DOI:10.32604/biocell.2022.021861

| BIOCELL DOI:10.32604/biocell.2022.021861 |  |
| Article |
Application of two forms of silicon and their impact on the postharvest and the content of bioactive compounds in cucumber (Cucumis sativus L.) fruits
1Departamento de Botánica, Universidad Autónoma Agraria Antonio Narro, Saltillo, 25315, México
2Maestría en Ciencias en Horticultura, Universidad Autónoma Agraria Antonio Narro, Saltillo, 25315, México
3Centro de Investigación en Química Aplicada, Saltillo, 25294, México
4Departamento de Horticultura, Universidad Autónoma Agraria Antonio Narro, Saltillo, 25315, México
*Address correspondence to: Antonio Juárez-Maldonado, antonio.juarez@uaaan.edu.mx
Received: 09 February 2022; Accepted: 18 April 2022
Abstract: The metabolic activity of the fruits continues even after harvest, which results in the loss of bioactive compounds, a decrease in the quality of the fruits, softening and browning, among other negative effects. The use of certain elements such as silicon can improve postharvest quality, since it is involved in the metabolic, physiological and structural activity of plants, moreover can increase the quality of the fruits. In addition, nanotechnology has had a positive impact on crop yield, nutritional value, fruit quality and can improve antioxidant activity. For these reasons, the use of beneficial elements such as silicon in the form of nanoparticles can be a viable option to improve the characteristics of the fruits. In the present study was evaluated the application of potassium silicate (125, 250 and 500 mg L−1) and SiO2 nanoparticles (125, 250 and 500 mg L−1) during the development of the crop. The results showed that the application of silicon (potassium silicate and silicon nanoparticles) increased the content of total soluble solids (up to 15.6% higher than control), titratable acidity (up to 38.8% higher than control), vitamin C (up to 78.2% higher than control), phenols (up to 22% higher than control), flavonoids (up to 64.6% higher than control), and antioxidant activity in lipophilic compounds (up to 56.2% higher than control). This study suggests that the use of silicon can be a good option to increase the content of bioactive compounds in cucumber fruits when they are applied during the development of the crop.
Keywords: Fruit quality; Nanotechnology; Silicon nanoparticles; Potassium silicate; Antioxidant compounds; Antioxidant capacity
Cucumber (Cucumis sativus L.) is one of the most commonly produced crops worldwide, and it has great value since it can be consumed fresh or processed. The fruits are used as a salad, they also have benefits for human health due to the content of biocompounds such as vitamins C, A and B complex, among others (Lim, 2012). However, after the harvest, a deterioration of the quality of the fruits begins, since the living tissues continue with their biological processes such as respiration (Gouda et al., 2020). In particular, cucumbers have a short shelf life, and quality decreases after harvest as the fruits lose a lot of moisture and are highly susceptible to physical damage during handling and storage (Maleki et al., 2018). A factor of great importance during postharvest is the content of bioactive compounds, but these can be degraded due to changes at the physiological level that the fruits maintain, such as respiration, which triggers oxidative reactions (Jia et al., 2018). For this reason, it is very important to look for management alternatives that allow increasing the quality of the fruits and especially the content of bioactive compounds during postharvest.
Silicon (Si) is the second most abundant element on the surface of the earth’s crust and in soils, it is present in various forms, especially quartz, silicates and oxides. The extractable forms of Si in the soil include amorphous Si, active Si and water-soluble Si, the latter being the only one in which it can be available to plants (Khan et al., 2021), since in the liquid phase of the soil, it is mainly found as monosilicic acid (H4SiO4) (also known as orthosilicic acid) and its concentration ranges between 0.1 and 0.6 mM. This element is involved in metabolic, physiological and structural activity in higher plants exposed to abiotic and biotic conditions (Thakral et al., 2021). Moreover, silicon has shown positive mechanical and physiological effects in plants, such as, increase resistance to strong wind and rain, promote the tolerance against drought, alleviation of salt stress, and improved the K and Ca absorption, which are fundamental elements in postharvest quality (Guntzer et al., 2012; Savvas and Ntatsi, 2015). Silicon also stimulates the antioxidant defense system, increasing the production and accumulation of enzymatic and non-enzymatic antioxidants in plants (Savvas and Ntatsi, 2015). Therefore, the use of this element during the production of different crops can positively impact the postharvest quality and shelf life of the fruits.
Nanotechnology can be applied from the production of crops to the processing and storage of vegetables, and other products during postharvest. It is known that nanomaterials (NMs) can increase crop yield, nutritional value, fruit quality, antioxidant activity and consequently the value of the products (Fu et al., 2014). Due to their size (1–100 nm), nanoparticles (NPs) can cross cell walls in various ways: endocytosis, pore formation, carrier proteins or through plasmodesmata (Zuverza-Mena et al., 2017). NPs are considered to have stimulating effects to induce antioxidant compounds, through the stimulation of the production of reactive oxygen species (ROS), which activate the defense system of the plant, which results in the production of antioxidant compounds (Fu et al., 2014). This can directly modify the quality of the fruits, especially the content of bioactive compounds during the postharvest life (Hernández-Fuentes et al., 2017).
Therefore, the objective of the work is to evaluate the effect of potassium silicate and SiO2 NPs on the postharvest behavior of this crop.
Experimental development and treatments
Cucumber fruits of the variety “Vitaly” (Syngenta AG, Switzerland) were used as plant material. This is a slicer type cucumber, parthenocarpic, dark green, strong and vigorous plant adapted to conditions in Mexico. The plants to obtain the fruits were established in a multi-tunnel greenhouse, with a polyethylene cover. The average of maximum temperature was 35°C and minimum 10°C, relative humidity of 40–90%, PAR maximum of 1600 µmol m−2 s−1, and 380 ppm of CO2 concentration. A mixture of peat moss and perlite (1:1) was used as substrate, the irrigation system was directed, and a Steiner solution was used for plant nutrition (Steiner, 1961). The Peat Moss (PREMIER®) is a moss that belongs to the Sphagnum genus, with a high water retention capacity, and a pH between 5.5 and 6. The expanded mineral perlite (HORTIPERL®), is an inert substrate, free of salts, neutral pH with high aeration capacity.
The treatments consisted in the application of two forms of silicon (potassium silicate and SiO2 nanoparticles) in the plants. The SiO2 NPs of 10–20 nm size had a spherical morphology, a surface area of 160 m2 g−1, and a bulk density of 0.08–0.1 g cm−3 (SkySpring Nanomaterials Inc., Houston, TX, USA).
Seven treatments were applied as follows (Table 1): T0 = Control, T1 = K2SiO3 at 125 mg L−1 (KS 125), T2 = K2SiO3 at 250 mg L−1 (KS 250), T3 = K2SiO3 at 500 mg L−1 (KS 500), T4 = SiO2 NPs at 125 mg L−1 (Si NPs 125), T5 = SiO2 NPs at 250 mg L−1 (Si NPs 250), and T6 = SiO2 NPs at 500 mg L−1 (Si NPs 500).

The application of the treatments was made through foliar application in plants at intervals of 15 days, starting 15 days after they emerged, and making a total of five applications during development of crop.
Physicochemical characteristics of the fruits
The fruit quality variables were evaluated in 6 fruits per treatment to determine the behavior of the variables during the shelf life, for which three sampling times were taken: (1) at harvest time, (2) at five days after harvest, and (3) ten days after harvest. The fruits were chosen considering the same size and state of commercial maturity, and it was verified that they had no damage. The fruits were stored at room temperature of 20 ± 1°C.
The percentage of weight loss by physiological activity was calculated. Potential of hydrogen (pH) was determined using a digital potentiometer (HI 98130, HANNA®). The total soluble solids (TSS, °Brix) were obtained with a refractometer (1940, ATAGO®), and the percentage of titratable acidity was determined according to the methodology of AOAC (AOAC, 1990), expressing the data as a percentage of citric acid (% CA). The firmness of the fruit was determined with a manual penetrometer (WAGNER INSTRUMENTS, model FDK 20, Greenwich, CT, USA). For this, measurements were made at three different points of the fruit and the average was obtained for each sample.
Bioactive compounds and antioxidant capacity
Fruit samples used to determine bioactive compounds and antioxidant capacity were sliced and stored in a −80°C deep freezer (3003 Ultrafreezer Thermo Scientific, USA). They were then lyophilized for 72 h at −84°C and 0.060 mbar in a lyophilizer (Labconco, FreeZone 2.5 L model, Kansas City, Mo, USA). Once the samples were lyophilized, they were ground manually in a porcelain mortar.
The vitamin C content was determined by the titration method with 2,6 dichlorophenolindophenol (Padayatty et al., 2003), expressing the results in mg 100 g−1 of fresh weight (FW). Glutathione quantification was performed using the spectrophotometric technique of (Xue et al., 2001), expressing the results in mmol 100 g−1 of dry weight (DW). The determination of phenols was carried out by the Folin-Ciocalteu method (Singleton et al., 1999), expressing the results in equivalent milligrams of gallic acid per 100 g of dry weight (mg of EGA 100 g−1 DW). Determination of flavonoids content was carried out by the method of (Arvouet-Grand et al., 1994), expressing the results in equivalent milligrams of quercetin per 100 g of dry weight (mg EQ 100 g−1 DW).
The antioxidant capacity was determined by the radical ABTS [2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)] (Re et al., 1999), expressing the results as Trolox equivalents in µmol per gram of dry weight (µmol g−1 DW). An extraction was carried out for lipophilic compounds, with a hexane: acetone solution. For hydrophilic compounds the extraction was carried out with phosphate buffer pH 7.0 (0.1 M). Total antioxidant capacity was de sum of both compounds.
The experiment was set up using a completely randomized experimental design. A total of seven treatments and six replicates per treatment were considered. For the statistical analysis was used the statistical program InfoStat (2016) with the analysis of variance and a Fisher’s Least Significant Difference means test were performed (α = 0.05).
Significant differences in TSS were found in the fruits treated, at the time of harvest the KS 125 treatment showed a concentration of TSS 8.5% higher than the control. Besides, after five days of storage an increase in TSS is observed, with a higher concentration being found in the control. However, ten days of storage, only the KS 250 treatment presents a concentration 15.6% higher than control (Fig. 1).
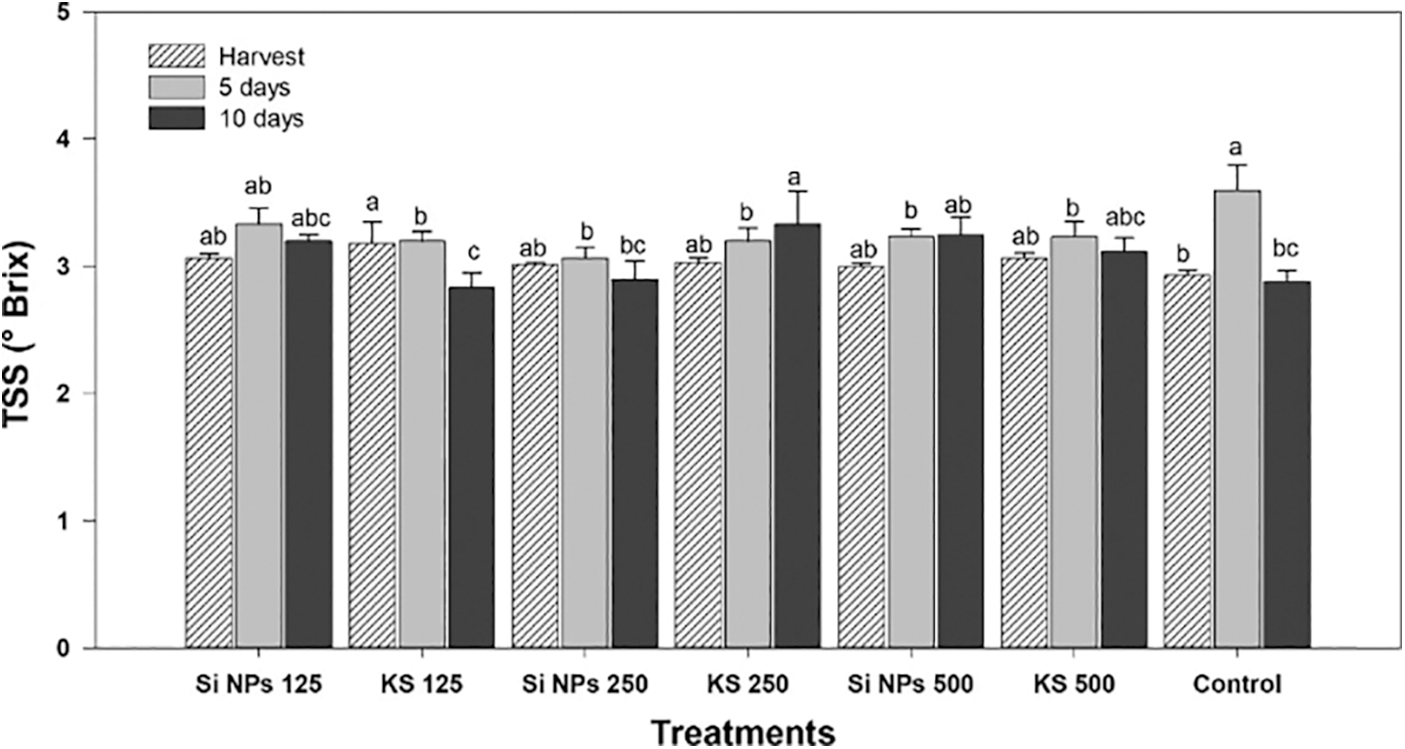
Figure 1: Content of total soluble solids (°Brix) in cucumber fruits of plants treated with K2SiO3 and SiO2 NPs. The fruits were evaluated at harvest time, 5 and 10 days of storage at room temperature 20°C ± 1. Different letters indicate significant differences according to the Fisher’s LSD test (α = 0.05). n = 6 ± standard error. KS 125, 250 and 500: K2SiO3 at 125, 250 and 500 mg L−1. Si NPs 125, 250 and 500: SiO2 NPs at 125, 250 and 500 mg L−1.
Significant differences were shown in titratable acidity. The highest acidity was observed in Si NPs 250 and Si NPs 500 treatments, being in the increase 28.8% and 27.8% higher than the control. However, at five days of storage there were not differences between treatments. At ten days of storage, the Si NPs 250 treatment showed an increase of 38.8% titratable acidity comparatively to the control (Fig. 2).
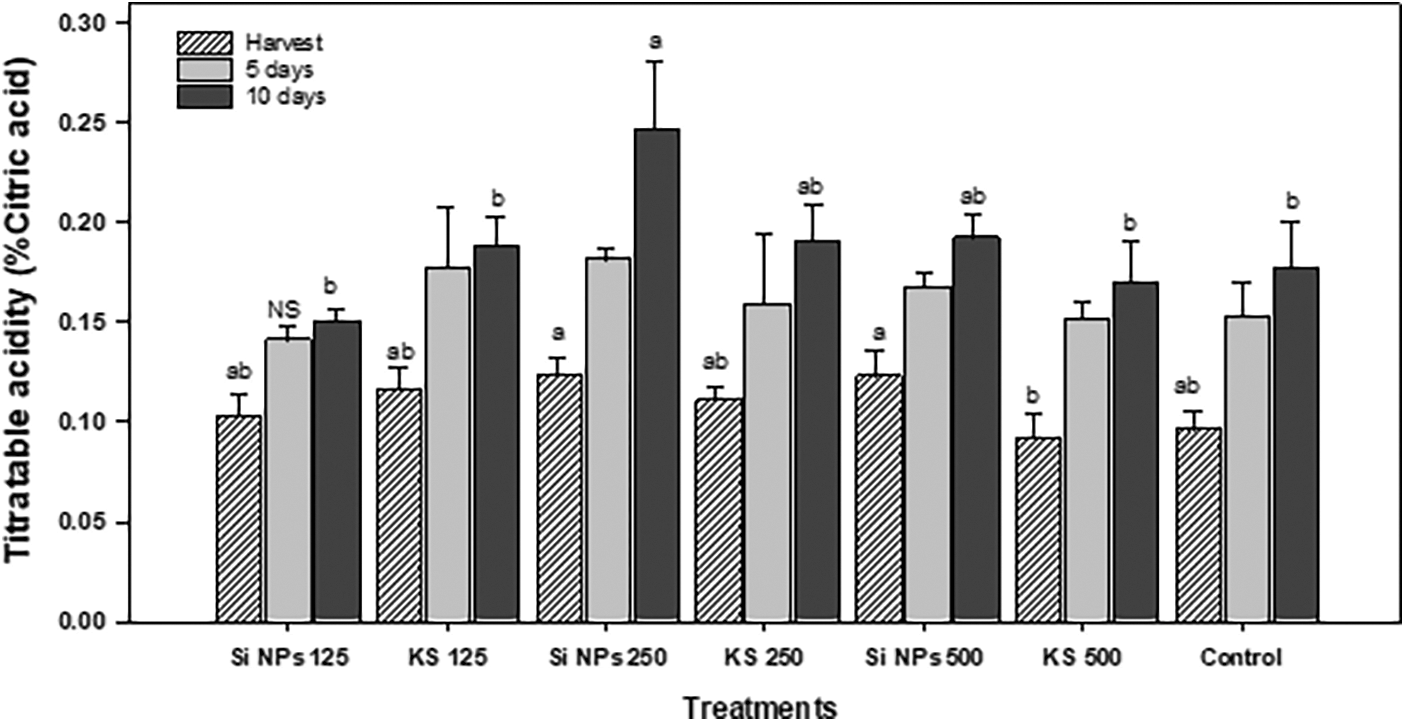
Figure 2: Titratable acidity (% citric acid) in cucumber fruits of plants treated with K2SiO3 and SiO2 NPs. The fruits were evaluated at harvest time, 5 and 10 days of storage at room temperature 20°C ± 1. Different letters indicate significant differences according to the Fisher’s LSD test (α = 0.05). n = 6 ± standard error. KS 125, 250 and 500: K2SiO3 at 125, 250 and 500 mg L−1. Si NPs 125, 250 and 500: SiO2 NPs at 125, 250 and 500 mg L−1.
Hydrogen potential (pH) results did not show statistical differences between the treatments applied in plants (Fig. 3).
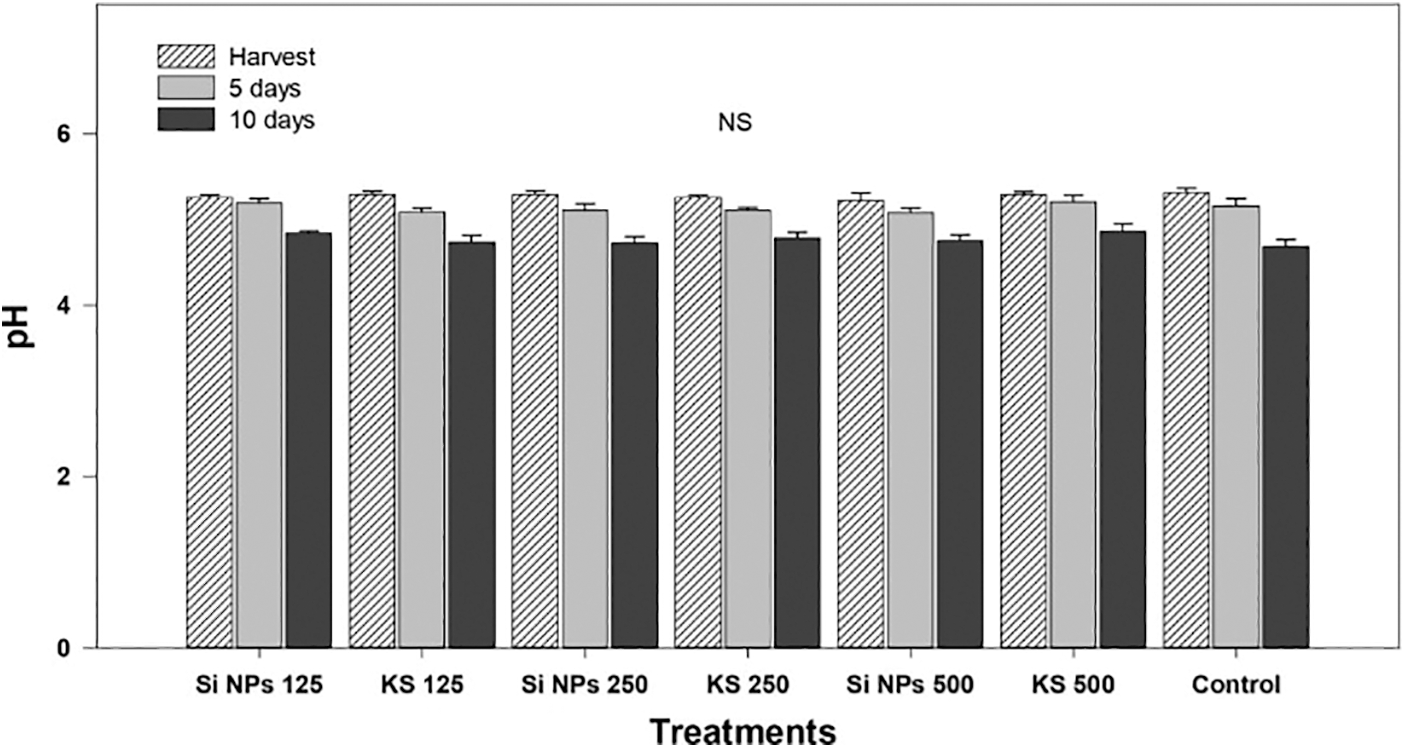
Figure 3: Potential of hydrogen (pH) in cucumber of plants treated with K2SiO3 and SiO2 NPs. The fruits were evaluated at harvest time, 5 and 10 days of storage at room temperature 20°C ± 1. Different letters indicate significant differences according to the Fisher’s LSD test (α = 0.05). n = 6 ± standard error. KS 125, 250 and 500: K2SiO3 at 125, 250 and 500 mg L−1. Si NPs 125, 250 and 500: SiO2 NPs at 125, 250 and 500 mg L−1.
On the other side, differences were found between treatments in the firmness of the fruits. At the time of harvest the greatest firmness was found in the Si NPs 250 treatment, presenting an increase about 22.5% than the control, contrarily at five days of storage, the control showed the greatest firmness. While at day ten, the treatments Si NPs 125, KS 125, KS 500 and the control had the highest firmness (Fig. 4).
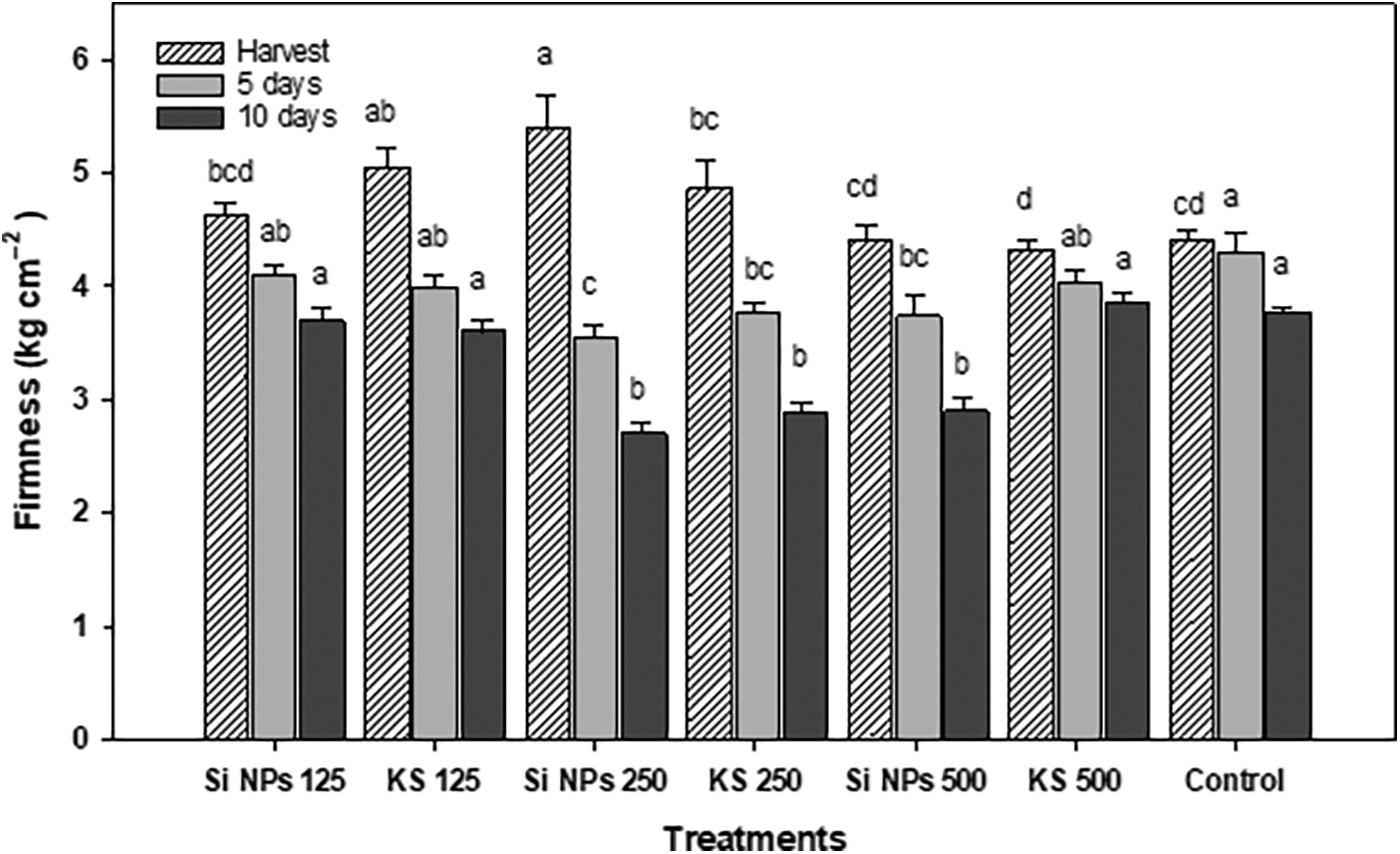
Figure 4: Firmness (kg cm−2) in cucumber fruits of plants treated with K2SiO3 and SiO2 NPs. The fruits were evaluated at harvest time, 5 and 10 days of storage at room temperature 20°C ± 1. Different letters indicate significant differences according to the Fisher’s LSD test (α = 0.05). n = 6 ± standard error. KS 125, 250 and 500: K2SiO3 at 125, 250 and 500 mg L−1. Si NPs 125, 250 and 500: SiO2 NPs at 125, 250 and 500 mg L−1.
Differences in physiological weight loss of the fruits were observed between treatments (Fig. 5). Data did not show significant differences between treatments at five days of storage. While at ten days of storage, the greatest weight loss was recorded by Si NPs 125 treatment, being this loss of 43% more than the control. Results showed that the control was the only one that showed the least weight loss throughout storage.
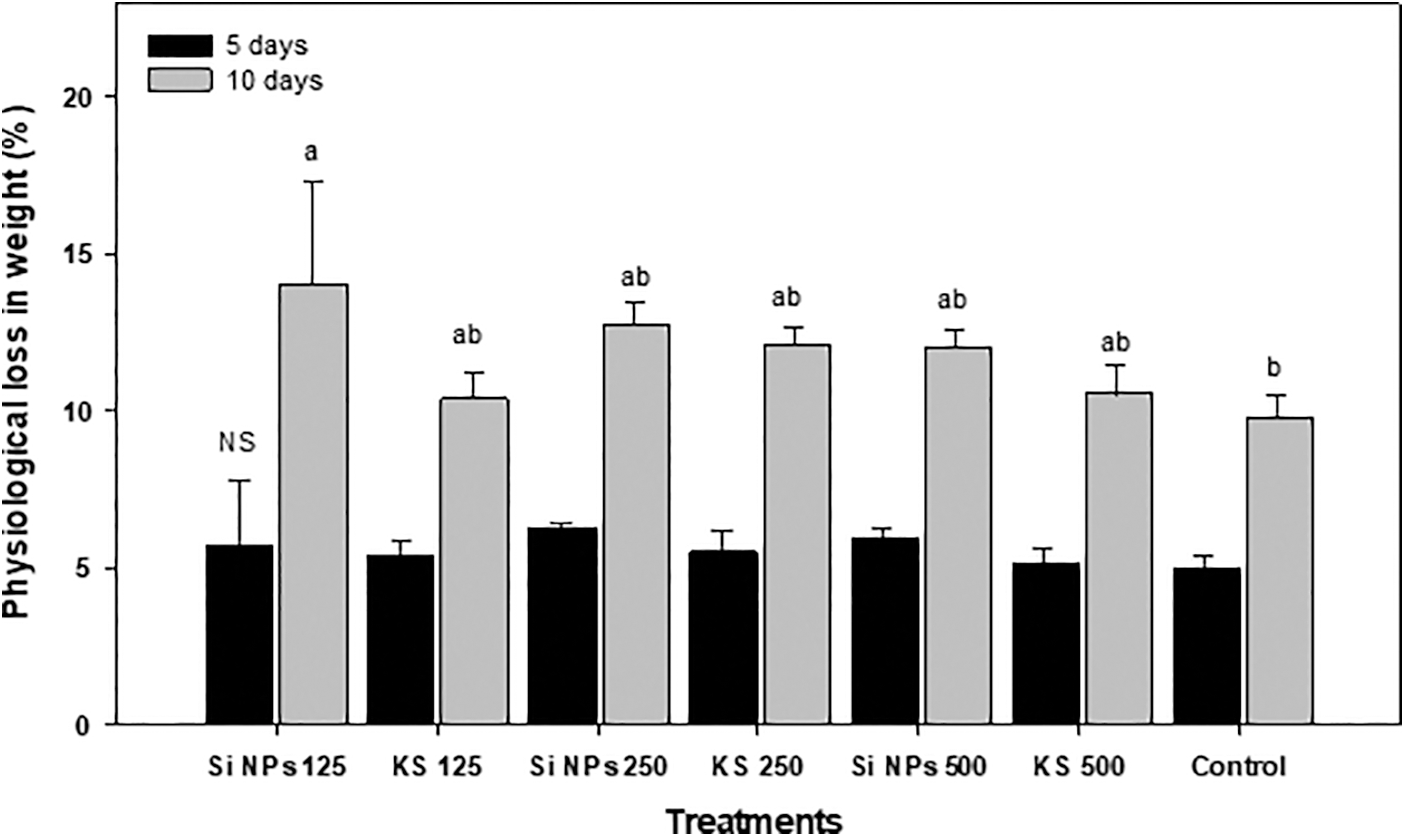
Figure 5: Physiological weight loss (%) in cucumber fruits of plants treated with K2SiO3 and SiO2 NPs. The fruits were evaluated at harvest time, 5 and 10 days of storage at room temperature 20°C ± 1. Different letters indicate significant differences according to the Fisher’s LSD test (α = 0.05). n = 6 ± standard error. KS 125, 250 and 500: K2SiO3 at 125, 250 and 500 mg L−1. Si NPs 125, 250 and 500: SiO2 NPs at 125, 250 and 500 mg L−1.
In vitamin C content, differences were observed between treatments (Fig. 6). At the time of harvest the control had the highest vitamin C content but after five days of storage, was the KS 125 treatment that presented an increase of 78.2% comparatively to the control. Moreover, at ten days of storage, the Si NPs 250 treatment presented 70.1% more vitamin C than the control.
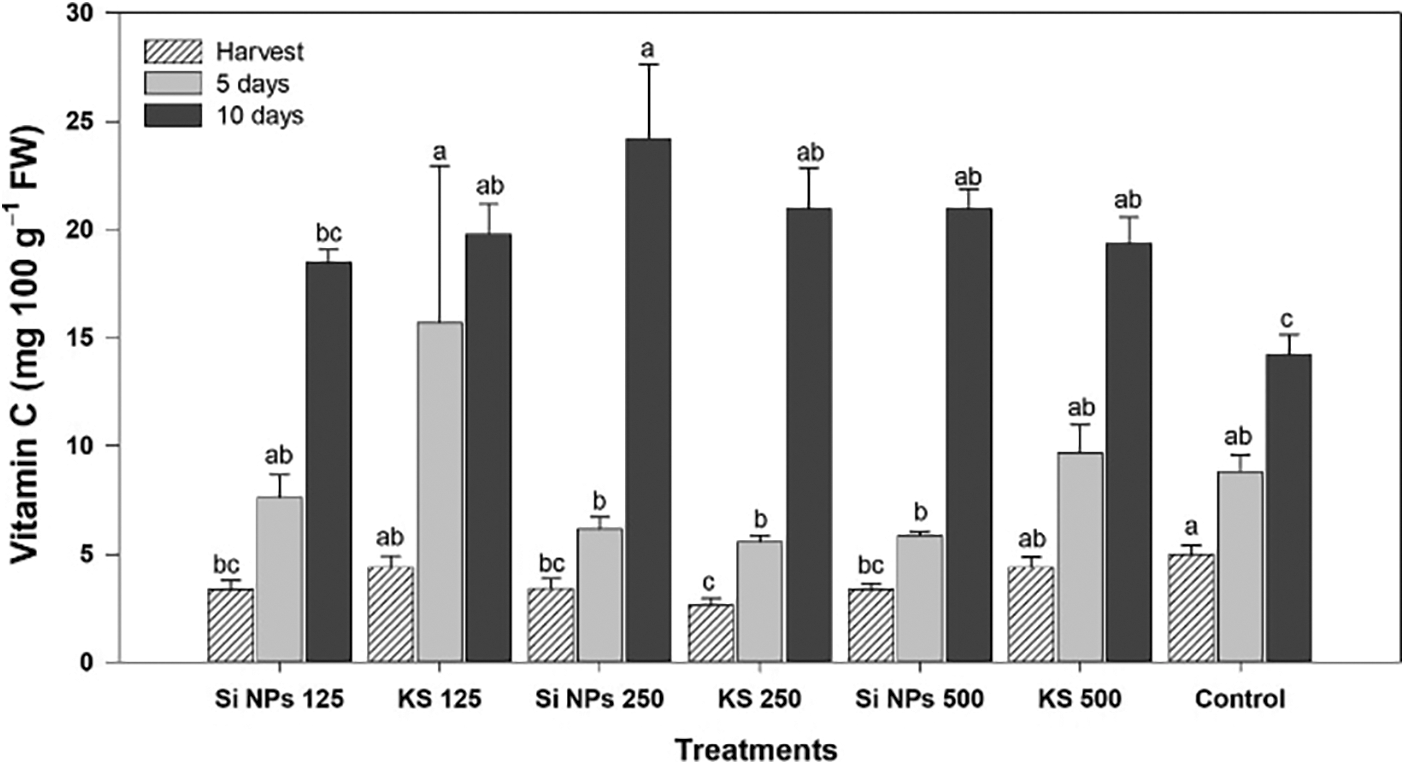
Figure 6: Content of vitamin C (mg 100 g−1 FW) in cucumber fruits of plants treated with K2SiO3 and SiO2 NPs. The fruits were evaluated at harvest time, 5 and 10 days of storage at room temperature 20°C ± 1. Different letters indicate significant differences according to the Fisher’s LSD test (α = 0.05). n = 6 ± standard error. KS 125, 250 and 500: K2SiO3 at 125, 250 and 500 mg L−1. Si NPs 125, 250 and 500: SiO2 NPs at 125, 250 and 500 mg L−1.
Significant differences were observed in the content of glutathione (Fig. 7). After five days of storage the Si NPs 125, Si NPs 250 and the control treatments presented the highest concentration of this compound. However, after ten days of storage, the control presented the highest concentration.
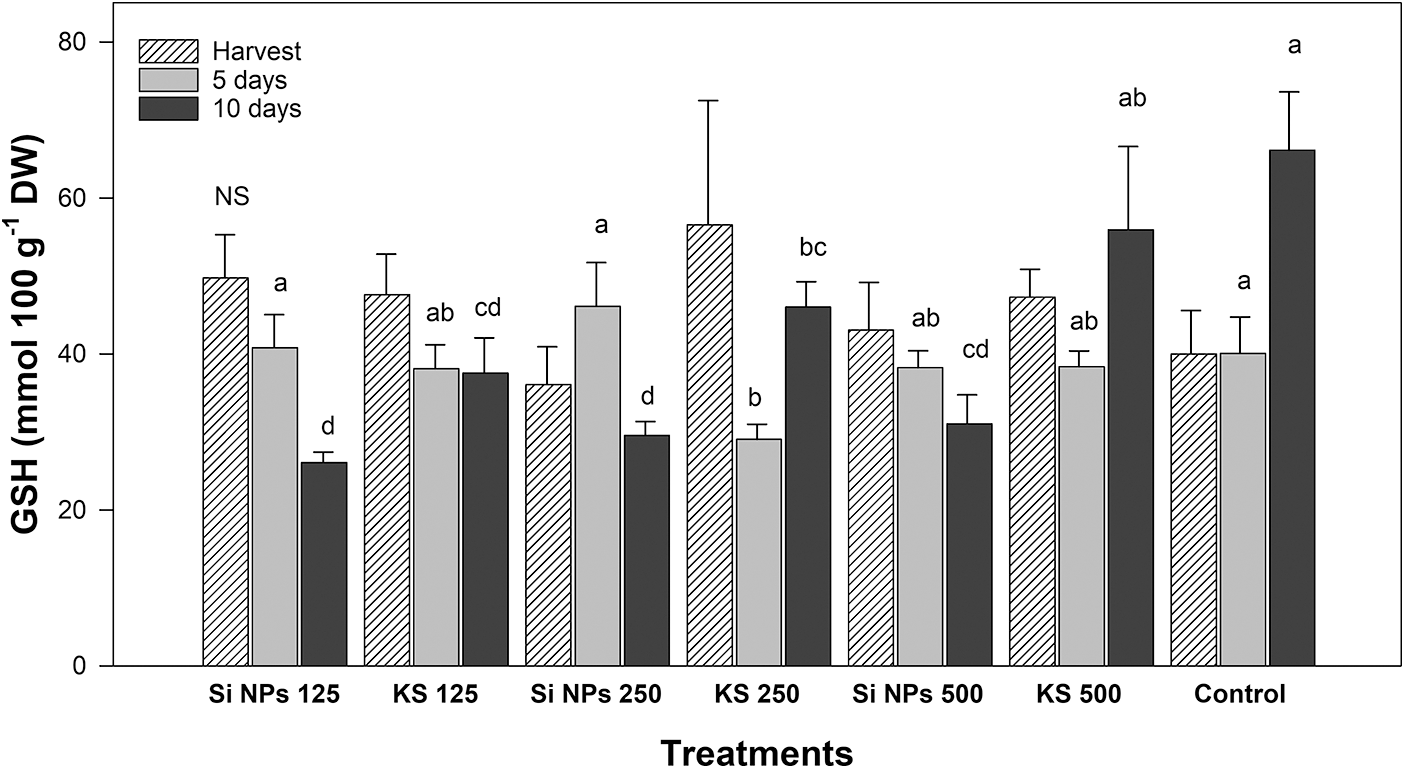
Figure 7: Content of glutathione (GSH, mmol 100 g−1 DW) in cucumber fruits of plants treated with K2SiO3 and SiO2 NPs. The fruits were evaluated at harvest time, 5 and 10 days of storage at room temperature 20°C ± 1. Different letters indicate significant differences according to the Fisher’s LSD test (α = 0.05). n = 6 ± standard error. KS 125, 250 and 500: K2SiO3 at 125, 250 and 500 mg L−1. Si NPs 125, 250 and 500: SiO2 NPs at 125, 250 and 500 mg L−1.
Significant differences between treatments were observed in phenol content (Fig. 8). Results showed that after five days of storage, the Si NPs 250 treatment presented a concentration of 13.04 mg g−1 DW, which representing an increase of 22% higher than the control. At ten days of storage, the Si NP 250 and KS 500 treatments had a concentration of 12.32 and 12.0 mg g−1 DW, representing an increase of 15.1% and 12% more than the control. It was observed that during storage phenols have a tendency to decrease as the time after harvest increase; however, some treatments (Si NPs 250 and KS 500) cause an increase as the storage time increases.
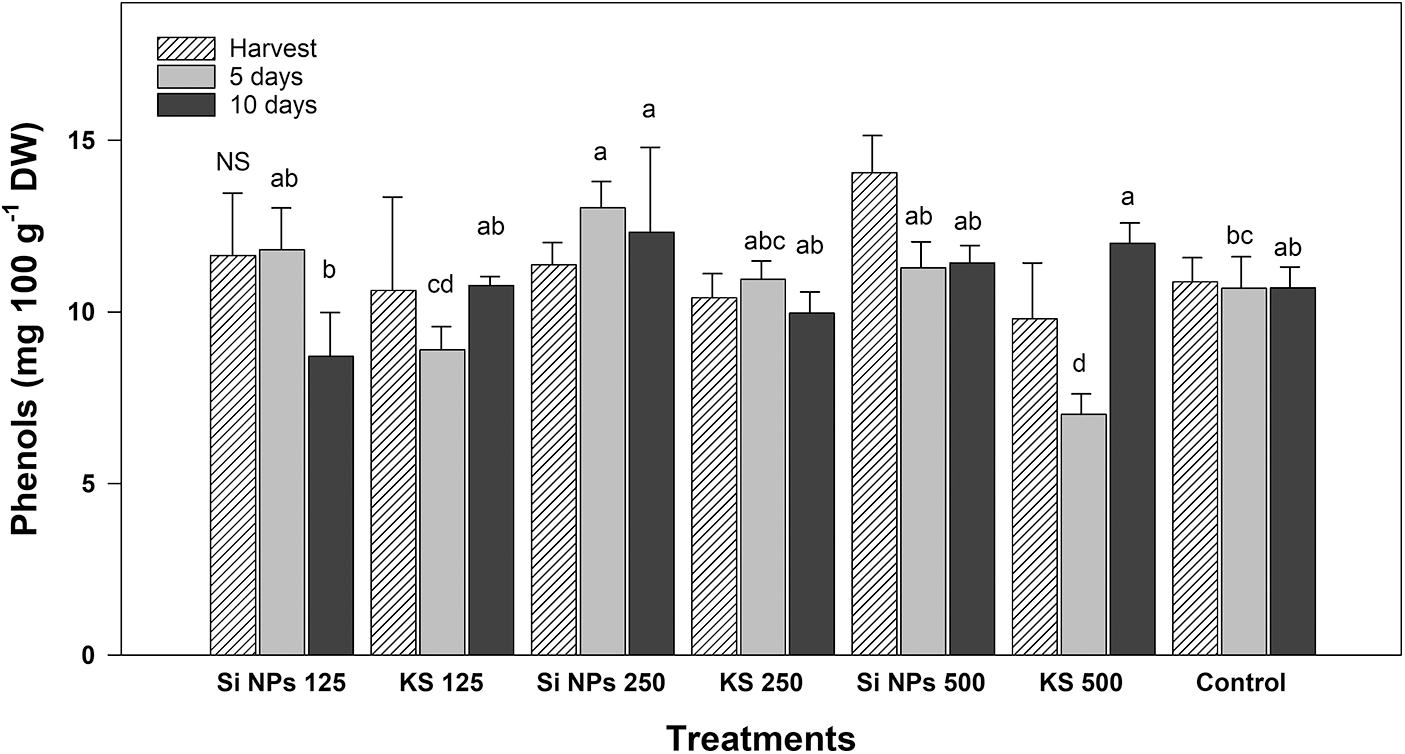
Figure 8: Content of phenols (mg 100 g−1 DW) in cucumber fruits of plants treated with K2SiO3 and SiO2 NPs. The fruits were evaluated at harvest time, 5 and 10 days of storage at room temperature 20°C ± 1. Different letters indicate significant differences according to the Fisher’s LSD test (α = 0.05). n = 6 ± standard error. KS 125, 250 and 500: K2SiO3 at 125, 250 and 500 mg L−1. Si NPs 125, 250 and 500: SiO2 NPs at 125, 250 and 500 mg L−1.
Fig. 9 showed significant differences between treatments in flavonoid content. In general, it was observed that flavonoids decrease as storage time progresses. However, at the time of harvest the Si NPs 125 treatment presented 35.7% more flavonoids content than the control. At five days of storage, the KS 125 and Si NPs 250 treatments recorded and increase of 52.4% and 64.6% more than fruits without silicon. At ten days of storage, the KS 125, KS 500 and control treatments presented the higher contents.
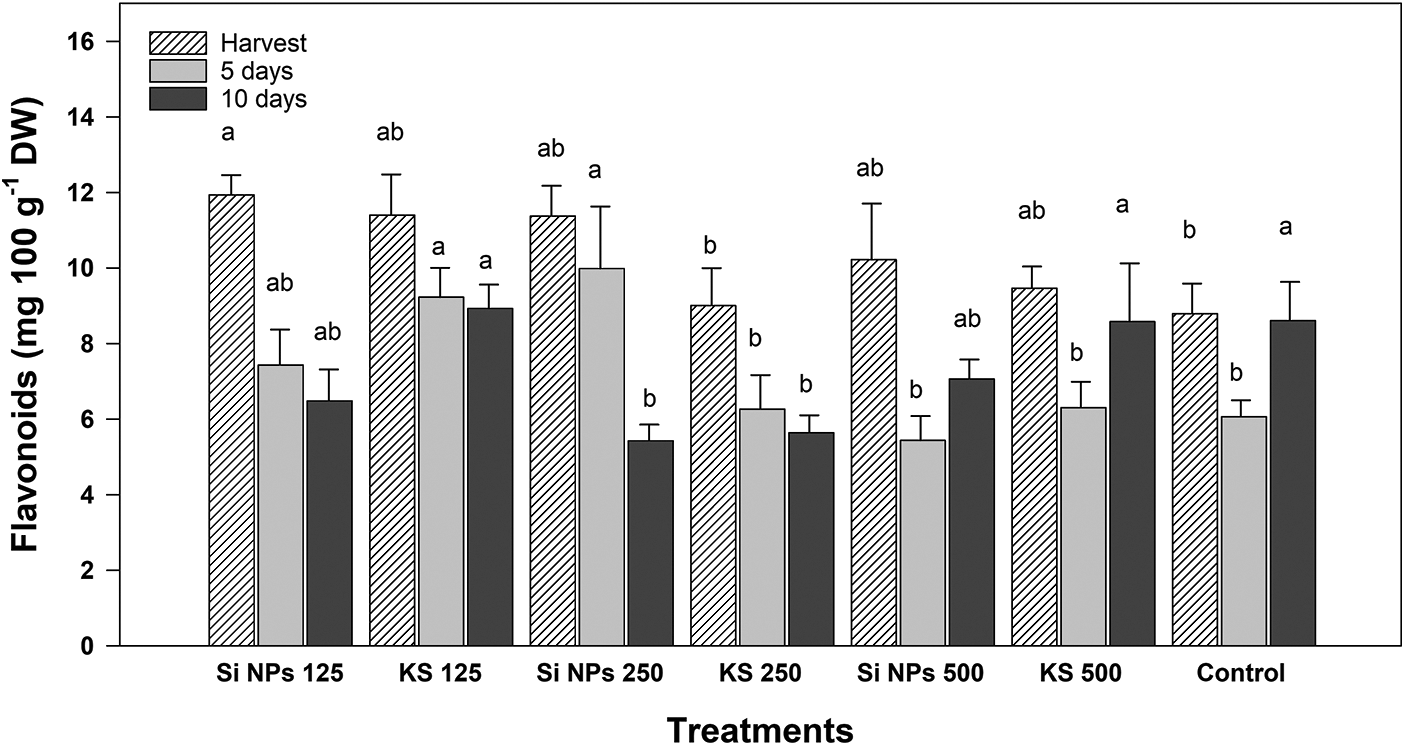
Figure 9: Content of flavonoids (mg 100 g−1 DW) in cucumber fruits of plants treated with K2SiO3 and SiO2 NPs. The fruits were evaluated at harvest time, 5 and 10 days of storage at room temperature 20°C ± 1. Different letters indicate significant differences according to the Fisher’s LSD test (α = 0.05). n = 6 ± standard error. KS 125, 250 and 500: K2SiO3 at 125, 250 and 500 mg L−1. Si NPs 125, 250 and 500: SiO2 NPs at 125, 250 and 500 mg L−1.
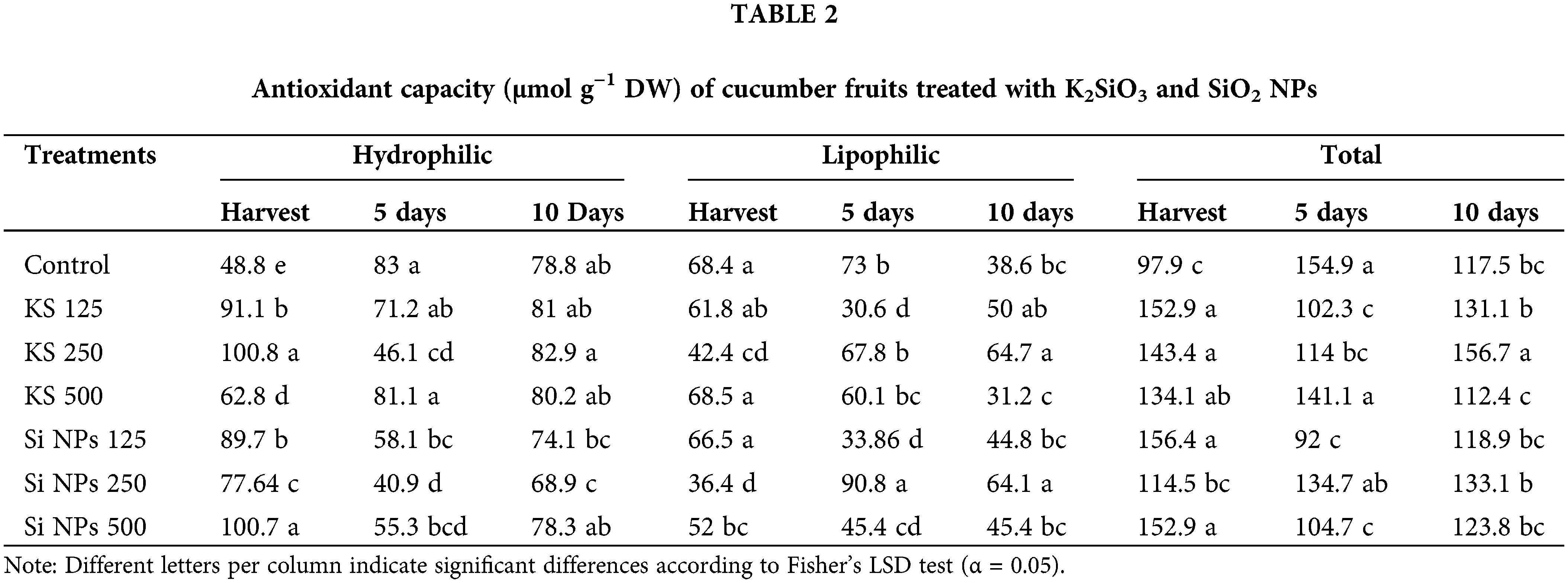
Differences were obtained in the antioxidant capacity of the cucumber fruits determined through the radical ABTS (Table 2). In the hydrophilic compounds at the time of harvest the KS 250 and Si NPs 500 treatments show values of 100.8 µmol g−1 DW, representing an increase of 106% more than the control. After five days of storage, a decrease in the antioxidant capacity was observed in all treatments, except control and the KS 500 treatment. While after ten days of storage, an increase in the antioxidant capacity is observed, being the KS 250 treatment which recorded the higher activity (82.9 µmol g−1 DW) with 5.2% more than the control.
In the lipophilic compounds, at the time of harvest the highest antioxidant capacity was observed in the KS 500 and Si NPs 125 treatments and in the control. After five days of storage, the Si NPs 250 treatment showed values of 93.8 µmol g−1 DW representing an increase of 24.3% more antioxidant capacity than the control. After ten days of storage, the KS 250 and Si NPs 250 treatments presented values of 64 µmol g−1 DW, representing an increase of 67% than the untreated plants.
The total antioxidant capacity at harvest was better in the treatments KS 125, KS 250, Si NPs 125 and Si NPs 500, with an increase of 56.1%, 46.4%, 59.7% and 56.2% respectively compared to the control. After five days of storage, the KS 500 treatment and the control had the highest total antioxidant capacity. At ten days of storage, the KS 250 treatment showed the highest antioxidant capacity, being 33.2% more than the control. These results suggests that the most of silicon treatments induce a greater antioxidant capacity in the cucumber fruits at the time of harvest, however, only the KS 250 treatment can maintain this capacity for the longest period of time.
An increasing trend was observed in TSS and titratable acidity as storage time passed. This is because TSS increase as the fruit ripens, due to the degradation and biosynthesis of polysaccharides and the accumulation of simple sugars (Ghasemnezhad et al., 2011). Some studies have shown that Si participates in the regulation and accumulation of soluble sugars and carbohydrates, which are an important factor for the quality of the fruits, since they determine the flavor (Peris-Felipo et al., 2020). In addition, these compounds serve as osmoprotectors, carbon sources and free radical scavengers in plants (Hassan et al., 2021). Zhu et al. (2016) showed that the application of 0.3 mM Na2SiO3 in the nutrient solution increased the content of soluble sugars (glucose, sucrose and fructose) in cucumber plants. Data was corroborated by Peris-Felipo et al. (2020) who reported the increase in glucose and fructose content in strawberry fruits (Fragaria x ananasa Duch. var. Fortuna), with the foliar and drench application of 1.5 mM SiO4H4. Moreover, Hu et al. (2022) referred the increase of sucrose and fructose in cucumber fruits when applied foliarly 1 mM of Na2SiO3.
Acids evaluated for titratable acidity serve as an energy reserve and participate in metabolic reactions for the synthesis of pigments, enzymes and other materials, as well as in the degradation of pectins and celluloses that are essential for the maturation processes (Ghasemnezhad et al., 2011). Therefore, titratable acidity is expected to increase with storage time and it results in a higher quality of the fruits.
In the present study, the application of Si NPs induced a greater firmness at harvest time. This is very important because one of the main problems in the quality of the fruits during postharvest is the respiration rate, which induces the “softening” of the tissues due to the loss of water and the decomposition of organic molecules (Gundewadi et al., 2018). When the water of the fruits is lost by 5–10%, most of the fruits are no longer commercial, in addition to loss of firmness, texture, flavor, accelerated senescence, susceptibility to cold damage, and membrane disintegration, and promotes “browning” (Lufu et al., 2020).
As in this study, Karagiannis et al. (2021) demonstrated that foliar application with 20 kg ha−1 of SiO2 in apple trees (Malus x domestica Borkh) increased fruit firmness. On the other side, Shalaby et al. (2021) verified that foliar application with 200 mg L−1 of Si NPs to cucumber plants induced an increase in fruit firmness. Mahmoud et al. (2022) observed the increase in firmness of potato tubers (Solanum tuberosum L.) when applying 20 and 55 mg L−1 of Si NPs in the nutrient solution.
Silicon is referred as an element that promotes fruit firmness because it plays an important role in stabilizing the cell wall, by promoting the formation of a double layer of silica in the cuticle, which reduces water loss through transpiration (Luyckx et al., 2017). This element also stimulates the deposition of cellulose and hemicellulose that reinforce cell walls and delays the softening process (Głazowska et al., 2018). Moreover, plays an important role in stabilizing the cell wall, protecting it from degrading enzymes, such as polygalacturonase, β-galactosidase, and pectin methylesterase (Karagiannis et al., 2021).
In this study it can be observed that the longer the storage time, the greater the loss of weight and firmness of the fruits (Kumar et al., 2021; Li et al., 2021). Over the time after harvest the fruits mature as part of their natural physiology, transpiration and respiration progress, and the fruits gradually lose water and firmness, decreasing their quality parameters (Kumar et al., 2021).
The content of bioactive compounds (such as vitamins, phenols, flavonoids, etc.) in cucumber fruits is one of the most important characteristics of this crop, so keeping them longer during postharvest is essential. For example, in our study was observed a higher vitamin C content in all Si treatments. Vitamin C is of great importance since humans must ingest vitamin C through rich sources such as fruits because the human body does not have the ability to produce it (Padayatty et al., 2003). Glutathione is one of the crucial non-enzymatic antioxidant compounds involved in defending against oxidative stress. In human health, it is important to maintain adequate levels of this compound to prevent health problems, since low levels have been associated with an increased risk of cancer, cardiovascular diseases, arthritis, diabetes, among others (Kalaras et al., 2017). Phenols are antioxidants that activate a series of secondary metabolites synthesized through the shikimic acid or malonic acid pathway, which exert cellular signaling functions under conditions of abiotic stress (Michalak, 2006). These compounds are a group of structurally diverse plant secondary metabolites that include terpenoids, phenylpropanoids, cinnamic acids, lignin precursors, hydroxybenzoic acids, catechols, coumarins, flavanoids, isoflavanoids, and tannins. Flavonoids in turn can be classified into flavones, flavonols, flavanones, isoflavones and anthocyanins (Fraga et al., 2019). They are a large and diverse group of low molecular weight secondary polyphenolic metabolites in plants and are related to stress (Klunklin and Savage, 2017). These compounds give the antioxidant capacity in fruits due to the reduction of oxidative changes in cells by reducing the levels of free radicals, they also have beneficial effects on cardiometabolic health and on cognitive functioning, they also have antidiabetic, anti-obesity and anticancer (Fraga et al., 2019; Klunklin and Savage, 2017). Therefore, an increase in the content of these compounds in food is beneficial for human health.
In this study, silicon in both forms of application induced positive responses, which could be enhanced with the application of Si NPs. This responses are because silicon (Si) can be involved in metabolic and physiological activity, and is associated with the components of cell walls so it can function as an elicitor (Savvas and Ntatsi, 2015). While NMs can stimulate the formation of ROS, which in turn activates the defense system of the plant, generating enzymatic and non-enzymatic antioxidant compounds (Juárez-Maldonado et al., 2021).
As observed here, it is known that nanomaterials during the development of crops can improve postharvest quality due to the changes it induces at the physiological level, it can improve the shelf life of crops, increasing the antioxidant system of plants and fruit (Achari and Kowshik, 2018). Also, the application of NMs in postharvest has positive effects, since semi-permeable barriers can form that inhibiting decomposition, reducing intracellular electrolyte leakage, lower respiration rate that results in lower weight and water loss (Shah et al., 2015).
The application of Si NPs can reduce lipid peroxidation preserving the integrity of the membrane through the activation of the antioxidant system (El-Serafy, 2019). The application of nano-calcium on apple trees (Malus domestica L. cv. Red Delicious) reduced the enzymatic activity of polygalacturonase, pectin methyl esterase and β-galactosidase, these enzymes are related to the hydrolysis of the cell wall that cause softening in climacteric fruits. Also, the application of nano-calcium could stabilize the cell membrane, maintaining the structure-rigidity-firmness of the cell wall of the fruit, derived from a greater penetration and better absorption of NPs, and as a result could reduce respiration, metabolism, and ethylene production, and hydrolysis of polysaccharides to monosaccharides (Ranjbar et al., 2018). The spraying of Zn nano-chelate on apple trees (Malus domestica L. cv. Delbard Estival and cv. Kohanz) in 3–4 weeks prior to harvest induces an improvement in the postharvest quality of fruits, increasing the content of ascorbic acid and SOD as well as a reduction of polyphenol oxidase (Rasouli and Saba, 2018). The application of Se NPs (3 and 10 mg L−1) on tomato seedlings (Solanum lycopersicum L.) increased the antioxidant capacity (catalase and peroxidase), as well as ascorbic acid, non-protein thiols, soluble phenols and higher enzymatic activity phenylalanine ammonia lyase, which could lead to a longer postharvest longevity of the fruit as well as a higher concentration of Se (biofortification) (Neysanian et al., 2020). Cu NPs in chitosan-polyvinyl alcohol (PVA) hydrogels before transplantation of jalapeño pepper plants (Capsicum annuum L.) they produce an improvement in the antioxidant activity of the fruit, which may a response to the stress caused by these compounds (Pinedo-Guerrero et al., 2017). These reports, like what was observed in this study, show that the use of nanomaterials positively influences the quality of the fruits, since it can improve the content of bioactive compounds and can also improve the shelf life of the fruits.
Antioxidants have an essential role in the shelf life of food, inhibit or prevent the oxidation of lipids and protect valuable food components such as proteins and vitamins (Rizwan et al., 2017). In addition to this, the antioxidant capacity is given by different types of antioxidants, either hydrophilic in nature such as vitamin C or lipophilic such as β-carotene, the most important with antioxidant activity being phenolic compounds, flavonoids and carotenoids (Arnao et al., 2001). Maintaining this antioxidant capacity in the fruits for a longer time after harvest is very important, since the nutritional quality of these is maintained, which has potential positive effects on human health.
The application of silicon in both forms had a positive effect on the postharvest quality of the cucumber fruits, increased the content of TSS, titratable acidity, vitamin C, maintaining their firmness during storage, increasing the quality of the fruits.
The present study suggests that Si treatments in ionic form and in NPs form had a positive effect on the antioxidants production, increasing the content of phenols, flavonoids and the antioxidant activity of lipophilic compounds.
The application of silicon in cucumber plants induces positive responses. Also, Si NPs are better than potassium silicate. However, for its use in agriculture, several aspects must be considered, such as the concentration used, and the possible transfer of nanomaterials in food chains.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: Yolanda González-García and Antonio Juárez-Maldonado; Data collection: Yolanda González-García and Valeria Flores-Robles; Analysis and interpretation of results: Gregorio Cadenas-Pliego, Adalberto Benavides-Mendoza, Marcelino Cabrera De La Fuente, Alberto Sandoval-Rangel, Antonio Juárez-Maldonado; Draft manuscript preparation: Yolanda González-García and Valeria Flores-Robles. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare no conflicts of interest.
Achari GA, Kowshik M (2018). Recent developments on nanotechnology in agriculture: Plant mineral nutrition, health, and interactions with soil microflora. Journal of Agricultural and Food Chemistry 66: 8647–8661. DOI 10.1021/acs.jafc.8b00691. [Google Scholar] [CrossRef]
AOAC (1990). Official Methods of Analysis of the AOAC, Methods 932.06, 925.09, 985.29, 923.03. Arlington, VA, USA: Association of Official Analytical Chemists. [Google Scholar]
Arnao MB, Cano A, Acosta M (2001). The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chemistry 73: 239–244. DOI 10.1016/S0308-8146(00)00324-1. [Google Scholar] [CrossRef]
Arvouet-Grand A, Vennat B, Pourrat A, Legret P (1994). Standardization of a propolis extract and identification of the main constituents. Journal of Pharmacie of Belgique 49: 462–468. [Google Scholar]
El-Serafy RS (2019). Silica nanoparticles enhances physio-biochemical characters and postharvest quality of Rosa hybrida L. cut flowers. Journal of Horticultural Research 27: 47–54. DOI 10.2478/johr-2019-0006. [Google Scholar] [CrossRef]
Fraga CG, Croft KD, Kennedy DO, Tomás-Barberán FA (2019). The effects of polyphenols and other bioactives on human health. Food & Function 10: 514–528. DOI 10.1039/C8FO01997E. [Google Scholar] [CrossRef]
Fu PP, Xia Q, Hwang HM, Ray PC, Yu H (2014). Mechanisms of nanotoxicity: Generation of reactive oxygen species. Journal of Food and Drug Analysis 22: 64–75. DOI 10.1016/j.jfda.2014.01.005. [Google Scholar] [CrossRef]
Ghasemnezhad M, Sherafati M, Payvast GA (2011). Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. Journal of Functional Foods 3: 44–49. DOI 10.1016/j.jff.2011.02.002. [Google Scholar] [CrossRef]
Głazowska S, Baldwin L, Mravec J, Bukh C, Hansen TH et al. (2018). The impact of silicon on cell wall composition and enzymatic saccharification of Brachypodium distachyon. Biotechnology for Biofuels 11: 17. DOI 10.1186/s13068-018-1166-0. [Google Scholar] [CrossRef]
Gouda MHB, Zhang C, Wang J, Peng S, Chen Y, Luo H, Yu L (2020). ROS and MAPK cascades in the post-harvest senescence of horticultural products. Journal of Proteomics & Bioinformatics 13: 1–7. DOI 10.35248/0974-276x.1000508. [Google Scholar] [CrossRef]
Gundewadi G, Reddy VR, Bhimappa B (2018). Physiological and biochemical basis of fruit development and ripening-a review. Journal of Hill Agriculture 9: 7–21. DOI 10.5958/2230-7338.2018.00003.4. [Google Scholar] [CrossRef]
Guntzer F, Keller C, Meunier JD (2012). Benefits of plant silicon for crops: A review. Agronomy for Sustainable Development 32: 201–213. DOI 10.1007/s13593-011-0039-8. [Google Scholar] [CrossRef]
Hassan H, Alatawi A, Abdulmajeed A, Emam M, Khattab H (2021). Roles of Si and Si NPs in improving thermotolerance of wheat photosynthetic machinery via upregulation of PsbH, PsbB and PsbD genes encoding PSII core proteins. Horticulturae 7: 16. DOI 10.3390/horticulturae7020016. [Google Scholar] [CrossRef]
Hernández-Fuentes A, López-Vargas E, Pinedo-Espinoza J, Campos-Montiel R, Valdés-Reyna J, Juárez-Maldonado A (2017). Postharvest behavior of bioactive compounds in tomato fruits treated with Cu nanoparticles and NaCl stress. Applied Sciences 7: 980. DOI 10.3390/app7100980. [Google Scholar] [CrossRef]
Hu W, Su Y, Zhou J, Zhu H, Guo J, Huo H, Gong H (2022). Foliar application of silicon and selenium improves the growth, yield and quality characteristics of cucumber in field conditions. Scientia Horticulturae 294: 110776. DOI 10.1016/j.scienta.2021.110776. [Google Scholar] [CrossRef]
Jia B, Zheng Q, Zuo J, Gao L, Wang Q et al. (2018). Application of postharvest putrescine treatment to maintain the quality and increase the activity of antioxidative enzyme of cucumber. Scientia Horticulturae 239: 210–215. DOI 10.1016/j.scienta.2018.05.043. [Google Scholar] [CrossRef]
Juárez-Maldonado A, Tortella G, Rubilar O, Fincheira P, Benavides-Mendoza A (2021). Biostimulation and toxicity: The magnitude of the impact of nanomaterials in microorganisms and plants. Journal of Advanced Research 31: 113–126. DOI 10.1016/j.jare.2020.12.011. [Google Scholar] [CrossRef]
Kalaras MD, Richie JP, Calcagnotto A, Beelman RB (2017). Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chemistry 233: 429–433. DOI 10.1016/j.foodchem.2017.04.109. [Google Scholar] [CrossRef]
Karagiannis E, Michailidis M, Skodra C, Molassiotis A, Tanou G (2021). Silicon influenced ripening metabolism and improved fruit quality traits in apples. Plant Physiology and Biochemistry 166: 270–277. DOI 10.1016/j.plaphy.2021.05.037. [Google Scholar] [CrossRef]
Khan I, Awan SA, Rizwan M, Ali S, Hassan MJ, Brestic M, Zhang X, Huang L (2021). Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotoxicology and Environmental Safety 222: 112510. DOI 10.1016/j.ecoenv.2021.112510. [Google Scholar] [CrossRef]
Klunklin W, Savage G (2017). Effect on quality characteristics of tomatoes grown under well-watered and drought stress conditions. Foods 6: 56. DOI 10.3390/foods6080056. [Google Scholar] [CrossRef]
Kumar N, Pratibha N, Ojha A, Upadhyay A, Singh R, Kumar S (2021). Effect of active chitosan-pullulan composite edible coating enrich with pomegranate peel extract on the storage quality of green bell pepper. LWT Food Science and Technology 138: 110435. DOI 10.1016/j.lwt.2020.110435. [Google Scholar] [CrossRef]
Li D, Zhou C, Zou N, Wu Y, Zhang J, An Q, Li JQ, Pan C (2021). Nanoselenium foliar application enhances biosynthesis of tea leaves in metabolic cycles and associated responsive pathways. Environmental Pollution 273: 116503. DOI 10.1016/j.envpol.2021.116503. [Google Scholar] [CrossRef]
Lim TK (2012). Cucumis sativus. In: Edible Medicinal and Non-Medicinal Plants, pp. 239–249. Dordrecht: Springer. [Google Scholar]
Lufu R, Ambaw A, Opara UL (2020). Water loss of fresh fruit: Influencing pre-harvest, harvest and postharvest factors. Scientia Horticulturae 272: 109519. DOI 10.1016/j.scienta.2020.109519. [Google Scholar] [CrossRef]
Luyckx M, Hausman JF, Lutts S, Guerriero G (2017). Silicon and plants: Current knowledge and technological perspectives. Frontiers in Plant Science 8: 1–8. DOI 10.3389/fpls.2017.00411. [Google Scholar] [CrossRef]
Mahmoud AWM, Samy MM, Sany H, Eid RR, Rashad HM, Abdeldaym EA (2022). Nanopotassium, nanosilicon, and biochar applications improve potato salt tolerance by modulating photosynthesis, water status, and biochemical constituents. Sustainability 14: 723. DOI 10.3390/su14020723. [Google Scholar] [CrossRef]
Maleki G, Sedaghat N, Woltering EJ, Farhoodi M, Mohebbi M (2018). Chitosan-limonene coating in combination with modified atmosphere packaging preserve postharvest quality of cucumber during storage. Journal of Food Measurement and Characterization 12: 1610–1621. DOI 10.1007/s11694-018-9776-6. [Google Scholar] [CrossRef]
Michalak A (2006). Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Plant Cell 15: 523–530. DOI 10.1016/j.fitote.2011.01.018. [Google Scholar] [CrossRef]
Neysanian M, Iranbakhsh A, Ahmadvand R, Ardebili ZO, Ebadi M (2020). Comparative efficacy of selenate and selenium nanoparticles for improving growth, productivity, fruit quality, and postharvest longevity through modifying nutrition, metabolism, and gene expression in tomato; potential benefits and risk assessment. PLoS One 15: 244207. DOI 10.1371/journal.pone.0244207. [Google Scholar] [CrossRef]
Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O et al. (2003). Vitamin C as an antioxidant: Evaluation of its role in disease prevention. Journal of the American College of Nutrition 22: 18–35. DOI 10.1080/07315724.2003.10719272. [Google Scholar] [CrossRef]
Peris-Felipo FJ, Benavent-Gil Y, Hernández-Apaolaza L (2020). Silicon beneficial effects on yield, fruit quality and shelf-life of strawberries grown in different culture substrates under different iron status. Plant Physiology and Biochemistry 152: 23–31. DOI 10.1016/j.plaphy.2020.04.026. [Google Scholar] [CrossRef]
Pinedo-Guerrero ZH, Hernández-Fuentes AD, Ortega-Ortiz H, Benavides-Mendoza A, Cadenas-Pliego G, Juárez-Maldonado A (2017). Cu nanoparticles in hydrogels of chitosan-PVA affects the characteristics of post-harvest and bioactive compounds of jalapeño pepper. Molecules 22: 926. DOI 10.3390/molecules22060926. [Google Scholar] [CrossRef]
Ranjbar S, Rahemi M, Ramezanian A (2018). Comparison of nano-calcium and calcium chloride spray on postharvest quality and cell wall enzymes activity in apple cv. Red Delicious. Scientia Horticulturae 240: 57–64. DOI 10.1016/j.scienta.2018.05.035. [Google Scholar] [CrossRef]
Rasouli M, Saba MK (2018). Pre-harvest zinc spray impact on enzymatic browning and fruit flesh color changes in two apple cultivars. Scientia Horticulturae 240: 318–325. DOI 10.1016/j.scienta.2018.06.05. [Google Scholar] [CrossRef]
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine 26: 1231–1237. DOI 10.1016/S0891-5849(98)00315-3. [Google Scholar] [CrossRef]
Rizwan M, Ali S, Qayyum MF, Ok YS, Adrees M, Ibrahim M, Zia-ur-Rehman M, Farid M, Abbas F (2017). Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. Journal of Hazardous Materials 322: 2–16. DOI 10.1016/j.jhazmat.2016.05.061. [Google Scholar] [CrossRef]
Savvas D, Ntatsi G (2015). Biostimulant activity of silicon in horticulture. Scientia Horticulturae 196: 66–81. DOI 10.1016/j.scienta.2015.09.010. [Google Scholar] [CrossRef]
Shah SWA, Jahangir M, Qaisar M, Khan SA, Mahmood T, Saeed M, Farid A, Liaquat M (2015). Storage stability of kinnow fruit (Citrus reticulata) as affected by CMC and guar gum-based silver nanoparticle coatings. Molecules 20: 22645–22661. DOI 10.3390/molecules201219870. [Google Scholar] [CrossRef]
Shalaby TA, Abd-Alkarim E, El-Aidy F, Hamed ES, Sharaf-Eldin M, Taha N, El-Ramady H, Bayoumi Y, Dos Reis AR (2021). Nano-selenium, silicon and H2O2 boost growth and productivity of cucumber under combined salinity and heat stress. Ecotoxicology and Environmental Safety 212: 111962. DOI 10.1016/j.ecoenv.2021.111962. [Google Scholar] [CrossRef]
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology 299: 152–178. DOI 10.1016/S0076-6879(99)99017-1. [Google Scholar] [CrossRef]
Steiner A (1961). A universal method for preparing nutrient solutions of a certain desired composition. Plant and Soil 15: 134–154. DOI 10.1007/BF01347224. [Google Scholar] [CrossRef]
Thakral V, Bhat JA, Kumar N, Myaka B, Sudhakaran S, Patil G, Sonah H, Shivaraj SM, Deshmukh R (2021). Role of silicon under contrasting biotic and abiotic stress conditions provides benefits for climate smart cropping. Environmental and Experimental Botany 189: 104545. DOI 10.1016/j.envexpbot.2021.104545. [Google Scholar] [CrossRef]
Xue T, Hartikainen H, Piironen V (2001). Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant and Soil 237: 55–61. DOI 10.1023/A:1013369804867. [Google Scholar] [CrossRef]
Zhu Y, Guo J, Feng R, Jia J, Han W, Gong H (2016). The regulatory role of silicon on carbohydrate metabolism in Cucumis sativus L. under salt stress. Plant and Soil 406: 231–249. DOI 10.1007/s11104-016-2877-2. [Google Scholar] [CrossRef]
Zuverza-Mena N, Martínez-Fernández D, Du W, Hernandez-Viezcas JA, Bonilla-Bird N, López-Moreno ML, Komárek M, Peralta-Videa JR, Gardea-Torresdey JL (2017). Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses–A review. Plant Physiology and Biochemistry 110: 236–264. DOI 10.1016/j.plaphy.2016.05.037. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |