DOI:10.32604/biocell.2021.015771

| BIOCELL DOI:10.32604/biocell.2021.015771 |  |
| Article |
Relationship between PON-1 enzymatic activity and risk factors for pesticide poisoning in farmers from the Cienega, Jalisco, Mexico
1Department of Medical and Life Sciences, University Center of La Cienega, University of Guadalajara (CUCI-UdeG), Ocotlán, CP 47810, Mexico
2Department of Biological Sciences, University Center of the Coast, University of Guadalajara (CUCosta-UdeG), Puerto Vallarta, CP 48280, Mexico
*Address correspondence to: Joel Salazar Flores, joelos12@hotmail.com
Received: 12 January 2021; Accepted: 18 March 2021
Abstract: Paraoxonase-1 (PON-1) is an enzyme that hydrolyzes organophosphate pesticides. The presence of polymorphisms in PON-1 (L55M and Q192R) decreases its enzyme activity and increases the risk of central nervous system (CNS) toxicity in occupationally exposed farmers, leading to chronic degenerative diseases and death. We studied 103 farmers in the region of Cienega Jalisco, Mexico, which were exposed mainly to organophosphate pesticides. We used serum and plasma samples to assay PON-1 activity and perform polymorphism analysis (L55M and Q192R) using qPCR and TaqMan probes, respectively. For both polymorphisms, there was high percentage of heterozygosity (55 LL = 0.19, LM = 0.75, MM = 0.06; 192 QQ = 0.12, QR = 0.72, RR = 0.16), while the allelic frequencies were more balanced (L = 0.56, M = 0.44; Q = 0.48, R = 0.52). There were no significant differences in enzyme activity of L55M polymorphism genotypes (LL = 179.27; LM = 192.11; MM = 122.11; QQ = 135.74; QR = 187.90; RR = 209; p > 0.05). But there was a slight decrease in enzyme activity for the Q192R polymorphism genotypes. The genotype and alcohol consumption associated with slight increases in enzyme activity. However, genotype and tobacco consumption did not have a significant effect on PON-1 activity (µU/mL) (p > 0.05). Overall, alcohol and tobacco consumption affected PON-1 enzyme activity (µU/mL) up to 21.1%. The data obtained in this study reveal that PON-1 activity is affected by genetic variants such as Q192R and alcohol consumption. This may influence the susceptibility of populations to organophosphate poisoning.
Keywords: Paraoxonase-1; Pesticides; Organophosphates; Polymorphisms
Pesticides are chemicals that prevent or destroy pests and are used primarily in agriculture to increase crop production and profitability; however, they are highly toxic to non-target organisms. Previous evidence indicates that exposure to pesticides decreases the enzymatic activity of enzymes such as carbonic anhydrase and glutathione-S-transferase. (Caglayan et al., 2019; Özaslan et al., 2018; Amr et al., 2015; Costa et al., 2015; Lozano-Paniagua et al., 2016; Muñoz-Quezada et al., 2016). Approximately 4 million tons/year are applied worldwide, out of which only 1% is estimated to have direct contact with pests (Amr et al., 2015). Pesticide toxicity in humans is mainly due to inappropriate use and mismanagement, as well as acute or chronic exposure, in addition to its multiple routes of human internalization such as a dermal route, inhalation, and ingestion (Lozano-Paniagua et al., 2016; Muñoz-Quezada et al., 2016).
According to the World Health Organization (WHO), about 3 million cases of intoxication and 220,000 deaths occur each year due to pesticides (Ali and Chia, 2008; Marsillach et al., 2016). The most widely used pesticides in the world are organophosphates (OPs), compounds derived from phosphoric acid esters. In humans, these compounds are activated via oxidation by the cytochrome P450 enzyme to ozons, toxic metabolites which are inhibitors of the enzyme acetylcholinesterase (AChE) (Dardiotis et al., 2019; Paul et al., 2017). Acetylcholine esterase catalyzes the hydrolysis of the neurotransmitter acetylcholine (ACh). Thus, the inhibition of AChE produces an increase in the level of the neurotransmitter substrate (ACh), causing a malfunction in the cholinergic synapses of the central nervous system (CNS) by increasing the transmission of electrical impulses (Gündoğdu et al., 2019). This produces what is known as a pesticide-induced cholinergic syndrome, which is characterized by symptoms such as excessive secretions from excretory glands, loss of consciousness, and respiratory and cardiac problems, which can cause death (Ali and Chia, 2008; Dardiotis et al., 2019; Sirivarasai et al., 2007; Durgun et al., 2020; Sever et al., 2020).
Paraoxonase-1 (PON-1) is an enzyme that hydrolyzes OP-type compounds. The first known substrate for the enzyme was paraoxon, a toxic metabolite of parathion, hence its name.
PON-1 hydrolyzes paraoxon to p-nitrophenol, but it also shows a high affinity for other molecules with free amino and hydroxyl groups (Demir et al., 2019; Demir, 2020). PON-1 is a multifunctional enzyme with the capacity to act as arylesterase, lactonase, and organophosphatase (Xotlanihua-Gervacio et al., 2019; Mitra et al., 2015). PON-1 is a 43-kDa calcium-dependent glycoprotein expressed by the 9-exon PON-1 gene located on the long arm of chromosome 7 (7q21); it is synthesized in the liver and secreted into the bloodstream via association with high-density lipoproteins (HDL) (Xotlanihua-Gervacio et al., 2019; López-Flores et al., 2009; Mitra et al., 2015; Mota et al., 2019; Demir, 2019). The binding of PON-1 to HDL depends on terminal amino residues that anchor to phospholipids. PON-1 has two critical calcium ions, one has a structural function, and the other is necessary for its catalytic activity. The active site of PON-1contains tryptophan, histidine, lysine, phenylalanine, and aspartate/glutamine residues. At the active site, calcium ion interacts with Asn 224, 270, 168, Asp 269, and Glu 53 residues. (Demir and Beydemir, 2015). Two polymorphisms in the coding region affect the activity of the enzyme: one at position L55M and the other at position Q192R. Polymorphism 192 (rs662 A > G) causes a change from glutamine (Q) to arginine (R) that affects the catalytic efficiency of the enzyme towards different substrates. For example, alloenzyme Q hydrolyzes diazoxon more effectively than alloenzyme R; whereas, alloenzyme R hydrolyzes paraoxon more effectively than alloenzyme Q. Polymorphism 55 (rs854560 A > T) has a substitution of leucine (L) by methionine (M), which is associated with a low serum concentration of the enzyme. This effect could be due to the low levels of PON-mRNA1, as a result of regulation by the ligation of the M variant with 108 C/T promoter region polymorphism (Costa et al., 2003; López-Flores et al., 2009; Pan et al., 2019; Sirivarasai et al., 2007). The objective of this study was to investigate the relationship between the enzymatic activity of PON-1 and its genetic polymorphisms (L55M and Q192R) and other variables, such as alcohol and tobacco consumption, in farmers exposed to pesticides from the region of the Cienega Jalisco, Mexico.
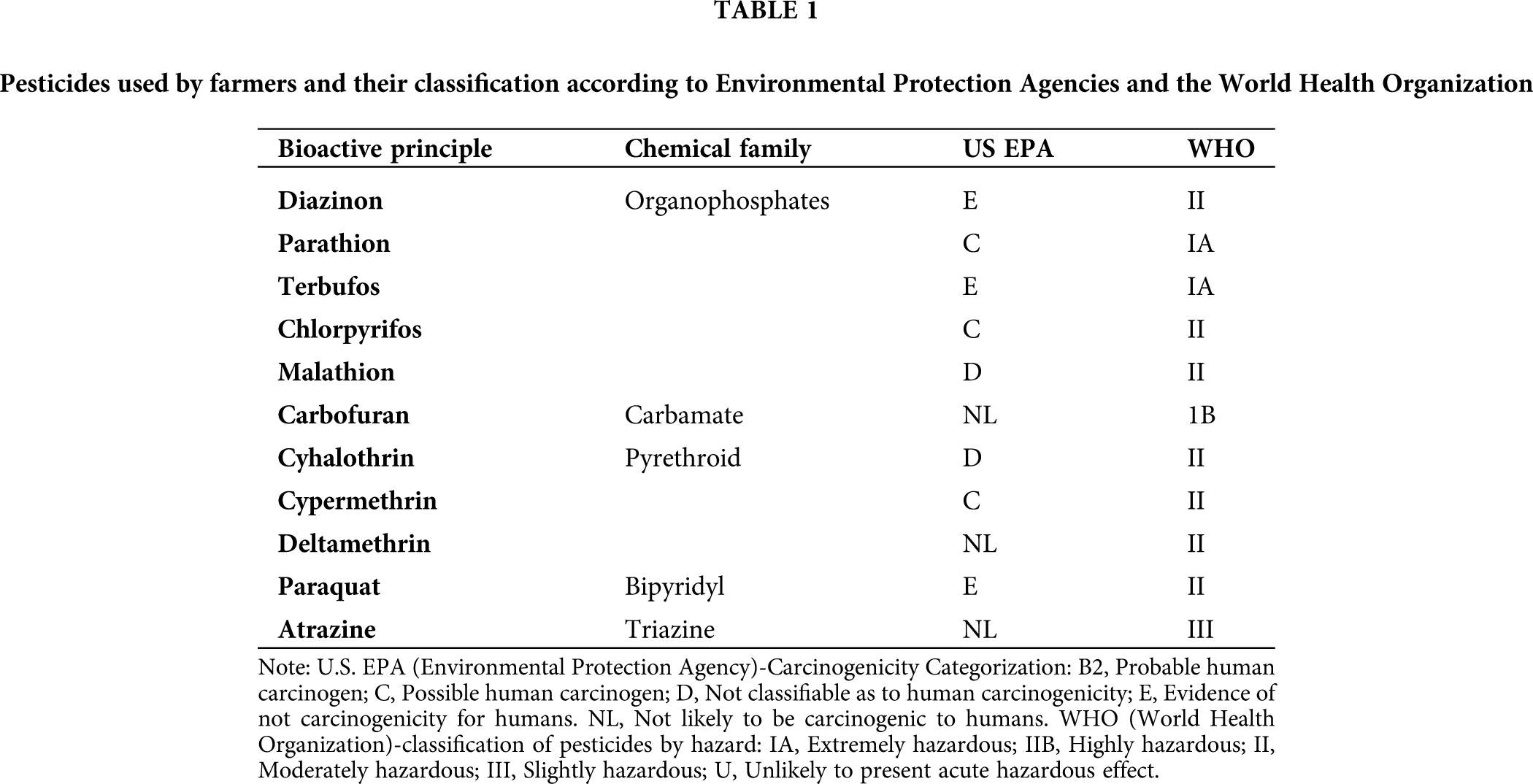
We collected 103 blood samples from farmers with occupational exposure to different pesticides (Tab. 1) from 14 localities in 3 municipalities in the region of the Cienega Jalisco Mexico. The exclusion criteria included subjects younger than 18 and older than 60 years of age, subjects with kidney and liver diseases, diabetes, hypertension, cardiovascular diseases, and cancer, on lipid-lowering treatment or illegal drug use. We were sampling during the months of April and May, which includes a period of intensive agriculture activity that involves preparations for the planting of corn in this region. We collected blood samples into sample tubes with or without EDTA (for plasma or serum samples, respectively). We used plasma and serum fractions for genotyping analysis and enzyme activity assays, respectively. In addition, we applied a survey to each farmer to identify the type of pesticide to which they are exposed.
In this study, DNA was extracted from the samples using Quick-DNATM Miniprep kit Catalog Nos. D3024 and D3025 of ZYMO RESEARCH CORP, in line with the manufacturer’s instructions. The PON-1 genotypes for polymorphisms 55 and 192 were determined with real-time PCR using Taqman® assays SNP Genotyping kit (ThermoFisher-Scientific), with identification number per test C_ _ _2259750_20 y C_ _ _ 2548962_20, respectively. Each amplification reaction mixture comprised 5 µl of TaqMan Universal PCR Master Mix from Applied Biosystems (containing AmpliTaq Gold DNA Polymerase UP, dNTPs with dUTP, ROX Passive Reference and optimized buffer components); 0.5 µL of Taqman® assay SNP Genotyping (containing wild and mutated TaqMan probe, and primers: forward and reverse); 0.5 µL genomic DNA containing approximately 20 ng, and 10 µL of DNase-free water. The reaction was amplified in a thermocycler LightCycler® Nano (Roche) under the following conditions: a preheating stage of 50°C per 2 min, a heating stage at 95°C for 10 min, and an amplification stage at 45 cycles of 95°C at 15 s, and 60°C for 1 min. At the end of the real-time PCR, the genotypes were analyzed with endpoint genotyping analysis that measured the distribution of fluorescence intensity of the TaqMan probe tags for detection of wild-type and mutated sequences (probe fluorophores: 6-FAM and VIC).
The activity of PON-1 in serum was measured using Paraoxonase-1 Activity Assay Kit (BioVision Incorporated) and paraoxon as substrate. The principle of the assay consists of the spectrofluorometric measurement (Ex/Em = 368/460 nm) of p-nitrophenol, which is formed as a yellow chromogenic product by the hydrolytic activity of PON-1. The reactions were performed in a volume of 80 µL, adding 5 µL of serum per sample. The plate was pre-incubated for 10 min at 37°C, and subjected to the kinetic mode for 10 min, taking readings every minute at 37°C. The test contained a positive control with PON-1. The negative control contained a 2-hydroxyquinoline inhibitor. Two time points (t1 and t2) in the linear phase of the progress curves of each reaction were used to determine PON-1 activity, obtaining the corresponding fluorescence values at those points (RFU1 and RFU2) to determine the change in fluorescence over time: FS = RFU2 − RFU1. The specific fluorescence (CS) was calculated by subtracting the control background (FBC) of each sample: CS = FS − FBC. The CS values were obtained from the standard fluorescence curve to obtain B pmol of the metabolized substrate during the reaction time and the enzymatic activity of PON-1 was calculated by the following formula:
• A = paraoxonase activity (pmol/min/mL = µU/mL).
• B = amount of metabolite produced calculated from the standard curve (pmol).
• ΔT = linear phase reaction time t2 − t1 (min).
• V = sample volume added in the sampling wells (mL).
• D = dilution factor of the sample if diluted to conform to the standard curve range (before preparation of the reaction well).
Appropriate statistical treatment of the data is essential. Allelic and genotypic frequencies, observed and expected heterozygosity, ligation imbalance, inbreeding coefficient (Fis), and Hardy–Weinberg equilibrium were determined through the Genetic Data Analysis (GDA) program (Lewis and Zaykin, 2001). The levels of significance were determined in 10000 simulations. Subjects were classified according to their genotypes (PON-1 55; LL, LM, MM and PON-1 192; QQ, QR and RR). The genotypes were tested for their association with enzymatic activity and various factors such as exposure to pesticides OPs, age groups (<20, 21–30, 31–40, 41–50, and >50 years), consumption of alcohol and tobacco. The comparison of enzymatic activity according to genotype was performed using ANOVA and Kruskal-Wallis tests. We also compared the mean values for the groups using the Student’s t-test and Mann Whitney’s U-test. Finally, the association between genotypes and different variables was determined using single and multiple linear regression analyses. All tests were performed using the statistical program IBM SPSS v.25.0. Values of p < 0.05 were indicative of statistical significance.
Pesticides used in the region of the Cienega Jalisco, Mexico
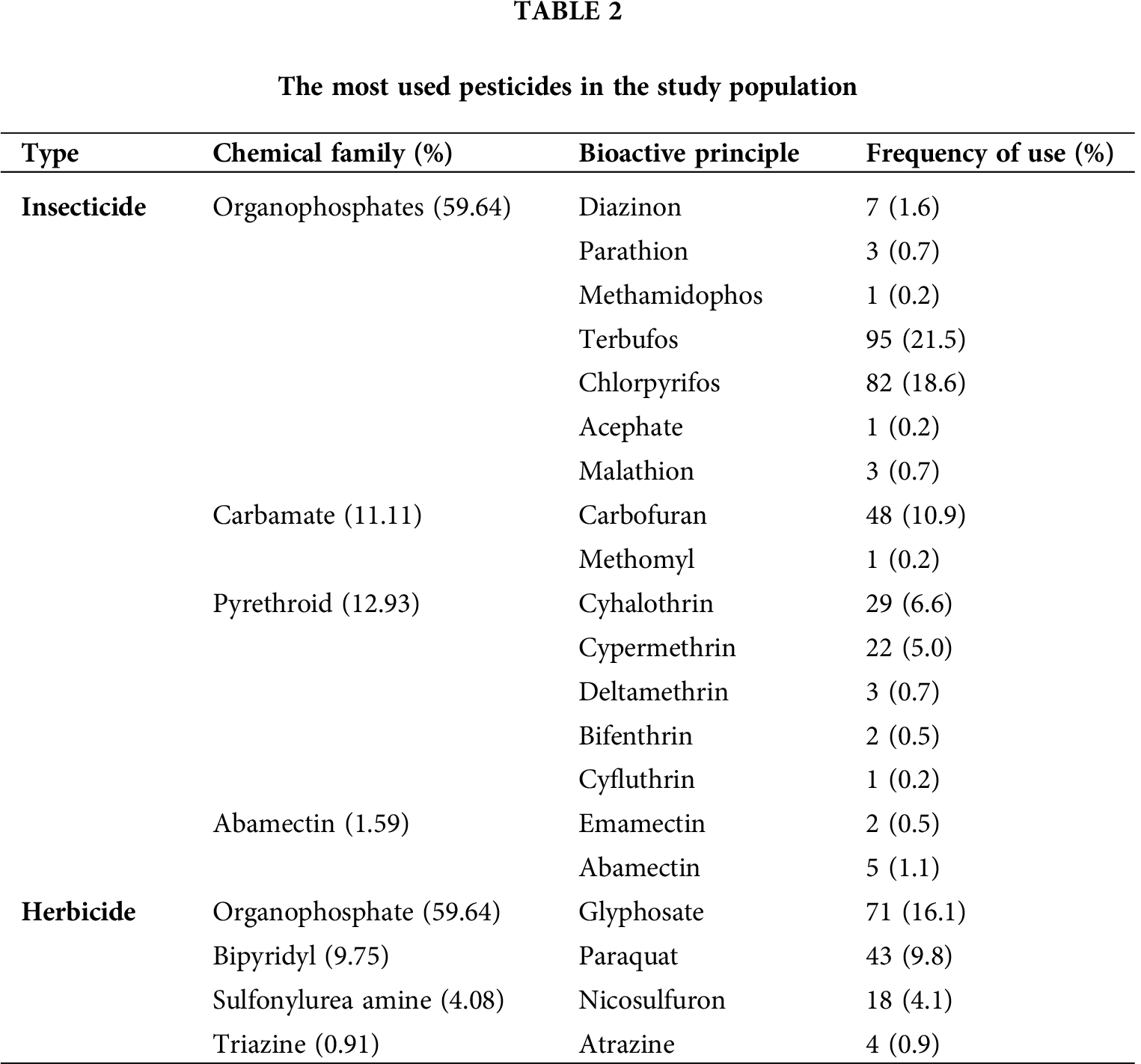
The participants indicated that they used 7 different classifications of pesticides, being organophosphate type the most used (59.64%), followed by pyrethroids (12.93%), carbamates (11.11%), and bipyridyls (9.75%). These results are shown in Tab. 2. The organophosphate pesticides most used were terbufos and chlorpyrifos (21.5% and 18.6%, respectively), while the most frequently used pesticide with herbicide function was glyphosate (16.1%).
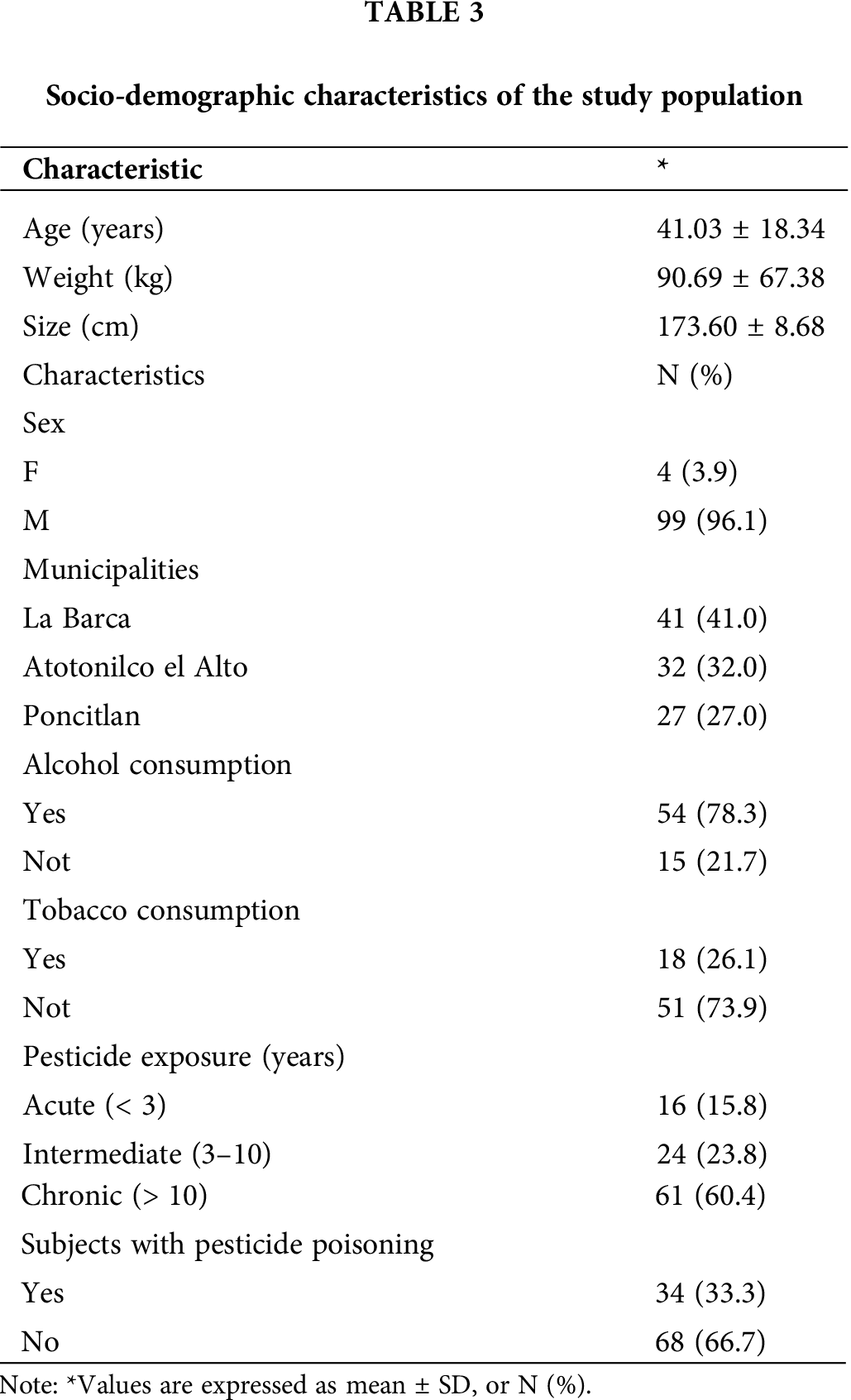
Characteristics of the study population
The study was carried out on farmers mainly exposed to OPs. The participants were mostly men (96.1%), with a mean age of 41 years. Approximately 84% of the participants had more than 3 years of pesticide exposure, while 33% of the farmers had experienced some form of pesticide poisoning. Tab. 3 shows the detailed characteristics of the study population, including other important parameters that directly affect the enzymatic activity of PON-1, such as alcohol consumption (78.3%) and tobacco use (26.1%).
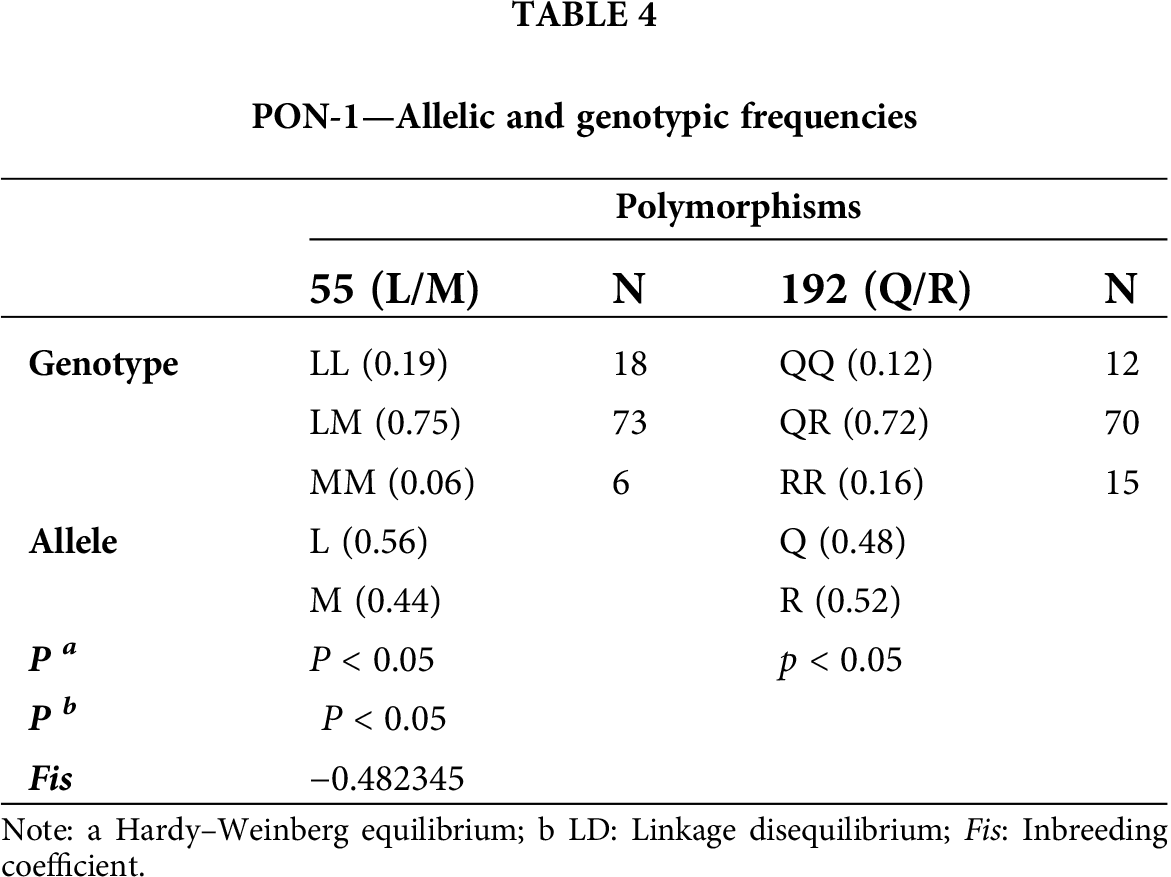
PON-1 genotypes and allelic frequencies
The allelic and genotypic frequencies of the PON1 coding region polymorphisms are shown in Tab. 4. We observed a high frequency of heterozygous genotypes for each polymorphism. The Hardy–Weinberg equilibrium was calculated, and statistical significance was obtained (p < 0.05). Therefore, the results suggest that the population was not in equilibrium. In addition,
the calculated ligation imbalance was statistically significant (p < 0.05). The value of the inbreeding coefficient Fis was −0.482345.
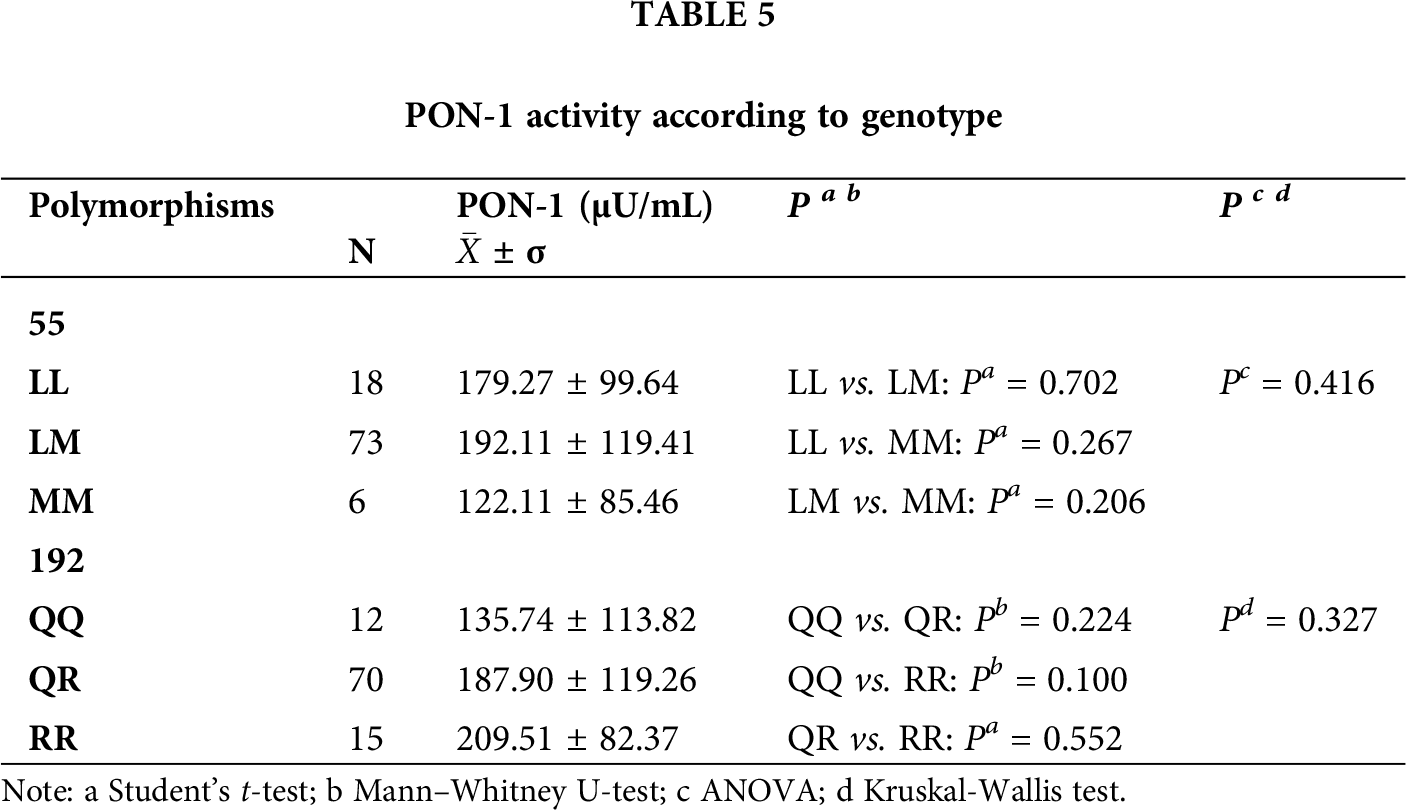
Enzymatic activity of PON-1 as a function of its genotypes
We classify the enzymatic activity of PON-1 according to its genotype (Tab. 5) and made comparisons between the L55M polymorphism and its genotypes, and the result did not show significant differences (p > 0.05). However, we observed that the mutated homozygous genotype had lower activity. Also, we did not find statistical significance (p > 0.05) between the Q192R polymorphism and its genotypes, but the wild-type homozygous genotype had lower enzymatic activity relative to the mutated genotype.
Enzymatic activity of PON-1 and non-genetic parameters
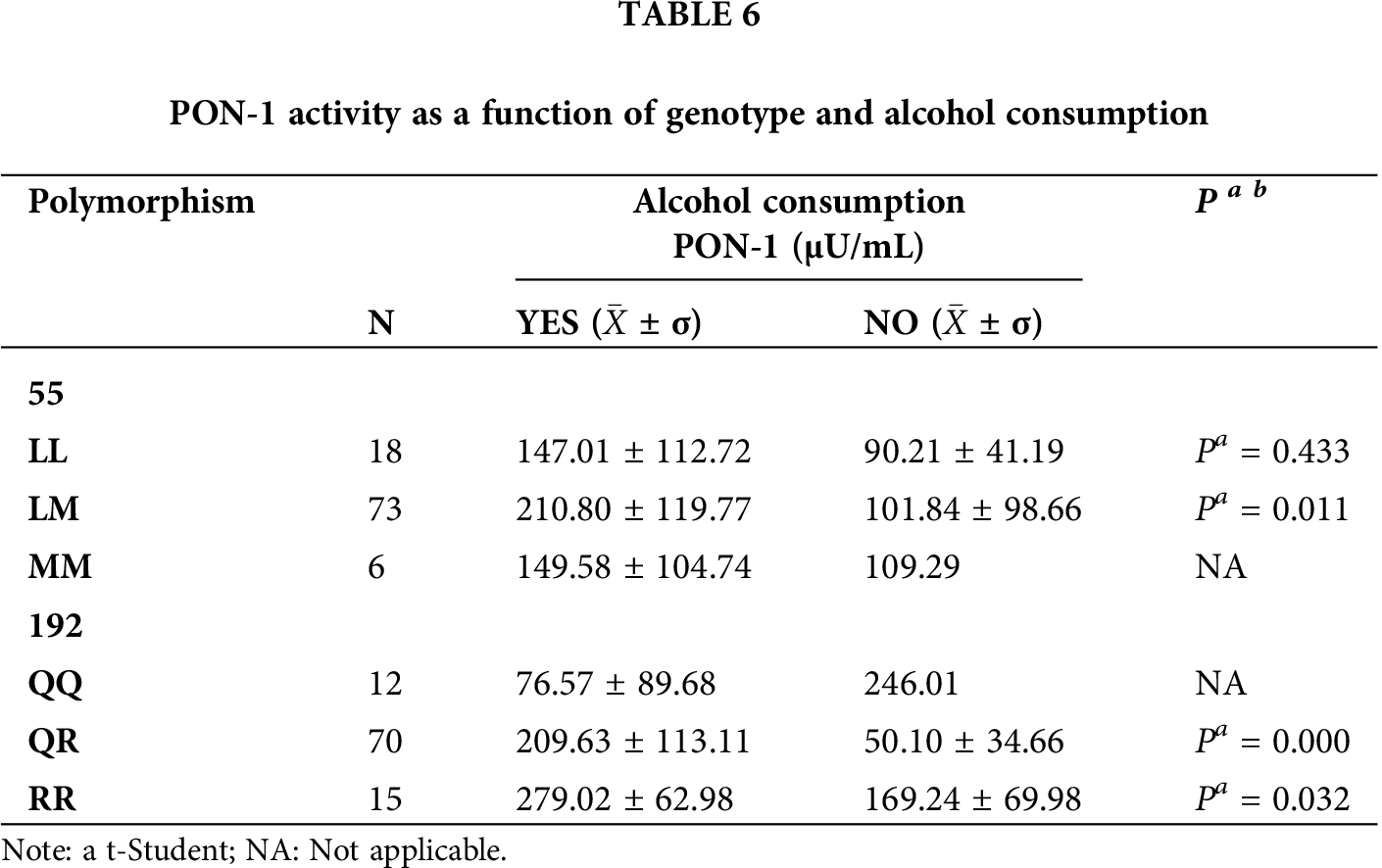
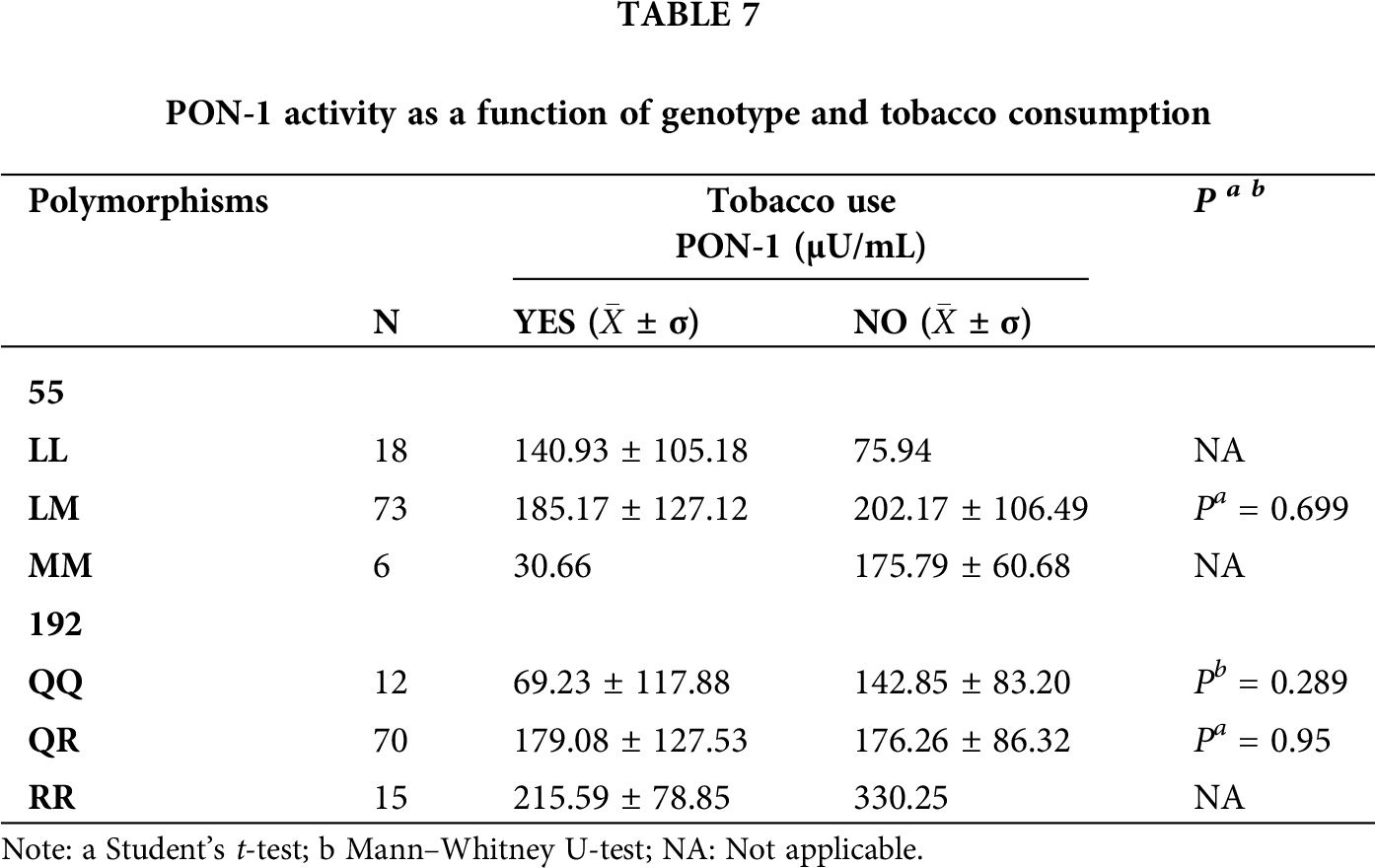
The enzymatic activity of PON-1 was compared with respect to the genotype of each polymorphism, alcohol consumption, tobacco use, age groups (<20, 21–30, 31–40, 41–50, and 50 years), and type of exposure (acute, intermediate, and chronic). For these comparisons, we found statistical significance only with alcohol consumption (p = 0.011) in relation to the LM genotype of the L55M polymorphism (Tab. 6). We also found statistical significance for the QR and RR genotypes of the Q192R polymorphism (p = 0.000 and p = 0.032, respectively) (Tab. 6). We also compared the enzyme activity as a function of genotype in tobacco consumers (Tab. 7), and in both polymorphisms, we did not find statistical significance (p > 0.05). However, we observed a reduction in enzyme activity in most genotypes.
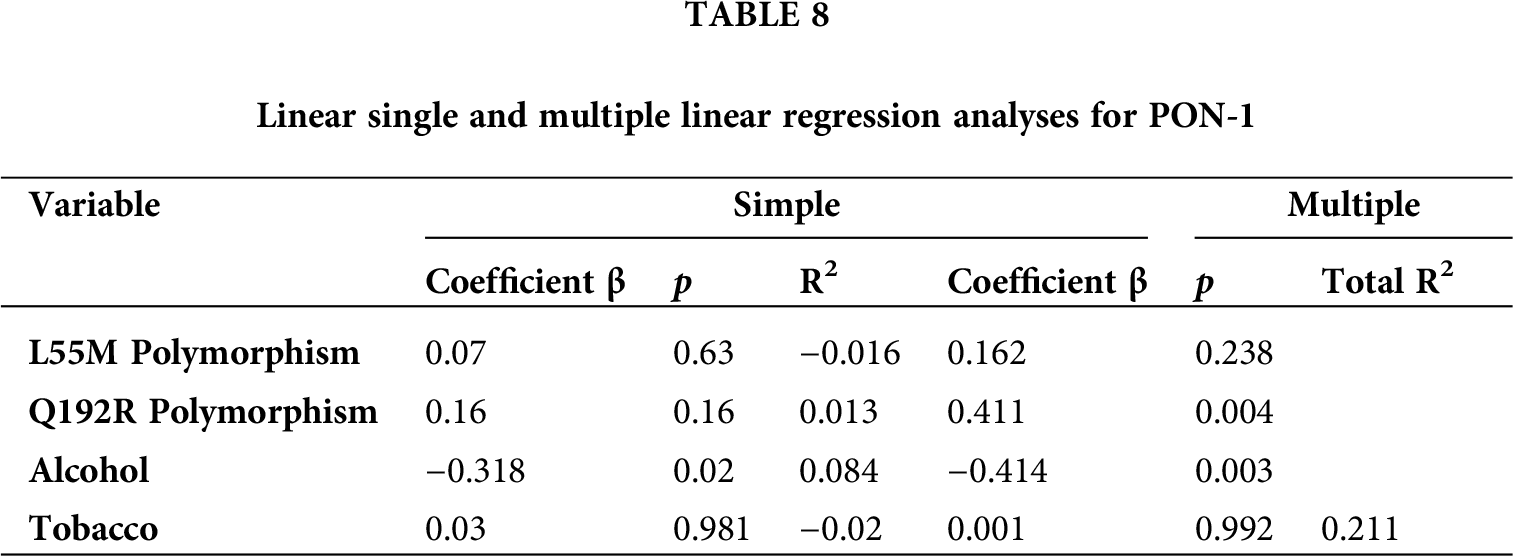
We used linear single and multiple linear regression models to determine the association between genotype, alcohol and tobacco consumption, and PON-1 activity. The multiple regression model shown an R2 value of 0.211, which indicated that the genotypes for both polymorphisms (alcohol and tobacco consumption) affected the enzymatic activity of PON-1; in contrast, the single linear regression model indicated a poor relationship (Tab. 8).
Pesticides used in the region of the Cienega Jalisco, Mexico
Most of the participants in this study were males (96.1%). This could be an advantage since there is less variability in the enzymatic activity of PON-1 with respect to the female sex (Fridman et al., 2001). Moreover, the ages of the participants ranged between 18 and 60 years, with a mean age of 41 years, which coincides with the age of greatest occupational exposure to pesticides in farmers. This is in agreement with a previous report in Mexico where the age range was 18 to 58 ± 5 years at the upper limit (López-Flores et al., 2009; Pérez-Herrera et al., 2008; Rojas-García et al., 2005). Sixty percent (60%) of participants were farmers with chronic occupational exposure (>10 years). The frequency of exposure to OPs was higher (approximately 60%) than that of other pesticides. This may be related to CNS toxicity, immunosuppression, genotoxicity and cancer (Androutsopoulos et al., 2011; Arévalo-Jaramillo et al., 2019; Ceja-Gálvez et al., 2020; Martínez-Valenzuela and Gómez-Arroyo, 2007; Reynoso et al., 2019; Suratman et al., 2015). The frequent use of OPs is due to their effectiveness in the control of corn pests (Murcia and Stashenko, 2008). The region of the Cienega is a major producer of this grain. Indeed, this region uses OPs more frequently than florists in the state of Mexico and Morelos, with a frequency of use of 49% (López-Flores et al., 2009). Thus, they are more susceptible to the health risks associated with these pesticides. Terbufos and chlorpyrifos are the most used. A study carried out in the region of Coquimbo, Chile, revealed that the most widely used pesticide was chlorpyrifos (Zúñiga-Venegas et al., 2015). The use of this pesticide may be responsible for the greater susceptibility to poisoning in farmers with the wild-type QQ genotype of the 192 polymorphism since the QQ alloenzyme presents a low capacity for metabolizing this substrate (Ceja-Gálvez et al., 2020; Torres-Sánchez et al., 2019). Regarding the use of herbicides, the Cienega in Jalisco and the states of Mexico and Morelos use mainly glyphosate, which is interesting, since the International Agency for Research on Cancer (IARC) decreed in 2015 that glyphosate is a potent carcinogen (Reynoso et al., 2019). A previous study suggested that glyphosate may be nephrotoxic when it interacts with metal compounds in hard water, which could be responsible for epidemics of chronic kidney disease (Jayasumana et al., 2014).
PON-1 genotypes and allelic frequencies
We found a high percentage of heterozygous genotypes for L55M polymorphism (0.75). This differs from the results of related studies in Mexico (Gamboa et al., 2006; López-Flores et al., 2009; Pérez-Herrera et al., 2008; Rojas-García et al., 2005; Torres-Sánchez et al., 2019) that showed heterozygosity >30% with the most common wild-type homozygous genotypes. However, it is possible that the heterozygosity of this polymorphism may be influenced by the genetic linkage it showed with the Q192R polymorphism. Allelic frequency was more balanced, but the wild-type allele was slightly more frequent (0.56). It is worth mentioning that in other studies carried out in Mexico, the wild-type allele presented a higher frequency, compared with the region of the Cienega (Gamboa et al., 2006; López-Flores et al., 2009; Pérez-Herrera et al., 2008; Rojas-García et al., 2005; Torres-Sánchez et al., 2019). We found a high percentage of heterozygous genotypes for Q192R (0.72). This is consistent with the results of studies carried out in Mexico and Peru. The Peruvian study showed more comparable results to the region of Cienega, with respect to genotypic frequency (0.61) (Cataño et al., 2006). Regarding allelic frequencies, we found a higher percentage of the mutated allele (0.52). This result is similar to that reported in a study carried out on farmers from Yucatan, Mexico, whose mutated allele had a frequency of 0.53 (Pérez-Herrera et al., 2008). It is worth mentioning that there are other studies that report different but relatively close allelic frequencies (Cataño et al., 2006; Gamboa et al., 2006; López-Flores et al., 2009; Pérez-Herrera et al., 2008; Rojas-García et al., 2005; Torres-Sánchez et al., 2019; Zúñiga-Venegas et al., 2015).
Regarding the heterozygotes of both polymorphisms, there was no Hardy–Weinberg equilibrium. Thus, the inbreeding coefficient Fis was calculated, resulting in a value of −0.482345, indicating that for both polymorphisms, there was an excess of 48% heterozygotes. Knowing that the high percentage of heterozygous may be due to technical genotyping problems, the procedure was verified in an external laboratory by sequencing selected samples for each genotype. This confirmed our results. However, it should be mentioned that there are other evolutionary factors such as gene flow, natural selection, and inbreeding, which can also influence allelic distribution (Mohammadi et al., 2015). The ligation imbalance was also calculated, obtaining a significant value (p < 0.05), which could explain the high percentage of heterozygotes.
Enzymatic activity of PON-1 as a function of its genotypes
The L55M polymorphism is known to result in reduced enzyme activity because there is a lower expression of the enzyme and therefore a lower concentration in the circulatory system. However, analysis of the relationship between genotypes and enzymatic activity did not show a significant trend (p > 0.05). In contrast, some trends were reported in other studies in which mutated homozygotes presented less PON-1 activity than wild-type homozygotes(Gamboa et al., 2006; López-Flores et al., 2009; Pérez-Herrera et al., 2008; Rojas-García et al., 2005; Torres-Sánchez et al., 2019). The Q192R polymorphism causes a switch from glutamine to arginine. This causes a conformational change in the active site of PON-1, resulting in changes in the enzyme activity. In addition, the activity of the enzyme is influenced by differences in the affinities towards different substrates to be metabolized. In our study, we did not find significant differences that indicate that the genotypes of this polymorphism affected enzymatic activity. In contrast, other studies carried out in Mexico, Chile and Peru have reported that mutated homozygotes had greater activity than wild-type ones, when using paraoxon as substrate (Cataño et al., 2006; Gamboa et al., 2006; López-Flores et al., 2009; Pérez-Herrera et al., 2008; Rojas-García et al., 2005; Torres-Sánchez et al., 2019; Zúñiga-Venegas et al., 2015).
Relationship between PON-1 activity and non-genetic parameters
In addition to genetic polymorphisms, the activity of PON-1 is affected by different variables such as diet, alcohol and tobacco consumption, environmental toxins, aging, pregnancy, and various pathologies (Ceja-Gálvez et al., 2020). We found that alcohol consumption affected PON-1 activity (p < 0.05), with respect to the heterozygotes of both polymorphisms and the mutated homozygous of the Q192R polymorphism, in which we observed increases in PON-1 activity. This is consistent with previous research where moderate alcohol consumption slightly increased PON-1 activity, while excessive alcohol exposure led to decreased enzyme activity (Gruppen et al., 2018; Rao et al., 2003; Xotlanihua-Gervacio et al., 2019). Similarly, a study in rats showed that light alcohol consumption regulated the expression of PON-1 mRNA (Rao et al., 2003). Additionally, a human study reported that the threshold for alcohol consumption to increase PON-1 activity ranged from 10 to 30 g/day (Gruppen et al., 2018). Comparisons between tobacco use and any of the genotypes of both polymorphisms did not show any statistically significant effect on enzyme activity (p > 0.05). However, we observed trends in which the genotype of tobacco users resulted in slightly reduced PON-1 activity. This may be due to oxidative stress produced by cigarette smoke which contains several oxidizing and pro-oxidant substances capable of producing reactive oxygen species with hydroxyl radicals, resulting in the inactivation or oxidization of PON-1, thus decreasing its activity (Bizoń et al., 2016; Kahraman et al., 2017; Marek et al., 2018; Xotlanihua-Gervacio et al., 2019). At the same time, it should be noted that there are studies that have shown that cigarette smoking affects the activity of the PON-1 enzyme by up to 40% (Bizoń et al., 2016).
The effect of tobacco and alcohol consumption on PON-1 enzyme activity and genetic polymorphism
PON-1 enzyme activity can vary in response to external and genetic factors, so we performed a single linear regression to find out if each of the variables (polymorphisms, alcohol, and tobacco consumption) affected the enzyme. The results showed a poor association between enzyme activity and these variables; only the Q192R polymorphism and alcohol consumption showed low correlation values (1.3% and 8.4%, respectively). However, multiple linear regression analysis showed that these variables affected PON-1 activity by up to 21.1%. A study carried out in Spain showed that polymorphisms (L55M and Q192R), alcohol consumption, tobacco use, gender, physical activity, cholesterol, triglycerides, HDL, body mass index, and nutrition (consumption of antioxidants and lipids) can influence 74% of PON-1 activity (Ferrè et al., 2003). In another study, in Mexico, it has been observed that four polymorphisms (−162, −108, L55M, and Q192R) caused a change in PON-1 of up to 25% (Rojas-García et al., 2005). This is a similar 21.1% change observed in this study when two polymorphisms (L55M and Q192R) were tested in relation to factors such as alcohol and tobacco consumption. Finally, our study shows that the additive effect of genetic variants of PON-1 and the consumption of alcohol and tobacco allow an association of susceptibility or risk of poisoning due to occupational exposure to pesticides. However, cohort studies are needed to assess whether genetic variants and other factors can improve the prediction of poisoning among farmers.
Acknowledgement: We thank CONACyT for the master’s scholarship to H.R.C.G.
Availability of Data and Materials: All the data and analyses can be made available by the corresponding author upon reasonable request.
Author Contributions: The authors confirm contribution to the paper as follows: Study conception and design: Joel Salazar Flores; data collection: Hazael Ramiro Ceja Gálvez, Erandis Dheni Torres Sánchez; analysis and interpretation of results: Joel Salazar Flores, Erandis Dheni Torres Sánchez, Juan Heriberto Torres Jasso; draft manuscript preparation: Hazael Ramiro Ceja Gálvez, Emmanuel Reyes Uribe. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: The Ethics Committee of the University Center of the Cienega, University of Guadalajara, approved the experimental protocol carried out in this project (Folio 2017–037). Each participant signed an informed consent letter guaranteeing the confidentiality of the data. The study was conducted in strict compliance with the principles of the Helsinki Declaration.
Funding Statement: The authors did not receive specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ali SM, Chia SE (2008). Interethnic variability of plasma paraoxonase (PON1) activity towards organophosphates and PON1 polymorphisms among Asian populations—A short review. Industrial Health 46: 309–317. DOI 10.2486/indhealth.46.309. [Google Scholar] [CrossRef]
Amr S, Dawson R, Saleh DA, Magder LS, George DM, El-Daly M, Squibb K, Mikhail NN, Abdel-Hamid M, Khaled H, Loffredo CA (2014). Pesticides, gene polymorphisms, and bladder cancer among Egyptian agricultural workers. Archives of Environmental & Occupational Health 70: 19–26. DOI 10.1080/19338244.2013.853646. [Google Scholar] [CrossRef]
Androutsopoulos VP, Kanavouras K, Tsatsakis AM (2011). Role of paraoxonase 1 (PON1) in organophosphate metabolism: Implications in neurodegenerative diseases. Toxicology and Applied Pharmacology 256: 418–424. DOI 10.1016/j.taap.2011.08.009. [Google Scholar] [CrossRef]
Arévalo-Jaramillo P, Idrobo A, Salcedo L, Cabrera A, Vintimilla A, Carrión M, Bailon-Moscoso N (2019). Biochemical and genotoxic effects in women exposed to pesticides in Southern Ecuador. Environmental Science and Pollution Research 26: 24911–24921. DOI 10.1007/s11356-019-05725-7. [Google Scholar] [CrossRef]
Bizoń A, Kepinska M, Snacki K, Milnerowicz H (2015). The impact of environmental and biological factors on paraoxonase 1 and γ-glutamyltranspeptydase activities in the blood of smelters. International Journal of Environmental Health Research 26: 222–238. DOI 10.1080/09603123.2015.1089533. [Google Scholar] [CrossRef]
Caglayan C, Taslimi P, Türk C, Kandemir FM, Demir Y, Gulcin I (2019). Purification and characterization of the carbonic anhydrase enzyme from horse mackerel (Trachurus trachurus) muscle and the impact of some metal ions and pesticides on enzyme activity. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 226: 108605. DOI 10.1016/j.cbpc.2019.108605. [Google Scholar] [CrossRef]
Cataño HC, Cueva JL, Cardenas AM, Izaguirre V, Zavaleta AI, Carranza E, Hernández AF (2006). Distribution of paraoxonase-1 gene polymorphisms and enzyme activity in a Peruvian population. Environmental and Molecular Mutagenesis 47: 699–706. DOI 10.1002/em.20259. [Google Scholar] [CrossRef]
Ceja-Gálvez HR, Torres-Sánchez ED, Torres-Jasso JH, Ornelas AV, Salazar-Flores J (2020). Effect of structure and function of paraoxonase-1 (PON-1) on organophosphate pesticides metabolism. BIOCELL 44: 363–370. DOI 10.32604/biocell.2020.09147. [Google Scholar] [CrossRef]
Costa C, Gangemi S, Giambo F, Rapisarda V, Caccamo D, Fenga C (2015). Oxidative stress biomarkers and paraoxonase 1 polymorphism frequency in farmers occupationally exposed to pesticides. Molecular Medicine Reports 12: 6353–6357. DOI 10.3892/mmr.2015.4196. [Google Scholar] [CrossRef]
Costa LG, Cole TB, Furlong CE (2003). Polymorphisms of paraoxonase (PON1) and their significance in clinical toxicology of organophosphates. Journal of Toxicology: Clinical Toxicology 41: 37–45. DOI 10.1081/CLT-120018269. [Google Scholar] [CrossRef]
Dardiotis E, Aloizou AM, Siokas V, Tsouris Z, Rikos D, Marogianni C, Aschner M, Kovatsi L, Bogdanos DP, Tsatsakis A (2019). Paraoxonase-1 genetic polymorphisms in organophosphate metabolism. Toxicology 411: 24–31. DOI 10.1016/j.tox.2018.10.012. [Google Scholar] [CrossRef]
Demir Y, Balcı N, Gürbüz M (2019). Differential effects of selective serotonin reuptake inhibitors on paraoxonase-1 enzyme activity: An in vitro study. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 226: 108608. DOI 10.1016/j.cbpc.2019.108608. [Google Scholar] [CrossRef]
Demir Y (2019). The behaviour of some antihypertension drugs on human serum paraoxonase-1: An important protector enzyme against atherosclerosis. Journal of Pharmacy and Pharmacology 71: 1576–1583. DOI 10.1111/jphp.13144. [Google Scholar] [CrossRef]
Demir Y (2020). Naphthoquinones, benzoquinones, and anthraquinones: Molecular docking, ADME and inhibition studies on human serum paraoxonase-1 associated with cardiovascular diseases. Drug Development Research 81: 628–636. DOI 10.1002/ddr.21667. [Google Scholar] [CrossRef]
Demir Y, Beydemir Ş. (2015). Purification, refolding, and characterization of recombinant human paraoxonase-1. Turkish Journal of Chemistry 39: 764–776. DOI 10.3906/kim-1501-51. [Google Scholar] [CrossRef]
Durgun M, Türkeş C, Işık M, Demir Y, Saklı A, Kuru A, Supuran CT (2020). Synthesis, characterisation, biological evaluation and in silico studies of sulphonamide Schiff bases. Journal of Enzyme Inhibition and Medicinal Chemistry 35: 950–962. DOI 10.1080/14756366.2020.1746784. [Google Scholar] [CrossRef]
Ferrè N, Camps J, Fernández-Ballart J, Arija V, Murphy MM, Ceruelo S, Biarnés E, Vilella E, Tous M, Joven J (2003). Regulation of serum paraoxonase activity by genetic, nutritional, and lifestyle factors in the general population. Clinical Chemistry 49: 1491–1497. DOI 10.1373/49.9.1491. [Google Scholar] [CrossRef]
Fridman O, Graciela A, Porcile R, Morales AV, Gariglio LO (2001). Archivos de cardiología de México. Archivos de Cardiología de México 81: 251–260. [Google Scholar]
Gamboa R, Zamora J, Rodríguez-Pérez JM, Fragoso JM, Cardoso G, Posadas-Romero C, Vargas-Alarcón G (2006). Distribution of paraoxonase PON1 gene polymorphisms in Mexican populations. Its role in the lipid profile. Experimental and Molecular Pathology 80: 85–90. DOI 10.1016/j.yexmp.2005.05.006. [Google Scholar] [CrossRef]
Gruppen EG, Bakker SJL, James RW, Dullaart RPF (2018). Serum paraoxonase-1 activity is associated with light to moderate alcohol consumption: The PREVEND cohort study. American Journal of Clinical Nutrition 108: 1283–1290. DOI 10.1093/ajcn/nqy217. [Google Scholar] [CrossRef]
Gündoğdu S, Türkeş C, Arslan M, Demir Y, Beydemir Ş. (2019). New isoindole-1, 3-dione substituted sulfonamides as potent inhibitors of carbonic anhydrase and acetylcholinesterase: Design, synthesis, and biological evaluation. Chemistry Select 4: 13347–13355. [Google Scholar]
Jayasumana C, Gunatilake S, Senanayake P (2014). Glyphosate, hard water and nephrotoxic metals: Are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? International Journal of Environmental Research and Public Health 11: 2125–2147. DOI 10.3390/ijerph110202125. [Google Scholar] [CrossRef]
Kahraman FU, Torun E, Osmanoğlu NK, Oruçlu S, Özer ÖF (2017). Serum oxidative stress parameters and paraoxonase-1 in children and adolescents exposed to passive smoking. Pediatrics International 59: 68–73. DOI 10.1111/ped.13073. [Google Scholar] [CrossRef]
Lewis PO, Zaykin D (2001). GDA (genetic data analysiscomputer program for the analysis of allelic data. University of Connecticut, Storrs, Versión 1.1, [Google Scholar]
López-Flores I, Lacasaña M, Blanco-Muñoz J, Aguilar-Garduño C, Sanchez-Villegas P, Pérez-Méndez OA, Gamboa-Ávila R, (2009). Relationship between human paraoxonase-1 activity and PON1 polymorphisms in Mexican workers exposed to organophosphate pesticides. Toxicology Letters 188: 84–90. DOI 10.1016/j.toxlet.2009.03.010. [Google Scholar] [CrossRef]
Lozano-Paniagua D, Gómez-Martín A, Gil F, Parrón T, Alarcón R, Requena M, Lacasaña M, Hernández AF (2016). Activity and determinants of cholinesterases and paraoxonase-1 in blood of workers exposed to non-cholinesterase inhibiting pesticides. Chemico-Biological Interactions 259: 160–167. DOI 10.1016/j.cbi.2016.04.008. [Google Scholar] [CrossRef]
Marek G, Ściskalska M, Grzebieniak Z, Milnerowicz H (2018). Decreases in paraoxonase-1 activities promote a pro-inflammatory effect of lipids peroxidation products in non-smoking and smoking patients with acute pancreatitis. International Journal of Medical Sciences 15: 1619–1630. DOI 10.7150/ijms.27647. [Google Scholar] [CrossRef]
Marsillach J, Costa LG, Furlong CE (2018). Paraoxonase-1 and early-life environmental exposures. Annals of Global Health 82: 100–110. DOI 10.1016/j.aogh.2016.01.009. [Google Scholar] [CrossRef]
Martínez-Valenzuela C, Gómez-Arroyo S (2007). Riesgo genotóxico por exposición a plaguicidas en trabajadores agrícolas. Revista Internacional de Contaminacion Ambiental 23: 185–200. [Google Scholar]
Mitra S, Khurana P, Panmei T, Kshatriya GK (2015). Allele frequencies of PON1 Q192R polymorphism in four populations of India. Environmental Toxicology and Pharmacology 39: 1051–1056. DOI 10.1016/j.etap.2015.03.001. [Google Scholar] [CrossRef]
Mohammadi M, Bababeik M, Barani S, Ebrahimzadeh F, Esmaeili M, Farjaddoost A, Hassanpour P, Kurdistani Z, Mohajerani N, Saberi S, Talebkhan Y, Oghalaie A, Trejaut J (2015). Distribution of cytokine gene single nucleotide polymorphisms among a multi-ethnic Iranian population. Advanced Biomedical Research 4: 160. DOI 10.4103/2277-9175.161809. [Google Scholar] [CrossRef]
Mota A, Hemati-Dinarvand M, Akbar-Taheraghdam A, Reza-Nejabati H, Ahmadi R, Ghasemnejad T, Hasanpour M, Valilo M (2019). Association of Paraoxonse1 (PON1) genotypes with the activity of PON1 in patients with Parkinson’s disease. Acta Neurologica Taiwanica 28: 66–74. [Google Scholar]
Muñoz-Quezada MT, Lucero BA, Iglesias VP, Muñoz MP, Cornejo CA, Achu E, Baumert B, Hanchey A, Concha C, Brito AM, Villalobos M (2016). Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: a review. International Journal of Occupational and Environmental Health 22: 68–79. DOI 10.1080/10773525.2015.1123848. [Google Scholar] [CrossRef]
Murcia AM, Stashenko E (2008). Determinación de plaguicidas organofosforados en vegetales producidos en Colombia. Agro Sur 36: 71–81. DOI 10.4206/agrosur.2008.v36n2-03. [Google Scholar] [CrossRef]
Özaslan MS, Demir Y, Aksoy M, Küfrevioğlu ÖI, Beydemir Ş (2018). Inhibition effects of pesticides on glutathione-S-transferase enzyme activity of Van Lake fish liver. Journal of Biochemical and Molecular Toxicology 32: e22196. DOI 10.1002/jbt.22196. [Google Scholar] [CrossRef]
Pan X, Huang L, Li M, Mo D, Liang Y, Liu Z, Huang Z, Huang L, Liu J, Zhu B (2019). The association between PON1 (Q192R and L55M) gene polymorphisms and risk of cancer: A meta-analysis based on 43 studies. BioMed Research International 2019: 1–14. DOI 10.1155/2019/5897505. [Google Scholar] [CrossRef]
Paul KC, Sinsheimer JS, Cockburn M, Bronstein JM, Bordelon Y, Ritz B (2017). Organophosphate pesticides and PON1 L55M in Parkinson’s disease progression. Environment International 107: 75–81. DOI 10.1016/j.envint.2017.06.018. [Google Scholar] [CrossRef]
Pérez-Herrera N, Polanco-Minaya H, Salazar-Arredondo E, Solís-Heredia MJ, Hernández-Ochoa I, Rojas-García E, Alvarado-Mejía J, Borja-Aburto VH, Quintanilla-Vega B (2008). PON1Q192R genetic polymorphism modifies organophosphorous pesticide effects on semen quality and DNA integrity in agricultural workers from southern Mexico. Toxicology and Applied Pharmacology 230: 261–268. DOI 10.1016/j.taap.2008.02.021. [Google Scholar] [CrossRef]
Rao MN, Marmillot P, Gong M, Palmer DA, Seeff LB, Strader DB, Lakshman MR (2003). Light, but not heavy alcohol drinking, stimulates paraoxonase by upregulating liver mRNA in rats and humans. Metabolism: Clinical and Experimental 52: 1287–1294. DOI 10.1016/S0026-0495(03)00191-4. [Google Scholar] [CrossRef]
Reynoso EC, Torres E, Bettazzi F, Palchetti I (2019). Trends and perspectives in immunosensors for determination of currently-used pesticides: The case of glyphosate, organophosphates, and neonicotinoids. Biosensors 9: 20. DOI 10.3390/bios9010020. [Google Scholar] [CrossRef]
Rojas-García AE, Solís-Heredia MJ, Piña-Guzmán B, Vega L, López-Carrillo L, Quintanilla-Vega B (2005). Genetic polymorphisms and activity of PON1 in a Mexican population. Toxicology and Applied Pharmacology 205: 282–289. DOI 10.1016/j.taap.2004.10.015. [Google Scholar] [CrossRef]
Sirivarasai J, Kaojarern S, Yoovathaworn K, Sura T (2007). Paraoxonase (PON1) polymorphism and activity as the determinants of sensitivity to organophosphates in human subjects. Chemico-Biological Interactions 168: 184–192. DOI 10.1016/j.cbi.2007.04.006. [Google Scholar] [CrossRef]
Sever B, Türkeş C, Altıntop MD, Demir Y, Beydemir Ş (2020). Thiazolyl-pyrazoline derivatives: In vitro and in silico evaluation as potential acetylcholinesterase and carbonic anhydrase inhibitors. International Journal of Biological Macromolecules 163: 1970–1988. DOI 10.1016/j.ijbiomac.2020.09.043. [Google Scholar] [CrossRef]
Suratman S, Edwards JW, Babina K (2015). Organophosphate pesticides exposure among farmworkers: Pathways and risk of adverse health effects. Reviews on Environmental Health 30: 65–79. DOI 10.1515/reveh-2014-0072. [Google Scholar] [CrossRef]
Torres-Sánchez L, Gamboa R, Bassol-Mayagoitia S, Huesca-Gómez C, Nava MP, Vázquez-Potisek JI, Yáñez-Estrada L, Mejía-Saucedo R, Blanco-Muñoz J (2019). Para-occupational exposure to pesticides, PON1 polymorphisms and hypothyroxinemia during the first half of pregnancy in women living in a Mexican floricultural area. Environmental Health: A Global Access Science Source 18: 1–10. [Google Scholar]
Xotlanihua-Gervacio MdelC, Herrera-Moreno JF, Medina-Díaz IM, Bernal-Hernández YY, Rothenberg SJ, Barrón-Vivanco BS, Rojas-García AE (2019). Relationship between internal and external factors and the activity of PON1. Environmental Science and Pollution Research 26: 24946–24957. DOI 10.1007/s11356-019-05696-9. [Google Scholar] [CrossRef]
Zúñiga-Venegas L, Aquea G, Taborda M, Bernal G, Pancetti F (2015). Determination of the genotype and phenotype of serum paraoxonase 1 (PON1) status in a group of agricultural and nonagricultural workers in the Coquimbo Region. Chile Journal of Toxicology and Environmental Health. Part A 78: 357–368. DOI 10.1080/15287394.2014.982843. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |