DOI:10.32604/biocell.2021.017016

| BIOCELL DOI:10.32604/biocell.2021.017016 |  |
| Article |
Propofol inhibits cells migration and invasion via HOTAIR/miR-93/HIF-1α-mediated lactate secretion in colon cancer
1Department of Cell Biology, School of Basic Medical Sciences, Southern Medical University, Guangzhou, 510515, China
2Department of Anesthesiology, Zhujiang Hospital, Southern Medical University, Guangzhou, 510282, China
3Department of Clinical Laboratory, The Fifth Affiliated Hospital, Southern Medical University, Guangzhou, 510920, China
*Address correspondence to: Fan Deng, fandeng@smu.edu.cn; Fangyin Zeng, zengfy@126.com
Received: 20 April 2021; Accepted: 18 June 2021
Abstract: Propofol, a common intravenous anesthetic used in clinical, has been shown to regulate cells proliferation, inflammation, angiogenesis and metastasis as well as exerting anti-cancer effect in several different types of cancer. However, the functions and mechanisms of propofol in lactate secretion and invasion of cancer cells remain unknown. The primary aim of this study was to investigate the role of propofol in cells migration and invasion of colon cancer (CRC). Scratches assay, Transwell assay were used to detect colon cells migration and invasion. Real-time Quantitative Polymerase Chain Reaction (RT-qPCR), Western blotting were utilized to detect the expression of related molecules. Dual-luciferase assay was performed to identify transcriptional activity of target genes, RNA pull-down assay was used to confirm the interactions of RNAs. Results indicated that propofol inhibited cell migration and invasion through suppressing lactic acid production in CRC. Moreover, propofol reduced lactic acid production by down-regulating the expression of hypoxia inducible factor-1α (HIF-1α) and monocarboxylate transporter 4 (MCT4). In addition, propofol inhibited the expression of HIF-1α and cells migration/invasion via suppressing the interaction of HOX transcript antisense RNA (HOTAIR) with miR-93. The present study indicated that propofol inhibited colon cancer cell migration and invasion by reducing lactic acid production and the expression of HIF-1α and MCT4 via HOTAIR/miR-93 axis. These data may provide potential metabolic targets for CRC treatment.
Keywords: Propofol; Colon cancer; Lactic acid; HIF-1α; HOTAIR; miR-93
Colorectal cancer (CRC) is the most common malignant tumor in digestive system. Surgical resection is the main treatment for CRC, however, prognosis of patients is still poor (Kryczka et al., 2020). The development of CRC is a multi-stage evolution process, due to postoperative recurrence and distant metastasis, which is the main cause of death in patients. To date, the mechanism of metastasis in CRC is not completely clear.
Cancer patients usually need to undergo anesthesia diagnosis and treatment when choosing surgical treatment (Sekandarzad et al., 2017). Numerous studies suggested that anesthetics may involve in tumor recurrence, metastasis, targeted therapy, and immunotherapy research (Bharati et al., 2016; Hu et al., 2017). Propofol (2,6-diisopropylphenol) is the most used sedative anesthetic in clinical (Smith et al., 1994; Walsh, 2018). Increasing evidence showed that propofol exerted many non-narcotic effects (Gao et al., 2020; Vasileiou et al., 2009). Recently, many studies have confirmed that propofol has potential anti-tumor properties through regulating cells proliferation, migration, and invasiveness (Du et al., 2018; Hovaguimian et al., 2020; Zhang et al., 2012). These studies have shown that propofol may affect the long-term prognosis of cancer patients, but the exact mechanism of propofol on tumors is still controversial (Neal et al., 2010).
Tumor cells rely on glycolysis, called Warburg effect (van der Heiden et al., 2009). Numerous studies have emphasized that lactic acid, as a highly active signal molecule with “hormone-like properties”, has an important role in tumor cells (Gottfried et al., 2012). Therefore, lactic acid production and relative proteins that regulate lactic acid metabolism providing new prospects for the research of new anti-cancer therapies (Doherty et al., 2014). HIF-1α/MCT4/lactate signaling pathway is essential for glycolysis (Luo et al., 2017; Philp et al., 2001). Up-regulation of HIF-1α can activate many key cancer events such as angiogenesis, cell proliferation, invasion, and metastasis (Ebright et al., 2020; Jang et al., 2013). The microenvironment formed by lactic acid deteriorates tumor cells invasion and metastasis (Parks et al., 2013). As previous studies confirmed, propofol can inhibit cancer cell proliferation, migration, and invasion (Du et al., 2018; Hovaguimian et al., 2020). However, the regulatory mechanism how propofol regulates lactic acid in tumor cell migration and invasion is unclear.
Recent studies have confirmed that non-coding RNAs are closely related to tumorigenesis and malignant progression, which may become a new class of potential drug targets (Matsui and Corey, 2017). Long non-coding RNA (lncRNA) is longer than 200 nucleotides and involved in tumorigenesis and malignant progression including angiogenesis, cells migration and invasion (Lv et al., 2020; Wang et al., 2017). HOX transcript antisense RNA (HOTAIR) in the HOXC cluster is the first lncRNA found to have trans transcriptional regulation. Some studies have shown that high expression of HOTAIR is related to the late progression and metastasis of gastrointestinal stromal tumors. Silencing HOTAIR can regulate the expression of target genes and inhibit tumor cells invasion (Wang et al., 2019). HOTAIR is considered as a competitive endogenous RNA (ceRNA) in colon cancer by functioning as miRNAs sponge (Xiao et al., 2018). Crosstalk between lncRNA and miRNA can control the development of cancer (Bayoumi et al., 2016). Micro RNAs (miRNAs) are composed of 18 to 25 nucleotides that is associated with many diseases, including cancer (Vishnoi and Rani, 2017). miRNAs act as suppressors or promoters in cancer, depending on whether they specifically target oncogenes (Shenouda and Alahari, 2009). For example, miR-93 promotes cell proliferation, invasion and migration in SiHa cells by up-regulating the expression of MMP-2 and MMP-9, down-regulating the expression of extracellular matrix (ECM) (Sun et al., 2019). Moreover, miR-93 can inhibit the activation of DAB2 via Akt/ERK1/2 signaling pathway to promote the progression and metastasis in prostate cancer (Yang et al., 2019). Another study has found that miR-93 is downregulated in human colon cancer tissues and colorectal cancer cell lines (Yu et al., 2017). In that way, HOTAIR and miR-93 are both involved in the development of colon cancer, whether there is a relationship between HOTAIR and miR-93, and whether they can affect cell migration and invasion by regulating lactic acid are unclear.
Therefore, this study aimed to investigate the effects and molecular mechanisms of propofol on colon cancer metastasis. Our study found that propofol inhibited colon cancer cell migration and invasion by reducing lactic acid production through suppressing HIF-1α/MCT4 pathway via HOTAIR/miR-93 axis.
HT29 and SW480 of human colon cancer cells obtained from American Type Culture Collection (ATCC, USA) were cultured in DMEM-F12 medium (Hyclone, Logan, UT, USA) containing 10% FBS (Hyclone, Logan, UT, USA). Cells are maintained at 37°C in a humidified atmosphere with 5% CO2. Besides, the lncRNA HOTAIR overexpression vector, HIF-1α overexpression vector and miR-93 inhibitor (details in the attachment) were constructed by HeChuang Biotech Inc. (Guangzhou, China). Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used to transfection following the manufacturer’s protocol.
Propofol pure product was dissolved with 0.1% dimethyl sulfoxide (DMSO) as stock solution and the stock solution was diluted to the required concentration (10 μg/mL) with basic medium (within clinically relevant concentration range) (Argano et al., 2019). HT29 and SW480 cells were treated with propofol, placed in a cell incubator (37°C, 5% CO2) and incubated for 24 h (Kang et al., 2019).
The concentration of lactic acid was identified by EnzyChrom™ L-Lactate Assay Kit (BioAssay Systems, Hayward, CA, USA) (for further details see Suppl. Methods S1).
Real-time quantitative polymerase chain reaction (RT-qPCR)
The details are described in Suppl. Methods S1.
Incubated with anti-HIF-1α (1:1000, Novus, Centennial, CO, USA), anti-MCT4 (1:1000, Abcam, Cambridge, MA USA), anti-GAPDH (1:10000, Kangcheng, Shanghai, China) overnight at 4°C. The bands were visualized by using the chemiluminescence detection kit (Thermo Scientific) and analyzed by Image J software (for further details see Suppl. Methods S1).
Pictures were taken at 0, 24, and 48 h after the scratch. Observe the healing of the scratches under an inverted microscope. Image Pro-Plus 6.0 software was utilized to measure the width of scratches at any eight locations at the same time point in each group of cells, and the cells migration distance and migration rate were calculated by the following formula: (0 h-other time points)/0 h (for further details see Suppl. Methods S1).
HT29 and SW480 cells were resuspended in 100 μL serum-free medium and planted in the top chamber of each insert (8 μm-pore size, Corning, NY, USA) with a Martrigel-coated membrane. Lower chambers of the inserts were filled with DMEM medium contained 10% FBS. 24 h later, cells invaded to the lower surface of the insert were fixed, stained, and counted under a light microscope.
Chromatin immunoprecipitation (ChIP)
ChIP was assessed by the ChIP-IT Express Enzymatic Chromatin Immunoprecipitation Kit (Active Motif, Carlsbad, CA, USA) and HIF-1α antibody (Novus, NB100-105) as described previously (Xu et al., 2018) (for further details see Suppl. Methods S1).
Dual-luciferase system was used to analyze the luciferase activity according to the manufacture’s protocol. HT29 cells were co-transfected with miR-93 inhibitor, HOTAIR overexpression vector, pmirGLO-hif-1α-wt or pmirGLO-hif-1α-mut luciferase reporter plus Renilla luciferase internal control reporter. Dual-luciferase system was used to analyze the luciferase activity according to the manufacture’s protocol (for further details see Suppl. Methods S1).
Biotin-labeled NC probe (UUGUACUACACAAAAGUACUG) and Biotin-labeled miR-93 probe were synthesized in vitro by Sangon Biotechnology Co., Ltd. (Shanghai, China). The RNA-pulldown was performed using the miRNA pulldown Kit (Hechuang Biotechnology Co., Ltd., Guangzhou, China) (for further details see Suppl. Methods S1).
All data analyses were performed by using the Graghpad software (version 8.0). All data were shown as mean ± standard deviation (SD). The significant differences between different groups were analyzed by t-test (comparison for two groups), One-way ANOVA, Dunnett’s multiple comparisons test (comparison for more than two groups). P < 0.05 was considered to be statistically significant.
ATCC American Type Culture Collection; ceRNA Competitive endogenous RNA; ChIP Chromatin immunoprecipitation; CRC Colorectal cancer; DMSO; Dimethyl sulfoxide; ECM Extracellular matrix; HIF-1α Hypoxia inducible factor-1α; HOTAIR HOX transcript antisense RNA; LncRNA Long non-coding RNA; MCT4; Monocarboxylate transporter 4; MiRNA Micro RNA; ORF Open reading frame; RT-qPCR Real-time quantitative polymerase chain reaction; 3’UTR 3’ Untranslated region.
Propofol inhibits migration and invasion by reducing the secretion of cellular lactic acid in colon cancer cells
To explore the effect of propofol on the glucose metabolism of colon cancer cells and tumor progression, first, we detected the lactate secretion of colon cancer cells with or without propofol treatment. As shown in Fig. 1A, propofol significantly reduced the production of lactic acid in HT29 and SW480 cells (Fig. 1A). Then, we explored the effect of propofol on migration and invasion in colon cancer cells by Scratch assay and Transwell assay. HT29 and SW480 cells were treated with propofol for 24 h and detected by Scratch assay and Transwell assay. Scratch assay showed that propofol inhibited migration in HT29 and SW480 cells (Fig. 1B). Similarly, invasion of HT29 and SW480 cells were also suppressed in response to propofol treatment by Transwell assay (Fig. 1C).
To clarify whether propofol suppressed colon cancer cells migration and invasion by reducing lactic acid production, exogenous lactic acid (2.5 mM) was used to treat cells. As shown in Figs. 1D and 1E, propofol inhibited HT29 cells migration and invasion, then exogenous lactic acid antagonized the inhibitory effect of propofol on cells migration and invasion. The results suggested that propofol may suppress colon cancer cells metastasis by down-regulating glycolysis and lactic acid production.
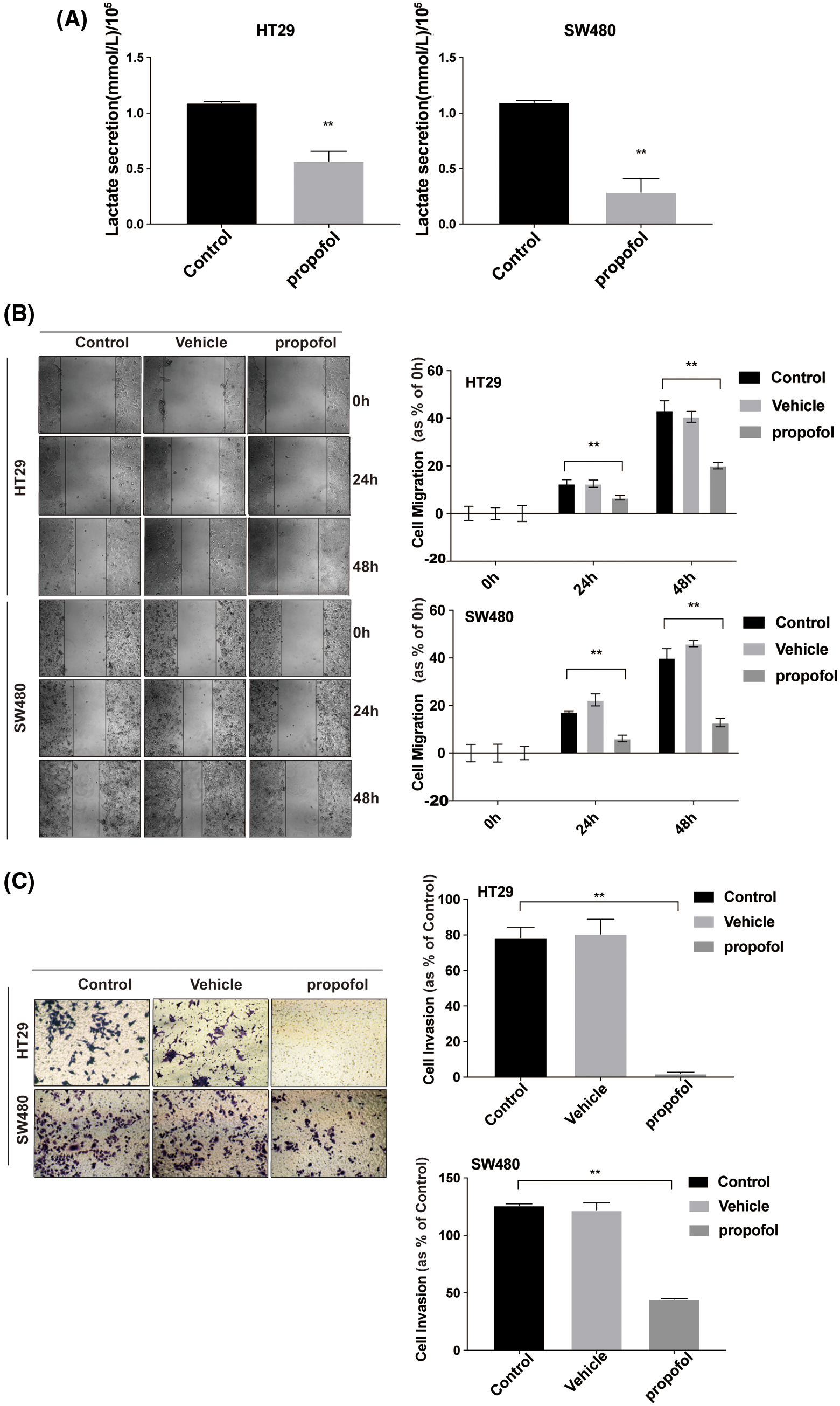
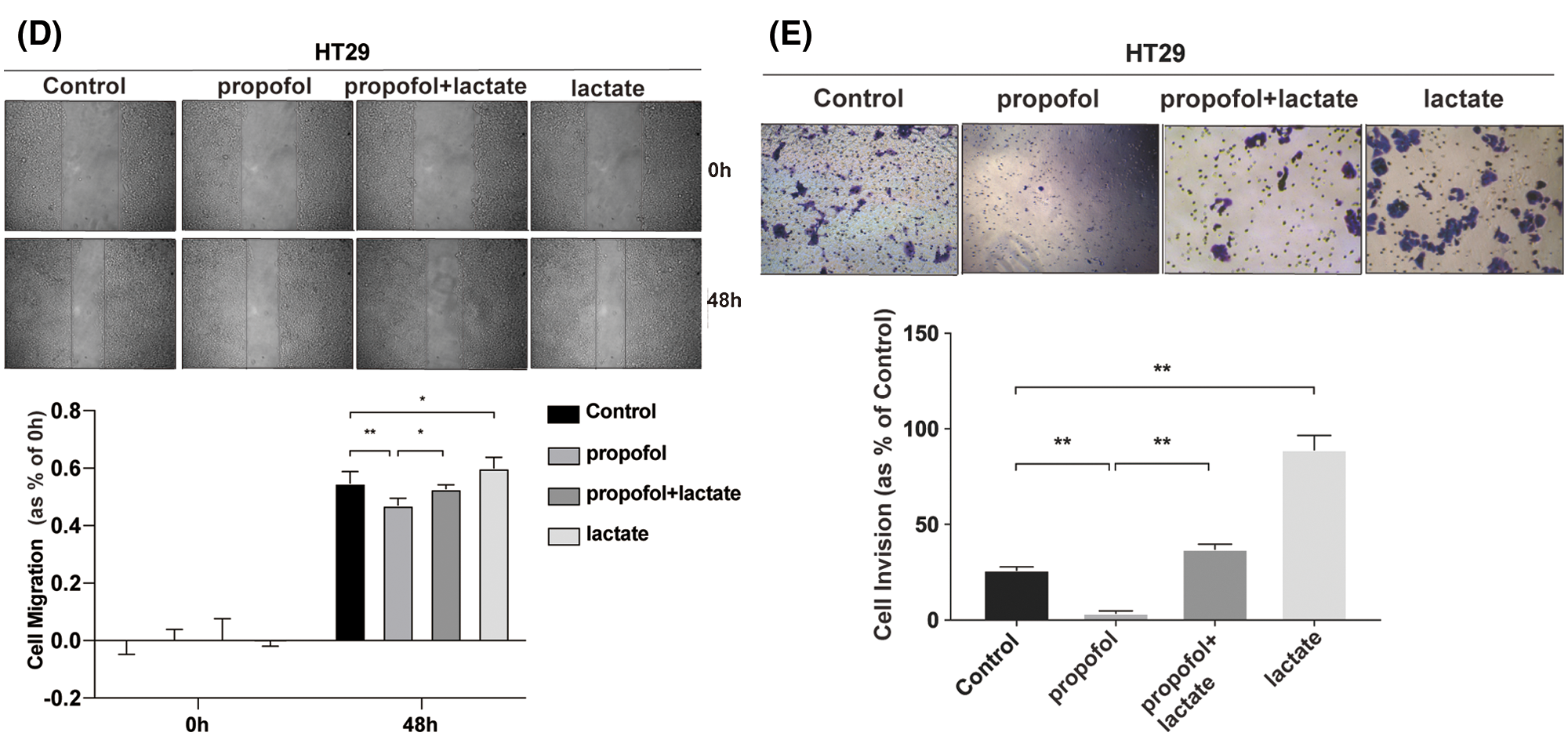
Figure 1: Propofol suppresses cells migration and invasion by reducing lactate secretion in colon cancer cells. (A) Lactic acid was detected in conditional medium from HT29 and SW480 cells with or without propofol (10 μg/mL) treatment for 24 h. (B) HT29 and SW480 cells were treated as (A) described, subsequently the cell migration was measured by Scratch assay at indicated time. (C) HT29 and SW480 cells were treated as (A) described, the cells invasion was measured by Transwell assay. HT29 cells migration (D) and invasion (E) were measured by Scratch assays or Transwell assay with propofol (10 μg/mL), exogenous lactic acid (2.5 mM) or combined treatment. Data represent the mean ± SD of three independent experiments or one-way ANOVA experiments (N = 3). *P < 0.05, **P < 0.01.
Overexpression of HIF-1α reverses the inhibitory effect of propofol on lactate secretion, cell migration and invasion in colon cancer cells
It has been reported that HIF-1α plays a crucial role in mediating glycolysis (Pavlova and Thompson, 2016). To explore the effect of propofol on the expression of HIF-1α and possible contribution of HIF-1α to the lactate secretion as well as cells migration and invasion in response to propofol, RT-qPCR showed that the mRNA level of HIF-1α was decreased significantly by propofol in HT29 and SW480 cells (Fig. 2A). Similarly, propofol also reduced the protein level of HIF-1α in HT29 and SW480 cells by Western blotting (Fig. 2B, Suppl. Fig. S1). Then we transfected pcDNA3.1 (control) or pcDNA3.1-HIF-1α plasmid into HT29 cells and treated with propofol. As shown in Fig. 2C, overexpression of HIF-1α rescued the lactic acid secretion in HT29 cells reduced by propofol. Similarly, inhibitory effect of propofol on migration and invasion were also recovered by overexpression of HIF-1α in HT29 cells (Figs. 2D and 2E). These data suggested that propofol mediated-expression of HIF-1α plays a crucial role in the lactate secretion and tumor cells migration/invasion.
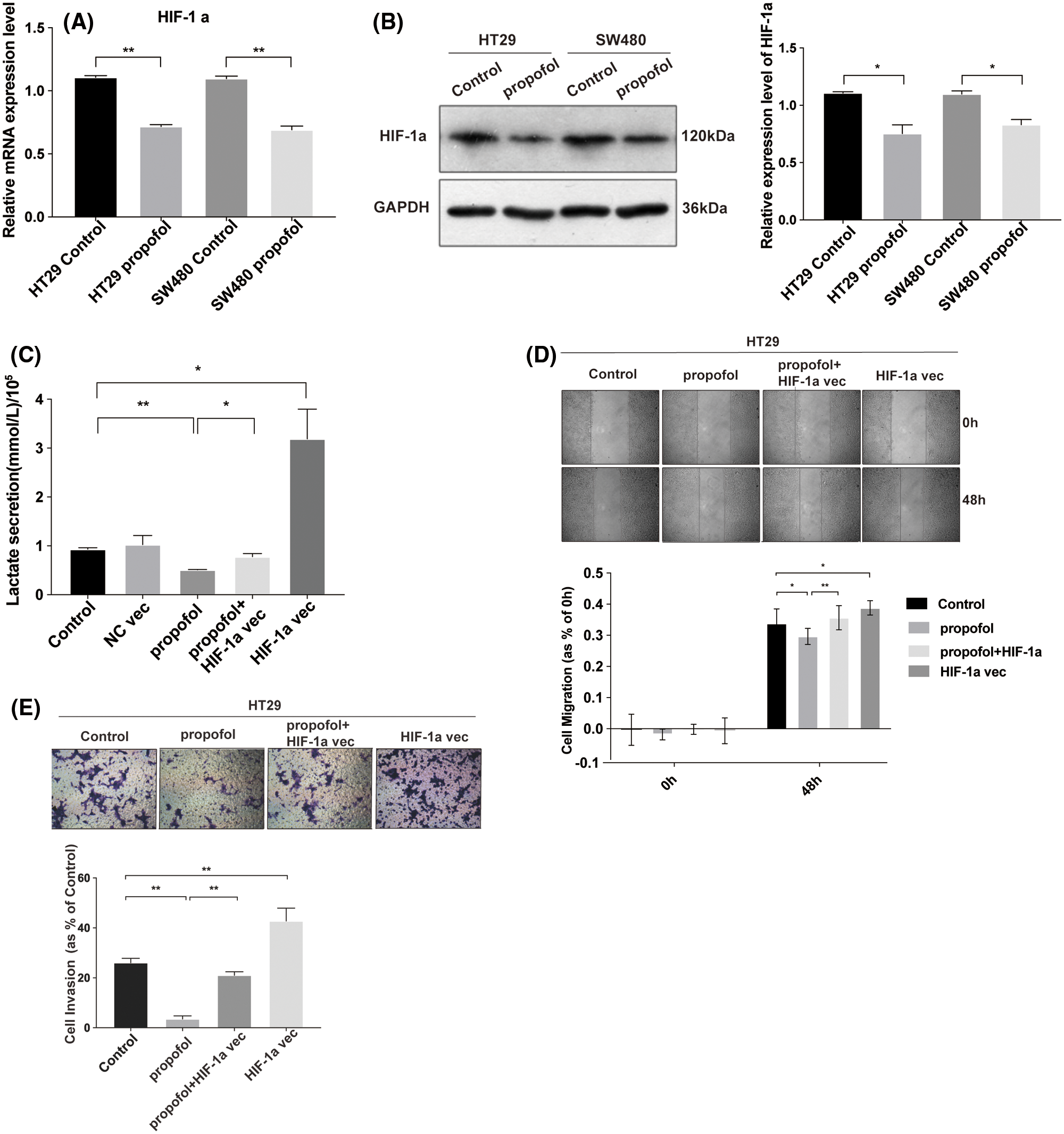
Figure 2: Propofol inhibits lactate secretion, cells migration and invasion via the downregulation of HIF-1α in colon cancer cells. The mRNA (A) and proteins (B) level of HIF-1α were measured by RT-qPCR and Western blotting in HT29 and SW480 cells with propofol (10 μg/mL) treatment. (C) The lactic acid secretion was detected in HT29 cells transfected with pcDNA3.1 (control), pcDNA3.1-HIF-1α plasmid and then treated with propofol (10 μg/mL). The migration (D) and invasion (E) of HT29 cells transfected with pcDNA3.1-HIF-1α plasmid and treated with propofol were measured by Scratch assays or Transwell assay. Data represent the mean ± SD of three independent experiments or one-way ANOVA experiments (N = 3). *P < 0.05, **P < 0.01.
Propofol inhibits the transcriptional activity and protein level of lactate transporter MCT4 by suppressing the binding of HIF-1α to the promoter of MCT4 in colon cancer cells
MCT4 is expressed prevalently in glycolytic cells that export large amounts of lactic acid and it is transcriptionally up-regulated by HIF-1α (Sanita et al., 2014). To ascertain whether propofol affects the expression of the lactate transporter MCT4, we performed RT-qPCR and Western blotting to find that the mRNA and protein expression levels of MCT4 were reduced by propofol in HT29 and SW480 cells (Figs. 3A and 3B). To further verify that HIF-1α is involved in downregulation of MCT4 induced by propofol, we detected the MCT4 expression in HT29 cells that transfected with pcDNA control vector or pcDNA-HIF-1α plasmid with or without propofol treatment. As shown in Fig. 3C, overexpression of HIF-1α remarkably rescued the protein expression of MCT4 decreased by propofol in HT29 cells. Moreover, we co-transfected pcDNA-HIF-1α plasmid and internal control of Renilla luciferase reporter with luciferase reporter of the promoter of MCT4 wild-type (wt) or mutant (mut) binding sites for HIF-1α. Luciferase reporter assay showed that propofol significantly decreased the transcriptional activity of MCT4 gene, whereas overexpression of HIF-1α reversed the downregulation of the transcriptional activity of MCT4 gene which was induced by propofol. By contrast, the transcriptional activity of the promoter of MCT4 mutant (mut) binding sites for HIF-1α was not affected by propofol and overexpression of HIF-1α (Fig. 3D). In agreement with luciferase data, ChIP experiments demonstrated that the binding of HIF-1α to the two putative sites of MCT4 promoter were decreased by propofol in HT29 cells (Fig. 3E). These data suggested that propofol contributes to tumor cells lactate secretion, migration, and invasion through down-regulation of HIF-1α/MCT4 signaling in colon cancer cells.
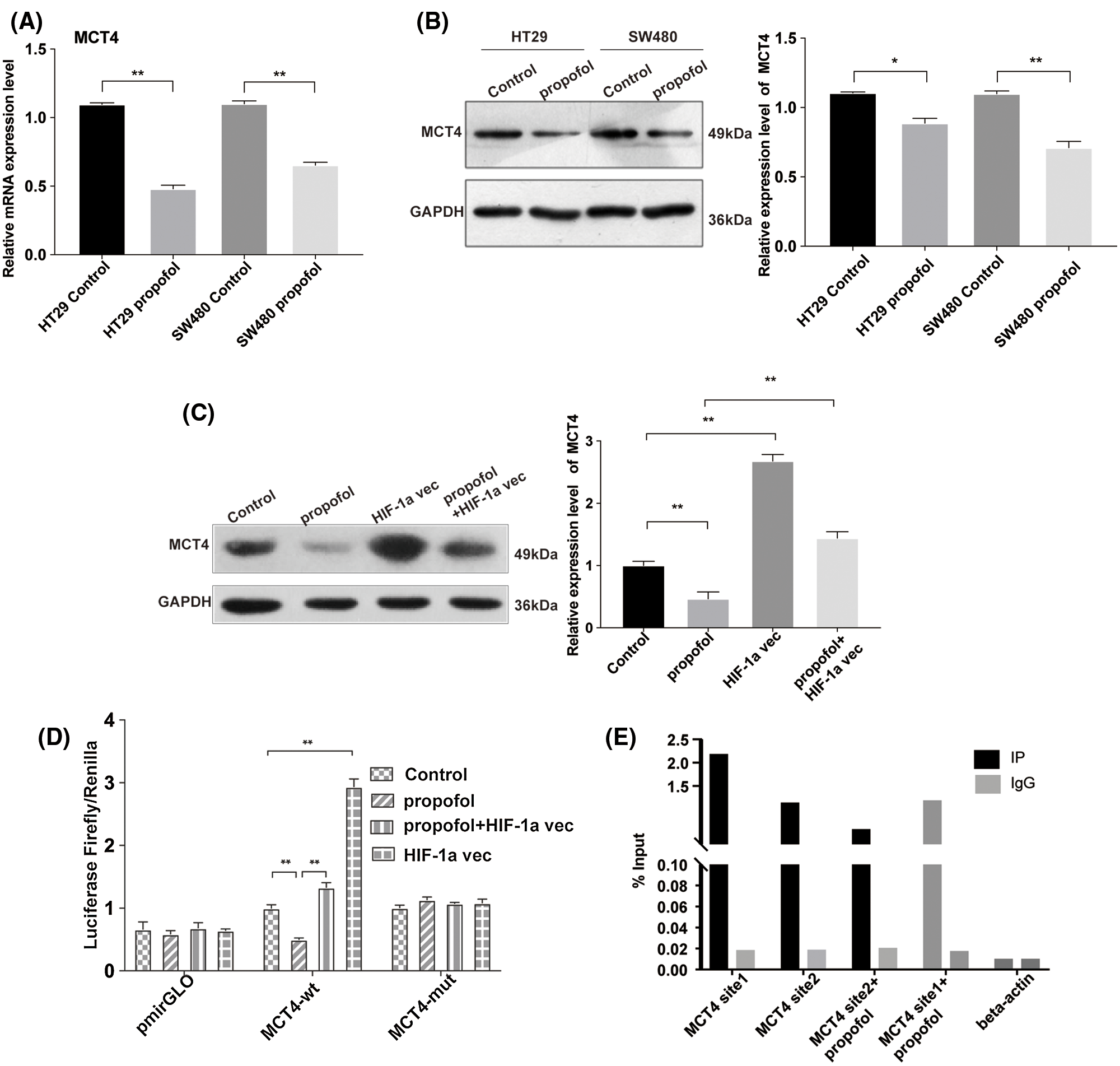
Figure 3: Propofol decreases the transcriptional activity of lactate transporter MCT4 via the downregulation of HIF-1α in colon cancer cells. The mRNA (A) and protein (B) level of MCT4 were measured by RT-qPCR and Western blotting in HT29 and SW480 cells with propofol (10 μg/mL) treatment. (C) The protein expression of MCT4 was detected by Western blotting in HT29 cells transfected with pcDNA3.1-HIF-1α plasmid and treated with propofol (10 μg/mL). (D) The transcription activity of MCT4 was detected by Dual-luciferase assay in HT29 cells co-transfected with pcDNA3.1 plasmid (control) and pmirGLO-mct4-wt or pmirGLO-mct4-mut, pcDNA3.1-HIF-1α plasmid and pmirGLO-mct4-wt or pmirGLO-mct4-mut, in response to propofol (10 μg/mL) treatment for 24 h. (E) The binding of HIF-1α to the two putative sites of mct4 promoter by ChIP and q-PCR in HT29 cells treated with 10 μg/mL propofol for 24 h. Data represent the mean ± SD of three independent experiments or one-way ANOVA.
Propofol inhibits the expression of HIF-1α by up-regulating miR-93
Next, we detected the effect of propofol on the expression of miR-93 by RT-qPCR. As shown in Fig. 4A, propofol dramatically enhanced the expression of miR-93 (Fig. 4A). Bioinformatics analysis by Targetscan online database (http://www.targetscan.org) further showed that there exist conserved sites for miR-93 in the 3’ untranslated region UTR (3’UTR) of human HIF-1α (Suppl. Fig. S2). To validate this prediction, we detected the interaction between miR-93 and HIF-1α mRNA. RNA-pulldown showed that propofol enhanced binding of miR-93 to the HIF-1α mRNA 3’UTR in HT29 cells compared with control (Fig. 4B). Moreover, Dual-luciferase assay revealed that miR-93 inhibitor markedly increased the luciferase activity of hif-1α-wt reporter and reversed the inhibitory effect of propofol on the luciferase activity of hif-1α-wt reporter but had no influence on the luciferase activity of hif-1α-mut reporter (Fig. 4C). In accordance with RNA-pulldown and Dual-luciferase assay, miR-93 inhibitor dramatically increased the protein expression level of HIF-1α and abolished the inhibitory effect of propofol on the protein expression of HIF-1α (Fig. 4D). These data indicated that propofol could inhibit the expression of HIF-1α through up-regulating miR-93 in colon cancer cells.
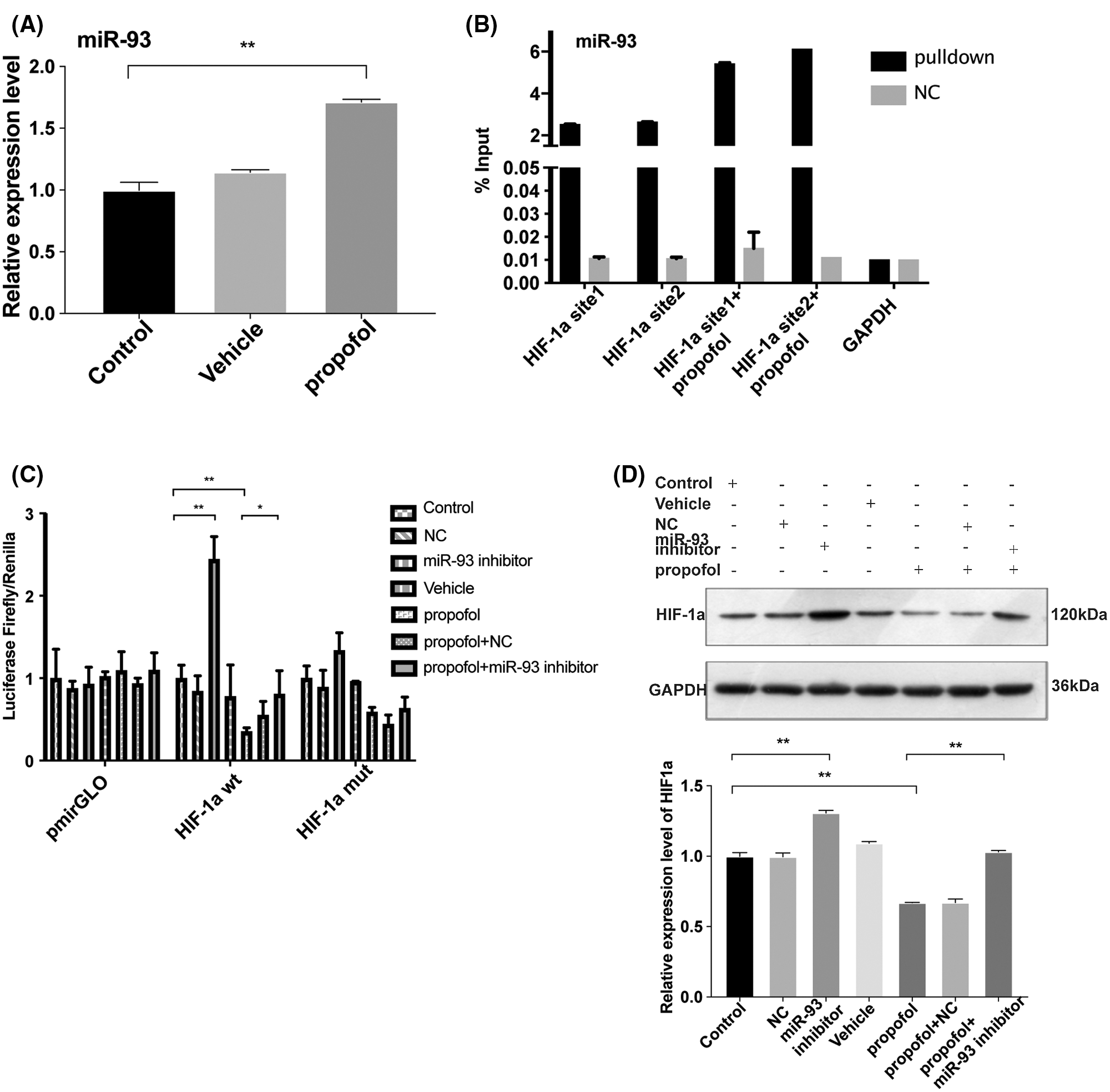
Figure 4: Propofol inhibits the expression of HIF-1α by upregulating miR-93. (A) The expression of miR-93 was detected by RT-qPCR in HT29 cells treated with 10 μg/mL propofol for 24 h. (B) RNA pull-down assay was performed to detect the binding of HIF-1α with miR-93 in HT29 cells using groups NC or miR-93 probe in response to propofol treatment. (C) The transcription activity of HIF-1α was detected by Dual-luciferase assay in HT29 cells transfected with pmirGLO-hif-1α-wt or pmirGLO-hif-1α-mut, and then treated with miR-93 inhibitor and propofol (10 μg/mL). (D) The protein expression of HIF-1α was evaluated by Western blotting in HT29 cells treated with miR-93 inhibitor and 10 μg/mL propofol. Data represent the mean ± SD of three independent experiments (N = 3). *P < 0.05, **P < 0.01.
Propofol suppresses the interaction between HOTAIR and miR-93 to decrease the expression of HIF-1α
LncRNAs usually serve as molecular sponges that sequester the expression of other RNAs (Bayoumi et al., 2016). Due to the cancer-promoting function lncRNA HOTAIR (Luo et al., 2016), we examined whether the expression of HOTAIR and recruitment of miR-93 to the site of HOTAIR could affect the expression of HIF-1α in response to propofol. By Starbase analysis (http://starbase.sysu.edu.cn/), we found that miR-93 can be recruited by HOTAIR (Suppl. Fig. S3). Thus, we speculated that propofol may regulate the interaction between HOTAIR and miR-93. To validate this hypothesis, we performed RNA-pulldown and found that miR-93 can bound to HOTAIR, while propofol suppressed the binding of miR-93 to two sites of HOTAIR (Fig. 5A) and reduced the expression of HOTAIR (Fig. 5B). Then, we further explored the effect of HOTAIR on the expression of HIF-1α mRNA. Dual-luciferase assay showed that overexpression of HOTAIR antagonized the inhibitory effect of propofol on the expression of HIF-1α mRNA 3’UTR (wt) (Fig. 5C). By contrast, the luciferase activity of the miR-93 binding site in the HIF-1α mRNA 3’UTR (mut) was not affected by propofol and overexpression of HOTAIR (Fig. 5C). In addition, we detected the effect of HOTAIR overexpression on the HIF-1α protein level triggered by propofol. Western blotting showed that overexpression of HOTAIR attenuated the protein expression of HIF-1α which was reduced by propofol (Fig. 5D). These data suggested that propofol reduced the interaction between HOTAIR and miR-93 to inhibit the expression of HIF-1α.
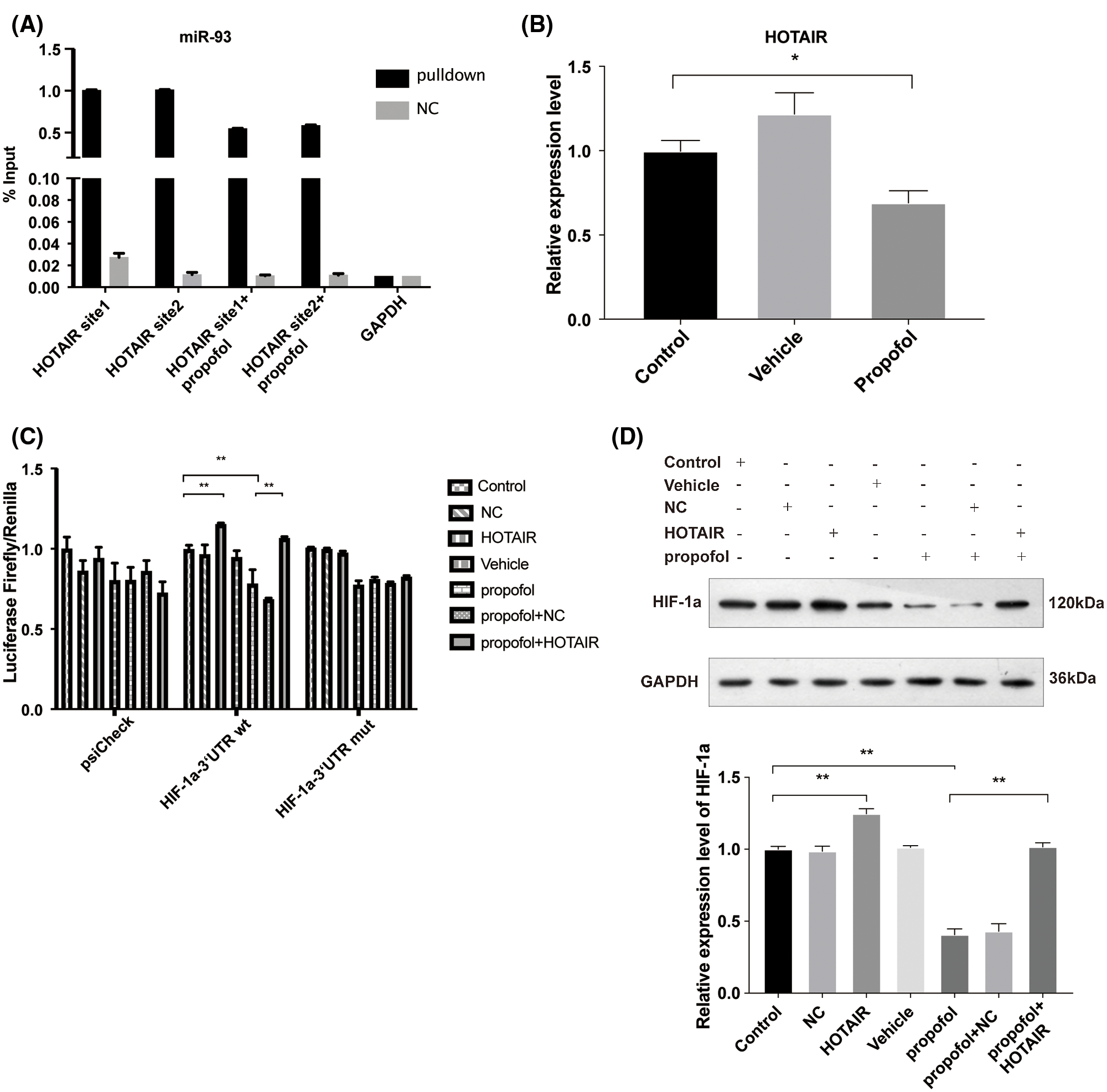
Figure 5: Propofol inhibits the expression of HIF-1α via downregulation of the interaction of HOTAIR with miR-93. (A) RNA-pulldown was performed in HT29 cells by using NC and miR-93 probe, followed by detection the combined level of HOTAIR and miR-93 with or without propofol treatment. (B) The expression of HOTAIR in HT29 cells treated with 10 μg/mL propofol was detected by RT-qPCR. (C) The transcription activity of HIF-1α was detected by Dual-luciferase assay in HT29 cells transfected with pmirGLO-hif-1α-wt or pmirGLO-hif-1α-mut and treated with HOTAIR overexpression vector and propofol (10 μg/mL). (D) The protein expression of HIF-1α was detected by Western blotting in HT29 cells transfected with HOTAIR overexpression vector and treated with 10 μg/mL propofol. Data represent the mean ± SD of three independent experiments (N = 3). *P < 0.05, **P < 0.01.
Propofol inhibits lactate production, cell migration and invasion via HOTAIR/miR-93 axis in colon cancer cells
To prove that propofol reduced colon cancer cells lactic acid production, migration, and invasion via HOTAIR/miR-93 axis, HOTAIR overexpression vector and miR-93 inhibitor were used. As shown in Figs. 6A and 6B, overexpression of HOTAIR or miR-93 inhibitor significantly increased lactate secretion compared with control, whereas both overexpression of HOTAIR and miR-93 inhibitor antagonized the inhibitory effect of propofol on lactic acid secretion. Moreover, both overexpression of HOTAIR and miR-93 inhibitor promoted colon cancer cells migration (Figs. 6C and 6D) and invasion (Figs. 6E and 6F), both overexpression of HOTAIR and miR-93 inhibitor abolished the inhibitory effect of propofol on colon cancer cells migration (Figs. 6C and 6D) and invasion (Figs. 6E and 6F). Altogether, these data revealed that propofol inhibited lactic acid production, migration, and invasion via HOTAIR/miR-93 axis in colon cancer cells.
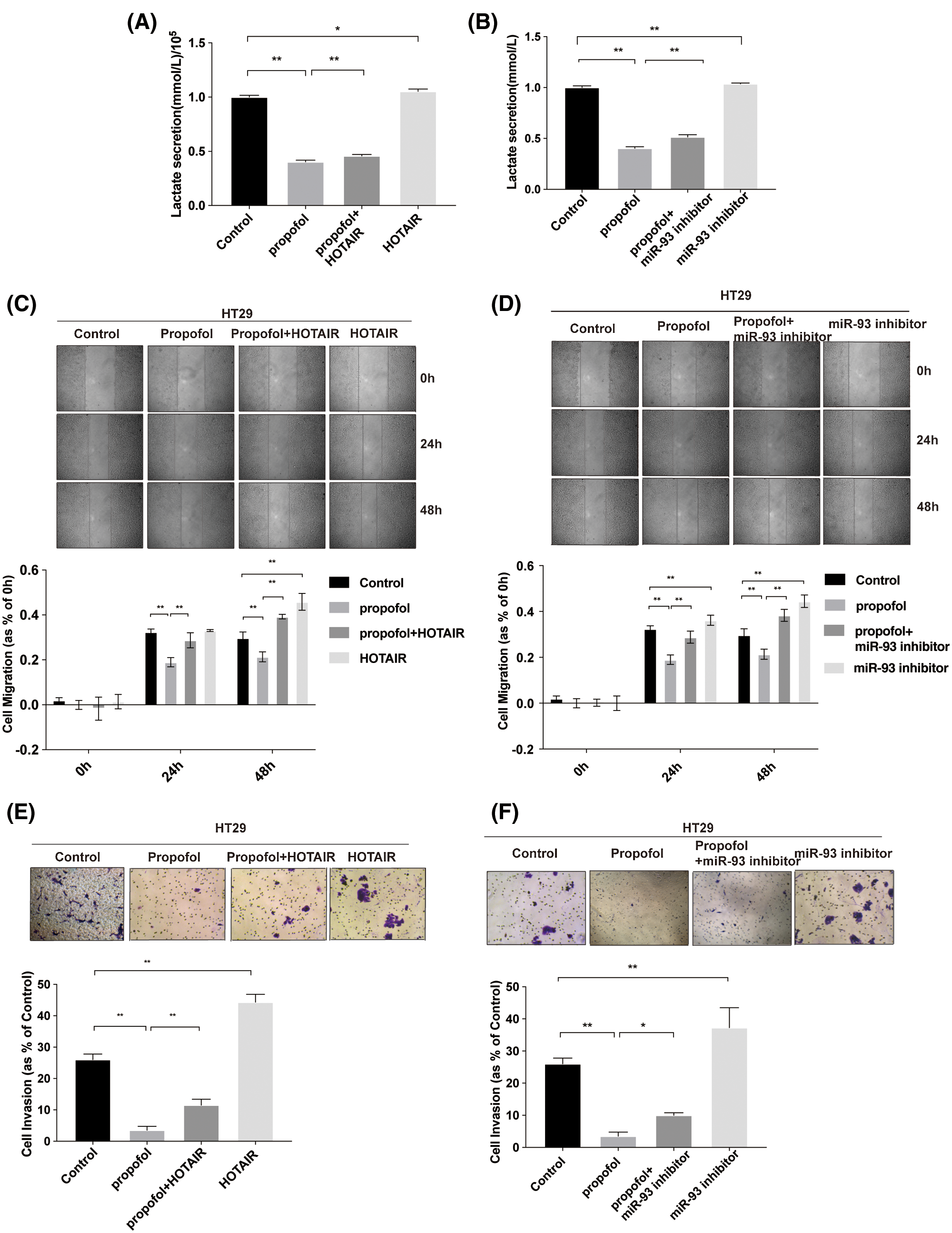
Figure 6: Propofol inhibits lactate secretion, migration, and invasion via HOTAIR/miR-93 axis in colon cancer cells. (A–B) The lactic acid secretion was analyzed in HT29 cells treated with miR-93 inhibitor and transfected with HOTAIR overexpression vector with or without 10 μg/mL propofol treatment. (C–D) Cell migration was measured by Scratch assays in HT29 cells treated with miR-93 inhibitor and transfected with HOTAIR overexpression vector with or without propofol. (E–F) Cell invasion was measured by Transwell assay for HT29 cells treated with miR-93 inhibitor and transfected with HOTAIR overexpression vector with or without propofol. Data represent the mean ± SD of three independent experiments (N = 3). *P < 0.05, **P < 0.01.
Anesthesia is a new direction in the field of tumor treatment, which is still in infancy. Perioperative anesthesia factors also have a non-negligible impact on the occurrence and development of tumors (Dubowitz et al., 2018). We found that propofol reduced the interaction between miR-93 and HOTAIR and inhibited HIF-1α/MCT4/lactate signaling, ultimately suppressed migration and invasion in colon cancer cells.
Cancer metastasis is induced by a series of complex cells biology events (Valastyan and Weinberg, 2011). We found that propofol can inhibit migration and invasion by reducing lactic acid production in colon cancer cells. It suggested that propofol may prevent the process of migration and invasion by reducing lactic acid secretion at the initial stage of cells metastasis cascade. Clinically, the accumulation of lactic acid often occurs in patients with systemic circulation or microcirculation disorders, indicating that various important organs of patients were poorly perfused (Hollyer et al., 2019). Poor organ perfusion and lactic acid accumulation could directly affect the disease prognosis (Mok et al., 2020). For this reason, we focused on exploring the effect of propofol on lactic acid production, which has potentially important clinical significance for the prevention and treatment of colon cancer.
To date, the relationship between propofol and lactic acid production remains unclear. Our study uncovered the inhibitory role of propofol in lactic acid secretion in cancer cells. A clinical study has indicated that short-term propofol infusion has no effect on the level of serum lactic acid in children (Indra et al., 2017). By contrast, another study revealed that propofol suppresses glycolysis and lactic acid in brain astrocyte (Hadjihambi et al., 2020), which is similar to our results in colon cancer. It is well known that HIF-1α/MCT4/lactate signaling pathway promotes cancer metastasis (Ferretta et al., 2016). Previous studies demonstrated that excessive lactic acid production can promote the development of cancer, including local tissue invasion and metastasis, new disordered angiogenesis, changing in tissue hypoxia resistance and changing in epigenetics (Choi et al., 2013). Lower pH provides a better microenvironment for protease activation, including MMP, urokinase-type plasminogen activator and cathepsins B, D and L, which induce ECM degradation and promote tumor cells metastasis (Gatenby et al., 2006). We found that propofol inhibited migration and invasion via down-regulating lactic acid production in colon cancer cells. It implies that propofol may also affect the balance of the internal environment by regulating lactic acid secretion in non-tumor cells, ensuring the microcirculation perfusion, maintaining the homeostasis of the environment, improving patient outcomes and long-term prognosis quality.
Furthermore, we elucidated the molecular mechanism how propofol regulates HIF-1α. According to database analysis, we found that miR-93 may bind to HIF-1α mRNA 3’UTR to suppress HIF-1α expression. Data showed that miR-93 can inhibit the growth of colon cancer stem cells (Zou et al., 2013), our results showed that propofol can up-regulate miR-93 to inhibit the expression of HIF-1α. Present study also suggested that miR-93 was a tumor suppressor. However, previous studies have revealed that miR-93 can activate the PI3K/Akt pathway by inhibiting LKB1/PTEN/CDKN1A and promote tumorigenesis and metastasis as well as promote the growth of cells by down-regulating DAB2 in lung cancer cells (Du et al., 2014; Li et al., 2017). Not only in lung cancer, miR-93 can also promote proliferation, invasion, and metastasis in other cancer cells (Li et al., 2019; Ma et al., 2017). Our work is different from previous studies, which may be attributed to some miRNAs act as oncogenes in one type of cancer but may be act as anti-oncogenes in other types of cancers (Spizzo et al., 2009). These have been observed species variability in earlier studies.
Due to the “sponge adsorption effect” of lncRNA, we found that miR-93 can be recruited by HOTAIR which expressed extremely high in colon cancer cells (Luo et al., 2016). To date, only one study has reported that HOTAIR reduces radiosensitivity of CRC by regulating miR-93 (Liu et al., 2020). Growing evidence have indicated abnormal expression of HOTAIR in multiple tumors, which is positively correlated with tumorigenesis, angiogenesis, drug resistance, tumor recurrence and poor prognosis (Chen et al., 2015; Shi et al., 2017). For patients with CRC, poor prognosis of primary tumors and diseases are related to the up-regulation of HOTAIR in the blood, which indicated that HOTAIR can be used as a potential biomarker for sporadic CRC (Svoboda et al., 2014). Our results showed that propofol down-regulated HOTAIR to free miR-93 and inhibited the expression of HIF-1α, further reduced lactic acid and cells migration and invasion. The HOTAIR/miR-93/HIF-1α axis may be used in the clinical diagnosis of colon cancer metastatic disease and may become a more sensitive marker than HOTAIR alone.
In our study propofol showed a significant effect on anti-tumor and provided new prospects for the prevention and treatment of cancers. If propofol is used as an anti-tumor drug, it might be delivered to the site of tumor directly in a targeted way. This problem needs to be solved by materials technology.
There are certain limitations and shortcomings in this study. The results showed that propofol up-regulated the expression of miR-93, indicating that propofol increased the expression of endogenous miR-93. However, the molecular mechanism is unclear. We speculated that miR-93 was sponge adsorbed to HOTAIR, and HOTAIR combined with methyltransferase to promote miRNA methylation, resulting in miRNA degradation and increased expression of endogenous miRNA. This part needs further research and discussion on molecular mechanism. We will further explore the molecular mechanism between HOTAIR, and miR-93 based on RNA epigenetics. Besides, the use of only in vitro system is another limitation of this study, and the nude mice is a potential topic of in vivo study for the effect of propofol on CRC in future.
This study indicated that propofol inhibits colon cancer cell migration and invasion through downregulating the expressions of HIF-1α and MCT4 to reduce lactic acid production via HOTAIR/miR-93 axis. These results may provide potential metabolic targets for CRC treatment.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: Ruonan Gu, Fan Deng, Fangyin Zeng; data collection: Ruonan Gu, Wenjing Guo, Wanlu Zhao, Zhicong Wu, Hua Chen, Wenyang Luo; analysis and interpretation of results: Ruonan Gu, Wenyang Wang, Guihuan Li, Xiaoju Lai, Zhibin Huang, Wanlu Zhao, Zhicong Wu, Hua Chen, Wenyang Luo; draft manuscript preparation: Ruonan Gu, Wenjing Guo, Fan Deng. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding Statement: This study is supported by the National Natural Science Foundation of China (81772761, 81672540).
Conflicts of Interest: The authors declare that they have no conflict of interest to report regarding of the present study.
Argano M, de Maria R, Rodlsberger K, Buracco P, Menzies MPL (2019). Use of a colorimetric assay to evaluate the proliferation of canine mammary tumor cells exposed to propofol. Canadian Journal of Veterinary Research 83: 149–153. [Google Scholar]
Bayoumi AS, Sayed A, Broskova Z, Teoh JP, Wilson J et al. (2016). Crosstalk between long noncoding RNAs and microRNAs in health and disease. International Journal of Molecular Sciences 17: 356. [Google Scholar]
Bharati SJ, Chowdhury T, Bergese SD, Ghosh S (2016). Anesthetics impact on cancer recurrence: What do we know? Journal of Cancer Research and Therapeutics 12: 464–468. [Google Scholar]
Chen J, Lin C, Yong W, Ye Y, Huang Z (2015). Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells. Cellular Physiology and Biochemistry 35: 722–728. [Google Scholar]
Choi SY, Collins CC, Gout PW, Wang Y (2013). Cancer-generated lactic acid: A regulatory, immunosuppressive metabolite? Journal of Pathology 230: 350–355. [Google Scholar]
Doherty JR, Yang C, Scott KE, Cameron MD, Fallahi M et al. (2014). Blocking lactate export by inhibiting the Myc target MCT1 Disables glycolysis and glutathione synthesis. Cancer Research 74: 908–920. [Google Scholar]
Du L, Zhao Z, Ma X, Hsiao TH, Chen Y et al. (2014). miR-93-directed downregulation of DAB2 defines a novel oncogenic pathway in lung cancer. Oncogene 33: 4307–4315. [Google Scholar]
Du Q, Liu J, Zhang X, Zhang X, Zhu H et al. (2018). Propofol inhibits proliferation, migration, and invasion but promotes apoptosis by regulation of Sox4 in endometrial cancer cells. Brazilian Journal of Medical and Biological Research 51: e6803. [Google Scholar]
Dubowitz JA, Sloan EK, Riedel BJ (2018). Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clinical & Experimental Metastasis 35: 347–358. [Google Scholar]
Ebright RY, Zachariah MA, Micalizzi DS, Wittner BS, Niederhoffer KL et al. (2020). HIF1A signaling selectively supports proliferation of breast cancer in the brain. Nature Communications 11: 6311. [Google Scholar]
Ferretta A, Maida I, Guida S, Azzariti A, Porcelli L et al. (2016). New insight into the role of metabolic reprogramming in melanoma cells harboring BRAF mutations. Biochimica et Biophysica Acta 1863: 2710–2718. [Google Scholar]
Gao X, Mi Y, Guo N, Luan J, Xu H et al. (2020). The mechanism of propofol in cancer development: An updated review. Asia-Pacific Journal of Clinical Oncology 16: e3–e11. [Google Scholar]
Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ (2006). Acid-mediated tumor invasion: A multidisciplinary study. Cancer Research 66: 5216–5223. [Google Scholar]
Gottfried E, Kreutz M, Mackensen A (2012). Tumor metabolism as modulator of immune response and tumor progression. Seminars in Cancer Biology 22: 335–341. [Google Scholar]
Hadjihambi A, Karagiannis A, Theparambil SM, Ackland GL, Gourine AV (2020). The effect of general anaesthetics on brain lactate release. European Journal of Pharmacology 881: 173188. [Google Scholar]
Hollyer TR, Bordoni L, Kousholt BS, Van Luijk J, Ritskes-Hoitinga M et al. (2019). The evidence for the physiological effects of lactate on the cerebral microcirculation: A systematic review. Journal of Neurochemistry 148: 712–730. [Google Scholar]
Hovaguimian F, Braun J, Z’graggen BR, Schlapfer M, Dumrese C et al. (2020). Anesthesia and circulating tumor cells in primary breast cancer patients: A randomized controlled trial. Anesthesiology 133: 548–558. [Google Scholar]
Hu Y, Qin X, Cao H, Yu S, Feng J (2017). Reversal effects of local anesthetics on P-glycoprotein-mediated cancer multidrug resistance. Anticancer Drugs 28: 243–249. [Google Scholar]
Indra S, Haddad H, O’riordan MA (2017). Short-term propofol infusion and associated effects on serum lactate in pediatric patients. Pediatric Emergency Care 33: e118–e121. [Google Scholar]
Jang M, Kim SS, Lee J (2013). Cancer cell metabolism: Implications for therapeutic targets. Experimental & Molecular Medicine 45: e45. [Google Scholar]
Kang FC, Wang SC, So EC, Chang MM, Wong KL et al. (2019). Propofol may increase caspase and MAPK pathways, and suppress the Akt pathway to induce apoptosis in MA10 mouse Leydig tumor cells. Oncology Reports 41: 3565–3574. [Google Scholar]
Kryczka J, Sochacka E, Papiewska-Pajak I, Boncela J (2020). Implications of ABCC4-mediated cAMP efflux for CRC migration. Cancers 12: 3547. [Google Scholar]
Li C, Lyu J, Meng QH (2017). MiR-93 promotes tumorigenesis and metastasis of non-small cell lung cancer cells by activating the PI3K/Akt pathway via inhibition of LKB1/PTEN/CDKN1A. Journal of Cancer 8: 870–879. [Google Scholar]
Li YJ, Yang Z, Wang YY, Wang Y (2019). Long noncoding RNA ZNF667-AS1 reduces tumor invasion and metastasis in cervical cancer by counteracting microRNA-93-3p-dependent PEG3 downregulation. Molecular Oncology 13: 2375–2392. [Google Scholar]
Liu Y, Chen X, Chen X, Liu J, Gu H et al. (2020). Long non-coding RNA HOTAIR knockdown enhances radiosensitivity through regulating microRNA-93/ATG12 axis in colorectal cancer. Cell Death & Disease 11: 175. [Google Scholar]
Luo F, Zou Z, Liu X, Ling M, Wang Q et al. (2017). Enhanced glycolysis, regulated by HIF-1α via MCT-4, promotes inflammation in arsenite-induced carcinogenesis. Carcinogenesis 38: 615–626. [Google Scholar]
Luo ZF, Zhao D, Li XQ, Cui YX, Ma N et al. (2016). Clinical significance of HOTAIR expression in colon cancer. World Journal of Gastroenterology 22: 5254–5259. [Google Scholar]
Lv J, Zhang S, Wu H, Lu J, Lu Y et al. (2020). Deubiquitinase PSMD14 enhances hepatocellular carcinoma growth and metastasis by stabilizing GRB2. Cancer Letters 469: 22–34. [Google Scholar]
Ma DH, Li BS, Liu JJ, Xiao YF, Yong X et al. (2017). miR-93-5p/IFNAR1 axis promotes gastric cancer metastasis through activating the STAT3 signaling pathway. Cancer Letters 408: 23–32. [Google Scholar]
Matsui M, Corey DR (2017). Non-coding RNAs as drug targets. Nature Reviews Drug Discovery 16: 167–179. [Google Scholar]
Mok G, Hendin A, Reardon P, Hickey M, Gray S et al. (2021). Macrocirculatory and microcirculatory endpoints in sepsis resuscitation. Journal of Intensive Care Medicine 2021: 885066620982585. [Google Scholar]
Neal CS, Michael MZ, Rawlings LH, van der Hoek MB, Gleadle JM (2010). The VHL-dependent regulation of microRNAs in renal cancer. BMC Medicine 8: 64. [Google Scholar]
Parks SK, Chiche J, Pouyssegur J (2013). Disrupting proton dynamics and energy metabolism for cancer therapy. Nature Reviews Cancer 13: 611–623. [Google Scholar]
Pavlova NN, Thompson CB (2016). The emerging hallmarks of cancer metabolism. Cell Metabolism 23: 27–47. [Google Scholar]
Philp NJ, Yoon H, Lombardi L (2001). Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. American Journal of Physiology-Cell Physiology 280: C1319–1326. [Google Scholar]
Sanita P, Capulli M, Teti A, Galatioto GP, Vicentini C et al. (2014). Tumor-stroma metabolic relationship based on lactate shuttle can sustain prostate cancer progression. BMC Cancer 14: 154. [Google Scholar]
Sekandarzad MW, van Zundert AAJ, Lirk PB, Doornebal CW, Hollmann MW (2017). Perioperative anesthesia care and tumor progression. Anesthesia & Analgesia 124: 1697–1708. [Google Scholar]
Shenouda SK, Alahari SK (2009). MicroRNA function in cancer: Oncogene or a tumor suppressor? Cancer and Metastasis Reviews 28: 369–378. [Google Scholar]
Shi J, Dong B, Cao J, Mao Y, Guan W et al. (2017). Long non-coding RNA in glioma: Signaling pathways. Oncotarget 8: 27582–27592. [Google Scholar]
Smith I, White PF, Nathanson M, Gouldson R (1994). Propofol. An update on its clinical use. Anesthesiology 81: 1005–1043. [Google Scholar]
Spizzo R, Nicoloso MS, Croce CM, Calin GA (2009). SnapShot: microRNAs in cancer. Cell 137: 586. [Google Scholar]
Sun XY, Han XM, Zhao XL, Cheng XM, Zhang Y (2019). MiR-93-5p promotes cervical cancer progression by targeting THBS2/MMPS signal pathway. European Review for Medical and Pharmacological Sciences 23: 5113–5121. [Google Scholar]
Svoboda M, Slyskova J, Schneiderova M, Makovicky P, Bielik L et al. (2014). HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis 35: 1510–1515. [Google Scholar]
Valastyan S, Weinberg RA (2011). Tumor metastasis: Molecular insights and evolving paradigms. Cell 147: 275–292. [Google Scholar]
Vander Heiden MG, Cantley LC, Thompson CB (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324: 1029–1033. [Google Scholar]
Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C et al. (2009). Propofol: A review of its non-anaesthetic effects. European Journal of Pharmacology 605: 1–8. [Google Scholar]
Vishnoi A, Rani S (2017). MiRNA biogenesis and regulation of diseases: An overview. In: Rani S (ed.MicroRNA profiling. Methods in molecular biology. New York: Humana Press. [Google Scholar]
Walsh CT (2018). Propofol: Milk of amnesia. Cell 175: 10–13. [Google Scholar]
Wang AH, Tan P, Zhuang Y, Zhang XT, Yu ZB, Li LN (2019). Down-regulation of long non-coding RNA HOTAIR inhibits invasion and migration of oesophageal cancer cells via up-regulation of microRNA-204. Journal of Cellular and Molecular Medicine 23: 6595–6610. [Google Scholar]
Wang L, Yang F, Jia LT, Yang AG (2017). Missing links in epithelial-mesenchymal transition: Long non-coding RNAs enter the arena. Cellular Physiology and Biochemistry 44: 1665–1680. [Google Scholar]
Xiao Z, Qu Z, Chen Z, Fang Z, Zhou K et al. (2018). LncRNA HOTAIR is a prognostic biomarker for the proliferation and chemoresistance of colorectal cancer via MiR-203a-3p-mediated Wnt/ss-catenin signaling pathway. Cellular Physiology and Biochemistry 46: 1275–1285. [Google Scholar]
Xu W, Zeng F, Li S, Li G, Lai X et al. (2018). Crosstalk of protein kinase C ε with Smad2/3 promotes tumor cell proliferation in prostate cancer cells by enhancing aerobic glycolysis. Cellular and Molecular Life Sciences 75: 4583–4598. [Google Scholar]
Yang K, Li YW, Gao ZY, Xiao W, Li TQ et al. (2019). MiR-93 functions as a tumor promoter in prostate cancer by targeting disabled homolog 2 (DAB2) and an antitumor polysaccharide from green tea (Camellia sinensis) on their expression. International Journal of Biological Macromolecules 125: 557–565. [Google Scholar]
Yu X, Mi L, Dong J, Zou J (2017). Long intergenic non-protein-coding RNA, 1567 (LINC01567) acts as a “sponge” against microRNA-93 in regulating the proliferation and tumorigenesis of human colon cancer stem cells. BMC Cancer 17: 716. [Google Scholar]
Zhang L, Wang N, Zhou S, Ye W, Jing G, Zhang M (2012). Propofol induces proliferation and invasion of gallbladder cancer cells through activation of Nrf2. Journal of Experimental & Clinical Cancer Research 31: 66. [Google Scholar]
Zou J, Mi L, Yu XF, Dong J (2013). Interaction of 14-3-3σ with KCMF1 suppresses the proliferation and colony formation of human colon cancer stem cells. World Journal of Gastroenterology 19: 3770–3780. [Google Scholar]
List of Supporting Information (Supplied as separate files)
Supplementary Methods S1. (Plasmid Construction, Detection of lactate, RT-qPCR, Western blotting, Scratches assay, Transwell assay, ChIP, Dual-Luciferase assay, RNA pull-down assay).
Materials and Methods
Plasmid construction
To overexpress lncRNA HOTAIR, the full-length HOTAIR transcript was amplified and cloned into pcDNA3.1 vector to construct the lncRNA HOTAIR overexpression vector. Besides, for the overexpression of HIF-1α, the open reading frame (ORF) of the hif-1α gene was amplified and cloned into pcDNA3.1 vector to generate the HIF-1α overexpression vector. Empty pcDNA3.1 vector was served as blank control.
Detection of lactate
Briefly, medium was collected and centrifuged at 10000 × g for 5 min to remove particles and polymers. Next, 20 μL of medium of each group and standard solution were placed in a 96-well plate, and 80 mL of working reagent was added into each well. Then the 96-well plate was put in the microplate reader immediately, the OD value of each well was measured at the working wavelength of 560 nm. After incubation at 25°C for 20 min, the 96-well plate was put in the microplate reader again to measure the OD value of each well at the working wavelength of 560 nm. The concentration of lactic acid was calculated according to the manufacturer’s protocol based on OD values.
Real-time quantitative polymerase chain reaction (RT-qPCR)
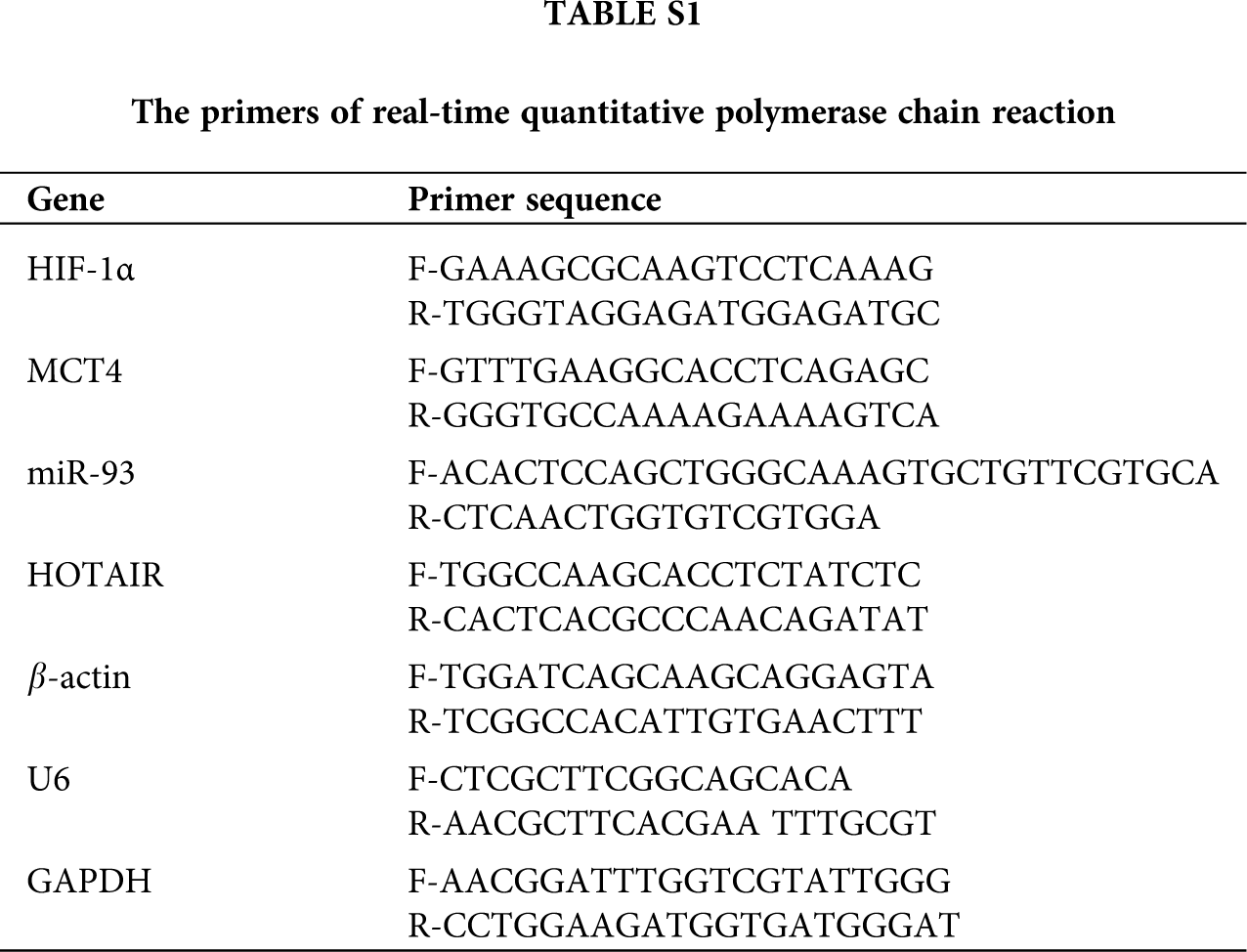
For the overexpression experiments, the overexpression efficiency is verified in transfected HT29 cells by real-time PCR. Total RNA of cells was extracted using Trizol (Invitrogen) according to the manufacturer’s instructions. The purity of RNA was measured by detecting the absorbance ratio of 260/280 nm using a Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Reverse transcription of RNA was performed via HiScript QRT SuperMix for qPCR (Vazyme, Nanjing, Jiangsu, China), and StepOnePlusTM Real-Time PCR System was used to detect the expression of target genes. The data were normalized to the levels of GAPDH and further analyzed using the 2-∆∆CT method. The primers used in the study were listed as Tab. 1.
Western blotting
Cells were lysed by lysis RIPA buffer. The extracted protein was then subjected to 10% SDS-PAGE electrophoresis and transferred onto the 0.22 μm PVDF membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat milk and incubated with anti-HIF-1α (1:1000, Novus, Centennial, CO, USA), anti-MCT4 (1:1000, Abcam, Cambridge, MA USA), anti-GAPDH (1:10000, Kangcheng, Shanghai, China,) overnight at 4°C. After being washed three times (5 min each time), membranes were incubated with HRP conjugated secondary antibodies (Southern biotech, Birmingham, AL, USA) for 2 h at room temperature (RT). The bands were visualized by using the chemiluminescence detection kit (Thermo Scientific) and analyze by Image J software.
Scratches assay
1 × 106 HT29 or SW480 cells were seeded into a well of 6-well plates and grown to reach 85% confluences. Next, a 100 μL pipette tip was used to scratch the cell monolayers. Subsequently, cells were washed with PBS three times gently and cultured with FBS-free medium. Pictures were taken at 0, 24, and 48 h after the scratch. Observe the healing of the scratches under an inverted microscope. Image Pro-Plus 6.0 software was utilized to measure the width of scratches at any eight locations at the same time point in each group of cells, and the cells migration distance and migration rate were calculated by the following formula: (0 h-other time points)/0 h.
Transwell assay
HT29 and SW480 cells were resuspended in 100 μL serum-free medium and planted in the top chamber of each insert (8-μm pore size, Corning, NY, USA) with a Martrigel-coated membrane. Lower chambers of the inserts were filled with DMEM medium contained 10% FBS. 24 h later, cells invaded to the lower surface of the insert were fixed, stained, and counted under a light microscope.
Chromatin immunoprecipitation (ChIP)
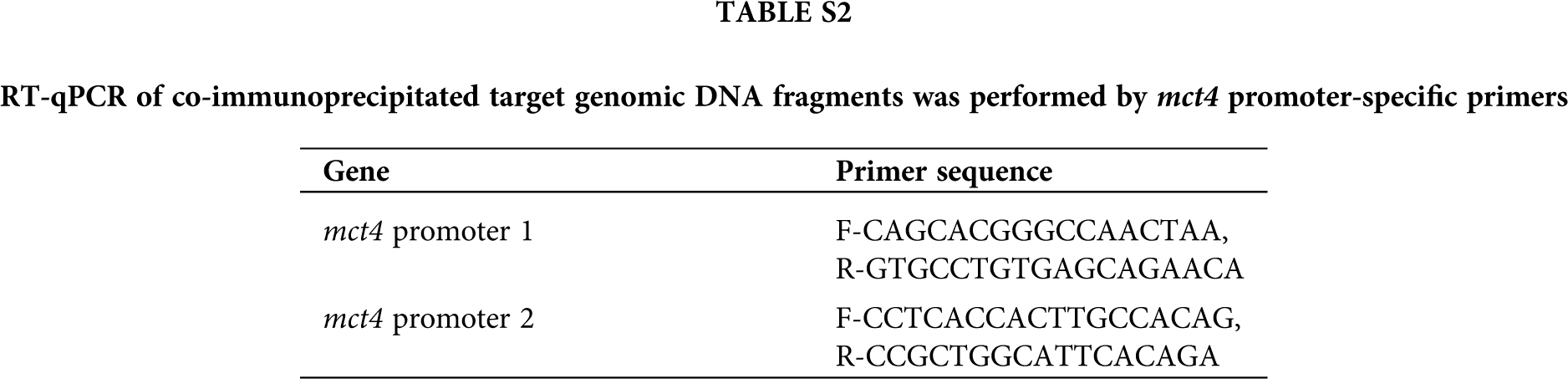
ChIP was assessed by the ChIP-IT Express Enzymatic Chromatin Immunoprecipitation Kit (Active Motif, Carlsbad, CA, USA) and HIF-1α antibody (Novus, NB100-105) as described previously. RT-qPCR of co-immunoprecipitated target genomic DNA fragments was performed by mct4 promoter-specific primers were listed as Tab. 2.
Luciferase Assay
The promoter of mct4 with wild-type (wt) or mutant (mut) binding sites for HIF-1α was amplified and cloned into the pmirGLO-vector (Promega, Madison, WI, USA) to generate the pmirGLO-mct4-wt or pmirGLO-mct4-mut plasmid. HT29 cells were co-transfected with HIF-1α overexpression vector and pmirGLO-mct4-wt or pmirGLO-mct4-mut plasmid, respectively. Dual-luciferase system was used to analyze the luciferase activity according to the manufacture’s protocol. The 3’-UTR region of hif-1α with wild-type (wt) or mutant (mut) binding sites for miR-93 was amplified and cloned into the pmirGLO-vector to generate the pmirGLO-hif-1α-wt or pmirGLO-hif-1α-mut plasmid. HT29 cells were co-transfected with miR-93 inhibitor, HOTAIR overexpression vector, pmirGLO-hif-1α-wt or pmirGLO-hif-1α-mut luciferase reporter plus Renilla luciferase internal control reporter. Dual-luciferase system was used to analyze the luciferase activity according to the manufacture’s protocol.
RNA Pull-Down Assay
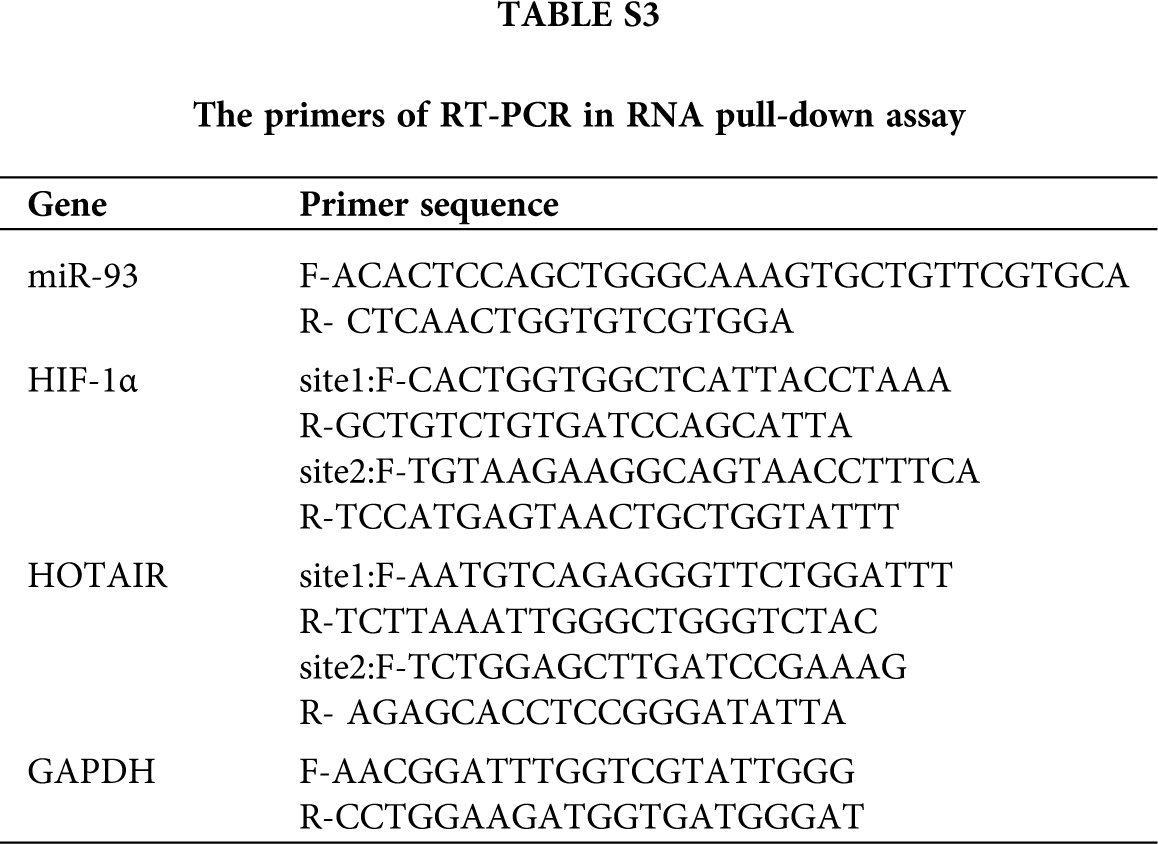
Biotin-labeled NC probe (UUGUACUACACAAAAGUACUG) and Biotin-labeled miR-93 probe were synthesized in vitro by Shanghai Sangon Biotechnology Co., Ltd.,Shanghai, China. The RNA-pulldown was performed using the miRNA pulldown Kit (He Chuang). In brief, HT29 cells were lysed with cell lysis buffer at 4°C for 1 h and centrifuged at 12 000 rpm for 10 min. The supernatant was then collected, transferred to an RNase-free centrifuge tube, washed twice with a combination of a low salt and a high-salt buffer each (10 min each), and incubated with 500 μL of streptavidin-agarose beads for 1 h at RT. After washing with a RIP buffer 5 times, RNA was eluted by elution buffer. Next, RT-PCR was performed to analyze the interaction between HIF-1α and miR-93, and HOTAIR and miR-93. Primers used in RT-PCR were listed as Tab. 3.
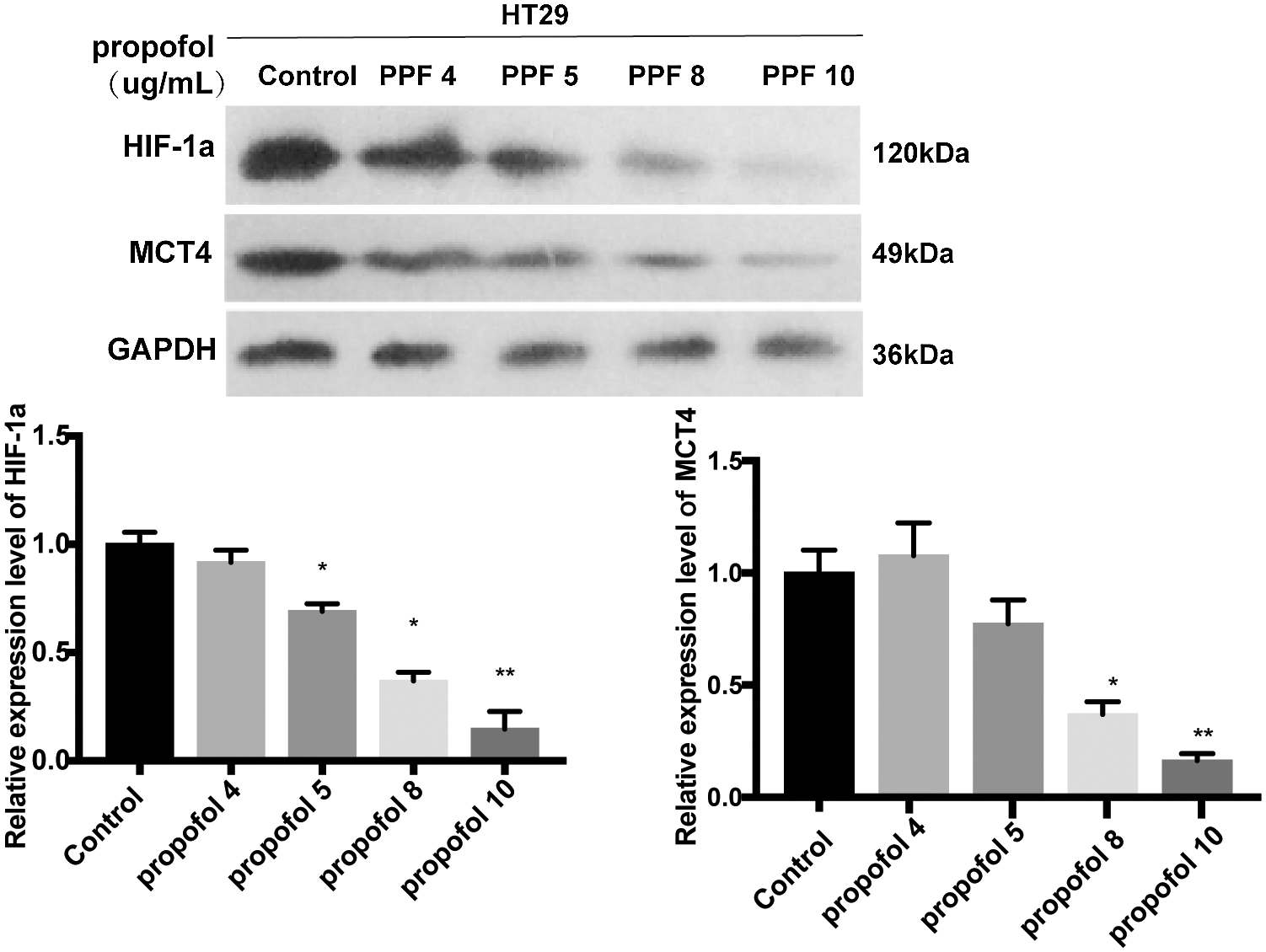
Supplementary figure S1: Different concentration of propofol have effect on the protein expression level of HIF-1α and MCT4 in HT29 cell.
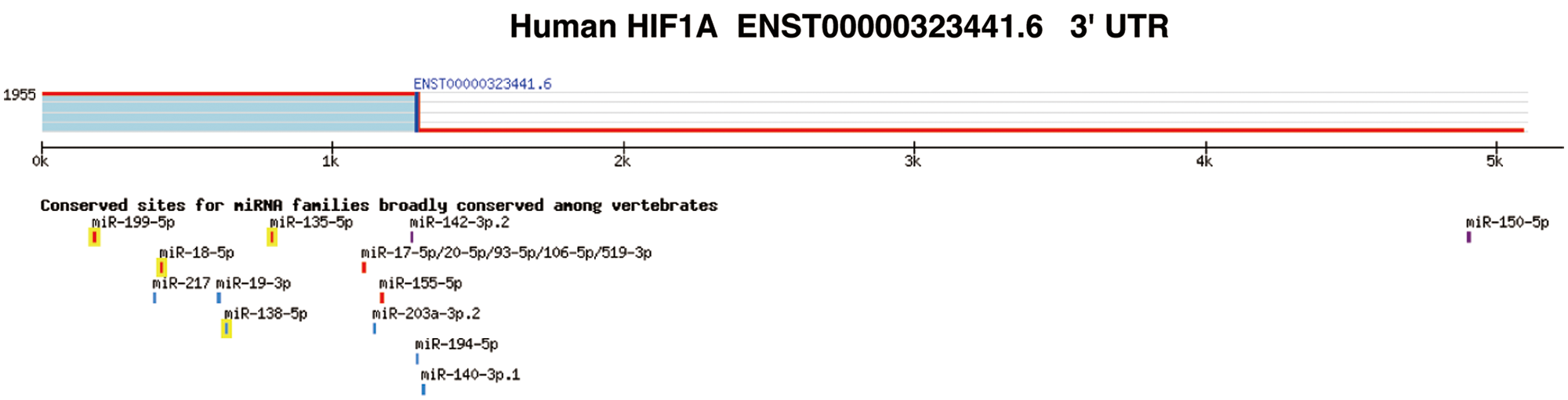
Supplementary figure S2: Targetscan analysis revealed that HIF-1α can be the target gene of multiple miRNAs.

Supplementary figure S3: Analysis of Starbase suggested that miR-93 can be recruited by HOTAIR.
Supplementary Table S1. The primers of RT-qPCR.
Supplementary Table S2. RT-qPCR of co-immunoprecipitated target genomic DNA fragments were performed by mct4 promoter-specific primers.
Supplementary Table S3. The primers of RT-PCR in RNA pull-down assay.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |