DOI:10.32604/biocell.2021.016869

| BIOCELL DOI:10.32604/biocell.2021.016869 |  |
| Article |
Investigation of the antioxidant defensive role of both AD-MSCs and BM-MSCs in modulating the alteration in the oxidative stress status in various STZ-diabetic rats’ tissues
1Zoology Department, Faculty of Science, Arish University, North Sinai, Egypt
2Biotechnology Department, Faculty of Science, Taif University, Taif, 21944, Saudi Arabia
3Clinical Laboratory Science Department, Turabah University College, Taif University, Taif, 21995, Saudi Arabia
4Department of Pharmacology and Biochemistry, Faculty of Pharmacy, Horus University-Egypt, New Damietta, 34518, Egypt
5Biology Department, College of Science, University of Bisha, Bisha, 61922, Saudi Arabia
6Fellow of Biochemistry, Genetic Unit, Children Hospital, Faculty of Medicine, Mansoura University, Mansoura, Egypt
7Biology Department, Faculty of Science, Taif University, Taif, Saudi Arabia
*Address correspondence to: Shady G. El-Sawah, dr.shadygamal83@gmail.com
Received: 04 April 2021; Accepted: 14 May 2021
Abstract: Diabetes mellitus (DM) could negatively affect patients’ health via inducing a lot of serious functional hazards in many tissues’ cells at molecular levels. Recently, many scientists had proposed stem cell therapy being an appropriate alternative treatment protocol for numerous health threatening issues including diabetes. Therefore, the current study was designed to investigate the antioxidant potentiality of two MSCs types in alleviating tissues’ oxidative stress dramatic elevation resulting as a consequence of Type 1 DM induction. In our 4 weeks study, animals were divided into four groups: control group, STZ-diabetic group (D), D+AD-MSCs group and D+BM-MSCs group. Data reported that diabetic rats treated with either AD-MSCs or BM-MSCs exhibited a marvelous body tissues (Pancreas, Liver and Kidney) enhancing capabilities in attenuating the oxidative stress status; as evidenced by XO, ROS, and MDA levels down-regulation; with a general concomitant elevation in the antioxidants’ content; evidenced by many enzymatic and non-enzymatic antioxidants up-regulation; relative to the diabetic untreated group. Interestingly, comparing both treatments with each other and to control group, most of the measured parameters were reverted back to near normal levels after AD-MSCs injection; which clearly point out their stunning health benefits and superiority as anti-diabetic agent in overcoming different tissues’ complications; owing to their marked cytoprotective and regenerative potentialities.
Keywords: Antioxidants; Diabetes; Mesenchymal stem cells; Oxidative stress
Abbreviations
| AD-MSCs: | adipose-derived mesenchymal stem cells |
| AGEs: | advanced glycation end products |
| BM-MSCs: | bone marrow-derived mesenchymal stem cells |
| CAT: | catalase |
| CD: | cluster of differentiation |
| D: | diabetic |
| DM: | diabetes mellitus |
| DMEM: | dulbecco’s modified Eagle’s medium |
| FBG: | fasting blood glucose |
| FBS: | fetal bovine serum |
| GPx: | glutathione peroxidase |
| GRd: | glutathione reductase |
| GSH: | glutathione |
| GST: | glutathione-S-transferase |
| H2O2: | hydrogen peroxide |
| HO-1: | heme-oxygenase 1 |
| iNOS: | inducible nitric oxide synthase |
| IPCs: | insulin producing cells |
| ISCT: | international Society for Cellular Therapy |
| LPO: | lipid peroxidation |
| MDA: | malondialdehyde |
| MSCs: | mesenchymal stem cells |
| NO: | nitric oxide |
| PBS: | phosphate-buffered saline |
| PUFA: | poly-unsaturated fatty acids |
| ROS: | reactive oxygen species |
| SEM: | standard error of mean |
| SOD: | superoxide dismutase |
| SPSS: | statistical Package for Social Scientists |
| STZ: | streptozotocin |
| T1DM: | type 1 diabetes mellitus |
| TAC: | total antioxidant capacity |
| TBARS: | thiobarbituric acid reactive substances |
| TOS: | total oxidative status |
| XO: | xanthine oxidase |
Diabetes mellitus (DM), a chronic metabolic disorder characterized with persistent elevated blood glucose level, is a growing disease in terms of the number of patients and the percentage of the population. A sudden increase in diabetic patients’ number was noticed throughout the world in last decades; particularly in the developing countries, although huge efforts made to suppress outspread of this metabolic disorder. The total number of diabetics worldwide has quadrupled in the last three decades. Interestingly, WHO recent estimations reported that DM prevalence rate among adults over 18 years was increased from 4.7% in 1980 to 8.5% in 2014. Moreover, International Diabetes Federation (IDF) last report expected that diabetic population count will rise from 415 million in 2015 reaching 642 million in 2040 (Peng et al., 2018; Laddha and Kulkarni, 2019; Gharib et al., 2020).
It was well known that pancreatic β-cells’ autoimmune destruction could induce Type 1 diabetes mellitus (T1DM) leading to a severe hypoinsulinemia and subsequent blood glucose level elevation. Such events, in turn, could increase advanced glycation end products (AGEs) formation with a marked oxidative stress progression leading to development of many pathological changes and complications in different organs; suggesting this disease one of the major leading causes of mortality worldwide (Laddha and Kulkarni, 2019; Aminzadeh et al., 2020). In patients with T1DM, continuous exogenous insulin therapy through daily injections is the most prominent therapeutic strategy that cannot be avoided. Despite being effective in most patients, sometimes diabetes and its associated complications progression might not be properly managed with insulin injection. Therefore, modern therapeutic approaches as regenerative medicine are advancing rapidly, breaking new ground in DM treatment.
Recently, stem cell therapy technologies; had gained a huge popularity as a promising therapeutic strategy for many degenerative disorders, including T1DM, owing to their self-renewal ability, multipotentiality, and immunomodulatory and regenerative capabilities (Päth et al., 2019; Aminzadeh et al., 2020; Bani Hamad et al., 2021). Since mesenchymal stem cells (MSCs) had taken the lead in β-cells regeneration, both adipose-derived (AD-MSCs) and bone-marrow (BM-MSCs) stem cells were proposed as more readily available types of stem cells that could be used in diabetes treatment (Brovkina et al., 2019). According to the minimal criteria laid down by Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) (Dominici et al., 2006), MSCs were defined by three parameters: the cells (1) they must be plastic-adherent and can be maintained in standard culture conditions; (2) express cluster of differentiation (CD105, CD73 and CD90 without expressing other lineage markers CD45 (panleukocyte), CD34 (hematopoietic and endothelial), CD14/CD11b (monocytic), CD79a/CD19 (B cell), or human leukocyte antigen (HLA) class II); and (3) show the classical trilineage differentiation into osteoblasts, adipocytes, and chondroblasts in vitro.
AD-MSCs success in translational medicine applications had been attributed to many reasons; such as their self-renewal, multipotency properties and the relatively simple and safe cell isolation protocol (Argentati et al., 2018; Si et al., 2019). However, hematopoietic stem cells and mesenchymal stem cells; are two distinct populations found in BM-MSCs; of which the later was considered more successful in adopting a pancreatic fate (Carlsson et al., 2015). Collectively, since T1DM was suggested as a probable candidate which might benefit from stem cell therapy procedure, this review outline the possible therapeutic benefits of both AD-MSC and BM-MSCs for the treatment of T1DM and its related complications via exploring their antioxidant capacity functionality in regulating and alleviating tissues’ oxidative stress status resulting as a consequence of DM induction in rats.
Streptozotocin (STZ) and culture media constituents of both BM-MSCs and AD-MSCs were purchased from Sigma Aldrich Co. (St. Louis, Mo 6, USA); and were of pure chemical gradient.
Serum glucose concentration was estimated by Trinder (1969) method using SPINREACT diagnostics kit, Spain. Insulin measurement occurred by ELISA kit purchased from Boehringer Mannheim, Germany, according to the method of Flier et al. (1976) using Boehringer Analyzer ES 300. C-peptide measurement occurred by enzyme immunoassay (EIA) kit purchased from Bio Vision, USA, according to the method of Flier et al. (1976). Glycosylated hemoglobin (HbA1c) was estimated according to the method of Gonen and Rubenstein (1978) by using glycosylated hemoglobin kit obtained from Teco Diagnostics, USA. Bio Diagnostic Company (Egypt) kits were used in estimating tissue levels of some oxidative stress markers [Xanthine oxidase (XO) and Reactive oxygen species (ROS) according to Young (2001), Malondialdehyde (MDA) according to Ohkawa et al. (1982)] in addition to many antioxidant markers [Catalase (CAT) according to Bock et al. (1980), Glutathione-S-transferase (GST) according to Habig et al. (1974), Superoxide dismutase (SOD) according to Nishikimi et al. (1972), Glutathione (GSH) according to Prins and Loose (1969), Heme-oxygenase 1 (HO-1) according to Gonen and Rubenstein (1978) and Total antioxidant capacity (TAC) according to Koracevic et al. (2001)], all according to the instructions of the supplier. Meanwhile, tissue G0/G1% in addition to surface markers % (for characterization of the isolated BM-MSCs and AD-MSCs phenotyping) was determined via Sigma Aldrich Company (St. Louis, Mo 6, USA) kits through flow cytometric analysis. Cultured cells were harvested and stained with antibodies against CD44, CD73, CD90, CD45, CD11b and CD31. using FACS caliber flow cytometer (Becton Dickinson, Sunnyvale, CA, USA), equipped with a compact air cooked low power 15 mW argon-iron laser beam (488 nm). Average evaluated nuclei per specimen are 20.000 (120 nuclei/s). Dean and Jett computer program for mathematical analysis is used to obtain the DNA histograms (Dean and Jett, 1974).
BM-MSCs and AD-MSCs preparation
Fresh bone marrow and subcutaneous adipose tissues were obtained from male 6–8-week-old rats and used to isolate BM-MSCs and AD-MSCs, respectively. To prepare BM-MSCs, each end of the femur and tibia was cut to expose the marrow cavity, then washed three times with phosphate-buffered saline (PBS). Fresh bone marrow was collected and centrifuged at 2000 rpm for 10 min. Pelleted cells were suspended in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin with 100 μg/mL streptomycin as an antibiotic at 37°C in a 5% CO2 atmospheric state with 95% humidity, then centrifuged at 2000 rpm for 10 min (Hamza et al., 2017). On the other hand, 1–2 mg fresh rats’ subcutaneous adipose tissues (epididymal fat) were harvested by lipoaspiration, minced, washed extensively three times in PBS and incubated into a digestion solution containing 0.075% collagenase Type I (prepared in PBS) at 37°C for 3 h, then centrifuged for 10 min at 2000 rpm. After discarding the supernatant, cells were collected as a pellet and suspended in DMEM (10% FBS, 2 mM L-glutamine, and 100 U/mL penicillin with 100 μg/mL streptomycin as an antibiotic) at 37°C in 5% CO2 with 95% humidity (Chen et al., 2020). The growth mediums of both MSCs types were changed every 3 days, and non-adherent cells were removed. All MSCs used in this study, were from passage 3–4, and transferred chilled for transplantation within 2 h.
BM-MSCs and AD-MSCs characterization
The inverted microscope has been used to perform both BM-MSCs and AD-MSCs morphological characterization for confirming their identity. Moreover, stemness of cultured cells should be confirmed by positive and negative surface markers (CD11b, CD34, CD45, CD73, CD90 and CD105) by flow cytometry to confirm retaining of their phenotype before performing the animal study; according to the minimal criteria defined by ISCT.
Experimental animals and maintenance
In this experimental study, twenty-four Rattus rattus male albino rats (6–8 weeks, 100–120 g) were kept throughout the study under a conventional laboratory conditions (a 12/12-h light/dark cycle, humidity of 50 ± 5%, and room temperature of 22 ± 2°C) in an aerated stainless cage. All rats were allowed free access to bottles of water, and standard pellet chow-containing plates. Following two weeks of acclimatization, four rat groups have been established randomly (n = 6). The study was continued for 4 consecutive weeks. All experimental procedures were approved and performed in accordance with the guidelines of the Animal Ethics Committee of the Faculty of Science, Arish University, North Sinai, Egypt. All efforts were made to minimize animal suffering.
A single STZ solution dose (45 mg/kg dissolved in citrate buffer, pH 4.6) were injected intraperitoneally in overnight fasting rats, while control rats received the vehicle alone. Animals were allowed to drink 5% glucose solution overnight to overcome drug-induced hypoglycemia. Diabetes was confirmed 3 days after induction via detecting level of blood glucose from tail vein, using Glukotest of diagnosis glucose level by ACCU–CHEKGo apparatus (Roche Company, Germany). Rats having fasting blood glucose (FBG) above 250 mg/dL were confirmed as diabetic and used for further experimentation (Kodidela et al., 2020).
1. Control group: Injected intraperitoneally with a single dose of sodium citrate buffer (pH 4.5).
2. Diabetic (D) untreated group: Injected intraperitoneally with a single dose of STZ (45 mg/kg bw) dissolved in sodium citrate buffer (pH 4.5).
3. Diabetic AD-MSCs treated group: Injected intravenously with a single dose of AD-MSCs (1 × 106 cell/rat).
4. Diabetic BM-MSCs treated group: Injected intravenously with single dose of BM-MSCs (1 × 106 cell/rat).
Before being dissected, diethyl ether was used to anesthetize rats and tissues’ specimens (pancreas, liver, and kidney) were quickly separated. An appropriate part was weighed and homogenized in cold distilled water forming 10% (w/v) homogenate, then labeled and kept at −20°C for latter biochemical estimations. However, the remnant parts were labeled and kept at −80°C for subsequent flowcytometric analysis.
Obtained data were statistically evaluated with ANOVA followed by post-hoc Tukey multiple range tests using Statistical Package for the Social Sciences (SPSS/17.5 software version) for Windows. All the results were expressed as the mean ± SEM (n = 6). The significance between groups was considered at the P ≤ 0.05.
Figs. 1A and 1B demonstrated the characterization of BM-MSCs and AD-MSCs based on the expression of their specific surface markers by fluorescence-activated cell sorting flow cytometry analysis. Data indicating that detected MSCs surface markers were highly expressed by both BM-MSCs and AD-MSCs. CD73, CD90 and CD105 expression were very high and considered positive. By contrast, CD11b, CD34 and CD45 expression were very low and considered negative. Such results are concomitants with the minimal criteria defined by ISCT.
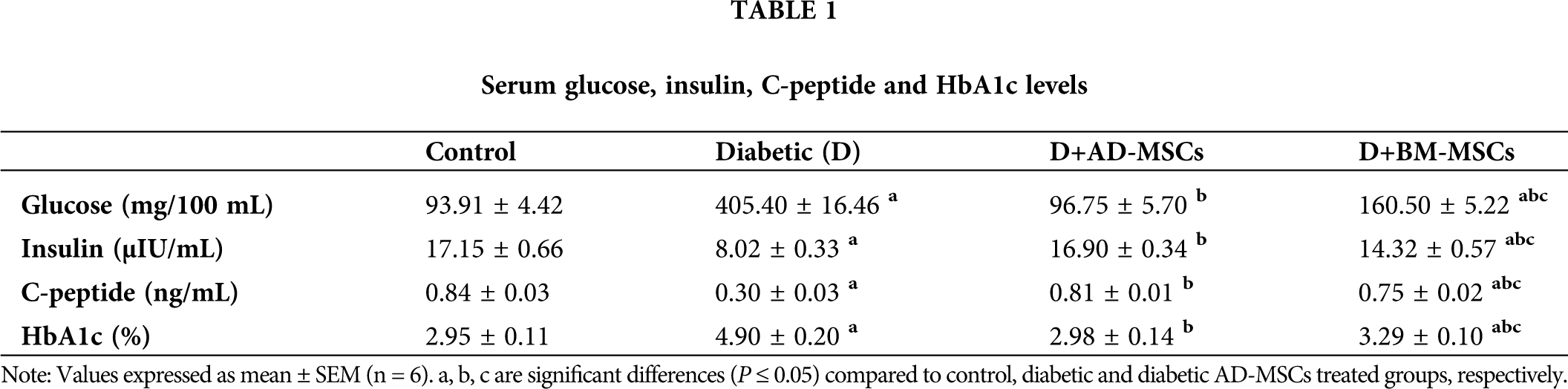
Tab. 1 showed a marked serum glucose and HbA1c elevation coupled with a significant insulin and C-peptide levels decline in diabetic group; compared to control. Regarding both MSCs-treated groups, all measured parameters of the diabetic rats revealed an obvious enhancement relative to diabetics, although values of BM-MSCs were still significantly variable relative to control. However, data of diabetic rats injected with AD-MSCs detected a marked hypoglycemic superiority when compared to those of AD-MSCs.
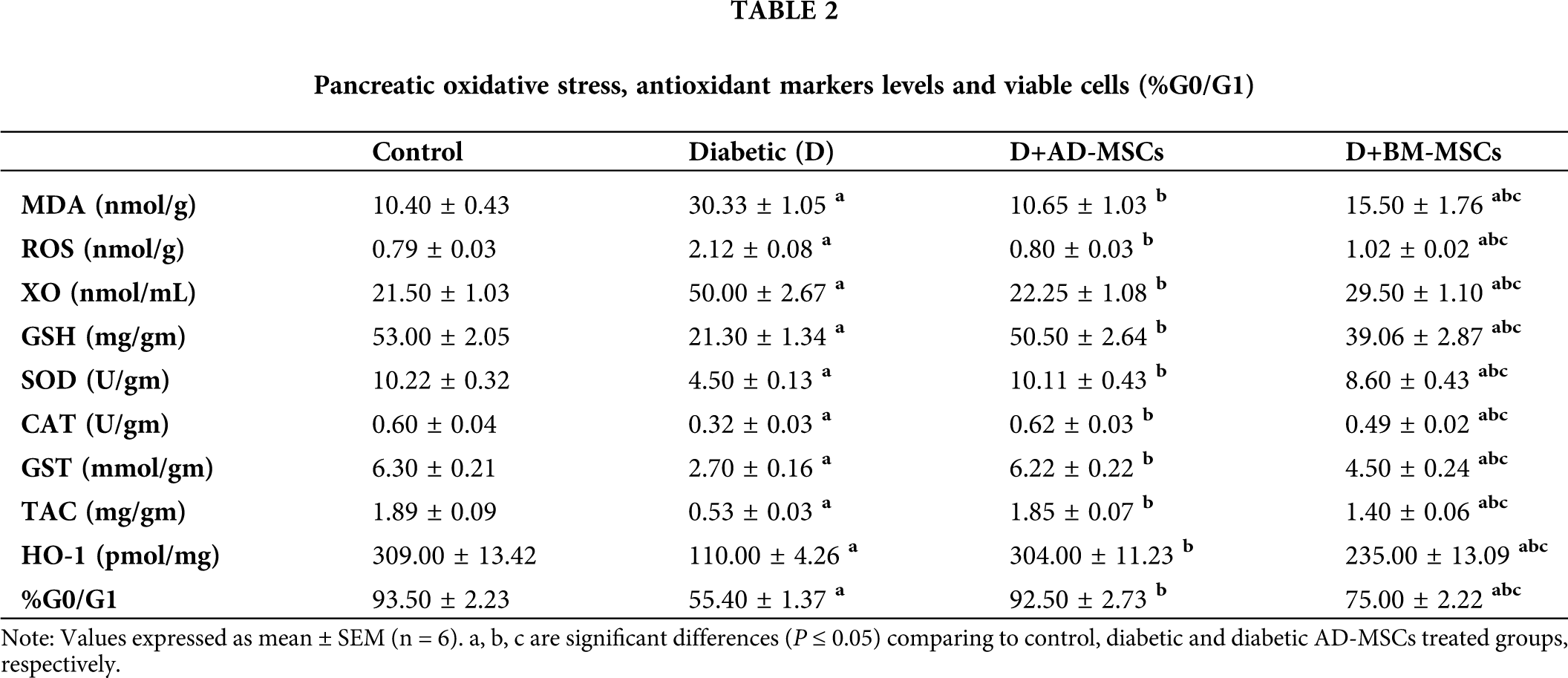
Tab. 2 illustrated that diabetic group exhibited significant increases in pancreatic oxidative stress markers (MDA, ROS, and XO) levels coupled with a marked decline in all antioxidant markers (GSH, SOD, CAT, GST, TAC and HO-1) levels in addition to pancreatic viable cells (%G0/G1), when compared to control group. However, present results revealed that diabetic rats treated with either AD-MSCs or BM-MSCs showed marked decrease in oxidative stress markers, while a significant increase in both antioxidant markers levels and G0/G1%; when compared to the diabetic group. On the other hand, AD-MSCs cleared non-significant changes in all tested parameters compared to control group; in contrast to BM-MSCs values which were still significantly variable. Interestingly, all results of diabetic rats treated with AD-MSCs recorded a much more enhancement than BM-MSCs treatment.
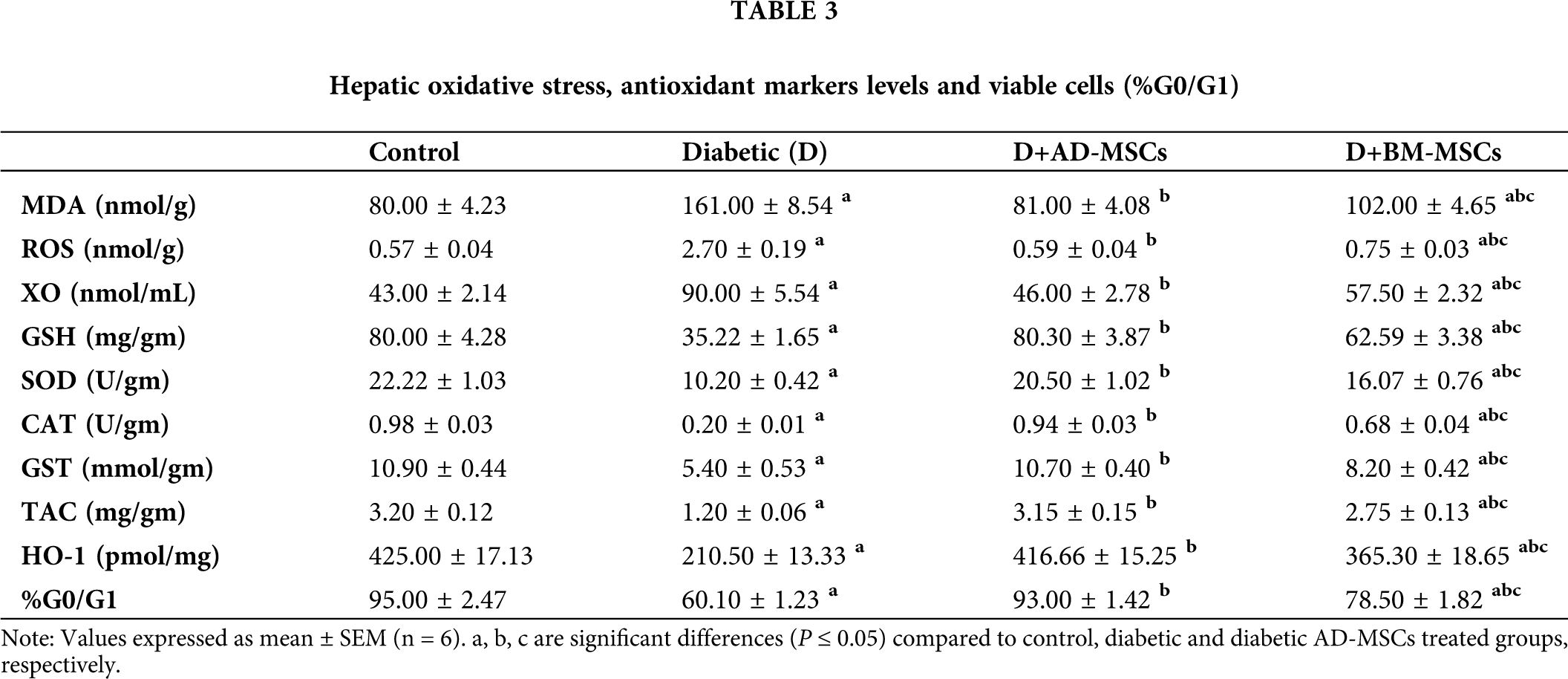
Tab. 3 summarized data indicated a significant increase in all hepatic oxidative stress parameters accompanied with a significant decrease in all antioxidant markers levels and %G0/G1 in diabetic group, regarding normal control one. On the other hand, relative to diabetic group, both treated groups’ results showed a significant enhancement in all tested parameters. Surprisingly, obtained results of the diabetic rats injected with AD-MSCs displayed an obvious improvement variation in all mentioned parameters, compared to those treated with BM-MSCs.
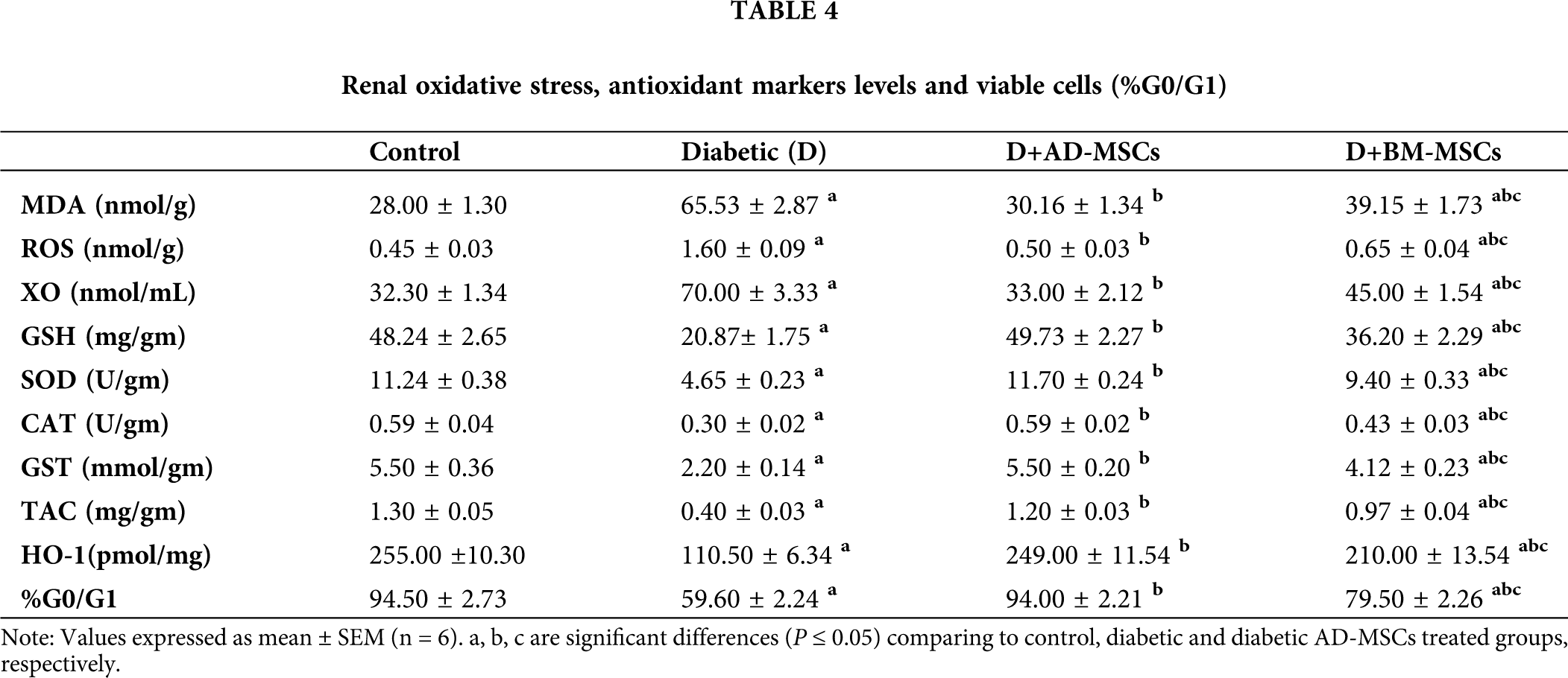
Tab. 4 obtained results revealed a marked oxidative stress status development in kidney tissues, while a marked antioxidant capacity decline in diabetic rats, in regard to control rats. Both MSCs treated groups tested indicators clearly demonstrated a significant improvement, when compared to the diabetic untreated group. Notably, diabetic rats injected with AD-MSCs recorded a great amelioration in all tested parameters; comparing to BM-MSCs treated rats.
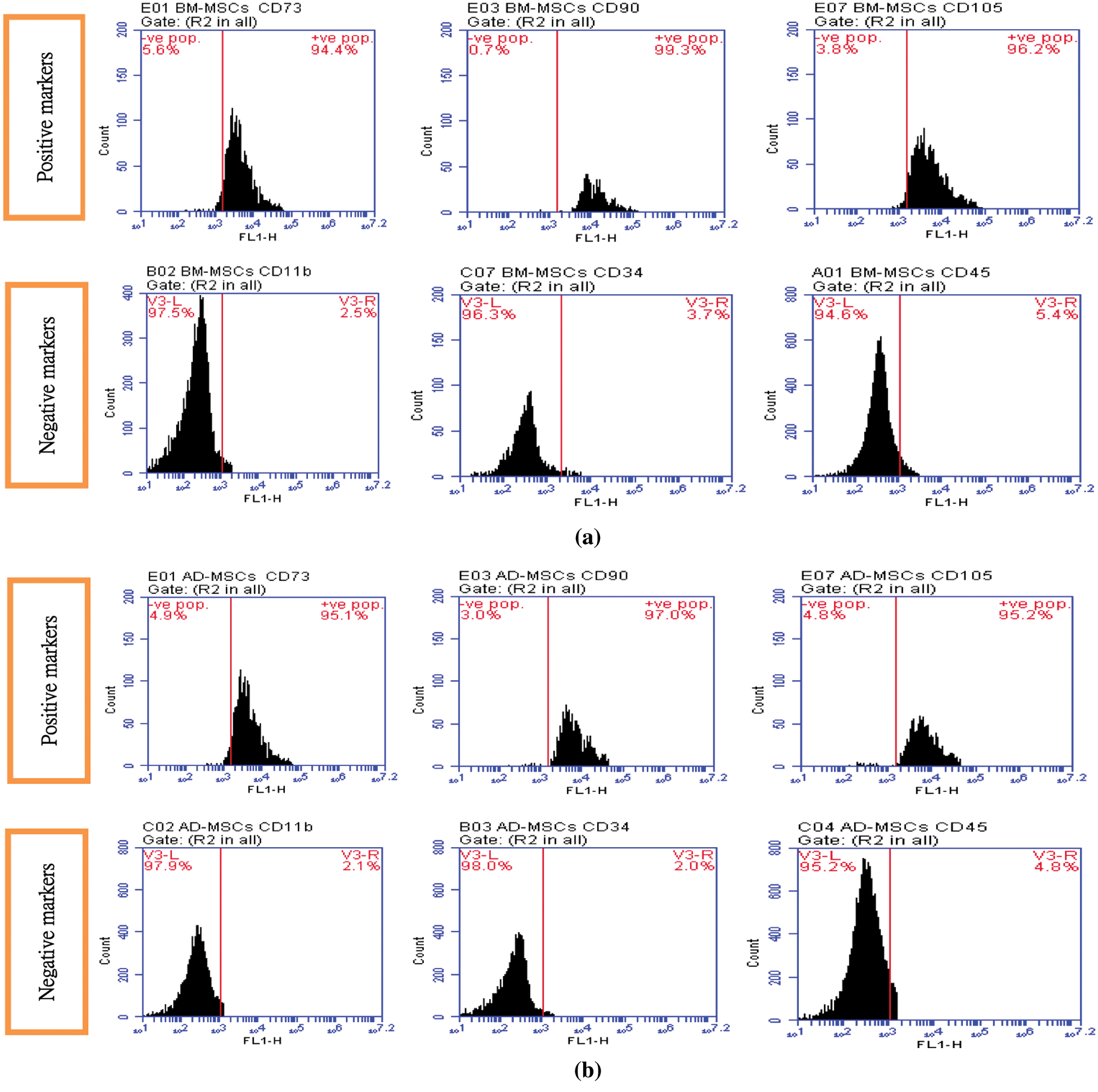
Figure 1: (A) BM-MSCs positive surface markers (CD 73: 94.4%, CD 90: 99.3%, CD 105: 96.2%) BM-MSCs negative surface markers (CD 11b: 2.5%, CD 34: 3.7%, CD 45: 5.4%), (B) AD-MSCs positive surface markers (CD 73: 95.1%, CD 90: 97.0%, CD 105: 95.2%). AD-MSCs negative surface markers (CD 11b: 2.1%, CD 34: 2.0%, CD 45: 4.8%)
DM impact on the human body is not restricted to a respective organ, since severe acute or chronic co-morbidities could take place if hyperglycemia persists and improperly controlled (Rogal et al., 2019). Since, current and traditional diabetes treatments only focused on to regulate the insulin level without alleviating the diabetic complications (Jamshidi et al., 2018), nowadays treatment protocols focus on the antioxidant protection of the pancreatic β-cells and other tissues’ cells; in order to facilitate their repair. In this line, cell-based therapy, in particular stem cells injection, was suggested as a promising therapeutic candidate against diabetic complications; owing to their regeneration potential and multilineage differentiation (Aminzadeh et al., 2020).
Course STZ administration was found to selectively targets and kills pancreatic β cells, causing a reduction in insulin production and subsequent prolonged hyperglycemia inducing an experimental model of T1DM; possibly via induction of cellular stress leading to excessive ROS production resulting in generation of lipid peroxidation (LPO) and DNA breaks, which plays a vital role in pancreatic dysfunction via causing inflammation, pancreatic islet cell death and diabetes (Hamza et al., 2017; Jamshidi et al., 2018). Herein, the increase in fasting blood glucose (FBG) in diabetic rats is well expected and agrees with earlier reports that cleared that diabetic subjects exhibited a huge serum glucose and HbA1c levels, coupled with a marked C-peptide and insulin levels decline; regarding control subjects (Amer et al., 2018; Samaha et al., 2020). These findings are also in agreement with Zang et al. (2017) and Nia et al. (2018) and their teams who reported that glucose level control deviation is sufficient to initiate a series of maladaptive processes resulting in an oxidative stress injury and elevated ROS and MDA production; which they were recognized as the major etiological factor in the development of diabetes; which might be a reflection of decreased antioxidant capability of the defensive systems and/or glucose oxidation. This would cause an inner endothelial tissue damage; that might eventually be directly responsible for the blood glucose level elevation. Similar assumption has been documented by Turkmen (2017) who stated that, the well-established features of STZ-diabetic complications include elevated blood glucose concentration accompanied by increased overproduction of LPO and ROS; mostly via mitochondrial electron transport chain; participating in the establishment of oxidative stress in many diabetic rats’ tissues compared to control. Many researchers attributed these disturbances to the marked decreased activities of many antioxidant enzymes, such as GSH-Px, SOD and CAT; suggesting that these antioxidants are exhausted to combat the deleterious effects of the increased oxidative stress (Mayyas et al., 2018; Kodidela et al., 2020). Such events could result in a notable cellular injury and to a point of no return in apoptosis when ROS scavenging and cytoprotective molecules are insufficiency present. Similar results were reported by El-Kholy et al. (2018) and El-Sawah et al. (2020).
On the other hand, owing to their ability to stimulate the damaged pancreatic β-cells proliferation and regeneration, MSCs hypoglycemic therapeutic effects on diabetes have been confirmed by many reports. Surprisingly, insulin producing cells (IPCs) differentiated from either AD-MSCs or BM-MSCs injection could obviously lower glucose and HbA1c serum levels and increase both C-peptide and insulin release; resulting in marked glucose and HbA1c reduction in diabetic rats (Gabr et al., 2017; El-Kholy et al., 2018) and in diabetic mice (Kono et al., 2014) as well as in diabetic patients (Thakkar et al., 2015); confirming MSCs hypoglycemic activity. In this regard, Amer et al. (2018) found that STZ-diabetic rats received IPCs differentiated from AD-MSCs; showed an apparent islet cells proliferation and regeneration that leads to a marked C-peptide increase accompanied with insulin secretion elevation; in glucose dependent manner. In this line, Zang et al. (2017) also reported that BM-MSCs showed a powerful glycemic control indicated by the 50% decline in insulin requirements; through restoring islet function, endogenous islet cells protection, induction of islet cell regeneration and differentiation into IPCs; followed by marked restoration of β-cell mass and significant hypoglycemia in STZ-diabetic mice.
Many reports attributed the obvious hypoglycemic capability of MSCs to their marked antioxidant activity in alleviating the resultant oxidative stress status due to prolonged hyperglycemia. Interestingly, according to Fattah et al. (2020) and Aminzadeh et al. (2020) administration of MSCs extremely ameliorated the elevated MDA and ROS levels while increasing the mRNA expression levels of many antioxidants, such as GSH, SOD-1 and 3, CAT, GSH-Px-1, 3 and 4, and TAC; in STZ-diabetic rats’ various tissues including pancreas; relative to diabetic untreated rats. In this regard, Chandravanshi and Bhonde (2017) also suggested a MSCs-protective effect on the pancreatic islet cells of diabetic rats against oxidative stress-mediated cellular injuries; as indicated by the reduced levels of ROS, nitric oxide (NO), and super oxide ions; after 48 h of MSCs transplantation. In addition, MSCs were found to suppress oxidative stress-induced renal damage through detoxifying ROS and increasing renal GST, GPx, SOD and CAT expression; which are considered as potential scavengers of free oxidative radicals; associated with renal cell inflammation and apoptosis inhibition in diabetic mouse kidneys. According to Chen et al. (2020), a marked ROS production decrease with a HO-1 increase were found in pancreas of STZ-diabetic rats after injection with AD-MSCs (1 × 106 cells/rat) for 2 months; compared to the untreated rats. Also, El-Kholy et al. (2018) and El-Sawah et al. (2020) results showed significant improvement in glucose, insulin, TAC, and MDA levels in STZ-diabetic rats injected with BM-MSCs (1 × 106 cell/rat); compared to the diabetic untreated rats.
Since our data reported the superiority of AD-MSCs injection over BM-MSCs in mitigating the prolonged hyperglycemic condition in diabetic subjects via increasing their tissues’ protective antioxidant contents; and thus, lowering the progress of the oxidative stress status; we could strongly propose AD-MSCs administration as a promising therapeutic strategy for T1DM; that may validate its co-transplantation utility with islet transplantation in managing diabetic complications.
Availability of Data and Materials: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ Contribution: SGE and FA conceived and designed the study. Experiments and lab work were done by SGE, MAA, HR, EIH, RMA and ES, while data tabulating and acquisition, searching for literature and preparing the first draft of the manuscript were performed by SGE, MAA, AA and EF. All authors have read and agreed to the published version of the manuscript.
Ethics Approval: All experimental procedures were approved and performed in accordance with the guidelines of the Animal Ethics Committee of the Faculty of Science, Arish University, North Sinai, Egypt.
Funding Statement: This study was funded by Taif University Researchers Supporting Project No. (TURSP-2020/222), Taif University, Taif, Saudi Arabia.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Amer MG, Embaby AS, Karam RA, Amer MA (2018). Role of adipose tissue derived stem cells differentiated into insulin producing cells in the treatment of Type I diabetes mellitus. Gene 654: 87–94. [Google Scholar]
Aminzadeh A, Maroof NT, Mehrabani M, Juybari KP, Sharifi AM (2020). Investigating the alterations of oxidative stress status, antioxidant defense mechanisms, MAP kinase and mitochondrial apoptotic pathway in adipose-derived mesenchymal stem cells from STZ diabetic rats. Cell Journal (Yakhteh) 22: 38–48. [Google Scholar]
Argentati C, Morena F, Bazzucchi M, Armentano I, Emiliani C, Martino S (2018). Adipose stem cell translational applications: from bench-to-bedside. International Journal of Molecular Sciences 19: 3475–3482. [Google Scholar]
Bani Hamad FR, Rahat N, Shankar K, Tsouklidis N (2021). Efficacy of stem cell application in diabetes mellitus: promising future therapy for diabetes and its complications. Cureus 13: e13563. [Google Scholar]
Bock PP, Karmer R, Paverka M (1980). A simple assay for catalase determination. Cell Biology Monographs 7: 44–74. [Google Scholar]
Brovkina O, Nikitin A, Khodyrev D, Shestakova E, Sklyanik I et al. (2019). Role of MicroRNAs in the regulation of subcutaneous white adipose tissue in individuals with obesity and without Type 2 diabetes. Frontiers in Endocrinology 10: 840–852. [Google Scholar]
Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K (2015). Preserved β-cell function in Type 1 diabetes by mesenchymal stromal cells. Diabetes 64: 587–592. [Google Scholar]
Chandravanshi B, Bhonde R (2017). Shielding engineered islets with mesenchymal stem cells enhance survival under hypoxia. Journal of Cellular Biochemistry 118: 2672–2683. [Google Scholar]
Chen TS, Lai PF, Kuo CH, Day CH, Chen RJ et al. (2020). Resveratrol enhances therapeutic effect on pancreatic regeneration in diabetes mellitus rats receiving autologous transplantation of adipose-derived stem cells. Chinese Journal of Physiology 63: 122–127. [Google Scholar]
Dean PN, Jett JH (1974). Mathematical analysis of DNA distributions derived from flow microfluorometry. Journal of Cell Biology 60: 523–524. [Google Scholar]
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwit EM (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317. [Google Scholar]
El-Kholy WM, Hussein RH, Khalil DY (2018). Antioxidant and anti-inflammatory therapeutic roles of BM-MSCs in enhancing pancreatic auto-immunity and apoptotic status in TIDM. Journal of American Science 14: 1–12. [Google Scholar]
El-Sawah SG, Nabil A, Rashwan H, Khalil DY, Khalil RY (2020). BM-MSCS immunomodulatory, anti-inflammatory, anti-apoptotic and antioxidant capacity roles in modulating the altered tissues oxidative stress status in STZ-diabetic rats: In comparison to the standard insulin treatment. International Journal of Advanced Research 8: 591–617. [Google Scholar]
Fattah SA, Waly H, El-enein AA, Kamel A, Labib H (2020). Mesenchymal stem cells versus curcumin in enhancing the alterations in the cerebellar cortex of streptozotocin-induced diabetic albino rats. The role of GFAP, PLC and α-synuclein. Journal of Chemical Neuroanatomy 109: 101842. DOI 10.1016/j.jchemneu.2020.101842. [Google Scholar] [CrossRef]
Flier J, Kahn C, Jarrett D, Roth J (1976). Characterization of antibodies to the insulin receptor: A cause of insulin-resistant diabetes in man. Journal of Clinical Investigation 58: 1442–1449. [Google Scholar]
Gabr MM, Zakaria MM, Refaie AF, Abdel-Rahman EA, Reda AM, Ali SS (2017). From human mesenchymal stem cells to insulin-producing cells: Comparison between bone marrow- and adipose tissue-derived cells. BioMed Research International 8, DOI 10.1155/2017/3854232. [Google Scholar] [CrossRef]
Gharib HM, Abajy MY, Omaren A (2020). Investigating the effect of some fluoroquinolones on C-reactive protein levels and ACh-Induced blood pressure reduction deviations after aging of diabetes in STZ-Induced diabetic Wistar rats. Heliyon 6: e03812. [Google Scholar]
Gonen B, Rubenstein AH (1978). Haemoglobin A1 and diabetes mellitus. Diabetologia 15: 1–8. [Google Scholar]
Habig H, Pabst J, Jakoby B (1974). Glutathione-S-transferase the first enzyme step in mercapturic acid formation. Journal of Biological Chemistry 1: 7139–7150. [Google Scholar]
Hamza AH, Al-Bishri WM, Damiati LA, Ahmed HH (2017). Mesenchymal stem cells: A future experimental exploration for recession of diabetic nephropathy. Renal Failure 39: 67–76. [Google Scholar]
Jamshidi M, Ziamajidi N, Khodadadi I, Dehghan A, Kalantarian G (2018). The effect of insulin-loaded trimethylchitosan nanoparticles on rats with diabetes Type I. Biomedicine and Pharmacotherapy 97: 729–735. [Google Scholar]
Kodidela S, BegumShaik F, Chinta V, AliMohammad S, Pasala C, Mittameedi S, Maddu N, Wudayagiri R, Nallanchakravarthula V (2020). Possible ameliorative role of green tea on chronic alcohol mediated renal toxicity of STZ-induced diabetic rats. Clinical Nutrition Experimental 34: 1–25. [Google Scholar]
Kono TM, Sims EK, Moss DR, Yamamoto W, Ahn G, Diamond J (2014). Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: Analysis of hASC-derived paracrine effectors. Stem Cells 32: 1831–1842. [Google Scholar]
Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001). Method for the measurement of antioxidant activity in human fluids. Journal of Clinical Pathology 54: 356–361. [Google Scholar]
Laddha AP, Kulkarni YA (2019). Tannins and vascular complications of diabetes: An update. Phytomedecine 56: 229–245. [Google Scholar]
Mayyas F, Jaradat R, Alzoubi KH (2018). Cardiac effects of fish oil in a rat model of streptozotocin-induced diabetes. Nutrition, Metabolism and Cardiovascular Diseases 28: 592–599. [Google Scholar]
Nia PH, Khorram S, Rezazadeh H, Safaiyan A, Tarighat-Esfanjani A (2018). The effects of natural clinoptilolite and nano-sized clinoptilolite supplementation on glucose levels and oxidative stress in rats with Type 1 diabetes. Canadian Journal of Diabetes 42: 31–35. [Google Scholar]
Nishikimi M, Roa NA, Yagi K (1972). The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communications 46: 849–854. [Google Scholar]
Ohkawa H, Wakatsuki A, Kaneda C (1982). Assay for lipid peroxides in animals’ tissues by thiobarbituric acid reaction. Analytical Biochemistry 95: 351–358. [Google Scholar]
Päth G, Perakakis N, Mantzoros CS, Seufert J (2019). Stem cells in the treatment of diabetes mellitus—Focus on mesenchymal stem cells. Metabolism-clinical and Experimental 90: 1–15. [Google Scholar]
Peng B, Dubey NK, Mishra VK, Tsai F, Dubey R, Deng W, Wei H (2018). Addressing stem cell therapeutic approaches in pathobiology of diabetes and its complications. Journal of Diabetes Research 2018: 7806435. DOI 10.1155/2018/7806435. [Google Scholar] [CrossRef]
Prins HK, Loose JA (1969). Glutathione. Biochemical Methods in Red Cell Genetics, pp. 115–137. New York: Academic Press. [Google Scholar]
Rogal J, Zbinden A, Schenke-Layland K, Loskill P (2019). Stem-cell based organ-on-a-chip models for diabetes research. Advanced Drug Delivery Reviews 140: 101–128. [Google Scholar]
Samaha MM, Said E, Salim HA (2020). Modulatory role of imatinib mesylate on pancreatic β-cells’ secretory functions in an STZ rat model of diabetes mellitus. Chemico-Biological Interaction 328. DOI 10.1016/j.cbi.2020.109197. [Google Scholar] [CrossRef]
Si Z, Wang X, Sun C, Kang Y, Xu J, Wang X, Hui Y (2019). Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomedicine & Pharmacotherapy 114: 108765. DOI 10.1016/j.biopha.2019.108765. [Google Scholar] [CrossRef]
Thakkar UG, Trivedi HL, Vanikar AV, Dave SD (2015). Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for Type 1 diabetes mellitus. Cytotherapy 17: 940–947. [Google Scholar]
Trinder P (1969). Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Annals of Clinical Biochemistry 6: 24–27. [Google Scholar]
Turkmen K (2017). Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: The Four Horsemen of the Apocalypse. International Urology and Nephrology 49: 837–844. [Google Scholar]
Young DS (2001). Effects of disease on clinical laboratory tests. American Association for Clinical Chemistry 4: 17–18. [Google Scholar]
Zang L, Hao H, Liu J, Li Y, Han W, Mu Y (2017). Mesenchymal stem cell therapy in Type II diabetes mellitus. Diabetology & Metabolic Syndrome 9: 36. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |