DOI:10.32604/biocell.2021.016524

| BIOCELL DOI:10.32604/biocell.2021.016524 |  |
| Article |
Data-independent acquisition mass spectrometry quantitative proteomic analysis reveals that skin aging-related proteins differ between men and women
1Department of Dermatology, No. 1 Hospital, Anhui Medical University, Hefei, 230022, China
2Institute of Dermatology, Anhui Medical University, Hefei, 230032, China
3Anhui Ferry Dermotological Institute, Hefei, 230031, China
4Department of Oncology, The Second Affiliated Hospital of Anhui Medical University, Hefei, 230601, China
*Address correspondence to: Liangdan Sun, ahmusld@163.com; Sen Yang, yang2004sen@163.com
#These authors contributed equally to this work
Received: 15 November 2020; Accepted: 15 March 2021
Abstract: The skin is the largest organ of the human body, and its aging is visible to the naked eye. The aging rate of men and women is slightly different. This study compared the protein expression of skin samples on the curved forearms of 11 healthy women and 9 healthy men. Quantitative proteomics analysis found that the expression of epidermal proteins in men and women of the same age group was different. Compared with female skin, in male skin, 20 proteins were upregulated, and 7 proteins were downregulated. These data suggest that men and women have differences in the speed of skin aging. For the first time in this experiment, a non-invasive mass spectrometer was used to detect 27 different-related epidermal proteins between men and women. Compared with women, among the 20 epidermal proteins upregulated in men, their functions can be classified into antioxidants, epidermal lipid metabolism, signal transduction, membrane transport, and cell biological processes; the 7 downregulated proteins are involved in a variety of biological processes and inflammatory reactions. Our experiments have discovered epidermal proteins related to the differences between men and women, enriching the library of epidermal differential proteins between men and women and enriching the mechanism of skin aging between men and women from the perspective of epidermal differential proteins.
Keywords: Epidermal protein; Mass spectrometer; Skin barrier; Gender-related skin aging; Proteome
The skin is the largest organ of the human body, covering the entire body surface and playing an important barrier function (Basler et al., 2016; Niehues et al., 2018). The stratum corneum provides a gas-liquid barrier, tight junctions in the granular layer provide a liquid-liquid barrier, and Langerhans cells provide an immune barrier (Taieb, 2018). Therefore, the epidermal barrier is essential for the health of the skin and the entire body. At the same time, the skin is also the first organ to appear to age (Pullar et al., 2017). Skin aging refers to the destruction of the skin’s function, which makes the skin’s ability to resist external stimuli and conditioning weakened so that it cannot adapt to changes in internal and external environments.
The natural aging of the skin is called endogenous aging, and there are many mechanisms: for example, matrix metalloproteinases (MMPs) gradually increase with age and can degrade collagen in the dermis (which maintains the skin tensile strength and firmness) and other macromolecules, such as fibronectin and elastin in the extracellular matrix (Kammeyer and Luiten, 2015; Quan and Fisher, 2015). Because of the continuous increase of MMPs, the degradation rate of collagen is faster than its synthesis rate, which ultimately leads to skin aging (Lee et al., 2016). Reactive oxygen species (ROS) are produced in the metabolism of aerobic cells and include O2-, H2O2, HO2•, and •OH. Studies have found that as age increases, active oxygen free radicals in the body continue to increase. ROS can be produced in various ways, such as by ultraviolet light, environmental pollution, and mitochondrial respiration, among which ultraviolet radiation is the most important (Li et al., 2018). ROS are also natural by-products of cellular respiration. The imbalance between ROS production and clearance leads to DNA mutations and cell damage, impedes protein synthesis, and induces skin cell apoptosis (Stuart et al., 2014). When autophagy-deficient keratinocytes are subjected to oxidative stress, DNA damage and aging are abnormally increased (Gu et al., 2020).
Inflammation leads to skin aging through a variety of mechanisms, including the formation of ROS. This process consists of a variety of inflammatory mediators, including the complement cascade, histamine, cytokines, kinin, fibrinopeptides, NF-κB, free radicals, and eicosanoids, such as prostaglandins, thromboxane, and leukotrienes. Ultraviolet rays and free radicals oxidize cell membrane lipids to cause the release of arachidonic acid, which plays an important role in skin inflammation because it activates cyclooxygenase 2 (COX-2) to produce prostaglandins and leukotrienes (Baumann, 2018). NF-κB is a key regulator that promotes the expression of interleukins IL-1β and IL-6, tumor necrosis factor-α (TNF-α), and other pro-inflammatory cytokines (Lai et al., 2017). Inflammation may increase the effects of ultraviolet rays, damage cells, and eventually lead to skin aging (Suggs et al., 2014).
Skin aging caused by external conditions is called exogenous aging. For example, if the body is in a state of stress for a long time, skin aging is more obvious (Clatici et al., 2017). Living long term in an environment with high particulate matter (PM), a main component of air pollution, promotes the production of ROS, thus activating MMPs, such as MMP-1, MMP-2, and MMP-9, leading to the degradation of collagen and accelerating the aging of the skin (Tobin, 2017). In addition, ultraviolet rays, electronic devices, and smoking all contribute to the aging of the skin (Arjmandi et al., 2018; Farage et al., 2008; Han et al., 2014; Hao et al., 2019; Megna et al., 2017).
Many studies have shown that the type and content of skin protein are closely related to skin barrier function. When the type and content (up or down) change, it can cause aging and impaired function (Breitenbach et al., 2015; Ma et al., 2019). The development of mass spectrometry has enabled large-scale proteomics research. In this study, we analyzed the differences in epidermal protein expression between healthy men and women to identify differentially expressed proteins and explore possible mechanisms of skin aging.
We selected 20 healthy Chinese volunteers to participate in this study, including 11 women (age range: 24–72 years old) and 9 men (age range: 25–65 years old). The average age of men was 46.7778 ± 5.02985, and the average age of women was 40.1818 ± 6.03749. An independent sample t-test showed no difference in age between the two groups (P = 0.425535965). The inclusion criteria were as follows: skin integrity at the sampling site; female donors were not pregnant and not breastfeeding; donors had no skin disease; donors had not used glucocorticoids in the past month and had not used immunosuppressive drugs in the past three months; donors had no other systemic diseases; donors had no allergic reaction to the tape; and donor did not use any moisturizer or other cosmetics on the day of the experiment. This study followed the recommendations of the Medical Ethics Committee of Anhui Medical University, and all subjects provided written informed consent.
We applied two consecutive strips of tape on the curved skin on the upper forearm of each subject to extract protein. Before peeling the tape, we gently wiped the skin of the forearm with a sterile cotton ball. Stratum corneum samples were collected using 3M medical tape, which was quickly pressed back and forth for 1 min under uniform pressure. At the same position, we performed peeling five times in sequence. The interval between each strip of tape was 25 ± 5 s. To minimize variability, the same technician performed the procedure for all volunteers during the study.
Protein extraction, protein extraction quality control, and proteolysis
Protein extraction: We used a razor to cut each tape sample on a glass plate into small 0.5 cm × 0.5 cm pieces, and then placed them into the corresponding 1.5-mL centrifuge tubes. We then added 50 μL L3 lysate without sodium dodecyl sulfate (SDS) and 1× cocktail containing ethylenediaminetetraacetic acid (EDTA) at a final concentration of 0.2 M. Samples were placed on ice for 5 min, and a final concentration of 10 mM dithiothreitol (DTT) was added for overnight incubation. Samples were then centrifuged at 25,000 g at 4°C for 15 min to collect the supernatant. DTT was then added at 10 mM final concentration and incubated at 56°C in a water bath for 1 h. We then added iodoacetamide (IAM) at a final concentration of 55 mM and incubated the samples in the dark for 45 min. Samples were then centrifuged at 25,000 g at 4°C for 15 min to collect the supernatant, which contained the protein solution.
Protein extraction quality control: We used Bradford analysis to measure protein concentration (Bradford, 1976). We performed sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with 30 μg of protein solution from each sample and 20 μL loading buffer, which were heated at 95°C for 5 min, centrifuged at 25,000 g for 5 min, and run on a 12% SDS polyacrylamide gel at 120 V constant voltage for 120 min. Gels were stained with Coomassie Brilliant Blue for 2 h and then decolorized (40% ethanol, 10% acetic acid) on a shaker 3 to 5 times for 30 min each.
For proteolysis, 100 μg protein/sample was hydrolyzed in 2.5 μg trypsin at 37°C for 4 h (protein:enzyme ratio = 40:1). Then, we added trypsin again in the same proportion and continued enzymatic hydrolysis at 37°C for 8 h. Next, the hydrolyzed peptide was desalted using a Strata X column and dried under a vacuum.
High pH reverse-phase separation
We took 10 μg of each sample for mixing. Then, we diluted 200 μg after mixing with 2 mL mobile phase A (5% ACN pH 9.8) and injected the sample into a Shimadzu LC-20AB liquid phase system with a Gemini C18 separation column of 5 μm and 4.6 × 250 mm. Elution was at a flow rate gradient of 1 mL/min: 5% mobile phase B (95% CAN, pH 9.8) for 10 min, 5% to 35% mobile phase B for 40 min, and 35% to 95% mobile phase B for 1 min. Phase B lasted 3 min, and 5% mobile phase B was equilibrated for 10 min. We monitored the elution peak at a wavelength of 214 nm and collected one component every minute. We combined the sample with the chromatographic elution peak to obtain 10 components and then freeze-dried the samples.
We reconstituted the dried peptide sample with mobile phase A (2% ACN, 0.1% FA), centrifuged at 20,000 g for 10 min, and injected the supernatant. We performed separation using a Thermo Corporation UltiMate 3000 ultra-high-performance liquid chromatographer (UHPLC). The sample was first enriched in a trap column and desalted and then connected in series with a self-packed C18 column (150 μm inner diameter, 1.8 μm column particle size, 25 cm column length). We then separated the sample at a flow rate of 500 nL/min through the following effective gradient: 5 min, 5% mobile phase B (98% ACN, 0.1% FA); 5–160 min, mobile phase B linearly increased from 5% to 35%; 160–170 min, mobile phase B increased from 35% to 80%; 170–175 min, 80% mobile phase B; and 176–180 min, 5% mobile phase B. The nanoliter liquid separation end was directly connected to the mass spectrometer.
Data-dependent and data-independent acquisition analysis
The dry peptide sample was re-dissolved in buffer A (2% ACN, 0.1% FA), centrifuged at 20,000 g for 10 min, and the supernatant was injected. We performed the separation using a Thermo Corporation UltiMate 3000 UHPLC. We first put the samples into the trap column for enrichment and desalination. Each sample was then connected in series with a self-assembled C18 column (150 µm inner diameter, 1.8 µm column diameter, 25 cm column length). We separated the peptide at a flow rate of 500 nL/min by the following effective gradient: 0–5 min, 5% buffer B (98% ACN, 0.1% FA); 5–160 min, 5–35% B buffer; 160–170 min, 35–80% B buffer; 170–175 min, 80% B buffer; and 176–180 min, 5% buffer B. The nL liquid separation end was directly connected to the mass spectrometer. The liquid-phase-separated peptides were sprayed into a nanometer ESI source and entered into a q-PRECISION HF tandem mass spectrometer for data-dependent and data-independent acquisition (DDA and DIA) analysis. We set the DDA MS parameters as follows: (1) MS: 350–1500 scanning range (m/z); 60,000 resolution; 3E6 AGC objectives; 50 MS maximum injection time (MIT); 30 cycle count; an NCE 28; (2) HCD-MS/MS: 15,000 resolution; 1E5 AGC objectives; 100 MS MIT; charge exclusion, exclusion 1, 7, 8, >8; Filter dynamic exclusion duration 30 s; 2.0 m/z isolation window. We used the same nanometer liquid chromatography system and gradient for analysis. We set the DIA MS parameters as follows: (1) MS: 350–1500 scanning range (m/z); 20 PPM MS tolerance; 120,000 resolution; 3E6 AGC objectives; 50 MS MIT; 50 cycle count; and (2) HCD-MS/MS: 1.7 m/z isolation window; 30,000 resolution; 1E5 AGC objectives; Automatic MIT; 50 cycle count; filter dynamic exclusion duration was 30 s; Step NCE: 22.5, 25, 27.5.
We used MaxQuant (version 1.5.3.30; (Cox and Mann, 2008) to identify DDA data. The final spectral library was constructed with peptide/protein entries that met the false discovery rate (FDR) ≤1%. Data were reviewed with the UniProtKB/SwissProt H. sapiens proteome database. We selected the following parameters: (1) Enzyme: trypsin; (2) Minimum peptide length: 7; (3) FDR and protein FDR at PSM level: 0.01; (4) Fixed modification: amino methylene (C); and (5) Variable modification: oxidation (M); Acetyl group (N terms of protein). We analyzed DIA data using Spectronaut (Bruderer et al., 2016), which calibrated the retention time using indexed retention time(iRT) peptides. We estimated FDR by the mProphet scoring algorithm, which accurately reflects the matching degree of ion pairs. Then, based on the target decoy model applicable to SWATH-MS, we accomplished false positive control with an FDR <1%. We used MSstats (Choi et al., 2014) to screen gender-differentiated proteins using differential multiples ≥1.5 and P < 0.05 as criteria.
Quantitative protein detection
Using conventional data correlation acquisition (DDA) mass spectrometry (MS), we established and analyzed a protein spectrum library of the anterior upper arms of 20 healthy people. We identified a total of 9005 peptides and 1631 proteins. Next, we used the DIA method for MS data acquisition. A total of 1,318 proteins were obtained (Supplementary Tab. S1), and then we used the limma software package to obtain 27 gender-related differential proteins with folding change ≥1.5 and P < 0.05 as the criteria for screening differentially expressed proteins (DEPs) (Supplementary Tab. S2). Among these differences, 20 epidermal proteins were upregulated, and 7 were downregulated in men compared with women (Fig. 1). Principal component analysis showed that the DEPs were divided into two independent clusters that could distinguish the male group from the female group (Fig. 2). Tabs. 1 and 2 provide the functional classification and Genecard information of the upregulated and downregulated DEPs.
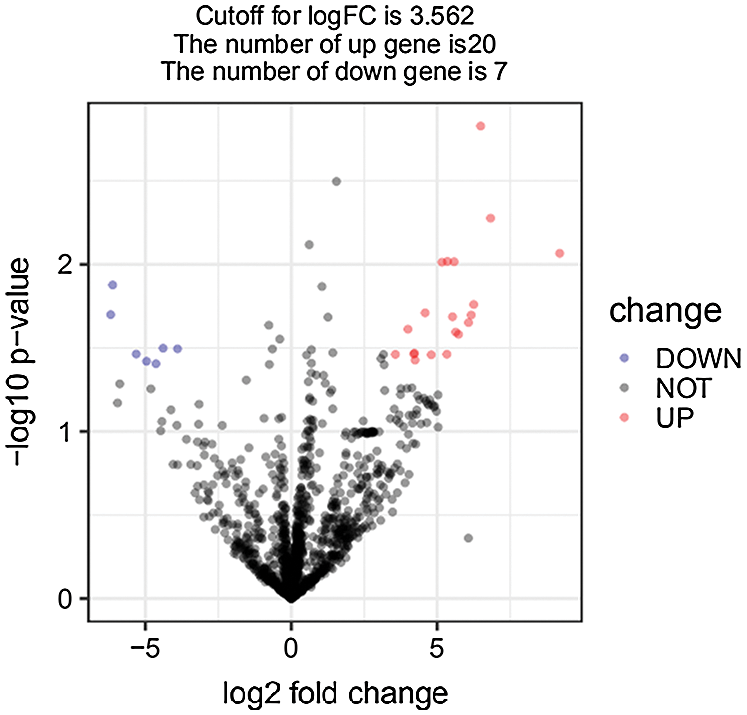
Figure 1: Identification of DEPs in male skin samples.
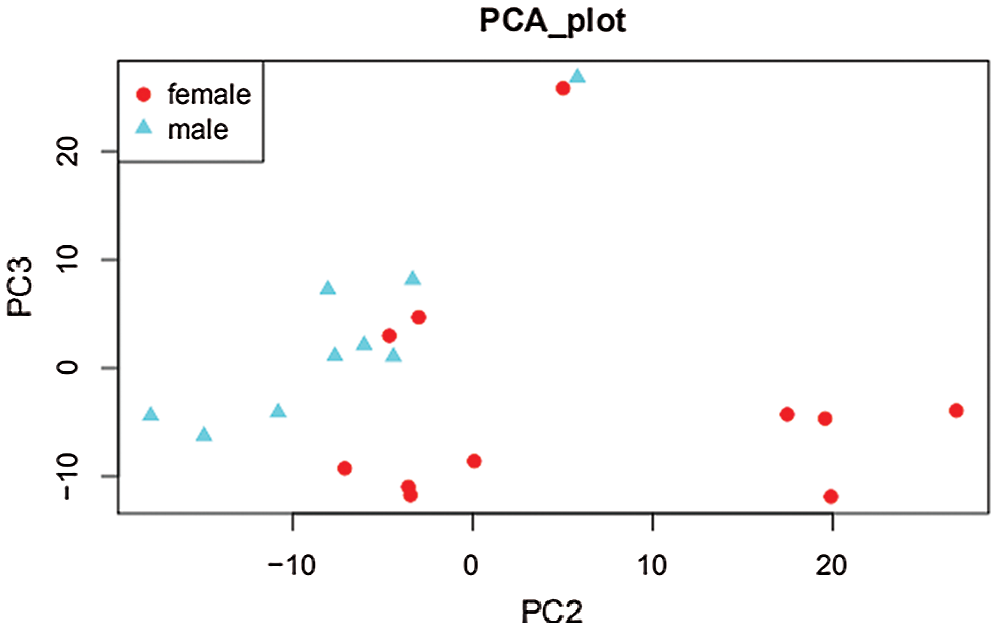
Figure 2: Principal component analysis.


Gene ontology enrichment analysis
To evaluate the functional importance of all identified proteins, we used Blast2GO software for gene ontology (GO) annotation (Fig. 3). “Signaling” was among the more meaningful biological processes, and the proteins included CAH2, S10AD, RAB25, VDAC3, STK25 ARGAL, DNPEP, LRC59, SEC13, and DNM1L. Significant molecular functions include “immune system processes”, the corresponding proteins are: CA2, FUCO2, S10AD, etc., “antioxidant activity”, including GPX4. “Molecular function modifiers” include proteins DNM1L, ARGAL (Supplementary Tab. S3).
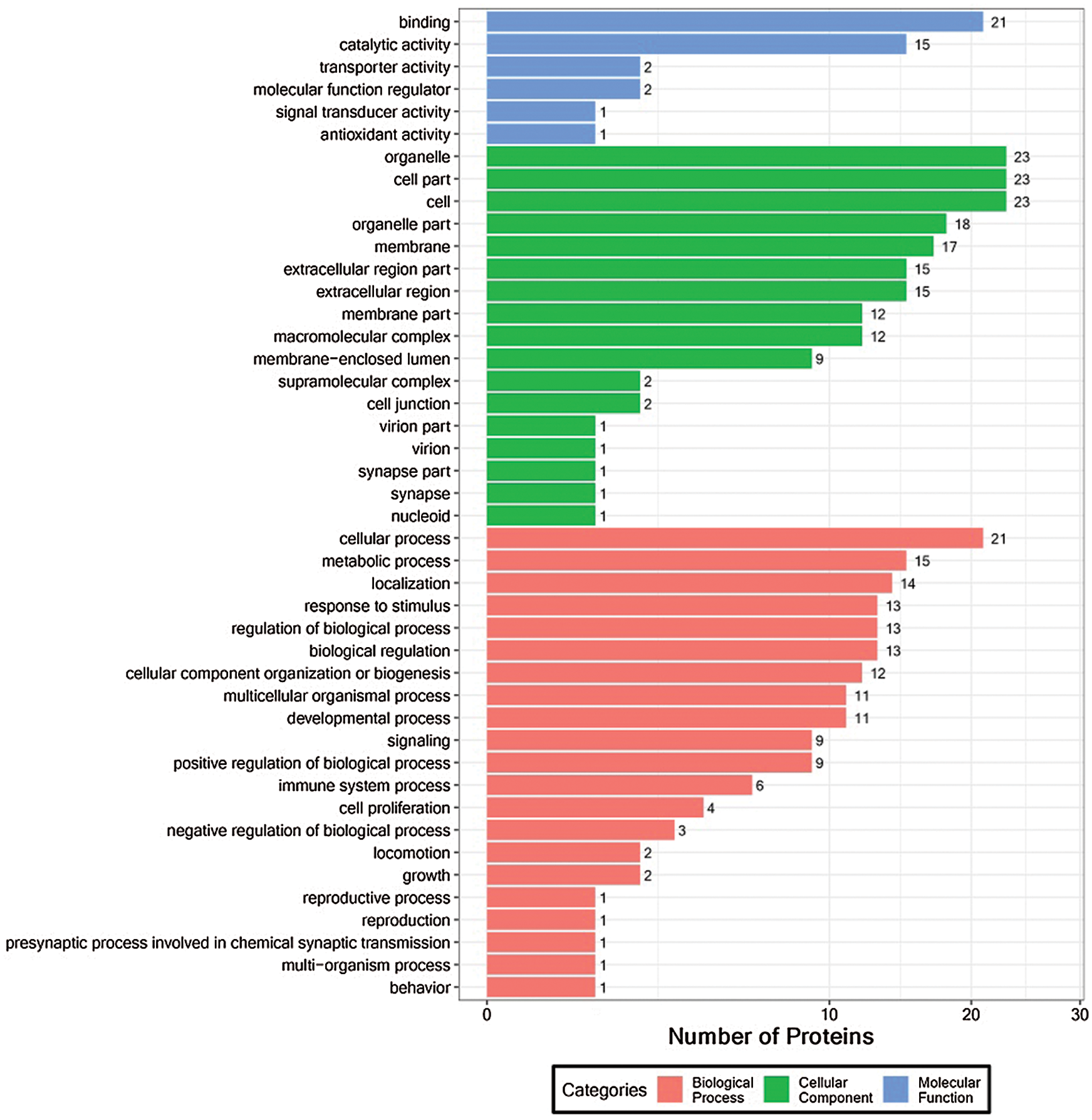
Figure 3: Functional GO classification of DEPs.
Next, on the basis of these results, we generated a GO functional classification map to represent all DEPs and to distinguish upregulated and downregulated proteins (Fig. 4). It was evident that, in general, both upregulated and downregulated DEPs participated in common structural or functional processes. For some GO terms, however, in the biological process and molecular function categories, we detected specific enrichment of proteins (such as “presynaptic process involved in chemical synaptic transmission, “reproduction,” and “antioxidant activity process”) or downregulation of proteins (such as the multi organism processes; Fig. 4; Supplementary Tab. S4).
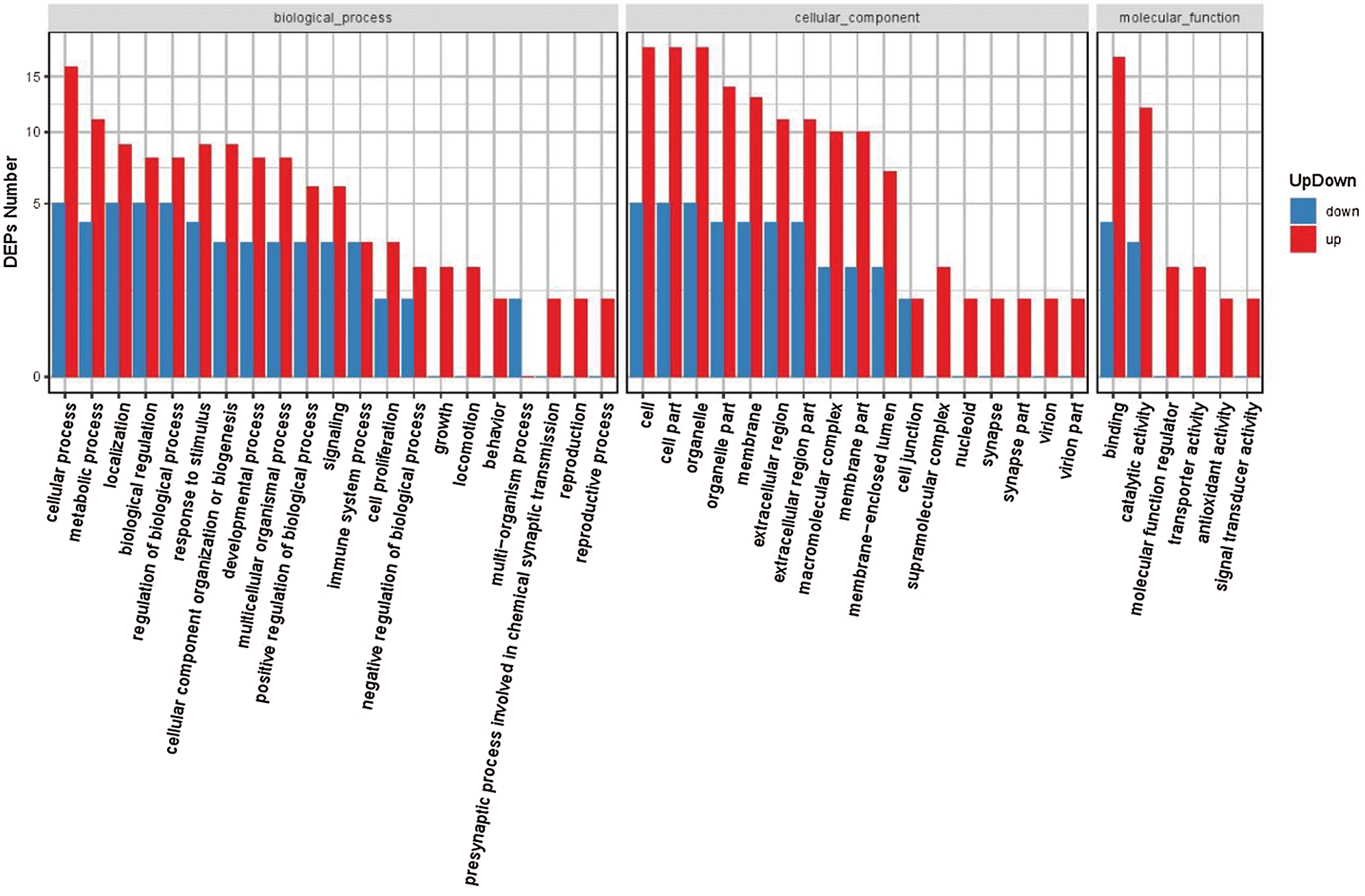
Figure 4: GO classification of upregulated and downregulated DEPs.
Karyotic orthologous groups classification
The Karyotic Orthologous Groups (KOGs) database attempts to classify the proteins into orthologous proteins. Each KOG entry contains a series of orthologs or paralogs. We compared the identified proteins with the KOG database to predict the potential functions of these proteins and perform functional classification statistics. The most representative KOG classification is “cell processes and signaling,” which showed predominant association of DEPs with intracellular trafficking, secretion, vesicular transport, and signal transduction mechanisms (Fig. 5; Supplementary Tab. S5).
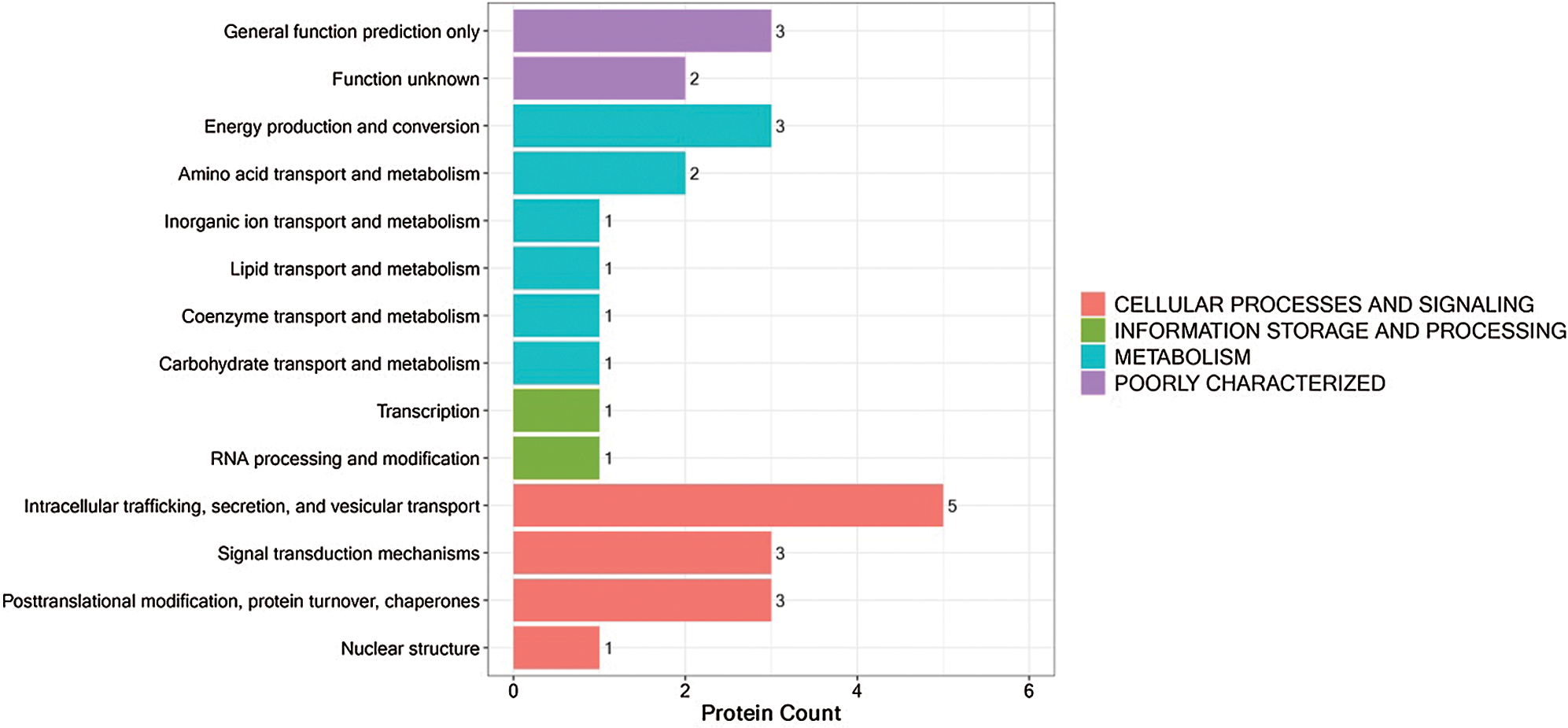
Figure 5: KOG functional annotation of DEPs.
Kyoto encyclopedia of genes and genomes pathway analysis
We performed Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis to further characterize the biological functions of identified DEPs (Fig. 6). Most DEPs participate in common pathways; some upregulated proteins participate in unique pathways, such as “mTOR signaling”; and some downregulated proteins participate in unique pathways, such as “P13K-Akt signaling” (Supplementary Tab. S6).
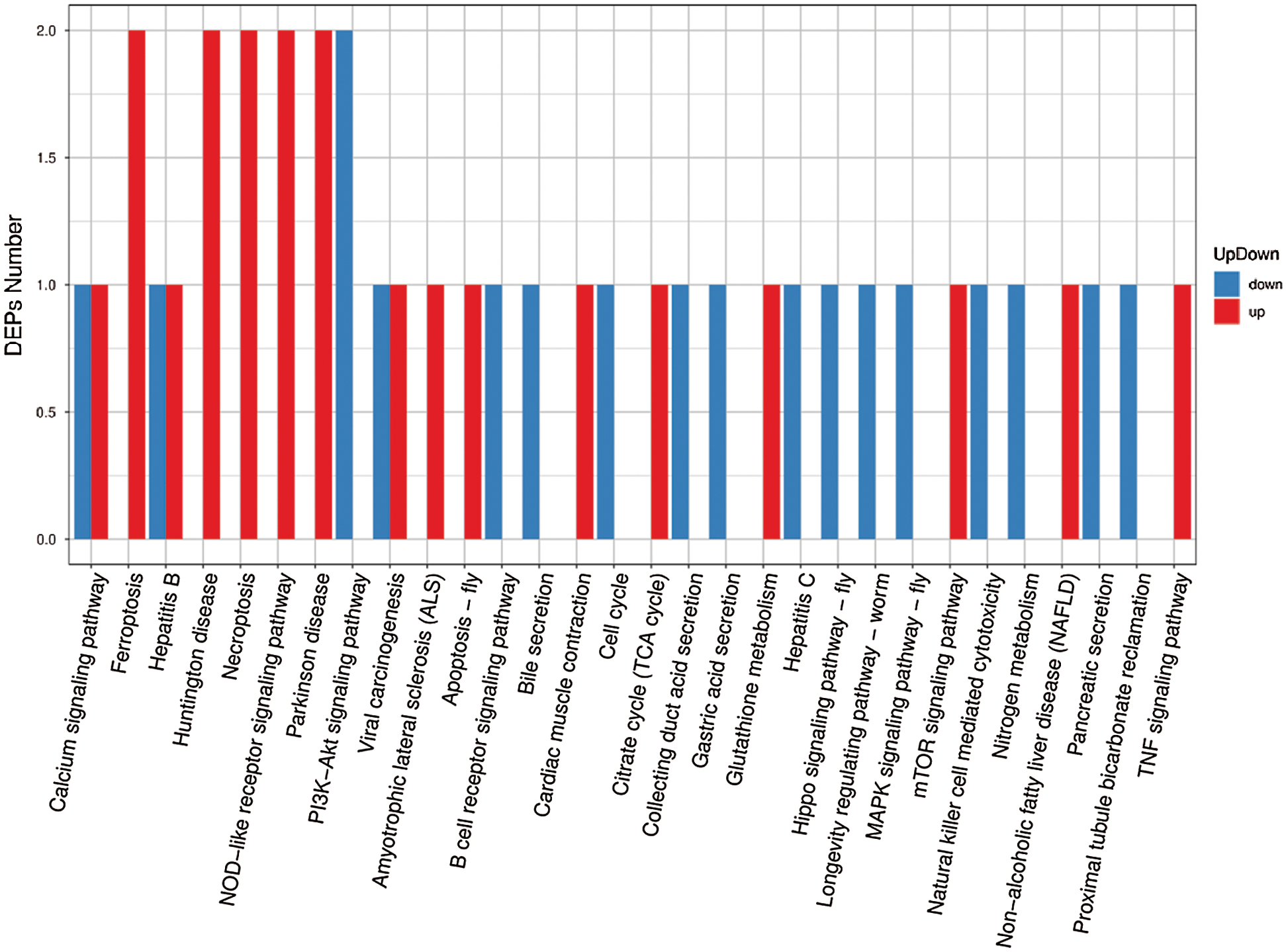
Figure 6: KEGG pathway classification of DEPs.
In organisms, different proteins coordinate with each other to perform their biological functions. Pathway-based analysis can help us further understand their biological functions. As shown in Fig. 7, the biological function of its down-regulated protein1433T is “aging.” (Supplementary Tab. S7).
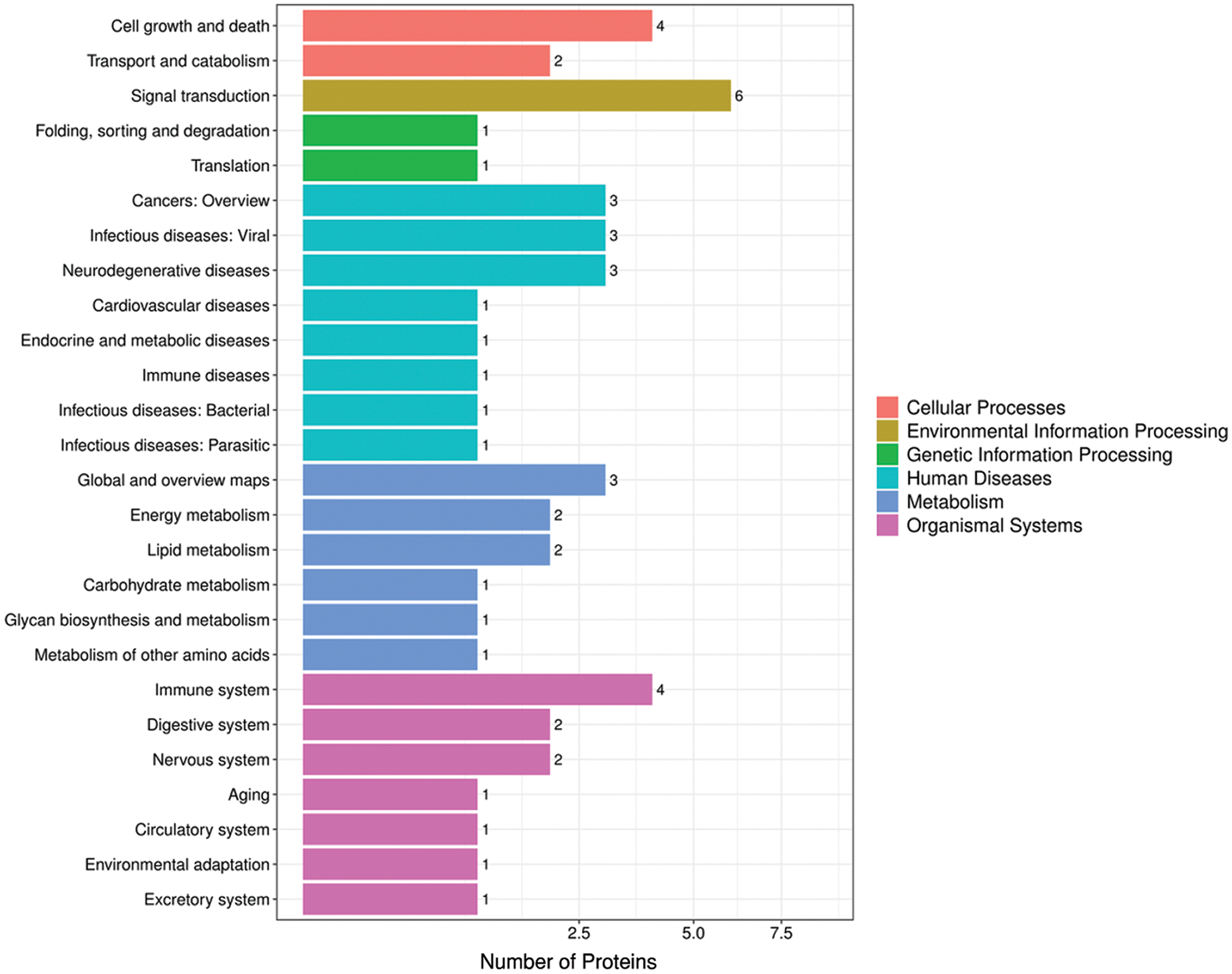
Figure 7: Pathway annotated histogram.
Fig. 8 shows the top three biological functions among our identified DEPs. Among these pathways, “Ferroptosis” was rich in GPX4 and VDAC3, which were both upregulated in men (Supplementary Tab. S8).
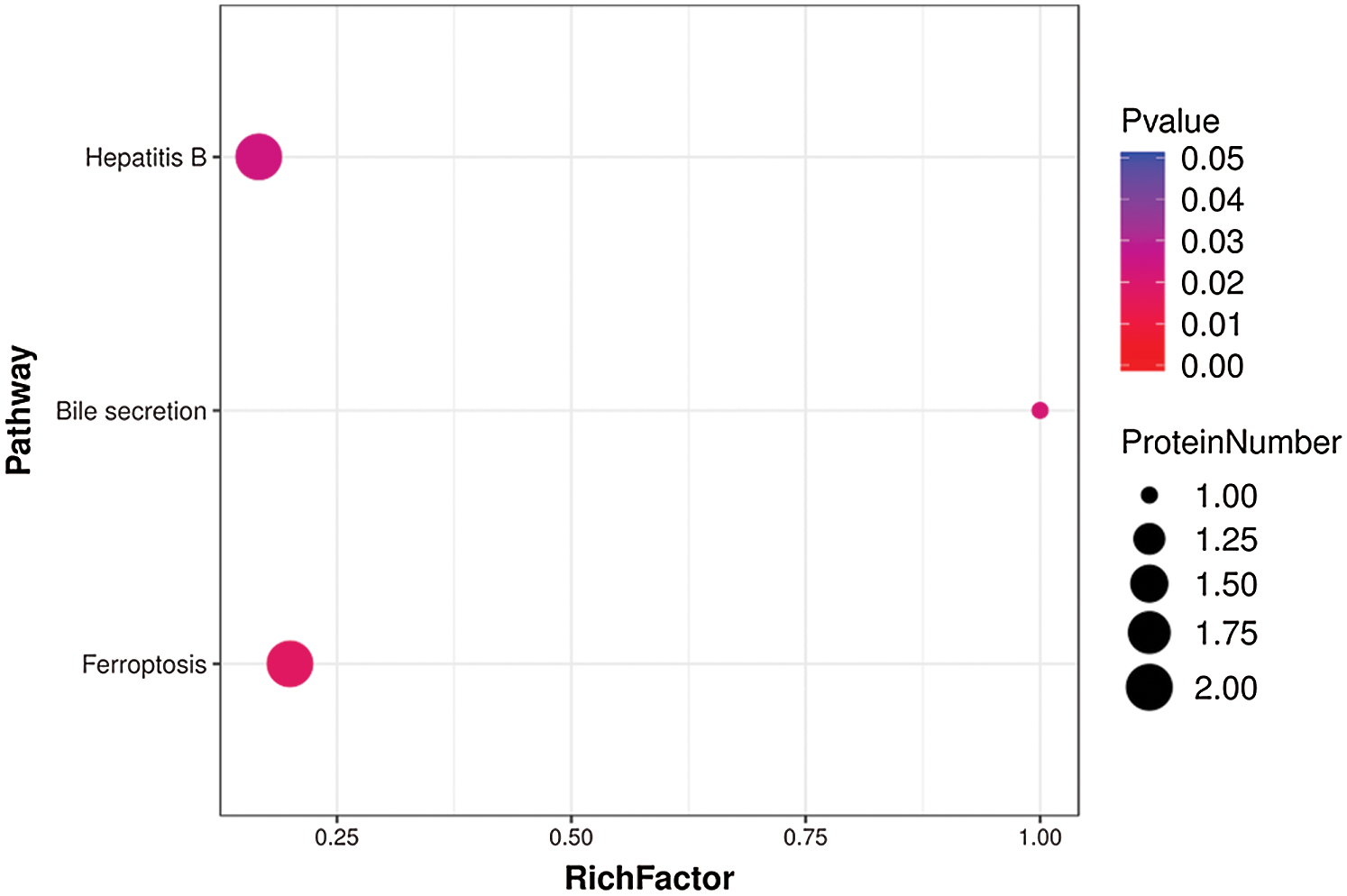
Figure 8: Top three pathways enriched in DEPs from aged skin.
Subcellular localization and protein-protein interaction network analyses
Next, we used WoLF-PSORT to predict the subcellular localization of the identified DEPs (Fig. 9). The results showed that nucleus, intracellular, extracellular, and mitochondria were the most representative locations (Supplementary Tab. S9).
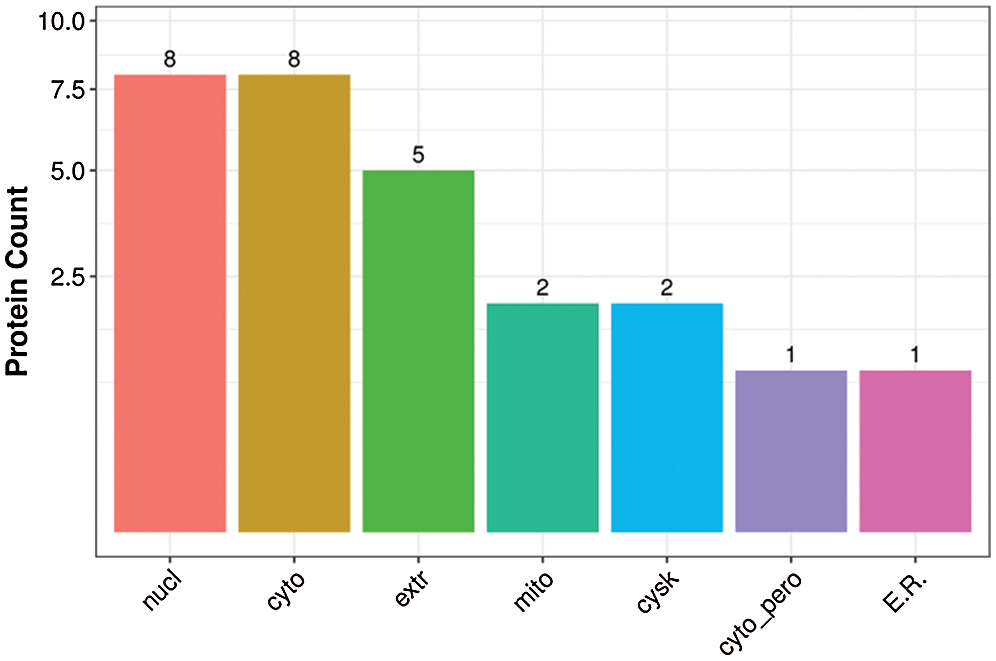
Figure 9: Subcellular localization of DEPs.
We then imported the DEPs into the STRING database (String11.0) and performed network interaction analysis of protein-protein relationships in the first 100 confidence intervals (Fig. 10). We identified one main cluster with a broad set of functions known as metabolic processes (including ACLY, GPDM, ILVB2, TOM40, QCR2, and DNPEP). This cluster also may have been related to “lipid metabolism” and “signal transducer activity” (Supplementary Tab. S10).
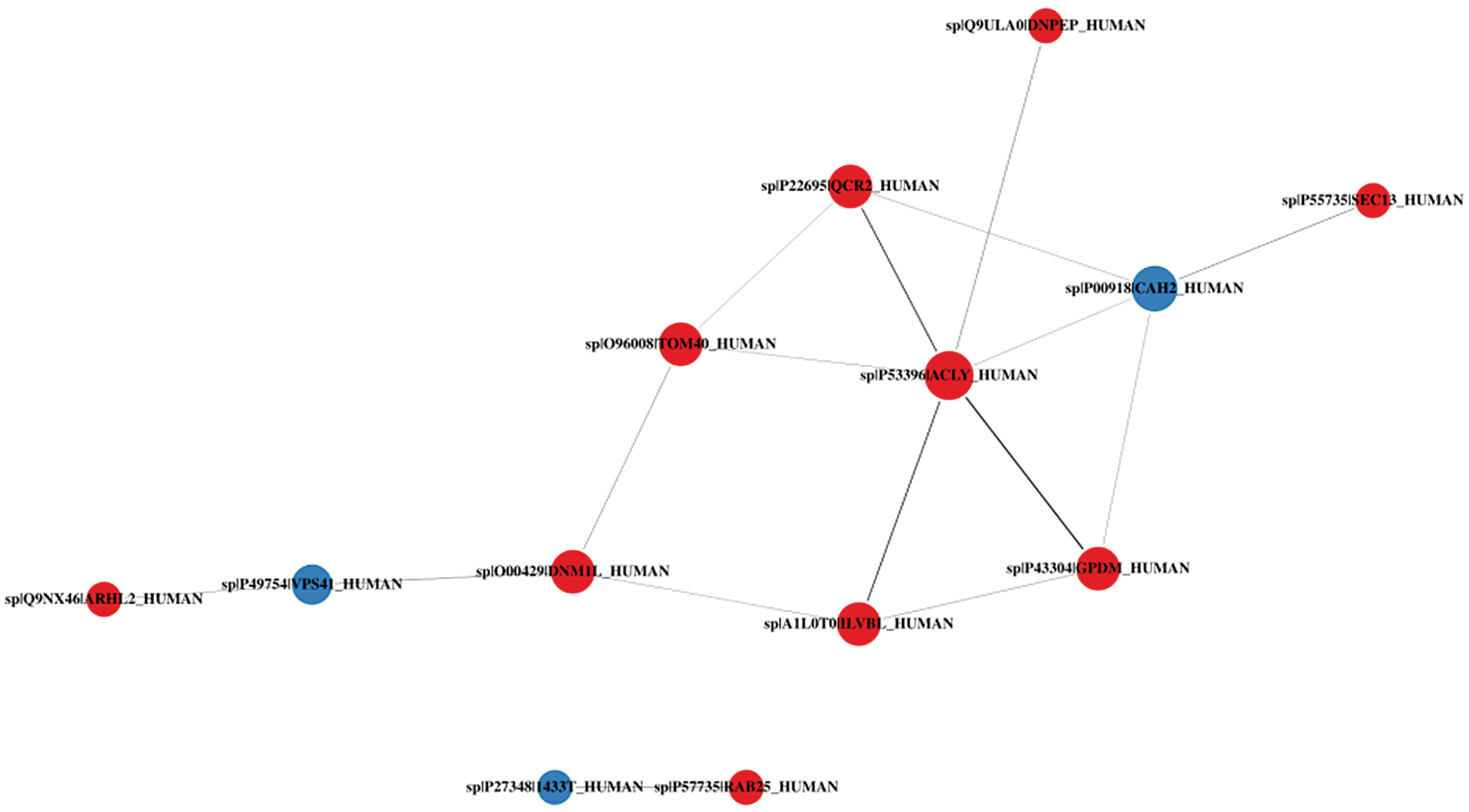
Figure 10: Protein-protein interaction network diagram of DEPs.
In this study, we used noninvasive MS to quantitatively analyze epidermal proteins. From 11 healthy Chinese female skin samples and 9 healthy Chinese male skin samples, we found a total of 27 types of epidermal proteins related to male and female differences. Previous studies on the differences in skin aging between men and women only studied the effects of estrogen, androgens, and their receptors (Lephart, 2018; Sator et al., 2004; Makrantonaki and Zouboulis, 2009; Zouboulis et al., 2007). Through dermoscopy and skin ultrasound examination of the skin conditions of men and women, it has been found that the number and density of superficial wrinkles in women were greater than in men (Dabrowska et al., 2018). Our study used MS to quantitatively analyze epidermal proteins and found 27 proteins (20 upregulated and 7 downregulated) that were differentially expressed in male skin.
We used GO enrichment analysis, KOG classification, and KEGG enrichment analysis to clarify the functional role of many upregulated proteins. For example, GPX4 enrichment is related to “antioxidant activity”. Antioxidants help fight free radical damage and help maintain healthy skin, reduce light damage, and prevent skin wrinkles and inflammation through intracellular signaling pathways involved in skin damage (Nguyen and Torres, 2012; Rinnerthaler et al., 2015). GPX4 belongs to the glutathione peroxidase family and is a key protein for the upstream regulation of ferritin metabolism (Seibt et al., 2019). It can catalyze the reduction of hydrogen peroxide, organic hydroperoxide, and lipid hydroperoxide, thereby protecting cells from oxidative damage. Studies have found that deletion of GPX4 reduced keratinocyte adhesion and increased lipid peroxidation and COX-2 levels in keratinocytes and in the entire skin, whereas the absence of GPX4 in the epidermis caused epidermal hyperplasia, inflammatory skin infiltration, hair follicle deformation, and hair loss (Sengupta et al., 2013). It is well known that skin surface lipids, such as squalene, sebacic acid, linoleic acid, and cholesterol (Niki, 2015; Kim and Karadeniz, 2012), have strong skin moisturizing and antioxidant effects and can effectively prevent free radical chain reactions and inhibit sebum peroxidation. Squalene by-products (mostly in the form of peroxidation) can cause acne and may cause skin sagging (wrinkles) (Pham et al., 2015). Once these lipids undergo oxidative stress reactions, the skin is prone to inflammatory reactions, destroying the skin barrier function and promoting skin aging (Mcdaniel et al., 2018). COX-2 is an inflammatory mediator (Tang et al., 2017), and its expression in keratinocytes is positively correlated with age (Surowiak et al., 2014). Ultraviolet-A and ultraviolet-B irradiation can induce increased COX-2 expression in keratinocytes (Chun and Langenbach, 2007), so GPX4 can reduce sebum peroxidation and COX-2 content to a certain extent, which is essential for maintaining skin barrier function and anti-aging.
LX15B, IAH1, and GPDM are related to “lipid transport and metabolism.” LX15B is a member of the lipoxygenase family of structurally related non-heme iron dioxygenases involved in the production of fatty acid hydroperoxides, converting arachidonic acid (AA) to 15S-hydroperoxodicarbon tetraenoic acid/(15S)-HPETE. It also acts on linoleic acid to produce 13-hydroxyoctadecadienoic acid/13-HPODE (Setkowicz et al., 2015). AA plays an important role in the development of disease in the epidermis and can synthesize the prostaglandin family through COX-1 and COX-2. AA can also synthesize the leukotriene family (Ruzicka and Printz, 1984) through 5-lipoxygenase. Many skin diseases are related to prostaglandins or related compounds. PGD2 and PDE2are related to inflammation and can promote skin capillary dilatation and erythema production (Ikai and Imamura, 1988). Therefore, some anti-inflammatory drugs currently targeted at the skin mainly inhibit skin microsomal prostaglandin synthase (Ziboh, 1973). B4 (LTB4) is also a well-known inflammatory factor that promotes methicillin-resistant Staphylococcus aureus (MRSA) infection through its receptor B leukotriene receptor 1 (BLT1) (Brandt et al., 2018). Additionally, 5-lipoxygenase (5-LO) product inhibition of leukotriene B4 (LTB4) and cysteinyl leukotriene (cysLTs) can regulate the inflammatory response and improve skin wound healing (Guimaraes et al., 2018). Therefore, the oxidative metabolism of AA in the epidermis leads to the formation of a variety of inflammatory mediators, causing inflammatory erythema, edema, and hyperalgesia in the skin, and has a destructive effect on skin barrier function (Aked et al., 1986). As a member of the lipoxygenase family, LX15B plays an important role in AA metabolism, reduces the excessive accumulation of AA in the epidermis, greatly reduces the inflammatory response, and is critical to maintaining skin barrier function. At the same time, LX15B can generate linoleic acid to produce 13-hydroxyoctadecadienoic acid/13-HPODE, and this process can be used to regulate the differentiation of skin cells, eventually forming a suitable water barrier layer (Nugteren and Kivits, 1987), thus maintaining normal skin barrier function.
IAH1 (isoamyl acetate hydrolyzing esterase 1) is involved mainly in lipid metabolism according to GO enrichment. Studies have found that it is widely distributed in the liver, kidney, lung, muscle, and accessory fat. The expression of Iah1 protein inhibits the expression levels of lipid metabolism-related genes (such as Cd36 and Dgat2). The increase of Cd36 mRNA level in the liver is related to the increase of hepatocellular carcinoma and the accumulation of hepatic triglycerides. Therefore, the expression of Iah1 can avoid the formation of fatty liver (Masuya et al., 2020; Kobayashi et al., 2016). Aging is related to the imbalance of glucose and lipid metabolism. The accumulation of liver lipids may lead to serious liver and systemic consequences, including steatohepatitis, cirrhosis, systemic glucose metabolism impairment, metabolic syndrome, and aging (Gong et al., 2017). The aging of the skin and the whole body is closely related to the decline of liver function and lipid disorders (Bertolotti et al., 2014). Fatty liver patients have decreased liver detoxification, endocrine disorders, and vitamin synthesis disorders. This results in skin melanin deposition, darkening, roughness, dullness, and loss of luster; fatty liver over the long term will make the skin lose elasticity and accelerate aging.
GPDM is enriched in “lipid transport and metabolism” and “energy production and conversion.” The gene card shows that the protein is localized in the inner mitochondrial membrane, and FAD is used as a cofactor to catalyze the conversion of 3-phosphoglyceride into phosphodihydroxyl acetone ester. Glycolysis is essential in the cell and can improve the tolerance of tissue cells to hypoxia (Wu et al., 2020). GPDM has a unique role in mediating glucose oxidation. During acute gram-negative bacilli (LPS) exposure, GPDM activity drives forward electron transport and the production of acetyl-CoA, thereby enhancing histone acetylation and inflammation. During long-term LPS exposure, however, continuous GPDM activity eventually will limit histone acetylation and inflammatory gene-induced acetyl-CoA production, so GPDM activity supports the inflammatory response after acute microbial exposure, but it helps suppress the inflammatory response after prolonged microbial exposure. Inflammation damages tissue defense capabilities, and GPDM plays a key role in maintaining such inflammatory balance (Langston et al., 2019).
Additionally, RAB25, VDAC3, STK25 ARGAL, DNPEP, LRC59, SEC13, and DNM1L are enriched in “signal transduction mechanisms.” RAB25 is a member of the Ras superfamily of small GTPases, which are involved in membrane transport and cell survival, regulate epithelial morphology, and create polarity in epithelial cells. Each layer of the epidermis expresses different markers, such as loricrin, involucrin, keratin family members, filaggrin, and integrins, which are essential for epidermal differentiation and functional maturation of the skin barrier. Among them, integrins α6, β4, and β1 are abundantly expressed on the basolateral side of the basal cell layer at the interface with the dermis. The absence of β1 integrin causes severe epidermal inflammation (Brakebusch et al., 2000). A lack of auxin results in abnormal expression of differentiated genes, reflecting the important role of integrins in the differentiation and proliferation of keratinocytes and their interaction with the extracellular matrix on the basement membrane. Deletion of Rab25 can impair the transport of integrin, so Rab25 plays an important role in regulating the differentiation and proliferation of keratinocytes. A lack of Rab25 will disrupt epidermal differentiation and skin barrier function, leading to increased transdermal water loss and reduced skin moisture (Jeong et al., 2019). Therefore, RAB25 is essential for maintaining skin barrier function.
VDAC3, a voltage-dependent anion channel (VDAC), belongs to the mitochondrial pore protein family. VDACs are small, intact membrane proteins that can cross the mitochondrial outer membrane and conduct ATP and other small metabolites. The VDAC3 carrying the VDAC1 N-terminus can complement mitochondrial respiration and ROS regulation of yeast cell protein deficiency. Such chimeras prolong life, suggesting a protective role in biological metabolism (Reina et al., 2010). In addition, VDAC3 is also involved in ferroptosis. The excessive accumulation of iron in the body can lead to aging (Wang et al., 2020). The upregulation of VDAC3 can reduce the production of lipid ROS and inhibit iron accumulation, thereby slowing down aging (Yang et al., 2020).
The enzyme encoded by STK25 plays a role in the serine-threonine liver kinase B1 (LKB1) signaling pathway, regulating the neuronal polarization and morphology of the Golgi body. The NF-κB pathway, which is closely related to skin aging, can be blocked by protein modification and signal transduction pathways (Adler et al., 2007).
As for the other proteins, ARGAL does not increase the risk of pigmentation and skin basal cell carcinoma (Stacey et al., 2008). DNPEP may play an important role in intracellular protein and peptide metabolism, but its role in the skin needs further study. LRC59 is an important positive regulator of DDX58-mediated type I IFN signal transduction. DDX58/RIG-I is a key pattern recognition receptor for viral RNA, which plays a vital role in antiviral immunity and maintains immune homeostasis (Xian et al., 2020).
SEC13 participates in the mTOR signaling pathway, is a component of the endoplasmic reticulum and nuclear pore complex, and plays an important role in protein transport. It can activate class I phosphoinositide 3-kinase (PI3K) and then activate the mechanistic target of rapamycin complex 1 (mTORC1) signaling network to guide metabolism and the growth of cells. The dysregulation of this pathway is related to many diseases, including cancer, diabetes, autism, and aging (Dibble and Cantley, 2015).
DNM1L mediates mitochondrial and peroxisomal division and is involved in developmental regulation of apoptosis and programmed necrosis. This protein plays a key role in mitochondrial fission (Sheffer et al., 2016).
ACLYis ATP citrate synthase and is the main enzyme responsible for the synthesis of cytoplasmic acetyl-CoA in many tissues. In previous studies, ACLY was found to be upregulated in normal human skin fibroblasts and normal human skin keratinocytes when a series of well-known purified antioxidants were applied for 24 h (Gruber and Holtz, 2010). Most antioxidants may affect the synthesis of skin lipids, and acetyl-CoA is an important component of the synthesis of sebum that can be used in many important biosynthetic pathways, including for fat formation and cholesterol generation. Cholesterol, as a keratinocyte lipid, is an important component to connect and stabilize the epidermis (Ramsing et al., 1995; Sochorova et al., 2019). It plays an important role in maintaining stability and moisturizing. We speculated that ACLY is involved mainly in the production of fat and cholesterol, which is essential for maintaining the stability of the skin barrier function and keeping the skin younger.
Mitochondrial outer membrane translocation enzyme-40 kD (TOMM40) is the key channel for various enzymes on the mitochondrial outer membrane to enter mitochondria, directly affecting mitochondrial function. The G/A polymorphism at locus 45395619 of the second intron of the TOMM40 gene is associated with longevity (Wang et al., 2020). ILVBL is related to “amino acid transport and metabolism” and may be involved in the metabolism of branched amino acids or pyruvate (Chang et al., 2017) and participate in the degradation of fatty acids (Kitamura et al., 2017). EMAL1 regulates the assembly and organization of the microtubule cytoskeleton and may play a role in regulating the direction of the mitotic spindle and the direction of the cell division plane. HNRH3 is related to “RNA processing and modification” and is involved in the splicing of messenger RNA, which is downregulated during aging (Holly et al., 2013). QCR2 is related to “energy production and conversion” and is a component of panthenol-cytochrome c oxidoreductase, a multi-subunit transmembrane complex that is part of the mitochondrial electron transport chain that drives oxidative phosphorylation.
Among the 7 downregulated proteins, KEGG results indicated that 1433T is involved in “aging”. It antagonizes growth factor signal transduction on the internal nuclear membrane, which is an important driver of pathological fibrosis in multiorgan systems. In humans, in heterozygotes of LEMD3 (LEM domain-containing protein 3), some skin collagenomas and elastomas (from abnormal TGF signaling) occur and affect dermal fibroblast metabolic processes (Chambers et al., 2018).
CAH2, IGHG3, and FUCO2 are related to the “immune system process”. Carbonic anhydrase (CA) is a universally expressed enzyme that reversibly synthesizes carbon dioxide and water into bicarbonate and protons. CAH2 is almost completely expressed in differentiated keratinocytes. This enzyme is involved in maintaining cell pH, water transport, and ion homeostasis. Many skin diseases are related to the destruction of the skin microbial environment. For example, S. aureus likes neutral pH. Skin disturbance of the surface pH value may cause colonization and infection by skin microorganisms, thereby promoting the occurrence of inflammatory skin diseases (Kamsteeg et al., 2007). Therefore, an increase in CAH2 will destroy the optimal PH value of the epidermis, leading to the destruction of the skin barrier. Studies have found that carbonic anhydrase (CAH1) is a candidate protein for human skin aging. Immunoglobulin secreted by IGHG3 mediates the response period of humoral immunity, leading to the elimination of binding antigens, and it participates in the P13K-Akt signaling pathway. Studies have shown that this protein is closely related to alopecia areata (a nonscarring inflammatory hair loss disease) and may be involved in the humoral immune mechanism (Subramanya et al., 2010). In addition, it is upregulated in vascular neuropathy and participates in B-cell antigen recognition (Steck et al., 2011). FUCO2 is a member of the glycosylhydrolase 29 family, and its mechanisms related to the skin have yet to be studied.
S10AD is related to “signaling”, is a member of the S100 protein family, and contains two EF-hand calcium-binding motifs. S100 protein is located in the cytoplasm or nucleus of a large number of cells and is involved in the regulation of many cellular processes, such as cell cycle progression and differentiation. Previous studies have shown that the expression level of S10AD in melanoma specimens is significantly higher than that of normal skin (Xiong et al., 2019). Human VPS41 protein is required for Golgi vacuole assembly and vacuole transport. VPS41 regulates vacuole transport and is thought to play a role in pigmentation (Ibarrola-Villava et al., 2015).
We used Q Exactive quadrupole MS to perform non-invasive quantitative analysis of skin epidermal proteins related to male and female differences. For the first time, we identified 27 kinds of epidermal proteins related to male and female differences and explored their possible functions in skin aging. Compared with women, the up-regulated epidermal proteins in men are: ILVBL, TOMM40, RAB25, VDAC3, EMAL1, LX15B, STK25, ARGAL, DNM1L, DNPEP, HNRH3, LRC59, BRI3B, IAH1, ACLY, SEC13, ARHL2, QCR2, GPDM, GPX4, their functions can be divided into antioxidants (GPX4), epidermal lipid metabolism (LX15B, IAH1, GPDM), signal transduction (RAB25, VDAC3, STK25, ARGAL, DNPEP, LRC59, SEC13, DNM1L), membrane transport (ILVBL, ACLY) and cell biology processes (TOMM40, QCR2, HNRH3, EMAL1), etc. Compared with women, the seven epidermal proteins downregulated in men are: S10AD, VPS41, 1433T, CAH2, IGHG3, FUCO2, and LORF1. They are involved in a variety of biological processes: for example, S10AD and VPS41 are related to pigmentation, and 1433T is related to aging, CAH2, IGHG3, FUCO2 are related to inflammation, and LORF1 is involved in the metabolism of skin fibroblasts. Although the sample size of our experiment is not large enough, our experiment detected the epidermal protein related to the difference between men and women for the first time, enriching the library of epidermal difference proteins between men and women and enriching the skin aging mechanism of men and women from the perspective of epidermal difference proteins. In the future, we will strive to use more samples for further research.
Acknowledgement: We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. Thanks to the First Affiliated Hospital of Anhui Medical University and the Institute of Dermatology of Anhui Medical University for their strong support. We would also like to thank BGI for its technical support.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Author Contribution: Experimental Design: SY, HZ, SR, LD, XJZ. Data analysis: XDZ, MJ, HZ, MTL, YCW, CX, BZ, Y-D H. Paper writing: HZ, SR.
Supplementary Material: The supplementary material is available online at DOI: 10.32604/biocell.2021.016524.
Ethics Approval: This study followed the recommendations of the Medical Ethics Committee of Anhui Medical University, and all subjects provided informed consent. Project: Research on skin barrier function and repair, Record No. PJ2017-11-15.
Funding Statement: This research was funded by the Science and Technology Action Plan for Prevention and Treatment of Major Diseases sponsored by the National Health and Family Planning Commission of the People’s Republic of China (Grant No. 2017ZX-01E-002) and Anhui Institute of Translational Medicine (ZHYX2020A005).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY (2007). Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes & Development 21: 3244–3257. DOI 10.1101/gad.1588507. [Google Scholar] [CrossRef]
Aked D, Foster SJ, Howarth A, Mccormick ME, Potts HC (1986). The inflammatory response of rabbit skin to topical arachidonic acid and its pharmacological modulation. British Journal of Pharmacology 89: 431–438. DOI 10.1111/j.1476-5381.1986.tb10277.x. [Google Scholar] [CrossRef]
Arjmandi N, Mortazavi G, Zarei S, Faraz M, Mortazavi S (2018). Can light emitted from smartphone screens and taking selfies cause premature aging and wrinkles? Journal of Biomedical Physics & Engineering 8: 447–452. [Google Scholar]
Basler K, Bergmann S, Heisig M, Naegel A, Zorn-Kruppa M, Brandner JM (2016). The role of tight junctions in skin barrier function and dermal absorption. Journal of Control Release 242: 105–118. DOI 10.1016/j.jconrel.2016.08.007. [Google Scholar] [CrossRef]
Baumann L (2018). How to use oral and topical cosmeceuticals to prevent and treat skin aging. Facial Plastic Surgery Clinics 26: 407–413. DOI 10.1016/j.fsc.2018.06.002. [Google Scholar] [CrossRef]
Bertolotti M, Lonardo A, Mussi C, Baldelli E, Pellegrini E, Ballestri S, Romagnoli D, Loria P (2014). Nonalcoholic fatty liver disease and aging: Epidemiology to management. World Journal of Gastroenterology 20: 14185–14204. DOI 10.3748/wjg.v20.i39.14185. [Google Scholar] [CrossRef]
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254. DOI 10.1016/0003-2697(76)90527-3. [Google Scholar] [CrossRef]
Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, Werner S, Fässler R (2000). Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO Journal 19: 3990–4003. DOI 10.1093/emboj/19.15.3990. [Google Scholar] [CrossRef]
Brandt SL, Wang S, Dejani NN, Klopfenstein N, Winfree S, Luciano Filgueiras L, McCarthy BP, Territo PR, Serezani CH (2018). Excessive localized leukotriene B4 levels dictate poor skin host defense in diabetic mice. JCI Insight 3: S27. DOI 10.1172/jci.insight.120220. [Google Scholar] [CrossRef]
Breitenbach JS, Rinnerthaler M, Trost A, Weber M, Klausegger A, Gruber C, Bruckner D, Reitsamer HA, Bauer JW, Breitenbach M (2015). Transcriptome and ultrastructural changes in dystrophic Epidermolysis bullosa resemble skin aging. Sedentary Life and Nutrition 7: 389–411. [Google Scholar]
Bruderer R, Bernhardt OM, Gandhi T, Reiter L (2016). High-precision iRT prediction in the targeted analysis of data-independent acquisition and its impact on identification and quantitation. Proteomics 16: 2246–2256. DOI 10.1002/pmic.201500488. [Google Scholar] [CrossRef]
Clatici VG, Racoceanu D, Dalle C, Voicu C, Tomas-Aragones L, Marron SE, Wollina U, Fica S (2017). Perceived age and life style. The specific contributions of seven factors involved in health and beauty. Maedica–A Journal of Clinical Medicine 12: 191–201. [Google Scholar]
Cox J, Mann M (2008). MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nature Biotechnology 26: 1367–1372. DOI 10.1038/nbt.1511. [Google Scholar] [CrossRef]
Chambers DM, Moretti L, Zhang JJ, Cooper SW, Chambers DM, Santangelo PJ, Barker TH (2018). LEM domain-containing protein 3 antagonizes TGFbeta-SMAD2/3 signaling in a stiffness-dependent manner in both the nucleus and cytosol. Journal of Biological Chemistry 293: 15867–15886. DOI 10.1074/jbc.RA118.003658. [Google Scholar] [CrossRef]
Chang HS, Park JS, Lee HS, Lyu J, Son JH, Choi IS, Shin HD, Choon-Sik P (2017). Association analysis of ILVBL gene polymorphisms with aspirin-exacerbated respiratory disease in asthma. BMC Pulmonary Medicine 17: 210. DOI 10.1186/s12890-017-0556-6. [Google Scholar] [CrossRef]
Choi M, Chang CY, Clough T, Broudy D, Killeen T, MacLean B, Vitek O (2014). MSstats: An R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics 30: 2524–2526. DOI 10.1093/bioinformatics/btu305. [Google Scholar] [CrossRef]
Chun KS, Langenbach R (2007). A proposed COX-2 and PGE(2) receptor interaction in UV-exposed mouse skin. Molecular Carcinogenesis 46: 699–704. DOI 10.1002/mc.20354. [Google Scholar] [CrossRef]
Dabrowska M, Mielcarek A, Nowak I (2018). Evaluation of sex-related changes in skin topography and structure using innovative skin testing equipment. Skin Research and Technology 24: 614–620. DOI 10.1111/srt.12473. [Google Scholar] [CrossRef]
Dibble CC, Cantley LC (2015). Regulation of mTORC1 by PI3K signaling. Trends in Cell Biology 25: 545–555. DOI 10.1016/j.tcb.2015.06.002. [Google Scholar] [CrossRef]
Farage KW, Elsner P, Maibach HI (2008). Intrinsic and extrinsic factors in skin ageing: A review. International Journal of Cosmetic Science 30: 87–95. DOI 10.1111/j.1468-2494.2007.00415.x. [Google Scholar] [CrossRef]
Gong Z, Tas E, Yakar S, Muzumdar R (2017). Hepatic lipid metabolism and non-alcoholic fatty liver disease in aging. Molecular and Cellular Endocrinology 455: 115–130. DOI 10.1016/j.mce.2016.12.022. [Google Scholar] [CrossRef]
Gruber JV, Holtz R (2010). Examining the genomic influence of skin antioxidants in vitro. Mediators of Inflammation 2010: 230450. [Google Scholar]
Gu Y, Han J, Jiang C, Zhang Y (2020). Biomarkers, oxidative stress and autophagy in skin aging. Ageing Research Reviews 59: 101036. DOI 10.1016/j.arr.2020.101036. [Google Scholar] [CrossRef]
Guimarães FR, Sales-Campos H, Nardini V, da Costa T A, Fonseca MTDC, Júnior VR, Sorgi CA, da Silva JS, Chica JEDL, Faccioli LAH, de Barros Cardoso C R (2018). The inhibition of 5-Lipoxygenase (5-LO) products leukotriene B4 (LTB4) and cysteinyl leukotrienes (cysLTs) modulates the inflammatory response and improves cutaneous wound healing. Clinical Immunology 190: 74–83. DOI 10.1016/j.clim.2017.08.022. [Google Scholar] [CrossRef]
Han A, Chien AL, Kang S (2014). Photoaging. Dermatologic Clinics 32: 291–299. DOI 10.1016/j.det.2014.03.015. [Google Scholar] [CrossRef]
Hao D, Wen X, Liu L, Wang L, Zhou X, Li Y, Zeng X, He G, Jiang X (2019). Sanshool improves UVB-induced skin photodamage by targeting JAK2/STAT3-dependent autophagy. Cell Death & Disease 10: 19. DOI 10.1038/s41419-018-1261-y. [Google Scholar] [CrossRef]
Holly AC, Melzer D, Pilling LC, Fellows AC, Tanaka T, Ferrucci L, Harries LW (2013). Changes in splicing factor expression are associated with advancing age in man. Mechanisms of Ageing and Development 134: 356–366. DOI 10.1016/j.mad.2013.05.006. [Google Scholar] [CrossRef]
Ibarrola-Villava M, Kumar R, Nagore E, Benfodda M, Guedj M, Gazal S, Hu HH, Guan J, Rachkonda P S, Descamps V, Basset-Seguin N, Bensussan A, Bagot M, Saiag P, Schadendorf D, Martin-Gonzalez M, Mayor M, Grandchamp B, Ribas G, Nadem S (2015). Genes involved in the WNT and vesicular trafficking pathways are associated with melanoma predisposition. International Journal of Cancer 136: 2109–2119. DOI 10.1002/ijc.29257. [Google Scholar] [CrossRef]
Ikai K, Imamura S (1988). Prostaglandin D2 in the skin. International Journal of Dermatology 27: 141–149. DOI 10.1111/j.1365-4362.1988.tb04917.x. [Google Scholar] [CrossRef]
Jeong H, Lim KM, Goldenring JR, Nam KT (2019). Rab25 deficiency perturbs epidermal differentiation and skin barrier function in mice. Biomolecules & Therapeutics 27: 553–561. DOI 10.4062/biomolther.2019.125. [Google Scholar] [CrossRef]
Kammeyer A, Luiten RM (2015). Oxidation events and skin aging. Ageing Research Reviews 21: 16–29. DOI 10.1016/j.arr.2015.01.001. [Google Scholar] [CrossRef]
Kamsteeg M, Zeeuwen PL, De Jongh GJ, Rodijk-Olthuis D, Zeeuwen-Franssen ME et al. (2007). Increased expression of carbonic anhydrase II (CA II) in lesional skin of atopic dermatitis: Regulation by Th2 cytokines. Journal of Investigative Dermatology 127: 1786–1789. DOI 10.1038/sj.jid.5700752. [Google Scholar] [CrossRef]
Kim SK, Karadeniz F (2012). Biological importance and applications of squalene and squalane. Advances in Food and Nutrition Research 65: 223–233. [Google Scholar]
Kitamura T, Seki N, Kihara A (2017). Phytosphingosine degradation pathway includes fatty acid alpha-oxidation reactions in the endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America 114: E2616–E2623. DOI 10.1073/pnas.1700138114. [Google Scholar] [CrossRef]
Kobayashi M, Suzuki M, Ohno T, Tsuzuki K, Taguchi C, Tateishi S, Kawada T, Kim YI, Murai A, Horio F (2016). Detection of differentially expressed candidate genes for a fatty liver QTL on mouse chromosome 12. BMC Genetics 17: 73. DOI 10.1186/s12863-016-0385-2. [Google Scholar] [CrossRef]
Lai JL, Liu YH, Liu C, Qi MP, Liu RN, Zhu XF, Zhou QG, Chen YY, Guo AZ, Hu CM (2017). Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation 40: 1–12. DOI 10.1007/s10753-016-0447-7. [Google Scholar] [CrossRef]
Langston PK, Nambu A, Jung J, Shibata M, Aksoylar HI, Lei J, Xu P, Doan MT, Jiang H, MacArthur MR, Gao X, Kong Y, Chouchani ET, Locasale JW, Snyder NW, Horng T (2019). Glycerol phosphate shuttle enzyme GPD2 regulates macrophage inflammatory responses. Nature Immunology 20: 1186–1195. DOI 10.1038/s41590-019-0453-7. [Google Scholar] [CrossRef]
Lee DH, Oh JH, Chung JH (2016). Glycosaminoglycan and proteoglycan in skin aging. Journal of Dermatological Science 83: 174–181. DOI 10.1016/j.jdermsci.2016.05.016. [Google Scholar] [CrossRef]
Lephart ED (2018). A review of the role of estrogen in dermal aging and facial attractiveness in women. Journal of Cosmetic Dermatology 17: 282–288. DOI 10.1111/jocd.12508. [Google Scholar] [CrossRef]
Li YF, Ouyang SH, Tu LF, Wang X, Yuan WL, Wang GE, Wu YP, Duan WJ, Yu HM, Fang ZZ, Kurihara H, Zhang Y, He RR (2018). Caffeine protects skin from oxidative stress-induced senescence through the activation of autophagy. Theranostics 8: 5713–5730. DOI 10.7150/thno.28778. [Google Scholar] [CrossRef]
Ma J, Xu S, Wang X, Zhang J, Wang Y, Liu M, Jin L, Wu M, Qian D, Li X, Zhen Q, Guo H, Gao J, Yang S, Zhang X (2019). Noninvasive analysis of skin proteins in healthy Chinese subjects using an Orbitrap Fusion Tribrid mass spectrometer. Skin Research and Technology 25: 424–433. DOI 10.1111/srt.12668. [Google Scholar] [CrossRef]
Makrantonaki E, Zouboulis CC (2009). Androgens and ageing of the skin. Current Opinion in Endocrinology, Diabetes and Obesity 16: 240–245. DOI 10.1097/MED.0b013e32832b71dc. [Google Scholar] [CrossRef]
Masuya T, Suzuki M, Tsujimura J, Kanamori S, Miyasaka Y, Ohno T, Murai A, Horio F, Kobayashi M, Guillou H (2020). Ablation of Iah1, a candidate gene for diet-induced fatty liver, does not affect liver lipid accumulation in mice. PLoS One 15: e0233087. DOI 10.1371/journal.pone.0233087. [Google Scholar] [CrossRef]
Mcdaniel D, Farris P, Valacchi G (2018). Atmospheric skin aging-Contributors and inhibitors. Journal of Cosmetic Dermatology 17: 124–137. DOI 10.1111/jocd.12518. [Google Scholar] [CrossRef]
Megna M, Lembo S, Balato N, Monfrecola G (2017). “Active” photoprotection: Sunscreens with DNA repair enzymes. Giornale Italiano di Dermatologia e Venereologia 152: 302–307. [Google Scholar]
Nguyen G, Torres A (2012). Systemic antioxidants and skin health. Journal of Drugs in Dermatology 11: e1–4. [Google Scholar]
Niehues H, Bouwstra JA, El Ghalbzouri A, Brandner JM, Zeeuwen P, Van Den Bogaard EH (2018). 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Experimental Dermatology 27: 501–511. DOI 10.1111/exd.13531. [Google Scholar] [CrossRef]
Niki E (2015). Lipid oxidation in the skin. Free Radical Research 49: 827–834. DOI 10.3109/10715762.2014.976213. [Google Scholar] [CrossRef]
Nugteren DH, Kivits GA (1987). Conversion of linoleic acid and arachidonic acid by skin epidermal lipoxygenases. Biochimica et Biophysica Acta 921: 135–141. DOI 10.1016/0005-2760(87)90179-2. [Google Scholar] [CrossRef]
Pham DM, Boussouira B, Moyal D, Nguyen QL (2015). Oxidization of squalene, a human skin lipid: A new and reliable marker of environmental pollution studies. International Journal of Cosmetic Science 37: 357–365. DOI 10.1111/ics.12208. [Google Scholar] [CrossRef]
Pullar JM, Carr AC, Vissers MCM (2017). The roles of vitamin C in skin health. Nutrients 9: 866. DOI 10.3390/nu9080866. [Google Scholar] [CrossRef]
Quan T, Fisher GJ (2015). Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontologia 61: 427–434. DOI 10.1159/000371708. [Google Scholar] [CrossRef]
Ramsing D, Agner E, Malinowski J, Meibom J, Agner T (1995). Effect of systemic treatment with cholesterol-lowering drugs on the skin barrier function in humans. Acta Dermato-Venereologica 75: 198–201. [Google Scholar]
Reina S, Palermo V, Guarnera A, Guarino F, Messina A, Mazzoni C, Pinto VD (2010). Swapping of the N-terminus of VDAC1 with VDAC3 restores full activity of the channel and confers anti-aging features to the cell. FEBS Letters 584: 2837–2844. DOI 10.1016/j.febslet.2010.04.066. [Google Scholar] [CrossRef]
Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K (2015). Oxidative stress in aging human skin. Biomolecules 5: 545–589. DOI 10.3390/biom5020545. [Google Scholar] [CrossRef]
Ruzicka T, Printz MP (1984). Arachidonic acid metabolism in skin: A review. Reviews of Physiology, Biochemistry and Pharmacology 100: 121–160. [Google Scholar]
Sator PG, Schmidt JB, Rabe T, Zouboulis CC (2004). Skin aging and sex hormones in women—Clinical perspectives for intervention by hormone replacement therapy. Experimental Dermatology 13: 36–40. DOI 10.1111/j.1600-0625.2004.00259.x. [Google Scholar] [CrossRef]
Seibt TM, Proneth B, Conrad M (2019). Role of GPX4 in ferroptosis and its pharmacological implication. Free Radical Biology and Medicine 133: 144–152. DOI 10.1016/j.freeradbiomed.2018.09.014. [Google Scholar] [CrossRef]
Sengupta A, Lichti UF, Carlson BA, Cataisson C, Ryscavage AO, Mikulec C, Conrad M, Fischer SM, Hatfield DL, Yuspa SH (2013). Targeted disruption of glutathione peroxidase 4 in mouse skin epithelial cells impairs postnatal hair follicle morphogenesis that is partially rescued through inhibition of COX-2. Journal of Investigative Dermatology 133: 1731–1741. DOI 10.1038/jid.2013.52. [Google Scholar] [CrossRef]
Setkowicz M, Mastalerz L, Gielicz A, Wojas-Pelc A, Sanak M (2015). Lack of association of ALOX12 and ALOX15B polymorphisms with psoriasis despite altered urinary excretion of 12(S)-hydroxyeicosatetraenoic acid. British Journal of Dermatology 172: 337–344. DOI 10.1111/bjd.13225. [Google Scholar] [CrossRef]
Sheffer R, Douiev L, Edvardson S, Shaag A, Tamimi K, Soiferman D, Meiner V, Saada A (2016). Postnatal microcephaly and pain insensitivity due to a de novo heterozygous DNM1L mutation causing impaired mitochondrial fission and function. American Journal of Medical Genetics Part A 170: 1603–1607. DOI 10.1002/ajmg.a.37624. [Google Scholar] [CrossRef]
Sochorova M, Audrlicka P, Cervena M, Kovacik A, Kopecna M, Opálka L, Pullmannová P, Vávrová K (2019). Permeability and microstructure of cholesterol-depleted skin lipid membranes and human stratum corneum. Journal of Colloid and Interface Science 535: 227–238. DOI 10.1016/j.jcis.2018.09.104. [Google Scholar] [CrossRef]
Stacey SN, Gudbjartsson DF, Sulem P, Bergthorsson JT, Kumar R, Thorleifsson G, Sigurdsson A, Jakobsdottir M, Sigurgeirsson B, Benediktsdottir KR, Thorisdottir K, Ragnarsson R, Scherer D, Rudnai P, Gurzau E, Koppova K, Höiom V, Botella-Estrada R, Soriano V, Juberías P, Grasa M, Carapeto FJ, Tabuenca P, Gilaberte Y, Gudmundsson J, Thorlacius S, Helgason A, Thorlacius T, Jonasdottir A, Blondal T, Gudjonsson S A, Jonsson GF, Saemundsdottir J, Kristjansson K, Bjornsdottir G, Sveinsdottir SG, Mouy M, Geller F, Nagore E, Mayordomo JI, Hansson J, Rafnar T, Kong A, Olafsson JH, Thorsteinsdottir U, Stefansson K (2008). Common variants on 1p36 and 1q42 are associated with cutaneous basal cell carcinoma but not with melanoma or pigmentation traits. Nature Genetics 40: 1313–1318. DOI 10.1038/ng.234. [Google Scholar] [CrossRef]
Steck A, Kinter J, Renaud S (2011). DNA microarray analysis in nerve biopsies of patients with vasculitic neuropathy. Revue Neurologique 167: 927–929. DOI 10.1016/j.neurol.2011.09.002. [Google Scholar] [CrossRef]
Stuart JA, Maddalena LA, Merilovich MR, Robb L (2014). A midlife crisis for the mitochondrial free radical theory of aging. Longevity & Healthspan 3: 4. DOI 10.1186/2046-2395-3-4. [Google Scholar] [CrossRef]
Subramanya RD, Coda AB, Sinha AA (2010). Transcriptional profiling in alopecia areata defines immune and cell cycle control related genes within disease-specific signatures. Genomics 96: 146–153. DOI 10.1016/j.ygeno.2010.05.002. [Google Scholar] [CrossRef]
Suggs A, Oyetakin-White P, Baron ED (2014). Effect of botanicals on inflammation and skin aging: Analyzing the evidence. Inflammation & Allergy-Drug Targets 13: 168–176. DOI 10.2174/1871528113666140526163052. [Google Scholar] [CrossRef]
Surowiak P, Gansukh T, Donizy P, Halon A, Rybak Z (2014). Increase in cyclooxygenase-2 (COX-2) expression in keratinocytes and dermal fibroblasts in photoaged skin. Journal of Cosmetic Dermatology 13: 195–201. DOI 10.1111/jocd.12103. [Google Scholar] [CrossRef]
Taieb A (2018). Skin barrier in the neonate. Pediatric Dermatology 35: s5–s9. DOI 10.1111/pde.13482. [Google Scholar] [CrossRef]
Tang SC, Liao PY, Hung SJ, Ge JS, Chen SM, Lai JC, Hsiao YP, Yang JH (2017). Topical application of glycolic acid suppresses the UVB induced IL-6, IL-8, MCP-1 and COX-2 inflammation by modulating NF-kappaB signaling pathway in keratinocytes and mice skin. Journal of Dermatological Science 86: 238–248. DOI 10.1016/j.jdermsci.2017.03.004. [Google Scholar] [CrossRef]
Tobin DJ (2017). Introduction to skin aging. Journal of Tissue Viability 26: 37–46. DOI 10.1016/j.jtv.2016.03.002. [Google Scholar] [CrossRef]
Wang L, Koenig HG, He Z, Sun X, Shohaib SA, Wang Z (2020). Religiosity and telomere length: Moderating effect of religiosity on the relationship between high-risk polymorphisms of the apolipoprotein E and TOMM40 gene and telomere length. Journal of Applied Gerontology 39: 627–634. DOI 10.1177/0733464819865415. [Google Scholar] [CrossRef]
Wu ST, Liu B, Ai ZZ, Hong ZC, You PT, Wu HZ, Yang YF (2020). Esculetin inhibits cancer cell glycolysis by binding tumor PGK2, GPD2, and GPI. Frontiers in Pharmacology 11: 379. DOI 10.3389/fphar.2020.00379. [Google Scholar] [CrossRef]
Xian H, Yang S, Jin S, Zhang Y, Cui J (2020). LRRC59 modulates type I interferon signaling by restraining the SQSTM1/p62-mediated autophagic degradation of pattern recognition receptor DDX58/RIG-I. Autophagy 16: 408–418. DOI 10.1080/15548627.2019.1615303. [Google Scholar] [CrossRef]
Xiong TF, Pan FQ, Li D (2019). Expression and clinical significance of S100 family genes in patients with melanoma. Melanoma Research 29: 23–29. DOI 10.1097/CMR.0000000000000512. [Google Scholar] [CrossRef]
Yang Y, Luo M, Zhang K, Zhang J, Gao T, Connell DO’, Yao F, Mu C, Cai B, Shang Y, Chen W (2020). Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nature Communications 11: 433. DOI 10.1038/s41467-020-14324-x. [Google Scholar] [CrossRef]
Ziboh VA (1973). Biosynthesis of prostaglandin E 2 in human skin: Subcellular localization and inhibition by unsaturated fatty acids and anti-inflammatory drugs. Journal of Lipid Research 14: 377–384. DOI 10.1016/S0022-2275(20)36869-3. [Google Scholar] [CrossRef]
Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R (2007). Sexual hormones in human skin. Hormone and Metabolic Research 39: 85–95. DOI 10.1055/s-2007-961807. [Google Scholar] [CrossRef]
Supplementary Materials
Supplementary Tab. 1 Dia-proteinSummary-noqc.xlsx
Supplementary Tab. 2 Sex-related epidermal difference protein.xlsx
Supplementary Tab. 3 Case-VS-control.GO2protein.xls
Supplementary Tab. 4 Case-VS-control_cfp.xls
Supplementary Tab. 5 Case-VS-control.kog2protein.xls
Supplementary Tab. 6 Case-VS-control pathway .xls
Supplementary Tab. 7 Case-VS-control.KEGG2protein.xls
Supplementary Tab. 8 Case-VS-control.path.edges.xls
Supplementary Tab. 9 Case-VS-control.subcellular2protein.xls
Supplementary Tab. 10 Case-VS-control.network.relation.xls
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |