DOI:10.32604/biocell.2022.019916

| BIOCELL DOI:10.32604/biocell.2022.019916 |  |
| Article |
3-epi-bufotalin suppresses the proliferation in colorectal cancer cells through the inhibition of the JAK1/STAT3 signaling pathway
1Guizhou Provincial College–Based Key Lab for Tumor Prevention and Treatment with Distinctive Medicines, Zunyi, 563000, China
2Life Science Institute of Zunyi Medical University, Zunyi, 563000, China
3School of Preclinical Medicine of Zunyi Medical University, Zunyi, 563000, China
*Address correspondence to: Lingjie Meng, menglj718@126.com; Yun Liu, liuyunzmu@126.com
#The authors contributed equally to the research
Received: 24 October 2021; Accepted: 06 April 2022
Abstract: Traditional Chinese medicine (TCM) has been increasingly employed in the last decades in China for both preventing and treating a variety of cancers. 3-epi-bufotalin is an active ingredient of TCM “Chanpi” with anti-tumor potential. However, the effect and mechanism of 3-epi-bufotalin on colorectal cancers were not well disclosed. The present study demonstrated that 3-epi-bufotalin could reduce viability, trigger apoptosis, and block the cell cycle at the G2/M stage in colorectal cancer cell lines HT29, RKO, and COLO205 in vitro. Moreover, 3-epi-bufotalin inhibited the JAK1/STAT3 signaling pathway. These results indicated the anti-proliferation ability of 3-epi-bufotalin in colorectal cancer cells.
Keywords: 3-epi-bufotalin; Colorectal cancer; JAK1/STAT3 signaling pathway; Apoptosis
Colorectal cancer (CRC) is one of the most common malignant tumors with high mortality (Feng et al., 2019; Sung et al., 2021; Zakaria et al., 2021). A large number of studies have focused on the pathogenic mechanism, effective prevention, and treatment of colorectal cancer. So far, while chemotherapy remains one of the important treatments for CRC, resistance to chemotherapeutic agents such as 5-fluorouracil (Vodenkova et al., 2020) and oxaliplatin (Martinez-Balibrea et al., 2015) remains a major problem. Thus, it is particularly important to discover more effective chemotherapeutic drugs.
Owing to its action on multiple signal pathways, only a few adverse effects, and systematic regulatory function, traditional Chinese medicine (TCM) has attracted growing appreciation in the clinical treatment of a variety of cancers (Wang et al., 2011; Wang et al., 2018). “Chanpi,” the skin of Bufo bufo gargarizans Cantor or Bufo melanostrictus Schneider, is a commonly used TCM in China for carbuncles, swelling, heatstroke coma, vomiting, diarrhea, and cancer (College, 1986). Furthermore, Cinobufacini (brand name: Huachansu), a water-soluble drug extracted from Chanpi, has been approved by the State of Food and Drug Administration of China to be applied to cancer patients in the clinical treatment and has shown promising efficacy in the treatment of hepatocellular carcinoma, lung cancer, colorectal carcinoma, and pancreatic cancer (Wang et al., 2018). However, the anti-tumor mechanisms of Chanpi and its processed products have not been studied exhaustively.
Bufadienolides are the predominant constituents of Chanpi and carry out anti-cancer activity through different mechanisms (Wang et al., 2011; Zhan et al., 2020). As a bufadienolide, bufotalin is rich in Chanpi and shows noticeable growth inhibition of hepatocellular carcinoma HepG2 cells (Zhang et al., 2012) and malignant melanoma A375 cells (Pan et al., 2019). In addition, 3-epi-bufotalin, with an opposite configuration of -OH at C-3bufotalin, is easily found in Chanpi or obtained via biotransformation from bufotalin. Bufotalin led to growth inhibition of HepG2 and breast cancer MCF-7cell lines (Zhang et al., 2011). However, the anti-tumor effects and mechanisms of 3-epi-bufotalin are unclear.
Here, we investigated the anti-proliferative effect of 3-epi-bufotalin on CRC cells. Our data indicated that 3-epi-bufotalin induced apoptosis and cell cycle arrest at the G2/M phase in CRC cells. Furthermore, 3-epi-bufotalin suppressed the growth of CRC cells via the Janus kinase 1/signal transducer and activator of transcription 3 (JAK1/STAT3) signaling pathway. These findings suggest that 3-epi-bufotalin could be a potential growth inhibitor for CRC cells.
Human CRC cell lines HT29, RKO, and COLO205, were purchased from Genechem (Shanghai, China). 3-epi-bufotalin was isolated from the aqueous extract of Chanpi, refined to 95% of purity by high-performance liquid chromatography, dissolved in DMSO as a stock concentration of 10 mM and stored at −20°C. Fetal bovine serum (BSA) was purchased from Biological Industries (Kibbutz Beit Haemek, Israel). Sulforhodamine B (SRB) was purchased from Sigma-Aldrich (Saint Louis, Missouri, USA). The phosphatase inhibitor was obtained from Roche (Mannheim, Germany). Phenylmethanesulfonyl fluoride (PMSF), FITC-Annexin V Apoptosis Detection Kit, and Cell Cycle Analysis Kit were obtained from Beyotime (Shanghai, China). Antibodies of JAK1 (#ET1705-84), STAT3 (#ET1605-45), p-STAT3 (S727) (#ET1607-39), B-cell lymphoma-2 (BCL-2; #ET1702-53), goat anti-Rabbit IgG-HRP antibody (#HA1001) and goat anti-Rat IgG (H+L)-HRP antibody (#HA1023) were purchased from Huabio (Hangzhou, China); antibodies for p-JAK1 (Y1022+Y1023) (#ab130085) and Tubulin (#ab6106) were purchased from Abcam (Cambridge, UK); antibodies for CyclinB1 (#12231), p-cell division control2 (p-CDC2; Tyr15) (#4529) and Bcl-2-associated X protein (BAX; #2772) were purchased from Cell Signal Technology (Boston, Massachusetts, USA); antibody for GAPDH (#10494-1-AP) was purchased from Proteintech (Rosemont, Minnesota, USA). PVDF transfer membranes were obtained from Millipore (Darmstadt, Germany).
The CRC cell lines HT29, RKO, and COLO205 were cultured in RPMI-1640 medium supplied with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C in a humidified incubator containing 5% CO2.
Cells were seeded in 96-well plates at 5 × 103 cells/well and cultured for 24 h to allow adherence. Then, cells were exposed to a series of concentrations of 3-epi-bufotalin while cells of the control group were added with a 1‰ volume of DMSO. After 24 to 72 h of incubation, cells were subjected to SRB colorimetry (Skehan et al., 1990), and the absorbance of each well was measured at 530 nm using SpectraMax i3X plate reader (Molecular Devices, USA).
Cell cycle and apoptosis analysis
Cells were plated into 6-well plates at 5 × 104 cells/well for 24 h and then incubated with 1.5 to 6 μM of 3-epi-bufotalin for 24 h. Cells were washed with cold PBS, collected in 500 μL binding buffer, then incubated with 10 μL RNase A (10 μg/mL) at 37°C in the dark for 30 min and combined with 20 μL propidium iodide (50 μg/mL) at 4°C in the dark for 20 min. Then the cell cycle phase was analyzed using a flow cytometer C6 (BD Bioscience, USA). For apoptosis analyses, cells were gently collected in cold PBS and resuspended in binding buffer, followed by treatment with 10 μL FITC-Annexin-V and 10 μL propidium iodide at room temperature in the dark for 15 min. Finally, cells were analyzed by flow cytometer C6.
Cells were plated into 6-well plates for culturing and then exposed to 1.5 to 6 μM 3-epi-bufotalinfor 24 h. The total protein of cells was extracted by RIPA buffer with proteinase inhibitor (PMSF) and phosphatase inhibitor. Protein samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 10% or 12% gel and transferred to PVDF membranes. After blocking with 5% BSA for 2 h at room temperature, membranes were incubated with primary antibodies (1:500 or 1:1000 dilution) at 4°C overnight and goat anti-Rat IgG-HRP antibody or goat anti-Rabbit IgG-HRP antibody (1:5000 dilution) for 2 h at room temperature. Protein bands were developed by an ECL chemiluminescence kit and visualized by ChemiDoc Imaging System (Bio-Rad, USA).
For statistical analyses, cells cultured at different passages were treated with drugs, and all of the experiments were performed three times independently. Differences between each 3-epi-bufotalin group and the control group were determined by ANOVA analysis using SPSS 17.0 (IBM, Armonk, New York, USA). P-value < 0.05 and P-value < 0.01 were considered statistically significant.
Effect of 3-epi-bufotalin on colorectal cancer cells proliferation
The structure of 3-epi-bufotalin is presented in Fig. 1A. To test the effect of 3-epi-bufotalin on human CRC cells, human CRC HT29, RKO, and COLO205 cell lines were treated with different concentrations of 3-epi-bufotalin (0, 1.25, 2.5, 5, 7.5, and 10 µM) for 24 to72 h. SRB assay showed that 3-epi-bufotalin significantly suppressed the viability of these cell lines in a dose and time dependent manner (Fig. 1), suggesting the anti-proliferation effect of 3-epi-bufotalin on CRC cell lines.
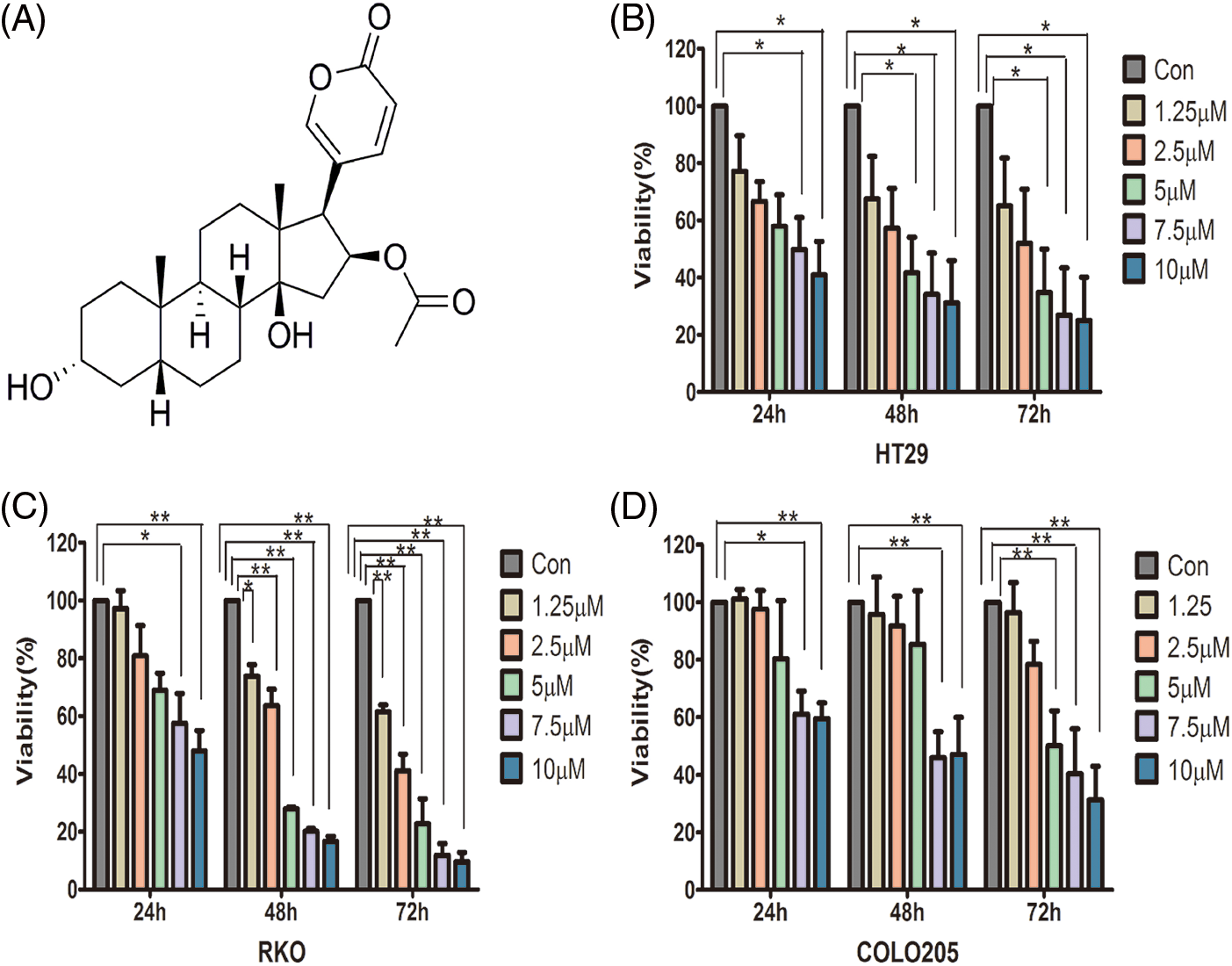
Figure 1: Inhibitory effect of different doses of 3-epi-bufotalin on colorectal cancer cells for different durations. Structure of 3-epi-bufotalin. (A), viability of HT29 cells (B), RKO cells (C), and COLO205 cells (D) (*P < 0.05 vs. control, **P < 0.01 vs. control).
3-epi-bufotalin induced apoptosis in colorectal cancer cells
To explore whether 3-epi-bufotalin induces the apoptosis in CRC cells, we detected the proportion of apoptotic cells stained with FITC-Annexin-V and propidium iodide by flow cytometry. The proportion of apoptotic cells (cells in the right upper quadrant are undergoing apoptosis at later stage and cells in the right lower quadrant are apoptosis at an early stage) markedly increased after treatment with 6 µM 3-epi-bufotalin in CRC cell lines (Figs. 2A–2C). Furthermore, we examined the expression of mitochondrial apoptosis-associated proteins BAX and BCL-2 after 3-epi-bufotalin treatment. Western blot analysis indicated an increase in the pro-apoptotic protein BAX after 3-epi-bufotalin treatment, while the anti-apoptotic protein BCL-2 decreased in CRC cells (Figs. 2D–2F). Overall, these results indicate the activation of intrinsic apoptosis pathway by 3-epi-bufotalin in CRC cells.
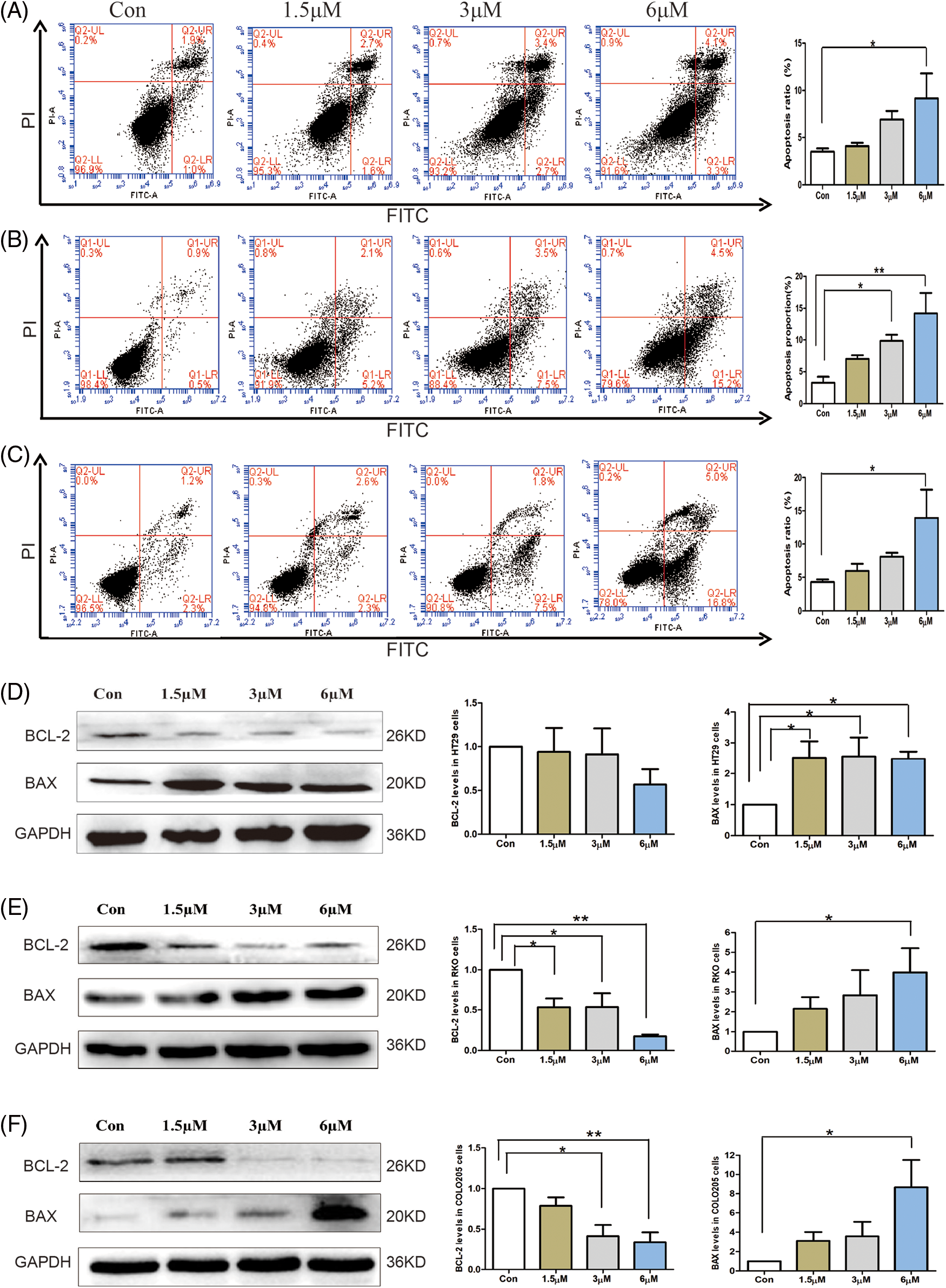
Figure 2: Apoptosis induced in colorectal cancer cells by 3-epi-bufotalin treating for 24 h. Apoptosis ratio of HT29 cells (A), RKO cells (B), and COLO205 cells (C). Mitochondrial apoptosis-associated protein BAX and BCL-2 were detected in HT29 cells (D), RKO cells (E), and COLO205 cells (F) (*P < 0.05 vs. control, **P < 0.01 vs. control).
3-epi-bufotalin induced cell cycle arrest in colorectal cancer cells
As continuous and precisely regulated progression of cell cycle ensures cell growth and proliferation, cell cycle arrest is a major indicator of cytotoxic effects of various chemical agents and always intimately coupled with apoptotic induction (Pucci et al., 2000). We detected the effect of 3-epi-bufotalin on cell cycle progression via flow cytometry, and found well-maintained at the G2/M stage after 3-epi-bufotalin treatment (Figs. 3A–3C). Furthermore, 3-epi-bufotalin also decreased the levels of G2/M phase-associated regulatory proteins, CyclinB1 and phosphorylated CDC2/CDK1 (Figs. 3D–3E). These results suggest that 3-epi-bufotalin could arrest the cell cycle at the G2/M stage in CRC cells.
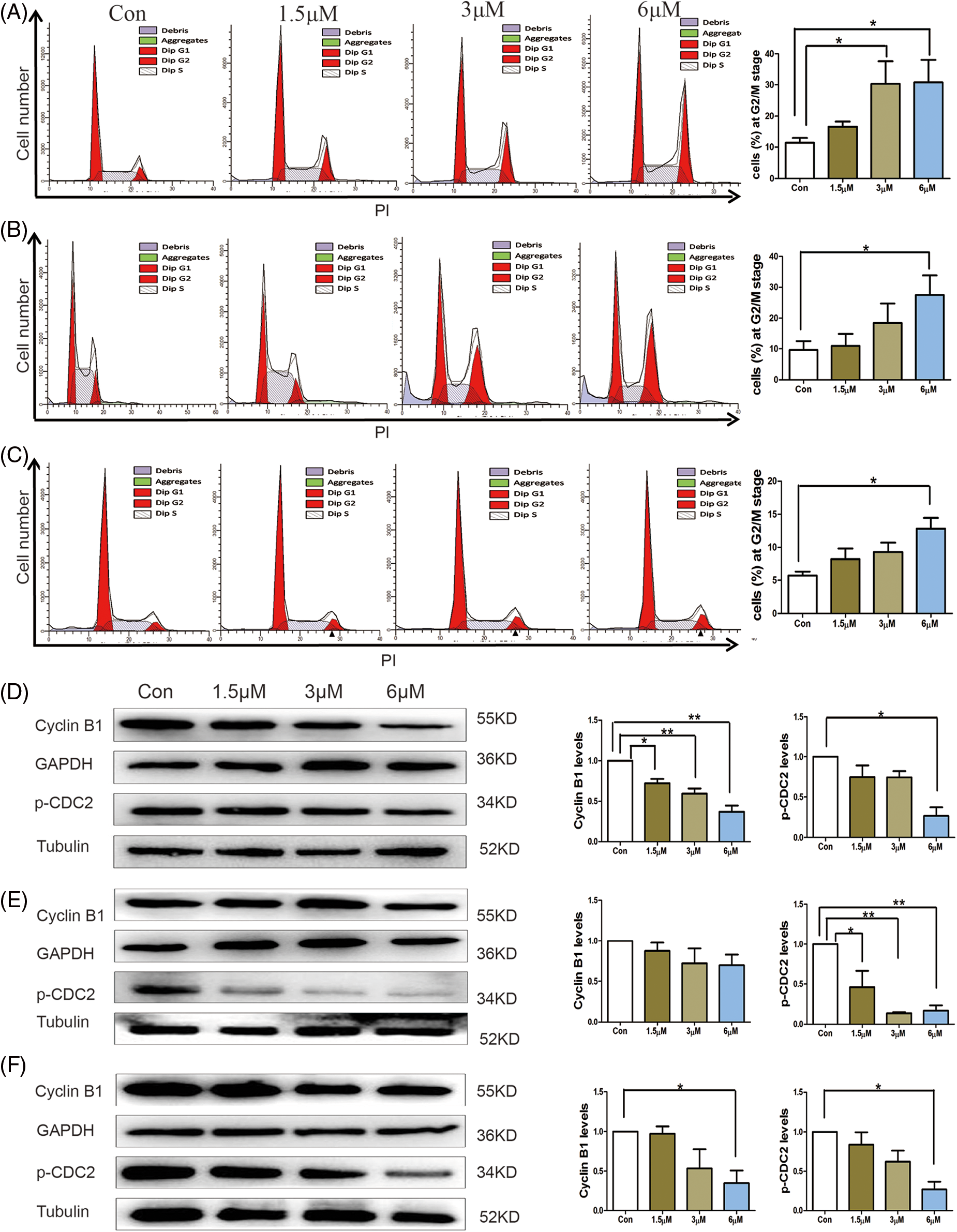
Figure 3: Cell cycle arrest in colorectal cancer cells after 24 h of 3-epi-bufotalin treatment. Cell cycle distribution of HT29 cells. (A), RKO cells (B), and COLO205 cells (C). Relative expression levels of cyclin B1 and p-CDC2 in HT29 cells (D), RKO cells (E), and COLO205 cells (F) (*P < 0.05 vs. control, **P < 0.01 vs. control).
3-epi-bufotalin inhibited the JAK1/STAT3 signaling pathway
The JAK/STAT signaling pathway is important for cell growth, proliferation, and differentiation, and excessive activation of the STAT3 pathway is considered a hallmark of many malignancies (Huynh et al., 2017). In view of this, we investigated whether 3-epi-bufotalin suppresses cell proliferation via inhibiting the JAK1/STAT3 signaling pathway. Western blotting revealed that total JAK1 and STAT3, as well as phosphorylated JAK1 and STAT3 were reduced after 3-epi-bufotalin treatment (Fig. 4). Furthermore, treatment of cells with 5 μM of AG490, a STAT3 inhibitor, indeed inhibited the activation of STAT3. Then, the effects of 3-epi-bufotalin (6 μM) on CRC cells after 24 h treatment revealed that AG490 decreased the levels of p-STAT3, and 3-epi-bufotalin did not further inhibit the proliferation or induce more apoptosis in CRC cells (Figs. S1A and S1B). Meanwhile, the expression of BAX and BCL-2 did not change noticeably after pre-treating with AG490 (Fig. S1C). These results suggested that the JAK1-STAT3 signal pathway participates in the inhibition effect of 3-epi-bufotalin on CRC cell proliferation. Thus, 3-epi-bufotalin inhibited the proliferation of colorectal cancer cells via down-regulation of the JAK1/STAT3 signal pathway.

Figure 4: Treatment with 3-epi-bufotalin for 24 h led to down-regulation of the JAK1/STAT3 signaling pathway in colorectal cancer cells. The expression levels of JAK1, STAT3, phosphorylated-JAK1, and phosphorylated-STAT3(A, B) (*P < 0.05 vs. control,**P < 0.01 vs. control).
Bufotalin is a bufadienolide with wide-spectrum anti-cancer activities (Qi et al., 2011; Wang et al., 2011; Wei et al., 2017). Toad compounds are highly toxic, and bufotalin is no exception. Bufotalin suppresses the proliferation of various cancer cells, such as hepatocellular carcinoma HepG2 cells (Zhang et al., 2012), osteoblastoma U2OS, SaOs-2 and MG-63 cells (Zhu et al., 2014), esophageal squamous cell carcinoma Eca-109 and EC9706 cells (Lin et al., 2018), and malignant melanoma A375 cells (Pan et al., 2019), via blocking cell cycle at G2/M phase and inducing apoptosis. Apoptosis was activated via the intrinsic pathway marked by the increase in BAX, and the decrease in BCL-2 expressions. Although the anti-cancer activities of bufotalin have been widely studied, the bioactivity of its epimer 3-epi-bufotalin remains unknown. This study also showed that 3-epi-bufotalin arrests the cell cycle at the G2/M phase and induces apoptosis in CRC cells.
The JAK/STAT signaling pathway plays a crucial role in the multi-step development of tumors, while excessive activation of this pathway results in tumor genesis, promoting metastatic ability and resistance to chemotherapy agents. JAK1 is widely expressed in various cells, and STAT3 is associated with the promotion of oncogenesis and immune suppression (Huynh et al., 2017; Yu et al., 2009). JAK/STAT3 is hyper-activated in many kinds of solid tumors, including colorectal cancer, and is associated with poor prognosis, invasion, metastasis, and multidrug resistance (Jin, 2020; Johnson et al., 2018). Therefore, JAK/STAT3 pathway is a promising target for the treatment of cancer. Several compounds of bufadienolides have been shown to inhibit JAK/STAT3 in cancer cells. For example, bufalin down-regulated anti-apoptotic protein myeloid leukemia 1 and B-cell lymphoma-Xl via inhibition of STAT3 in breast cancer cells (Dong et al., 2011). Likewise, BF211, a derivative of bufalin, induced apoptosis via inhibition of the IL-6/JAK2/STAT3 signaling pathway in myeloma cells (Wu et al., 2018). Cinobufagin suppressed the viability of osteosarcoma cancer U2OS/MG-63 cells by inhibiting the IL-6-OPN-STAT3 signaling pathway (Zhang et al., 2019). In this study, we found that 3-epi-bufotalin reduced the levels of total JAK1 and STAT3, as well as phosphorylated JAK1 and STAT3 in CRC cells. Our work indicates that 3-epi-bufotalin could be a new JAK/STAT3 inhibitor for treating CRC.
To conclude, our results demonstrated that 3-epi-bufotalin inhibited the growth of CRC cells via suppressing the JAK1/STAT3 signaling pathway and arrested the cell cycle at the G2/M stage to induce intrinsic apoptosis in CRC cells.
Availability of Data and Materials:The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contribution:Yun Liu, Lingjie Meng, and Sanhua Li conceptualized and designed the study; Sanhua Li, Qinghong Kong, Xiaoke Zhang, Xinting Zhu, Chunbo Yu, Changyan Yu, Nian Jiang, and Jing Hui performed the experiments. Yun Liu, Lingjie Meng, and Sanhua Li revised the manuscript. All authors have read and approved the final manuscripts.
Ethics Approval:Not applicable.
Funding Statement:This work was supported by the Guizhou Provincial Science and Technology Program (QKHZC[2020]4Y154), Science and Technology Plan of Zunyi (ZSKHSZ [2018]18), Science and Technology Plan of Zunyi (ZSKHHZ[2020]83), the Xinmiao Funding of Zunyi Medical University (QKPTRC[2019]022), City School Joint Fund of Zunyi (ZSKHHZ [2021] 277), and the Innovation Talent Team of Zunyi (ZSKRC[2019]1).
Conflicts of Interest:The authors declare that they have no conflicts of interest to report regarding the present study.
College JNM (1986). Dictionary of Traditional Chinese Medicine. Shanghai, China: Shanghai Scientific and Technical Publishing House. [Google Scholar]
Dong Y, Yin S, Li J, Jiang C, Ye M, Hu H (2011). Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis 16: 394–403. DOI 10.1007/s10495-011-0573-5. [Google Scholar] [CrossRef]
Feng RM, Zong YN, Cao SM, Xu RH (2019). Current cancer situation in China: Good or bad news from the 2018 global cancer statistics? Cancer Communications 39: 22. DOI 10.1186/s40880-019-0368-6. [Google Scholar] [CrossRef]
Huynh J, Etemadi N, Hollande F, Ernst M, Buchert M (2017). The JAK/STAT3 axis: A comprehensive drug target for solid malignancies. Seminars in Cancer Biology 45: 13–22. DOI 10.1016/j.semcancer.2017.06.001. [Google Scholar] [CrossRef]
Jin W (2020). Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial-mesenchymal transition. Cells 9: 217. DOI 10.3390/cells9010217. [Google Scholar] [CrossRef]
Johnson DE, O’Keefe RA, Grandis JR (2018). Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nature Reviews Clinical Oncology 15: 234–248. DOI 10.1038/nrclinonc.2018.8. [Google Scholar] [CrossRef]
Lin S, Lv J, Peng P, Cai C, Deng J, Deng H, Li X, Tang X (2018). Bufadienolides induce p53-mediated apoptosis in esophageal squamous cell carcinoma cells in vitro and in vivo. Oncology Letters 15: 1566–1572. DOI 10.3892/ol.2017.7457. [Google Scholar] [CrossRef]
Martinez-Balibrea E, Martínez-Cardús A, Ginés A, Ruiz de Porras V, Moutinho C et al. (2015). Tumor-related molecular mechanisms of oxaliplatin resistance. Molecular Cancer Therapy 14: 1767–1776. DOI 10.1158/1535-7163.MCT-14-0636. [Google Scholar] [CrossRef]
Pan Z, Qu C, Chen Y, Chen X, Liu X et al. (2019). Bufotalin induces cell cycle arrest and cell apoptosis in human malignant melanoma A375 cells. Oncology Reports 41: 2409–2417. DOI 10.3892/or.2019.7032. [Google Scholar] [CrossRef]
Pucci B, Kasten M, Giordano A (2000). Cell cycle and apoptosis. Neoplasia 2: 291–299. DOI 10.1038/sj.neo.7900101. [Google Scholar] [CrossRef]
Qi F, Li A, Inagaki Y, Kokudo N, Tamura S, Nakata M, Tang W (2011). Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. International Immunopharmacology 11: 342–349. DOI 10.1016/j.intimp.2010.12.007. [Google Scholar] [CrossRef]
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute 82: 1107–1112. DOI 10.1093/jnci/82.13.1107. [Google Scholar] [CrossRef]
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer Journal for Clinicians 71: 209–249. DOI 10.3322/caac.21660. [Google Scholar] [CrossRef]
Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V (2020). 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacology and Therapeutics 206: 107447. DOI 10.1016/j.pharmthera.2019.107447. [Google Scholar] [CrossRef]
Wang DL, Qi FH, Tang W, Wang FS (2011). Chemical constituents and bioactivities of the skin of Bufo bufo gargarizans Cantor. Chemistry and Biodiversity 8: 559–567. DOI 10.1002/cbdv.201000283. [Google Scholar] [CrossRef]
Wang Z, Qi F, Cui Y, Zhao L, Sun X, Tang W, Cai P (2018). An update on Chinese herbal medicines as adjuvant treatment of anticancer therapeutics. BioScience Trends 12: 220–239. DOI 10.5582/bst.2018.01144. [Google Scholar] [CrossRef]
Wei X, Si N, Zhang Y, Zhao H, Yang J, Wang H, Wang L, Han L, Bian B (2017). Evaluation of bufadienolides as the main antitumor components in cinobufacin injection for liver and gastric cancer therapy. PLoS One 12: e0169141. DOI 10.1371/journal.pone.0169141. [Google Scholar] [CrossRef]
Wu XY, Tian F, Su MH, Wu M, Huang Y, Hu LH, Jin L, Zhu XJ (2018). BF211, a derivative of bufalin, enhances the cytocidal effects in multiple myeloma cells by inhibiting the IL-6/JAK2/STAT3 pathway. International Immunopharmacology 64: 24–32. DOI 10.1016/j.intimp.2018.08.016. [Google Scholar] [CrossRef]
Yu H, Pardoll D, Jove R (2009). STATs in cancer inflammation and immunity: A leading role for STAT3. Nature Reviews Cancer 9: 798–809. DOI 10.1038/nrc2734. [Google Scholar] [CrossRef]
Zakaria A, Abdullah S, Nahar Z, Snigdha HJ, Murshed T et al. (2021). A short review of the genes involved in the development and progression of colorectal cancer.Biocell 45: 483–487. [Google Scholar]
Zhan X, Wu H, Wu H, Wang R, Luo C, Gao B, Chen Z, Li Q (2020). Metabolites from Bufo gargarizans (Cantor, 1842A review of traditional uses, pharmacological activity, toxicity and quality control. Journal of Ethnopharmacology 246: 112178. DOI 10.1016/j.jep.2019.112178. [Google Scholar] [CrossRef]
Zhang C, Ma K, Li WY (2019). Cinobufagin suppresses the characteristics of osteosarcoma cancer cells by inhibiting the IL-6-OPN-STAT3 pathway. Drug Design, Development and Therapy 13: 4075–4090. DOI 10.2147/dddt.S224312. [Google Scholar] [CrossRef]
Zhang DM, Liu JS, Tang MK, Yiu A, Cao HH, Jiang L, Chan JY, Tian HY, Fung KP, Ye WC (2012). Bufotalin from Venenum Bufonis inhibits growth of multidrug resistant HepG2 cells through G2/M cell cycle arrest and apoptosis. European Journal of Pharmacology 692: 19–28. DOI 10.1016/j.ejphar.2012.06.045. [Google Scholar] [CrossRef]
Zhang X, Ye M, Dong YH, Hu HB, Tao SJ, Yin J, Guo DA (2011). Biotransformation of bufadienolides by cell suspension cultures of Saussurea involucrata. Phytochemistry 72: 1779–1785. DOI 10.1016/j.phytochem.2011.05.004. [Google Scholar] [CrossRef]
Zhu YR, Xu Y, Fang JF, Zhou F, Deng XW, Zhang YQ (2014). Bufotalin-induced apoptosis in osteoblastoma cells is associated with endoplasmic reticulum stress activation. Biochemical and Biophysical Research Communications 451: 112–118. DOI 10.1016/j.bbrc.2014.07.077. [Google Scholar] [CrossRef]
Supplementary Materials
Figure S1: HT29 and RKO cells were treated with AG490 (5 μM) for 2 h and followed with treating with 3-epi-bufotalin (6 μM) for 24 h. Viability of cells (A). Apoptosis ratios of cells (B). Changes of JAK1 protein, p-STAT3 protein, apoptosis relative proteins BAX and BCL-2 (C) (ns: no statistical significance).
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |