DOI:10.32604/biocell.2022.019937

| BIOCELL DOI:10.32604/biocell.2022.019937 |  |
| Review |
Cancer combination therapy with carnosic acid
1Hacettepe University, Faculty of Pharmacy, Department of Pharmacognosy, Ankara, 06100, Turkey
2Johannes Gutenberg University, Institute of Pharmaceutical and Biomedical Sciences, Department of Pharmaceutical Biology, Mainz, 55128, Germany
*Address correspondence to: Thomas Efferth, efferth@uni-mainz.de
Received: 25 October 2021; Accepted: 24 January 2022
Abstract: Carnosic acid (CA) is a natural phenolic diterpene mainly occurring in some species of the Lamiaceae family. Numerous studies described the cytotoxicity of CA towards different types of cancer both in vitro and in vivo. Particularly, the influence of CA in combination with other drugs, vitamins or natural products through affecting various targets has raised interest. Current experimental in vivo data suggested that CA may cooperate with clinically used anticancer drugs promoting their activity against cancer. From this point of view, CA gained importance, because it may alter pharmacodynamic profiles of various agents in the case of their co-administration, and thereby, act in a potentially synergistic manner, which can provide a basis for potential applications of CA in the management of cancer. In the present review, we give an overview of CA as well as CA co-treatment regimens with a special focus on cancer. In this context, the role of CA as an adjuvant treatment alternative is highlighted.
Keywords: Carnosic acid; Cancer; Combination therapy; Synergism
Carnosic acid (salvin, CA) is a phenolic diterpene with the chemical formula of C20H28O4 (Fig. 1). CA has been identified in some species of the Lamiaceae family, among which Rosmarinus officinalis L. is the most abundant in CA, followed by diverse Salvia species (Birtić et al., 2015). CA is mainly known for its antioxidant and antimicrobial properties and has been used in foods, in vivo and clinical studies as a chemoprotective, an antioxidant or an anti-inflammatory agent (Birtić et al., 2015). Besides, assorted studies have unraveled the potential of CA against carcinogenesis through diverse actions both in vitro and in vivo as of yet (Allegra et al., 2020; Moore et al., 2016). To exemplify, anti-angiogenic (López-Jiménez et al., 2013), anti-invasive (Huang et al., 2005), chemosensitive, chemopreventive and chemoadjuvant roles of CA in various cancer types have been demonstrated (Nabekura et al., 2010; Shanmugam et al., 2010).
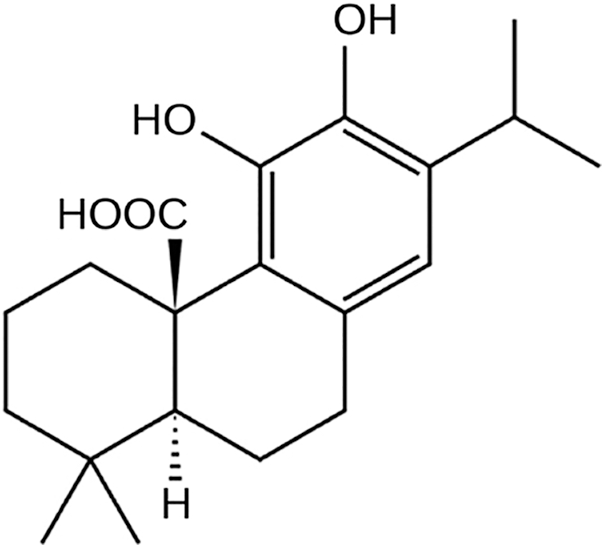
Figure 1: Chemical structure of carnosic acid.
Numerous proteins or signaling pathways are involved in the emergence of CA-related effects in many cancer types. Many in vitro investigations pointed out CA’s mechanism in cancer types from different origins. For instance, it exhibited antiproliferative effect on HT-29 colon cells and induced cell cycle arrest. Altered function of detoxifying enzymes and metabolites as well as modified expression of transport and biosynthesis genes were attributed as mechanisms behind its antiproliferative capacity (Valdés et al., 2014). In another example, CA suppressed proliferation of breast cancer cells and improved apoptosis in vitro (Yesil-Celiktas et al., 2010; Einbond et al., 2012; Han et al., 2017). Ngo et al. (2011) reported numerous preclinical findings on the relevance of CA for cancer prevention, indicating in vitro and in vivo antitumor effects addressing different molecular targets (Ngo et al., 2011). CA significantly induced tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human renal carcinoma (Caki, ACHN, and A498), human hepatocellular carcinoma (SK-HEP-1), and human breast carcinoma (MDA-MB-231) cells. Besides, it boosted sensitization against TRAIL-linked apoptosis through downregulating c-FLIP and Bcl-2 expression and upregulating ER stress-mediated DR5, Bim, and PUMA expression at the transcriptional level in human carcinoma Caki cells (Jung et al., 2015).
We consider that if CA would ever be used clinically to treat cancer patients, then it would not be used in monotherapy but with all probability as part of combination therapy regimens. Therefore, the question raises, how CA may perform in combination with other drugs. If combined with anticancer drugs, vitamins or natural products, CA indeed exhibited synergistic activity affecting their pharmacodynamic profiles (Fig. 2) (Einbond et al., 2012; Han et al., 2017; Zhang et al., 2019; Ayaz et al., 2019). Therefore, in the present mini-review, we give an overview of the literature deposited in the PubMed database as of May 2021 with a special interest to CA combination therapy. Still, we also mentioned the anticancer potential of CA alone, in order to draw a general concept of the anti-cancer potential of CA and relevant mechanism for a better explanation of CA- associated cytotoxicity in the case of cancer combination therapy.
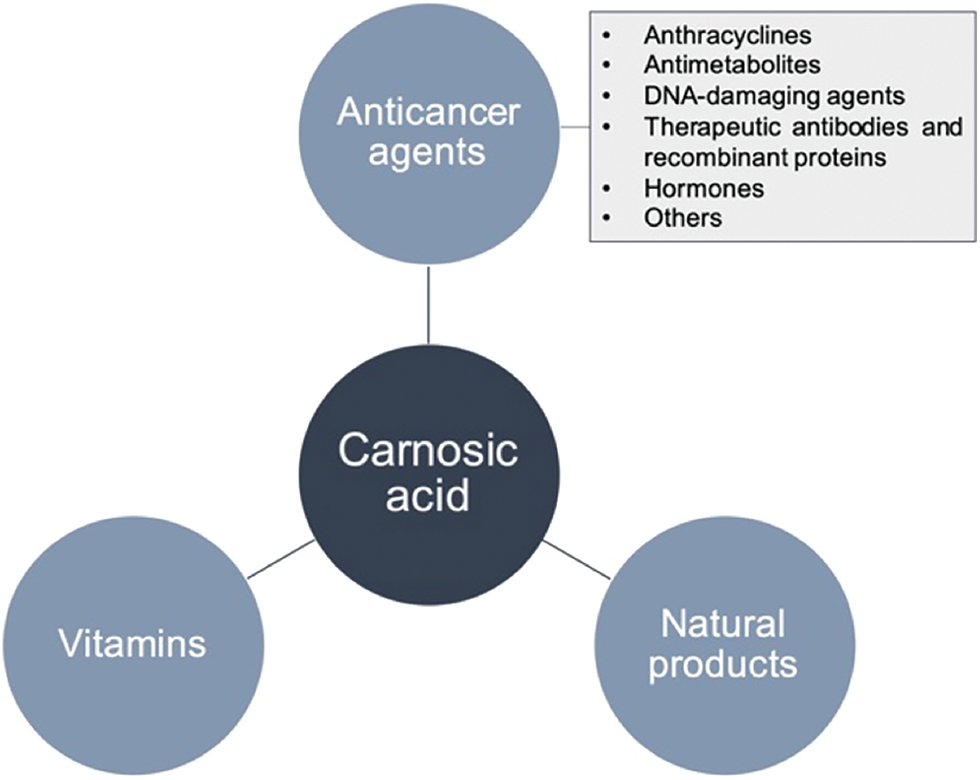
Figure 2: Schematic representation of the potential agents with a capability of synergism when combined with carnosic acid.
Anticancer potential and modes of action of carnosic acid monotherapy
The effect of carnosic acid (CA) against various types of cancer has been studied and unraveled in assorted preclinical models, which may provide a basis for forthcoming clinical studies. For instance, recent investigations conducted by El-Huneidi et al. (2021) unraveled that CA repressed proliferation and resulted in apoptosis in human gastric cancer cell lines by influencing Akt/mTOR pathway (El-Huneidi et al., 2021). Evaluating the cytotoxicity of CA against a number of known mechanisms of anticancer drug resistance, Mahmoud et al. (2020) discovered the capability of CA to induce apoptosis in leukemia cells in addition to the induction of genes as molecular determinants of classical drug resistance (Mahmoud et al., 2020). Likewise, the anticancer potential of CA on chronic myeloid leukemia cells was investigated by Liu et al. (2018a), who observed the induction of apoptosis and cell cycle arrest (Liu et al., 2018a).
Jiang et al. (2021) observed that CA suppressed cell proliferation, apoptosis, migration, and colony formation of esophageal squamous cell carcinoma in a dose-dependent manner. Cell cycle arrest at the G2/M phase, apoptosis, DNA damage, and MAPK signaling inhibition were detected as associated mechanisms behind its cytotoxicity (Jiang et al., 2021).
In another study conducted by Kar et al. (2012), CA inhibited the proliferation of androgen-independent human prostate cancer PC-3 cells via apoptosis through activating serine/threonine protein phosphatase 2A resulting from the AKT/IKK/NF-κB pathway. CA further induced apoptosis in androgen refractory prostate cancer DU145 cells via the activation of caspase-3, enhancement of Bax:Bcl-2 ratio, and cytochrome-c release (Kar et al., 2012).
Lin et al. (2018) demonstrated the anticancer potential of CA in melanoma in vitro and in vivo. CA significantly inhibited the growth of B16F10 melanoma cells and induced cell cycle arrest in addition to its enhancing effect on carmustine- and lomustine-mediated cytotoxicity in the B16F10 tumor model in vivo (Lin et al., 2018).
In vivo evidence of the anticancer property of CA further exists in the literature. Petiwala et al. (2016) unraveled that CA caused the degradation of androgen receptors and enhanced the expression of endoplasmic reticulum proteins such as CHOP in human prostate cancer cells in vitro, which likely contributed to its in vivo antiproliferative effect on prostate cancer xenograft tumors, all proving the anticancer property of CA against prostate cancer (Petiwala et al., 2016).
Although the anticancer potential and modes of action of CA have been intensively studied in assorted cancer types, the focus of many studies has been more on the combinatorial application of CA rather than monotherapy.
Synergistic or additive cytotoxic effects in combination with anticancer drugs
Additive cytotoxic properties have been described for CA-anthracycline combinations. A remarkably reduced number of leukemia cells and a higher percentage of apoptotic cells were observed in a mouse model of acute myeloid leukemia (AML) upon co-administration of CA and doxorubicin compared to mice treated with doxorubicin alone. Thus, the potential of CA as a promising adjuvant anti-cancer drug was emphasized (Wang et al., 2015). Another study investigated daunorubicin accumulation in the presence of various rosemary phytochemicals including CA in a P-glycoprotein (P-gp)-overexpressing multidrug-resistant human KB-C2 cervical carcinoma cell line. CA enhanced the cellular accumulation of daunorubicin in a concentration-dependent manner through inhibiting P-gp-mediated efflux of daunorubicin, improving the efficacy of chemotherapy regimens (Nabekura et al., 2010; Khan et al., 2015). Similarly, CA alleviated the 50% inhibition concentration (IC50) of doxorubicin by enhancing its cellular concentration and, thereby, increased the sensitivity of K562/AO2 cells through downregulation of MDR1 and inhibition of P-gp-mediated drug efflux (Yu et al., 2008).
Cytarabine or cytosine arabinoside (1-β-D-arabinofuranosylcytosine) is a pyrimidine nucleoside analog substantially used for myeloid or lymphoid leukemias as well as Hodgkin or Non-Hodgkin lymphomas (di Francia et al., 2021). Doxercalciferol (1-D2) in combination with CA boosted the therapeutic activity of cytarabine in patient-derived AML blasts and triggered apoptotic signals in cells sustaining from DNA injury upon cytarabine treatment. This was caused by activation of the vitamin D-upregulated protein TXNIP (Wang et al., 2019). Likewise, cytarabine-induced cell death in HL-60 and U937 AML cells was remarkably enhanced by the addition of 1α-hydroxyvitamin D2 and CA to cytarabine in comparison to cytarabine monotherapy. The enhanced cell death was induced by apoptosis and necrosis and caused by increased DNA injury with higher levels of the DNA damage response activated marker Chk1 (Wang et al., 2016). Cytarabine in combination with 1-D2 and CA exhibited selective and enhanced cell death in malignant AML blasts, which was linked to the activation of the monocytic differentiation program as well as the elevated expression levels of the vitamin D receptor. Besides, caspase-dependent apoptosis with enhanced expression of the apoptosis modulator Bim was displayed as the background mechanism in association with this combination regimen (Harrison et al., 2016).
A number of studies have proved the accelerative effect of CA in combination with DNA-damaging agents against cancer. If combined with cisplatin, CA exhibited greater anti-growth and pro-apoptotic effects by discouraging myeloid-derived suppressor cells in Lewis lung cancer xenografts with fewer side effects than those of cisplatin (Liu et al., 2018b). Shao et al. (2019) established the cellular mechanisms of CA in combination with the alkylating agent temozolomide (TMZ). The cytotoxicity of TMZ was enhanced in glioma cells in addition to the increment in TMZ-associated suppression of colony formation and cell migration as well as TMZ-associated cell cycle arrest and cellular apoptosis. Furthermore, autophagy was induced upon their co-administration. Cyclin B1 inhibition and activation of poly (ADP-ribose) polymerase and caspase-3 were attributed as mechanisms underlying TMZ-induced cell cycle arrest and cellular apoptosis, while TMZ-induced cellular autophagy was encouraged by CA by the inhibition of p-AKT, the downregulation of p62, and the transition of LC3-I to LC3-II (Shao et al., 2019).
Therapeutic antibodies and recombinant proteins
The humanized monoclonal antibody trastuzumab cooperatively inhibited migration of oncoprotein ERBB2-positive breast cancer cells and induced cell cycle arrest in G0/G1 if combined with CA. Furthermore, CA reversibly improved trastuzumab-associated inhibition of cell survival. These effects were linked to the downregulation of ERBB2, the upregulation of both CDKN1A/p21 WAF1 and CDKN1B/p27 KIP1, and the modulation of the PI3K/AKT/mTOR signaling pathway. Besides, CA partially enhanced sensitivity towards trastuzumab in SKBR-3-trastuzumab-resistant cells (D’Alesio et al., 2017).
CA induced apoptosis in breast cancer cells in vitro by the caspase-3 signaling pathway/TRAIL activation if administered with the selective estrogen receptor modulator tamoxifen. Besides, the cooperation of CA and tamoxifen displayed a better inhibition of breast cancer growth in a mouse xenograft model in vivo in comparison to the treatments of CA or tamoxifen alone (Han et al., 2017). In combination with the corticosteroid dexamethasone, CA exerted better cytotoxicity levels than that of CA alone towards pulmonary adenocarcinoma (Coyne and Narayanan, 2019).
CA reversed P-gp-linked multi-drug resistance and sensitized KB-C2 cells to vinblastine (Nabekura et al., 2010). CA and arsenic trioxide co-treatment possessed a greater rate of apoptosis in a mice model of AML, which was attributed to the modulated expression levels of cleaved caspase-3, PTEN, p27 gene mRNA, and proteins compared with CA or arsenic trioxide monotherapy. Besides, the longest survival time was observed in mice receiving CA and arsenic trioxide combination therapy, indicating the synergistic potency against leukemia (Li et al., 2016). Together with arsenic trioxide, CA promoted the cytotoxic activity of arsenic trioxide and caused G1 arrest and apoptosis in HL-60 leukemia cells by modulation of PTEN/AKT signaling pathway and displaying synergistic effects (Wang et al., 2012b). CA activated nuclear transcription factor E2-related factor 2 (NRF2) in AML, alleviated the cytotoxic effects of arsenic trioxide through stimulation of the glutathione biosynthetic pathway and, thereby, accelerated arsenic excretion from the cells (Nishimoto et al., 2016). CA stimulated the expression of NAD(P)H quinone oxidoreductase (NQO1) and markedly augmented the cytotoxicity of β-lapachone (β-lap) in all of the tested melanoma cell lines through stabilization of NRF2. This can be taken as a hint that CA enhances the clinical response to NQO1-linked antitumor drugs (Arakawa et al., 2018).
Synergistic or additive cytotoxic effects in combination with vitamins
CA combined with the physiologically active form of vitamin D3, 1,25-dihydroxyvitamin D3 (1,25D), potentiated differentiation of HL-60 myeloid leukemia cells and exerted anticancer effects by attenuating the cellular reactive oxygen species content and advancing glutathione levels (Danilenko et al., 2003; Danilenko and Studzinski, 2004). The potentiated pro-differentiation effect of 1,25D was associated with an activation of the JNK pathway as well as increased expression of early growth response-1 (EGR-1) and c-FOS (Wang et al., 2005b). Similarly, 1,25D and CA combination treatment activated the NRF2/antioxidant response element pathway and acted cooperatively to differentiate myeloid leukemia cells (Bobilev et al., 2011).
CA and CA-rich ethanolic extracts of rosemary leaves markedly augmented the in vitro differentiating and antiproliferative effects of 1,25D and its low-calcemic analog, 1,25-dihydroxy-16-ene-5,6-trans-cholecalciferol (Ro25-4020) in WEHI-3BD- murine myelomonocytic leukemia cells by cell cycle arrest in the G0/G1 phase and pronounced cell growth inhibition. Furthermore, the combined administration of rosemary extract and Ro25-4020 strongly exhibited cooperative antitumor effects without causing hypercalcemia (Sharabani et al., 2006). In another study, 1,25D in combination with CA and the p38 MAPK inhibitor SB202190 boosted the differentiation of OCI-AML3 (p53 wild type) and HL60 (p53 null) AML cells by enhancing the inhibitory phosphorylation levels of MEK-1. On the other hand, this combination treatment decreased the levels of activated ERK1/2 (Thompson et al., 2010). 1α,25-Dihydroxyvitamin D2, and its analogs PRI-1916 and PRI-1917 cooperated with CA to induce cell differentiation and G1/S cell cycle inhibition (Nachliely et al., 2016a). Likewise, the in vitro differentiation of HL-60 and freshly obtained leukemic cells induced by 1,25D analogs was enhanced by 1,25D-CA co-treatment ex vivo through the JNK pathway (Wang et al., 2005a). Hematopoietic progenitor kinase 1 (HPK1) holds a dual role as (1) positive regulator of 1,25D-linked differentiation and cell cycle arrest of AML cells and as (2) mediator of vitamin D resistance. A combination of CA and a selective inhibitor of the α and β isoforms of p38 MAPK (SB202910) increased the sensitization of 40AF cells to 1,25D through increasing HPK1 protein levels if simultaneously added to 1,25D (Chen-Deutsch and Studzinski, 2012). In another study, an analog of 1,25D, 1-D2, exhibited antitumor activity with significantly reduced calcemic effects compared with those of 1,25D. In comparison to 1-D2 monotherapy, the combination of CA and 1-D2 enhanced monocytic differentiation of HL-60 and U937 cells and induced cell cycle arrest, which was attributed to the modulated expression of microRNA181a (Duggal et al., 2012). If administered with the novel double-point modified analogs of 1,25-dihydroxyvitamin D2 (PRI-5201 and PRI-5202), CA augmented the antileukemic effects with upregulated vitamin D receptor protein levels, which was probably linked to increased activation of differentiation-associated vitamin D response elements (Nachliely et al., 2016b). Administration of CA together with 1,25D and a kinase inhibitor SB202190 jointly enhanced the intensity of differentiation through upregulating activated JNK1p46 and the transcription factors modulated by the JNK pathway, c-JUN, JUNB, ATF2 as well as C/EBP β (Chen-Deutsch et al., 2009).
Synergistic or additive cytotoxic effects in combination with natural products
Many studies confirmed the combinatorial use of CA with natural products as exemplified below.
Co-treatment of CA and the flavonoid fisetin exerted cytotoxicity towards lung cancer in vitro and in vivo. The combination therapy of CA and fisetin suppressed lung cancer growth even more in comparison to CA and fisetin monotherapy through caspase-3-dependent apoptosis (Shi et al., 2017). If combined with curcumin, CA exhibited potentialized growth inhibitory effects compared to CA alone in MDA-MB-468 triple-negative breast cancer cells, indicating varying synergy rates ranging from slight to strong based on their concentration through inhibiting Na-K-ATPase activity (Einbond et al., 2012). Likewise, curcumin-CA co-treatment each combined at low, non-cytotoxic doses, synergistically induced apoptosis through disrupting cellular Ca2+ homeostasis in AML cells but not in non-neoplastic hematopoietic cells, proving its cancer-selective action. Even more, co-administration of curcumin and CA significantly alleviated disease progression in mice suffering from AML, suggesting a model for a new Ca2+-targeted pharmacological approach (Pesakhov et al., 2016). Another study by Pesakhov et al. (2010) described the synergistic antiproliferative effect and apoptosis induction by curcumin-CA combination treatment in HL-60 and KG-1a human AML cells at non-cytotoxic concentrations of each agent. The caspases-8, -9, and -3 and the proapoptotic protein Bid were activated in curcumin-CA-induced apoptosis (Pesakhov et al., 2010). If rats with bleomycin-induced lung fibrosis were treated with a combination of rosemary leaf extracts (RLE) abundant in CA or RLE abundant in rosmarinic acid following industrial elimination of essential oils, an enhanced antifibrotic effect was observed compared with RLE abundant in CA or RLE abundant in rosmarinic acid monotherapy (Bahri et al., 2021).
The use of CA in food, in vivo, and clinical studies has been sufficiently documented so far. The function of CA towards assorted conditions was diverse ranging from chemoprotective to anti-inflammatory (Birtić et al., 2015; Gomez-Garcia et al., 2013; Park and Mun, 2013). Despite increased utilization of CA in food, nutritional health, and cosmetic industries, it may exhibit a side effect profile. For instance, high-dose CA was proved to be relatively safe due to the fact that short-term oral administration exhibited a low toxicity profile in different organs of rats (Wang et al., 2012a). Evaluated its potential harm in primary human hepatocytes and microsomes, a dose-dependent enhancement in hepatotoxicity and increased CYP3A enzyme activity in comparison to rifampicin were observed (Dickmann et al., 2012). On the other hand, Lin et al. (2018) unraveled that CA alleviated the values of aspartate aminotransferase and alanine aminotransferase in vivo, pointing out the safety of CA as a likely chemotherapeutic agent. Still, to stay on the safe side, CA needs a safety assessment prior to its planned use.
Nature is a unique source of new chemical entities and provides selective and effective drug leads with fewer side effects. For instance, 83% of approved drugs between 1981 to 2004 in the field of anticancer drugs were natural products, natural product derivatives, and natural product mimics (Newman and Cragg, 2016). In this context, natural compounds hold vital importance with improved pharmacological features in terms of the discovery of new drug leads and the design of prospective (semi)synthetic derivatives. CA certainly deserves further preclinical validation to move to the clinics, specifically in the field of oncology.
Numerous investigations demonstrated the beneficial effects of co-treatment regimens of CA together with clinically established anticancer drugs, vitamins, hormones, or natural compounds. Preclinical studies pointed to the synergistic (or at least additive) potency of combined applications of CA as its probable future applications. Therefore, CA may represent a useful alternative (1) to improve cytotoxic treatment with classical anticancer drugs, (2) for differentiation therapy with vitamin D derivatives, (3) for phytotherapy, and (4) for reducing side effects of classical anticancer drugs. Clinical trials are warranted confirming its potential use as an adjuvant and efficient agent in cancer therapy.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: N. Özenver, T. Efferth; data collection: N. Özenver, T. Efferth; analysis and interpretation of results: N. Özenver; draft manuscript preparation: N. Özenver. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Allegra A, Tonacci A, Pioggia G, Musolino C, Gangemi S (2020). Anticancer activity of Rosmarinus officinalis L.: Mechanisms of action and therapeutic potentials. Nutrients 12: 1739. DOI 10.3390/nu12061739. [Google Scholar] [CrossRef]
Arakawa N, Okubo A, Yasuhira S, Takahashi K, Amano H et al. (2018). Carnosic acid, an inducer of NAD(P)H quinone oxidoreductase 1, enhances the cytotoxicity of β-lapachone in melanoma cell lines. Oncology Letters 15: 2393–2400. [Google Scholar]
Ayaz M, Ullah F, Sadiq A, Ullah F, Ovais M et al. (2019). Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chemico-Biological Interactions 308: 294–303. DOI 10.1016/j.cbi.2019.05.050. [Google Scholar] [CrossRef]
Bahri S, Ali RB, Abdennabi R, Nahdi A, Mlika M et al. (2021). Industrial elimination of essential oils from Rosmarinus officinalis: In support of the synergic antifibrotic effect of rosmarinic and carnosic acids in bleomycin model of lung fibrosis. Nutrition and Cancer 73: 2376–2387. [Google Scholar]
Birtić S, Dussort P, Pierre FX, Bily AC, Roller M (2015). Carnosic acid. Phytochemistry 115: 9–19. DOI 10.1016/j.phytochem.2014.12.026. [Google Scholar] [CrossRef]
Bobilev I, Novik V, Levi I, Shpilberg O, Levy J et al. (2011). The Nrf2 transcription factor is a positive regulator of myeloid differentiation of acute myeloid leukemia cells. Cancer Biology & Therapy 11: 317–329. DOI 10.4161/cbt.11.3.14098. [Google Scholar] [CrossRef]
Chen-Deutsch X, Garay E, Zhang J, Harrison JS, Studzinski GP (2009). c-Jun N-terminal kinase 2 (JNK2) antagonizes the signaling of differentiation by JNK1 in human myeloid leukemia cells resistant to vitamin D. Leukemia Research 33: 1372–1378. DOI 10.1016/j.leukres.2009.03.003. [Google Scholar] [CrossRef]
Chen-Deutsch X, Studzinski GP (2012). Dual role of hematopoietic progenitor kinase 1 (HPK1) as a positive regulator of 1α,25-dihydroxyvitamin D-induced differentiation and cell cycle arrest of AML cells and as a mediator of vitamin D resistance. Cell Cycle 11: 1364–1373. DOI 10.4161/cc.19765. [Google Scholar] [CrossRef]
Coyne CP, Narayanan L (2019). Carnosic acid, tangeretin, and ginkgolide-B anti-neoplastic cytotoxicity in dual combination with dexamethasone-[anti-EGFR] in pulmonary adenocarcinoma (a549). anticancer agents. Medicinal Chemistry 19: 802–819. [Google Scholar]
D’Alesio C, Bellese G, Gagliani MC, Aiello C, Grasselli E et al. (2017). Cooperative antitumor activities of carnosic acid and trastuzumab in ERBB2(+) breast cancer cells. Journal of Experimental Clinical Cancer Research 36: 154. DOI 10.1186/s13046-017-0615-0. [Google Scholar] [CrossRef]
Danilenko M, Studzinski GP (2004). Enhancement by other compounds of the anti-cancer activity of vitamin D3 and its analogs. Experimental Cell Research 298: 339–358. DOI 10.1016/j.yexcr.2004.04.029. [Google Scholar] [CrossRef]
Danilenko M, Wang Q, Wang X, Levy J, Sharoni Y et al. (2003). Carnosic acid potentiates the antioxidant and prodifferentiation effects of 1alpha,25-dihydroxyvitamin D3 in leukemia cells but does not promote elevation of basal levels of intracellular calcium. Cancer Research 63: 1325–1332. [Google Scholar]
Dickmann LJ, VandenBrink BM, Lin YS (2012). In vitro hepatotoxicity and cytochrome P450 induction and inhibition characteristics of carnosic acid, a dietary supplement with antiadipogenic properties. Drug Metabolism and Disposition 40: 1263–1267. [Google Scholar]
di Francia R, Crisci S, de Monaco A, Cafiero C, Re A et al. (2021). Response and toxicity to cytarabine therapy in leukemia and lymphoma: From dose puzzle to pharmacogenomic biomarkers. Cancers 13: 966. [Google Scholar]
Duggal J, Harrison JS, Studzinski GP, Wang X (2012). Involvement of microRNA181a in differentiation and cell cycle arrest induced by a plant-derived antioxidant carnosic acid and vitamin D analog doxercalciferol in human leukemia cells. MicroRNA 1: 26–33. [Google Scholar]
Einbond LS, Wu HA, Kashiwazaki R, He K, Roller M et al. (2012). Carnosic acid inhibits the growth of ER-negative human breast cancer cells and synergizes with curcumin. Fitoterapia 83: 1160–1168. [Google Scholar]
El-Huneidi W, Bajbouj K, Muhammad JS, Vinod A, Shafarin J et al. (2021). Carnosic acid induces apoptosis and inhibits Akt/mTOR signaling in human gastric cancer cell lines. Pharmaceuticals 14: 230. [Google Scholar]
Gomez-Garcia FJ, Lopez-Jomet MP, Alvarez-Sanchez N, Castillo-Sanchez J, Benavente-Garcia O et al. (2013). Effect of the phenolic compounds apigenin and carnosic acid on oral carcinogenesis in hamster induced by DMBA. Oral Diseases 19: 279–286. [Google Scholar]
Han NN, Zhou Q, Huang Q, Liu KJ (2017). Carnosic acid cooperates with tamoxifen to induce apoptosis associated with Caspase-3 activation in breast cancer cells in vitro and in vivo. Biomedicine & Pharmacotherapy 89: 827–837. [Google Scholar]
Harrison JS, Wang X, Studzinski GP (2016). The role of VDR and BIM in potentiation of cytarabine-induced cell death in human AML blasts. Oncotarget 7: 36447–36460. [Google Scholar]
Huang SC, Ho CT, Lin-Shiau SY, Lin JK (2005). Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochemical Pharmacology 69: 221–232. DOI 10.1016/j.bcp.2004.09.019. [Google Scholar] [CrossRef]
Jiang S, Qiu Y, Wang Z, Ji Y, Zhang X et al. (2021). Carnosic acid induces antiproliferation and anti-metastatic property of esophageal cancer cells via MAPK signaling pathways. Journal of Oncology 4451533: 1–11. DOI 10.1155/2021/4451533. [Google Scholar] [CrossRef]
Jung KJ, Min KJ, Bae JH, Kwon TK (2015). Carnosic acid sensitized TRAIL-mediated apoptosis through down-regulation of c-FLIP and Bcl-2 expression at the post translational levels and CHOP-dependent up-regulation of DR5, Bim, and PUMA expression in human carcinoma Caki cells. Oncotarget 6: 1556–1568. DOI 10.18632/oncotarget.2727. [Google Scholar] [CrossRef]
Kar S, Palit S, Ball WB, Das PK (2012). Carnosic acid modulates Akt/IKK/NF-κB signaling by PP2A and induces intrinsic and extrinsic pathway mediated apoptosis in human prostate carcinoma PC-3 cells. Apoptosis 17: 735–747. DOI 10.1007/s10495-012-0715-4. [Google Scholar] [CrossRef]
Khan M, Maryam A, Mehmood T, Zhang Y, Ma T (2015). Enhancing activity of anticancer drugs in multidrug resistant tumors by modulating P-glycoprotein through dietary nutraceuticals. Asian Pacific Journal of Cancer Prevention 16: 6831–6839. DOI 10.7314/APJCP.2015.16.16.6831. [Google Scholar] [CrossRef]
Li H, Wang R, Li XX, Wang LQ, Yu XN (2016). Carnosic acid-combined arsenic trioxide antileukaemia cells in the establishment of NB4/SCID mouse model. Basic & Clinical Pharmacology & Toxicology 119: 259–266. [Google Scholar]
Liu D, Wang B, Zhu Y, Yan F, Dong W (2018a). Carnosic acid regulates cell proliferation and invasion in chronic myeloid leukemia cancer cells via suppressing microRNA-708. Official Journal of the Balkan Union of Oncology 23: 741–746. [Google Scholar]
Liu W, Wu TC, Hong DM, Hu Y, Fan T et al. (2018b). Carnosic acid enhances the anti-lung cancer effect of cisplatin by inhibiting myeloid-derived suppressor cells. Chinese Journal of Natural Medicines 16: 907–915. DOI 10.1016/S1875-5364(18)30132-8. [Google Scholar] [CrossRef]
Lin KI, Lin CC, Kuo SM, Lai JC, Wang YQ et al. (2018). Carnosic acid impedes cell growth and enhances anticancer effects of carmustine and lomustine in melanoma. Bioscience Reports 38: BSR20180005. DOI 10.1042/BSR20180005. [Google Scholar] [CrossRef]
López-Jiménez A, García-Caballero M, Medina MÁ, Quesada AR (2013). Anti-angiogenic properties of carnosol and carnosic acid, two major dietary compounds from rosemary. European Journal of Nutrition 52: 85–95. DOI 10.1007/s00394-011-0289-x. [Google Scholar] [CrossRef]
Mahmoud N, Saeed EM, Sugimoto Y, Klinger A, Fleischer E et al. (2020). Putative molecular determinants mediating sensitivity or resistance towards carnosic acid tumor cell responses. Phytomedicine 77: 153271. DOI 10.1016/j.phymed.2020.153271. [Google Scholar] [CrossRef]
Moore J, Yousef M, Tsiani E (2016). Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols. Nutrients 8: 731. DOI 10.3390/nu8110731. [Google Scholar] [CrossRef]
Nabekura T, Yamaki T, Hiroi T, Ueno K, Kitagawa S (2010). Inhibition of anticancer drug efflux transporter P-glycoprotein by rosemary phytochemicals. Pharmacological Research 61: 259–263. DOI 10.1016/j.phrs.2009.11.010. [Google Scholar] [CrossRef]
Nachliely M, Sharony E, Bolla NR, Kutner A, Danilenko M (2016a). Prodifferentiation activity of novel vitamin D2 analogs PRI-1916 and PRI-1917 and their combinations with a plant polyphenol in acute myeloid leukemia cells. International Journal of Molecular Sciences 17: 1068. DOI 10.3390/ijms17071068. [Google Scholar] [CrossRef]
Nachliely M, Sharony E, Kutner A, Danilenko M (2016b). Novel analogs of 1,25-dihydroxyvitamin D2 combined with a plant polyphenol as highly efficient inducers of differentiation in human acute myeloid leukemia cells. Journal of Steroid Biochemistry and Molecular Biology 164: 59–65. DOI 10.1016/j.jsbmb.2015.09.014. [Google Scholar] [CrossRef]
Newman DJ, Cragg GM (2016). Natural products as sources of new drugs from 1981 to 2014. Journal of Natural Products 79: 629–661. DOI 10.1021/acs.jnatprod.5b01055. [Google Scholar] [CrossRef]
Ngo SN, Williams DB, Head RJ (2011). Rosemary and cancer prevention: Preclinical perspectives. Critical Reviews in Food Science and Nutrition 51: 946–954. DOI 10.1080/10408398.2010.490883. [Google Scholar] [CrossRef]
Nishimoto S, Suzuki T, Koike S, Yuan B, Takagi N et al. (2016). Nrf2 activation ameliorates cytotoxic effects of arsenic trioxide in acute promyelocytic leukemia cells through increased glutathione levels and arsenic efflux from cells. Toxicology and Applied Pharmacology 305: 161–168. DOI 10.1016/j.taap.2016.06.017. [Google Scholar] [CrossRef]
Park MY, Mun ST (2013). Dietary carnosic acid suppresses hepatic steatosis formation via regulation of hepatic fatty acid metabolism in high-fat diet-fed mice. Nutrition Research and Practise 7: 294–301. DOI 10.4162/nrp.2013.7.4.294. [Google Scholar] [CrossRef]
Pesakhov S, Khanin M, Studzinski GP, Danilenko M (2010). Distinct combinatorial effects of the plant polyphenols curcumin, carnosic acid, and silibinin on proliferation and apoptosis in acute myeloid leukemia cells. Nutrition and Cancer 62: 811–824. DOI 10.1080/01635581003693082. [Google Scholar] [CrossRef]
Pesakhov S, Nachliely M, Barvish Z, Aqaqe N, Schwartzman B et al. (2016). Cancer-selective cytotoxic Ca2+ overload in acute myeloid leukemia cells and attenuation of disease progression in mice by synergistically acting polyphenols curcumin and carnosic acid. Oncotarget 7: 31847–31861. DOI 10.18632/oncotarget.7240. [Google Scholar] [CrossRef]
Petiwala SW, Li G, Bosland MC, Lantvit DD, Petukhov PA et al. (2016). Carnosic acid promotes degradation of the androgen receptor and is regulated by the unfolded protein response pathway in vitro and in vivo. Carcinogenesis 37: 827–838. [Google Scholar]
Shanmugam M, Vasanthaselvan M, Silvan S, Baskaran N, Singh A et al. (2010). Carnosic acid: A potent chemopreventive agent against oral carcinogenesis. Chemico-Biological Interactions 188: 616–622. [Google Scholar]
Shao N, Mao J, Xue L, Wang R, Zhi F et al. (2019). Carnosic acid potentiates the anticancer effect of temozolomide by inducing apoptosis and autophagy in glioma. Journal of Neuro-Oncology 141: 277–288. [Google Scholar]
Sharabani H, Izumchenko E, Wang Q, Kreinin R, Steiner M et al. (2006). Cooperative antitumor effects of vitamin D3 derivatives and rosemary preparations in a mouse model of myeloid leukemia. International Journal of Cancer 118: 3012–3021. [Google Scholar]
Shi B, Wang LF, Meng WS, Chen L, Meng ZL (2017). Carnosic acid and fisetin combination therapy enhances inhibition of lung cancer through apoptosis induction. International Journal of Oncology 50: 2123–2135. [Google Scholar]
Thompson T, Danilenko M, Vassilev L, Studzinski GP (2010). Tumor suppressor p53 status does not determine the differentiation-associated G1 cell cycle arrest induced in leukemia cells by 1,25-dihydroxyvitamin D3 and antioxidants. Cancer Biology Therapy 10: 344–350. DOI 10.4161/cbt.10.4.12366. [Google Scholar] [CrossRef]
Valdés A, García-Cañas V, Simó C, Ibáñez C, Micol V et al. (2014). Comprehensive foodomics study on the mechanisms operating at various molecular levels in cancer cells in response to individual rosemary polyphenols. Analytical Chemistry 86: 9807–9815. DOI 10.1021/ac502401j. [Google Scholar] [CrossRef]
Wang LQ, Wang R, Li XX, Yu XN, Chen XL et al. (2015). The anti-leukemic effect of carnosic acid combined with adriamycin in a K562/A02/SCID leukemia mouse model. International Journal of Clinical and Experimental Medicine 8: 11708–11717. [Google Scholar]
Wang Q, Harrison JS, Uskokovic M, Kutner A, Studzinski GP (2005a). Translational study of vitamin D differentiation therapy of myeloid leukemia: Effects of the combination with a p38 MAPK inhibitor and an antioxidant. Leukemia 19: 1812–1817. DOI 10.1038/sj.leu.2403916. [Google Scholar] [CrossRef]
Wang Q, Salman H, Danilenko M, Studzinski GP (2005b). Cooperation between antioxidants and 1,25-dihydroxyvitamin D3 in induction of leukemia HL60 cell differentiation through the JNK/AP-1/Egr-1 pathway. Journal of Cellular Physiology 204: 964–974. DOI 10.1002/(ISSN)1097-4652. [Google Scholar] [CrossRef]
Wang QL, Li H, Li XX, Cui CY, Wang R et al. (2012a). Acute and 30-day oral toxicity studies of administered carnosic acid. Food and Chemical Toxicology 50: 4348–4355. DOI 10.1016/j.fct.2012.08.057. [Google Scholar] [CrossRef]
Wang R, Cong WH, Guo G, Li XX, Chen XL et al. (2012b). Synergism between carnosic acid and arsenic trioxide on induction of acute myeloid leukemia cell apoptosis is associated with modulation of PTEN/Akt signaling pathway. Chinese Journal of Integrative Medicine 18: 934–941. DOI 10.1007/s11655-012-1297-z. [Google Scholar] [CrossRef]
Wang X, Harrison JS, Studzinski GP (2016). Enhancement of arabinocytosine (AraC) toxicity to AML cells by a differentiation agent combination. Journal of Steroid Biochemistry and Molecular Biology 164: 72–78. DOI 10.1016/j.jsbmb.2015.08.023. [Google Scholar] [CrossRef]
Wang X, Nachliely M, Harrison JS, Danilenko M, Studzinski GP (2019). Participation of vitamin D-upregulated protein 1 (TXNIP)-ASK1-JNK1 signalosome in the enhancement of AML cell death by a post-cytotoxic differentiation regimen. Journal of Steroid Biochemistry and Molecular Biology 187: 166–173. DOI 10.1016/j.jsbmb.2018.11.015. [Google Scholar] [CrossRef]
Yesil-Celiktas O, Sevimli C, Bedir E, Vardar-Sukan F (2010). Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods for Human Nutrition 65: 158–163. DOI 10.1007/s11130-010-0166-4. [Google Scholar] [CrossRef]
Yu XN, Chen XL, Li H, Li XX, Li HQ et al. (2008). Reversion of P-glycoprotein-mediated multidrug resistance in human leukemic cell line by carnosic acid. Chinese Journal of Physiology 51: 348–356. [Google Scholar]
Zhang QL, Yang JJ, Zhang HS (2019). Carvedilol (CAR) combined with carnosic acid (CAA) attenuates doxorubicin-induced cardiotoxicity by suppressing excessive oxidative stress, inflammation, apoptosis and autophagy. Biomedicine & Pharmacotherapy 109: 71–83. DOI 10.1016/j.biopha.2018.07.037. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |