DOI:10.32604/biocell.2022.021059

| BIOCELL DOI:10.32604/biocell.2022.021059 |  |
| Review |
Identification of SARS-CoV-2 by gold nanoparticles
1Department of Pharmaceutical Sciences, University of Milan, Milano, 20133, Italy
2National Institute of Molecular Genetics (INGM), Milano, 20122, Italy
3Institute for Bioengineering of Catalonia (IBEC), The Barcelona Institute of Science and Technology, Barcelona, 08028, Spain
4Department of Mathematics and Physics “Ennio De Giorgi”, via per Arnesano, Lecce, 73100, Italy
*Address Correspondence to: Valeria De Matteis, valeria.dematteis@unisalento.it
Received: 28 December 2021; Accepted: 18 April 2022
Abstract: The SARS-CoV-2 outbreaks highlighted the need for effective, reliable, fast, easy-to-do and cheap diagnostics procedures. We pragmatically experienced that an early positive-case detection, inevitably coupled with a mass vaccination campaign, is a milestone to control the COVID-19 pandemic. Gold nanoparticles (AuNPs) can indeed play a crucial role in this context, as their physicochemical, optics and electronics properties are being extensively used in photothermal therapy (PTT), radiation therapy (RT), drug delivery and diagnostic. AuNPs can be synthesized by several approaches to obtain different sizes and shapes that can be easily functionalized with many kinds of molecules such as antibodies, proteins, probes, and lipids. In addition, AuNPs showed high biocompatibility making them useful tool in medicine field. We thus reviewed here the most relevant evidence on AuNPs as effective way to detect the presence of SARS-CoV-2 antigens. We trust future diagnostic efforts must take this ‘old-fashioned’ nanotechnology tool into consideration for the development and commercialization of reliable and feasible detection kits.
Keywords: SARS-CoV-2; Gold nanoparticles; Diagnosis; Therapy; Physicochemical properties; Pandemic disease
The Coronaviruses, belonging to the Coronaviridae family, are characterized by an enveloped, non-segmented, positive-sense RNA (Yang et al., 2020; Liu et al., 2020b). In humans, they can induce severe symptoms at respiratory, hepatic, and enteric level. In December 2019, a novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) quickly spread from China to the whole world (Mi et al., 2020), inducing the well-known Covid-19 disease. As of 4 April 2022, time of this article drafting, there were approximately 486 million confirmed infection cases worldwide (https//:covid19.who.it; Dong et al., 2020). The easy human-to-human transmission is ascribed to the virus airborne nature, which makes it easy to spread through air droplets especially in the last two virus variants, namely Omicron and Omicron 2 (Rowe et al., 2022; Dance, 2022). Also, the biomolecular interaction has been characterized straight after the beginning of the pandemic outbreak and explained by the selective binding of the virus spike protein to the angiotensin-converting enzyme 2 (ACE2) receptors, which are over-expressed by type 2 pneumocytes in human airways (Kumar et al., 2021). The further virus uptake is due to the lysis of spike proteins induced by specific proteases of pneumocytes, which favors the entry by endocytosis or membrane fusion (Mirastschijski et al., 2020). Once within cells, the SARS-CoV-2 releases its positive-sense RNA within the host cytoplasm, which triggers the production of pp1a and pp1ab polyproteins, and promotes the replication and transcription of viral RNA. The further transcription of viral mRNA induces the production of viral structural components like membrane, spike and nucleocapsid proteins (Zhang et al., 2021). The viral particles are transported to the proximity of the host plasma membrane through the Golgi, and the new formed viruses escape by exocytosis, a specific mechanism promoting the infection of neighboring cells (Nakagawa et al., 2016; Mason, 2020) (Fig. 1).
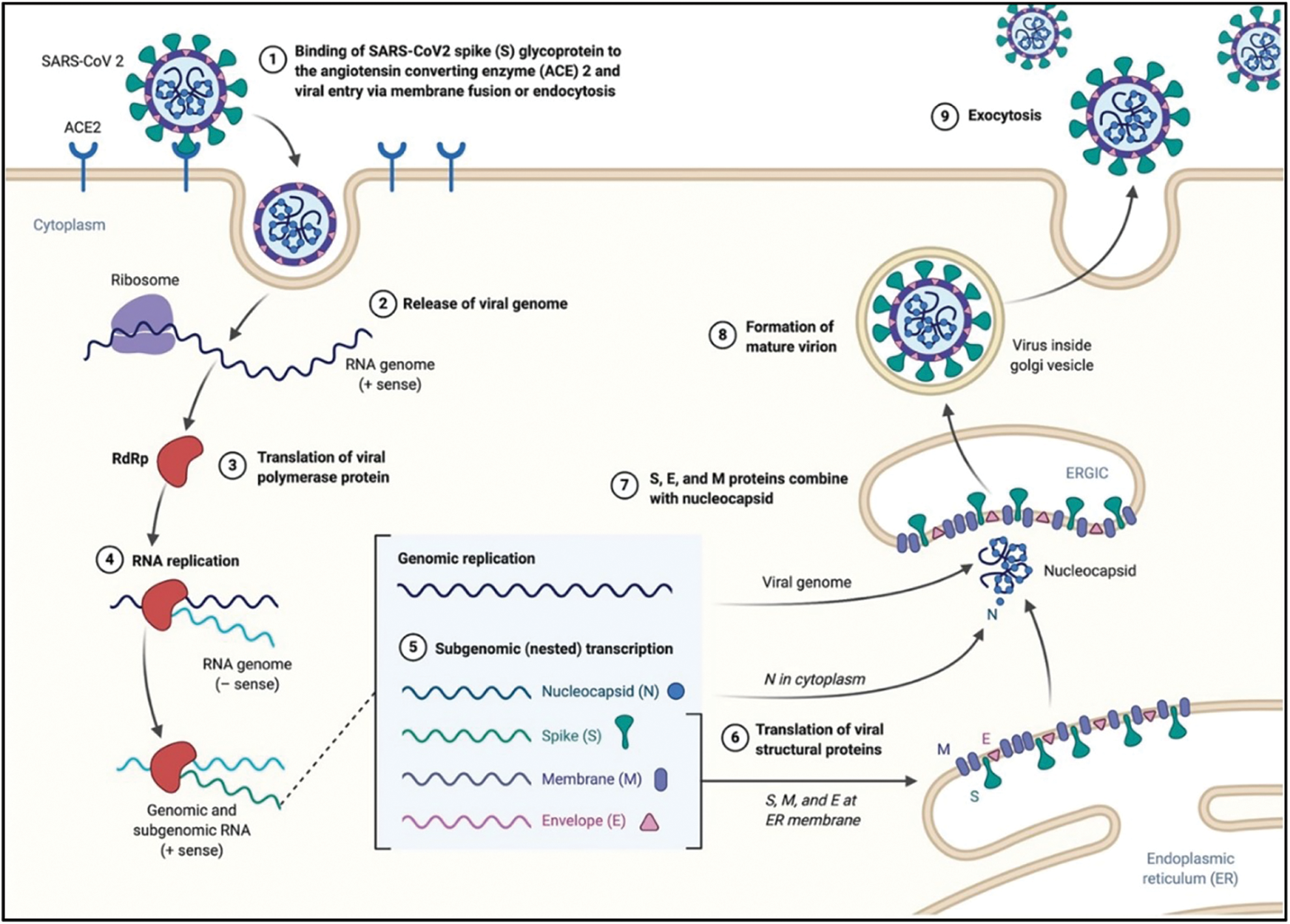
Figure 1: Mechanism of infection and life cycle of SARS-CoV-19 (Alanagreh et al., 2020).
The massive inoculation of different kinds of vaccines worldwide has provided a reduction of Covid-19 outbreaks; in addition to the more conventional vaccines containing a weakened or killed virus, mRNA vaccines, such as the BNT162b2 vaccine, produced by BioNTech (in collaboration with Pfizer) delivers a small segment of mRNA loaded in lipidic nanovesicles into the cells (Kitchin et al., 2020). This mRNA portion has the particularity to have the instructions to produce the spike proteins activating immune cells producing antibodies (Teijaro and Farber, 2021). The lipidic nanostructures are constituted by different types of lipidic molecules, but one of these is positive charged. Then, these molecules bind mRNA (negatively charged) but lose their positive charge when the basic conditions of the bloodstream occurred to reduce their adverse effects in human body (Dolgin, 2021). However, scientists were developing new strategies for enhancing the endosomal release of therapeutic nucleic acids, such as the use of new surfactants that enhance the delivery of mRNA into the cells (Røise et al., 2022). Nevertheless, the scientific community also focused on the early diagnosis of the virus, to develop remedies that can support the active vaccination campaign. Indeed, the diagnostic assays can be a powerful tool to facilitate the surveillance after the vaccination as vaccinated people can also be infected by the virus (Fresco-Taboada et al., 2022). In this scenario, nanotechnologies could be an optimal alternative for early SARS-CoV-2 diagnostic, especially using metallic NPs. Among these, gold (Au) based nanomaterials are probably the best choice for its unique physicochemical properties as it allows a combination of reliable detection (for diagnostic) together with a low toxicity (de Matteis and Rizzello, 2020; Silva Lopes et al., 2014; de Matteis et al., 2017).
Physicochemical properties of AuNPs
The physicochemical properties of AuNPs are several. However, some of them turn out to be suitable with a view to using these nano-objects as a diagnostic or therapeutic agent (Dykman and Khlebtsov, 2011; Yeh et al., 2012). First, the Localized Surface Plasmon Resonance (LSPR) is by far the ‘hallmark’ among all the AuNPs physical properties. LSPR is an optical phenomenon occurring when polarized light triggers the oscillation of free conduction electrons on the AuNPs surface (de Matteis et al., 2019). This specific feature of AuNPs makes possible a strong improvement of the optical extinction compared to the conventional organic molecules.
In general, the position of the LSPR peak is strongly dependent on the nanoparticle’s physical properties such as such shape and size. In addition, the dielectric properties (Saison-Francioso et al., 2015) as well as the local environment, namely surface-confined molecules substrate, solvent, and substrate, influence the LSPR phenomenon (Peixoto de Almeida et al., 2014) (Fig. 2).
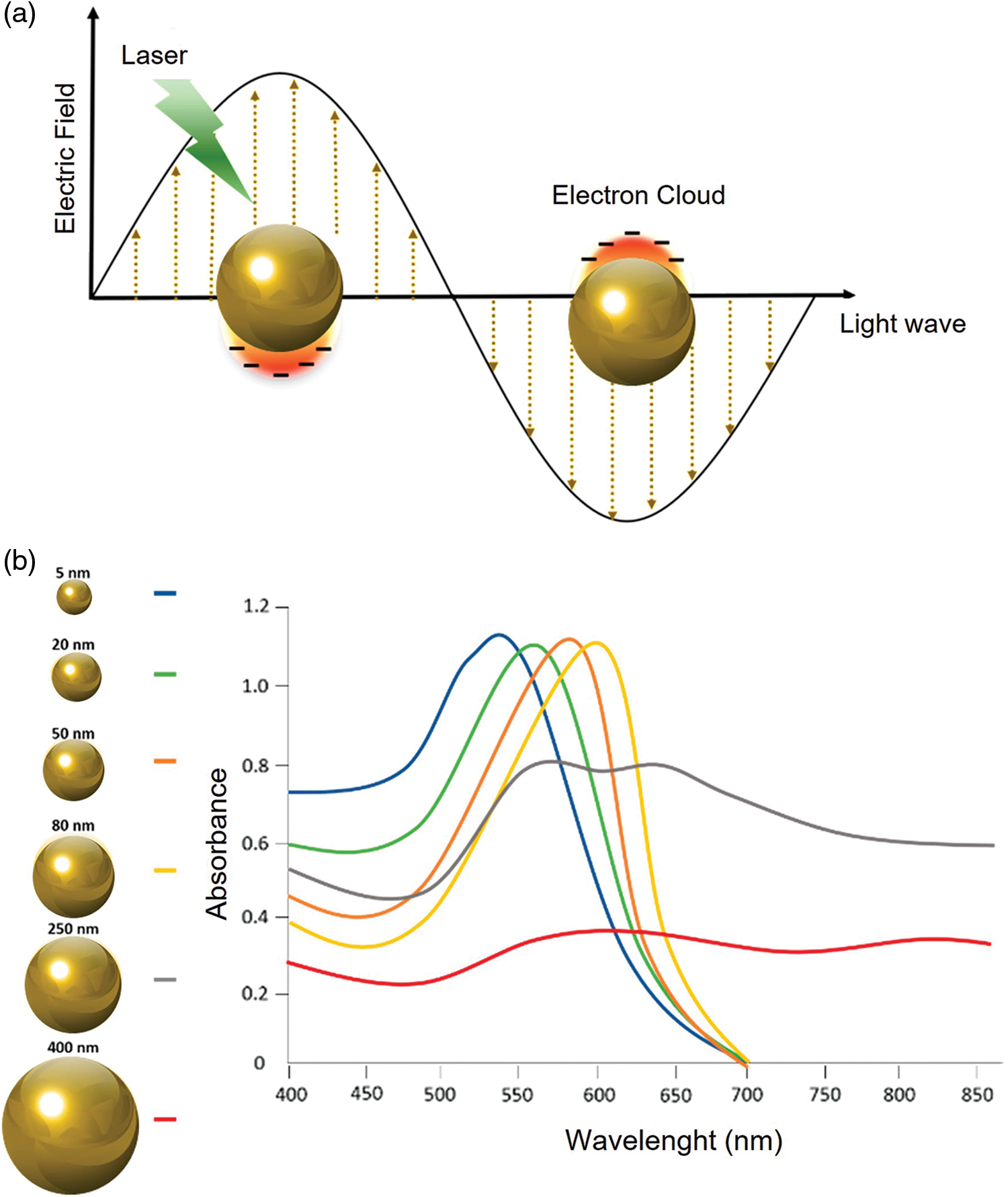
Figure 2: LSPR of AuNPs (a); Different UV-visible absorption spectra based on AuNPs size (b). Adapted from Kim and Lee (2018).
Moreover, the enhanced absorption of light by AuNPs has tremendous implications in different medicine sectors such as cancer treatment (Dykman and Khlebtsov, 2011) namely photothermal therapy applications (Bai et al., 2020; De Puig et al., 2015) and NP-based biosensors using colorimetric point-of-care devices (Iarossi et al., 2018). In the latter case, the color of NPs solution changes due to plasmonic coupling between the NPs, as the color shift from red to purple color is due to aggregation in solution (Ghosh and Pal, 2007).
In addition to this, the use of Au in the body is tolerated due to its high reduction potential which makes it less ionizable and then stable in the body (Albanese and Chan, 2011). Several studies performed in living cells and animal models demonstrated the biocompatibility of AuNPs (Zhang et al., 2011; de Matteis et al., 2020) which therefore is strongly dependent on NPs size and concentrations (Kang et al., 2020) as well as on the cell types. In particular, the small and high doses correspond to the high toxicity (Vecchio et al., 2012; Sani et al., 2021).
The surface functionalization of AuNPs with several types of polymers, proteins, peptides, surfactant is a good approach to reduce the impact of NPs in cells (Mahato et al., 2019).
In a typical photothermal treatment, the cancer cells are heated using temperatures between 41°C to 43°C by NIR stimulation after the uptake of Au nanomaterials (such as Au nanorods or Au nanoshells); as consequence, malignant cells can be destroyed (Zaho et al., 2014; de Matteis et al., 2021). As well as being a therapeutic agent, AuNPs can be used as diagnostic tools by exploiting other intrinsic characteristics of Au at the nanoscale.
For example, it is well known that the Raman scattering, representing the footprint of a certain molecule, has several applications in different fields (Cheng et al., 2018). However, its use is limited by the small cross section associated to the Raman scattering that is 10–30 to 10−25 cm2 per molecule (Nie and Emory, 1997). To improve the scattering, the molecules should be in the proximity of the AuNPs in order to exploit their local optical fields; this triggers a signal enhancement called Surface-Enhanced Raman Scattering (SERS) (Shuker and Gammon, 1970; Lee et al., 2011). The latter is dependent on the AuNPs physicochemical properties such as shape, size, and aggregation. In particular, the best enhancement can be obtained on AuNPs aggregates or cluster having a size ranging from 20 nm to 60 nm (Kneipp et al., 1997).
LSPR can also modulates the quantum yield of fluorescent dyes near their surface, thus inducing fluorescent enhancement or, alternatively, quenching, a process known as Metal Enhanced Fluorescence (MEF) (Geddes and Lakowicz, 2002; Shankar et al., 2009). The two distinct effects (enhancement or quenching) are functions of the distance between the Au surface and the dye. This phenomenon is based on the fluorescence resonance energy transfer (FRET), responsible of the fluorescence quenching. FRET regards the energy transfer between fluorophores. It is widely used to study the potential interaction of biological molecules (such as proteins, lipids, or nucleic acids) located in proximity. The mechanism exploits the presence of two fluorescent molecules, called donor and acceptor. The donor can be excited at a specific wavelength. This molecule emits energy which, in turn, can be transmitted to the acceptor, that consequently emits fluorescence at a different wavelength of emission compared to the donor. To this respect, AuNPs can improve the sensitivity and efficiency of FRET due to their large molar extinction coefficients (Chen et al., 2012).
The latter is also extremely useful for enhancing the sensitivity of standard colorimetric assays (Brennan et al., 2009). One advantage is the possibility to make a real-time monitoring with a good reproducibility and sensitivity, making AuNPs suitable for point of care (POC) diagnostics (Mariani and Minunni, 2014). For example, the plasmon-induced color switch has been exploited to detect altered levels of cytokines, proteins, DNA in cancer (Reddy et al., 2012), to monitor the bacteria contamination in food (Waswa et al., 2007) and in laboratory medicine (Helmerhorst et al., 2012).
In the context of X-ray-based diagnostic, AuNPs are applied as alternative tools to the traditional contrast agents like iodine or barium sulfate (Künzel et al., 2013). The need to replace the standard contrast agents is attributable to their high kidney toxicity (Kaller and An, 2022). AuNPs are particularly proper for this application due to the high atomic number (Z = 79) and density (=19.3 g/cm3) (Cutler et al., 2012). Furthermore, AuNPs show high X-ray attenuation coefficients providing sharper images enhancing the time of blood circulation (Hernandez-Rivera et al., 2017; Leung et al., 2011).
For biomedical, forensic, and environmental applications is crucial to detect chemical and biological molecules. Developing affordable, highly sensitive, miniature sensors is the only way to achieve this goal. As a rule, sensors comprise two parts: a recognition element for selectively binding the target analyte, and a transducer able to highlight the binding event (Naresh and Lee, 2021). The performance of a capable sensor is deeply dependent on these two elements based on selectivity, response time, and limits of detection (LOD) (Mathew et al., 2021). It is well known that nanomaterials have remarkable physicochemical properties that can be of great assistance in creating new recognition and transduction processes for chemical and biological sensors miniaturizing sensor elements (Malik et al., 2021). AuNPs are remarkably interesting for their several properties as described above and, in addition, high oxidative stability and low toxicity compared to others types of inorganic materials (Bakand et al., 2012). They can use alone or in combination with other types of nanomaterials such as covalent organic frameworks-AuNPs composite (COFs-AuNPs) to monitor kidney injury (Boyacıoğlu et al., 2022) Tumor Necrosis Factor-alpha (TNF-α) (Yola and Atar, 2021) or to detect toxins potentially dangerous for living organisms and environment (Karaman et al., 2021).
Diagnosis of COVID-19 Using AuNPs
It is indeed accepted that an early diagnosis represents the best method to prevent the uncontrolled virus transmission. Three broad groups of diagnostic tests are developed to monitor the spread of COVID-19 (Jarrom et al., 2022):
(i) Tests able to detect the existence of coronavirus in the respiratory secretion consisting in the amplification of viral nucleic acid by Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) (Kevadiya et al., 2021);
(ii) Antigen tests (rapid diagnostic tests) to directly detect SARS-CoV-2 proteins produced by virus in respiratory secretions (Interim guidance WHO, 2020). Today, represents the golden standard for a rapid, yet effective way to monitor the positive cases.
(iii) Serum tests revealing the presence of antibodies against SARS-CoV-2 in blood.
Specialized laboratories process the samples from nasopharyngeal swabs but, as the spread of the virus progresses, self-screening tests have also been developed. Then, the antigenic as well as the serological approaches have been developed as laboratory-based tests. Serological tests can be useful to indirectly identify the virus, that is through the detection of antibodies produced in the serum following the interaction with the pathogen. Rapid in vitro diagnostic tests for the qualitative detection of IgG and IgM antibodies against SARS-CoV-2 in human serum, plasma, venous whole blood, and acupuncture were developed by some companies such as Abbott BinaxNow (https://www.abbott.com/), bioMérieux SA (https://www.biomerieux.it/), AESKU Diagnostics (https://www.aesku.com/), Roche (https://www.roche.com/) and other ones. However, the limitation about sensitivity and specificity as well as the LOD quantity highlights the need for different and more sensitive approach able to monitor the COVID-19 spread in the population.
In this framework, a lot of nanomaterials, thanks to the aforementioned properties seems to be the best choice (Hasanzadeh et al., 2021).
The WHO guidelines reported the methodology of Point-Of-Care (POC) devices based on the detection of viral genes and proteins (Amer et al., 2013) These “pieces” of virus are anchored on the NPs surface and the detection is based on electrical, optical, and electrochemical strategies (Lee et al., 2018). The physicochemical properties of AuNPs have been thus investigated in terms of both detection and treatment of the SARS-CoV-2 (Bidram et al., 2021). In particular, the AuNPs-based colorimetric detection is particularly suitable for the red-to-blue shift as consequence of LSPR coupling among NPs (Li et al., 2015) permitting a rapid screening without the use of expensive instruments. Some works improved AuNPs using anti-spike (Pramanik et al., 2021) or anti-Nucleocapsid (N) antibodies attached on the AuNPs surface by colorimetric reading. SERS was used for the virus identification, using 4-aminothiophenol as reporter molecule linked on AuNPs by Au-S bonds (Pramanik et al., 2021). The addition of the virus with the AuNPs in the reaction mixture induced the aggregation of NPs, which was immediately distinguished even by naked eye thanks to the solution color change from pink to blue. The aggregated AuNPs created “hot-spots” showing a strong SERS signal derived from 4-aminothiophenol and antibody attached on the NPs surface. This methodology allowed detecting up to 4 pg/mL of viral proteins in five minutes. Moreover, the effectiveness of the nano system on the virus spread was demonstrated using Cellosaurus HEK293T cells, known to express angiotensin-converting enzyme 2 (ACE2) receptor. Then, anti-spike antibodies functionalized AuNPs effectively blocked the SARS-CoV-2 infection by inhibiting its replication. In a recent strategy (Aithal et al., 2022), AuNPs were decorated by a certain aptamer able to bind the SARS-CoV-2 spike proteins. The spikes were recognized upon addition of a coagulant. According to this approach, the AuNPs that did not recognize the spike underwent an aggregation process, whereas the binding event was detected by SPR. This system allowed detecting a concentration down to 16 nM of free spike proteins.
Femtomolar detection of spike proteins was carried out using plasmonic metasensor technology which can compress electromagnetic fields (Ahmadivand et al., 2021). The advantages of these structures were the low-radiative losses and a strong electromagnetic fields confinement. A plasmonic label-free toroidal metasurfaces at terahertz frequencies were fabricated (~0.4 THz) coupled with colloidal AuNPs functionalized by specific monoclonal antibody to improve the binding features of the molecules. After excitation, the resonance shifts based on the different concentrations of spike proteins binding antibodies. The LOD of this device was about 4.2 fM. Engineered terahertz (THz) plasmonic metamaterials, in particular toroidal materials, are new technologies for diagnosis for their low impact in vivo and in vitro systems and in addition, they are non-destructive platforms (Ahmadivand et al., 2018). As firstly described, the coupling with AuNPs is very useful for the detection of proteins due to the opportunity to detect several targets observing distinct resonance peaks (Xu et al., 2016).
In this context, smart devices were developed for IgG detection, using AuNPs (Li et al., 2015; Liu et al., 2020a; Pohanka, 2021; Ahmadi et al., 2021). Among these, an interesting platform based on a lateral flow immunoassay strip (LFIAs) has been recently created (Wen et al., 2020; Gupta et al., 2020) (Fig. 3a). The nucleocapsid proteins of SARS-CoV-2 were immobilized on the strip to specifically bind IgG-functionalized AuNPs. The goodness and reproducibility of the assay was finally assessed using serum samples from clinically diagnosed cases of COVID-19. The presence of target proteins was evaluated following the color footprint of AuNPs, making this test particularly suitable to equip the conventional test and to use it in low-resource countries. In addition, LFIAs can be potentially applied in mass screening or home tests. A colorimetric assay was developed to detect IgGs in plasma using AuNPs functionalized by antigenic epitopes (Lew et al., 2021). These immunodominant linear B-cell epitopes are present on spike and nucleocapsid proteins of SARS-CoV-2 showing great affinity with IgG. For this reason, the AuNPs surface was decorated with epitopes to bind specific antibodies. The binding event induced NPs aggregation triggering the plasmonic absorbance change, thus allowing detection of the virus with a LOD of 3.2 nM. The clinical tests, performed on human plasma medium, showed the identification of coronavirus with 100% of specificity, also in circulating COVID-19 variants (Lew et al., 2021).
Another work focused on the recognition of SARS-CoV-2 using serological test implemented with dot-blot assay (FT-DBA) for the detection of IgG (Sil et al., 2021). In this approach, AuNPs were functionalized with anti-human IgG (hIgG-AuNPs) able to bind the virus antigens immobilized on nitrocellulose membrane.
The selectivity and sensibility were higher compared to ELISA standard tests, obtaining results within two minutes, with sensitivity and specificity of about 98% (Fig. 3b).
Recently, Yano et al. (2022) created a platform in which the SPR of AuNPs, conveniently functionalized by antibodies, was used to find the nucleocapsid proteins of the SARS-CoV-2. The large size of the AuNPs (150 nm) enhanced the sensitivity detection with respect to the smaller AuNPs allowing to found protein virus at fM levels. The experimental data were both theoretical and experimental comparing size of 40 nm and 150 nm showing an increase of SPR angle of 0.94 degree for larger NPs; the smaller exhibited only a 0.06 degree (Figs. 3c and 3d).
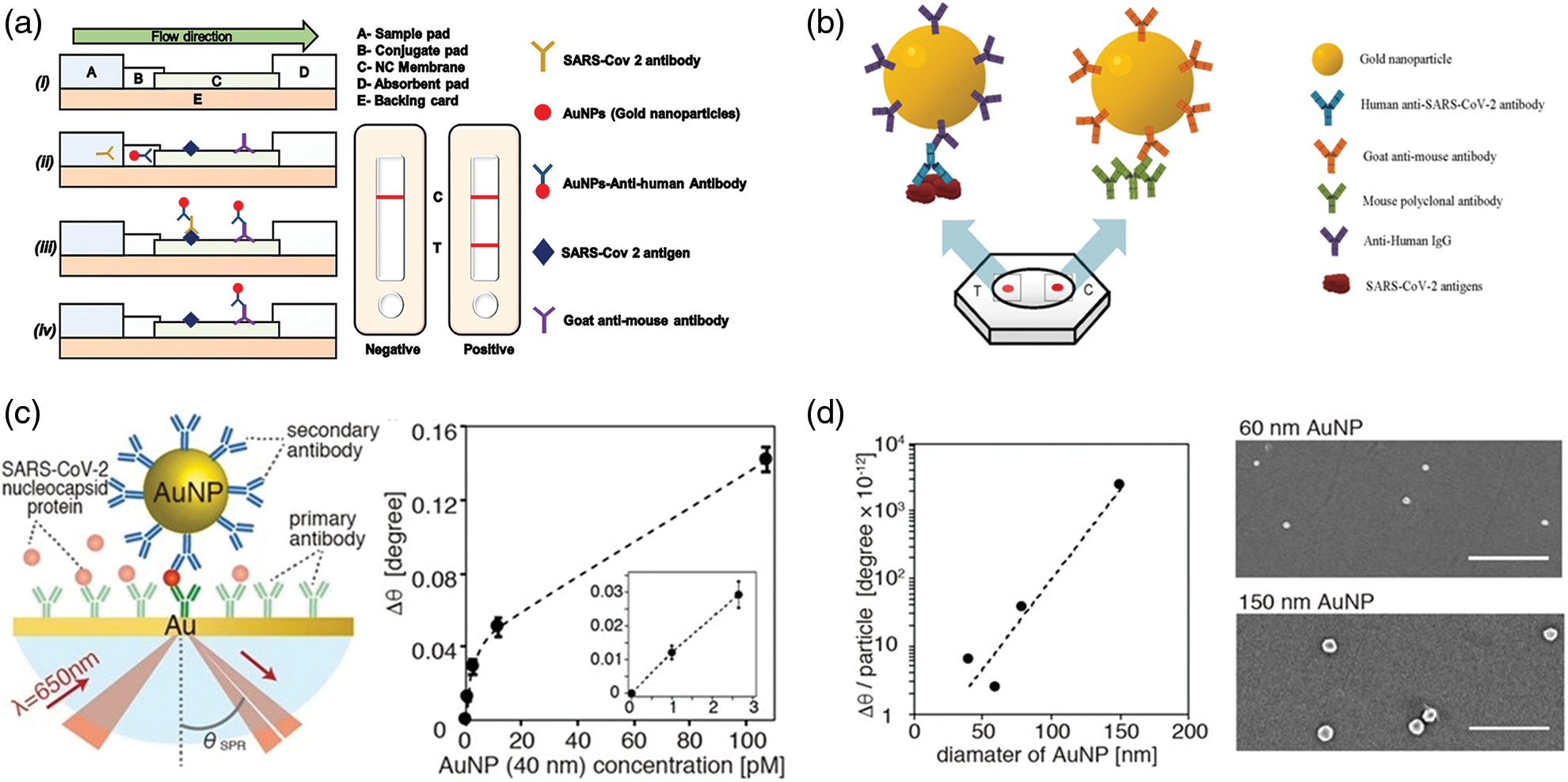
Figure 3: (a) Schematic representation of Lateral flow assay (LFA) using AuNPs. In the figure is reported the conjugation between the antibody and antigen as well as the relative positive results on the test (Gupta et al., 2020); (b) Rapid flow-through dot-blot immunoassay (FT-DBA) using AuNPs functionalized by anti-human IgG. AuNPs with goat anti-mouse can bind to mouse polyclonal antibody (control) (Sil et al., 2021); (c) identification of nucleocapsid protein of SARS-CoV-2 using AuNPs and SPR angular shift (∆θ) degree related to 40 nm AuNPs at different concentrations (pM); (d) Angular shift as function of different sizes of AuNPs and SEM acquisitions of AuNPs after SPR measurements (Yano et al., 2022).
The nasal swab are the most popular tests. In a recent experimental work, an Au antigen−antibody nanoplatform was developed showing a sensitivity and specificity higher than 95% (Della Ventura et al., 2020). The photochemical immobilization technique (PIT), a surface functionalization procedure, was employed to absorb antibodies on the AuNPs surface. Then, the nanoplatform was used to bind three surface viral proteins, namely the spike, the envelope, and the membrane proteins (S, E, and M, respectively). The AuNPs-protein interaction triggered the red shift of the absorption spectra. Alafeef et al. (2020) developed a paper-based colorimetric sensors to digitally detect SARS-CoV-2 by its viral RNA. The sensors were created using AuNPs functionalized by antisense oligonucleotides (ssDNA) able to bind nucleocapsid coronavirus protein, which generated electrochemical response by graphene-ssDNA-AuNP surface (Fig. 4a).
Hyperspectral microscopic data, together with the evidence on clinical samples of positive COVID-19 and healthy asymptomatic subjects, confirmed the effectiveness of this sensors with 100% of accuracy and a sensitivity of 231 copies μL−1. The LOD was 6.9 copies/μL without other amplification cycles. A detailed protocol for a nano-amplified colorimetric test to relieve SARS-CoV-2 without the need of RNA extraction was recently reported (Alafeef et al., 2021). This test was very effective due to the nano-amplification of plasmonic AuNPs previously functionalized by antisense oligonucleotides (ASOs), used as a colorimetric reporter to bind and amplify the viral genetic material. The use of ASOs was crucial for their ability to bind nucleocapsid proteins; the NPs aggregation induced a change in plasmonic response of the NPs upon interaction with the virus. The LOD was recorded to 10 copies/μL showing high specificity and sensitivity without using expensive equipment. In addition, this test can be used for a quantitative response using a handheld optical reader. Rodríguez-Díaz (Rodríguez-Díaz et al., 2022) produced a colorimetric test based on AuNPs (15–38 nm) and PCR. In this specific conformation, the cholesterol is folded inside and the AuNPs solution takes on a red color. On the other hand, when the target is present, the molecules change their conformation anchoring the target, thus determining the aggregation of the nanostructures. The result was spectrophotometrically quantifiable using patient respiratory swab samples. Finally, the authors implemented PCR procedure to complete the detection in 1 h. Recently (Alfassam et al., 2021), AuNPs were conjugated with sialic acid, a glycoprotein binding lung epithelial cell. This molecule represented a common target of respiratory virus, as it binds the viral surface protein hemagglutinin. Nasopharyngeal swabs containing SARS-CoV-2 were immersed in the solution containing the SA-AuNPs and, after 20 min, the authors observed a change in color detected by the UV-Vis spectrophotometry (Figs. 4b–4d).
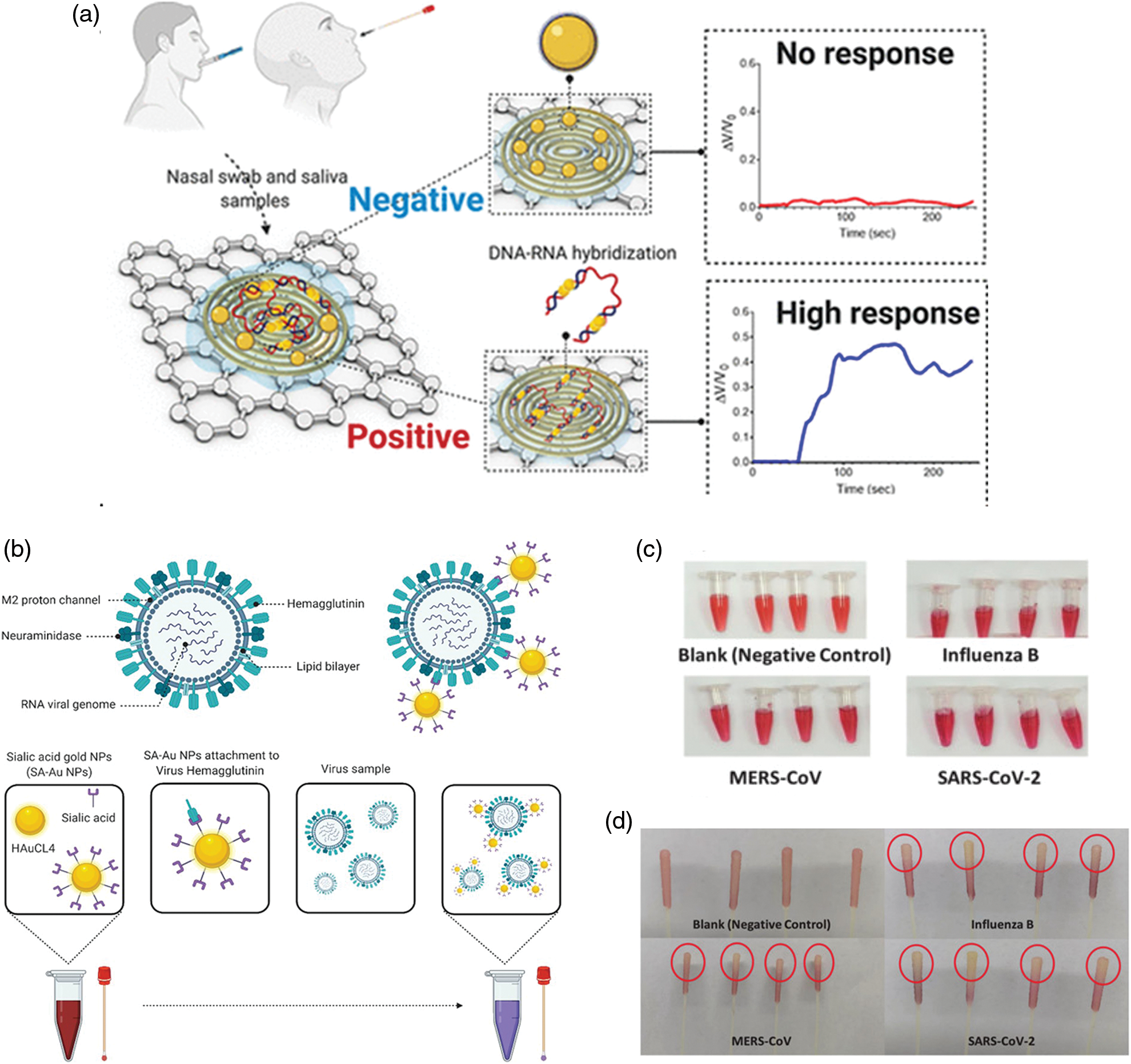
Figure 4: (a) Schematic principle of paper based colorimetric sensors with AuNPs-ssDNA. In the presence of SARS-CoV-2, the AuNPs-ssDNA bind viral RNA triggering a strong electrochemical signal. Reprinted by permission of American Chemical Society (Alafeef et al., 2020); (b) Colorimetric detection of SARS-CoV-2 based on AuNPs conjugated with sialic acid, (c) positive swabs of different kind of viruses included SARS-CoV-2 after immersion in solution containing AuNPs and (d) colored tips of samples compared to the negative control (Alfassam et al., 2021).
AuNPs Combined Machine Learning to Detect SARS-CoV-2
The validation of the SARS-CoV-2 devices is a critical challenge to verify the optimal performance of diagnostic, especially the one carried out at home for self-monitoring (Vandenberg et al., 2021). In this perspective, a recent work developed a Dense Neural Network (DNN) as a machine learning (ML) approach (Beduk et al., 2022) to validate the accuracy of POC device in which AuNPs were associated with a graphene sensor. Firstly, the device can recognize the ACE2 enzyme (due to its affinity to bind spike proteins of coronavirus) and it was finally integrated in a potentiostat connected with a smartphone by wireless.
The nasopharyngeal swabs were collected by several patients affected by alpha (B.1.1.7), beta (B.1.351), delta (B.1.617.2) virus variants as well as negative patients. The SARS-CoV-2 was find using the machine learning model showing an accuracy of 99.37% (Figs. 5a and 5b). Similar approach was used by Liang et al. (2022) to overcome the limitation in the use of SPR based AuNPs for the detection of novel coronavirus. In particular, the authors focused on the disadvantages emerging in the analysis by SPR since the information of spatial and temporal distribution during the analysis were ignored reducing the accuracy and sensitivity. The authors proposed a test based on the analysis of images acquired by microscopy. These images were extracted following interaction between spike proteins adsorbed on the sensors and AuNPs decorated with anti-SARS-CoV-2 antibodies. Binding results in transmission inhibition in the far field. Consequently, a reduction in gray values can be observed that is proportional with virus concentration that was detected in the range of 125.28 to 106 vp/mL with a LOD of 100 vp/mL. At this point, following further extractions of other images with different shades of colors corresponding to certain concentrations of pathogen, these were inserted into the machine learning program useful to detect SARS-CoV-2 quickly and safely (Figs. 5c and 5d).
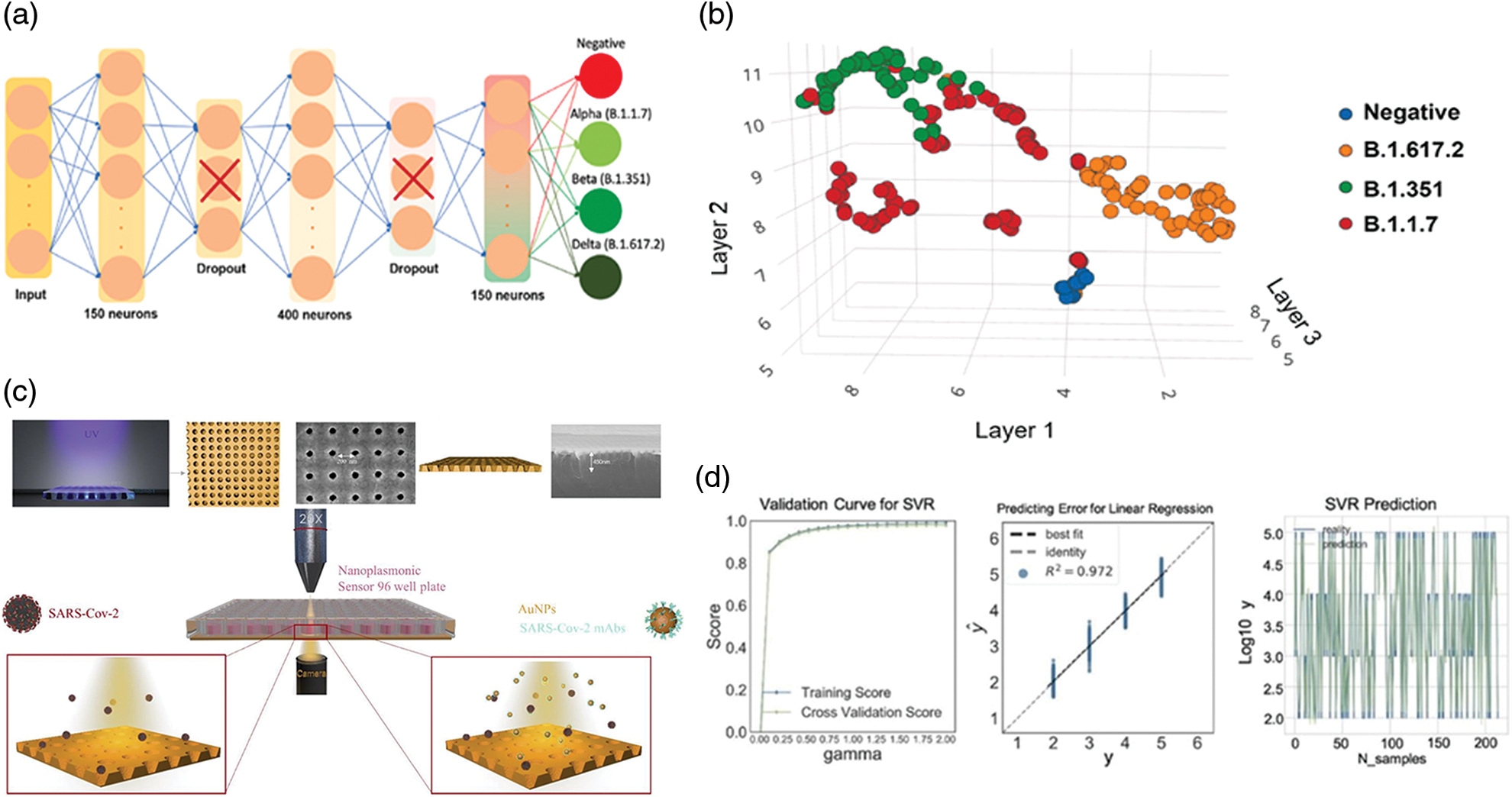
Figure 5: Graphical representations of The Dense Neural Network (DNN) (a) and records acquired from positive e negative tests performed using nasopharyngeal swabs (b), Adapted from Beduk et al. (2022); (c) summary scheme of the plasmonic device for SARS-CoV-2 detection using microscope. Machine learning SVM regression models to predict the virus concentration (R2 > 95%), (d) Adapted from Liang et al. (2022).
Behrouzi and Lin (2021) developed a convolutional neural learning able to increase the efficiency of SARS-Cov-2 test by analysis of images. The method is based on the double-coffee ring phenomenon using LSPR by the presence of AuNPs functionalized using specific antibodies against the coronavirus permitting to detect virus with a LOD of 5 ng/mL. With this aim, as sensing system a hydrophilic membrane characterized by a porous pattern and hydrophobic barriers was used. When AuNPs bond to viral units, a specific image characterized by a double coffee ring can be displayed. In this way, the neural network will be able to detect this interaction. Indeed, a small ring was formed in the center due to the interaction between the NPs surrounded by a second ring produced instead by the hydrophobic barrier.
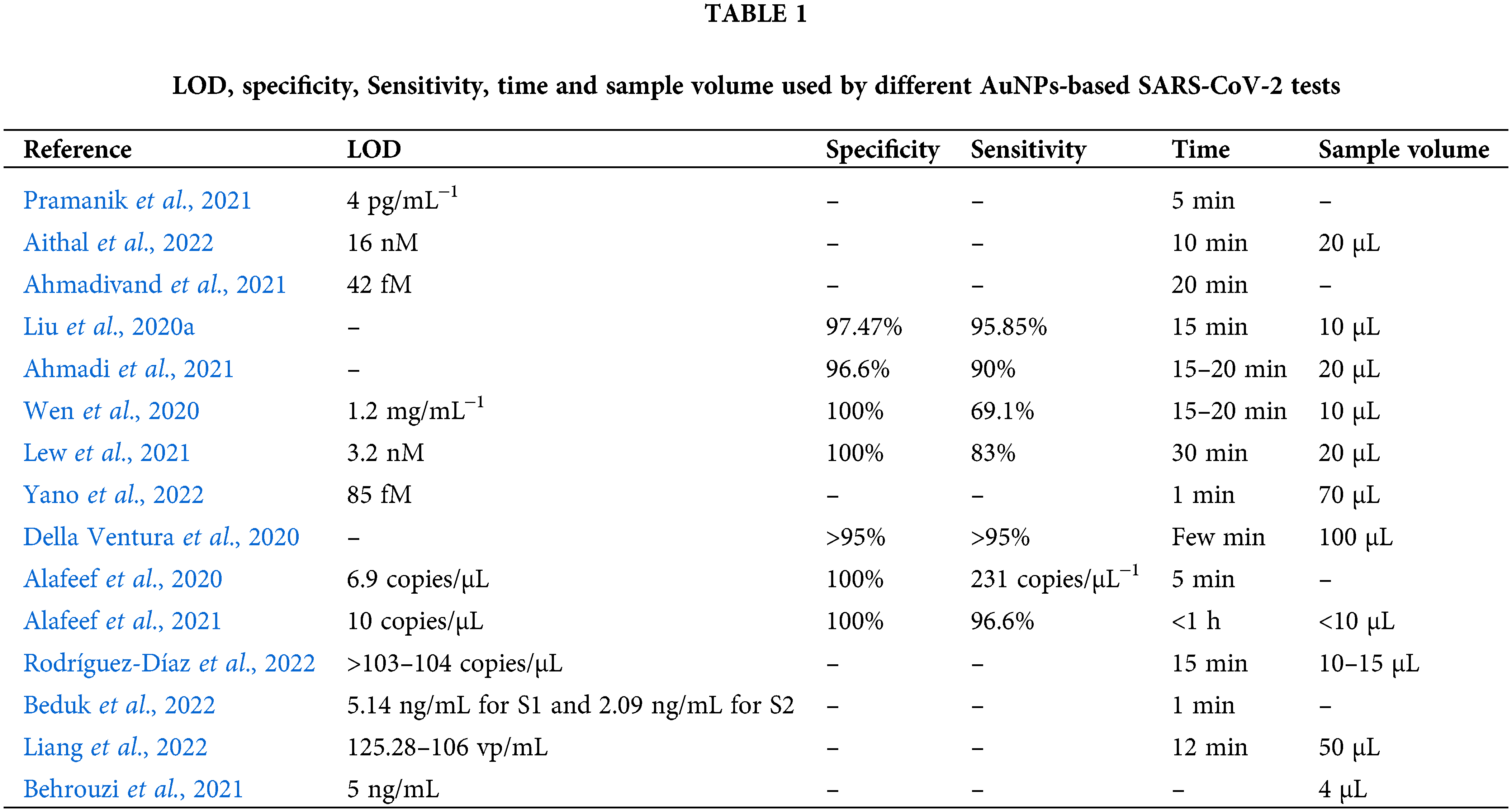
In the Table 1 are reported the most important characteristic of the sensors described in this review.
In the last year we witnessed a huge and common human effort towards the fight of the life-threatening COVID-19 infections. If on one side mass vaccination surely stopped the uncontrolled circulation of the virus, on the other a huge diagnostic campaign helped tracking positive cases, which can be then easily quarantined. With respect to the latter approach, we have witnessed a fast development of several different diagnostic kits, used both in the clinic as well as within common pharmacies. In this work, we described the most recent efforts to produce SARS-CoV-2 diagnostic tool based on AuNPs. Thanks to their unique physicochemical properties, AuNPs allowed indeed the detection of viral antigens even at picograms level, without the need of specialized equipment, in a cheap way. Since the pandemic situation is it is constantly evolving, we believe the development of next generation diagnostic kits should take into consideration the use of nanomaterials, to develop a nanoscale personalized platform able to detect viral RNA in specific manner with an optimal limit of detection and high sensitivity.
Acknowledgement: V. D. M. kindly acknowledges Programma Operativo Nazionale (PON) Ricerca e Innovazione 2014-20202014-azione IV.6 “Contratti su tematiche green”-DM 1062/2021 for sponsoring her salary and work. L. R. kindly acknowledges the ERC-2019-StG (Grant No. 850936) and Fondazione Cariplo (Grant No. 2019-4278) for supporting his research activities.
Author Contribution: Conceptualization and writing: V. D. M.; editing: L. R.
Ethics Approval: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare no conflicts of interest.
Ahmadi A, Mirzaeizadeh Z, Omidfar K (2021). Simultaneous detection of SARS-CoV-2 IgG/IgM antibodies, using gold nanoparticles-based lateral flow immunoassay. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy 40: 210–218. DOI 10.1089/mab.2021.0027. [Google Scholar] [CrossRef]
Ahmadivand A, Gerislioglu B, Ramezani Z, Kaushik A, Manickam P, Ghoreishi SA (2021). Functionalized terahertz plasmonic metasensors: Femtomolar-level detection of SARS-CoV-2 spike proteins. Biosensors and Bioelectronics 177: 112971. DOI 10.1016/j.bios.2021.112971. [Google Scholar] [CrossRef]
Ahmadivand A, Gerislioglu B, Tomitaka A, Manickam P, Kaushik A et al. (2018). Extreme sensitive metasensor for targeted biomarkers identification using colloidal nanoparticles-integrated plasmonic unit cells. Biomedical Optics Express 9: 373–386. DOI 10.1364/BOE.9.000373. [Google Scholar] [CrossRef]
Aithal S, Mishriki S, Gupta R, Sahu RP, Botos G, Tanvir S, Hanson RW, Puri IK (2022). SARS-CoV-2 detection with aptamer-functionalized gold nanoparticles. Talanta 236: 122841. DOI 10.1016/j.talanta.2021.122841. [Google Scholar] [CrossRef]
Alafeef M, Moitra P, Dighe K, Pan D (2021). RNA-extraction-free nano-amplified colorimetric test for point-of-care clinical diagnosis of COVID-19. Nature Protocols 16: 3141–3162. DOI 10.1038/s41596-021-00546-w. [Google Scholar] [CrossRef]
Alafeef M, Dighe K, Moitra P, Pan D (2020). Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano 14: 17028–17045. DOI 10.1021/acsnano.0c06392. [Google Scholar] [CrossRef]
Alanagreh L, Alzoughool F, Atoum M (2020). The human coronavirus disease COVID-19: Its origin, characteristics, and insights into potential drugs and its mechanisms. Pathogens 9: 331. DOI 10.3390/pathogens9050331. [Google Scholar] [CrossRef]
Albanese A, Chan W (2011). Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 5: 5478–5489. DOI 10.1021/nn2007496. [Google Scholar] [CrossRef]
Alfassam HA, Nassar MS, Almusaynid MM, Khalifah BA, Alshahrani AS et al. (2021). Development of a colorimetric tool for SARS-CoV-2 and other respiratory viruses detection using sialic acid fabricated gold nanoparticles. Pharmaceutics 13: 502. DOI 10.3390/pharmaceutics13040502. [Google Scholar] [CrossRef]
Amer HM, Abd El Wahed A, Shalaby MA, Almajhdi FN, Hufert FT, Weidmann M (2013). A new approach for diagnosis of bovine coronavirus using a reverse transcription recombinase polymerase amplification assay. Journal of Virological Methods 193: 337–340. DOI 10.1016/j.jviromet.2013.06.027. [Google Scholar] [CrossRef]
Bai X, Wang Y, Song Z, Feng Y, Chen Y et al. (2020). The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. International Journal of Molecular Sciences 21: 2480. DOI 10.3390/ijms21072480. [Google Scholar] [CrossRef]
Bakand S, Hayes A, Dechsakulthorn F (2012). Nanoparticles: A review of particle toxicology following inhalation exposure. Inhalation Toxicology 24: 125–135. DOI 10.3109/08958378.2010.642021. [Google Scholar] [CrossRef]
Beduk D, Ilton de Oliveira Filho J, Beduk T, Harmanci D, Zihnioglu F et al. (2022). All in one SARS-CoV-2 variant recognition platform: Machine learning-enabled point of care diagnostics. Biosensors and Bioelectronics: X 10: 1–7. DOI 10.1016/j.biosx.2022.100105. [Google Scholar] [CrossRef]
Behrouzi K, Lin L (2021). Double-coffee ring nanoplasmonic effects with convolutional neural learning for Sars-Cov-2 detection. 2021 21st International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), pp. 381–384. Orlando, FL, USA. DOI 10.1109/Transducers50396.2021.9495602 [Google Scholar] [CrossRef]
Bidram E, Esmaeili Y, Amini A, Sartorius R, Tay FR, Shariati L, Makvandi P (2021). Nanobased platforms for diagnosis and treatment of COVID-19: From benchtop to bedside. ACS Biomaterials Sciences and Engineering 14: 2150–2176. DOI 10.1021/acsbiomaterials.1c00318. [Google Scholar] [CrossRef]
Boyacıoğlu H, Yola B, Karaman C, Karaman O, Atar N et al. (2022). A novel electrochemical kidney injury molecule-1 (KIM-1) immunosensor based covalent organic frameworks-gold nanoparticles composite and porous NiCo2S4@CeO2 microspheres: The monitoring of acute kidney injury. Applied Surface Science 578: 152093. DOI 10.1016/j.apsusc.2021.152093. [Google Scholar] [CrossRef]
Brennan D, Justice J, Corbett B, McCarthy T, Galvin P (2009). Emerging optofluidic technologies for point-of-care genetic analysis systems: A review. Analytical and Bioanalytical Chemistry 3: 621–636. DOI 10.1007/s00216-009-2826-5. [Google Scholar] [CrossRef]
Chen NT, Cheng SH, Liu CP, Souris JS, Chen CT et al. (2012). Recent advances in nanoparticle-based förster resonance energy transfer for biosensing, molecular imaging and drug release profiling. International Journal of Molecular Sciences 13: 16598. DOI 10.3390/ijms131216598. [Google Scholar] [CrossRef]
Cheng C, Li J, Lei H, Baojun L (2018). Surface enhanced Raman scattering of gold nanoparticles aggregated by a gold-nanofilm-coated nanofiber. Photonics Research 6: 357–362. DOI 10.1364/PRJ.6.000357. [Google Scholar] [CrossRef]
Cutler CS, Hennkens HM, Sisay N, Huclier-Markai S, Jurisson S (2012). Radiometals for combined imaging and therapy. Chemical Review 113: 858–883. DOI 10.1021/cr3003104. [Google Scholar] [CrossRef]
Dance BA (2022). Omicron’s lingering mysteries. Nature 603. [Google Scholar]
De Matteis V, Cascione M, Rizzello L, Manno DE, Diguglielmo C, Rinaldi R (2021). Synergistic effect induced by gold nanoparticles with polyphenols shell during thermal therapy: Macrophage inflammatory response and cancer cell death assessment. Cancers 13: 3610. DOI 10.3390/cancers13143610. [Google Scholar] [CrossRef]
de Matteis V, Cascione M, Toma CC, Rinaldi R (2019). Engineered gold nanoshells killing tumor cells: New perspectives. Current Pharmaceutical Design 25: 1477–1489. DOI 10.2174/1381612825666190618155127. [Google Scholar] [CrossRef]
de Matteis V, Rizzello L, Cascione M, Liatsi-Douvitsa E, Apriceno A et al. (2020). Green plasmonic nanoparticles and bio-inspired stimuli-responsive vesicles in cancer therapy application. Nanomaterials 10: 1083. DOI 10.3390/nano10061083. [Google Scholar] [CrossRef]
de Matteis V, Rizzello L, Di Bello MP, Rinaldi R (2017). One-step synthesis, toxicity assessment and degradation in tumoral pH environment of SiO2@Ag core/shell nanoparticles. Journal of Nanoparticle Research 19: 14. DOI 10.1007/s11051-017-3870-2. [Google Scholar] [CrossRef]
de Matteis V, Rizzello L (2020). Noble metals and soft bio-inspired nanoparticles in retinal diseases treatment: A perspective. Cells 9: 679. DOI 10.3390/cells9030679. [Google Scholar] [CrossRef]
de Puig H, Tam JO, Yen CW, Gehrke L, Hamad-Schifferli K (2015). Extinction coefficient of gold nanostars. The Journal of Physical Chemistry C 119: 17408–17415. DOI 10.1021/acs.jpcc.5b03624. [Google Scholar] [CrossRef]
Della Ventura B, Cennamo M, Minopoli A, Campanile R, Bolletti Censi S, Terracciano D, Portella G, Velotta R (2020). Colorimetric test for fast detection of SARS-CoV-2 in nasal and throat swabs. ACS Sensors 5: 3043–3048. DOI 10.1021/acssensors.0c01742. [Google Scholar] [CrossRef]
Dolgin E (2021). The tangled history of mRNA vaccines. Nature 597: 318–324. DOI 10.1038/d41586-021-02483-w. [Google Scholar] [CrossRef]
Dong E, Du H, Gardner L (2020). An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infectious Diseases 20: 533–534. DOI 10.1016/S1473-3099(20)30120-1. [Google Scholar] [CrossRef]
Dykman LA, Khlebtsov NG (2011). Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Naturae 3: 34–55. DOI 10.32607/20758251-2011-3-2-34-55. [Google Scholar] [CrossRef]
Fresco-Taboada A, García-Durán M, Aira C, López L, Sastre P et al. (2022). Diagnostic performance of two serological assays for the detection of SARS-CoV-2 specific antibodies: Surveillance after vaccination. Diagnostic Microbiology and Infectious Disease 102: 115650. DOI 10.1016/j.diagmicrobio.2022.115650. [Google Scholar] [CrossRef]
Geddes CD, Lakowicz JR (2002). Editorial: Metal-enhanced fluorescence. Journal of Fluorescence 12: 121–129. DOI 10.1023/A:1016875709579. [Google Scholar] [CrossRef]
Ghosh SK, Pal T (2007). Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chemical Reviews 107: 4797–4862. DOI 10.1021/cr0680282. [Google Scholar] [CrossRef]
Gupta R, Sagar P, Priyadarshi N, Kaul S, Sandhir R et al. (2020). Nanotechnology-based approaches for the detection of SARS-CoV-2. Frontiers in Nanotechnology 2: 143. DOI 10.3389/fnano.2020.589832. [Google Scholar] [CrossRef]
Hasanzadeh A, Alamdaran M, Ahmadi S, Nourizadeh H, Bagherzadeh MA et al. (2021). Nanotechnology against COVID-19: Immunization, diagnostic and therapeutic studies. Journal of Controlled Release 336: 354–374. DOI 10.1016/j.jconrel.2021.06.036. [Google Scholar] [CrossRef]
Helmerhorst E, Chandler DJ, Nussio M, Mamotte CD (2012). Real-time and label-free bio-sensing of molecular interactions by surface plasmon resonance: A laboratory medicine perspective. The Clinical Biochemist. Review 33: 161–173. [Google Scholar]
Hernandez-Rivera M, Kumar I, Cho SY, Cheong BY, Pulikkathara MX, Moghaddam SE, Whitmire KH, Wilson LJ (2017). High-performance hybrid bismuth-carbon nanotube based contrast agent for X-ray CT imaging. ACS Applied Materials and Interfaces 9: 5709–5716. DOI 10.1021/acsami.6b12768. [Google Scholar] [CrossRef]
https://www.abbott.com/. [Google Scholar]
https://www.aesku.com/. [Google Scholar]
https://www.biomerieux.it/. [Google Scholar]
https://www.roche.com/. [Google Scholar]
Iarossi M, Schiattarella C, Rea I, Stefano LD, Fittipaldi R et al. (2018). Colorimetric immunosensor by aggregation of photochemically functionalized gold nanoparticles. ACS Omega 3: 3805–3812. DOI 10.1021/acsomega.8b00265. [Google Scholar] [CrossRef]
World Health Organization (2020). Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays: Interim guidance. WHO/2019-nCoV/Antigen Detection/2021.1. [Google Scholar]
Jarrom D, Elston L, Washington J, Prettyjohns M, Groves P (2022). Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: A rapid systematic review. BMJ Evidence-Based Medicine 27: 33–45. DOI 10.1136/bmjebm-2020-111511. [Google Scholar] [CrossRef]
Kaller MO, An J (2022). Contrast Agent Toxicity. Treasure Island (FLStatPearls Publishing. [Google Scholar]
Kang MS, Lee SY, Kim KS, Han DW (2020). State of the art biocompatible gold nanoparticles for cancer theragnosis. Pharmaceutics 12: 701. DOI 10.3390/pharmaceutics12080701. [Google Scholar] [CrossRef]
Karaman C, Karaman O, Yola BB, Ülker İ, Atar N et al. (2021). A novel electrochemical aflatoxin B1 immunosensor based on gold nanoparticle-decorated porous graphene nanoribbon and Ag nanocube-incorporated MoS2 nanosheets. New Journal of Chemistry 45: 11222–11233. DOI 10.1039/D1NJ02293H. [Google Scholar] [CrossRef]
Kevadiya BD, Machhi J, Herskovitz J, Oleynikov MD, Blomberg WR et al. (2021). Diagnostics for SARS-CoV-2 infections. Nature Materials 20: 593–605. DOI 10.1038/s41563-020-00906-z. [Google Scholar] [CrossRef]
Kim HS, Lee DY (2018). Near-infrared-responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers 10: 961. DOI 10.3390/polym10090961. [Google Scholar] [CrossRef]
Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K et al. (2020). Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. The New England Journal of Medicine 383: 2439–2450. DOI 10.1056/NEJMoa2027906. [Google Scholar] [CrossRef]
Kneipp K, Wang Y, Kneipp H, Perelman LT, Itzkan I et al. (1997). Single molecule detection using surface-enhanced raman scattering (SERS). Physical Review Letters 78: 1667–1670. DOI 10.1103/PhysRevLett.78.1667. [Google Scholar] [CrossRef]
Kumar V, Doshi KU, Khan WH, Rathore AS (2021). COVID-19 pandemic: Mechanism, diagnosis, and treatment. Chemical Technology and Biotechnology 96: 299–308. DOI 10.1002/jctb.6641. [Google Scholar] [CrossRef]
Künzel R, Okuno E, Levenhagen RS, Umisedo NK (2013). Evaluation of the X-ray absorption by gold nanoparticles solutions. International Scholarly Research Notices 2013: 1–5. DOI 10.1155/2013/865283 ID 865283. [Google Scholar] [CrossRef]
Lee J, Morita M, Takemura K, Park ET (2018). A multi-functional gold/iron-oxide nanoparticle-CNT hybrid nanomaterial as virus DNA sensing platform. Biosensors and Bioelectronics 102: 425–431. DOI 10.1016/j.bios.2017.11.052. [Google Scholar] [CrossRef]
Lee M, Lee S, Lee JH, Lim HW, Seong GH et al. (2011). Highly reproducible immunoassay of cancer markers on a gold-patterned microarray chip usingsurface-enhanced Raman scattering imaging. Biosensors and Bioelectronics 26: 2135–2141. DOI 10.1016/j.bios.2010.09.021. [Google Scholar] [CrossRef]
Leung MKK, Chow JCL, Chithrani D, Lee MJG, Oms B et al. (2011). Irradiation of gold nanoparticles by X-rays: Monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Medical Physics 38: 624–631. DOI 10.1118/1.3539623. [Google Scholar] [CrossRef]
Lew TTS, Aung KMM, Ow SY, Amrun SN, Sutarlie L, Ng LFP, Su X (2021). Epitope-functionalized gold nanoparticles for rapid and selective detection of SARS-CoV‐2IgG antibodies. ACS Nano 15(7). DOI 10.1021/acsnano.1c04091. [Google Scholar] [CrossRef]
Li M, Cushing SK, Wu N (2015). Plasmon-enhanced optical sensors: A review. Analyst 140: 3. DOI 10.1039/C4AN01079E. [Google Scholar] [CrossRef]
Li X, Zhang Q, Hou P, Chen M, Hui W et al. (2015). Gold magnetic nanoparticle conjugate-based lateral flow assay for the detection of IgM class antibodies related to TORCH infections. International Journal of Molecular Medicine 36: 1319–1326. DOI 10.3892/ijmm.2015.2333. [Google Scholar] [CrossRef]
Liang J, Zhang W, Qin Y, Li Y, Liu GL et al. (2022). Applying machine learning with localized surface plasmon resonance sensors to detect SARS-CoV-2 particles. Biosensors 12: 173. DOI 10.3390/bios12030173. [Google Scholar] [CrossRef]
Liu C, Mao B, Martinez V, Chen X, Li Y et al. (2020a). A facile assay for rapid detection of COVID-19 antibodies. RSC Advances 10: 28041–28048. DOI 10.1039/D0RA04107F. [Google Scholar] [CrossRef]
Liu C, Zhou Q, Li Y, Garner LV, Watkins SP et al. (2020b). Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Central Sciences 6: 315–331. DOI 10.1021/acscentsci.0c00272. [Google Scholar] [CrossRef]
Mahato K, Nagpal S, Shah MA, Srivastava A, Maurya PK et al. (2019). Gold nanoparticle surface engineering strategies and their applications in biomedicine and diagnostics. 3 Biotech 9: 2440. DOI 10.1007/s13205-019-1577-z. [Google Scholar] [CrossRef]
Malik P, Gupta R, Malik V, Ameta RK (2021). Emerging nanomaterials for improved biosensing. Measurement: Sensors 16: 100050. DOI 10.1016/j.measen.2021.100050. [Google Scholar] [CrossRef]
Mariani S, Minunni M (2014). Surface plasmon resonance applications in clinical analysis. Analytical and Bioanalytical Chemistry 406: 2303–2323. DOI 10.1007/s00216-014-7647-5. [Google Scholar] [CrossRef]
Mason RJ (2020). Pathogenesis of COVID-19 from a cell biology perspective. European Respiratory Journal 55: 2000607. DOI 10.1183/13993003.00607-2020. [Google Scholar] [CrossRef]
Mathew M, Radhakrishnan S, Vaidyanathan A, Chakraborty B, Rout CS (2021). Flexible and wearable electrochemical biosensors based on two-dimensional materials: Recent developments. Analytical and Bioanalytical Chemistry 413: 727–762. DOI 10.1007/s00216-020-03002-y. [Google Scholar] [CrossRef]
Mi YN, Huang TT, Zhang JX, Qin Q, Gong YX, Liu SY, Xue HM, Ning CH, Cao L, Cao YX (2020). Estimating the instant case fatality rate of COVID-19 in China. International Journal of Infectious Diseases 97: 1–6. DOI 10.1016/j.ijid.2020.04.055. [Google Scholar] [CrossRef]
Mirastschijski U, Dembinski R, Maedler K (2020). Lung surfactant for pulmonary barrier restoration in patients with COVID-19 pneumonia. Frontiers in Medicine 7: 254. DOI 10.3389/fmed.2020.00254. [Google Scholar] [CrossRef]
Nakagawa K, Lokugamage KG, Makino S (2016). Viral and cellular mRNAtranslation in coronavirus-infected cells. Advances in Virus Research 96: 165–192. DOI 10.1016/bs.aivir.2016.08.001. [Google Scholar] [CrossRef]
Naresh V, Lee NA (2021). Review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 21: 1109. DOI 10.3390/s21041109. [Google Scholar] [CrossRef]
Nie S, Emory SR (1997). Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275: 1102–1106. DOI 10.1126/science.275.5303.1102. [Google Scholar] [CrossRef]
Peixoto de Almeida M, Pereira E, Baptista P, Gomes I, Figueiredo S et al. (2014). Chapter 13–Gold nanoparticles as (Bio) chemical sensors. Comprehensive Analytical Chemistry 66: 529–567. DOI 10.1016/B978-0-444-63285-2.00013-4. [Google Scholar] [CrossRef]
Pohanka M (2021). Point-of-care diagnoses and assays based on lateral flow test. International Journal of Analytical Chemistry 2021: 1–9. DOI 10.1155/2021/6685619. [Google Scholar] [CrossRef]
Pramanik A, Gao Y, Patibandla S, Mitra D, Mccandless MG et al. (2021). The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Advances 3: 1588–1596. DOI 10.1039/D0NA01007C. [Google Scholar] [CrossRef]
Reddy PJ, Sadhu S, Ray S, Srivastava S (2012). Cancer biomarker detection by surface plasmon resonance biosensors. Clinics in Laboratory Medicine 32: 47–72. DOI 10.1016/j.cll.2011.11.002. [Google Scholar] [CrossRef]
Rodríguez-Díaz C, Lafuente-Gómez N, Coutinho C, Pardo D, Alarcón-Iniesta H et al. (2022). Development of colorimetric sensors based on gold nanoparticles for SARS-CoV-2 RdRp, E and S genes detection. Talanta 243: 123393. DOI 10.1016/j.talanta.2022.123393. [Google Scholar] [CrossRef]
Røise J, Han H, Li J, Kerr DL, Taing C et al. (2022). Acid-Sensitive surfactants enhance the delivery of nucleic acids. Molecular Pharmaceutics 19: 67–79. DOI 10.1021/acs.molpharmaceut.1c00579. [Google Scholar] [CrossRef]
Rowe BR, Canosa A, Meslem A, Rowe F (2022). Increased airborne transmission of COVID-19 with new variants implications for health policies. medRxiv 133: 915. DOI 10.1101/2022.01.13.22269234. [Google Scholar] [CrossRef]
Saison-Francioso O, Lévêque G, Boukherroub R, Szunerits S, Akjouj A (2015). Dependence between the refractive-index sensitivity of metallic nanoparticles and the spectral position of their localized surface plasmon band: A numerical and analytical study. The Journal of Physical Chemistry C 119: 28551–28559. DOI 10.1021/acs.jpcc.5b08357. [Google Scholar] [CrossRef]
Sani A, Cao C, Cui D (2021). Toxicity of gold nanoparticles (AuNPsA review. Biochemistry and Biophysics Reports 26: 100991. DOI 10.1016/j.bbrep.2021.100991. [Google Scholar] [CrossRef]
Shankar S, Rizzello L, Cingolani R, Rinaldi R, Pompa PP (2009). Micro/Nanoscale patterning of nanostructured metal substrates for plasmonic applications. ACS Nano 3: 893–900. DOI 10.1021/nn900077s. [Google Scholar] [CrossRef]
Shuker R, Gammon R (1970). Raman-scattering selection-rule breaking and the density of states in amorphous materials. Physical Review Letters 25: 222–225. DOI 10.1103/PhysRevLett.25.222. [Google Scholar] [CrossRef]
Sil BK, Jamiruddin MR, Haq MA, Khondoker MU, Jahan N et al. (2021). Aunp coupled rapid flow-through dot-blot immuno-assay for enhanced detection of SARS-COV-2 specific nucleocapsid and receptor binding domain igg. International Journal of Nanomedicine 16: 4739–4753. DOI 10.2147/IJN.S313140. [Google Scholar] [CrossRef]
Silva Lopes T, Gomes Alves G, Pereira R, Granjeiro JM, Correa Leite PA (2014). Advances and potential application of gold nanoparticles in nanomedicine. Journal of Cellular Biochemistry 120: 16370–16378. DOI 10.1002/jcb.29044. [Google Scholar] [CrossRef]
Teijaro JR, Farber DL (2021). COVID-19 vaccines: Modes of immune activation and future challenges. Nature Review Immunology 21: 195–197. DOI 10.1038/s41577-021-00526-x. [Google Scholar] [CrossRef]
Vandenberg O, Martiny D, Rochas O, Belkum AV, Kozlakidis Z (2021). Considerations for diagnostic COVID-19 tests. Nature Review Microbiology 19: 171–183. DOI 10.1038/s41579-020-00461-z. [Google Scholar] [CrossRef]
Vecchio G, Galeone A, Brunetti V, Maiorano G, Sabella S et al. (2012). Concentration-dependent, size-independent toxicity of citrate capped AuNPs in Drosophila melanogaster. PLoS One 7: e29980. DOI 10.1371/journal.pone.0029980. [Google Scholar] [CrossRef]
Waswa J, Irudayaraj J, DebRoyc C (2007). Direct detection of E. coli O157: H7 in selected food systems by a surface plasmon resonance biosensor. LWT-Food Science and Technology 40: 187–192. DOI 10.1016/j.lwt.2005.11.001. [Google Scholar] [CrossRef]
Wen T, Huang C, Shi FJ, Zeng XY, Lu T et al. (2020). Development of a lateral flow immunoassay strip for rapid detection of IgG antibody against SARS-CoV-2 virus. Analyst 145: 5345–5352. DOI 10.1039/D0AN00629G. [Google Scholar] [CrossRef]
WHO. Health Emergency Dashboard. https://covid19.who.int/. [Google Scholar]
Xu W, Xie L, Zhu J, Xu X, Ye Z, Wang C, Ma Y, Ying Y (2016). Gold nanoparticle-based terahertz metamaterial sensors: Mechanisms and applications. ACS Photonics 3: 2308–2314. DOI 10.1021/acsphotonics.6b00463. [Google Scholar] [CrossRef]
Yang X, Yu Y, Xu J, Shu H, Liu H et al. (2020). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. The Lancet Respiratory Medicine 8: 475–481. DOI 10.1016/S2213-2600(20)30079-5. [Google Scholar] [CrossRef]
Yano T, Kajisa T, Ono M, Miyasaka Y, Hasegawa Y (2022). Ultrasensitive detection of SARS‐CoV‐2 nucleocapsid protein using large gold nanoparticle‐enhanced surface plasmon resonance. Scientific Report, 12, 1060. DOI 10.1038/s41598-022-05036-x. [Google Scholar] [CrossRef]
Yeh YC, Creran B, Rotello V (2012). Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 4: 1871–1880. DOI 10.1039/C1NR11188D. [Google Scholar] [CrossRef]
Yola ML, Atar N (2021). Novel voltammetric tumor necrosis factor-alpha (TNF-α) immunosensor based on gold nanoparticles involved in thiol-functionalized multi-walled carbon nanotubes and bimetallic Ni/Cu-MOFs. Analytical and Bioanalytical Chemistry 413: 2481–2492. DOI 10.1007/s00216-021-03203-z. [Google Scholar] [CrossRef]
Zaho J, Wallace M, Melancon MP (2014). Cancer theranostics with gold nanoshells. Nanomedicine 9: 2041–2057. DOI 10.2217/nnm.14.136. [Google Scholar] [CrossRef]
Zhang Q, Xiang R, Huo S, Zhou Y, Jiang S et al. (2021). Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduction and Targeted Therapy 6: 233. DOI 10.1038/s41392-021-00653-w. [Google Scholar] [CrossRef]
Zhang XD, di Wu XS, Liu PX, Yang N, Zhao B et al. (2011). Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. International Journal of Nanomedicine 6: 2071–2081. DOI 10.2147/IJN.S21657. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |