DOI:10.32604/biocell.2021.016502

| BIOCELL DOI:10.32604/biocell.2021.016502 |  |
| Review |
Nanoscale interactions between the nicotinic acetylcholine receptor and cholesterol
Laboratory of Molecular Neurobiology, Institute of Biomedical Research (BIOMED) UCA-CONICET, Faculty of Medical Sciences, Buenos Aires, Argentina
*Address correspondence to: Francisco J. Barrantes, francisco_barrantes@uca.edu.ar
#This is a solicited review summarizing the plenary lecture presented at the joint international meeting of the Pan-American Association for Biochemistry and Molecular Biology and the Argentine Society for Biochemical and Molecular Biology Investigations (SAIB) in Salta, Argentina
Received: 12 March 2021; Accepted: 29 April 2021
Abstract: Cholesterol is a major lipid in biological membranes. It not only plays a structural role but also modulates a wide range of functional properties of neurotransmitter and hormone receptors and ion channels. The membrane-embedded segments of the paradigm neurotransmitter receptor for acetylcholine (nAChR) contain linear sequences of amino acids with the capacity to recognize cholesterol. These cholesterol consensus domains have been designated as “CARC” and its mirror sequence “CRAC”. CARC preferentially occurs in the exoplasmic-facing membrane leaflet, and CRAC, in the cytoplasmic-facing hemilayer. Both motifs are highly conserved among ion-channel and neurotransmitter receptor proteins in vertebrate nervous systems, where they recognize cholesterol, and in prokaryotic homologues in bacteria, where they recognize hopanoids. This phylogenetically conserved trait is an indication that the hopanoids in some bacteria and cholesterol in eukaryotes subserve analogous functions, probably contributing to the stability of membrane-embedded protein domains. Structural studies from our laboratory using superresolution optical microscopy (“nanoscopy”) have disclosed other interrelated functional and structural properties exerted by cholesterol on the nAChR. The neutral lipid content at the cell surface influences both the macromolecular organization of the receptor and its translational mobility (diffusion) in the plane of the membrane.
Keywords: Cholesterol; Pentameric ligand-gated ion channel; Nicotinic acetylcholine receptor; Membrane proteins; Evolution; Nanoscopy; Cholesterol-recognition domains
A wealth of accumulated evidence in recent years supports the notion that cholesterol binds to neurotransmitter and hormone receptors and to ligand- and voltage-gated ion channels, exerting pleiotropic modulatory functions on their ligand-recognition, gating, and ion permeation properties (Levitan and Barrantes, 2012; Levitan et al., 2014). Probably one of the most extensively studied properties of cholesterol in membrane proteins is its ability to modify the compartmentalization of such proteins through their inclusion or exclusion in/from specialized membrane domains of microscopic/nanoscopic dimensions, commonly termed “lipid rafts”. Cholesterol effects have been divided into those exerted in a non-specific, indirect manner, e.g., changes in the physical state of the bulk lipid bilayer such as membrane fluidity changes (El Battari et al., 1985; Lazar and Medzihradsky, 1992; Maguire and Druse, 1989) or curvature (Lee, 2004; Yesylevskyy et al., 2013) or, alternatively, more specific effects involving direct interactions with the membrane-spanning segments of the membrane proteins (Baier et al., 2011; Barrantes, 2004; Fantini and Barrantes, 2013; Popot et al., 1977; Posada et al., 2014).
The sterol biosynthetic machinery may have appeared on this planet roughly 2.7–2.4 billion years ago, because of the emergence of oxygenic photosynthesis with the resultant oxygenation of the atmosphere and oceans. Sterols may have initially played a protective role in eukaryotes, shielding cells from harmful oxidative stress induced by the higher oxygen levels on Earth (Brown and Galea, 2010; Galea and Brown, 2009). Once the cholesterol biosynthetic enzymes became integral constituents of eukaryotes, the endogenous de novo biosynthesis became the main source of cholesterol in cells, with only a small fraction originating from dietary sources (Bloch, 1965; van Meer, 1989). The mechanisms involved in the uptake of dietary cholesterol are strictly regulated and involve the participation of plasma lipoproteins (Barter et al., 1982; Mahley et al., 1984). Cholesterol efflux, and its subsequent transport in plasma via high-density lipoproteins, is also highly regulated (Rothblat et al., 1999).
In addition to being an essential structural building block in the plasmalemma of most cells (Lange and Steck, 2020; Van Meer et al., 2008), cholesterol performs more specialized functions in cell-surface membranes that harbor neurotransmitter and hormone receptors. The postsynaptic membrane of the neuromuscular junction is a representative example; in this membrane, cholesterol modulates the distribution and several other functional properties of the muscle-type nAChR (Barrantes, 2010, 2012). The homologous postsynaptic membrane of the electric fish electromotor synapse is another example of crosstalk between cholesterol and the nAChR: the receptor protein restricts the mobility of lipids in its annular belt region relative to bulk membrane lipid (Marsh and Barrantes, 1978), with sterols forming part of this protein-immobilized pool (reviewed in Barrantes, 2007). One of the most remarkable effects of cholesterol on muscle type nAChR is exerted on its stability at the cell surface. Modification of cholesterol levels affects the lifetime of the receptor at the plasma membrane and the rate (Borroni et al., 2007; Kumari et al., 2008) and endocytic mechanism (Borroni and Barrantes, 2011) of receptor internalization. These are important modulatory effects of cholesterol that affect the metabolic stability of active nicotinic receptors at the synapse.
Cholesterol recognition motifs in transmembrane proteins
The strategy behind the approach
The number of macromolecules whose structure has been solved at the atomic level by X-ray diffraction and other physical techniques constitutes only a small number (about 2,000) of proteins considering that the genes coding for membrane proteins constitute around 25% of the total. This is probably the main reason why direct structural evidence of the occurrence of cholesterol recognition sites in membrane proteins is relatively scarce. Given such shortage of atomic structures, in-silico approaches have provided a valid alternative for the uncovering of cholesterol recognition motifs in membrane proteins. This entails an initial search in protein sequence data banks to identify the occurrence of specific linear sequences of transmembrane (TM) protein regions, eventually resulting in the successful identification of consensus motifs (Baier et al., 2011; Epand et al., 2006; Epand, 2008; Epand et al., 2010; Jamin et al., 2005) in a variety of membrane proteins. The first such consensus motif was defined by the linear array (L/V)-X1−5-(Y)-X1−5-(K/R), and was termed “cholesterol recognition amino acid consensus” (CRAC) (Jamin et al., 2005). My collaborator Javier Baier subsequently introduced the algorithm (K/R)-X1−5-(Y/F)-X1−5-(L/V), i.e., the mirror sequence of CRAC, which we coined “CARC” (Baier et al., 2011).
The cholesterol consensus motifs in the nAChR
The cholesterol consensus-motif strategy was initially applied to the study of the nAChR (Baier et al., 2011), the paradigm pentameric ligand-gated ion channel (pLGIC) (Barrantes, 2015) in an attempt to characterize the structural and thermodynamic properties of the interactions between sterol molecules and the nAChR macromolecule. As shown in Fig. 1, several cholesterol molecules (~15, Mantipragada et al., 2003) can be accommodated around the membrane-embedded region of the nAChR. The total free energy of interaction between the nAChR and cholesterol was found to be in the order of −510/−530 kJ/mol, i.e., above −100 kJ/mol per subunit of this pentameric macromolecule. The transmembrane 4 segment in the human nAChR γ subunit (428RVCFLAML435) displayed an exceptionally high energy of interaction with cholesterol (−60 kJ/mol), i.e., ~60% of the total energy contributed by the entire subunit.
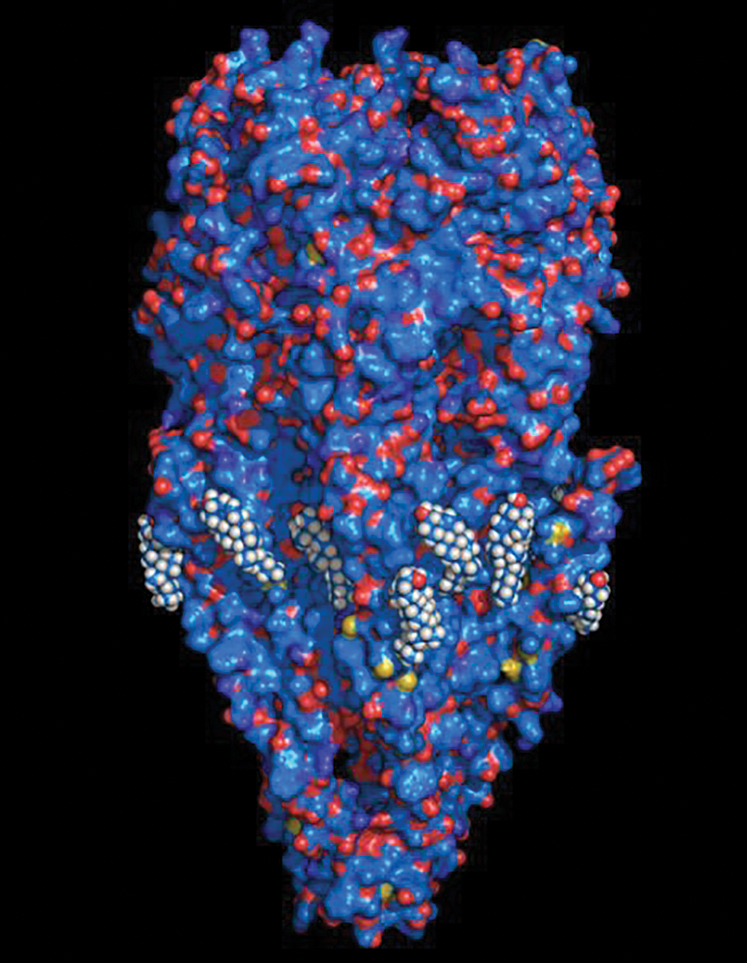
Figure 1: Surface-rendering molecular representation of the nicotinic acetylcholine receptor (nAChR) macromolecule with cholesterol molecules surrounding the lipid-belt region of its transmembrane region.
Experimental demonstration of direct contacts between cholesterol and proteins was missing for membrane proteins other than G protein-coupled receptors (GPCRs). We therefore studied the interaction of cholesterol with the CARC motif in the nAChR by resorting to two biophysical techniques: lipid monolayer studies and nuclear magnetic resonance (NMR) spectroscopy. We analyzed cholesterol interactions with the wild-type or a mutated cholesterol-recognition motif in the transmembrane segment 4 of the nAChR. The CARC wild-type motif was found to exhibit a high affinity for the sterol, which was largely abolished upon substituting phenylalanine for alanine (F-452/A mutant). Phenylalanine 452 is the central residue in the CARC motif. When this residue was replaced by tryptophan (F-452/W mutation) the interaction with cholesterol remained intact, indicating that aromatic residues in the mid region of the motif are key for the interaction with cholesterol. We also demonstrated that the motif is cholesterol specific (CARC did not recognize phosphatidylcholine) and showed the concentration dependence of the binding (Fantini et al., 2016b). For the NMR experiments, we employed a synthetic 13C/15N-labeled peptide (Asp464-Val492 in Torpedo marmorata γ-subunit transmembrane domain 4). MAS triple resonance magic-angle spinning deuterium NMR was performed using deuterated Ala471. Addition of cholesterol reduced the rotational motion of the peptide immersed in the phospholipid bilayer, consistent with the cholesterol-mediated peptide oligomerization, as previously found in collaborative work with Manuel Prieto’s laboratory (de Almeida et al., 2006; de Almeida et al., 2004).
We subsequently extended the thermodynamic analyses to several other membrane proteins. We found that the CARC motif displays higher affinity for cholesterol than the CRAC motif (Fantini and Barrantes, 2013). The reason for this is the “snorkeling” effect exerted by Lys/Arg residues (Strandberg and Killian, 2003) in combination with the structure of cholesterol itself (Fantini and Barrantes, 2009, 2013; Fantini et al., 2016a).
Emergence of cholesterol consensus motifs during phylogenetic evolution
In another series of studies, we asked ourselves whether (and if so when) consensus cholesterol-recognition motifs appeared in the course of phylogenetic evolution of the pLGICs. We found that occurrence of these domains covered a wide evolutionary span, from ion channel proteins present in bacteria to channels in Homo sapiens brains. We explored in detail the presence of these domains in the structural homologs of vertebrate pLGIC in the ion-channel protein GLIC from cyanobacterium Gloebacter violaceous and its homologue from Erwinia chrysanthemi. We found that Cyanobacteria exhibit affinity for hopanoids, sterol-like molecules with high structural similarity to sterols (Fig. 2).
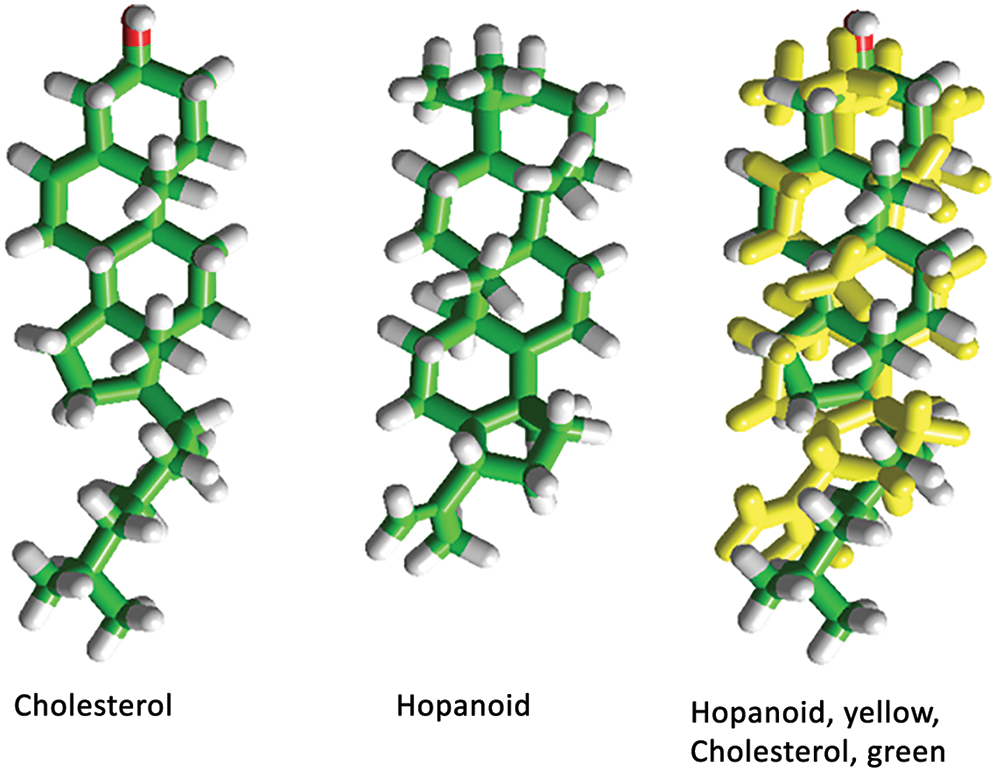
Figure 2: 3D chemical structure of cholesterol and diploptene, a typical hopanoid sterol-like molecule.
The overlapped structures on the right point to the similarities between the two evolutionarily distant molecules.
The conservation of the cholesterol-recognition motifs along millions of years of phylogenetic evolution led us to suggest that these motifs convey functional and/or structural properties (Barrantes, 2015; Barrantes and Fantini, 2016). Strong evidence in support of this concept is provided by experimental work from our laboratory and other groups showing that mutations in amino acid residues in the membrane-embedded regions containing or close to CARC/CARC-like domains of the nAChR alter its ion permeation properties (review in Barrantes, 2007).
Functional modulation of the nAChR cell-surface distribution by cholesterol
A further functional modulatory role of cholesterol was explored in a mammalian cell model system robustly expressing adult-type muscle nAChR using optical superresolution microscopy. Static imaging of fixed cells was initially conducted using stimulated emission depletion (STED) nanoscopy (Kellner et al., 2007). The nAChR was found to be distributed in a non-random fashion at the cell surface: clusters of nanometric dimensions were observed in the first study of this kind, showing the nanoscale packing of a neurotransmitter receptor imaged beyond the diffraction limit. Acute cholesterol depletion mediated by methyl-β-cyclodextrin modified this pattern, altering the short- and the long-range organization of the receptors at the cell surface. At the short-range, the nanoclusters became larger, from 55 nm mean diameter to 116 nm after cholesterol reduction, possibly resulting from the coalescence of smaller nanoclusters due to cholesterol-dependent changes in receptor-receptor interactions. The long-range changes consisted of an increase in the ordering of the nAChR clusters at spacings of 600 to 2,000 nm, i.e., one order of magnitude larger, which we interpreted as resulting from cholesterol effect on the cortical cytoskeletal network (Kellner et al., 2007). The influence of cholesterol-sensitive interactions with the actin cytoskeleton extends over the range of a few microns (Kwik et al., 2003).
Dynamics of the nAChR translational motion studied by single-molecule localization microscopy
More recently, we studied dynamic aspects of cholesterol modulation of the nAChR in living cells using total internal reflection fluorescence (TIRF) microscopy combined with single-particle tracking (SPT) methods (Almarza et al., 2014). This study recorded the lateral translation of cell-surface nAChR nanoclusters in real time, disclosing the wide range of motional regimes exhibited by the cell-surface nAChR. In spite of the excellent signal-to-noise ratio afforded by the TIRF technique, the study did not provide sufficient resolution to follow the dynamics of individual constituents of the nanoclusters.
Stochastic optical reconstruction microscopy (STORM) is a form of single-molecule localization microscopy. Application of this method allowed us to track the motion of individual nAChR molecules labeled with fluorescent α-bungarotoxin (Mosqueira et al., 2018). Essentially all nAChR single-molecule trajectories consisted of free Brownian walks interrupted by confinement episodes with average durations of ~400 ms (Fig. 3). The nAChR translational motion covers an enormous span of diffusional regimes, from highly subdiffusive to superdiffusive. Notice that even the superdiffusive trajectories experience confinement stop-overs. Fig. 3 also shows the influence of cholesterol on diffusional properties of the receptor.
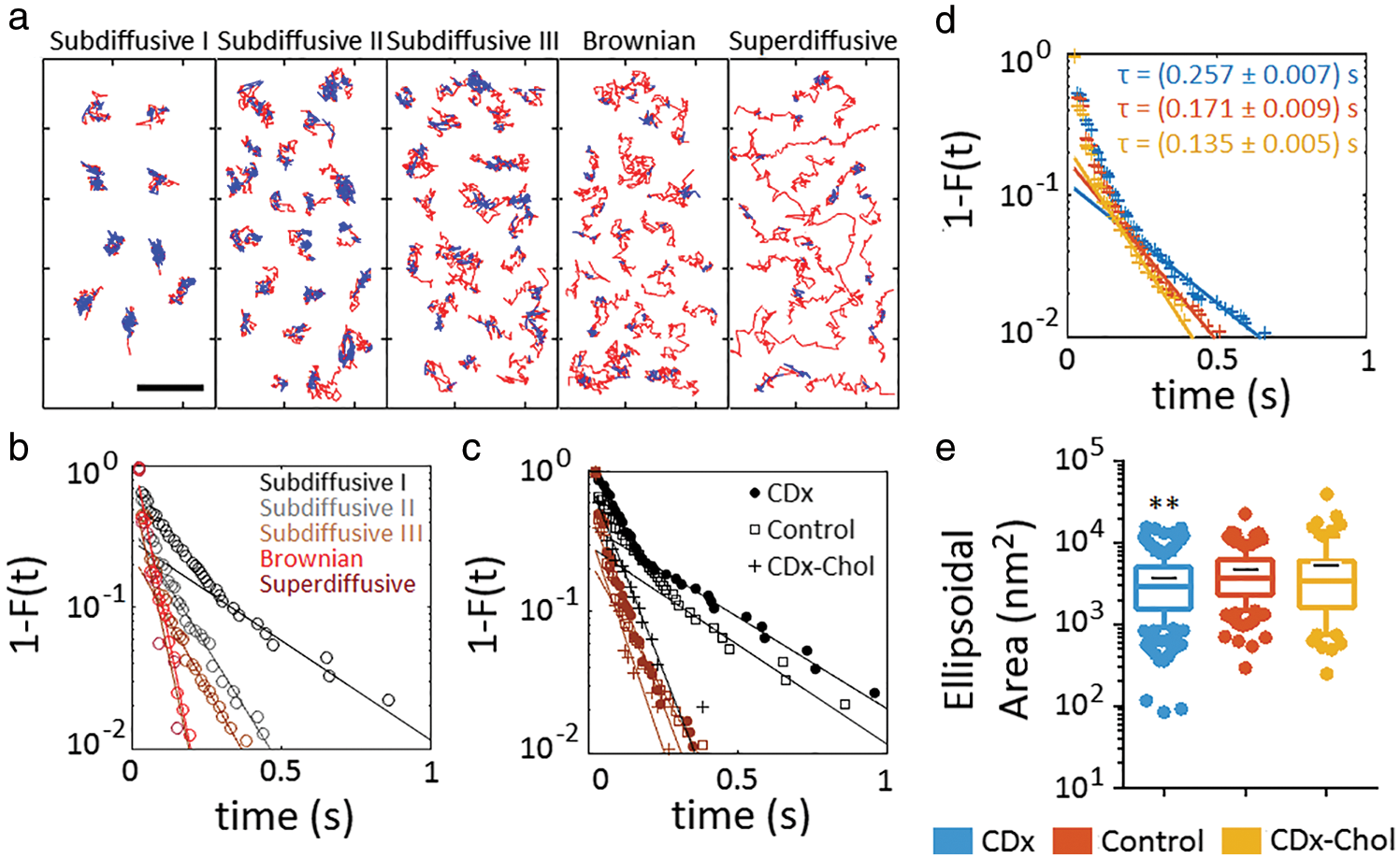
Figure 3: Microscopic heterogeneity of nAChR single-molecule trajectories. Single molecules exhibit transient confinement within individual trajectories.
(a) Recurrence analysis of individual trajectories (Sikora et al., 2017) identifies the free (unconfined) diffusing portion of each individual trajectory (red) and transient confinement sojourns (blue) within the trajectories under control conditions. Notice the decreasing immobilization (confinement) from subdiffusive I to the superdiffusive subpopulation. Bar: 1 µm. (b) Complementary cumulative distribution function of the residence times in the confined state of each subpopulation (control conditions). (c) The same as in (b) but showing the effect of cholesterol modification on the subdiffusive I and III subpopulation (same colour code as in (b)). (d) The same as in (c), but for the total population of nAChRs after cholesterol enrichment (CDx-cholesterol) and cholesterol depletion mediated by methyl-β-cyclodextrin (CDx). (e) Ellipsoidal area of the trajectories´ portion in the confined state, obtained by fitting the confinement domain in the trajectory with an elliptic surface. Whiskers in box plots correspond to 95% confidence intervals; the limits indicate 75% confidence intervals; the black–symbols indicate the mean and the horizontal lines the median in each case. The dots outside the confidence intervals are outliers. **P < 0.01. From reference Mosqueira et al. (2018) under a Creative Commons Attribution 4.0 International License.
Possible implications of highly subdiffusive nAChR motions in myasthenia gravis
Myasthenia gravis is an autoimmune disease that compromises the function of the peripheral cholinergic synapse because the autoantibodies against the nAChR and other constituents of the neuromuscular junction accelerate the disappearance of the targeted molecules and the complement-mediated destruction of the synaptic apparatus. A subsequent work undertaken on nAChRs labeled with the monoclonal antibody mAb35 enabled us to explore some of the actions exerted by antibody crosslinking of the receptor, mimicking the initial events met when pathogenic autoantibodies attack the neuromuscular junction in myasthenia gravis (see review in Paz and Barrantes, 2019). Antibody-tagged nAChRs (Mosqueira et al., 2020) differed in their translational diffusion from those receptors labeled with fluorescent α-bungarotoxin (Mosqueira et al., 2018). Antibody-tagged nAChR spent more time in the confined state, some of them essentially motionless in terms of translation in the plane of the membrane. We interpret these results as an indication that multivalent antibody-induced crosslinking of the receptor leads to the formation of supramolecular self-aggregates that obstruct its free diffusion, as schematically shown in Fig. 4.
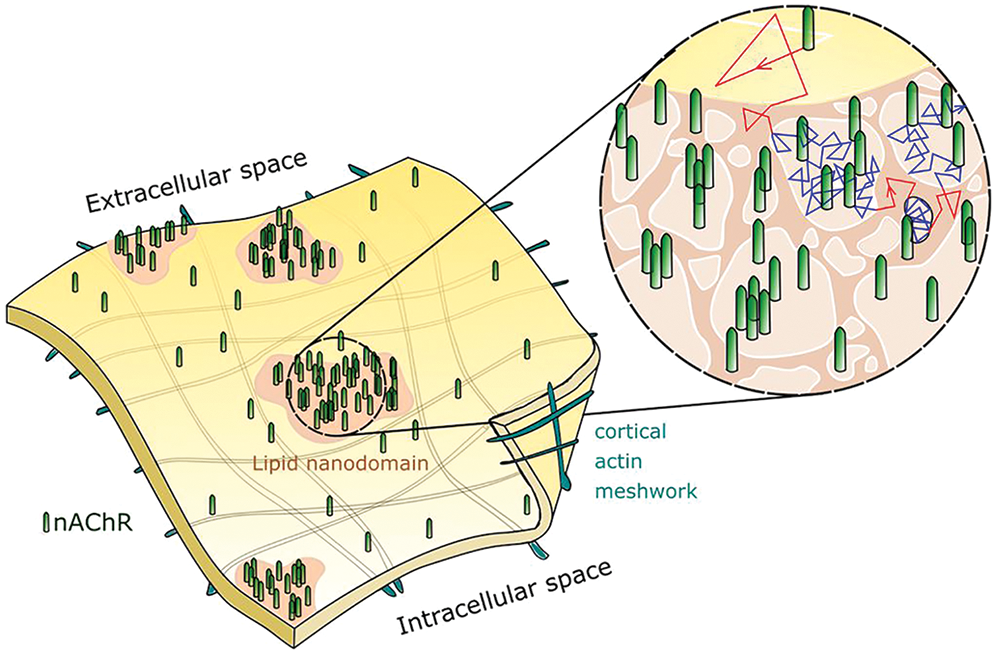
Figure 4: Schematic diagram depicting nicotinic acetylcholine receptor molecules (nAChR, green cylinders) distributed in a non-random fashion at the cell surface; some nAChRs occur as isolated single molecules whereas others aggregate in the form of nanometer-sized clusters. The sensitivity of these supramolecular assemblies to changes in cholesterol levels (see Fig. 3) suggests that nanocluster-aggregated nAChRs predominantly reside in cholesterol-rich liquid-ordered lipid nanodomains. From a dynamic perspective, nAChRs undergo free Brownian diffusion (red traces) in liquid-disordered regions of the membrane (yellow surfaces) and slow down their motion (blue traces) within the liquid-ordered domains (light-brown areas), where they remain confined for millisecond-long periods until regaining thermally driven lateral motion. The sub-membrane actin meshwork that compartmentalizes the cytoplasmic face of the plasma membrane is another source of hindrance to translational motion of transmembrane proteins. However, the actin corrals occur at mesoscale distances (μm-scale), whereas single-molecule confinements cover linear distances of less than 100 nm. Illustration made by Alejo Mosqueira.
Moreover, we have experimentally demonstrated that mAb35 accelerates the internalization of nAChRs in the same cell line used for the nanoscopy experiments, as well as in C2C12 developing myotubes (Kumari et al., 2008). This is a cholesterol-dependent phenomenon; man-tailored depletion of the cell-surface cholesterol content by acute methyl-β-cyclodextrin treatment accelerates the rate of nAChR internalization and, more importantly, changes the endocytic route involved; the process no longer depends on the submembrane actin cytoskeleton network and the small kinases involved in endocytosis are replaced by other enzymatic cascades (Borroni and Barrantes, 2011). Enhanced endocytic rates are also characteristically observed at the neuromuscular synapse in myasthenia gravis (Engel and Fumagalli, 1982; Fumagalli et al., 1982; Kuncl et al., 1993).
Availability of Data and Materials: Data sharing is not applicable to this article as no datasets were generated or analyzed in this review article.
Author Contribution: The author confirms sole responsibility for the following: study conception and design, data collection, analysis and interpretation of results, and manuscript preparation.
Funding Statement: Experimental work quoted in this review was supported by Grants PICT 2011-0604 from FONCYT, Ministry of Science and Technology and PIP No. N° 112-201101-01023 from the National Scientific and Technical Research Council of Argentina (CONICET).
Conflicts of Interest: The author declares that there are no conflicts of interest regarding the present study.
Almarza G, Sanchez F, Barrantes FJ (2014). Transient cholesterol effects on nicotinic acetylcholine receptor cell-surface mobility. PLoS One 9: e100346. [Google Scholar]
Baier CJ, Fantini J, Barrantes FJ (2011). Disclosure of cholesterol recognition motifs in transmembrane domains of the human nicotinic acetylcholine receptor. Scientific Reports 1: 69. [Google Scholar]
Barrantes FJ (2004). Structural basis for lipid modulation of nicotinic acetylcholine receptor function. Brain Research Reviews 47: 71–95. [Google Scholar]
Barrantes FJ (2007). Cholesterol effects on nicotinic acetylcholine receptor. Journal of Neurochemistry 103: 72–80. [Google Scholar]
Barrantes FJ (2010). Cholesterol effects on nicotinic acetylcholine receptor: cellular aspects. In: Harris JR (ed.Cholesterol Binding and Cholesterol Transport Proteins, pp. 467–487. Springer Verlag. Dordrecht Heidelberg: London, New York. [Google Scholar]
Barrantes FJ (2012). Regulation of the nicotinic acetylcholine receptor by cholesterol as a boundary lipid. In: Levitan I, Barrantes FJ (eds.Cholesterol Regulation of Ion Channels and Receptors. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
Barrantes FJ (2015). Phylogenetic conservation of protein-lipid motifs in pentameric ligand-gated ion channels. Biochimica et Biophysica Acta (BBA)-Biomembranes 1848: 1796–1805. [Google Scholar]
Barrantes FJ, Fantini J (2016). From hopanoids to cholesterol: Molecular clocks of pentameric ligand-gated ion channels. Progress in Lipid Research 63: 1–13. [Google Scholar]
Barter PJ, Hopkins GJ, Calvert GD (1982). Transfers and exchanges of esterified cholesterol between plasma lipoproteins. Biochemical Journal 208: 1–7. [Google Scholar]
Bloch K (1965). The biological synthesis of cholesterol. Science 150: 19–28. [Google Scholar]
Borroni V, Baier CJ, Lang T, Bonini I, White MM, Garbus I, Barrantes FJ (2007). Cholesterol depletion activates rapid internalization of submicron-sized acetylcholine receptor domains at the cell membrane. Molecular Membrane Biology 24: 1–15. [Google Scholar]
Borroni V, Barrantes FJ (2011). Cholesterol modulates the rate and mechanism of acetylcholine receptor internalization. Journal of Biological Chemistry 286: 17122–17132. [Google Scholar]
Brown AJ, Galea AM (2010). Cholesterol as an evolutionary response to living with oxygen. Evolution 64: 2179–2183. [Google Scholar]
de Almeida RFM, Loura LM, Prieto M, Watts A, Fedorov A, Barrantes FJ (2006). Structure and dynamics of the γM4 transmembrane domain of the acetylcholine receptor in lipid bilayers: insights into receptor assembly and function. Molecular Membrane Biology 23: 305–315. [Google Scholar]
de Almeida RFM, Loura LMS, Prieto M, Watts A, Fedorov A, Barrantes FJ (2004). Cholesterol modulates the organization of the γM4 transmembrane domain of the muscle nicotinic acetylcholine receptor. Biophysical Journal 86: 2261–2272. [Google Scholar]
El Battari A, Ah-Kye E, Muller JM, Sari H, Marvaldi J (1985). Modification of HT 29 cell response to the vasoactive intestinal peptide (VIP) by membrane fluidization. Biochimie 67: 1217–1223. [Google Scholar]
Engel AG, Fumagalli G (1982). Mechanisms of acetylcholine receptor loss from the neuromuscular junction. Ciba Foundation Symposium 90: 197–224. [Google Scholar]
Epand RF, Thomas A, Brasseur R, Vishwanathan SA, Hunter E, Epand RM (2006). Juxtamembrane protein segments that contribute to recruitment of cholesterol into domains. Biochemistry 45: 6105–6114. [Google Scholar]
Epand RM (2008). Proteins and cholesterol-rich domains. Biochimica et Biophysica Acta (BBA)-Biomembranes 1778: 1576–1582. [Google Scholar]
Epand RM, Thomas A, Brasseur R, Epand RF (2010). Cholesterol interaction with proteins that partition into membrane domains: an overview. Subcellular Biochemistry 51: 253–278. [Google Scholar]
Fantini J, Barrantes FJ (2009). Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochimica et Biophysica Acta (BBA)-Biomembranes 1788: 2345–2361. [Google Scholar]
Fantini J, Barrantes FJ (2013). How cholesterol interacts with membrane proteins: an exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Frontiers in Physiology 4: 31. [Google Scholar]
Fantini J, Di Scala C, Baier CJ, Barrantes FJ (2016a). Molecular mechanisms of protein-cholesterol interactions in plasma membranes: Functional distinction between topological (tilted) and consensus (CARC/CRAC) domains. Chemistry and Physics of Lipids 199: 52–60. [Google Scholar]
Fantini J, Di Scala C, Evans LS, Williamson PTF, Barrantes FJ (2016b). A mirror code for protein-cholesterol interactions in the two leaflets of biological membranes. Scientific Reports 6: 21907. [Google Scholar]
Fumagalli G, Engel AG, Lindstrom J (1982). Ultrastructural aspects of acetylcholine receptor turnover at the normal end-plate and in autoimmune myasthenia gravis. Journal of Neuropathology & Experimental Neurology 41: 567–579. [Google Scholar]
Galea AM, Brown AJ (2009). Special relationship between sterols and oxygen: were sterols an adaptation to aerobic life? Free Radical Biology and Medicine 47: 880–889. [Google Scholar]
Jamin N, Neumann JM, Ostuni MA, Vu TK, Yao ZX et al. (2005). Characterization of the cholesterol recognition amino acid consensus sequence of the peripheral-type benzodiazepine receptor. Molecular Endocrinology 19: 588–594. [Google Scholar]
Kellner RR, Baier CJ, Willig KI, Hell SW, Barrantes FJ (2007). Nanoscale organization of nicotinic acetylcholine receptors revealed by stimulated emission depletion microscopy. Neuroscience 144: 135–143. [Google Scholar]
Kumari S, Borroni V, Chaudhry A, Chanda B, Massol R, Mayor S, Barrantes FJ (2008). Nicotinic acetylcholine receptor is internalized via a Rac-dependent, dynamin-independent endocytic pathway. Journal of Cell Biology 181: 1179–1193. [Google Scholar]
Kuncl RW, Wittstein I, Adams RN, Wiggins WW, Avila O, Pestronk A, Mcintosh K, Lucas D, Desilva S, Lehar M, Drachman DB (1993). A novel therapy for myasthenia gravis by reducing the endocytosis of acetylcholine receptors. Annals of the New York Academy of Sciences 681: 298–302. [Google Scholar]
Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M (2003). Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proceedings of the National Academy of Sciences of the United States of America 100: 13964–13969. [Google Scholar]
Lange Y, Steck TL (2020). Active cholesterol 20 years on. Traffic 21: 662–674. [Google Scholar]
Lazar DF, Medzihradsky F (1992). Altered microviscosity at brain membrane surface induces distinct and reversible inhibition of opioid receptor binding. Journal of Neurochemistry 59: 1233–1240. [Google Scholar]
Lee AG (2004). How lipids affect the activities of integral membrane proteins. Biochimica et Biophysica Acta 1666: 62–87. [Google Scholar]
Levitan I, Barrantes FJ (2012). Cholesterol Regulation of Ion Channels and Receptors. Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
Levitan I, Singh DK, Rosenhouse-Dantsker A (2014). Cholesterol binding to ion channels. Frontiers in Physiology 5: 65. [Google Scholar]
Maguire PA, Druse MJ (1989). The influence of cholesterol on synaptic fluidity, dopamine D1 binding and dopamine-stimulated adenylate cyclase. Brain Research Bulletin 23: 69–74. [Google Scholar]
Mahley RW, Innerarity TL, Rall SC,Jr., Weisgraber KH (1984). Plasma lipoproteins: apolipoprotein structure and function. Journal of Lipid Research 25: 1277–1294. [Google Scholar]
Mantipragada SB, Horvath LI, Arias HR, Schwarzmann G, Sandhoff K, Barrantes FJ, Marsh D (2003). Lipid-protein interactions and effect of local anesthetics in acetylcholine receptor-rich membranes from Torpedo marmorata electric organ. Biochemistry 42: 9167–9175. [Google Scholar]
Marsh D, Barrantes FJ (1978). Immobilized lipid in acetylcholine receptor-rich membranes from Torpedo marmorata. Proceedings of the National Academy of Sciences of the United States of America 75: 4329–4333. [Google Scholar]
Mosqueira A, Camino PA, Barrantes FJ (2018). Cholesterol modulates acetylcholine receptor diffusion by tuning confinement sojourns and nanocluster stability. Scientific Reports 8: 11974. [Google Scholar]
Mosqueira A, Camino PA, Barrantes FJ (2020). Antibody-induced crosslinking and cholesterol-sensitive, anomalous diffusion of nicotinic acetylcholine receptors. Journal of Neurochemistry 152: 663–674. [Google Scholar]
Paz ML, Barrantes FJ (2019). Autoimmune attack of the neuromuscular junction in myasthenia gravis: nicotinic acetylcholine receptors and other targets. ACS Chemical Neuroscience 10: 2186–2194. [Google Scholar]
Popot JL, Demel RA, Sobel A, van Deenen LL, Changeux JP (1977). Preferential affinity of acetylcholine receptor protein for certain lipids studied using monolayer cultures. Comptes Rendus Hebdomadaires des Seances de l’Academie des Sciences. Serie D: Sciences Naturelles 285: 1005–1008. [Google Scholar]
Posada IM, Fantini J, Contreras FX, Barrantes F, Alonso A, Goni FM (2014). A cholesterol recognition motif in human phospholipid scramblase 1. Biophysical Journal 107: 1383–1392. [Google Scholar]
Rothblat GH, Llera-Moya MDL, Arger V, Kellner-Weibel G, Wlliams DL, Phillips MC (1999). Cell cholesterol efflux: integration of old and new observations provides new insights. Journal of Lipid Research 40: 781–796. [Google Scholar]
Sikora G, Wilomanska A, Gajda J, Sole L, Akin EJ, Tamkun MM, Krapf D (2017). Elucidating distinct ion channel populations on the surface of hippocampal neuros via single-particle tracking recurrence analysis. Physical Review E 96: 062404. [Google Scholar]
Strandberg E, Killian JA (2003). Snorkeling of lysine side chains in transmembrane helices: how easy can it get? FEBS Letters 544: 69–73. [Google Scholar]
van Meer G (1989). Lipid traffic in animal cells. Annual Review of Cell Biology 5: 247–275. [Google Scholar]
van Meer G, Voelker DR, Feigenson GW (2008). Membrane lipids: where they are and how they behave. Nature Reviews Molecular Cell Biology 9: 112–124. [Google Scholar]
Yesylevskyy SO, Demchenko AP, Kraszewski S, Ramseyer C (2013). Cholesterol induces uneven curvature of asymmetric lipid bilayers. Scientific World Journal 2013: 965230. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |