DOI:10.32604/biocell.2022.020218

| BIOCELL DOI:10.32604/biocell.2022.020218 |  |
| Article |
L-Selenocystine induce HepG2 cells apoptosis through ROS-mediated signaling pathways
1Center Laboratory, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
2Nanfang Hospital, Southern Medical University, Guangzhou, China
3Department of Laboratory Medicine, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
*Address correspondence: Bing Zhu, zhubing@gzhmu.edu.cn; Yinghua Li, liyinghua@gzhmu.edu.cn
#These authors contributed equally to this work
Received: 11 November 2021; Accepted: 15 February 2022
Abstract: At present, Hepatocarcinoma is one of the main causes of tumor related death all over the world. However, there are still many clinical restrictions on the treatment of liver cancer. Recently, L-Selenocystine has been shown to be a novel treatment for tumors, especially human glioma cells. But, the mechanism of L-Selenocystine against hepatocellular carcinoma remains unclear. Therefore, the main objective of this study was to investigate the effects of L-Selenocystine on HepG2 cell proliferation and activation of reactive oxygen species (ROS) mediated signaling pathway. L-Selenocystine can significantly inhibit HepG2 cell proliferation by activating caspase-3 and cleaving PARP to induce apoptosis. Moreover, the excessive production of ROS and the influence of Bax signaling pathway which can promote cell apoptosis are key factors for L-Selenocystine to induce HepG2 cell apoptosis. Therefore, the date of this study suggest that ROS mediated signal transduction mechanism may provide certain reference significance for L-Selenocystine induced HepG2 cell apoptosis.
Keywords: Selenium; Apoptosis; L-Selenocystine; Anticancer activity; ROS
Hepatocellular carcinoma is one of the three majority common cancers in the general population and the fourth leading cause of cancer-related death (Fitzmaurice et al., 2018; Mattiuzzi and Lippi, 2019). Although the prognosis of patients with early or intermediate liver cancer has improved significantly in recent years due to advances in diagnosis and treatment, most liver cancers are diagnosed as advanced when treatment is not feasible (Hilmi et al., 2019). At the same time, the overall prognosis of HCC is also influenced by important factors such as its strong metastatic potential, strong resistance to traditional drugs and poor sensitivity to chemotherapeutic drugs. Thus, the development of effective chemotherapy has become a major challenge in clinical treatment and is imperative (Cao et al., 2021; Marin et al., 2020). In addition, most of the current anti-cancer drugs have some common shortcomings, such as short effective time and poor solubility in water, which affect the effectiveness of chemotherapy (Yingchoncharoen et al., 2016; Glasgow and Chougule, 2015). Lately, L-Selenocystine has been diffusely used because of its unique biological characteristics, including that it is the main form of selenium in proteins, the only amino acid containing metalloid elements, and is mostly located in the active centers of selenium-containing enzymes or selenoproteins (Seale, 2019; Evans et al., 2019). With the further study of L-Selenocystine, people have hope for L-Selenocystine as an alternative anticancer drug (Radomska et al., 2021; Álvarez-Pérez et al., 2018).
L-Selenocystine is less toxic than other forms of selenium and is one of the forms of selenium that enter cells to perform biological functions. Compared with cysteine, L-Selenocystine has attracted extensive attention in consumer and medical products because of its selenium element. Selenium is also an essential trace element in human body, which plays an important role in immune regulation and antioxidant (Álvarez-Pérez et al., 2018; Misra et al., 2015; Spallholz, 1994). Furthermore, L-Selenocystine is very low toxic in humans and has different applications in in vitro and in vivo (Hoefig et al., 2011; Wallenberg et al., 2014; Kumar et al., 2009; Ostádalová and Babický, 1980). Therefore, the emergence of L-Selenocystine as a promising anticancer selenium species has attracted increasingly attention.
ROS is a single-electron reduction product of oxygen produced in cells, including hydroxyl radical, superoxide anion radical, hydrogen peroxide, oxygen free radicals and singlet oxygen (Zorov et al., 2014; Li et al., 2016a). As an obvious imbalance, oxidative stress was often described between ROS consumption and cellular defense mechanisms. (Moloney and Cotter, 2018; Klaunig, 2018; Wu et al., 2019). Previous studies have also shown that ROS have important effects on many physiological processes in the body. Redox imbalances have been linked to many serious diseases, such as cancer, skin disease, chronic kidney disease, neurodegenerative diseases, etc. (Bjørklund et al., 2020; Limongi and Baldelli, 2016; Xian et al., 2019; Poulianiti et al., 2016). Previous studies have shown that selenocysteine can interact with other compounds to induce apoptosis of HepG2 cells (Su et al., 2013; Fan et al., 2014). However, it has not been reported that different concentrations of selenocysteine can induce apoptosis of HepG2 cells under the premise of ensuring high survival rate of normal liver cells. It is greatly interesting to clarify the mechanism of L-Selenocystine. This study aims to investigate how changes in L-Selenocystine-related redox balance induced HepG2 cell apoptosis.
Human hepatocellular carcinoma cell line, HepG2 and hepatic cell line, L02 were maintained in our laboratory. Dulbecco modified Eagle Culture Medium (DMEM) was purchased from Gibco. Fetal Bovine serum (FBS) was purchased from ExCell. Bcl-2, Bax, Bid, c-PARP and caspase-3 and caspase-9 antibody were obtained from Cell Signaling Technology. L-Selenocystine were obtained from Sigma. 2’,7’-dichlorofluorescein diacetate (DCF-DA), thiazolyl blue tetrazolium bromide (MTT) and bicinchoninic acid (BCA) were obtained from Sigma. RIPA (protein lysis buffer) and enhanced mitochondrial membrane potential assay kit with JC-1 was obtained from Beyotime. Milli-Q Water used in all experiments was provided by Water Purification System of Millipore Company.
IC50 of L-Selenocystine in HepG2 cell line and L02 cell line
This routine work for apoptosis research is only carried out in HepG2 cells, not in normal liver cells L02. The MTT experiment was used to detect cell survival rate, and GraphPad prism was used to calculate IC50 values and draw graphs.
HepG2 and L02 cells were cultured in DMEM supplemented with 10% FBS and antibiotics (penicillin and streptomycin), and placed in a humidified incubator at 37°C and 5% CO2. The cytotoxicity of L-Selenocystine was detected by MTT test as described by us earlier (Zhu et al., 2016). In short, the classic MTT method was used to detect cell viability. HepG2 cells (6 × 104 cells/well) and L02 cells (6 × 104 cells/well) were inoculated in 96-well cell culture plate and incubated at 37°C with 5% CO2 for 24 h. Then, the cells were treated with 8 μM, 16 μM, and 32 μM L-selenocystine for 24 h, respectively. After treatment, 180 µl/well DMEM containing 1% FBS and 20 µl/well MTT solution was added and incubated for another 4 h. The medium was removed and 150 µl/well DMSO was added to dissolve the formazan crystals. The 96-well plates were then placed on a 37°C shaker and shaken for 10 min. Finally, the color intensity of solution was measured at 570 nm using thermo Flyer’s microplate reader (Varioskan). The test was repeated three times.
Determination of ROS production in HepG2 cells
As previously reported, ROS production in HepG2 cells treated with L-Selenocystine was detected by DCF fluorescence staining (Zhu et al., 2016; Li et al., 2016b; Li et al., 2018). Briefly, DCFH-DA was added to fully cover the cells after the medium was removed, and incubated at 37°C for 20 min. The cells were repeatedly washed twice with DMEM to completely remove the extracellular DCFH-DA, and the fluorescence intensity was observed with a fluorescence microscope. The experiment was repeated three times.
Mitochondrial membrane potential measurement (ΔΨm)
Changes in mitochondrial membrane potential in HepG2 cells exposed to L-Selenocystine were assessed using JC-1. The experiment steps follow the instructions. HepG2 cells were inoculated on 6-well plates and cultured for 24 h. After 24 h, the medium was removed and the cells were washed once with PBS or other suitable solution as required by the specific experiment. Then 1 mL DMEM and JC-1 staining solution were added to the six-well plate and fully mixed, and the cells were kept at 37°C for 20 min. During incubation, 4 mL distilled water was added to each 1 mL JC-1 staining buffer (5X) to prepare an appropriate amount of JC-1 staining buffer (1X) and put it in an ice bath. After incubation at 37°C, the supernatant was removed and washed twice with JC-1 staining buffer (1X). Add 2 mL cell culture solution and observe under inverted fluorescence microscope.
As mentioned earlier, the classical Western Blot method was used to detect the effect of L-Selenocystine on the expression levels of various intracellular proteins in HepG2 cells, including the classic P53 pathway, Bcl-2 family and caspase family, etc. (Guo et al., 2017; Li et al., 2016c). RIPA was used to digest HepG2 cells treated with L-Selenocystine to obtain total protein. The BCA assay was used to detect protein concentration. 10 µg protein was taken from each group. The transferred PVDF membrane was sealed in 5% skimmed milk powder for 2 h, then incubated overnight with 1:1000 diluted primary antibody at 4°C, and finally incubated with 1:1000 diluted secondary antibody at 4°C for 2 h. Enhanced chemiluminescence detection reagent (ECL) was used to visualize the protein bands.
Each experiment was repeated at least three times. All data were expressed as mean ± standard deviation. Two-tailed student T test was used to analyze the differences between the two groups. P < 0.05(*) or P < 0.01(**) were judged to be statistically significant. Multiple groups were compared using one-way analysis of variance. These analyses were performed using SPSS26.0 for Windows software.
The IC50 of HepG2 and L02 cell line
As shown in Fig. 1A, as the IC50 of L-Selenocystine in L02 is >70 μM, it is not considered that L-Selenocystine is cytotoxic to normal liver cells. However, the IC50 in HepG2 cells is 5.444 µM (Fig. 2B). This means that L-Selenocystine has a higher proliferation inhibitory activity on HepG2 cells while ensuring the survival of L02 cells. Therefore, 8, 16 and 32 µM L-Selenocystine were selected for follow-up studies.
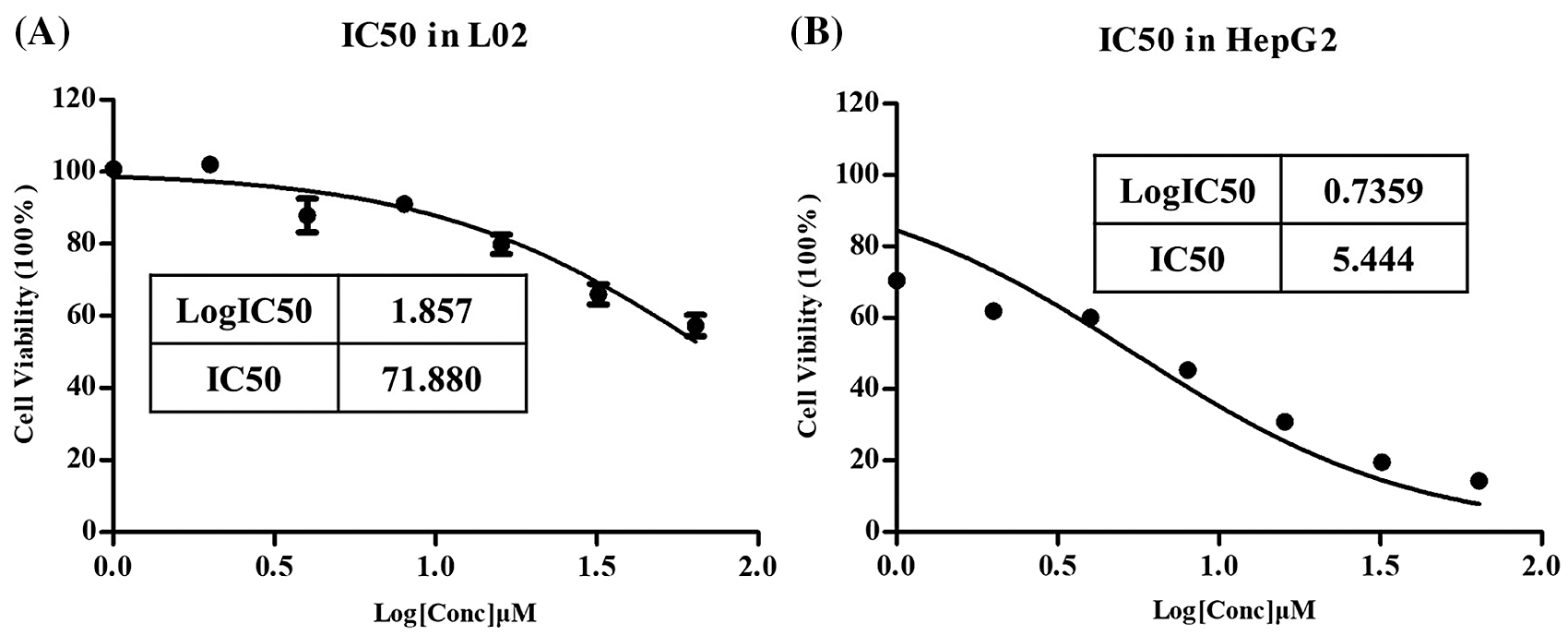
Figure 1: Percentages of cell viability of HepG2 cells treated with L-Selenocystine using serial dilutions from 64 to 1 µM.
Anticancer activity of L-Selenocystine in vitro
MTT assay was used to detect the toxicity of L-Selenocystine on HepG2 cells. As shown in Fig. 2A, L-Selenocystine significantly inhibited HepG2 cell growth with increasing concentration. HepG2 cells were treated with 8 μM L-selenocystine for 24 h, the cell viability was decreased to 43.37%. At the concentration of 16 μM and 32 μM, L-Selenocystine obviously reduced the cell viability (29.50% and 18.04%). As shown in Fig. 2B, the anticancer activity of L-Selenocystine was further confirmed. L-selenocystine made HepG2 cells significantly smaller and round, intercellular contact disappeared, and intercellular space increased. In conclusion, these outcomes suggest that L-selenocystine inhibits tumor cell growth in a dose-dependent manner.
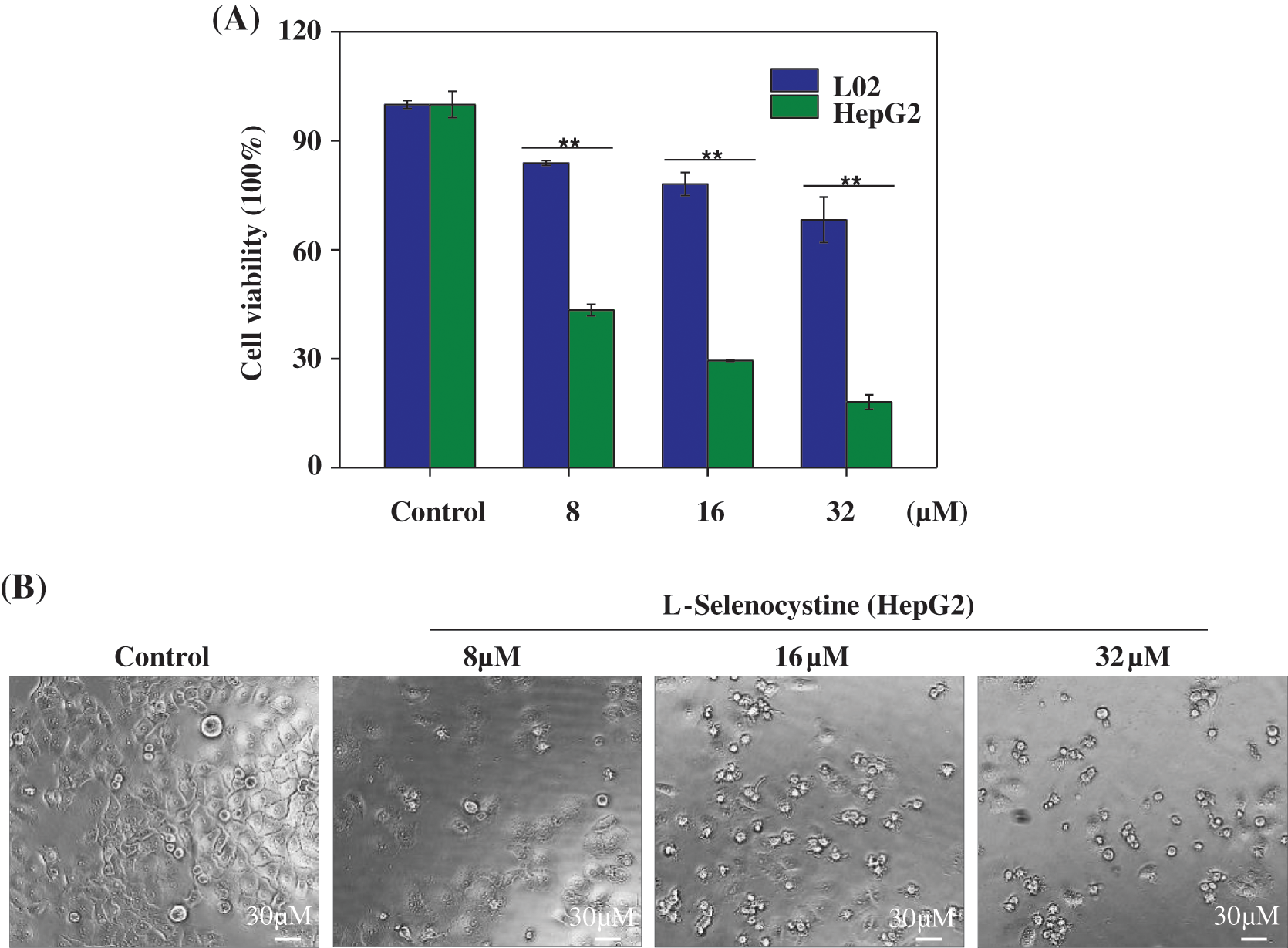
Figure 2: Cytotoxic effects of L-Selenocystine on HepG2 cells. (A) Cell viability of HepG2 and L02 cells with the treatment of L-Selenocystine were determined by MTT assay. (B) Morphological changes in HepG2 cells. Bars with different characters are statistically different at P < 0.05 (*) or P < 0.01(**).
ROS production induced by L-Selenocystine
ROS is a well-known key stimulator of apoptosis, especially in apoptosis induced by many anti-tumor drugs (Srinivas et al., 2019; Perillo et al., 2020). Consequently, the production of ROS was determined by DCF fluorescence method in this study to disclose its role in the antitumor activity of L-selenocystine. As illustrated in Fig. 3, the production of ROS in HepG2 cells was increased little by little under the action of L-Selenocystine, and the fluorescence intensity was found to be stronger in HepG2 cells with a concentration of 32 µM.
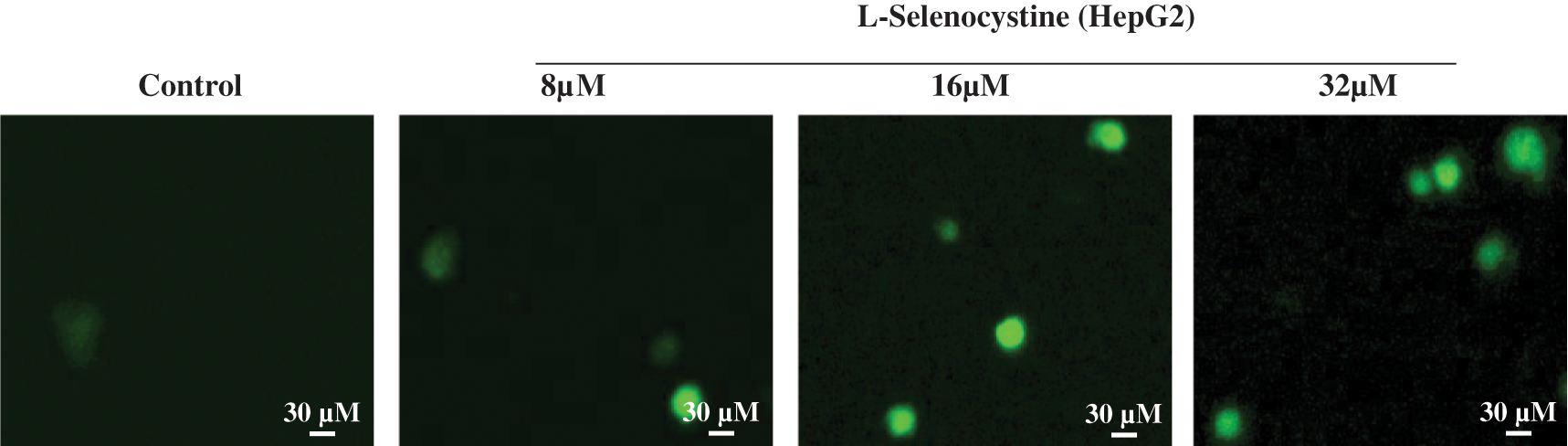
Figure 3: ROS overproduction induced by L-Selenocystine in HepG2 cells. HepG2 cells were incubated with 10 μM DCF-DA in PBS for 30 min and then the fluorescence intensity was observed under microscope.
Depletion of mitochondrial membrane potential (ΔΨm)
Mitochondrial membrane potential (MMP, ΔΨm) was used to detect the occurrence of apoptosis by fluorescence inverted microscope (Ma et al., 2018). As shown in Fig. 4, red fluorescence represents complete MMP, while green fluorescence represents lost MMP. Compared with the control group, the fluorescence of HepG2 cells treated with different concentrations of L-Selenocystine changed from red to green, indicating that mitochondrial depolarization of HepG2 cells was enhanced and MMP was significantly decreased.

Figure 4: Depletion of mitochondrial membrane potential induced by L-Selenocystine. JC-1 exists in the mitochondria of normal HepG2 cells in the form of polymer, showing bright red fluorescence and weak green fluorescence. After treatment with L-Selenocystine, JC-1 could not exist in the mitochondrial membrane potential in the form of polymer, so the red fluorescence intensity in mitochondria was significantly decreased, while the green fluorescence was significantly enhanced.
Activation of ROS-mediated signaling pathways by L-Selenocystine
Intracellular ROS overproduction could trigger DNA damage and cell apoptosis by activating of Bax signaling pathways. The western blot assay was used to detect its effects on ROS-mediated downstream pathways. As illustrated in Fig. 5A, the expression level of Bax protein in HepG2 cells was significantly increased after treated with L-Selenocystine. Moreover, the effects of L-Selenocystine treatment on Bcl-2 and Bid were different, which significantly increased Bid and inhibited Bcl-2. Meanwhile, the expression levels of caspase family and cleaved PARP with the group of L-Selenocystine treatment were significantly higher than those in the control group. The signaling pathway of ROS-mediated regulation of DNA damage and Bax as shown in Fig. 5B.

Figure 5: Activation of apoptotic signaling pathways by L-Selenocystine in HepG2 cells. The expression level of Bax, Bcl-2, Bid and caspase in HepG2 cells (A). The signaling pathway of ROS-mediated regulation of DNA damage and Bax (B). The relative expression levels of apoptosis-related proteins in L-selenocystine treatment group and blank control group (C) and (D). The expression of β-actin was measured as control. Abbreviations: PARP, poly (ADP-ribose) polymerase; ROS, reactive oxygen species. Casp 9, Caspase 9; C-C9, Cleaved Caspase 9; Casp 3, Caspase 3; C-C3, Cleaved Caspase 3.
HCC is a primary cancer with a very high mortality rate. It is the most common malignancy worldwide, especially in Asia, Africa and parts of Europe. There are many factors leading to HCC, such as chronic HBV/HCV infection, epigenetic changes, aflatoxin exposure, etc. (Wu et al., 2021). For the treatment of HCC, there are no effective drugs to curb the progression of the disease, and the prognosis is mostly very poor, prone to recurrence and metastasis, and eventually ends in death. Therefore, it is necessary to explore new and effective drugs to resist the growth of HCC cells.
Our results shown that the cytoplasm of HepG2 cells treated with L-Selenocystine was significantly atrophied, the contact between cells was lost, and the number of cells was evidently reduced. These data indicate that L-Selenocystine could significantly inhibit the replication and proliferation of HepG2 cells in a dose-dependent manner in vitro. In addition, MMP decreased and ROS increased in HepG2 cells with L-Selenocystine treatment group, which are the key factors to induce apoptosis. ROS is a kind of substance with free radical chemical activity. In cells, ROS is mainly produced by mitochondria through the respiratory chain (Wang et al., 2020). Its balance plays an important role in maintaining the normal physiological function of cells (Mirzaei et al., 2021). Previous study show that the increase of intracellular ROS production will break the redox balance, induce changes in cell function and participate in the occurrence and development of diseases (Butterfield and Halliwell, 2019). Mitochondrial membrane potential is a necessary index to evaluate mitochondrial function. As one of the key organelles in cells, mitochondria have a wide range of physiological functions, including energy metabolism, signal transduction, maintaining intracellular redox balance and so on. Mitochondrial damage plays a very important role in triggering cell apoptosis (Romero-Cordero et al., 2021). These data intensively make clear that L-Selenocystine can induce HepG2 cell apoptosis through mitochondrial damage and ROS production.
Bcl-2 protein family is an important protein regulating cell survival, including pro-apoptotic proteins bid, Bad, Bax and pro-survival proteins Bcl-2, BFL-1, Bcl-XL (Fairlie and Lee, 2021). Under normal circumstances, these two kinds of proteins will maintain a certain balance in cells. If this balance was broken, it will lead to cell apoptosis (Ye et al., 2021). Compare with the control group, the expression of Bid and Bax protein in HepG2 cells treated with L-Selenocystine increased significantly, while the expression of Bcl-2 protein decreased significantly in this study. The Caspase family plays a critical role in the modulation of apoptosis. In particular, Caspase-3 plays an important central regulatory role in the signal network (Liu et al., 2022; Wang et al., 2010). Therefore, Caspase-3 and Cleaved PARP were also detected to evaluate their role in apoptosis in Figs. 5A and 5D. L-Selenocystine significantly activated the expression of caspase-3 and the downstream effect of PARP cleavage. Take it all together, these dates confirm that L-Selenocystine induces HepG2 cells apoptosis by regulation of ROS-mediated Bax signaling pathways (Fig. 5B).
In summary, this study describes the ability of L-Selenocystein to inhibit HepG2 cell proliferation through cellular oxidative stress. L-Selenocystine significantly increased apoptosis in a dose-dependent manner. Molecular mechanism studies showed that L-Selenocystein promotes caspase-3-mediated apoptosis through its involvement in ROS production. Further studies showed that Bax signaling pathway was the main apoptotic signaling pathway triggered by L-Selenocystine in HepG2 cells. In a word, our results suggest that L-Selenocystine is an amino acid with potential anticancer properties.
Availability of Data and Material: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Author’s Contribution: Study design: BZ and YHL; experiments carry out: JYS, DYC and YYD; data collection: RLZ and QLD; analysis of results: QQD; draft manuscript preparation: HYC. All authors reviewed the final version of the manuscript.
Ethics Approval: This project was approved by the Ethics Committee of Guangzhou Women and Children Medical Center (Approval No. 2017021803).
Funding Statement: This work was supported by the fund from Open Project of Guangdong Key Laboratory of Marine Materia Medica (LMM2020-7), the fund from Guangzhou Institute of Pediatrics/Guangzhou Women and Children’s Medical Center (Nos. Pre-NSFC-2019-016, YIP-2019-059, IP-2019-019), the Project of Traditional Chinese Medicine Bureau of Guangdong Province (No. 20192075), the technology planning projects of Guangdong province (No. 202102010202).The Guangdong Natural Science Foundation (2020A1515110648), the Open Fund of Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications (2020A03).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Alvarez-Perez, M., Ali, W., Marc, M.A., Handzlik, J., Dominguez-Alvarez, E (2018). Selenides and diselenides: A review of their anticancer and chemopreventive activity. Molecules 23: 1–19. DOI 10.3390/molecules23030628. [Google Scholar] [CrossRef]
Bjørklund G, Tinkov AA, Hosnedlová B, Kizek R, Ajsuvakova OP et al. (2020). The role of glutathione redox imbalance in autism spectrum disorder: A review. Free Radical Biology & Medicine 160: 149–162. DOI 10.1016/j.freeradbiomed.2020.07.017. [Google Scholar] [CrossRef]
Butterfield DA, Halliwell B (2019). Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nature Reviews. Neuroscience 20: 148–160. DOI 10.1038/s41583-019-0132-6. [Google Scholar] [CrossRef]
Cao W, Liu X, Zhang Y, Li A, Xie Y et al. (2021). BEZ235 increases the sensitivity of hepatocellular carcinoma to sorafenib by inhibiting PI3K/AKT/mTOR and inducing autophagy. BioMed Research International 2021: 5556306. DOI 10.1155/2021/5556306. [Google Scholar] [CrossRef]
Evans SO, Jacobson GM, Goodman HJB, Bird S, Jameson MB (2019). Comparative safety and pharmacokinetic evaluation of three oral selenium compounds in cancer patients. Biological Trace Element Research 189: 395–404. DOI 10.1007/s12011-018-1501-0. [Google Scholar] [CrossRef]
Fairlie WAO, Lee EAO (2021). Co-operativity between MYC and BCL-2 pro-survival proteins in cancer. International Journal of Molecular Sciences 22: 2841. DOI 10.3390/ijms22062841. [Google Scholar] [CrossRef]
Fan C, Zheng W, Fu X, Li X, Wong YS et al. (2014). Strategy to enhance the therapeutic effect of doxorubicin in human hepatocellular carcinoma by selenocystine, a synergistic agent that regulates the ROS-mediated signaling. Oncotarget 5: 2853–2863. DOI 10.18632/oncotarget.1854. [Google Scholar] [CrossRef]
Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R et al. (2018). Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncology 4: 1553–1568. DOI 10.1001/jamaoncol.2018.2706. [Google Scholar] [CrossRef]
Glasgow MD, Chougule MB (2015). Recent developments in active tumor targeted multifunctional nanoparticles for combination chemotherapy in cancer treatment and imaging. Journal of Biomedical Nanotechnology 11: 1859–1898. DOI 10.1166/jbn.2015.2145. [Google Scholar] [CrossRef]
Guo M, Li Y, Lin Z, Zhao M, Xiao M et al. (2017). Surface decoration of selenium nanoparticles with curcumin induced HepG2 cell apoptosis through ROS mediated p53 and AKT signaling pathways. RSC Advances 7: 52456–52464. DOI 10.1039/C7RA08796A. [Google Scholar] [CrossRef]
Hilmi M, Vienot A, Rousseau B, Neuzillet C (2019). Immune therapy for liver cancers. Cancers 12: 1–23. DOI 10.3390/cancers12010077. [Google Scholar] [CrossRef]
Hoefig CS, Renko K, Köhrle J, Birringer M, Schomburg L (2011). Comparison of different selenocompounds with respect to nutritional value vs. toxicity using liver cells in culture. Journal of Nutritional Biochemistry 22: 945–955. DOI 10.1016/j.jnutbio.2010.08.006. [Google Scholar] [CrossRef]
Klaunig JE (2018). Oxidative stress and cancer. Current Pharmaceutical Design 24: 4771–4778. DOI 10.2174/1381612825666190215121712. [Google Scholar] [CrossRef]
Kumar BS, Kunwar A, Ahmad A, Kumbhare LB, Jain VK et al. (2009). In vitro radioprotection studies of organoselenium compounds: Differences between mono- and diselenides. Radiation and Environmental Biophysics 48: 379–384. DOI 10.1007/s00411-009-0240-1. [Google Scholar] [CrossRef]
Li R, Jia Z, Trush MA (2016a). Defining ROS in biology and medicine. Reactive Oxygen Species 1: 9–21. DOI 10.20455/ros.2016.803. [Google Scholar] [CrossRef]
Li Y, Guo M, Lin Z, Zhao M, Xiao M et al. (2016b). Polyethylenimine-functionalized silver nanoparticle-based co-delivery of paclitaxel to induce HepG2 cell apoptosis. International Journal of Nanomedicine 11: 6693–6702. DOI 10.2147/IJN. [Google Scholar] [CrossRef]
Li Y, Lin Z, Zhao M, Xu T, Wang C et al. (2016c). Multifunctional selenium nanoparticles as carriers of HSP70 siRNA to induce apoptosis of HepG2 cells. International Journal of Nanomedicine 11: 3065–3076. DOI 10.2147/IJN. [Google Scholar] [CrossRef]
LiY, LinZ,GuoM, ZhaoM,Xia Y et al. (2018) Inhibition of H1N1 influenza virus-induced apoptosis by functionalized selenium nanoparticles with amantadine through ROS-mediated AKT signaling pathways. International Journal of Nanomedicine 13: 2005–2016. DOI 10.2147/IJN.S155994. [Google Scholar] [CrossRef]
LimongiD, Baldelli SAO (2016). Redox imbalance and viral infections in neurodegenerative diseases. Oxidative Medicine and Cellular Longevity 2016: 6547248. DOI 10.1155/2016/6547248. [Google Scholar] [CrossRef]
LiuX, ChenD, SuJ, ZhengR, NingZet al. (2022) Selenium nanoparticles inhibited H1N1 influenza virus-induced apoptosis by ROS-mediated signaling pathways. RSC Advances, 12: 3862. DOI 10.1039/d1ra08658h. [Google Scholar] [CrossRef]
Ma ZJ, Lu L, Yang JJ, Wang XX, Su G et al. (2018). Lariciresinol induces apoptosis in HepG2 cells via mitochondrial-mediated apoptosis pathway. European Journal of Pharmacology 821: 1–10. DOI 10.1016/j.ejphar.2017.12.027. [Google Scholar] [CrossRef]
Marin JJG, Macias RIR, Monte MJ, Romero MR, Asensio M et al. (2020). Molecular bases of drug resistance in hepatocellular carcinoma. Cancers 12: 1663. DOI 10.3390/cancers12061663. [Google Scholar] [CrossRef]
Mattiuzzi C, Lippi G (2019). Current cancer epidemiology. Journal of Epidemiology and Global Health 9: 217–222. DOI 10.2991/jegh.k.191008.001. [Google Scholar] [CrossRef]
Mirzaei S, Hushmandi K, Zabolian A, Saleki HAO, Torabi SAO et al. (2021). Elucidating role of reactive oxygen species (ROS) in cisplatin chemotherapy: A focus on molecular pathways and possible therapeutic strategies. Molecules 26: 2382. DOI 10.3390/molecules26082382. [Google Scholar] [CrossRef]
Misra S, Boylan M, Selvam A, Spallholz JE, Björnstedt M (2015). Redox-active selenium compounds--from toxicity and cell death to cancer treatment. Nutrients 7: 3536–3556. DOI 10.3390/nu7053536. [Google Scholar] [CrossRef]
Moloney JN, Cotter TG (2018). ROS signalling in the biology of cancer. Seminars in Cell & Developmental Biology 80: 50–64. DOI 10.1016/j.semcdb.2017.05.023. [Google Scholar] [CrossRef]
Ostádalová I, Babický A (1980). Toxic effect of various selenium compounds on the rat in the early postnatal period. Archives of Toxicology 45: 207–211. DOI 10.1007/BF02419000. [Google Scholar] [CrossRef]
Perillo B, di Donato M, Pezone A, di Zazzo E, Giovannelli P et al. (2020). ROS in cancer therapy: The bright side of the moon. Experimental & Molecular Medicine 52: 192–203. DOI 10.1038/s12276-020-0384-2. [Google Scholar] [CrossRef]
Poulianiti KAO, Kaltsatou A, Mitrou GAO, Jamurtas AZ, Koutedakis Y et al. (2016). Systemic redox imbalance in chronic kidney disease: A systematic review. Oxidative Medicine and Cellular Longevity 2016: 8598253–8598219. DOI 10.1155/2016/8598253. [Google Scholar] [CrossRef]
Radomska D, Czarnomysy R, Radomski D, Bielawski K (2021). Selenium compounds as novel potential anticancer agents. International Journal of Molecular Sciences 22: 1009. DOI 10.3390/ijms22031009. [Google Scholar] [CrossRef]
Romero-Cordero S, Kirwan R, Noguera-Julian AAO, Cardellach F, Fortuny C et al. (2021). A mitocentric view of the main bacterial and parasitic infectious diseases in the pediatric population. International Journal of Molecular Sciences 22: 3272. DOI 10.3390/ijms22063272. [Google Scholar] [CrossRef]
Seale LA (2019). Selenocysteine β-Lyase: Biochemistry, regulation and physiological role of the selenocysteine decomposition enzyme. Antioxidants 8: 357. DOI 10.3390/antiox8090357. [Google Scholar] [CrossRef]
Spallholz JE (1994). On the nature of selenium toxicity and carcinostatic activity. Free Radical Biology & Medicine 17: 45–64. DOI 10.1016/0891-5849(94)90007-8. [Google Scholar] [CrossRef]
Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD (2019). ROS and the DNA damage response in cancer. Redox Biology 25: 101084. DOI 10.1016/j.redox.2018.101084. [Google Scholar] [CrossRef]
Su J, Lai H, Chen J, Li L, Wong YS et al. (2013). Natural borneol, a monoterpenoid compound, potentiates selenocystine-induced apoptosis in human hepatocellular carcinoma cells by enhancement of cellular uptake and activation of ROS-mediated DNA damage. PLoS One 8: e63502. DOI 10.1371/journal.pone.0063502. [Google Scholar] [CrossRef]
Wallenberg M, Misra S, Wasik AM, Marzano C, Björnstedt M et al. (2014). Selenium induces a multi-targeted cell death process in addition to ROS formation. Journal of Cellular and Molecular Medicine 18: 671–684. DOI 10.1111/jcmm.12214. [Google Scholar] [CrossRef]
Wang YB, Qin J, Zheng XY, Bai Y, Yang K et al. (2010). Diallyl trisulfide induces Bcl-2 and caspase-3-dependent apoptosis via downregulation of Akt phosphorylation in human T24 bladder cancer cells. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 17: 363–368. DOI 10.1016/j.phymed.2009.07.019. [Google Scholar] [CrossRef]
WangC, ChenH, ChenD,ZhaoM,LinZ et al.(2020) The inhibition of H1N1 influenza virus-induced apoptosis by surface decoration of selenium nanoparticles with β-thujaplicin through reactive oxygen species-mediated AKT and p53 signaling pathways. ACS Omega 5: 30633–30642. DOI 10.1021/acsomega.0c04624. [Google Scholar] [CrossRef]
Wu HL, Fu XY, Cao WQ, Xiang WZ, Hou YJ et al. (2019). Induction of apoptosis in human glioma cells by fucoxanthin via triggering of ROS-Mediated oxidative damage and regulation of MAPKs and PI3K-AKT pathways. Journal of Agricultural and Food Chemistry 67: 2212–2219. DOI 10.1021/acs.jafc.8b07126. [Google Scholar] [CrossRef]
Wu M, Xia X, Hu JAO, Fowlkes NAO, Li SAO (2021). WSX1 act as a tumor suppressor in hepatocellular carcinoma by downregulating neoplastic PD-L1 expression. Nature communications 12: 3500. DOI 10.1038/s41467-021-23864-9. [Google Scholar] [CrossRef]
Xian D, Lai R, Song JAO, Xiong X, Zhong JAO (2019). Emerging perspective: Role of increased ros and redox imbalance in skin carcinogenesis. Oxidative Medicine and Cellular Longevity 2019: 8127362–8127311. DOI 10.1155/2019/8127362. [Google Scholar] [CrossRef]
YeZ, ChenD, ZhengR, ChenH, XuT et al. (2021) Curcumin induced G2/M cycle arrest in SK-N-SH neuroblastoma cells through the ROS-mediated p53 signaling pathway. Journal of Food Biochemistry 45: e13888. DOI 10.1111/jfbc.13888. [Google Scholar] [CrossRef]
Yingchoncharoen P, Kalinowski DS, Richardson DR (2016). Lipid-based drug delivery systems in cancer therapy: What is available and what is yet to come. Pharmacological Reviews 68: 701–787. DOI 10.1124/pr.115.012070. [Google Scholar] [CrossRef]
Zhu B, Li Y, Lin Z, Zhao M, Xu T et al. (2016). Silver nanoparticles induce HepG2 cells apoptosis through ROS-mediated signaling pathways. Nanoscale Research Letters 11: 198. DOI 10.1186/s11671-016-1419-4. [Google Scholar] [CrossRef]
Zorov DB, Juhaszova M, Sollott SJ (2014). Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological Reviews 94: 909–950. DOI 10.1152/physrev.00026.2013. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |