DOI:10.32604/biocell.2022.022290

| BIOCELL DOI:10.32604/biocell.2022.022290 |  |
| Viewpoint |
Osteocyte pericellular and perilacunar matrices as markers of bone–implant mechanical integrity
1Université de Lyon, CNRS, INSA de Lyon, Université Claude Bernard Lyon 1, MATEIS, UMR5510, Villeurbanne, F-69621, France
2Université de Lyon, Université Claude Bernard Lyon 1, INSERM, LYOS UMR 1033, Lyon, F-69008, France
3Université de Lyon, INSA-Lyon, CNRS UMR5259, LaMCoS, Villeurbanne, F-69621, France
4Université de Lyon, Université Claude Bernard Lyon 1, UMR CNRS 5615, Laboratoire des Multimatériaux et Interfaces, Villeurbanne, F-69622, France
5Université de Lyon, Université Claude Bernard Lyon 1, Faculté d’Odontologie, Lyon, F-69008, France
6Université de Lyon, Université Gustave Eiffel, Univ Claude Bernard Lyon 1, LBMC UMR_T 9406, Lyon, F-69622, France
*Address correspondence to: Rémy Gauthier, remy.gauthier@cnrs.fr
Received: 02 March 2022; Accepted: 10 March 2022
Abstract: To develop durable bone healing strategies through improved control of bone repair, it is of critical importance to understand the mechanisms of bone mechanical integrity when in contact with biomaterials and implants. Bone mechanical integrity is defined here as the adaptation of structural properties of remodeled bone in regard to an applied mechanical loading. Accordingly, the authors present why future investigations in bone repair and regeneration should emphasize on the matrix surrounding the osteocytes. Osteocytes are mechanosensitive cells considered as the orchestrators of bone remodeling, which is the biological process involved in bone homeostasis. These bone cells are trapped in an interconnected porous network, the lacunocanalicular network, which is embedded in a bone mineralized extracellular matrix. As a consequence of an applied mechanical loading, the bone deformation results in the deformation of this lacunocanalicular network inducing a shift in interstitial fluid pressure and velocity, thus resulting in osteocyte stimulation. The material environment surrounding each osteocyte, the so called perilacunar and pericellular matrices properties, define its mechanosensitivity. While this mechanical stimulation pathway is well known, the laws used to predict bone remodeling are based on strains developing at a tissue scale, suggesting that these strains are related to the shift in fluid pressure and velocity at the lacunocanalicular scale. While this relationship has been validated through observation in healthy bone, the fluid behavior at the bone-implant interface is more complex. The presence of the implant modifies fluid behavior, so that for the same strain at a tissue scale, the shift in fluid pressure and velocity will be different than in a healthy bone tissue. In that context, new markers for bone mechanical integrity, considering fluid behavior, have to be defined. The viewpoint exposed by the authors indicates that the properties of the pericellular and the perilacunar matrices have to be systematically investigated and used as structural markers of fluid behavior in the course of bone biomaterial development.
Keywords: Bone mechanical integrity; Osteocytes; Pericellular; Perilacunar; Mechanosensitivity
Understanding how human cells respond to stimuli is of great importance for developing durable healing and tissue engineering strategies. Cellular activity is mainly investigated in the field of biology, and involves the strong multi-physic coupling between different biological, biomechanical, biochemical, or bioelectrical mechanisms. In a biological system that is made up of cells embedded in an extracellular matrix, the different elements interact together to coordinate cell activity and thus insure system viability. Bone tissue plays various roles within our body. Beyond its well-known mechanical function, bone is the main regulator for both phosphate and calcium. These two chemical species play a major role in organism homeostasis. Bone cells are thus sensitive to many kinds of stimuli, from hormonal to biomechanical stimuli.
As an example, it is well known that bone cells are sensitive to biomechanical stimuli that can tune bone properties and ensure biomechanical function (Turner, 1998). This viewpoint will mainly focus on this type of stimuli. This mechanosensitive nature is of great importance in the course of bone remodeling around implants (Li et al., 2018). Among other factors, mechanical loading plays a major role in the risk for peri-implant osteolysis (Amirhosseini et al., 2017; Goodman and Gallo, 2019). While mechanical loading is known to be involved in the stress shielding mechanism (Sumner, 2015), bone resorption can also be associated with a mechanically-induced inflammatory response (Amirhosseini et al., 2017). The bone-implant system thus appears to promote the development of a complex mechanical environment in which specific tissue properties develop (Fraulob et al., 2020; Le Cann et al., 2020; Li et al., 2018). Whether or not these properties are suitable for ensuring the mechanical integrity of the bone-implant system is still an open question. In order to provide some answers, it is important to first understand how bone ensures its own mechanical integrity.
What is bone mechanical integrity?
This paper defines bone mechanical integrity as the adaptation of bone structure in response to biomechanical loading that is experienced during life (Fig. 1). Bone mechanical behavior is closely associated with its structure at different scales (Zimmermann and Ritchie, 2015).
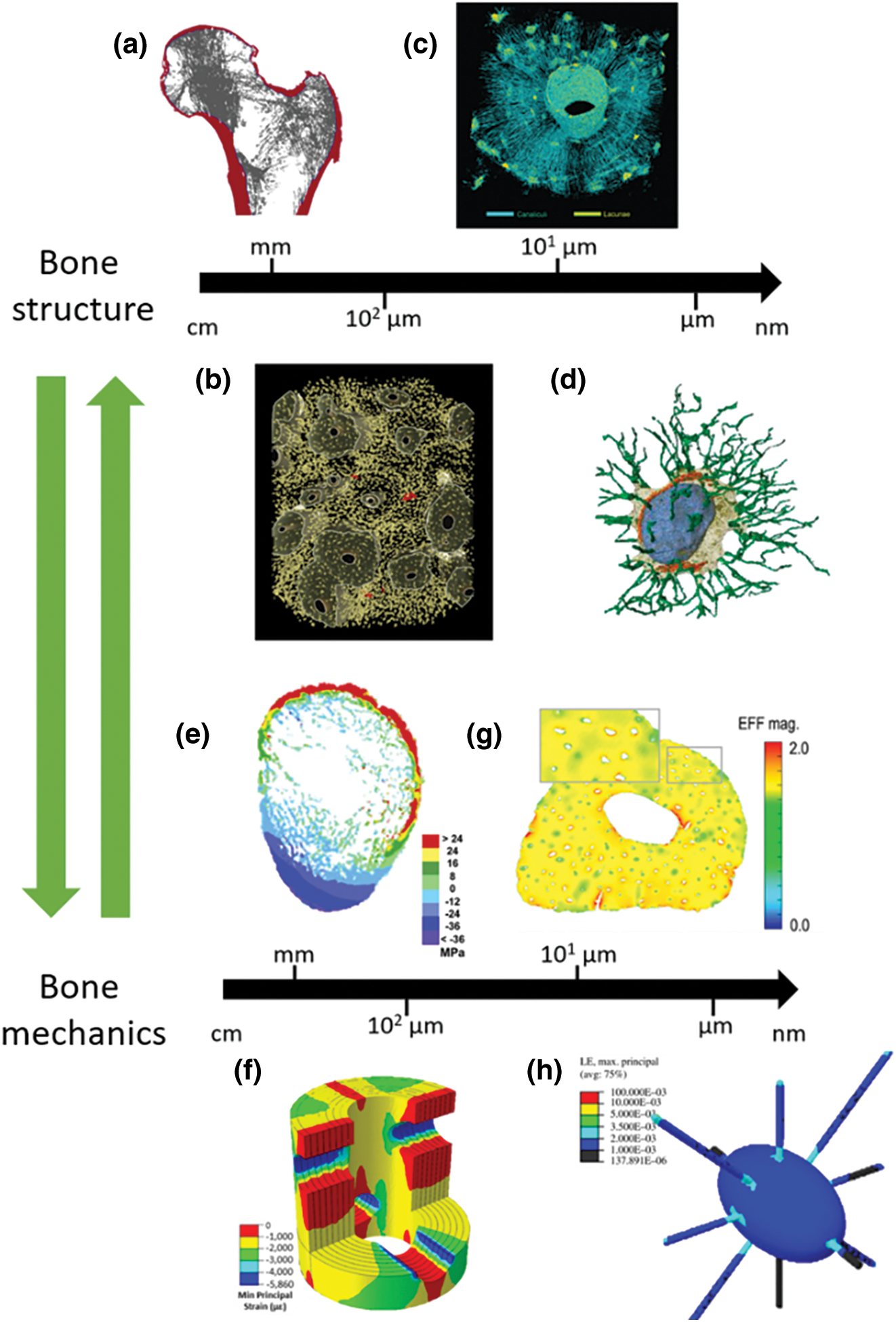
Figure 1: Illustration of bone mechanical integrity. At all length scale, bone structural elements are associated with the tissue mechanical response. a., projection of a proximal femur, with cortical bone in red, and trabecular bone in grey (reprinted from Nawathe et al. (2015)). 3D X-rays micrography reconstruction of human cortical bone with voxel size of 0.7 µm (b., reprinted from Gauthier et al. (2019)) and 0.28 µm (c., reprinted from Pacureanu et al. (2012)). d., 3D reconstruction of a lacuna through electronic microscopy based tomography (reprinted from Goggin et al. (2020)). From e. to h., stress (e., reprinted from Nawathe et al. (2015)) or strain maps (f., reprinted from Vaughan et al. (2013), g., reprinted from Hemmatian et al. (2021), and h., reprinted from Verbruggen et al. (2012)) at different bone length scales. For more accurate images, the readers are referred to the original articles.
Although bone is a complex material that presents heterogeneity at all length scales associated with mechanical properties (Bala et al., 2012; Rho et al., 1998; Rux et al., 2022), the following investigation will focus only on the bone porous network. At the macroscale, two bone tissues can be distinguished in terms of their porosities: trabecular bone being porous, and cortical bone being compact ((Nawathe et al., 2015), Fig. 1a). Interestingly, bone loading and load distribution follow the same pattern as bone mass distribution ((Nawathe et al., 2015), Fig. 1e). Bone macroporosity is mainly made of vascular canals. In cortical bone, these canals account for less than 10% of the tissue volume with a diameter between 50 and 100 µm (Gauthier et al., 2019, Fig. 1c). Vascular canals influence mechanical stress distribution within the tissue (Vaughan et al., 2013, Fig. 1f). At a smaller scale, an interconnected network, called the lacunocanalicular network (LCN), is distributed within the bone matrix (Goggin et al., 2020; Pacureanu et al., 2012, Figs. 1c and 1d). This LCN is made of micrometer ellipsoidal lacunae that are spread within the tissue with a density higher than 20,000 mm3 (Gauthier et al., 2019, depicted in yellow in Fig. 1b). These lacunae are all connected together through canaliculi, 400 nm in diameter (Varga et al., 2014; Yu et al., 2020). It is estimated that one lacuna is connected to 58 canaliculi, on average, in human femoral diaphysis (Yu et al., 2021). Even with their micrometer and nanometer scales, both lacunae and canaliculi play a role in the tissue mechanical response (Hemmatian et al., 2021; Verbruggen et al., 2012, Figs. 1g and 1h). Fig. 1 shows an overview of bone mechanical integrity, with a specific mechanical answer, in terms of stress distribution, being associated with bone structural organization at all length scales.
How does bone ensure its mechanical integrity?
Bone mechanical integrity is maintained through a balance between bone resorption and bone formation. The process ensuring this homeostasis is known as bone remodeling. Remodeling occurs to allow bone to adapt to its mechanical environment and to repair damaged tissue (Burr, 2002). Bone remodeling involves different types of cells:
• Osteoclasts are recruited to remove targeted tissues through an acidic dissolution of bone mineral and proteolytic digestion of organic matrix.
• Osteoblasts are recruited to deposit a new tissue by synthesizing an organic template for further nucleation and the growth of bone minerals.
• Osteocytes are former osteoblasts that have been trapped and embedded within the mineralized extracellular bone matrix. It has been estimated that 10% to 30% of osteoblasts become osteocytes (Franz-Odendaal et al., 2006). They are believed to orchestrate bone remodeling through the regulation of both osteoclasts and osteoblasts (Robling and Bonewald, 2020). Osteocytes are able to sense a change in bone mechanical integrity. They can then secrete and send biochemical mediators towards the osteoclasts and osteoblasts and hence control bone remodeling. From the different signaling pathways of the osteocytes, their mechanosensitivity is determinant to ensure bone mechanical integrity (Cowin et al., 1991; Delgado-Calle and Bellido, 2022; Palumbo and Ferretti, 2021). It is believed that abnormal mechanical stimulation explains the complex peri-implant bone organization (Gramanzini et al., 2016; Li et al., 2018; Okawara et al., 2021).
How does the osteocyte sense a mechanical signal?
The structural organization of osteocytes within the bone matrix is of great importance to understanding their mechanical stimulation. In vivo, the connected osteocytes are trapped in the LCN. This porous network allows for the transport of interstitial fluid from the vascular canals to the cells. Within this porous network, the cells are surrounded by a glycoproteic pericellular matrix less than 100 nm in thickness (PCM) (You et al., 2004), mainly made of perlecan (Thompson et al., 2011), and are attached to the wall of the lacuna through tethering perlecan fibers (Bertacchini et al., 2017; McNamara et al., 2009) with an average spacing of 40 nm (You et al., 2004). This leaves a space, the pericellular space, between the PCM and the lacunar wall, where interstitial fluids can flow from the vascular canals to the osteocytes. In summary, the osteocyte and its canaliculi are surrounded by a perilacunar matrix (PLM) around the porosity and a PCM between a bone mineralized matrix and the cell (Fig. 2). The lacunocanalicular network irrigates all the bone volume so that any tissue damage can be detected and processed by the cells. In addition to this complex micro- and nanostructural organization, the composition of these PCM and PLM also present some heterogeneity. As an example, it has recently been measured that there is a decrease in elastic modulus with an increasing distance to the lacunar wall. Interestingly, the gradient magnitude is believed to be associated with the age of the osteocyte trapped within the studied lacuna (Rux et al., 2022). Similarly, if the PCM is mainly composed of perlecan, other components could influence the fluid behavior within the LCN (Wang, 2018).
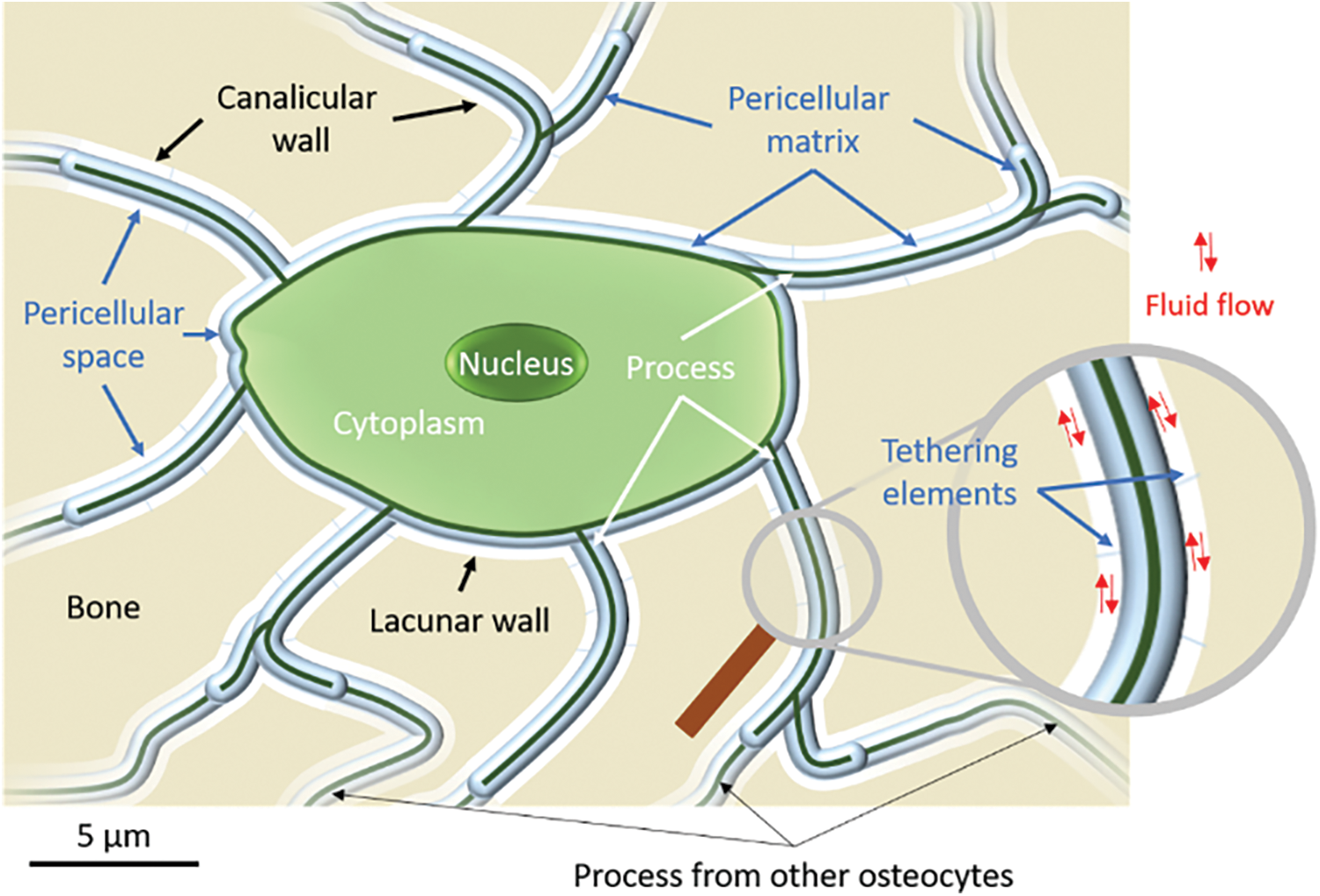
Figure 2: Schematic of an osteocyte structural organization within bone mineral matrix. Interstitial fluid flows through the pericellular space dragging the tethering elements. The elements of the perilacunar, pericellular, and cellular matrices are written in black, blue, and white, respectively.
When bone is subjected to a mechanical loading, the whole lacunocanalicular network is deformed together with its surrounding mineralized matrix. Due to its particular organization within this network, osteocytes can experience the mechanical strain, or deformation, through different mechanisms.
The strain of the lacunae can directly be transmitted to the cell through hydrostatic pressure that can be up to the MPa (Cowin et al., 2009; Gardinier et al., 2010; Zhang et al., 1998). It is known that a direct low pressure as low as 68 kPa applied on osteocytes induces their expression of bone remodeling mediators (Henstock et al., 2013; Liu et al., 2010). Due to their ellipsoidal morphological feature, lacunae play the role of strain concentrators within the tissue (Hemmatian et al., 2021; Inglis, 1913). It has been measured that an apparent 0.2% deformation leads to a local deformation of up to 1.5% in the vicinity of a lacuna in an in vitro bovine bone (Nicolella et al., 2006). This feature can increase the pressure developed within on lacuna and thus a compressive strain on the osteocyte.
The deformation of the lacunocanalicular network also induces pressure gradients within the porous canals, resulting in the flow of the interstitial fluid (Cowin, 1999; Lemaire et al., 2011). Osteocytes are sensitive to fluid flow (Chen et al., 2021; Tan et al., 2007). The lacunae, through their ability to generate deformation concentrations, may locally modify the pressure gradient and thus the fluid velocity within this interconnected porous network. Pressure variations can also be induced by the variation of the canaliculi pericellular space during bone deformation. The induced fluid flow can drag the tethering elements attaching the PCM to the lacunar wall resulting in the deformation of the PCM and cell process (Wijeratne et al., 2016; Yokoyama et al., 2021). Hence, the properties of this PCM are a major factor involved in the mechanical stimulation of osteocytes (Thompson et al., 2011; Wang et al., 2014; Wijeratne et al., 2016).
The deformation of an osteocyte and its related processes could stimulate intra-cellular mechanosensors, such as integrin or ion channels (Qin et al., 2020), which then induce the secretion of bone remodeling mediators such as nitric oxide (Tan et al., 2007), calcium ions (Lewis et al., 2017), or sclerostin (Nishiyama et al., 2014). These mediators are then transported through interstitial fluids to the osteoblasts, osteoclasts, and other biological actors of bone remodeling that are located in the vascular porosities. Such solute transport is also related to LCN mechanical stimulation (Fan et al., 2016), with for example bigger molecules being transported only under mechanical stimulation. The diffusion of different chemical species involved in bone metabolism, and more generally in our organism metabolism, is largely influenced by the properties of the PCM (Wang, 2018).
These results highlight the importance of fluid behavior (i.e., fluid pressure in the lacunae and fluid velocity in the canaliculi) surrounding the osteocytes and their processes on the mechanotransduction pathway of bone tissue. Osteocyte-based bone remodeling is activated through a shift in such fluid pressure and velocity.
What is the marker of bone mechanical integrity?
Mechanical loading applied at the organ scale induces mechanical strains at the tissue level that subsequently produces the development of hydrostatic pressure and pressure gradient-induced fluid flow within the LCN (Palumbo and Ferretti, 2021; Zhang et al., 1998). This pathway defines how osteocytes sense a mechanical stimulation and hence regulate bone mechanical integrity through tissue remodeling.
Such a pathway has led some scientists to develop a theoretical model of bone remodeling based on tissue deformation. In that context, major progress has been made by Frost who developed the mechanostat mechanism theory (Frost, 1987). In this theory, Frost defined different thresholds, known as minimum effective strains (MES), as the strains developed at a tissue scale below which bone resorption occurs (MES for bone remodeling, MESr), and above which bone formation occurs (MES for bone modeling, MESm). This also implies that there is a range of deformations, between MESr and MESm, in which bone structure remains the same (Frost, 1983).
This strain-based principle of bone remodeling is attractive, because bone tissue strains can be routinely estimated using numerical tools such as finite element modeling (Hemmatian et al., 2021; van Rietbergen et al., 2003; Werner et al., 2019). Bone tissue strain has hence been used to predict the course of bone remodeling (McNamara and Prendergast, 2007; Schulte et al., 2013), and can thus be considered as a marker of bone mechanical integrity.
What about the bone-implant system mechanical integrity?
While this strain-based theory has also been widely investigated in the context of peri-implant tissue remodeling (Huiskes et al., 1987; Mirulla et al., 2021), its relevance is not obvious.
Considering an osteocyte mechanical stimulation pathway, the strain-based theory could indirectly suggest that strains at a tissue scale promote fluid movement within the LCN. All the tissue elements on which strain is calculated are thus considered as equivalent in terms of fluid behavior. Nevertheless, in the vicinity of an implant, fluid behavior is different from what may occur in the bulk (Fahlgren et al., 2010), with a direct incidence on tissue remodeling, and hence on the bone-implant system mechanical integrity. The shift in fluid behavior will be different between the bone surrounding the implant and in the bulk due to tissue strain.
This means that there is a need for additional markers to help understand strain-induced fluid behavior close to the bone-implant interface.
Osteocyte perilacunar and pericellular matrices as markers of bone-implant system mechanical integrity
The viewpoint exposed by the authors is that PCM and PLM properties can be considered as suitable markers, and that these matrices have to be systematically investigated to validate the efficiency of future bone implants. There is increasing evidence that PCM and PLM properties and remodeling are associated with bone mechanical function (Dole et al., 2017; Milovanovic and Busse, 2019; van Tol et al., 2020b). Interestingly, it has recently been shown that a mechanical loading (in vitro and in vivo) influences the turnover of this PCM (Pei et al., 2021). Such results further support the need to have a better understanding the roles of both PCM and PLM on bone mechanosensitivy.
Within the physiological window defined by Frost, strains do not promote either bone formation or resorption (Frost, 1987). Within this physiological window, the strain-based shift in fluid pressure or velocity is not high enough to induce bone remodeling. The current viewpoint thus considers that PCM and PLM reach specific properties while bone tissue lies within this physiological strain window. In other words, the limits of this physiological window are defined by PCM and PLM properties.
Considering this viewpoint, and due to different fluid behavior, the physiological window close to an implant should thus be different from the bulk. Investigating both PCM and PLM properties is thus necessary to define the physiological range during which bone remodeling does not occur.
It is known that the fluid behavior within the LCN depends on the distance to vascular canals in cortical bone (van Tol et al., 2020a) and to canaliculi interconnectivity (Bortel et al., 2021). Accordingly, it has been observed that both lacunar and canalicular morphological parameters depend on their location between the vascular canal and the cement line of an osteon (Gauthier et al., 2019; Repp et al., 2017).
This viewpoint is also interesting when considering the chemoregulator role of osteocytes within our body (Bonewald, 2017). The properties of the LCN do not depend only on bone mechanical integrity. Osteocytes also act as regulators for both calcium and phosphate metabolism to maintain systemic mineral homeostasis in physiological conditions (Cheng and Hulley, 2010; Delgado-Calle and Bellido, 2022; Horner, 2004). During specific adaptation cases, for example during lactation, changes in lacunar morphology have already been highlighted (Qing et al., 2012), and are associated with a decrease in the effective elastic properties of bone tissue (Kaya et al., 2017). It is known that their activity is partly regulated through hormonal pathways. For example, parathyroid hormone (PTH) is very important in osteocyte functions, and hence in bone homeostasis (Bellido et al., 2013). PTH inhibits sclerostin expression that hence prevents the osteoblast from synthetizing new bone. Nevertheless, sclerostin can be expressed by bone cells through mechanical loading and independently of PTH (Spatz et al., 2015). Furthermore, it is known that osteocyte apoptosis can be induced through mechanical loading (Hughes and Petit, 2010; Nakao et al., 2021). Such results highlight that osteocytes need to be in a quiescent state, or equilibrium state, and within the physiological strain window, even when considering their chemical regulator role.
In contrast to peri-implant bone, the variations in PLM properties are not related to a modification in fluid behavior (i.e., fluid pressure and velocity), but instead to a metabolic need for calcium or phosphate. Nevertheless, with different PCM and PLM properties, the strain-induced shift in fluid pressure and velocity necessary to activate osteocyte–based bone remodeling is also different. As for peri-implant bone, the physiological strain window evolves with PCM and PLM properties. This may explain the decrease in bone mechanical properties in the case of lactation. As the external mechanical loading remains the same, the strain at the tissue level does not evolve. However, with different limits in the physiological window, bone remodeling is not activated at the same strain magnitude. This results in the development of a different structural organization, and thus a different result in mechanical integrity.
This viewpoint highlights that the consideration of bone remodeling as just a result of strains at the tissue level may not be accurate enough to cover different abnormal cases. While such remodeling is true in healthy bone, it might not be accurate in the vicinity of an implant, where fluid behavior is unknown, or in the case of a biological pathology or aging, which can induce an evolution in PCM and PLM properties. According to the present hypothesis, the strain-based shift in fluid pressure and velocity is the real determinant in bone remodeling and mechanical integrity. Hence, in addition to tissue strain, PCM and PLM properties have to be considered as major features involved in this fluid behavior strain-based shift.
To better understand this relationship between strain and fluids, further investigations of the LCN has to be performed considering their precise location in regard to the vascular network, and how this acts as the main fluid reservoir. Are there particular patterns in PCM and PLM distribution properties in relation to the distance to a vascular canal? Is there a difference between trabecular or cortical LCN? Those questions remain unanswered. Similarly, there is no data on PCM and PLM properties in the vicinity of an implant. Furthermore, while bone implant efficiency is currently defined as its capacity to induce suitable strain in the peri-implant bone, major efforts have to be made regarding the implant’s ability to influence interstitial fluid behavior in its surrounding LCN.
Authors’ Contribution: RG conceived the first draft and all of the authors critically reviewed the draft.
Ethics Approval: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Amirhosseini M, Andersson G, Aspenberg P, Fahlgren A (2017). Mechanical instability and titanium particles induce similar transcriptomic changes in a rat model for periprosthetic osteolysis and aseptic loosening. Bone Reports 7: 17–25. DOI 10.1016/j.bonr.2017.07.003. [Google Scholar] [CrossRef]
Bala Y, Depalle B, Farlay D, Douillard T, Meille S et al. (2012). Bone micromechanical properties are compromised during long-term alendronate therapy independently of mineralization. Journal of Bone and Mineral Research 27: 825–834. DOI 10.1002/jbmr.1501. [Google Scholar] [CrossRef]
Bellido T, Saini V, Pajevic PD (2013). Effects of PTH on osteocyte function. Bone 54: 250–257. DOI 10.1016/j.bone.2012.09.016. [Google Scholar] [CrossRef]
Bertacchini J, Benincasa M, Checchi M, Cavani F, Smargiassi A et al. (2017). Expression and functional proteomic analyses of osteocytes from Xenopus laevis tested under mechanical stress conditions: Preliminary observations on an appropriate new animal model. Journal of Anatomy 231: 823–834. DOI 10.1111/joa.12685. [Google Scholar] [CrossRef]
Bonewald LF (2017). The role of the osteocyte in bone and nonbone disease. Endocrinology and Metabolism Clinics of North America 46: 1–18. DOI 10.1016/j.ecl.2016.09.003. [Google Scholar] [CrossRef]
Bortel E, Grover LM, Eisenstein N, Seim C, Suhonen H et al. (2021). Interconnectivity explains high canalicular network robustness between neighboring osteocyte lacunae in human bone. Advanced NanoBiomed Research 27: 2100090. DOI 10.1002/anbr.202100090. [Google Scholar] [CrossRef]
Burr DB (2002). Targeted and nontargeted remodeling. Bone 30: 2–4. DOI 10.1016/S8756-3282(01)00619-6. [Google Scholar] [CrossRef]
Chen M, Li M, Kwoon S, Chow H, Man R et al. (2021). The role of osteocytes-specific molecular mechanism in regulation of mechanotransduction–A systematic review. Journal of Orthopaedic Translation 29: 1–9. DOI 10.1016/j.jot.2021.04.005. [Google Scholar] [CrossRef]
Cheng F, Hulley P (2010). The osteocyte—A novel endocrine regulator of body phosphate homeostasis. Maturitas 67: 327–338. DOI 10.1016/j.maturitas.2010.08.011. [Google Scholar] [CrossRef]
Cowin SC, Moss-Salentijn L, Moss ML (1991). Candidates for the mechanosensory system in bone. Journal of Biomechanical Engineering 113: 191–197. DOI 10.1115/1.2891234. [Google Scholar] [CrossRef]
Cowin SC (1999). Bone poroelasticity. Journal of Biomechanics 32: 217–238. DOI 10.1016/S0021-9290(98)00161-4. [Google Scholar] [CrossRef]
Cowin SC, Gailani G, Benalla M (2009). Hierarchical poroelasticity: Movement of interstitial fluid between porosity levels in bones. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 367: 3401–3444. DOI 10.1098/rsta.2009.0099. [Google Scholar] [CrossRef]
Delgado-Calle J, Bellido T (2022). The osteocyte as a signaling cell. Physiological Reviews 102: 379–410. DOI 10.1152/physrev.00043.2020. [Google Scholar] [CrossRef]
Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA et al. (2017). Osteocyte-Intrinsic TGF-β signaling regulates bone quality through perilacunar/canalicular remodeling. Cell Reports 21: 2585–2596. DOI 10.1016/j.celrep.2017.10.115. [Google Scholar] [CrossRef]
Fahlgren A, Bostrom MP, Yang X, Johansson L, Edlund U et al. (2010). Fluid pressure and flow as a cause of bone resorption. Acta Orthopaedica 81: 508–516. DOI 10.3109/17453674.2010.504610. [Google Scholar] [CrossRef]
Fan L, Pei S, Lu L, Wang L (2016). A multiscale 3D finite element analysis of fluid/solute transport in mechanically loaded bone. Bone Research 4: 344. DOI 10.1038/boneres.2016.32. [Google Scholar] [CrossRef]
Franz-Odendaal TA, Hall BK, Witten PE (2006). Buried alive: How osteoblasts become osteocytes. Developmental Dynamics 235: 176–190. DOI 10.1002/(ISSN)1097-0177. [Google Scholar] [CrossRef]
Fraulob M, Le Cann S, Voumard B, Yasui H, Yano K et al. (2020). Multimodal evaluation of the spatio-temporal variations of periprosthetic bone properties. Journal of Biomechanical Engineering 142: 217. DOI 10.1115/1.4048399. [Google Scholar] [CrossRef]
Frost HM (1983). A determinant of bone architecture. The minimum effective strain. Clinical Orthopaedics and Related Research 175: 286–292. DOI 10.1097/00003086-198305000-00047. [Google Scholar] [CrossRef]
Frost HM (1987). Bone mass and the mechanostat: A proposal. Anatomical Record 219: 1–9. DOI 10.1002/(ISSN)1097-0185. [Google Scholar] [CrossRef]
Gardinier JD, Townend CW, Jen KP, Wu Q, Duncan RL et al. (2010). In situ permeability measurement of the mammalian lacunar-canalicular system. Bone 46: 1075–1081. DOI 10.1016/j.bone.2010.01.371. [Google Scholar] [CrossRef]
Gauthier R, Follet H, Olivier C, Mitton D, Peyrin F (2019). 3D analysis of the osteonal and interstitial tissue in human radii cortical bone. Bone 127: 526–536. DOI 10.1016/j.bone.2019.07.028. [Google Scholar] [CrossRef]
Goggin P, Ho EML, Gnaegi H, Searle S, Oreffo ROC et al. (2020). Development of protocols for the first serial block-face scanning electron microscopy (SBF SEM) studies of bone tissue. Bone 131: 115107. DOI 10.1016/j.bone.2019.115107. [Google Scholar] [CrossRef]
Goodman SB, Gallo J (2019). Periprosthetic osteolysis: Mechanisms, prevention and treatment. Journal of Clinical Medicine 8: 2091. DOI 10.3390/jcm8122091. [Google Scholar] [CrossRef]
Gramanzini M, Gargiulo S, Zarone F, Megna R, Apicella A et al. (2016). Combined microcomputed tomography, biomechanical and histomorphometric analysis of the peri-implant bone: A pilot study in minipig model. Dental Materials 32: 794–806. DOI 10.1016/j.dental.2016.03.025. [Google Scholar] [CrossRef]
Hemmatian H, Bakker AD, Klein-Nulend J, van Lenthe GH (2021). Alterations in osteocyte lacunar morphology affect local bone tissue strains. Journal of the Mechanical Behavior of Biomedical Materials 123: 104730. DOI 10.1016/j.jmbbm.2021.104730. [Google Scholar] [CrossRef]
Henstock JR, Rotherham M, Rose JB, El Haj AJ (2013). Cyclic hydrostatic pressure stimulates enhanced bone development in the foetal chick femur in vitro. Bone 53: 468–477. DOI 10.1016/j.bone.2013.01.010. [Google Scholar] [CrossRef]
Horner JH (2004). Bone structure and calcium metabolism. Surgery 22: 24a–24d. DOI 10.1383/surg.22.1.24.27039. [Google Scholar] [CrossRef]
Hughes JM, Petit MA (2010). Biological underpinnings of frost’s mechanostat thresholds: The important role of osteocytes. Journal of Musculoskeletal Neuronal Interactions 10: 128–135. [Google Scholar]
Huiskes R, Weinans H, Grootenboer HJ, Dalstra M, Fudala B et al. (1987). Adaptive bone-remodeling theory applied to prosthetic-design analysis. Journal of Biomechanics 20: 1135–1150. DOI 10.1016/0021-9290(87)90030-3. [Google Scholar] [CrossRef]
Inglis CE (1913). Stresses in a plate due to the presnce of cracks and sharp corners. In: Transactions of the Institute of Naval Architects, vol. 55, pp. 219–241. London, UK. [Google Scholar]
Kaya S, Basta-Pljakic J, Seref-Ferlengez Z, Majeska RJ, Cardoso L et al. (2017). Lactation-induced changes in the volume of osteocyte lacunar-canalicular space alter mechanical properties in cortical bone tissue. Journal of Bone and Mineral Research 32: 688–697. DOI 10.1002/jbmr.3044. [Google Scholar] [CrossRef]
Le Cann S, Törnquist E, Silva Barreto I, Fraulob M, Albini Lomami H et al. (2020). Spatio-temporal evolution of hydroxyapatite crystal thickness at the bone-implant interface. Acta Biomaterialia 116: 391–399. DOI 10.1016/j.actbio.2020.09.021. [Google Scholar] [CrossRef]
Lemaire T, Capiez-Lernout E, Kaiser J, Naili S, Sansalone V (2011). What is the importance of multiphysical phenomena in bone remodelling signals expression? A multiscale perspective. Journal of the Mechanical Behavior of Biomedical Materials 4: 909–920. DOI 10.1016/j.jmbbm.2011.03.007. [Google Scholar] [CrossRef]
Lewis KJ, Frikha-Benayed D, Louie J, Stephen S, Spray DC et al. (2017). Osteocyte calcium signals encode strain magnitude and loading frequency in vivo. PNAS 114: 11775–11780. DOI 10.1073/pnas.1707863114. [Google Scholar] [CrossRef]
Li Z, Müller R, Ruffoni D (2018). Bone remodeling and mechanobiology around implants: Insights from small animal imaging. Journal of Orthopaedic Research 36: 584–593. DOI 10.1002/jor.23758. [Google Scholar] [CrossRef]
Liu C, Zhao Y, Cheung WY, Gandhi R, Wang L et al. (2010). Effects of cyclic hydraulic pressure on osteocytes. Bone 46: 1449–1456. DOI 10.1016/j.bone.2010.02.006. [Google Scholar] [CrossRef]
McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB (2009). Attachment of osteocyte cell processes to the bone matrix. Anatomical Record 292: 355–363. DOI 10.1002/ar.20869. [Google Scholar] [CrossRef]
McNamara LM, Prendergast PJ (2007). Bone remodelling algorithms incorporating both strain and microdamage stimuli. Journal of Biomechanics 40: 1381–1391. DOI 10.1016/j.jbiomech.2006.05.007. [Google Scholar] [CrossRef]
Milovanovic P, Busse B (2019). Inter-site variability of the human osteocyte lacunar network: Implications for bone quality. Current Osteoporosis Reports 17: 105–115. DOI 10.1007/s11914-019-00508-y. [Google Scholar] [CrossRef]
Mirulla AI, Pinelli S, Zaffagnini S, Nigrelli V, Ingrassia T et al. (2021). Numerical simulations on periprosthetic bone remodeling: A systematic review. Computer Methods and Programs in Biomedicine. 204: 106072.10.1016/j.cmpb.2021.106072 [Google Scholar] [CrossRef]
Nakao N, Mori I, Sunaga J, Adachi T (2021). Large magnitude of force leads to NO-mediated cell shrinkage in single osteocytes implying an initial apoptotic response. Journal of Biomechanics 117: 110245. DOI 10.1016/j.jbiomech.2021.110245. [Google Scholar] [CrossRef]
Nawathe S, Nguyen BP, Barzanian N, Akhlaghpour H, Bouxsein ML et al. (2015). Cortical and trabecular load sharing in the human femoral neck. Journal of Biomechanics 48: 816–822. DOI 10.1016/j.jbiomech.2014.12.022. [Google Scholar] [CrossRef]
Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J (2006). Osteocyte lacunae tissue strain in cortical bone. Journal of Biomechanics 39: 1735–1743. DOI 10.1016/j.jbiomech.2005.04.032. [Google Scholar] [CrossRef]
Nishiyama Y, Matsumoto T, Lee JW, Saitou T, Imamura T et al. (2014). Changes in the spatial distribution of sclerostin in the osteocytic lacuno-canalicular system in alveolar bone due to orthodontic forces, as detected on multimodal confocal fluorescence imaging analyses. Archives of Oral Biology 60: 45–54. DOI 10.1016/j.archoralbio.2014.08.013. [Google Scholar] [CrossRef]
Okawara H, Arai Y, Matsuno H, Marcián P, Borák L et al. (2021). Effect of load-induced local mechanical strain on peri-implant bone cell activity related to bone resorption and formation in mice: An analysis of histology and strain distributions. Journal of the Mechanical Behavior of Biomedical Materials 116: 104370. DOI 10.1016/j.jmbbm.2021.104370. [Google Scholar] [CrossRef]
Pacureanu A, Langer M, Boller E, Tafforeau P, Peyrin F (2012). Nanoscale imaging of the bone cell network with synchrotron X-ray tomography: Optimization of acquisition setup. Medical Physics 39: 2229–2238. DOI 10.1118/1.3697525. [Google Scholar] [CrossRef]
Palumbo C, Ferretti M (2021). The osteocyte: From Prisoner to Orchestrator. Journal of Functional Morphology and Kinesiology 6: 28. DOI 10.3390/jfmk6010028. [Google Scholar] [CrossRef]
Pei S, Wang S, Martinez JR, Parajuli A, Kirn-Safran CB et al. (2021). Osteocytic Pericellular Matrix (PCMAccelerated degradation under in vivo loading and unloading conditions using a novel imaging approach. Genes 13: 72. DOI 10.3390/genes13010072. [Google Scholar] [CrossRef]
Qin L, Liu W, Cao H, Xiao G (2020). Molecular mechanosensors in osteocytes. Bone Research 8: 1–24. DOI 10.1038/s41413-020-0099-y. [Google Scholar] [CrossRef]
Qing H, Ardeshirpour L, Divieti Pajevic P, Dusevich V, Jähn K et al. (2012). Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. Journal of Bone and Mineral Research 27: 1018–1029. DOI 10.1002/jbmr.1567. [Google Scholar] [CrossRef]
Repp F, Kollmannsberger P, Roschger A, Kerschnitzki M, Berzlanovich A et al. (2017). Spatial heterogeneity in the canalicular density of the osteocyte network in human osteons. Bone Reports 6: 101–108. DOI 10.1016/j.bonr.2017.03.001. [Google Scholar] [CrossRef]
Rho JY, Kuhn-Spearing L, Zioupos P (1998). Mechanical properties and the hierarchical structure of bone. Medical Engineering and Physics 20: 92–102. DOI 10.1016/S1350-4533(98)00007-1. [Google Scholar] [CrossRef]
Robling AG, Bonewald LF (2020). The osteocyte: New insights. Annual Review of Physiology 82: 485–506. DOI 10.1146/annurev-physiol-021119-034332. [Google Scholar] [CrossRef]
Rux CJ, Vahidi G, Darabi A, Cox LM, Heveran CM (2022). Perilacunar bone tissue exhibits sub-micrometer modulus gradation which depends on the recency of osteocyte bone formation in both young adult and early-old-age female C57Bl/6 mice. Bone 157: 116327. DOI 10.1016/j.bone.2022.116327. [Google Scholar] [CrossRef]
Schulte FA, Zwahlen A, Lambers FM, Kuhn G, Ruffoni D et al. (2013). Strain-adaptive in silico modeling of bone adaptation–A computer simulation validated by in vivo micro-computed tomography data. Bone 52: 485–492. DOI 10.1016/j.bone.2012.09.008. [Google Scholar] [CrossRef]
Spatz JM, Wein MN, Gooi JH, Qu Y, Garr JL et al. (2015). The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. Journal of Biological Chemistry 290: 16744–16758. DOI 10.1074/jbc.M114.628313. [Google Scholar] [CrossRef]
Sumner DR (2015). Long-term implant fixation and stress-shielding in total hip replacement. Journal of Biomechanics 48: 797–800. DOI 10.1016/j.jbiomech.2014.12.021. [Google Scholar] [CrossRef]
Tan SD, de Vries TJ, Kuijpers-Jagtman AM, Semeins CM, Everts V, Klein-Nulend J (2007). Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone 41: 745–751. DOI 10.1016/j.bone.2007.07.019. [Google Scholar] [CrossRef]
Thompson WR, Modla S, Grindel BJ, Czymmek KJ, Kirn-Safran CB et al. (2011). Perlecan/Hspg2 deficiency alters the pericellular space of the lacunocanalicular system surrounding osteocytic processes in cortical bone. Journal of Bone and Mineral Research 26: 618–629. DOI 10.1002/jbmr.236. [Google Scholar] [CrossRef]
Turner CH (1998). Three rules for bone adaptation to mechanical stimuli. Bone 23: 399–407. DOI 10.1016/S8756-3282(98)00118-5. [Google Scholar] [CrossRef]
van Rietbergen B, Huiskes R, Eckstein F, Rüegsegger P (2003). Trabecular bone tissue strains in the healthy and osteoporotic human femur. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research 18: 1781–1788. DOI 10.1359/jbmr.2003.18.10.1781. [Google Scholar] [CrossRef]
van Tol AF, Roschger A, Repp F, Chen J, Roschger P et al. (2020a). Network architecture strongly influences the fluid flow pattern through the lacunocanalicular network in human osteons. Biomechanics and Modeling in Mechanobiology 19: 823–840. DOI 10.1007/s10237-019-01250-1. [Google Scholar] [CrossRef]
van Tol AF, Schemenz V, Wagermaier W, Roschger A, Razi H et al. (2020b). The mechanoresponse of bone is closely related to the osteocyte lacunocanalicular network architecture. Proceedings of the National Academy of Sciences of the United States of America 117: 32251–32259. DOI 10.1073/pnas.2011504117. [Google Scholar] [CrossRef]
Varga P, Hesse B, Langer M, Schrof S, Männicke N et al. (2014). Synchrotron X-ray phase nano-tomography-based analysis of the lacunar-canalicular network morphology and its relation to the strains experienced by osteocytes in situ as predicted by case-specific finite element analysis. Biomechanics and Modeling in Mechanobiology 14: 267–282. DOI 10.1007/s10237-014-0601-9. [Google Scholar] [CrossRef]
Vaughan TJ, Verbruggen SW, Mcnamara LM (2013). Are all osteocytes equal? Multiscale modelling of cortical bone to characterise the mechanical stimulation of osteocytes. International Journal for Numerical Methods in Biomedical Engineering 29: 1361–1372. DOI 10.1002/cnm.2578. [Google Scholar] [CrossRef]
Verbruggen SW, Vaughan TJ, McNamara LM (2012). Strain amplification in bone mechanobiology: A computational investigation of the in vivo mechanics of osteocytes. Journal of the Royal Society Interface 9: 2735–2744. DOI 10.1098/rsif.2012.0286. [Google Scholar] [CrossRef]
Wang B, Lai X, Price C, Thompson WR, Li W et al. (2014). Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. Journal of Bone and Mineral Research 29: 878–891. DOI 10.1002/jbmr.2105. [Google Scholar] [CrossRef]
Wang L (2018). Solute transport in the bone Lacunar-Canalicular System (LCS). Current Osteoporosis Reports 16: 32–41. DOI 10.1007/s11914-018-0414-3. [Google Scholar] [CrossRef]
Werner B, Ovesy M, Zysset PK (2019). An explicit micro-FE approach to investigate the post-yield behaviour of trabecular bone under large deformations. International Journal for Numerical Methods in Biomedical Engineering 35: 1–16. DOI 10.1002/cnm.3188. [Google Scholar] [CrossRef]
Wijeratne SS, Martinez JR, Grindel BJ, Frey EW, Li J et al. (2016). Single molecule force measurements of perlecan/HSPG2: A key component of the osteocyte pericellular matrix. Matrix Biology 50: 27–38. DOI 10.1016/j.matbio.2015.11.001. [Google Scholar] [CrossRef]
Yokoyama Y, Kameo Y, Kamioka H, Adachi T (2021). High-resolution image-based simulation reveals membrane strain concentration on osteocyte processes caused by tethering elements. Biomechanics and Modeling in Mechanobiology 20: 2353–2360. DOI 10.1007/s10237-021-01511-y. [Google Scholar] [CrossRef]
You LD, Weinbaum S, Cowin SC, Schaffler MB (2004). Ultrastructure of the osteocyte process and its pericellular matrix. Anatomical Record–Part A Discoveries in Molecular, Cellular, and Evolutionary Biology 278: 505–513. DOI 10.1002/ar.a.20050. [Google Scholar] [CrossRef]
Yu B, Pacureanu A, Olivier C, Cloetens P, Peyrin F (2020). Assessment of the human bone lacuno-canalicular network at the nanoscale and impact of spatial resolution. Scientific Reports 10: 4567. DOI 10.1038/s41598-020-61269-8. [Google Scholar] [CrossRef]
Yu B, Pacureanu A, Olivier C, Cloetens P, Peyrin F (2021). Quantification of the bone lacunocanalicular network from 3D X-ray phase nanotomography images. Journal of Microscopy 282: 30–44. DOI 10.1111/jmi.12973. [Google Scholar] [CrossRef]
Zhang D, Weinbaum S, Cowin SC (1998). Estimates of the peak pressures in bone pore water. Journal of Biomechanical Engineering 120: 697–703. DOI 10.1115/1.2834881. [Google Scholar] [CrossRef]
Zimmermann EA, Ritchie RO (2015). Bone as a structural material. Advanced Healthcare Materials 4: 1287–1304. DOI 10.1002/adhm.201500070. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |