DOI:10.32604/biocell.2021.015411

| BIOCELL DOI:10.32604/biocell.2021.015411 |  |
| Article |
GC/MS-based differential metabolic profiling of human peptic ulcer disease to study Helicobacter pylori-induced metabolic perturbations
1Department of Biotechnology, Punjabi University, Patiala, 147002, India
2Department of General Medicine, Government Medical College and Hospital, Chandigarh, 160047, India
3Department of Clinical Pharmacy, College of Pharmacy, King Saud University, Riyadh, 11451, Saudi Arabia
4Department of Zoology, College of Science, King Saud University, Riyadh, 11451, Saudi Arabia
*Address correspondence to: Baljinder Kaur, baljinderkaur@pbi.ac.in
Received: 17 December 2020; Accepted: 16 February 2021
Abstract: Helicobacter pylori infection has been significantly linked to Peptic Ulcer Disease and Gastric Cancer. Metabolomic fingerprinting may offer a principal way of early diagnosis and to understand the molecular mechanism of H. pylori-induced pathogenicity. The rationale of the study is to explore the underlying distinct metabolic mechanisms of H. pylori-induced PUD and to identify potential biomarkers for disease diagnosis and associated risks using Gas chromatography/mass spectrometry. GC/MS-based analytical method was used to compare metabolic profiles of healthy controls (N = 20) and peptic ulcer patients (N = 45). Acquired metabolomic data were analyzed by constructing a diagnostic model using principal component analysis and a non-parametric two-tailed paired Wilcoxon analysis to identify disease-specific metabolic biomarkers. A total of 75 low-molecular-weight endogenous metabolites were detected during comparative metabolomic analysis of PUD vs. healthy gut tissues, among which 16 metabolites are being proposed to be diagnostic markers of Human PUD. Perturbations related to amino acids, carbohydrates, fatty acids, organic acids, and sterol metabolism were significantly revealed during this differential metabolomic profiling. Results convincingly suggest that metabolic profiles can contribute immensely in early diagnosis of the disease and understanding molecular mechanisms of disease progression for predicting novel drug targets for prophylactic and anaphylactic measures.
Keywords: Peptic ulcer disease; Gastric cancer; Helicobacter pylori; Metabolic profiling; Perturbations; Stomach ulcers
A peptic ulcer results from an imbalance between damaging forces of gastric acid-pepsin and gastro-duodenal mucosal defense barriers (Chung and Shelat, 2017). Peptic ulcer disease (PUD), being most prevalent in third-world countries, is responsible for 4% of the total mortality among digestive diseases. In addition, as per the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), 14.5 million Americans and about 350,000 new PUD cases are diagnosed with PUD every year (Chai, 2011). Among various endogenous and exogenous stress factors, smoking, nutritional deficiencies, gastric acid, dyspepsia, prolonged intake of non-steroidal anti-inflammatory drug use (NSAIDs), and alcohol are the factors associated with peptic ulcers (Li et al., 2014). In 70–90% of cases, peptic ulcers are found to be associated with spiral-shaped Helicobacter pylori bacteria that reside in the acidic environment of the stomach (Ali, 2013). Interestingly, H. pylori infection leads to the development of antral-predominant gastritis that is being linked with gastric hyperchlorydria and duodenal ulcer (DU). Further, it can develop corpus gastritis, gastric atrophy, peptic ulcer, and an increased risk of non-cardia gastric cancer (Roesler et al., 2014). Hence, early H. pylori eradication is documented with decreased risk of carcinoma in patients with PUD (Take et al., 2005; Wu et al., 2009). At present, the most frequently used technique for early cancer diagnosis is the endoscopy-based pathohistological study of tissue biopsies which is quite complicated (Trivanovic et al., 2019). Hereby, empirical knowledge of disease progression and biomarkers at the molecular level are attractive propositions in order to diagnose and treat PUD patients at an early stage (Song et al., 2012). Thus, metabolomics offers a robust opportunity for understanding various metabolic perturbations and identifying possible biomarkers for early diagnosis of PUD and further might assist in proposing efficient therapeutics/drug targets for the treatment of the PUD at an early stage before its progression into gastric cancer.
Metabolomics refers to the study of endogenous and exogenous metabolites in biological systems to provide comparative semi-quantitative information about all metabolites in the system ranging from cell cultures to human biological fluids such as urine, saliva, tissue, and blood. Metabolomics is an emerging and potentially powerful tool in discovering biomarkers and identifying perturbed pathways due to disease, which can clarify the mechanism of action (Song et al., 2011; Tan et al., 2016). For metabolomic profiling, gas chromatography/mass spectrometry (GC/MS) has proven to be among the best analytical technique due to its high sensitivity, peak resolution, and reproducibility (Jonsson et al., 2004; Griffin and Shockcor, 2004).
In the present study, we hypothesized that the GC/MS-based inclusive analysis of tissue metabolites symbolizes the markers that can discriminate the PUD group from the normal mucosal group. Our study demonstrated major metabolic perturbations in the antral gastric regions of H. pylori-infected PUD patients that may contribute to the identification of metabolic biomarkers indicating the development of PUD.
Institutional Ethics Committee at Punjabi University, Patiala, has approved the protocol of this study (vide IEC approval no. IEC/05/09/15). As per protocol approved by the review board, written informed consent was taken from all the subjects prior to sample collection. The study using human subjects was performed in accordance with the approved ICMR guidelines and regulations.
Patient recruitment, sample collection and histopathological evaluation
Antral biopsies from peptic ulcer patients (N = 72) and healthy volunteers with normal mucosa (N = 20) were collected from freshly isolated resections using Upper Gastrointestinal Endoscopy (UGIE) carried out by trained professionals at Government Medical College & Hospital, Sector-32, Chandigarh (Punjab, India).
Inclusion criteria: H. pylori-infected PUD patients, as identified in the histopathological evaluation, were enrolled for the present study. Complete medical history of the enrolled patients was recorded as they should not be suffering from any kind of metabolic disorder such as diabetes, hyperthyroidism, or cancer. In addition, healthy subjects were also carefully monitored with no signs of any peptic ulcer/infection and other dietary habits like smoking, drinking alcohol, or stress factor.
Exclusion criteria: Pregnant women, children, immuno-compromised patients, drug abusers (NSAIDs or antibiotics), illiterate and mentally challenged were not considered for the present study.
The tissue biopsies were collected from the margin of the ulcer, cleaned using saline solution, and finally using liquid nitrogen were snapped-frozen within 30 min of collection. The collected biopsies were then investigated for H. pylori infection using hematoxylin and eosin (H&E) stain as stated by Fischer et al. (2008) at the Department of Pathology, Government Medical College & Hospital, Chandigarh. Total cases (normal 20, PUD 45) enrolled for the study included 43 males (66%) and 22 females (34%), ages ranged from 20 to 80 years (patient demographics are given in Suppl. Tab. S1). For further study, biopsies of normal healthy mucosa and H. pylori-infected antral ulcer tissue were considered. Samples were then labeled and stored at −80°C until processing.
Sample preparation for gas chromatography/mass-spectrometry analysis of the gastric tissue specimens
Fifteen milligrams of antral biopsy samples were transferred to 2.0 mL Eppendorf tube and extracted with 500 μL methanol/chloroform solvent in a ratio of 3:1 (v/v) and vortexed for 30 s. The samples were then ultrasonicated (30% amplitude, with pulses 30 s ON, 10 s OFF) for 15 min and vortex-mixed for 30 s. Samples were stored for 10 min at −20°C and then centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant was collected and transferred to a fresh vial and stored at −20°C until analysis. For GC/MS analysis, samples were prepared by adding 100 μL of ethyl acetate (EA) to each tissue extract and vortex-mixed for 1 min. Then, 100 μL of derivatization reagent, consisting of a mixture (3:1:1, v/v/v) of N, O-bis(trimethylsilyl) trifluoroacetamide (BSTFA), pyridine, and EA, was added, and samples were incubated for 16 h at room temperature. The resulting solution was then vortexed for 1 min, and GC/MS analysis was performed.
Using Perkin Elmer Clarus 500 Gas Chromatography system outfitted with DB-5MS capillary column (25 × 0.2 mm ID, 0.25 μm film thickness) having Perkin Elmer Clarus 500 annual injector analysis was performed. Helium at a constant flow rate of 1.0 mL/min was used as the carrier gas. An aliquot (1 μL) of each of the derivatized solutions was injected in splitless mode. The temperature of the inlet (270°C), transfer line (260°C), ion source (200°C) and quadrupole (150°C) were maintained, respectively. The chromatogram temperature program was run at 80°C (isothermal heating) for 2 min, followed by the sequential rise in oven temperature at the rate of 10 °C/min to 180°C, 5 °C/min to 240°C, 10 °C/min to 260°C and finally maintained at 280°C for 5 min. Data acquisition in MS was achieved in an electron impact mode at 60 eV and scanned for m/z values ranging from 5 to 400 with an acquisition rate of 20 spectra/s with 6 min of solvent delay time (Song et al., 2011).
After analysis, each sample was symbolized by a GC/MS total ion current (TIC) chromatogram constituting various peaks. For further study, peaks detected in at least 80% of the samples were considered and analyzed by comparing their respective MS spectra and retention indices with reference data in the Mass Spectral Library of the National Institute of Standards and Technology (NIST) (Wiley Registry, 9th edition, 2008). The mass spectra of each metabolite were vigilantly investigated, and compounds with matching probability >80% were considered for further study. The data in context to ion intensities of respective detected peaks were normalized and then exported to PAST (PAleontological Statistics, Version 3.24) to perform principal component analysis (PCA) with 95% confidence ellipses, where distinctly separate alignment trend was observed in the data. Further, using statistically significant metabolites only (P-value < 0.05) to identify marker metabolites that differentiated PUD and normal groups, a model was proposed. In order to study the statistical significance of the data, a non-parametric two-tailed paired Wilcoxon analysis was performed with SPSS version13.0. P-values of <0.05 were considered statistically significant. False Discovery Rate (FDR)-correction (q values < 0.1) was applied to the P-values to estimate false-positive results according to the standard method of Benjamini and Hochberg. Also, fold change (FC) was calculated by considering a deviation from the median values of 45 PUD specimens relative to that of 20 normal specimens.
For the present study, 20 healthy volunteers were enrolled, keeping in view the exclusion criteria viz a viz no previous medical history related to metabolic disorders or diet and stress-related illness and no intake of NSAIDs, antibiotics, or any kind of drug for at least six months prior to the current investigation. On the other hand, a total of 45 H. pylori-associated PUD patients were considered, including 35 males (78%) and 10 females (22%) with a mean age of 46.27 ± 14.8 years. Among the PUD group, the most affected age group was found to be 30–50-year-old (51.1%), and most of them were underweight as per their BMI. However, among 35 infected males, 46% were reported to be non-vegetarian, 43% were regular smokers, and surprisingly 63% were found to be alcoholic; whereas, in the case of females, 80% were having non-vegetarian diets, 20% were smoking, and 30% have been reported to be suffering from psychological stress as mentioned in Suppl. Tab. S1. The study does not provide direct correlations of age, gender, diet, and smoking with PUD development, as the cohort size was very limited in the study.
Histopathology of antral biopsy specimens
The presence of H. pylori was not observed in any of the antral biopsies collected from normal healthy volunteers. However, H. pylori infection was reported in the gastric mucosa of the peptic ulcer test group by histopathological evaluation, whereas the ulcer biopsies with H. pylori-negative evaluation were not considered for further study. Intraepithelial lymphocytes were usually absent in gastric mucosa. However, as evidenced from Fig. 1, breaches in the gastric epithelium were observed in H. pylori-infected tissue specimens. Necrotic changes were noticed at the base of the ulcer with no morphological changes at the periphery. In comparison to normal healthy mucosa, H. pylori-infected epithelium was reportedly showing signs of dense lymphomononuclear infiltrate migration of mononuclear and polymorphonuclear leukocyte and aggregation of lymphocytes which is generally followed by H. pylori proliferation in the necrotic debris. The deterioration of gastric epithelium was observed in the H. pylori-infected PUD patients that may increase the risk of its progression to gastric carcinoma due to damaging forces of the gastric secretions and the activity of the pathogenic strain if the peptic ulcer remains untreated for a long time.
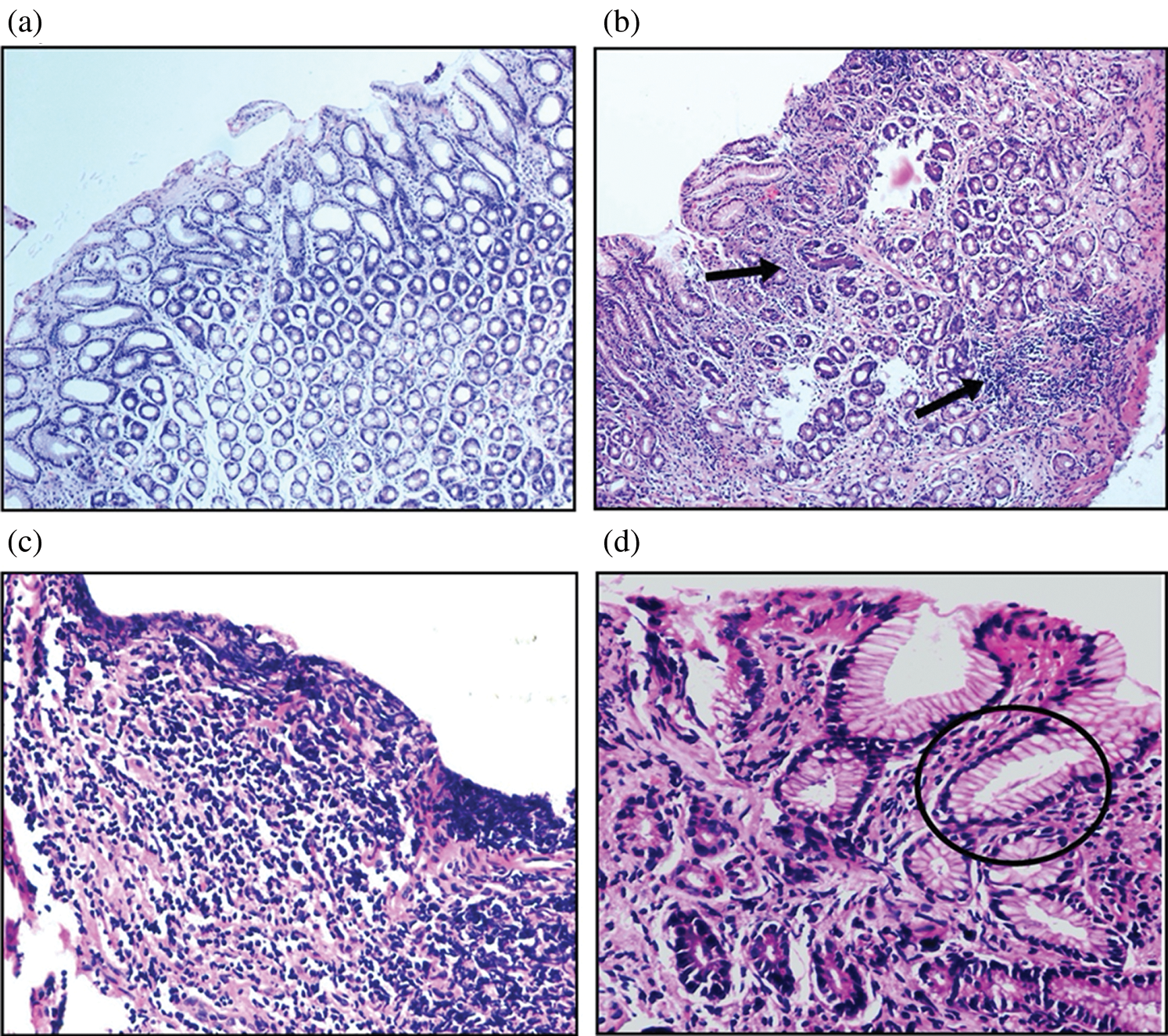
Figure 1: Histopathological evaluation of antral gastric biopsies of normal and H. pylori-infected PUD patients (H&E-stained), where (a) healthy mucosa in normal patients (100×), (b) mucosal damage due to necrosis of cells, acute inflammation, proliferation with necrotic debris (40×), (c) area of ulceration with the presence of dense lymphomononuclear infiltrate (400×) and (d) showing the presence of microorganisms conforming to the morphology of H. pylori within the gastric glands (400×).
Metabolomic profiling of healthy and H. pylori-infected PUD tissue specimens
Under optimal GC/MS conditions as mentioned earlier, typical representative total ion chromatograms (TIC) of tissue samples were derived for peptic ulcer patients and the normal controls as presented in Fig. 2. Within one TIC chromatogram, consistently, an average of 90 signals were sensed, of which some peaks were not taken into consideration due to low abundance and poor spectral quality to be declared as metabolites. In the study, the majority of peaks were identified as low-molecular-weight endogenous compounds based on NIST Mass Spectral Library. As mentioned in Tab. 1, a total of 75 compounds, such as amino acids, carbohydrates, fatty acids, organic acids, and steroids, being involved in various biochemical pathways like amino acid, energy and lipid metabolism were detected in the ulcer and normal mucosal tissues.
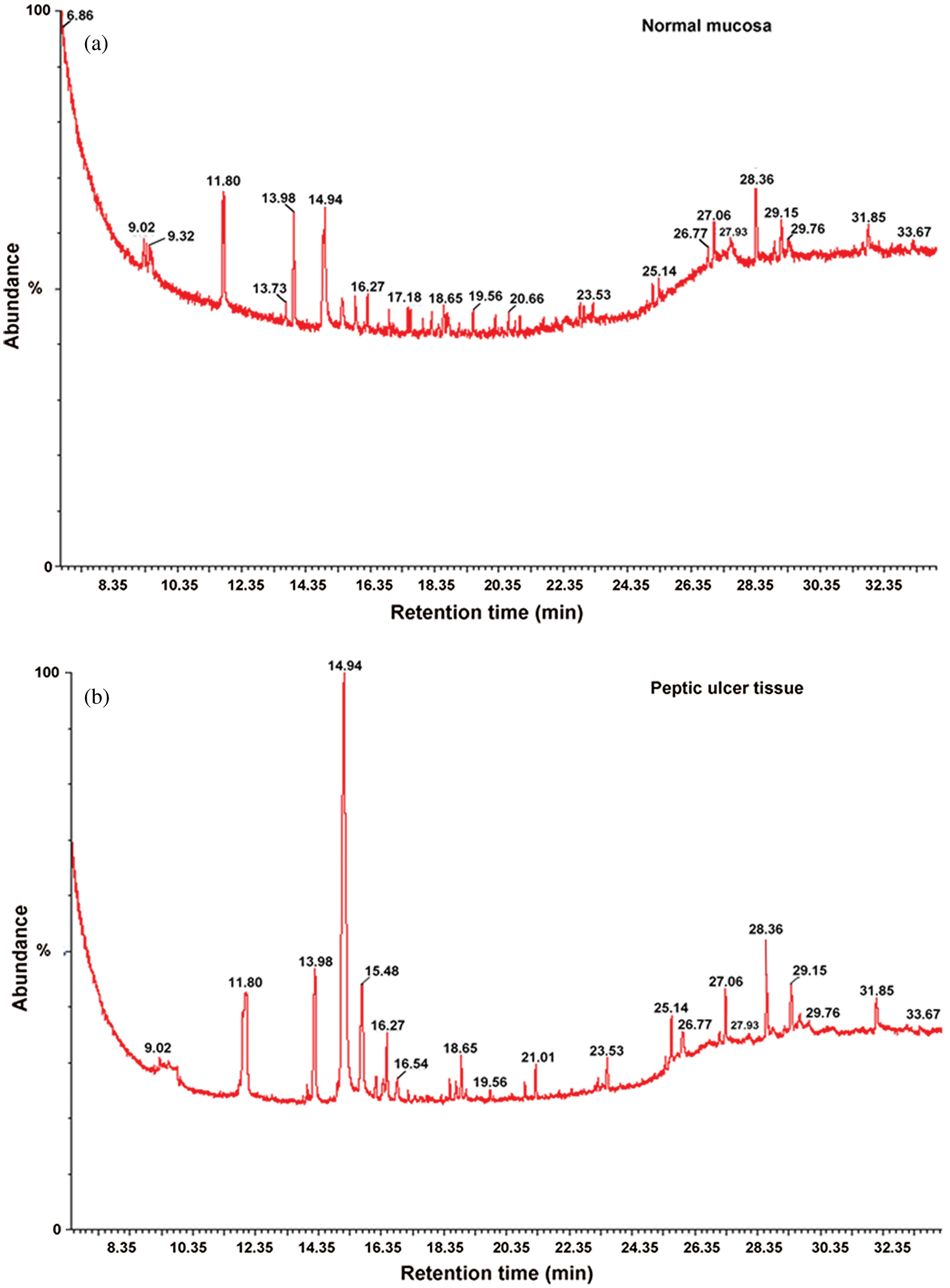
Figure 2: Typical GC/MS total ion current (TIC) chromatograms.
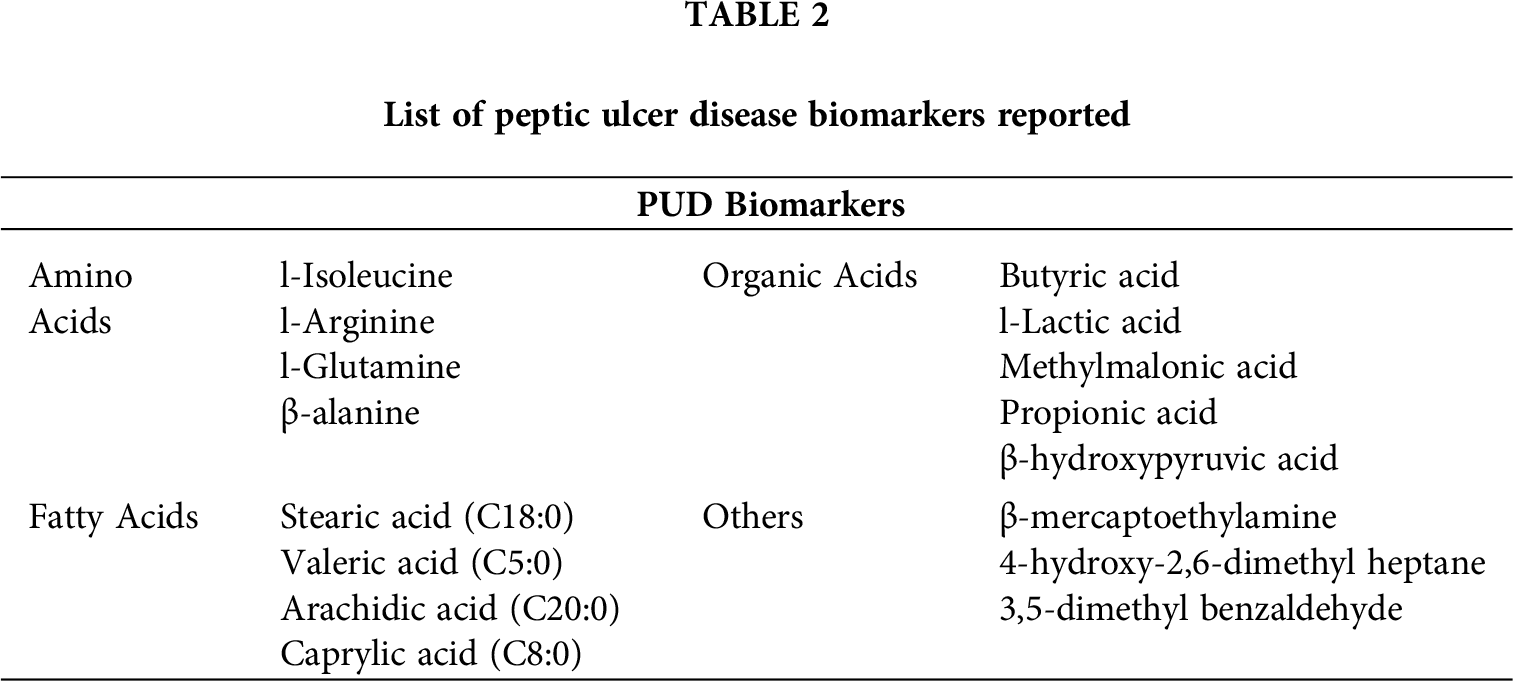
Contrastingly, 15 metabolites viz a viz 4-hydroxy-2,6-dimethyl heptane, arachidic acid (C20:0), butyric acid (C4:0), caprylic acid (C8:0), D-mannose, l-(+)-lactic acid, l-arginine, methylmalonic acid, naphthalene, norephedrine, oleanitrile, peroxyergosterol, R-(ar)-tumerone and cysteamine were generated in ulcer tissues but absent in normal mucosal tissue while cholesterol is the most depleted metabolite in ulcer tissues. However, four metabolites, i.e., naphthalene, oleanitrile, peroxyergosterol and R-(ar)-tumerone were not considered for further study as these are main constituents of different food sources and moreover not reported in human gastric metabolic profile yet. Further, the PCA scatter plot, as shown in Fig. 3, satisfactorily confirmed the statistical model to discriminate between the PUD and normal groups when plotted for the obtained.
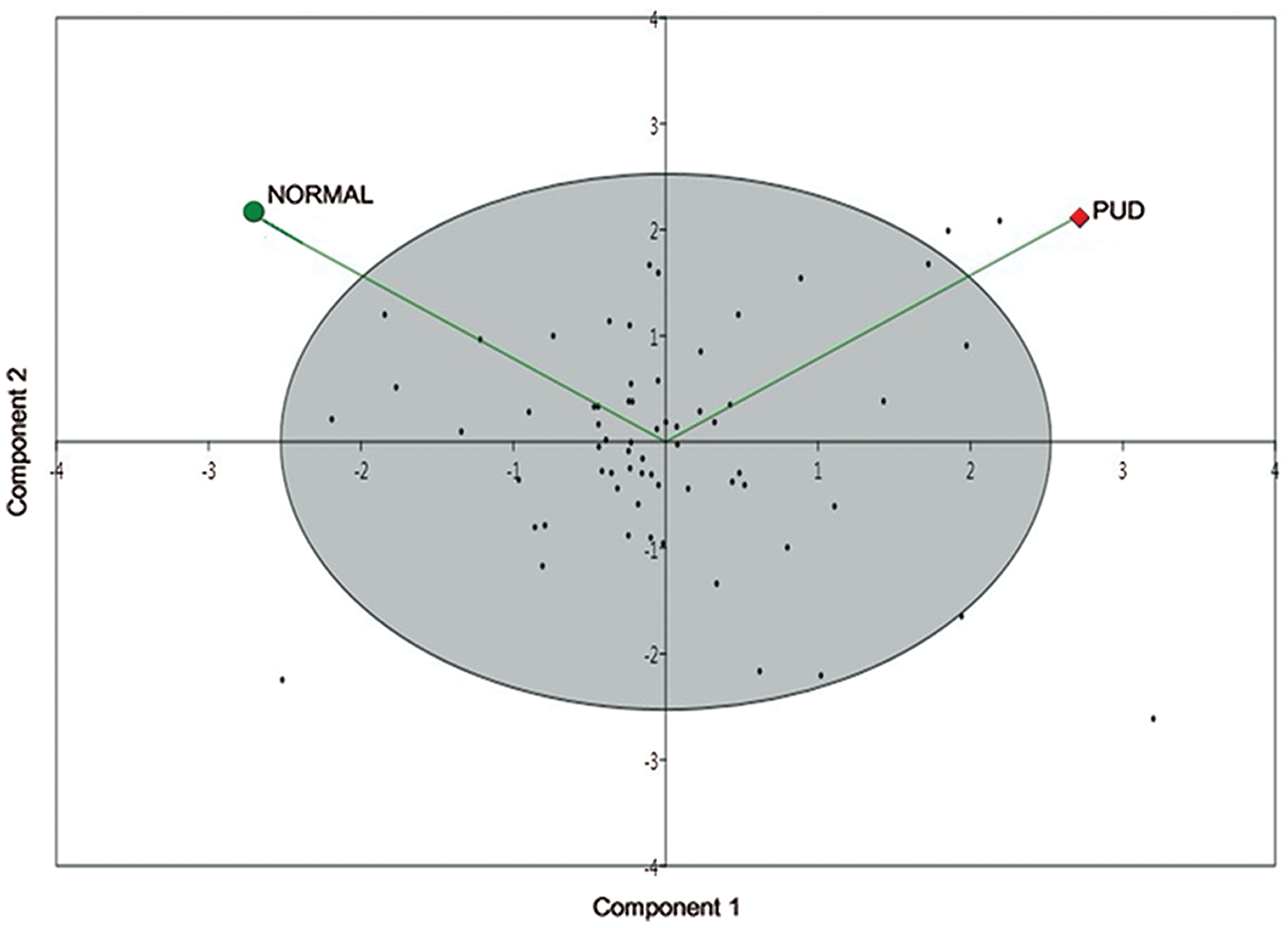
Figure 3: Establishment of the PCA model.
Analysis of the perturbed metabolites in PUD
To investigate differences in the biomolecular composition of the peptic ulcer and normal mucosal samples, the Wilcoxon test was performed on statistically significant hits (P < 0.05) that resulted in the distinct separation of tissues in the PCA plot. Consequently, a total of 45 metabolites were found to have a significant probability (P < 0.05) that discriminated metabolic profiles of the two groups as shown in Tab. 1. Interestingly, 29 metabolites were found to be upregulated, while 16 were down-regulated in ulcer tissues. Additionally, l-isoleucine (FC, 13.353), β-hydroxy pyruvic acid (FC, 9.961), stearic acid (FC, 6.042), valeric acid (FC, 3.857), propionic acid (FC, 3.024), and palmitic acid (FC, 0.021) were significantly upregulated in the ulcer tissues, while metabolites like arachidic acid (C20:0), butyric acid (C4:0), caprylic acid (C8:0), l-(+)-lactic acid, l-arginine, methylmalonic acid, norephedrine, and cysteamine were detected only in the PUD tissue specimens; whereas, cholesterol was the most depleted metabolite found in PUD tissue specimens.
Metabolic profiling of gastric cancer using various analytical techniques has been reported earlier (Abbassi-Ghadi et al., 2013; Chan et al., 2014), while none have actually focused on the exploration of the differential gastric metabolic profile of the peptic ulcer in humans. The present study thus aimed at investigating comparative tissue metabolomic fingerprinting of PUD patients vis-a-vis healthy controls. In view of the fact that plenty of variables are interconnected with the metabolomic data, so in order to screen and select differential metabolites, PCA was performed.
From the GC/MS analysis, several marker metabolites related to peptic ulcer disease have been discovered. Several intermediates of “Aerobic Glycolysis” (glucose, β-D-galactopyranoside, l-(+)-lactic and β-hydroxypyruvic acid) were found to be up-regulated in the peptic ulcer tissue, which is also supported by a previous study on gastric cancer (Chan et al., 2014). Under aerobic conditions, cancer cells prefer aerobic metabolism over the most efficient oxidative phosphorylation for the synthesis of ATP, stated as the ‘Warburg Effect’ (Alfarouk et al., 2014). Despite the ample availability of oxygen, pyruvate in the gastric tissues is converted into lactate and other metabolites that may particularly benefit the proliferating cancerous cells (vander Heiden et al., 2009). In addition, it has also been reported that lactic acid downregulates the cytotoxic T-cell/NK cell function, thereby inhibiting the differentiation of monocytes to dendritic cells resulting in the immune escape of the cancer cells (Xiao and Zhou, 2017).
Under excess glucose conditions, rapidly proliferating cancer cells tend to be highly glycolytic (Crabtree Effect). Due to disruption of oxidative phosphorylation, they tend to become more sensitive to mitochondrial dysfunctioning (Aguer et al., 2011). In the normal cells, endogenous galactose is phosphorylated to galactose-1-phosphate by the activity of galactokinase (GALK1). Galactose-1-phosphate uridylyltransferase (GALT) further converts galactose-1-phosphate to glucose-1-phosphate that enters glycolysis to yield energy. UDP-galactose is consumed as galactosyl donor for the biosynthesis of glycoproteins and glycolipids, which are crucial for cellular metabolism (Fig. 4). However, in the case of GALT deficiency, a lethal disease called Galactosemia that is frequently observed in cancer patients, galactose is accumulated in the cells leading to various syndromes like hepatomegaly, bleeding disorder, E. coli sepsis, and death. In addition, excess galactose is also converted into toxic metabolites like galactitol and galactonate. Galactitol further results in the accumulation of hydrogen peroxide and other free radicals, causing oxidative damage to the cells (Lai et al., 2008).
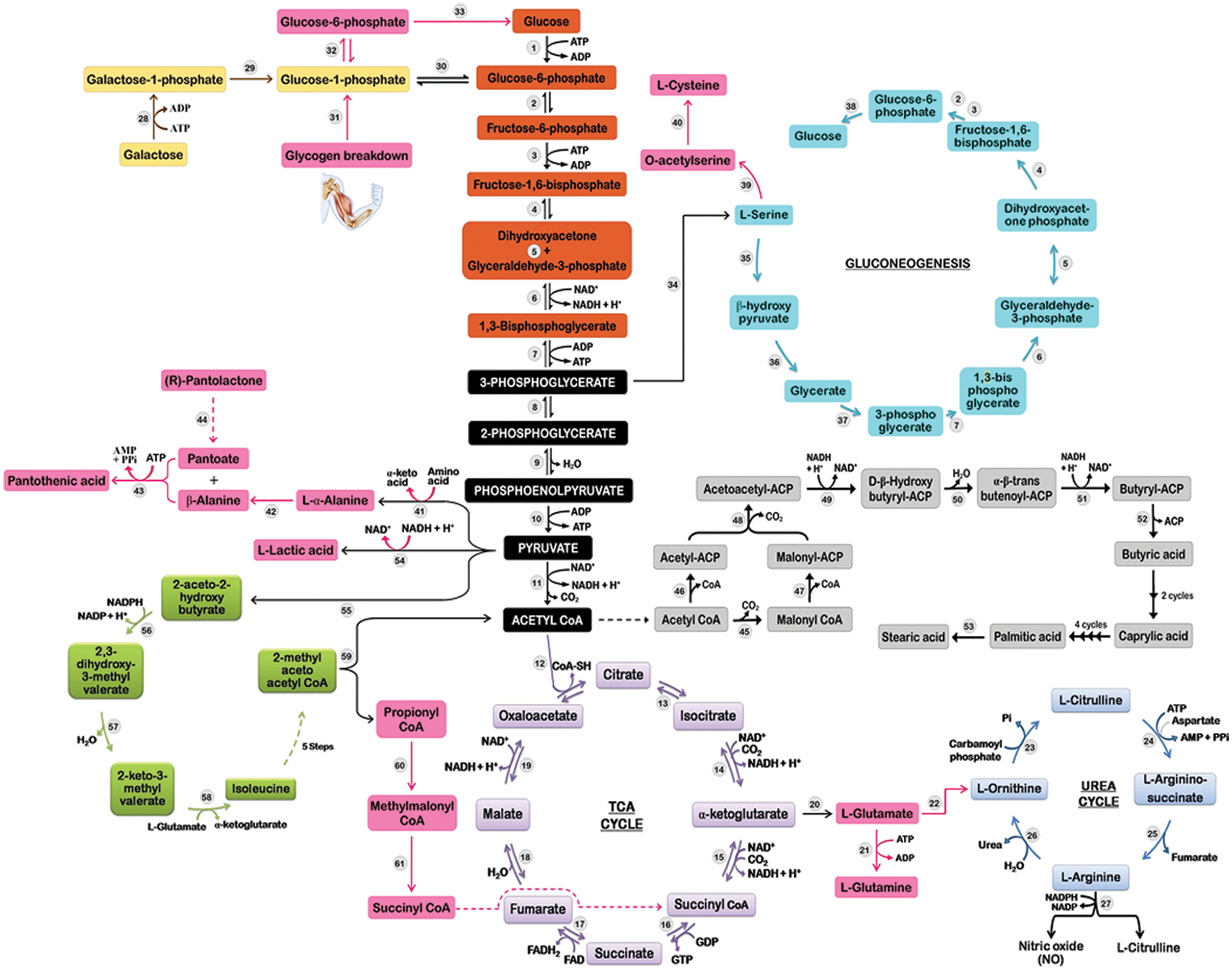
Figure 4: Perturbed metabolic pathway analysis in PUD patients using GC/MS; where enzymes catalyzing the respective reactions are as follows: hexokinase (1), phosphoglucokinase (2), phosphofructokinase (3), aldolase (4), triosephosphate isomerase (5), glyceraldehyde-3-phosphate dehydrogenase (6), phosphoglycerate kinase (7), phosphoglycerate mutase (8), enolase (9), pyruvate kinase (10), pyruvate dehydrogenase (11), citrate synthase (12), aconitase (13), isocitrate dehydrogenase (14), α-ketoglutarate dehydrogenase (15), succinyl-CoA synthetase (16), succinate dehydrogenase (17), fumarase (18), malate dehydrogenase (19), glutamate dehydrogenase (20), glutamine synthetase (21), ornithine-oxo acid transaminase (22), ornithine carbamoyl transferase (23), argininosuccinate synthase (24), argininosuccinate lyase (25), arginase (26), NOS (27), galactokinase (28), GALT (29), phosphoglucomutase (30), glycogen phosphorylase (31), phosphoglucomutase (32), glucose-6-phosphatase (33), phosphoglycerate dehydrogenase (34), serine aminotransferase (35), hydroxypyruvate reductase (36), glycerate kinase (37), glucose-6-phosphatase (38), serine acetyl transferase (39), O-acetyl serine sulfhydrolase (40), transaminase (41), alanine-2,3-amino mutase (42), pantothenate synthetase (43), unknown (44), ACCase (45), acetyl-CoA-ACP transacylase (46), malonyl-CoA-ACP transacylase (47), β-ketoacyl-ACP synthase (48), β-ketoacyl-ACP reductase (49), β-hydroxyacyl-ACP dehydrase (50), enoyl-ACP reductase (51), thioesterase (52), elongase (53), lactic dehydrogenase (54), 2-aceto-2-hydroxy butyrate synthase (55), acetohydroxyacid isomeroreductase (56), dihydroxyacid dehydratase (57), isoleucine transaminase (58), 2-methyl acetoacetyl CoA thiolase (59), propionic carboxylase (60), and methylmalonic acid mutase (61).
Oxidative stress is involved in natural aging and some human diseases like neurodegeneration, multiple sclerosis, cardiovascular disease, cancer, and rheumatoid arthritis. Elevated levels of reactive oxygen species (ROS), producing free radicals, results in molecular and tissue damage triggering a chain of destruction to promote oncogenic transformation; thus, free radicals have proven to be a useful index for depicting biological markers in vivo (Katerji et al., 2019). In the present study, several oxidative stress markers viz. β-hydroxy pyruvic acid, 4-hydroxy-2,6-dimethyl heptane, β-mercaptoethylamine, and 3,5-dimethyl benzaldehyde have been detected in PUD tissue which indicates the possibility of oxidative DNA damage and hormonal dysbiosis in the diseased patients (Tab. 2). Earlier also, it was reported that 3,5-dimethyl benzaldehyde, obtained from lipid peroxidation, synergizes with DNA damage to induce accelerated stress. Its endogenous routes involve phenyl acetaldehyde through β-oxidation or cinnamic acid through β-oxidation (Flor and Kron, 2016).
Elevated amino acids are the major contributing factors in cancer cells; specifically, many cancer cell lines require l-glutamine, a well-known nutrient for their survival even under metabolic stress (Choi and Park, 2018). Therefore, following a general trend, the level of amino acid biosynthesis is upregulated in cancerous tissues as reported earlier in esophagogastric cancer (Abbassi-Ghadi et al., 2013). A similar disturbance in the amino acid metabolism was also observed in the present study as levels of l-glutamine (FC, 1.831), l-isoleucine (FC, 13.353), and β-alanine (FC, 3.14) were significantly upregulated in the H. pylori-infected gastric tissue. Amino acids have proved to be an alternative source of energy generated through anaplerotic reactions. Glutamine, a prime example of anaplerotic reaction, enters the TCA cycle by its conversion into glutamate and α-ketoglutarate (Chan et al., 2014). But in the present study, disruption of the TCA cycle was observed, and subsequent levels of α-ketoglutarate declined in ulcer tissues, which indicate glutamate bioconversion to arginine (via urea cycle) which is detected only in the PUD (Fig. 4). The gluconeogenetic β-alanine (FC, 3.14) and l-glutamine (FC, 1.831) were found to be linked previously with hepatocellular carcinoma (Brosnan, 2003; Xue et al., 2008). The endogenous levels of amino acids cysteine, l-threonine, l-alanine, l-glycine, and l-aspartate showed depletion due to disruption in metabolic changes associated with gastric dysbiosis were previously linked with the etiology of gastric cancer by Wu et al. (2010). l-arginine is exclusively reported as an amino acid biomarker of PUD that represents the progression of the disease as observed earlier in arginine-dependent tumors, i.e., hepatocellular carcinoma, lymphomas, melanoma, sarcomas, and urological cancers (Phillips et al., 2013). Further, branched-chain amino acids (l-isoleucine, l-leucine and l-valine) are reported to be essential for tumor proliferation as they are incorporated directly into proteins or being metabolized. The amino group of branched-chain amino acids is being transferred to α-ketoglutarate in presence of branched-chain amino acid transferase in order to produce glutamate and a branched-chain α-ketoacid that eventually enters the TCA cycle and thus is suggested as a prognostic cancer indicator (Selwan and Edinger, 2017). He et al. (2012) stated that perturbations in glutamine and arginine metabolic pathways may contribute to the pathomechanisms and memory deficits associated with Schizophrenia.
Glutamine basically tends to control the master regulator of protein translation mTORC1 required for anabolic growth of cells. In addition, it acts as a nitrogen donor for various metabolic enzymes and de novo synthesis of purines and pyrimidines required for proliferating cancer cells (DeBerardinis and Cheng, 2010). l-arginine is required by the proliferating cancer cells for protein biosynthesis, growth and survival. Under the restricted exogenous l-arginine supply, cancer cells may suffer from increased autophagy and apoptosis, and thus, cancer cells are bound to produce an ample amount of l-arginine (Szlosarek, 2014). Additionally, l-arginine is also a precursor of nitric oxide (NO), which is essential for maintaining healthy endothelium. Interestingly, l-arginine can also be catabolized to urea which inhibits the activity of nitric oxide synthase (NOS). Prior studies have demonstrated that NOS inhibition is a risk factor for cardiovascular mortality, diabetes, hypertension, and renal dysfunction (Finkelman et al., 2017).
The homeostasis of amino acids has been regulated by two protein kinases, namely mechanistic target of rapamycin complex 1 (mTORC1) and general control non-derepressable 2 (GCN2), required for cell growth and proliferation in response to numerous growth and stress signals. Increased endogenous levels of l-arginine and l-cysteine significantly activate mTORC1 (sensor of arginine and leucine) and GCN2 kinases. In response to amino acid-uncharged tRNA, kinases result in inhibition of protein translation that further induce the elevation of the cellular amino acid pool (Bar-Peled and Sabatini, 2014; Vučetić et al., 2017). Consequently, glutathione (GSH), linked with increased endogenous levels of l-cysteine, imparts a significant role in cell differentiation, proliferation, and progression to various diseases, including cancer (Traverso et al., 2013).
Organic acids such as l-(+)-lactic acid, methylmalonic acid and butyric acid (fatty acid intermediate) emerged as important biomarkers of PUD. Accumulation of lactic acid and disruption of TCA cycle as reported earlier in cases of colorectal, gastric, liver, and invasive ovarian cancers by Tan et al. (2016). Previous studies have demonstrated that elevated levels of glycolytic flux and lactate excretion are associated with malignant transformation; however, lactate accumulation alone is sufficient to enhance the degree of tumor malignancy and impairment of mitochondrial oxidative metabolism (Redjems-Bennani et al., 1998; Walenta and Mueller-Klieser, 2004). Earlier, Frye et al. (2016) reported that elevated endogenous levels of propionic acid (studied in Autism Spectrum Disorders in children) were metabolized in multiple reactions to succinyl-CoA that inhibited the activity of first (citrate synthase) and fourth (α-ketoglutarate dehydrogenase) enzymes, thus blocking TCA cycle that results in decreased production of NADH.
Another prominent observation recorded in the present study was the perturbation of fatty acid metabolism in ulcer tissues. Metabolomic fingerprinting revealed significant enhancement in the levels of saturated fatty acids (arachidic acid, caprylic acid, nonahexacontanoic acid, stearic acid and valeric acid) significantly increases in PUD tissues; while the reduction in the endogenous level of unsaturated fatty acid (arachidonic acid) was reported as previously observed in case of lung cancer and non-Hodgkin lymphoma (Song et al., 2011; Song et al., 2012). According to the scientific evidence, cancer cells show dysfunction of the β-oxidation pathway of fatty acid biosynthesis and cell membrane synthesis (Chan et al., 2014). Stearic acid (FC, 6.042) and valeric acid (FC, 3.857) were the most elevated saturated fatty acids, while arachidonic acid (FC, −0.645) was the most depleted one. Li et al. (2014) reported the role of stearic acid (also known as Octadecanoic acid) in fatty acid metabolism disorder and rehabilitation of peptic ulcers. Stearic acid promotes ulcer formation by increasing the inflammatory response and mitochondrial dysfunction.
Elevated endogenous levels of methylmalonic acid (MMA) observed in PUD tissue specimens might be because of its inability to convert into succinyl CoA signifying vitamin B12 deficiency (Vashi et al., 2016). Schloss et al. (2013) stated that malabsorption, gastrointestinal surgeries, and intake of medications (proton pump inhibitors, etc.) precede cancer patients susceptible to vitamin B12 deficiency. In PUD, gastric mucosa is not intact, thus unable to produce intrinsic factors leading to vitamin B12 malabsorption. Hence, vitamin B12 was marked as a potential biomarker for atrophic gastritis that increases the risk of gastric adenocarcinoma (Miranti et al., 2017). Further, accumulation of MMA may also result in renal failure, thyroid disease, implicated in the pathogenesis of neuronal damage and Alzheimer’s disease (Bjørke Monsen and Ueland, 2003; Kolker et al., 2000; Serot et al., 2005). While, butyric acid has proven to be an indicator for compromised gut wall integrity, as observed in AIDS subjects (Stein et al., 1997). Short-chain fatty acids such as acetic, butyric, and propanoic acids cause a decrease in the functioning of the mucosal barrier, which may contribute to increased pathogenesis of gastric ulcers through multiple mechanisms such as alternate energy reservoir, acidification of cellular content, impairment of Na-transport channels, and cellular swelling as observed in animal model studies (Nadeau et al., 2003; Załęski et al., 2013). An interesting finding is the depletion of cholesterol in the case of peptic ulcer tissues. The decline in cholesterol synthesis (linked with genes present in chromosome 5) might be due to translational competition, which increases the concentration of adenocarcinoma-associated biomarkers leading to the progression of adenoma to gastric cancer (Song et al., 2011). (R)-Pantolactone is a precursor of pantothenic acid, needed to synthesize coenzyme A and acyl carrier protein (ACP) portion of fatty acid synthetase (Rawalpally, 2001). It is therefore hypothesized that altered levels of coenzyme A and ACP have major implications on fatty acid biosynthesis and the progression of PUD. So, pantothenic acid (vitamin B5) deficiency may be caused in the animals (symptoms common with PUD).
Cysteamine (also known as β-mercaptoethylamine) is a biological compound produced in the gastrointestinal tract and hypothalamus of all animals acting upon the somatotrophic axis. It helps in improving nutrient digestion and absorption by enhancing portal drained visceral blood flow and net portal absorption. Further, cysteamine also downregulates the secretion of gastroenteropancreatic plasma and an inhibitory hormone named somatostatin (Barnett and Hegarty, 2016). In the gastrointestinal tract, somatostatin is present in abundance that helps in the suppression of gastric acid secretion. Altered expression of somatostatin results in increased levels of gastric acid secretion leading to various gastrointestinal diseases/dysbiosis (Minalyan et al., 2017). Also, cysteamine emerged as an important oxidative biomarker of PUD, which has been previously linked with the etiology of Ehlers-Danlos syndrome, severe skin rashes, duodenal ulcers, or bleeding in the intestine (Weintraub, 2015). It also tends to concentrate in the duodenum and generate reactive oxygen species (ROS), resulting in the progression of the ulcer by decreasing the defense activity of superoxide dismutase (SOD), as stated by Elberry (2013). Contrastingly, Borrell-Pagès et al. (2006) reported cysteamine to be a part of treatment for Huntington’s disease (HD), as it elevates the level of heat shock DnaJ-containing protein 1b (HSJ1b), which is present in low concentration in HD patients. Norephedrine, an α, β-adrenergic receptor agonist, enhances the secretion of nor-epinephrine hormone that further elevates blood pressure, heart rate, breakdown of galactose to release glucose, inhibits voiding of bladder and gastrointestinal motility or bowel movements (Deedwania, 2015; Konturek et al., 2004; Mareev and Cleland, 2015). As these conditions are frequently reported in PUD patients, thus occurrence of endogenous levels of cysteamine and norephedrine is pointing towards hormonal dysbiosis in PUD patients.
In summary, by using a combinational approach involving highly sensitive analytical techniques and multivariate and univariate statistical tests, significant metabolic shifts and perturbations of amino acids, carbohydrate, fatty acids, organic acids, and sterols were identified in the case of peptic ulcer tissues. The results obtained in the study revealed the potential of the MS-based robust metabolic profiling approach for early diagnosis of PUD and contributed substantial evidence to understand pathophysiological responses during the development of PUD in humans. However, the study could not provide a direct correlation of age, gender, diet, and smoking with the development of PUD, as the cohort size was very limited.
The results obtained in the study could be utilized to develop serosurvey kits for early diagnosis of PUD using marker metabolites excreted in blood or urine of the H. pylori-infected PUD patients. However, a detailed evaluation of the abundance and occurrence of these marker metabolites in blood and urine samples needs a thorough investigation using PUD subjects. In addition, perturbed metabolites detected in the PUD group could lay the foundation for finding novel therapeutic targets and discovering new multi-target drugs. It is hereby proposed that enzymatic dysbiosis is also linked with PUD and differential expression of several candidate genes expressing adenylate kinase, AMP kinase, pyruvate kinase, lactate dehydrogenase, GALT, GCN2 kinase, GSH, branched-chain aminotransferases, SOD, ROS, coenzyme A synthetase, and acyl carrier protein could be explored for understanding a detailed mechanism of development of H. pylori-induced PUD and its progression to gastric carcinoma, if untreated.
Acknowledgement: Authors also acknowledge the Department of Medicine and Department of Pathology, Government Medical College & Hospital, Chandigarh for providing biopsy samples of concerned patients and histopathology investigation with informed consent for the research purpose; and Sophisticated Analytical Instrument Lab, Thapar University, Patiala (PB), India for providing GC/MS facility. The authors would like to extend their sincere appreciation to the Researchers Supporting Project No. (RSP-2020/19), King Saud University, Riyadh, Saudi Arabia.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Authors’ Contribution: Research work, results interpretation and manuscript preparation was done by Gaganjot Gupta; research idea and guidance by Dr. Baljinder Kaur; endoscopy and biopsy sectioning were performed by Dr. Deepak Bansal and Dr. Atul Sachdev; Anshula Sharma, Ajaz Ahmad, Tawseef Ahmad and Hamed A. El-Serehy helped in manuscript preparation.
Ethics Approval: All procedures performed in the study were in accordance with the ethical standards of the Institutional Ethics Committee and as per Indian Council of Medical Research (ICMR), New Delhi (India) guidelines and regulations with approval No. IEC/05/09/15 on 10th September, 2015.
Funding Statement: This work was supported by the UGC, New Delhi awarding Maulana Azad National Fellowship to Ms. Gaganjot [Grant No. F1-17.1/2015-16/MANF-2015-17-PUN-53869]. The authors would like to extend their sincere appreciation to the Researchers Supporting Project No. (RSP-2021/19), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abbassi-Ghadi N, Kumar S, Huang J, Goldin R, Takats Z, Hanna GB (2013). Metabolomic profiling of oesophago-gastric cancer: A systematic review. European Journal of Cancer 49: 3625–3637. DOI 10.1016/j.ejca.2013.07.004. [Google Scholar] [CrossRef]
Aguer C, Gambarotta D, Mailloux RJ, Moffat C, Dent R, McPherson R, Harper ME (2011). Galactose enhances oxidative metabolism and reveals mitochondrial dysfunction in human primary muscle cells. PLoS One 6: e28536. DOI 10.1371/journal.pone.0028536. [Google Scholar] [CrossRef]
Alfarouk KO, Verduzco D, Rauch C, Muddathir AK, Adil HH, Elhassan GO, Ibrahim ME, David Polo Orozco J, Cardone RA, Reshkin SJ, Harguindey S (2014). Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question. Oncoscience 1: 777–802. DOI 10.18632/oncoscience.109. [Google Scholar] [CrossRef]
Ali MZ (2013). Peptic ulcer disease. Khwaja Yunus Ali Medical College Journal 3: 270–272. [Google Scholar]
Bar-Peled L, Sabatini DM (2014). Regulation of mTORC1 by amino acids. Trends in Cell Biology 24: 400–406. DOI 10.1016/j.tcb.2014.03.003. [Google Scholar] [CrossRef]
Barnett M, Hegarty RS (2016). Cysteamine: A human health dietary additive with potential to improve livestock growth rate and efficiency. Animal Production Science 56: 1330–1338. DOI 10.1071/AN15339. [Google Scholar] [CrossRef]
Bjørke Monsen AL, Ueland PM (2003). Homocysteine and methylmalonic acid in diagnosis and risk assessment from infancy to adolescence. American Journal of Clinical Nutrition 78: 7–21. DOI 10.1093/ajcn/78.1.7. [Google Scholar] [CrossRef]
Borrell-Pagès M, Canals JM, Cordelières FP, Parker JA, Pineda JR, Grange G, Bryson EA, Guillermier M, Hirsch E, Hantraye P, Cheetham ME, Néri C, Alberch J, Brouillet E, Saudou F, Humbert S (2006). Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. Journal of Clinical Investigation 116: 1410–1424. DOI 10.1172/JCI27607. [Google Scholar] [CrossRef]
Brosnan JT (2003). Interorgan amino acid transport and its regulation. Journal of Nutrition 133: 2068S–2072S. DOI 10.1093/jn/133.6.2068S. [Google Scholar] [CrossRef]
Chai J (2011). Gastric ulcer healing—Role of serum response factor. In: Chai J (ed.Peptic ulcer disease, pp. 143–164. 1st edition. 14.5 million Americans and about 350,000 new PUD casesare diagnosed with PUD every year. IntechOpen, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). [Google Scholar]
Chan AW, Gill RS, Schiller D, Sawyer MB (2014). Potential role of metabolomics in diagnosis and surveillance of gastric cancer. World Journal of Gastroenterology 20: 12874–12882. DOI 10.3748/wjg.v20.i36.12874. [Google Scholar] [CrossRef]
Choi YK, Park KG (2018). Targeting glutamine metabolism for cancer treatment. Biomolecules and Therapeutics 26: 19–28. DOI 10.4062/biomolther.2017.178. [Google Scholar] [CrossRef]
Chung KT, Shelat VG (2017). Perforated peptic ulcer—An update. World Journal of Gastrointestinal Surgery 9: 1–12. DOI 10.4240/wjgs.v9.i1.1. [Google Scholar] [CrossRef]
DeBerardinis RJ, Cheng T (2010). Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29: 313–324. DOI 10.1038/onc.2009.358. [Google Scholar] [CrossRef]
Deedwania PC (2015). Management of patients with stable angina and type 2 diabetes. Reviews in Cardiovascular Medicine 16: 105–113. [Google Scholar]
Elberry AA (2013). Protective effect of sildenafil against cysteamine induced duodenal ulcer in Wistar rats. African Journal of Pharmacy and Pharmacology 7: 2352–2357. DOI 10.5897/AJPP2013.3571. [Google Scholar] [CrossRef]
Finkelman BS, Putt M, Wang T, Le W, Narayan H, Domchek S, DeMichele A, Fox K, Matro J, Shah P, Clark A, Bradbury A, Narayan V, Carver JR, Tang WHW, Ky B (2017). Arginine-nitric oxide metabolites and cardiac dysfunction in patients with breast cancer. Journal of the American College of Cardiology 70: 152–162. DOI 10.1016/j.jacc.2017.05.019. [Google Scholar] [CrossRef]
Fischer AH, Jacobson KA, Rose J, Zeller R (2008). Hematoxylin and eosin staining of tissue and cell sections. CSH Protocols 1: 2008:pdb.prot4986. [Google Scholar]
Flor A, Kron S (2016). Lipid-derived reactive aldehydes link oxidative stress to cell senescence. Cell Death & Disease 7: e2366. DOI 10.1038/cddis.2016.275. [Google Scholar] [CrossRef]
Frye RE, Rose S, Chacko J, Wynne R, Bennuri SC, Slattery JC, Tippett M, Delhey L, Melnyk S, Kahler SG, MacFabe DF (2016). Modulation of mitochondrial function by the microbiome metabolite propionic acid in autism and control cell lines. Translational Psychiatry 6: e927. DOI 10.1038/tp.2016.189. [Google Scholar] [CrossRef]
Griffin JL, Shockcor JP (2004). Metabolic profiles of cancer cells. Nature Reviews Cancer 4: 551–561. DOI 10.1038/nrc1390. [Google Scholar] [CrossRef]
He Y, Yu Z, Giegling I, Xie L, Hartmann AM, Prehn C, Adamski J, Kahn R, Li Y, Illig T, Wang-Sattler R, Rujescu D (2012). Schizophrenia shows a unique metabolomics signature in plasma. Translational Psychiatry 2: e149. DOI 10.1038/tp.2012.76. [Google Scholar] [CrossRef]
Jonsson P, Gullberg J, Nordström A, Kusano M, Kowalczyk M, Sjöström M, Moritz T (2004). A strategy for identifying differences in large series of metabolomic samples analyzed by GC/MS. Analytical Chemistry 76: 1738–1745. DOI 10.1021/ac0352427. [Google Scholar] [CrossRef]
Katerji M, Filippova M, Duerksen-Hughes P (2019). Approaches and methods to measure oxidative stress in clinical samples: Research applications in the cancer field. Oxidative Medicine and Cellular Longevity 2019: 1–29. DOI 10.1155/2019/1279250. [Google Scholar] [CrossRef]
Kolker S, Ahlemeyer B, Krieglstein J, Hoffmann GF (2000). Methylmalonic acid induces excitotoxic neuronal damage in vitro. Journal of Inherited Metabolic Disease 23: 355–358. DOI 10.1023/A:1005631230455. [Google Scholar] [CrossRef]
Konturek SJ, Konturek JW, Pawlik T, Brzozowski T (2004). Brain-gut axis and its role in the control of food intake. Journal of Physiology and Pharmacology 55: 137–154. [Google Scholar]
Lai K, Tang M, Yin X, Klapper H, Wierenga K, Elsas L (2008). ARHI: A new target of galactose toxicity in Classic Galactosemia. Bioscience Hypotheses 1: 263–271. DOI 10.1016/j.bihy.2008.06.011. [Google Scholar] [CrossRef]
Li TJ, Wang S, Meng XS, Bao YR, Guan SS, Liu B, Chen L, Wang L, Ran XR (2014). Metabolomics coupled with multivariate data and pathway analysis on potential biomarkers in gastric ulcer and intervention effects of Corydalis yanhusuo alkaloid. PLoS One 9: e82499. DOI 10.1371/journal.pone.0082499. [Google Scholar] [CrossRef]
Mareev Y, Cleland JG (2015). Should β-blockers be used in patients with heart failure and atrial fibrillation? Clinical Therapeutics 37: 2215–2224. DOI 10.1016/j.clinthera.2015.08.017. [Google Scholar] [CrossRef]
Minalyan A, Gabrielyan L, Scott D, Jacobs J, Pisegna JR (2017). The gastric and intestinal microbiome: role of proton pump inhibitors. Current Gastroenterology Reports 19: 42. DOI 10.1007/s11894-017-0577-6. [Google Scholar] [CrossRef]
Miranti EH, Stolzenberg-Solomon R, Weinstein SJ, Selhub J, Männistö S, Taylor PR, Freedman ND, Albanes D, Abnet CC, Murphy G (2017). Low vitamin B12 increases risk of gastric cancer: A prospective study of one-carbon metabolism nutrients and risk of upper gastrointestinal tract cancer. International Journal of Cancer 141: 1120–1129. DOI 10.1002/ijc.30809. [Google Scholar] [CrossRef]
Nadeau JA, Andrews FM, Patton CS, Argenzio RA, Mathew AG, Saxton AM (2003). Effects of hydrochloric, acetic, butyric, and propionic acids on pathogenesis of ulcers in the nonglandular portion of the stomach of horses. American Journal of Veterinary Research 64: 404–412. DOI 10.2460/ajvr.2003.64.404. [Google Scholar] [CrossRef]
Phillips MM, Sheaff MT, Szlosarek PW (2013). Targeting arginine-dependent cancers with arginine-degrading enzymes: Opportunities and challenges. Cancer Research and Treatment 45: 251–262. DOI 10.4143/crt.2013.45.4.251. [Google Scholar] [CrossRef]
Rawalpally TR (2001). Pantothenic acid. Kirk-Othmer encyclopedia of chemical technology. New York, NY: John Wiley & Sons. [Google Scholar]
Redjems-Bennani N, Jeandel C, Lefebvre E, Blain H, Vidailhet M, Guéant JL (1998). Abnormal substrate levels that depend upon mitochondrial function in cerebrospinal fluid from Alzheimer patients. Gerontologia 44: 300–304. DOI 10.1159/000022031. [Google Scholar] [CrossRef]
Roesler B, Rabelo-Gonçalves E, Zeitune J (2014). Helicobacter pylori and upper gastrointestinal diseases: A review. Health 6: 263–273. DOI 10.4236/health.2014.64039. [Google Scholar] [CrossRef]
Schloss JM, Colosimo M, Airey C, Masci PP, Linnane AW, Vitetta L (2013). Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPNA systematic review. Clinical Nutrition 32: 888–893. DOI 10.1016/j.clnu.2013.04.007. [Google Scholar] [CrossRef]
Selwan EM, Edinger AL (2017). Branched chain amino acid metabolism and cancer: The importance of keeping things in context. Translational Cancer Research 6: S578–S584. DOI 10.21037/tcr.2017.05.05. [Google Scholar] [CrossRef]
Serot JM, Barbe F, Arning E, Bottiglieri T, Franck P, Montagne P, Nicolas JP (2005). Homocysteine and methylmalonic acid concentrations in cerebrospinal fluid: Relation with age and Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry 76: 1585–1587. DOI 10.1136/jnnp.2004.056119. [Google Scholar] [CrossRef]
Song H, Peng JS, Yao DS, Yang ZL, Liu HL, Zeng YK, Shi XP, Lu BY (2012). Serum metabolic profiling of human gastric cancer based on gas chromatography/mass spectrometry. Brazilian Journal of Medical and Biological Research 45: 78–85. DOI 10.1590/S0100-879X2011007500158. [Google Scholar] [CrossRef]
Song H, Wang L, Liu HL, Wu XB, Wang HS, Liu ZH, Li Y, Diao DC, Chen HL, Peng JS (2011). Tissue metabolomic fingerprinting reveals metabolic disorders associated with human gastric cancer morbidity. Oncology Reports 26: 431–438. [Google Scholar]
Stein TP, Koerner B, Schluter MD, Leskiw MJ, Gaprindachvilli T, Richards EW, Cope FO, Condolucci D (1997). Weight loss, the gut and the inflammatory response in aids patients. Cytokine 9: 143–147. DOI 10.1006/cyto.1996.0148. [Google Scholar] [CrossRef]
Szlosarek PW (2014). Arginine deprivation and autophagic cell death in cancer. Proceedings of the National Academy of Sciences of the United States of America 111: 14015–14016. DOI 10.1073/pnas.1416560111. [Google Scholar] [CrossRef]
Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Yokota K, Oguma K, Okada H, Shiratori Y (2005). The effect of eradicating Helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. American Journal of Gastroenterology 100: 1037–1042. DOI 10.1111/j.1572-0241.2005.41384.x. [Google Scholar] [CrossRef]
Tan SZ, Begley P, Mullard G, Hollywood KA, Bishop PN (2016). Introduction to metabolomics and its applications in ophthalmology. Eye 30: 773–783. DOI 10.1038/eye.2016.37. [Google Scholar] [CrossRef]
Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA (2013). Role of glutathione in cancer progression and chemoresistance. Oxidative Medicine and Cellular Longevity 2013: 1–10. DOI 10.1155/2013/972913. [Google Scholar] [CrossRef]
Trivanovic D, Plestina S, Honovic L, Dobrilla-Dintinjana R, Tanaskovic JV (2019). Final results of early non-invasive gastric cancer detection study using the serum pepsinogen test method in Croatian patients. Journal of Clinical Oncology 37: e15551. DOI 10.1200/JCO.2019.37.15_suppl.e15551. [Google Scholar] [CrossRef]
Vander Heiden MG, Cantley LC, Thompson CB (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324: 1029–1033. DOI 10.1126/science.1160809. [Google Scholar] [CrossRef]
Vashi P, Edwin P, Popiel B, Lammersfeld C, Gupta D (2016). Methylmalonic acid and homocysteine as indicators of vitamin B-12 deficiency in cancer. PLoS One 11: e0147843. DOI 10.1371/journal.pone.0147843. [Google Scholar] [CrossRef]
Vučetić M, Cormerais Y, Parks SK, Pouysségur J (2017). The central role of amino acids in cancer redox homeostasis: Vulnerability points of the cancer redox code. Frontiers in Oncology 7: 319. DOI 10.3389/fonc.2017.00319. [Google Scholar] [CrossRef]
Walenta S, Mueller-Klieser WF (2004). Lactate: Mirror and motor of tumor malignancy. Seminars in Radiation Oncology 14: 267–274. DOI 10.1016/j.semradonc.2004.04.004. [Google Scholar] [CrossRef]
Weintraub L (2015). Procysbi (delayed-release cysteamine bitartrate capsules)–Clinical Review. Supplemental NDA 203389/(b)(4)S-10. [Google Scholar]
Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, Lin JT (2009). Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology 137: 1641–1648. DOI 10.1053/j.gastro.2009.07.060. [Google Scholar] [CrossRef]
Wu H, Xue R, Tang Z, Deng C, Liu T, Zeng H, Sun Y, Shen X (2010). Metabolomic investigation of gastric cancer tissue using gas chromatography/mass spectrometry. Analytical and Bioanalytical Chemistry 396: 1385–1395. DOI 10.1007/s00216-009-3317-4. [Google Scholar] [CrossRef]
Xiao S, Zhou L (2017). Gastric cancer: Metabolic and metabolomics perspectives (Review). International Journal of Oncology 51: 5–17. DOI 10.3892/ijo.2017.4000. [Google Scholar] [CrossRef]
Xue R, Lin Z, Deng C, Dong L, Liu T, Wang J, Shen X (2008). A serum metabolomic investigation on hepatocellular carcinoma patients by chemical derivatization followed by gas chromatography/mass spectrometry. Rapid Communications in Mass Spectrometry 22: 3061–3068. DOI 10.1002/rcm.3708. [Google Scholar] [CrossRef]
Załęski A, Banaszkiewicz A, Walkowiak J (2013). Butyric acid in irritable bowel syndrome. Przegląd Gastroenterologiczny 8: 350–353. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |