DOI:10.32604/biocell.2022.020141

| BIOCELL DOI:10.32604/biocell.2022.020141 |  |
| Review |
Navigating the genomic instability mine field of osteosarcoma to better understand implications of non-coding RNAs
Biomedical and Pharmaceutical Science, Idaho State University, Pocatello, 83209, USA
*Address correspondence to: Jared Barrott, jaredbarrott@isu.edu
Received: 25 February 2022; Accepted: 30 March 2022
Abstract: Osteosarcoma is one of the most genomically complex cancers and as result, it has been difficult to assign genomic aberrations that contribute to disease progression and patient outcome consistently across samples. One potential source for correlating osteosarcoma and genomic biomarkers is within the non-coding regions of RNA that are differentially expressed. However, it is unsurprising that a cancer classification that is fraught with genomic instability is likely to have numerous studies correlating non-coding RNA expression and function have been published on the subject. This review undertakes the formidable task of evaluating the published literature of non-coding RNAs in osteosarcoma. This is not the first review on this topic and will certainly not be the last. The review is organized with an introduction into osteosarcoma and the epigenetic control of gene expression before reviewing the molecular function and expression of long non-coding RNAs, circular RNAs, and short non-coding RNAs such as microRNAs, piwi RNAs, and short-interfering RNAs. The review concludes with a review of the literature and how the biology of non-coding RNAs can be used therapeutically to treat cancers, especially osteosarcoma. We conclude that non-coding RNA expression and function in osteosarcoma is equally complex to understanding the expression differences and function of coding RNA and proteins; however, with the added lens of both coding and non-coding genomic sequence, researchers can begin to identify the patterns that consistently associate with aggressive osteosarcoma.
Keywords: Osteosarcoma; Epigenetics; Long non-coding RNA; Circular RNA; MicroRNA
Osteosarcoma is the most common form of bone cancer and is the third most common cancer among adolescents. It is an aggressive cancer that frequently metastasizes within a year of forming (Faisham et al., 2017; Herzog, 2005; Mirabello et al., 2009; Tang et al., 2008). Over 40 years ago, long-term, disease-free survival of patients with high-grade osteosarcoma radically improved from less than 20% to greater than 60% with the advent of combinatorial, cytotoxic chemotherapy (Link et al., 1986). The most common cocktail of adjuvant chemotherapy to treat high-grade osteosarcoma consists of cisplatin, methotrexate, and doxorubicin (Carrle and Bielack, 2006). Despite the herculean reversal in dismal outcomes 40 years ago, there remains an enigmatic fraction of patients who fail to exhibit a durable response. Efforts have been made to genomically identify responders vs. non-responders in attempts to guide non-responders early to other therapeutic alternatives. However, due to the high level of genomic complexity, this has largely been unproductive. Osteosarcoma is the quintessential example of genomic instability, with numerous point mutations, INDELS, and structural variants throughout the entire genomic landscape. Most of the identified genomic variations are seen as inconsequential; although, the non-coding variants might hold the key to unlocking the mystery underlying the differences between survivors and non-survivors. Herein this review, the most common epigenetic mechanisms that can contribute to chemosensitivity are discussed; including numerous mechanisms that involve non-coding RNAs.
Epigenetic Control of Gene Expression
Precision medicine is centralized around the dogma of molecular biology. This states that DNA is the fundamental coding material that transcribes sequences into RNA, and RNA is translated into protein resulting in phenotypic cellular behavior (Crick, 1970). Thus, changes in any step along the process can impact how cells act. While DNA mutations can alter this pattern, factors outside of DNA mutations can equally impact gene expression levels to change cell behavior. This is referred to as epigenetic control (Baylin and Jones, 2016). Although DNA methylation and histone modifications are the most commonly studied and well-understood mechanisms of epigenetic control over gene expression, another new angle that has been under investigation in recent times is regulation by non-coding RNAs (Yang et al., 2020). With progress made in personalized medicine and epigenetic therapeutics in cancer, harnessing this unique interplay between non-coding RNAs and the other epigenetic enzymes might be an excellent alternative to address the therapeutic challenges in osteosarcoma.
The entire mammalian genome can be transcribed to some level, yet there remain thousands of RNA transcripts that do not code for proteins. These are known as non-coding RNAs (ncRNAs) (Palazzo and Lee, 2015; Yan and Bu, 2021; Yang et al., 2020; van Bakel et al., 2010). Their expression level and the function are very controversial. Previously known as non-functional, ‘noise’ or ‘junk’ of transcriptome, according to recent reports, much of those transcriptions are likely functional (Richard Boland, 2017) and associated with disease pathogenesis and progression, especially in cancers and neurodegenerative disorders. They can alter gene expression at pre-transcription, transcription, and post-transcription levels and regulate numerous cellular processes related to cancer initiation and progression (Schmitt and Chang, 2016; Yang et al., 2020; Zhu et al., 2019). A growing body of evidence emphasize that ncRNA’s control over genes and chromosomal modifications are attributed to their interactions with the chromatin remodeling complexes, histone modifiers, or DNA methyltransferases (Chen and Xue, 2016; Costa, 2008; Peschansky and Wahlestedt, 2014; Ramassone et al., 2018; Yu, 2009). Differential expression and stability of ncRNAs, especially in blood or urine hold great diagnostic and prognostic biomarker potential in various cancer types including osteosarcoma (di Fiore et al., 2013; Wu et al., 2019). Indeed, some ncRNAs have already been proposed to be the circulating biomarkers owing to their correlation with osteosarcoma progression and metastasis, clinical stage, and patient outcome (Botti et al., 2019).
Based on their size, shape and genomic location, ncRNAs are divided into three major classes, long ncRNAs (lncRNAs), circular RNAs (circRNAs) and short ncRNAs (micro RNAs, short interfering RNAs, piwi RNAs) (Palazzo and Lee, 2015; Wu et al., 2019; Yan and Bu, 2021). Long ncRNAs (lncRNA) are the linear gene transcripts with limited protein-coding capacities, over 200 NT long and regulate gene expression at pre-transcriptional, transcriptional, and post-transcriptional levels (Yan and Bu, 2021). Depending on their expression pattern and biological function, lncRNAs can be classified as sense, antisense, bidirectional, intron, intergenic, or enhancer-lncRNAs (Al-Rugeebah et al., 2019; Palazzo and Lee, 2015). Circular RNAs (circRNA) are another long transcript type but unlike lncRNAs, these single-stranded RNAs form a covalently closed continuous loop (Chen and Huang, 2018). Regulation of gene expression by both lncRNAs and circRNAs includes miRNA decoy/sponging, therefore interacting with DNA, RNA, and proteins (Zhang et al., 2020c). MicroRNAs (miRNA) are the small, 19 to 22 nucleotide base pair sequences, they can inhibit translation or result in the degradation of target mRNA by forming RNA-induced silencing protein complex (RISC) (Llobat and Gourbault, 2021). PIWI-interacting RNAs (piRNA) are the 24 to 32 long transcripts mainly expressed in the germline, derived from single-stranded RNA, and processed by Dicer-independent process. piRNAs are very well known for their function in repressing transposable elements and epigenetic regulation of gene expression (Costa, 2008; Zeng et al., 2020).
Non-coding RNAs and genomic instability
DNA damage response, mediated by a well-constructed regulatory network, is a vital part of maintaining genomic integrity. Decades of research in ncRNAs led to a significant bidirectional regulatory loop between the differential expression of ncRNAs and regulation of DDR-associated genes expression (Malakoti et al., 2021; Zhang and Peng, 2015). ncRNAs, especially lncRNAs, miRNAs and circRNAs, have been found to play multifaceted roles in DDR such as acting as DDR sensor or transducer; thereby, repairing DNA, causing cell cycle arrest, or inducing apoptosis (Khanduja et al., 2016; Malakoti et al., 2021; Zhang and Peng, 2015). It is not surprising that many of these pathways are also interlinked with chromatin remodeling or histone modifications. However, even with most extensive research and understanding of ncRNAs and DDR pathways, it remains questionable how ncRNAs and DDR pathways align together in maintaining cellular integrity. Thus, in this review, we sought to understand whether there is any correlation between epigenetic regulation of the ncRNAs with one of the major hallmarks of cancer: genomic instability.
lncRNAs can directly or indirectly affect almost all of the hallmarks of cancer (Hanahan and Weinberg, 2011; Yan and Bu, 2021) and their oncogenic or tumor-suppressive role can be regulated genetically and/or epigenetically (Zhang et al., 2020c). Since discovery of the regulatory mechanisms of the earliest lncRNAs (Lo et al., 2014), many functional lncRNAs were found to work through epigenetic mechanisms, such as, H19 (imprinted maternally expressed transcript), Xist (X-inactive-specific transcript), HOTAIR (Hox transcript antisense intergenic RNA), etc. (Chan et al., 2014; Clemson et al., 1996; Fazi et al., 2018; Zhang et al., 2015; Zhang et al., 2020c; Zhao et al., 2008).
The expression pattern of lncRNAs and their function in tumorigenesis is well investigated in osteosarcoma (Table 1). A recent transcriptome profiling study based on the TARGET data detected a total of 13,903 expressed lncRNAs and their integrative gene expressions and SCNA analysis revealed 167 novel driver lncRNAs (including 2 previously reported lncRNA PVT1 and ZFAS1) to be associated with osteosarcoma (Luo et al., 2019). Another microarray analysis noted 25,733 expressed lncRNAs in human osteosarcoma, among which 403 were upregulated and 798 were downregulated when comparing osteosarcoma tissues to adjacent normal tissues (Li et al., 2018). Some lncRNAs were also highlighted as circulating biomarkers due to their stability, significantly higher expression in body fluids (especially in serum/plasma of osteosarcoma patients) and their correlation to disease stage or metastatic potential; for example, lncRNA TUG1, UCA1, HNF1A-AS1, MALAT-1, FAL-1 and ATB, etc. (Botti et al., 2019). All of these data imply that many lncRNAs are involved in osteosarcoma occurrence, chemoresistance and metastasis or relapse which could be exploited as potential diagnostic, prognostic biomarkers, and therapeutic targets.
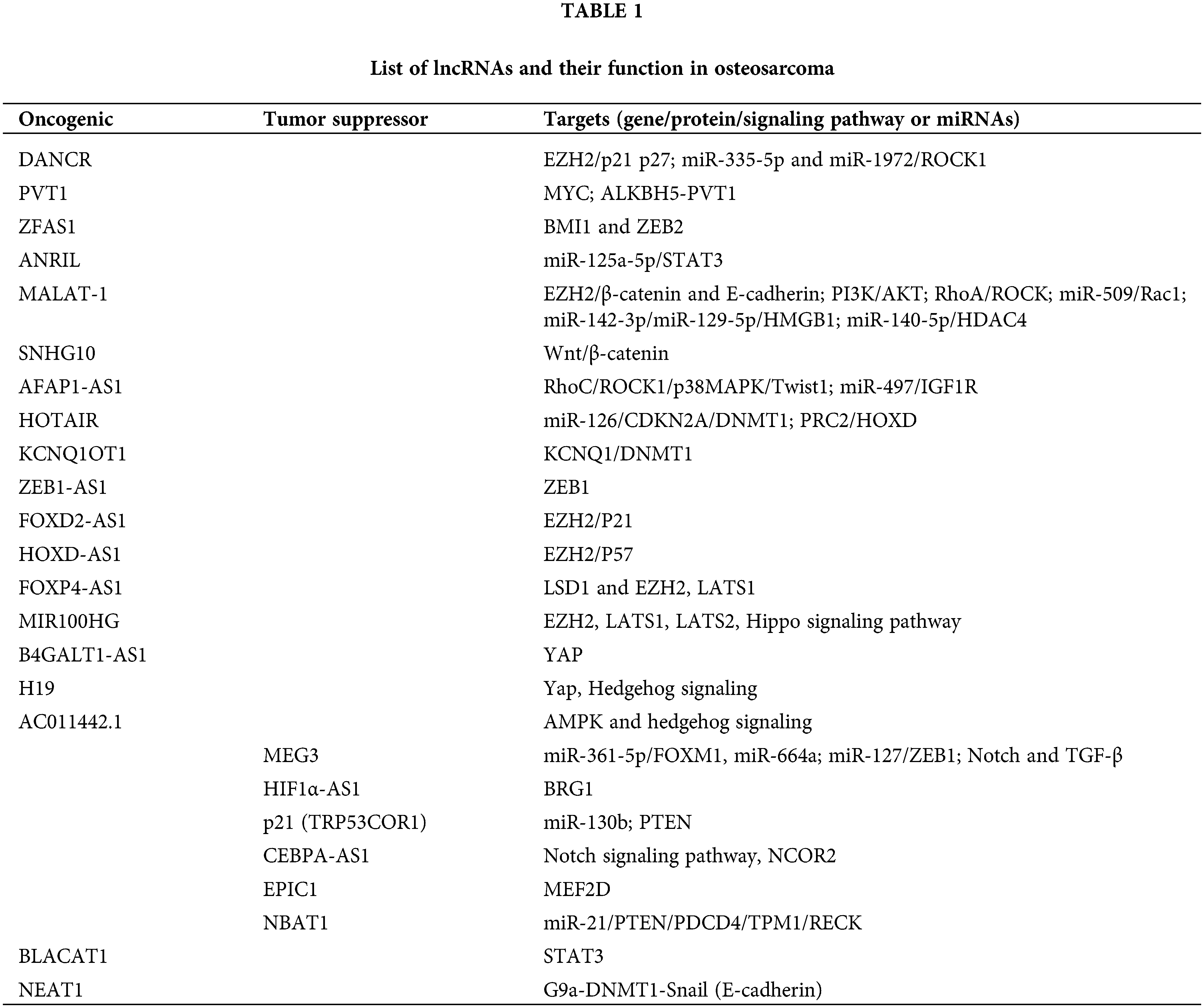
Until recently, several differentially expressed lncRNAs have been identified in different cancers owing to their association and interaction through epigenetic modifications such as histone modification and/or chromatin remodeling (e.g., lncRNA XIST, MALAT1, HOTAIR, ANRIL, HULC, GCLnc1, FENDRR, UCA1, TCF7, GAS5, NEAT1, PVT1); DNA methylation (e.g., lncRNA H19, DACOR1, PTENP1); and CpG Island methylation at the Imprinting Control Regions (e.g., lncRNA TP53TG1, MEG3), competing endogenous RNA (ceRNA) networks (e.g., lncRNA CASC2/miR-183/Sprouty2; NKAPP1-miR-21-5p-PRDM11, MSTO2P-miR-29c-3p-EZH2 and RPLP0P2-miR-29c-3p-EZH2) (Gao et al., 2019; Lou et al., 2020; Wei et al., 2017). lncRNAs are generally found to carry out their epigenetic modifications via-(a) chromatin modification and remodeling, (b) histone modification and nuclear body localization, (c) altering chromosome structures by interacting with the SWI/SNF complex, (d) inducing DNA methylation and/or (e) through interactions with micro RNAs by acting as miRNA sponges or via ceRNA (competitive endogenous RNA) networking (Shin et al., 2020; Zhang et al., 2020c) (Fig. 1).
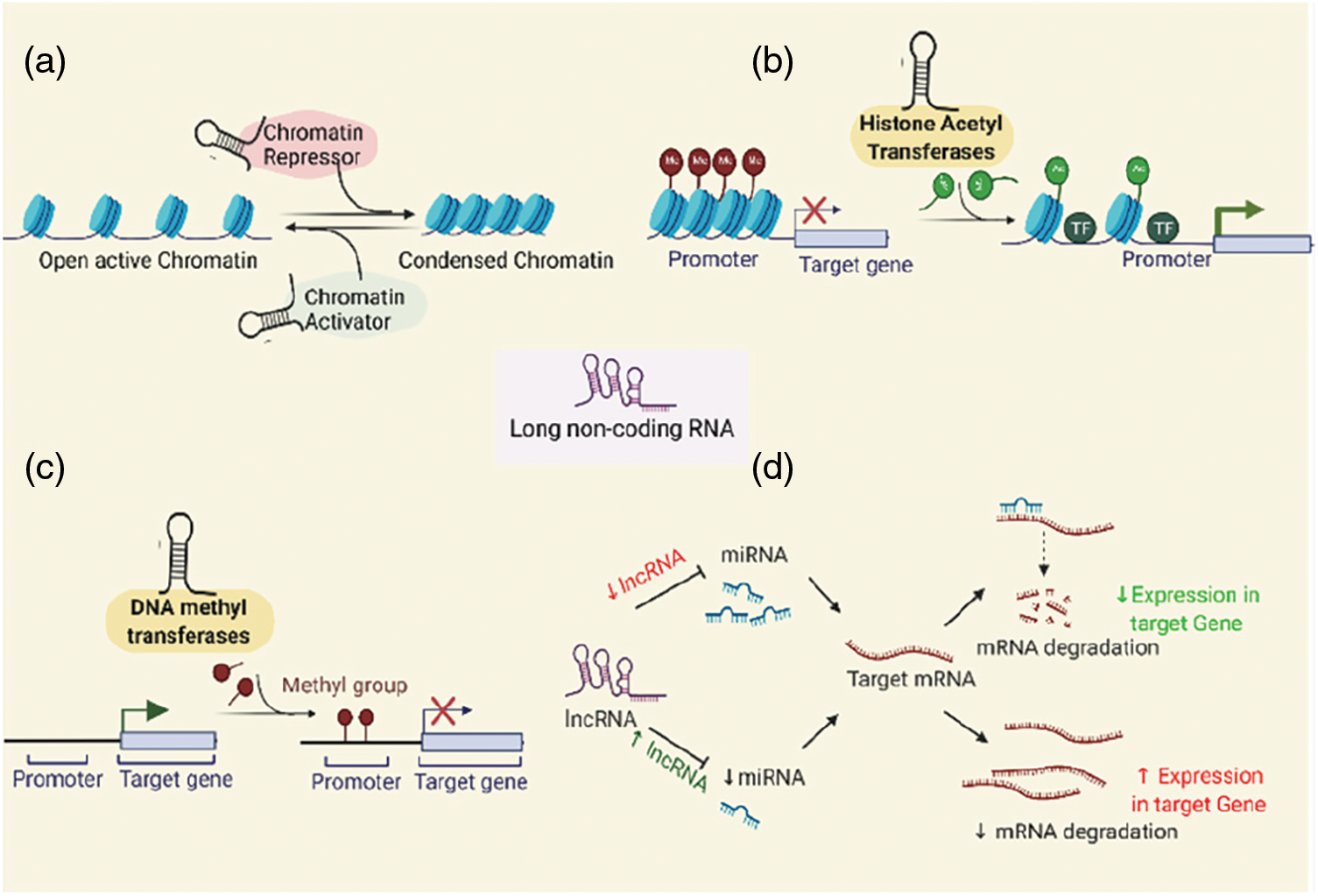
Figure 1: Epigenetic modifications of the lncRNAs to regulate gene expression: (a) via chromatin modification and remodeling by interacting with the epigenetic activator or repressive complex members, (b) histone modifications to alter chromatin structure, (c) via Promoter DNA methylation, (d) via ceRNA networking (miRNA sponging).
Chromatin modifications and DNA methylation patterns
Around 25% of all the intergenic lncRNAs have been found to interact with the chromatin-modifying proteins especially via interacting with Histone modifying enzymes (e.g., histone acetyl transferases, histone deacetylases) and/or incorporating PRC2 complex members (e.g., EED, SUZ12 and EZH2), and facilitate transcriptional and post-transcriptional regulation of target genes (Cantile et al., 2020; Ren et al., 2019a). EZH2 (Enhancer of Zeste Homolog 2), which is a histone methyltransferase, a critical element of the multiplex-suppression complex called Polycomb Repressive Complex 2 (PRC2). EZH2 functions through the trimethylation of lysine in histone H3 and its aberrant expression has been heavily investigated in cancers (Czermin et al., 2002; Yang et al., 2018). Several studies have documented the involvement of EZH2 with lncRNAs in osteosarcoma. In addition to these, the alteration in the methylation patterns (by binding DNMTs, e.g., DNMT1, DNMT3A, and DNMT3B) mediated by lncRNAs have also been associated with the pathogenesis and progression of osteosarcoma. Other mechanisms involve ubiquitination or phosphorylation in important onco- or tumor suppressor genes, and a strong regulatory network amongst the lncRNAs and miRNAs could also be exploited given their implicit regulation of genes expression. The following sections list some of the most significant lncRNAs in osteosarcoma that have been implicated for their biomarker potential.
lncRNA HOTAIR is one of the broadly studied ncRNAs. HOTAIR is an important EMT regulator and has been implicated in the pathogenesis of several cancers. HOTAIR inhibits HOXD transcription through PRC2 recruitment, forming a heterochromatin and transcriptional gene suppression via H3K9 trimethylation (Zhang et al., 2015). In osteosarcoma, HOTAIR was found to positively regulate the global DNA methylation level and specifically DNMT1 expression, making it an interesting diagnostic marker and therapeutic target (Li et al., 2017b). lncRNA MALAT1 (Metastasis Associated Lung Adenocarcinoma Transcript 1) located at chromosome 11q13.1, is suggested to be an oncogenic lncRNA in other cancers. Epigenetic regulation of MALAT1 in osteosarcoma has been investigated (Zhang et al., 2018b) especially with respect to the expression pattern of EZH2 (Li et al., 2017a). Zhang et al. (2018b) identified that MALAT1 regulates the expression of β-catenin and E-cadherin via the MALAT1/EZH2 axis which in turn changes the gene expression downstream of β-catenin. In another latest study, MALAT1 was also found to serve as a ceRNA network for HDAC4 (histone deacetylase 4), where it regulates osteosarcoma proliferation and apoptosis by upregulating HDAC4 via decoying miR-140-5p (Sun and Qin, 2018).
A majority of the antisense-lncRNAs influenced dysregulation by either methylation pattern or chromatin conformational changes, typically found to regulate expression of their opposite strand gene. For instance, lncRNA KCNQ1OT1 (KCNQ1-opposite strand/antisense transcript-1) negatively regulates KCNQ1 gene via promoting DNMT1 expression in the KCNQ1 gene promoter region (Qi et al., 2019). lncRNA FOXD2-AS1 (FOXD2 Adjacent Opposite Strand RNA 1) is robustly expressed in the osteosarcoma tissue specimens and cell lines (induced by transcription factor HIF-1α). FOXD2-AS1 was found to play a critical role in hypoxia-induced osteosarcoma tumorigenesis by interacting with the EZH2 and repressing p21 protein expression (Ren et al., 2019b; Zhang et al., 2021). The oncogenic lncRNA DANCR (Differentiation Antagonizing non-coding RNA) is overexpressed in many cancers and also promotes proliferation, migration and invasion in osteosarcoma (Pan et al., 2020). Several studies found the interaction between DANCR and EZH2 in many tumor types including osteosarcoma (Cheng et al., 2021; Wang et al., 2019; Zhang and Peng, 2017). When DANCR was knocked down, it lowered the EZH2 expression and activated both p21 and p27, hence inhibiting the osteosarcoma cell proliferation, migration, and invasion (Zhang and Peng, 2017). lncRNA HOXD-AS1 epigenetically inhibits p57 by interacting with EZH2, thereby repressing the expression of p57 and aggravating osteosarcoma oncogenesis (Gu et al., 2018).
lncRNA interactions with EZH2 were seen again with FOXP4-AS1 (forkhead box P4-AS1). Overexpression of FOXP4-AS1 was found to regulate osteosarcoma progression by downregulating LATS1 (large tumor suppressor 1) through binding LSD1 (lysine-specific demethylase 1) and EZH2 (Yang et al., 2018). The oncogenic lncRNA MIR100HG is another potential prognostic marker that promotes osteosarcoma progression via interacting with EZH2. MIR100HG epigenetically silences both LATS1 and LATS2 kinases by binding with EZH2 and thereby inactivating the Hippo signaling pathway (Su et al., 2019).
Hippo is an evolutionarily highly conserved pathway for the control of organ development and other cellular functions that are vital in oncogenesis (Calses et al., 2019; Harvey et al., 2013). The transcriptional activity of one of the downstream effectors of Hippo signaling, YAP (Yes-associated protein 1) was proved to be governed by lncRNA B4GALT1-AS1(Li et al., 2018) and XIST to maintain osteosarcoma tumor progression. Interestingly, YAP overexpression is also induced by aberrant Hedgehog signaling, which in turn causes overexpression of lncRNA H19 and is responsible for the pathogenesis of osteoblastic osteosarcoma (Chan et al., 2014). Recently, in a comprehensive study characterizing prognostic lncRNA, correlation analysis of copy number alteration (CNA) and lncRNA expression identified AC011442.1, predicted to regulate AMPK and hedgehog signaling pathway thereby acting as an oncogenic driver in osteosarcoma (Gao et al., 2020).
Oncogenic lncRNA ZEB1-AS1 (ZEB1 Antisense-1) has been implicated as a potential biomarker and therapeutic target due to its association with the opposite strand gene, ZEB1. ZEB1-AS1 can recruit histone acetyltransferase p300 to the promoter region of ZEB1 that results in an open chromatin structure and active transcription of ZEB1 promoting osteosarcoma proliferation and migration (Liu and Lin, 2016; Cheng et al., 2020). lncRNA HIF1α-AS1 (hypoxia-inducible factor 1α-antisense-1) interacts with BRG1 (Brahma-related gene-1), this was suggested as a novel therapeutic agent for bone diseases as it was found to be an essential mediator of osteoblast differentiation regulated by acetylation (histone deacetylase sirtuin 1) (Xu et al., 2015).
Crosstalk between lncRNAs and miRNAs
One of the MEG3 (maternally expressed gene 3) gene transcripts, a 1.6 kb lncRNA situated in 14q32, is a very well-known tumor suppressor lncRNA in many cancer types (Li et al., 2013; Shen et al., 2019). The lost or reduced expression of MEG3 in different cancers has been associated with promoter hypermethylation and hypermethylation of the intergenic region (Al-Rugeebah et al., 2019; Modali et al., 2015; Sellers et al., 2019; Zhou et al., 2012). In osteosarcoma, MEG3 expression and function were mainly associated with ceRNA network or miRNA sponging mechanisms, e.g., sponging onco-miR664a, MEG3/miR-361-5p/FoxM1 axis, MEG3/miR-127/ZEB1 axis, etc. In most of the cases, MEG3 was suggested to be acting as a tumor suppressor and thereby a potential prognostic biomarker for osteosarcoma. Its overexpression was also able to prevent cell growth and metastasis by targeting oncogenes or by inhibiting signaling pathways like Notch and TGF-β (Shen et al., 2019; Sun et al., 2020; Zhang et al., 2017a). lncRNA CEBPA-AS1 (CCAAT enhancer-binding protein alpha, aka LOC80054), that is usually downregulated in osteosarcoma (GSE16088) and other cancers (Ke et al., 2017), has recently been reported to inhibit osteosarcoma cell proliferation, differentiation, and enhance apoptosis by repressing the Notch signaling pathway via upregulating the expression of miR-10b-5p-mediated nuclear receptor corepressor 2 (NCOR2) (Xia et al., 2020). lncRNA-p21 (also known as TRP53COR1-tumor protein p53 pathway corepressor 1) has been reported to have in vivo and in vitro antitumor properties against wide range of tumors especially via cell cycle checkpoint regulation or regulating energy metabolism, or p53 and B-catenin pathway (Dimitrova et al., 2014; Wang et al., 2014; Yang et al., 2014; Yang et al., 2015; Yang et al., 2016). In osteosarcoma it was downregulated, and when overexpressed, it could upregulate the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) level (Han and Liu, 2018). The tumor suppressor function of p-21 was via sponging miR-130b that significantly inhibited osteosarcoma proliferation (Han and Liu, 2018). The lncRNA SNHG10 (lncRNA small nucleolar RNA host gene 10) plays an important role in osteosarcoma growth via miR-182-5p sponging and the SNHG10/miR-182-5p/FZD3 axis maintain the activation of the Wnt/β-catenin signaling pathway (Zhu et al., 2020).
The oncogenic lncRNA AFAP1-AS1 (actin fiber-associated protein 1 antisense RNA 1) has been proposed as a promising therapeutic target in osteosarcoma as it is found to be overexpressed and promoting the epithelial-mesenchymal transition of osteosarcoma through RhoC/ROCK1/p38MAPK/Twist1 signaling pathway (Shi et al., 2019). Recently, Fei et al. (2020) further strengthened this fact showing that AFAP1-AS1 promotes osteosarcoma progression by regulating the miR-497/IGF1R axis and targeting it could inhibit tumorigenesis both in vitro and in vivo. The lncRNA NBAT1 (neuroblastoma-associated transcript 1), is recognized as a tumor suppressor lncRNA in some cancers. In osteosarcoma, the lower NBAT1 expression was associated with distant metastasis and poor prognosis, as it interacts with miR-21 and its downstream gene targets including PTEN, PDCD4, TPM1 and RECK (Yang et al., 2017). lncRNA PVT1 (Plasmacytoma Variant Translocation 1) is a well-studied oncogenic lncRNA that was found to function as competing endogenous RNA or interact and stabilize the MYC protein (Sun et al., 2019; Tseng et al., 2014). Recently Chen et al. (2020) investigated the regulatory mechanism of PVT1 in osteosarcoma and identified that m6A demethylase ALKBH5-mediated demethylation of the PVT1 promotes osteosarcoma growth. The authors proposed PVT1 as a potential prognostic marker and ALKBH5-PVT1 to be a promising therapeutic target.
A novel interplay between lncRNA HOTAIR, miR-126, and DNA methylation in osteosarcoma has been reported by Li et al. (2017b), where they found that HOTAIR can repress CDKN2A gene expression through DNA hypermethylation by suppressing miR-126 expression (a negative regulator of DNMT1). Therefore, the lncRNAHOTAIR-miR126-DNMT1-CDKN2A axis was proposed to be a novel therapeutic alternative, especially targeting HOTAIR due to its potential to increase osteosarcoma chemosensitivity toward DNMT1 inhibitors (Li et al., 2017b). lncRNA ANRIL (antisense non-coding RNA in the INK4 locus) is upregulated across many cancers. It is a 3.8 kB-long transcript expressed in the opposite direction from INK4A-ARF-INK4B which represses the expression of tumor suppressor gene p15 (INK4B) via recruiting PRC2 complexes (Kotake et al., 2011). The epigenetic crosstalk between ANRIL and the microRNAs was first documented in gastric cancer (Zhang et al., 2014a), its overexpression affects osteosarcoma cell proliferation, invasion, apoptosis (Cheng et al., 2017; Yu et al., 2018) and metastasis (Guan et al., 2018). Knockdown of ANRIL in the osteosarcoma cell lines was able to increase the Cisplatin-induced chemosensitivity via the upregulation of miR-125a-5p and reduction of its target gene STAT3 (Li and Zhu, 2019). Consistent with other studies, this research indeed reveals the potential for targeting lncRNA-ANRIL/miR-125a-5p axis in the treatment of the chemoresistant osteosarcoma. The lncRNA DANCR has been documented to function by decoying miR-335-5p and miR-1972 in osteosarcoma and to facilitate ROCK1-mediated proliferation and metastasis (Pan et al., 2020). The major signaling pathways and ceRNA network that have been found to be associated with lncRNA MALAT1 are the PI3K/AKT pathway, RhoA/ROCK signal transduction pathway, MALAT1/miR-509/Rac1 axis, MALAT1/miR-142-3p/miR-129-5p/HMGB1 axis (Cai et al., 2016; Dong et al., 2015; Liu et al., 2017a; Zhang et al., 2018a).
lncRNA EPIC1 (Epigenetically Induced lncRNA-1), despite playing an oncogenic role in other cancers through interacting with the oncogenic c-MYC protein (Wang et al., 2018), it has shown to have an opposite effect on osteosarcoma. It is able to inhibit the cell viability and invasion in vitro as well as suppress tumor growth in the osteosarcoma xenograft model by ubiquitin-mediated degradation of myocyte-specific enhancer factor 2, MEF2D (Zhao et al., 2019). lncRNA BLACAT1 (Bladder cancer associated transcript 1) interacts with STAT3 and regulates the phosphorylation of STAT3 and contributes to the proliferation and migration of osteosarcoma cells (Dong and Wang, 2019).
Another emerging new class of biomarkers for cancer development and progression are the circular RNAs (circRNAs). CircRNAs are the covalently closed/looped single-stranded non-coding RNA molecules, created by back-splicing of the pre-mRNA regulated by specific RNA-binding proteins (Bach et al., 2019; Zhang et al., 2020b). Previously they were considered as splicing errors, but recently many circRNAs are being discovered taking part in the post-transcriptional regulation of gene expression via different mechanisms.
In osteosarcoma, circRNAs modulate cell proliferation, migration and invasive properties mostly via circRNA-miRNA-mRNA interaction to regulate expression of specific onco- or tumor suppressor genes; for example, hsa_circ_0001564 (sponge miR-29c-3p), hsa-circ-0016347 (sponge miR-214), circGLI2 (sponge miR-125b-5p), circ-03955 (sponge miR-3662), circ-0001785 (sponge miR-1200), circPVT1 (circPVT1/miR-52b/FOXC2 axis), circ-NT5C2, hsa_circ_0092509, hsa_circ_0009910 (Chen and Huang, 2018; Fatema and Barrott, 2022; Jin et al., 2017; Liu et al., 2021; Wu et al., 2021; Wu et al., 2019). However, their function is not confined to sponging miRNA or proteins only. Accumulating evidence suggests a clear connection between the differential expression of circRNAs and the enzymes regulating DNA methylation or histone proteins (Bach et al., 2019; Chen and Huang, 2018; Jin et al., 2017; Lee et al., 2019) (Fig. 2).
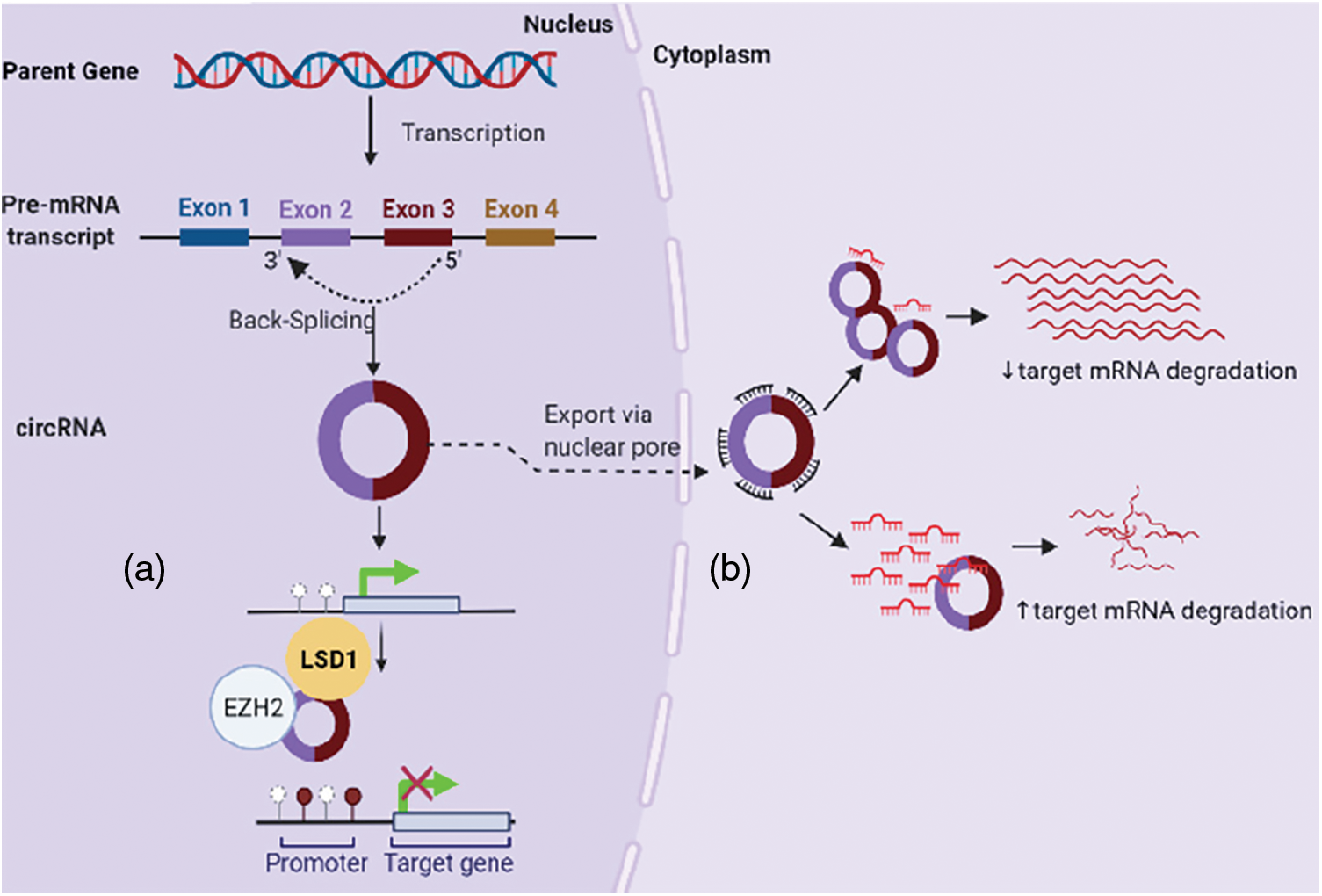
Figure 2: CircRNAs in osteosarcoma regulate gene expression: (a) by interfering with DNA methylation (e.g., binding EZH2), (b) via circRNA-miRNA-mRNA interaction.
A comprehensive characterization of circRNAs in around 1000 human cancer cell lines identified a strong association between the circRNAs and drug response, especially circMYC (associated with breast cancer cell proliferation) has shown a great sensitivity towards the drugs targeting histone acetylation (i.e., HDAC inhibitors Belinostat and Vorinostat) (Ruan et al., 2019).
Positive correlation between the histones and circRNAs has also been documented in several osteosarcoma cases (Table 2). For example, novel circRNA, circHIPK3 (homeodomain interacting protein kinase-3) which has been found to promote HDAC4 expression via sponging of miR-637 to regulate osteosarcoma cell proliferation, migration, and invasion (Wen et al., 2021). However, earlier reports of circHIPK3 state that it has a tumor suppressor function and a clinical correlation where decreased expression associates with shorter overall survival (Long et al., 2018). Oncogenic circLRP6 is highly expressed in osteosarcoma and its overexpression was associated with shorter patient survival (both disease-free and overall). Functionally, circ-LRP6 binds to both LSD1 and EZH2 and inhibits APC and KLF2 expression thereby promoting osteosarcoma progression (Zheng et al., 2019). In another study, Wu et al. (2019) focused on the underlying mechanism of circTADA2A which is abundantly expressed in osteosarcoma. TADA2A gene is part of the PCAF histone acetyltransferase complex and plays important role in chromatin remodeling, TP53 transcriptional activity, and regulating apoptosis via XRCC6 acetylation (Huang et al., 2012). This group concluded that circTADA2A targets an oncogene, CREB3 to promote osteosarcoma progression and metastasis via sponging to miR-203a-3p and emphasized circTADA2A-miR-203a-3p-CREB3 axis as a potent osteosarcoma-targeted therapy.
Short Non-Coding RNAs–Micro RNAs
According to the miRBase v.22.1, the human genome encodes for approximately 2,654 mature microRNAs (Gregorova et al., 2021) and any single microRNA is capable of regulating the expression of hundreds of different genes (Kim et al., 2008; Plotnikova et al., 2019). Depending on their expression pattern and molecular targets in different cancer types, miRNAs can have either oncogenic or tumor suppressive function in tumorigenesis (di Leva et al., 2014). miRNAs can regulate gene expression via RNA interference (RNAi) mechanism, miRNA sponging mechanism (ceRNA network), other epigenetic processes such as interfering with DNA methylation, especially targeting DNA methyltransferases (Fabbri et al., 2007; Garzon et al., 2009; Llobat and Gourbault, 2021) as well as the histone-modifying complex members (Alvarez-Saavedra et al., 2011; Garzon et al., 2009).
miRNAs can initiate transcriptional gene silencing or induce re-expression of methylation-silenced genes through restricting chromatin remodeling enzymes activity and/or altering DNA methylation status (Benetti et al., 2008; Fabbri et al., 2007; Wei et al., 2017; Yuan et al., 2011). Epigenetic mechanisms and chromosomal abnormalities have also been highlighted as the trigger to the aberrant expression of miRNAs in different cancers (di Leva and Croce, 2013; Vicentini et al., 2019). In 23 different types of tumors, 12% of all the miRNA genes associated with CpG islands were found inactivated by methylation (Gregorova et al., 2021). Moreover, the epigenetic modulators, such as histone methyltransferases, methyl CpG binding proteins, chromatin domain proteins, and histone deacetylases are also identified as potential targets of the miRNAs (Gregorova et al., 2021; Kim et al., 2008; Plotnikova et al., 2019). They have been found to modulate components of Polycomb complexes, e.g., targeting the EZH2 (miRNA-101, miR-26a), inhibiting stem cell factor BMI-1 (miR-128, miR-200c), promoting skeletal muscle differentiation (miR-214) (Peschansky and Wahlestedt, 2014). Differentially expressed histone deacetylases are also targets of miRNAs, but the conclusions often vary among tumor types (e.g., miR-449) (Buurman et al., 2012; Noonan et al., 2009).
miRNA clusters and families in the cancer epigenetics
25% of all human miRNA genes are organized in clusters based on their genomic location (within <10 kB range) and expression profiles (Kabekkodu et al., 2018). These clusters may contain the smallest to the highest number of miRNAs with similar biological function; whereby intergenic regions contain the most clusters compared to other locations. Two or more miRNAs with high sequence similarity are referred to as miRNA gene family and each family can be part of the same or different miRNA clusters depending on their function (Guo et al., 2014).
miRNAs within the same cluster regulate the expression of the onco- and tumor suppressor genes to promote carcinogenesis both genetically (e.g., SNPs) and epigenetically (e.g., CpG island hyper- and hypomethylation, etc.) (Kabekkodu et al., 2018). Gregorova et al. (2021) highlighted the epigenetic mechanisms in different cancers that are found associated with the dysregulation of miRNA clusters or miRNA gene families such as-global or site-specific hypomethylation, CpG island promoter hypermethylation, and histone modifications, etc. According to that report, the most highlighted cancer associated miRNA clusters/families are-Let-7-5p/98-5p Family, miR-125-5p Family, miR-99-5p/100-5p Family; miR-34-5p/449-5p Family, miR-34b-5p/449c-5p Family; The miR-141-3p/200a-3p Family, miR-200ab-5p Family, miR-200bc-3p/429 Family, miR-200c-5p/550a-3p Family; miR-17~92a-1 Cluster, miR-106a~363 Cluster; miR-15-5p/16-5p/195-5p/424-5p/497-5p Family; miR-23-3p Family, miR-23b~24-1 Cluster, miR-23a~24-2 Cluster; miR-130-3p/301-3p/454-3p Family and miR-29-3p Family (Gregorova et al., 2021).
Several miRNAs found deregulated in osteosarcoma compared to normal bone, osteoblasts, and mesenchymal stem cells, and some are also selectively expressed in osteosarcoma (Baumhoer et al., 2012; Jones et al., 2012; Llobat and Gourbault, 2021; Maire et al., 2011; Ramassone et al., 2018; Sarver et al., 2010; Thayanithy et al., 2012; Ziyan et al., 2011). miRNAs may well play both oncogenic and tumor suppressive roles depending on their target genes and pathways in osteosarcoma (Llobat and Gourbault, 2021). In a recent review on miRNAs in human osteosarcoma, Llobat and Gourbault (2021) compiled the miRNAs involved in osteosarcoma progression, in particular the clusters which have pivotal role in cancer hallmarks, for example the miR-17-92 and miR-106b-25 clusters. In a global microarray analysis of a panel of 19 human osteosarcoma cell lines, Namløs et al. (2012) identified 177 differentially expressed miRNAs relative to normal bone, nearly half of which overlapped with two earlier studies, including the common miR-150. Among these, miR-126/miR-126*, miR-142-3p, miR-150, miR-223, miR-486-5p, and members of the miR-1/miR-133a, miR-144/miR-451, miR-195/miR-497 and miR-206/miR-133b clusters were found to be downregulated; miR-9/miR-9*, miR-21*, miR-31/miR-31*, miR-196a/miR-196b, miR-374a and members of the miR-29 and miR-130/301 families were found to be upregulated (Table 3).
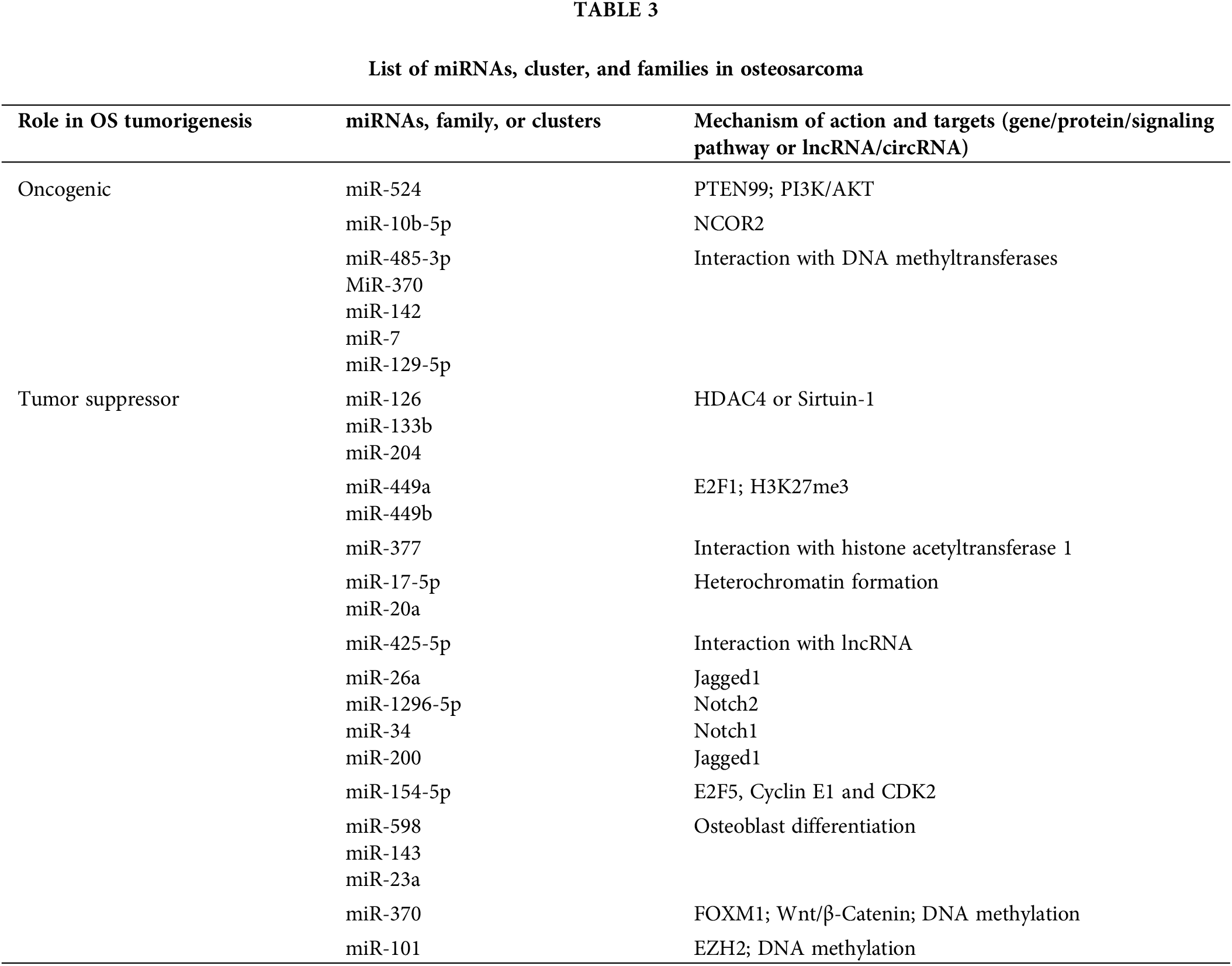
All such differentially expressed miRNAs, miRNA clusters and families - having the similar biological function with the potential to regulate specific mRNAs of target genes-are promising diagnostic and prognostic markers for osteosarcoma (Lei et al., 2020; Ramassone et al., 2018; Zhang et al., 2015). A large number of miRNAs hold biomarker potential due to their differential expression in body fluids (especially in patients’ blood), their correlation with the response to chemotherapy and patient survival; for instance, miR-Let7A (Hua et al., 2018). Botti et al. (2019) recently in a review summarized the circulating miRNAs with biomarker potential in the diagnosis, prognosis, and clinical monitoring of osteosarcoma patients. According to them, miR-29 family members (miR-29a, miR-29b, miR-29c), miR-199a-3p, miR-196a, miR-196b, miR-214, miR-574, miR-335, miR-9, miR-191, miR-221, miR-148, miR-195-5p, miR 320a, miR-421, miR-542, miR-95-3p, miR-21, miR-27a and miR-253-p are found highly expressed in patient serum and the miR-34 family members (miR-34a and miR-34b), miR-124, miR-205-5p, miR-133b, miR-206, miR-195, miR-152, miR-326, miR-145, miR-558, miR-497, miR-491 and miR-375 are significantly downregulated. Zhang et al. (2020a); Wu et al. (2011) proposed a novel diagnostic marker for predicting osteosarcoma metastasis, the plasma EV-packaged miR-101 (EV-miR-101), which the author indicated might serve as a useful circulating biomarker. Furthermore, the following sections will delineate the major markers of epigenetic control of miRNAs in osteosarcoma.
DNA methylation and chromatin modifying miRNAs
An assessment of the clinical utility of miRNA sets and their association with methylation status (Hill et al., 2017) found that most prognostic miRNAs affecting gene expression via DNA methylation, cluster in 14q32-a region which was also previously reported to encode more than 40 miRNAs including imprinted genes important in osteogenic differentiation and inhibiting cancer (Thayanithy et al., 2012). This report suggests that miRNAs and modulation in methylation patterns may offer prognostic and therapeutic strategies in osteosarcoma treatment (Lietz et al., 2020). The hypothesis that miRNA may regulate gene expression epigenetically was reinforced by the relationship of certain miRNAs and DNA methyltransferases, for example-miR-485-3p, MiR-370, miR-142, miR-7, miR-129-5p (Ding et al., 2015; Du et al., 2018; Fabbri et al., 2007; Khraiwesh et al., 2010; Wu et al., 2010; Zhang and Peng, 2017; Zhang et al., 2017a; Zhang et al., 2019) (Fig. 3).
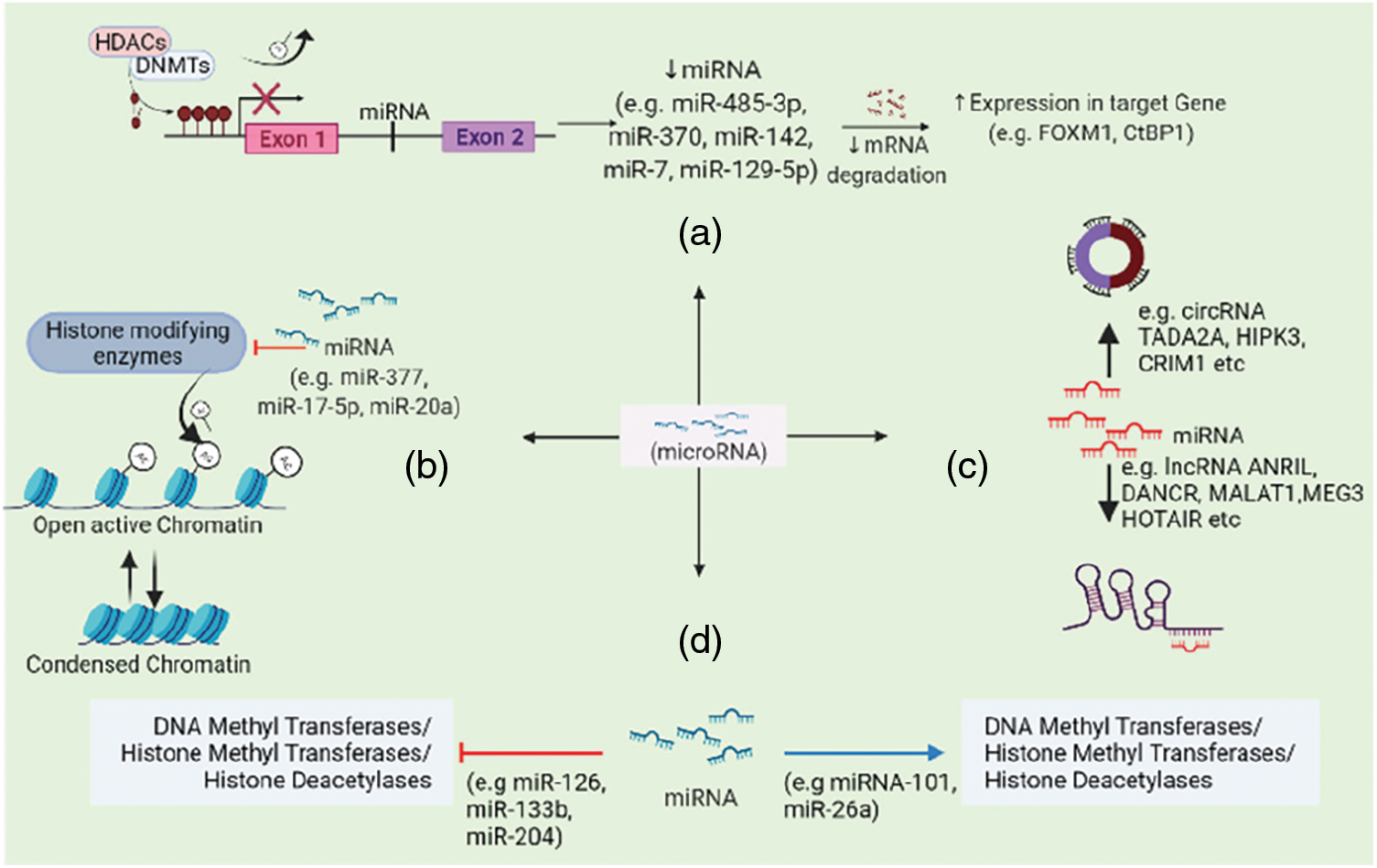
Figure 3: miRNAs affect gene expression by (a) regulating histone modifying enzymes activity and DNA methylation, (b) altering chromatin structure, (c) ceRNA networking mechanisms, (d) regulating onco- or tumor-suppressor genes expression via miRNA sponging.
Few other miRNAs have been found targeting the histone modifying enzymes such as HDAC4 or Sirtuin-1 and thus inhibiting proliferation, migration, invasion and epithelial-mesenchymal transition of osteosarcoma cells, for example, miR-126, miR-133b, miR-204 (Liao et al., 2021; Shi et al., 2017; Tang et al., 2017; Ying et al., 2017). Tumor suppressor miR-449a and miR-449b were epigenetically repressed in the osteosarcoma cell line via H3K27me3 resulting in E2F1 deregulation. And their expression could be restored when targeted with a combination of small-molecule histone methylation inhibitor Deazaneplanocin A (DZNep) and HDAC inhibitor trichostatin-A (TSA) (Yang et al., 2009).
Another proposed mechanism of action is through forming heterochromatin (e.g., miR-377, miR-17-5p and miR-20a) (Gonzalez et al., 2008; Xia et al., 2019). miR-377, which is a well-recognized tumor suppressor miRNA in many cancers, has been found to target HAT1 (histone acetyltransferase 1) in osteosarcoma (Xia et al., 2019). And apparently upregulation of miR-377 or inhibition of HAT1 prevented osteosarcoma progression via inactivating Wnt pathway thereby providing a therapeutic alternative.
Interaction with lncRNA and circRNA
Several miRNAs have been reported to interact with the lncRNAs as well as circRNAs to serve their oncogenic or tumor suppressive role in osteosarcoma (described in the previous sections). Significantly reduced serum miR-425-5p expression makes it a potential prognostic marker in osteosarcoma, and when overexpressed it decreases the expression of very well-known oncogenic lncRNA MALAT1 and TUG1 in addition to suppressed tumor growth in-vivo (Yang et al., 2019).
Other signaling pathways governed by miRNAs
Several miRNAs have been reported to be involved in the Notch signaling pathway in the initiation and progression of osteosarcoma, of which some are tumor suppressor miRNAs such as miR-26a (targets Jagged1), miR-1296-5p (targets Notch2), miR-34 and miR-200 (targets Notch1) and some play oncogenic role for example, miR-10b-5p (targets NCOR2) (Lei et al., 2020; Xia et al., 2020). miR-154-5p acts as tumor suppressor in osteosarcoma and its upregulation inhibits the proliferation, migration and invasion in vitro as well as in-vivo tumor growth via the dysregulation in the pro-apoptotic proteins’ expression and the cell cycle regulators such as E2F5, Cyclin E1 and CDK2 (Tian et al., 2020). Another miRNA, miR-524 activates PI3K/AKT pathway and induces proliferation in osteosarcoma via directly inhibiting PTEN expression (Zhuang et al., 2018). Some miRNAs such as miR-598, miR-143, and miR-23a, also found to play a role in osteoblast differentiation in osteosarcoma progression (Grilli et al., 2015; Liu et al., 2017b).
Short Non-Coding RNAs–piRNAs and siRNAs
piRNAs are approximately 26–30 NT long, consisting of more than 50,000 different species. Recent studies demonstrated possible involvement of aberrant piRNAs expression in tumorigenesis and all the hallmarks of cancer, and thereby suggested as a diagnostic and prognostic marker (e.g., piR-L-163, piR-823) (Wei et al., 2017; Wu et al., 2020). piRNAs been speculated to have a role in epigenetic regulation as they bind to Piwi proteins, a known epigenetic regulator functioning by binding to Polycomb group genes (Lin, 2007). piR-39980, which has been reported to have an oncogenic function in human osteosarcoma cells (Das et al., 2020), has been found to have strong anti-tumor activity in fibrosarcoma (early metastatic lethal tumor) by repressing RRM2 (Das et al., 2019). siRNAs can result in transcriptional gene silencing via DNA methylation and histone modifications in cells especially through interfering with EZH2 (Bayne and Allshire, 2005; Morris et al., 2004; Zhou et al., 2015).
Non-Coding RNAs as Therapeutic Targets for Epigenetics-Driven Personalized Medicine
Epigenetic therapeutics in combination with the selective targeting of the ncRNAs might hold a great key for treating the cancers that are more chemoresistant and more prone to relapse after chemotherapy. In fact, studies focused on DNA methylation pattern for drug repurposing in osteosarcoma (Chaiyawat et al., 2020) identified a significant increase in DNMT1-dependent chemosensitivity toward Cisplatin therapies when treated with Decitabine (DNMT inhibitor).
The lncRNA HOTAIR (discussed in earlier sections) is an outstanding therapeutic target for anticancer therapies (Cantile et al., 2020; Li et al., 2017b). Recently, a computer-aided structure-based drug design method has been able to develop small molecule inhibitor of HOTAIR (e.g., AC1NOD4Q) which particularly interferes with the HOTAIR/EZH2 interaction and prevents tumor metastasis in breast cancer models (Ren et al., 2019a). Moreover, suppressing HOTAIR in combination with other epigenetic drugs (e.g., DZNep and AC1Q3QWB) showed a great promise in treatments for breast cancer and glioblastoma (Li et al., 2019; Sun et al., 2015). Its unique expression pattern in osteosarcoma, regulation of DNA methylation, exploiting chromatin remodelers, functioning via ceRNA network, and also promising outcomes in other cancers; all of these shows a great potential for HOTAIR to be an excellent candidate for epigenetic therapeutics in osteosarcoma (Cantile et al., 2020; Li et al., 2017b).
lncRNA NEAT1 (nuclear enriched abundant transcript 1) is another oncogenic transcript involved in the osteosarcoma metastasis and EMT regulation (Li and Cheng, 2018). NEAT1 induces epigenetic suppression of E-cadherin (CDH1) expression by mediating CDH1 promoter methylation via G9a methyltransferase. And when knocked down, it can significantly reduce G9a-DNMT1-Snail complex association in CDH1 promoter. Consequently, NEAT1 is yet another promising target in the treatment of metastatic osteosarcoma via epigenetic-derived therapeutics. Previously described lncRNA MEG3 was also suggested to be a potential therapeutic target in osteosarcoma due to its negative regulation of the well-known oncogene FOXM1 through sponging miR-361-5p (Shen et al., 2019). Other oncogenic lncRNAs such as AFAP-AS1 and MALAT1 (discussed previously) have also been proposed to be a therapeutic target due to their effects on osteosarcoma progression and metastasis (Fei et al., 2020).
Preclinical studies on osteosarcoma emphasized the potentials of different miRNAs as therapeutic targets (e.g., miR-146b-5p) (Jiang et al., 2019). Several miRNAs expression has also been associated with abnormal methylation that could be targeted with DNMT inhibitors to suppress osteosarcoma progression (e.g., miR-485-3p, miR-370, miR-142, miR-7, miR-129-5p) (Ding et al., 2015; Du et al., 2018; Long et al., 2015; Zhang and Peng, 2017; Zhang et al., 2019). Indeed, osteosarcoma cells when treated with DNMT inhibitor DAC, increased the levels of tumor suppressor microRNA miR-370. Not only that, DAC treatment also enhanced its inhibitory effect on FOXM1 by suppressing FOXM1-β-catenin interaction and inhibiting Wnt/β-Catenin signaling (Zhang et al., 2017b).
In a recent review, Lei et al. (2020) outlined the miRNA-based therapeutics in clinical trials as well as the miRNA mimics that are currently under development for targeting osteosarcoma both in vitro and in vivo. These includes nanoparticles, bioengineered prodrugs, and exosome-mediated delivery of miRNA mimics of miR-199a-3p, let-7a, miR-34a, miR-145, miR-143, and miR-101. A prodrug designed for miR-34a (tRNA/miR-34a) has shown substantial antitumor activity in preclinical canine model of osteosarcoma cell lines and in vivo xenograft model (Alegre et al., 2018), providing evidence for the potential of the miRNA-based therapies in the treatment of human osteosarcoma.
miR-101 is a well-known tumor suppressor miRNA in several cancers and in osteosarcoma, it was found functioning through repressing EZH2 expression to decrease metastasis (Zhang et al., 2014b). Recently, Zhang et al. (2020a) designed an exosomal delivery of miR-101 with EV derived from engineered AD-MSCs, and their study showed that miR-101 had the potential to inhibit metastatic osteosarcoma, possibly via regulation of EZH2 and BCL6.
Identifying biomarkers that differentiate responders/survivors from non-responders remains enigmatic and could overcome the stagnate survival statistics that have persisted for the past 40 years. While biomarkers in the coding RNA and DNA have been unreliable, there is potential to investigate the ncRNA for consistent biomarkers. As discussed in this review, there are a number of potential biomarkers among the different classes of ncRNAs that not only serve as biomarkers to differentiate more aggressive osteosarcomas, but help to explain the biological processes that drive the hallmarks of cancer. By understanding the underpinnings of how ncRNAs drive transformation and progression, they can then become pharmacological targets to modulate cancer pathways and drive favorable outcomes for patients. The most advanced therapeutics in this field involve the complementary targeting of miRNAs that regulate numerous cell processes that regulate protumorigenic behavior. Currently several clinical trials are underway to investigate their therapeutic potential. However, as with most targeted therapies already applied to osteosarcoma, these therapies will also likely fail unless combined with other approaches. The heterogeneity and genomic instability that exists in the DNA coding regions is likely to complicate the interpretation of the ncRNAs, but the potential is there to discover something truly ground-breaking.
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Author Contribution: The authors confirm contribution to the paper as follows: draft manuscript preparation: K. Fatema. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: Not applicable.
Funding Statement: This research and publication were funded by The Pardee Foundation, INBRE Program NIH [Grant No. P20 GM103408] (National Institute of General Medical Sciences), and Idaho State Career Path Internships.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Alegre F, Ormonde AR, Snider KM, Woolard K, Yu AM, Wittenburg LA (2018). A genetically engineered microRNA-34a prodrug demonstrates anti-tumor activity in a canine model of osteosarcoma. PLoS One 13: e0209941. DOI 10.1371/journal.pone.0209941. [Google Scholar] [CrossRef]
Al-Rugeebah A, Alanazi M, Parine NR (2019). MEG3: An oncogenic long non-coding RNA in different cancers. Pathology & Oncology Research 25: 859–874. DOI 10.1007/s12253-019-00614-3. [Google Scholar] [CrossRef]
Alvarez-Saavedra M, Antoun G, Yanagiya A, Oliva-Hernandez R, Cornejo-Palma D, Perez-Iratxeta C, Sonenberg N, Cheng HY (2011). miRNA-132 orchestrates chromatin remodeling and translational control of the circadian clock. Human Molecular Genetics 20: 731–751. DOI 10.1093/hmg/ddq519. [Google Scholar] [CrossRef]
Bach DH, Lee SK, Sood AK (2019). Circular RNAs in cancer. Molecular Therapy–Nucleic Acids 16: 118–129. DOI 10.1016/j.omtn.2019.02.005. [Google Scholar] [CrossRef]
Baumhoer D, Zillmer S, Unger K, Rosemann M, Atkinson MJ et al. (2012). MicroRNA profiling with correlation to gene expression revealed the oncogenic miR-17-92 cluster to be up-regulated in osteosarcoma. Cancer Genetics 205: 212–219. DOI 10.1016/j.cancergen.2012.03.001. [Google Scholar] [CrossRef]
Baylin SB, Jones PA (2016). Epigenetic determinants of cancer. Cold Spring Harbor Perspectives in Biology 8: a019505. DOI 10.1101/cshperspect.a019505. [Google Scholar] [CrossRef]
Bayne EH, Allshire RC (2005). RNA-directed transcriptional gene silencing in mammals. Trends in Genetics 21: 370–373. DOI 10.1016/j.tig.2005.05.007. [Google Scholar] [CrossRef]
Benetti R, Gonzalo S, Jaco I, Muñoz P, Gonzalez S et al. (2008). A mammalian microRNA cluster controls DNA methylation and telomere recombination via Rbl2-dependent regulation of DNA methyltransferases. Nature Structural & Molecular Biology 15: 268–279. DOI 10.1038/nsmb.1399. [Google Scholar] [CrossRef]
Botti G, Giordano A, Feroce F, de Chiara AR, Cantile M (2019). Noncoding RNAs as circulating biomarkers in osteosarcoma patients. Journal of Cellular Physiology 234: 19249–19255. DOI 10.1002/jcp.28744. [Google Scholar] [CrossRef]
Buurman R, Gürlevik E, Schäffer V, Eilers M, Sandbothe M, Kreipe H, Wilkens L, Schlegelberger B, Kühnel F, Skawran B (2012). Histone deacetylases activate hepatocyte growth factor signaling by repressing microRNA-449 in hepatocellular carcinoma cells. Gastroenterology 143: 811–820.e815. DOI 10.1053/j.gastro.2012.05.033. [Google Scholar] [CrossRef]
Cai X, Liu Y, Yang W, Xia Y, Yang C, Yang S, Liu X (2016). Long noncoding RNA MALAT1 as a potential therapeutic target in osteosarcoma. Journal of Orthopaedic Research 34: 932–941. DOI 10.1002/jor.23105. [Google Scholar] [CrossRef]
Calses PC, Crawford JJ, Lill JR, Dey A (2019). Hippo pathway in cancer: Aberrant regulation and therapeutic opportunities. Trends in Cancer 5: 297–307. DOI 10.1016/j.trecan.2019.04.001. [Google Scholar] [CrossRef]
Cantile M, di Bonito M, Cerrone M, Collina F, de Laurentiis M, Botti G (2020). Long non-coding RNA HOTAIR in breast cancer therapy. Cancers 12: 1197. DOI 10.3390/cancers12051197. [Google Scholar] [CrossRef]
Carrle D, Bielack SS (2006). Current strategies of chemotherapy in osteosarcoma. International Orthopaedics 30: 445–451. DOI 10.1007/s00264-006-0192-x. [Google Scholar] [CrossRef]
Chaiyawat P, Sirikaew N, Budprom P, Klangjorhor J, Phanphaisarn A, Teeyakasem P, Settakorn J, Pruksakorn D (2020). Expression profiling of DNA methyl transferase I (DNMT1) and efficacy of a DNA-hypomethylating agent (decitabine) in combination with chemotherapy in osteosarcoma. Journal of Bone Oncology 25: 100321. DOI 10.1016/j.jbo.2020.100321. [Google Scholar] [CrossRef]
Chan LH, Wang W, Yeung W, Deng Y, Yuan P, Mak KK (2014). Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene 33: 4857–4866. DOI 10.1038/onc.2013.433. [Google Scholar] [CrossRef]
Chen B, Huang S (2018). Circular RNA: An emerging non-coding RNA as a regulator and biomarker in cancer. Cancer Letters 418: 41–50. DOI 10.1016/j.canlet.2018.01.011. [Google Scholar] [CrossRef]
Chen J, Xue Y (2016). Emerging roles of non-coding RNAs in epigenetic regulation. Science China Life Sciences 59: 227–235. DOI 10.1007/s11427-016-5010-0. [Google Scholar] [CrossRef]
Chen S, Zhou L, Wang Y (2020). ALKBH5-mediated m6A demethylation of lncRNA PVT1 plays an oncogenic role in osteosarcoma. Cancer Cell International 20: 34. DOI 10.1186/s12935-020-1105-6. [Google Scholar] [CrossRef]
Cheng C, Dong Y, Ru X, Xia Y, Ji Y (2021). LncRNA ANCR promotes glioma cells invasion, migration, proliferation and inhibits apoptosis via interacting with EZH2 and repressing PTEN expression. Cancer Gene Therapy 28: 1025–1034. DOI 10.1038/s41417-020-00263-8. [Google Scholar] [CrossRef]
Cheng S, Guo S, He H, Kaminga AC, Xu H (2020). Clinical value of long noncoding RNA ZEB1 anti-sense1 in cancer patients: A meta-analysis. Medicine 99: e21307. DOI 10.1097/MD.0000000000021307. [Google Scholar] [CrossRef]
Cheng S, Huang T, Li P, Zhang W, Wang Z, Chen Y (2017). Long non-coding RNA ANRIL promotes the proliferation, migration and invasion of human osteosarcoma cells. Experimental and Therapeutic Medicine 14: 5121–5125. DOI 10.3892/etm.2017.5123. [Google Scholar] [CrossRef]
Clemson CM, McNeil JA, Willard HF, Lawrence JB (1996). XIST RNA paints the inactive X chromosome at interphase: Evidence for a novel RNA involved in nuclear/chromosome structure. Journal of Cell Biology 132: 259–275. DOI 10.1083/jcb.132.3.259. [Google Scholar] [CrossRef]
Costa FF (2008). Non-coding RNAs, epigenetics and complexity. Gene 410: 9–17. DOI 10.1016/j.gene.2007.12.008. [Google Scholar] [CrossRef]
Crick F (1970). Central dogma of molecular biology. Nature 227: 561–563. DOI 10.1038/227561a0. [Google Scholar] [CrossRef]
Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V (2002). Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111: 185–196. DOI 10.1016/S0092-8674(02)00975-3. [Google Scholar] [CrossRef]
Das B, Jain N, Mallick B (2020). piR-39980 promotes cell proliferation, migration and invasion, and inhibits apoptosis via repression of SERPINB1 in human osteosarcoma. Biology of the Cell 112: 73–91. DOI 10.1111/boc.201900063. [Google Scholar] [CrossRef]
Das B, Roy J, Jain N, Mallick B (2019). Tumor suppressive activity of PIWI-interacting RNA in human fibrosarcoma mediated through repression of RRM2. Molecular Carcinogenesis 58: 344–357. DOI 10.1002/mc.22932. [Google Scholar] [CrossRef]
di Fiore R, Fanale D, Drago-Ferrante R, Chiaradonna F, Giuliano M et al. (2013). Genetic and molecular characterization of the human osteosarcoma 3AB-OS cancer stem cell line: A possible model for studying osteosarcoma origin and stemness. Journal of Cellular Physiology 228: 1189–1201. DOI 10.1002/jcp.24272. [Google Scholar] [CrossRef]
di Leva G, Croce CM (2013). miRNA profiling of cancer. Current Opinion in Genetics & Development 23: 3–11. DOI 10.1016/j.gde.2013.01.004. [Google Scholar] [CrossRef]
di Leva G, Garofalo M, Croce CM (2014). MicroRNAs in cancer. Annual Review of Pathology 9: 287–314. DOI 10.1146/annurev-pathol-012513-104715. [Google Scholar] [CrossRef]
Dimitrova N, Zamudio JR, Jong RM, Soukup D, Resnick R et al. (2014). LincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Molecular Cell 54: 777–790. DOI 10.1016/j.molcel.2014.04.025. [Google Scholar] [CrossRef]
Ding M, Hu J, Ni J, Zheng Z, Song D, Wang J (2015). Demethylation of microRNA-142 induced by demethylation agents plays a suppressive role in osteosarcoma cells. Oncology Letters 9: 2261–2267. DOI 10.3892/ol.2015.3036. [Google Scholar] [CrossRef]
Dong Y, Liang G, Yuan B, Yang C, Gao R, Zhou X (2015). MALAT1 promotes the proliferation and metastasis of osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biology 36: 1477–1486. DOI 10.1007/s13277-014-2631-4. [Google Scholar] [CrossRef]
Dong Z, Wang Y (2019). LncRNA BLACAT1 accelerates the proliferation and migration of osteosarcoma cells through regulating STAT3. Pathology–Research and Practice 215: 571–579. DOI 10.1016/j.prp.2019.01.017. [Google Scholar] [CrossRef]
Du K, Zhang X, Lou Z, Guo P, Zhang F, Wang B, Chen L, Zhang C (2018). MicroRNA485-3p negatively regulates the transcriptional co-repressor CtBP1 to control the oncogenic process in osteosarcoma cells. International Journal of Biological Science 14: 1445–1456. DOI 10.7150/ijbs.26335. [Google Scholar] [CrossRef]
Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N et al. (2007). MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proceedings of the National Academy of Sciences 104: 15805–15810. DOI 10.1073/pnas.0707628104. [Google Scholar] [CrossRef]
Faisham WI, Mat Saad AZ, Alsaigh LN, Nor Azman MZ, Kamarul Imran M et al. (2017). Prognostic factors and survival rate of osteosarcoma: A single-institution study. Asia-Pacific Journal of Clinical Oncology 13: e104–e110. DOI 10.1111/ajco.12346. [Google Scholar] [CrossRef]
Fatema K, Barrott JJ (2022). Circling back to epigenetic regulation in osteosarcoma: Comment on ‘Hsa_circ_0088212-mediated miR-520h/APOA1 axis inhibits the osteosarcoma progression’ by ‘Hao Peng’. Translational Oncology 15: 101271. DOI 10.1016/j.tranon.2021.101271. [Google Scholar] [CrossRef]
Fazi B, Garbo S, Toschi N, Mangiola A, Lombari M et al. (2018). The lncRNA H19 positively affects the tumorigenic properties of glioblastoma cells and contributes to NKD1 repression through the recruitment of EZH2 on its promoter. Oncotarget 9: 15512–15525. DOI 10.18632/oncotarget.24496. [Google Scholar] [CrossRef]
Fei D, Zhang X, Lu Y, Tan L, Xu M, Zhang Y (2020). Long noncoding RNA AFAP1-AS1 promotes osteosarcoma progression by regulating miR-497/IGF1R axis. American Journal of Translational Research 12: 2155–2168. [Google Scholar]
Gao H, Guo Y, Zhang M, Yi Z (2020). Comprehensive characterization of prognostic long noncoding RNAs in osteosarcoma. BioMed Research International 2020: 1–12. DOI 10.1155/2020/6725753. [Google Scholar] [CrossRef]
Gao W, Lin S, Cheng C, Zhu A, Hu Y, Shi Z, Zhang X, Hong Z (2019). Long non-coding RNA CASC2 regulates Sprouty2 via functioning as a competing endogenous RNA for miR-183 to modulate the sensitivity of prostate cancer cells to docetaxel. Archives of Biochemistry and Biophysics 665: 69–78. DOI 10.1016/j.abb.2018.01.013. [Google Scholar] [CrossRef]
Garzon R, Liu S, Fabbri M, Liu Z, Heaphy CE et al. (2009). MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood 113: 6411–6418. DOI 10.1182/blood-2008-07-170589. [Google Scholar] [CrossRef]
Gonzalez S, Pisano DG, Serrano M (2008). Mechanistic principles of chromatin remodeling guided by siRNAs and miRNAs. Cell Cycle 7: 2601–2608. DOI 10.4161/cc.7.16.6541. [Google Scholar] [CrossRef]
Gregorova J, Vychytilova-Faltejskova P, Sevcikova S (2021). Epigenetic regulation of MicroRNA clusters and families during tumor development. Cancers 13: 1333. DOI 10.3390/cancers13061333. [Google Scholar] [CrossRef]
Grilli A, Sciandra M, Terracciano M, Picci P, Scotlandi K (2015). Integrated approaches to miRNAs target definition: Time-series analysis in an osteosarcoma differentiative model. BMC Medical Genomics 8: 34. DOI 10.1186/s12920-015-0106-0. [Google Scholar] [CrossRef]
Gu W, Zhang E, Song L, Tu L, Wang Z, Tian F, Aikenmu K, Chu G, Zhao J (2018). Long noncoding RNA HOXD-AS1 aggravates osteosarcoma carcinogenesis through epigenetically inhibiting p57 via EZH2. Biomedicine & Pharmacotherapy 106: 890–895. DOI 10.1016/j.biopha.2018.06.173. [Google Scholar] [CrossRef]
Guan H, Mei Y, Mi Y, Li C, Sun X, Zhao X, Liu J, Cao W, Li Y, Wang Y (2018). Downregulation of lncRNA ANRIL suppresses growth and metastasis in human osteosarcoma cells. Onco Targets and Therapy 11: 4893–4899. DOI 10.2147/OTT. [Google Scholar] [CrossRef]
Guo L, Zhao Y, Zhang H, Yang S, Chen F (2014). Integrated evolutionary analysis of human miRNA gene clusters and families implicates evolutionary relationships. Gene 534: 24–32. DOI 10.1016/j.gene.2013.10.037. [Google Scholar] [CrossRef]
Han W, Liu J (2018). LncRNA-p21 inhibited the proliferation of osteosarcoma cells via the miR-130b/PTEN/AKT signaling pathway. Biomedicine & Pharmacotherapy 97: 911–918. DOI 10.1016/j.biopha.2017.11.014. [Google Scholar] [CrossRef]
Hanahan D, Weinberg RA (2011). Hallmarks of cancer: The next generation. Cell 144: 646–674. DOI 10.1016/j.cell.2011.02.013. [Google Scholar] [CrossRef]
Harvey KF, Zhang X, Thomas DM (2013). The Hippo pathway and human cancer. Nature Reviews Cancer 13: 246–257. DOI 10.1038/nrc3458. [Google Scholar] [CrossRef]
Herzog CE (2005). Overview of sarcomas in the adolescent and young adult population. Journal of Pediatric Hematology/Oncology 27: 215–218. DOI 10.1097/01.mph.0000161762.53175.e4. [Google Scholar] [CrossRef]
Hill KE, Kelly AD, Kuijjer ML, Barry W, Rattani A et al. (2017). An imprinted non-coding genomic cluster at 14q32 defines clinically relevant molecular subtypes in osteosarcoma across multiple independent datasets. Journal of Hematology & Oncology 10: 107. DOI 10.1186/s13045-017-0465-4. [Google Scholar] [CrossRef]
Hua J, Liu D, Cao L, Wang D, Wu T, Lin F, Su P, Niu Y, Sun Y (2018). Diagnostic and prognostic values of blood microRNA-Let7A for osteosarcoma. Journal of Bone Oncology 12: 65–68. DOI 10.1016/j.jbo.2018.05.001. [Google Scholar] [CrossRef]
Huang J, Zhang L, Liu W, Liao Q, Shi T, Xiao L, Hu F, Qiu X (2012). CCDC134 interacts with hADA2a and functions as a regulator of hADA2a in acetyltransferase activity, DNA damage-induced apoptosis and cell cycle arrest. Histochemistry and Cell Biology 138: 41–55. DOI 10.1007/s00418-012-0932-5. [Google Scholar] [CrossRef]
Jiang M, Lu W, Ding X, Liu X, Guo Z, Wu X (2019). p16INK4a inhibits the proliferation of osteosarcoma cells through regulating the miR-146b-5p/TRAF6 pathway. Bioscience Reports 39: 104. DOI 10.1042/BSR20181268. [Google Scholar] [CrossRef]
Jin H, Jin X, Zhang H, Wang W (2017). Circular RNA hsa-circ-0016347 promotes proliferation, invasion and metastasis of osteosarcoma cells. Oncotarget 8: 25571–25581. DOI 10.18632/oncotarget.16104. [Google Scholar] [CrossRef]
Jones KB, Salah Z, Del Mare S, Galasso M, Gaudio E et al. (2012). miRNA signatures associate with pathogenesis and progression of osteosarcoma. Cancer Research 72: 1865–1877. DOI 10.1158/0008-5472.CAN-11-2663. [Google Scholar] [CrossRef]
Kabekkodu SP, Shukla V, Varghese VK, D’Souza J, Chakrabarty S, Satyamoorthy K (2018). Clustered miRNAs and their role in biological functions and diseases. Biological Reviews of the Cambridge Philosophical Society 93: 1955–1986. DOI 10.1111/brv.12428. [Google Scholar] [CrossRef]
Ke D, Li H, Zhang Y, An Y, Fu H, Fang X, Zheng X (2017). The combination of circulating long noncoding RNAs AK001058, INHBA-AS1, MIR4435-2HG, and CEBPA-AS1 fragments in plasma serve as diagnostic markers for gastric cancer. Oncotarget 8: 21516–21525. DOI 10.18632/oncotarget.15628. [Google Scholar] [CrossRef]
Khanduja JS, Calvo IA, Joh RI, Hill IT, Motamedi M (2016). Nuclear noncoding RNAs and genome stability. Molecular Cell 63: 7–20. DOI 10.1016/j.molcel.2016.06.011. [Google Scholar] [CrossRef]
Khraiwesh B, Arif MA, Seumel GI, Ossowski S, Weigel D, Reski R, Frank W (2010). Transcriptional control of gene expression by microRNAs. Cell 140: 111–122. DOI 10.1016/j.cell.2009.12.023. [Google Scholar] [CrossRef]
Kim DH, Saetrom P, Snøve, OJr., Rossi JJ (2008). MicroRNA-directed transcriptional gene silencing in mammalian cells. PNAS 105: 16230–16235. DOI 10.1073/pnas.0808830105. [Google Scholar] [CrossRef]
Kotake Y, Nakagawa T, Kitagawa K, Suzuki S, Liu N, Kitagawa M, Xiong Y (2011). Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene 30: 1956–1962. DOI 10.1038/onc.2010.568. [Google Scholar] [CrossRef]
Lee WJ, Moon J, Jeon D, Shin YW, Yoo JS et al. (2019). Possible epigenetic regulatory effect of dysregulated circular RNAs in Alzheimer’s disease model. Scientific Reports 9: 11956. DOI 10.1038/s41598-019-48471-z. [Google Scholar] [CrossRef]
Lei, Y, Chen JX, Huang YC, Liu XG, Yu BS (2020). Role of microRNAs in the crosstalk between osteosarcoma cells and the tumour microenvironment. Journal of Bone Oncology, 25, 100322–100322. DOI 10.1016/j.jbo.2020.100322. [Google Scholar] [CrossRef]
Li G, Zhu Y (2019). Effect of lncRNA ANRIL knockdown on proliferation and cisplatin chemoresistance of osteosarcoma cells in vitro. Pathology - Research and Practice 215: 931–938. DOI 10.1016/j.jbo.2020.100322. [Google Scholar] [CrossRef]
Li JP, Liu LH, Li J, Chen Y, Jiang XW, Ouyang YR, Liu YQ, Zhong H, Li H, Xiao T (2013). Microarray expression profile of long noncoding RNAs in human osteosarcoma. Biochemical and Biophysical Research Communications 433: 200–206. DOI 10.1016/j.bbrc.2013.02.083. [Google Scholar] [CrossRef]
Li P, Zhang X, Wang H, Wang L, Liu T, Du L, Yang Y, Wang C (2017a). MALAT1 is associated with poor response to oxaliplatin-based chemotherapy in colorectal cancer patients and promotes chemoresistance through EZH2. Molecular Cancer Therapeutics 16: 739–751. DOI 10.1158/1535-7163.MCT-16-0591. [Google Scholar] [CrossRef]
Li X, Lu H, Fan G, He M, Sun Y, Xu K, Shi F (2017b). A novel interplay between HOTAIR and DNA methylation in osteosarcoma cells indicates a new therapeutic strategy. Journal of Cancer Research Clinical Oncology 143: 2189–2200. DOI 10.1007/s00432-017-2478-3. [Google Scholar] [CrossRef]
Li Y, Cheng C (2018). Long noncoding RNA NEAT1 promotes the metastasis of osteosarcoma via interaction with the G9a-DNMT1-Snail complex. American Journal of Cancer Research 8: 81–90. [Google Scholar]
Li Y, Ren Y, Wang Y, Tan Y, Wang Q et al. (2019). A compound AC1Q3QWB selectively disrupts HOTAIR-mediated recruitment of PRC2 and enhances cancer therapy of DZNep. Theranostics 9: 4608–4623. DOI 10.7150/thno.35188. [Google Scholar] [CrossRef]
Li Z, Wang Y, Hu R, Xu R, Xu W (2018). LncRNA B4GALT1-AS1 recruits HuR to promote osteosarcoma cells stemness and migration via enhancing YAP transcriptional activity. Cell Proliferation 51: e12504. DOI 10.1111/cpr.12504. [Google Scholar] [CrossRef]
Liao L, Chen Y, Zhou J, Ye J (2021). MicroRNA-133b inhibits ntumor cell proliferation, migration and invasion by targeting SUMO1 in endometrial carcinoma. Technology in Cancer Research & Treatment 20: 15330338211065241. DOI 10.1177/15330338211065241. [Google Scholar] [CrossRef]
Lietz CE, Garbutt C, Barry WT, Deshpande V, Chen YL et al. (2020). MicroRNA-mRNA networks define translatable molecular outcome phenotypes in osteosarcoma. Scientific Reports 10: 4409. DOI 10.1038/s41598-020-61236-3. [Google Scholar] [CrossRef]
Lin H (2007). piRNAs in the germ line. Science 316: 397. DOI 10.1126/science.1137543. [Google Scholar] [CrossRef]
Link MP, Goorin AM, Miser AW, Green AA, Pratt CB et al. (1986). The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. New England Journal of Medicine 314: 1600–1606. DOI 10.1056/NEJM198606193142502. [Google Scholar] [CrossRef]
Liu C, Lin J (2016). Long noncoding RNA ZEB1-AS1 acts as an oncogene in osteosarcoma by epigenetically activating ZEB1. American Journal of Translational Research 8: 4095–4105. [Google Scholar]
Liu F, Zhang X, Wu F, Peng H (2021). Hsa_circ_0088212-mediated miR-520 h/APOA1 axis inhibits osteosarcoma progression. Translational Oncology 14: 101219. DOI 10.1016/j.tranon.2021.101219. [Google Scholar] [CrossRef]
Liu K, Huang J, Ni J, Song D, Ding M, Wang J, Huang X, Li W (2017a). MALAT1 promotes osteosarcoma development by regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle 16: 578–587. DOI 10.1080/15384101.2017.1288324. [Google Scholar] [CrossRef]
Liu K, Sun X, Zhang Y, Liu L, Yuan Q (2017b). MiR-598: A tumor suppressor with biomarker significance in osteosarcoma. Life Sciences 188: 141–148. DOI 10.1016/j.lfs.2017.09.003. [Google Scholar] [CrossRef]
Llobat L, Gourbault O (2021). Role of MicroRNAs in human osteosarcoma: Future perspectives. Biomedicines 9: 463. DOI 10.3390/biomedicines9050463. [Google Scholar] [CrossRef]
Lo WW, Wunder JS, Dickson BC, Campbell V, McGovern K, Alman BA, Andrulis IL (2014). Involvement and targeted intervention of dysregulated Hedgehog signaling in osteosarcoma. Cancer 120: 537–547. DOI 10.1002/cncr.28439. [Google Scholar] [CrossRef]
Long XH, Zhou YF, Peng AF, Zhang ZH, Chen XY, Chen WZ, Liu JM, Huang SH, Liu ZL (2015). Demethylation-mediated miR-129-5p up-regulation inhibits malignant phenotype of osteogenic osteosarcoma by targeting Homo sapiens valosin-containing protein (VCP). Tumour Biology 36: 3799–3806. DOI 10.1007/s13277-014-3021-7. [Google Scholar] [CrossRef]
Lou W, Ding B, Fu P (2020). Pseudogene-derived lncRNAs and their miRNA sponging mechanism in human cancer. Frontiers in Cell and Developmental Biology 8: 78. DOI 10.3389/fcell.2020.00085. [Google Scholar] [CrossRef]
Luo Z, Xiao L, Li J, Dong B, Wang C (2019). Integrative analysis reveals driver long non-coding RNAs in osteosarcoma. Medicine 98: e14302. DOI 10.1097/MD.0000000000014302. [Google Scholar] [CrossRef]
Maire G, Martin JW, Yoshimoto M, Chilton-MacNeill S, Zielenska M, Squire JA (2011). Analysis of miRNA-gene expression-genomic profiles reveals complex mechanisms of microRNA deregulation in osteosarcoma. Cancer Genetics 204: 138–146. DOI 10.1016/j.cancergen.2010.12.012. [Google Scholar] [CrossRef]
Malakoti F, Alemi F, Younesi S, Majidinia M, Yousefi B, Morovat P, Khelghati N, Maleki M, Karimian A, Asemi Z (2021). The cross-talk between signaling pathways, noncoding RNAs and DNA damage response: Emerging players in cancer progression. DNA Repair 98: 103036. DOI 10.1016/j.dnarep.2020.103036. [Google Scholar] [CrossRef]
Mirabello L, Troisi RJ, Savage SA (2009). Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer 115: 1531–1543. DOI 10.1002/cncr.24121. [Google Scholar] [CrossRef]
Modali SD, Parekh VI, Kebebew E, Agarwal SK (2015). Epigenetic regulation of the lncRNA MEG3 and its target c-MET in pancreatic neuroendocrine tumors. Molecular Endocrinology 29: 224–237. DOI 10.1210/me.2014-1304. [Google Scholar] [CrossRef]
Morris KV, Chan SW, Jacobsen SE, Looney DJ (2004). Small interfering RNA-induced transcriptional gene silencing in human cells. Science 305: 1289–1292. DOI 10.1126/science.1101372. [Google Scholar] [CrossRef]
Namløs HM, Meza-Zepeda LA, Barøy T, Østensen IH, Kresse SH, Kuijjer ML, Serra M, Bürger H, Cleton-Jansen AM, Myklebost O (2012). Modulation of the osteosarcoma expression phenotype by microRNAs. PLoS One 7: e48086. DOI 10.1371/journal.pone.0048086. [Google Scholar] [CrossRef]
Noonan EJ, Place RF, Pookot D, Basak S, Whitson JM, Hirata H, Giardina C, Dahiya R (2009). miR-449a targets HDAC-1 and induces growth arrest in prostate cancer. Oncogene 28: 1714–1724. DOI 10.1038/onc.2009.19. [Google Scholar] [CrossRef]
Palazzo AF, Lee ES (2015). Non-coding RNA: What is functional and what is junk? Frontiers in Genetics 6: 2. DOI 10.3389/fgene.2015.00002. [Google Scholar] [CrossRef]
Pan L, Xiao X, Zhao Y, Yin L, Fu M, Zhang X, Jiang P (2020). The functional roles of long noncoding RNA DANCR in human cancers. Journal of Cancer 11: 6970–6981. DOI 10.7150/jca.44384. [Google Scholar] [CrossRef]
Peschansky VJ, Wahlestedt C (2014). Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics 9: 3–12. DOI 10.4161/epi.27473. [Google Scholar] [CrossRef]
Plotnikova O, Baranova A, Skoblov M (2019). Comprehensive analysis of human microRNA-mRNA interactome. Frontiers in Genetics 10: 689. DOI 10.3389/fgene.2019.00933. [Google Scholar] [CrossRef]
Qi X, Yu XJ, Wang XM, Song TN, Zhang J, Guo XZ, Li GJ, Shao M (2019). Knockdown of KCNQ1OT1 Suppresses cell invasion and sensitizes osteosarcoma cells to CDDP by upregulating DNMT1-mediated Kcnq1 expression. Molecular Therapy. Nucleic Acids 17: 804–818. DOI 10.1016/j.omtn.2019.06.010. [Google Scholar] [CrossRef]
Ramassone A, Pagotto S, Veronese A, Visone R (2018). Epigenetics and microRNAs in cancer. International Journal of Molecular Sciences 19: 459. DOI 10.3390/ijms19020459. [Google Scholar] [CrossRef]
Ren Y, Wang YF, Zhang J, Wang QX, Han L, Mei M, Kang CS (2019a). Targeted design and identification of AC1NOD4Q to block activity of HOTAIR by abrogating the scaffold interaction with EZH2. Clinical Epigenetics 11: 29. DOI 10.1186/s13148-019-0624-2. [Google Scholar] [CrossRef]
Ren Z, Hu Y, Li G, Kang Y, Liu Y, Zhao H (2019b). HIF-1α induced long noncoding RNA FOXD2-AS1 promotes the osteosarcoma through repressing p21. Biomedicine & Pharmacotherapy 117: 109104. DOI 10.1016/j.biopha.2019.109104. [Google Scholar] [CrossRef]
Richard Boland C (2017). Non-coding RNA: It’s not junk. Digestive Diseases and Sciences 62: 1107–1109. DOI 10.1007/s10620-017-4506-1. [Google Scholar] [CrossRef]
Ruan H, Xiang Y, Ko J, Li S, Jing Y et al. (2019). Comprehensive characterization of circular RNAs in ~1000 human cancer cell lines. Genome Medicine 11: 55. DOI 10.1186/s13073-019-0663-5. [Google Scholar] [CrossRef]
Sarver AL, Phalak R, Thayanithy V, Subramanian S (2010). S-MED: Sarcoma microRNA expression database. Laboratory Investigation 90: 753–761. DOI 10.1038/labinvest.2010.53. [Google Scholar] [CrossRef]
Schmitt AM, Chang HY (2016). Long noncoding RNAs in cancer pathways. Cancer Cell 29: 452–463. DOI 10.1016/j.ccell.2016.03.010. [Google Scholar] [CrossRef]
Sellers ZP, Bolkun L, Kloczko J, Wojtaszewska ML, Lewandowski K, Moniuszko M, Ratajczak MZ, Schneider G (2019). Increased methylation upstream of the MEG3 promotor is observed in acute myeloid leukemia patients with better overall survival. Clinical Epigenetics 11: 50. DOI 10.1186/s13148-019-0643-z. [Google Scholar] [CrossRef]
Shen B, Zhou N, Hu T, Zhao W, Wu D, Wang S (2019). LncRNA MEG3 negatively modified osteosarcoma development through regulation of miR-361-5p and FoxM1. Journal of Cellular Physiology 234: 13464–13480. DOI 10.1002/jcp.28026. [Google Scholar] [CrossRef]
Shi D, Wu F, Mu S, Hu B, Zhong B, Gao F, Qing X, Liu J, Zhang Z, Shao Z (2019). LncRNA AFAP1-AS1 promotes tumorigenesis and epithelial-mesenchymal transition of osteosarcoma through RhoC/ROCK1/p38MAPK/Twist1 signaling pathway. Journal of Experimental & Clinical Cancer Research 38: 375. DOI 10.1186/s13046-019-1363-0. [Google Scholar] [CrossRef]
Shi Y, Huang JJ, Yi X, Yu SW, Zhang LY, Wang J, Cheng LX (2017). MicroRNA-133b inhibits cell proliferation and invasion in osteosarcoma by targeting Sirt1. Oncology Research 25: 1421–1430. DOI 10.3727/096504016X14826089198805. [Google Scholar] [CrossRef]
Shin TJ, Lee KH, Cho JY (2020). Epigenetic mechanisms of LncRNAs binding to protein in carcinogenesis. Cancers 12: 2925. DOI 10.3390/cancers12102925. [Google Scholar] [CrossRef]
Su X, Teng J, Jin G, Li J, Zhao Z et al. (2019). ELK1-induced upregulation of long non-coding RNA MIR100HG predicts poor prognosis and promotes the progression of osteosarcoma by epigenetically silencing LATS1 and LATS2. Biomedicine & Pharmacotherapy 109: 788–797. DOI 10.1016/j.biopha.2018.10.029. [Google Scholar] [CrossRef]
Sun F, Lee L, Zhang Z, Wang X, Yu Q, Duan X, Chan E (2015). Preclinical pharmacokinetic studies of 3-deazaneplanocin A, a potent epigenetic anticancer agent, and its human pharmacokinetic prediction using GastroPlusTM. European Journal of Pharmaceutical Sciences 77: 290–302. DOI 10.1016/j.ejps.2015.06.021. [Google Scholar] [CrossRef]
Sun H, Peng G, Wu H, Liu M, Mao G, Ning X, Yang H, Deng J (2020). Long non-coding RNA MEG3 is involved in osteogenic differentiation and bone diseases (Review). Biomedical Reports 13: 15–21. DOI 10.3892/br.2020.1305. [Google Scholar] [CrossRef]
Sun Y, Qin B (2018). Long noncoding RNA MALAT1 regulates HDAC4-mediated proliferation and apoptosis via decoying of miR-140-5p in osteosarcoma cells. Cancer Medicine 7: 4584–4597. DOI 10.1002/cam4.1677. [Google Scholar] [CrossRef]
Sun ZY, Jian YK, Zhu HY, Li B (2019). lncRNAPVT1 targets miR-152 to enhance chemoresistance of osteosarcoma to gemcitabine through activating c-MET/PI3K/AKT pathway. Pathology–Research and Practice 215: 555–563. DOI 10.1016/j.prp.2018.12.013. [Google Scholar] [CrossRef]
Tang F, Choy E, Tu C, Hornicek F, Duan Z (2017). Therapeutic applications of histone deacetylase inhibitors in sarcoma. Cancer Treatment Reviews 59: 33–45. DOI 10.1016/j.ctrv.2017.06.006. [Google Scholar] [CrossRef]
Tang N, Song WX, Luo J, Haydon RC, He TC (2008). Osteosarcoma development and stem cell differentiation. Clinical Orthopaedics and Related Research 466: 2114–2130. DOI 10.1007/s11999-008-0335-z. [Google Scholar] [CrossRef]
Thayanithy V, Sarver AL, Kartha RV, Li L, Angstadt AY, Breen M, Steer CJ, Modiano JF, Subramanian S (2012). Perturbation of 14q32 miRNAs-cMYC gene network in osteosarcoma. Bone 50: 171–181. DOI 10.1016/j.bone.2011.10.012. [Google Scholar] [CrossRef]
Tian Q, Gu Y, Wang F, Zhou L, Dai Z et al. (2020). Upregulation of miRNA-154-5p prevents the tumorigenesis of osteosarcoma. Biomedicine & Pharmacotherapy 124: 109884. DOI 10.1016/j.biopha.2020.109884. [Google Scholar] [CrossRef]
Tseng YY, Moriarity BS, Gong W, Akiyama R, Tiwari A et al. (2014). PVT1 dependence in cancer with MYC copy-number increase. Nature 512: 82–86. DOI 10.1038/nature13311. [Google Scholar] [CrossRef]
van Bakel H, Nislow C, Blencowe BJ, Hughes TR (2010). Most dark matter transcripts are associated with known genes. PLoS Biology 8: e1000371. DOI 10.1371/journal.pbio.1000371. [Google Scholar] [CrossRef]
Vicentini C, Galuppini F, Corbo V, Fassan M (2019). Current role of non-coding RNAs in the clinical setting. Non-coding RNA Research 4: 82–85. DOI 10.1016/j.ncrna.2019.09.001. [Google Scholar] [CrossRef]
Wang G, Li Z, Zhao Q, Zhu Y, Zhao C, Li X, Ma Z, Li X, Zhang Y (2014). LincRNA-p21 enhances the sensitivity of radiotherapy for human colorectal cancer by targeting the Wnt/β-catenin signaling pathway. Oncology Reports 31: 1839–1845. DOI 10.3892/or.2014.3047. [Google Scholar] [CrossRef]
Wang N, Zhang C, Wang W, Liu J, Yu Y, Li Y, Zhang M, Ge X, Li Q, Miao L (2019). Long noncoding RNA DANCR regulates proliferation and migration by epigenetically silencing FBP1 in tumorigenesis of cholangiocarcinoma. Cell Death & Disease 10: 585. DOI 10.1038/s41419-019-1810-z. [Google Scholar] [CrossRef]
Wang Z, Yang B, Zhang M, Guo W, Wu Z et al. (2018). lncRNA Epigenetic landscape analysis identifies EPIC1 as an oncogenic lncRNA that interacts with MYC and promotes cell-cycle progression in cancer. Cancer Cell 33: 706–720.e709. DOI 10.1016/j.ccell.2018.03.006. [Google Scholar] [CrossRef]
Wei JW, Huang K, Yang C, Kang CS (2017). Non-coding RNAs as regulators in epigenetics. Oncology Reports 37: 3–9. DOI 10.3892/or.2016.5236. [Google Scholar] [CrossRef]
Wen Y, Li B, He M, Teng S, Sun Y, Wang G (2021). circHIPK3 promotes proliferation and migration and invasion via regulation of miR‐637/HDAC4 signaling in osteosarcoma cells. Oncology Reports 45: 169–179. DOI 10.3892/or.2020.7833. [Google Scholar] [CrossRef]
Wu ZY, Yang SH, Weng XF, Liu XY (2011). MicroRNA-21 is involved in osteosarcoma cell invasion and migration. Medical Oncology 28: 1469–1474. DOI 10.1007/s12032-010-9563-7. [Google Scholar] [CrossRef]
Wu L, Zhou H, Zhang Q, Zhang J, Ni F, Liu C, Qi Y (2010). DNA methylation mediated by a microRNA pathway. Molecular Cell 38: 465–475. DOI 10.1016/j.molcel.2010.03.008. [Google Scholar] [CrossRef]
Wu P, Kong Y, Dai Z, Liu W, Zhao Z (2021). The circular RNA circCRIM1 inhibits osteosarcoma progression through sponging miR-513. Mammalian Genome 32: 495–502. DOI 10.1007/s00335-021-09903-2. [Google Scholar] [CrossRef]
Wu X, Pan Y, Fang Y, Zhang J, Xie M, Yang F, Yu T, Ma P, Li W, Shu Y (2020). The biogenesis and functions of piRNAs in human diseases. Molecular Therapy-Nucleic Acids 21: 108–120. DOI 10.1016/j.omtn.2020.05.023. [Google Scholar] [CrossRef]
Wu Y, Xie Z, Chen J, Chen J, Ni W et al. (2019). Circular RNA circTADA2A promotes osteosarcoma progression and metastasis by sponging miR-203a-3p and regulating CREB3 expression. Molecular Cancer 18: 73. DOI 10.1186/s12943-019-1007-1. [Google Scholar] [CrossRef]
Wu ZY, Yang SH, Weng XF, Liu XY (2011). MicroRNA-21 is involved in osteosarcoma cell invasion and migration. Medical Oncology, 28, 1469–1474. DOI 10.1007/s12032-010-9563-7. [Google Scholar] [CrossRef]
Xia P, Gu R, Zhang W, Shao L, Li F, Wu C, Sun Y (2019). MicroRNA-377 exerts a potent suppressive role in osteosarcoma through the involvement of the histone acetyltransferase 1-mediated Wnt axis. Journal of Cellular Physiology 234: 22787–22798. DOI 10.1002/jcp.28843. [Google Scholar] [CrossRef]
Xia P, Gu R, Zhang W, Sun YF (2020). lncRNA CEBPA-AS1 overexpression inhibits proliferation and migration and stimulates apoptosis of OS cells via notch signaling. Molecular Therapy-Nucleic Acids 19: 1470–1481. DOI 10.1016/j.omtn.2019.10.017. [Google Scholar] [CrossRef]
Long MX, Zhu KP, Zhang CL (2018). Circular RNA circ_HIPK3 is down-regulated and suppresses cell proliferation, migration and invasion in osteosarcoma. Journal of Cancer 9: 1856–1862. DOI 10.7150/jca.24619. [Google Scholar] [CrossRef]
Xu Y, Wang S, Tang C, Chen W (2015). Upregulation of long non-coding RNA HIF 1α-anti-sense 1 induced by transforming growth factor-β-mediated targeting of sirtuin 1 promotes osteoblastic differentiation of human bone marrow stromal cells. Molecular Medicine Reports 12: 7233–7238. DOI 10.3892/mmr.2015.4415. [Google Scholar] [CrossRef]
Yan H, Bu P (2021). Non-coding RNA in cancer. Essays in Biochemistry 65: 625–639. DOI 10.1042/EBC20200032. [Google Scholar] [CrossRef]
Yang C, Wang G, Yang J, Wang L (2017). Long noncoding RNA NBAT1 negatively modulates growth and metastasis of osteosarcoma cells through suppression of miR-21. American Journal of Cancer Research 7: 2009–2019. [Google Scholar]
Yang F, Zhang H, Mei Y, Wu M (2014). Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the Warburg effect. Molecular Cell 53: 88–100. DOI 10.1016/j.molcel.2013.11.004. [Google Scholar] [CrossRef]
Yang G, Zhang C, Wang N, Chen J (2019). miR-425-5p decreases LncRNA MALAT1 and TUG1 expressions and suppresses tumorigenesis in osteosarcoma via Wnt/β-catenin signaling pathway. International Journal of Biochemistry and Cell Biology 111: 42–51. DOI 10.1016/j.biocel.2019.04.004. [Google Scholar] [CrossRef]
Yang L, Ge D, Chen X, Qiu J, Yin Z, Zheng S, Jiang C (2018). FOXP4-AS1 participates in the development and progression of osteosarcoma by downregulating LATS1 via binding to LSD1 and EZH2. Biochemistry and Biophysical Research Communications 502: 493–500. DOI 10.1016/j.bbrc.2018.05.198. [Google Scholar] [CrossRef]
Yang N, Fu Y, Zhang H, Sima H, Zhu N, Yang G (2015). LincRNA-p21 activates endoplasmic reticulum stress and inhibits hepatocellular carcinoma. Oncotarget 6: 28151–28163. DOI 10.18632/oncotarget.4661. [Google Scholar] [CrossRef]
Yang W, Yu H, Shen Y, Liu Y, Yang Z, Sun T (2016). MiR-146b-5p overexpression attenuates stemness and radioresistance of glioma stem cells by targeting HuR/lincRNA-p21/β-catenin pathway. Oncotarget 7: 41505–41526. DOI 10.18632/oncotarget.9214. [Google Scholar] [CrossRef]
Yang X, Feng M, Jiang X, Wu Z, Li Z, Aau M, Yu Q (2009). miR-449a and miR-449b are direct transcriptional targets of E2F1 and negatively regulate pRb-E2F1 activity through a feedback loop by targeting CDK6 and CDC25A. Genes and Development 23: 2388–2393. DOI 10.1101/gad.1819009. [Google Scholar] [CrossRef]
Yang X, Liu M, Li M, Zhang S, Hiju H, Sun J, Mao Z, Zheng M, Feng B (2020). Epigenetic modulations of noncoding RNA: A novel dimension of cancer biology. Molecular Cancer 19: 64. DOI 10.1186/s12943-020-01159-9. [Google Scholar] [CrossRef]
Ying B, Huang H, Li H, Song M, Wu S, Ying H (2017). Procaine inhibits proliferation and migration and promotes cell apoptosis in Osteosarcoma Cells by upregulation of MicroRNA-133b. Oncology Research, 25, 1463–1470. DOI 10.3727/096504017X14878518291077. [Google Scholar] [CrossRef]
Yu G, Liu G, Yuan D, Dai J, Cui Y, Tang X (2018). Long non-coding RNA ANRIL is associated with a poor prognosis of osteosarcoma and promotes tumorigenesis via PI3K/Akt pathway. Journal of Bone Oncology 11: 51–55. DOI 10.1016/j.jbo.2018.02.002. [Google Scholar] [CrossRef]
Yu H (2009). Epigenetics: Advances of non-coding RNAs regulation in mammalian cells. Hereditas 31: 1077–1086. DOI 10.3724/sp.j.1005.2009.01077. [Google Scholar] [CrossRef]
Yuan JH, Yang F, Chen BF, Lu Z, Huo XS, Zhou WP, Wang F, Sun SH (2011). The histone deacetylase 4/SP1/microrna-200a regulatory network contributes to aberrant histone acetylation in hepatocellular carcinoma. Hepatology 54: 2025–2035. DOI 10.1002/hep.24606. [Google Scholar] [CrossRef]
Zeng Q, Wan H, Zhao S, Xu H, Tang T, Oware KA, Qu S (2020). Role of PIWI-interacting RNAs on cell survival: Proliferation, apoptosis, and cycle. IUBMB Life 72: 1870–1878. DOI 10.1002/iub.2332. [Google Scholar] [CrossRef]
Zhang C, Peng G (2015). Non-coding RNAs: An emerging player in DNA damage response. Mutation Research/Reviews in Mutation Research 763: 202–211. DOI 10.1016/j.mrrev.2014.11.003. [Google Scholar] [CrossRef]
Zhang EB, Kong R, Yin DD, You LH, Sun M et al. (2014a). Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget 5: 2276–2292. DOI 10.18632/oncotarget.1902. [Google Scholar] [CrossRef]
Zhang F, Peng H (2017). LncRNA-ANCR regulates the cell growth of osteosarcoma by interacting with EZH2 and affecting the expression of p21 and p27. Journal of Orthopaedic Surgery and Research 12: 103. DOI 10.1186/s13018-017-0599-7. [Google Scholar] [CrossRef]
Zhang K, Dong C, Chen M, Yang T, Wang X et al. (2020a). Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics 10: 411–425. DOI 10.7150/thno.33482. [Google Scholar] [CrossRef]
Zhang K, Sun X, Zhou X, Han L, Chen L et al. (2015). Long non-coding RNA HOTAIR promotes glioblastoma cell cycle progression in an EZH2 dependent manner. Oncotarget 6: 537–546. DOI 10.18632/oncotarget.2681. [Google Scholar] [CrossRef]
Zhang K, Zhang Y, Ren K, Zhao G, Yan K, Ma B (2014b). MicroRNA-101 inhibits the metastasis of osteosarcoma cells by downregulation of EZH2 expression. Oncology Reports 32: 2143–2149. DOI 10.3892/or.2014.3459. [Google Scholar] [CrossRef]
Zhang M, Xu K, Fu L, Wang Q, Chang Z, Zou H, Zhang Y, Li Y (2020b). Revealing epigenetic factors of circRNA expression by machine learning in various cellular contexts. iScience 23: 101842. DOI 10.1016/j.isci.2020.101842. [Google Scholar] [CrossRef]
Zhang QQ, Xu SL, Ding C, Ma CC, Yuan TS, Hua CC, Wang XH (2021). LncRNA FOXD2-AS1 knockdown inhibits the resistance of human osteosarcoma cells to cisplatin by inhibiting miR-143 expression. European Review for Medical and Pharmacological Sciences 25: 678–686. DOI 10.26355/eurrev_202101_24629. [Google Scholar] [CrossRef]
Zhang SZ, Cai L, Li B (2017a). MEG3 long non-coding RNA prevents cell growth and metastasis of osteosarcoma. Bratislava Medical Journal 118: 632–636. DOI 10.4149/BLL_2017_121. [Google Scholar] [CrossRef]
Zhang W, Duan N, Zhang Q, Song T, Li Z, Zhang C, Chen X, Wang K (2017b). DNA methylation mediated down-regulation of miR-370 regulates cell growth through activation of the Wnt/β-catenin signaling pathway in human osteosarcoma cells. International Journal of Biological Sciences 13: 561–573. DOI 10.7150/ijbs.19032. [Google Scholar] [CrossRef]
Zhang Y, Dai Q, Zeng F, Liu H (2018a). MALAT1 promotes the proliferation and metastasis of osteosarcoma cells by activating the Rac1/JNK pathway via targeting MiR-509. Oncology Research. DOI 10.3727/096504017X14957939026111. [Google Scholar] [CrossRef]
Zhang Y, Pu Y, Wang J, Li Z, Wang H (2020c). Research progress regarding the role of long non-coding RNAs in osteosarcoma. Oncology Letters 20: 2606–2612. DOI 10.3892/ol.2020.11807. [Google Scholar] [CrossRef]
Zhang Z, Zhao M, Wang G (2019). Upregulation of microRNA-7 contributes to inhibition of the growth and metastasis of osteosarcoma cells through the inhibition of IGF1R. Journal of Cellular Physiology 234: 22195–22206. DOI 10.1002/jcp.28787. [Google Scholar] [CrossRef]
Zhang ZC, Tang C, Dong Y, Zhang J, Yuan T, Li XL (2018b). Targeting LncRNA-MALAT1 suppresses the progression of osteosarcoma by altering the expression and localization of β-catenin. Journal of Cancer 9: 71–80. DOI 10.7150/jca.22113. [Google Scholar] [CrossRef]
Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT (2008). Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322: 750–756. DOI 10.1126/science.1163045. [Google Scholar] [CrossRef]
Zhao W, Zhang D, Qin P, Zhang J, Cui X, Gao J, Wang J, Li J (2019). Long non-coding RNA EPIC1 inhibits viability and invasion of osteosarcoma cells by promoting MEF2D ubiquitylation. International Journal of Biological Macromolecules 128: 566–573. DOI 10.1016/j.ijbiomac.2019.01.156. [Google Scholar] [CrossRef]
Zheng S, Qian Z, Jiang F, Ge D, Tang J et al. (2019). CircRNA LRP6 promotes the development of osteosarcoma via negatively regulating KLF2 and APC levels. American Journal of Translational Research 11: 4126–4138. [Google Scholar]
Zhou W, Wang J, Man WY, Zhang QW, Xu WG (2015). siRNA silencing EZH2 reverses cisplatin-resistance of human non-small cell lung and gastric cancer cells. Asian Pacific Journal of Cancer Prevention 16: 2425–2430. DOI 10.7314/APJCP.2015.16.6.2425. [Google Scholar] [CrossRef]
Zhou Y, Zhang X, Klibanski A (2012). MEG3 noncoding RNA: A tumor suppressor. Journal of Molecular Endocrinology 48: R45–53. DOI 10.1530/JME-12-0008. [Google Scholar] [CrossRef]
Zhu KP, Zhang CL, Ma XL, Hu JP, Cai T, Zhang L (2019). Analyzing the interactions of mRNAs and ncRNAs to predict competing endogenous RNA networks in osteosarcoma chemo-resistance. Molecular Therapy 27: 518–530. DOI 10.1016/j.ymthe.2019.01.001. [Google Scholar] [CrossRef]
Zhu S, Liu Y, Wang X, Wang J, Xi G (2020). lncRNA SNHG10 promotes the proliferation and invasion of osteosarcoma via Wnt/β-catenin signaling. Molecular Therapy-Nucleic Acids 22: 957–970. DOI 10.1016/j.omtn.2020.10.010. [Google Scholar] [CrossRef]
Zhuang M, Qiu X, Cheng D, Zhu C, Chen L (2018). MicroRNA-524 promotes cell proliferation by down-regulating PTEN expression in osteosarcoma. Cancer Cell International 18:114. DOI 10.1186/s12935-018-0612-1. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |