DOI:10.32604/biocell.2021.016484

| BIOCELL DOI:10.32604/biocell.2021.016484 |  |
| Review |
Delineating the role of phytocompounds against anti-bacterial drug resistance–An update
1Department of Biotechnology, Sir J. C. Bose Technical Campus, Kumaun University, Nainital, India
2Department of Medical Laboratory Sciences, University of Bisha, Bisha, Saudi Arabia
3Department of Chemistry, Kumaun University, Nainital, India
4Dhanvantari Nano Ayushadi Pvt., Ltd., Chennai, India
5Department of Medical Laboratory Sciences (Pathology), College of Applied Medical Sciences, Prince Sattam Bin Abdulaziz University, Riyadh, Saudi Arabia
6Department of Life Sciences, School of Basic Sciences and Research, Sharda University, Greater Noida, India
7Department of Biotechnology, Rama Devi Women’s University, Bhubaneswar, India
8Department of Botany, Mahatma Gandhi Central University, Motihari, India
*Address correspondence to: Piyush Kumar Gupta, piyush.kumar1@sharda.ac.in; Dillip Kumar Bishi, dillipkumarbishi@rdwu.ac.in
#These authors have contributed equally to this work
Received: 09 March 2021; Accepted: 25 April 2021
Abstract: Antibacterial resistance developed by bacteria due to the unlimited use of antibiotics has posed a challenge for human civilization. This kind of problem is not limited to India only, but it is a global concern. Nowadays, many treatments and medicines for bacterial diseases have been developed. However, they possess some drawbacks. Therefore, the alternative medicine has been used to target the drug resistant mechanisms and such medicines have less side effects which is becoming necessary. Natural products have traditionally or historically been of importance for the development of antibacterial agents and are also known to overcome bacterial drug resistance by directly targeting the drug resistance mechanisms in bacteria. In recent years, researchers have also focused on new drug discovery from plant-based research. They have looked on various phytocompounds as antibacterial agents. In the current review, we report various classes of secondary metabolites such as phenolic compounds, flavonoids, alkaloids, saponins, terpenes, quinones, and some essential oils that have been used as an antibacterial agent. In addition, we also discuss several mechanisms behind bacterial multi-drug resistance that are used during bacterial pathogenesis.
Keywords: Drug-resistance; Phytocompounds; Antibacterial; Mechanisms; Medicinal plants; Natural products
Anti-bacterial drug resistance is a serious concern during the treatment of infectious bacterial diseases. Globally, about one-half of the human deaths are caused by infectious diseases and mainly bacterial diseases are of great concern. They can be transmitted from one person to another person by direct contact and indirectly transmitted through air, water, food, insect, and other environmental factors. Before 19th century, many bacterial diseases did exist and many people from the same village died because there was no cure for these diseases (Lederberg, 2000).
Nowadays, various drugs have been developed for bacterial disease treatments and they have potential benefits but they also possess some drawbacks. The larger use of anti-bacterial drugs and antibiotics leads to the development of drug resistance in bacteria, which has become one of the serious concerns in biomedical science (Lederberg, 2000). At present, the anti-bacterial drug resistance remains a challenge to the researchers worldwide. The continuous and excessive use of anti-bacterial drugs and antibiotics has led to the origin of the new drug resistant microbes known as ‘superbug’. This is a result of adaptation or evolution of bacteria, which increases their potency to tolerate or degrade the drugs as compared to the parent strain (Chandra et al., 2017). Many antibiotics were discovered in 19th and 20th centuries which majorly belong to the class of beta-lactam and glycol-peptides respectively. These antibiotics inhibit the peptidoglycan synthesis in bacteria. Some antibiotics inhibit the replication, transcription, or protein translation in bacteria (Kumar et al., 2018). However, many of these antibiotics do not show any therapeutic effect against infectious bacterial diseases as a result of bacteria have developed the resistance. According to the World Health Organization (WHO) report (WHO, 2017), the list of antibiotic-resistant bacteria was presented based on the available evidence and priority. This list includes the species of different bacterial strains such as Acinetobacter, Pseudomonas, Enterobacter, Enterococcus, Staphylococcus, Helicobacter, Neisseria, Salmonella, Campylobacter, Haemophilus, and Shigella which were resistant to antibiotics like penicillin, ampicillin, fluoroquinolone, vancomycin, and carbapenem. Most of the diseases caused by these bacterial species were difficult to control by available anti-bacterial drugs (WHO, 2017). In the WHO 2002 report, the drug resistance was the emerging cause of deadly infectious diseases like tuberculosis, respiratory tract diseases, meningitis, and diarrhoea (WHO, 2002).
In 2016, Tsitsopoulos et al. (2016) demonstrated the nosocomial bloodstream infections (NBSI) due to the antibacterial drug resistance and analysed the neurosurgical patients for 10 years from 2003 to 2013. A total of 236 patients were identified with NBSI and 378 cases were recovered from the blood culture. In this case, Gram-negative bacteria were predominant compared to the Gram-positive bacteria. They found some common drug resistant bacteria such as Staphylococci, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii, where Staphylococcus aureus was found resistant for methicillin. Likewise, Acinetobacter baumannii and Pseudomonas aeruginosa were found resistant for carbapenem. Similarly, in 2020, Naderi et al. (2020) also reported the pattern of antibacterial drug resistance of non-fermenting Gram-negative bacteria from Burn wound. It is a long-term major infection with thermal injuries associated with mortality. They examined a total of 100 samples of wounds, where Acinetobacter baumannii and Pseudomonas aeruginosa were identified to be the most common type of pathogens. Pseudomonas aeruginosa showed resistance against amikacin, imipenem, ciprofloxacin, gentamicin, ceftazidime, and piperacillin-tazobactam. Similarly, Acinetobacter baumannii showed 100% resistance for ceftazidime followed by other antibiotics such as imipenem, amikacin, ciprofloxacin, gentamicin, etc. Escherichia coli and Staphylococcus aureus were also reported in the drug resistance list against several antibiotics. Like, Escherichia coli showed resistance against ciprofloxacin, ampicillin, ceftriaxone, amoxicillin, and trimethoprim. While, Staphylococcus aureus exhibited resistance for erythromycin, trimethoprim-sulfamethoxazole, and methicillin. From the last 5-year periods (2012–2017), the augmentation in the antibacterial drug resistance rate against these two species was found to be a major global concern (Monteiro et al., 2020).
According to the Centers for Disease Control and Prevention (CDC), more than 2 million people were affected by antibiotic-resistant bacteria each year (Pourmand et al., 2017). Similarly, WHO states that the Global Antimicrobial Surveillance System (GLASS) provided an evidence for the occurrence of antibiotic-resistant bacterial infection in 500,000 people across 22 countries. Till now, total 52 countries have enrolled in GLASS. Out of 52, 40 countries have provided the information of the national surveillance system and furthermore, 22 countries gave the data on antibiotic resistant bacteria. The commonly reported bacterial strains were Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli, Mycobacterium tuberculosis, and Staphylococcus pneumoniae. Therefore, GLASS encouraged to each country for the surveillance of antibacterial drug resistance which will be helpful for their prevention as well as it will be useful in new drug discovery (WHO, 2019).
Thus, for the development of a new class of antibiotics, there is a prerequisite to understand the relation between the human-associated bacteria and the mechanisms behind the pathogenesis of infectious diseases. Presently, researchers attempt to fight against these drug resistant pathogens and to eliminate them at rapid pace. Currently, the use of traditional medicine or phytomedicine has drastically increased because phytomedicinal compounds are natural, inexpensive, non-toxic, and able to overcome the drug resistance problem easily. Some phytocompounds alone or in combination with antibiotics have shown a good therapeutic efficacy against antibacterial drug resistance by targeting different mechanisms that bacteria generally use to develop resistance. Some phytocompounds also inhibit the bacterial biofilm formation which is responsible for drug resistance in bacteria. Therefore, it is imperative to prevent the resistance developing mechanisms in bacteria and there is need of continuous search for discovering novel antibacterial drugs that have less side-effects. Nowadays, traditional medicine which is described in Ayurveda, Sushruta Samhita and Charak Samhita is largely explored by researchers all over the world. The traditional medicines have numerous medicinal properties with negligible side-effects. Therefore, the market size of their usage is continuously increasing day-by-day. In this review, we have comprehensively discussed different types of natural phytocompounds like phenolic, tannins, coumarins, alkaloids, flavonoids, terpenes, etc., and their therapeutic role in the elimination of anti-bacterial drug resistance condition. These phytocompounds are elaborated in brief using suitable examples. Further, we described different mechanisms of anti-bacterial drug resistance that are targeted by natural phytocompounds. Besides it, recent challenges and future perspectives in this research area are also discussed.
Phytocompounds and Anti-Bacterial Drug Resistance
Traditionally, medicinal plants are regarded as a treasure of phytocompounds that have excellent therapeutic properties. The crude phytoextracts or purified phytocompounds show good antibacterial activity by which they can inhibit the bacterial growth or completely kill the bacterial pathogens. In recent years, the use of phytomedicine has increased tremendously. Nowadays, the antibacterial drug resistance is a serious threat to humans. It is speculated that we would soon run out of antibiotics because bacteria get evolved and later develop resistance to the antibiotics that are being continuously used. Therefore, natural products are a good source for new anti-bacterial drug discovery. In the past two decades, many researchers have focused on natural products and ethnomedicines for the discovery of new antibacterial drugs. The active phytocompounds isolated from the medicinal plants which are responsible for bactericidal and bacteriostatic actions are flavonoids, terpenes, alkaloids, tannins, lignans, phenols, saponins, and other secondary metabolites as listed in Tab. 1 and shown in Fig. 1. The essential oil of some of the medicinal plants also shows the antibacterial activity and prevents the bacterial drug resistance.
Phenolic compounds are a large class of secondary metabolites which contain benzene ring with a hydroxyl group. In 2019, Jafri et al. (2019) reported that Streptococcus mutans displayed a resistance to azithromycin, ampicillin, vancomycin, and ceftriaxone antibiotics. For inhibiting this anti-bacterial drug resistance, they used eugenol (4-allyl-2-methoxyphenol), which is a phenolic compound extracted from the essential oil of Syzygium aromaticum. Also, the eugenol exhibited the concentration-dependent biofilm inhibition activity in the bacterial drug-resistant population. It showed 36.37% growth inhibition in S. mutans and the cell viability of bacteria decreased from 6.4 to 3.8 CFU/mL (Jafri et al., 2019). Some of the phenolic compounds are discussed in brief which are as follows.
Tannins are large polyphenolic compounds containing both carboxyl and hydroxyl groups. This is an abundant organic compound found in many plants. Tannins possess an astringent property and cause dry and puckery feeling when taken orally. It is also used to clean the skin and tighten the skin pore. For example-Persimmon fruit is a rich source of tannins, found in many countries. The extract of persimmon fruit has shown a strong antibacterial activity against Methicillin-Resistant Staphylococcus aureus (MRSA) (Liu et al., 2019). Generally, Tannins are a good source of tannic acid. Like, Psidium guajava is a rich source of tannins, which has good antibacterial activity (Biswas et al., 2013).
Coumarins are found in many medicinal plants. It is basically a phenolic compound used in the pharmaceutical industries for medicinal uses. For example-Scopoletin is the best example of coumarins that is found in Morinda citrifolia seeds. It exhibits a strong antibacterial activity with a MIC value of 100 μg/mL against the MRSA (de La Cruz-Sánchez et al., 2019).
Lignans are a group of polyphenolic compounds found in various medicinal plants. For example–8-hydroxypinoresinol, a lignin compound isolated from Strombosia grandifolia. This compound has shown the potent antibacterial activity against Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, and Salmonella typhi. In disk diffusion method, the zone of inhibition was measured to be between 11 and 17 mm for this compound in different bacterial culture. The MIC and MBC values of 8-hydroxypinoresinol was calculated and the MIC values was found between 0.73 and 3.0 mg/mL and the MBC values were 1.5 mg/mL (Ekalu et al., 2019). Lignans are also present in Sesamum indicum honey which showed a strong antibacterial activity against Escherichia coli, Salmonella typhimurium, and Vibrio cholerae (Das et al., 2015; Kumar and Singh, 2015).
Flavonoids are an important class of plant secondary metabolites. These compounds are commonly found in the leaves, flowers, seeds, and other parts of the medicinal plants. In 2018, Chew et al. (2018) reported that the flowers of the Bauhinia kockiana have potent antibacterial activity against MRSA. They confirmed the presence of the flavonoids in the flower of this plant during phytochemical analysis. They found gallic acid and methyl gallate in the flowers of Bauhinia kockiana plant and showed a strong antibacterial activity with changes in cell membrane structure, cell membrane fluidity, and cell membrane attachment. In 2013, Mun et al. (2013) used curcumin, a natural flavonoid which has numerous traditionally well-known medicinal values. They determined the synergistic antibacterial effect of curcumin against MRSA by standard microdilution method. In 2015, Abreu et al. (2015) demonstrated the synergistic antibacterial effect of five different flavonoids with antibiotics against Staphylococcus aureus. The examples of some other flavonoids are quercetin, kaempferol, myricetin, luteolin, licochalcone, and flavones. These compounds also exhibited potent antibacterial activity (Farhadi et al., 2019).
Alkaloids are nitrogen-containing phytocompounds present in the flowering plants and rarely in gymnosperms. Alkaloids have strong antibacterial medicinal property as these natural compounds can inhibit the biofilm formation in bacterial population. In 2016, Abreu et al. (2016) studied the antibacterial effect of some alkaloids like quinine, pyrrolidine, and reserpine in the presence of few antibiotics against biofilm forming bacteria Staphylococcus aureus. Their results showed a significant reduction in the biofilm formation and reduced the antibacterial drug resistance developed by this bacterium to the antibiotics. In 2015, AL-Ani et al., (2015) reported the synergistic bactericidal activity of sanguinarine, berberine, carvacrol, and benzyl isothiocyanate alkaloids with bee venom and some antibiotics against multi-drug resistant Gram-positive and Gram-negative bacteria. In 2015, Peng et al. (2015) showed the strong antibacterial activity of berberine alkaloid (isolated from Berberis sp.) against Streptococcus agalactiae. This alkaloid can intercalate with DNA, disrupt the bacterial cell membrane structure, and increase the cell membrane permeability in bacteria.
Terpenes constitute the large class of phytocompounds which contain five-carbon isoprene units. It is the primary ingredient of essential oil and has numerous medicinal properties. Terpenes group has approximately 30,000 compounds, in which around 400 are monoterpenes. The derivatives of monoterpenes such as nitrogen-containing terpenoids showed the significant antibacterial activity against Staphylococcus aureus and Bacillus subtilis (Kozioł et al., 2020). In 2017, Gupta et al. (2017) discussed about Citral (3, 7-dimethyl-2,6-octadienal) monoterpene which is present in the essential oil of many medicinal plants. Citral exhibited a bactericidal activity in combination with norfloxacin against drug-resistant Staphylococcus aureus. This compound inhibited the drug resistance by closing the efflux pump, damaging the bacterial cell membrane, and changing the membrane potential. Hence, Citral is a strong anti-staphylococcal agent but in combination with antibiotic, it reduces the antibiotic drug resistance. In 2019, Costa et al. (2019) discussed about Limonene, a monoterpene found in the essential oil of many plant species. This compound has strong antibacterial activity against both Gram-positive and Gram-negative bacteria.
Saponins are glycosides which contain steroid linked to the carbohydrate moieties by a glycosidic bond. It has a detergent property. In 2019, Lall et al. (2019) discussed about the saponins. They extracted these glycosides from Argania spinosa plant and they observed a potent antibacterial activity against Cutibacterium acnes and Prevotella intermedia bacteria. In 2019, Fleck et al. (2019) reported about Quillaja saponaria plant which is a rich source of saponin. They showed a high antibacterial activity against Salmonella typhimurium, Staphylococcus aureus, and Escherichia coli.
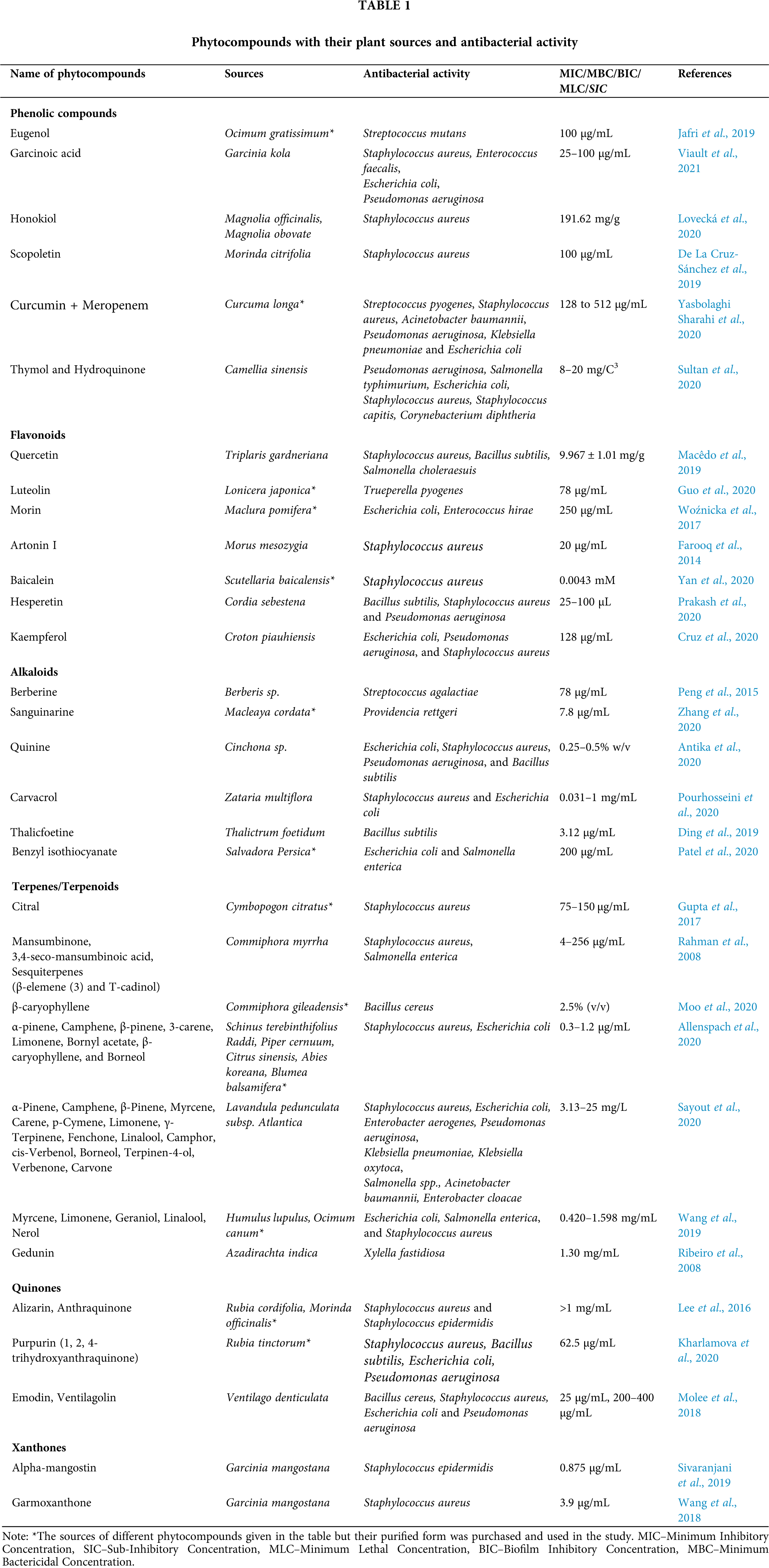
Quinones
Quinones have numerous biological and pharmacological activities. In 2016, Lee et al. (2016) described about quinones and their inhibitory role in biofilm formation. These quinones were anthraquinones, alizarin, purpurin and quinalizarin. These phytocompounds prevented the antibacterial drug resistance induced by infectious bacteria. During the post-transcriptional analysis, they found that Alizarin repress the rbf, spα, and psmα genes responsible for biofilm formation and they also found the expression of cid/led genes responsible for reducing the biofilm formation.
Essential Oil and Other Phytochemicals
In the last few decades, health care system gradually moved towards the use of traditional medicines and plant-based drugs. Most of the research communities are trying to learn from nature for the discovery of new drugs based on the medicinal plants. In 2016, Moussaoui and Alaoui (2016) showed the antibacterial activity of the essential oils of five medicinal plants such as Thymus willdenowii, Chrysanthemum coronarium, Origanum compactum, Origanum majorana, and Melissa officinalis against 10 bacterial strains. They observed a synergistic effect of essential oils with the antibiotic. This study unequivocally demonstrated that these bacterial strains show antagonistic effect when treated with antibiotics alone but the combination of antibiotics with the essential oils resulted in the synergistic effect and reduced the antibacterial drug resistance.
In 2017, Mishra et al. (2017) described the antibacterial activity of the crude extract of some medicinal plants against multi-drug resistant bacteria. In this study, the crude extracts of nine medicinal plants namely Azadirachta indica, Anogeissus acuminate, Boerhaavia diffusa, Bauhinia variegate, Soymida febrifuga, Punica granatum, Terminalia chebula, Tribulus terrestris and Tinospora cordifolia were tested against 11 multidrug-resistant bacteria, isolated directly from the urine samples of the urinary tract infected patients. These bacteria were resistant to 17 antibiotics. This study highlighted that these three plants namely Anogeissus acuminate, Punica granatum and Soymida febrifuga contain stigmasterol and luteolin-7-O glucoside as major phytocompounds, due to which they possess a potent antibacterial activity against multi-drug resistant bacteria. In 2015, Kouidhi et al. (2015) demonstrated the antibacterial drug resistance in the dental care which was developed due to the biofilm forming bacteria like Streptococcus, Lactobacillus, Actinomycetes, and other Gram-negative/Gram-positive bacteria within the oral cavity. They used different plant extracts to prevent the biofilm formation in the dental cavities. In this study, the extracts of total 21 medicinal plants containing numerous phytocompounds exhibited the strong antibacterial activity against the multi-drug resistant bacteria via the inhibition of biofilm formation. In 2015, Tankeo et al. (2015) reported the antibacterial activity of different phytoextracts of Polyscias fulva, Newbouldia laevis, Beilschmedia acuta, and Clausena anisate against different bacterial strains like Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, and Enterobacter aerogenes. Among these extracts, the phytoextract of Beilschmedia acuta plant showed better potency against multi-drug resistant bacterial infection. Similarly, in 2016, Barreto et al. (2016) examined the use of aminoglycosides isolated from Anadenanthera colubrine plant along with antibiotics against the multidrug-resistant bacteria and they observed the inhibition of the drug efflux pump which reduced the drug resistance in bacteria. Further, in 2015, Fankam et al. (2015) revealed that the extracts of Allanblackia gabonensis, Combretum mole, and Gladiolus quartinianus increased the antibacterial activity against the drug-resistant Gram-negative bacteria and these extracts inhibited the drug-resistant mechanisms in the tested bacteria.
In 2017, Zacchino et al. (2017a) comprehensively discussed the combinations of 17 antibiotics with phytocompounds. They showed the synergistic action of antibiotics with natural products in the prevention of bacterial biofilm formation. Further, some of the natural products such as kibdelomycin, hymenosetin, hunanamycin, batumin, tetarimycin, artonin, baulamycin, viridicatumtoxin, hongoquercin, diorcinol, baicalein, propylgallate, honokiol, and acteoside are active phytocompounds with good antibacterial activity. These natural products are the best candidates for the new antibacterial drug discovery (Moloney, 2016; Zacchino et al., 2017b). In 2017, Chandra et al., collected the data on the use of plant based medicinal compounds for controlling the antibacterial drug resistance. This study concluded that active phytocompounds such as quinones, alkaloids, flavonoids, coumarins, terpenoids, tannins, lignans, glucosinolates, and essential oils participate in modulating the drug resistance (Chandra et al., 2017). As plants are the major source of derived phytocompounds for therapeutic use, however most of the higher plants have not been explored completely. Out of near 3 lakh species of higher plants, only 6% plants have been investigated pharmacologically for their medicinal values and about 15% based on the phytochemical analysis (Borges et al., 2016). Thus, natural products are the best sources for the new drug discovery in order to overcome the antibacterial drug resistance condition. These products inhibit such condition by targeting key signaling pathways which are described in detail in the next section.
Mechanisms Behind Antibacterial Drug Resistance
Antibiotics have been largely used in the treatment of the bacterial infections. These antibiotics can be classified as broad or narrow spectrum depending on whether it targets the wide-range or specific groups of bacteria respectively. In current scenario, the pharmacological activity of these antibiotics became limited due to their excess and unwanted use against bacteria.
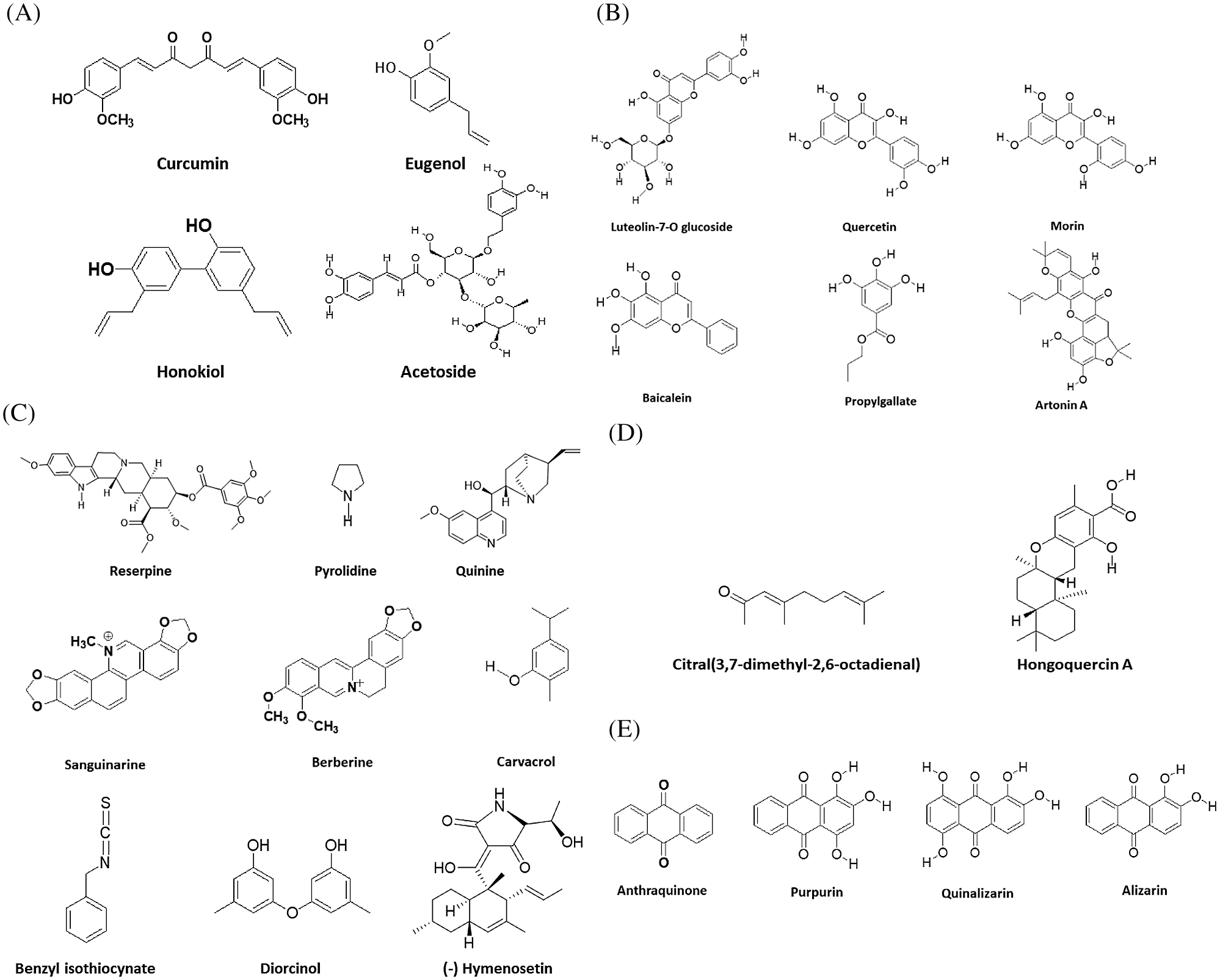
Figure 1: Chemical structure of phytocompounds known for inhibiting anti-bacterial drug resistance (A) Phenolics, (B) Flavonoids, (C) Alkaloids, (D) Terpenes, and (E) Quinones.
Globally, a large population of the drug-resistant bacteria is continuously increasing. These bacteria are existing in nature due to their continuous evolutionary process and adaptation. In recent time, the antibacterial drug resistance has become a major clinical challenge and worldwide concern. To meet this challenge, traditional medicines are being used which delivers a promising solution in the clinics without any toxic side-effects. Phytomedicines are well-known pharmacological compounds that can directly target the antibacterial drug resistance mechanisms. Some of these mechanisms are described in detail as follows.
The efflux pump is a key mechanism which is present in almost all the drug-resistant bacteria. In bacteria, the efflux pump helps in reducing the concentration of toxic substances like drugs that kill the bacteria. These pumps also regulate the quorum sensing signals (Soto, 2013). The efflux pumps are found in both Gram-positive and Gram-negative bacteria. The overexpression of more than one efflux pumps lead to the antibacterial drug resistance in clinics. Based on the bacterial cell membrane composition and type of drug molecules, the efflux pumps get activated (Chandra et al., 2017). These multidrug efflux pumps are classified into five different families as shown in Fig. 2.
• Multidrug-Antimicrobial Extrusion protein (MATE): MATE is also known as multidrug and toxic compound extrusion transporter. Such pump is usually found in both Gram-positive and Gram-negative bacteria. ArcAB, MdtK are the few examples of MATE found in E. coli and MepA protein is present in Staphylococcus aureus (Sun et al., 2014). These transporters usually lead to the efflux of several cationic drugs such as fluoroquinolones, aminoglycosides (Soto, 2013).
• Resistance-Nodulation-Division (RND) protein: RND transporters are mostly found in the Gram-negative bacteria. It is a type of efflux pump which majorly leads to the drug resistance in bacteria. For example- MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY are RND family proteins which are commonly present in Pseudomonas aeruginosa (Gupta et al., 2017). In 2014, Sun et al. (2014) reported ArcAB-ToIC, AcrD, AcrEF, CusCFBA, MtdABC, and MtdEF efflux pumps which are present in Escherichia coli. Similarly, other bacteria also have different types of efflux pump such as CmeABC (Campylobacter jejuni), AdeABC (Acinetobacter baumannii), and AmeABC (Agrobacterium tumefaciens). Likewise, Neisseria gonorrhoeae has FarAB and MtrCDE. Salmonella typhimurium has ArcAB, ArcD, ArcEF, MdsABC, and MdtABC efflux pumps. Also, Pseudomonas putida has SrpABC, TgtABC, and ArpAB type of efflux pumps. These RND proteins efflux many antibiotics such as chloramphenicol, novobiocin, and β-lactams.
• Small Multidrug Resistance (SMR)/Drug Metabolite Transporter (DMT) protein: SMR transporters are present in the inner membrane of Gram-negative bacteria. For example–NepAB, EbrAB, and SsmE are present in Arthronobacter nicotinovorans, Bacillus subtilis, and Serratia marcescens respectively. Likewise, EmrE is present in Pseudomonas aeruginosa and Escherichia coli (Blanco et al., 2016; Srinivasan et al., 2009). These DMT proteins actively pump the methylamine, ciprofloxacin, amikacin, cefepime, and many others drugs which are known to cause drug resistance in bacteria.
• ATP Binding Cassette (ABC) proteins: ABC transporters bind to the small drug molecules and efflux them outside the bacterial cell through ATP hydrolysis. The ABC pump is present in both Gram-positive and Gram-negative bacteria which actively export the macrolides. Also, this transporter led to the biofilm formation in Listeria monocytogenes bacteria (Soto, 2013). MacABCsm and MacAB are some of the examples of ABC protein, present in Stenotrophomonas maltophilia and Salmonella typhimurium bacteria respectively. ABC proteins are also overexpressed in most of the drug resistant bacteria (Lin et al., 2014).
• Major Facilitator Superfamily (MFS): MFS membrane proteins are the largest group of transporters, present in both Gram-positive and Gram-negative bacteria. In Gram-positive strains, MFS proteins have been shown to export cetrimide and acriflavine whereas in Gram-negative bacteria, these proteins have been shown to export the novobiocin and nalidixic acid. The MFS superfamily contains numerous types of efflux pumps like Bmr and Blt in Bacillus subtilis, EmrAB and EmrKY in Escherichia coli, QacA and NorA in Staphylococcus aureus, and PmeA in Streptococcus pneumoniae (Soto, 2013).
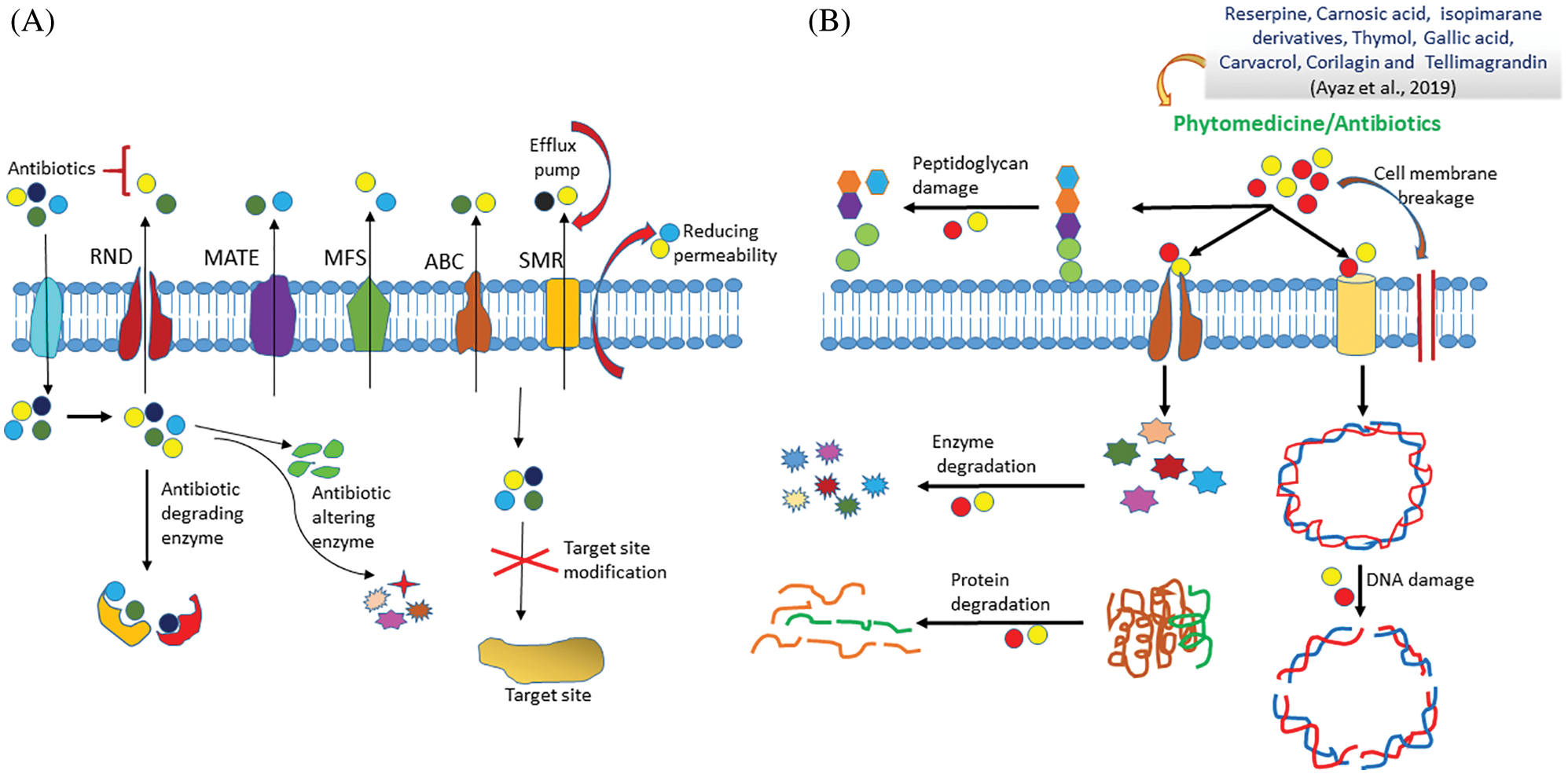
Figure 2: Mechanism of drug resistance in resistant and non-resistant bacterial cell.
From ancient times, the herbal products have been used to cure several bacterial infections. The herbal medicine has the potential to directly target the bacterial drug-resistant mechanisms. Like, 4’, 5’-O-decaffeoylquinicacid (4’, 5’-ODCQA) isolated from Artemisia absinthium, showed the strong antibacterial activity by directly targeting the MFS transporters in Staphylococcus aureus, Bacillus cereus, and Enterococcus faecalis (Fiamegos et al., 2011). In 2018, Shin et al., discussed about several flavonoids like myricetin, epigallocatechin, and robinetin which directly inhibit the DNA synthesis whereas, quercetin blocks the ATPase activity in Escherichia coli by binding with GyrB protein (Shin et al., 2018). Similarly, other phytocompounds such as reserpine, gallotannin, curcumin, piperine, chalcones, berberine, and carnisic acid directly inhibit the ABC efflux pump (Shriram et al., 2018). Likewise, phytocompounds such as sarothrin, capsaicin, lanatoside, cathecol, olympicin, daidzein, lysergol, and ursolic acid also inhibit the efflux pump in many pathogenic bacterial strains (Prasch and Bucar, 2015).
Inactivation and Modification of Antibacterial Agents
This is another mechanism in which the antibacterial agents become inactive by its modifications which takes place by the drug-resistant bacteria. Generally, antibiotics target the bacteria at different levels such as inhibition of DNA and protein synthesis and prevention of bacterial cell wall formation. These antibiotics such as aminoglycosides, macrolides, chloramphenicol, ampicillin, novobiocin can be modified or degraded by the bacterial hydrolytic enzymes like transferases and β-lactamase. Also, bacteria can utilize other mechanisms like redox process, enzymatic hydrolysis, and group transfer to degrade these antibiotics. For example-Metallo-β-lactamase is present in Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae. Cephalosporinases is found in Enterobacter sp. and penicillinase is found in Staphylococcus aureus. These enzymes can degrade or alter the chemical structure of antibiotics (Bush and Fisher, 2011). Usually, the inactivation of antibiotics takes place by phosphorylation, acetylation, and adenylation of the drug molecule using phosphoryl transferases, nucleotidyl transferases, and acetyltransferases enzymes. Hence, bacteria have potential to inactivate the antibacterial agents and become resistant for that particular drug (Borges et al., 2016; Chandra et al., 2017).
Modification in the Target Site of Antibacterial Agent
The modification in the target site is also an important mechanism by which pathogenic bacteria prevent the drug to reach their target site. Mutation is the main factor for the modification of the drug target site. Usually, antibiotics such as chloramphenicol, streptomycin, and tetracycline block protein synthesis in the pathogenic bacteria by targeting 23S rRNA of the 50S ribosomal subunit. Due to mutation in 23S rRNA, Enterococcus sp. shows resistance against oxazolidinones. Also, the mutation in 16S rRNA induce the resistance in bacteria against aminoglycosides. However, the drug resistance in Mycobacterium tuberculosis occurs due to the mutation in the ribosomal protein S12 encoding rpsL gene (Chandra et al., 2017). It has been shown that post-transcriptional or post-translational modifications lead to the changes in the target site of antibiotics responsible for antibiotic resistance in bacteria (Kuiper and Conn, 2014). Bacteria can also alter the binding sites of drug molecules. The spontaneous mutations in the chromosome also lead to the alternation in the amino acids sequence by which the encoded protein sequence changes. These mutations also modify the recombination sites in the bacteria by which they become drug resistant (Borges et al., 2016). For example - Penicillin, a β-lactam antibiotic, targets the penicillin-binding protein (PBP) which is a transpeptidase found in the bacterial cell wall. A mutation in PBP leads to the changes in the binding site of penicillin and causes resistance in bacteria (Cabot et al., 2016).
In bacteria, the transfer of genetic material occurs by the process of horizontal transfer which is mainly of three types: conjugation, transformation, and transduction. If the genetic material transfers from one species to other species of bacteria, then it is vertical gene transfer and if the transfer occurs from the parent to the progeny, then it is horizontal gene transfer. Generally, the resistant genes transfer in bacteria from the parent to the progeny by the process of conjugation using pilus. In 2017, Chandra et al. (2017) investigated about the pheromone–responsive plasmids which are constituted of mobile genetic elements that facilitate the transfer of antibiotic-resistant genes.
Together, the antibacterial drug resistance is a major and serious global issue which persists due to one or more drug resistance mechanisms. To encounter this, there is an urgent need to develop novel antibacterial drugs which can target the bacteria in multiple ways. At the same time, the development of plant-based medicines to combat the antibacterial drug resistance is still at underway.
Recent Challenges and Future Prospective
As of today, the development of new antibacterial drug to prevent the bacterial drug resistance is a major challenge. Many pharmaceutical companies take part in the synthesis of the new antibacterial drug but due to the drug resistance problem, these drugs are no longer effective. Therefore, developing new drugs which can fight against antibacterial drug resistance is the need of the hour. Currently, many pharmaceutical companies and academic institutes have invested major resources for the research and development of new antibacterial drugs and few others are using natural compounds as medicines but it is still a big challenge. The invention of new technologies on day by day gives a better hope for developing the effective drugs against these drug resistant pathogens. For instance, the nanotechnology-based techniques have revolutionized the field biomedicine as drug delivery systems. Due to the advent of nano-carriers, several parameters such as drug solubility, permeability, blood circulation time, and therapeutic efficiency of poorly water-soluble drugs and phytocompounds have been improved. Moreover, the site-specific drug delivery is achieved due to the introduction of actively targeted nanoparticles. The physical entrapment or stimuli-responsive conjugation of these anti-bacterial drugs could be possible inside the nanoparticles. However, some metal nanoparticles display potent bactericidal activity. When these metal nanoparticles were conjugated with phytocompounds then it could become an ideal choice for the development of new antibacterial drug which have prevented the bacterial drug resistance. In 2018, Lakshminarayanan et al. (2018) reported that both metallic and non-metallic nanoparticles show antibacterial activity and target the drug resistant mechanisms of bacteria. Especially, non-metallic nanoparticles such as lipid-based and polymer-based nanoparticles were used as a carrier for the delivery of antimicrobial drugs to increase their therapeutic efficacy and reduce the systemic cytotoxicity. It is worthy to mention that the numbers of publications on nanoparticles based antimicrobial drugs have drastically increased from 2002 to 2017. In 2018, Hussain et al. (2018) synthesized the antibiotic loaded nanoparticles against bacterial infections for improving the antibacterial activity of antibiotics. In 2018, Escárcega-González et al. (2018) demonstrated the antibacterial activity of silver nanoparticles synthesized using the medicinal plant Acacia rigidula against Gram-positive and Gram-negative bacterial strains. Thus, the synthesis of nanoparticles using medicinal plants and their derivatives, as reducing and stabilizing agents has led to the advancements in the biomedical science field.
The synergistic combinatorial therapeutic effects of antibiotics with one or more phytochemicals are the perspective for decreasing the bacterial drug resistance (Ayaz et al., 2019). In 2018, Cheypratub et al. (2018) displayed the antibacterial activity of Cyperus rotundus extract with ampicillin against the ampicillin resistant strain Staphylococcus aureus. This combination increased the cytoplasmic permeability of the bacteria. In 2018, Vambe et al. (2018) reported that the mixed phytoextracts of seven medicinal plants (Solanum panduriforme, Prunus africana, Protea caffra, Searsia lancea, Cucumis myriocarpus, Bolusanthus speciosus, and Ekebergia capensis) exhibited strong antibacterial activity. On the other hand, some phytocompounds showed the inhibitory effects against bacterial drug resistance as listed in Tab. 1. Reserpine, pyrrolidine, quinine, morin, quercetin, alizarin, and anthraquinones have been reported as an antibacterial compound against Staphylococcus aureus (Abreu et al., 2016; Lee et al., 2016). Similarly, cinnamaldehyde displayed the strong antibacterial activity against Aeromonas hydrophila with a MIC value of 256 and 512 μg/mL (Yin et al., 2020). Further, eugenol also showed the potent antibacterial activity against Salmonella typhimurium, Mycobacterium tuberculosis, and Listeria monocytogenes (Jafri et al., 2019). In addition, carvacrol, benzyl isothiocyanate, sanguinarine, and berberine are also reported as antibacterial phytocompounds against Staphylococcus aureus, Streptococcus thermophilus, Enterococcus casseliflavus, and Mycobacterium sp. (AL-Ani et al., 2015), where these phytocompounds also prevent the bacterial drug resistance. Similarly, artonin and baulamycin showed a potent antibacterial effect against Bacillus subtilis, Listeria sp., Enterococcus sp., and Staphylococcus aureus (Moloney, 2016).
Medicinal plants are nature’s gift to humans and they are well known for their antimicrobial, antioxidant, anti-inflammatory, antipyretic, and anticancer properties. Due to these properties, medicinal plants have been used in a wide range of biomedical applications. Most of the pharmaceuticals available in the market are known for treating bacterial diseases. However, these bacteria can develop resistance against these pharmaceuticals and cannot be killed so easily. There are several bacterial diseases like tuberculosis and pneumonia where, the complete treatment is not available due to the development of continuous drug resistance. Therefore, the concept of using traditional medicine to overcome such bacterial drug resistance is the solution at present. The medicinal plants have active phytocompounds such as flavonoids, alkaloids, terpenes, saponin, glycosides, polyphenols, and others, which have involved in the inhibition of drug resistance mechanism. These phytocompounds directly target the drug resistant mechanisms in bacteria and form the base for researchers to search for new antibacterial drugs. Moreover, the relations between the antibiotics, phytocompounds, and the cocktail of extracts increase the potential to fight against drug resistant bacterial pathogens. This review concludes that several phytocompounds were discussed in this paper that directly target the bacterial drug resistance mechanisms and in recent years, traditional medicine has gained more popularity and been the focus area of research due to the fewer side effects and promising efficacy results.
Acknowledgement: Dr. Piyush Kumar Gupta is thankful to the Department of Life Sciences, School of Basic Sciences and Research, Sharda University. Ms. Rekha Gahtori is thankful to the Senior Research Fellowship (Award No. 45/4/2019/MP/BMS) awarded by Indian Council of Medical Research, New Delhi, India.
Author Contributions: Ms. Rekha Gahtori contributed in the collection of the literature, writing the first draft of the manuscript. Ms. Nidhi Negi contributed in the drawing the chemical structure of phytocompounds. Dr. Saravanan Krishnan, Dr. KanuPriya, Dr. Soumya Pandit, Dr. Sugapriya Dhanasekaran, Dr. Ram Prasad, and Dr. Dillip K Bishi contributed in editing the draft and provided critical inputs in the review discussion. Dr. Piyush Kumar Gupta conceptualized, planned and finalized the manuscript.
Ethical Statement: This article does not contain any studies with animals performed by any of the authors.
Funding Statement: DKB acknowledges the funding support from Science and Engineering Research Board (SERB), Department of Science and Technology, Govt. of India for the “Start-up Research Grant-2019” (SRG/2019/001995).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abreu AC, Saavedra MJ, Simões LC, Simões M (2016). Combinatorial approaches with selected phytochemicals to increase antibiotic efficacy against Staphylococcus aureus biofilms. Biofouling 329: 1103–1114. DOI 10.1080/08927014.2016.1232402. [Google Scholar] [CrossRef]
Abreu AC, Serra SC, Borges A, Saavedra MJ, Mcbain AJ, Salgado AJ, Simões M (2015). Combinatorial activity of flavonoids with antibiotics against drug-resistant Staphylococcus aureus. Microbial Drug Resistance 21: 600–609. DOI 10.1089/mdr.2014.0252. [Google Scholar] [CrossRef]
AL-Ani I, Zimmermann S, Reichling J, Wink M (2015). Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 22: 245–255. DOI 10.1016/j.phymed.2014.11.019. [Google Scholar] [CrossRef]
Allenspach MD, Valder C, Steuer C (2020). Absolute quantification of terpenes in conifer-derived essential oils and their antibacterial activity. Journal of Analytical Science and Technology 11: 12. DOI 10.1186/s40543-020-00212-y. [Google Scholar] [CrossRef]
Antika LD, Triana D, Ernawati T (2020). Antimicrobial activity of quinine derivatives against human pathogenic bacteria. IOP Conference Series: Earth and Environmental Science 462: 012006. DOI 10.1088/17551315/462/1/012006. [Google Scholar] [CrossRef]
Ayaz M, Ullah F, Sadiq A, Ullah F, Ovais M, Ahmed J, Devkota HP (2019). Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chemico-Biological Interactions 308: 294–303. DOI 10.1016/j.cbi.2019.05.050. [Google Scholar] [CrossRef]
Barreto HM, Coelho KMRN, Ferreira JHL, dos Santos BHC, de Abreu APL, Coutinho HDM, da Silva RAC, de Sousa TO, Citó AM das GL, Lopes JAD (2016). Enhancement of the antibiotic activity of aminoglycosides by extracts from Anadenanthera colubrine (Vell.) Brenan var. Cebil against multi-drug resistant bacteria. Natural Product Research 30: 1289–1292. DOI 10.1080/14786419.2015.1049177. [Google Scholar] [CrossRef]
Biswas B, Rogers K, McLaughlin F, Daniels D, Yadav A (2013). Antimicrobial activities of leaf extracts of Guava (Psidium guajava L.) on two gram-negative and gram-positive bacteria. International Journal of Microbiology 2013: 746165. DOI 10.1155/2013/746165. [Google Scholar] [CrossRef]
Blanco P, Hernando-Amado S, Reales-Calderon J, Corona F, Lira F, Alcalde-Rico M, Bernardini A, Sanchez M, Martinez J (2016). Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 4: 14. DOI 10.3390/microorganisms4010014. [Google Scholar] [CrossRef]
Borges A, Abreu A, Dias C, Saavedra M, Borges F, Simões M (2016). New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 21: 877. DOI 10.3390/molecules21070877. [Google Scholar] [CrossRef]
Bush K, Fisher JF (2011). Epidemiological expansion, structural studies, and clinical challenges of new β-Lactamases from gram-negative bacteria. Annual Review of Microbiology 65: 455–478. DOI 10.1146/annurev-micro-090110-102911. [Google Scholar] [CrossRef]
Cabot G, Zamorano L, Moyà B, Juan C, Navas A, Blázquez J, Oliver A (2016). Evolution of Pseudomonas aeruginosa antimicrobial resistance and fitness under low and high mutation rates. Antimicrobial Agents and Chemotherapy 60: 1767–1778. DOI 10.1128/AAC.02676-15. [Google Scholar] [CrossRef]
Chandra H, Bishnoi P, Yadav A, Patni B, Mishra A, Nautiyal A (2017). Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials—A review. Plants 6: 16. DOI 10.3390/plants6020016. [Google Scholar] [CrossRef]
Chew YL, Mahadi AM, Wong KM, Goh JK (2018). Anti-methicillin-resistance Staphylococcus aureus (MRSA) compounds from Bauhinia kockiana Korth. and their mechanism of antibacterial activity. BMC Complementary and Alternative Medicine 18: 70. DOI 10.1186/s12906-018-2137-5. [Google Scholar] [CrossRef]
Cheypratub P, Leeanansaksiri W, Eumkeb G (2018). The synergy and mode of action of Cyperus rotundus L. extract plus ampicillin against ampicillin-resistant Staphylococcus aureus. Evidence-Based Complementary and Alternative Medicine 2018: 3438453. DOI 10.1155/2018/3438453. [Google Scholar] [CrossRef]
Costa MDS, Rocha JE, Campina FF, Silva ARP, Da Cruz RP, Pereira RLS, Quintans-Júnior LJ, De Menezes IRA, De S. Araújo AA, De Freitas TS, Teixeira AMR, Coutinho HDM (2019). Comparative analysis of the antibacterial and drug-modulatory effect of d-limonene alone and complexed with β-cyclodextrin. European Journal of Pharmaceutical Sciences 128: 158–161. DOI 10.1016/j.ejps.2018.11.036. [Google Scholar] [CrossRef]
Cruz BG, Dos Santos HS, Bandeira PN, Rodrigues THS, Matos MGC et al. (2020). Evaluation of antibacterial and enhancement of antibiotic action by the flavonoid kaempferol 7-O-β-D-(6’’-O-cumaroyl)-glucopyranoside isolated from Croton piauhiensis Müll. Microbial Pathogenesis 143: 104144. DOI 10.1016/j.micpath.2020.104144. [Google Scholar] [CrossRef]
Das A, Datta S, Mukherjee S, Bose S, Ghosh S, Dhar P (2015). Evaluation of antioxidative, antibacterial and probiotic growth stimulatory activities of Sesamum indicum honey containing phenolic compounds and lignans. LWT–Food Science and Technology 61: 244–250. DOI 10.1016/j.lwt.2014.11.044. [Google Scholar] [CrossRef]
de La Cruz-Sánchez NG, Gómez-Rivera A, Alvarez-Fitz P, Ventura-Zapata E, Pérez-García MD, Avilés-Flores M, Gutiérrez-Román AS, González-Cortazar M (2019). Antibacterial activity of Morinda citrifolia Linneo seeds against Methicillin-Resistant Staphylococcus spp. Microbial Pathogenesis 128: 347–353. DOI 10.1016/j.micpath.2019.01.030. [Google Scholar] [CrossRef]
Ding CF, Qin XJ, Yu HF, Liu YP, Wang XH, Luo XD (2019). Thalicfoetine, a novel isoquinoline alkaloid with antibacterial activity from Thalictrum foetidum. Tetrahedron Letters 60: 151135. DOI 10.1016/j.tetlet.2019.151135. [Google Scholar] [CrossRef]
Ekalu A, Ayo RGO, Habila JD, Hamisu I (2019). In vitro antimicrobial activity of lignan from the stem bark of Strombosia grandifolia Hook. f. ex Benth. Bulletin of the National Research Centre 43: 115. DOI 10.1186/s42269-019-0159-x. [Google Scholar] [CrossRef]
Escárcega-González CE, Garza-Cervantes JA, Vazquez-Rodríguez A, Montelongo-Peralta LZ, Treviño-Gonzalez MT, Díaz Barriga Castro E, Saucedo-Salazar EM, Chávez Morales RM, Regalado-Soto DI, Treviño-González FM, Carrazco Rosales JL, Villalobos Cruz R, Morones-Ramirez JR (2018). In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. International Journal of Nanomedicine 13: 2349–2363. DOI 10.2147/IJN.S160605. [Google Scholar] [CrossRef]
Fankam AG, Kuiate JR, Kuete V (2015). Antibacterial and antibiotic resistance modifying activity of the extracts from Allanblackia gabonensis, Combretum molle and Gladiolus quartinianus against Gram-negative bacteria including multi-drug resistant phenotypes. BMC Complementary and Alternative Medicine 15: 206. DOI 10.1186/s12906-015-0726-0. [Google Scholar] [CrossRef]
Farhadi F, Khameneh B, Iranshahi M, Iranshahy M (2019). Antibacterial activity of flavonoids and their structure-activity relationship: An update review: Antibacterial activity of flavonoids. Phytotherapy Research 33: 13–40. DOI 10.1002/ptr.6208. [Google Scholar] [CrossRef]
Farooq S, Wahab AT, Fozing CDA, Rahman AU, Choudhary MI (2014). Artonin I inhibits multidrug resistance in Staphylococcus aureus and potentiates the action of inactive antibiotics in vitro. Journal of Applied Microbiology 117: 996–1011. DOI 10.1111/jam.12595. [Google Scholar] [CrossRef]
Fiamegos YC, Kastritis PL, Exarchou V, Han H, Bonvin AMJJ, Vervoort J, Lewis K, Hamblin MR, Tegos GP (2011). Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS One 6: e18127. DOI 10.1371/journal.pone.0018127. [Google Scholar] [CrossRef]
Fleck JD, Betti AH, Da Silva FP, Troian EA, Olivaro C, Ferreira F, Verza SG (2019). Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular chemical characteristics and biological activities. Molecules 24: 171. DOI 10.3390/molecules24010171. [Google Scholar] [CrossRef]
Guo Y, Liu Y, Zhang Z, Chen M, Zhang D, Tian C, Liu M, Jiang G (2020). The antibacterial activity and mechanism of action of luteolin against Trueperella pyogenes. Infection and Drug Resistance 13: 1697–1711. DOI 10.2147/IDR.S253363. [Google Scholar] [CrossRef]
Gupta P, Patel DK, Gupta VK, Pal A, Tandon S, Darokar MP (2017). Citral, a monoterpenoid aldehyde interacts synergistically with norfloxacin against methicillin resistant Staphylococcus aureus. Phytomedicine 34: 85–96. DOI 10.1016/j.phymed.2017.08.016. [Google Scholar] [CrossRef]
Hussain S, Joo J, Kang J, Kim B, Braun GB, She ZG, Kim D, Mann AP, Mölder T, Teesalu T, Carnazza S, Guglielmino S, Sailor MJ, Ruoslahti E (2018). Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nature Biomedical Engineering 2: 95–103. DOI 10.1038/s41551-017-0187-5. [Google Scholar] [CrossRef]
Jafri H, Khan MSA, Ahmad I (2019). In vitro efficacy of eugenol in inhibiting single and mixed-biofilms of drug-resistant strains of Candida albicans and Streptococcus mutans. Phytomedicine 54: 206–213. DOI 10.1016/j.phymed.2018.10.005. [Google Scholar] [CrossRef]
Kharlamova ТV, Gabdrakipov AV, Seidakhmetova PB, Praliyev KD (2020). Antibacterial activity of synthesized derivatives of purpurin containing cyсlopropane and cyclobutane fragment. Eurasian Chemico–Technological Journal 22: 213–218. DOI 10.18321/ectj972. [Google Scholar] [CrossRef]
Kouidhi B, Al Qurashi YMA, Chaieb K (2015). Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microbial Pathogenesis 80: 39–49. DOI 10.1016/j.micpath.2015.02.007. [Google Scholar] [CrossRef]
Kozioł A, Grela E, Macegoniuk K, Grabowiecka A, Lochyński S (2020). Synthesis of nitrogen-containing monoterpenoids with antibacterial activity. Natural Product Research 34: 1074–1079. DOI 10.1080/14786419.2018.1548456. [Google Scholar] [CrossRef]
Kuiper EG, Conn GL (2014). Binding induced RNA conformational changes control substrate recognition and catalysis by the Thiostrepton resistance methyltransferase (Tsr). Journal of Biological Chemistry 289: 26189–26200. DOI 10.1074/jbc.M114.574780. [Google Scholar] [CrossRef]
Kumar M, Curtis A, Hoskins C (2018). Application of nanoparticle technologies in the combat against anti-microbial resistance. Pharmaceutics 10: 11. DOI 10.3390/pharmaceutics10010011. [Google Scholar] [CrossRef]
Kumar CM, Singh SA (2015). Bioactive lignans from sesame (Sesamum indicum L.Evaluation of their antioxidant and antibacterial effects for food applications. Journal of Food Science and Technology 52: 2934–2941. DOI 10.1007/s13197-014-1334-6. [Google Scholar] [CrossRef]
Lakshminarayanan R, Ye E, Young DJ, Li Z, Loh XJ (2018). Recent advances in the development of antimicrobial nanoparticles for combating resistant pathogens. Advanced Healthcare Materials 7: 1701400. DOI 10.1002/adhm.201701400. [Google Scholar] [CrossRef]
Lall N, Canha MD, Szuman K, Charrouf Z, Davids LM, Rademan S (2019). The anti-proliferative and anti-bacterial activity of argan oil and crude saponin extract from Argania spinosa (L.) Skeels. Pharmacognosy Journal 11: 26–31. DOI 10.5530/pj.2019.1.5. [Google Scholar] [CrossRef]
Lederberg J (2000). Infectious history. Science 288: 287–293. DOI 10.1126/science.288.5464.287. [Google Scholar] [CrossRef]
Lee JH, Kim YG, Yong Ryu S, Lee J (2016). Calcium-chelating alizarin and other anthraquinones inhibit biofilm formation and the hemolytic activity of Staphylococcus aureus. Scientific Reports 6: 19267. DOI 10.1038/srep19267. [Google Scholar] [CrossRef]
Lin YT, Huang YW, Liou RS, Chang YC, Yang TC (2014). MacABCsm, an ABC-type tripartite efflux pump of Stenotrophomonas maltophilia involved in drug resistance, oxidative and envelope stress tolerances and biofilm formation. Journal of Antimicrobial Chemotherapy 69: 3221–3226. DOI 10.1093/jac/dku317. [Google Scholar] [CrossRef]
Liu M, Yang K, Wang J, Zhang J, Qi Y, Wei X, Fan M (2019). Young astringent persimmon tannin inhibits methicillin-resistant Staphylococcus aureus isolated from pork. LWT Food Science and Technology 100: 48–55. DOI 10.1016/j.lwt.2018.10.047. [Google Scholar] [CrossRef]
Lovecká P, Svobodová A, Macůrková A, Vrchotová B, Demnerová K, Wimmer Z (2020). Decorative magnolia plants: A comparison of the content of their biologically active components showing antimicrobial effects. Plants 9: 879. DOI 10.3390/plants9070879. [Google Scholar] [CrossRef]
Macêdo SK, Almeida TS, Alencar Filho JMT, Lima KSB, Libório RC et al. (2019). Phytochemical identification and quantification of quercetin in Triplaris gardneriana wedd. leaves by HPLC-DAD with evaluation of antibacterial activity. Natural Product Research, 1–6. DOI 10.1080/14786419.2019.1682573. [Google Scholar] [CrossRef]
Mishra MP, Rath S, Swain SS, Ghosh G, Das D, Padhy RN (2017). In vitro antibacterial activity of crude extracts of 9 selected medicinal plants against UTI causing MDR bacteria. Journal of King Saud University–Science 29: 84–95. DOI 10.1016/j.jksus.2015.05.007. [Google Scholar] [CrossRef]
Molee W, Phanumartwiwath A, Kesornpun C, Sureram S, Ngamrojanavanich N, Ingkaninan K, Mahidol C, Ruchirawat S, Kittakoop P (2018). Naphthalene derivatives and quinones from Ventilago denticulata and their nitric oxide radical scavenging, antioxidant, cytotoxic, antibacterial, and phosphodiesterase inhibitory activities. Chemistry & Biodiversity 15: 1700537. DOI 10.1002/cbdv.201700537. [Google Scholar] [CrossRef]
Moloney MG (2016). Natural products as a source for novel antibiotics. Trends in Pharmacological Sciences 37: 689–701. DOI 10.1016/j.tips.2016.05.001. [Google Scholar] [CrossRef]
Monteiro T, Wysocka M, Tellez E, Monteiro O, Spencer L, Veiga E et al. (2020). A five-year retrospective study shows increasing rates of antimicrobial drug resistance in Cabo Verde for both Staphylococcus aureus and Escherichia coli. Journal of Global Antimicrobial Resistance 22: 483–487. DOI 10.1016/j.jgar.2020.04.002. [Google Scholar] [CrossRef]
Moo CL, Yang SK, Osman MA, Yuswan MH, Loh JY, Lim WM, Lim SHE, Lai KS (2020). Antibacterial activity and mode of action of β-caryophyllene on Bacillus cereus. Polish Journal of Microbiology 69: 1–6. DOI 10.33073/pjm-2020-007. [Google Scholar] [CrossRef]
Moussaoui F, Alaoui T (2016). Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants. Asian Pacific Journal of Tropical Biomedicine 6: 32–37. DOI 10.1016/j.apjtb.2015.09.024. [Google Scholar] [CrossRef]
Mun SH, Joung DK, Kim YS, Kang OH, Kim SB, Seo YS, Kim YC, Lee DS, Shin DW, Kweon KT, Kwon DY (2013). Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 20: 714–718. DOI 10.1016/j.phymed.2013.02.006. [Google Scholar] [CrossRef]
Naderi N, Safdarpour A, Hakemi-Vala M, Masoomi H (2020). Antimicrobial resistance pattern and prevalence of extended spectrum beta-lactamase in non-fermenting Gram-negative bacteria, isolated from burn wounds: A prospective study from a tertiary burn center. Journal of Burn Care & Research 41: S59–S60. DOI 10.1093/jbcr/iraa024.093. [Google Scholar] [CrossRef]
Patel J, Yin HB, Bauchan G, Mowery J (2020). Inhibition of Escherichia coli O157: H7 and Salmonella enterica virulence factors by benzyl isothiocyanate. Food Microbiology 86: 103303. DOI 10.1016/j.fm.2019.103303. [Google Scholar] [CrossRef]
Peng L, Kang S, Yin Z, Jia R, Song X, Li L, Li Z, Zou Y, Liang X, Li L, He C, Ye G, Yin L, Shi F, Lv C, Jing B (2015). Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. International Journal of Clinical and Experimental Pathology 8: 5217–5223. [Google Scholar]
Pourhosseini SH, Ahadi H, Aliahmadi A, Mirjalili MH (2020). Chemical composition and antibacterial activity of the carvacrol-rich essential oils of Zataria multiflora Boiss. (Lamiaceae) from southern natural habitats of Iran. Journal of Essential Oil-Bearing Plants 23: 779–787. DOI 10.1080/0972060X.2020.1824688. [Google Scholar] [CrossRef]
Pourmand A, Mazer-Amirshahi M, Jasani G, May L (2017). Emerging trends in antibiotic resistance: Implications for emergency medicine. The American Journal of Emergency Medicine 35: 1172–1176. DOI 10.1016/j.ajem.2017.03.010. [Google Scholar] [CrossRef]
Prakash S, Elavarasan N, Subashini K, Kanaga S, Dhandapani R, Sivanandam M, Kumaradhas P, Thirunavukkarasu C, Sujatha V (2020). Isolation of hesperetin—A flavonoid from Cordia sebestena flower extract through antioxidant assay guided method and its antibacterial, anticancer effect on cervical cancer via in vitro and in silico molecular docking studies. Journal of Molecular Structure 1207: 127751. DOI 10.1016/j.molstruc.2020.127751. [Google Scholar] [CrossRef]
Prasch S, Bucar F (2015). Plant derived inhibitors of bacterial efflux pumps: An update. Phytochemistry Reviews 14: 961–974. DOI 10.1007/s11101-015-9436-y. [Google Scholar] [CrossRef]
Rahman MM, Garvey M, Piddock LJV, Gibbons S (2008). Antibacterial terpenes from the oleoresin of Commiphora molmol (Engl.). Phytotherapy Research 22: 1356–1360. DOI 10.1002/ptr.2501. [Google Scholar] [CrossRef]
Ribeiro AB, Abdelnur PV, Garcia CF, Belini A, Severino VGP et al. (2008). Chemical characterization of Citrus sinensis grafted on C. limonia and the effect of some isolated compounds on the growth of Xylella fastidiosa. Journal of Agricultural and Food Chemistry 56: 7815–7822. DOI 10.3390/molecules25030493. [Google Scholar] [CrossRef]
Sayout A, Ouarhach A, Rabie R, Dilagui I, Soraa N, Romane A (2020). Evaluation of antibacterial activity of Lavandula pedunculata subsp. atlantica (BRAUN-BLANQ) Romo essential oil and selected terpenoids against resistant bacteria strains-structure–activity relationships. Chemistry & Biodiversity 17: e1900496. DOI 10.1002/cbdv.201900496. [Google Scholar] [CrossRef]
Shin J, Prabhakaran VS, Kim K (2018). The multi-faceted potential of plant-derived metabolites as antimicrobial agents against multidrug-resistant pathogens. Microbial Pathogenesis 116: 209–214. DOI 10.1016/j.micpath.2018.01.043. [Google Scholar] [CrossRef]
Shriram V, Khare T, Bhagwat R, Shukla R, Kumar V (2018). Inhibiting bacterial drug efflux pumps via phyto-therapeutics to combat threatening antimicrobial resistance. Frontiers in Microbiology 9: 2990. DOI 10.3389/fmicb.2018.02990. [Google Scholar] [CrossRef]
Sivaranjani M, Leskinen K, Aravindraja C, Saavalainen P, Pandian SK, Skurnik M, Ravi AV (2019). Deciphering the antibacterial mode of action of alpha-mangostin on Staphylococcus epidermidis RP62A through an integrated transcriptomic and proteomic approach. Frontiers in Microbiology 10: 150. DOI 10.3389/fmicb.2019.00150. [Google Scholar] [CrossRef]
Soto SM (2013). Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 4: 223–229. DOI 10.4161/viru.23724. [Google Scholar] [CrossRef]
Srinivasan VB, Rajamohan G, Gebreyes WA (2009). Role of AbeS, a novel efflux pump of the SMR family of transporters, in resistance to antimicrobial agents in Acinetobacter baumannii. Antimicrobial Agents and Chemotherapy 53: 5312–5316. DOI 10.1128/AAC.00748-09. [Google Scholar] [CrossRef]
Sultan FI, Khorsheed AC, Khalel AMS (2020). Separation of four fatty acids and two phenolic compounds from Camellia sinensis using chromatographic techniques and evaluation of their antibacterial activity. EurAsian Journal of BioSciences 14: 2123–2129. [Google Scholar]
Sun J, Deng Z, Yan A (2014). Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochemical and Biophysical Research Communications 453: 254–267. DOI 10.1016/j.bbrc.2014.05.090. [Google Scholar] [CrossRef]
Tankeo SB, Tane P, Kuete V (2015). In vitro antibacterial and antibiotic-potentiation activities of the methanol extracts from Beilschmiedia acuta, Clausena anisata, Newbouldia laevis and Polyscias fulva against multidrug-resistant Gram-negative bacteria. BMC Complementary and Alternative Medicine 15: 412. DOI 10.1186/s12906-015-0944-5. [Google Scholar] [CrossRef]
Tsitsopoulos PP, Iosifidis E, Antachopoulos C, Anestis DM, Karantani E, Karyoti A, Papaevangelou G, Kyriazidis E, Roilides E, Tsonidis C (2016). Nosocomial bloodstream infections in neurosurgery: A 10-year analysis in a center with high antimicrobial drug-resistance prevalence. Acta Neurochirurgica 158: 1647–1654. DOI 10.1007/s00701-016-2890-5. [Google Scholar] [CrossRef]
Vambe M, Aremu AO, Chukwujekwu JC, Finnie JF, Van Staden J (2018). Antibacterial screening, synergy studies and phenolic content of seven South African medicinal plants against drug-sensitive and -resistant microbial strains. South African Journal of Botany 114: 250–259. DOI 10.1016/j.sajb.2017.11.011. [Google Scholar] [CrossRef]
Viault G, Kempf M, Ville A, Alsabil K, Perrot R, Richomme P, Helesbeux JJ, Seraphin D (2021). Semisynthetic vitamin E derivatives as potent antibacterial agents against resistant gram-positive pathogens. ChemMedChem 16: 881–890. DOI 10.1002/cmdc.202000792. [Google Scholar] [CrossRef]
Wang CY, Chen YW, Hou CY (2019). Antioxidant and antibacterial activity of seven predominant terpenoids. International Journal of Food Properties 22: 230–238. DOI 10.1080/10942912.2019.1582541. [Google Scholar] [CrossRef]
Wang W, Liao Y, Huang X, Tang C, Cai P (2018). A novel xanthone dimer derivative with antibacterial activity isolated from the bark of Garcinia mangostana. Natural Product Research 32: 1769–1774. DOI 10.1080/14786419.2017.1402315. [Google Scholar] [CrossRef]
World Health Organization (2002). Antimicrobial resistance, Fact sheet no. 194. Geneva, Switzerland. [Google Scholar]
World Health Organization (2017). Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Geneva, Switzerland. [Google Scholar]
World Health Organization (2019). High levels of antibiotic resistance found worldwide, new data shows. Geneva, Switzerland. [Google Scholar]
Woźnicka E, Zapała L, Pieniążek E, Kosińska M, Ciszkowicz E, Lecka-Szlachta K, Pusz J, Maciołek U, Dronka J (2017). Synthesis, characterization and antibacterial studies of Tm(IIIYb(III) and Lu(III) complexes of morin. Journal of Coordination Chemistry 70: 1451–1463. DOI 10.1080/00958972.2017.1291935. [Google Scholar] [CrossRef]
Yan T, Ji M, Sun Y, Yan T, Zhao J, Zhang H, Wang Z (2020). Preparation and characterization of baicalein/hydroxypropyl-β-cyclodextrin inclusion complex for enhancement of solubility, antioxidant activity and antibacterial activity using supercritical antisolvent technology. Journal of Inclusion Phenomena and Macrocyclic Chemistry 96: 285–295. DOI 10.1007/s10847-019-00970-2. [Google Scholar] [CrossRef]
Yasbolaghi Sharahi J, Aliakbar Ahovan Z, Taghizadeh Maleki D, Riahi Rad Z, Riahi Rad Z, Goudarzi M, Shariati A, Bostanghadiri N, Abbasi E, Hashemi A (2020). In vitro antibacterial activity of curcumin-meropenem combination against extensively drug-resistant (XDR) bacteria isolated from burn wound infections. Avicenna Journal of Phytomedicine 10: 3–10. [Google Scholar]
Yin L, Chen J, Wang K, Geng Y, Lai W, Huang X, Chen D, Guo H, Fang J, Chen Z, Tang L, Huang C, Li N, Ouyang P (2020). Study the antibacterial mechanism of cinnamaldehyde against drug resistant Aeromonas hydrophila in vitro. Microbial Pathogenesis 145: 104208. DOI 10.1016/j.micpath.2020.104208. [Google Scholar] [CrossRef]
Zacchino SA, Butassi E, Cordisco E, Svetaz LA (2017a). Hybrid combinations containing natural products and antimicrobial drugs that interfere with bacterial and fungal biofilms. Phytomedicine 37: 14–26. DOI 10.1016/j.phymed.2017.10.021. [Google Scholar] [CrossRef]
Zacchino SA, Butassi E, Liberto MD, Raimondi M, Postigo A, Sortino M (2017b). Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine 37: 27–48. DOI 10.1016/j.phymed.2017.10.018. [Google Scholar] [CrossRef]
Zhang Q, Lyu Y, Huang J, Zhang X, Yu N, Wen Z, Chen S (2020). Antibacterial activity and mechanism of sanguinarine against Providencia rettgeri in vitro. PeerJ 8: e9543. DOI 10.7717/peerj.9543. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |