DOI:10.32604/biocell.2021.016281

| BIOCELL DOI:10.32604/biocell.2021.016281 |  |
| Article |
Effects of docosahexaenoic acid or arachidonic acid supplementation on gene expression and contractile force of rat cardiomyocytes in primary culture
1Graduate School of Science and Engineering, Yamagata University, Yonezawa, 9928510, Japan
2Graduate School of Medical Science, Yamagata University, Yamagata, 9909585, Japan
3Department of Materials and Applied Chemistry, College of Science and Technology, Nihon University, Tokyo, 1018308, Japan
4Department of Health and Nutrition, Yamagata Prefectural Yonezawa University of Nutrition Sciences, Yonezawa, 9920025, Japan
*Address correspondence to: Daisuke Sato, d_sato@yz.yamagata-u.ac.jp; Zhonggang Feng, zhgfeng@yz.yamagata-u.ac.jp
Received: 23 February 2021; Accepted: 26 April 2021
Abstract: While fatty acids play essential roles in the physiology of the myocardium, conventional culture media contain little lipid. We previously revealed that rat neonatal myocardium mainly contains docosahexaenoic (DHA), linoleic (LA), and arachidonic (AA) acids as polyunsaturated fatty acids (PUFAs), and these contents in cultured cardiomyocytes derived from fetal rats were markedly lower than those in the neonatal myocardium. In this study, we first assessed the effects of supplementation of DHA, LA, or AA on the fatty acid contents and the percentage change of contractile area in primarily cultured rat cardiomyocytes. Based on this assessment, we then evaluated the effects of DHA or AA supplementation on mRNA expression and further directly measured the contractile force of cardiomyocytes with the supplementations. This study revealed that percentage change of contractile area was maximized under 20 μM DHA or 50 μM AA supplementation while LA supplementation did not affect this contraction index, and that a widespread upregulation tendency of the mRNA expression related to differentiation, maturity, fatty acid metabolism, and cell adhesion was seen in the cultured cardiomyocytes with supplementation of DHA or AA. In particular, upregulation of the gene expression of cellular adhesion molecules connexin43 and N-cadherin were remarkable, whereas the effects on differentiation and maturation were less pronounced. Correspondingly, the increase of the percentage change of the contractile area of cardiomyocyte clusters in culture dishes with the supplementations was significant, whereas the enhancement of the contractile force was modest. These results suggest that supplementation of DHA or AA to the fetal cardiomyocyte culture may play effective roles in preventing the de-differentiation of the cardiomyocytes in culture and that the enhancement of the contractile performance may be mainly attributed to the improvement of intercellular connection.
Keywords: Cardiomyocyte culture; Polyunsaturated fatty acid; mRNA expression; Contractile force; Intercellular connection
Heart failure makes up one of the major causes of mortality and morbidity worldwide. The development of effective therapies for heart failure has always been a big challenge in cardiovascular medicine. Nowadays, regenerative medicine is regarded as a promising alternative treatment to drug therapies and donor heart or artificial heart transplantations. However, it is known that the myocardial tissue equivalently constructed in vitro is significantly inferior in mechanical properties to its counterpart in vivo. For example, twitch stress generated in three-dimensional myocardial tissue engineered from fetal rat cardiomyocytes by using collagen scaffold is only ca. 2.0 kPa (Eschenhagen et al., 2002), while the stress generated by myocardial tissue in vivo is ca. 22.0 kPa (Vahl et al., 1994; Yamada et al., 2017).
Fatty acids are important components of lipids in living bodies and classified into saturated and unsaturated fatty acids. Unsaturated fatty acids are further divided into monounsaturated, and n–3 and n–6 polyunsaturated fatty acids (PUFAs). While saturated and monounsaturated fatty acids can be biosynthesized by mammals in vivo (Iso et al., 2006; Matsuzaka et al., 2007), PUFAs cannot because mammals do not have the desaturation enzyme necessary for making a double bond at n–3 or n–6 binding site. Hence, PUFAs have to be consumed from food (Schmitz and Ecker, 2008). Mammalian hearts shift their energetic substrates from lactate and glucose to fatty acids during the neonatal period (Horikoshi et al., 2019; Isu et al., 2019). Moreover, PUFAs have various physiological activities and are known as physiological ligands of transcriptional factors (Yamada et al., 2017). In particular, n–3 PUFAs are known to have cardioprotective effects (Shysh et al., 2016). In spite of the facts, the lipids supplied to the culture medium are quite limited as only contained in fetal bovine serum (FBS) supplements. Therefore, fatty acids in cultured cardiomyocytes might be considerably insufficient.
In our preceding research, to clarify the effects of lipids on cardiomyocytes in culture, we compared the fatty acid composition in cultured rat fetal cardiomyocytes with that in myocardial tissues collected from rat neonates. The results showed a considerable shortage of n–3 and n–6 PUFAs in cultured cells (Karimata et al., 2013). In particular, the contents of linoleic acid (18:2n–6, LA), docosahexaenoic acid (22:6n–3, DHA), and arachidonic acid (20:4n–6, AA) were significantly lower than those in the neonatal tissue.
In this study, we intended to reveal the effects of PUFAs supplementations on mRNA expression and contractile force of the cultured cardiomyocytes. We firstly screened the most promising PUFAs and supplementation concentrations for the enhancement of the contractile function of cardiomyocytes in culture by assessing the percentage change of the contractile area of the cardiomyocyte clusters in culture dishes with PUFA supplementations; then, we evaluated the effects of the promising supplementations on mRNA expression and contractile force of cultured cardiomyocytes. The investigated genes fell into three classifications related to “differentiation & maturity” (Nkx2.5, Srf, P300, cTnT, Mlc2v, and Errγ), “fatty acid metabolism” (Cd36, Pparα, and Pparδ) and “cell adhesion” (Cdh2 (N-cadherin) and Cx43 (connexin43)). The direct measurement of the contractile force of the cardiomyocytes cultured with the supplementations was conducted by means of a lab-made vision-based device measuring the dynamic deflection of an L-shape cantilever attached to a re-configured circular cardiomyocyte-collagen gel. This research is a succeeding investigation corresponding to our previous report (Karimata et al., 2013) and presents further insight into the effects of PUFA supplementations on the fetal cardiomyocytes in culture.
The animal handling and experimental methods used in this study were approved in advance by the Yamagata University Animal Experiment Committee.
Female Wistar rats, obtained from an in-house breeding colony, were housed in a room maintained at 21 ± 1°C and 12-h light/dark cycle, and allowed free access to chow and tap water. The details of the harvest and primary culture of rat embryonic cardiomyocytes are described elsewhere (Karimata et al., 2013). In brief, approximately 20 days after impregnation, pregnant rats were anesthetized with 4% isoflurane, and the jugular vein and carotid artery were cut. Fetal rats were taken out at laparotomy and their ventricles were harvested by thoracotomy after lumber fracture. The ventricles were digested to isolate cardiomyocytes with 0.1% type I collagenase and 0.1% D-glucose in PBS over five cycles, each lasted for 40 min.
The cells were cultured at the seeding density of 1 × 106 per 60 mm dish for 7 days with DMEM/F12-Ham (Sigma-Aldrich, St. Louis, USA) containing 10% FBS (Nichirei Biosciences, Tokyo, Japan), 1% penicillin-streptomycin (Sigma-Aldrich), and 260 mU/mL insulin (Humulin R; Eli Lilly Japan, Tokyo, Japan) in a 5%-CO2 incubator at 37°C. The culture medium was changed firstly 24 h after the cell seeding and then every other day.
In the experiment of fatty acid measurement, neonatal heart tissue was used to obtain in vivo reference data. The method to harvest neonatal tissue may refer Karimata et al. (2013).
Determination of supplementation doses of PUFAs
To optimize supplementation doses of PUFAs, cardiomyocytes were supplemented with one of the following PUFAs via culture medium. DHA, LA (Sigma-Aldrich), or AA (Tokyo Chemical Industry) was mixed with and conjugated to bovine serum albumin (Sigma-Aldrich) at a ratio of 2:1 (mol/mol) (Oliveira et al., 2005) and supplemented (DHA: 10–40 μM; LA: 10–30 μM; AA: 20–60 μM) at every medium change.
Measurement of fatty acid composition
On the 14th day of the culture, cardiomyocytes were harvested by scraping them in 0.01 M PBS, and centrifuged at 1,600 × g for 2 min. The pelleted cells were weighted and stored at −80°C. As described elsewhere (Karimata et al., 2013), fatty acids of the cells were extracted in chloroform/methanol solution (2:1, vol/vol), and the contents of methylated 22 fatty acids (Tab. 1) were measured with a gas chromatography system (6890GC; Agilent Technologies, Santa Clara, CA) equipped with a flame ionization detector.
The fatty acid contents were compared with data of cardiomyocytes cultured without any fatty acid supplementation (control group; N = 8), and with those of neonatal myocardium (9 days of age, N = 11) reported elsewhere (Karimata et al., 2013) to determine the supplemental amount closest to the neonatal cellular content.
Assessment of the percentage change of contractile area
On the fourth day after the onset of supplementation of DHA, LA, or AA, we arbitrarily selected several colonies of beating cardiomyocytes under a microscope, and we recorded their video image on a personal computer. Beat rate was manually counted for 10 s on the video image, and then the rate was sextupled to obtain beat rate per minute. Percentage change of contractile area (CCA (%)) was calculated from the change in beating area in a beating cycle of several colonies as a formula shown below:
We arbitrarily selected four points around each beating colony on the video image as shown in Fig. 1, and calculated box area formed with the points. The areas were calculated using ImageJ. We traced the points during several beating cycles to detect the image frames having maximal or minimal area.
Fatty acid supplementation for measurement of gene expression and contractile force
In the experiment of mRNA extraction, DHA or AA was supplemented in the culture medium (DHA at 20 µM concentration [DHA20]; AA at 50 µM [AA50]) in the same manner as described above. The supplementations of DHA and AA at the above concentrations were determined by the CCA (%) assessment. In the experiment of contractile force measurement, DHA or AA was supplemented in the same manner except for an additional medium change with the supplementation one hour before the contractile force measurement.
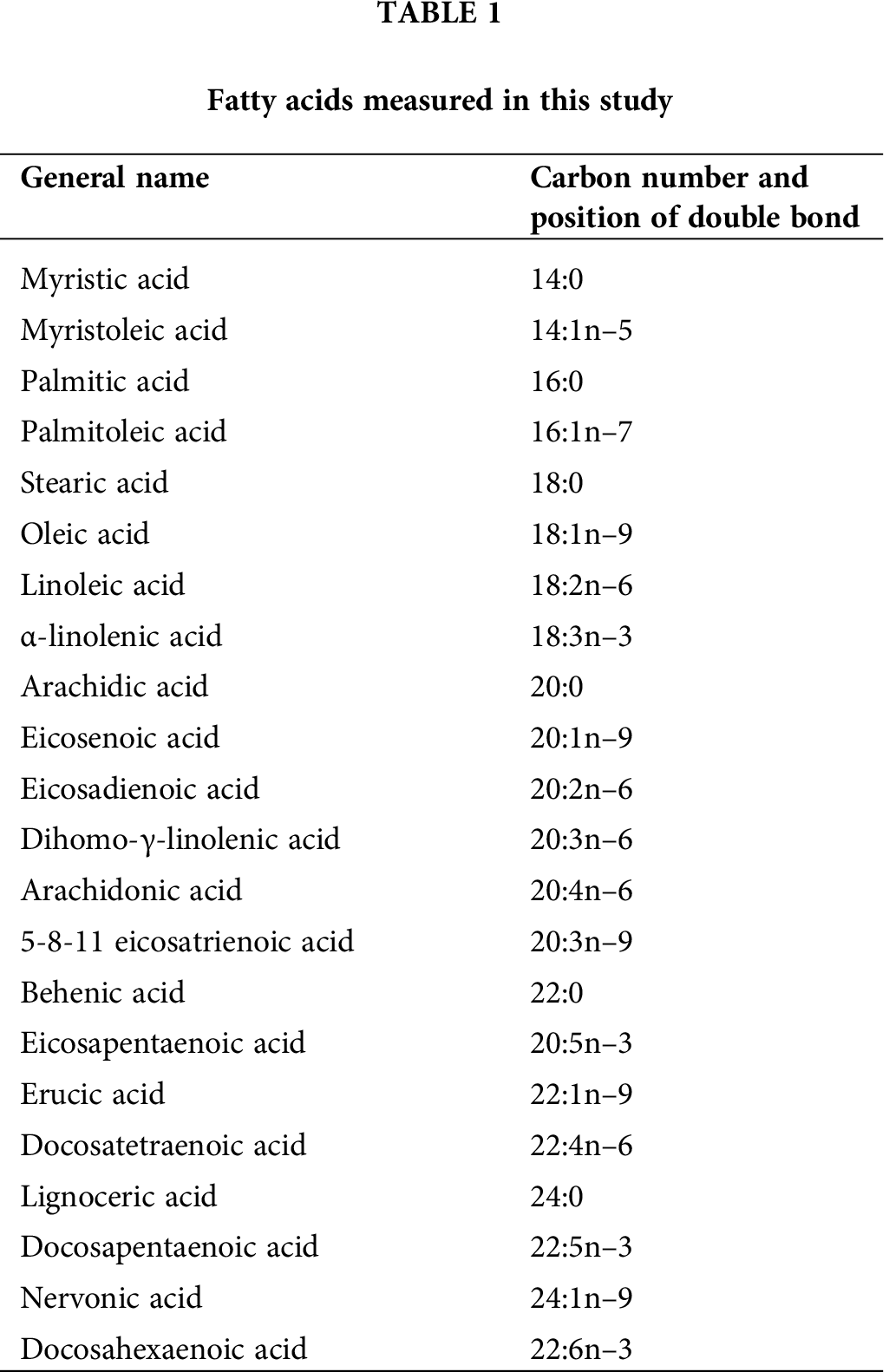

Figure 1: An example of microscopic image for the measurement of percentage change of contractile area of a beating colony. We arbitrarily selected four points around each beating colony on the video image, and calculated box area formed by the points. We traced the points during several beating cycles to detect the image flames having maximal and minimal area.
Reverse-transcription polymerase chain reaction (PCR)
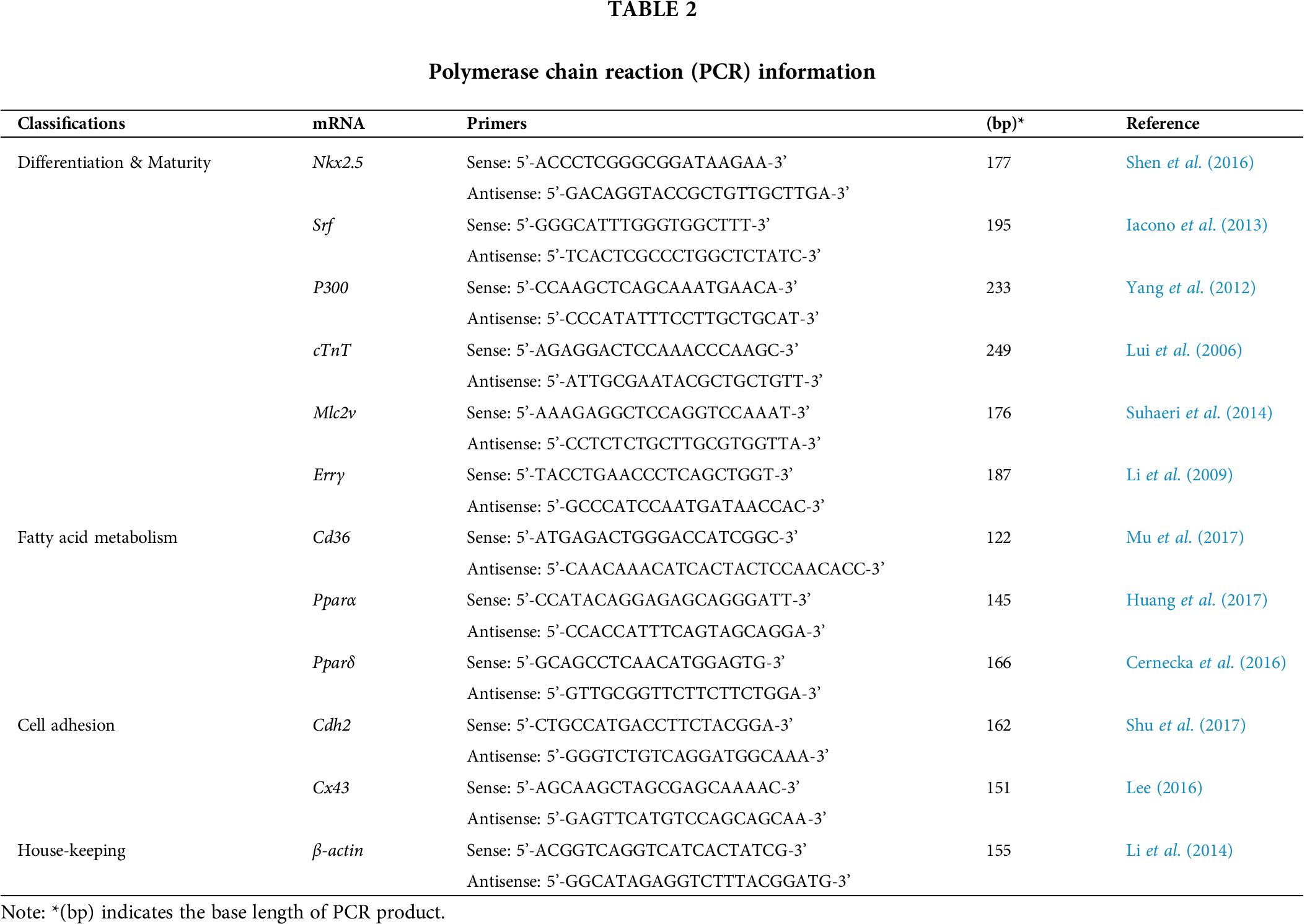
Tab. 2 shows investigated genes and their primers for real-time PCR. The genes were classified into three classifications related to “differentiation & maturity” (Nkx2.5, Srf, P300, cTnT, Mlc2v, and Errγ), “fatty acid metabolism” (Cd36, Pparα, and Pparδ) and “cell adhesion” (Cdh2 and Cx43). β-actin was used for internal reference.
Nkx2.5 is a critical cardiac-specific transcriptional factor. It is required for the terminal differentiation of cardiomyocytes to establish and maintain a ventricular gene expression program and it is used as cardiac differentiation marker in numerous studies (Bruneau, 2002). Although Srf is not a myocardial-specific gene, it is known as a cofactor gene expressed in myocardium to regulate the expression of α-actin, α-heavy chain, and β-myosin heavy chain and used to assess cardiac differentiation (Parlakian et al., 2004; Psichari et al., 2002; Nakamura et al., 2008). P300 is a cofactor for myocardial transcription factors (Backs and Olson, 2006; Shen et al., 2016). It also has histone transferase (HAT) activity, activates the myocardial-specific transcription factor GATA4 and promotes cardiomyocyte hypertrophy (Takaya et al., 2008) and its correlation with myocyte growth is also reported (Yanazume et al., 2003). cTnT is a muscular sarcomere gene among those expressed during early cardiac differentiation, persisting and increasing its expression level in adult human cardiomyocytes compared to the prenatal expression level (Anderson et al., 1991; Li et al., 2011). Mlc2v is another sarcomere gene known as specifically expressed in ventricular muscle as a marker for ventricular muscle differentiation (O’Brien et al., 1993). These two genes were chosen to evaluate the level of the cardiac differentiation and the sarcomere maturation. Errγ is a transcriptional regulator of postnatal mitochondrial biogenesis and function, serve a role in the broader cardiac maturation program (Sakamoto et al., 2020) as a maturation marker.
Cd36 is known to act as a fatty acid transporter (JFC Glatz et al., 2016), while Pparα and Pparδ regulate fatty acid oxidation in the myocardium (Steinmetz et al., 2005; Cheng et al., 2004). They were measured for investigating the effects of DHA and AA on the fatty acid metabolism in cultured cardiomyocytes.
Cdh2 and Cx43 are the critical intercalated disc constituent proteins of the myocardium (Salameh et al., 2004; Zuppinger et al., 2000) and used to evaluate intercellular adhesion in this investigation.
mRNA was extracted on days 1, 2, and 7 of the culture by means of a conventional phenol-based extraction method (Sambrook and Russell, 2006). cDNA was synthesized using PrimeScript RT reagent Kit (Takara Bio). The real-time polymerase chain reaction (rtPCR) was conducted with Thermal Cycler (Thermal Cycler Dice® Real Time System II, Takara) under the condition of 95°C (30 s) initial denaturation and repetitive 45-cycle of dissociation and annealing at 95°C (5 s) and 60°C (30 s), respectively.
The ΔΔCt method was used to evaluate the relative expression level of each gene (Livak and Schmittgen, 2001). The Ct values provided from real-time PCR instrumentation were imported into a Micro Excel spreadsheet. The threshold cycle (Ct) was the fractional cycle number at which the amount of amplified target reached a fixed threshold. ΔCt was equal to the difference in threshold cycles for target gene and reference (β-actin) (Cttarget gene – Ctβ-actin). The fold change in each target gene relative to the No-PUFAs-supplementation group (control) on each extraction day was determined by 2−ΔΔCt, where ΔΔCt = (Cttarget – Ctβ-actin)DHA or AA – (Cttarget – Ctβ-actin) control.
Fabrication of circular cardiomyocyte-collagen gels
Circular collagen gels with 10 mm inner diameter and 20 mm outer diameter (i.e., 5 mm width) were fabricated by casting collagen solution into silicone rubber molds. The collagen solution was formed by mixing type I collagen from rat tails (3.77 mg/mL, 0.02 M acetic acid solution, Corning) with DMEM/F12 culture medium and 1 N NaOH to obtain a neutralized solution at 1.5 mg/mL collagen concentration. Each circular collagen gel had an initial volume of 0.4 mL so that the initial thickness of the gel was 1.2 mm. Gelation was allowed in a 5%-CO2, 37°C incubator for 40 min. After the gelation, 1 mL crosslinker solution, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) in distilled water (DW) at 5 mM concentration, was added into the mold to modulate the mechanical properties of the gel (Fujita et al., 2018). The crosslinking was conducted under room temperature for 48 h. After crosslinking, gels were washed with DW (30 min × 6 times) and PBS supplemented with 1% penicillin (24 h × 2 times). After washing, the gels were coated with 1 mL solution of Matrigel (Matrigel® hESC-qualified Matrix, Corning) in DMEM/F12 at 10.9 µL/mL volume concentration in a 5%-CO2, 37°C incubator for one hour to enhance cellular adhesion to the gel. The cardiomyocytes harvested by the aforementioned method were additionally washed one more time with 10% FBS DMEM/F12 medium and seeded on the circular collagen gels at a density of 80,000 cells/mm2. The spontaneous beating of the circular cardiomyocyte-collagen gels can be observed 24 h later after the cell seeding.
Measurement of the contractile force of cultured cardiomyocytes
Fig. 2 shows our lab-made vision-based measurement system for cardiomyocyte contractile force. The spontaneously beating circular cardiomyocyte-collagen gel was installed into the measurement device and set into an equilateral triangle with its two vertices fixed by two stainless hooks (with 1 mm wire diameter), respectively; and the rest vertex (beating vertex) attached to an L-shape cantilever made of a silver wire with 0.2 mm diameter (Figs. 2a–2b). The sample was immersed into the culture medium. Once the installation was finished, the sample with the device was incubated for 30 min in a 5% CO2 incubator (37°C) to make the sample adapt to the new configuration. The displacement of the beating vertex was measured under an inverted microscope (X71 Olympus, Japan) at different stretch strains of the cardiomyocyte-collagen gel. The stretch strain was achieved by pulling the silver cantilever at 5%, 10%, and 15% stretch ratio to the initial side length, respectively (Fig. 2c).
The force exerted from the gel onto the cantilever was calculated by multiplying the maximum beating displacement of the beating vertex (Fig. 2d) with the cantilever deflection coefficient. To obtain the spontaneous contractile force of the cardiomyocytes, we must take the gel deformation into account. Since cardiomyocytes were cultured on the gel, their contractile force was equal to the sum of the force exerted onto the cantilever and the force deforming the gel during the beating as described by the following Eq. (2):
where fc is the contractile force generated by the cardiomyocytes; fL the maximal force exerted onto the L-shape cantilever calculated by Eq. (2b): α the angle of the beating vertex; E is the elasticity of the sample, which is measured after the force measurement as described in the next subsection; Sg is the cross-sectional area of the collagen gel calculated by Eq. (2c); and εb the maximal beating strain of the gel calculated by Eq. (2d). In Eq. (2b), KL is the cantilever deflection coefficient, which was calibrated in advance of each measurement; and b is the maximal displacement of the tip of the L-shaped cantilever (Fig. 2d). In Eq. (2c), ϕ1 and ϕ2 are the inner and outer diameters of the gel sample, respectively, which were measured before the sample was set into the device; and h is the sample thickness measured with the gel elasticity measurement. In Eq. (2d), l is the side length as shown in Fig. 2c.
The first term on the right side of Eq. (2a) is the force component exerted from cardiomyocytes which aligns with the gel (denoted as fg in Fig. 2c) and pulls the cantilever to produce the beating displacement b (Fig. 2d). The last term in Eq. (2a) is the force component exerted from cardiomyocytes which deforms the collagen gel during the beating; it is thus calculated by multiplying the beating strain εb of the gel with the gel elastic coefficient ESg.
Measurement of the elasticity of circular cardiomyocyte-collagen gels
For the above analysis of the contractile force exerted by cardiomyocytes on the circular collagen gels, the elasticity E of the gels in Eq. (2) must be obtained. It was conducted as follows. After the measurement of the cardiomyocyte-collagen gel beating as described above, the gel was immediately subjected to an indentation test. The indentation test device was developed in our laboratory. As schematically shown in Fig. 3, the device has a moving stage driven by a step motor, which elevates the tested sample on the stage to approach and contact against an indenter. The indenter is an L-shape cantilever and its tip indenting into the sample is a lead sphere with 1.0 mm diameter.
In the test, two laser displacement sensors (CD22-15, OPTEX FA CO., Ltd., Kyoto, Japan) were employed to measure the stage and the indenter tip displacements, respectively. The outputs of the sensors were sampled into a personal computer for the succeeding indent and force analysis, i.e., the depth of the indent was equal to the difference between the stage displacement and the indenter tip displacement after the contact occurred; and the contact force can be calculated by multiplying the tip displacement with the cantilever deflection coefficient, which was calibrated in advance of the test. The indent depth and force were regressed against the following Hertz contact theory within the indent depth from 0 to 0.2 mm.
where u is the depth of the indent; F is the contact force; R the radius of the indenter tip; E the elasticity of the sample; and ν the Poisson ratio of the sample set as 0.5 in this study.
In this study, the velocity of the moving stage was set at 0.008 mm/s and the depth of the indent at ca. 0.2 mm. For each sample, the indentation test was conducted at three separated randomly chosen sites on the sample and the average of the elasticity at the three sites was taken as the elasticity of the sample.
Experimental data was expressed as mean ± SE. Multiple comparison tests were performed using Fisher’s least significant difference method (BellCurve for Excel 3.20) to compare the data in the DHA/AA groups with respect to No-PUFAs-suppl. group (control). P < 0.05 was regarded as significant.

Figure 2: The lab-made vision-based measurement system for cardiomyocyte contractile force. (a) Photograph of the device with microscope; (b) schematic drawing of the device; (c) schematic drawing of the equilateral triangle setting of the circular cardiomyocyte-collagen gel and the stretch strain to the gel, the decomposition of the contractile force is illustrated as well; (d) measurement of the displacement of beating vertex under microscope.

Figure 3: Schematic drawing of the indentation test device.
Fatty acid contents in cardiomyocytes under DHA supplementation (Fig. 4)
The contents of palmitic (16:0) and stearic (18:0) acids in the control group were significantly lower than those in the neonatal myocardium (palmitic: 2.10 ± 0.42 vs. 4.93 ± 0.90 μmol/g wet tissue, P < 0.05; stearic: 1.72 ± 0.28 vs. 4.31 ± 0.72 μmol/g wet tissue, P < 0.01). The contents of mylistic (14:0) and palmitic acids in 30 μM DHA-supplemented cardiomyocytes were significantly higher than those, respectively, in the control group (0.43 ± 0.03 vs. 0.18 ± 0.02 μmol/g wet tissue, P < 0.05) (Fig. 4a).
In monounsaturated fatty acids (MUFAs), contents of palmitoleic (16:1n–7) and oleic (18:1n–9) acids in the control group was significantly higher than those in the neonatal group (palmitoleic: 0.19 ± 0.03 vs. 0.07 ± 0.02 μmol/g wet tissue, P < 0.05; oleic: 3.08 ± 0.53 vs. 1.23 ± 0.27 μmol/g wet tissue, P < 0.01). Oleic acid content in the supplemented groups tended to be lower than that in the control group, and the content in the cells supplemented with 40 μM (1.58 ± 0.08 μmol/g wet tissue) was closest to the level of the neonatal group (1.23 ± 0.27 μmol/g wet tissue) (Fig. 4b).
In regard to n–3 PUFAs, contents of docosapentaenoic acid (22:5n–3) and DHA in the control group were significantly lower than those in the neonatal group (docosapentaenoic: 0.20 ± 0.04 vs. 0.55 ± 0.07 μmol/g wet tissue, P < 0.01; DHA: 0.26 ± 0.05 vs. 1.90 ± 0.20 μmol/g wet tissue, P < 0.01). Eicosapentaenoic acid (20:5n–3, EPA) content in each supplemented group was significantly higher (P < 0.01) than that in the control and neonatal groups. DHA content in the supplemented groups were generally higher than those in the control group. The content was close to the neonatal level under 10–20 μM supplementation (10 μM: 1.72 ± 0.25 μmol/g wet tissue; 20 μM: 2.30 ± 0.26 μmol/g wet tissue). Docosapentaenoic acid content tended to be higher than that in the control group under 30–40 μM supplementation although significant difference was not detected (Fig. 4c).
n–6 PUFA contents in the cultured cells were generally lower than those in the neonatal group regardless of the DHA supplementation. Eicosadienoic acid (20:2n–6) content was significantly lower (P < 0.05) than that in the control group under all the supplemented doses (Fig. 4d).

Figure 4: Effects of docosahexaenoic acid supplementation on contents of saturated (a), monounsaturated (b), n–3 polyunsaturated (c), and n–6 polyunsaturated (d) fatty acids in rat fetal cardiomyocytes in primary culture. Data are expressed as mean ± SE. **P < 0.01, *P < 0.05.
Fatty acid contents in cardiomyocytes under LA supplementation (Fig. 5)
No significant difference in contents of saturated fatty acids (SFAs) was observed among each supplemented group and control group. Palmitic acid content became close to that in the neonatal group whereas stearic acid content remained lower (Fig. 5a). In MUFAs, contents of palmitoleic and oleic acids in each supplemented group was generally higher than those in the neonatal group (Fig. 5b).
In regard to n–3 PUFAs, EPA content in each supplemented group was significantly lower (P < 0.05) than that in the control and neonatal groups. Docosapentaenoic acid and DHA contents was still lower (P < 0.01) in each supplemented group than in the neonatal group (Fig. 5c).
Regarding n–6 PUFAs, contents of LA and AA in the supplemented groups were dose-dependent, and under 20–30 μM LA supplementation, LA (20 μM: 1.86 ± 0.16 μmol/g wet tissue; 30 μM: 2.02 ± 0.28 μmol/g wet tissue) and docosatetraenoic acid (22:4n–6) contents (20 μM: 0.25 ± 0.06 μmol/g wet tissue; 30 μM: 0.34 ± 0.06 μmol/g wet tissue) were close to those contents in the neonatal group (0.24 ± 0.03 μmol/g wet tissue). In spite of the LA supplementation, AA content in the cardiomyocytes still remained lower than that in the neonatal group (Fig. 5d).

Figure 5: Effects of linoleic acid supplementation on contents of saturated (a), monounsaturated (b), n–3 polyunsaturated (c), and n–6 polyunsaturated (d) fatty acids in rat fetal cardiomyocytes in primary culture. Data are expressed as mean ± SE. **P < 0.01, *P < 0.05. The data of neonatal and control groups are the same as those shown in Fig. 4.
Fatty acid contents in cardiomyocytes under AA supplementation (Fig. 6)
The contents of mylistic and palmitic acid in the AA-supplemented cardiomyocytes were generally higher than those in the control group (Fig. 6a). The content of oleic acid in the supplemented cells tended to be lower than the control group whereas little difference was observed in palmitoleic acid content (Fig. 6b).
Although the AA supplementation did not affect docosapentaenoic acid and DHA contents in the cardiomyocytes, EPA content was significantly lower in the cells cultured with AA than in the neonatal and control groups (P < 0.01) (Fig. 6c).
The supplementation did not affect LA content, and the content was significantly lower (P < 0.01) than that in the neonatal group. Eicosadienoic acid content in AA-supplemented cells was significantly lower than that in the neonatal and control groups under 30–60 μM supplementation (P < 0.05 vs. neonatal group; P < 0.01 vs. control group). Dihomo-γ-linolenic acid (20:3n–6) contents in the supplemented cells under 60 μM supplementation (0.54 ± 0.10 μmol/g wet tissue) were supplemented-dose-dependent, and the content was higher than that in the neonatal (0.26 ± 0.04 μmol/g wet tissue, P < 0.05) and control (0.16 ± 0.03 μmol/g wet tissue, P < 0.01) cells. AA content in the cardiomyocytes cultured with AA were supplemented-dose-dependent, and each content was significantly higher than that in the control group (P < 0.05 under 20 μM supplementation; P < 0.01 under 30–60 μM supplementations). Docosatetraenoic acid content in the cells cultured with AA was significantly higher than those in the neonatal and control groups under 30–60 μM supplementation (Fig. 6d).
Percentage change of contractile area
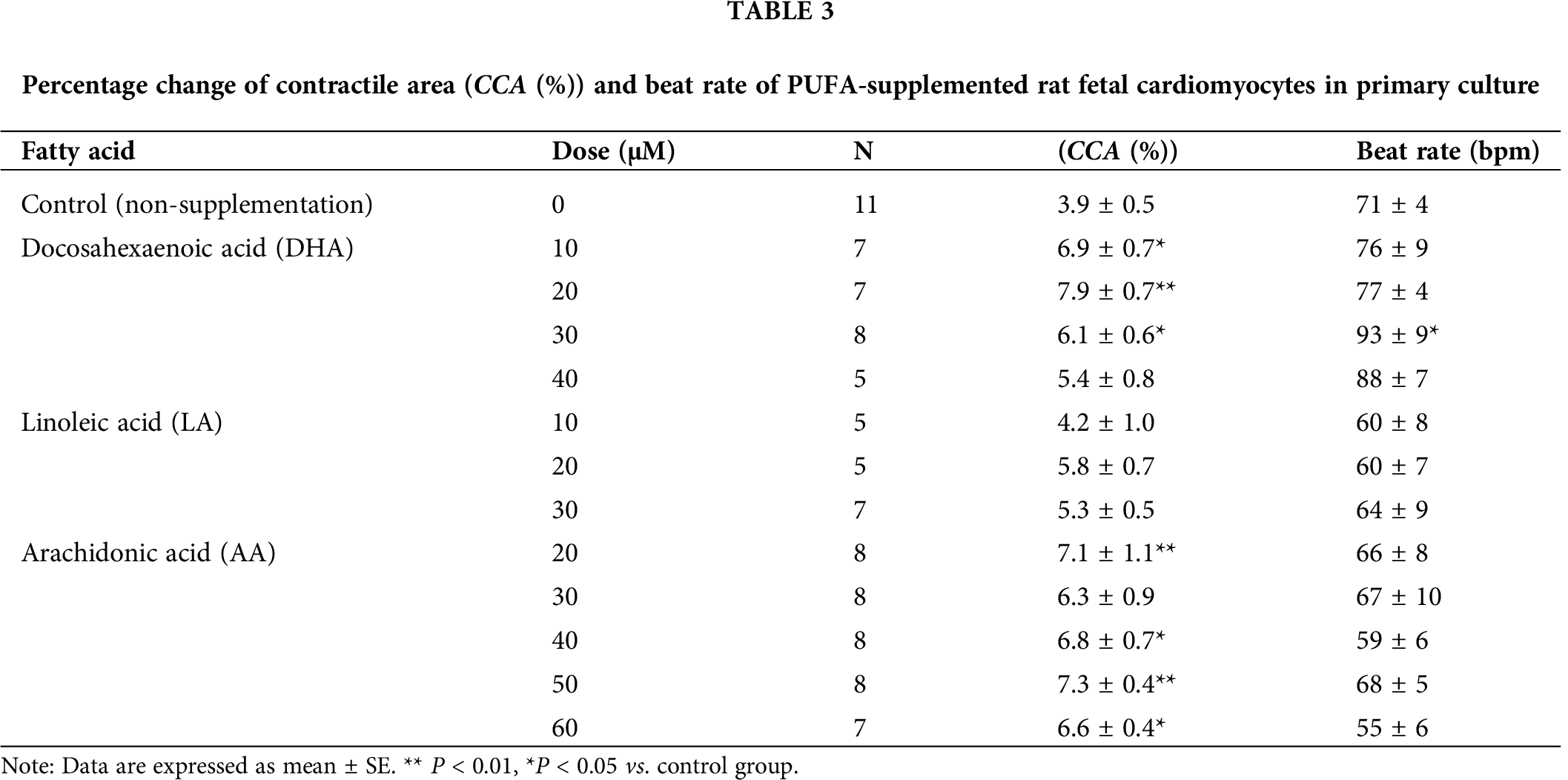
As shown in Tab. 3, the percentage change of contractile area in DHA-supplemented groups were significantly higher (P < 0.05) than that in the control group, except for 40 μM supplementation group. The maximal percentage change of contractile area was obtained in 20 μM supplemented cells (approximately 200% of the control group). The percentage change of contractile area in AA-supplemented cells was also higher (P < 0.05) than the control group except for 30 μM supplemented group. The percentage change of contractile area was maximum under 50 μM supplementation (approximately 190% of the control group). On the other hand, no significant difference was observed between the LA-supplemented cells and control group.
While no significant difference in beat rate was observed between the LA- or AA-supplemented cells and control group, the rate was significantly higher (P < 0.05) than that in the control group only under 30 μM DHA supplementation.
Based on the above results, we chose the most promising supplementation conditions for improving the cardiomyocyte performance, i.e., DHA and AA at 20 μM and 50 μM concentrations, respectively, to do the further mRNA and contractile force investigations.
Fig. 7 shows time series data of the gene expression related with “differentiation & maturity” (a–f), “fatty acid metabolism” (g–i), and “cell adhesion” (j, k) in DHA20 or AA50 supplementation relative to the control.

Figure 6: Effects of arachidonic acid supplementation on contents of saturated (a), monounsaturated (b), n–3 polyunsaturated (c), and n–6 polyunsaturated (d) fatty acids in rat fetal cardiomyocytes in primary culture. Data are expressed as mean ± SE. **P < 0.01, *P < 0.05. The data of neonatal and control groups are the same as those shown in Fig. 4.
For the effects of DHA supplementation, the relative expression of the “differentiation” marker Nkx2.5 (Fig. 7a) was slightly higher from day 1 and the data on day 2 was significantly higher than the control (P < 0.05). The higher level kept till day 7. The expression level of Srf (Fig. 7b) was also slightly higher (>1) from day 1, and then without obvious change on day 7. The expression of P300 (Fig. 7c) showed a transient increase on day 1 though without significance. The expression of the cardiac structural marker cTnT (Fig. 7d) tended to be at the similar level compared to that of the control. Another cardiac structural and ventricular-specific marker Mlc2v (Fig. 7e) increased approximately 3-fold from day 2 to day 7, and the difference became significant on day 7 (P < 0.05). These structural markers indicate the development of the cardiomyocytes and can also be regarded as the indicators for cardiomyocyte maturity. The final marker in this classification, a maturity marker Errγ (Fig. 7f), showed a significant transient higher expression on day 1.
The expression of the “fatty acid metabolism” marker Cd36 (Fig. 7g) was approximately twice of that in the control on day 1, and further increased to approximately five-fold that of control on day 7 (P < 0.01 or 0.05). The Pparα expression (Fig. 7h) tended to increase due to the culture, whereas the Pparδ expression (Fig. 7i) kept stable throughout the culture period.

Figure 7: Relative expression of genes related with cardiomyocyte differentiation & maturity (a–f), fatty acid metabolism (g–i), and cell adhesion (j, k). n = 5, mean ± SE; *P < 0.05 and **P < 0.01 vs. control; †P < 0.05 and ‡P < 0.01 between DHA20 and AA50.
The expression of the “cell adhesion” factor Cdh2 (Fig. 7j) tended to keep a higher level during the culture and the significance could be detected on day 7 (P < 0.05). The expression of Cx43 (Fig. 7k) was approximately twice of that in the control on day 2 (P < 0.01) and tended to maintain at this level though without significance.
As for the effects of AA supplementation, the expression of “differentiation” markers Nkx2.5 (Fig. 7a) and Srf (Fig. 7b) tended to increase at the later period of the culture. The expression of P300 (Fig. 7c) showed a transient increase on day 1 as that with the DHA supplementation. cTnT (Fig. 7d) tended to be at higher level on day 7 and so did Mlc2v (Fig. 7e). The expression of Errγ (Fig. 7f) was slightly higher than that of the control throughout the culture period. However, all the above-mentioned expression differences were not significant.
The expression of the “Fatty acid metabolism” marker Cd36 (Fig. 7g) was at the significantly higher level than that in the control and showed a similar profile to the effect of the DHA supplementation. The expression level of Pparα was significantly higher on day 1 and day 2 (Fig. 7h), and the data on day 2 was also significantly higher than that for the DHA20 group (P < 0.01). For Pparδ (Fig. 7i), its significant higher expression on day 1 and day 2 declined to the expression level of the control on day 7.
For one of the “cell adhesion” molecules, Cdh2 (Fig. 7i), the significant increases of the expression on day 1 and day 2 could be detected and the expression tended to drop back to the level of the control on day 7. For Cx43 (Fig. 7k), the expression level significantly increased on day 1 and day 2 and the higher level kept till day 7 (P < 0.05).
The feature of the phenotype of the cardiomyocytes cultured with the fatty acid supplementation is that upregulation dominated the expression of the investigated genes; most of the genes in the study exhibited relative expressions greater than 1. Tab. 4 shows the significantly upregulated expressions with heat colors to indicate the extents of the increases. It can be seen that both the PUFAs had stronger influence on the expression of genes in the “fatty acid metabolism” and “cell adhesion” classifications rather than that in the “differentiation & maturity” category. In particular, the significant enhancement on the “fatty acid metabolism” and “cell adhesion” by AA is remarkable.
Contractile force of the cardiomyocytes on the circular collagen gels
Tab. 5 presents geometries and the elasticity of the cardiomyocyte-collagen gels at the time of contractile force measurement. It can be seen that the collagen gels were compacted due to the interaction between collagen fibrils and the cardiomyocytes cultured on them. The compaction phenomenon of collagen gels when mesenchymal cells are cultured in or on the gels has been well investigated (Bell et al., 1979; Vernon and Sage, 1996; Feng et al., 2014), and as shown in Fig. 8 the compaction enhanced the elasticity of the gels. It should be noted that the PUFAs in this experiment tended to elevate the mechanical elasticity of the gels greater than that of the control.
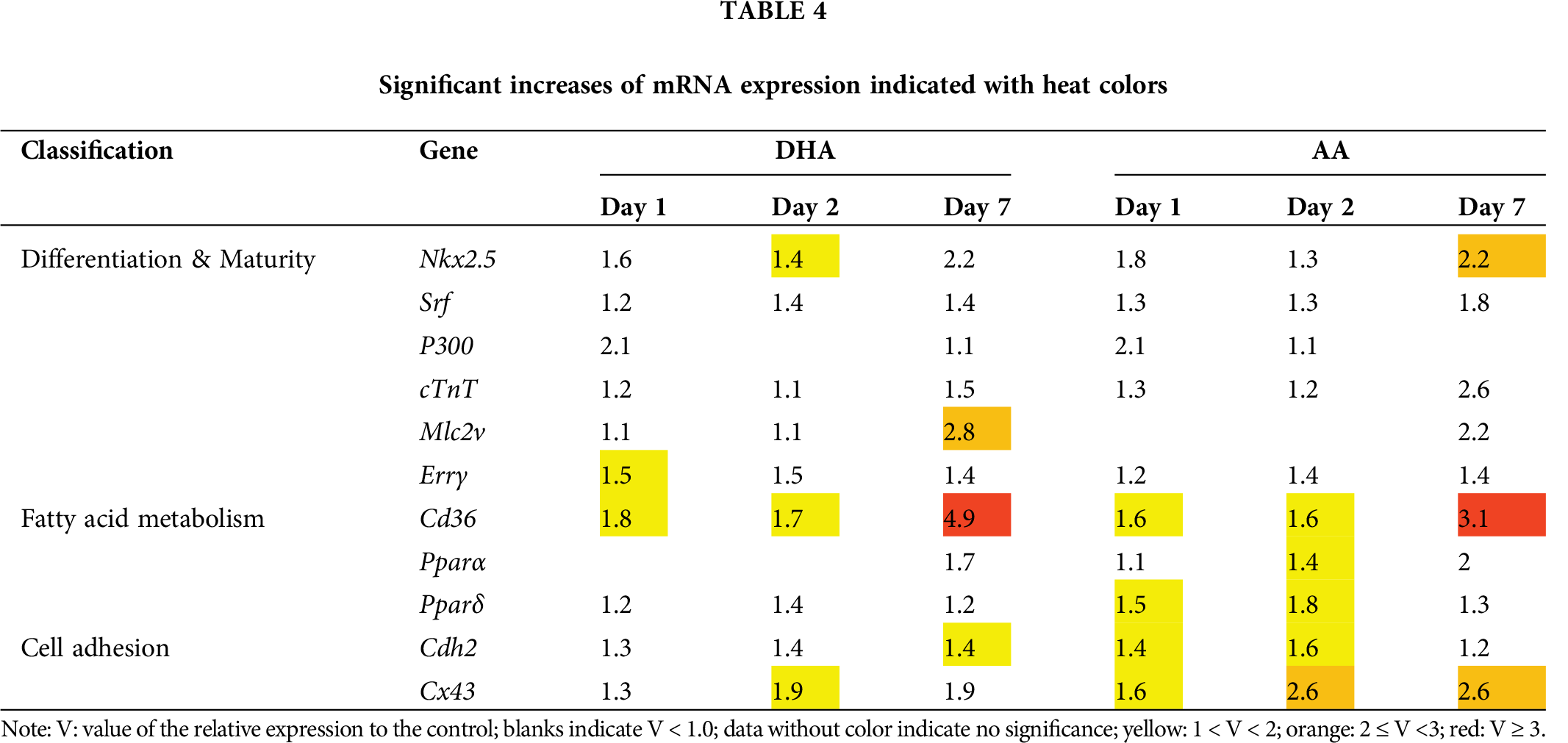
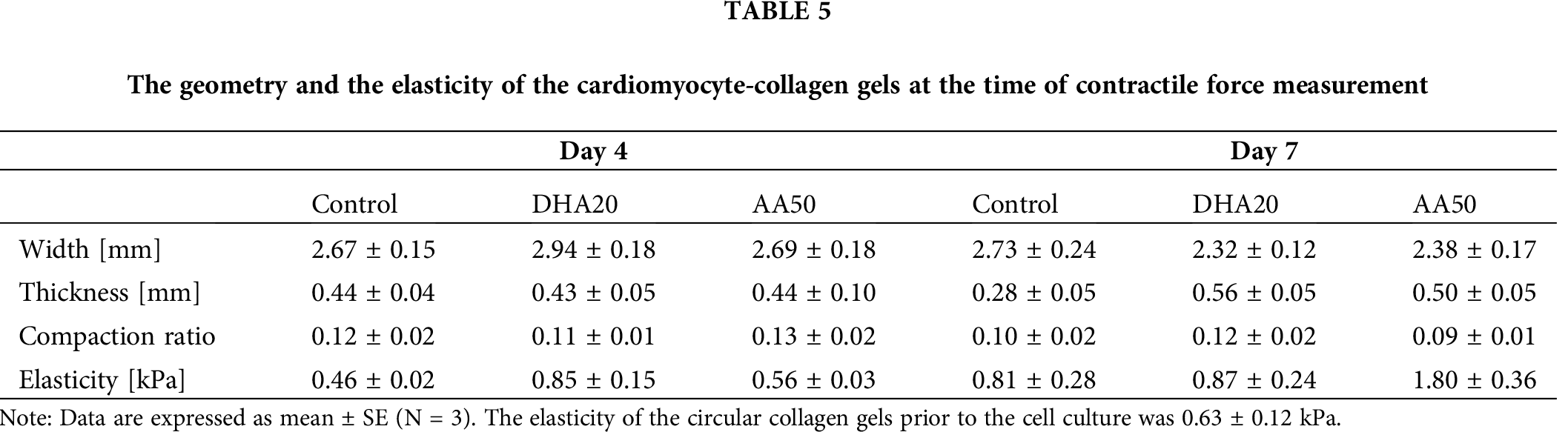
Fig. 9 shows the contractile force generated by the fetal cardiomyocytes cultured on the gels under different stretch strain on day 4 and day 7. The supplementation of the PUFAs had modest augment effect on the contractile force of the cardiomyocytes because no significance was detected on the data larger than the corresponding data of the control, which was in contrast to the result of percentage change of contractile area, therein the significant increase had been found. Supplementation of AA tended to increase the contractile force at the slack state (zero strain) of the gels on day 4 and to increase the force over the whole strain range on day 7. Notably, contractile force of the control on day 4 exhibited a biphasic profile with respect to the stretch strain of the gel, showing a maximum around 5% strain (Fig. 9a). However, cardiomyocytes in the control at the later period (day 7) or with the PUFAs supplementation on both day 4 and day 7 presented monotonic declined contractile force with the increase of the tensile strain.
Cardiac regenerative medicine faces the challenges in deriving cardiomyocytes from stem cells with phenotypic fidelity (Lian et al., 2013; Zhao et al., 2019) and in generating the mature cardiomyocytes with enough contractile force to fulfill cardiac pump function (Bouchard et al., 2018; Karbassi et al., 2020). Due to the shift of energetic substrates from lactate and glucose to fatty acids during the neonatal period of mammalian hearts (Horikoshi et al., 2019; Isu et al., 2019) and the discovered myocardial physiological functions of fatty acids (Yamada et al., 2017; Shysh et al., 2016), it is reasonable to expect beneficial effects of the supplementation of fatty acids in the culture medium on the heart cells (Horikoshi et al., 2019; Isu et al., 2019).

Figure 8: Elasticity of the cardiomyocyte-collagen gels versus gel compaction ratio (N = 3, mean ± SE).

Figure 9: Contractile force exerted from the fetal cardiomyocytes in the cardiomyocyte-collagen gel constructs (N = 5–7, mean ± SE).
In order to ultimately impact the differentiation and maturation of stem cells into cardiomyocytes by means of the supplementation of PUFAs, the aim of the present study is to firstly investigate the effects of the supplementation on the differentiated embryonic cardiomyocytes and to tease out the proper PUFAs for the further study on the differentiation of stem cells. Based on our previous investigations (Karimata et al., 2013), we targeted three PUFAs (DHA, LA, and AA) in this study and further investigated the effects on the phenotypic expression and the contractile force of the fetal cardiomyocytes cultured with the most promising supplementation conditions.
Mylistic, palmitic, and stearic acids in any of the PUFA-supplemented cardiomyocytes tended to be higher than those in the control group (not significant, Figs. 4a, 5a, and 6a). Although fatty acids are major energy source of cardiomyocytes (Berg et al., 2002; Pascual and Coleman, 2016), SFAs which are produced from glucose in the medium via de novo synthesis could have been used less than usual in the present study because supplemented PUFA might have alternatively been used.
In contrast, oleic acid content tended to be somewhat lower than that in control group under the supplementation of DHA, LA, or AA. While palmitic acid induces oxidative stress in cardiomyocytes, oleic acid mitigates the stress (Al-Shudiefat et al., 2013; Miller et al., 2005). Oleic acid can be supplied by desaturation of SFAs and/or elongation induced by stearoyl-CoA desaturase (SCD-1) and ELOVL6, respectively. In the present study, however, the supply pathway might be limited because supplemented PUFAs might have suppressed SCD-1 activity and caused a profound attenuation of Elovl6 mRNA expression (Ntambi, 1999; Matsuzaka et al., 2002). Thus, oleic acid might have been utilized more in response to increasing trend of palmitic acid accumulation.
Under the DHA supplementation, DHA content was increased in a dose-dependent manner in cardiomyocytes and tended to be higher than those in the control cells (P < 0.01 under 20–40 μM supplementations, Fig. 4c). This result may imply that supplemented DHA could have successfully been incorporated into the cardiomyocytes. In addition, DHA supplementation also resulted in higher EPA contents in the cardiomyocytes. Hence, the relatively high EPA might be attributed to oxidation of supplemented DHA.
Regarding n–6 PUFAs, the relatively lower content in eicosadienoic acid was observed in DHA-supplemented cells (Fig. 4d). It was reported that DHA has an inhibitory effect on LA elongation or desaturation activity at least in cultured cardiomyocytes derived from neonatal rats (Hrelia et al., 1995; Bordoni et al., 1996). Therefore, in the present study as well, eicosadienoic acid could have been utilized to synthesize longer n–6 PUFAs more under the effect of supplemented DHA shown above.
Under the LA supplementation, low EPA content was seen in the supplemented cells while AA content tended to be higher than that in the control cells (not significant). Bordoni et al. (1996) reported that γ-linolenic acid (18:3n–6) supplementation decreases the conversion of α-linolenic acid to longer n–3 PUFAs in rat cardiomyocytes in vitro. Although we did not cover γ-linolenic acid in the present study, γ-linolenic acid may be synthesized from LA on the way of AA synthesis. Thus, the supplemented LA might inhibit elongation of α-linolenic acid to EPA under the effect of γ-linolenic acid, and EPA content decreased due to its conversion to longer n–3 PUFAs.
The contents of LA, AA, and docosatetraenoic acid in the LA-supplemented cells were increased in a dose-dependent manner, and generally, were higher than those in the control cells (Fig. 5d). LA and docosatetraenoic acid contents reached to close levels of those in the neonatal tissue under the high dose condition. These results suggest that supplemented LA was elongated even in cultured cardiomyocytes. However, AA content was still lower than that in the neonatal myocardium, suggesting that LA supplementation cannot increase AA content up to the in vivo level.
As a result of the AA supplementation, contents of dihomo-γ-linolenic acid, AA, and docosatetraenoic acid in the cardiomyocytes were elevated in a dose-dependent manner to levels more than those in the control group (Fig. 6d). Since fatty acid was gradually degraded via β-oxidation, the oxidation of AA may result in somewhat increase in dihomo-γ-linolenic acid content. On the other hand, Leroy et al. (2008) reported the possibility of fatty acid elongation in cardiomyocytes, and thus the relatively high contents of docosatetraenoic acid in the AA-supplemented cells might be attributed to the elongation of AA. Thus, the supplemented AA might be incorporated into the cells and led to increase in the contents of dihomo-γ-linolenic and docosatetraenoic acids via enhancement of oxidation and elongation, respectively, of AA.
Hagve and Sprecher (1989) reported that isolated cardiomyocytes derived from rats incorporate approximately 66 and 70 nmol of LA and AA, respectively, within 120 min per 2.5 mg protein (2.5 mg protein correspond to 431,000 cardiomyocytes). Therefore, most of supplemented 50–150 nmol/dish LA or 100–300 nmol/dish AA (corresponding to 10–30 μM or 20–60 μM, respectively) could be easily incorporated into 2 million cardiomyocytes seeded in the present study.
Relatively low EPA content observed in the LA-supplemented cells was seen in the AA-supplemented cells as well (Fig. 6d). However, the mechanisms of the lowering of EPA content have not been clarified yet.
The AA supplementation resulted in lower eicosadienoic acid content in comparison with the control group (Fig. 6d), as seen in DHA supplementation, which may imply that the supplementation may have enhanced utilization of eicosadienoic acid. AA is a precursor to a number of potent pro-inflammatory mediators including prostaglandins and leukotrienes (Innes and Calder, 2018), while eicosadienoic acid suppresses production of nitric oxide and inflammatory cytokines such as prostaglandin E2, TNF-α, and so forth at least in macrophages (Huang et al., 2011). Therefore, the AA supplementation may enhance utilization of eicosadienoic acid in response to increase in inflammatory mediators. On the other hand, AA supplementation led to relatively high content of docosatetraenoic acid. Thus, the supplementation may not inhibit elongation of AA to longer n–6 PUFAs.
The percentage change of contractile area in DHA- or AA-supplemented cells were higher than that in the control group (Tab. 3). Xiao et al. (1998) suggested that cytochrome P450 enzymes (CYPs) modulate cardiac contraction. CYPs metabolize a number of substances including fatty acids in mammalian cells. Inhibition of CYPs suppresses the L-type Ca2+ current (ICa) in rat ventricular myocytes. Extracellular administration of a metabolite of AA, which is produced via CYPs, elevates intracellular cAMP level and ICa. Thus, AA may contribute enhancement of cardiac contractile function via CYP activity. Since CYPs are also involved in metabolism of DHA and EPA (Arnold et al., 2010), high percentage change of contractile area seen in the DHA-supplemented cells in the present study may be relevant in part to CYPs activity although the mechanism is not clear. Therefore, at least DHA and AA may exert cultured cardiomyocytes on improvement of contractile performance, and effective doses to maximize the percentage change of contractile area were 20 μM and 50 μM, respectively, where the content of supplemented PUFA in the cardiomyocytes was closest to that in the neonatal tissue.
Figs. 4a and 6a show a significant increase of palmitic acid content with the supplementation of DHA and AA, respectively. It may be asked whether this increase in palmitic acid might result in the outcomes in the experiments for the contractile performance and mRNA expression. We excluded the impact of palmitic acid on the outcomes because it was known that polyunsaturated fatty acids such as DHA and AA can be act as physiological ligands of transcriptional factors (Yamada et al., 2017) rather than saturated fatty acid such as palmitic acid. The increase of palmitic acid with the supplementation of DHA or AA was regarded as the secondary downstream effect of the supplementation since palmitic acid, synthesized from glucose de nova, may be consumed less under the supplementations as aforementioned. Most critically, the increases of palmitic acid under the supplementation of DHA at 20 μM or AA at 50 μM was found no significance.
Regarding the beat rate, we observed relatively higher rate in the 30–40 μM DHA-supplemented cells whereas slightly lower rate was seen under the AA supplementation (Tab. 3). DHA induces positive chronotropic action, and the effect is higher than that of EPA (Grynberg et al., 1995; Grynberg et al., 1996; Mauricio et al., 2016). Conversely, AA induces negative chronotropy (Mackay and Mochly-Rosen, 2001). Therefore, in the present study, the beat rate might directly be affected by the PUFA supplementation.
In this study, eleven genes were chosen and classified into three categories by their functions in cardiomyocytes and their mRNA expressions were investigated by real-time PCR. We realized that the culture was a mixture of cardiomyocytes and fibroblasts. However, because one-week culture is a relatively short period, besides the cells have a lag phase to recover from the cell harvest; the proliferation of the cells was limited and there was no obvious difference of the cellular fractions among different dishes in each experiment, which also can be implied by the relatively stable beat frequency of the cardiomyocytes under different conditions as shown in Tab. 3. Therefore, the mixture with cardiac fibroblasts did not result in considerable deviation between the mRNA data of the control and of the supplementations.
As a result, this study revealed that the supplementation of 20 μM DHA or 50 μM AA induced a wide-spread upregulation tendency across the investigated mRNAs in the cultured cardiomyocytes. The significant upregulations aggregated in the classifications for fatty acid metabolism and cell adhesion (Tab. 4). To the differentiated heart cells at the late stage, the effects of the PUFAs supplementation on differentiation and maturity of the fetal cardiomyocytes may become limited compared with that on differentiation and maturity of stem cells (Horikoshi et al., 2019; Sharma et al., 2018).
The significant upregulation of those genes in charge of the fatty acid metabolism was understandable since their ligand substance was supplemented and the upregulation suggested that DHA and AA did behave as energetic substrates in the cardiomyocytes. However, since the sake of inferior contractile performance of the cultured cardiomyocytes is not considered as lack of energetic source; the other physiological activities of DHA and AA are much more attractive for the purpose of the study.
Interestingly, this study unveiled the enhancement of the cardiomyocyte connection with the supplementation of the PUFAs in culture. In particular, the effect of AA supplementation on the cardiomyocyte connection was manifest. It has been reported by our previous study that the expressions of Cx43 and Cdh2 in cultured cardiomyocytes were significantly lower than that in fetal and neonatal myocardium (Nakamura et al., 2008). In this paper, we firstly reported that DHA or AA could prevent the decline and further promote the expressions of the two vital adhesion molecules for cardiac function.
As for the mechanism by which the supplementation with DHA and AA augments the expression of connexin 43, DHA was reported to suppress the loss of Cx43 function by inhibiting signal transduction of the inflammatory cytokine interleukin-1β (IL-1β) in rat neonatal cardiomyocytes (Baum et al., 2012) and to stabilize connexins within the membrane by intercalation of the cellular membrane (Adkins and Kelley, 2010). It should also be noted that the content of EPA (20:5n–3) was significantly higher in the DHA group than that in the control (Fig. 4c). EPA has been reported to enhance gap junctions by suppressing hypoxia-induced activation of tyrosine kinases (Zhang et al., 1999; Zhang et al., 2002). Regarding the supplementation of AA, there have been no reports on AA’s effect to directly increase Cx43 expression in cultured cardiomyocytes. However, it has been reported that AA-derived metabolite 11,12-epoxyeicosatrienoic acid may transiently enhance cell-cell coupling (Spector et al., 2004), and n–6 PUFAs γ-linolenic acid increase Cx43-gap junction channel in human vascular endothelial cells (Jiang et al., 1997). These direct interactions or indirect metabolite functions may be implied for the mechanism of the connexin43 enhancement and need further investigation.
In view of the above analysis and our previous research (Nakamura et al., 2008), which investigated the mRNA expression of SRF, p300, Nkx2.5, myocardin, Cdh2, and Cx43 in fetal cardiomyocytes under conventional monolayer culture and found the tendency of cardiac de-differentiation in terms of the attenuation of the mRNA expressions; so that the role of the PUFA supplementation may be at first suggested as preventing the cardiomyocytes under culture from the de-differentiation.
In this study, we constructed a cardiomyocyte-collagen gel assay to directly assess the contractile force generated by the cardiomyocytes cultured on the collagen gel. Compared to the existing 3-dimensional engineered tissue for the contractile force assessment (Boudou et al., 2012; Hansen et al., 2010), this construct has the following advantages. First, the cultured cardiomyocytes were directly exposed to the culture medium so as to avoid the intervening effects of substance diffusion on cellular metabolism in the case of 3-dimensional culture; this advantage is critical for the purpose of this study. Second, the contractile force generated by the cardiomyocytes in the top layer of the construct can be exclusively assessed by using Eq. (2) due to the separated two-layer structure of the cardiomyocyte-collagen gels. Recently, Sasaki et al. (2018) developed another approach to measure the contractile force of cultured cardiomyocytes based on cell-sheet technology, which also had the merit of the separated configuration between cell and substrate components.
It comes out that the supplementation of DHA or AA in the culture medium had modest enhancement effect on the contractile force of the cardiomyocytes since no significance was detected in the force data with the supplementation larger than that of the control. This result was in accordance with the insignificance of the most upregulations of the genes for cardiomyocyte maturity with the supplementation. In view of the significant augment of the phenotypic expression related with the cell connection and the significant increase of the percentage change of contractile area, we speculate that the enhancement of the beating performance of the fetal cardiomyocytes with the PUFAs supplementation may mainly result from the enhancement of the cellular connection; because the connection enhancement may promote the syncytium of the cardiomyocyte cluster in culture dishes so as to manifest the percentage change of contractile area but not increase the essential contractile force therein the significant reinforcement of the cardiomyocyte maturity is needed.
The biphasic feature of the cardiomyocyte contractile force with the passive stretch strain is regarded as the fundamental at the cellular level for the cardiac Frank-Starling law (Allen and Kentish, 1985), and on the top of which the structure and arrangement of the cardiac tissue also account for this well-known cardiological mechanism (Buckberg et al., 2008). The loss of the biphasic feature in contractile force may implicate the deviation of myofilament structure in the cardiomyocytes under the culture conditions from the structure in vivo. The experiment showed that the biphasic profile of the contractile force with respect to stretch strain only resided in the control cardiomyocytes at the early culture period (Fig. 9a). It is difficult to retain the biphasic feature under the free culture condition without coordinating the cardiomyocyte orientation. Oriented cardiomyocyte culture can be realized by applying increment strain to the gel or by unidirectional constrained compaction of the collagen gels as reported in literature (Eschenhagen et al., 2002; Feng et al., 2005). However, the reason why the cardiomyocytes cultured with the PUFA supplementation lost the biphasic feature at the early period (Fig. 9a) deserves further investigation.
The study unveiled substantial upregulation effect of the PUFAs supplementation on the expression of genes spreading at the differentiation & maturity, fatty acid metabolism, and cell adhesion classifications. In particular, the effect to enhance cardiomyocyte connection is firstly reported. For the differentiated fetal cardiomyocytes used in this study, it suggests the role of the PUFAs supplementation in the cell culture may be more plausible to prevent the de-differentiation of the cardiomyocytes rather than to promote the essential contractile function. Upon the current results, investigation of the effects of DHA and AA supplementation on the differentiation and maturity of the human iPS cell-derived cardiomyocytes is ongoing at our laboratory.
Availability of Data and Materials: The dataset generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ Contribution: The authors confirm contribution to the paper as follows: study conception and design: M. Yano, T. Nakamura, D. Sato, Z. Feng; data collection: M. Yano, Y. Umehara, T. Kudo; analysis and interpretation of results: M. Yano, T. Nakamura, T. Kosawada, A. Nishina, M. Sazuka, D. Sato, Z. Feng; draft manuscript preparation: M. Yano, T. Nakamura, D. Sato, Z. Feng. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: This study and all experiments involved are under approval of the Yamagata University Animal Experiment Committee.
Funding Statement: The present study was supported financially in part by Challenging Exploratory Research (16K12864), Grants-in-Aid for Scientific Research (C) (23500539) and (21K12661) from the Japan Society for the Promotion of Science.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Adkins Y, Kelley DS (2010). Mechanisms under lying the car-dioprotective effects of omega-3 polyunsaturated fatty acids. The Journal of Nutritional Biochemistry 21: 781–792.
Al-Shudiefat AA, Sharma AK, Bagchi AK, Dhingra S, Singal PK (2013). Oleic acid mitigates TNF-α-induced oxidative stress in rat cardiomyocytes. Molecular and Cellular Biochemistry 372: 75–82.
Allen DG, Kentish JC (1985). The cellular basis of the length-tension relation in cardiac muscle. Journal of Molecular and Cellular Cardiology 17: 821–840.
Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD (1991). Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circulation Research 69: 1226–1233.
Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, Rothe M, Schunk WH (2010). Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of ω-3 fatty acids. Journal of Biological Chemistry 285: 32720–32733.
Backs J, Olson EN (2006). Control of cardiac growth by histone acetylation/deacetylation. Circulation Research 98: 15–24.
Baum JR, Dolmatova E, Tan A, Duffy HS (2012). Omega 3 fatty acid inhibition of inflammatory cytokine-mediated Connexin43 regulation in the heart. Frontiers in Physiology 3: 272.
Bell E, Ivarsson B, Merrill C (1979). Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proceedings of the National Academy of Sciences of the United States of America 76: 1274–1278.
Berg JM, Tymoczko JL, Stryer L (2002). Each organ has a unique metabolic profile. Biochemistry, 5th edition, pp. 851–853. New York: W. H. Freeman.
Bordoni A, Lopez-Jimenez JA, Spanò C, Biagi P, Horrobin DF, Hrelia S (1996). Metabolism of linoleic and α-linolenic acids in cultured cardiomyocytes: Effect of different N-6 and N-3 fatty acid supplementation. Molecular and Cellular Biochemistry 157: 217–222.
Bouchard KR, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Novakovic GV (2018). Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556: 239–243.
Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS (2012). A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Engineering Part A 18: 910–919.
Bruneau BG (2002). Transcriptional regulation of vertebrate cardiac morphogenesis. Circulation Research 90: 509–519.
Buckberg G, Hoffman JIE, Mahajan A, Saleh S, Coghlan C (2008). Cardiac mechanics revisited: The relationship of cardiac architecture to ventricular function. Circulation 118: 2571–2587.
Cernecka H, Doka G, Srankova J, Pivackova L, Malikova E, Galkova K, Kyselovic J, Krenek P, Klimas J (2016). Ramipril restoresPPARβ/δ and PPARγ expressions and reduces cardiac NADPH oxidase but fail stores to recardiac function and accompanied myosin heavy chain ratio shift in severe anthracycline-induced cardiomyopathy in rat. European Journal of Pharmacology 791: 244–253.
Cheng L, Ding G, Qin Q, Xiao Y, Woods D, Chen YE, Yang Q (2004). Peroxisome proliferator-activated receptor δ activates fatty acid oxidation in cultured neonatal and adult cardiomyocytes. Biochemical and Biophysical Research Communications 313: 277–286.
Eschenhagen T, Didié M, Heubach J, Ravens U, Zimmermann WH (2002). Cardiac tissue engineering. Transplant Immunology 9: 315–321.
Feng Z, Matsumoto T, Nomura Y, Nakamura T (2005). An electro-tensile bioreactor for 3-D culturing of cardiomyocytes. IEEE Engineering in Medicine and Biology Magazine 24: 73–79.
Feng Z, Wagatsuma Y, Kikuchi M, Kosawada T, Nakamura T, Sato D, Shirasawa N, Kitajima T, Umezu M (2014). The mechanisms of fibroblast-mediated compaction of collagen gels and the mechanical niche around individual fibroblasts. Biomaterials 35: 8078–8091.
Fujita K, Feng Z, Sato D, Kosawada T, Nakamura T, Shiraishi Y, Umezu M (2018). Modulation of the mechanical properties of ventricular extracellular matrix hydrogels with a carbodiimide crosslinker and investigation of their cellular compatibility. AIMS Materials Science 5: 54–74.
Glatz JFC, Nabben M, Heather LC, Bonen A, Luiken JJFP (2016). Regulation of the subcellular trafficking of CD36, a major determinant of cardiac fatty acid utilization. Biochimica et Biophysica Acta 1861: 1461–1471.
Grynberg A, Fournier A, Sergiel JP, Athias P (1995). Effect of docosahexaenoic acid and eicosapentaenoic acid in the phospholipids of rat heart muscle cells on adrenoceptor responsiveness and mechanism. Journal of Molecular and Cellular Cardiology 27: 2507–2520.
Grynberg A, Fournier A, Sergiel JP, Athias P (1996). Membrane docosahexaenoic acid vs. eicosapentaenoic acid and the beating function of the cardiomyocyte and its regulation through the adrenergic receptors. Lipids 31: S205–S210.
Hagve TA, Sprecher H (1989). Metabolism of long-chain polyunsaturated fatty acids in isolated cardiac myocytes. Biochimica et Biophysica Acta 1001: 338–344.
Hansen A, Eder A, Bönstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwörer A, Uebeler J, Eschenhagen T (2010). Development of a drug screening platform based on engineered heart tissue. Circulation Research 107: 35–44.
Horikoshi Y, Yan Y, Terashvili M, Wells C, Horikoshi H, Fujita S, Bosnjak ZJ, Bai X (2019). Fatty acid-treated induced pluripotent stem cell-derived human cardiomyocytes exhibit adult cardiomyocyte-like energy metabolism phenotypes. Cells 8: 1095.
Hrelia S, Lopez Jimenez JA, Bordoni A, Nvarro SZ, Horrobin DF, Rossi CA, Biagi PL (1995). Essential fatty acid metabolism in cultured rat cardiomyocytes in response to either N-6 or N-3 fatty acid supplementation. Biochemical and Biophysical Research Communications 216: 11–19.
Huang Y, Ye T, Liu C, Fang F, Chen Y, Dong Y (2017). Maternal high-fat diet during pregnancy and lactation affects hepatic lipid metabolism in early life of offspring rat. Journal of Biosciences 42: 311–319.
Huang YS, Huang WC, Li CW, Chuang LT (2011). Eicosadienoic acid differentially modulates production of pro-inflammatory modulators in murine macrophages. Molecular and Cellular Biochemistry 358: 85–94.
Iacono G, Altafini C, Torre V (2013). Early phase of plasticity-related gene regulation and SRF dependent transcription in the hippocampus. PLoS One 8: e68078.
Innes JK, Calder PC (2018). ω-6 fatty acids and inflammation. Prostaglandins, Leukotrienes, and Essential Fatty Acids 132: 41–48.
Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, Kokubo Y, Tsugane S (2006). Intake of fish and n-3 fatty acids and risk of coronary heart disease among Japanese. Circulation 133: 195–202.
Isu G, Diaz DR, Grussenmeyer T, Gaudiello E, Eckstein F, Brink M, Marsano A (2019). Fatty acid-based monolayer culture to promote in vitro neonatal rat cardiomyocyte maturation. Biochimica et Biophysica Acta—Molecular Cell Research 1867: 118561.
Jiang WG, Bryce RP, Mansel RE (1997). Gamma linolenic acid regulates gap junction communication in endothelial cells and their interaction with tumour cells. Prostaglandins, Leukotrienes & Essential Fatty Acids 56: 307–316.
Karbassi E, Fenix A, Marchiano S, Muraoka N, Nakamura K, Yang X, Murry CE (2020). Cardiomyocyte maturation: Advances in knowledge and implications for regenerative medicine. Nature Reviews Cardiology 17: 341–359.
Karimata T, Sato D, Seya D, Sato D, Wakatsuki T, Feng Z, Nishina A, Kusunoki M, Nakamura T (2013). Fatty acid composition in fetal, neonatal, and cultured cardiomyocytes in rats. Vitro Cellular & Developmental Biology—Animal 49: 798–804.
Lee KH (2016). Changes in expression of connexin isoforms in the caudal epididymis of adult Sprague-Dawley rats exposed to estradiol benzoate or flutamide at the neonatal age. Development & Reproduction 20: 237–245.
Leroy C, Tricot S, Lacour B, Grynberg A (2008). Protective effect of eicosapentaenoic acid on palmitate-induced apoptosis in neonatal cardiomyocytes. Biochimica et Biophysica Acta 1781: 685–693.
Li J, Fu D, Hong G, Chen J, Kang H, Chen Z (2009). Skeletal muscle-derived stem cells exhibit cardiocyte competences. Journal of Huazhong University of Science and Technology—Medical Science 29: 741–744.
Li Y, Wang J, Gu T, Yamahara J, Li Y (2014). Oleanolic acid supplement attenuates liquid fructose-induced adipose tissue insulin resistance through the insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt signaling pathway in rats. Toxicology and Applied Pharmacology 277: 155–163.
Li Z, Guo X, Matsushita S, Guan J (2011). Differentiation of cardiosphere-derived cells into a mature cardiac lineage using biodegradable poly(N-isopropylacrylamide) hydrogels. Biomaterials 32: 3220–3232.
Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP (2013). Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nature Protocols 8: 162–175.
Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408.
Lui K, Huang Y, Choi HL, Yu S, Wong KB, Chen S, Chan FL (2006). Molecular cloning and functional study of rat estrogen receptor-related receptor gin rat prostatic cells. The Prostate 66: 1600–1619.
Mackay K, Mochly-Rosen D (2001). Arachidonic acid protects neonatal rat cardiac myocytes from ischaemic injury through ε protein kinase C. Cardiovascular Research 50: 65–74.
Matsuzaka T, Shimano H, Yahagi N, Kato T, Atsumi A, Yamamoto T, Inoue N, Ishikawa M, Okada S, Ishigaki N, Iwasaki H, Iwasaki Y, Karasawa T, Kumadaki S, Matsui T, Sekiya M, Ohashi K, Hasty AH, Nakagawa Y, Takahashi A, Suzuki H, Yatoh S, Sone H, Toyoshima H, Osuga J, Yamada N (2007). Crucial role of a long chain fatty acid elongase. Nature Medicine 13: 1193–1202.
Matsuzaka T, Shimano H, Yahagi N, Yoshikawa T, Amemiya-Kudo M, Hasty AH, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Osuga J, Takahashi A, Yato S, Sone H, Ishibashi S, Yamada N (2002). Cloning and characterization of a mammalian fatty acyl-CoA elongase as a lipogenic enzyme regulated by SREBPs. Journal of Lipid Research 43: 911–920.
Mauricio AF, Pereira JA, Neto HS, Marques MJ (2016). Effects of fish oil containing eicosapentaenoic acid and docosahexaenoic acid on dystrophic mdx mice hearts at later stages of dystrophy. Nutrition 32: 855–862.
Miller TA, LeBrasseur NK, Cote GM, Trucillo MP, Pimentel DR, Ido Y, Ruderman NB, Sawyer DB (2005). Oleate prevents palmitate-induced cytotoxic stress in cardiac myocytes. Biochemical and Biophysical Research Communications 336: 309–315.
Mu O, Wang L, Hang H, Liu C, Wu G (2017). Rosiglitazone pretreatment influences thrombin-induced phagocytosis by rat microglia via activating PPAR and CD36. Neuroscience Letters 651: 159–164.
Nakamura T, Feng Z, Honda T, Nomura Y, Kitajima T, Umezu M (2008). Comparison of mRNA expression of transcriptional factors and intercalated disk constituent proteins between in vivo and cultured cardiomyocytes. Journal of Artificial Organs 11: 134–140.
Ntambi JM (1999). Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. Journal of Lipid Research 40: 1549–1558.
O’Brien TX, Lee KJ, Chien KR (1993). Positional specification of ventricular myosin light chain 2 expression in the primitive murine heart tube. Proceedings of the National Academy of Sciences of the United States of America 90: 5157–5161.
Oliveira AF, Cunha DA, Ladriere L, Igoillo-Esteve M, Bugliani M, Marchetti P, Cnop M (2005). In vitro use of free fatty acids bound to albumin: A comparison of protocols. Biotechniques 58: 228–233.
Parlakian A, Tuil D, Hamard G, Tavernier G, Hentzen D, Concordet JP, Paulin D, Li Z, Daegelen D (2004). Targeted in activation of serum response factor in the developing heart results in myocardial defects and embryonic lethality. Molecular and Cellular Biology 24: 5281–5289.
Pascual F, Coleman RA (2016). Fuel availability and fate in cardiac metabolism: A tale of two substrates. Biochimica et Biophysica Acta 1861: 1425–1433.
Psichari E, Balmain A, Plows D, Zoumpourlis V, Pintzas A (2002). High activity of serum response factor in the mesenchymal transition of epithelial tumor cells is regulated by RhoA signaling. Journal of Biological Chemistry 277: 29490–29495.
Sakamoto T, Matsuura TR, Wan S, Ryba DM, Kim J, Won KJ, Lai L, Petucci C, Petrenko N, Musunuru K, Vega RB, Kelly DP (2020). A critical role for estrogen-related receptor signaling in cardiac maturation. Circulation Research 126: 1685–1702.
Salameh A, Schneider P, Muhlberg K, Hagendorff A, Dhein S, Pfeiffer D (2004). Chronic regulation of the expression of gap junction proteins connexin40, connexin43, and connexin45 in neonatal rat cardiomyocytes. European Journal of Pharmacology 503: 9–16.
Sambrook J, Russell DW (2006). The condensed protocols from molecular cloning: A laboratory manual. USA: Cold Spring Harbor Laboratory Press.
Sasaki D, Matsuura K, Seta H, Haraguchi Y, Okano T, Shimizu T (2018). Contractile force measurement of human induced pluripotent stem cell-derived cardiac cell sheet-tissue. PLoS One 13: e0198026.
Schmitz G, Ecker J (2008). The opposing effects of n-3 and n-6 fatty acids. Progress in Lipid Research 47: 147–155.
Sharma A, Zhang Y, Buikema JW, Serpooshan V, Chirikian O, Kosaric N, Churko JM, Dzilic E, Shieh A, Burridge PW, Wu JC, Wu SM (2018). Stage-specific effects of bioactive lipids on human iPSC cardiac differentiation and cardiomyocyte proliferation. Scientific Reports 8: 6618.
Shen P, Feng X, Zhang X, Huang X, Liu S, Lu X, Li J, You J, Lu J, Li Z, Ye J, Liu P (2016). SIRT6 suppresses phenylephrine-induced cardiomyocyte hypertrophy though inhibiting p300. Journal of Pharmacological Sciences 132: 31–40.
Shu DY, Wojciechowski MC, Lovicu FJ (2017). Bone morphogenetic protein-7 suppresses TGFb2-induced epithelial-mesenchymal transition in the lens: Implications for cataract prevention. Investigative Ophthalmology and Visual Science 58: 781–796.
Shysh AM, Nagibin VS, Kaplinskii SP, Dosenko VE (2016). N-3 long chain polyunsaturated fatty acids increase he expression of PPARγ-target genes and resistance of isolated heart and cultured cardiomyocytes to ischemic injury. Pharmacological Reports 68: 1133–1139.
Spector AA, Fang X, Snyder GD, Weintraub NL (2004). Epoxyeicosatrienoic acids (EETsMetabolism and biochemical function. Progress in Lipid Research 43: 55–90.
Steinmetz M, Quentin T, Poppe A, Paul T, Jux C (2005). Changes in expression levels of genes involved in fatty acid metabolism: Upregulation of all three members of the PPAR family (α, γ, δ) and the newly described adiponectin receptor 2, but not adiponectin receptor 1 during neonatal cardiac development of the rat. Basic Research in Cardiology 100: 263–269.
Suhaeri M, Subbiah R, van SY, Du P, Kim IG, Lee K, Park K (2014). Cardiomyoblast (h9c2) differentiation on tunable extracellular matrix microenvironment. Tissue Engineering Part A 21: 1940–1451.
Takaya T, Kawamura T, Morimoto T, Ono K, Kita T, Shimatsu A, Hasegawa K (2008). Identification of p300-targeted acetylated residues in GATA4 during hypertrophic responses in cardiac myocytes. Journal of Biological Chemistry 283: 9828–9835.
Vahl CF, Bonz A, Timek T, Hagl S (1994). Intracellular calcium transient of working human myocardium of seven patients transplanted for congestive heart failure. Circulation Research 74: 952–958.
Vernon RB, Sage EH (1996). Contraction of fibrillar type I collagen by endothelial cells: A study in vitro. Journal of Cellular Biochemistry 60: 185–197.
Xiao YF, Huang L, Morgan JP (1998). Cytochrome P450: A novel system modulating Ca2+ channels and contraction in mammalian heart cells. Journal of Physiology 508: 777–792.
Yamada K, Sato D, Nakamura T, Amano H, Morimoto Y (2017). Unknown biological effects of L-glucose, ALA, and PUFA. Journal of Physiological Sciences 67: 539–548.
Yanazume T, Hasegawa K, Morimoto T, Kawamura T, Wada H, Matsumori A, Kawase Y, Hirai M, Kita T (2003). Cardiac p300 is involved in myocyte growth with decompensated heart failure. Molecular and Cellular Biology 23: 3593–3606.
Yang G, Tian J, Feng C, Zhao L, Liu Z, Zhu J (2012). Trichostatin a promotes cardiomyocyte differentiation of rat mesenchymal stem cells after 5-azacytidine induction or during coculture with neonatal cardiomyocytes via a mechanism independent of histone deacetylase inhibition. Cell Transplantation 21: 985–996.
Zhang YW, Morita I, Yao XS, Murota S (1999). Pretreatment with eicosapentaenoic acid prevented hypoxia/reoxygenation-induced abnormality in endothelial gap junctional intercellular communication through inhibiting the tyrosine kinase activity. Prostaglandins, Leukotrienes & Essential Fatty Acids 61: 33–40.
Zhang YW, Yao XS, Murota S, Morita I (2002). Inhibitory effects of eicosapentaenoic acid (EPA) on the hypoxia/reoxygenation-induced tyrosine kinase activation in cultured human umbilical vein endothelial cells. Prostaglandins, Leukotrienes & Essential Fatty Acids 67: 253–261.
Zhao M, Tang Y, Zhou Y, Zhang J (2019). Deciphering role of Wnt signalling in cardiac mesoderm and cardiomyocyte differentiation from human iPSCs: Four-dimensional control of Wnt pathway for hiPSCCMs differentiation. Scientific Reports 9: 19389.
Zuppinger C, Eberhardt ME, Eppenberger HM (2000). N-Cadherin: Tructure, function and importance in the formation of new intercalated disc-like cell contacts in cardiomyocytes. Heart Failure Reviews 5: 251–257.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |