DOI:10.32604/biocell.2022.017406

| BIOCELL DOI:10.32604/biocell.2022.017406 |  |
| Viewpoint |
Mechanotransduction-The relationship between gravity, cells and tensile loading in extracellular matrix
Department of Pathology and Laboratory Medicine, Robert Wood Johnson Medical School, Rutgers, The State University of New Jersey, Piscataway, 08854, USA
*Address correspondence to: Frederick H. Silver, silverfr@rutgers.edu
Received: 08 May 2021; Accepted: 07 June 2021
Abstract: Gravity plays a central role in vertebrate development and evolution. Mechanotransduction involves the tensile tethering of veins and arteries, connections between the epidermis and dermis in skin, tensile stress concentrations that occur at tissue interfaces, cell-cell interactions, cell-collagen fiber stress transfer in extracellular matrix and fluid shear flow. While attention in the past has been directed at understanding the myriad of biochemical players associated with mechanotransduction pathways, less attention has been focused on determining the tensile mechanical behavior of tissues in vivo. Fibroblasts sit on the surface of collagen fibers in living skin and exert a retractile force on the fibers. This retractile force pulls against the tension in collagen fibers in skin. After fibroblast-collagen fiber interactions are altered either by changes in fibroblast adhesion or after formation of cancer associated fibroblasts, and changes in cell junctions, alterations in the retractive force leads to changes in mechanotransduction. The purpose of this paper is to present a model of tensile forces that occur at the fibroblast-collagen fiber interface and how these forces are important in extracellular matrix physiology in health and disease.
Keywords: Collagen; Mechanotransduction; Fibroblasts; Gravity; Tensile forces
Gravity plays a central role in vertebrate development and evolution. Gravitational forces acting on mammalian tissues cause the net muscle forces required for locomotion to be higher on earth than on a body subjected to a microgravitational field (Silver, 2006). Thus mechanical forces required to do the work (mechanical energy) of locomotion must be sensed by cells and converted into chemical energy (synthesis of new tissue) when tissues experience moderate increases in loading over a period of time (Silver and Siperko, 2003). Mechanotransduction extends far beyond biochemical and biophysical musculoskeletal events that occur in vivo. Mechanotranduction involves the tensile tethering of veins and arteries in the cardiovascular system (Silver et al., 2021), connections between the epidermis and dermis in skin (Silver et al., 2002a), tensile stress concentrations that occur at interfaces between implants and natural tissues (Silver et al., 2018), cell-cell interactions at cell attachments (Dasgupta and McCollum, 2019), cell-collagen fiber stress transfer in extracellular matrix (ECM) (Silver and Siperko, 2003), and fluid shear flow in chondrocytes and bone cells (Pattappa et al., 2019; Jin et al., 2020). Mechanotransduction involves activation of a complex series of pathways including MAP kinase and other pathways that leads to a balance between tissue anabolism and catabolism that is influenced by genetic changes within the cell nucleus (Silver and Siperko, 2003; Silver et al., 2003; Dasgupta and McCollum, 2019).
While much attention has been directed to understand the myriad of biochemical players associated with mechanotransduction pathways and their behaviors in model systems, less attention has been focused on the tensile mechanical behavior of cells and tissues in vivo. Shear behavior of cardiovascular tissue, bone and other tissues where mechanotransduction has been shown to be influenced by fluid shear forces gives valuable information (Pattappa et al., 2019; Li et al., 2019; Jin et al., 2020); however, for most tissues the tensile forces that exist between cells and the collagen fibers provide a feed-back system that may be a factor that shifts the balance between tissue anabolism and catabolism.
Examples of the importance of tensile forces in tissues exist in skin, cardiovascular tissue and cartilage. Excision of these tissues results in changes in their geometry. Skin is under tension and contracts when it is excised (Silver et al., 2002). Arterial tissue also contracts and shrinks as much as 50% when removed from the cardiovascular system (Silver et al., 2021) while articular cartilage curls when it is released from a normal joint surface (Silver et al., 2002).
The existence of Langer’s lines of tension in skin is well known to Plastic Surgeons and has been reviewed in the past and leads to a model of this interaction (Silver et al., 2002a) (see Figs. 1 and 2). It was concluded that the collagen fibers in skin have preferred lines of tension (Silver et al., 2002a) that lead to circular holes in cadaver skin elongating along the lines of tension when the surrounding skin is cut. However, Langer failed to address the observation that circular holes made in living skin remain circular after skin is excised (Silver and Siperko, 2003). The only explanation for this difference is that the cells in the living portion of skin also provide tension. Therefore, the fibroblasts that sit on the collagen fibers must exert contractile forces that oppose the tensile forces acting on the fibers to cause the skin around the circular defect to remain circular in living skin. Thus the relationship between cellular forces and the forces applied by collagen fibers of the extracellular matrix are important factors in tissue metabolism and changes seen in cardiovascular, cartilage and skin diseases to name a few examples.

Figure 1: Polarized light micrograph of normal human dermis taken approximately parallel to the skin surface after the skin is processed for routine histology but not stained. The tissue was not stretched during fixation or sample preparation allowing the tissue to relax and lose some of its orientation. Note the bright white collagen fibers that run approximately orthogonal to each other and appear to be interwoven at several points. These points act as crosslinks holding the tissue together when the fibers are cut. It is likely the angle between collagen fibers is less than 900 in stretched skin in vivo. The bar shown has a length of 95 μm.
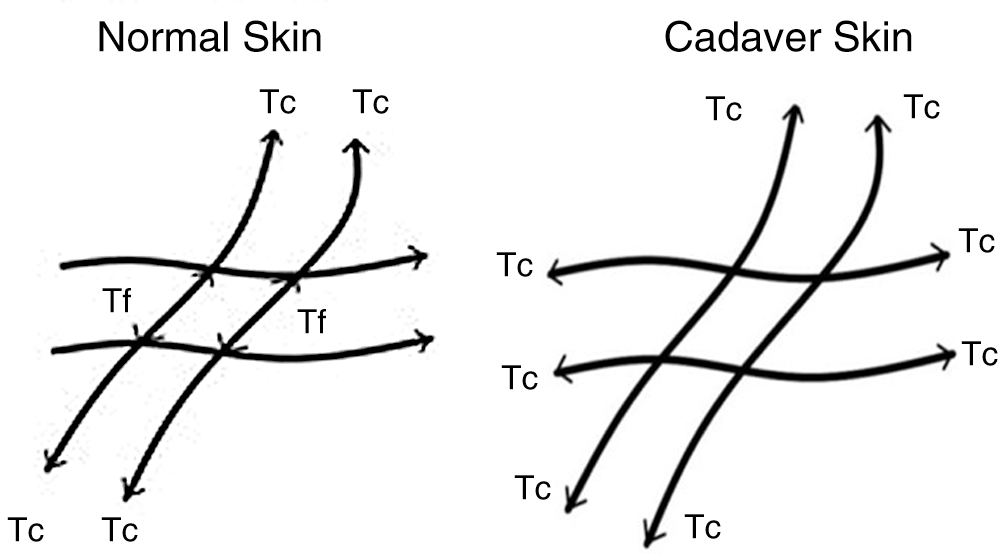
Figure 2: In living skin (left), the fibroblasts provide a retractive force (Tf) that prevents the skin from elongating after the edges of the skin are cut as diagrammed. In cadaver skin (right) the tension in the collagen fibers (Tc) causes a circular hole to become elliptical as a result of the forces acting along the collagen fibers. The ellipse forms as a result of the collagen fiber tensile forces that attempt to elongate the skin when the edges are cut.
Implantation of stiff cardiovascular implants and stents result in stress concentrations and overloading of vascular tissue (Snowhill et al., 2001). This contributes to intimal hyperplasia and stenosis especially in small diameter synthetic vascular grafts (Silver et al., 2018). In osteoarthritis, loss of chondrocyte differentiation and synthesis of proteoglycans and type II collagen results in a soft matrix that no longer stores elastic energy or can dissipate it efficiently (Silver et al., 2002). Increased cellular stiffness associated with basal cell and squamous cell carcinomas result in increased turnover of the dermal collagen and increased collagen deposition of fibrotic tissue (Silver et al., 2019). In this case changes in the cellular and tissue stiffness are associated with malignancy and perhaps eventual metastasis (Silver et al., 2019; Stanisavljevic et al., 2015). Chemical and thermal burns lead to increased stiffness of the granulation tissue and remodeled fibrous collagen deposited in the skin (Silver et al., 2020; Silver and Shah, 2020) as does the genetic changes associated with the disease Scleroderma (Odell et al., 2020). The observation that negative pressure applied to wounds (Huang et al., 2014) and suturing the edges of the wound together support the idea that stretching and tensile mechanical stress promotes healing. Providing tensile forces at the wound edges restores the balance of forces acting at the collagen fiber-fibroblast interface. This balance is somehow lost due to dysregulation in hypertrophic scarring (Dunn et al., 1985) and keloid formation.
All of the these examples underscore the need to further study the relationship between forces that exist between cells and collagen fibers in tissues in vivo in order to better understand the pathogenesis and diagnoses of a variety of diseases including cardiovascular diseases and cancer.
The relationship between tension in the parenchyma of many mammalian tissues and the cellular retractive forces holding the tissues together, suggest that the force equilibrium may play a widespread role in tissue metabolism under physiologic conditions. This equilibrium can be altered via changes in tissue external loading, cellular mutations, tissue injury and changes associated with aging. Of critical importance, considering the complexity of the possible biochemical actors involved in mechanotransduction in vitro, is to understand if the same pathways occur in vivo under normal tensile loading.
Further studies of the biochemical mechanotransduction pathways are needed under conditions that mimic the normal tensile loading patterns seen in vivo. This requires the development of new techniques to understand the level of tensile forces acting in vivo in each tissue. One new technique termed vibrational optical coherence tomography has been used to measure the tensile elastic modulus of several tissues in vivo (Silver et al., 2020, 2020a, 2021). These measurements are necessary to relate changes in the mechanical properties of tissues and alterations of mechanotransduction that occur in vivo that is associated with cardiovascular disease and metastatic cancer.
Acknowledgement: The author thanks Nikita Kelkar and Tanmay Desmukh for drawing the figures.
Author Contribution: The author confirms sole responsibility for the following: study conception and design, data collection, analysis and interpretation of results, and manuscript preparation.
Funding Statement: The author received no specific funding for this study.
Conflicts of Interest: The author declares that he has no conflicts of interest to report regarding the present study.
Dasgupta I, McCollum D (2019). Control of cellular responses to mechanical cues through YAP/TAZ regulation. Journal of Biological Chemistry 294: 17693–17706. DOI 10.1074/jbc.REV119.007963. [Google Scholar] [CrossRef]
Dunn MG, Silver FH, Swann DA (1985). Mechanical analysis of hypertrophic scar tissue: Structural basis for apparent increased rigidity. Journal of Investigative Dermatology 84: 9–13. [Google Scholar]
Huang C, Leavitt T, Bayer LR, Orgill DP (2014). Effect of negative pressure wound therapy on wound healing. Current Problems in Surgery 51: 301–331. [Google Scholar]
Jin J, Jaspers RT, Gang W, Korfage JAM, Klein-Nulend J, Bakker AD (2020). Shear stress modulates osteoblast cell and nucleus morphology and volume. International Journal of Molecular Sciences 21: 8361. DOI 10.3390/ijms21218361. [Google Scholar] [CrossRef]
Li X, Han L, Nookaew I, Mannen E, Silva MJ, Almeida M, Xiong J (2019). Stimulation of Piezo1 by mechanical signals promotes bone anabolism. eLife 8: e49631. DOI 10.7554/eLife.49631.001. [Google Scholar] [CrossRef]
Odell ID, Flavell RA, Silver FH (2020). Vibrational optical coherence tomography detects unique skin fibrotic states: Preliminary results of animal and human studies. Journal of the American Academy of Dermatology 26: SO190–9622(20) 32488-9. [Google Scholar]
Pattappa G, Zellner J, Johnstone B, Docheva D, Angele P (2019). Cells under pressure–The relationship between hydrostatic pressure and mesenchymal stem cell chondrogenesis. European Cells and Materials 37: 360–381. DOI 10.22203/eCM.v037a22. [Google Scholar] [CrossRef]
Silver FH, Bradica G, Tria A (2002). Elastic energy storage in human articular cartilage: Estimation of the elastic spring constant for type II collagen and changes associated with osteoarthritis. Matrix Biology 21: 129–137. [Google Scholar]
Silver FH, Siperko LM, Seehra GP (2002a). Mechanobiology of force transduction in dermis. Skin Research and Technology 8: 1–21. [Google Scholar]
Silver FH (2006). Mechanosensing and mechanochemical transduction in extracellular matrix. USA: Springer. [Google Scholar]
Silver FH, Siperko LM (2003). Mechanosensing and mechanochemical transduction. Critical Reviews in Biomedical Engineering 31: 255–331. [Google Scholar]
Silver FH, Freeman J, Seehra GP (2003). Collagen self-assembly and development of matrix mechanical properties. Journal of Biomechanics 36: 1529–1553. [Google Scholar]
Silver FH, Shah RG, Silver LL (2018). The use of vibrational optical coherence tomography in matching host tissue and implant mechanical properties. Journal of Biomaterials and Medical Applications 2. DOI 10.4172/2577-0268.1000115. [Google Scholar] [CrossRef]
Silver FH, Shah RG, Richard M, Benedetto D (2019). Comparative virtual biopsies of normal skin and skin lesions using vibrational optical coherence tomography. Skin Research and Technology 25: 743–749. [Google Scholar]
Silver FH, Kelkar N, Desmukh T, Horvath I, Shah RG (2020). Mechano-vibrational spectroscopy of tissues and materials using vibrational optical coherence tomography: A new non-invasive and non-destructive technique. Recent Progress in Materials 2. DOI 10.21926/rpm.2002010. [Google Scholar] [CrossRef]
Silver FH, Horvath I, Kelkar N, Deshmukh T, Shah R (2020a). In vivo biomechanical analysis of human tendon using vibrational optical coherence tomography: Preliminary results. Journal of Clinical Cases and Reports 4: 12–19. [Google Scholar]
Silver FH, Shah R (2020). “Virtual biopsies” of normal skin and thermal and chemical burn wounds. Advances in Skin and Wound Care 33: 307–312. [Google Scholar]
Silver FH, Kelkar N, Deshmukh T (2021). In vivo non-invasive analysis of the mechanical properties of vessel walls using vibrational optical coherence tomography. Online Journal of Cardiology Research and Reports 5. DOI 10.33552/OJCR.2021.05.000603. [Google Scholar] [CrossRef]
Snowhill PB, Noshir JL, Siegel RL, Silver FH (2001). Characterization of radial forces in Z stents. Investigative Radiology 36: 521–530. [Google Scholar]
Stanisavljevic J, Loubat-Casanovas J, Hererra M, Luque T, Pena R et al. (2015). Snail1-expressing fibroblasts in the tumor microenvironment display mechanical properties that support metastasis. Cancer Research 75: 285–295. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |