DOI:10.32604/biocell.2022.020013

| BIOCELL DOI:10.32604/biocell.2022.020013 |  |
| Viewpoint |
Mesenchymal stem cells, the secretome and biomaterials: Regenerative medicine application
Department of Biosystems Engineering, Interdisciplinary Program in Smart Agriculture, Kangwon National University, Chuncheon, 341, Korea
*Address correspondence to: Ki-Taek Lim, ktlim@kangwon.ac.kr
#These two authors contributed equally to this manuscript
Received: 07 December 2021; Accepted: 10 March 2022
Abstract: Mesenchymal stem cells (MSCs) are multipotent cells usually isolated from bone marrow, endometrium, adipose tissues, skin, and dental pulp. MSCs played a crucial role in regenerative therapy and have been introduced as an interdisciplinary field between cell biology and material science. Recently, MSCs have been widely explored for their application in regenerative medicine and COVID-19 treatment. Different approaches to evaluate the future of biomaterials and stem cell properties have been developed. However, misconceptions and ethical issues still exist, such as MSCs being non-angiogenic, anti-apoptotic, and immunoregulatory competencies. Embryonic stem cells isolation primarily requires the consent of donors and can include the killing of fertilized eggs. These issues generate questions related to ethical and moral issues. However, MSCs have gained considerable attention for tissue regeneration owing to their differentiation ability with immunomodulatory effects. They are capable of secreting a broad range of biomolecules such as proteins, nucleic acids, exosomes, microRNAs, and membrane vesicles, collectively known as secretomes. Secretomes are released in response to the surrounding microenvironment. In this article, we briefly address topics related to the therapeutic potential of MSCs as an advanced approach in the field of regenerative medicine and various perspectives.
Keywords: Mesenchymal Stem Cells; Secretome; Biomaterials; Regenerative Medicine
Abbreviations
| Angiopoietin-1: | Ang-1 |
| Angiopoietin-2: | Ang-2 |
| Cardiac Troponin-I: | C-TnI |
| Vascular Endothelial Growth Factor: | VEGF |
| von Willebrand Factor: | vWF |
| Spinal Muscular Atrophy: | SMA |
| Interleukin-6: | IL-6 |
| Insulin-like growth factor 1: | IGF-1 |
| Transforming growth factor β: | TGF-β |
Mesenchymal stem cells (MSCs) are multipotent stromal cells capable of self-renewal and exhibit multilineage differentiation properties. MSCs are isolated from various human body tissues, including skin and bone marrow, which shows multipotent differentiation competencies (Marofi et al., 2019a). However, some ethical issues exist owing to the related properties of MSCs, such as pro-angiogenic, anti-apoptotic, and immunomodulatory attributes (Tavakoli et al., 2020). Many studies related to MSCs therapeutic potential in which MSCs derived extracellular vesicles accomplished a vital role. Additionally, a significant investigation revealed the potential applications of MSCs in a range of disorders, including cardiomyopathy, neurodegenerative diseases, cancers, and injuries related to the spinal cord, kidney, liver, and lungs (Bodart-Santos et al., 2019; Marofi et al., 2019b). Bone marrow resident c-kit+ stem cells (BMSCc+) were previously cultured with L-carnitine (LC) for cardiac cell therapy (Fathi et al., 2020). Upregulation of mRNA and protein associated with cardiac markers, including Ang-1, Ang-2, C-TnI, VEGF, vWF, and SMA, was observed in BMSCc+ with LC.
Enhanced expression of IL-6, IGF-1, TGF-β, and VEGF has also been observed in LC treated BMSCc+ groups, suggested the cardiac differentiation of BMSCc+, and can be utilized in tissue engineering for cardiac cell therapy. MSCs can secrete various immunomodulatory factors that create a regenerative microenvironment (Vasanthan et al., 2021). The Mesenchymal and Tissue Stem Cell Committee, which belongs to the International Society for Cellular Therapy, stated that plastic-adherent properties are the minimum criteria for determining MSCs. Furthermore, they should express proteins CD105, CD90, and CD73, suppress the expression of proteins CD45, CD79α, CD34, CD11b, CD14, CD19, and human leukocyte antigen-DR isotype (HLA-DR) surface biomolecules, and differentiate into adipocytes, osteoblasts, muscle cells, neurons, and chondrocytes (Dominici et al., 2006). In in vivo, MSCs are the source of trophic factors that modulate the immune system in the body and induce innate stem cells to repair injured tissues (Rodríguez-Fuentes et al., 2021). However, it can be challenging to differentiate when they are connected with wounded tissue, opposed to when they instruct tissue-specific progenitor cells responsible for the redevelopment of damaged tissue.
Biomaterials evolve from inert materials that overcome the lack of interaction with the body to continue biological activities (i.e., informational materials that can act as hosts and provide signals to surrounding cells and tissues) (Rodrigo-Navarro et al., 2021). These well-engineered living materials contain living cells and polymeric matrices, functioning as actively responding biomaterials. These biomaterials can be prepared using various technologies such as electrospinning, 3D printing, coating, and freeze-drying (Chen et al., 2021; Mishra and Srivastava, 2021). For instance, Chandramohan et al. (2021) isolated MSCs from human ovarian follicular fluid and grew them on a chitosan-polycaprolactone-zinc scaffold to study their application in bone tissue engineering (Chandramohan et al., 2021). The biological outcome confirmed that the engineered biomaterial promoted osteoblast differentiation at the molecular and cellular levels. The expression of secretomes (osteoblast markers) such as RunX family transcription factor 2 (Runx2) and type 1 collagen messenger RNAs (mRNAs), osteonectin, and osteocalcin were enhanced in the presence of the scaffold. In these studies, MSCs were successfully utilized as initial cells to differentiate into tissue cells that provide essential cell sources and practical support for tissue regeneration treatment.
For a wide variety of clinical applications, MSCs require the host cells to migrate to target tissues (de Becker and Riet, 2016). However, biomaterials can act as hosts for MSCs. Improved angiogenesis, cell proliferation, and scarless wound healing applications were clinically observed in adipose-derived MSCs with or without the presence of biomaterials (Gentile et al., 2021a). Shilan and coworkers developed the exosome-loaded bioactive alginate scaffold for wound healing application. They found that the developed scaffolds facilitated enhanced collagen synthesis, angiogenesis, and improved wound repair (Shafei et al., 2020). The clinical trials were conducted to explore the potential use of MSCs in acute lung injury or acute respiratory distress syndrome (ARDS), which opened a new possibility for the treatment of post-COVID-19 patients (Loke et al., 2021). Gentile and Sterodimas published an article suggesting the emergency and successive use of MSCs in COVID-19 treatment (Gentile and Sterodimas, 2020a). The authors recommend inserting Adipose-derived stromal stem cells (ACSs) and stromal vascular fraction cells (SVFs) in patients along with the conventional therapy as an immediate step of cure. Another alternative could be to produce SVFs and ASCs from MSCs, and isolate the secretomes to reinfuse in certified drugs or directly into patients. Flow diagram of AD-MSCs biomolecular pathway and mechanism in COVID-19 induced pneumonia is presented in Fig. 1. In another study, intravenous incorporation of MSCs into patients can improve the respiratory activity in COVID-19 patients (Gentile and Sterodimas, 2020b). The MSCs therapy enriched the lung microenvironment after the SARS-CoV-2 infection. Immune-modulation effects of SVFs and ASCs help form new micro-capillary networks, which mediates the improved delivery of proper nutrients and oxygen for fast dealing in COVID-19 patients. MSCs and AD-MSCs are also used as anti-viral drug delivery agents in the virus-infected microenvironment (Gentile et al., 2020). AD-MSCs have the homing ability, lower risk of complications, potent anti-inflammatory and immune-modulatory ability, which improves its potential as regenerative medicine in this critical pandemic situation of COVID-19.

Figure 1: Biomolecular pathway od AD-MSCs and its mechanism for treatment in COVID-19 induced pneumonia (ESC: Epidermal stem cells; PGE2: prostaglandin E2; LIF: leukemia-inhibiting factor; ECM: extracellular matrix; TGF-1: transforming growth factor-1; HGF: hepatocyte growth factors; INF-γ: interferon-γ; VEGF: vascular endothelial growth factor; PDGF: platelet-derived growth factors; GF: growth factors) (Gentile and Sterodimas, 2020a).
In the present article, we briefly addressed the current knowledge about MSC-secreting molecules with biomaterials. We have also concisely illustrated the possible applications in the field of regeneration medicine and tissue engineering. A schematic presentation for different sources, immunomodulation and differentiation of MSCs is presented in Fig. 2.

Figure 2: Overview of various sources of Mesenchymal Stem Cells (MSCs), their further culturing for the development of engineered living biomaterial, and release of secretome for immunomodulation and differentiation in another kind of cells (neuron, chondrocytes, osteocytes, adipocytes, hepatocytes).
Biomolecules derived from MSCs
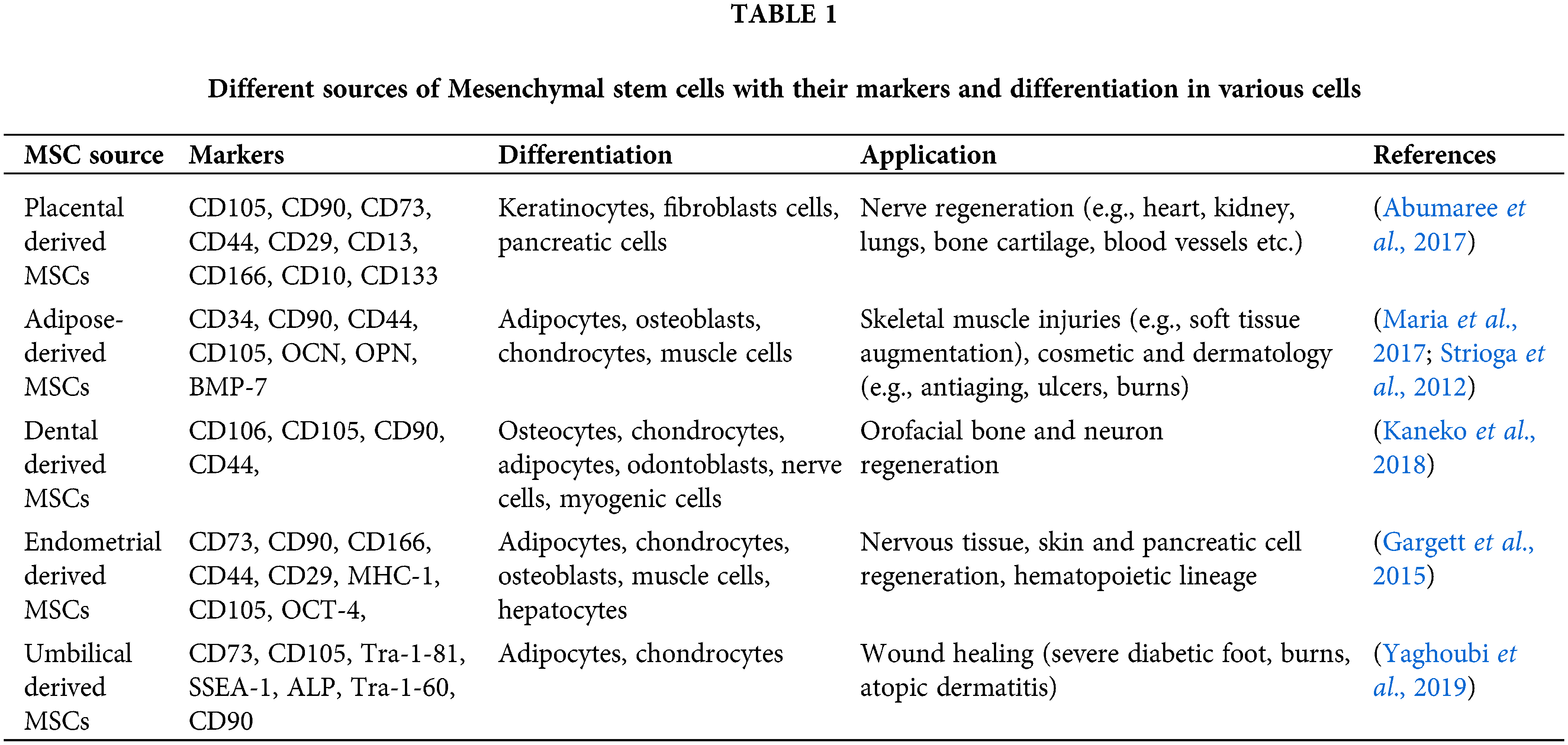
Mesenchymal stem cells (MSCs) can be initially isolated from the shin, bone marrow, adipose tissue, umbilical cord, and placental tissues (Rungsiwiwut et al., 2021). MSCs secrete a broad range of active biomolecules, comprising cytokines, messenger RNAs (mRNAs), growth factors, proteins, microRNAs (miRNAs), and membrane vesicles. Exosomes are also secreted by MSCs and are widely used in regenerative medicine and tissue engineering (Pant et al., 2021). MSCs, along with their benefits, also have fewer moral, ethical, and safety issues than embryonic stem cells. Additionally, they possess poor immunogenicity and desirable immunomodulatory properties that can alter the function of lymphocytes (Natural killer cells, T cells, B cells), macrophages, and monocytes (Ahangar et al., 2020). For bone regeneration, MSCs secrete proteins such as VEGF, TGF-β, and bone morphogenic proteins (BMPs) in the conditioned medium or inside extracellular vesicles (El Moshy et al., 2020). The composition of the MSC secretome is highly influenced by the microenvironment to which it is exposed. In the presence of inflammatory signals such as interferon-γ (IFN-γ) and interleukin-1β (IL-1β), MSCs release the immunomodulator granulocyte colony-stimulating factor (G-CSF), galectin-9, and factor H (Wangler et al., 2021). A list of various sources of MSCs with their secretomes and applications is presented in Table 1.
Use of stem cells in regenerative medicine
Stem cells have multifunctional potentials and show different effects on different cell lines. Fathi et al. examined the impact of MSCs on MolT-4 cell lines as acute lymphoblastic leukemia cells (Fathi and Vietor, 2021). A dramatic decrease in telomerase activity was observed in MSCs co-cultured with MolT-4 cells. Whereas an enhancement in β-galactosidase activity occurred in MSCs co-cultured with MolT-4 cells. A significant enhancement in pro-caspase-8 and cleaved-caspase 8 and 9 expressions have also appeared in MSCs co-culture with MolT-4 cells. The co-cultured groups demonstrated increased apoptosis and senescence in Molt-4 cells via the caspase-8, 9 cascades, as well as the GSK-3α/β and ERK1/2 signaling pathways (Fathi and Vietor, 2021). In another study, Fathi and coworkers also investigated the effects of BMSCs on the proliferation of chronic myeloid leukemia (CML) cell lines (K562) via the Extracellular signal-regulated kinase (ERK) pathway (Ezzatollah et al., 2020). A lowering in BMSCs induced cell line growth has occurred in K562 cells co-cultured with BMSCs via decreasing ERK protein expression. BMSCs produce CD444 and CD90, but not CD34 or CD56 hematopoietic markers. Many cytokines and growth factors are secreted in the co-culture of BMSCs and K562 cells, which restricted the proliferation of CML cell lines via the ERK pathway.
It is essential to address the ethical issues associated with the clinical applications of cell transplantation. The protection of donor privacy is mandatory for such applications. It is also essential to understand how gametes, embryos, and somatic cells of donors will be used in the future and how donors’ privacy will be protected (Charitos et al., 2021). The guidelines have been updated related to the ethical issues regularly from the International Society for Stem Cell Research (ISSCR). These recommendations should be studied for information on cell and tissue donors and the permission process and data collection. Two major issues, including donor’s agreement and their information, are associated with isolating stem cells from the umbilical cord. Pain and hazards related to the donor should also consider during the isolation of BMSCs. These issues are also associated with the somatic cell collection process.
In vitro fertilization is used to obtain the overabundance of fertilized eggs. Following the isolation of pluripotent stem cells, these fertilized eggs are destroyed, raising moral concerns about the death of fertilized eggs with the potential to develop into human beings. However, according to medical and biological sciences, fertilized eggs from the first fourteen days do not contain life. As a result, the termination of life does not include pluripotent cell separation in the first fourteen days. Also, in the event of a deliberate or spontaneous abortion, a pregnant woman’s consent is required to isolate stem cells from cadaveric embryonic tissue. However, it is illegal to create human embryos only for research purposes (Charitos et al., 2021). According to Ballini et al. (2019) there are no ethical concerns with stem cells produced from the dental bud and pulp and hence can be used for research without any issues (Ballini et al., 2019).
According to previous studies (Moghadasi et al., 2021), MSC-based tissue engineering still has considerable limitations, including the fact that among the injected cells, only a few can survive and grow on the desired tissue area and play a medicinal role. MSCs possess self-renewal and multilineage differentiation ability; however, they were not frequently utilized in tissue regenerative medicine applications. Although the cell-free approach is a promising therapy, various problems have arisen before the clinical transformation. Establishing therapeutic and clinical regimens is complicated due to the intricate interactions among secretome components during tissue restoration (Rahimi et al., 2021). Another limitation of the secretome is related to its collection purity, preparation, and analysis workflow. Some secreted factors have low concentrations because of dilution by the culture media. Moreover, dead cells or proteins secreted by apoptotic cells contaminate the secretome. Therefore, MSC-conditioned media should be prepared cautiously to ensure it is free from impurities. Another drawback is that proteomic analysis interfaces with salt and other compounds in the culture media, making specific protein precipitation necessary for proper proteomic analysis.
Biomaterials must possess different properties according to the demand of the treatment area. The developed material is expected to occupy the anatomy of the defective site, providing pleasing surroundings to recruit and promote host stem cells. For example, scaffolds for bone regeneration should have good tensile and compressive strengths to fulfill the osteoconductive and osteoinductive criteria. Osteoconductive properties stimulate bone cell growth, and osteoinductive properties promote the proliferation and differentiation of MSCs (Ercal and Pekozer, 2020). Various materials such as ceramics, natural and synthetic polymers, and metallic nanoparticles are used widely to synthesize scaffolds. Collagen is a natural polymer with a low antigenic reaction, high tensile strength, and flexibility. Biomaterials and MSCs-based regenerative therapy essentially preserve cell viability while properly guiding their fate and functionalization toward the wounded site. MSCs’ phenotypic functioning and viability can quickly be changed if isolated from their surroundings. As a result, for MSCs to preserve their characteristics and optimally demonstrate therapeutic benefits, highly imitating native room biomaterials are required. MSC behavior is influenced by various biophysical and biochemical parameters, including stiffness, topography, porosity, growth factors, and tiny bioactive compounds (Li et al., 2021). Incorporating biophysical and biochemical cues into next-generation biomaterials could assist in regulating MSCs in the futuristic approach of regenerative medicine and cell therapy. Biomaterial development for regenerative medicine must be paired with multidisciplinary research advancements and the biophysical and biochemical parameters that influence MSCs’ behavior. The parameters must be accurately integrated and altered according to the needs of the wounded area. Yasamin et al. (2020) coupled multi-material 3D bio-printing with electronic platform technology in a prior study to develop a hybrid device that reconstructs the mechanical structure of nasal cartilage and detects odor.
Before utilizing MSCs, a selection of procedures should be performed, and the particular properties and types of proteins, material wettability, mechanical and chemical properties, and topography should be determined (Ferreira et al., 2012; Ventura Ferreira et al., 2012). The selection of more specific biomaterials and optimized parameters can make a significant difference in regenerative medicine and tissue engineering results. However, polymers such as acrylamide (AAM) possess drawbacks, including low elasticity, which is lower than many tissues in the human body (Engler et al., 2006). However, several clinical trials have been conducted or are ongoing. It is necessary that the comparative response of biomaterial-modified MSCs be investigated within different research groups for similar types of experiments and to generalize the knowledge and opinions about more important parameters (cytotoxicity/compatibility, protein adhesion layers, proliferation, and differentiation), as well as the treatment steps for standard models (Moghadasi et al., 2021). The most appropriate biomaterial to be used with MSCs for regenerative medicine should be discovered faster and more reliable. This is necessary to improve the efficiency of MSC-based therapy as well as the parameters for cell culture conditions, the procedure for manufacturing, storage, optimized therapeutic doses and schedules, and dependable potency that can be generalized for particular therapy (Schaap-Oziemlak et al., 2014). MSCs can be used against infections and inflammatory diseases such as cholera. Bahroudi and coworkers demonstrated the immune-modulation effect of lipopolysaccharide (LPS)-MSC-conditioned media as a vaccine candidate against Vibrio cholera LPS immunization in a mice model (Bahroudi et al., 2021). The LPS-MSC-conditioned media regulated the anti-inflammatory response and induced the formation of vibriocidal antibodies to fight against the cholera disease. In another study, they showed robust bactericidal and antibiofilm performance of MSCs supernatant to inhibit the cholera infection (Bahroudi et al., 2020). Another approach for inhibiting bacterial infection is by culturing the MSCs with toll-like receptor (TLR)3 ligands (Johnson et al., 2017). This helps neutrophils to survive for a long time and kill the bacteria.
The benefits and capabilities of MSC biomaterials are receiving growing attention. MSC-exosomes (Harrell et al., 2020) are being broadly utilized for more advanced innovative regenerative approaches for several treatments. This includes inflammatory responses, targeting of the pivotal signaling axis of immune cells, inhibition of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) protein activation and pro-fibrotic molecule expression, suppression, and expression of matrix metalloproteinases (MMPs) to promote the generation of pro-angiogenic factors, and activation of pro-fibrotic molecules in various treatments. However, MSC-based applications must still overcome several challenges. Many researchers currently use growth factors to facilitate stem cell differentiation and tissue engineering. Most research is in the preclinical stage, and their translation into clinical applications necessitates additional testing to demonstrate safety and efficacy. Furthermore, it was discovered that the same elements have distinct impacts on MSCs from other sources. Current and future research will continue to focus on the origin of MSCs as well as the desirable surface microenvironment.
MSC adhesion to biomaterials should also be considered. New strategies need to be developed to improve the in vitro adhesion of MSCs. This can be achieved by modifying the extracellular matrix (ECM) through using natural, artificial, and smart polymers, joining adhesion peptides, designing cytocompatible scaffolds, nanopatterning, and using molecular signals. Understanding the mechanism, involved molecules, and factors influencing adhesion could help manage MSC survival and proper manipulation for regenerative applications. Several issues must be considered in the future to develop regenerative medicine better. These approaches include the following: MSCs should retain their properties when exposed to modified surfaces of biomaterials, physical properties should promote the growth of MSCs, and cells should maintain their properties for an extended period.
Innovative platforms with customizable biophysical properties, such as biomaterial diversified multiscale topography, 3D engineering microenvironment, and 4D reversibly responsive systems, which may react to internal or external stimuli, have recently been developed. These can perfectly replicate the stem cell’s dynamic milieu and give practical tools for studying the impact of biophysical stimuli on stem cell behavior in a spatiotemporal manner (Wan et al., 2021). Therefore, a complete understanding of the roles of biophysical cues in cell stimulation within multiple dimensions will boost basic cell biology and biomaterial design for tissue regeneration. Furthermore, the MSC culture process might affect the amount of expression of the resultant factors. Wound healing, photoprotection, hair growth encouragement, psoriasis treatment, and other antimicrobial uses are the secretome’s skin regeneration applications. Given the many components of the secretome, it has a lot of potential in various diseases, but it will require additional in-depth research to be entirely relevant.
The application of MSCs in wound healing, scar treatment, and soft tissue repair is also studied extensively. In recent years, lipofilling has emerged as a popular scar treatment method. The regenerative capacity of adipose-derived stem cells (AD-MSCs) suspended in an extracellular matrix called stromal vascular fraction (SVF) has significant benefits for scar treatment. Similarly, fat tissue is also used as a bioactive material in regenerative surgery. Lipofilling/fat graft-nano-fat technique act as a bioactive scaffold for wound healing and scar treatment (Gentile et al., 2021b). Foot ulceration in diabetic patients can lead to high disability and even mortality. Platelet-rich plasma (PRP) therapy is used widely for diabetic wound healing due to its high content of growth factors. To overcome the rapid degradation of growth factors in PRP, AD-MSCs are combined with PRP therapy to promote diabetic wound healing by downregulation of the notch signaling pathway (Ebrahim et al., 2021). The combined therapy showed improved re-epithelialization and granulation tissue formation with a significant increase in collagen, epidermal thickness, and angiogenesis. Autologous stem cell therapy, including PRP, human follicles stem cells, and AD-MSCs, are effective for hair regrowth in patients affected by androgenetic alopecia and for wound healing (Gentile et al., 2021a). The skin acts as a natural shield against the sun’s ultraviolet (UV) rays (Gentile et al., 2021b). However, extended exposure to the sun and UV radiation can harm the skin’s structure, reduce collagen formation, and speed up the aging process (called photoaging). Photoaging indications, wrinkles, loss of elasticity, and soft tissue abnormalities in the face are all treated with AD-MSCs. AD-MSCs are used because of their migratory activity, paracrine effects, and in vivo-ex vivo outcomes, such as dermal fibroblast proliferation, antioxidant impact, and reduction of matrix metalloproteinase (MMPs) (Gentile et al., 2021b). In conclusion, considering the benefits of MSCs, these cells have wide applications in pure form as well as combined with biomaterials or other various therapies to treat diseases.
Acknowledgement: Funders are thanked for support of this writing.
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Authors Contribution: The authors confirm contribution to the paper as follows: study conception and design: KTL, TVP; data collection: KTL, TVP; Data analysis and interpretation: TVP, DKP, SDD, KG, AR; Drafting the article: TVP; Critical revision of the article: KTL, DKP; Final approval of the version to be published: KTL.
Ethics Approval: No committees were required for this study.
Funding Statement: This research was supported by the Basic Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2018R1A6A1A03025582) and the National Research Foundation of Korea (NRF-2019R1D1A3A03103828).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abumaree MH, Abomaray FM, Alshabibi MA, AlAskar AS, Kalionis B (2017). Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta 59: 87–95. DOI 10.1016/j.placenta.2017.04.003. [Google Scholar] [CrossRef]
Ahangar P, Mills SJ, Cowin AJ (2020). Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. International Journal of Molecular Sciences 21: 7038. DOI 10.3390/ijms21197038. [Google Scholar] [CrossRef]
Bahroudi M, Bakhshi B, Soudi S, Najar-Peerayeh S (2020). Antibacterial and antibiofilm activity of bone marrow-derived human mesenchymal stem cells secretome against Vibrio cholerae. Microbial Pathogenesis 139: 103867. DOI 10.1016/j.micpath.2019.103867. [Google Scholar] [CrossRef]
Bahroudi M, Bakhshi B, Soudi S, Najar-peerayeh S (2021). Immunomodulatory effects of mesenchymal stem cell-conditioned media on lipopolysaccharide of Vibrio cholerae as a vaccine candidate. Stem Cell Research & Therapy 12: 564. DOI 10.1186/s13287-021-02622-0. [Google Scholar] [CrossRef]
Ballini A, Cantore S, Scacco S, Perillo L, Scarano A et al. (2019). A comparative study on different stemness gene expression between dental pulp stem cells vs. dental bud stem cells. European Review for Medical and Pharmacological Sciences 23: 1626–1633. DOI 10.26355/eurrev_201902_17122. [Google Scholar] [CrossRef]
Bodart-Santos V, de Carvalho LRP, de Godoy MA, Batista AF, Saraiva LM et al. (2019). Extracellular vesicles derived from human Wharton’s jelly mesenchymal stem cells protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. Stem Cell Research & Therapy 10: 332. DOI 10.1186/s13287-019-1432-5. [Google Scholar] [CrossRef]
Chandramohan Y, Jeganathan K, Sivanesan S, Koka P, Amritha TMS et al. (2021). Assessment of human ovarian follicular fluid derived mesenchymal stem cells in chitosan/PCL/Zn scaffold for bone tissue regeneration. Life Sciences 264: 118502. DOI 10.1016/j.lfs.2020.118502. [Google Scholar] [CrossRef]
Charitos IA, Ballini A, Cantore S, Boccellino M, Di Domenico M et al. (2021). Stem cells: A historical review about biological, religious, and ethical issues. Stem Cells International 2021: 9978837. DOI 10.1155/2021/9978837. [Google Scholar] [CrossRef]
Chen L, Cheng L, Wang Z, Zhang J, Mao X et al. (2021). Conditioned medium-electrospun fiber biomaterials for skin regeneration. Bioactive Materials 6: 361–374. DOI 10.1016/j.bioactmat.2020.08.022. [Google Scholar] [CrossRef]
de Becker A, Riet IV (2016). Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World Journal of Stem Cells 8: 73–87. DOI 10.4252/wjsc.v8.i3.73. [Google Scholar] [CrossRef]
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317. DOI 10.1080/14653240600855905. [Google Scholar] [CrossRef]
Ebrahim N, Dessouky AA, Mostafa O, Hassouna A, Yousef MM et al. (2021). Adipose mesenchymal stem cells combined with platelet-rich plasma accelerate diabetic wound healing by modulating the Notch pathway. Stem Cell Research & Therapy 12: 392. DOI 10.1186/s13287-021-02454-y. [Google Scholar] [CrossRef]
El Moshy S, Radwan IA, Rady D, Abbass MMS, El-Rashidy AA et al. (2020). Dental stem cell-derived secretome/conditioned medium: The future for regenerative therapeutic applications. Stem Cells International 2020: 7593402. DOI 10.1155/2020/75934022020. [Google Scholar] [CrossRef]
Engler AJ, Sen S, Sweeney HL, Discher DE (2006). Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689. DOI 10.1016/j.cell.2006.06.044. [Google Scholar] [CrossRef]
Ercal P, Pekozer GG (2020). A current overview of scaffold-based bone regeneration strategies with dental stem cells. In: Turksen K (eds.Cell Biology and Translational Medicine, Vol. 9, pp. 61–85. Cham: Springer International Publishing. [Google Scholar]
Ezzatollah F, Behnaz V, Raheleh F (2020). Targeting the proliferation inhibition of chronic myeloid leukemia cells by bone marrow derived-mesenchymal stem cells via ERK pathway as a therapeutic strategy. Acta Medica Iranica 58: 199. DOI 10.18502/acta.v58i5.3952. [Google Scholar] [CrossRef]
Fathi E, Farahzadi R, Vietor I, Javanmardi S (2020). Cardiac differentiation of bone-marrow-resident c-kit+ stem cells by L-carnitine increases through secretion of VEGF, IL6, IGF-1, and TGF-β as clinical agents in cardiac regeneration. Journal of Biosciences 45: 1036. DOI 10.1007/s12038-020-00063-0. [Google Scholar] [CrossRef]
Fathi E, Vietor I (2021). Mesenchymal stem cells promote caspase expression in molt-4 leukemia cells via GSK-3α/B and ERK1/2 signaling pathways as a therapeutic strategy. Current Gene Therapy 21: 81–88. DOI 10.2174/1566523220666201005111126. [Google Scholar] [CrossRef]
Ferreira MV, Labude N, Piroth D, Jahnen-Dechent W, Knüchel R et al. (2012). Compatibility of different polymers for cord blood-derived hematopoietic progenitor cells. Journal of Materials Science: Materials in Medicine 23: 109–116. DOI 10.1007/s10856-011-4483-4. [Google Scholar] [CrossRef]
Gargett CE, Schwab KE, Deane JA (2015). Endometrial stem/progenitor cells: the first 10 years. Human Reproduction Update 22: 137–163. DOI 10.1093/humupd/dmv051. [Google Scholar] [CrossRef]
Gentile P, Alves R, Cole JP, Andjelkov K, van Helmelryck T et al. (2021a). AIRMESS-Academy of International Regenerative Medicine & Surgery Societies: recommendations in the use of platelet-rich plasma (PRPautologous stem cell-based therapy (ASC-BT) in androgenetic alopecia and wound healing. Expert Opinion on Biological Therapy 21: 1443–1449. DOI 10.1080/14712598.2021.1908995. [Google Scholar] [CrossRef]
Gentile P, Sterodimas A (2020a). Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID-19)-induced pneumonia. Expert Opinion on Biological Therapy 20: 711–716. DOI 10.1080/14712598.2020.1761322. [Google Scholar] [CrossRef]
Gentile P, Sterodimas A (2020b). Adipose Stem Cells (ASCs) and Stromal Vascular Fraction (SVF) as a potential therapy in combating (COVID-19)-disease. Aging and Disease 11: 465–469. DOI 10.14336/AD.2020.0422. [Google Scholar] [CrossRef]
Gentile P, Sterodimas A, Calabrese C, Garcovich S (2021b). Systematic review: Advances of fat tissue engineering as bioactive scaffold, bioactive material, and source for adipose-derived mesenchymal stem cells in wound and scar treatment. Stem Cell Research & Therapy 12: 318. DOI 10.1186/s13287-021-02397-4. [Google Scholar] [CrossRef]
Gentile P, Sterodimas A, Pizzicannella J, Calabrese C, Garcovich S (2020). Research progress on Mesenchymal Stem Cells (MSCsAdipose-Derived Mesenchymal Stem Cells (AD-MSCsDrugs, and Vaccines in Inhibiting COVID-19 Disease. Aging and Disease 11: 1191–1201. DOI 10.14336/AD.2020.0711. [Google Scholar] [CrossRef]
Harrell, CR, Jovicic, BP, Djonov, V, Volarevic, V (2020). Therapeutic potential of mesenchymal stem cells and their secretome in the treatment of SARS-CoV-2-induced acute respiratory distress syndrome. Analytical Cellular Pathology 2020: 1939768. DOI 10.1155/2020/1939768. [Google Scholar] [CrossRef]
Jodat YA, Kiaee K, Vela Jarquin D, de la Garza Hernández RL, Wang T et al. (2020). A 3D-printed hybrid nasal cartilage with functional electronic olfaction. Advanced Science 7: 1901878. DOI 10.1002/advs.201901878. [Google Scholar] [CrossRef]
Johnson V, Webb T, Norman A, Coy J, Kurihara J et al. (2017). Activated mesenchymal stem cells interact with antibiotics and host innate immune responses to control chronic bacterial infections. Scientific Reports 7: 9575. DOI 10.1038/s41598-017-08311-4. [Google Scholar] [CrossRef]
Kaneko T, Gu B, Sone PP, Zaw SYM, Murano H et al. (2018). Dental pulp tissue engineering using mesenchymal stem cells: A review with a protocol. Stem Cell Reviews and Reports 14: 668–676. DOI 10.1007/s12015-018-9826-9. [Google Scholar] [CrossRef]
Li J, Liu Y, Zhang Y, Yao B, Enhejirigala et al. (2021). Biophysical and biochemical cues of biomaterials guide mesenchymal stem cell behaviors. Frontiers in Cell and Developmental Biology 9: 3547. DOI 10.3389/fcell.2021.640388. [Google Scholar] [CrossRef]
Loke XY, Imran SAM, Tye GJ, Wan Kamarul Zaman WS, Nordin F (2021). Immunomodulation and regenerative capacity of MSCs for long-COVID. International Journal Molecular Science 22: 12421. DOI 10.3390/ijms222212421. [Google Scholar] [CrossRef]
Maria ATJ, Maumus M, Le Quellec A, Jorgensen C, Noël D et al. (2017). Adipose-derived mesenchymal stem cells in autoimmune disorders: State of the art and perspectives for systemic sclerosis. Clinical Reviews in Allergy & Immunology 52: 234–259. DOI 10.1007/s12016-016-8552-9. [Google Scholar] [CrossRef]
Marofi F, Hassanzadeh A, Solali S, Vahedi G, Mousavi Ardehaie R et al. (2019a). Epigenetic mechanisms are behind the regulation of the key genes associated with the osteoblastic differentiation of the mesenchymal stem cells: The role of zoledronic acid on tuning the epigenetic changes. Journal of Cellular Physiology 234: 15108–15122. DOI 10.1002/jcp.28152. [Google Scholar] [CrossRef]
Marofi F, Vahedi G, hasanzadeh A, Salarinasab S, Arzhanga P et al. (2019b). Mesenchymal stem cells as the game-changing tools in the treatment of various organs disorders: Mirage or reality? Journal of Cellular Physiology 234: 1268–1288. DOI 10.1002/jcp.27152. [Google Scholar] [CrossRef]
Mishra A, Srivastava V (2021). Biomaterials and 3D printing techniques used in the medical field. Journal of Medical Engineering & Technology 45: 290–302. DOI 10.1080/03091902.2021.1893845. [Google Scholar] [CrossRef]
Moghadasi S, Elveny M, Rahman HS, Suksatan W, Jalil AT et al. (2021). A paradigm shift in cell-free approach: The emerging role of MSCs-derived exosomes in regenerative medicine. Journal of Translational Medicine 19: 302. DOI 10.1186/s12967-021-02980-6. [Google Scholar] [CrossRef]
Pant T, Juric M, Bosnjak ZJ, Dhanasekaran A (2021). Recent insight on the non-coding RNAs in mesenchymal stem cell-derived exosomes: Regulatory and therapeutic role in regenerative medicine and tissue engineering. Frontiers in Cardiovascular Medicine 8: 136. DOI 10.3389/fcvm.2021.737512. [Google Scholar] [CrossRef]
Rahimi B, Panahi M, Saraygord-Afshari N, Taheri N, Bilici M et al. (2021). The secretome of mesenchymal stem cells and oxidative stress: Challenges and opportunities in cell-free regenerative medicine. Molecular Biology Reports 48: 5607–5619. DOI 10.1007/s11033-021-06360-7. [Google Scholar] [CrossRef]
Rodrigo-Navarro A, Sankaran S, Dalby MJ, del Campo A, Salmeron-Sanchez M (2021). Engineered living biomaterials. Nature Reviews Materials 6: 1175–1190. DOI 10.1038/s41578-021-00350-8. [Google Scholar] [CrossRef]
Rodríguez-Fuentes DE, Fernández-Garza LE, Samia-Meza JA, Barrera-Barrera SA, Caplan AI et al. (2021). Mesenchymal stem cells current clinical applications: A systematic review. Archives of Medical Research 52: 93–101. DOI 10.1016/j.arcmed.2020.08.006. [Google Scholar] [CrossRef]
Rungsiwiwut R, Virutamasen P, Pruksananonda K (2021). Mesenchymal stem cells for restoring endometrial function: An infertility perspective. Reproductive Medicine and Biology 20: 13–19. DOI 10.1002/rmb2.12339. [Google Scholar] [CrossRef]
Schaap-Oziemlak AM, Kühn PT, van Kooten TG, van Rijn P (2014). Biomaterial-stem cell interactions and their impact on stem cell response. RSC Advances 4: 53307–53320. DOI 10.1039/C4RA07915A. [Google Scholar] [CrossRef]
Shafei S, Khanmohammadi M, Heidari R, Ghanbari H, Taghdiri Nooshabadi V et al. (2020). Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. Journal of Biomedical Materials Research Part A 108: 545–556. DOI 10.1002/jbm.a.36835. [Google Scholar] [CrossRef]
Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J (2012). Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells and Development 21: 2724–2752. DOI 10.1089/scd.2011.0722. [Google Scholar] [CrossRef]
Tavakoli S, Ghaderi Jafarbeigloo HR, Shariati A, Jahangiryan A, Jadidi F et al. (2020). Mesenchymal stromal cells; a new horizon in regenerative medicine. Journal of Cellular Physiology 235: 9185–9210. DOI 10.1002/jcp.29803. [Google Scholar] [CrossRef]
Vasanthan J, Gurusamy N, Rajasingh S, Sigamani V, Kirankumar S et al. (2021). Role of human mesenchymal stem cells in regenerative therapy. Cells 10: 54. DOI 10.3390/cells10010054. [Google Scholar] [CrossRef]
Ventura Ferreira MS, Jahnen-Dechent W, Labude N, Bovi M, Hieronymus T et al. (2012). Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials 33: 6987–6997. DOI 10.1016/j.biomaterials.2012.06.029. [Google Scholar] [CrossRef]
Wan X, Liu Z, Li L (2021). Manipulation of stem cells fates: The master and multifaceted roles of biophysical cues of biomaterials. Advanced Functional Materials 31: 2010626. DOI 10.1002/adfm.202010626. [Google Scholar] [CrossRef]
Wangler S, Kamali A, Wapp C, Wuertz-Kozak K, Häckel S et al. (2021). Uncovering the secretome of mesenchymal stromal cells exposed to healthy, traumatic, and degenerative intervertebral discs: a proteomic analysis. Stem Cell Research & Therapy 12: 11. DOI 10.1186/s13287-020-02062-2. [Google Scholar] [CrossRef]
Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A et al. (2019). Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sciences 233: 116733. DOI 10.1016/j.lfs.2019.116733. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |