DOI:10.32604/biocell.2021.015090

| Biocell DOI:10.32604/biocell.2021.015090 |  |
| Article |
Thymoquinone as a potential therapeutic for Alzheimer’s disease in transgenic Drosophila melanogaster model
1Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Kattankulathur, 603203, India
2Department of Physics and Nanotechnology, School of Bioengineering, SRM Institute of Science and Technology, Kattankulathur, 603203, India
*Address correspondence to: G. Devanand Venkatsubbu, devanang@srmist.edu.in; Sahabudeen Sheik Mohideen, sahabuds@srmist.edu.in
Received: 20 November 2020; Accepted: 18 February 2021
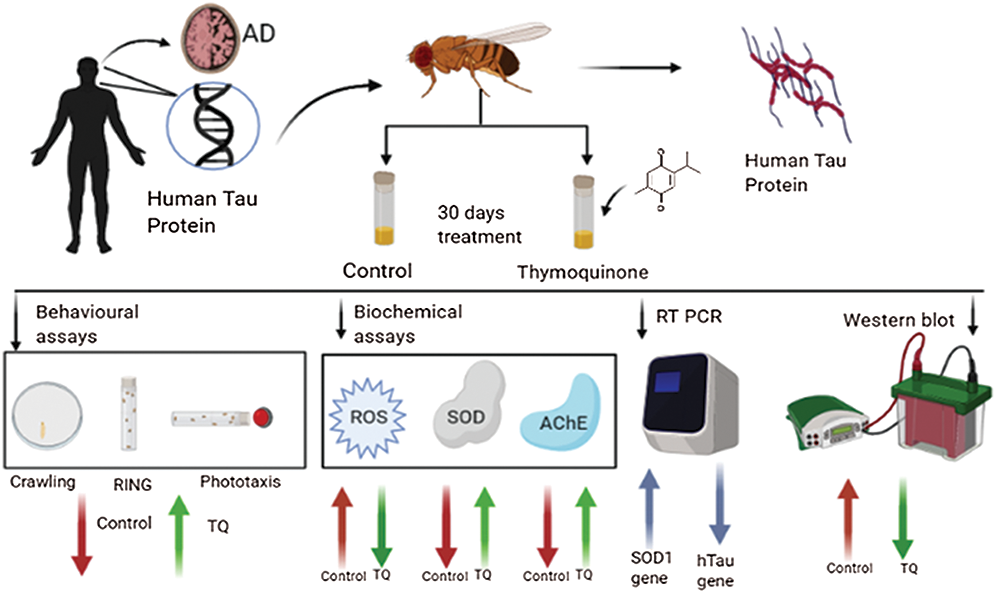
Abstract: Alzheimer’s disease (AD) is one of the most common forms of dementia. Cognitive dysfunction and memory loss are the two main clinical symptoms of AD. Drosophila melanogaster models of AD, which are based on overexpression of human amyloid β (Aβ) or human tau (hTau) protein, have been used to study the mechanism underlying AD and to screen potential therapeutic compounds. Drugs that are currently available for AD provide only symptomatic relief. Huge unmet medical needs exists to slow, stop, or reverse the progression of AD. Thymoquinone (TQ) is an active ingredient isolated from Nigella sativa (NS) and possesses various pharmacological activities, and it is also a potential neuropharmacological agent. The current study was performed to investigate the effect of dietary administration of TQ at concentrations of 12.5 μM and 25 μM for 15 and 30 days on biochemical and behavioral parameters, gene, and protein expression of hTau, using Drosophila models of AD. Transgenic Drosophila models exhibiting pan-neuronal and eye-specific expression of hTau were generated using the GAL4/UAS system. Treatment with TQ at both concentrations resulted in a significant increase in behavioral activity, a significant reduction in the amount of reactive oxygen species (ROS), and restoration of depleted superoxide dismutase (SOD) and acetylcholine esterase (AChE) activities. A significant decrease in gene and protein expression of hTau was also observed at the molecular level for both concentrations of TQ. Therefore, TQ has the potential to be investigated as a potential therapeutic phytochemical for the treatment of AD in future studies.
Keywords: hTau; GAL4/UAS system; Nigella sativa
Graphical Abstract
Abbreviations
| AChE: | Acetyl choline esterase |
| AChI: | Acetylthiocholine iodide |
| AD: | Alzheimer’s disease |
| APP: | Amyloid precursor protein |
| Aβ: | Amyloid beta |
| BACE: | β-Secretase |
| DCHF-DA: | 2’, 7’-dichlorofluorescein diacetate |
| DTNB: | 5,5’-dithiobis-2-nitrobenzoic acid |
| elav: | Embryonic lethal abnormal vision |
| FDA: | Food and Drug Administration |
| GMR: | Glass multiple reporter |
| MAPT: | Microtubule-associated Tau |
| NADH: | Nicotinamide adenine dinucleotide |
| NBT: | Nitroblue tetrazolium |
| NFT: | Neurofibrillary tangles |
| NS: | Nigella sativa |
| OPS: | Orthophosphoric acid |
| PD: | Parkinson’s disease |
| PMS: | Phenazonium methosulphate |
| PUFA: | Polyunsaturated fatty acids |
| ROS: | Reactive oxygen species |
| TQ: | Thymoquinone |
| UAS: | Upstream activating sequence |
| α-syn: | α synuclein |
Alzheimer’s disease (AD) is a chronic-non curative irreversible neurodegenerative disease caused due to damage to neurons and synapses in the cerebral cortex and brain stem. It is one of the fatal disease forms related to dementia associated with aging and cannot be cured or prevented using existing therapies. The five drugs that have been approved by the Food and Drug Administration (FDA) to date only help to improve cognitive functions and provide temporary symptomatic relief (Kumar et al., 2015). Over the past decade, the number of deaths from AD has increased to 146.2%, and it is estimated that by 2050, the total prevalence of the disease will surpass 13.8 million (Alzheimer’s Association, 2020). Neurodegenerative diseases consist of several pathological processes that lead to progressive neuronal cell death in specific regions of the brain (Fu et al., 2018). The major causative factors for neuronal death are the aggregation of proteins such as microtubule-associated tau (MAPT) and β-amyloid (Aβ) [Alzheimer’s disease], Lewy bodies [Dementia], α-synuclein (α-syn) [Parkinson’s disease (PD)], Huntingtin [Huntington’s disease], and TDP-43 [frontotemporal lobar degeneration and amyotrophic lateral sclerosis] (Fu et al., 2018; Dugger and Dickson, 2017). AD and PD are considered the most widespread neurodegenerative diseases (Erkkinen et al., 2018; Mayeux and Stern, 2012). In the Aβ pathology of AD, there is an abnormal production of Aβ protein, which results in the excessive accumulation of Aβ plaques extracellularly. On the other hand, tau pathology is characterized mainly by the hyperphosphorylation of tau protein and the subsequent destabilization of microtubules leading to abnormal tau aggregation and formation of intra-neuronal tangles. Currently, therapeutic hypotheses mainly focus on these two proteins, neurotransmitters and neuroinflammatory molecules (Sanabria-Castro et al., 2017; Buée et al., 2000).
The hypothesis of the amyloid cascade has been extensively researched and has led to the production of newer drugs that target neuronal plaques. However, the failure of these drugs to elicit any significant beneficial effects has opened research prospects to explore non-amyloid targeting strategies (Iqbal et al., 2010). The intense exploration of non-amyloid targets revealed numerous research openings based on the hypothesis regarding aggregation of hyperphosphorylated tau proteins in the brain. However, Aβ proteins are involved in various pathways and have many physiological functions, and it is likely that targeting Aβ protein therapeutically may also cause detrimental effects. It is also evident from various in-vitro experiments and neuronal cell line assays that abnormality and alterations in Aβ protein directly induce tau pathologies (Wang et al., 2003; Zheng et al., 2002; Götz et al., 2001). The Aβ oligomers, especially Aβ-42, induce hyperphosphorylation of tau protein, leading to the formation of neurofibrillary tangles (NFTs), and this concludes the major event in the onset of molecular AD pathology. Existing therapeutic options mainly provide symptomatic relief by targeting the neurotransmitters that are secondary to the pathology of AD. As AD involves complex pathology, it is essential to consider several molecular divergent factors apart from the events that result in the production of toxic plaques and NFTs (Hanger et al., 2009). Therefore, drugs and therapeutic agents that can target the secondary factors involved in the pathogenesis of AD and inhibit the hyperphosphorylation of tau are being considered as promising candidates for the treatment of AD (Kawamata et al., 2008).
Tau is a phosphorylated, soluble microtubule-associated protein encoded by 17q21 of the human chromosome having a native unfolded structure. It contains potential phosphorylation sites for binding serine (S), threonine (T), and tyrosine (Y) residues. Most of the phosphorylated residues on tau are seen in the proline-rich domain, adjoining the microtubule-binding domain. Hyperphosphorylation of the tau protein or a mutation caused by the dysregulation of the gene encoding for the tau protein leads to a disruption in axonal transport, destabilization of the neuronal structure, and impairments in neurite outgrowth, resulting in a pathological condition known as Tauopathy. Recent evidence suggests that targeting tau phosphorylation via inhibition of the protein kinases could represent a valid therapeutic approach to reduce tau aggregation and the associated neuronal death (Churcher, 2006).
Apart from problems such as lethality and toxicity, several factors such as high costs and prolonged time consumption in the phase of drug development delay the discovery of synthetic therapeutics for the treatment of neurological disorders. Hence, the numerous potential benefits and safety of phytochemicals and herbal medicines enable their investigation as an alternative drug treatment for disorders of the nervous system. The trend seen in the last five years indicates the increased focus of researchers to investigate the ameliorating effects of various natural compounds and phytoconstituents for the treatment of AD. These traditional medicinal herbs and their constituents are immensely proven to possess antioxidant, anti-inflammatory, and neuroprotective properties (Duraiswamy et al., 2019). The use of natural compounds also has other advantages, such as the reduction in side effects and the ability to target multiple pathological factors, which is particularly useful for the treatment or prevention of neurodegenerative diseases (Pohl and Kong Thoo Lin, 2018; Bagli et al., 2016). Since polyphenolic compounds such as curcumin, resveratrol, epigallocatechin gallate, and capsaicin possess antioxidant properties, they are currently being investigated for their neuroprotective effects (Habtemariam, 2019; Zhang et al., 2017).
Drosophila melanogaster is a powerful model organism in the field of developmental biology and genetics. The insect is approximately 3 mm in length and weighs 0.5 mg. Drosophila is commonly used as an in-vivo model organism to study neurodegenerative diseases due to the feasibility to perform many types of genetic manipulations. The genes of Drosophila are approximately 60% homologous when compared with human genes, and they have been widely studied to gain a better understanding of the disease processes in human beings. The flies can be grown and maintained with minimal resources such as a cornmeal agar and occupy very little space. Compared to other in vivo models, drug screening and different assays can be performed at a faster rate in Drosophila, since its reproductive cycle is 12–14 days at 25°C (Wangler et al., 2015). Transgenic flies have been used in several studies to express the human amyloid β-precursor protein (APP) and β-secretase (BACE) to investigate the pathways involved in Aβ-induced neurodegeneration. Overexpression of BACE and APP caused the neuronal loss (Muhammad et al., 2008). Age-dependent neurodegeneration in neurons caused by the overexpression of wild-type or mutant hTau is characterized by vacuole formation and nuclear fragmentation. Currently used fly models of human tauopathy include those that overexpress tau mutants (R406W & V337M) or the wild-type hTau (Nishimura et al., 2004; Wittmann et al., 2001; Jackson et al., 2002). The expression of tau protein in the eyes induces retinal degradation, which is characterized by the reduction in retinal thickness and degradation of neuropils in the medulla (Jackson et al., 2002; Nishimura et al., 2004). Transgenic flies expressing tau have a reduced lifespan, and the pathological phenotypes are severe than the wild type due to the expression of mutant tau (Wittmann et al., 2001). Moreover, expression of hTau in the mushroom body neurons can result in impaired memory and olfactory learning prior to the onset of neurodegeneration (Mershin et al., 2004). The GAL4/UAS system is used in Drosophila to drive the expression of a gene of interest. Thus, over-expression or misexpression of various genes at different sites in the body can be studied using tissue-specific promoters. The fly is an apt model to investigate the tau-associated AD pathology due to similarities with humans in tau homology and signaling pathways involved in neurotoxicity (Heidary and Fortini, 2001). It also exhibits the tau-induced alterations in both behavioral and cognitive abilities, thus making it a powerful tool in tau pathology. Drosophila is a perfect, proven tool for therapeutic screening, drug testing, and deciphering the biological mechanisms of AD. Drosophila can also be used as an ideal model organism to test natural products for the treatment of AD (Chen et al., 2007; Prüßing et al., 2013). Nigella sativa (NS) (black cumin) is the seed of a flowering plant, belonging to the Ranunculaceae family, commonly seen in the Middle East, Southwest Asia, and Africa. NS and its derivatives are commonly used as spices and preservatives in the food industry. It is one of the most valued nutrient-rich medicinal plants possessing enormous therapeutic properties such as anti-inflammatory, anti-diabetic, anti-hypertensive, and anti-tumor activity (Ramadan, 2007). Thymoquinone (TQ) is one of the major bioactive constituents present in the seeds of NS and possesses inherent antioxidant and other curative properties, particularly related to cell damage. NS oil (NSO) contains high amounts of fatty acids such as polyunsaturated fatty acids (PUFA), phytosterol, TQ (30% in volatile NSO), carvacrol, terpinol, and t-anethole. It has also been reported to possess the ability to reduce the levels of intracellular reactive oxygen species (ROS) in neuronal cells (Akram Khan and Afzal, 2016).
The neuroprotective effect of TQ is mediated via mechanisms such as ROS scavenging, inhibition of lipid peroxidation, downregulation of proinflammatory cytokines, and inhibition of apoptosis (Isaev et al., 2020). It has been reported that when cultured rat primary neuronal cells were subjected to Aβ1–42-induced neurotoxicity and subsequently treated with TQ, it elicited protective effects, reduced Aβ-induced toxicity, and inhibited synaptic vesicle recycling (Alhibshi et al., 2019). TQ has been reported to exhibit the ability to restore the learning function in a rat model of AD. While Aβ proteins elicited neurotoxic effects, treatment with TQ decreased the formation of plaques in the hippocampus, increased neuronal survivability, and protected the pyramidal cells (Poorgholam et al., 2018). The ameliorative and inhibitory effects of TQ on Parkinson’s disease have also been recently proven, thus paving the way for a wider scope of research into the neuroprotective role of nutraceuticals (Samarghndian and Farkhondeh, 2018). Various in-vitro studies using cell lines and in-vivo studies on rats have been reported for the pathological improvements in AD following treatment with TQ. TQ elicited beneficial effects via mechanisms such as ROS scavenging, decreasing the levels of Aβ, inhibition of acetylcholine esterase (AChE) activity, prevention of neurotoxicity memory enhancement, preventing the depletion of hippocampal neuronal cells, and behavioral changes, respectively (Cascella et al., 2018). Even though TQ has been recently reported to possess neuroprotective and curative effects, the influence and therapeutic potential of this phytochemical have so far not been investigated in a tau model of AD. Therefore, the current study was performed to investigate the efficacy of TQ in reducing the levels of aggregated tau protein using behavioral and biochemical assays, gene, and protein expression studies in hTau-induced Drosophila models of AD.
Sodium phosphate monobasic, sodium phosphate dibasic, ethanol, and agar-agar type I were obtained from HiMedia, Mumbai. D-glucose, 5,5’-dithiobis-2-nitrobenzoic acid (DTNB), copper sulfate, sodium carbonate, sodium potassium tartrate, potassium chloride, sodium hydroxide, acetylthiocholine iodide, potassium chloride, methanol, yeast extract powder, chloroform, nicotinamide adenine dinucleotide (NADH), nitro blue tetrazolium (NBT), phenazonium methosulphate (PMS), glacial acetic acid, and bovine serum albumin were purchased from SRL Chemicals, Mumbai. Propionic acid was procured from Merck Life Sciences, Mumbai, while methyl para-hydroxy benzoate was obtained from Rankem, New Delhi. 2’,7’-Dichlorofluorescein diacetate (DCHF-DA) and thymoquinone were obtained from Sigma Aldrich, China. Orthophosphoric acid was purchased from Thermo Fischer Scientific, Mumbai. Acetylthiocholine iodide (AChI) and antibodies for hTau and β-actin were obtained from DSHB. All the chemicals and reagents utilized were of standard analytical grade.
Transgenic strains used in this study, such as elav-GAL4, UAS R406W, and GMR-GAL4, were procured from Bloomington (IU, Indiana). The flies were reared in a 12-hour dark-light cycle at 25°C and 60% humidity on a standard cornmeal agar diet (consisting of corn flour, sugar, agar-agar, D-glucose, and yeast). Anti-fungal chemicals such as TEGO (methyl para-hydroxy benzoate dissolved in ethanol), propionic acid, and orthophosphoric acid (OPS) were added to the food medium after it was autoclaved at 121°C for 15 min.
The experimental group consisted of control, 12.5 μM, and 25 μM of TQ/mL of food. A stock concentration of TQ (25 mM) was prepared in 0.1% DMSO and diluted accordingly to obtain the working concentrations. 0.1% DMSO was used as the control vehicle for elav-GAL4 and untreated AD flies. After 24 h, 10 male flies and 20 female flies were transferred to the vials. After 48 h, the flies were discarded, and the F1 males were used for performing different experiments.
The larvae obtained by the mating of elav-GAL4 virgin females and UAS R406W males in control and TQ-containing food at concentrations of 12.5 μM and 25 μM were allowed to grow till the third instar stage, following which they were used for performing the crawling assay based on a previously described method. Briefly, nine third instar larvae from control and treatment groups were collected, washed with 1X PBS to remove any food traces, and transferred onto a glass Petri plate coated with 2% agarose. The larvae were allowed to crawl thrice on the agar surface placed over a graph sheet for one minute, and a video was recorded. The number of grid lines crossed in one minute was counted, and the distance covered in 1 min per treatment group was obtained by calculating the mean of the distance covered by 9 larvae (Sundararajan et al., 2019; Nichols et al., 2012).
elav- GAL4 virgin females were crossed with UAS- hTau R406W male flies in a standard cornmeal agar medium with TQ, and the agar medium devoid of TQ served as the control group. Twenty F1 male flies (3 replicates per group) having a pan-neuronal expression of hTau were collected and maintained in TQ containing food for 15 days and 30 days, respectively, for performing the climbing assay. The flies were transferred into 10-cm-long vials that contained a mark at 3 cm from the bottom, and the other end of the vial was sealed using cotton. The vials were tapped three times, and flies were allowed to climb for 10 s. The number of flies that climbed beyond the 3 cm mark within 10 s was noted. Triplicates were done for each group. The mean number of flies that climbed beyond the marked distance was used to express the result in the form of percentage.
The experimental and control groups consisted of GMR- GAL4 flies crossed with UAS hTau R406W flies in standard cornmeal agar medium, with and without TQ, respectively. The F1 flies (GMR; hTau R406W) were assessed for their phototaxis activity. The F1 flies were exposed to TQ-containing food for 15 days, following which they were transferred into a 30-cm-long vial. The vials were tapped three times, and a light source was placed at the opposite end of the 30-cm-vial to attract the flies (Vang et al., 2012). The percentage of flies that moved toward the light was calculated in triplicates per treatment group. The attraction of flies toward three different colors, namely blue, green, and red, was analyzed, and the mean value was used to express the result in the form of percentage.
Quantification of ROS generation
The amount of ROS produced in the flies was estimated using DCHF-DA. Twenty fly heads were dissected from elav; R406W flies exposed to TQ-containing-food for 15 days and 30 days, respectively, and homogenized separately in 1 mL of 20 mM Tris buffer (pH 7). This was followed by centrifugation at 5000 rpm for 10 min at 4°C. The supernatant was incubated with DCHF-DA (10 mM) in the dark for 1 h at room temperature. A microplate fluorometer was used to measure the intensity of the product formed as a result of the reaction (488 nm excitation, 525 nm emission).
Quantification of acetylcholine esterase (AChE) activity
AChE activity in male fly heads (3 replicates and 20 flies per treatment group) after exposure to TQ-containing-food for 15 days and 30 days, respectively, was estimated using Ellman’s method. The fly heads were homogenized in 0.5 mL of 0.1 M sodium phosphate buffer containing 150 mM KCl (pH 7.4) and centrifuged at 5000 rpm for 10 min at 4°C. From the supernatant, 0.2 mL was aspirated and added to a mixture containing 0.65 mL of 100 mM sodium phosphate buffer (7.4 pH). This was followed by the addition of 0.2 mL of 10 mM DTNB and 0.02 mL of 75 mM acetylthiocholine iodide (AChI). The change in absorbance per minute for 10 min was read at 412 nm, and the enzyme activity was expressed per mg of protein.
Quantification of SOD activity
SOD activity was estimated by the homogenization of 20 fly heads (500 µL of 100 mM PB buffer in 150 mM KCl (pH 7.5) followed by centrifugation at 5000 rpm for 10 min at 4°C. The reaction was performed at room temperature by adding 200 μL of 780 μM NADH to the reaction mixture (1000 μL of 0.052 M sodium pyrophosphate buffer (pH 8), 100 μL of 186 μM PMS, 300 μL of NBT, 200 μL of water, and 200 μL of the supernatant from the fly head homogenate). The reaction was stopped after 1 min at room temperature by adding 1 mL of acetic acid. The intensity of the product formed was then measured at 560 nm. One unit of enzyme activity is denoted as the activity of the enzyme required to inhibit chromogen formation by 50 percentage. The final result was expressed as Units/mg of protein.
Quantitative Real Time PCR (qRT-PCR) studies for gene expression analysis
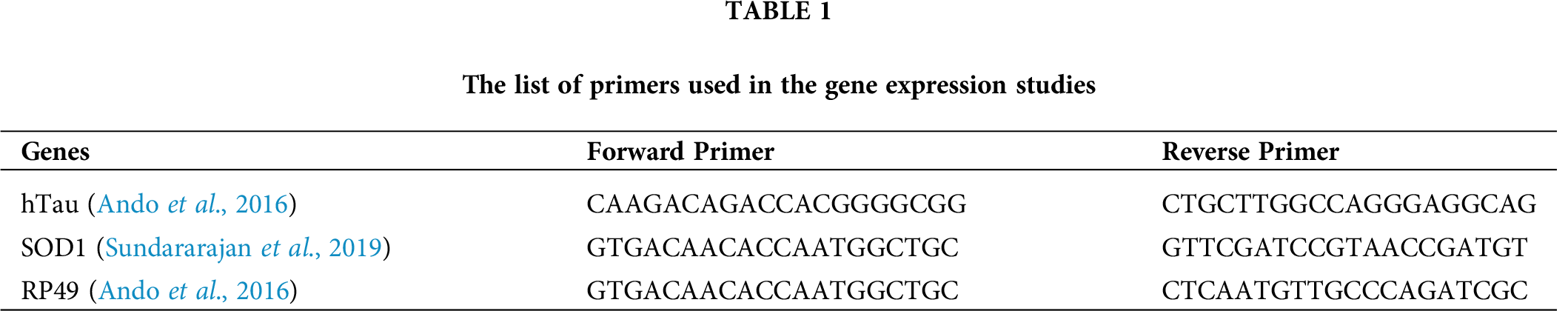
Twenty transgenic fly heads (3 replicates per treatment group) dissected from different treatment groups were homogenized in TRIZOL reagent (Invitrogen) to isolate RNA. The isolated RNA was quantified and checked for purity using Nanodrop lite (Thermo Fisher). By using 500 ng of the isolated RNA, cDNA was synthesized (iScript cDNA synthesis kit, Bio-Rad) under the following conditions: 42°C for 1 h and then 85°C for 5 min. The comparative CT (ΔΔCT) method was used to analyze the relative mRNA expression of hTau and SOD on a QuantStudio 5 Real-Time PCR system (Applied Biosystems), and RP49 was used as the internal control. The reaction mixture included 1 μL of the gene-specific forward and reverse primers, 2 μL of cDNA, 6 μL of nuclease-free water, and 10 μL of 2 X SYBR Green master mixes (TB Green® Premix Ex Taq™ II). Each sample was run in duplicates. The following reaction conditions were used for all the genes: 95°C for 5 min, 40 cycles of 95°C for 15 s, and 60°C for 35 s. The melt curve stage consisted of 95°C for 15 s, 60°C for 1 min, and 95°C for 1 s. The primers used in the study are mentioned in Tab. 1.
Western blotting was performed to determine the expression of hTau protein following dietary administration of TQ for one month. Twenty fly heads from each treatment group were dissected and homogenized in sample lysis buffer (containing 10 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, and 1X PIC) to extract the TS soluble fraction (Sheik Mohideen et al., 2015). The samples were stored in −20°C in 2X Laemmli buffer (containing 2 M Tris, 10% SDS, 1% glycerol, and 2-mercaptoethanol). The samples were run on SDS-PAGE gel at 100 V for 60 min, then soaked in transfer buffer for 5 min and finally transferred onto a PVDF membrane. The transfer was carried out at 100 mA for 240 min. The membrane was then blocked with 10% skim milk for 1 h, followed by overnight incubation with the primary antibody. The membrane was then washed three times, each for 15 min, followed by incubation with the secondary antibody for 60 min. The bands were visualized using ClarityTM Western ECL substrate (BioRad), and the image was captured using Multi Imaging System (Cell Biosciences, Santa Clara, CA).
The difference in estimated parameters between control and treatment groups was analyzed using one-way ANOVA followed by Bonferroni’s post-test, using GraphPad Prism 5 (San Diego, CA). The data were expressed as mean ± SEM, and all parameters were analyzed at 95% confidence intervals. P < 0.05 was considered statistically significant.
The crawling activity of 3rd instar larvae is considered one of the behavioral activities, and the distance covered by larvae is generally used as a parameter to assess the effect of drug treatment on the locomotor activity. Fig. 1a shows the distance covered in one minute by the 3rd instar larvae. It was observed that pan-neuronal expression of hTau reduced the locomotor activity when compared with the elav-GAL4 strain. The distance covered by control larvae with hTau expression in one minute was 31.78 mm, while that of elav-GAL4 was 46.17 mm. Moreover, TQ treatment at both concentrations elicited a significant increase (P < 0.001) in locomotor activity. The distance covered by larvae with hTau expression, treated with TQ at concentrations of 12.5 and 25 μM, was 54.05 mm and 77.6 mm, respectively.
The ability of flies to move toward a light source can be affected due to damaged photoreceptors as a result of hTau expression in the eyes. The percentage of flies that moved toward various light sources was analyzed, and it was observed that there was a significant reduction (P < 0.001) in the ability of flies to move toward the light source. The percentage of flies that moved towards different light sources, white, red, blue, and green, after one-month dietary exposure to TQ, is shown in Fig. 1b. The flies were attracted more toward the green color and were not attracted toward the red color. In assays using white, green, and blue colors, flies treated with TQ at concentrations of 12.5 and 25 µM exhibited a significant increase (P < 0.001) in their ability to move toward the light source.
Rapid Iterative Negative Geotaxis (RING) assay
The RING assay is a behavioral assay and is commonly used to assess the climbing activity of flies. The climbing activity of control and AD flies with a pan-neuronal expression of hTau was examined on day 15 and day 30 following dietary administration of TQ, and the results revealed a significant increase in climbing activity for both durations of the treatment. The percentage of flies above the 3 cm mark decreased from 98.25% to 51.19% (P < 0.001) on day 15 and 94.2% to 31.03% (P < 0.001) on day 30, respectively, for elav GAL4 and elav; R406W flies. Thus, pan-neuronal expression of hTau resulted in a significant reduction in the climbing activity, and TQ treatment at both concentrations helped in restoring the activity. The percentage of flies that climbed beyond the 3 cm mark following dietary administration of TQ at a concentration of 25 µM was 89.39 on day 15 and 59.21 on day 30 (P < 0.001). Figs. 1c and 1d show the comparison of the percentage of elav-control, Tau R406W control, and TQ-treated flies that climbed beyond the 3 cm mark.
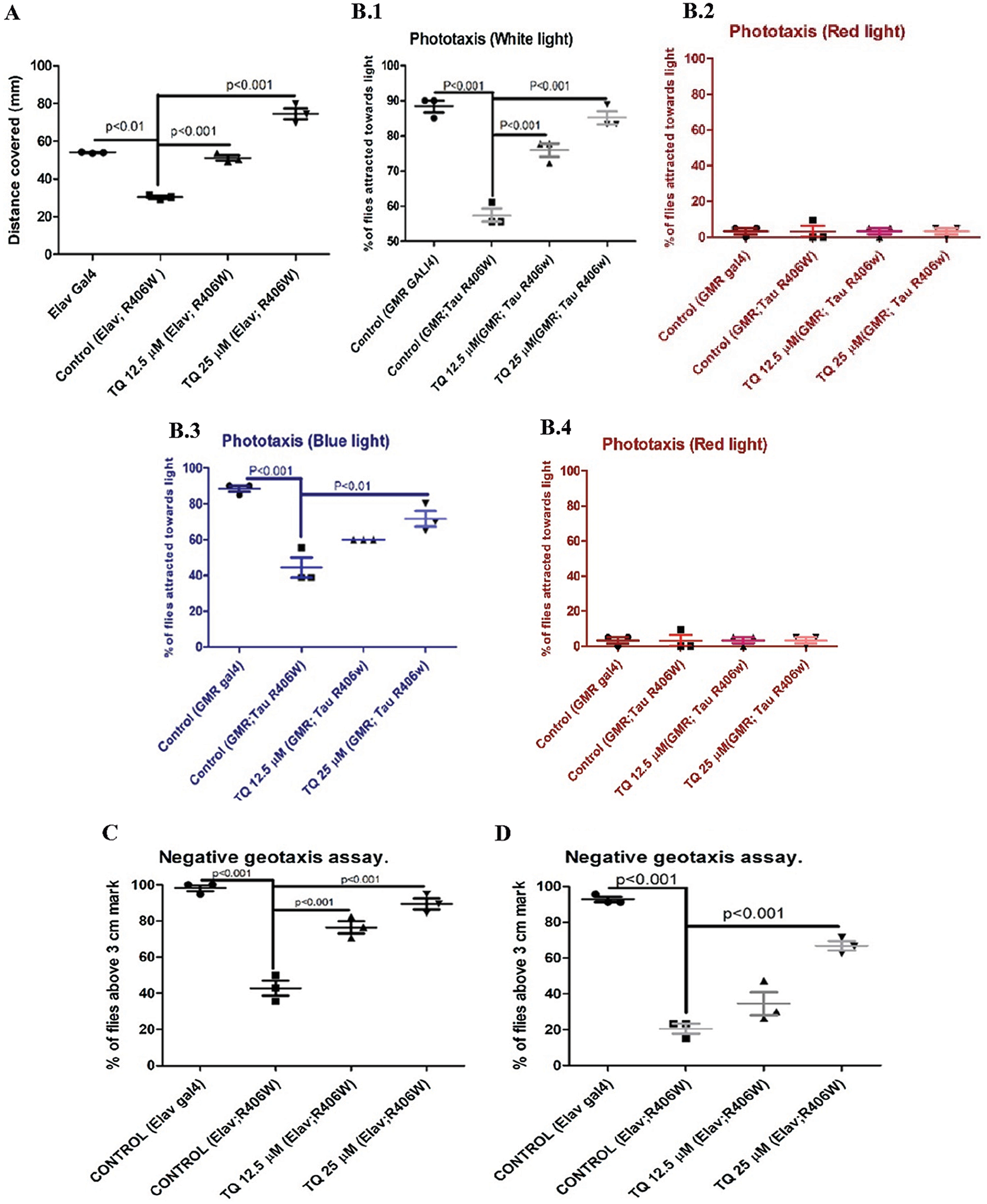
Figure 1: (a) Crawling assay. (b.1–4) Phototaxis assay (15 days). (c) Geotaxis assay (15 days). (d) Geotaxis assay (30 days). Effect of TQ on various behavioral activities (a) Distance covered by 3rd instar larvae, (b) Percentage of flies attracted towards lights of different colors, i.e., white, green, blue and red, respectively, (c) Comparison of percentage of flies above the 3 cm height on 15 days, (d) Comparison of percentage of flies above the 3 cm height on 30 days (N = 3, 20 flies per group). All comparisons were done for larvae or flies fed on different treatments, i.e., TQ 12.5 μM and TQ 25μM with respect to elav or GMR GAL4 control and hTau R406W control.
Effect of TQ on ROS generation
The amount of ROS was elevated in flies with hTau expression than elav control flies reared on standard cornmeal food. The ROS generation was 411.25 and 177.57 relative fluorescence unit (RFU) on day 15 and day 30 for tauopathy flies, which was highly significant (P < 0.001) compared to normal flies that had 48.3 and 198.72 RFU on day 15 and day 30, respectively. There was a significant reduction (P < 0.01) in ROS levels in flies that fed on both concentrations of TQ- containing food when compared with control. Figs. 2a and 2b show the ameliorative effect of TQ on ROS generation in flies on day 15 and day 30.
Effect of TQ on antioxidant enzyme activity
The activity of the antioxidant enzyme SOD was reduced in tauopathy flies compared to control flies. The reduction in the enzyme activity was 32.15% on day 15 and 33.2% on day 30 (P < 0.01), respectively. Flies that were exposed to TQ at a concentration of 25 µM exhibited a significant increase (P < 0.01) in SOD activity compared to tauopathy flies. A 27.16% increase on day 15 and a 45.05% increase on day 30 in SOD activity were observed in flies exposed to TQ at a concentration of 25 µM. The restoration of SOD activity shows the antioxidant effect of TQ in a tauopathy model. Figs. 2c and 2d show the SOD activity in flies exposed to TQ. SOD activity was analyzed based on the inhibition of reduction in NBT by superoxide anions. 1 SOD unit is equivalent to 50% inhibition of NBT reduction per min. Therefore, TQ treatment for two weeks and one month helped in elevating the level of SOD similar to control values.
AChE activity was estimated to gain more insight into the neuroprotective effect of TQ. There was a significant reduction in and 59.38% in AChE activity in tauopathy flies on day 15 (53.39% decrease, P < 0.05) and day 30 (59.38% decrease, P < 0.01), when compared with control flies. The neuroprotective effect of 25 µM TQ was significantly evident in AChE activity since the enzyme activity was restored to 75.38% on day 15 and 92.23% on day 30 (P < 0.01). Figs. 2e and 2f show the activity of AChE in response to dietary administration of TQ for a period of two weeks and one month. The TQ- treated flies exhibited an increase in AChE activity compared to the elav; hTau control.
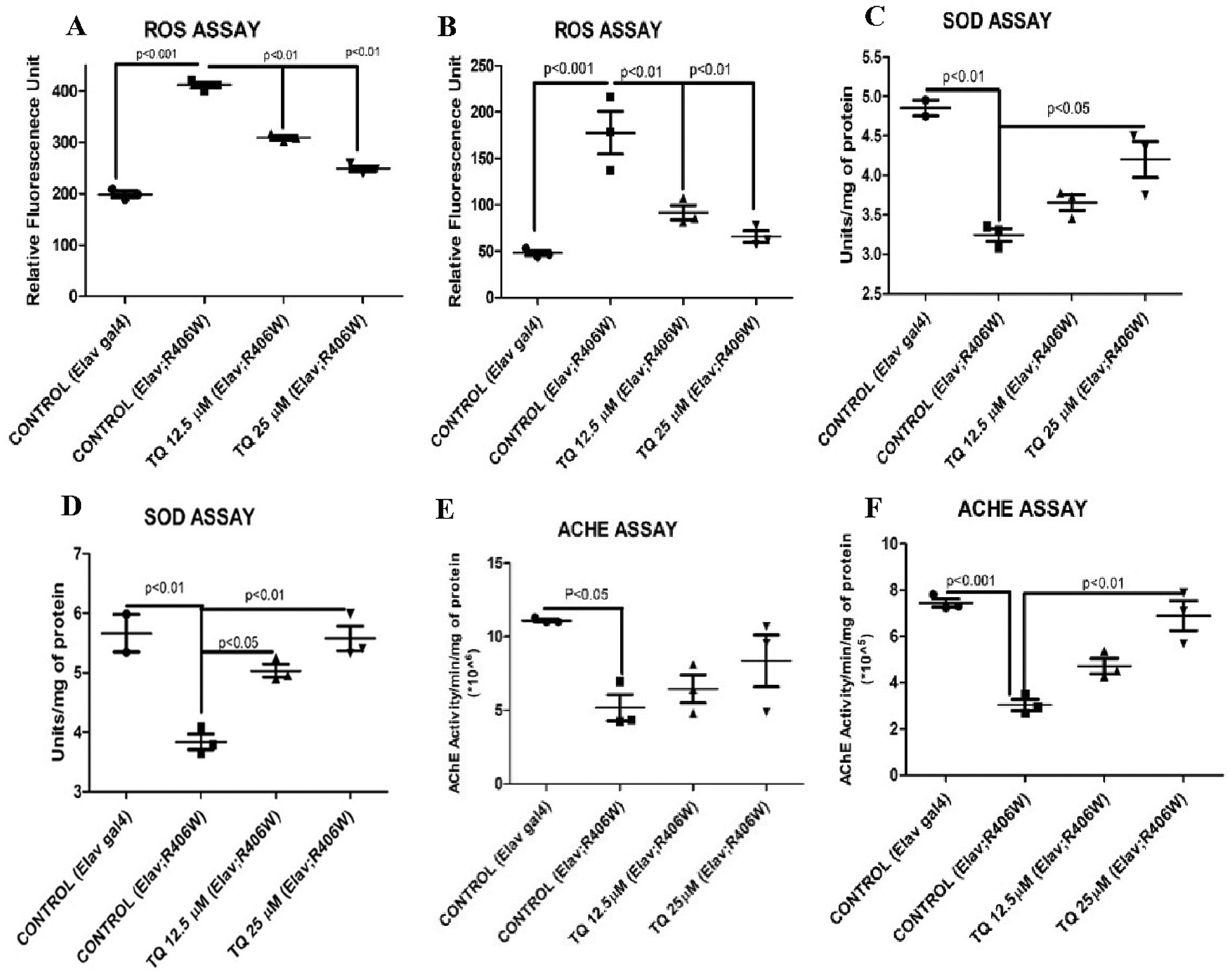
Figure 2: (a) ROS assay (15 days). (b) ROS assay (30 days). (c) SOD assay (15 days). (d) SOD assay (30 days). (e) ACHE assay (15 days). (f) ACHE assay (30 days). Biochemical assays for the evaluation of TQ activity, (a), (b), (c), (d), (e), (f) shows the estimation of ROS, SOD level, and AChE activity on 15th and 30th day respectively in control, tauopathy flies and flies fed on TQ supplemented food. Mean ± SEM values are depicted (N = 3, 20 fly head per group). Statistical significance was compared by ANOVA, followed by Bonferroni’s test, P < 0.05 was set as significant.
The mRNA expression of hTau and SOD1 genes was studied using qRT-PCR. The gene expression of hTau showed a significant decrease and following treatment with TQ at concentrations of 12.5 μM (68% decrease, P < 0.01) and 25 μM (77.12% decrease, P < 0.001) concentrations, with respect to tauopathy flies (Fig. 3a). Dietary administration of TQ at a concentration of 25µM helped in significantly elevating (P < 0.01) the SOD gene expression, which was decreased in GMR; hTau flies. The SOD1 gene expression in the heads of tauopathy flies was reduced by 27% compared to GMR control, and treatment with TQ at a concentration of 25 µM significantly increased the expression of SOD1 (P < 0.01) compared to tauopathy flies (Fig. 3).
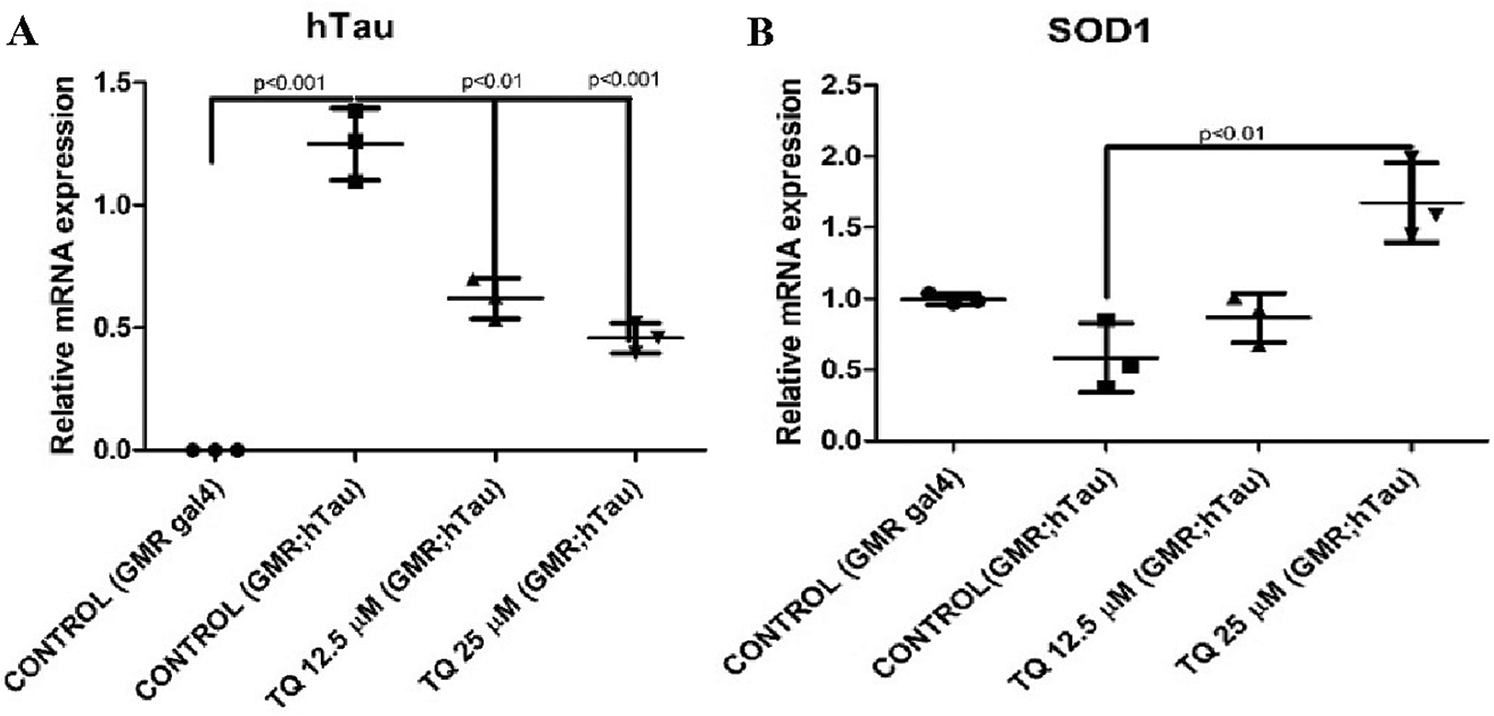
Figure 3: (a) Relative mRNA expression-hTau. (b) Relative mRNA expression-SOD1. The relative mRNA expression of (a) hTau and (b) SOD1 gene in the heads of male GMR; hTau R406W flies newly born in and exposed to dietary TQ for one month. The gene expressions were normalized using RP49 and presented compared to control values (N = 5, 20 fly heads per group). Statistical significance was compared by ANOVA, followed by Bonferroni’s test, P < 0.05 was set as significant.
Western blotting was performed using β actin (42 kDa) as the housekeeping protein and Tau5 (55 kDa) as the target protein. There was a concentration-dependent decrease in hTau protein expression compared to the GMR; hTau control flies. GMR-GAL4 flies were used as a negative control. Thus, dietary administration of TQ for one month helped in reducing the amount of soluble hTau protein in GMR; R406W hTau fly heads (Fig. 4).
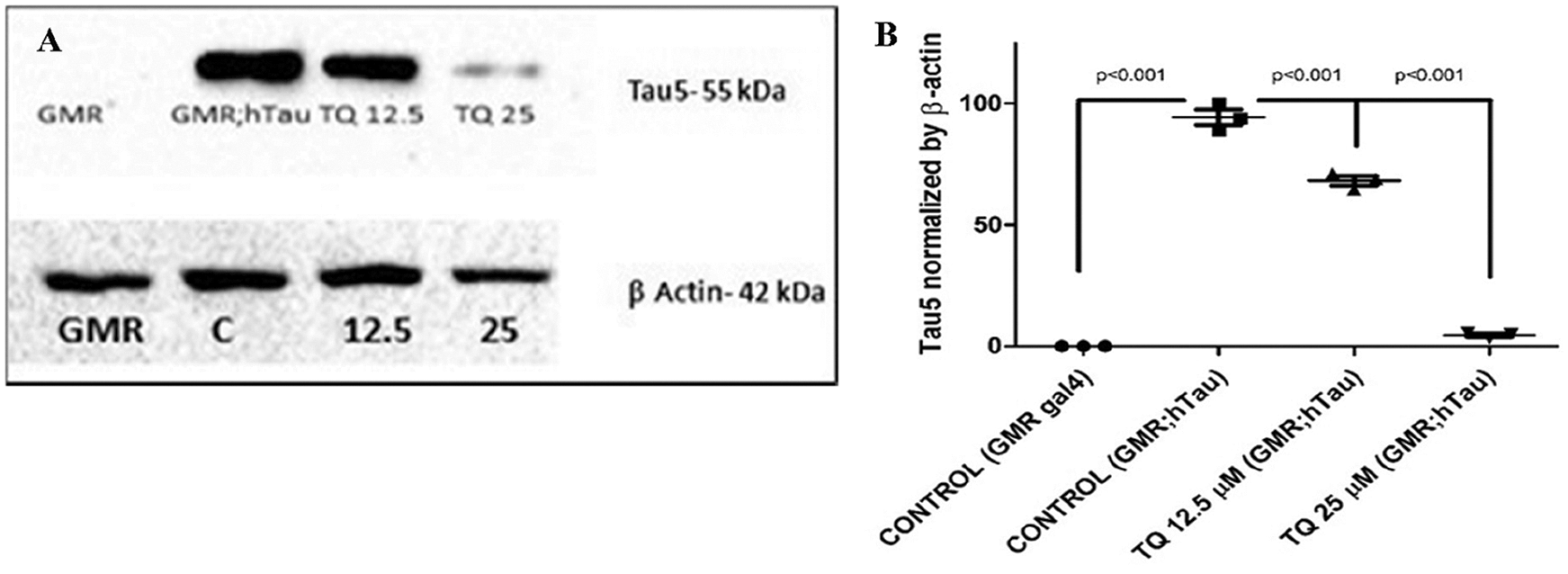
Figure 4: (a) Western blot analysis of transgenic protein, β actin (42 kDa), was used as a housekeeping protein and Tau5 (55 kDa) as a target protein. (a) The bands represent the protein contents for the following genotypes: GMR GAL4 (control), GMR; hTau R406W as a negative control group, and TQ-treated flies as treatment groups. (b) Quantitative representation of Tau5 normalized with β Actin. Mean ± SEM values are depicted (20 fly head per group). Statistical significance was compared by ANOVA, followed by Bonferroni’s test, P < 0.05 was set as significant.analysis. (b) Quantitative representation of a western blot.
Drosophila melanogaster is commonly used as an in vivo model to study neurodegenerative diseases such as AD and is considered a powerful model to study disorders that occur due to aging. This is mainly due to the short life span and ease of handling Drosophila (Caesar et al., 2012; Iijima et al., 2010). Over the last decade, the primary focus of research has been to investigate the therapeutic effect of drugs that can target important pathological markers of tau protein-induced degenerative changes in AD (Panza et al., 2016). Using Drosophila models of tauopathy, various phytochemicals and plant extract-based studies are currently being performed (Anupama et al., 2017; Zhang et al., 2016), and TQ has been reported to exert neuroprotective effects against Aβ-induced toxicity in Aβ models of AD (Elibol et al., 2019; Alhibshi et al., 2019). To the best of the authors’ knowledge, this is the first study to investigate the protective effects of TQ on hTau models of AD. Previous studies using N. sativa had reported that the main constituents present in it were amino acids, proteins, fatty acids, volatile oils, vitamins, carbohydrates, crude fiber, alkaloids, saponins, and minerals. The TQ content in volatile NS oil is approximately 25%, and NS oil also contains phytosterol, polyunsaturated fatty acids (PUFA), terpinol, t-anethole, and sesquiterpene longifolene (Liu et al., 2011; Akram Khan and Afzal, 2016). TQ also possesses antioxidant and anti-inflammatory properties (Ragheb et al., 2009), free radical scavenging activity (Badary et al., 2003), anticancer activity (Kundu et al., 2014), and hepatoprotective activity (Yildiz et al., 2008).
To achieve overexpression of hTau in D. melanogaster models, the UAS-GAL4 system was used in the current study. Crossbreeding of UAS R406W with GMR GAL4 driver and elav GAL4 driver line results in the expression of the UAS target sequence in a spatiotemporal manner. While GMR-GAL4 drives the expression of the protein of interest in the eyes, elav-GAL4 drives the pan-neuronal expression of the protein of interest. The binding of the GAL4 protein to the UAS enhancer sequence results in the expression of UAS target sequences (Duffy, 2002), as shown in Fig. 5.
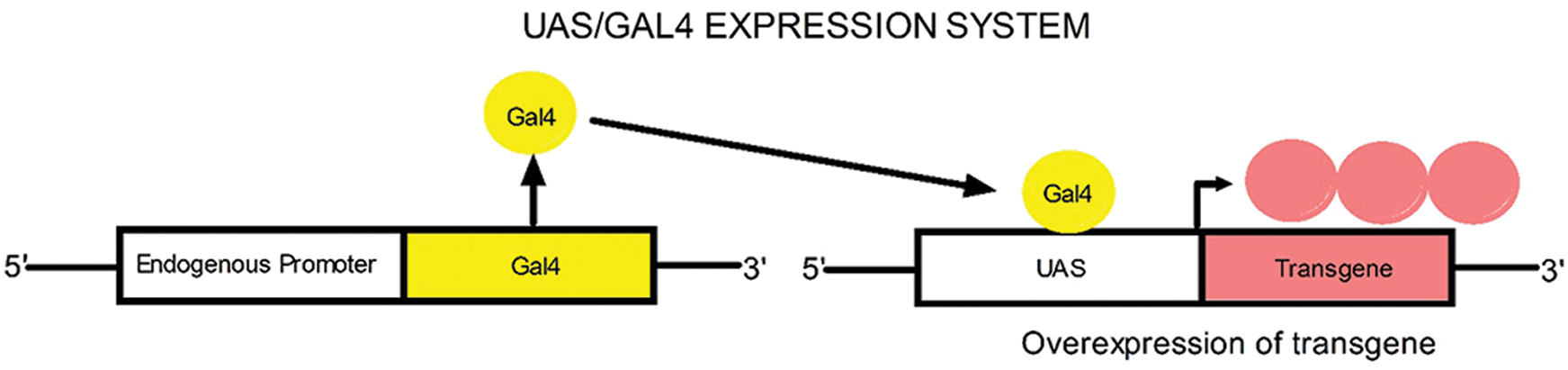
Figure 5: UAS/GAL4 expression system.
Neurodegenerative disorders and diseases often affect locomotor activity. Progressive deterioration in learning, memory, and motor disorders are the clinical manifestations of AD (Kurlan et al., 2000). In this study, behavioral studies were performed to understand the effect of TQ on locomotor activities such as movement, climbing activity, and attraction toward light. The locomotor activity of Drosophila larvae can be assessed by calculating the distance covered by larvae in a specific period of time. Therefore, the ability to cover more distance can be used as a preliminary assessment of the effect of tau expression in the specific enhancer region (Mudher et al., 2004). The 3rd instar larvae that were reared on TQ-supplemented food exhibited a significant increase in the crawling activity compared to larvae from normal food. The pan-neuronal expression of hTau elicited a significant decrease in the climbing activity of flies. The loss of photoreceptors in the eyes results in a decline in the ability of flies to get attracted toward a light source. The phototaxis activity of flies can be considered as a behavioral activity, which can decline in AD (Kain et al., 2012; Luo et al., 1992). The phototaxis activity of flies can be easily assessed in order to determine the variation in activity between treatment groups (Vang et al., 2012). The results of the current study indicated that the ability of flies to move toward the light source increased with TQ supplementation and the response varied according to the color of the light used. It demonstrated a positive response toward white, blue, and green light. There was a significant decrease in the phototaxis activity of tauopathy flies when compared with normal flies. Color vision in D. melanogaster has been extensively studied over recent years (Yamaguchi et al., 2010; Paulk et al., 2013; Schnaitmann et al., 2013). Drosophila species are considered to be most sensitive to green, blue, and ultraviolet light (Kelber and Henze, 2013; Bertholf, 1932). The visual sensitivity in D. melanogaster is consistent and stable from 406 nm to 525 nm. However, the sensitivity decreases by 25 times at a longer wavelength (Hernández de Salomon and Spatz, 1983). Thus, D. melanogaster is less sensitive to infrared, red, and orange (longer wavelength) and more sensitive to green, blue, and ultraviolet (shorter wavelength) (Kelber and Henze, 2013). The climbing assay is one of the commonly used assays to assess locomotor defects in Drosophila, and neurodegenerative diseases such as AD often significantly affect the climbing activity of flies. The decline in locomotor activity is considered a phenotypic marker of aging and neurodegenerative disorders (Iijima et al., 2004). Administration of TQ at a concentration of 25 μM elicited a significant improvement in the climbing activity of AD flies compared to control tauopathy flies. In this study when TQ is administered from the larval stage, the effects elicited by TQ could be considered as preventive as the hTau effect on is taking place and while the treatment is done after eclosion, the treatment is done also to induce a therapeutic effect. Thus, the neuroprotective effect of TQ helped in ameliorating the climbing defects in AD flies.
Biochemical analyses were performed in the current study to investigate the modulatory effect of TQ on the levels of ROS, SOD, and AChE in AD flies. Generally, oxidative stress is higher in tau-induced AD flies than normal flies due to the downregulation of antioxidant enzymes (Dias-Santagata et al., 2007). Moreover, neurodegeneration in the elderly population is also due to the significant reduction in the levels of antioxidant enzymes (Fabian et al., 2011). The amount of ROS increased significantly (P < 0.001) in tauopathy flies compared to non-AD flies, and dietary administration of TQ helped in significantly reducing (P < 0.01) the amount of ROS. Also, it is evident from the current study that the activities of SOD and AChE were regained following dietary administration of TQ when compared with tauopathy flies. It has been reported that depletion in the levels of AChE can affect cholinergic neurotransmission, which is considered one of the cognitive symptoms of AD (Rinne, 2003). Thus, it is clear that dietary administration of TQ helps in scavenging the free radicals and enhances the activity of SOD.
The relative mRNA expression of hTau and SOD genes was analyzed in AD flies following dietary administration of TQ for one month. The SOD enzyme helps in the reduction of oxidative stress and in a Drosophila model (Elmaci and Altinoz, 2016). In the current study, dietary administration of TQ at a concentration of 25 µM resulted in a significant increase (P < 0.01) in the mRNA expression of the SOD gene and a significant reduction in the mRNA expression of the hTau gene (P < 0.001). Moreover, a preliminary analysis of the effect of dietary administration of TQ on soluble hTau protein fraction revealed a concentration-dependent decrease.
In summary, dietary administration of TQ to AD flies improved their climbing activities, restored the activities of SOD and AChE, decreased ROS production, and also elicited a significant decrease in hTau expression at the transcriptional and translational levels. Therefore, further investigations are warranted in Drosophila and other disease models of AD to elucidate the mechanisms underlying the neuroprotective effects of TQ.
Acknowledgement: The authors thank the SRM-DBT Platform and Research Facility for providing the required facilities for qRT-PCR experiments.
Authors’ Contributions: Narayanan Nampoothiri V. P. contributed to the experimental part, analysis of obtained data, and drafting of the manuscript. Vignesh Sundararajan contributed also with experimental part to the whole study. Pallavi Dan contributed with the experimental analysis part. Sheik Mohideen Sahabudeen contributed to the supervision, project administration and execution. All authors read and approved the final version of the manuscript.
Availability of Data and Materials: All the data sets provided in the present study are available with the corresponding author and can be shared on request basis.
Ethics Approval: The current study used Drosophila melanogaster model for analysis. The use of this model organism does not entail for any kind of ethical clearance. Prior approval for handling transgenic flies was obtained from RCGM, DBT, India via IBSL committee.
Funding Statement: This work was funded and supported by Department of Biotechnology, School of Bioengineering, SRM Institute of Science & Technology, Tamil Nadu, India.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Akram Khan M, Afzal M (2016). Chemical composition of Nigella sativa Linn: Part 2 recent advances. Inflammopharmacology 24: 67–79. DOI 10.1007/s10787-016-0262-7. [Google Scholar] [CrossRef]
Alhibshi AH, Odawara A, Suzuki I (2019). Neuroprotective efficacy of thymoquinone against amyloid beta-induced neurotoxicity in human induced pluripotent stem cell-derived cholinergic neurons. Biochemistry and Biophysics Reports 17: 122–126. DOI 10.1016/j.bbrep.2018.12.005. [Google Scholar] [CrossRef]
Alzheimer’s Association (2020). Alzheimer’s disease facts and figures. Alzheimer’s Dement 16: 391–460. DOI 10.1002/alz.12068. [Google Scholar]
Ando K, Oka M, Ohtake Y, Hayashishita M, Shimizu S, Hisanaga S, Iijima KM (2016). Tau phosphorylation at Alzheimer’s disease-related Ser356 contributes to tau stabilization when PAR-1/MARK activity is elevated. Biochemical and Biophysical Research Communications 478: 929–934. DOI 10.1016/j.bbrc.2016.08.053. [Google Scholar] [CrossRef]
Anupama KP, Shilpa O, Anet A, Siddama TK, Gurushankara HP (2017). Convolvulus pluricaulis (Shankhapushpi) ameliorates human microtubule-associated protein tau (hMAPτ) induced neurotoxicity in Alzheimer’s disease Drosophila model. Journal of Chemical Neuroanatomy 95: 115–122. [Google Scholar]
Badary OA, Taha RA, el-Din Gamal AM, Abdel-Wahab MH (2003). Thymoquinone is a potent superoxide anion scavenger. Drug and Chemical Toxicology 26: 87–98. DOI 10.1081/DCT-120020404. [Google Scholar] [CrossRef]
Bagli E, Goussia A, Moschos MM, Agnantis N, Kitsos G (2016). Natural compounds and neuroprotection: Mechanisms. In vivo 30: 535–548. [Google Scholar]
Bertholf LM (1932). The extent of the spectrum for Drosophila and the distribution of stimulative efficiency in it. Zeitschrift für vergleichende Physiologie 18: 32–64. [Google Scholar]
Buée L, Bussiere T, Buée-Scherrer V, Delacourte A, Hof PR (2000). Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Research Reviews 33: 95–130. DOI 10.1016/S0165-0173(00)00019-9. [Google Scholar] [CrossRef]
Caesar I, Jonson M, Nilsson KPR, Thor S, Hammarström P (2012). Curcumin promotes A-beta fibrillation and reduces neurotoxicity in transgenic Drosophila. PLoS One 7: e31424. DOI 10.1371/journal.pone.0031424. [Google Scholar] [CrossRef]
Cascella M, Bimonte S, Barbieri A, Del Vecchio V, Muzio M R, Vitale A, Benincasa G, Ferriello AB, Azzariti A, Arra C, Cuomo A (2018). Dissecting the potential roles of Nigella sativa and its constituent thymoquinone on the prevention and on the progression of Alzheimer’s Disease. Frontiers in Aging Neuroscience 10: 10–16. DOI 10.3389/fnagi.2018.00016. [Google Scholar] [CrossRef]
Chen X, Li Y, Huang J, Cao D, Yang G, Liu W, Lu H, Guo A (2007). Study of tauopathies by comparing Drosophila and human tau in Drosophila. Cell and Tissue Research 329: 169–178. DOI 10.1007/s00441-007-0401-y. [Google Scholar] [CrossRef]
Churcher I (2006). Tau therapeutic strategies for the treatment of Alzheimer’s disease. Current Topics in Medicinal Chemistry 6: 579–595. DOI 10.2174/156802606776743057. [Google Scholar] [CrossRef]
Dias-Santagata D, Fulga TA, Duttaroy A, Feany MB (2007). Oxidative stress mediates tau-induced neurodegeneration in Drosophila. Journal of Clinical Investigation 117: 236–245. DOI 10.1172/JCI28769. [Google Scholar] [CrossRef]
Duffy JB (2002). GAL4 system in Drosophila: A fly geneticist’s Swiss Army knife. Genesis 34: 1–15. DOI 10.1002/gene.10150. [Google Scholar] [CrossRef]
Dugger BN, Dickson DW (2017). Pathology of neurodegenerative diseases. Cold Spring Harbor Perspectives in Biology 9: a028035. DOI 10.1101/cshperspect.a028035. [Google Scholar] [CrossRef]
Duraiswamy B, Nehru C, Kumar SS, Mohan KS (2019). Phytoconstituents and their possible mechanistic profile for Alzheimer’s disease—A literature review. Current Drug Targets 20: 263–291. DOI 10.2174/1389450119666180813095637. [Google Scholar] [CrossRef]
Elibol B, Terzioglu-Usak S, Beker M, Sahbaz C (2019). Thymoquinone (TQ) demonstrates its neuroprotective effect via an anti-inflammatory action on the Aβ (1-42)-infused rat model of Alzheimer’s disease. Psychiatry and Clinical Psychopharmacology 29: 379–386. DOI 10.1080/24750573.2019.1673945. [Google Scholar] [CrossRef]
Elmaci I, Altinoz MA (2016). Thymoquinone: An edible redox-active quinone for the pharmacotherapy of neurodegenerative conditions and glial brain tumors. A short review. Biomedicine & Pharmacotherapy 83: 635–640. DOI 10.1016/j.biopha.2016.07.018. [Google Scholar] [CrossRef]
Erkkinen MG, Kim M, Geschwind MD (2018). Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harbor Perspectives in Biology 10: a033118. DOI 10.1101/cshperspect.a033118. [Google Scholar] [CrossRef]
Fabian E, Bogner M, Elmadfa I (2011). Age-related modification of antioxidant enzyme activities in relation to cardiovascular risk factors. European Journal of Clinical Investigation 42: 42–48. DOI 10.1111/j.1365-2362.2011.02554.x. [Google Scholar] [CrossRef]
Fu H, Hardy J, Duff KE (2018). Selective vulnerability in neurodegenerative diseases. Nature Neuroscience 21: 1350–1358. DOI 10.1038/s41593-018-0221-2. [Google Scholar] [CrossRef]
Götz J, Chen F, van Dorpe J, Nitsch RM (2001). Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293: 1491–1495. DOI 10.1126/science.1062097. [Google Scholar] [CrossRef]
Habtemariam S (2019). Natural products in Alzheimer’s disease therapy: Would old therapeutic approaches fix the broken promise of modern medicines? Molecules 24: E1519. DOI 10.3390/molecules24081519. [Google Scholar] [CrossRef]
Hanger DP, Anderton BH, Noble W (2009). Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends in Molecular Medicine 15: 112–119. DOI 10.1016/j.molmed.2009.01.003. [Google Scholar] [CrossRef]
Heidary G, Fortini ME (2001). Identification and characterization of the Drosophila tau homolog. Mechanisms of Development 108: 171–178. DOI 10.1016/S0925-4773(01)00487-7. [Google Scholar] [CrossRef]
Hernández de Salomon C, Spatz HC (1983). Colour vision in Drosophila melanogaster: Wavelength discrimination. Journal of Comparative Physiology 150: 31–37. DOI 10.1007/BF00605285. [Google Scholar] [CrossRef]
Iijima K, Gatt A, Iijima-Ando K (2010). Tau Ser262 phosphorylation is critical for A 42-induced tau toxicity in a transgenic Drosophila model of Alzheimer’s disease. Human Molecular Genetics 19: 2947–2957. DOI 10.1093/hmg/ddq200. [Google Scholar] [CrossRef]
Iijima K, Liu HP, Chiang AS, Hearn SA, Konsolaki M, Zhong Y (2004). Dissecting the pathological effects of human A 40 and A 42 in Drosophila: A potential model for Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America 101: 6623–6628. DOI 10.1073/pnas.0400895101. [Google Scholar] [CrossRef]
Iqbal K, Liu F, Gong CX, Grundke-iqbal I (2010). Tau in Alzheimer disease and related tauopathies. Current Alzheimer Research 7: 656–664. DOI 10.2174/156720510793611592. [Google Scholar] [CrossRef]
Isaev NK, Chetverikov NS, Stelmashook EV, Genrikhs EE, Khaspekov LG, Illarioshkin SN (2020). Thymoquinone as a potential neuroprotector in acute and chronic forms of cerebral pathology. Biochemistry 85: 167–176. [Google Scholar]
Jackson GR, Wiedau-Pazos M, Sang TK, Wagle N, Brown CA, Massachi S, Geschwind DH (2002). Human Wild-Type Tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron 34: 509–519. DOI 10.1016/S0896-6273(02)00706-7. [Google Scholar] [CrossRef]
Kain JS, Stokes C, de Bivort BL (2012). Phototactic personality in fruit flies and its suppression by serotonin and white. Proceedings of the National Academy of Sciences of the United States of America 109: 19834–19839. DOI 10.1073/pnas.1211988109. [Google Scholar] [CrossRef]
Kawamata T, Kamada Y, Kabeya Y, Sekito T, Ohsumi Y (2008). Organization of the pre-autophagosomal structure responsible for autophagosome formation. Molecular Biology of the Cell 19: 2039–2050. DOI 10.1091/mbc.e07-10-1048. [Google Scholar] [CrossRef]
Kelber A, Henze MJ (2013). Colour vision: Parallel pathways intersect in Drosophila. Current Biology 23: R1043–R1045. DOI 10.1016/j.cub.2013.10.025. [Google Scholar] [CrossRef]
Kumar A, Singh A, Ekavali A (2015). A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacological Reports 67: 195–203. DOI 10.1016/j.pharep.2014.09.004. [Google Scholar] [CrossRef]
Kundu J, Chun KS, Aruoma OI, Kundu JK (2014). Mechanistic perspectives on cancer chemoprevention/chemotherapeutic effects of thymoquinone. Mutation Research 768: 22–34. DOI 10.1016/j.mrfmmm.2014.05.003. [Google Scholar] [CrossRef]
Kurlan R, Richard IH, Papka M, Marshall F (2000). Movement disorders in Alzheimer’s disease: More rigidity of definitions is needed. Movement Disorders 15: 24–29. DOI 10.1002/1531-8257(200001)15:1<24::AID-MDS1006>3.0.CO;2-X. [Google Scholar] [CrossRef]
Liu X, Abd El-Aty M, Shim JH (2011). Various extraction and analytical techniques for isolation and identification of secondary metabolites from Nigella sativa seeds. Mini-Reviews in Medicinal Chemistry 11: 947–955. DOI 10.2174/138955711797068472. [Google Scholar] [CrossRef]
Luo L, Tully T, White K (1992). Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for appl gene. Neuron 9: 595–605. DOI 10.1016/0896-6273(92)90024-8. [Google Scholar] [CrossRef]
Mayeux R, Stern Y (2012). Epidemiology of Alzheimer disease. Cold Spring Harbor Perspectives in Medicine 2: a006239. DOI 10.1101/cshperspect.a006239. [Google Scholar] [CrossRef]
Mershin A, Pavlopoulos E, Fitch O, Braden BC, Nanopoulos DV, Skoulakis EM (2004). Learning and memory deficits upon TAU accumulation in Drosophila mushroom body neurons. Learning & Memory 11: 277–287. DOI 10.1101/lm.70804. [Google Scholar] [CrossRef]
Mudher A, Shepherd D, Newman TA, Mildren P, Jukes JP, Squire A, Mears A, Berg S, MacKay D, Asuni AA, Bhat R, Lovestone S (2004). GSK-3β inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Molecular Psychiatry 9: 522–530. DOI 10.1038/sj.mp.4001483. [Google Scholar] [CrossRef]
Muhammad A, Flores I, Zhang H, Yu R, Staniszewski A, Planel E, Herman M, Ho L, Kreber R, Honig LS, Ganetzky B, Duff K, Arancio O, Small SA (2008). Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proceedings of the National Academy of Sciences of the United States of America 105: 7327–7332. DOI 10.1073/pnas.0802545105. [Google Scholar] [CrossRef]
Nichols CD, Becnel J, Pandey UB (2012). Methods to assay Drosophila behavior. Journal of Visualized Experiments 2012: e3795. [Google Scholar]
Nishimura I, Yang Y, Lu B (2004). PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell 116: 671–682. DOI 10.1016/S0092-8674(04)00170-9. [Google Scholar] [CrossRef]
Panza F, Solfrizzi V, Seripa D, Imbimbo BP, Lozupone M, Santamato A, Logroscino G (2016). Tau-centric targets and drugs in clinical development for the treatment of Alzheimer’s disease. BioMed Research International 2016: 3245935. [Google Scholar]
Paulk A, Millard SS, van Swinderen B (2013). Vision in Drosophila: Seeing the world through a model’s eyes. Annual Review of Entomology 58: 313–332. DOI 10.1146/annurev-ento-120811-153715. [Google Scholar] [CrossRef]
Pohl F, Kong Thoo Lin P (2018). The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 23: 3283. [Google Scholar]
Poorgholam P, Yaghmaei P, Hajebrahimi Z (2018). Thymoquinone recovers learning function in a rat model of Alzheimer’s disease. Avicenna Journal of Phytomedicine 8: 188–197. [Google Scholar]
Prüßing K, Voigt A, Schulz JB (2013). Drosophila melanogaster as a model organism for Alzheimer’s disease. Molecular Neurodegeneration 8: 1. DOI 10.1186/1750-1326-8-1. [Google Scholar] [CrossRef]
Ragheb A, Attia A, Eldin W, Elbarbry F, Gazarin S, Shoker A (2009). The protective effect of thymoquinone, an anti-oxidant and anti-inflammatory agent, against renal injury: A review. Saudi Journal of Kidney Diseases and Transplantation 20: 741–752. [Google Scholar]
Ramadan MF (2007). Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.An overview. International Journal of Food Science & Technology 42: 1208–1218. DOI 10.1111/j.1365-2621.2006.01417.x. [Google Scholar] [CrossRef]
Rinne JO (2003). Brain acetylcholinesterase activity in mild cognitive impairment and early Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry 74: 113–115. DOI 10.1136/jnnp.74.1.113. [Google Scholar] [CrossRef]
Samarghndian S, Farkhondeh T (2018). A review on possible therapeutic effect of Nigella sativa and thymoquinone in neurodegenerative diseases. CNS & Neurological Disorders-Drug Targets 6: 420–460. [Google Scholar]
Sanabria-Castro A, Alvarado-Echeverría I, Monge-Bonilla C (2017). Molecular pathogenesis of Alzheimer’s disease: An update. Annals of Neuroscience 24: 46–54. DOI 10.1159/000464422. [Google Scholar] [CrossRef]
Schnaitmann C, Garbers C, Wachtler T, Tanimoto H (2013). Color discrimination with broadband photoreceptors. Current Biology 23: 2375–2382. DOI 10.1016/j.cub.2013.10.037. [Google Scholar] [CrossRef]
Sheik Mohideen S, Yamasaki Y, Omata Y, Tsuda L, Yoshiike Y (2015). Nontoxic singlet oxygen generator as a therapeutic candidate for treating tauopathies. Scientific Reports 5: 10821. DOI 10.1038/srep10821. [Google Scholar] [CrossRef]
Sundararajan V, Dan P, Kumar A, Venkatasubbu GD, Ichihara S, Ichihara G, Sheik Mohideen S (2019). Drosophila melanogaster as an in vivo model to study the potential toxicity of cerium oxide nanoparticles. Applied Surface Science 490: 70–80. DOI 10.1016/j.apsusc.2019.06.017. [Google Scholar] [CrossRef]
Vang LL, Medvedev AV, Adler J (2012). Simple ways to measure behavioral responses of Drosophila to stimuli and use of these methods to characterize a novel mutant. PLoS One 7: e37495. DOI 10.1371/journal.pone.0037495. [Google Scholar] [CrossRef]
Wang HY, Li W, Benedetti NJ, Lee DH (2003). Alpha 7 nicotinic acetylcholine receptors mediate beta-amyloid peptide-induced tau protein phosphorylation. Journal of Biological Chemistry 278: 31547–31553. DOI 10.1074/jbc.M212532200. [Google Scholar] [CrossRef]
Wangler MF, Yamamoto S, Bellen HJ (2015). Fruit flies in biomedical research. Genetics 199: 639–653. DOI 10.1534/genetics.114.171785. [Google Scholar] [CrossRef]
Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB (2001). Tauopathy in Drosophila: Neurode-generation without neurofibrillary tangles. Science 293: 711–714. DOI 10.1126/science.1062382. [Google Scholar] [CrossRef]
Yamaguchi S, Desplan C, Heisenberg M (2010). Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 107: 5634–5639. DOI 10.1073/pnas.0809398107. [Google Scholar] [CrossRef]
Yildiz F, Coban S, Terzi A, Ates M, Aksoy N, Cakir H, Ocak AR, Bitiren M (2008). Nigella sativa relieves the deleterious effects of ischemia reperfusion injury on liver. World Journal of Gastroenterology 14: 5204–5209. DOI 10.3748/wjg.14.5204. [Google Scholar] [CrossRef]
Zhang B, Li Q, Chu X, Sun S, Chen S (2016). Salidroside reduces tau hyperphosphorylation via up-regulating GSK-3β phosphorylation in a tau transgenic Drosophila model of Alzheimer’s disease. Translational Neurodegeneration 5: 21. DOI 10.1186/s40035-016-0068-y. [Google Scholar] [CrossRef]
Zhang H, Bai L, He J, Zhong L, Duan X, Ouyang L, Zhu Y, Wang T, Zhang Y, Shi J (2017). Recent advances in discovery and development of natural products as source for anti-Parkinson’s disease lead compounds. European Journal of Medicinal Chemistry 141: 257–272. DOI 10.1016/j.ejmech.2017.09.068. [Google Scholar] [CrossRef]
Zheng WH, Bastianetto S, Mennicken F, Ma W, Kar S (2002). Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 115: 201–211. DOI 10.1016/S0306-4522(02)00404-9. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |