DOI:10.32604/biocell.2021.016193

| BIOCELL DOI:10.32604/biocell.2021.016193 |  |
| Article |
Biosynthesis of raw starch degrading β-cyclodextrin glycosyltransferase by immobilized cells of Bacillus licheniformis using potato wastewater
1Department of Biology, College of Science, King Khalid University, Abha, 9004, Saudi Arabia
2Prince Sultan Bin Abdulaziz Center for Environmental and Tourism Research and Studies, King Khalid University, Abha, 9004, Saudi Arabia
3Environmental Biotechnology Department, Genetic Engineering and Biotechnology Research Institute, City of Scientific Research & Technological Applications, New Borg El-Arab City, 21934, Egypt
4Department of Botany and Microbiology, Faculty of Science, Assiut University, Assiut, 71515, Egypt
5Department of Botany and Microbiology, Faculty of Science, South Valley University, Qena, 83523, Egypt
6Department of Microbiology and Immunology, Faculty of Pharmacy, October University for Modern Science and Art, Giza, 12451, Egypt
*Address correspondence to: Yasser S. Mostafa, ysolhasa1969@hotmail.com
Received: 16 February 2021; Accepted: 23 April 2021
Abstract: The study was sought to enhance the synthesis of thermal stable β-cyclodextrin glycosyltransferase (β-CGTase) using potato wastewater as a low-cost medium and assess the degree to which it is efficient for industrial production of β-cyclodextrin (β-CD) from raw potato starch. Thermophilic bacteria producing β-CGTase was isolated from Saudi Arabia and the promising strain was identified as Bacillus licheniformis using phylogenetic analysis of the 16S rRNA gene. Alginate-encapsulated cultures exhibited twice-fold of β-CGTase production more than free cells. Scanning electron microscopy (SEM) of polymeric capsules indicated the potential for a longer shelf-life, which promotes the restoration of activity in bacterial cells across semi-continuous fermentation of β-CGTase production for 252 h. The optimal conditions for β-CGTase synthesis using potato wastewater medium were at 36 h, pH of 8.0, and 50°C with 0.4% potato starch and 0.6% yeast extract as carbon and nitrogen sources, respectively. The purified enzyme showed a specific activity of 63.90 U/mg with a molecular weight of ∼84.6 kDa as determined by SDS-PAGE analysis. The high enzyme activity was observed up to 60°C, and complete stability was achieved at 75°C. High levels of activity and stability were shown at pH 8.0, and the pH range from 7.0–10.0, respectively. The enzyme has an appreciable affinity for raw potato starch with a Km of 5.7 × 10−6 M and a Vmax of 87.71 µmoL/mL/min. β-CD production was effective against 25 U/g of raw potato starch. The outcomes demonstrated its feasibility to develop a fermentation process by integrating the cost-effective production of β-CGTase having distinctive properties for β-CD production with eco-friendly utilization of potato wastewater.
Keywords: Potato wastewater; Bacillus licheniformis; β-CGTase; 16S rDNA gene; Semi-continuous fermentation; β-cyclodextrin
Cyclodextrins (CDs) are cyclic molecules synthesized by the intramolecular transglycosylation reaction of microbial cyclodextrin glucanotransferase (CGTase) on starch forming a hydrophilic external surface and a hydrophobic internal cavity (Rakmai and Cheirsilp, 2016). The prominent group of glycoside hydrolase is the family 13 (GH13) that catalyzes the conversion of starch to malto-oligosaccharides (Stam et al., 2006). The CGTases (EC 2.4.1.19) and α-amylases (EC 3.2.1.1.) are members of the GH13 family. Dependent on their protein sequence in the Carbohydrate-Active Enzyme (CAZy) database, GH13 family is divided into 43 subfamilies (Lombard et al., 2014). The CGTases are the main members of the subfamily GH13_2, while α-amylases (3.2.1.1.) are members of other subfamilies.
Because CDs are shaped like rings, they can contain guest particles and produce inclusion complexes with both inorganic and organic compounds.
As a result, their applications are widespread in various industries; pharmaceutical, food, cosmetics, chemical, agriculture, emulsifiers, nanotechnology, and biomedical (Rakmai and Cheirsilp, 2016). The CGTase acts explicitly on α-glycosidic bonds in starch to produce various types of CDs through transglycosylation reaction relying upon the enzyme sources (Amiri et al., 2015). The CGTase cleaves α-1,4 glycosidic bond to 6, 7, or 8 glucose units of the non-reducing end of starch. The formed reducing end glucose was then transferred to the hydroxyl group of the non-reducing end glucose unit of the same chain of glucose residue to form a cyclic compound of α-, β- or γ- CDs with 6, 7, or 8 glucose units, respectively (Janeˇcek et al., 2019). Various industries are interested in the use of β-CGTase due to its ability to produce large quantities of β-CD from starch as well as the preparation of inclusion complexes that can straightforwardly take place, and their low solubility offers genuine steadiness (Wang et al., 2018).
The highly β-CGTase production can take place using specific bacteria, including Bacillus spp. (B. macerans, B. megaterium, B. circulans, B. stearothermophilus, B. ohbensis), Klebsiella oxytoca, Micrococcus luteus, and Thermococcus sp. (Martins and Hatti-Kaul, 2002; Rajput et al., 2016b). The main challenge for β-CD production is a commercial one, which stems from the high cost of enzymes used, and physical treatments for starchy substrates, and the feedback inhibition associated with the end products (Moriwaki et al., 2007). Subsequently, the search for thermophilic bacteria that produce high amounts of the enzyme has been driven by the industrial demand for thermostable β-CGTase (Wang et al., 2017).
When fermentation processes are designed, it is possible to use immobilized bacterial cells several times with a reduced impact of inhibitory compounds and nutrient exhaustion. Also, the dense cell culture, and straightforward nature of product separation, which gives rise to an efficient bioprocess (Eş et al., 2016; Ravinder et al., 2012a; Ravinder et al., 2012b). For almost all β-CGTase, it is only possible to convert gelatinized starch to β-cyclodextrin, and the physical treatments have been employed to improve the efficacy of the enzyme. β-CGTase from some microbial sources has proven viable to degrade raw starch, thus avoiding physical treatment and increasing the efficiency of CD production (Gawande and Patkar, 2001; Rajput et al., 2016a). The considerable promise is associated with the use of starchy residues in fermentation procedures to improve the enzyme production cost and productivity, as well as a notable rise in environmental issue (Coelho et al., 2016).
Potato wastewater is one of the most prevalent starch-rich by-products of potato industrial processes (Guo et al., 2018). It is an obvious candidate for use in the production of high-value components given its presence of inorganic and organic compounds, e.g., proteins, starch, ammonium, and phosphate (Yuliani et al., 2011). The large quantities of potato wastewater are produced from the potato-chips factories in Saudi Arabia that can be used for the fermentation process such as lactic acid (Huang et al., 2003), bio-flocculants (Guo et al., 2018), and activated sludge system (Azab, 2008).
With these issues in mind, the purpose of the present study was to isolate thermophilic bacteria that produce β-CGTase and to develop a fermentation process employing potato wastewater in a semi-continuous fermentation process. The properties of β-CGTase and their activity concerning gelatinized and raw starch were examined.
Soil samples were collected from two unexplored areas of the southern region, Saudi Arabia: Abha city (18°13’1”N 42°30’19”E, 21°C), and Jazan city (16°53’21”N 42°33’40”E, 44°C). The potato wastewater was collected after the peeling, slicing, and washing process of potato from the Saudi Snack Foods Company, Jeddah, KSA. The same batch of wastewater was used to carry out the experiments. The chemical analysis of potato wastewater was performed for dry matter, nitrogen, starch, reducing sugars, and total ash according to Baird et al. (2017) and Evers et al. (1984).
Isolation and screening of β-CGTase-producing bacteria
A 1.0-g soil sample underwent serial dilution (10−1–10−5), 0.1 mL of which was spread onto the agar plates medium containing soluble starch (2%), peptone (0.5%), yeast extract (0.5%), MgSO4.7H2O (0.02%), K2HPO4 (0.1%), phenolphthalein (0.03%), methyl orange (0.01%), and agar (1.5%) at pH 7.5 (Salva et al., 1997). The plates were incubated at 50°C for 48 h. The potential colonies surrounded by the clear zone in the rapid plate assay method were selected for further investigation based on enzymatic activity index, a ratio of the zone of clearance diameter to colony diameter (More et al., 2012).
Molecular identification and phylogenetic tree construction
The growth of the promising bacterial isolate took place for 24 h in a nutrient broth medium, after which centrifuging was undertaken for 20 min at 6573 × g (Hermle, Z 326 K, Germany). To facilitate genomic DNA extraction, QIAamp DNA Mini kit (Qiagen Inc., Valencia, CA) was employed (Neilan, 1995).
The PCR amplification of 16S rRNA was performed by PCR Master Mix kit (TAKARA, Japan) using the universal primers 27 F (5’-CCA GCA GCC GCG GTA ATA CG-3’) and 1492 R (5’-ATC GG(C/T) TAC CTT GTT ACG ACT TC-3’). The PCR components were incubated at 94°C for 10 min, followed by 35 cycles of 94°C for 1 min, 55°C for 30 s, and 72°C for 54 s, followed by a final extension step at 72°C for 10 min. Using 1.5% agarose gel in a 1 × TBE running buffer with an electrophoresis unit, the migration of the PCR product was observed. The confirmation of the presence of the PCR product was facilitated after 30 min and shot-utilizing gel documentation was undertaken (BIORAD, USA). The 16S rRNA gene PCR product was sequenced by Macrogen, Korea. The obtained sequence was aligned and contrasted with the genes saved in GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi). In turn, accommodation in GenBank was facilitated using an explicit accession number. A phylogenetic tree was constructed with MEGA v. 5.1 (Abdel-Razek et al., 2020). The phylogenetic relationship among the sequenced gene and other related genes in the GenBank database was constructed depending on the neighbor-joining method and based on the Jukes-Cantor distances of the used software.
Immobilization of bacterial cell using sodium alginate
The bacterial cell immobilization was carried out according to Ravinder et al. (2012a). The bacterial cells of the free-cell inoculum were centrifuged at 6573 × g for 20 min, and mixed with 10.0 mL 2% (w/v) sodium alginate, then stirred for 10 min. This enabled the achievement of a consistent texture. The drop-wise extrusion of the mixture then took place using 2 mL syringe into 25 mL of 3.5% (w/v) CaCl2 solution. The hardening of the alginate drops was observed after contacting the solution, thereby leading to the formation of capsules that entrapped the bacterial cells. The solidification of the capsules occurred for 30 min, after which excess Ca2+ and cells were eliminated through washing in a sterile saline solution several times. The capsules were preserved in a refrigerator using CaCl2 solution. The immobilization process was carried out in an aseptic manner in the laminar flow unit.
The free cells inoculum was prepared in nutrient broth medium at 50°C, 200 rpm, for 24 h. Erlenmeyer flasks (250 mL) containing 50 mL of potato wastewater medium (pH 7.5) were inoculated with 5.0 mL (1 × 108 CFU/mL) to produce the β-CGTase by free cell cultures. This amount of inoculum was equal to that used in immobilized cultures (4.0 g alginate gel capsules). The production of β-CGTase by free and immobilized cultures were performed at 50°C, 200 rpm for 48 h. Cultures were centrifuged (6573 × g for 20 min) and the supernatant was used as the enzyme source. The biomass of free and immobilized cells was determined as cell dry weight at 70°C (Costa et al., 2015). The optimization of β-CGTase production was carried out by changing single factors, including time (12–96 h), initial pH (6.0–10), temperature (35–65°C). Different potato starch concentrations (0.1–0.8%) were added to potato wastewater. The control was the native potato starch in potato wastewater. Also, various nitrogen sources (yeast extract, peptone, ammonium sulfate, ammonium nitrate, ammonium chloride, sodium nitrate, and potassium nitrate) were added to the potato wastewater medium to fortify the medium for β-CGTase production in the same nitrogen concentration of 0.06% (w/v).
Scanning electron microscopy (SEM)
In line with Costa et al.’s description (Costa et al., 2015), energy-dispersive analysis (Joel Jsm 6360 LA, Japan) was used to characterize the highly amplified morphological appearance of the alginate capsules and the immobilized bacterial cells. After solidification of the prepared capsules, some plain and immobilized capsules were divided into two hemispheres using sharp razor. Both whole and divided spheres were kept at room temperature for 24 h until dryness. The capsules were then submitted for the coating process followed by photographing using scanning electron microscope with multiple magnifications.
The fermentation process was executed under optimal conditions for 10 cycles to achieve semi-continuous fermentation. The recovery of the production medium was undertaken once each cycle had finished. The curing of the immobilized cells occurred with 2% (w/v) CaCl2 following the end of every cycle, after which a new production medium was inserted to continue the fermentation procedure (Abdel-Naby et al., 2011).
Purification of β-CGTase by gel-filtration chromatography
The enzyme protein of the cell-free extract was precipitated overnight by 70% (NH4)2SO4. The precipitated mixture after centrifugation (8586 × g for 20 min) was resolved in 0.1 M phosphate buffer and then dialyzed at 4°C. The dialyzed fraction was loaded onto a Sepharose 6B column and the fractions were collected and estimated for protein and enzyme activity (Laszlo et al., 1981). The molecular weight of the purified enzyme was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 15% SDS polyacrylamide gel and run at 150 V for 3.0 h at 25°C (Laemmli, 1970; Guru et al., 2012). The extracted protein was stained with Coomassie Blue, and the gel was treated by de-staining solution to remove unbound dye and was imaged by a Gel Documentation System (BIORAD, USA).
Kinetics properties of β-CGTase
The effect of pH and temperature on β-CGTase activity was investigated under several pH and temperature conditions (from pH 4.0 to 12.0 and 40–90°C, respectively). The following buffers were used: 50 mM sodium acetate (pH 4.0–6.0); 50 mM Tris-HCl (pH 7.0–8.0); 50 mM glycine-NaOH buffer (pH 9.0–10.0); 50 mM carbonate-bicarbonate buffer (pH 11.0–12.0). An assessment of the thermo-stability of β-CGTase occurred through the pre-incubation of the enzyme in 50 mM Tris-HCl (pH 8.0) at 40–90°C for 60 min. Additionally, pH stability was evaluated through the incubation of the enzyme in various buffers for 60 min. The relative activity of β-CGTase (%) was expressed as the ratio of the enzyme activity at different parameters (pH, temperature) to the activity of the control sample under standard assay conditions. The kinetic parameters of β-CGTase (Km and Vmax) were determined using potato starch as a substrate at concentrations ranging from 0.0 to 0.1 M. The Michaelis–Menten parameters were determined from Lineaweaver-Burk plots using the equation derived from linear regression analysis of the curve using Sigma Plot ver. 14.0 (Mora et al., 2012).
The production of β-CD was studied under various β-CGTase concentrations with gelatinized and raw potato starch as the substrates. The gelatinized potato starch was prepared by heating 10 mL starch (20 g/L) in 50 mM phosphate buffer (pH 8.0) in a water bath for 10 min. The reaction mixture of 100 g/L substrate in 10 mL phosphate buffer (50 mM, pH 8.0) and 100 μL of different enzyme concentrations were incubated for 1 h at 60°C. The samples were inactivated by boiling for 5 min, then centrifuged at 9693 × g for 10 min. The β-CD was assessed with the phenolphthalein method (Kaneko et al., 1987) based on the standard curve of 0–0.8 g/L β-CD. 0.5 mL of 0.02% (w/v) phenolphthalein in 0.005 M Na2CO3 was added. The absorbance was measured using a double beam UV/Vis scanning spectrophotometer (Model: Shimadzu, 1601PC) at 550 nm.
The phenolphthalein method was employed to estimate β-CGTase activity (Kaneko et al., 1987). The reaction mixture containing 1.0 mL of 40 mg soluble potato starch in 0.1 M phosphate buffer (pH 8.0), and 0.1 mL enzyme was incubated at 50°C for 10 min in a water bath. The reaction was stopped by adding 3.5 mL of 0.03 M NaOH solution. A 0.5 mL of 0.02% (w/v) phenolphthalein in 0.005 M Na2CO3 was added to the reaction mixture. The decrease in color intensity was assessed after 15 min using a spectrophotometer at 550 nm after 15 min. The unit of enzyme activity was defined as the rate of the enzyme catalyzing the production of l.0 µmoL of β-CD/min under the reaction conditions. The protein concentration was estimated using Folin-Ciocalteu reagent (Lowry et al., 1951). All experiments were performed in triplicate.
Data were first investigated for their normal distribution of errors with Shapiro-Wilk’s W test, and Levene’s test was employed to facilitate an investigation of the homogeneity of variances. One-way analysis of variance (ANOVA) was then used to examine the significance of variation. To examine significant differences between the means of the treatments, the least significant difference (LSD) was employed, where P < 0.05 was used as the cut-off for statistical significance.
Isolation and screening of thermophilic β-CGTase-producing bacteria
Out of 112 thermophilic bacterial isolates were isolated from the soil samples of the two regions: Jazan and Abha, Saudi Arabia. All purified bacterial isolates were screened for β-CGTase production by rapid plate assay method. Forty-three of β-CGTase-producing bacterial isolates (38.39%) were detected by a clearance zone around the colonies. The highest number of β-CGTase-producing bacteria was isolated from the Jazan region (N = 32), and the lowest isolates were from Abha region (N = 11). Only ten promising isolates showed a high clear zone around the colony in rapid plate assay reached 1.8 cm for promising bacterial isolate, JA-16 (Fig. 1A). The quantitative screening of ten potentially effective isolates for β-CGTase production was undertaken with submerged fermentation. The large spectrum of enzyme production ability was detected by the tested isolates (Fig. 1B). The optimum β-CGTase production (9.6 U/mL) was obtained by bacterial isolate, JA-16 strain isolated from the Jazan region.
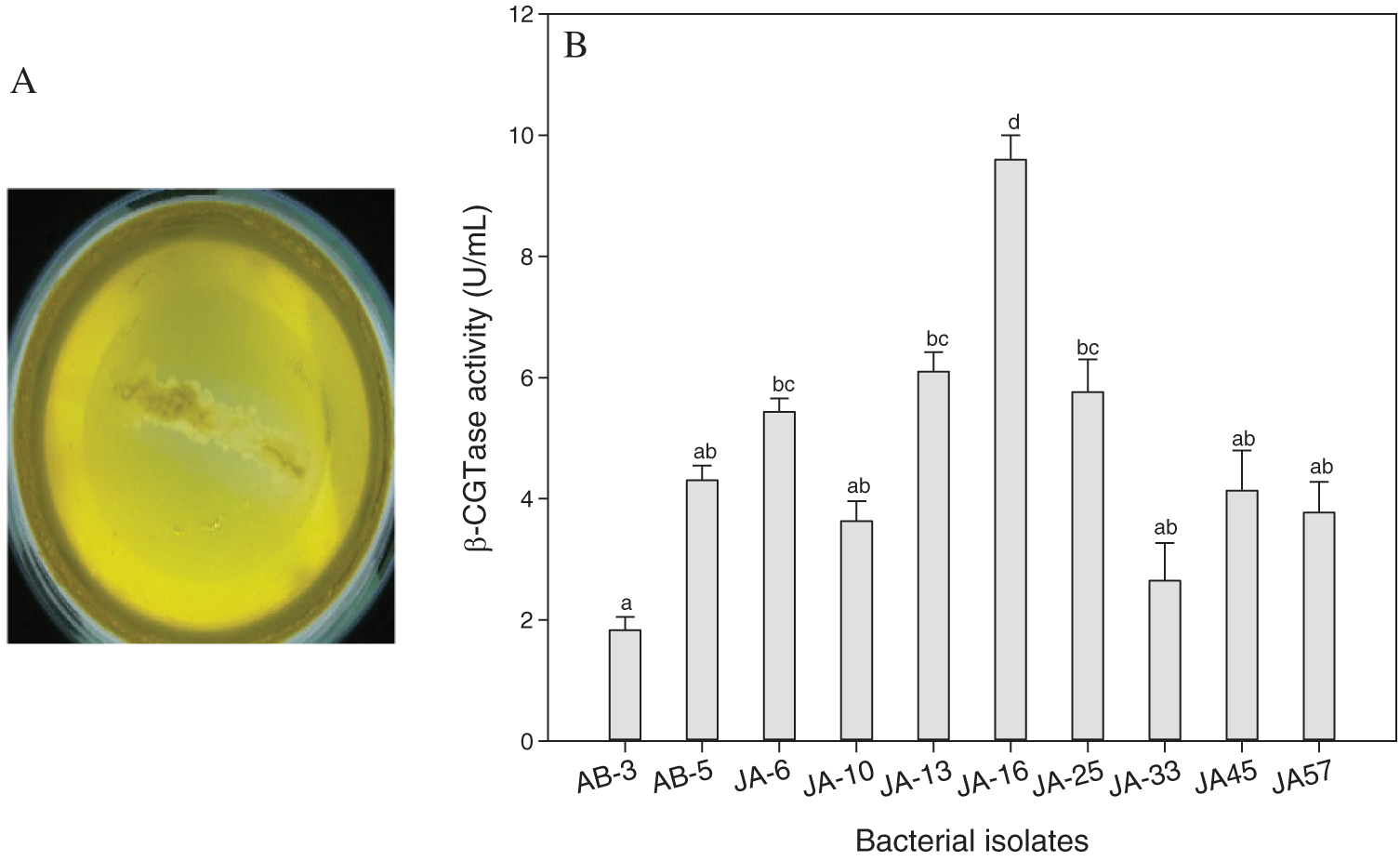
Figure 1: Rapid plate assay for promising bacterial isolate, JA-16 for β-CGTase production (A), and quantitative screening of promising bacterial isolates for β-CGTase production at pH 7.5, 50°C, 200 rpm, and 48 h (B).
Vertical bars indicate the standard errors of the means. Means followed by different letters are significantly different at P < 0.05. Bacterial isolates were collected from Abha (AB), Bacterial isolates were collected from Jazan (JA).
Molecular identification of potential β-CGTase-producing bacterial isolate
A 99% of the promising isolate, JA-16 was comparable to Bacillus licheniformis, as revealed by the sequencing and amplification of the 16S rRNA gene. The isolate’s sequence was submitted to GenBank with an explicit accession number (MK693006.1). The isolate’s phylogenetic relationship to the other species was identified, and contemporary taxonomy for distinguishing between rarely seen bacterial strains relied on the use of molecular techniques (foremost among which was the examination of the 16S rRNA gene (Fig. 2).
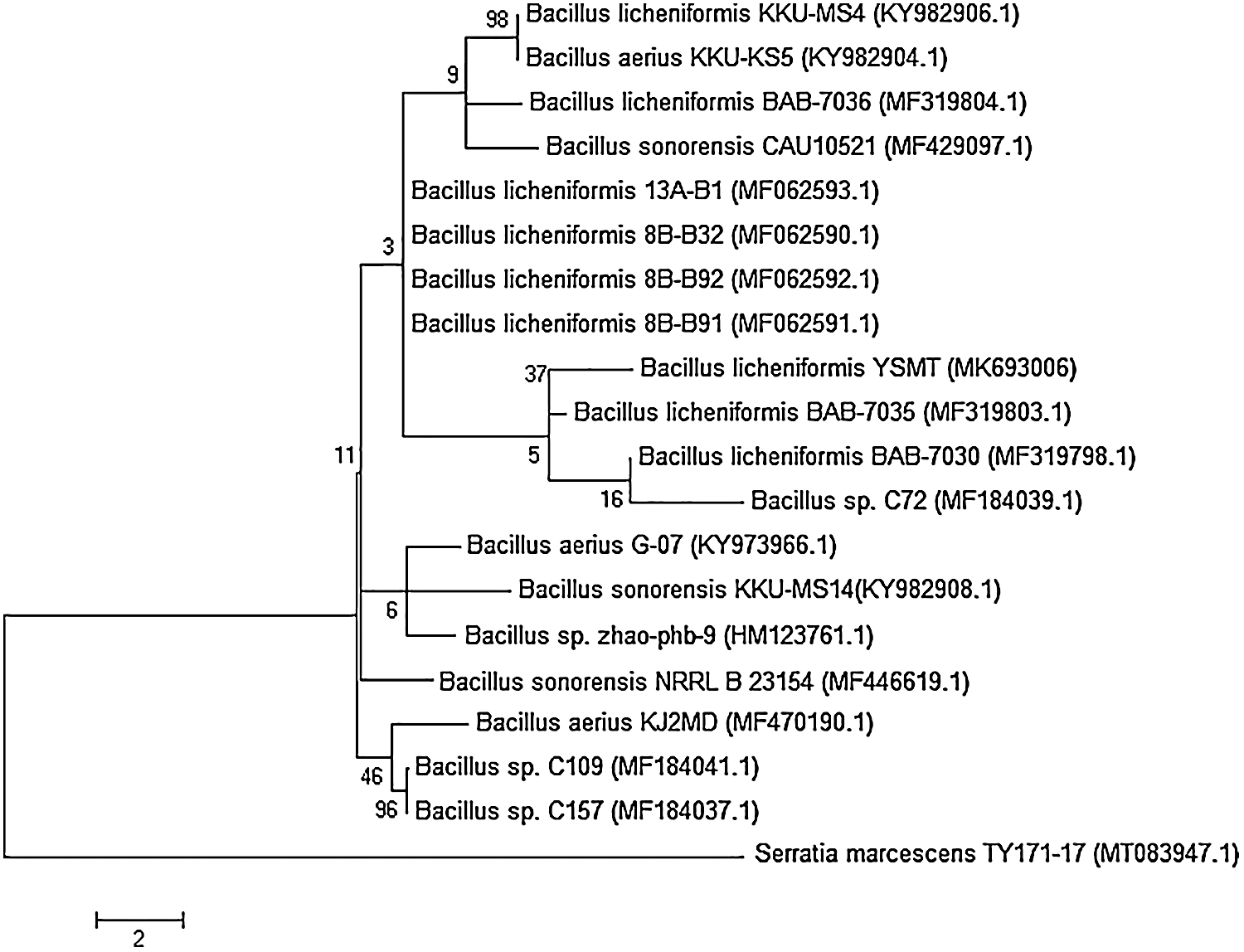
Figure 2: Phylogenetic relationship of Bacillus licheniformis YSMT and other related 16S rRNA gene sequences of published strains generated by the Neighbour-Joining method using MEGA 5.1 software.
The bootstrap values (%) at the nodes. The accession number in GenBank put in parentheses.
Effect of incubation time on β-CGTase production with free and immobilized cells
The immobilized cells of Bacillus licheniformis were more effective for β-CGTase production that gave about two-fold higher than the free cells (Fig. 3). A significant relationship was observed between β-CGTase production and bacterial growth, where the highest level of enzyme production occurred during the late exponential phase and, after this, a reduction in bacterial growth took place. A high rate of cell growth was observed in the primary 12 h of free cell cultures, and once 48 h had elapsed, a cell concentration of 3.6 mg/mL was associated with optimal enzyme production (9.6 U/mL). On the other hand, the rapid increase in biomass contained in gel capsules occurred over 36 h, thereby reaching the highest level of growth, and enzyme production reached to 4.5 mg/mL and 17.2 U/mL, respectively (Fig. 3). The subsequent fermentation experiments were performed by immobilized cells of Bacillus licheniformis.
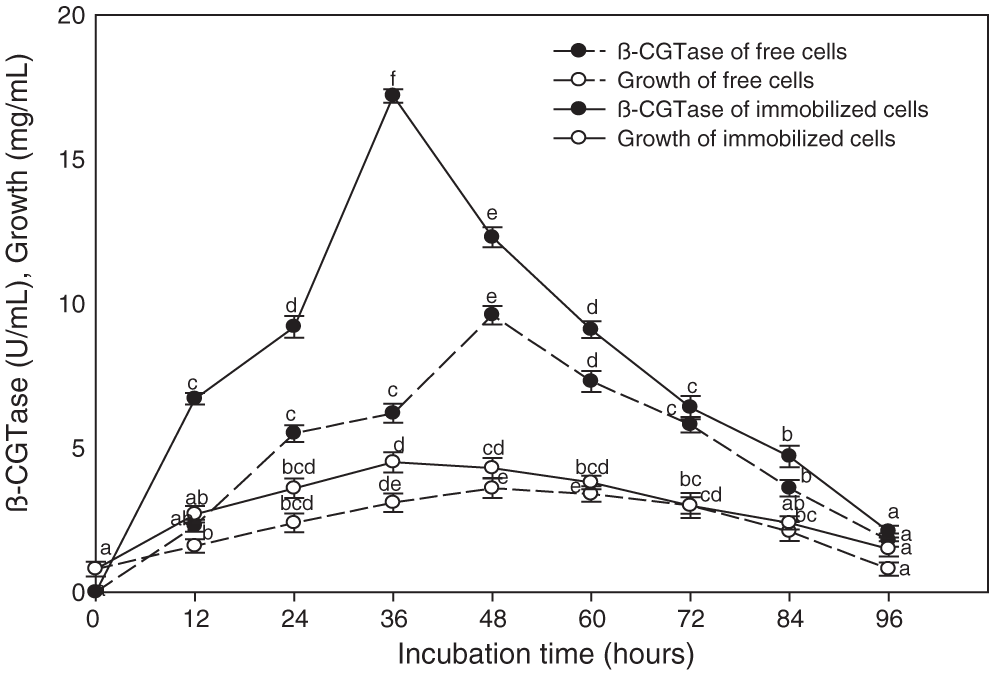
Figure 3: Time course of β-CGTase production using free and immobilized cells of Bacillus licheniformis YSMT at pH 7.5, 50°C, and 200 rpm.
Mean ± SEM (N = 3) was presented. Vertical bars indicate the standard errors of the means. Means followed by different letters are significantly different at P < 0.05.
Scanning electron microscopy (SEM)
SEM was used to examine the internal and external appearances of the cells immobilized in alginate capsules. The dried alginate capsule displayed a solid, elongated structure while, under standard conditions, the alginate capsules were consistently round and hollow in terms of their structure (Fig. 4A). The alginate gel was encapsulated and safeguarded the bacterial cells that enabled nutrients from the outside to enter and exit the capsules without restriction. Furthermore, the encapsulated bacterial cells were specified as intact bacilli shapes that appeared not to have sustained damage, thereby suggesting that alginate capsules had been safeguarded from extreme environmental conditions (Fig. 4B).
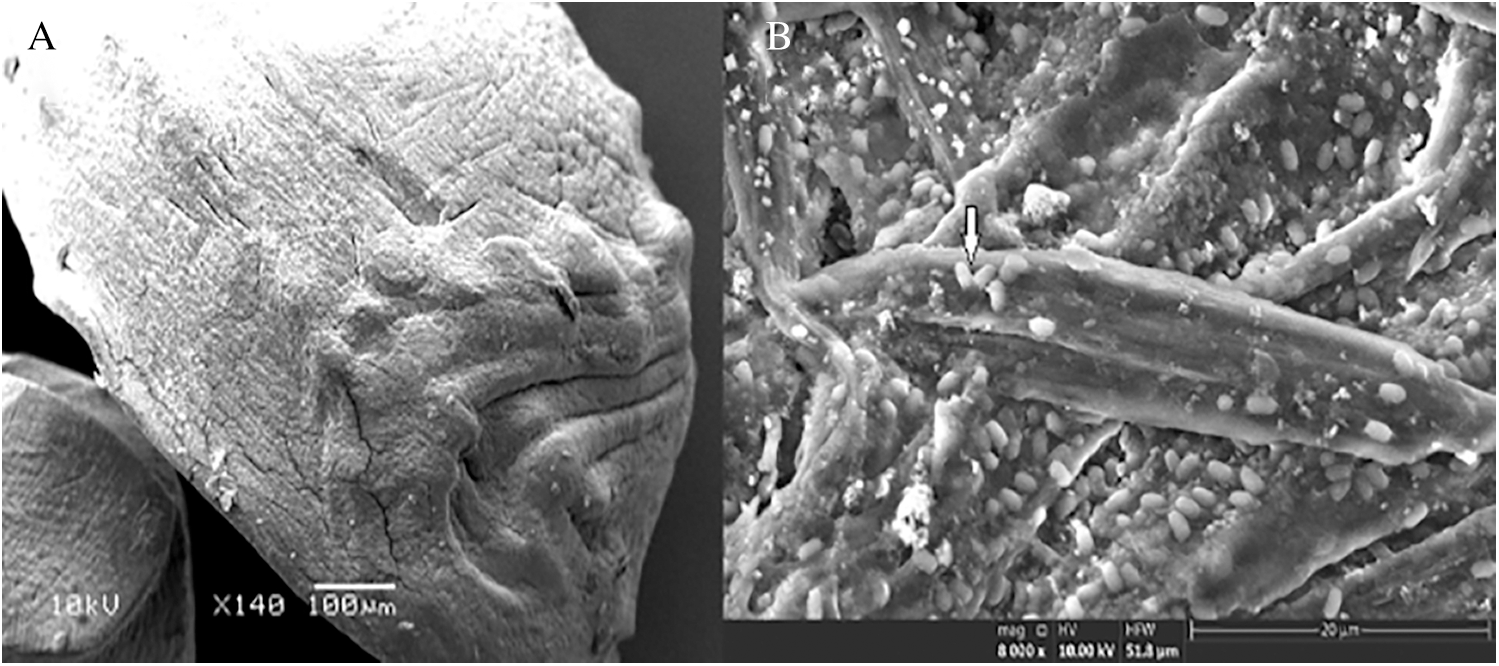
Figure 4: SEM of alginate capsules externally (A), and internally (B).
Effect of initial pH and incubation temperature on β-CGTase
The highest level of β-CGTase production was observed at an initial pH of 8.0 reaching 25.40 U/mL (Fig. 5A). The reduction in enzyme activity in the alkaline conditions was not high compared to acidic conditions. The decrease of 44.09% in β-CGTase production was observed at pH 9.0, whereas a greater reduction of 91.33% was related to pH 6.0. In respect to the effect of the incubation temperature, a positive correlation was observed between temperature and β-CGTase production up to 50°C (Fig. 5B). The incubation temperature changes on both sides of the optimal value resulted in a decrease in β-CGTase production. The rapid reduction in β-CGTase production was noticed at temperature conditions that exceeded 60°C.
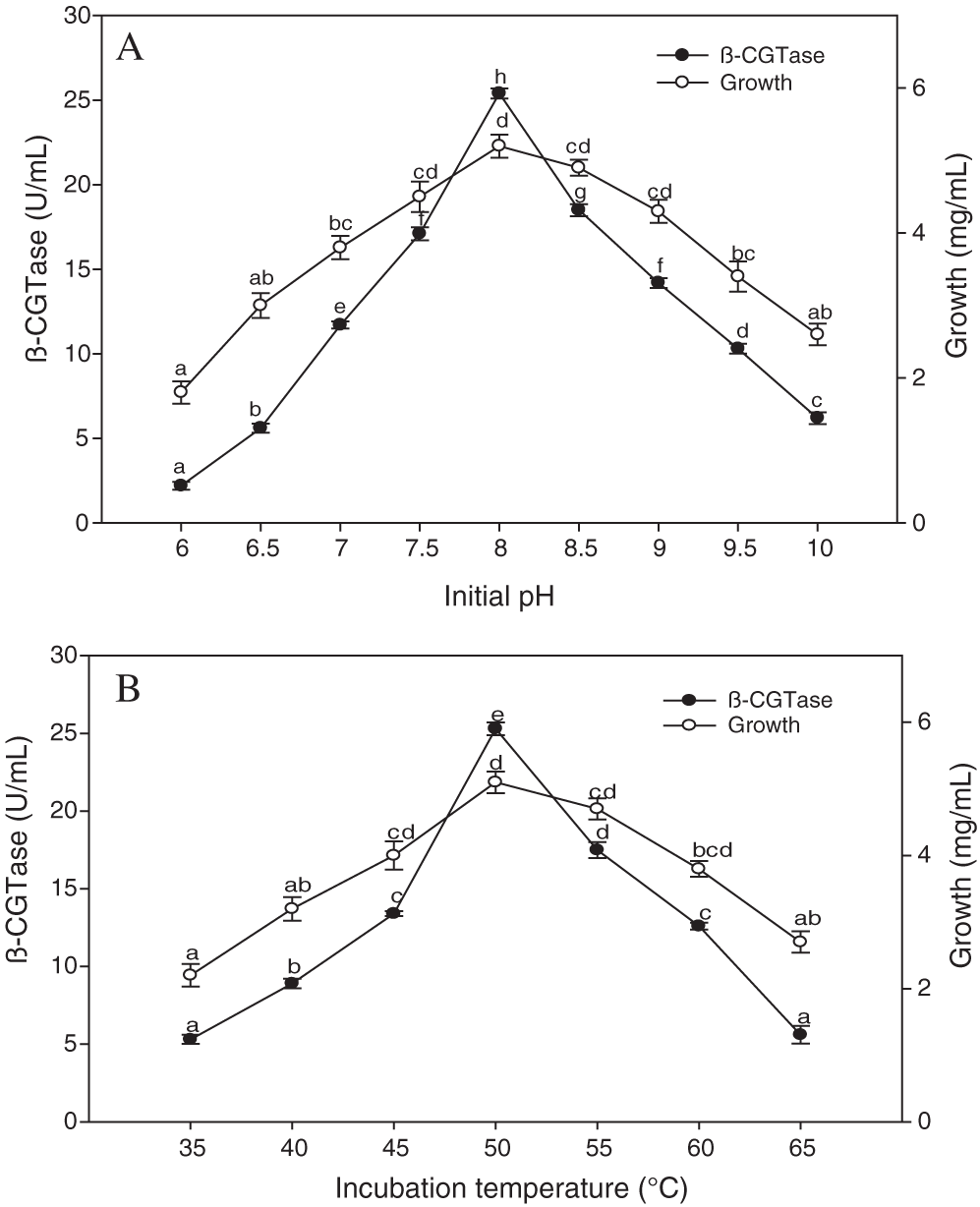
Figure 5: Effect of initial pH on β-CGTase production at 50°C, 200 rpm, and 36 h (A), the effect of incubation temperature on β-CGTase production at pH 8.0, 200 rpm, and 36 h (B).
Mean ± SEM (N = 3) was presented. Vertical bars indicate the standard errors of the means. Means followed by different letters are significantly different at P < 0.05.
Effect of potato starch concentration on β-CGTase production
The chemical analysis of potato wastewater showed beneficial compounds for the fermentation process, including dry matter (2.87%), nitrogen (0.33%), starch (1.94%), reducing sugars (0.1%), and total ash (0.29%). Various potato starch concentrations were added to potato wastewater to elucidate the best concentration for maximum bacterial growth and β-CGTase production. The control is the native potato starch in potato wastewater. The results showed that adding 0.4% of potato starch to potato wastewater medium gave the highest β-CGTase production (Fig. 6). Further increasing the amount of starch was unfavorable to produce β-CGTase.
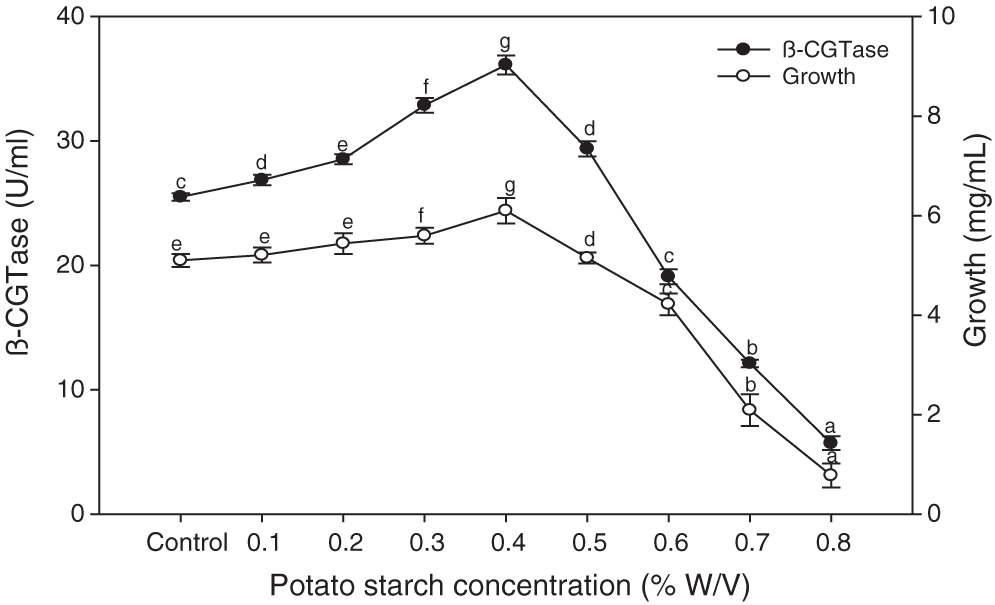
Figure 6: Effect of potato starch concentration on β-CGTase production at pH 8.0, 50°C, 200 rpm, and 36 h.
Mean ± SEM (N = 3) was presented. Vertical bars indicate the standard errors of the means. Means followed by different letters are significantly different at P < 0.05. (Control is the potato wastewater without additions).
Effect of nitrogen sources on β-CGTase production
Different organic and inorganic nitrogen sources were added to the potato wastewater medium in the same nitrogen concentration to support the enzyme production. The results refer to that organic nitrogen sources promoted β-CGTase production and bacterial growth than inorganic sources (Fig. 7A). The high bacterial performances for both growth and β-CGTase production were noticed using yeast extract as the optimum nitrogen source with a 17.33% increase compared to the control medium. The inorganic nitrogen sources had a reductive impact on β-CGTase production. The nitrates salts lead to a decrease in β-CGTase production (47.13% to 70.26%), while ammonium salts led to a reduction from 28.01% to 54.21%. Furthermore, the effect of different yeast extract concentrations, as an ideal nitrogen source on β-CGTase production was evaluated. The high enzyme production was obtained by adding 0.6% yeast extract (Fig. 7B).
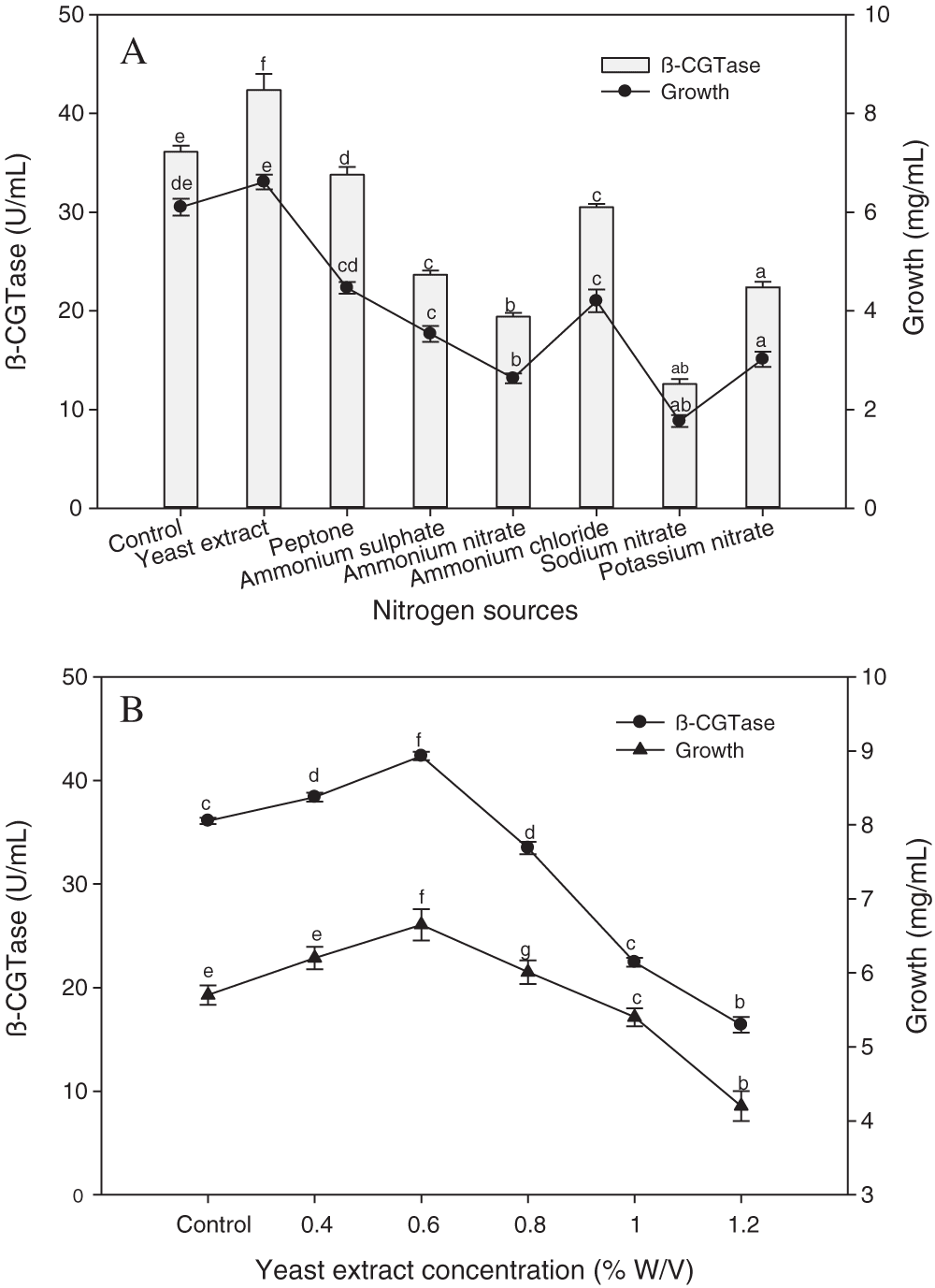
Figure 7: Effect of nitrogen sources on β-CGTase production at pH 8.0, 50°C, 200 rpm, 36 h, and 4.0% (w/v) potato starch (A), effect of yeast extract concentration at pH 8.0, 50°C, 200 rpm, 36 h and 4.0% (w/v) potato starch (B).
Mean ± SEM (N = 3) was presented. Vertical bars indicate the standard errors of the means. Means followed by different letters are significantly different at P < 0.05. (Potato wastewater without additions of nitrogen source was used as control).
Semi-continuous β-CGTase production
In repeated batch cultures, the immobilized cells were transferred from the fermented medium into a sterile medium once a 36-hour period had elapsed, and the process was carried out for ten cycles. The culture activity was attained for β-CGTase production until the seventh cycle since enzyme production remained between 40.10–50.60 U/mL over the entire optimum period of operation (Fig. 8). The β-CGTase production remained stable in a semi-continuous fermentation over a period of 252 h, then the enzyme production decreased extraordinarily. The positive relationship with the biomass content of the beads and β-CGTase production was observed when biomass concentration in the beads increased with the first few batches which remained almost steady for the subsequent batches.
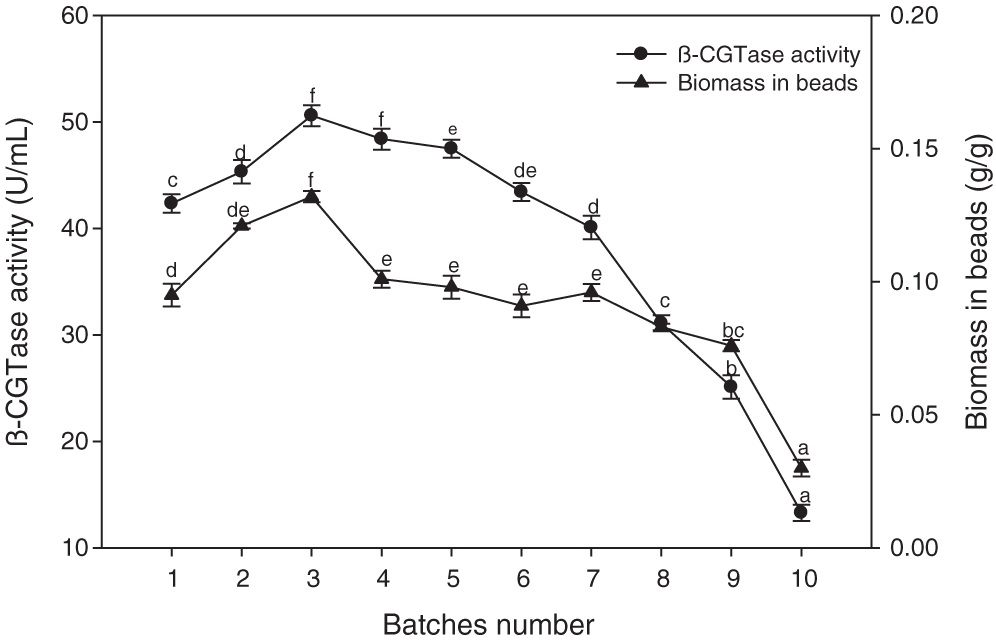
Figure 8: Semi-continuous β-CGTase production by repeated batch culture for 10 batches.
Mean ± SEM (N = 3) was presented. Vertical bars indicate the standard errors of the means. Means followed by different letters are significantly different at P < 0.05.
Purification and kinetic properties of β-CGTase
The enzyme was purified by (NH4)2SO4 precipitation, dialysis, and gel filtration chromatography using a Sepharose 6B column. The purification results of each step were as shown in Tab. 1. One portion from 21 fractions showed β-CGTase activity. After purification to homogeneity, the specific activity was 63.90 U/mg and the fold purification and yield were 19.84 and 41.77%, respectively.

The molecular weight of the purified enzyme was determined by SDS-PAGE analysis. The single protein band with a molecular weight of ∼84.4 kDa was detected (Fig. 9).
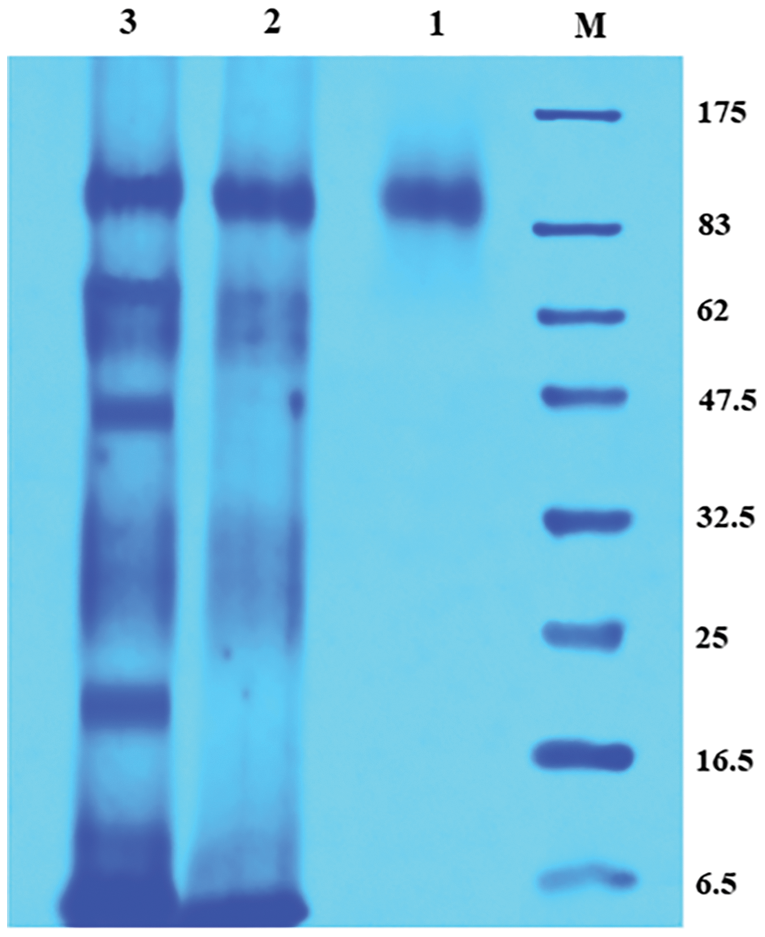
Figure 9: SDS-polyacrylamide gel electrophoresis of the purified enzyme.
M: Protein marker; 1: Sepharose 6B purified enzyme; 2: (NH4)2SO4 precipitated enzyme; 3: Cell-free crude enzyme.
The activity and stability of β-CGTase were studied as an effect of pH and temperature. The enzyme activity was observed in the neutral-to-alkaline pH (7.0 to 11.0), with the highest level at pH 8.0, while 85.8% of the enzyme’s activity was retained until pH 10 (Fig. 10A). A broad range of pH values (7.0 to 11.0) for 1.0 h was associated with maintenance of the enzyme’s stability (91–100%). Under acidic conditions, the stability of the enzyme was lower, with residual activity of 62.16% and 75.16% at pH 5.0 and 6.0, respectively. In respect to the temperature effect on enzyme activity and stability, the temperature from 40°C to 60°C was significant for β-CGTase activity. The highest level of enzyme activity was obtained at 50–55°C, which reduced to 93.4% at 60°C, and fell to 45.83% with an increase in the reaction temperature to 70°C (Fig. 10B). The thermal stability of the enzyme was completely retained in terms of initial activity following thermal treatment to 75°C for 1 h, while at 80°C retained about 93.26% of the activity. The Km and Vmax were estimated using the Michaelis-Menten equation and the Lineweaver-Burk plot. The β-CGTase has a high affinity toward the potato starch, with a lower Km value of 5.7 × 10−6 M with a Vmax of 87.71 μmol/mL/min (Fig. 10C).
β-cyclodextrin production with raw and gelatinized potato starch
The purified β-CGTase was used to produce β-CD using both raw and gelatinous potato starch as the substrate to evaluate the enzyme action. The raw potato starch granules were optimal for β-CD production than gelatinous form. The raw potato starch at an enzyme concentration of 25 U/g achieved 33.3 g/L β-CD while using gelatinous potato starch granules, 17.60 g/L β-CD was obtained at an enzyme concentration of 15 U/g (Fig. 11).
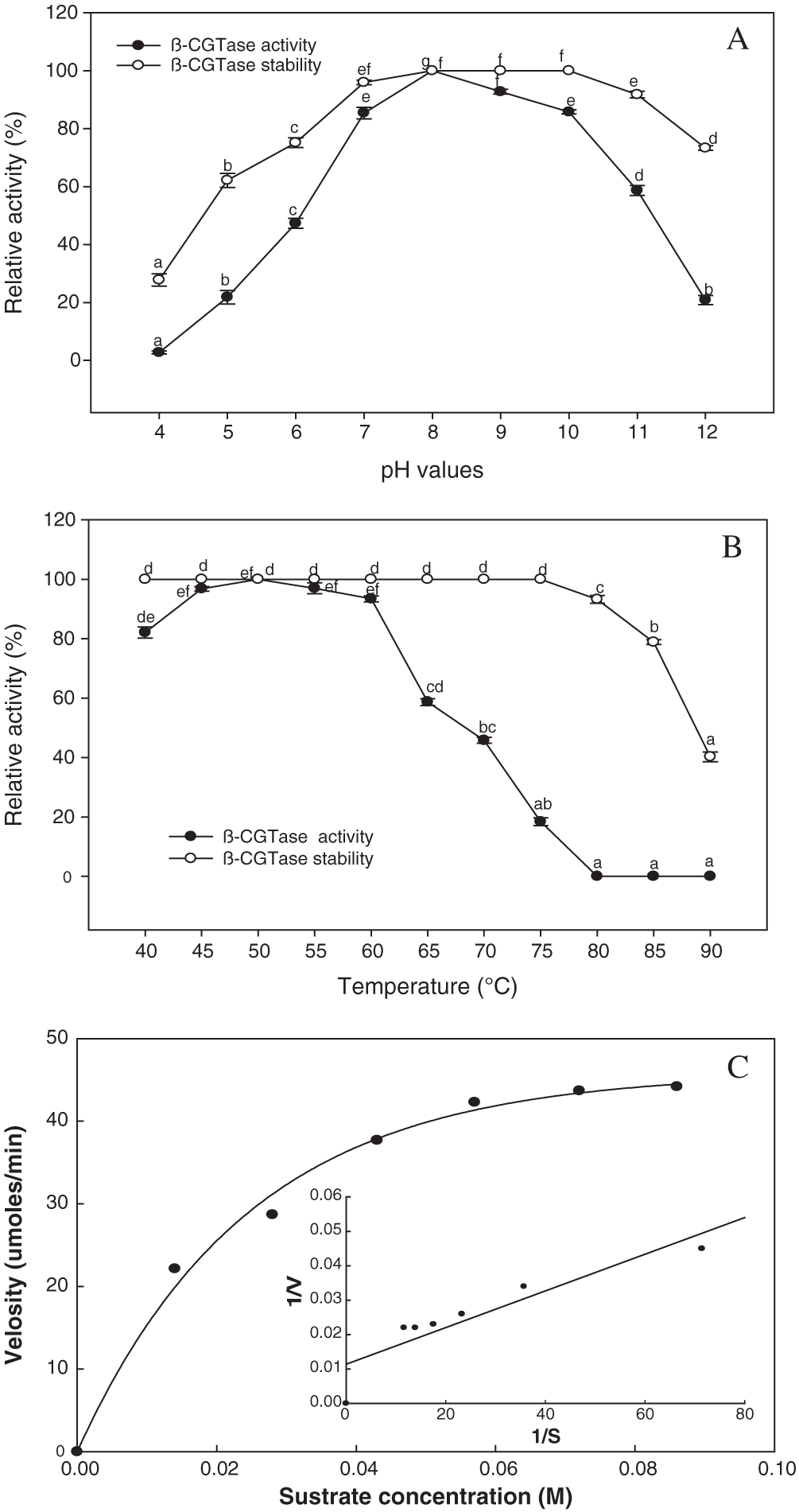
Figure 10: Kinetic parameters of purified β-CGTase.
Effect of pH value from 4.0 to 12 (A) and temperature from 40°C to 90°C (B), on β-CGTase activity and stability. Mean ± SEM (N = 3) was presented. Vertical bars indicate the standard errors of the means. Means followed by different letters are significantly different at P < 0.05. The plot of the reaction velocities (V) vs. substrate concentration fitted to the Michaelis-Menten equation and determination of the Km and Vmax values of the purified enzyme by Lineweaver-Burk plot (C).
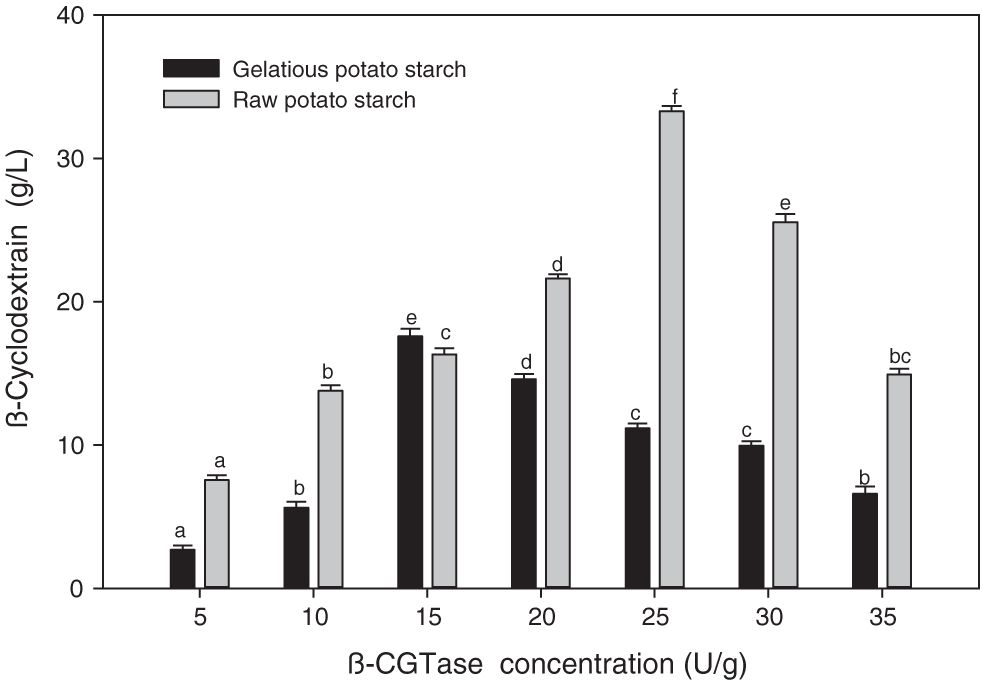
Figure 11: Effect of β-CGTase concentration on β-CD production using raw and gelatinized potato starch.
Mean ± SEM (N = 3) was presented. Vertical bars indicate the standard errors of the means. Means followed by different letters are significantly different at P < 0.05.
The thermophilic β-CGTase -producing bacterial isolates were isolated from soil samples of Abha and Jazan regions, Saudi Arabia. The phenolphthalein inclusion with the production of cyclodextrin due to microbial efficiency in producing β-CGTase was indicted by the observation of a clearance zone around the colonies (Kitcha et al., 2008). The acclimatization of bacterial strains in the hot region is one of the ways to ensure their survival, which are regarded as the optimal areas for their isolation. This may explain the increase in the number of isolates in the Jazan region (44°C) compared to the Abha region (21°C). Despite various research reported the existence of thermophilic bacteria in permanently hot habitats, thermophilic bacteria have been isolated from a range of temperate and cold soil environments worldwide (Marchant et al., 2002). The diversity of these bacteria and their geochemical impact on their environment need more investigation where it may have an effective role in humus hydrolysis accompanied by exothermic reactions (Rahman et al., 2004). The soil properties and environmental conditions can influence the presence of bacterial isolates in a particular area (Bibi et al., 2017). The thermophilic bacteria usually produced distinctive enzymes that operate in the context of extreme conditions, which are analogous to industrial conditions relating to specific processes (Letsididi et al., 2011). Among ten promising isolates examined by submerged fermentation, the JA-16 strain obtained from the Jazan region was associated with the greatest rate of β-CGTase production (9.6 U/mL). This isolate was comparable for β-CGTase production when compared with the strains that have already been documented (Amiri et al., 2015; Costa et al., 2015).
The molecular identification of the promising isolate, JA-16 by the sequencing and amplification of the 16S rRNA gene proved high genetic similarity to Bacillus licheniformis. The widely recognized genetic markers were used due to their appropriate length (1500 bp), which includes steady locals that sign in the planning of amplification primers (Bibi et al., 2017). Various Bacillus strains producing β-CGTase have been identified using molecular basis as Bacillus sp N-227 (Wang et al., 2017); Bacillus cereus NRC7 (Abdel-Naby et al., 2011); Bacillus circulans DF 9R (Costa et al., 2015), and Bacillus firmus (Higuti et al., 2004).
The fermentation procedure for enzyme production has oscillated between immobilized and free cells techniques that play the main role in the high production of β-CGTase. The immobilized cells of Bacillus licheniformis were more active for β-CGTase production in an abbreviated cultivation time (36 h) compared with free cells (48 h), where gave two-fold higher during the late exponential phase. The same results were obtained previously by Eş et al. (2016). The reduction in bacterial growth and enzyme production after the optimum fermentation period was due to the consumption of nutrients (Rajput et al., 2016b). The presence of Bacillus sp. cells within the alginate gel capsules enhanced the efficacy of β-CGTase production reached 68.50 U/mL at 36 h (Ravinder et al., 2012a). The positive change in enzyme synthesis kinetics in the immobilized state can be due to the modifications in macro-environmental conditions and stress conditions (Costa et al., 2015).
Scanning electron microscopy (SEM) for alginate capsules indicates the dried capsule displayed a solid, elongated structure. This can be attributed to the dryness of the air and the coating parameters needed for a valid SEM evaluation. The alginate capsules safeguarded the bacterial cells and enabled nutrients to enter without restriction. The immobilized cells appeared not to have sustained damage, thereby suggesting that alginate capsules had been safeguarded from extreme environmental conditions. The same trend was mentioned in previous studies where SEM has been employed to verify the accuracy and efficient immobilization of Bacillus sp. (Eş et al., 2016), and Bacillus firmus (Pazzetto et al., 2012).
The optimum β-CGTase production was obtained at an initial pH of 8.0 (25.40 U/mL), which was following Bacillus licheniformis BS 7 (Sivakumar and Banu, 2011). However, the optimum pH value for β-CGTase production by Bacillus licheniformis strains were varied from pH 6.0–9.0 (Letsididi et al., 2011). The pH exhibits a potent effect on enzyme production by influence the bioavailability of the trace elements and permeability of nutrients which vary depending on the microbial strain (Coelho et al., 2016). A significant correlation was observed between incubation temperature and β-CGTase production up to 50°C. Based on the bacterial strain, the ideal temperature range for β-CGTase production was varied, at 37°C for Bacillus circulans DF 9R (Costa et al., 2015), and Bacillus licheniformis (Sivakumar and Banu, 2011), while at 60°C for Geobacillus thermoglucosidans (Jia et al., 2018) and 65°C for Thermoanaerobacter kivui (Avci and Dönmez, 2009). The incubation temperature changes other than optimal value resulted in a decrease in β-CGTase production due to the reduced activity of the metabolic enzymes (Eş et al., 2016).
The highest β-CGTase production was achieved by supporting the potato wastewater medium with 0.4% of potato starch. These results may be explained by the result of potato wastewater analysis which found that it had a lot of remaining starch (1.94%). These results were in accordance with those of Azab (2008), Yuliani et al. (2011), and Guo et al. (2018). They reported that the useful compounds for the fermentation process were detected in potato wastewater as organic and inorganic compounds, e.g., proteins, starch, ammonium, and phosphate. Different starch concentrations were reported for high β-CGTase productivity; 1.5% for Bacillus halodurans (More et al., 2012), and Bacillus alkalophilic CGII (Freitas et al., 2004), while at 3.0% the efficient enzyme production was accomplished by Bacillus sp. TPR71H (Ravinder et al., 2012c). Also, Nishida et al. (1997) found that the high β-CGTase production was achieved in the presence of starch, while the β-CGTase gene (cgt) was completely repressed by glucose. On the contrary, Wang et al. (2018) mentioned that β-CGTase synthesis was not dependent on the presence of an explicit inducer which asserts that the metabolic synthesis of β-CGTase varied among microbial strains. The decline of enzyme production with a high concentration of starch could be explained by the medium’s high viscosity which influenced the accessibility of oxygen required in the bacterial growth (Sivakumar and Banu, 2011).
The production of β-CGTase is a process affected strongly by nitrogen because it plays a main role in protein and cell wall component synthesis (Wang et al., 2018). Based on the results, yeast extract promoted β-CGTase production and bacterial growth than inorganic sources. Consistent results have been noted that yeast extract incorporates the basic micronutrients; minerals, vitamins, amino acids, and nitrogen components that are required to initiate bacterial growth and β-CGTase production (Yap et al., 2010). Nevertheless, the current study found that inorganic nitrogen sources, nitrates, and ammonium salts had a reductive impact on β-CGTase production.
Despite comparable results regarding the impact of inorganic nitrogen sources (Amiri et al., 2015), the difference between inorganic and organic nitrogen sources on β-CGTase production was negligible in other reports (Higuti et al., 2004). On the other hand, the enzyme production was optimum with 0.6% yeast extract which attributed to the nature of potato wastewater used as a basic medium contains about 0.33% nitrogen. The results agreed with those of Yuliani et al. (2011)., they found out that the potato wastewater contained about 3.47 g/L total nitrogen and could be exploited for the production of industrial enzymes. Also, the availability of minerals and nitrogen in potato wastewater was essential to promote enzyme production (Huang et al., 2003). The maximum β-CGTase production by Bacillus licheniformis (4.5 U/mL) (Sivakumar and Banu, 2011), Bacillus sp. TPR71H (30.34 U/mL) (Ravinder et al., 2012c), and Bacillus sp. C26 (25.7 U/mL) (Eş et al., 2016) were obtained using 0.4%, 0.5% and 1.0% (w/v) yeast extract, respectively. The decline in β-CGTase production at high yeast extract concentration could be explained by its effects on the surface charge, hydrophobicity, and the bacterial cell wall’s carbon/nitrogen ratio (Schär-Zammaretti et al., 2005).
The ability of immobilized cells to produce β-CGTase in a stable manner in the semi-continuous fermentation is the essential advantage. The culture activity was attained for β-CGTase production until the seventh cycle since enzyme production remained between 40.10–50.60 U/mL over the entire optimum period of operation for about 252 hours. Both Ravinder et al. (2012b) and Costa et al. (2015) reported comparable results, demonstrating that Bacillus sp. and Bacillus circulans immobilized cells had greater efficacy in β-CGTase production for repeated batch fermentation up to 6 and 12 batches, respectively. Another study reported that β-CGTase production by immobilized Bacillus firmus and Bacillus sphaericus gave the higher rates of β-CGTase production for three cycles (Moriwaki et al., 2014). The reduction in β-CGTase production from cycle to cycle can be clarified by referencing the diminishing availability of nutrients and product transport and capsule mechanical degradation (Rakmai and Cheirsilp, 2016).
The specific activity of the purified enzyme was about 63.90 U/mg with the fold purification and yield of 19.84% and 41.77%, respectively. Considering the previous studies of purification techniques, it was established that the yield gained was appreciable. The purification yield of CGTase from Bacillus halodurans by starch adsorption was 49.44% (More et al., 2012), while from Bacillus circulans by Sepharose 6B was 55.14% (Guru et al., 2012), besides, a yield of 36.9% for CGTase of Geobacillus thermoglucosidans by Sephadex G-100 (Jia et al., 2018). The molecular weight of the purified enzyme was determined by SDS-PAGE analysis and the single protein band with a molecular weight of ∼84.4 kDa was detected. The result was in accordance with β-CGTase produced by Bacillus licheniformis with one protein of 85.2 kDa (Bonilha et al., 2006). The molecular weights of β-CGTase vary according to the bacterial species, 83 kDa for Bacillus circulans (Guru et al., 2012), and 33 kDa for Bacillus halodurans (More et al., 2012).
The highest level of enzyme activity was observed at a pH 8.0 with a broad range of pH stability (7.0–11.0) with optimum activity at 50–60°C, and the thermal stability was completely retained at 75°C. Consistent results have been reported by Bonilha et al. (2006). A broad pH range of 5.0–11.0 and temperature of 45–75°C was mentioned for CGTase of Amphibacillus sp. NPST-10 (Ibrahim et al., 2012). Similarly, the highest β-CGTase activity of Bacillus sp. N-227 was at pH 6.0 and 70°C, with stability maintained at a range of pH 5.0–7.0 (Wang et al., 2017). The β-CGTase obtained in the present study was associated with higher thermal stability than the β-CGTase of Klebsiella pneumoniae AS-22 (Gawande and Patkar, 2001) and Bacillus agaradhaerens (Martins and Hatti-Kaul, 2002), in both cases, the highest level of stability observed was 40°C. The stability of β-CGTase at alkaline pH and high temperatures are favorable for β-CD production in industrial settings. The enzyme’s thermal stability can support its reaction with raw starch, and alkaline pH can lower the viscosity problem with elevated concentrations of substrate, thereby promoting the cost-effectiveness of cyclodextrin (Jia et al., 2018).
The Km and Vmax of purified enzyme were estimated using Michaelis-Menten equation and Lineweaver-Burk plot (Mora et al., 2012). The purified β-CGTase has a high affinity toward the raw potato starch, with a lower Km value of 5.7 × 10−6 M with a Vmax of 87.71 μmol/mL/min (Fig. 10C). These results can be efficient compared with Km and Vmax values for β-CGTase from Bacillus licheniformis (Bonilha et al., 2006), Bacillus firmus (Moriwaki et al., 2007).
Different industries are concerned about the use of β-CGTase due to its ability to produce a high amount of β-CD from starch, preparation of inclusion complexes, and their low solubility that offers genuine steadiness (Wang et al., 2018). β-CD production with raw and gelatinized potato starch using diverse β-CGTase concentrations was evaluated. Raw potato starch granules were optimal for β-CD production, offering a 1.89-fold advantage over gelatinous potato starch. The β-CD production using raw potato starch offers benefits at high enzyme concentrations (25 U/g) where the weak binding affinity of β-CGTase for raw potato starch is a possibility that accounts for the relevance of high enzyme concentrations (Ibrahim et al., 2013). Also, the raw potato starch was the optimal substrate for β-CD production (13.46 g/L) with β-CGTase of Microbacterium terrace, which bypassed the need to gelatinize starch (Rajput et al., 2016b). Another study demonstrated that raw corn starch played a key role in elevated β-CD production with the β-CGTase of Bacillus megaterium, noting that α-amylase pre-treatment was unnecessary (Pishtiyski and Zhekova, 2006). Besides, the unfavorable effect of physical treatment on the starch structure at temperatures above 75°C adversely affects β-CD production (Kim et al., 1997). Also, Jia et al. (2018) stated that the thermal stability of β-CGTase could support its interaction with raw starch which improves β-CD production through efficient use of β-CGTase without degradation of the produced β-CD and avoid uses of physical treatment of starch substrates. These notices confirm our results as the obtained β-CGTase has thermal stability at 75°C which supports its interaction with raw potato starch.
The results indicated that potato wastewater could be used as a new inexpensive medium to produce of β-CGTase by Bacillus licheniformis YSMT. When compared to free cells, alginate encapsulated cells were associated with higher enzyme production at the optimized conditions of 36 h, pH 8.0, and 50°C, with adding the potato starch (0.4%) and yeast extract (0.6%) to the potato wastewater medium. The seventh-batch semi-continuous repeated cultivation process is utilized for maximized the cost-effectiveness of β-CGTase. The highest level of purified enzyme activity was observed for the enzyme at 50°C and pH 8.0, and full stability was retained until 75°C. Additionally, it was effective when considered in relation to raw potato starch. The potato wastewater as a cost-effective fortify medium for β-CGTase production and the high thermal stability with an efficiency of the enzyme for β-CD synthesis from raw starch is intrinsic for industrial issues.
Author Contribution: Conceptualization, (YSM and SAA); Data curation, (SAA, MH, MM); Formal analysis, (SAA, THT, LIF, MH); Funding acquisition, (YSM and SAA); Methodology, (THT, LIF, SA and MH.); Writing–Original draft, (SAA, YSM, MM, and MH); Writing–Review and editing, (YSM, SA and SAA). All authors have read and agreed to the published version of the manuscript.
Availability of Data and Materials: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Funding Statement: This research was funded by Deanship of Scientific Research at King Khalid University through research groups program, Grant No. R.G.P. 1/241/41.
Conflicts of Interest: The authors declare no conflict of interest.
Abdel-Naby M, El-Refai H, Abdel-Fattah A (2011). Biosynthesis of cyclodextrin glucosyltransferase by the free and immobilized cells of Bacillus cereus NRC7 in batch and continuous cultures. Journal of Applied Microbiology 111: 1129–1137. [Google Scholar]
Abdel-Razek AS, El-Sheikhb HH, Suleimanb WB, Taha TH, Mohameda MK (2020). Bioelimination of phenanthrene using degrading bacteria isolated from petroleum soil: safe approach. Desalination and Water Treatment 181: 131–140. [Google Scholar]
Amiri A, Mohamad R, Rahim RA, Illias RM, Namvar F, Tan JS, Abbasiliasi S (2015). Cyclodextrin glycosyltransferase biosynthesis improvement by recombinant Lactococcus lactis NZ: NSP: CGT: medium formulation and culture condition optimization. Biotechnology & Biotechnological Equipment 29: 555–563. [Google Scholar]
Avci A, Dönmez S (2009). A novel thermophilic anaerobic bacteria producing cyclodextrin glycosyltransferase. Process Biochemistry 44: 36–42. [Google Scholar]
Azab MS (2008). Waste-waste treatment technology and environmental management using sawdust bio-mixture. Journal of Taibah University for Science 1: 12–22. [Google Scholar]
Bibi F, Ullah I, Alvi S, Bakhsh S, Yasir M, Al-Ghamdi A, Azhar E (2017). Isolation, diversity, and biotechnological potential of rhizo-and endophytic bacteria associated with Mangrove plants from Saudi Arabia. Genetics and Molecular Research 16: gmr16029657. DOI 10.4238/gmr16029657. [Google Scholar] [CrossRef]
Baird B, Eaton D, Rice W (2017). Standard methods for the examination of water and wastewater. 23rd edition. USA: American Public Health Association, American Water Works Association, Water Environment Federation. [Google Scholar]
Bonilha PRM, Menocci V, Goulart AJ, de Moraes Polizeli MLT, Monti R (2006). Cyclodextrin glycosyltransferase from Bacillus licheniformis: optimization of production and its properties. Brazilian Journal of Microbiology 37: 317–323. [Google Scholar]
Coelho SL, Magalhães VC, Marbach PA, Cazetta ML (2016). A new alkalophilic isolate of Bacillus as a producer of cyclodextrin glycosyltransferase using cassava flour. Brazilian Journal of Microbiology 47: 120–128. [Google Scholar]
Costa H, Gastón JR, Lara J, Martinez CO, Moriwaki C, Matioli G, Ferrarotti SA (2015). Cyclodextrin glycosyltransferase production by free cells of Bacillus circulans DF 9R in batch fermentation and by immobilized cells in a semi-continuous process. Bioprocess and Biosystems Engineering 38: 1055–1063. [Google Scholar]
Eş I, Ribeiro MC, dos Santos Júnior SR, Khaneghah AM, Rodriguez AG, Amaral AC (2016). Production of cyclodextrin glycosyltransferase by immobilized Bacillus sp. on chitosan matrix. Bioprocess and Biosystems Engineering 39: 1487–1500. [Google Scholar]
Evers D, Baker L, Stevens J (1984). Production and measurement of starch damage in flour. Staerke 36: 309–312. [Google Scholar]
Freitas TLd, Monti R, Contiero J (2004). Production of CGTase by a Bacillus alkalophilic CGII strain isolated from wastewater of a manioc flour industry. Brazilian Journal of Microbiology 35: 255–260. [Google Scholar]
Gawande B, Patkar A (2001). Purification and properties of a novel raw starch degrading-cyclodextrin glycosyltransferase from Klebsiella pneumoniae AS-22. Enzyme and Microbial Technology 28: 735–743. [Google Scholar]
Guo J, Liu J, Yang Y, Zhou Y, Jiang S, Chen C (2018). Fermentation and kinetics characteristics of a bioflocculant from potato starch wastewater and its application. Scientific Reports 8: 3631. [Google Scholar]
Guru MMS, Rajakumari SDM, Jayashree S, Fauzia M, Kumar DM, Kalaichelvan P (2012). Production and purification of CGTase of alkalophilic Bacillus isolated from Marneri pond in Tirunelveli District, Tamil Nadu. Journal of Academia and Industrial Research 2: 101–105. [Google Scholar]
Higuti I, Silva P, Papp J, Okiyama V, Andrade E, Marcondes A, Nascimento A (2004). Colorimetric determination of α and β-cyclodextrins and studies on optimization of CGTase production from B. firmus using factorial designs. Brazilian Archives of Biology and Technology 47: 837–841. [Google Scholar]
Huang LP, Jin B, Lant P, Zhou J (2003). Biotechnological production of lactic acid integrated with potato wastewater treatment by Rhizopus arrhizus. Journal of Chemical Technology & Biotechnology 78: 899–906. [Google Scholar]
Ibrahim A, Al-Salamah A, El-Tayeb M, El-Badawi Y, Antranikian GA (2012). Novel Cyclodextrin glycosyltransferase from alkaliphilic Amphibacillus sp. NPST-10: purification and properties. International Journal of Molecular Science 13: 10505–10522. DOI 10.3390/ijms130810505. [Google Scholar] [CrossRef]
Ibrahim AS, Al-Salamah AA, El-Tayeb MA, El-Badawi YB (2013). Enhancement of Amphibacillus sp NPST-10 cyclodextrin glucanotransferase production by optimizing physio-environmental factors. Journal of Pure and Applied Microbiology 7: 2597–2606. [Google Scholar]
Janeček S, Mareček F, MacGregor EA, Svensson B (2019). Starch-binding domains as CBM families-history, occurrence, structure, function and evolution. Biotechnology Advances 37: 107451. DOI 10.1016/j.biotechadv.2019.107451. [Google Scholar] [CrossRef]
Jia X, Ye X, Chen J, Lin X, Vasseur L, You M (2018). Purification and biochemical characterization of a cyclodextrin glycosyltransferase from Geobacillus thermoglucosidans CHB1. Starch-Stärke 70: 1700016. DOI 10.1002/star.201700016. [Google Scholar] [CrossRef]
Kaneko T, Kato T, Nakamura N, Horikoshi K (1987). Spectrophotometric determination of cyclization activity of β-cyclodextrin-forming cyclomaltodextrin glucanotransferase. Journal of the Japanese Society of Starch Science 34: 45–48. [Google Scholar]
Kim TJ, Kim BC, Lee HS (1997). Production of cyclodextrin using raw corn starch without a pretreatment. Enzyme and Microbial Technology 20: 506–509. [Google Scholar]
Kitcha S, Cheirsilp B, Maneerat S (2008). Cyclodextrin glycosyltransferase from a newly isolated alkalophilic Bacillus sp. C26. Songklanakarin Journal of Science and Technology 30: 723–728. [Google Scholar]
Laemmli UK (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [Google Scholar]
Laszlo E, Banky B, Seres G, Szejtli J (1981). Purification of cyclodextringlycosyl transferase enzyme by affinity-chromatography. Starch-Stärke 33: 281–283. [Google Scholar]
Letsididi R, Sun T, Mu W, Kessy N, Djakpo O, Jiang B (2011). Production of a thermoactive β-cyclodextrin glycosyltransferase with a high starch hydrolytic activity from an alkalitolerant Bacillus licheniformis SK 13.002 strain. Asian Journal of Biotechnology 3: 214–225. [Google Scholar]
Lombard V, Ramulu HG, Drula E, Coutinho PM, Henrissat B (2014). The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Research 42: 490–495. [Google Scholar]
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 193: 265–275. [Google Scholar]
Marchant R, Banat IM, Rahman TJ, Berzano M (2002). The frequency and characteristics of highly thermophilic bacteria in cool soil environments. Environmental Microbiology 4: 595–602. [Google Scholar]
Martins RF, Hatti-Kaul R (2002). A new cyclodextrin glycosyltransferase from an alkaliphilic Bacillus agaradhaerens isolate: purification and characterisation. Enzyme and Microbial Technology 30: 116–124. [Google Scholar]
Mora M, Sánchezl K, Santana R, Rojas A, Ramírez H, Torres-Labandeira J (2012). Partial purification and properties of cyclodextrin glycosiltransferase (CGTase) from alkalophilic Bacillus species. SpringerPlus 1: 61. DOI 10.1186/2193-1801-1-61. [Google Scholar] [CrossRef]
More SS, Niraja R, Evelyn C, Byadgi AM, Shwetha V, Das Mangaraj S (2012). Isolation, purification and biochemical characterization of CGTase from Bacillus halodurans. Croatian Journal of Food Technology, Biotechnology and Nutrition 7: 90–97. [Google Scholar]
Moriwaki C, Costa GL, Pazzetto R, Zanin GM, Moraes FF, Portilho M, Matioli G (2007). Production and characterization of a new cyclodextrin glycosyltransferase from Bacillus firmus isolated from Brazilian soil. Process Biochemistry 42: 1384–1390. [Google Scholar]
Moriwaki C, Mangolim CS, Ruiz GB, de Morais GR, Baesso ML, Matioli G (2014). Biosynthesis of CGTase by immobilized alkalophilic bacilli and crystallization of beta-cyclodextrin: effective techniques to investigate cell immobilization and the production of cyclodextrins. Biochemical Engineering Journal 83: 22–32. [Google Scholar]
Neilan BA (1995). Identification and phylogenetic analysis of toxigenic cyanobacteria by multiplex randomly amplified polymorphic DNA PCR. Applied and Environmental Microbiology 61: 2286–2291. [Google Scholar]
Nishida T, Nakamura A, Masaki H, Uozumi T (1997). Regulation of cyclodextrin glucanotransferase synthesis in Bacillus ohbensis. FEMS Microbiology Letters 149: 221–226. [Google Scholar]
Pazzetto R, Ferreira SBdS, Santos EJS, Moriwaki C, Guedes TA, Matioli G (2012). Preservation of Bacillus firmus strain 37 and optimization of cyclodextrin biosynthesis by cells immobilized on loofa sponge. Molecules 17: 9476–9488. [Google Scholar]
Pishtiyski I, Zhekova B (2006). Effect of different substrates and their preliminary treatment on cyclodextrin production. World Journal of Microbiology and Biotechnology 22: 109–114. [Google Scholar]
Rahman TJ, Marchant R, Banat IM (2004). Distribution and molecular investigation of highly thermophilic bacteria associated with cool soil environments. Biochemical Society Transactions 32: 209–213. [Google Scholar]
Rajput KN, Patel KC, Trivedi UB (2016a). β-cyclodextrin production by cyclodextrin glucanotransferase from an alkaliphile Microbacterium terrae KNR 9 using different starch substrates. Biotechnology Research International 2016: 2034359. DOI 10.1155/2016/2034359. [Google Scholar] [CrossRef]
Rajput KN, Patel KC, Trivedi UB (2016b). Screening and selection of medium components for cyclodextrin glucanotransferase production by new alkaliphile Microbacterium terrae KNR 9 using Plackett-Burman design. Biotechnology Research International 2016: 3584807. DOI 10.1155/2016/3584807. [Google Scholar] [CrossRef]
Rakmai J, Cheirsilp B (2016). Continuous production of β-cyclodextrin by cyclodextrin glycosyltransferase immobilized in mixed gel beads: Comparative study in continuous stirred tank reactor and packed bed reactor. Biochemical Engineering Journal 105: 107–113. [Google Scholar]
Ravinder K, Prabhakar T, Prasanthkumar K, Venuka N (2012a). Immobilization of cyclodextrin glycosyltransferase from newly isolated, mutated Bacillus sp. PR71HNA6 by Entrapment technique. Advances in Applied Science Research 3: 2288–2298. [Google Scholar]
Ravinder K, Prabhakar T, Prasanthkumar K, Venuka N (2012b). Immobilization of cyclodextrin glycosyltransferase from newly isolated, mutated Bacillus sp. TPR71HNA6 by adsorption technique. Journal of Scientific Research in Pharmacy 1: 95–99. [Google Scholar]
Ravinder K, Prabhakar T, Bhavanidevi R (2012c). Optimization of process parameters for the production of cyclodextrin glycosyltransferase by newly isolated Bacillus sp. Tpr71h by conventional method. International Journal of Advanced Biotechnology and Research 3: 578–584. [Google Scholar]
Salva T, Lima V, Pagan AP (1997). Screening of alkalophilic bacteria for cyclodextrin glycosyltranferase production. Revista de Microbiologia 28: 157–164. [Google Scholar]
Schär-Zammaretti P, Dillmann ML, D’Amico N, Affolter M, Ubbink J (2005). Influence of fermentation medium composition on physicochemical surface properties of Lactobacillus acidophilus. Applied and Environmental Microbiology 71: 8165–8173. [Google Scholar]
Sivakumar N, Banu S (2011). Standardization of optimum conditions for cyclodextrin glycosyltransferase production. International Conference on Food Engineering and Biotechnology. IPCBEE, vol. 9. Singapoore: IACSIT Press. [Google Scholar]
Stam MR, Danchin EG, Rancurel C, Coutinho PM, Henrissat B (2006). Dividing the large glycoside hydrolase family 13 into subfamilies: towards improved functional annotations of alpha-amylase-related proteins. Protein Engineering Design & Selection 19: 555–562. [Google Scholar]
Wang H, Zhou W, Li H, Rie B, Piao C (2017). Improved activity of β-cyclodextrin glycosyltransferase from Bacillus sp. N-227 via mutagenesis of the conserved residues. 3 Biotech 7: 149. DOI 10.1007/s13205-017-0725-6. [Google Scholar] [CrossRef]
Wang H, Zhou W, Li H, Bu R (2018). Optimization of the fermentation conditions for the mutant strain of β-cyclodextrin glycosyltransferase H167C to produce cyclodextrins. 3 Biotech 8: 165. DOI 10.1007/s13205-018-1182-6. [Google Scholar] [CrossRef]
Yap P, Ariff A, Woo K, Hii S (2010). Production of cyclodextrin glycosyltransferase (CGTase) by Bacillus lehensis S8 using sago starch as carbon source. Journal of Biological Sciences 10: 676–681. [Google Scholar]
Yuliani E, Imai T, Teeka J, Tomita S, Suprayogi (2011). Exopolysaccharide production from sweet potato-shochu distillery wastewater by Lactobacillus sakei CY1. Biotechnology & Biotechnological Equipment 25: 2329–2333. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |