528.x. [Google Scholar] [CrossRef]
Sniegocka M, Podgórska E, Płonka PM, Elas M, Romanowska-Dixon B, Szczygieł M (2018). Transplantable melanomas in hamsters and gerbils as models for human melanoma. Sensitization in melanoma radiotherapy—From animal models to clinical trials. International Journal of Molecular Sciences 19: 1048. DOI 10.3390/ijms19041048. [Google Scholar] [CrossRef]
Solano F (2017). Melanin and melanin-related polymers as materials with biomedical and biotechnological applications—Cuttlefish ink and mussel foot proteins as inspired biomolecules. International Journal of Molecular Sciences 18: 1561. DOI 10.3390/ijms18071561. [Google Scholar] [CrossRef]
Sparsa A, Faucher K, Sol V, Durox H, Boulinguez S, Doffoel-Hantz V, Calliste CACook-Moreau JC, Krausz P, Sturtz FG, Bedane C, Jauberteau-Marchan MO, Ratinaud MH, Bonnetblanc JM (2010). Blue light is phototoxic for B16F10 murine melanoma and bovine endothelial cell lines by direct cytocidal effect. Anticancer Research 30: 143–147. [Google Scholar]
Stockert JC (2020). Lipid peroxidation assay using BODIPY-phenylbutadiene probes: A methodological overview. Methods in Molecular Biology 2202: 199–214. [Google Scholar]
Stockert JC, Blázquez-Castro A (2017). Fluorescence Microscopy in Life Sciences, E-Book. Sharjah, U.A.E: Bentham Science Publishers. [Google Scholar]
Stockert JC, Juarranz A, Villanueva A, Nonell S, Horobin RW, Soltermann AT, Durantini EN, Rivarola V, Colombo LL, Espada J, Cañete M (2004). Photodynamic therapy: selective uptake of photosensitizing drugs into tumor cells. Current Topics in Pharmacology 8: 185–217. [Google Scholar]
Stockert JC, Vanzulli SI, Cañete M, Villanueva A, Juarranz A, Nonell S, Colombo LL (2012). Regression of the murine LM3 tumor by repeated photodynamic therapy with meso-tetra (4-N,N,N-trimethylanilinium) porphine. Journal of Porphyrins and Phthalocyanines 13: 560–566. DOI 10.1142/S1088424609000577. [Google Scholar] [CrossRef]
Swan GA (1974). Structure, chemistry, and biosynthesis of the melanins. Fortschrift für Chemie und Organische Naturstuffe 31: 521–552. [Google Scholar]
Swetter S (2008). Malignant melanoma. Journal of the American Academy of Dermatology 45: 579–586. www.emedicine.com/DERM/topic257.htm. [Google Scholar]
Tan L, Sandhu S, Lee RJ, Li J, Callahan J, Ftouni S, Dhomen N, Middlehurst P, Wallace A, Raleigh J, Hatzimihalis A, Henderson MA, Shackleton M, Haydon A, Mar V, Gyorki DE, Oudit D, Dawson MA, Hicks RJ, Lorigan P, McArthur GA, Marais R, Wong SQ, Dawson SJ (2019). Prediction and monitoring of relapse in stage III melanoma using circulating tumor DNA. Annals of Oncology 30: 804–814. DOI 10.1093/annonc/mdz048. [Google Scholar] [CrossRef]
Testori A, Ribero S, Bataille V (2017). Diagnosis and treatment of in-transit melanoma metastases. European Journal of Surgical Oncology (EJSO) 43: 544–560. DOI 10.1016/j.ejso.2016.10.005. [Google Scholar] [CrossRef]
Urbanska K, Romanowska-Dixon B, Matuszak Z, Oszajca J, Nowak-Sliwinska P, Stochel G (2002). Indocyanine green as a prospective sensitizer for photodynamic therapy of melanomas. Acta Biochimica Polonica 49: 387–391. DOI 10.18388/abp.2002_3797. [Google Scholar] [CrossRef]
Valli F, García Vior MC, Roguin LP, Marino J (2019). Oxidative stress generated by irradiation of a zinc(II) phthalocyanine induces a dual apoptotic and necrotic response in melanoma cells. Apoptosis 24: 119–134. DOI 10.1007/s10495-018-01512-w. [Google Scholar] [CrossRef]
Valli F, García Vior MC, Roguin LP, Marino J (2020). Crosstalk between oxidative stress-induced apoptotic and autophagic signaling pathways in Zn(II) phthalocyanine photodynamic therapy of melanoma. Free Radical Biology and Medicine 152: 743–754. DOI 10.1016/j.freeradbiomed.2020.01.018. [Google Scholar] [CrossRef]
Viator JA, Hazur M, Sajewski A, Tarhini A, Sanders ME, Edgar RH (2020). Photoacoustic detection of circulating melanoma cells in late stage patients. Journal of Innovative Optical Health Sciences 13: 2050023. DOI 10.1142/S1793545820500236. [Google Scholar] [CrossRef]
Visús C, Andresa R, Mayordomo JI, Martinez-Lorenzo MJ, Murillo L, Sáez-Gutiérrez B, Diestre C, Marcos I, Astier P, Godino J, Carapeto-Marquez de Prado FJ, Larrad L, Tresa A (2007). Prognostic role of circulating melanoma cells detected by reverse transcriptase-polymerase chain reaction for tyrosinase mRNA in patients with melanoma. Melanoma Research 17: 83–89. DOI 10.1097/CMR.0b013e3280a60878. [Google Scholar] [CrossRef]
Wain E, Stefanato C, Barlow R (2008). A clinicopathological surprise: amelanotic malignant melanoma. Clinic and Experimental Dermatology 33: 365–366. DOI 10.1111/j.1365-2230.2008.02707.x. [Google Scholar] [CrossRef]
Walker GJ, Hayward NK (2018). Pathways to melanoma development: lessons from the mouse. International Journal of Molecular Sciences 19: 1566. DOI 10.3390/ijms19061566. [Google Scholar] [CrossRef]
Wang SQ, Setlow R, Berwick M, Polsky D, Marghoob AA, Kopf AW, Bart RS (2001). Ultraviolet A and melanoma: a review. Journal of the American Academy of Dermatology 44: 837–846. DOI 10.1067/mjd.2001.114594. [Google Scholar] [CrossRef]
Weinstock MA (1996). Controversies in the role of sunlight in the pathogenesis of cutaneous melanoma. Photochemistry and Photobiology 63: 406–410. DOI 10.1111/j.1751-1097.1996.tb03056.x. [Google Scholar] [CrossRef]
Wiriyasermkul P, Moriyama S, Nagamori S (2020). Membrane transport proteins in melanosomes: Regulation of ions for pigmentation. Biochimica Biophysica Acta Biomembranes 1862: 183318. DOI 10.1016/j.bbamem.2020.183318. [Google Scholar] [CrossRef]
Yang TD, Choi W, Yoon TH, Lee KJ, Lee JS, Joo JH, Lee MG, Yim HS, Choi KM, Kim B, Lee JJ, Kim H, Young Lee DH, Jung KY, Baek SK (2016). In vivo photothermal treatment by the peritumoral injection of macrophages loaded with gold nanoshells. Biomedical Optics Express 7: 185–193. DOI 10.1364/BOE.7.000185. [Google Scholar] [CrossRef]
Zheng R, Wang S, Tian Y, Jiang X, Fu D, Shen S, Yang W (2015). Polydopamine-coated magnetic composite particles with an enhanced photothermal effect. ACS Applied Materials & Interfaces 7: 15876–15884. DOI 10.1021/acsami.5b03201. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |