DOI:10.32604/biocell.2022.021016

| BIOCELL DOI:10.32604/biocell.2022.021016 |  |
| Article |
Isolation and species diversity of arbuscular mycorrhizal fungi in the rhizosphere of Puccinellia tenuiflora of Songnen saline-alkaline grassland, China
College of Landscape Architecture, Northeast Forestry University, Harbin, 150040, China
*Address correspondence to: Chunxue Yang, senxiu99@163.com
Received: 23 December 2021; Accepted: 06 April 2022
Abstract: Salinization has led to the deterioration of the ecological environment, affected the growth of plants, and hindered the development of agriculture and forestry. Arbuscular mycorrhizal (AM) fungi, as important soil microorganisms, play significant physiological and ecological roles in promoting plant nutrient absorption and improving soil structure. Puccinellia tenuiflora (Turcz.) Scribn. et Merr. in Songnen saline-alkaline grassland was selected as the research object to observe AM fungal colonization of the roots and explore the species and diversity of AM fungi in symbiotic association with P. tenuiflora. This study showed that AM fungi colonized in P. tenuiflora roots and formed a typical Arum–type mycorrhizal structure. A significant correlation was observed between vesicular abundance and the colonization intensity of mycorrhiza. Isolation and identification revealed 40 species of AM fungi in the rhizosphere of P. tenuiflora, belonging to 14 genera, of which two species could not be identified. The richness of the genus Glomus was the highest, accounting for 30% of the total species. Funneliformis mosseae and Rhizophagus intraradices were isolated from all the samples and were the species with the widest distribution in the rhizosphere of P. tenuiflora. Correlation analysis showed that pH only had a significant impact on the distribution of a few species, such as Glomus pustulatum, Diversispora spurca, Glomus aggregatum, Rhizophagus clarum, and Acaulospora foveata. The present study provides a theoretical basis for further exploring the resources of AM fungi in saline-alkaline soil.
Keywords: Morphological identification; Spore density; Species richness; Diversity indexes; Colonization intensity
Songnen Plain is the primary distribution area of soda saline-alkali soil in China and also one of the three soda saline soil distribution areas in the world (Zhang and Feng, 2009; Wang et al., 2018), with a saline-alkali land area of 3.93 × 106 hm2 (Li and Zhang, 2005). More seriously, due to the rapid development of agriculture, animal husbandry, overexploitation, and utilization of resources, as well as the increasingly arid climate, the salinized land in Songnen Plain have increased by about 1.7% every year since the second half of the 20th century (Lin et al., 1999). To make full use of the resources of saline-alkali land and for the sustainable development of the ecological environment, several measures for improving soil physical, chemical, biological, agronomic, and hydraulic engineering have been undertaken and have provided good results (Zhang et al., 2002). Among them, the application of arbuscular mycorrhizal (AM) fungi as a “biological fertilizer” to the saline-alkali land is of great help to improve plant tolerance and improve soil structure; it is considered to be a green and efficient method and plays an irreplaceable role in the ecosystem (Zhang et al., 2019; Deng et al., 2019).
AM fungi are one of the largest biomass components of soil microbial community (Wang et al., 2015); about 80% of bryophytes, pteridophytes, and spermatophytes, can be colonized by AM fungi to form a symbiotic system (Brundrett, 2009). This mycorrhizal symbiotic system has become a new type of bioremediation to cope with global change, which can shorten the restoration cycle of damaged and degraded ecosystems, improve the success rate of restoration and ensure the stability of restoration effects (van der Heijden et al., 2008). AM fungi play an important role in the remediation of saline soil in the following aspects: (1) improve the quality of saline-alkali soil by improving the physical and chemical properties of soil (Rilling et al., 2002), (2) significantly promote the absorption and utilization of water, mineral elements, and phosphorus to promote plant growth (Metwally and Abdelhameed, 2018; Parvin et al., 2020), (3) operate nutrients between mycorrhizal fungi and plants, mycorrhizal fungi and microorganisms, and plants and microorganisms through their huge mycelium network, to form a complete biological community (Yang et al., 2015), etc.
Abundant AM fungi resources exist naturally in saline habitats of terrestrial ecosystems; for example, 19, 6, and 24 AM fungi species were found in the saline soil of Argentina, northern Portugal, and the salt marsh of Cabo de Gata Natural Park in Europe (Becerra et al., 2014; Estrada et al., 2013b; Oliveira et al., 2005). In addition, 18, 33, and 26 AM fungal species were identified from the rhizosphere of plants in saline soils of Gansu, Ningxia, and Inner Mongolia Province, China, respectively (Huang, 2007; Liu et al., 2017; Zhang, 2007). Importantly, the composition of AM fungal communities is strongly influenced by the high pH of salinized soils and is often specific in such soils, which may contain AM fungi with special functions, especially strong stress resistance (Carvalho et al., 2003; Adenan et al., 2021; Estrada et al., 2013a). Therefore, exploring the composition of AM fungi population in the different rhizosphere of different plants in different areas is conducive to the development of professional AM fungal agents (He et al., 2020).
Puccinellia tenuiflora, a perennial herbaceous plant of the Poaceae, is an excellent forage that grows well in soils with high Na+ and pH (Greenway and Munns, 1980; Chen et al., 2018; Zhao et al., 2016), widely distributed in the Songnen saline grassland (Yu et al., 2011). Yang et al. (2019) showed that the growth of P. tenuiflora could promote the downward leaching of salt and reduce the evaporation of water, which is important for the restoration of alkaline soil and is the pioneer of the restoration of degraded land in Songnen saline grassland. As for the salt-alkali tolerance mechanism of P. tenuiflora, many scholars have carried out a series of studies (Gao et al., 2005; Wang et al., 2008; Kobayashi et al., 2015). More importantly, the degradation of saline-alkali soil has been controlled to some extent through the application of cultivated P. tenuiflora in Heilongjiang Province and other areas, which also confirms the higher tolerance and ecological value of P. tenuiflora (Sun et al., 1997; Yan and Sun, 2000).
In an earlier study, we found that AM fungi positively affect the tolerance of P. tenuiflora under NaCl and NaHCO3 stresses with a concentration of up to 400 mmol/L. That is, AM fungi inoculation can improve the resistance to stress to a certain extent by increasing the activity of antioxidant enzymes, the content of metabolites, and plant hormones in P. tenuiflora (Zhang and Yang, 2018; Yang et al., 2020b). Therefore, it seems reasonable to use AM fungi to further improve the saline-alkali tolerance of P. tenuiflora and then use this symbiont in saline-alkali soil remediation. More investigations on AM fungi symbiotic with P. tenuiflora are worth carrying out. However, in our previous study, only three dominant AM fungi were identified in the rhizosphere soil of P. tenuiflora in songnen saline-alkali grassland by the molecular biological method, including Rhizoglomus intraradices, Claroideoglomus etunicatum, and Funneliformis mosseae (Yang et al., 2020a). The extraction of AM fungal spore DNA is often affected by the composition of microflora and growth cycle, and the DNA is prone to contamination in the amplification process, making it difficult to obtain correct detection results (Clapp et al., 2002). Therefore, the in-depth study of AM fungal diversity is still inseparable from the traditional morphological method for spore identification. Based on these, considering P. tenuiflora in Songnen saline-alkali grassland as the research object, in this study, we investigated the colonization of AM fungi in the rhizosphere of P. tenuiflora, isolated AM fungal spores in the rhizosphere soil, and identified the species and diversity of AM fungi symbiotic with P. tenuiflora through morphological characteristics. It is expected to provide a theoretical basis for studying AM fungal resources in saline-alkaline habitats and promoting the application of AM fungi in improving the saline-alkali tolerance of P. tenuiflora, especially for the strain screening in the production of mycorrhizal seedlings.
Sampling site and samples collection
Zhaodong City (125°–125°42’ E, 46°16’–46°17’ N) in Heilongjiang Province is located in the middle of the Songnen Plain and has a temperate continental monsoon climate. Precipitation is concentrated during specific times of the year, with annual precipitation of 350–550 mm, which primarily occurs from July to September.
Referring to the method described by Meng (1996), nine sites were randomly selected from four different directions, where large clumps of single species of P. tenuiflora were distributed. After removing the impurities of dead leaves and 5 cm thick topsoil, the soil was dug to a depth of 10–20 cm to collect 1.5 kg of the intact root system and rhizosphere soil of P. tenuiflora per site. Then, 1 kg of the sample was selected by quartering for air-drying and preservation. The roots were separated from each sample, cut into approximately 1 cm-long segments, and soaked in FAA solution (5 mL formalin, 5 mL glacial acetic acid, and 90 mL of 70% ethyl alcohol) after being repeatedly washed with distilled water. Finally, the remaining soil and roots samples were stored at 4°C.
The soil pH was measured using a pH meter (METTLER TOLEDO FE20). From each sample, 20 g of air-dried soil was taken and fully dissolved in 20 mL distilled water, and the adjusted pH meter was immersed into the mixed suspension to read the pH value; this was repeated three times.
Assessment of natural colonization of arbuscular mycorrhizal fungi
Trypan-blue staining method was used to stain the structure of AM fungi in root segments. First, the root segments soaked in the FAA solution were taken out, washed with distilled water, and incubated in a 10% KOH solution (90°C, 60 min) to make them soft and transparent. After that, the root segments were neutralized for 5–10 min with 2% hydrochloric acid and then stained at 90°C for 30 min in 0.05% trypan-blue reagent. The decolorization was carried out with glycerin lactate solution. The morphology of the arbuscle, vesicles, and hyphae was examined under the microscope (OLYMPUS-DSX500), and 10 root segments were repeated for each sample. The colonization status of P. tenuiflora was evaluated and graded by the root segment observation method (Trouvelot et al., 1986); that is, the colonization intensities of the root segments were categorized into five grades: (1) 0%–1%: Grade 1, (2) 1%–10%: Grade 2, (3) 11%–50%: Grade 3, (4) 51%–90%: Grade 4, and (5) 91%–100%: Grade 5. Similarly, the arbuscular abundance was categorized into three grades: (1) Grade 1: the number of arbuscles was <5%, (2) Grade 2: the number of arbuscles was 5%–50%, and (3) Grade 3: number of arbuscles was >50%. The evaluation method of vesicles was the same. MYCOCALC software was used to enter grade parameters to obtain colonization rate (%), colonization intensity (%), arbuscular abundance (%), and vesicle abundance (%). The calculation formulae were as follows:
Colonization rate (%) = the number of colonized root segments/the total number of root segments × 100.
Colonization intensity (%) = (0.95 × N5 + 0.70 × N4 + 0.30 × N3 + 0.05 × N2 + 0.01 × N1)/total number of root segments × 100. Note: 0.95, 0.70, 0.30, 0.05, and 0.01 represent the weight of each grade, respectively, the same below. N5 = the number of the root segments colonized at Grade 5, N4, N3, N2, and N1 had the same meaning.
Arbuscular abundance (%) = (mA3 + 0.5 × mA2 + 0.1 × mA1)/100, where m = Colonization intensity (%) × the total number of root segments/the number of colonized root segments. Note: A3 = the number of root segments with arbuscular abundance of Grade 3.
Separation and morphological identification of arbuscular mycorrhizal fungi spores
The spores were isolated by wet screening and sucrose density-gradient centrifugation method. Fifty grams of soil from each sampling point was passed through three standard soil sieves (the aperture from top to bottom is 76, 50, and 38.5 μm); the soil on the sieve was washed in running water until the filtrate was clear. The residue in the lower two sieves was transferred into a 50 mL centrifuge tube containing 60% sucrose solution, allowed to stand for 1.5 h, centrifuged at 4500 r/min for 20 min. The obtained supernatant was quickly poured onto the sieve (aperture 38.5 μm) and washed with water for 2 min to obtain AM fungal spores.
The spores were counted under the anatomical microscope. Spore density (SD) was calculated as the number of spores per 50 g of air-dried soil from direct counts. For the morphological identification of spore species, spores were picked under the anatomical microscope, and then polyvinyl alcohol-lactic acid-glycerine (PVLG), as well as Melzer’s reagent was added to prepare a permanent slide. The diameter, color, surface decoration, and thickness of single spores were observed under an optical microscope (OLYMPUS-DSX500). Fungal species were identified based on original and recent species descriptions, as well as the pictures and species classification provided by INVAM (http://fungi.invam.wvu.edu/the-fungi/species-descriptions.html).
Diversity indexes were calculated, including indexes of separation frequency, relative abundance, and importance value. The equations used to calculate these indexes were as follows (Yang et al., 2011):
Separation frequency (F) = Occurrence frequency of certain species/total sample number × 100%
Relative abundance (RA) = Spore number of certain species/total quantity of AM fungal spores × 100%
Importance value (IV) = (F + RA)/2 × 100%
The diversity function of the “Vegan” package of R and RStudio (version 4.1.1) were used to calculate the diversity index of the four genera with the largest abundance. The “TreeMap” package and the “d3Tree” package were used to build the tree diagram. The genescloud platform (https://www.genescloud.cn) was used to make the chord diagram. The “Psych” package of R and RStudio was used to construct the correlation matrix between pH value and AM fungi distribution, as well as to assess the correlation between the colonization indexes. The decorana function in RStudio was used to examine the axis lengths of the community data, followed by RDA analysis to explore the relationship between pH and AM fungal communities. SPSS (Statistical Product and Service Solutions) 25.0 was used for one-way ANOVA and correlation analysis. Spearman correlation coefficient was used to describe the correlation, and when p < 0.05, the difference was statistically significant. Excel (2019) was used to process the experimental data, and all the data were expressed as mean ± standard deviation (n = 3).
Natural colonization of arbuscular mycorrhizal fungi
A typical Arum-type (A-type) mycorrhizal structure was observed in the roots (Fig. 1). The structural characteristics of P. tenuiflora rhizosphere were as follows: the hyphae colonized on the root surface (Fig. 1a), grew in cortical cell spaces of the host plant, generated many intercellular hyphae (Fig. 1b), which could branch laterally into the cells (Fig. 1c). A portion of hyphae grew into dichotomous branching (Fig. 1e) and formed the arbuscules (Fig. 1d), a typical AM structures with a cauliflower shape. The apices of the endophytic hyphae expanded to form vesicles and were consistently presented as a circle (Fig. 1g), oval (Figs. 1h and 1i), or irregular shapes (Figs. 1c and 1f).
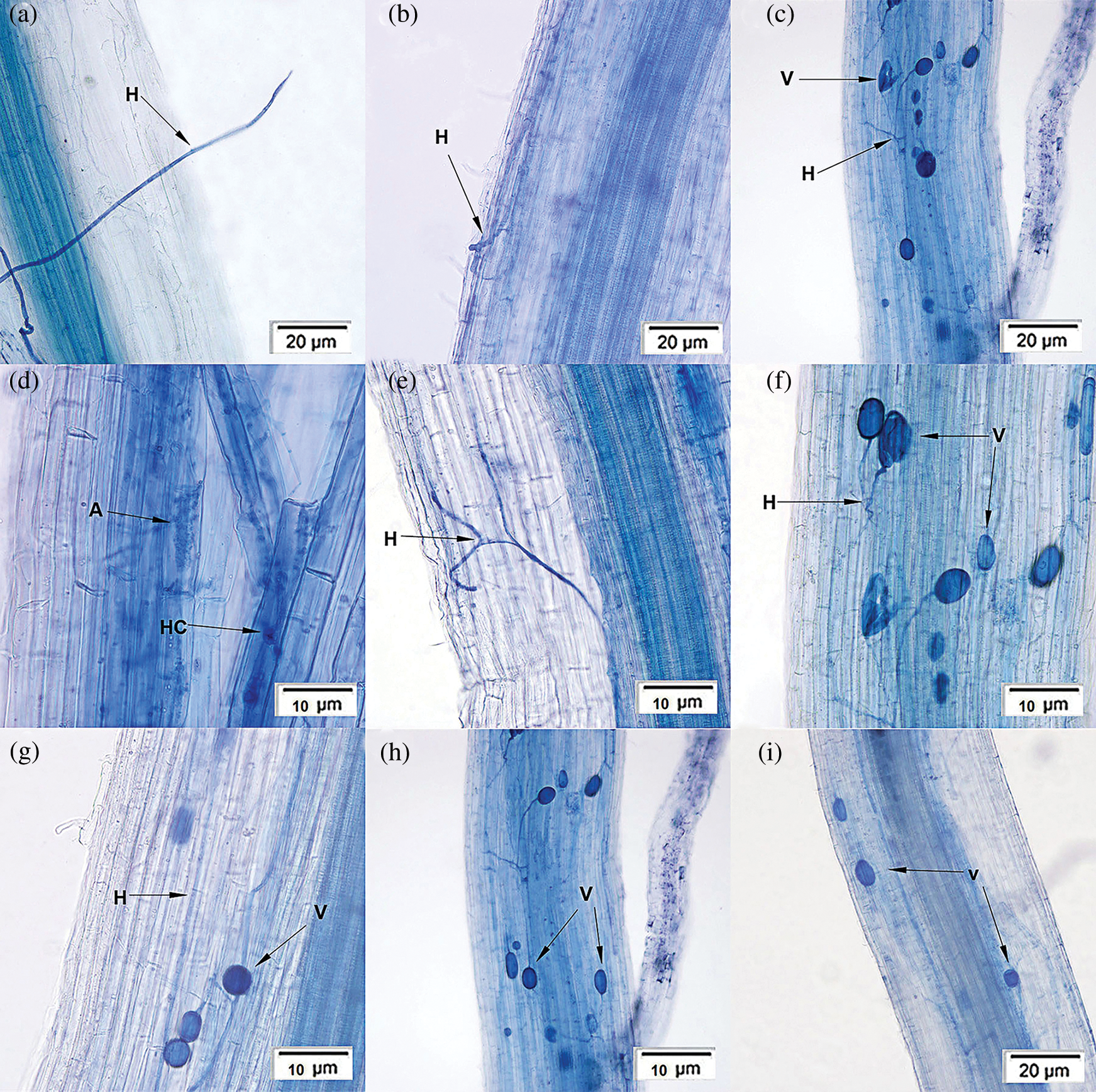
Figure 1: Symbiotic structure characteristics of Puccinellia tenuiflora rhizosphere. Notes: Hypha (H); Vesicle (V); Hypha coil (HC); Arbuscule (A).
The investigation showed that the soil of the nine sampling sites in Songnen saline-alkali grassland belonged to severe alkaline soil (pH > 8.5, Table 1), and the ANOVA test indicated significant differences among different sites. The roots of P. tenuiflora could be colonized in different pH soil habitats, but the value of colonization indexes was not normally distributed. As shown in Table 1, when the soil pH was at the lowest value (pH = 9.35), the colonization rate was 80%, and the spore density was 25.14/g, reaching the maximum value. When the soil pH was the highest (pH = 9.81), the colonization rate was the lowest (40%). In addition, the maximum colonization rate (93.3%) was observed when the soil pH reached 9.72, the maximal vesicle abundance (13.31%), and the maximal colonization intensity (30.87%) were observed at a soil pH of 9.50. The values of arbuscular abundance were relatively low (<1%) in all examined samples, and even no arbuscule was detected in most samples.

The morphological identification of AM fungi
Through morphological identification, 40 AM fungi species belonging to 14 genera were isolated from the rhizosphere soil of P. tenuiflora (Fig. 2), including 12 species of Glomus, 11 species of Acaulospora, five species of Rhizophagus, two species of Ambispora, as well as one species each of Septoglomos, Funneliformis, Entrophospora, Diversispora, Sclerocystis, Scutellospora, Pacispora, Claroideoglomus, Racocetra, and Halonatospora.
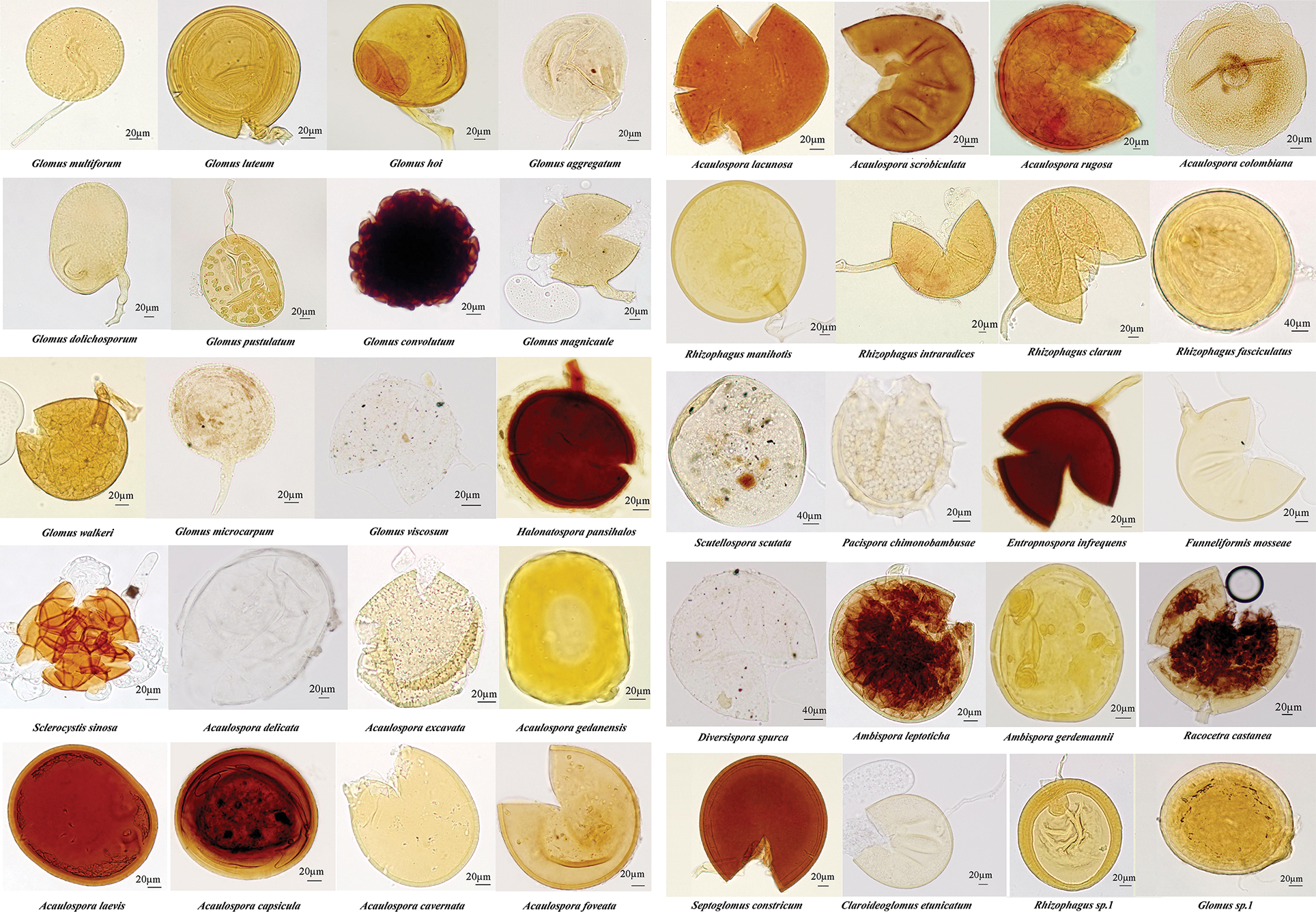
Figure 2: Spore morphology of arbuscular mycorrhizal fungi in the rhizosphere of Puccinellia tenuiflora.
The diversity indexes of arbuscular mycorrhizal fungal species
As shown in Table 2 and Fig. 3a, among the nine soil samples, the largest diversity were identified in Nos. 2, 1, and 9, with 20, 19, and 19 species, accounting for 50.00%, 47.50%, and 47.50% of the total, respectively. In addition, the complex relationship between the distribution and quantity of AM fungi and sampling sites can be seen in Fig. 3a, indicating a complex association network in the community, with a strong adoptive ability of the community to the changeable saline-alkali environment. As shown in Table 2 and Fig. 3b, Glomus was the most widely distributed genus with the highest richness, accounting for 30% of all species, followed by Acaulospora, Rhizophagus, and Ambispora. What’s more, Funneliformis mosseae and Rhizophagus intraradices were isolated from all soil samples. Acaulospora laevis, Acaulospora scrobiculata, and Claroideoglomus etunicatum were common species in the rhizosphere of P. tenuiflora and were observed in all six soil samples. Acaulospora colombiana, Acaulospora lacunosa, and Sclerocystis sinuosa were occasional species and were only isolated from soil samples 3, 5, and 1, respectively.

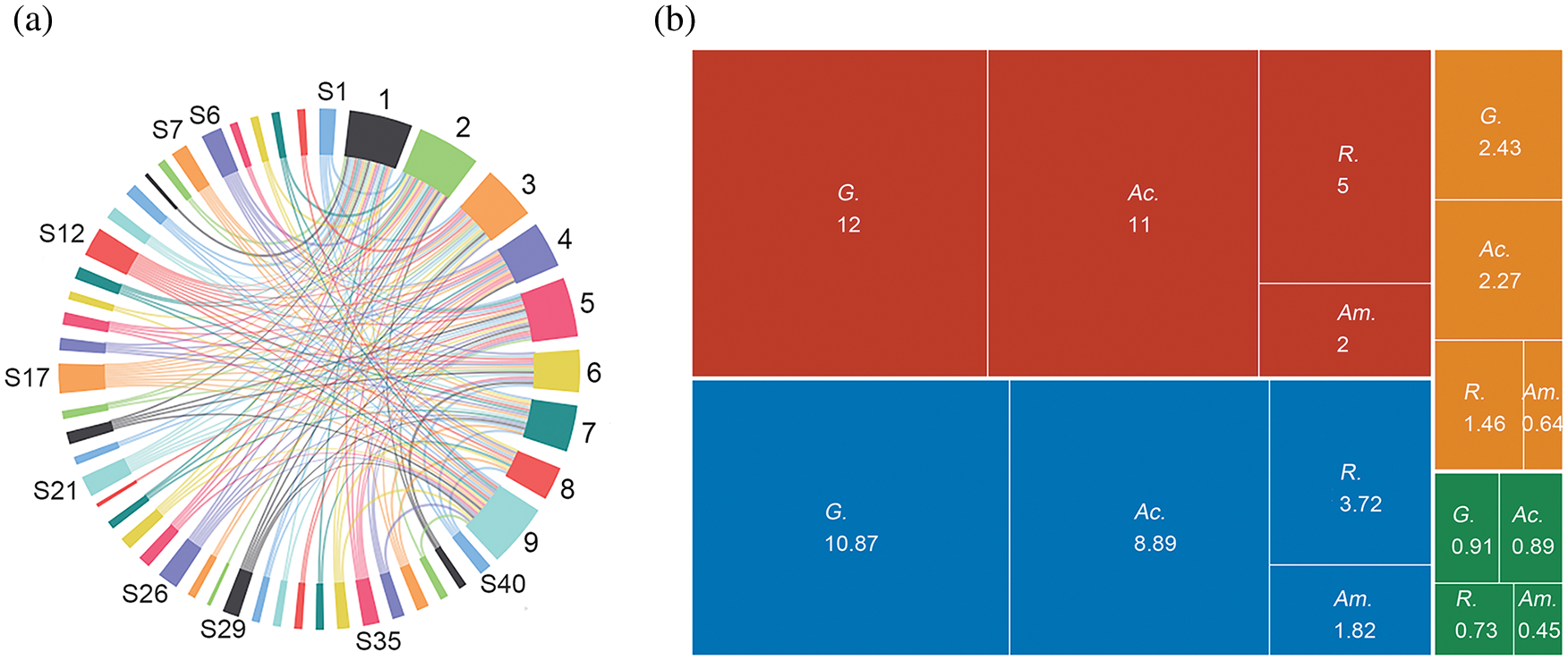
Figure 3: Arbuscular mycorrhizal (AM) fungal species distribution (a) and Alpha diversity of the four most abundant genera (b). Note: (a), S1–S40: 40 AM fungal species (The numbering of some species is omitted); 1–9: 9 sample sites. The proportion of each node segment shows the overall proportion of the number of species or sample sites. (b), Red area: Total species; Blue area: Inv; Orange area: Shannon index; Green area: Simpson index; G.: Glomus; Ac.: Acaulospora; R.: Rhizophagus; Am.: Ambispora.
Spearman correlation coefficient (Fig. 4) showed that vesicle abundance and arbuscular abundance were significantly correlated with colonization intensity at the level of 0.05.
The RDA analysis (Axis length = 1.9679) results showed a significant effect of the soil pH on the variation of AM fungal community between different sampling sites (r2 = 0.903, p = 0.001).
In addition, Correlation matrix analysis showed that pH was correlated with the distribution of multiple species. For example, pH was negatively correlated with Glomus pustulatum (R = −0.693, p = 0.039), Diversispora spurca (R = −0.6928, p = 0.039), and Glomus aggregatum (R = −0.725, p = 0.0272). On the contrary, pH was positively correlated with Rhizophagus clarum (R = 0.693, p = 0.0385) and Acaulospora foveata (R = 0.822, p = 0.007).
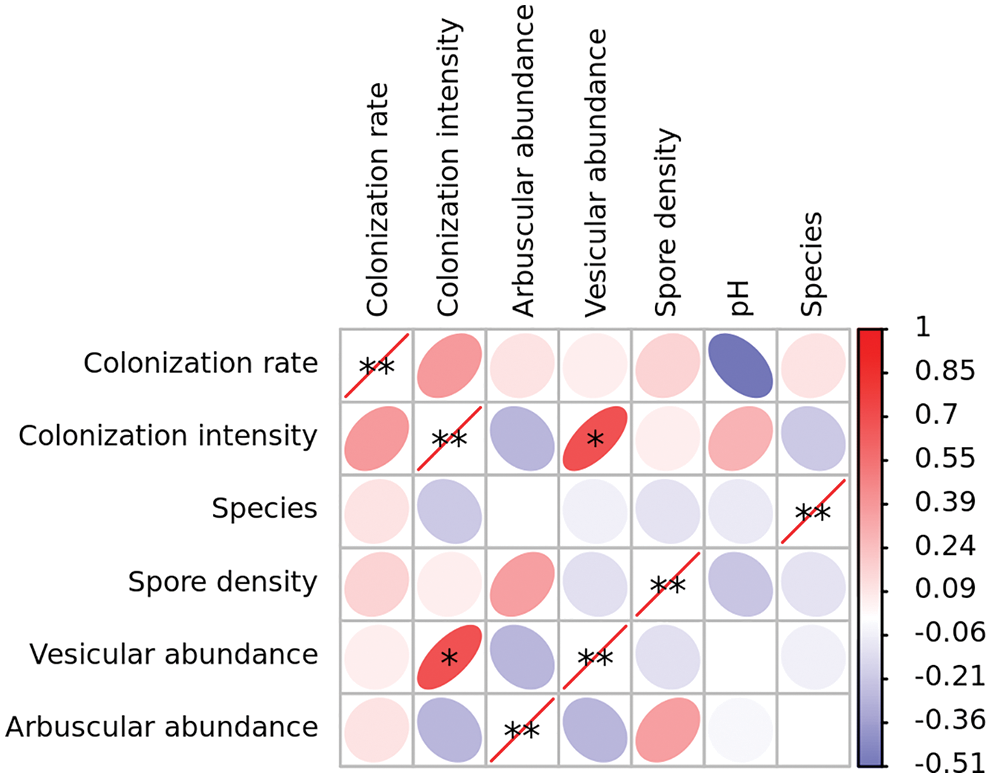
Figure 4: Correlations between pH, arbuscular mycorrhizal (AM) fungal colonization, and diversity in rhizosphere soil of Puccinellia tenuiflora. * indicated the significance at p < 0.05 and R > 0.6.
Arbuscular mycorrhizal fungi naturally colonized the root system of Puccinellia tenuiflora
As is well-known, the mycorrhizal colonization rate describes the colonization degree of plant roots by AM fungi and also reflects the arbuscular mycorrhiza formation and the affinity of AM fungi to plants. In the natural state, the high colonization rate may be due to the highly harmonious symbiotic relationship between AM fungi and host plants in the long–term coevolution process. The structures of arbuscle, hyphae, and vesicles observed in this study were similar to those of Moreira-Souza et al. (2003), and these structural characteristics are the main indicators for assessing mycorrhiza formation. Among them, vesicles usually exist in roots for a longer period, while the formation and decomposition of arbuscle are relatively fast (Wu et al., 2009), which may be one of the reasons why the abundance of vesicles detected in all samples was higher than that of arbuscle. In addition, P. tenuiflora often grows in alkaline spots, where there is temporary shallow water accumulation on the surface in the rainy season (Zhao et al., 2000). AM fungi are sensitive to excess water and hypoxia, which is also an important factor that inhibits the formation of arbuscle.
Interestingly, we found a significant correlation between vesicle abundance and colonization intensity, possibly because vesicles, as nutrient storage organs (Yang et al., 2015), play a unique role in the mycorrhizal colonization of plant roots (Smith and Read, 2008).
Arbuscular mycorrhizal fungi resources are abundant in the rhizosphere of P. tenuiflora of Songnen saline-alkaline grassland
Recent studies have shown that the species richness of Glomeraceae is higher in saline soils (Estrada et al., 2013; Krishnamoorthy et al., 2020), and Glomus is the dominant genus (Bonfim et al., 2016; Wang et al., 2004). Our study also confirmed the conclusion. The spore-forming patterns of Glomus species may be more adaptive to environmental conditions in long–term saline stress, or soil nutrient and climate change enhance the dominance of Glomus (Bonfim et al., 2016).
According to statistics, Funneliformis mosseae and Rhizophagus intraradices, were the most widely distributed species in the rhizosphere of P. tenuiflora, and these two species were also found in saline habitats of many other countries (Estrada et al., 2013b; Evelin et al., 2012; Oliveira et al., 2005), which suggests that these two species possibly have a higher level of adaptability. Besides, our previous studies have shown that both Funneliformis mosseae and Rhizophagus intraradices formed a symbiotic association with P. tenuiflora and improved the salt-alkali tolerance of P. tenuiflora (Yang et al., 2017; Zhao et al., 2020). Moreover, previous studies have verified the effects of Funneliformis mosseae and Rhizophagus intraradices on the salt tolerance of plants (Wang et al., 2020; Qiu et al., 2020). By increasing the nutrient content in roots and leaves (e.g., N, P, K, Ca, and Mg), maintaining a good ion balance (e.g., K+/Na+), increasing the activity of antioxidant enzymes, and the accumulation of antioxidant compounds in plants, mycorrhiza could improve the tolerance of plants to salt stress. Therefore, it could be preliminary concluded that Funneliformis mosseae and Rhizophagus intraradices might show a certain degree of competitiveness and efficiency in a saline-alkali environment.
Notably, among the 40 AM fungi species isolated in our study, the two species, which distributed widely, belonging to Glomus and Rhizophagus, respectively, could not be identified and needed to be propagated for further identification. In addition, the abundant AM fungi resources distributed in Songnen saline-alkali grassland need to be explored further in the future.
Effects of high pH of saline-alkali soil on arbuscular mycorrhizal fungi
Previous studies on species composition of AM fungi in saline soils showed that soil pH directly affects the species distribution and sporulation of AM fungi, as well as the formation and effectiveness of mycorrhiza (Yang et al., 2020c; Wang et al., 2010). However, it should be noted that in the current study, we did not find any significant association of pH with AM fungal colonization, species richness, and spore density. However, it had a significant impact on the distribution of partially AM fungal species; this might be related to the adaptability of these AM fungal species formed in the long-term evolution, making them more vulnerable to the influence of alkalinity, and the impact of other factors needs to be further studied.
This study showed abundant AM fungal resources in the rhizosphere of P. tenuiflora growing in Songnen saline-alkaline grassland. AM fungi colonize the roots of P. tenuiflora to form Arum–type arbuscular mycorrhiza, and the colonization intensity is significantly correlated with the abundance of vesicle structure. Genus Glomus had the widest distribution and the dominant position. Funneliformis mosseae and Rhizophagus intraradices might be the efficient AM fungal species in saline-alkali habitats. High soil pH value only affected the distribution of some AM fungi and had no significant correlation with colonization, species richness, and spore density. These findings provide a theoretical basis for further understanding of the highly-efficient AM fungal resources in saline-alkali soils and promote the application of mycorrhizal symbionts in the ecological restoration of saline-alkali lands. However, the molecular diversity of AM fungi in Songnen saline-alkali grassland and the role of soil physical and chemical properties on diversity needs further exploration and will be the focus of our future research.
Availability of Data and Materials:All data generated or analyzed during this study are included in this published article.
Author Contribution:The authors confirm contribution to the paper as follows: study conception and design: Chunxue Yang; data collection: Fei Chen; analysis and interpretation of results: Yajie Liu, Fei Chen; problem-solving of the research: Wenna Zhao, Yudan Wang; draft manuscript preparation: Yunhui Zhou. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval:Not applicable.
Funding Statement:This work was supported by the National Natural Science Foundation of China (31601986), the Fundamental Research Funds for the Central Universities (2572018BK02), and Heilongjiang Postdoctoral Scientific Research Developmental Fund (LBH–Q16005).
Conflicts of Interest:The authors declare that they have no conflicts of interest to report regarding the present study.
Adenan S, Oja J, Alatalo JM, Shraim AM, Alsafran M, Tedersoo L, Zobel M, Ahmed T (2021). Diversity of arbuscular mycorrhizal fungi and its chemical drivers across dryland habitats. Mycorrhiza 31: 685–697. DOI 10.1007/s00572-021-01052-3. [Google Scholar] [CrossRef]
Becerra A, Bartolon N, Cofre N, Soteras F, Cabello M (2014). Arbuscular mycorrhizal fungi in saline soils: Vertical distribution at different soil depth. Brazilian Society for Microbiology 45: 585–594. DOI 10.1590/s1517-83822014000200029. [Google Scholar] [CrossRef]
Bonfim JA, Vasconcellos RLF, Gumiere T, Mescolotti DDC, Oehl F, Cardoso EJB (2016). Diversity of arbuscular mycorrhizal fungi in a Brazilian Atlantic Forest Toposequence. Microbial Ecology 71: 164–177. DOI 10.1007/s00248-015-0661-0. [Google Scholar] [CrossRef]
Brundrett M (2009). Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant and Soil 320: 37–77. DOI 10.1007/s11104-008-9877-9. [Google Scholar] [CrossRef]
Chen F, Wang N, Huang SC, Gao XM, Zhang L, Yang CX (2018). Effects of dominant fungi species in rhizosphere of Puccinellia tenuiflora on its salt and salinity tolerance. Northern Horticulture 14: 91–97. DOI 10.11937/bfyy.20173351. [Google Scholar] [CrossRef]
Carvalho LM, Correia PM, Cacador I, Martins-Loucao MA (2003). Effects of salinity and flooding on the infectivity of salt marsh arbuscular mycorrhizal fungi in Aster tripolium L. Biology and Fertility of Soils 38: 137–143. DOI 10.1007/s00374-003-0621-6. [Google Scholar] [CrossRef]
Clapp IP, Rodriguez A, Dodd JC (2002). Glomales rRNA gene diversity—all that glistens is not necessarily glomalean? Mycorrhiza 12: 269–270. [Google Scholar]
Deng J, Li F, Gu LJ, Duan TY (2019). Effect of arbuscular mycorrhizal fungi on alfalfa seedling growth at different soil pH. Pratacultural Science 36: 2854–2862. [Google Scholar]
Estrada B, Barea JM, Aroca R, Ruiz-Lozano JM (2013a). A native Glomus intraradices strain from a Mediterranean saline area exhibits salt tolerance and enhanced symbiotic efficiency with maize plants under salt stress conditions. Plant and Soil 366: 333–349. DOI 10.1007/s11104-012-1409-y. [Google Scholar] [CrossRef]
Estrada B, Beltrán-Hermoso M, Palenzuela J, Iwase K, Ruiz-Lozano JM, Barea J, Oehl F (2013b). Diversity of arbuscular mycorrhizal fungi in the rhizosphere of Asteriscus maritimus (L.) Less., a representative plant species in arid and saline Mediterranean ecosystems. Journal of Arid Environments 97: 170–175. DOI 10.1016/j.jaridenv.2013.05.019. [Google Scholar] [CrossRef]
Evelin H, Giri B, Kapoor R (2012). Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 22: 203–217. DOI 10.1007/s00572-011-0392-0. [Google Scholar] [CrossRef]
Gao HW, Wang JB, Sun GR (2005). Further study of physiological mechanism of the saline-alkali tolerance of Puccinellia tenuiflora. Acta Botanica Boreali-Occidentalia Sinica 25: 1589–1594. [Google Scholar]
Greenway H, Munns R (1980). Mechanism of salt tolerance in non-halophytes. Annual Review of Plant Physiology 31: 149–190. DOI 10.1146/annurev.pp.31.060180.001053. [Google Scholar] [CrossRef]
He F, Li DH, Bu F (2020). Analysis of arbuscular mycorrhizal fungal community structure in the rhizosphere of different tea cultivars. Journal of Tea Science 40: 319–327. [Google Scholar]
Huang YH (2007). The Diversity, Distribution and Improvement Salt- Resistance of Host Plants of AM Fungi in Saline Alkaline Soil of Inner Mongolia (Master Thesis). Northwest Agriculture & Forestry University. [Google Scholar]
Kobayashi S, Satone H, Tan EK, Kurokochi H, Asakawa S, Liu SK, Takano T (2015). Transcriptional responses of a bicarbonate-tolerant monocot, Puccinellia tenuiflora, and a related bicarbonate-sensitive species, Poa annua, to NaHCO3 stress. International Journal of Molecular Sciences 16: 496–509. DOI 10.3390/ijms16010496. [Google Scholar] [CrossRef]
Krishnamoorthy R, Anandham R, Senthilkumar M, Sa T (2020). Diversity and community structure of arbuscular mycorrhizal fungi in the rhizosphere of salt-affected soils. Rhizosphere Microbes 23: 453–470. DOI 10.1007/978-981-15-9154-9_18. [Google Scholar] [CrossRef]
Li XY, Zhang SW (2005). Tempo-spatial dynamics and driving factors of saline-alkali land in Da’an City of Jilin Province. Resource Science 27: 92–97. DOI 10.3321/j.issn:1007-7588.2005.03.015. [Google Scholar] [CrossRef]
Lin NF, Tang J, Bian JM, Yang JQ (1999). The quaternary environmental evolution and the problem of desertification in northeast Plain. Quaternary Sciences 5: 448–455. [Google Scholar]
Liu HG, Wang YJ, Tang M (2017). Arbuscular mycorrhizal fungi diversity associated with two halophytes Lycium barbarum L. and Elaeagnus angustifolia L. in Ningxia. China Archives of Agronomy and Soil Science 63: 796–806. DOI 10.1080/03650340.2016.1235783. [Google Scholar] [CrossRef]
Meng FR (1996). Forest Mycorrhizology. Harbin: Northeast Forestry University Press. [Google Scholar]
Metwally RA, Abdelhameed RE (2018). Synergistic effect of arbuscular mycorrhizal fungi on growth and physiology of salt-stressed Trigonella foenum-graecum plants. Biocatalysis and Agricultural Biotechnology 16: 538–544. DOI 10.1016/j.bcab.2018.08.018. [Google Scholar] [CrossRef]
Moreira-Souza M, Trufem SFB, Gomes-da-Costa SM, Cardoso EJB (2003). Arbuscular mycorrhizal fungi associated with Araucaria angustifolia (Bert.) O. Ktze. Mycorrhiza 13: 211–215. DOI 10.1007/s00572-003-0221-1. [Google Scholar] [CrossRef]
Oliveira RS, Vosatk M, Dodd JC, Castro PML (2005). Studies on the diversity of arbuscular mycorrhizal fungi and the efficacy of two native isolates in a highly alkaline anthropogenic sediment. Mycorrhiza 16: 23–31. DOI 10.1007/s00572-005-0010-0. [Google Scholar] [CrossRef]
Parvin S, Geel MV, Yeasmin T, Verbruggen E, Honnay O (2020). Effects of single and multiple species inocula of arbuscular mycorrhizal fungi on the salinity tolerance of a Bangladeshi rice (Oryza sativa L.) cultivar. Mycorrhiza 30: 431–444. DOI 10.1007/s00572-020-00957-9. [Google Scholar] [CrossRef]
Qiu YJ, Zhang NL, Zhang LL, Zhang XL, Wu AP, Huang JY, Yu SQ, Wang YH (2020). Mediation of arbuscular mycorrhizal fungi on growth and biochemical parameters of Ligustrum vicaryi in response to salinity. Physiological and Molecular Plant Pathology 112: 101522. DOI 10.1016/j.pmpp.2020.101522. [Google Scholar] [CrossRef]
Rilling MC, Wright SF, Eviner VT (2002). The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: Comparing effects of five plant species. Plant and Soil 238: 325–333. DOI 10.1023/A:1014483303813. [Google Scholar] [CrossRef]
Smith SE, Read D (2008). Mineral nutrition, toxic element accumulation and water relations of arbuscular mycorrhizal plants. In: Mycorrhizal Symbiosis, Third edition, pp. 145–148. London: Academic Press. [Google Scholar]
Sun GR, Yan XF, Xiao W (1997). Preliminary study on physiological mechanism of saline-alkaline tolerance of Puccinellia tenuiflora. Plant Science Journal 15: 162–166. [Google Scholar]
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986). Mesure du Taux de Mycorhization VA d’un Systeme Radiculaire. Recherche de Methodes d’estimation Ayant une Signification Fonctionnelle. Paris: INRA Press. [Google Scholar]
van der Heijden MGA, Verkade S, De Bruin SJ (2008). Mycorrhizal fungi reduce the negative effects of nitrogen enrichment on plant community structure in dune grassland. Global Change Biology 14: 2626–2635. DOI 10.1111/j.1365-2486.2008.01691.x. [Google Scholar] [CrossRef]
Wang FY, Liu RJ, Lin XG, Zhou JM (2004). Arbuscular mycorrhizal status of wild plants in saline-alkaline soils of the Yellow River Delta. Mycorrhiza 14: 133–137. DOI 10.1007/s00572-003-0248-3. [Google Scholar] [CrossRef]
Wang CM, Zhang JL, Liu XS, Li Z, Wu GQ, Cai JY, Timothy JF, Wang SM (2009). Puccinellia tenuiflora maintains a low Na+ level under salinity by limiting unidirectional Na+ influx resulting in a high selectivity for K+ over Na+. Plant, Cell & Environment 32: 486–496. DOI 10.1111/j.1365-3040.2009.01942.x. [Google Scholar] [CrossRef]
Wang HF, Zhao X, Yan XF (2010). Primary survey for plant arbuscular mycorrhiza in Songnen alkaline grassland. Chinese Journal of Soil Science 6: 1380–1385. DOI 10.19336/j.cnki.trtb.2010.06.019. [Google Scholar] [CrossRef]
Wang JP, Zhai L, Ma JY, Zhang JC, Wang GG, Liu X, Zhang SF, Song J, Wu YK (2020). Comparative physiological mechanisms of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects on leaves and roots of Zelkova serrata. Mycorrhiza 30: 341–355. DOI 10.1007/s00572-020-00954-y. [Google Scholar] [CrossRef]
Wang MM, Rengasamy P, Wang ZC, Yang F, Ma HY et al. (2018). Identification of the most limiting factor for rice yield using soil data collected before planting and during the reproductive stage. Land Degradation and Development 29: 2310–2320. DOI 10.1002/ldr.3026. [Google Scholar] [CrossRef]
Wang Q, Wang Q, Wang XJ, Zhang L, Jin L (2015). Research progress on ecological function of arbuscular mycorrhizal network. Chinese Journal of Applied Ecology 7: 2192–2202. DOI 10.13287/j.1001-9332.20150506.017. [Google Scholar] [CrossRef]
Wu YQ, Liu TT, He XL (2009). Mycorrhizal and dark septate endophytic fungi under the canopies of desert plants in Mu Us Sandy Land of China. Frontiers of Agriculture in China 3: 164–170. DOI 10.1007/s11703-009-0026-x. [Google Scholar] [CrossRef]
Yan XF, Sun GR (2000). Physiology and Ecology of Puccinellia tenuiflora. China: Science Press. [Google Scholar]
Yang CX, Huang SC, Wang YD, Chen F, Wang N, Lin JX (2017). Effects of two kinds of AMF on the antioxidant enzyme activity of Puccinellia tenuiflora seedlings under salt-alkali stress. Fresenius Environmental Bulletin 26: 5420–5427. [Google Scholar]
Yang CX, Zhao WN, Wang YD (2020a). Isolation and identification of three dominant arbuscular mycorrhizal fungi in the rhizosphere of Puccinellia tenuiflora from saline-alkaline grassland of Songnen Plain. Sydowia 71: 247–253. DOI 10.12905/0380.sydowia71-2019-0247. [Google Scholar] [CrossRef]
Yang CX, Zhao WN, Wang YN, Zhang L, Huang SC et al. (2020b). Metabolomics analysis reveals the alkali tolerance mechanism in Puccinellia tenuiflora plants inoculated with arbuscular mycorrhizal fungi. Microorganisms 8: 327. DOI 10.3390/microorganisms8030327. [Google Scholar] [CrossRef]
Yang HT, An FH, Zhang L, Zhao DD, Zhu WD, Yang F, Wang ZC (2019). Effects of different ameliorations on the physical properties of saline-sodic soil on the Songnen Plain. Chinese Journal of Ecology 38: 3416–3424. DOI 10.13292/j.1000-4890.201911.001. [Google Scholar] [CrossRef]
Yang HX, Guo SX, Liu RJ (2015). Characteristics of arbuscular mycorrhizal fungal diversity and functions in saline-alkali land. Chinese Journal of Applied Ecology 26: 311–320. DOI 10.13287/j.1001-9332.20141124.002. [Google Scholar] [CrossRef]
Yang J, He XL, Zhao LL (2011). Species diversity of arbuscular mycorrhizal fungi in the rhizosphere of Salix psammophila in Inner Mongolia desert. Biodiversity Science 19: 377–385. [Google Scholar]
Yang R, Qin ZF, Wang JJ, Xu S, Zhao W, Zhang XX, Huang ZY (2020c). Salinity changes root occupancy by arbuscular mycorrhizal fungal species. Pedobiologia 81–82: 150665. DOI 10.1016/j.pedobi.2020.150665. [Google Scholar] [CrossRef]
Yu JJ, Chen SX, Zhao Q, Wang T, Yang CP, Diaz C, Sun GR, Dai SJ (2011). Physiological and proteomic analysis of salinity tolerance in Puccinellia tenuiflora. Journal of Proteome Research 10: 3852–3870. DOI 10.1021/pr101102p. [Google Scholar] [CrossRef]
Zhang FF (2007). Resources and Salinity-Resistance of Mycorrhizal Fungi at Common Plants in Saline Alkaline Soil of Gansu Province (Master’s Thesis). Northwest A&F University. [Google Scholar]
Zhang JF, Song YM, Xing SJ, Ma BY, Xi JB (2002). Saline soil amelioration and forestation techniques. Journal of Northeast Forestry University 30: 124–129. DOI 10.3969/j.issn.1000-5382.2002.06.037. [Google Scholar] [CrossRef]
Zhang JF, Xie JH, Tian L, Ji L, Chen DG, Tian CJ (2019). Advances in mycorrhizal fungi to improve salt tolerance of plants. Northern Horticulture 23: 146–152. DOI 10.11937/bfyy.20191224. [Google Scholar] [CrossRef]
Zhang L, Yang CX (2018). Enzyme activities and free amino acids of Puccinellia tenuiflora-arbuscular mycorrhizal symbiont under saline-alkali Stress. Journal of Northeast Forestry University 46: 91–96. DOI 10.3969/j.issn.1000-5382.2018.11.019. [Google Scholar] [CrossRef]
Zhang W, Feng YJ (2009). Physicochemical properties and ecological recovery of saline-alkaline soil in Songnen Plain. Acta Pedologica Sinica 46: 169–172. DOI 10.3321/j.issn:0564-3929.2009.01.025. [Google Scholar] [CrossRef]
Zhao LP, Shang QC, Li CL (2000). Research status and problems of soda alkali-saline soil improvement and utilization in Songliao Plain. Journal of Jilin Agricultural University s1: 79–83, 85. [Google Scholar]
Zhao WN, Huang SC, Wang YD, Yang CX (2020). Effects of two AMF on physiological indexes and soil enzyme activities of Puccinellia tenuiflora seedlings under salt (NaCl) and alkali (NaHCO3) stress. Fresenius Environmental Bulletin 29: 6378–6385. [Google Scholar]
Zhao Q, Suo JW, Chen SX, Jin YD, Ma XL et al. (2016). Na2CO3-responsive mechanisms in halophyte Puccinellia tenuiflora roots revealed by physiological and proteomic analyses. Scientific Reports 6: 32717. DOI 10.1038/srep32717. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |