DOI:10.32604/biocell.2022.020899

| BIOCELL DOI:10.32604/biocell.2022.020899 |  |
| Article |
Co-ordinated combination of Embden-Meyerhof-Parnas pathway and pentose phosphate pathway in Escherichia coli to promote L-tryptophan production
1The Key Laboratory of Industrial Biotechnology, Ministry of Education, Ministry of Education, School of Biotechnology, Jiangnan University, Wuxi, 214122, China
2National Engineering Laboratory for Cereal Fermentation Technology (NELCF), Jiangnan University, Wuxi, 214122, China
*Address correspondence to: Jianzhong Xu, xujianzhong@jiangnan.edu.cn; Weiguo Zhang, zhangwg@jiangnan.edu.cn
Received: 17 December 2021; Accepted: 17 March 2022
Abstract: In this study, phosphoenolpyruvate and erythrose-4-phosphate are efficiently supplied by collaborative design of Embden-Meyerhof-Parnas (EMP) pathway and pentose phosphate (PP) pathway in Escherichia coli, thus increasing the L-tryptophan production. Firstly, the effects of disrupting EMP pathway on L-tryptophan production were studied, and the results indicated that the strain with deletion of phosphofructokinase A (i.e., E. coli JW-5 ΔpfkA) produced 23.4 ± 2.1 g/L of L-tryptophan production. However, deletion of phosphofructokinase A and glucosephosphate isomerase is not conducive to glucose consumption and cell growth, especially deletion of glucosephosphate isomerase. Next, the carbon flux in PP pathway was enhanced by introduction of the desensitized glucose-6-phosphate dehydrogenase (zwf) and 6-phosphogluconate dehydrogenase (gnd) and thus increasing the L-tryptophan production (i.e., 26.5 ± 3.2 g/L vs. 21.7 ± 1.3 g/L) without obviously changing the cell growth (i.e., 0.41 h−1 vs. 0.44 h−1) as compared with the original strain JW-5. Finally, the effects of co-modifying EMP pathway and PP pathway on L-tryptophan production were investigated. It was found that the strain with deletion of phosphofructokinase A as well as introduction of the desensitized zwf and gnd (i.e., E. coli JW-5 zwf243 gnd361 ΔpfkA) produced 31.9 ± 2.7 g/L of L-tryptophan, which was 47.0% higher than that of strain JW-5. In addition, the glucose consumption rate of strain JW-5 zwf243 gnd361 ΔpfkA was obviously increased despite of the bad cell growth as compared with strain JW-5. The results of this study have important reference value for the following application of metabolic engineering to improve aromatic amino acids producing strains.
Keywords: Escherichia coli; L-tryptophan production; Phosphoenolpyruvate; Erythrose-4-phosphate; Collaborative design
L-tryptophan, as one of the essential amino acids for human and livestock, plays an important role in various biological activities, which has been widely used as feed additive or nutritional supplement in feed or food industry (Schoppel et al., 2021; Trondle et al., 2020). In addition, it is also widely used as medical intermediate in pharmaceutical industry (Fricke et al., 2017; Trondle et al., 2018). By reason of its commercial importance, more and more researchers are committed to promote the efficient synthesis of L-tryptophan. At present, microbial fermentation using Escherichia coli as work-horse is the mainstream method to produce L-tryptophan in industry because of more environmentally friendly and more economic benefit (Liu et al., 2020). Thus, an L-tryptophan producing strain with excellent fermentability is important to increase the final titer and yield as well as to reduce the production cost. As shown in Fig. 1, the L-tryptophan biosynthetic pathway in E. coli can be divided into the following three modules: the central metabolic pathway, the common biosynthetic pathway of aromatic amino acid and the terminal biosynthetic pathway of L-tryptophan. The central metabolic pathway mainly divides into four parts, i.e., Embden-Meyerhof-Parnas (EMP) pathway, tricarboxylic acid (TCA) cycle, pentose phosphate (PP) pathway and glyoxylate cycle. For L-tryptophan biosynthesis, EMP pathway is responsible for providing phosphoenolpyruvate (PEP) while PP pathway provides erythrose-4-phosphate (E4P), phosphoribosyl pyrophosphate (PRPP) and reducing cofactor NADPH. In addition, the other precursor in L-tryptophan biosynthesis, i.e., L-glutamine, is synthesized in the TCA cycle. Many studies have been conducted to increase these above mentioned precursors supply by modification of EMP pathway or PP pathway. For example, Liu et al. (2017) pointed out that modification of phosphoenolpyruvate:glucose phosphotransferase system (PTS) increases the supply of PEP and thus to increase the L-tryptophan production in E. coli . To increase the E4P supply, moreover, the gene zwf1 (encoding glucose 6-phosphate dehydrogenase) was deleted and the gene tkl1 (encoding transketolase) was overexpressed at the same time to enhance the carbon flux in non-oxidative PP pathway (Trondle et al., 2020). However, the initial step for L-tryptophan biosynthesis is a stereo-specific condensation of E4P and PEP to generate 3-deoxy-d-arobino-heptulosonate 7-phosphate (DAHP) (Fig. 1). Given that the low concentration of intracellular PEP would result in declining the carbon flux in the TCA cycle, the final titer would be decreased because of the poor cell growth (Li et al., 2020). By contrast, the weak PP pathway results in the shortage of E4P (Ikeda et al., 1999; Koendjbiharie et al., 2020). To construct an L-tryptophan high-yielding strain, therefore, it not only needs to increase the carbon flux in EMP pathway, but also needs to enhance the PP pathway. However, how to balance the carbon flux in EMP pathway and PP pathway is important for constructing L-tryptophan producing strains with excellent fermentability.

Figure 1: The biosynthetic pathway of L-tryptophan in E. coli. Different color lines represent the different pathway. The symbols “×” and “⇒” represent the deletion and replacement, respectively.
In order to address the above mentioned problem, the objective of this study was to try to construct an L-tryptophan high-producing strain based on coordinated combination of EMP pathway and PP pathway in an L-tryptophan producing strain E. coli JW-5. The main methods of this study involved in three aspects: (1) Individually reconstructing the EMP pathway or PP pathway in strain E. coli JW-5 via deletion of the key enzymes in EMP pathway or introduction of the desensitized zwf (i.e., zwfA243T, encoded by gene zwf243) and gnd (i.e., gndS361F, encoded by gene gnd361) from C. glutamicum. These results indicated that disruption of EMP pathway or enhancement of PP pathway plays a positive effect on increasing L-tryptophan production but individual disruption of EMP pathway limits the glucose consumption rate and cell growth; (2) Collaboratively modifying the EMP pathway and PP pathway via introduction of the zwfA243T and gndS361F in the recombinant strains with the disturbed EPM pathway. Compared with the strain with the only modification of EMP pathway or PP pathway, the strain with the co-modification of EMP pathway and PP pathway showed the excellent fermentability, i.e., the high L-tryptophan yield and cell growth as well as the quick glucose consumption rate. As a result, the resultant high-yielding strain E. coli JW-5 zwf243 gnd361 ΔpfkA produced 31.9 ± 2.7 g/L of L-tryptophan in 40-h fed-batch fermentation. Here, we report an effective strategy to construct an L-tryptophan high-producing strain by collaboratively modifying EMP pathway and PP pathway. The design-based strategy for constructing L-tryptophan high-yielding strain reported here could serve as a general concept for constructing the other fine chemicals high-yielding strain, in which the biosynthesis of this fine chemical needs no fewer than two precursors.
Strains, growth medium and culture conditions
Strains used in this study are listed in Table 1. The parental strain E. coli JW-5 (i.e., E. coli PFPr 5-FTr AzaSerr SGr Tyr−) was an L-tryptophan producing strain derived from the wild-type strain E. coli MG1655, which was mutagenized by Atmospheric and Room Temperature Plasma (ARTP) mutagenesis breeding system (Wuxi Institute of Applied Technology, Tsinghua University, Wuxi, China) and screened from p-fluorophenylalanine (PFP)-, 5-fluoro-tryptophan (5-FT)-, azaserine (AzaSer) and sulfanaidine (SG)-resistance M9 minimal agar medium without L-tyrosine (Tyr). E. coli was cultivated in Luria-Bertani (LB) medium at 37°C (Xu et al., 2016). M9 minimal medium was prepared according to the previous report (Siedler et al., 2012). The SOC medium was used for cell recovery after electro-transformation, which was prepared according to the description of Chung et al. (2017). Appropriately, 50 μg/mL kanamycin (Kan) and 50 μg/mL streptomycin (Sm) solution was used to screen the target plasmids and strains.
Fed-batch fermentation was carried out in 5-L fermenter with 2.5 L of fermentation medium, and was performed according to the methods described by Gu et al. (2013). The seed medium was prepared according to the following formula (per liter): 15 g glucose, 2.5 g (NH4)2HPO4, 5 g yeast extract powder, 1.5 g KCl, 1.5 g MgSO4·7H2O, 30 mg FeSO4, 2 mg thiamine·HCl. Inoculum was from a seed culture with ∆OD562 = 0.45–0.50 (at a dilution of 25-fold), and the inoculation amount was 10%. The fermentation medium was prepared according to the following formula (per liter): 30 g glucose, 5 g corn steep powder, 5 g (NH4)2SO4, 2 g yeast extract powder, 2 g citric acid, 1 g KH2PO4, 2 g MgSO4·7H2O, 0.01 g MnSO4·H2O, 50 mg FeSO4, 160 µg thiamine·HCl, 50 µg biotin and 5 g CaCO3.
Two genes (i.e., pgi and pfkA) were individually deleted via CRISPR-Cas9-assited genome-editing technique (Marco et al., 2022). The oligonucleotide primers used in this study are listed in Table 2. The two-plasmid system including pCas and pTarget was used for genome editing. pCas was initially introduced into the L-tryptophan producers. 20 mmol/L of L-arabinose was supplemented in twice when competent cell was prepared to balance cell growth and RED proteins. pTarget-Δpgi and pTarget-ΔpfkA were separately constructed by whole plasmid PCR using the original pTargetF as template, just adjusting N20 sequence embedded into primers at 5’ end. Homologous arms including the upstream and downstream regions of the targeted locus were fused by overlap extension PCR. The detail progress of gene deletion was referred to the previous methods (Jiang et al., 2015).
Site-directed mutagenesis of zwf and gnd gene was performed using a commercial Site-Directed Mutagenesis Kit (Mut Express® MultiS Fast Mutagenesis Kit V2, Vazyme, Nanjing, China). Base replacement was introduced into oligonucleotide primers. The specific steps of the method are referred to the manufacturer’s instructions. The oligonucleotide primers used in this study are listed in Table 2. The base replacements in genes zwf and gnd were confirmed via sequencing by GENEWIZ (Suzhou), Inc. (Suzhou, China).

Construction of E. coli recombinant strains
Plasmids used in this study are listed in Table 1. Plasmid pTarget-Δpgi, pTarget-ΔpfkA were used for gene pgi and pfkA deletion, respectively. In addition, plasmids pTarget-zwf and pTarget-gnd were used for replacing the native genes zwf and gnd in E. coli. The detail methods used for gene deletion and gene replacement were referred to the published method (Jiang et al., 2015). The resultant plasmids were transferred into E. coli by electroporation, and the target recombinants were selected referring to the published method (Fang et al., 2020). In order to confirm the gene replacements in E. coli genome, the genes zwf and gnd were cloned by PCR using the target E. coli recombinant genome as template and using zwf234-F/zwf234-R and gnd361-F/gnd361-R as primer pairs, respectively.
Preparation of crude enzyme and enzyme activity assays
The cells in the logarithmic post-growth were collected by centrifuge for 10 min at 6000 r/min, and then disrupted using sonication. Following, fragmentized liquid was separated by centrifuge at 10000 r/min and 4°C for 30 min. Thus, the cell-free supernatants were used as crude enzyme to determine the enzyme activities. The enzyme activity assays were done in triplicate, and the analytical methods of zwf and gnd were based on the protocol of Wang et al. (2011).
A sample was taken from the fermenter at the right time. These samples were used to analyze cell growth, glucose concentration and L-tryptophan concentration. For analyzing cell growth, optical density at 562 nm (i.e., OD562) was detected using a spectrophotometer after an appropriate dilution. In addition, glucose concentration was detected using an SBA-40E immobilized enzyme biosensor (Shandong, China). The concentration of L-tryptophan was monitored by high performance liquid chromatography (HPLC) using Agilent 1200 system (Agilent Technologies, Santa Clara, CA, USA) according to the procedure described by Trondle et al. (2020).
Disturbance of EMP pathway to increase L-tryptophan production but to limit glucose consumption and cell growth
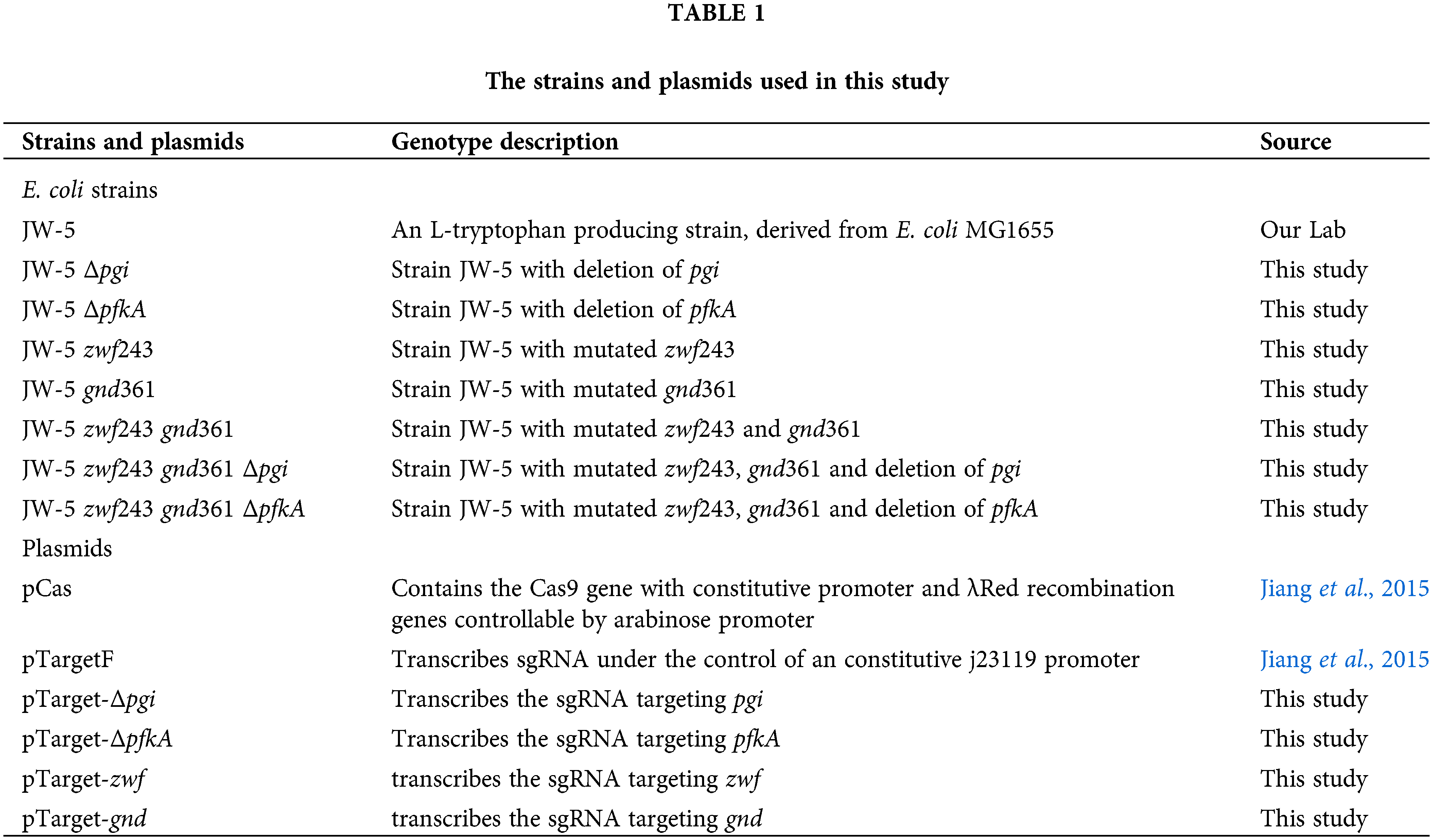
To disturb the EMP pathway, glucosephosphate isomerase (encoded by gene pgi) or phosphofructokinase A (encoded by pfkA) were deleted. The resultant strains E. coli JW-5 Δpgi and E. coli JW-5 ΔpfkA showed the bad glucose consumption and cell growth as compared with the original strain E. coli JW-5 in M9 minimal medium with 0.1 g/L L-tyrosine, especially for strain JW-5 Δpgi (Fig. 2). The similar results were also found in the next study, in which strains JW-5 Δpgi and JW-5 ΔpfkA were incubated in the fermentation medium by fed-batch culture. As can be seen from Figs. 3a and 3b and Table 3, the glucose consumption and cell growth of strains JW-5 Δpgi and JW-5 ΔpfkA were obviously decreased as compared with the strain JW-5. It is worth noting that deletion of phosphofructokinase A has lower effect on glucose consumption and cell growth than deletion of glucosephosphate isomerase, especially cultivated in M9 minimal medium (Fig. 2, Figs. 3a and 3b). In fed-batch fermentation, the highest cell growth rate (μ) was found in original strain JW-5 (i.e., 0.44 h−1), and the next was strain JW-5 ΔpfkA (i.e., 0.29 h−1) and strain JW-5 Δpgi (i.e., 0.12 h−1) (Table 3). In addition, the original strain JW-5 showed the highest glucose consumption rate (i.e., 0.75 ± 0.05 g/(h·g DCW)) followed by strain JW-5 ΔpfkA (i.e., 0.63 ± 0.04 g/(h·g DCW)) and strain JW-5 Δpgi (i.e., 0.32 ± 0.07 g/(h·g DCW)) (Table 3). Although strain JW-5 ΔpfkA showed the worse glucose consumption and cell growth than that of strain JW-5, it accumulated the highest L-tryptophan titer (i.e., 23.4 ± 2.1 g/L) (Fig. 3c and Table 3). However, the strain JW-5 Δpgi showed the lowest L-tryptophan titer (i.e., 10.2 ± 1.6 g/L) because of the worst glucose consumption and cell growth (Fig. 3c and Table 3). Thus, deletion of phosphofructokinase A is the optional strategy to disturb the EMP pathway for constructing L-tryptophan high-producing strain.

Figure 2: Growth situation of recombinant and original strains in M9 medium. (a) Dry cell weight (DCW) of the test strains. (b) The glucose consumption of the test strains. The data represent mean values and standard deviations obtained from three independent cultivations.

Figure 3: Fermentation performance of original strain and recombinant strains in fermentation medium. (a) DCW of the test strains. (b) Glucose consumption of the test strains. (c) L-tryptophan production of the test strains. The data represent mean values and standard deviations obtained from three independent cultivations.

Enhancement of PP pathway to increase the L-tryptophan production rather than to obviously change glucose consumption and cell growth
To enhance the carbon flux in PP pathway, the desensitized zwf (i.e., zwfA243T) and/or gnd (i.e., gndS361F) from C. glutamicum replaced the native zwf and gnd in E. coli JW-5. The results of enzymes analysis confirmed that zwfA243T-coding gene zwf243 and/or gndS361F-coding gene gnd361 were successfully introduced into E. coli JW-5 chromosome (Table 4). Michaelis-Menten affinity constants of zwf for glucose6-phosphate (G6P) and NADP (Km(zwf)G6P and Km(zwf)NADP) of strains JW-5 zwf243 and JW-5 zwf243 gnd361 were all significantly lower than that of strain JW-5 (Table 4). Similar results were also found on Michaelis-Menten affinity constants of gnd for 6-phosphogluconate (6GP) and NADP (Km(gnd)6GP and Km(gnd)NADP) of strains JW-5 gnd361 and JW-5 zwf243 gnd361 (Table 4). Although the activity of zwfA243T and gndS361F slight lower than that of the native zwf and gnd in strain JW-5, they showed the lower sensitivity against inhibition by ATP, NADPH and fructose-1, 6-diphosphate (FBP) than that of the native zwf and gnd, respectively (Fig. 4).


Figure 4: The enzymatic properties of the wild-type and mutated zwf and gnd. (a) The specific activity of zwf and gnd in different strains. (b) Relative activity (%) of the wild-type and the mutated variant by addition of ATP as inhibitor. (c) Relative activity (%) of the wild-type and the mutated variant by addition of NADPH as inhibitor. (d) Relative activity (%) of the wild-type and the mutated variant by addition of FBP as inhibitor. The data represent mean values and standard deviations obtained from three independent cultivations.
The cell growth of the original and modified strains was monitored in fermentation medium and M9 minimal medium with 0.1 g/L L-tyrosine, and there is not a sharp distinction between the original strain and the modified strains, whether in fermentation medium or in M9 medium (Figs. 5a and 5b). The original strain JW-5 and strain JW-5 gnd361 had the highest μ (i.e., 0.44 h−1) and was followed by the strain JW-5 zwf243 (i.e., 0.43 h−1) and the strain JW-5 zwf243 gnd361 (i.e., 0.41 h−1) in fermentation medium (Table 3). In addition, the modified strains also showed the similar glucose consumption to the original strain in M9 medium (Fig. 5c and Table 3). The modified strain JW-5 zwf243 gnd361 had the highest glucose consumption rate (i.e., 0.76 ± 0.08 g/(h·g DCW)), which was increased no more than 1% than that of the original strain JW-5 (i.e., 0.75 ± 0.05 g/(h·g DCW)) in fermentation medium (Table 3). However, the L-tryptophan production was significantly increased with introduction of the desensitized zwfA243T and/or gndS361F in strain JW-5 in fermentation medium (Fig. 5d). L-tryptophan production of strain JW-5 zwf243 gnd361 increased by 22.1% over the strain JW-5 (i.e., 26.5 ± 3.2 g/L vs. 21.7 ± 1.3 g/L), while that of strain JW-5 zwf243 (i.e., 24.8 ± 1.8 g/L) and strain JW-5 gnd361 (i.e., 23.3 ± 2.5 g/L) increased by 14.3% and 7.4%, respectively (Table 3). As can be seen from Table 3, the strain JW-5 zwf243 gnd361 showed the highest L-tryptophan yield (i.e., 0.190 g/g) among the above mentioned strains including the strains with disruption of EMP pathway and the other strains with enhancement of PP pathway.

Figure 5: Fermentation performance of original strain and recombinant strains in M9 medium and fermentation medium. (a) DCW of the test strains in M9 medium. (b) DCW of the test strains in fermentation medium. (c) The glucose consumption of the test strains in M9 medium. (d) The L-tryptophan production of the test strains in fermentation medium. The data represent mean values and standard deviations obtained from three independent cultivations.
Collaboratively modification of the EMP pathway and PP pathway to balance the cell growth and erythrose-4-phosphate supply
As the mention above (Figs. 3 and 5 and Table 3), disruption of EMP pathway (e.g., strain JW-5 ΔpfkA) limited the cell growth and glucose consumption, whereas enhancement of PP pathway (e.g., strain JW-5 zwf243 gnd361) did not obviously change the cell growth and glucose consumption. It is worth noting that disruption of EMP pathway (i.e., deletion of pfkA) and enhancement of PP pathway (i.e., introduction of the desensitized zwf234 and gnd361) benefits to increase L-tryptophan production because of increasing the carbon flux in PP pathway and thus increasing of the E4P supply. These results implied that the E4P supply is an important limited factor for increasing L-tryptophan production in strain JW-5. As the other precursor for L-tryptophan biosynthesis, PEP is synthesized at the end of the EMP pathway (Fig. 1). In theory, increasing the carbon flux in PP pathway did not change the concentration of PEP (Patnaik and Liao, 1994). Thus, in order to efficiently supply E4P for L-tryptophan production, EMP pathway and PP pathway were co-modified to increase E4P supply rather than to significantly limit the cell growth. In this study, the phosphofructokinase A-coding gene pfkA in strain JW-5 zwf243 gnd361 was deleted to disturb the EMP pathway. And the resultant strain JW-5 zwf243 gnd361 ΔpfkA showed the decreased cell growth rate as compared with the strain JW-5 zwf243 gnd361 in fermentation medium (i.e., 0.32 h−1 vs. 0.41 h−1) (Table 3). However, the final dry cell weight (DCW) of strain JW-5 zwf243 gnd361 ΔpfkA (i.e., 14.2 ± 1.3 g/L) was similar with the strain JW-5 zwf243 gnd361 (i.e., 13.9 ± 0.8 g/L) (Fig. 6a). In addition, the strain JW-5 zwf243 gnd361 ΔpfkA had the highest glucose consumption rate (i.e., 0.83 ± 0.04 g/(h·g DCW)) followed by the strain JW-5 zwf243 gnd361 and the strain JW-5 with 0.76 ± 0.08 g/(h·g DCW) and 0.75 ± 0.05 g/(h·g DCW), respectively (Table 3 and Fig. 6b). As can be seen from Fig. 6c and Table 3, the strain JW-5 zwf243 gnd361 ΔpfkA produced 31.9 ± 2.7 g/L of L-tryptophan, which was 20.4% and 47.0% higher than that of strain JW-5 zwf243 gnd361 and strain JW-5, respectively.
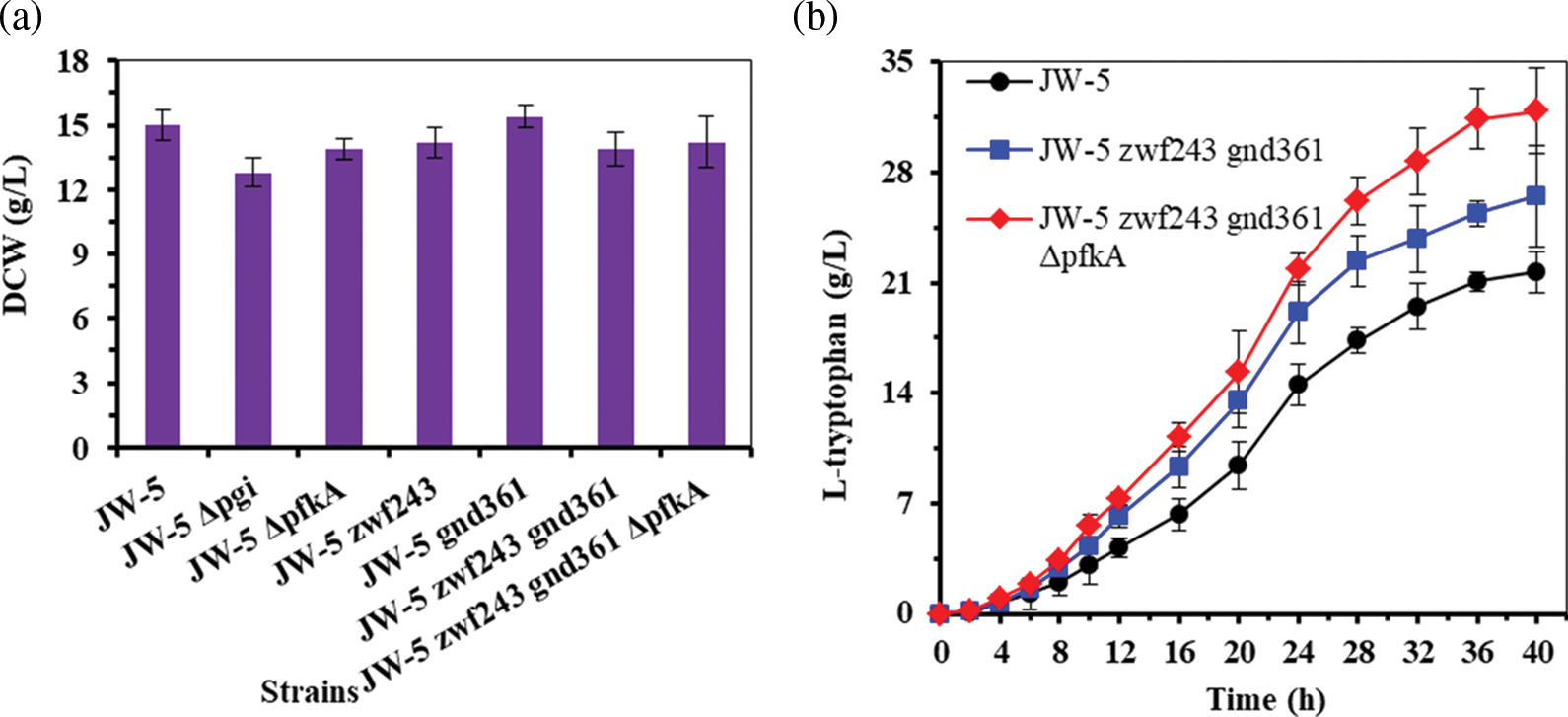
Figure 6: Fermentation performance of original strain and recombinant strains in fermentation medium. (a) DCW of the test strains. (b) The L-tryptophan production of the test strains. The data represent mean values and standard deviations obtained from three independent cultivations.
To develop an L-tryptophan high-producing strain, increasing the accessible amounts of precursors has been considered as an indispensable strategy. According to Eq. (1) reported by Bai et al. (2019), there are five precursors (i.e., PEP, E4P, Gln, Ser and PRPP) and two co-factors (i.e., NADPH and ATP) involved in L-tryptophan biosynthesis. Normally, increasing the supply of PEP or E4P was deemed as a preferential option for enhancing
production of DAHP, which was the key intermediate for L-tryptophan biosynthesis (Xiong et al., 2021). Two categories of metabolic engineering strategies are used to increase PEP supply in developing L-tryptophan high-producing strain, i.e., reasonable control the local PEP-Pyruvate metabolic note (e.g., overexpression of ppsA and deletion of pykAF or ppc) and reasonable control the PTS (e.g., replacement of PTS transport system by PTS-independent transport system) (Chen et al., 2018; Liu et al., 2017; Ruan et al., 2020; Xiong et al., 2021). In order to increase E4P supply, inactivation or suppression of glucosephosphate isomerase and/or overexpression of glucose-6-phosphate dehydrogenase and phosphoketolase have been reported (Li et al., 2021; Na et al., 2013; Xiong et al., 2021). However, few researches focused on maintaining the supply of PEP and E4P by collaboratively modifying EMP pathway and PP pathway in Escherichia coli to promote L-tryptophan production as far as we know. In this study, we collaboratively modified EMP pathway and PP pathway to construct an L-tryptophan high-producing strain, and the resultant strain E. coli JW-5 zwf243 gnd361 ΔpfkA produced 31.9 ± 2.7 g/L of L-tryptophan with a yield of 0.213 g/g in 40-h fed-batch fermentation. The results of this study have important reference value for constructing the other fine chemicals high-yielding strain, in which the biosynthesis of this fine chemical needs no fewer than two precursors Glucosephosphate isomerase (encoded by pgi) and phosphofructokinase A (encoded by pfkA) are key enzymes in EMP pathway (Fig. 1). Deletion of pgi or pfkA strongly decreased the cell growth and glucose consumption rate (Fig. 2). The similar results were also found in previous results (Li et al., 2021; Siedler et al., 2012), and this is because the high glucose-6-phosphate pool will destabilize the ptsG mRNA in pgi-deleted strain (Morita et al., 2003). In addition, deletion of pgi or pfkA reduces PEP concentration and thus limits the PTS (Roehl and Vinopal, 1976). As compared with the strain JW-5 Δpgi, the strain JW-5 ΔpfkA showed the high cell growth, glucose consumption rate and L-tryptophan production in fermentation medium (Fig. 3). According to the flux analysis reported by Siedler et al. (2012), the pfkA-deleted strain possessed the higher carbon flux in EMP pathway and PP pathway than that in pgi-deleted strain. Therefore, this may be the reason why the strain JW-5 ΔpfkA shows the better fermentability than strain JW-5 Δpgi. zwf (encoded by zwf) and gnd (encoded by gnd) are the limit enzymes in PP pathway, which involve in the NADPH regeneration (Chen et al., 2019). However, the activity of zwf and gnd was inhibited by NADPH, ATP and fructose-1,6-bisphosphate (Becker et al., 2007; Ohnishi et al., 2005). In addition, the activity of gnd was additionally inhibited by glyceraldehyde-3-phosphate, E4P and ribulose 5-phosphate (Wang et al., 2011). Becker et al. (2007) pointed out that replacement of L-alanine residue by L-threonine residue at 243 site (i.e., A243T) relieves the inhibition by ATP, PEP and fructose-1,6-bisphosphate as well as increases the affinity of zwf towards NADP+. In addition, introduction of point mutation (i.e., S361F) in gnd resulted in reducing the sensitivity against inhibition by fructose-1,6-bisphosphate, glyceraldehyde-3-phosphate, PRPP, ATP, and NADPH (Ohnishi et al., 2005). Based on these findings, the native zwf and gnd were replaced by the mutated zwf (i.e., zwfA234T) and gnd (i.e., gndS361F) from C. glutamicum in E. coli JW-5, and the resultant strains showed the significantly lower Km for substrate than that of the originals strain JW-5 (Table 4). The similar results were also found in previous results, in which the zwfA243T and gndS361F were introduced into Bacillus subtilis (Wang et al., 2011). Our results (Table 3 and Fig. 5) and previous results (Meng et al., 2016; Wang et al., 2011) confirmed that the introduction of zwfA234T and gndS361F did not obviously change glucose consumption rate and cell growth rate but significant increase the production of the target products. These results indicated that increase the carbon flux in PP pathway benefits to increase L-tryptophan production and the E4P supply is an important limited factor for increasing L-tryptophan production in strain JW-5. In addition, Xiong et al. (2021) found that heterologous expression of phosphoketolase from Bifidobacterium adolescentis to strengthen E4P formation benefits to increase L-tryptophan production, and these results confirmed E4P supply is an important limited factor for L-tryptophan biosynthesis. In order to enhancement the PP pathway, EMP pathway and PP pathway were co-modified to increase E4P supply. As can be seen from Fig. 6c and Table 3, the resultant strain JW-5 zwf243 gnd361 ΔpfkA produced 31.9 ± 2.7 g/L of L-tryptophan, which was 20.4% and 47.0% higher than that of strain JW-5 zwf243 gnd361 and strain JW-5, respectively. Although the strain JW-5 zwf243 gnd361 ΔpfkA showed the decreased cell growth rate as compared with the strain JW-5 zwf243 gnd361 in fermentation medium (Table 3), its final DCW was similar with the strain JW-5 zwf243 gnd361 (Fig. 6a). This is probably due to the low PEP supply for cell growth. Li et al. (2021) indicated that the mutated E. coli strains show the different growth situation during suppressing of pfkA with different repression range using CRISPRi. It should be noted that the strain JW-5 zwf243 gnd361 ΔpfkA had the highest glucose consumption rate(i.e., 0.83±0.04 g/(h·g DCW)) among these tested strains (Table 3). In addition, the cell growth rate and DCW of strain JW-5 zwf243 gnd361 ΔpfkA were also higher than that of strain JW-5 ΔpfkA in fermentation medium (Fig. 3a, Fig. 6a and Table 3). Morita et al. (2003) pointed out that the high glucose-6-phosphate pool will destabilize the ptsG mRNA in pgi-deleted strain and thus limit the PTS. However, the strain JW-5 zwf243 gnd361 ΔpfkA with the desensitized zwfA234T and gndS361F ensure that glucose-6-phosphate could effectively entry in PP pathway. As compared with original strain JW-5, the strain JW-5 zwf243 gnd361 ΔpfkA with co-modification of EMP pathway and PP pathway showed the high L-tryptophan titer (i.e., 31.9 ± 2.7 g/L vs. 21.7 ± 1.3 g/L), the high L-tryptophan yield (i.e., 0.213 g/g vs. 0.146 g/g) and the high glucose consumption rate (i.e., 0.83 ± 0.04 g/(h·g DCW) vs. 0.75 ± 0.05 g/(h·g DCW)) despite of the bad cell growth rate (i.e., 0.32 h−1 vs. 0.44 h−1). These are because enhancement of PP pathway results in the increase of intermediate metabolites (e.g., NADPH, PRPP and E4P) supply and thus to increase the production of L-tryptophan and the other products, for example, xylitol (Yuan et al., 2021).
In conclusion, an L-tryptophan high-yielding strain E. coli JW-5 zwf243 gnd361 ΔpfkA was constructed by collaboratively modifying EMP pathway and PP pathway for the first time, which produced 31.9 ± 2.7 g/L of L-tryptophan with a yield of 0.213 g/g in 40-h fed-batch fermentation. Compared with the strain with the only modification of EMP pathway or PP pathway, the strain E. coli JW-5 zwf243 gnd361 ΔpfkA showed the excellent fermentability, i.e., the high L-tryptophan titer and yield as well as the quick glucose consumption rate.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: Shuai Liu, Jian-Zhong Xu, Zhi-Ming Rao, Wei-Guo Zhang; data collection: Shuai Liu, Ting-Shan Liu; analysis and interpretation of results: Shuai Liu; Jian-Zhong Xu; draft manuscript preparation: Jian-Zhong Xu, Shuai Liu. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: Not applicable.
Funding Statement: This work as financially supported by the National Key Research and Development Program of China (2021YFC2100900), the Key Laboratory of Industrial Biotechnology, Ministry of Education, Jiangnan University (KLIB-KF 202004), and Fundamental Research Funds for the Central Universities [No. JUSRP115A19].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Bai Y, Lang EJM, Nazmi AR, Parker EJ (2019). Domain cross-talk within a bifunctional enzyme provides catalytic and allosteric functionality in the biosynthesis of aromatic amino acids. Journal of Biological Chemistry 294: 4828–4842. DOI 10.1074/jbc.RA118.005220. [Google Scholar] [CrossRef]
Becker J, Klopprogge C, Herold A, Zelder O, Bolten CJ et al. (2007). Metabolic flux engineering of L-lysine production in Corynebacterium glutamicum-over expression and modification of G6P dehydrogenase. Journal of Biotechnology 132: 99–109. DOI 10.1016/j.jbiotec.2007.05.026. [Google Scholar] [CrossRef]
Chung ME, Yeh IH, Sung LY, Wu MY, Chao YP et al. (2017). Enhanced integration of large DNA into E. coli chromosome by CRISPR/Cas9. Biotechnology and Bioengineering 114: 172–183. DOI 10.1002/bit.26056. [Google Scholar] [CrossRef]
Chen L, Zhang ZY, Hoshino A, Zheng HD, Morley M et al. (2019). NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nature Metabolism 1: 404–415. [Google Scholar]
Chen YY, Liu YF, Ding DQ, Cong LN, Zhang DW (2018). Rational design and analysis of an Escherichia coli strain for high-efficiency tryptophan production. Journal of Industrial Microbiology & Biotechnology 45: 357–367. DOI 10.1007/s10295-018-2020-x. [Google Scholar] [CrossRef]
Fricke J, Blei F, Hoffmeister D (2017). Enzymatic ssynthesis of psilocybin. Angewandte Chemie International Edition 56: 12352–12355. DOI 10.1002/anie.201705489. [Google Scholar] [CrossRef]
Fang Y, Wang J, Ma W, Yang J, Zhang H et al. (2020). Rebalancing microbial carbon distribution for L-threonine maximization using a thermal switch system. Metabolic Engineering 61: 33–46. DOI 10.1016/j.ymben.2020.01.009. [Google Scholar] [CrossRef]
Gu PF, Yang F, Li FF, Liang QF, Qi QS (2013). Knocking out analysis of tryptophan permeases in Escherichia coli for improving L-tryptophan production. Applied Microbiology and Biotechnology 97: 6677–6683. DOI 10.1007/s00253-013-4988-5. [Google Scholar] [CrossRef]
Ikeda M, Okamoto K, Katsumata R (1999). Cloning of the transketolase gene and the effect of its dosage on aromatic amino acid production in Corynebacterium glutamicum. Applied Microbiology and Biotechnology 51: 201–206. DOI 10.1007/s002530051382. [Google Scholar] [CrossRef]
Jiang Y, Chen B, Duan C, Sun B, Yang J et al. (2015). Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Applied Environment Microbiology 81: 2506–2514. DOI 10.1128/AEM.04023-14. [Google Scholar] [CrossRef]
Koendjbiharie JG, Hon S, Pabst M, Hooftman R, Stevenson DM et al. (2020). The pentose phosphate pathway of cellulolytic clostridia relies on 6-phosphofructokinase instead of transaldolase. Journal of Biological Chemistry 295: 1867–1878. DOI 10.1074/jbc.RA119.011239. [Google Scholar] [CrossRef]
Li Y, Xian H, Xu Y, Zhu Y, Sun ZJ et al. (2021). Fine tuning the glycolytic flux ratio of EP-bifido pathway for mevalonate production by enhancing glucose-6-phosphate dehydrogenase (zwf) and CRISPRi suppressing 6-phosphofructose kinase (PfkA) in Escherichia coli. Microbial Cell Factories 20: 32. DOI 10.1186/s12934-021-01526-1. [Google Scholar] [CrossRef]
Li Z, Ding DQ, Wang HY, Liu LX, Fang H et al. (2020). Engineering Escherichia coli to improve tryptophan production via genetic manipulation of precursor and cofactor pathways. Synthetic and Systems Biotechnology 5: 200–205. DOI 10.1016/j.synbio.2020.06.009. [Google Scholar] [CrossRef]
Liu LN, Chen S, Wu J (2017). Phosphoenolpyruvate: glucose phosphotransferase system modification increases the conversion rate during L-tryptophan production in Escherichia coli. Journal of Industrial Microbiology & Biotechnology 44: 1385–1395. DOI 10.1007/s10295-017-1959-3. [Google Scholar] [CrossRef]
Liu XZ, Niu H, Huang ZS, Li Q, Gu PF (2020). Construction of a switchable synthetic Escherichia coli for aromatic amino acids by a tunable switch. Journal of Industrial Microbiology & Biotechnology 47: 233–242. DOI 10.1007/s10295-020-02262-y. [Google Scholar] [CrossRef]
Marco T, Luisa L, Rosa MM, Alessandra V, Angela T et al. (2022). Clustered Regularly Interspaced Short Palindromic Repeats (CRISPRA critical overview on the most promising applications of molecular scissors in oral medicine. BIOCELL 46: 1–6. DOI 10.32604/biocell.2022.020570. [Google Scholar] [CrossRef]
Meng J, Wang BY, Liu DY, Chen T, Wang ZW et al. (2016). High-yield anaerobic succinate production by strategically regulating multiple metabolic pathways based on stoichiometric maximum in Escherichia coli. Microbial Cell Factories 15: 141. DOI 10.1186/s12934-016-0536-1. [Google Scholar] [CrossRef]
Morita T, El-Kazzaz W, Tanaka Y, Inada T, Aiba H (2003). Accumulation of glucose 6-phosphate or fructose 6-phosphate is responsible for destabilization of glucose transporter mRNA in Escherichia coli. Journal of Biological Chemistry 278: 15608–15614. DOI 10.1074/jbc.M300177200. [Google Scholar] [CrossRef]
Na D, Yoo SM, Chung H, Park H, Park JH et al. (2013). Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nature Biotechnology 31: 170–174. DOI 10.1038/nbt.2461. [Google Scholar] [CrossRef]
Ohnishi J, Katahira R, Mitsuhashi S, Kakita S, Ikeda M (2005). A novel gnd mutation leading to increased L-lysine production in Corynebacterium glutamicum. FEMS Microbiology Letters 242: 265–274. DOI 10.1016/j.femsle.2004.11.014. [Google Scholar] [CrossRef]
Patnaik R, Liao JC (1994). Engineering of Escherichia coli central metabolism for aromatic metabolite production with near theoretical yield. Applied and Environmental Microbiology 60: 3903–3908. DOI 10.1128/AEM.60.11.3903-3908.1994. [Google Scholar] [CrossRef]
Roehl RA, Vinopal RT (1976). Lack of glucose phosphotransferase function in phosphofructokinase mutants of Escherichia coli. Journal of Bacteriology 126: 852–860. DOI 10.1128/jb.126.2.852-860.1976. [Google Scholar] [CrossRef]
Ruan HZ, Yu B, Xu JZ (2020). The glucose uptake systems in Corynebacterium glutamicum: A review. World Journal of Microbiology and Biotechnology 36: 126–134. DOI 10.1007/s11274-020-02898-z. [Google Scholar] [CrossRef]
Schoppel K, Trachtmann N, Mittermeier F, Sprenger GA, Weuster-Botz D (2021). Metabolic control analysis of L-tryptophan producing Escherichia coli applying targeted perturbation with shikimate. Bioprocess and Biosystems Engineering 44: 2591–2613. DOI 10.1007/s00449-021-02630-7. [Google Scholar] [CrossRef]
Siedler S, Bringer S, Blank LM, Bott M (2012). Engineering yield and rate of reductive biotransformation in Escherichia coli by partial cyclization of the pentose phosphate pathway and PTS-independent glucose transport. Applied Microbiology and Biotechnology 93: 1459–1467. DOI 10.1007/s00253-011-3626-3. [Google Scholar] [CrossRef]
Trondle J, Schoppel K, Bleidt A, Trachtmann N, Sprenger GA et al. (2020). Metabolic control analysis of L-tryptophan production with Escherichia coli based on data from short-term perturbation experiments. Journal of Biotechnology 307: 15–28. DOI 10.1016/j.jbiotec.2019.10.009. [Google Scholar] [CrossRef]
Trondle J, Trachtmann N, Sprenger GA, Weuster-Botz D (2018). Fed-batch production of L-tryptophan from glycerol using recombinant Escherichia coli. Biotechnology and Bioengineering 115: 2881–2892. DOI 10.1002/bit.26834. [Google Scholar] [CrossRef]
Wang ZW, Chen T, Ma XH, Shen Z, Zhao XM (2011). Enhancement of riboflavin production with Bacillus subtilis by expression and site-directed mutagenesis of zwf and gnd gene from Corynebacterium Glutamicum. Bioresource Technology 102: 3934–3940. DOI 10.1016/j.biortech.2010.11.120. [Google Scholar] [CrossRef]
Xiong B, Zhu YD, Tian DG, Jiang S, Fan XG et al. (2021). Flux redistribution of central carbon metabolism for efficient production of L-tryptophan in Escherichia coli. Biotechnology and Bioengineering 118: 1393–1404. DOI 10.1002/bit.27665. [Google Scholar] [CrossRef]
Xu J, Han M, Ren X, Zhang W (2016). Modification of aspartokinase III and dihydrodipicolinate synthetase increases the production of L-lysine in Escherichia coli. Biochemical Engineering Journal 114: 79–86. DOI 10.1016/j.ymben.2010.03.005. [Google Scholar] [CrossRef]
Yuan X, Mao Y, Tu S, Lin J, Shen H et al. (2021). Increasing NADPH availability for xylitol production via pentose phosphate pathway gene overexpression and Embden-Meyerhof-Parnas pathway gene deletion in Escherichia coli. Journal of Agricultural and Food Chemistry 69: 9625–9631. DOI 10.1021/acs.jafc.1c03283. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |