DOI:10.32604/biocell.2022.016586

| BIOCELL DOI:10.32604/biocell.2022.016586 |  |
| Article |
Calcium supplementation in colorectal cancer prevention: A systematic meta-analysis of adverse events
1Department of General Surgery, Mianzhu People’s Hospital of Sichuan, Mianzhu, China
2Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
3Department of Biosciences, Shri Ram Group of College (SRGC), Muzaffarnagar, India
4Department of Health Sciences, Novel Global Community Educational Foundation, Sydney, Australia
5Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia
6Department of Biology, College of Science, Imam Mohammad Ibn Saud Islamic University (IMSUI), Riyadh, Saudi Arabia
7Oncology Center, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
8Shiraz University of Medical Sciences, Shiraz, Iran
*Address correspondence to: Shahanavaj Khan, sdkhan@ksu.edu.sa
Received: 27 March 2021; Accepted: 20 May 2021
Abstract: Despite the multiple systematic reviews and meta-analyses accumulating evidence on the preventive effect of calcium supplementation for colorectal cancer, most of the associated adverse effects are not systematically analyzed. The aim of the study is evaluating adverse events associated with calcium supplementation for colorectal cancer prevention through a systematic meta-analysis. We searched Medline, PubMed Central, EMBASE (Excerpta Medica database), Scopus, Cochrane Central Register of Controlled Trials, and Web of Science published in English from database inception up to 31 July 2019. In the current systematic meta-analysis, we included human studies (including cohort studies, clinical trials, case-control studies) on supplementation of calcium in patients with or at risk of colorectal cancer. Assessment of the quality of included studies was performed by Jadad score. Information on the patient population, number of enrolled subjects in each group, dose of calcium supplementation, duration of calcium supplementation, and reported adverse events were gathered. The data were pooled for incidence rates for any adverse event during the study period regardless of causality association. We identified 6 studies, comprising 4583 participants that met the inclusion criteria. Meta-analysis on pooled incidence rates for adverse event during study period showed no statistically significant increased risk for cancer (OR = 0.92, 95% CI: 0.70–1.21, P = 0.577; I2 = 0.0%, P = 0.731), coronary revascularization (OR = 1.12, 95% CI: 0.79–1.59, P = 0.492; I2 = 0.0%, P = 0.957), myocardial infarction (OR = 0.81, 95% CI: 0.34–1.91, P = 0.634; I2 = 67.9%, P = 0.047), stroke (OR = 0.75, 95% CI: 0.42–1.33, P = 0.332, I2 = 0.00%, P = 0.717), Transient Ischemic Attack (TIA) (OR = 1.37, 95% CI: 0.28–6.51, P = 0.692, I2 = 81.9%, P = 0.002), urolithiasis (OR = 1.23, 95% CI: 0.75–2.01, P = 0.410; I2 = 0.0%, P = 0.851), fracture (OR = 0.98, 95% CI: 0.70–1.37, P = 0.938; I2 = 37.8%, P = 0.152) and death (OR = 1.05, 95% CI: 0.71–1.56, P = 0.786, I2 = 12.2%, P = 0.317) in patients receiving calcium supplementation for colorectal cancer prevention compared to control. Based on the results of Egger test, publication bias was not observed among the studies (P = 0.262). The current result of the meta-analysis on human studies reporting adverse events associated with calcium supplementation for the prevention of colorectal cancer demonstrated no statistically significant increased risk for the development of adverse events compared to control groups.
Keywords: Colorectal cancer; Calcium; Supplement; Adverse event; Systematic reviewed; Meta-analysis
List of Abbreviations
| EMBASE: | Excerpta Medica database |
| PICOS: | population, intervention, control, outcome, study |
| CaSR: | calcium-sensing receptors |
| PRISMA: | preferred reporting items for systematic reviews and meta-analyses |
| RCTs: | randomized controlled trials |
| RevMan: | review manager |
Colorectal cancer incidence varies markedly worldwide. Globally, it is the third most common detected cancer in males and the second in females (Favoriti et al., 2016). In 2018, 1.8 million new patients and 861,000 deaths from colorectal cancer is reported worldwide (Macrae, 2016). Lifestyle and infections are considered more important factors in development of colorectal cancers (Chapkin et al., 2020). Obesity, lack of physical activity, smoking, and alcohol are known risk factors for colorectal cancer (Abdifard et al., 2016). Minority of cases are associated with a specific genetic susceptibility such as familial adenomatous polyposis and hereditary non-polyposis colon cancer. Most of cases begin as benign lesions like polyp undergoing malignant transformation through the time (Rodrigues et al., 2020).
Different interventions are suggested for modifying the colorectal cancer risk (Janne and Mayer, 2000). Dietary supplements, physical activity, non-steroidal anti-inflammatory drugs, aspirin, and hormones are among the preventive measures with the most supporting evidence (Afrin et al., 2018; Barry et al., 2018; Shaw et al., 2018; Wilkins et al., 2018). Fibers, folic acid, pyridoxine, garlic, fish oil, vitamin D, and calcium are the most studied supplements for colorectal cancer prevention (Murphy et al., 2019; Waluga et al., 2018).
Calcium is an essential molecule to maintain human body health. Calcium is needed for different physiologic functions in human body including skeletal, neurologic, cardiologic, and muscular system functions (Pravina et al., 2013). The most popular function of calcium is in maintaining skeletal system (Power et al., 1999). Hypocalcemia may lead to osteoporosis or osteomalacia. Increased contractility of the muscles is caused by the raised extracellular ionized Calcium. This effect is not limited to skeletal muscles and is also present in cardiac muscle. Thus, problems in calcium hemostasis may lead to weakness, muscle spasm and heart failure or arrhythmia. Calcium also affects nervous system excitability. Calcium affects cellular permeability which cause its important role in allergic conditions (Pu et al., 2016).
Calcium supplementation is largely studied for its effect on colorectal cancer risk reduction in patients at risk, with promising results. These studies vary on the level of evidence and include case controls, cohorts, and clinical trials (Flood et al., 2005; Grau et al., 2003; Jenab et al., 2010). A systematic review and meta-analysis of 3 studies on the role of calcium supplementation for 3–4 years in preventing recurrence of colorectal adenomas in 1,279 patients found a significantly lower recurrence rate in patients receiving calcium supplementation compared to placebo (RR: 0.80, CI: 0.68, 0.93; P = 0.004) (Shaukat et al., 2005). Another recent systematic review and meta-analysis of 5 trials assessing the preventive effect of calcium supplementation in patients at risk of colorectal cancer including 2234 patients revealed the positive effect of calcium supplementation (RR: 0.88) (Veettil et al., 2017). This study also demonstrated the superior effect of elemental calcium dose of more than 1600 mg/day (RR, 0.74) compared to less than 1200 mg/day (RR, 0.84). Multiple biologically probable mechanisms are supposed for the protective effect of calcium supplementation in colorectal cancer. Calcium ameliorates inflammation and bile acid irritation on the colon wall. Moreover, intracellular calcium suppresses neoplastic promoting pathways in colon epithelial cells. It is also suggested that calcium can repress bile acid toxicity in the colon (Han et al., 2015).
Despite the beneficial preventive effects of calcium supplementation on colorectal cancer, some studies suggested health risks and adverse events associated with calcium supplementation. In a randomized controlled trial on 1471 postmenopausal women receiving calcium supplementation, the adjusted rate of myocardial infarction in the calcium group was significantly higher than the rate in the placebo group (RR: 2.12, 95% CI: 1.01–4.47) (Bolland et al., 2008). The result of a prospective cohort study on 91,731 women suggested an increased risk of kidney stones in patients receiving non-dietary calcium supplementation (RR: 1.20, 95% CI: 1.02–1.41) (Curhan et al., 1997). Additionally, other potential adverse effects of calcium supplementation such as constipation, metabolic syndrome, and age-related macular degeneration were reported (Kakigi et al., 2015; Noe et al., 2015; Prince et al., 2006).
Despite the multiple systematic reviews and meta-analyses accumulating evidence on the preventive effect of supplementation of calcium on the risk of colorectal cancer, the associated adverse events were not systematically reviewed. The aim of this study to evaluates the adverse events associated with calcium supplementation for colorectal cancer prevention in a systematic meta-analysis.
A comprehensive search was performed to retrieve any reported adverse event associated with calcium supplementation in patients with or at risk of colorectal cancer from published literature. The following databases were searched since initiation up to 31 July 2019; Medline, PubMed Central, EMBASE (Excerpta Medica database), Scopus, Cochrane Central Register of Controlled Trials, and Web of Science. Keywords including “trial”, “cohort”, “case-control”, “observational”, “interventional”, “patients” were added to “colorectal”, “colon” plus “calcium” and were used for database search. Our search was limited to studies reported in the English language.
To meet the study objectives, human studies (including cohort studies, clinical trials, and case-control studies) on supplementation of calcium in patients with or at risk of colorectal cancer were included. To be included in the meta-analysis, studies should include information on the observed adverse events in two study groups (the active group receiving calcium supplementation and the control group not receiving calcium supplementation) (Table 1).

The following studies were excluded: (1) non-original reports; (2) experimental models and in-vitro studies; (3) reports not mentioning any information about adverse events; (4) studies on the concomitant use of calcium with other supplementation in all patients; (5) studies on only dietary calcium intake without calcium supplementation; and (6) studies in which full text could not be sourced.
Bibliographic information of all manuscripts retrieved through database search was transferred to Endnotes V.X6. Data extraction was performed by two independent reviewers. Disagreements were resolved by a third reviewer.
Data were documented in predefined forms. Information on the patient population, numbers in each study group, dose of calcium supplementation, duration of calcium supplementation, reported adverse events in each group were extracted from each included study. The data were pooled for incidence rates for any adverse event during the study period regardless of causality association.
Study quality and risk of bias
Assessment of the quality of included studies was performed by Jadad score.
A comparison of reported adverse events was made between interventions by collecting data from studies by direct meta-analysis technique. Meta-analysis of the available data was performed using Review Manager (RevMan V.5.1) software. Dichotomous outcomes were summarized as risk (relative) ratios.
After retrieving data from various international databases, 210 articles were retrieved. Omitting duplicate articles, 162 articles were remained which sent for evaluation of the topics and abstracts. Passing this stage, 69 articles entered the full texts review and finally, 6 eligible articles entered the final analysis. It should be noted that the references of the included articles were also reviewed to add relevant studies. The flowchart of the included studies is displayed in Fig. 1.
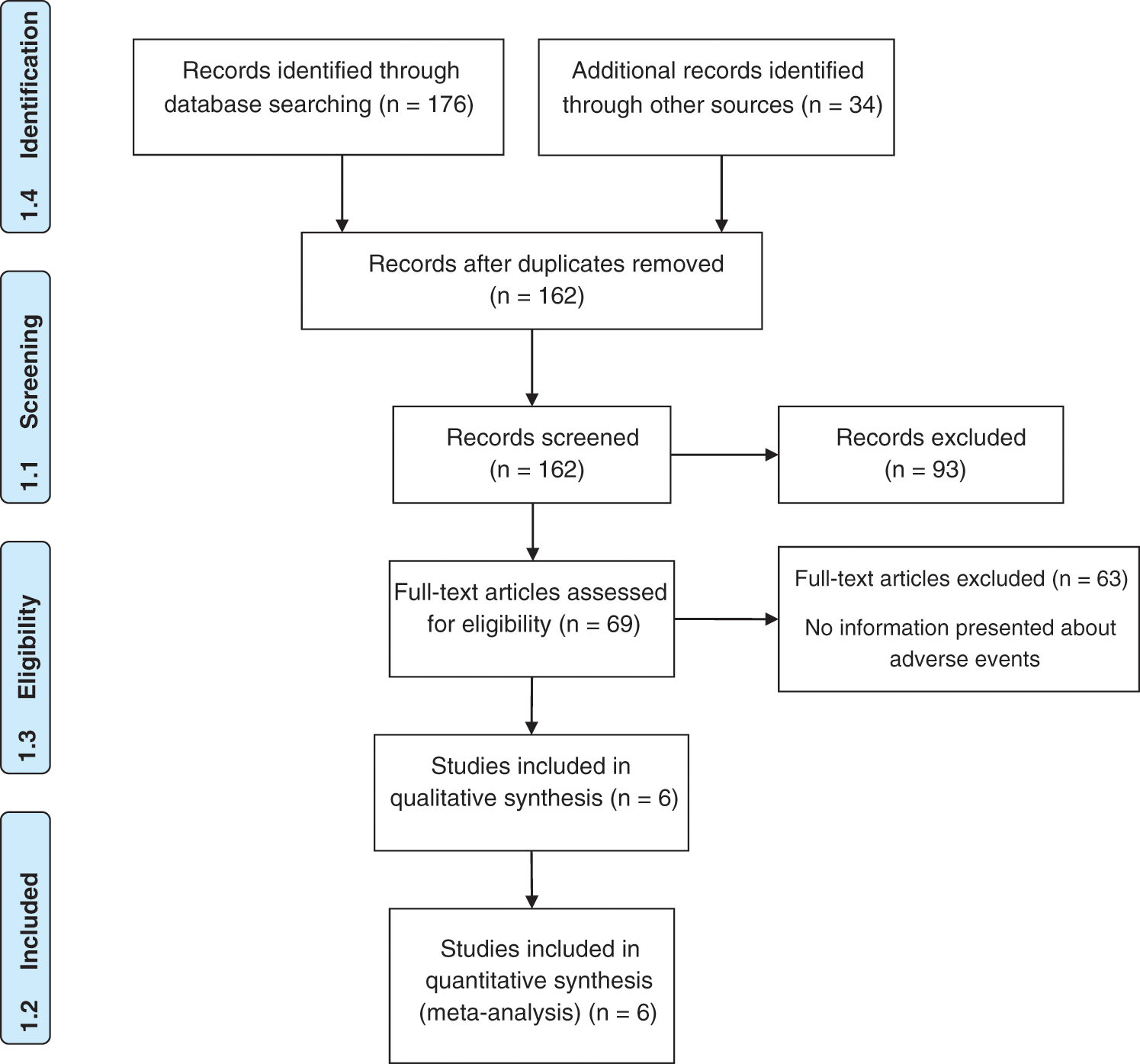
Figure 1: PRISMA flow diagram for included studies.
Characteristics of the included studies
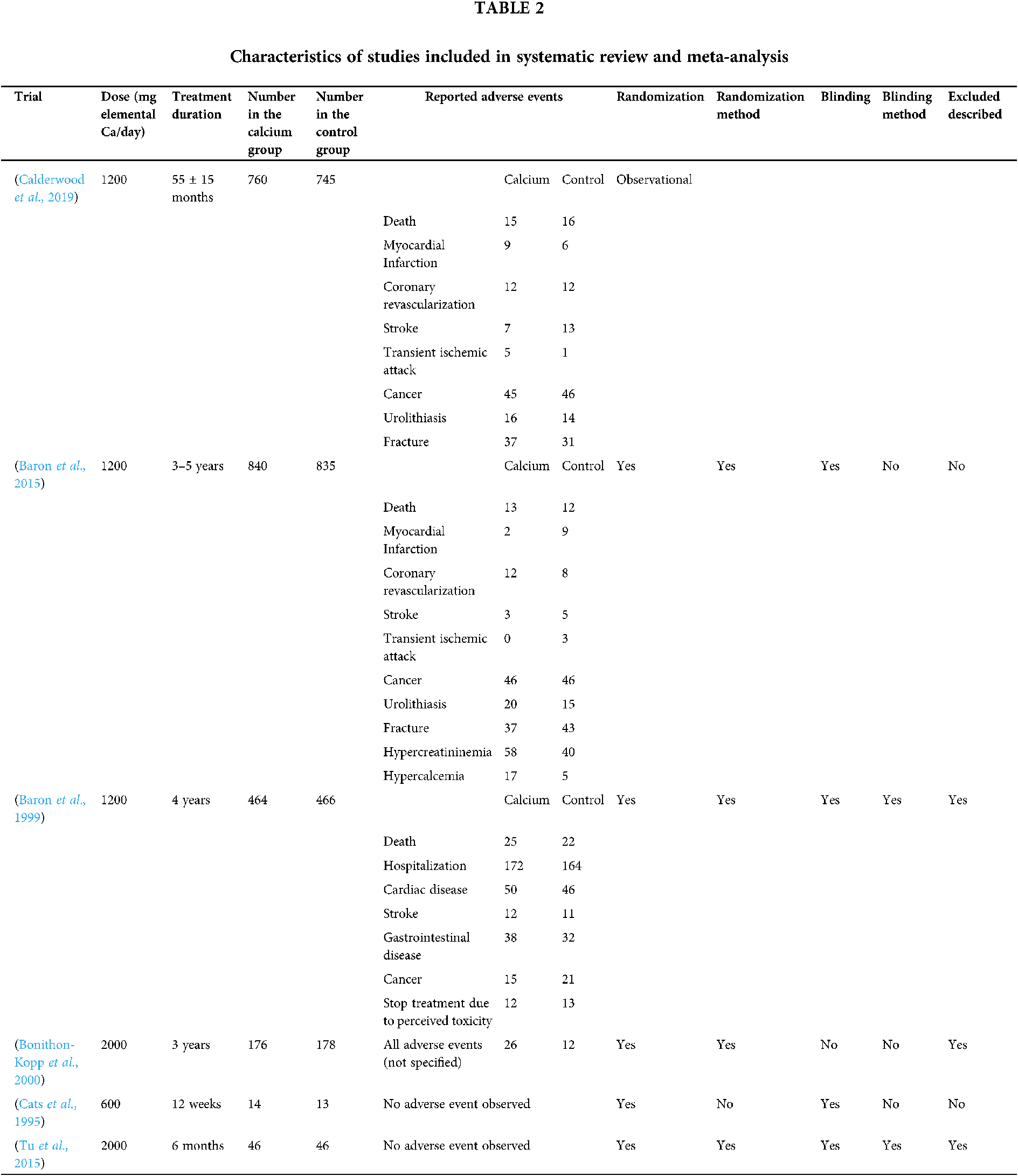
The characteristics of the studies included in the meta-analysis are presented in Table 2. According to the geographical area, four studies were performed on the North American population and two on the French and Dutch population.
Meta-analysis and data synthesis
Among the included studies, three randomized controlled trials (RCTs) assessed the risk of development of cancer in patients receiving calcium supplementation. Meta-analysis revealed less incidence of cancer in intervention group compared to placebo group, but these findings were not statistically significant (OR = 0.92, 95% CI: 0.70–1.21, P = 0.577; I2 = 0.0%, P = 0.731).
Three studies reported risk of coronary revascularization. No overall significant effect was observed in the intervention compared to control group (OR = 1.12, 95% CI: 0.79–1.59. P = 0.492; I2 = 0.0%, P = 0.957).
Among the included studies, two RCTs assessed the risk of myocardial infarction. Meta-analysis revealed lower incidence of myocardial infarction in patients receiving calcium supplementation compared to control, but these findings were not statistically significant (OR = 0.81, 95% CI: 0.34–1.91, P = 0.634; I2 = 67.9%, P = 0.047).
Three studies assessed the risk of stroke in studied patients. Pooled effect of intervention compared to control showed not significant association (OR = 0.75, 95% CI: 0.42–1.33, P = 0.332, I2 = 0.00%, P = 0.717).
Transient Ischemic Attack (TIA)
Two studies assessed the effect of calcium supplementation on the risk of TIA. Despite the increased risk, the statistical analysis indicated no significant effect of calcium supplement compared to control (OR = 1.37, 95% CI: 0.28– 6.51, P = 0.692; I2 = 81.9%, P = 0.002).
Two studies reported this adverse event in patients who received calcium supplementation. No overall significant effect was observed in the intervention compared to control group (OR = 1.23, 95% CI: 0.75–2.01, P = 0.410; I2 = 0.0%, P = 0.851).
Two studies assessed the effect of calcium supplementation on the risk of fractures. The results indicated no significant effect of calcium supplementation compared to control (OR = 0.98, 95% CI: 0.70–1.37, P = 0.938, I2 = 37.8%, P = 0.152).
Three studies assessed the risk of death in the patient population. Pooled effect of intervention compared to control showed no significant association (OR = 1.05, 95% CI: 0.71–1.56, P = 0.786; I2 = 12.2%, P = 0.317).
Based on the results of the Egger test, publication bias was not observed among the included studies (P = 0.262). Fig. 2 shows the funnel plot for each adverse event included in the meta-analysis of these studies.
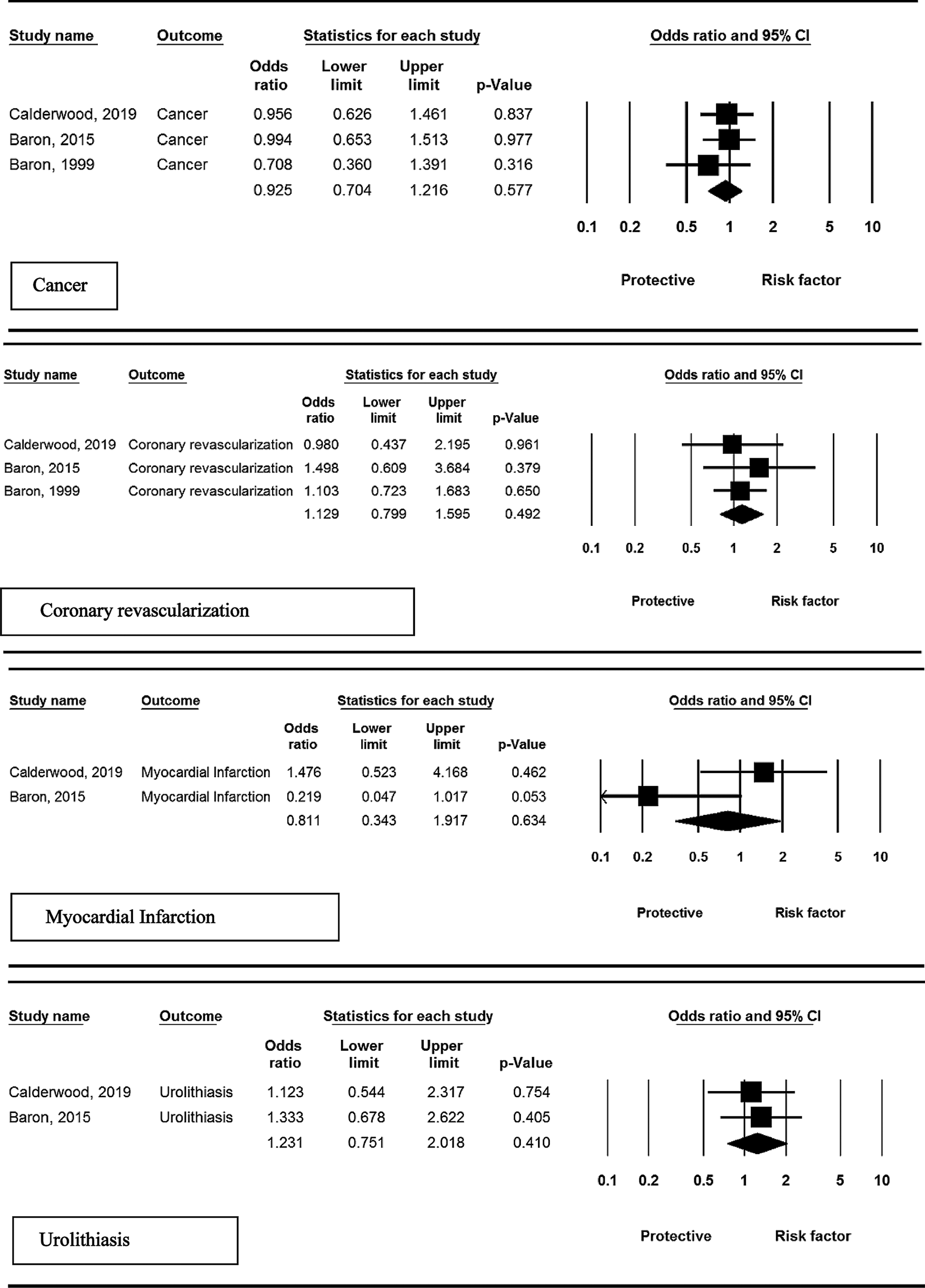
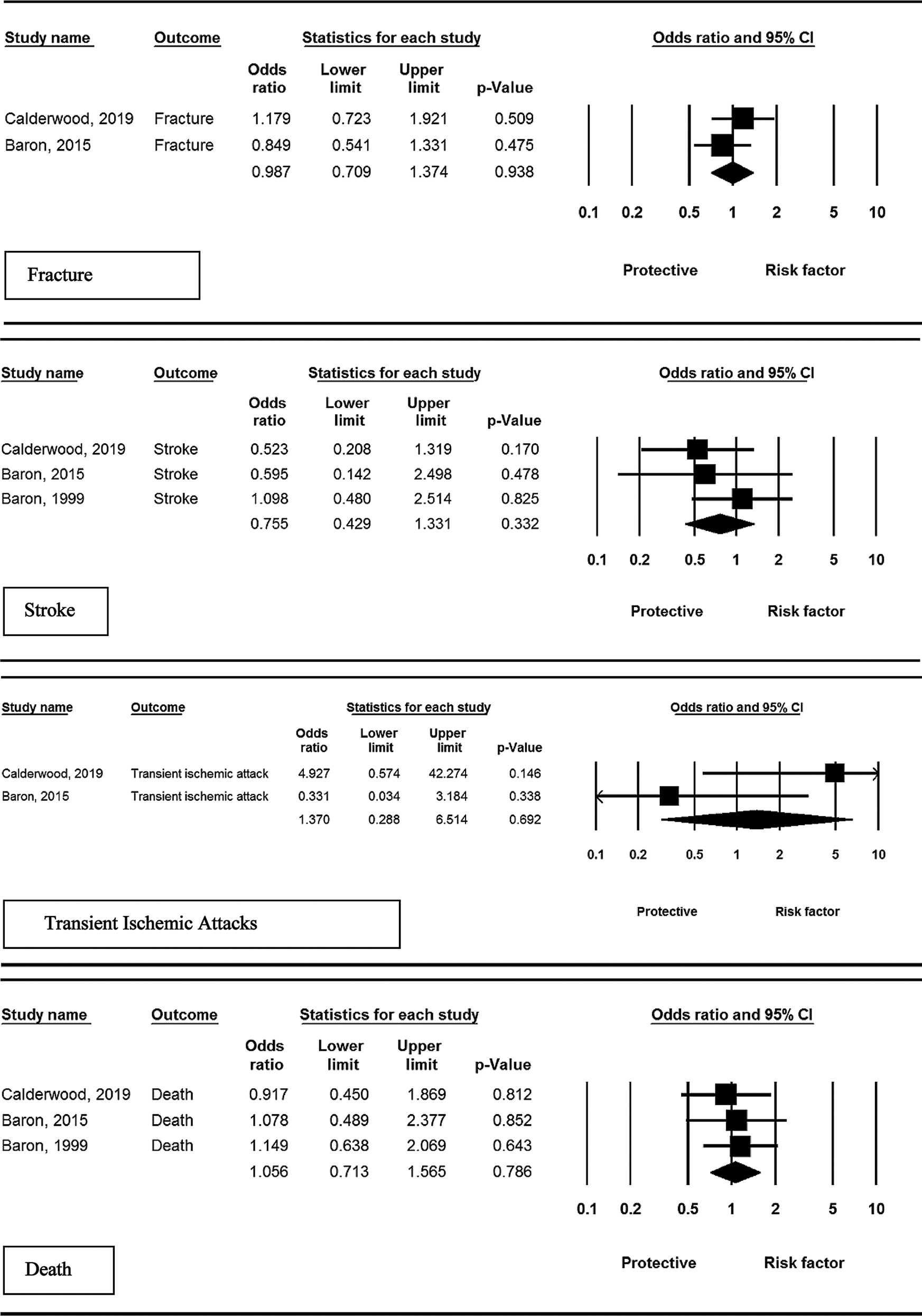
Figure 2: Results of meta-analysis, any adverse events: calcium vs. placebo.
Calcium homeostasis play important roles in different systems of our body, including skeletal, hormonal, cardiovascular, neurologic, and gastrointestinal systems (Peacock, 2010). Dietary calcium is absorbed throughout the small intestine. Absorbed calcium is mostly deposited into bones with excessive amounts excreted in the urine and feces. The main pathways in the homeostasis of calcium are regulated by parathyroid hormone and calcitonin (Veldurthy et al., 2016).
The preventive effect of calcium in colorectal cancer is considered to be through extracellular calcium-sensing receptors (CaSR) in the colon. The CaSR is involved in cell proliferation and differentiation. Accumulating evidence suggests that the CaSR might play a protective role against colorectal cancer. CaSR expression was shown to be reduced in colorectal cancer (Iamartino et al., 2018).
Different mechanisms are proposed for the potential adverse effects of calcium supplementation. Transiently elevated calcium levels by calcium supplements may contribute to cardiovascular risk. This risk is considered to be associated with calcium-sensing receptors on platelets that are activated with elevated serum calcium and cause increased blood coagulability. (Chin et al., 2017) It is also supposed that calcium supplementation may increase the urinary calcium excretion which can lead to stone formation or progression through hypercalciuria. However, this effect is not observed in patients receiving dietary calcium (Sorensen, 2014).
Notwithstanding the proposed mechanisms for adverse events associated with calcium supplementation, our results demonstrated no significant increase in the mentioned side effects in patients receiving calcium supplementation for colorectal cancer prevention compared to control groups.
The main strength of this study was its novelty. Despite the popular use of calcium supplementation in patients at the risk of colorectal cancer, no research has previously accumulated the evidence for potential risks of this intervention. This study has evaluated the risk of calcium supplementation in this population of patients for the first time. The use of the meta-analysis method was another important point of study which let us accumulate the observed adverse events in 2300 patients of 6 different human studies and compare it with 2283 controls. The use of control patients for statistical comparison made it possible to evaluate the potential causality of observed adverse events to calcium supplementation. The use of control comparison in the evaluation of adverse effects is of special importance in high-risk patient populations like patients with colorectal cancer which different adverse events may be observed in them without a causal relationship with the specific intervention.
There are some limitations to this systematic study. The majority of studies on calcium supplementation in colorectal cancer did not report information on adverse events. Studies included in this review were not similar in follow-up duration and the dose of calcium supplementation. The interaction between calcium and other supplementation used in colorectal cancer prevention like vitamin D, folic acid, pyridoxine, garlic, and fish oil was not evaluated in this study. Publication bias is another source of bias in the meta-analysis. Studies with a higher rate of adverse events are more prone not to be published in the literature. Language limitation of included studies is another potential source of bias in this systematic review.
A meta-analysis of human studies reporting adverse events associated with calcium supplementation for the prevention of colorectal cancer demonstrated no statistically significant increased risk for the development of adverse events compared to control groups.
Acknowledgement: The authors extend their appreciation to the College of Applied Medical Sciences Research Center and the Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia for their kind support.
Authors’ Contribution: XL and SK conceived of the presented idea. SK also supervised the current work. AM and MA developed the theoretical section and suggested for necessary corrections; AAC, SB and FT designed and conducted the computational All authors have read and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abdifard E, Amini S, Bab S, Masroor N, Khachian A, Heidari M (2016). Incidence trends of colorectal cancer in Iran during 2000-2009: A population-based study. Medical Journal of the Islamic Republic of Iran 30: 382. [Google Scholar]
Afrin S, Giampieri F, Gasparrini M, Forbes-Hernández TY, Cianciosi D et al. (2018). Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnology Advances 38: 107322. [Google Scholar]
Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS et al. (2015). A trial of calcium and vitamin D for the prevention of colorectal adenomas. New England Journal of Medicine 373: 1519–1530. [Google Scholar]
Baron JA, Beach M, Mandel JS, Van Stolk RU, Haile RW et al. (1999). Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. New England Journal of Medicine 340: 101–107. [Google Scholar]
Barry EL, Fedirko V, Baron JA (2018). NSAIDs and colorectal cancer phenotypes: What now? Journal of the National Cancer Institute 111: 440–441. [Google Scholar]
Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A et al. (2008). Vascular events in healthy older women receiving calcium supplementation: Randomised controlled trial. BMJ 336: 262. [Google Scholar]
Bonithon-Kopp C, Kronborg O, Giacosa A, Rath U, Faivre J (2000). Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: A randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet 356: 1300–1306. [Google Scholar]
Calderwood AH, Baron JA, Mott LA, Ahnen DJ, Bostick RM et al. (2019). No evidence for posttreatment effects of vitamin D and calcium supplementation on risk of colorectal adenomas in a randomized trial. Cancer Prevention Research 12: 295–304. [Google Scholar]
Cats A, Kleibeuker JH, van der Meer R, Kuipers F, Sluiter WJ et al. (1995). Randomized, double-blinded, placebo-controlled intervention study with supplemental calcium in families with hereditary nonpolyposis colorectal cancer. Journal of the National Cancer Institute 87: 598–603. [Google Scholar]
Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ (1997). Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Annals of Internal Medicine 126: 497–504. [Google Scholar]
Chapkin RS, Navarro SL, Hullar MA, Lampe JW (2020). Diet and gut microbes act coordinately to enhance programmed cell death and reduce colorectal cancer risk. Digestive Diseases and Sciences 65: 840–851. [Google Scholar]
Chin K, Appel LJ, Michos ED (2017). Vitamin D, calcium, and cardiovascular disease: A “D”vantageous or “D”etrimental? An era of uncertainty. Current Atherosclerosis Reports 19: 5. [Google Scholar]
Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi REM, Corcione F (2016). Worldwide burden of colorectal cancer: A review. Updates in Surgery 68: 7–11. [Google Scholar]
Flood A, Peters U, Chatterjee N, Lacey JV, Schairer C, Schatzkin A (2005). Calcium from diet and supplements is associated with reduced risk of colorectal cancer in a prospective cohort of women. Cancer Epidemiology and Prevention Biomarkers 14: 126–132. [Google Scholar]
Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML et al. (2003). Vitamin D, calcium supplementation, and colorectal adenomas: Results of a randomized trial. Journal of the National Cancer Institute 95: 1765–1771. [Google Scholar]
Han C, Shin A, Lee J, Lee J, Park JW et al. (2015). Dietary calcium intake and the risk of colorectal cancer: A case control study. BMC Cancer 15: 966. [Google Scholar]
Iamartino L, Elajnaf T, Kallay E, Schepelmann M (2018). Calcium-sensing receptor in colorectal inflammation and cancer: Current insights and future perspectives. World Journal of Gastroenterology 24: 4119–4131. [Google Scholar]
Janne PA, Mayer RJ (2000). Chemoprevention of colorectal cancer. New England Journal of Medicine 342: 1960–1968. [Google Scholar]
Jenab M, Bueno-De-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T et al. (2010). Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: A nested case-control study. BMJ 340: b5500. [Google Scholar]
Kakigi CL, Singh K, Wang SY, Enanoria WT, Lin SC (2015). Self-reported calcium supplementation and age-related macular degeneration. JAMA Ophthalmology 133: 746–754. [Google Scholar]
Macrae FA (2016). Colorectal cancer: Epidemiology, risk factors, and protective factors. Clinics in Colon and Rectal Surgery 18: 133–140. [Google Scholar]
Murphy N, Moreno V, Hughes DJ, Vodicka L, Vodicka P et al. (2019). Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Molecular Aspects of Medicine 69: 2–9. [Google Scholar]
Noe EB, Chon SJ, Kim MK, Yun BH, Lee BS et al. (2015). Associations between dietary calcium intake with prevalence of metabolic syndrome and changes in bone mineral density among Korean population. Maturitas 81: 171. [Google Scholar]
Peacock M (2010). Calcium metabolism in health and disease. Clinical Journal of the American Society of Nephrology 5: S23–S30. [Google Scholar]
Power ML, Heaney RP, Kalkwarf HJ, Pitkin RM, Repke JT et al. (1999). The role of calcium in health and disease. American Journal of Obstetrics and Gynecology 181: 1560–1569. [Google Scholar]
Pravina P, Sayaji D, Avinash M (2013). Calcium and its role in human body. International Journal of Research in Pharmaceutical and Biomedical Sciences 4: 659–668. [Google Scholar]
Prince RL, Devine A, Dhaliwal SS, Dick IM (2006). Effects of calcium supplementation on clinical fracture and bone structure: Results of a 5-year, double-blind, placebo-controlled trial in elderly women. Archives of Internal Medicine 166: 869–875. [Google Scholar]
Pu F, Chen N, Xue S (2016). Calcium intake, calcium homeostasis and health. Food Science and Human Wellness 5: 8–16. [Google Scholar]
Rodrigues R, Geyl S, Albouys J, de Carvalho C, Crespi M et al. (2020). Effect of implementing a regional referral network on surgical referral rate of benign polyps found during a colorectal cancer screening program: A population-based study. Clinics and Research in Hepatology and Gastroenterology 45: 101488. [Google Scholar]
Shaukat A, Scouras N, Schunemann HJ (2005). Role of supplemental calcium in the recurrence of colorectal adenomas: A metaanalysis of randomized controlled trials. American Journal of Gastroenterology 100: 390–394. [Google Scholar]
Shaw E, Farris MS, Stone CR, Derksen JW, Johnson R et al. (2018). Effects of physical activity on colorectal cancer risk among family history and body mass index subgroups: A systematic review and meta-analysis. BMC Cancer 18: 71. [Google Scholar]
Sorensen MD (2014). Calcium intake and urinary stone disease. Translational Andrology and Urology 3: 235–240. [Google Scholar]
Tu H, Flanders WD, Ahearn TU, Daniel CR, Gonzalez-Feliciano AG et al. (2015). Effects of calcium and vitamin D3 on transforming growth factors in rectal mucosa of sporadic colorectal adenoma patients: A randomized controlled trial. Molecular Carcinogenesis 54: 270–280. [Google Scholar]
Veettil SK, Ching SM, Lim KG, Saokaew S, Phisalprapa P, Chaiyakunapruk N (2017). Effects of calcium on the incidence of recurrent colorectal adenomas: A systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Medicine 96: e7661. [Google Scholar]
Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, Christakos S (2016). Vitamin D, calcium homeostasis and aging. Bone Research 4: 16041. [Google Scholar]
Waluga M, Zorniak M, Fichna J, Kukla M, Hartleb M (2018). Pharmacological and dietary factors in prevention of colorectal cancer. Journal of Physiology and Pharmacology 69: 325–336. [Google Scholar]
Wilkins T, Mcmechan D, Talukder A (2018). Colorectal cancer screening and prevention. American Family Physician 97: 658–665. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |