DOI:10.32604/biocell.2022.021314

| BIOCELL DOI:10.32604/biocell.2022.021314 |  |
| Article |
In vivo protective effect of late embryogenesis abundant protein (ApSK3 dehydrin) on Agapanthus praecox to promote post-cryopreservation survival
1Department of Landscape Science and Engineering, School of Design, Shanghai Jiao Tong University, Shanghai, 200240, China
2Institute for Agri-Food Standards and Testing Technology, Shanghai Academy of Agricultural Sciences, Shanghai, 201403, China
*Address correspondence to: Di Zhang, zhangdi2013@sjtu.edu.cn
#Authors contributed equally to this work
Received: 08 January 2022; Accepted: 11 April 2022
Abstract: Dehydrins (DHNs), as members of the late embryogenesis abundant protein family, play critical roles in the protection of seeds from dehydration and plant adaptation to multiple abiotic stresses. Vitrification is a basic method in plant cryopreservation and is characterized by forming a glassy state to prevent lethal ice crystals produced during cryogenic storage. In this study, ApSK3 type DHN was genetically transformed into embryogenic calluses (EC) of Agapanthus praecox by overexpression (OE) and RNA interference (RNAi) techniques to evaluate the in vivo protective effect of DHNs during cryopreservation. The cell viability showed a completely opposite trend in OE and RNAi cell lines, the cell relative death ratio was decreased by 20.0% in ApSK3-OE EC and significantly increased by 66.15% in ApSK3-RNAi cells after cryopreservation. Overexpression of ApSK3 increased the content of non-enzymatic antioxidants (AsA and GSH) and up-regulated the expression of CAT, SOD, POD, and GPX genes, while ApSK3-RNAi cells decreased antioxidant enzyme activities and FeSOD, POD, and APX genes expression during cryopreservation. These findings suggest that ApSK3 affects ROS metabolism through chelating metal ions (Cu2+ and Fe3+), alleviates H2O2 and OH· excessive generation, activates the antioxidant system, and improves cellular REDOX balance and membrane lipid peroxidation damage of plant cells during cryopreservation. DHNs can effectively improve cell stress tolerance and have great potential for in vivo or in vitro applications in plant cryopreservation.
Keywords: Dehydrin; Embryogenic callus; Cryopreservation; RNAi; Gene overexpression
Cryopreservation provides a safe and cost-effective in vitro method for the long-term preservation of plant genetic resources (Ren et al., 2021). Vitrification, dehydration-encapsulation, and controlled freezing are commonly used methods for plant cryopreservation (Reed, 2001). Vitrification is widely used in plant cryopreservation because of its rapid and convenient operation (Ren et al., 2013). The basic principle of the vitrification procedure is to prevent the formation of lethal ice crystals within the cell (Sakai et al., 2008; Zamecnik et al., 2021). Cryoprotectant treatment can lead to continuous dehydration of plant cells, increase cytoplasmic viscosity and achieve a glassy state, which is important for cell survival during vitrification cryopreservation (Meryman, 2007). However, cryopreservation treatments can also cause severe complex stresses to preserved cells, including dehydration and osmotic damage, oxidative stress, lipid membrane damage, protein aggregation, and ion toxicity (Meryman, 2007; Ren et al., 2013; Ren et al., 2021; Fayter et al., 2020; Elliott et al., 2017).
Antioxidant systems, including superoxide dismutase (SOD), peroxidase (POD), the ascorbic acid (AsA)-glutathione (GSH) cycle, and the glutathione peroxidase (GPX) cycle, are involved in removing excessive reactive oxygen species (ROS) and alleviating oxidative stress damage during cryopreservation procedure (Ren et al., 2015; Zhang et al., 2015). Ren et al. (2014) used some exogenous regulatory substances to alleviate the cell oxidative stress and optimize the cryopreservation system and found that GSH, AsA, abscisic acid, and glycine betaine can effectively increase the rate of survival post-cryopreservation. Nowadays, the promotion of the post-cryopreservation survival or regeneration of plants after cryopreservation is still a significant scientific issue in cryobiology.
Living cells are not tolerant of dehydration. Orthodox seeds (desiccation-tolerant seeds), as a special case, lose most water achieving the glassy state and can be stored for a long term (Hoekstra et al., 2001; Smolikova et al., 2020). Late embryogenesis abundant (LEA) proteins are highly abundant during the later stages of seed maturation to endow the seeds with drought tolerance ability (Liu et al., 2013; Avelange-Macherel et al., 2015; Bao et al., 2017). In the last decade, some studies found that in addition to protecting the seeds from dehydration, LEA protein is also induced by water-related abiotic stress in a wide range of vegetative tissues and organisms (Saibi et al., 2015; Saucedo et al., 2017). Dehydrins (DHNs), the group II LEA proteins, are one of the most functional members of the LEA family (Close, 1997). DHNs are disordered proteins and have highly hydrophilic and flexible structures (Hara et al., 2016; Hughes et al., 2013). This special structure enables DHNs to maintain stability even at high temperatures, such as in boiling water or at freezing temperatures (Livernois et al., 2009; Saucedo et al., 2017; Yang et al., 2019). DHNs protect biomacromolecules by nonspecific binding mode, called ‘molecular shield’ (Hughes et al., 2013). Several studies have reported that under different environmental stresses, DNH is able to directly bind to the metal ions to reduce the ROS, prevent membrane and protein aggregation (Halder et al., 2016, 2017; Rakhra et al., 2017), and protect the integrity of biomolecules (Abdul et al., 2021). In our previous studies, two DHNs (ApSK3 and ApY2SK2) as protective proteins were screened in embryogenic callus (EC) of Agapanthus praecox during cryopreservation by combined RNA-seq and proteomics analysis. In vitro protein functional analysis indicated that ApY2SK2 and ApSK3 can effectively protect enzyme activity and significantly inhibit hydroxyl radical (OH·) generation during the freeze-thaw process (Yang et al., 2019). Furthermore, we purified ApY2SK2 and ApSK3 proteins by prokaryotic expression method and added them to PVS2. The results showed that the survival rate of Arabidopsis thaliana seedlings increased approximately by 100% after adding DHNs compared to the control group after cryopreservation (Zhang et al., 2021). However, in vivo protective effects of DHNs on cryopreservation have not yet been fully elucidated.
In this study, ECs of Agapanthus praecox were genetically transformed for ApSK3 overexpression or silencing through RNA interference (RNAi). Cryopreservation and stress physiological tests were performed on transgenic EC to evaluate the in vivo protective effect of DHNs during cryopreservation and obtain a novel insight for the in vivo or in vitro application of DHNs to optimize cryopreservation techniques.
The pHB-YFP-ApSK3 (Overexpression) and pTCK303-ApSK3-GUS (RNAi) were transferred into Agrobacterium tumefaciens (GV3101), which was then co-cultured and allowed to infect EC of Agapanthus praecox for 5 d (Figs. 1a and 1e). The transgenic cells were screened using carboxypenicillin and hygromycin. Most ECs showed browning and gradually died after 4–8 weeks of hygromycin screening (Figs. 1b, 1c, 1f and 1g). The transgenic EC proliferated gradually and were clearly differentiated from the browning cells after 12 weeks of hygromycin screening (Figs. 1d and 1h).
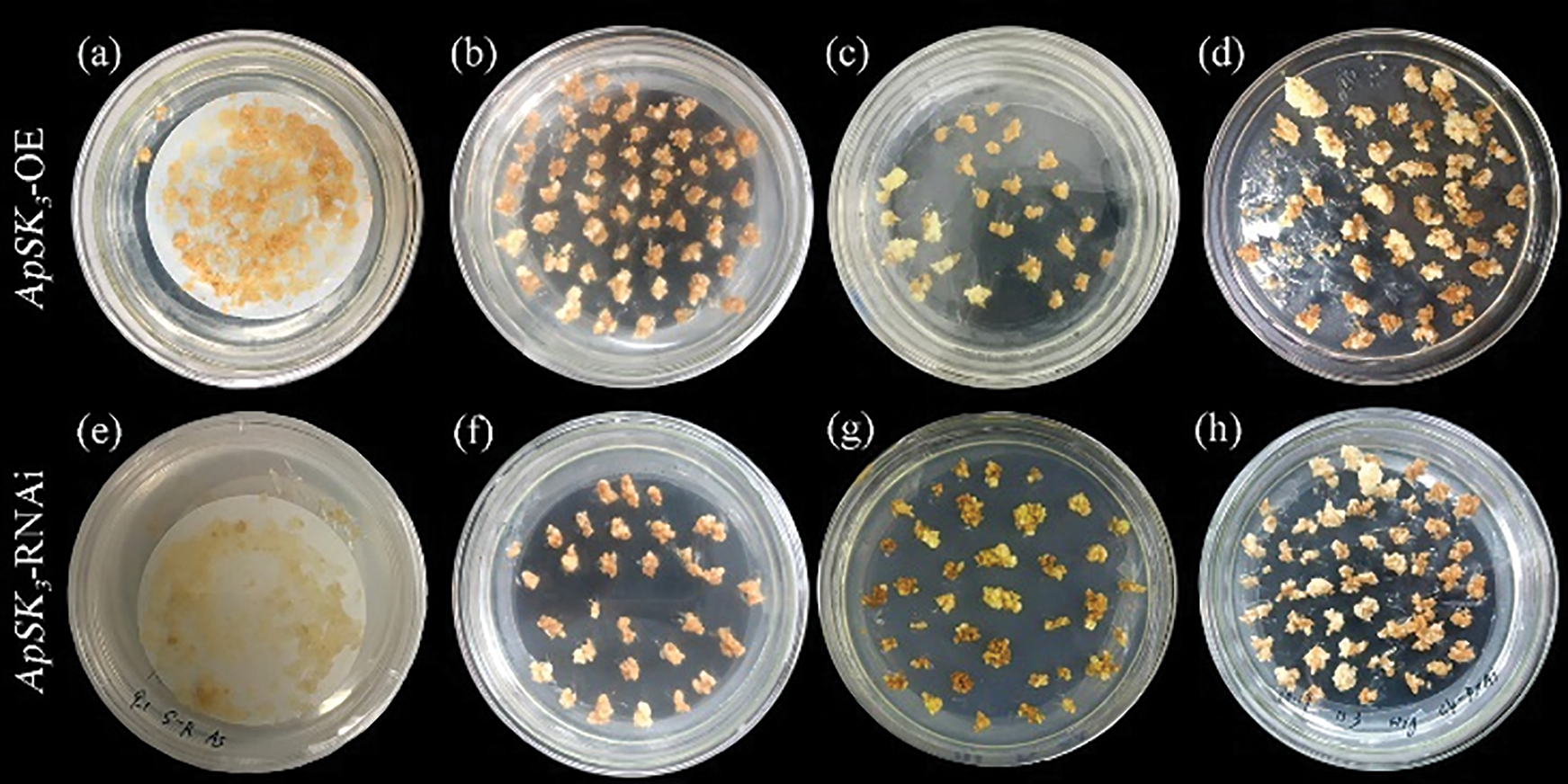
Figure 1: Transgenic embryogenic callus of Agapanthus praecox screening with hygromycin. (a–d) Overexpression of late embryogenesis abundant protein ApSK3 (ApSK3-OE); (e–f) RNA interference of ApSK3; (a, e) Embryogenic callus (EC) and Agrobacterium tumefaciens co-culture stage; (b, f) Hygromycin resistance was screened for 4 weeks; (c, g) Hygromycin resistance was screened for 8 weeks; (d, h) Hygromycin resistance was screened for 12 weeks.
Yellow fluorescence protein (YFP) signals and histochemical β-glucuronidase (GUS) staining results showed that ApSK3 was successfully transformed and stably expressed in EC cells (Figs. 2a and 2b). The gene expression level of ApSK3-OE and ApSK3-RNAi cell lines were 126%–162% and 71%–84% of that of control EC, respectively (Fig. 2c). The western blot test showed stable expression of ApSK3 protein in each transgenic cell line (Fig. 2d). These results suggested that ApSK3-OE and ApSK3-RNAi transgenic EC could be used for subsequent experiments on the evaluation of cryopreservation and stress physiology.

Figure 2: Detection of ApSK3 overexpression and RNAi transgenic Agapanthus praecox embryogenic callus. (a) Confocal microscopy images of overexpressed ApSK3-YFP fusion protein in embryogenic callus (EC). Bar = 50 μm; (b) GUS staining of ApSK3 RNAi transgenic EC; (c) Real-time quantitative polymerase chain reaction detection of ApSK3 overexpression and RNAi transgenic Agapanthus praecox EC, the dotted line represents the expression level of the housekeeping gene Actin; (d) Western blot results of ApSK3 overexpression transgenic Agapanthus praecox EC (lanes 1–3: overexpressing cell lines). *P < 0.05.
ApSK3 can significantly improve the cell viability and antioxidant system, and alleviate oxidative stress damage during cryopreservation. The results of 2,3,5-triphenyltetrazolium chloride (TTC) staining showed that the cell viability of the three genotypes had no significant difference before cryopreservation. However, the cell viability of ApSK3-OE EC was significantly higher than that of the control group, while that in the ApSK3-RNAi EC was noticeably lower than the control group after cryopreservation (Fig. 3a). Evan’s blue detection showed that the cell relative death ratio of the control EC group was 50.43%, while the mortality of ApSK3-OE EC was significantly reduced to 40.57%, and that ApSK3-RNAi cells was significantly increased to 83.79% after cryopreservation treatment (Fig. 3b). The content of malondialdehyde (MDA), a product of membrane lipid peroxidation, increased continuously in EC during cryopreservation. The MDA content of ApSK3-RNAi cells was significantly higher than that of control cells at osmo-protection and quick thawing stages, and the MDA content of ApSK3-OE EC was significantly lower than the control group after cryopreservation (Fig. 3c). These results suggest that ApSK3 can alleviate damage due to membrane lipid peroxidation during cryopreservation. Furthermore, ROS metabolism also changed significantly between the three EC genotypes (Fig. 3). H2O2 and OH· levels in ApSK3-RNAi cells were significantly higher than in the other two EC genotypes. However, H2O2 and OH· had no significant difference between the control and ApSK3-OE groups during cryopreservation treatments (Figs. 3d and 3e). Additionally, antioxidant enzyme SOD activity was significantly decreased in ApSK3-RNAi cells (Fig. 3g), and non-enzymatic antioxidants AsA and GSH contents increased significantly in the ApSK3-OE group during the whole cryopreservation process (Figs. 3h and 3i).
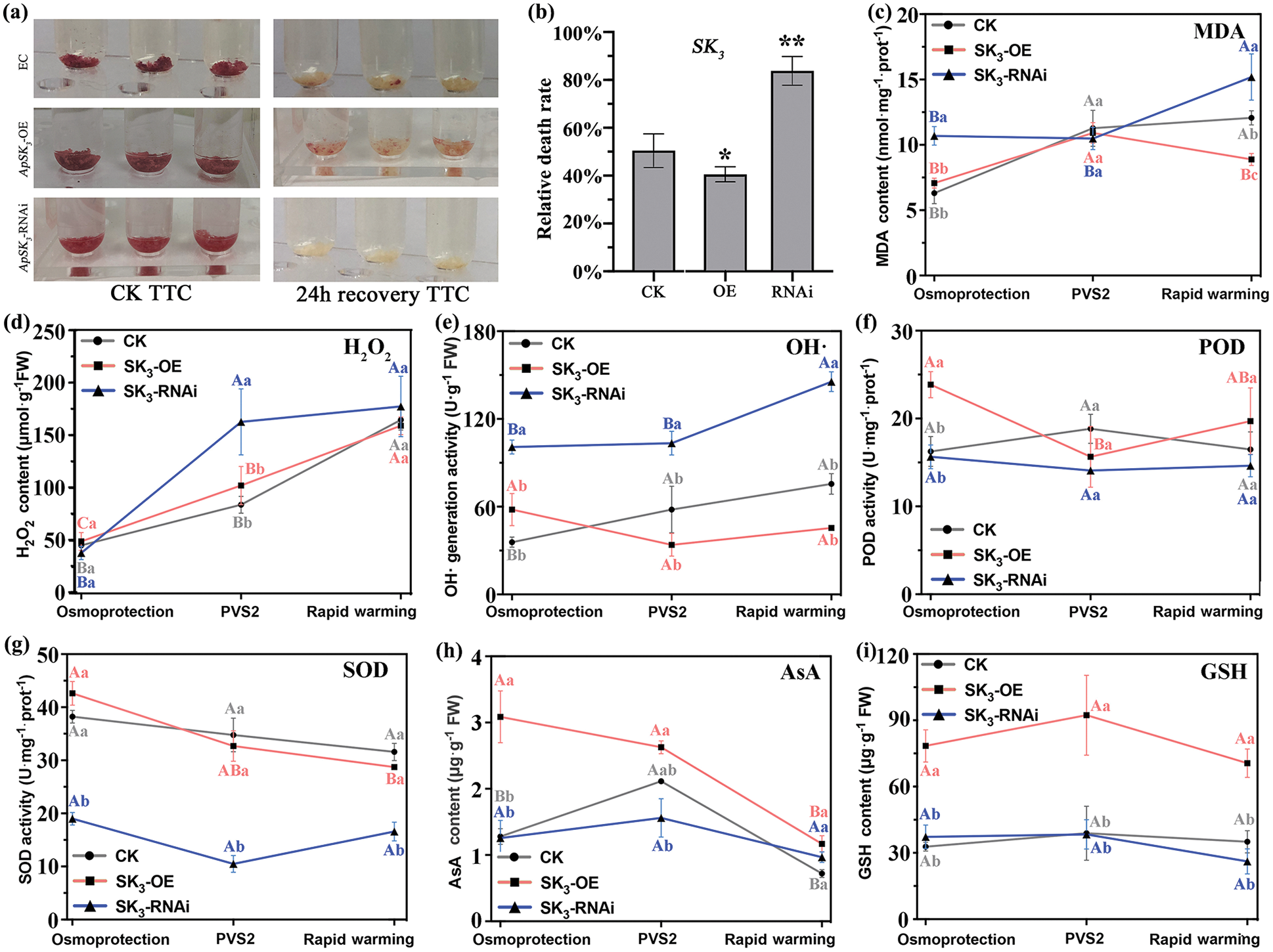
Figure 3: Changes of cell viability and stress physiological indexes of different genotypic Agapanthus praecox embryogenic callus during cryopreservation. Capital letters indicate that the same genotype embryogenic calluses have significant differences at different steps of cryopreservation; lower-case letters represent that the different genotype embryogenic calluses have significant differences at the same steps of cryopreservation. *P < 0.05, **P < 0.01.
To evaluate the effect of ApSK3 on the response of antioxidant system-related genes during cryopreservation, catalase (CAT), Cu/ZnSOD, FeSOD, POD, glutathione peroxidase (GPX), and ascorbate peroxidase (APX) were selected for gene quantitative expression analysis. Cu/ZnSOD, FeSOD, POD, and GPX of ApSK3-OE EC were significantly up-regulated than in other groups at dehydration and quick thawing stages, and the expression of these genes did not differ significantly between the control and ApSK3-RNAi groups (Fig. 4). CAT and APX were up-regulated in the ApSK3-OE sample only during the quick thawing stage (Figs. 4a and 4f), and APX and GPX of ApSK3-RNAi cells were significantly down-regulated compared to other groups in the later stages of cryopreservation (Figs. 4e and 4f). These results indicate that antioxidant systems related genes in ApSK3-OE EC responded positively to cryopreservation complex stresses, and inhibition of ApSK3 expression affects the GPX cycle and AsA–GSH cycle-related genes.

Figure 4: Real-time PCR quantitative analysis of antioxidant system-related genes of ApSK3 transgenic embryogenic callus (EC). CAT: catalase; SOD: superoxide dismutase; POD: peroxidase; GPX: glutathione peroxidase; APX: ascorbate peroxidase. OP: osmo-protection; PVS2: immersed in PVS2 (dehydration); RW: rapid warming (quick thawing). Capital letters indicate that ECs of the same genotype have significant differences at different steps of cryopreservation; lower-case letters represent that ECs of different genotypes have significant differences at the same steps of cryopreservation.
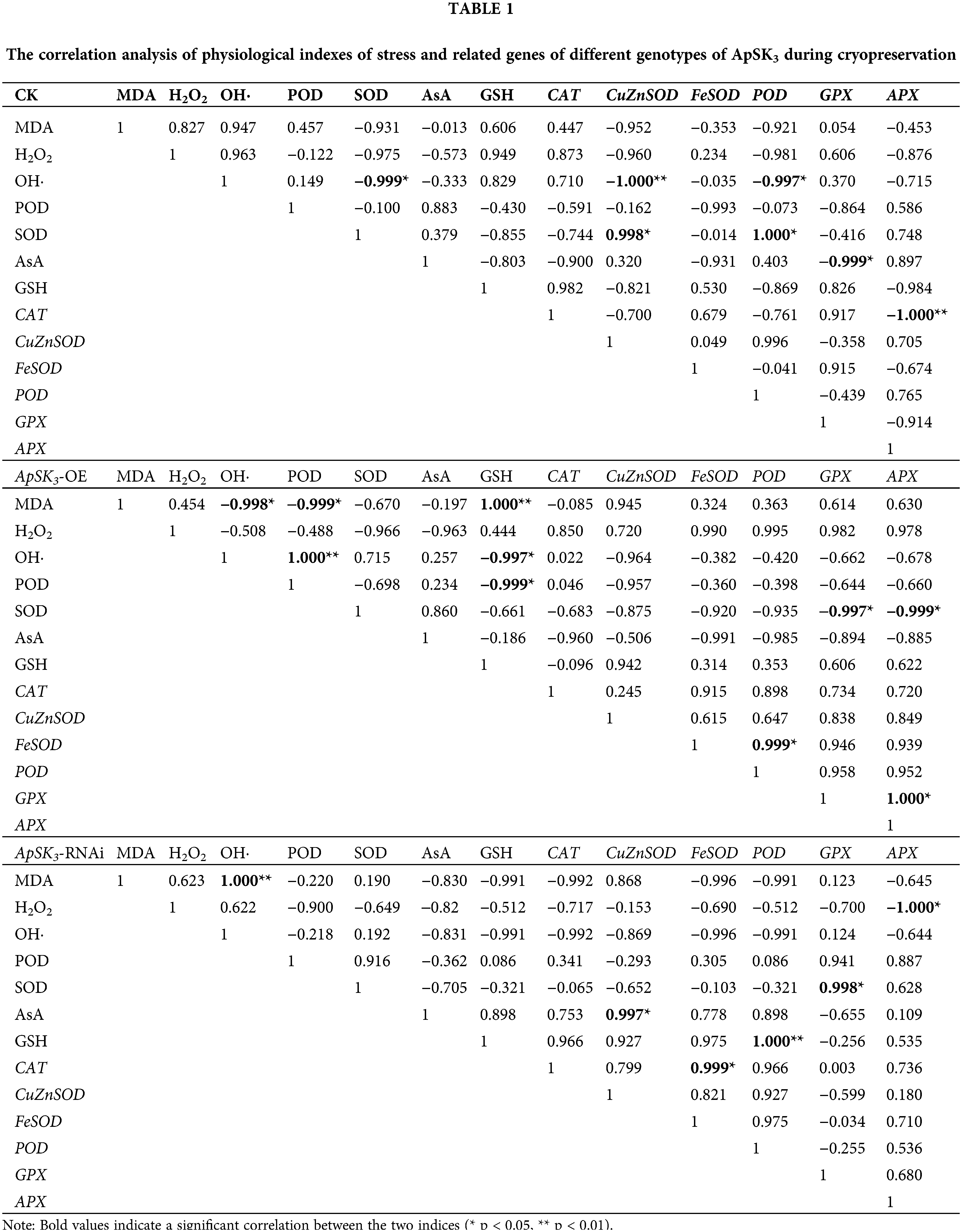
Correlation analysis of stress physiological indexes between the three genotypic cells was performed to reveal the direct or indirect protective function of ApSK3 in cryopreservation (Table 1). The results show that, in the control group, the level of OH· correlated negatively with SOD, Cu/ZnSOD, and POD. SOD activity had a significant positive correlation to Cu/ZnSOD and POD expression level, and GPX was negatively correlated with AsA. However, in the ApSK3-OE group, MDA contents presented a significant negative correlation with OH· and POD and a significant positive correlation with GSH. Strikingly, in the ApSK3-RNAi group, MDA contents had a significant positive correlation with OH· generation, H2O2 was negatively correlated with APX, and SOD was positively correlated with GPX (Table 1). Therefore, the correlation of membrane lipid peroxidation level and OH· generation activity during cryopreservation had significant differences among the three different ApSK3 genotypic cells. The gene expression levels of SOD, GPX, and APX had a positive response to the above-mentioned changes.
DHNs are accumulated during the late stages of seed development and are involved in various environmental stresses (Dure and Galau, 1981; Hernández-Sánchez et al., 2014). DHNs response to stress protection has been well studied in several plant species, such as Oryza sativa (Lee et al., 2005), Citrus unshiu (Hara et al., 2005), Hordeum vulgare (Kosová et al., 2008), Populus trichocarpa (Liu et al., 2012), Vitis vinifera (Yang et al., 2012), Solanum habrochaites (Liu et al., 2015), Hevea brasiliensis (Cao et al., 2017), and Arabidopsis thaliana (Nguyen et al., 2020). Various investigations have indicated that the SKn type DHNs impart plant stress tolerance by improving ROS detoxification and reducing lipid peroxidation (Cao et al., 2017; Riyazuddin et al., 2021). Overexpression of two SKn type DHNs from Hevea brasiliensis increased proline accumulation, reduced H2O2 content, and alleviated electrolyte leakage in Arabidopsis thaliana under osmotic and drought stress (Cao et al., 2017). In Solanum habrochaites, the SK3-type DHN (ShDHN) is regulated by drought, salt, and cold stress, and its ectopic expression reduces H2O2 accumulation, alters the expression of several antioxidant genes including POD, SOD, and GST, and alleviated membrane damage in tomato (Liu et al., 2015). In Agapanthus praecox, ApSK3 was differentially expressed at both transcription and protein levels during the cryopreservation procedure and considered associated with the complex stress response. Ectopic expression of ApSK3 in Arabidopsis thaliana could alleviate oxidative damage and promote post-cryopreservation survival. The ApSK3 transgenic plants showed better growth status than wild-type Arabidopsis thaliana under osmotic and salt stress, with less ROS accumulation, higher antioxidant enzyme activity, greater accumulation of proline, and lower degrees of membrane lipid peroxidation (Yang et al., 2019). Additionally, in vitro addition of ApSK3 protein to PVS2 (a cryoprotectant) doubled the survival rate of Arabidopsis thaliana seedlings and significantly decreased the content of MDA and H2O2 (Zhang et al., 2021). However, in vitro application of dehydrin, whether these proteins can cross the cell membrane into the plant cells and play a physiological protective role is still unclear. In this study, the cell viability of Agapanthus praecox EC, after cryopreservation, showed a completely opposite trend between overexpression and RNAi transgenic cell lines. Overexpression of ApSK3 enhanced the cell viability, non-enzymatic antioxidant (AsA and GSH) content, the expression level of antioxidant system-related genes, and affected the ROS metabolism during cryopreservation (Figs. 3 and 4). Down-regulated ApSK3 reduced the cell viability, antioxidant enzyme activities, and the expression level of antioxidant system-related genes, and significantly increased OH· generation activity during cryopreservation. Therefore, in vivo protective function of ApSK3 can significantly improve the cell viability and antioxidant system, and alleviate oxidative stress damage during cryopreservation.
The molecular shielding model is an important hypothesis explaining the mechanism of the protective functions of biomacromolecules such as enzymes and proteins (Chakrabortee et al., 2012; Hara et al., 2016; Hughes et al., 2013). ApSK3 dehydrin genetic transformation experiment in cryopreservation complex stresses supports the above model hypothesis (Yang et al., 2019). Hara et al. (2003) investigated the effects of DHNs on lipid peroxidation and found that CuCOR19 of Citrus unshiu could prevent the oxidation of liposomes most likely by scavenging ROS. Several research groups have also demonstrated that, in addition to membranes, diverse DHNs are able to bind to many small ions and ligands (Kovacs et al., 2008) and buffer the increase in ion concentration during dehydration stress. In this study, H2O2 and OH· levels had no significant difference between control and ApSK3-OE groups during cryopreservation. However, OH· levels in ApSK3-RNAi cells were significantly higher than in control and ApSK3-OE ECs. Additionally, there was a significant positive correlation between MDA and OH· generation activity in the ApSK3-RNAi group, while a significant negative correlation was found in the ApSK3-OE group. ApSK3 has a stronger Cu2+ and Fe3+ binding function and effectively inhibits the Fenton Reaction (H2O2 to OH·) (Yang et al., 2019). Zhang et al. (2015) reported that OH· is a highly reactive and toxic chemical species in cells, and H2O2 is the main ROS component mediating oxidative damage and affecting cell viability during plant cryopreservation. Accordingly, these clues indicate that ApSK3 can affect ROS metabolism through chelating metal ions and decreasing the damage of H2O2 and OH· to plant cells during cryopreservation.
We compared the effects of in vitro addition (Zhang et al., 2021) and in vivo genetic transformation of ApSK3 on cell viability after cryopreservation. Both methods could significantly improve cell viability and promote post-cryopreservation survival, and the protective mechanisms of dehydrin were similar in vitro and in vivo. The main difference between the above two methods, including in vitro added ApSK3 to PVS, has a better effect on reducing intracellular H2O2 content, and ApSK3 transgenic cells have more beneficial in reducing OH· damage and protecting the activity of antioxidant enzymes during cryopreservation. These results indicate that ApSK3 dehydrin can effectively enhance the stress resistance of plants and has great potential for both in vitro and in vivo applications in plant cryopreservation.
EC of Agapanthus praecox was induced from pedicel tissue, according to Zhang et al. (2015). EC was continuously sub-cultured in the dark on Murashige and Skoog (MS) medium supplemented with 1.5 mg·L−1 picloram (Sangon Biotech, Shanghai, China) at 25°C.
Plasmid construction and genetic transformation
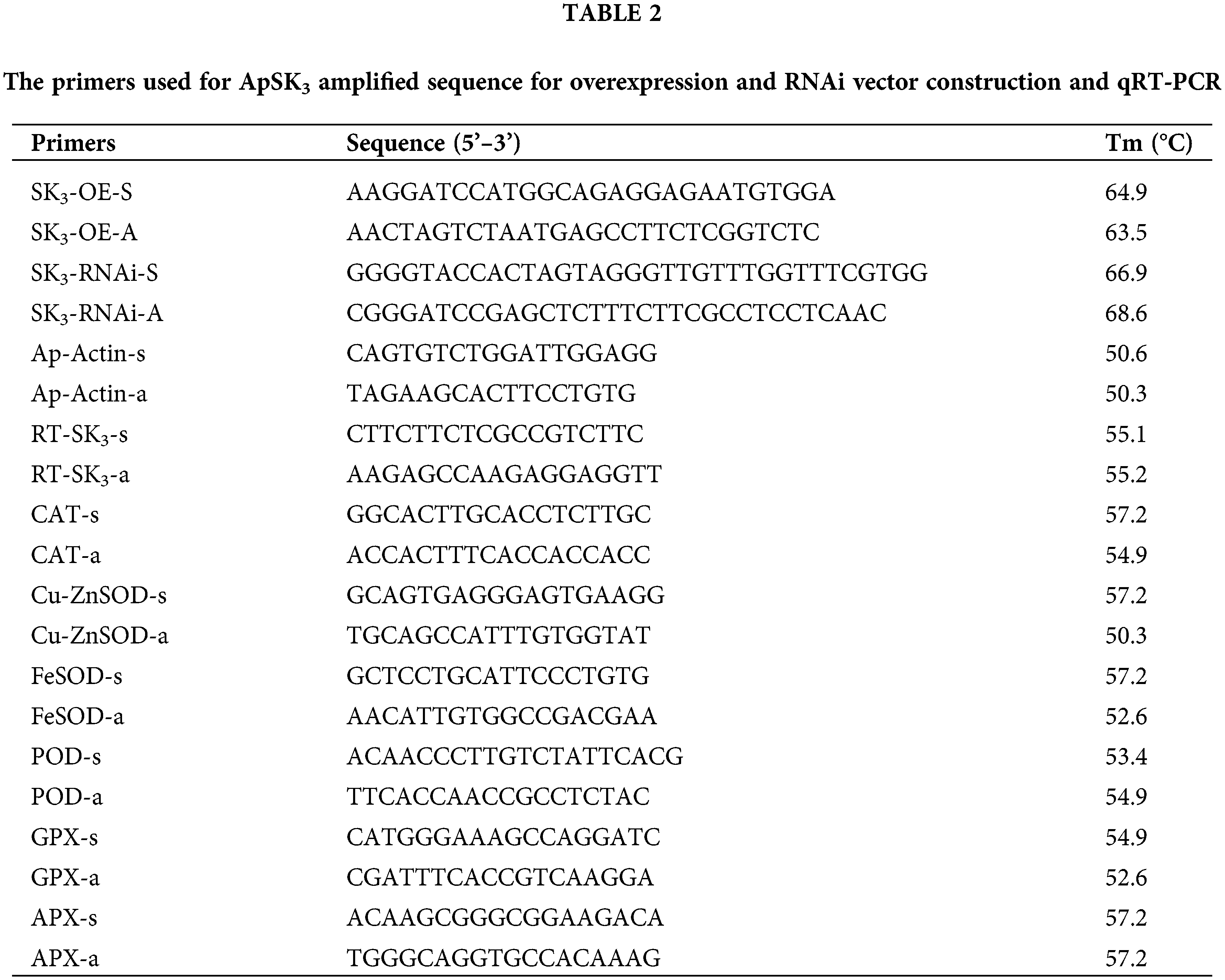
The gene sequence of SK3 was obtained from Yang et al. (2019). The gene open reading frame (ORF) and RNAi sequence of ApSK3 were amplified by gene-specific primers. The digestion sites of the CDS sequence are SpeI and BamHI, and the digestion sites of the ApSK3 RNAi sequence are SacI/BamHI and KpnI/SpeI (Table 2). RNAi and ORF sequence of ApSK3 were ligated to pTCK303 and pHB vectors, respectively, by enzyme digestion (Chen et al., 2021; Yuan et al., 2014). The recombinant plasmids (pHB-YFP-ApSK3 and pTCK303-ApSK3) were transferred into the EC by the GV3101-mediated method. The transgenic materials were screened for hygromycin resistance (Sangon Biotech, Shanghai, China), and stable growth calluses were obtained for further study. Quantitative real-time PCR and western blot analysis were used to verify the mRNA and protein level of ApSK3 in all transgenic lines. The YFP signals of overexpression transgenic EC were observed by laser-scanning confocal microscopy (Leica TCS SP5II, Wetzlar, Germany), according to Yang et al. (2019). The GUS staining of RNAi transgenic EC was performed by Jefferson et al. (1987).
Pre-culture: the EC of Agapanthus praecox was placed on the pre-culture solid medium (0.5 M sucrose MS medium), 4°C, in the dark for 2 days. For loading: 0.2 g of EC was placed onto the cryopreservation tube, and 2 mL loading solution was added and treated at 25°C for 1 h. For dehydration: the loading liquid was completely removed, and 2 mL of cryoprotectant (PVS2) was added and treated at 0°C on ice for 40 min. For rapid freezing: the cryopreservation tube was placed in liquid nitrogen for 1 h; for quick thawing, it was placed in a 40°C water bath for 90 s. This was washed using a washing solution to remove PVS2 solution for 30 min, and fresh washing solution was replaced every 10 min. For the recovery of culture: ECs were transferred to a solid subculture medium, and the residual washing solution was sucked and treated at 25°C in the dark for 1 d. The preparation of media and solutions were according to Zhang et al. (2015).
Western blot analysis was performed as previously described (Yang et al., 2019). To resolve the proteins, sodium dodecyl sulfate-polyacrylamide gel (12% for separation and 8% stacking gel) was prepared. Twenty microliters of protein samples were added to the well and separated at 90 V for 30 min and then at 80 V for 90 min. After electrophoresis, the gel was removed, rinsed with deionized water, placed in the film transfer fluid, and balanced for 15 min. For membrane transfer, a 0.22 μM PVDF membrane (Amersham Pharmacia, Shanghai, China) was used to transfer the protein at 30 V and 4°C for 3 h. The membrane was blocked with 5% nonfat milk for 3 h, and GFP primary antibody (Sangon Biotech, Shanghai, China) was added to the diluent containing the membrane for 3 h. The membrane was washed with TBST buffer for 30 min. Then, the secondary antibody diluent (Sangon Biotech, Shanghai, China) was added.
Assay for cell viability and cell death
The survival of cryopreserved cells was assessed by the TTC method. EC tissue (100 mg) was immersed into 2 mL of TTC (Sangon Biotech, Shanghai, China) buffer (0.8% TTC in 0.05 M PBS) and incubated in the dark at 25°C for 20 h. EC cells were rinsed thrice with 2 mL sterile water. The staining state of the EC was captured using a Nikon camera.
Evan’s blue assay was performed according to Chen et al. (2021). EC (100 mg) was suspended in 0.05% Evan’s blue solution and incubated for 15 min at 25°C. Cells were collected by centrifugation at 16,000 g for 5 min and washed with distilled water until no more dye was eluted. The trapped dye was then released by adding 1.0 mL of 1% SDS at 80°C for 1 h. The supernatant was detected at an absorbance of 600 nm by a spectrophotometer (Thermo Biomate160).
Detection of physiological indices
The contents of MDA, H2O2, OH·, and GSH and the activity of POD, SOD, AsA and GSH were detected using their respective test kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) (Yang et al., 2019).
Total RNA extraction and quantitative real-time PCR
The total RNA was extracted using the TaKaRa plant RNA extraction kit (TaKaRa, Shanghai, China). RNA was then reverse transcribed to cDNA using the RT reagent kit (TaKaRa, Shanghai, China). qRT-PCR was performed in an ABI 7900 HT RT-PCR detection system (Thermo Fisher Scientific, Boston, USA). Amplification was carried out using Brilliant SYBR Green QPCR Master Mix (Takara, Shanghai, China) according to the manufacturer’s instructions. The relative quantification of transcript abundance was calculated using the 2−ΔΔCT method. The ID of genes related to antioxidant system are as follows: CAT (CL12359.Contig2), Cu/Zn SOD (CL2674.Contig5), FeSOD (Unigene1850), POD (Unigene3194), GPX (CL12033.Contig1), and APX (Unigene3543). The primer sequences used for qRT-PCR are listed in Table 2. ApActin was used as the endogenous control.
All experiments had more than three different biological replicates, and experimental data were expressed as the mean ± SD. The correlation analyses and statistical comparisons were determined by SPSS (Version 20.0, USA). Differences in parameters were analyzed, and their level of significance was calculated by using one-way ANOVA. Data were visualized using GraphPad Prism (Version 9.0, USA).
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Author Contribution: Study conception and design: Di Zhang; data collection: Tingting Huang and Shan Deng; analysis and interpretation of results: Tingting Huang and Jiangyuan Sheng; draft manuscript: Di Zhang and Tingting Huang. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: Not applicable.
Funding Statement: This work was supported by the National Natural Science Foundation of China [Grant Nos. 31870686, 31971705, and 31670693] and the Natural Science Foundation of Shanghai [Grant No. 21ZR1434200].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abdul AM, Sabeem M, Mullath SK, Brini F, Masmoudi K (2021). Plant group II LEA proteins: Intrinsically disordered structure for multiple functions in response to environmental stresses. Biomolecules 9: 1662. [Google Scholar]
Avelange-Macherel MH, Payet N, Lalanne D, Neveu M, Tolleter D, Burstin J, Macherel D (2015). Variability within a pea core collection of LEAM and HSP22, two mitochondrial seed proteins involved in stress tolerance. Plant Cell and Environment 38: 1299–1311. [Google Scholar]
Bao F, Du D, An Y, Yang W, Wang J, Cheng T, Zhang Q (2017). Overexpression of Prunus mume dehydrin genes in tobacco enhances tolerance to cold and drought. Frontiers in Plant Science 8: 1299–1311. [Google Scholar]
Cao Y, Xiang X, Geng M, You Q, Huang X (2017). Effect of HbDHN1 and HbDHN2 genes on abiotic stress responses in Arabidopsis. Frontiers in Plant Science 8: 470. [Google Scholar]
Chakrabortee S, Tripathi R, Watson M, Schierle GS, Kurniawan DP et al. (2012). Intrinsically disordered proteins as molecular shields. Molecular BioSystems 8: 210–219. [Google Scholar]
Chen G, Zhang D, Pan J, Yue J, Shen X (2021). Cathepsin B-like cysteine protease ApCathB negatively regulates cryo-injury tolerance in transgenic Arabidopsis and Agapanthus praecox. Plant Science 308: 110928. [Google Scholar]
Close TJ (1997). Dehydrins: A commonality in the response of plants to dehydration and low temperature. Plant Physiology 100: 291–296. [Google Scholar]
Dure L, Galau GA (1981). Developmental biochemistry of cotton seed embryogenesis and germination XIII. Regulation of biosynthesis of principal storage proteins. Plant Physiology 68: 187–194. [Google Scholar]
Elliott GD, Wang S, Fuller BJ (2017). Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 76: 74–91. [Google Scholar]
Fayter AER, Hasan M, Congdon TR, Kontopoulou I, Gibson MI (2020). Ice recrystallisation inhibiting polymers prevent irreversible protein aggregation during solvent-free cryopreservation as additives and as covalent polymer-protein conjugates. European Polymer Journal 140: 110036. [Google Scholar]
Halder T, Agarwal T, Ray S (2016). Isolation, cloning, and characterization of a novel Sorghum dehydrin (SbDhn2) protein. Protoplasma 253: 1475–1488. [Google Scholar]
Hara M, Terashima S, Fukaya T, Kuboi T (2003). Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta 217: 290–298. [Google Scholar]
Hara M, Fujinaga M, Kuboi T (2005). Metal binding by citrus dehydrin with histidine-rich domains. Journal of Experimental Botany 56: 2695–2703. [Google Scholar]
Hara M, Monna S, Murata T, Nakano T, Amano S, Nachbar M, Wätzig H (2016). The Arabidopsis KS-type dehydrin recovers lactate dehydrogenase activity inhibited by copper with the contribution of his residues. Plant Science 245: 135–142. [Google Scholar]
Hernández-Sánchez IE, Martynowicz DM, Rodríguez-Hernández AA, Pérez-Morales MB, Graether SP, Jimenez-Bremont JF (2014). A dehydrin-dehydrin interaction: The case of SK3 from Opuntia streptacantha. Frontiers in Plant Science 5: 520. [Google Scholar]
Hoekstra FA, Golovina EA, Buitink J (2001). Mechanisms of plant desiccation tolerance. Trends in Plant Science 6: 431–438. [Google Scholar]
Hughes SL, Schart V, Malcolmson J, Hogarth KA, Martynowicz DM, Tralman-Baker E, Patel SN, Graether SP (2013). The importance of size and disorder in the cryoprotective effects of dehydrins. Plant Physiology 163: 1376–1386. [Google Scholar]
Jefferson RA, Kavanagh TA, Bevan MW (1987). GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. The EMBO Journal 6: 3901–3907. [Google Scholar]
Kosová K, Holková L, Prásil IT, Prásilová P, Bradácová M, Vítámvás P, Capková V (2008). Expression of dehydrin 5 during the development of frost tolerance in barley (Hordeum vulgare). Journal of Plant Physiology 165: 1142–1151. [Google Scholar]
Kovacs D, Kalmar E, Torok Z, Tompa P (2008). Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiology 147: 381–390. [Google Scholar]
Lee SC, Lee MY, Kim SJ, Jun SH, An G, Kim SR (2005). Characterization of an abiotic stress-inducible dehydrin gene, OsDhn1, in rice (Oryza sativa L.). Molecules and Cells 19: 212–218. [Google Scholar]
Liu CC, Li CM, Liu BG, Ge SJ, Dong X (2012). Genome-wide identification and characterization of a dehydrin gene family in poplar (Populus trichocarpa). Plant Molecular Biology Reporter 30: 848–859. [Google Scholar]
Liu H, Yu C, Li H, Ouyang B, Wang T, Zhang J, Wang X, Ye Z (2015). Overexpression of ShDHN, a dehydrin gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses in tomato. Plant Science 231: 198–211. [Google Scholar]
Liu Y, Wang L, Xing X, Sun L, Pan J, Kong X, Zhang M, Li D (2013). ZmLEA3, a multifunctional group 3 LEA protein from maize (Zea mays L.is involved in biotic and abiotic stresses. Plant and Cell Physiology 54: 944–959. [Google Scholar]
Livernois AM, Hnatchuk DJ, Findlater EE, Graether SP (2009). Obtaining highly purified intrinsically disordered protein by boiling lysis and single step ion exchange. Analytical Biochemistry 392: 70–76. [Google Scholar]
Meryman HT (2007). Cryopreservation of living cells: Principles and practice. Transfusion 47: 935–945. [Google Scholar]
Nguyen PN, Tossounian MA, Kovacs DS, Thu TT, Stijlemans B et al. (2020). Dehydrin ERD14 activates glutathione transferase Phi9 in Arabidopsis thaliana under osmotic stress. Biochimica et Biophysica Acta (BBA)-General Subjects 3: 129506. [Google Scholar]
Rakhra G, Kaur T, Vyas D, Sharma AD, Singh J, Ram G (2017). Molecular cloning, characterization, heterologous expression and in-silico analysis of disordered boiling soluble stress-responsive wBsSRP protein from drought tolerant wheat cv.PBW 175. Plant Physiology and Biochemical Journal 112: 29–44. [Google Scholar]
Reed BM (2001). Implementing cryogenic storage of clonally propagated plants. Cryo-Letters 22: 97–104. [Google Scholar]
Ren L, Zhang D, Jiang X, Gai Y, Wang W, Reed BM, Shen X (2013). Peroxidation due to cryoprotectant step is a vital factor for cell survival in Arabidopsis cryopreservation. Plant Science 212: 37–47. [Google Scholar]
Ren L, Zhang D, Shen X, Reed BM (2014). Antioxidants and anti-stress compounds improve the survival of cryopreserved Arabidopsis seedlings. Acta Horticulturae 1039: 57–62. [Google Scholar]
Ren L, Zhang D, Chen G, Reed BM, Shen X, Chen H (2015). Transcriptomic profiling revealed the regulatory mechanism of Arabidopsis seedlings response to oxidative stress from cryopreservation. Plant Cell Reports 34: 2161–2178. [Google Scholar]
Ren L, Wang MR, Wang QC (2021). ROS-induced oxidative stress in plant cryopreservation: Occurrence and alleviation. Planta 254: 124. [Google Scholar]
Riyazuddin R, Nisha N, Singh K, Verma R, Gupta R (2021). Involvement of dehydrin proteins in mitigating the negative effects of drought stress in plants. Plant Cell Reports 41: 519–533. DOI 10.1007/s00299-021-02720-6. [Google Scholar] [CrossRef]
Saibi W, Feki K, Mahmoud RB, Brini F (2015). Durum wheat dehydrin (DHN-5) confers salinity tolerance to transgenic Arabidopsis plants through the regulation of proline metabolism and ROS scavenging system. Planta 242: 1187–1194. [Google Scholar]
Sakai A, Dai H, Niino T (2008). Development of pvs-based vitrification and encapsulation-vitrification protocols. In: Plant Cryopreservation: A Practical Guide, pp. 33–57. New York: Springer. [Google Scholar]
Saucedo AL, Hernández-Domínguez EE, de Luna-Valdez LA, Guevara-García AA, Escobedo-Moratilla A et al. (2017). Insights on structure and function of a late embryogenesis abundant protein from Amaranthus cruentus: An intrinsically disordered protein involved in protection against desiccation, oxidant conditions, and osmotic stress. Frontiers in Plant Science 8: 497. [Google Scholar]
Smolikova G, Leonova T, Vashurina N, Frolov A, Medvedev S (2020). Desiccation tolerance as the basis of long-term seed viability. International Journal of Molecular Medicine 22: 101. [Google Scholar]
Yang Y, He M, Zhu Z, Li S, Xu Y et al. (2012). Identification of the dehydrin gene family from grapevine species and analysis of their responsiveness to various forms of abiotic and biotic stress. BMC Plant Biology 12: 140. [Google Scholar]
Yang Z, Sheng J, Lv K, Ren L, Zhang D (2019). Y2SK2 and SK3 type dehydrins from Agapanthus praecox can improve plant stress tolerance and act as multifunctional protectants. Plant Science 284: 143–160. [Google Scholar]
Yuan F, Chen M, Yang J, Leng B, Wang B (2014). A system for the transformation and regeneration of the recretohalophyte Limonium bicolor. In Vitro Cellular and Developmental Biology 50: 610–617. [Google Scholar]
Zamecnik J, Faltus M, Bilavcik A (2021). Vitrification solutions for plant cryopreservation: Modification and properties. Plants 10: 2623. [Google Scholar]
Zhang D, Ren L, Chen G, Zhang J, Reed BM, Shen X (2015). ROS-induced oxidative stress and apoptosis-like event directly affect the cell viability of cryopreserved embryogenic callus in Agapanthus praecox. Plant Cell Reports 34: 1499–1513. [Google Scholar]
Zhang D, Yang T, Ren L (2021). Y2SK2 and SK3 type dehydrins from Agapanthus praecox act as protectants to improve plant cell viability during cryopreservation. Plant Cell, Tissue and Organ Culture 144: 271–279. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |