DOI:10.32604/biocell.2022.021032

| BIOCELL DOI:10.32604/biocell.2022.021032 |  |
| Review |
Effect of demethyltransferase FTO on tumor progression
1School of Stomatology, Weifang Medical University, Weifang, 261035, China
2Department of Oral and Maxillofacial Surgery, Shenzhen Hospital, Peking University, Shenzhen, 518036, China
*Address correspondence to: Hongyu Yang, Yang-Hongyu192@hotmail.com
Received: 27 December 2021; Accepted: 10 March 2022
Abstract: N6-methyladenosine (m6A) modification is the most widespread and conserved internal mRNA modification in mammalian cells. It greatly affects genetic regulation by enhancing the involvement of diverse cellular enzymes and thus, plays a significant role in basic life processes. Numerous studies on m6A modification identified FTO as a crucial demethylase that participates in various biological processes. Not only does FTO play a pivotal role in obesity-related conditions, but it also influences the occurrence, development, and prognosis of several cancers, such as acute myeloid leukemia, breast cancer, liver cancer, and lung cancer. Moreover, FTO also shows a close association with immunity and viral infections. This article summarized the molecular mechanism of FTO in tumorigenesis and tumor progression.
Keywords: m6A modification; Fat mass and obesity-associated protein; Molecular functional mechanism
The N6-methyladenosine (m6A) modification is considered the most abundant internal modification of mammalian mRNAs identified in the 1970s (Wu et al., 2019) and plays a significant role in regulating several cellular mechanisms involved in RNA stability, localization, splicing, transcription, and translation, as well as their interactions with other RNAs and proteins (Roundtree et al., 2017; Genenncher et al., 2018; Ke et al., 2015; Li et al., 2017a; Delaunay and Frye, 2019; Chen et al., 2017). The abnormalities in m6A modification may profoundly influence tumorigenesis and the development of multiple tumors. Although the exact molecular mechanism of m6A modification is still ambiguous, due to the discovery of m6A demethylases (FTO, ALKB5) and sequencing technologies (m6A-seq, MeRIP-seq) (Molinie and Giallourakis, 2017; Zhang and Hamada, 2020), the molecular mechanism of m6A modification in several cancers has been widely reported in recent years (Su et al., 2018; Vu et al., 2017; Ma et al., 2017; Li et al., 2017a).
The m6A modification process is dynamic and reversible due to the combined actions of methylases, demethylasea, and m6A-binding proteins (Duan et al., 2019). As FTO was previously thought to be associated with debilitating diseases such as obesity and diabetes, it was first identified as a key demethylase instigating the m6A modification in 2011 (Fig. 1), thus playing a modulatory role in multiple biological processes in a demethylase activity-dependent manner. For example, the FTO dysregulation might even aggravate major diseases like acute myeloid leukemia (AML) by carcinogenesis (Paris et al., 2019), glioblastoma cell proliferation (Su et al., 2018), and increased food intake and obesity by FTO overexpression (Jia et al., 2008). The molecular structure characteristics, biological properties, and potential molecular functional mechanisms of FTO in various cancers were discussed in this article (Fig. 2).
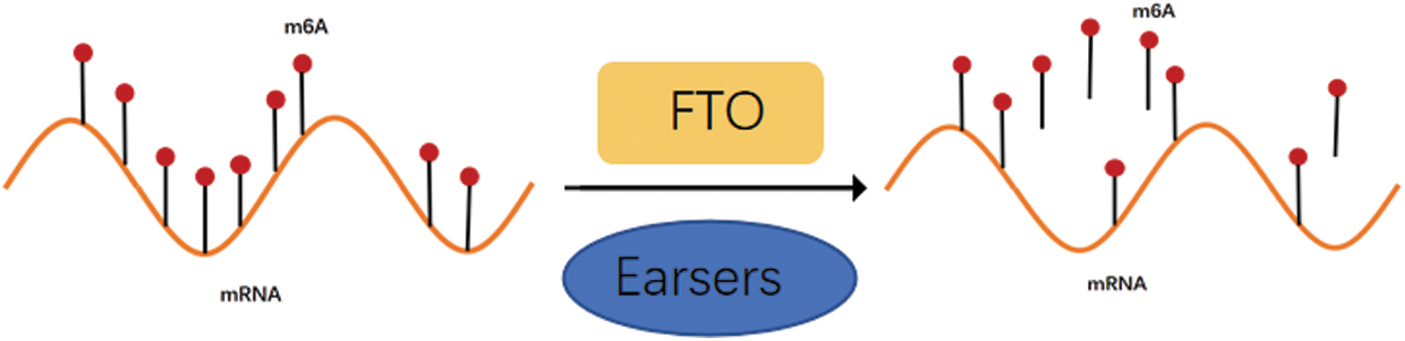
Figure 1: Demethylase FTO can reverse m6A modification.
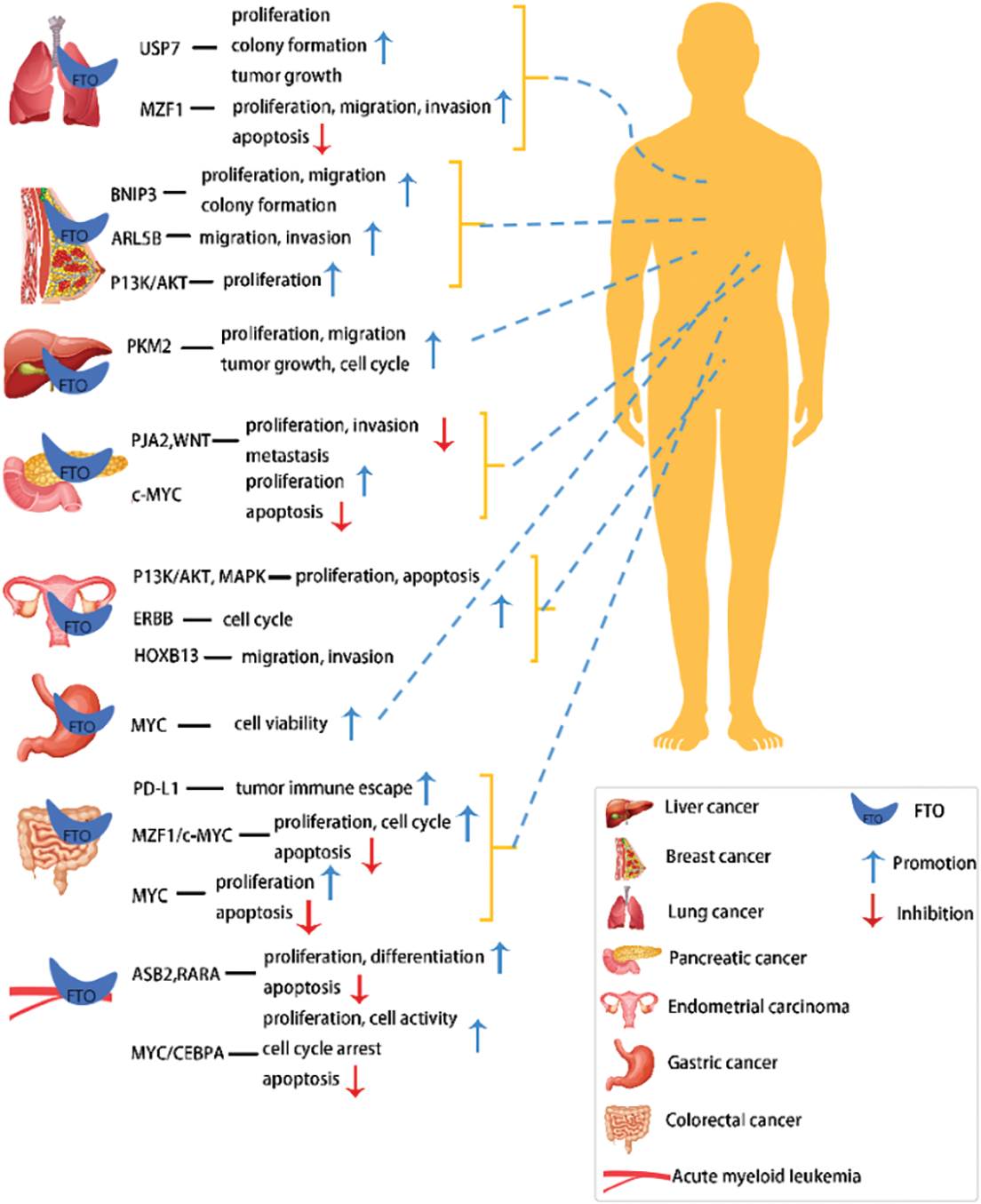
Figure 2: Targets and biological functions of FTO in different tumors.
Discovery, molecular structure, and functions of FTO
In 1994, van der Hoeven et al. (1994), for the first time, observed the deficiency of one of the six genes in the fused toe on chromosome 8 in mutant mice. A subsequent study in 2007 revealed a close association between a group of single nucleotide polymorphisms (SNPs) present in FTO with obesity in type2 diabetes patients by using genomic data and stated that obesity poses an influence on body mass index (BMI) (Frayling et al., 2007). The fat mass and obesity-associated protein (FTO) is located on the long arm of chromosome 16 on position 12.2 and possesses a double helix structure that is 410.509 bp long with 9 exons and 410507 bases (Stratigopoulos et al., 2008; Müller et al., 2008). Robbens et al. (2008) revealed the widespread existence of FTO in vertebrates, Ostreococcus, and diatom species, while no signs of FTO were observed in other invertebrates (such as insects and fungi). Frayling et al. (2007) reported that FTO is extensively expressed in human systemic tissues, especially in the hypothalamus.
According to bioinformatics analysis, the protein encoded by FTO is a member of the AlkB dioxygenase family, further categorized as deoxyribonucleic acid demethylase (Han et al., 2010). Unlike other members, FTO mainly exerts its effects on single-stranded DNAs and RNAs and produces a distinctive demethylating effect on 3-methylthymine and 3-methyluracil in single-stranded DNAs and RNAs, respectively (Jia et al., 2008; Han et al., 2010). However, there is a substrate specificity of FTO that is exhibited in different demethylase activities and is dominated by m6A and N6, 2-O-dimethyladenosine (m6Am) in the cytoplasm and m6A in the nucleus (Wei et al., 2018).
Obesity, as one of the major risk factors for cancer development, induces tumorigenesis and progression of diverse tumors such as colorectal cancer, breast cancer, pancreatic cancer, and endometrial cancer (Akbari et al., 2018). Deng et al. (2016) revealed that obesity-related inflammatory factors could facilitate tumor cell production, growth, and migration. It is suggested that SNPs in the FTO gene show a possible correlation with tumorigenesis and tumor development; obesity-related FTO polymorphism rs8050136 is specifically associated with endometrial carcinogenesis, while rs9939609 is related to pancreatic cancer (Huang et al., 2017; Lurie et al., 2011). The regulatory mechanism of FTO gene variation remains elusive to date. The FTO post-transcriptionally regulates the expression of its critical target genes; thus, affecting the occurrence and development of tumors as depicted in Table 1.

Functional mechanism of FTO in tumors
Acute myeloid leukemia (AML) is the most common malignant tumor in the bone marrow and lymphatic system. FTO plays a key role in oncogene-mediated cell transformation and apoptosis of blood cells. FTO is overexpressed in certain AML subtypes carrying several oncogenes, like PML-RARA, FLT3-ITD, and NPM1 mutations leading to an accelerated AML onset and development. Subsequently, it also promotes tumor cell growth in AML and suppresses the differentiation induced by all-trans retinoic acid (ATRA) by targeting ankyrin repeat and SOCS box containing 2 (ASB2) protein-coding gene and retinoic acid receptor alpha (RARA) (Li et al., 2017b), that show anti-leukemic effects (Guibal et al., 2002; Glasow et al., 2005).
Additionally, the FTO inhibitor, R-2-hydroxyglutarate(R-2HG), elevates m6A mRNA levels, reduces the stability of c-MYC-CEBPA transcripts, inhibits FTO demethylase activity, and limits AML cell proliferation and survival by directly binding to FTO protein; thus, exerting anti-leukemia activity in tumor cells with FTO low expression (Glasow et al., 2005). Hence, it can be inferred that R-2HG has an innate potential to effectively suppress leukemia and produce evident anti-tumor effects in patients with FTO overexpression. Thus, the production of corresponding FTO inhibitors (Huang et al., 2019) that inhibit FTO demethylase activity decrease m6A demethylation, and suppress AML cell proliferation, and growth lead to the development of novel therapeutic targets exhibiting anti-leukemic activity.
Huang et al. (2019) developed two likely inhibitors of FTO, namely FB23 and FB23-2, which after binding to FTO, specifically inhibited FTO demethylase activity, among which FB23-2 showed better anti-proliferative ability than FB23 and could repress cancer cell proliferation, promote normal cell differentiation, and delay AML progression. Therefore, it was suggested that FTO inhibitors in AML downstream target genes such as c-MYC, CEBPA, ASB2, and RARA that might help in formulating effective therapies against AML (Su et al., 2018; Li et al., 2017b). According to recent evidence, Rhein, an FTO inhibitor, can competitively bind to the FTO active site and directly jeopardize FTO binding to the m6A substrate, thereby inhibiting FTO-dependent m6A demethylation (Chen et al., 2012). In addition, Zheng et al. (2014) also designed multiple chemical compounds designated as FTO inhibitors which selectively suppressed FTO demethylase activity to increase cellular m6A methylase activity.
In light of the significance of FTO in carcinogenesis and drug resistance, the best therapeutic strategy for combating AML might be the implementation of stable and effective FTO inhibitors. Although clinical FTO inhibitors for therapeutic application are available nowadays, more selective and effective FTO inhibitors are greatly needed to control AML pathogenesis and progression.
An estimated 19.3 million new cancer cases and almost 10.0 million cancer deaths occurred in 2020, as indicated by the Global Cancer Statistics database, 2020 (Sung et al., 2021). Breast cancer (BC) ranked first in cancer incidence worldwide, with an estimated 2.3 million newly emerged cases (11.7%) in 2020. In China, BC is now considered as the second leading health burden in terms of incidence next to lung cancer, colorectal cancer, and gastric cancer. The risk factors of BC included genetic predisposition (Braithwaite et al., 2018), age (Spreafico et al., 2020), reproductive and hormonal factors (Fan et al., 2014; Nelson et al., 2012; Sun et al., 2017), obesity (Eliassen et al., 2006; Jiralerspong and Goodwin, 2016), and unhealthy lifestyles (Nelson et al., 2012; Kerr et al., 2017). Distant metastases and chemoresistance are a few major contributors to the surging mortality rate of BC patients. Moreover, incorporating early targeted cancer interventional therapy in current BC treatment modalities might prevent invasive metastasis; thus, increasing the survival rates and projecting a favorable prognosis.
Niu et al. (2019) observed that a higher FTO expression in MCF7 and MDA-MB231 cell lines facilitated BC cell proliferation and colony formati-on, inhibited cancer cell apoptosis, and promoted tumorigenesis and development by suppressing m6A demethylation of a pro-apoptotic gene, BNIP3 3’ untranslated regions(3’UTRs). Consequently, the inhibition of FTO expression in BC can inhibit tumor cell proliferation and migration (Niu et al., 2019). However, beyond that, a study by Kaklamani et al. (2011) confirmed a significant association of rs1477196 SNP in the intron 1 region of the FTO gene with BC carcinogenesis, which was further suggestive of a direct correlation between FTO SNPs with BC risks. Moreover, FTO promotes BC cell migration and invasion by upregulating ARL5B that shows carcinogenic activity in BC cells (Xu et al., 2020). Therefore, the clinical applications of FTO can be utilized as the potential novel therapeutic target as well as a prognostic biomarker for BC treatment.
Endometrial cancer (EC) is the most commonly diagnosed invasive gynecological cancer and a leading cause of cancer-related deaths in American females. More than 60,000 women were diagnosed with endometrial cancer in 2018, with nearly 10,000 deaths reported annually (Brooks et al., 2019). Recent years have seen a more remarkable surge in the incidence and mortality rates of EC, which might be due to the decline of resection rates in benign uterine diseases. Relative to other countries, China has reported fewer EC cases but has witnessed a significant rise in its incidence lately. Despite recent advances, the exploration for additional reliable therapeutic targets in EC is of utmost importance for reducing EC incidence and mortality rates.
The genome-wide association studies revealed a close correlation between obesity-related SNPs and EC (Delahanty et al., 2011). The bioinformatics analysis on multiple databases by Zhang and Yang (2021) reflected the abnormal expression of enzymes involved in m6A modification in EC, among which YTHDF1, YTHDF2, and METTL3 had upregulated expressions, whereas METTL14, FTO, and ALKBH5 showed downregulated expressions. A subsequent Cox survival analysis demonstrated that aberrant FTO expression was negatively correlated with the total survival rates. Although these database findings are beneficial for exploring other novel biological targets, their effectiveness and potency may require further validation in experimental and clinical research.
Additionally, FTO overexpression can also enhance proliferative and invasive abilities of tumor cells through the PI3K/AKT and MAPK pathways (Zhang et al., 2012; Zhu et al., 2016), promote tumor progression by activating the WNT pathway (Zhang and Hamada, 2020), and alter the tumor cell cycle in EC via the ERBB pathway (Zhang and Yang, 2021). Endometrial cancer, as one of the most common gynecologic cancer (Siegel et al. 2019; Ito et al., 2007), requires further investigation into the intrinsic molecular mechanisms of its tumorigenesis and progression. There is an increased demand for new therapeutic targets that will improve the prevention and treatment of EC.
Hepatocellular carcinoma (HCC) is the most common primary liver cancer (European Association for the Study of the Liver, 2018), with a high incidence and mortality rate worldwide (Gong and Qin, 2018; Torre et al., 2015). There are several etiologic factors for HCC like chronic hepatitis B virus infection (Rawla et al., 2018), liver cirrhosis, alcoholic liver disease, non-alcoholic fatty liver disease, aflatoxin exposure (Piñeros et al., 2018), obesity, and metabolic syndrome, reported in the Western countries (Younossi et al., 2019). Despite the recent diagnostic, therapeutic, and preventive advances, most patients are diagnosed at an advanced stage of the disease, making them more susceptible to relapse. Liver cancer remains the second leading cause of cancer-related death due to an 18% 5-year survival rate (Torre et al., 2015; Forner et al., 2018; Jemal et al., 2017), which necessitates the search for new prognostic biomarkers and therapeutic targets for reducing cancer-related mortality.
Ye et al. (2020) observed a significantly higher expression of FTO in 330 HCC cases than the normal liver tissues through immunohistochemical analysis and revealed that FTO facilitated cancer cell proliferation, migration, and invasion, inhibited apoptosis, and accelerated liver cancer progression in the human liver cancer cell line (HepG2). Additionally, Li et al. (2019b) also found that FTO overexpression in HCC cells could modulate m6A level in PKM2 mRNA to enhance the PKM2 expression and facilitate tumor cell proliferation and migration. A subsequent Kaplan-Meier analysis also confirmed an important correlation between the FTO overexpression and poor prognosis of HCC patient.
Presently, lung cancer remains the leading cause of cancer death and a global threat to public health. Non-small cell lung cancer (NSCLC) accounts for nearly 80% of all lung cancer types (Siegel et al., 2020; Siegel et al., 2018) and consists of two major subtypes, squamous cell carcinoma of the lung and pulmonary adenocarcinoma. Due to an unsatisfactory survival rate, 21 million people were newly diagnosed with lung cancer in 2018 and 18 million deaths worldwide in 2019 (Siegel et al., 2018). Moreover, the functional mechanisms of FTO in lung squamous cell carcinoma are still not fully understood and require in-depth investigation for further validation.
In lung cancer, FTO enhances the stability of deubiquitinated ubiquitin-specific protease 7 (USP7) mRNA level and accelerates the progression of NSCLC (Li et al., 2019a), while FTO demethylase activity plays a key role in increasing USP7 expression, thus, denoting a close association of FTO-USP7 signaling axis with NSCLC occurrence and its potential as a therapeutic target in lung cancer treatment. Furthermore, Liu et al. (2018) investigated the association of FTO expression with poor prognosis in patients with non-squamous cell carcinoma in the TCGA database and discovered FTO overexpression in cancer tissues. Furthermore, FTO facilitates the proliferation, migration, and colony formation of lung cancer cells and produces a carcinogenic effect by inhibiting m6A levels in myeloid zinc finger protein 1 (MZF1) mRNA transcripts as well as upregulating the MZF1 expression.
Gastric cancer (GC) is the fifth most common cancer globally and the third most common cause of cancer-related mortality (McGuire et al., 2016; Figueiredo et al., 2017; Ferro et al., 2014). Although GC incidence is relatively low in China, it remains a common malignancy accounting for 10% of all malignant tumors (Jackson and Giraud, 2009). Various genetic and environmental factors, including GC family history, Helicobacter pylori infection, excessive salt intake, and chronic gastropathy, play vital roles in inducing GC (Fuccio et al., 2010). Due to the development of the latest biological technology, the discovery of intrinsic molecular mechanisms in inducing GC occurrence and progression along with sustained investigation and exploration will contribute substantially to the early diagnosis and prognosis of GC.
As indicated in a previous study (Xu et al., 2017), FTO overexpression plays a contributory role in GC cell proliferation, migration, and invasion, while a Kaplan-Meier analysis on the same a negative correlation between the 5-year survival rate of GC patients and FTO expression. Similarly, Li et al. (2019c) and Guan et al. (2020), through Kaplan-Meier analysis and TCGA databases, revealed a close association between low FTO expressions and the shorter overall survival rate of GC patients. Hence, it can be duly inferred that FTO overexpression is directly correlated with the development and poorer prognosis of GC.
The forkhead transcription factor (Foxa2) directly binds to the FTO promoter region and inversely modulates the activity and expression of the FTO promoter (Guo et al., 2012). Furthermore, histone deacetylase 3 (HDAC3) overexpression in GC significantly inhibits Foxa2 protein expression and activates the FTO/m6A/MYC axis, promoting tumor cell proliferation, migration, and migration invasion, consequently contributing to GC (Yang et al., 2021). These findings confirmed that HDAC3 induces tumor cell activity in GC by modulating Foxa2-mediated FTO/m6A/MYC axis and provided a theoretical foundation for targeted genetic modification therapy.
According to a study by Su et al. (2019), m6A modified demethylase FTO can serve as a potential prognostic marker for GC. Based on GO enrichment and TCGA database analysis results, FTO expression is related to various immune cell genotypes, which further affects the immune microenvironment of tumor cells (Xu et al., 2021). There is increasing evidence that FTO has the potential to become a promising noninvasive biomarker for GC treatment leading to a better prognosis and survival rate.
Oral cancer is the sixth most common cancer globally, with a high mortality rate (Hussein et al., 2017). Oral squamous cell carcinoma (OSCC) is the most common malignancy in the mouth and accounts for 90% of all oral cancers. Due to inconspicuous clinical manifestations in the early stages, most patients are diagnosed in the later stages (Valdez and Brennan, 2018), accompanied by cervical lymph node and distant metastasis (Mupparapu and Shanti, 2018). Although several progressions have been made in the treatment strategies, the 5-year survival rate remains as low as 50% due to a higher recurrence rate (Diao et al., 2018; Zanoni et al., 2019). Therefore, the functional mechanism of tumorigenesis and tumor progression should be accurately investigated for better patient outcomes.
Wang et al. (2021) observed a significant rise in FTO expression in OSCC cells while FTO knockdown leads to downregulated eIF4G1, increased autophagy flux, apoptosis inhibition, and consequently, tumorigenesis. Furthermore, FTO upregulation in oral squamous cell carcinoma causes further tumor progression, whereas FTO downregulation remarkably represses tumor cell proliferation, colony formation and tumor growth (Li et al., 2021a). The in vivo assays in mice indicated that FTO knockdown slows down tumor growth (Li et al., 2021b). Subsequently, FTO might act as an effective therapeutic target against OSCC. However, the regulatory action of FTO in OSCC has not been fully clarified and requires further exploratory measures.
Glioma is the most common primary intracranial malignant tumor, accounting for 80% of malignant brain tumors (Ostrom et al., 2014; Neglia et al., 2006). It mainly originates from neuroglial stem cells or progenitor cells (Weller et al., 2015; Jones et al., 2018). Pediatric low-grade gliomas and WHO grade I or II gliomas are generally benign tumors. The WHO grade I or II gliomas rarely show malignant transformation with a good prognosis. However, WHO grade III or IV high-grade gliomas are usually malignant tumors with a rapid rate of progression and poor prognosis, with a five-year survival rate of 20% (Bush et al., 2017; Louis et al., 2016).
Most researchers argue that high-dose ionizing radiation is the cause of the disease (Vienne-Jumeau et al., 2019; Davis, 2018). The effect of other pathogenic factors such as lifestyle, dietary habits, and genetic factors on glioma should also be further studied (Davis, 2018).
Many genetic factors were determined to be glioma risk factors. Genome-wide association studies (GWASs) have found a large number of SNPs related to glioma risk-related susceptibility polymorphisms, such as CDKN2B, RTEL1, CCDC26, PHLDB1, EGFR, and TP53 (Wrensch et al., 2009; Shete et al., 2009; Melin et al., 2017; Kinnersley et al., 2015). Studies on the mechanism of the effect of FTO on glioma are increasing. Some studies have shown that SNPs of the FTO gene can affect tumor risk (Sigurdson, et al., 2016). He et al. (2021) detected a significant negative correlation between FTO rs8047395 and glioma susceptibility. Cui et al. (2017) showed that knocking out FTO inhibits the growth and self-renewal of glioblastoma stem cells. In glioma, ectopic high expression of FTO can improve the stability of MYC and promote the proliferation of glioma cells (Wang et al., 2018). Tao et al. (2020) found that the protein level of FTO in glioma tissues was significantly lower compared to that in brain tissues. Subsequent experiments revealed that the inhibitory effect of FTO on glioma might be related to its interaction with FOXO3a, thus promoting the expression of FTO target genes. The above results indicate that FTO can be used as an effective biological therapeutic target for treating gliomas and further improving the survival rate of gliomas.
FTO not only regulates the occurrence, development, and prognosis of tumors but also plays an important role in non-neoplastic diseases. Epidemiological studies showed that type 2 diabetes is a risk factor for Alzheimer’s disease (Vagelatos and Eslick, 2013). In vitro animal experiments showed that FTO expression was significantly lower in the brain tissues of diabetic, aged, and Alzheimer’s disease (AD) mice, which indicated that FTO might be closely related to diabetes and AD (Li et al., 2018). Additionally, bioinformatics analysis showed that FTO may also be associated with chronic obstructive pneumonia (COPD). Furthermore, correlation analyses showed that FTO expression was negatively correlated with the expression of several genes, such as BCL2A1, CYP1A1, and CYP1B1. These genes are enriched in signaling pathways that promote the development of COPD and are closely related to the development and progression of chronic obstructive pulmonary disease (Huang et al., 2020). In bronchial asthma, FTO deletion decreases the stability of the mRNA encoding the ciliary transcription factor FOXJ1 (Kim et al., 2021), which induces the development of asthma. Through mouse experiments, Mathiyalagan et al. (2019) demonstrated that FTO expression levels were significantly downregulated in mice within 4 h of myocardial infarction caused by the coronary arteries on the left side of the heart. Cardiac remodeling was performed a short while after myocardial infarction, and high expression of FTO for about a week was shown to repair the function of cardiomyocytes (Mathiyalagan et al., 2019). Shen et al. (2021) showed that overexpression of FTO enhanced cardiac function and inhibited cardiomyocyte apoptosis, thereby delaying heart failure. This demonstrated an important role of FTO in regulating cardiac function and cardiac remodeling in heart failure and suggested that the role of epigenetics of FTO in myocardial infarction needs to be further studied.
To summarize, FTO exhibits dual effects such as carcinogenic and anti-carcinogenic manifestation in human tissues. For instance, FTO participates in liver cancer cell proliferation, migration, and cell cycle and is associated with tumor growth. Similarly, FTO downregulation as an anti-tumor gene inhibits the WNT pathway and suppresses pancreatic cancer cell proliferation and migration, while FTO upregulation as an oncogene activates the c-MYC pathway and promotes cancer cell proliferation. Correspondingly, FTO as an oncogene facilitates AML progression, whereas several effective FTO inhibitors like Rhein, FB23-2, and R-2HG delay the development of AML.
With the advances made in the high-throughput sequencing and bioinformatics technique, m6A modification has attracted a lot of attention due to its universal existence in mammals and close associations with certain diseases, especially malignant tumors. Recent explorations have documented different roles of diverse regulatory factors like METTL3, METTL14, WTAP, FTO, and YTH families in different tumors and their corresponding pathways. Hence, m6A modification is a complex process of molecular interaction with indispensable functions in tumorigenesis and development. Multiple regulatory factors related to m6A modification can be applied as noninvasive biomarkers for detecting oncogenesis as well as a better prognosis. On the one hand, oncogenesis is a complex pathologic process manipulated by a variety of molecules, which makes it difficult for tumors to be fully cured; on the other hand, it might be feasible to activate the tumor-suppressive pathways and inhibit the oncogenic pathways via certain regulatory factors for arresting tumor progression and increasing the survival rates.
m6A modification has now been proved as a double-edged sword in tumor progression, excessive m6A modification of several genes can promote tumorigenesis and development, while the lack of m6A modification in some genes facilitates tumor cell proliferation, migration, and invasion. The role of m6A methylation in non-neoplastic diseases is similar to that in cancer. This article elucidated the role of FTO in several tumors but still faced some limitations in the investigation of some diseases. For example, studies regarding FTO expression in GC are rare. The complex molecular mechanism of FTO as an oncogene in GC requires further investigations to provide reliable biological therapeutic targets for its clinical management and prognosis. This article also identified several carcinogenic and anti-carcinogenic effects of FTO via activating and inhibiting some common tumor-related pathways (PI3K/AKT, WNT, and c-MYC) yet the regulatory role of FTO in other tumor-related pathways remains unknown. Owing to the complex causes of non-neoplastic diseases, new effective therapeutic targets can significantly improve the quality of life of non-neoplastic patients. Furthermore, it is imperative to conduct several in-depth studies for studying the m6A modification in tumors or non-tumors to extract potential therapeutic targets for novel disease treatment strategies.
Author Contribution: The authors confirm contribution to the paper as follows: article conception and design: Ling Sheng, Yuehong Shen; draft manuscript preparation: Ling Sheng; draft manuscript revision: Yuehong Shen, Hongyu Yang. All authors reviewed the article and approved the final version of the manuscript.
Funding Statement: This study was supported by the Natural Science Foundation of Guangdong Provincial (2019A1515011911), the Research Program of Shenzhen Innovation Council (JCYJ20200109140208058), the Sanming Project of Medicine in Shenzhen (SZSM202111012) and the Oral and Maxillofacial Surgery Team, Professor Yu Guangyan, Stomatological Hospital Peking University, Guangdong Provincial High-Level Clinical Key Specialty (Shenzhen Matching Construction Fund) (Grant No. SZGSP008).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present article.
Akbari ME, Gholamalizadeh M, Doaei S, Mirsafa F (2018). FTO gene affects obesity and breast cancer through similar mechanisms: A new insight into the molecular therapeutic targets. Nutrition and Cancer 70: 30–36. DOI 10.1080/01635581.2018.1397709. [Google Scholar] [CrossRef]
Braithwaite D, Miglioretti DL, Zhu WW, Demb J, Trentham-Dietz A et al. (2018). Family history and breast cancer risk among older women in the breast cancer surveillance consortium cohort. JAMA Internal Medicine 178: 494–501. DOI 10.1001/jamainternmed.2017.8642. [Google Scholar] [CrossRef]
Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW et al. (2019). Current recommendations and recent progress in endometrial cancer. CA: A Cancer Journal for Clinicians 69: 258–279. DOI 10.3322/caac.21561. [Google Scholar] [CrossRef]
Bush NA, Chang SM, Berger MS (2017). Current and future strategies for treatment of glioma. Neurosurgical Review 40: 1–14. DOI 10.1007/s10143-016-0709-8. [Google Scholar] [CrossRef]
Chen BE, Ye F, Yu L, Jia GF, Huang XT et al. (2012). Development of cell-active N6-methyladenosine RNA demethylase FTO inhibitor. Journal of the American Chemical Society 134: 17963–17971. DOI 10.1021/ja3064149. [Google Scholar] [CrossRef]
Chen JX, Sun YC, Xu X, Wang DW, He JB et al. (2017). YTH domain family 2 orchestrates epithelial-mesenchymal transition/proliferation dichotomy in pancreatic cancer cells. Cell Cycle 16: 2259–2271. DOI 10.1080/15384101.2017.1380125. [Google Scholar] [CrossRef]
Cui Q, Shi HL, Ye P, Li L, Qu QH et al. (2017). m6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Reports 18: 2622–2634. DOI 10.1016/j.celrep.2017.02.059. [Google Scholar] [CrossRef]
Cui YB, Zhang CY, Ma SS, Li Z, Wang WJ et al. (2021). RNA m6A demethylase FTO-mediated epigenetic up-regulation of LINC00022 promotes tumorigenesis in esophageal squamous cell carcinoma. Journal of Experimental & Clinical Cancer Research: CR 40: 294. DOI 10.1186/s13046-021-02096-1. [Google Scholar] [CrossRef]
Davis ME (2018). Epidemiology and overview of gliomas. Seminars in Oncology Nursing 34: 420–429. DOI 10.1016/j.soncn.2018.10.001. [Google Scholar] [CrossRef]
Delahanty RJ, Beeghly-Fadiel A, Xiang YB, Long JR, Cai QY et al. (2011). Association of obesity-related genetic variants with endometrial cancer risk: A report from the Shanghai Endometrial Cancer Genetics Study. American Journal of Epidemiology 174: 1115–1126. DOI 10.1093/aje/kwr233. [Google Scholar] [CrossRef]
Delaunay S, Frye M (2019). RNA modifications regulating cell fate in cancer. Nature Cell Biology 21: 552–559. DOI 10.1038/s41556-019-0319-0. [Google Scholar] [CrossRef]
Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA (2016). Obesity, inflammation, and cancer. Annual Review of Pathology 11: 421–449. DOI 10.1146/annurev-pathol-012615-044359. [Google Scholar] [CrossRef]
Diao PF, Wu YP, Li J, Zhang W, Huang R et al. (2018). Preoperative systemic immune-inflammation index predicts prognosis of patients with oral squamous cell carcinoma after curative resection. Journal of Translational Medicine 16: 365. DOI 10.1186/s12967-018-1742-x. [Google Scholar] [CrossRef]
Duan HC, Wang Y, Jia GF (2019). Dynamic and reversible RNA N6-methyladenosine methylation. Wires RNA 10: e1507. DOI 10.1002/wrna.1507. [Google Scholar] [CrossRef]
Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE (2006). Adult weight change and risk of postmenopausal breast cancer. JAMA 296: 193–201. DOI 10.1001/jama.296.2.193. [Google Scholar] [CrossRef]
European Association for the Study of the Liver (2018). EASL clinical practice guidelines: Management of hepatocellular carcinoma. Journal of Hepatology 69: 182–236. DOI 10.1016/j.jhep.2018.03.019. [Google Scholar] [CrossRef]
Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM et al. (2014). Breast cancer in China. Lancet Oncology 15: e279–e289. DOI 10.1016/S1470-2045(13)70567-9. [Google Scholar] [CrossRef]
Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P et al. (2014). Worldwide trends in gastric cancer mortality (1980–2011with predictions to 2015, and incidence by subtype. European Journal of Cancer 50: 1330–1344. DOI 10.1016/j.ejca.2014.01.029. [Google Scholar] [CrossRef]
Figueiredo C, Camargo MC, Leite M, Fuentes-Pananá EM, Rabkin CS et al. (2017). Pathogenesis of gastric cancer: Genetics and molecular classification. Current Topics in Microbiology and Immunology 400: 277–304. DOI 10.1007/978-3-319-50520-6_12. [Google Scholar] [CrossRef]
Forner A, Reig M, Bruix J (2018). Hepatocellular carcinoma. Lancet 391: 1301–1314. DOI 10.1016/S0140-6736(18)30010-2. [Google Scholar] [CrossRef]
Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM et al. (2007). A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316: 889–894. DOI 10.1126/science.1141634. [Google Scholar] [CrossRef]
Fuccio L, Eusebi LH, Bazzoli F (2010). Gastric cancer, helicobacter pylori infection and other risk factors. World Journal of Gastrointestinal Oncology 2: 342–347. DOI 10.4251/wjgo.v2.i9.342. [Google Scholar] [CrossRef]
Genenncher B, Durdevic Z, Hanna K, Zinkl D, Mobin MB et al. (2018). Mutations in cytosine-5 tRNA methyltransferases impact mobile element expression and genome stability at specific DNA repeats. Cell Reports 22: 1861–1874. DOI 10.1016/j.celrep.2018.01.061. [Google Scholar] [CrossRef]
Gholamalizadeh M, Jarrahi AM, Akbari ME, Bourbour F, Mokhtari Z et al. (2020). Association between FTO gene polymorphisms and breast cancer: The role of estrogen. Expert Review of Endocrinology & Metabolism 15: 115–121. DOI 10.1080/17446651.2020.1730176. [Google Scholar] [CrossRef]
Glasow A, Prodromou N, Xu K, von Lindern M, Zelent A (2005). Retinoids and myelomonocytic growth factors cooperatively activate RARA and induce human myeloid leukemia cell differentiation via MAP kinase pathways. Blood 105: 341–349. DOI 10.1182/blood-2004-03-1074. [Google Scholar] [CrossRef]
Gong XL, Qin SK (2018). Study progression of anti-angiogenetic therapy and its combination with other agents for the treatment of advanced hepatocellular carcinoma. Hepatobiliary Surgery and Nutrition 7: 466–474. DOI 10.21037/hbsn.2018.11.04. [Google Scholar] [CrossRef]
Guan KL, Liu X, Li JH, Ding YX, Li J et al. (2020). Expression status and prognostic value of m6A-associated genes in gastric cancer. Journal of Cancer 11: 3027–3040. DOI 10.7150/jca.40866. [Google Scholar] [CrossRef]
Guibal FC, Moog-Lutz C, Smolewski P, di Gioia Y, Darzynkiewicz Z et al. (2002). ASB-2 inhibits growth and promotes commitment in myeloid leukemia cells. Journal of Biological Chemistry 277: 218–224. DOI 10.1074/jbc.M108476200. [Google Scholar] [CrossRef]
Guo JJ, Ren W, Ding Y, Li A, Jia L et al. (2012). Fat mass and obesity associated gene (FTO) expression is regulated negatively by the transcription factor Foxa2. PLoS One 7: e51082. DOI 10.1371/journal.pone.0051082. [Google Scholar] [CrossRef]
Han ZF, Niu TH, Chang JB, Lei XG, Zhao MY et al. (2010). Crystal structure of the FTO protein reveals basis for its substrate specificity. Nature 464: 1205–1209. DOI 10.1038/nature08921. [Google Scholar] [CrossRef]
He J, Yuan L, Lin HR, Lin A, Chen HT et al. (2021). Genetic variants in m6A modification core genes are associated with glioma risk in Chinese children. Molecular Therapy Oncolytics 20: 199–208. DOI 10.1016/j.omto.2020.12.013. [Google Scholar] [CrossRef]
Huang XW, Lv DJ, Yang X, Li M, Zhang H (2020). m6A RNA methylation regulators could contribute to the occurrence of chronic obstructive pulmonary disease. Journal of Cellular and Molecular Medicine 24: 12706–12715. DOI 10.1111/jcmm.15848. [Google Scholar] [CrossRef]
Huang XY, Zhao J, Yang MY, Li M, Zheng JM (2017). Association between FTO gene polymorphism (rs9939609 T/A) and cancer risk: A meta-analysis. European Journal of Cancer Care 26: e12464. DOI 10.1111/ecc.12464. [Google Scholar] [CrossRef]
Huang Y, Su R, Sheng Y, Dong L, Dong Z et al. (2019). Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell 35: 677–691. DOI 10.1016/j.ccell.2019.03.006. [Google Scholar] [CrossRef]
Hussein AA, Helder MN, de Visscher JG, Leemans CR, Braakhuis BJ et al. (2017). Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: A systematic review. European Journal of Cancer 82: 115–127. DOI 10.1016/j.ejca.2017.05.026. [Google Scholar] [CrossRef]
Ito K, Utsunomiya H, Yaegashi N, Sasano H (2007). Biological roles of estrogen and progesterone in human endometrial carcinoma--new developments in potential endocrine therapy for endometrial cancer. Endocrine Journal 54: 667–679. DOI 10.1507/endocrj.KR-114. [Google Scholar] [CrossRef]
Jackson CB, Giraud AS (2009). STAT3 as a prognostic marker in human gastric cancer. Journal of Gastroenterology and Hepatology 24: 505–507. DOI 10.1111/j.1440-1746.2009.05822.x. [Google Scholar] [CrossRef]
Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J et al. (2017). Annual report to the nation on the status of cancer, 1975-2014, featuring survival. Journal of the National Cancer Institute 109: djx030. DOI 10.1093/jnci/djx030. [Google Scholar] [CrossRef]
Jia GF, Yang CG, Yang SD, Jian X, Yi CQ et al. (2008). Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Letters 582: 3313–3319. DOI 10.1016/j.febslet.2008.08.019. [Google Scholar] [CrossRef]
Jiralerspong S, Goodwin PJ (2016). Obesity and breast cancer prognosis: Evidence, challenges, and opportunities. Journal of Clinical Oncology 34: 4203–4216. DOI 10.1200/JCO.2016.68.4480. [Google Scholar] [CrossRef]
Jones DTW, Kieran MW, Bouffet E, Alexandrescu S, Bandopadhayay P et al. (2018). Pediatric low-grade gliomas: Next biologically driven steps. Neuro-Oncology 20: 160–173. DOI 10.1093/neuonc/nox141. [Google Scholar] [CrossRef]
Kaklamani V, Yi NJ, Sadim M, Siziopikou K, Zhang K et al. (2011). The role of the fat mass and obesity associatedgene (FTO) in breast cancer risk. BMC Medical Genetics 12: 52. DOI 10.1186/1471-2350-12-52. [Google Scholar] [CrossRef]
Ke SD, Alemu EA, Mertens C, Gantman EC, Fak JJ et al. (2015). A majority of m6A residues are in the last exons, allowing the potential for 3’ UTR regulation. Genes & Development 29: 2037–2053. DOI 10.1101/gad.269415.115. [Google Scholar] [CrossRef]
Kerr J, Anderson C, Lippman SM (2017). Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncology 18: 457–471. DOI 10.1016/S1470-2045(17)30411-4. [Google Scholar] [CrossRef]
Kim H, Lee YS, Kim SM, Jang S, Choi H et al. (2021). RNA demethylation by FTO stabilizes the FOXJ1 mRNA for proper motile ciliogenesis. Developmental Cell 56: 1118–1130. DOI 10.1016/j.devcel.2021.03.006. [Google Scholar] [CrossRef]
Kinnersley B, Labussière M, Holroyd A, Di Stefano AL, Broderick P et al. (2015). Genome-wide association study identifies multiple susceptibility loci for glioma. Nature Communications 6: 8559. DOI 10.1038/ncomms9559. [Google Scholar] [CrossRef]
Li DQ, Huang CC, Zhang G, Zhou LL (2021a). FTO demethylates YAP mRNA promoting oral squamous cell carcinoma tumorigenesis. Neoplasma 69: 71–79. DOI 10.4149/neo_2021_210716N967. [Google Scholar] [CrossRef]
Li HJ, Ren Y, Mao KS, Hua F, Yang YL et al. (2018). FTO is involved in Alzheimer’s disease by targeting TSC1-mTOR-Tau signaling. Biochemical and Biophysical Research Communications 498: 234–239. DOI 10.1016/j.bbrc.2018.02.201. [Google Scholar] [CrossRef]
Li J, Han Y, Zhang HM, Qian Z, Jia WY et al. (2019a). The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochemical and Biophysical Research Communications 512: 479–485. DOI 10.1016/j.bbrc.2019.03.093. [Google Scholar] [CrossRef]
Li J, Zhu LJ, Shi YH, Liu JN, Lin L et al. (2019b). m6A demethylase FTO promotes hepatocellular carcinoma tumorigenesis via mediating PKM2 demethylation. American Journal of Translational Research 11: 6084–6092. [Google Scholar]
Li X, Tang J, Huang W, Wang F, Li P et al. (2017a). The m6A methyltransferase METTL3: Acting as a tumor suppressor in renal cell carcinoma. Oncotarget 8: 96103–96116. DOI 10.18632/oncotarget.21726. [Google Scholar] [CrossRef]
Li X, Xie XL, Gu YC, Zhang JM, Song J et al. (2021b). Fat mass and obesity-associated protein regulates tumorigenesis of arecoline-promoted human oral carcinoma. Cancer Medicine 10: 6402–6415. DOI 10.1002/cam4.4188. [Google Scholar] [CrossRef]
Li Y, Zheng DY, Wang F, Xu YX, Yu HY et al. (2019c). Expression of demethylase genes, FTO and ALKBH1, is associated with prognosis of gastric cancer. Digestive Diseases and Sciences 64: 1503–1513. DOI 10.1007/s10620-018-5452-2. [Google Scholar] [CrossRef]
Li ZJ, Weng HY, Su R, Weng XC, Zuo ZX et al. (2017b). FTO plays an oncogenic role in acute myeloid leukemia as a N6-methyladenosine RNA demethylase. Cancer Cell 31: 127–141. DOI 10.1016/j.ccell.2016.11.017. [Google Scholar] [CrossRef]
Liu JQ, Ren DL, Du ZH, Wang HK, Zhang H et al. (2018). m6A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochemical and Biophysical Research communications 502: 456–464. DOI 10.1016/j.bbrc.2018.05.175. [Google Scholar] [CrossRef]
Liu SX, Huang M, Chen ZQ, Chen JD, Chao Q et al. (2020). FTO promotes cell proliferation and migration in esophageal squamous cell carcinoma through up-regulation of MMP13. Experimental Cell Research 389: 111894. DOI 10.1016/j.yexcr.2020.111894. [Google Scholar] [CrossRef]
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D et al. (2016). The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathologica 131: 803–820. DOI 10.1007/s00401-016-1545-1. [Google Scholar] [CrossRef]
Lurie G, Gaudet MM, Spurdle AB, Carney ME, Wilkens LR et al. (2011). The obesity-associated polymorphisms FTO rs9939609 and MC4R rs17782313 and endometrial cancer risk in non-Hispanic white women. PLoS One 6: e16756. DOI 10.1371/journal.pone.0016756. [Google Scholar] [CrossRef]
Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH et al. (2017). METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N6-methyladenosine-dependent primary MicroRNA processing. Hepatology 65: 529–543. DOI 10.1002/hep.28885. [Google Scholar] [CrossRef]
Mathiyalagan P, Adamiak M, Mayourian J, Sassi Y, Liang Y et al. (2019). FTO-Dependent N6-Methyladenosine regulates cardiac function during remodeling and repair. Circulation 139: 518–532. DOI 10.1161/CIRCULATIONAHA.118.033794. [Google Scholar] [CrossRef]
McGuire S (2016). World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Advances in Nutrition 7: 418–419. DOI 10.3945/an.116.012211. [Google Scholar] [CrossRef]
Melin BS, Barnholtz-Sloan JS, Wrensch MR, Johansen C, Il’yasova D et al. (2017). Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nature Genetics 49: 789–794. DOI 10.1038/ng.3823. [Google Scholar] [CrossRef]
Molinie B, Giallourakis CC (2017). Genome-wide location analyses of N6-methyladenosine modifications (m6A-Seq). Methods in Molecular Biology 1562: 45–53. DOI 10.1007/978-1-4939-6807-7. [Google Scholar] [CrossRef]
Müller TD, Hinney A, Scherag A, Nguyen TT, Schreiner F et al. (2008). ‘Fat mass and obesity associated’ gene (FTONo significant association of variant rs9939609 with weight loss in a lifestyle intervention and lipid metabolism markers in German obese children and adolescents. BMC Medical Genetics 9: 85. DOI 10.1186/1471-2350-9-85. [Google Scholar] [CrossRef]
Mupparapu M, Shanti RM (2018). Evaluation and staging of oral cancer. Dental Clinics of North America 62: 47–58. DOI 10.1016/j.cden.2017.08.003. [Google Scholar] [CrossRef]
Neglia JP, Robison LL, Stovall M, Liu Y, Packer RJ et al. (2006). New primary neoplasms of the central nervous system in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Journal of the National Cancer Institute 98: 1528–1537. DOI 10.1093/jnci/djj411. [Google Scholar] [CrossRef]
Nelson HD, Zakher B, Cantor A, Fu R, Griffin J et al. (2012). Risk factors for breast cancer for women aged 40 to 49 years: A systematic review and meta-analysis. Annals of Internal Medicine 156: 635–648. DOI 10.7326/0003-4819-156-9-201205010-00006. [Google Scholar] [CrossRef]
Niu Y, Lin ZY, Wan A, Chen HL, Liang H et al. (2019). RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Molecular Cancer 218: 46. DOI 10.1186/s12943-019-1004-4. [Google Scholar] [CrossRef]
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y et al. (2014). CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-Oncology 16: 1–63. DOI 10.1093/neuonc/nou223. [Google Scholar] [CrossRef]
Paris J, Morgan M, Campos J, Spencer GJ, Shmakova A et al. (2019). Targeting the RNA m6A reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell 25: 137–148. DOI 10.1016/j.stem.2019.03.021. [Google Scholar] [CrossRef]
Piñeros M, Frech S, Frazier L, Laversanne M, Barnoya J et al. (2018). Advancing reliable data for cancer control in the central America four region. Journal of Global Oncology 4: 1–11. DOI 10.1200/JGO.2016.008227. [Google Scholar] [CrossRef]
Rawla P, Thandra KC, Vellipuram A, Ali CDM (2018). Efficacy and safety of megestrol in the management of hepatocellular carcinoma: A systematic review of the literature. Contemporary Oncology 22: 209–214. DOI 10.5114/wo.2018.82641. [Google Scholar] [CrossRef]
Robbens S, Rouzé P, Cock JM, Spring J, Worden AZ et al. (2008). The FTO gene, implicated in human obesity, is found only in vertebrates and marine algae. Journal of Molecular Evolution 66: 80–84. DOI 10.1007/s00239-007-9059-z. [Google Scholar] [CrossRef]
Roundtree IA, Evans ME, Pan T, He C (2017). Dynamic RNA modifications in gene expression. Cell 69: 1187–1200. DOI 10.1016/j.cell.2017.05.045. [Google Scholar] [CrossRef]
Shen W, Li HQ, Su H, Chen KY, Yan J (2021). FTO overexpression inhibits apoptosis of hypoxia/reoxygenation-treated myocardial cells by regulating m6A modification of Mhrt. Molecular and Cellular Biochemistry 476: 2171–2179. DOI 10.1007/s11010-021-04069-6. [Google Scholar] [CrossRef]
Shete S, Hosking FJ, Robertson LB, Dobbins SE, Sanson M et al. (2009). Genome-wide association study identifies five susceptibility loci for glioma. Nature Genetics 41: 899–904. DOI 10.1038/ng.407. [Google Scholar] [CrossRef]
Siegel F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 68: 394–424. DOI 10.3322/caac.21492. [Google Scholar] [CrossRef]
Siegel RL, Miller KD, Jemal A (2020). Cancer statistics, 2020. CA: A Cancer Journal for Clinicians 70: 7–30. DOI 10.3322/caac.21590. [Google Scholar] [CrossRef]
Song W, Yang K, Luo JJ, Gao ZY, Gao YL (2021). Dysregulation of USP18/FTO/PYCR1 signaling network promotes bladder cancer development and progression. Sedentary Life and Nutrition 13: 3909–3925. DOI 10.18632/aging.202359. [Google Scholar] [CrossRef]
Spreafico FS, Cardoso-Filho C, Cabello C, Sarian LO, Zeferino LC et al. (2020). Breast cancer in men: clinical and pathological analysis of 817 cases. American Journal of Men’s Health 14: 1557988320908109. DOI 10.1177/1557988320908109. [Google Scholar] [CrossRef]
Stratigopoulos G, Padilla SL, LeDuc CA, Watson E, Hattersley AT et al. (2008). Regulation of Fto/Ftm gene expression in mice and humans. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 294: 1185–1196. DOI 10.1152/ajpregu.00839.2007. [Google Scholar] [CrossRef]
Su R, Dong L, Li CY, Nachtergaele S, Wunderlich M et al. (2018). R-2HG exhibits anti-tumor activity by targeting FTO/m6A/MYC/CEBPA signaling. Cell 172: 90–105. DOI 10.1016/j.cell.2017.11.031. [Google Scholar] [CrossRef]
Su YS, Wei X, Hu JC (2019). m6A RNA methylation regulators contribute to malignant progression and have clinical prognostic impact in gastric cancer. Frontiers in Oncology 9: 1038. DOI 10.3389/fonc.2019.01038. [Google Scholar] [CrossRef]
Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ et al. (2017). Risk factors and preventions of breast cancer. International Journal of Biological Sciences 13: 1387–1397. DOI 10.7150/ijbs.21635. [Google Scholar] [CrossRef]
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 71: 209–249. DOI 10.3322/caac.21660. [Google Scholar] [CrossRef]
Tang XD, Liu SH, Chen DW, Zhao ZG, Zhou JH (2019). The role of the fat mass and obesity-associated protein in the proliferation of pancreatic cancer cells. Oncology Letters 17: 2473–2478. DOI 10.3892/ol.2018.9873. [Google Scholar] [CrossRef]
Tao BB, Huang XH, Shi JH, Liu J, Li S et al. (2020). FTO interacts with FOXO3a to enhance its transcriptional activity and inhibits aggression in gliomas. Signal Transduction and Targeted Therapy 5: 130. DOI 10.1038/s41392-020-00234-3. [Google Scholar] [CrossRef]
Tao L, Mu XY, Chen HG, Jin D, Zhang RY et al. (2021). FTO modifies the m6A level of MALAT and promotes bladder cancer progression. Clinical and Translational Medicine 11: e310. DOI 10.1002/ctm2.310. [Google Scholar] [CrossRef]
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J et al. (2015). Global cancer statistics. 2012 CA: A Cancer Journal for Clinicians 65: 87–108. DOI 10.3322/caac.21262. [Google Scholar] [CrossRef]
Tsuruta N, Tsuchihashi K, Ohmura H, Yamaguchi K, Ito M et al. (2020). RNA N6-methyladenosine demethylase FTO regulates PD-L1 expression in colon cancer cells. Biochemical and Biophysical Research Communications 530: 235–239. DOI 10.1016/j.bbrc.2020.06.153. [Google Scholar] [CrossRef]
Vagelatos NT, Eslick GD (2013). Type 2 diabetes as a risk factor for Alzheimer’s disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiologic Reviews 35: 152–160. DOI 10.1093/epirev/mxs012. [Google Scholar] [CrossRef]
Valdez JA, Brennan MT (2018). Impact of oral cancer on quality of life. Dental Clinics of North America 62: 143–154. DOI 10.1016/j.cden.2017.09.001. [Google Scholar] [CrossRef]
van der Hoeven F, Schimmang T, Volkmann A, Mattei MG, Kyewski B et al. (1994). Programmed cell death is affected in the novel mouse mutant Fused toes (Ft). Development 20: 2601–2607. DOI 10.1242/dev.120.9.2601. [Google Scholar] [CrossRef]
Vienne-Jumeau A, Tafani C, Ricard D (2019). Environmental risk factors of primary brain tumors: A review. Revue Neurologique 175: 664–678. DOI 10.1016/j.neurol.2019.08.004. [Google Scholar] [CrossRef]
Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D et al. (2017). The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nature Medicine 23: 1369–1376. DOI 10.1038/nm.4416. [Google Scholar] [CrossRef]
Wang F, Liao Y, Zhang M, Zhu Y, Wang W et al. (2021). N6-methyladenosine demethyltransferase FTO-mediated autophagy in malignant development of oral squamous cell carcinoma. Oncogene 40: 3885–3898. DOI 10.1038/s41388-021-01820-7. [Google Scholar] [CrossRef]
Wang SY, Chai PW, Jia RB, Jia RB (2018). Novel insights on m6A RNA methylation in tumorigenesis: A double-edged sword. Molecular Cancer 17: 101. DOI 10.1186/s12943-018-0847-4. [Google Scholar] [CrossRef]
Wei JB, Liu FG, Lu ZK, Fei QL, Ai YX et al. (2018). Differential m6A, m6Am, and m1A demethylation mediated by FTO in the cell nucleus and cytoplasm. Molecular Cell 71: 973–985.e5. DOI 10.1016/j.molcel.2018.08.011. [Google Scholar] [CrossRef]
Weller M, Wick W, Aldape K, Brada M, Berger M et al. (2015). Glioma. Nature Reviews Disease Primers 1: 15017. DOI 10.1038/nrdp.2015.17. [Google Scholar] [CrossRef]
Wrensch M, Jenkins RB, Chang JS, Yeh RF, Xiao Y et al. (2009). Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nature Genetics 41: 905–908. DOI 10.1038/ng.408. [Google Scholar] [CrossRef]
Wu LP, Wu DY, Ning JF, Liu W, Zhang DH (2019). Changes of N6-methyladenosinemodulators promote breast cancer progression. BMC Cancer 19: 326. DOI 10.1186/s12885-019-5538-z. [Google Scholar] [CrossRef]
Xu D, Shao WW, Jiang YS, Wang X, Liu Y et al. (2017). FTO expression is associated with the occurrence of gastric cancer and prognosis. Oncology Reports 38: 2285–2292. DOI 10.3892/or.2017.5904. [Google Scholar] [CrossRef]
Xu X, Zhou E, Zheng J, Zhang CH, Zou YH et al. (2021). Prognostic and predictive value of m6A. Frontiers in Oncology 11: 631803. DOI 10.3389/fonc.2021.631803. [Google Scholar] [CrossRef]
Xu Y, Ye S, Zhang N, Zheng S, Liu H et al. (2020). The FTO/miR-181b-3p/ARL5B signaling pathway regulates cell migration and invasion in breast cancer. Cancer Communications 40: 484–500. DOI 10.1002/cac2.12075. [Google Scholar] [CrossRef]
Yang Z, Jiang XD, Zhang ZH, Zhao ZT, Xing WJ et al. (2021). HDAC3-dependent transcriptional repression of FOXA2 regulates FTO/m6A/MYC signaling to contribute to the development of gastric cancer. Cancer Gene Therapy 28: 141–155. DOI 10.1038/s41417-020-0193-8. [Google Scholar] [CrossRef]
Ye ZQ, Wang SB, Chen WY, Zhang X, Chen J et al. (2020). Fat mass and obesity-associated protein promotes the tumorigenesis and development of liver cancer. Oncology Letters 20: 1409–1417. DOI 10.3892/ol.2020.11673. [Google Scholar] [CrossRef]
Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E et al. (2019). Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clinical Gastroenterology and Hepatology 17: 748–755. DOI 10.1016/j.cgh.2018.05.057. [Google Scholar] [CrossRef]
Yue CF, Chen JR, Li ZY, Li LS, Chen JG et al. (2020). microRNA-96 promotes occurrence and progression of colorectal cancer via regulation of the AMPKα2-FTO-m6A/MYC axis. Journal of Experimental & Clinical Cancer Research 39: 240. DOI 10.1186/s13046-020-01731-7. [Google Scholar] [CrossRef]
Zanoni DK, Montero PH, Migliacci JC, Shah JP, Wong RJ et al. (2019). Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncology 90: 115–121. DOI 10.1016/j.oraloncology.2019.02.001. [Google Scholar] [CrossRef]
Zeng J, Zhang HY, Tan YG, Wang Z, Li YW et al. (2021). m6A demethylase FTO suppresses pancreatic cancer tumorigenesis by demethylating PJA2 and inhibiting Wnt signaling. Molecular Therapy-Nucleic Acids 25: 277–292. DOI 10.1016/j.omtn.2021.06.005. [Google Scholar] [CrossRef]
Zhang L, Wan YC, Zhang ZH, Jiang Y, Lang JH et al. (2021a). FTO demethylates m6A modifications in HOXB13 mRNA and promotes endometrial cancer metastasis by activating the WNT signalling pathway. RNA Biology 18: 1265–1278. DOI 10.1080/15476286.2020.1841458. [Google Scholar] [CrossRef]
Zhang YL, Yang YJ (2021). Effects of m6A RNA methylation regulators on endometrial cancer. Journal of Clinical Laboratory Analysis 35: e23942. DOI 10.1002/jcla.23942. [Google Scholar] [CrossRef]
Zhang YQ, Hamada M (2020). MoAIMS efficient software for detection of enriched regions of MeRIP-Seq. BMC Bioinformatics 21: 103. DOI 10.1186/s12859-020-3430-0. [Google Scholar] [CrossRef]
Zhang ZB, Zhou DM, Lai YL, Liu YJ, Tao X et al. (2012). Estrogen induces endometrial cancer cell proliferation and invasion by regulating the fat mass and obesity-associated gene via PI3K/AKT and MAPK signaling pathways. Cancer Letters 319: 89–97. DOI 10.1016/j.canlet.2011.12.033. [Google Scholar] [CrossRef]
Zhang ZY, Gao QF, Wang SC (2021b). Kinase GSK3β functions as a suppressor in colorectal carcinoma through the FTO-mediated MZF1/c-Myc axis. Journal of Cellular and Molecular Medicine 25: 2655–2665. DOI 10.1111/jcmm.16291. [Google Scholar] [CrossRef]
Zheng GQ, Cox T, Tribbey L, Wang GZ, Iacoban P et al. (2014). Synthesis of a FTO inhibitor with anticonvulsant activity. ACS Chemical Neuroscience 5: 658–665. DOI 10.1021/cn500042t. [Google Scholar] [CrossRef]
Zhou G, Yan K, Liu J, Gao L, Jiang X et al. (2021). FTO promotes tumour proliferation in bladder cancer via the FTO/miR-576/CDK6 axis in an m6A-dependent manner. Cell Death Discovery 7: 329. DOI 10.1038/s41420-021-00724-5. [Google Scholar] [CrossRef]
Zhu YP, Shen JQ, Gao LY, Feng YJ (2016). Estrogen promotes fat mass and obesity-associated protein nuclear localization and enhances endometrial cancer cell proliferation via the mTOR signaling pathway. Oncology Reports 35: 2391–2397. DOI 10.3892/or.2016.4613. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |