DOI:10.32604/biocell.2022.013432

| BIOCELL DOI:10.32604/biocell.2022.013432 |  |
| Article |
The role of baicalin on carbon tetrachloride induced liver fibrosis
Department of Gastroenterology, the Affiliated People’s Hospital of Ningbo University, Ningbo, 315040, China
*Address correspondence to: Mengting Li, limengting1992@foxmail.com
Received: 07 August 2020; Accepted: 25 September 2020
Abstract: The effect of the baicalin, a bio-active flavonoid extracted from Scutellaria baicalensis Georgi, on the carbon tetrachloride (CCl4) induced liver fibrosis was investigated. To compare the effect of baicalin on the liver fibrosis, five different groups of rats treated by 100, 200, and 400 mg/kg baicalin were studied. Upon CCl4 treatment, the levels of procollagen type III, aspartate aminotransferase, aminotransferase, hyaluronic acid, and hydroxyproline were significantly increased, whereas the superoxide dismutase and glutathione peroxidase content were decreased. These changes in the biochemical parameters, which are associated with liver function, were significantly attenuated by the baicalin treatment, suggesting that baicalin can suppress the liver fibrosis induced by CCl4. Moreover, the histological staining analysis demonstrated that baicalin could effectively inhibit the degree of liver cell injury. The protein expression of AKT/JAK2/ERK in the serum were markedly increased by CCl4 but suppressed by the treatment of baicalin in a dose-dependent manner, implying that baicalin can attenuated cell apoptosis induced by CCl4. Overall, these results suggest that baicalin effectively protects hepatocytes from the CCl4 oxidative damage, likely due to the inhibition of free radical generation and cell apoptosis during the liver injury.
Keywords: Liver Fibrosis; Baicalin; Cell Apoptosis
Liver disease is one of the major global challenges. According to the Office for National Statistics in the United Kingdom, liver disease is the fifth most common cause (Toledano et al., 2019). Liver fibrosis is a common chronic liver disease, and it is considered as a critical issue due to its high rate of morbidity and mortality (Huang et al., 2014; Sun et al., 2007). Hepatitis viral infection is the most common cause. Drug abuse, autoimmune disorders, metabolic disorders, and biliary obstruction due to mineral overload are attributed to the liver fibrosis (Friedman, 2003; Kisseleva and Brenner, 2006). Many environmental toxins can also cause liver injury and fibrosis, which is often characterized as the accumulation of extracellular matrix protein and collagen in the perisinusoidal space (Dai et al., 2009; Sun et al., 2012). Moreover, it was found that serious liver fibrosis leads to cirrhosis and liver failure eventually (Are et al., 2020; Zimmermann et al., 2019). However, treatment of liver diseases still remains as a therapeutic challenge. Therefore, there is a need to develop effective therapeutic strategies that will decrease the extent of liver fibrosis or enhance the liver regeneration. Despite technology advances in medicine, only few medical treatment approaches are available for the liver fibrosis (Sun et al., 2012). Meanwhile, modern medicine lacks reliable and effective liver protective activities. Therefore, there has been considerable interest in the development of alternative and complementary medicine for the treatment of liver diseases.
Although the pathogenesis of liver fibrosis is quite complicate, the understanding of biochemical and cellular factors associated with liver fibrosis is gradually increasing. Several reports have suggested that oxidative stress can play a key role in the liver fibrosis (Cederbaum et al., 2009; Poli, 2000). For instance, free radicals, as found during the oxidative process in cells, can attack unsaturated fatty acids of cell membranes, resulting in cell peroxidation, destructing DNA and protein (Sastre et al., 2007). These processes eventually cause various liver injuries. It has been shown that traditional herb medicine, which contains antioxidants, can be an effective approach to prevent liver fibrogenesis (Gebhardt, 2002; Lin et al., 2018).
For example, the radix of Scutellaria baicalensis is an eastern traditional medicine, and it has been used as a relaxant for smooth muscle and an anti-inflammatory reagent (Huang et al., 2018; Liu et al., 2019). It has also been used as an ingredient of herb mixture for the hepatoprotective agent (Dong et al., 2020; Gao et al., 1995; Huang et al., 2012; Liu et al., 2015a; Liu et al., 2015b; Wu et al., 2018; Yin et al., 2018; Yu et al., 2020; Zhou et al., 2018). Baicalin is the major active component of the isolated root of Scutellaria baicalensis, and it acts as a free radical scavenger for reactive oxygen species and has an anti-inflammatory activity (Gao et al., 1995; Zhou et al., 2018). It was found that the baicalin can inhibit the H2O2-induced liver injury caused by suppression of oxidation in the cell (Yu et al., 2020). It can also inhibit the activation of redox sensitive nuclear factor-κB (NF-κB) in kidney from the old rats (Liu et al., 2015a; Yin et al., 2018). However, information about the hepatoprotective effect of baicalin is very limited.
Carbon tetrachloride (CCl4) is commonly employed as an inducer of the liver injury in small animals (Huang et al., 2014; Yu et al., 2020). It is known that CCl4 is hepatotoxic as well as nephrotoxic to humans (Kodavanti et al., 1989; Ritesh et al., 2015). It has been reported that hepatic necrosis induced by CCl4 involves the activation by a microsomal cytochrome P450-depentent (Sun et al., 2007) monooxygenase system, resulting in the generation of ROS and trichloromethyl free radicals. It eventually leads to damage of cell membrane (Mccay et al., 1984), following by the release of inflammatory mediators from the activated macrophages. They are believed to be the cause of the CCl4-induced hepatic injury. In this work, we investigated the role of baicalin in CCl4 induced liver injury and how it can protect the liver injury. The possible molecular mechanism and the effect of inhibition of the oxidative stress and inflammation will be discussed.
Carbon tetrachloride (CCl4) was purchased from Aladdin (part no.: C112043, China). Commercial kits for SOD, GSH-Px, hydroxyproli, MDA, AST/GOT, and ALT/GPT were purchased from Jiancheng Co. (Nanjing, China). 85% Baicalin was purchased from Fusion Biology Co. (Part No. 21967-41-9, China).
Experiments were carried out on 17 ICR male rats (Jestier, China) of 18–22 g body weight. They were housed at 22–24°C and exposed to alternate cycles of 12 h light and dark. They were given free access to a standard pellet diet and tap water. The animal study was approved by the Laboratory Animal’s Ethic Committee of the Hospital. It was carried out throughout the experiment following the international laboratory animal use and care guideline.
Experimental model and treatment
We first prepared a 2% CCl4 solution by mixing CCl4 and corn oil thoroughly mixed for 2 min. Then the CCl4-corn oil solution was administered subcutaneously at a dose of 0.1 mL/kg of body weight, twice per week and continued for 6 weeks, in order to induce the liver fibrosis.
The animals were randomly divided into 5 groups: (1) Control group (normal rats), in which 3 rats were injected subcutaneously with corn oil with no CCl4. (2) CCl4 control group, in which 5 rats were administered 2% CCl4 without adding baicalin. (3) CCl4 + low dose baicalin group, in which 3 rats were administered with 2% CCl4 and baicalin (100 mg/kg). (4) CCl4 + intermediate dose baicalin group, in which 3 rats were administered with 2% CCl4 and baicalin mixture (200 mg/kg) (5) CCl4 + high dose baicalin group, in which 3 rats were administered with 2% CCl4 and baicalin (400 mg/kg).
At the end of six weeks, animals were scarified. The blood samples were obtained from orbital veins. The serum samples were collected by centrifugation in a Sorvall RC centrifuge (Thermo Scientific, Germany) of the whole blood at 4000 rpm for 10 min. The samples were stored frozen at −80°C until further use. The livers were immediately removed and were fixed in 10% formalin for histological analysis.
Liver index, kidney index, and spleen index
Relative weights of liver, kidney and spleen were represented as the percentage of the total body weight in gram.
Biochemical metabolic parameters
The activities of ALT and AST were measured by spectrophotometry using commercial kits, according to the manufacturer’s instructions. The levels of LN, HA, PC III were obtained by radio-immunoassay kits, according to the manufacturer’s instructions. TGF-beta1 was determined by the rat ELISA kit.
Evaluation of oxidative stress and antioxidant status
The tissue homogenates of livers were used to determine SOD, MDS, and GDH-PX levels, according to the manufacturer’s instructions.
Liver collage concentration was determined by measuring the level of hydroxyproline, according to the method published previously (Sun et al., 2007).
Livers were isolated from rats and the tissues were fixed using 10% formalin for 24 h. The fixed tissues were then dehydrated and immersed in paraffin solution, followed by cutting into small sections for staining. The hematoxylin-eosin staining was used to observe liver injury. The tissues were stained with Masson staining reagents to detect the deposition of collagen and stained with Sirius red staining reagents to measure the type and content of collagen. The pathological observation was evaluated by a Nikon microscope with a digital camera and polarization microscope.
Western immunoblotting analysis of liver tissue was undertaken using the following monoclonal antibodies: β-actin was purchased from Zsbio (Beijing, China). α-SMA was purchased from Abcam Inc. (Shanghai, China). JAK2, phosphor-JAK2 (or p-JAK2), phosphor-ErK (or p-ErK), Erk, Akt, phosphor-Akt (or p-Akt) and cleaved Caspase 3 were purchased from CST (Beverly, MA). Rock1, P-53, Bcl-2, and Bax were purchased from Santa Cruz biotechnology (Santa Cruz, CA). These antibodies were used to identify proteins expressed. The extracted proteins were subjected to electrophoresis on a 10% SDS-PAGE after normalization for protein content. After resolution, the protein samples were electrotransferred onto PVDF.
The membrane was blocked for 1 h in 0.1% nonfat dry milk in PBS. Membranes were incubated overnight at room temperature with the primary antibody in TBS. Membranes were washed twice for 5 min in PBS before the addition of the secondary antibody in TBS containing 0.1% nonfat dry milk for 1 h. The membranes were then washed in TBS for 5 min followed by water for 5 min. Reactive bands were identified using enhanced chemiluminescence and autoradiography according to the manufacturer’s instructions.
Quantitative data was demonstrated as the mean ± standard deviation (SD). The significance of the difference vs. the CCl4 group was analyzed by a Student’s t-test. A Mann–Whitney rank sum test was used to measure the degree of histopathological liver fibrosis. The P-value less than 5% (P < 0.05) was considered to be statistically significant.
The effect of baicalin on body weight, liver, spleen, and kidney index
The roles of baicalin on the rats’ body weight, and the index of liver, spleen and kidney were evaluated, as shown in Fig. 1. The liver, kidney and spleen index were measured as the percentage of the total body weight. The results in Figs. 1B and 1C show that the body weight, the liver, kidney, and spleen index do not exhibit any statistically difference between normal and liver-fibrosis rats. The baicalin treatment does not have a significant impact on these factors.
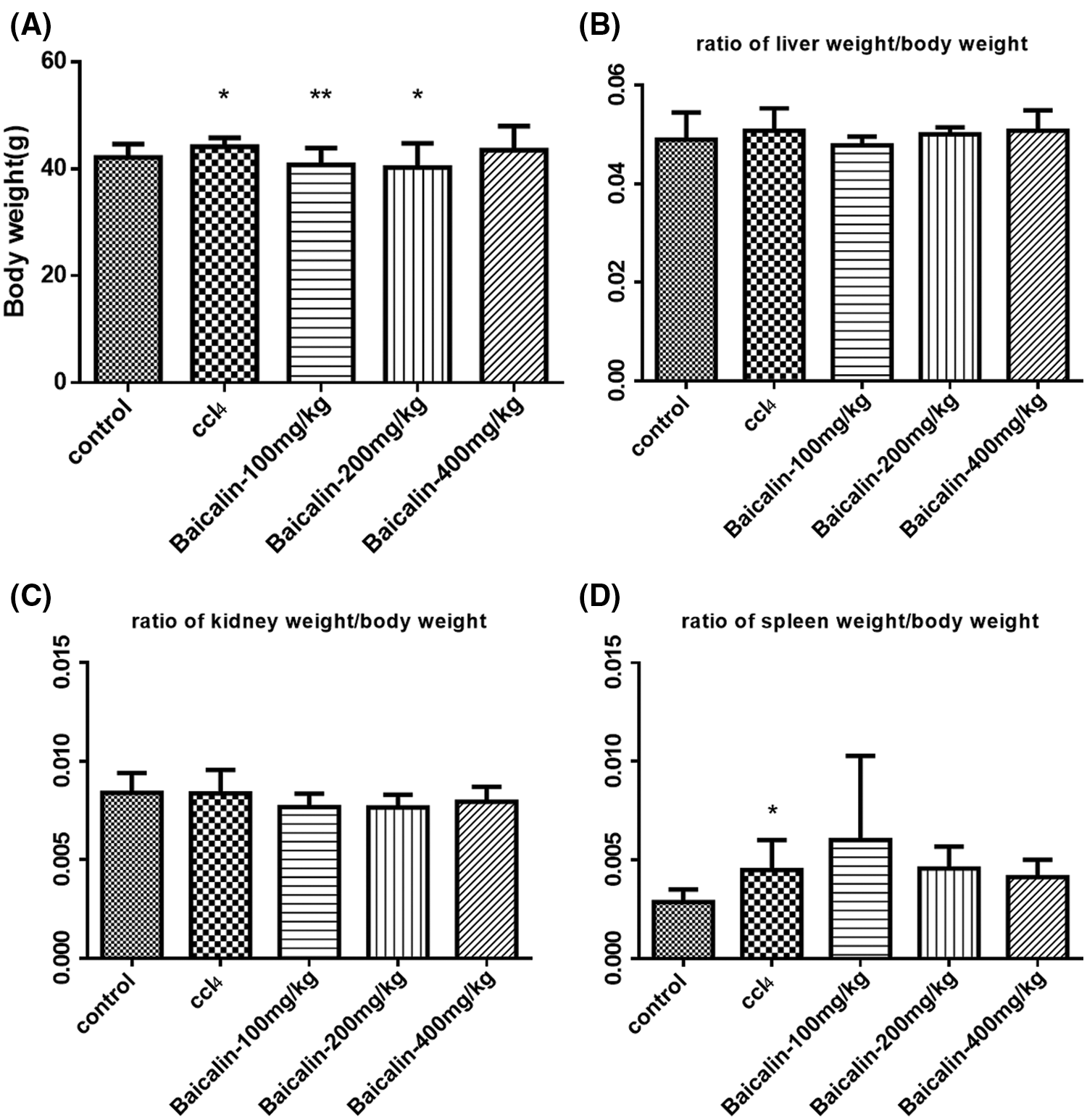
Figure 1: Body weight (A), liver (B), kidney (C) and spleen (D) index in the different groups.
Data were presented as mean ± SD. * and **represent the significant difference from the control group with P < 0.5, and P < 0.01, respectively.
The effects of baicalin on liver function
The effect of baicalin on the liver function was evaluated by measuring the therapeutic serum regimens, hyaluronic acid (HA), Procollagen type III (HPCIII), Aspartate aminotransferase (ALT), and Alanine aminotransferase (AST), on the hepatic fibrosis in CCl4 rat model (Hu et al., 2010; Ozer et al., 2010). The results are summarized in Fig. 2. The significantly higher levels of HA, HPCIII, AST and ALT in the rats’ serum in the CCl4 group were observed than that of the control group. However, HA and HPCIII levels (Figs. 2A and 2B) were effectively reduced at a higher dose of baicalin (above 100 mg/kg). For example, by treating with 400 mg/kg baicalin, the HA and HPCII levels were reduced significantly by approximately 80% and 40%, respectively. Additionally, baicalin also significantly suppressed CCl4-induced increase in AST level at relatively lower baicalin dose (Fig. 2D), while ALT level does not show a notably change upon treating baicalin (Fig. 2C). This result indicated that baicalin has a protective effect for the CCl4-induced liver injuries.
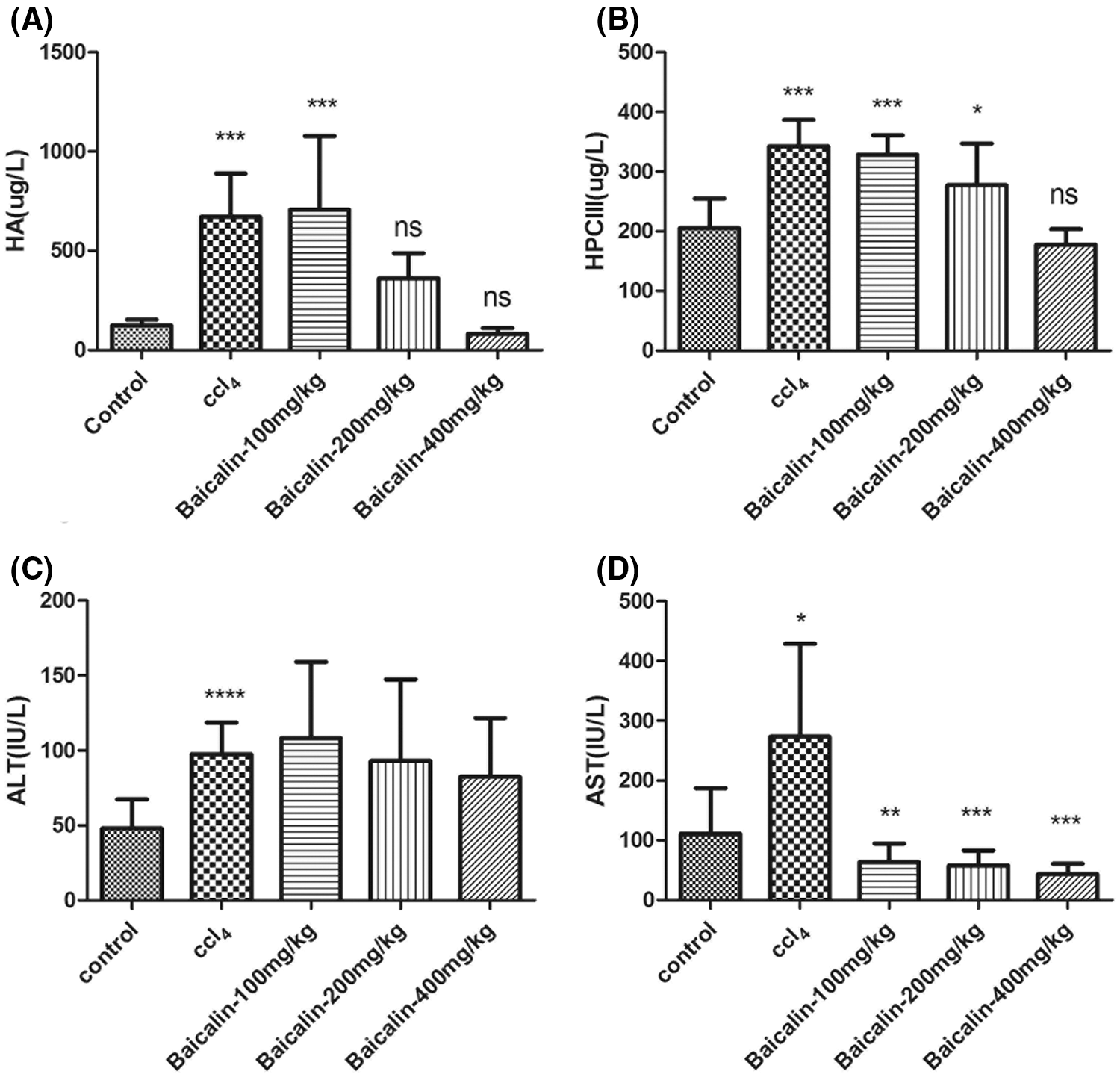
Figure 2: Hyaluronic acid (HA, A), Procollagen type III (HPCIII, B), Aspartate aminotransferase (ALT, C) and Alanine aminotransferase (AST, D) in different experimental groups.
Data were expressed as mean ± SD. *, **, ***, and **** represent the significant difference from the control group with P < 0.5, P < 0.01, P < 0.001, and P < 0.0001, respectively.
The baicalin effect of hepatic activities
Next, we investigated the effect of baicalin on the hepatic anti-fibrotic and anti-oxygenation capability by evaluating the hydroxyproline (HyP), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD) in liver. HyP is an amino acid, and it exists almost exclusively in collagens (Alcock et al., 2019; Katayama et al., 1997; Kim et al., 2009). Measurement of HyP in fibrotic tissues has been used as a reliable method to study fibrosis and to investigate the potentially anti-fibrotic agent (Kim et al., 2009). As shown in Fig. 3A, the content of HyP in the livers after treating CCl4 were significantly higher than those in the normal livers, suggesting the liver fibrosis has been formed in the CCl4 treated rats. After treating different doses of baicalin, the hepatic HyP levels were markedly decreased. This finding suggests that baicalin is an effective anti-fibrotic agent in CCl4 injury rats.
Moreover, MDA, SOD, and GSH-Px are commonly used as biomarkers to evaluate the oxidative stress and the antioxidant status in liver injuries (Katayama et al., 1997; Romani et al., 1988; Sampey et al., 2003). As can be seen in Figs. 3C and 3D, GSH-Px and SOD levels are reduced in CCl4-groups, suggesting that the end-product of lipid peroxidation due to the oxygenation process is higher in CCl4 group than in the control group. As increasing the dose of baicalin treatment (Figs. 3C and 3D), the SOD and GSH-Px levels effectively increase. In contract, MDA level does not display a statistical difference among the group studied (Fig. 3B).
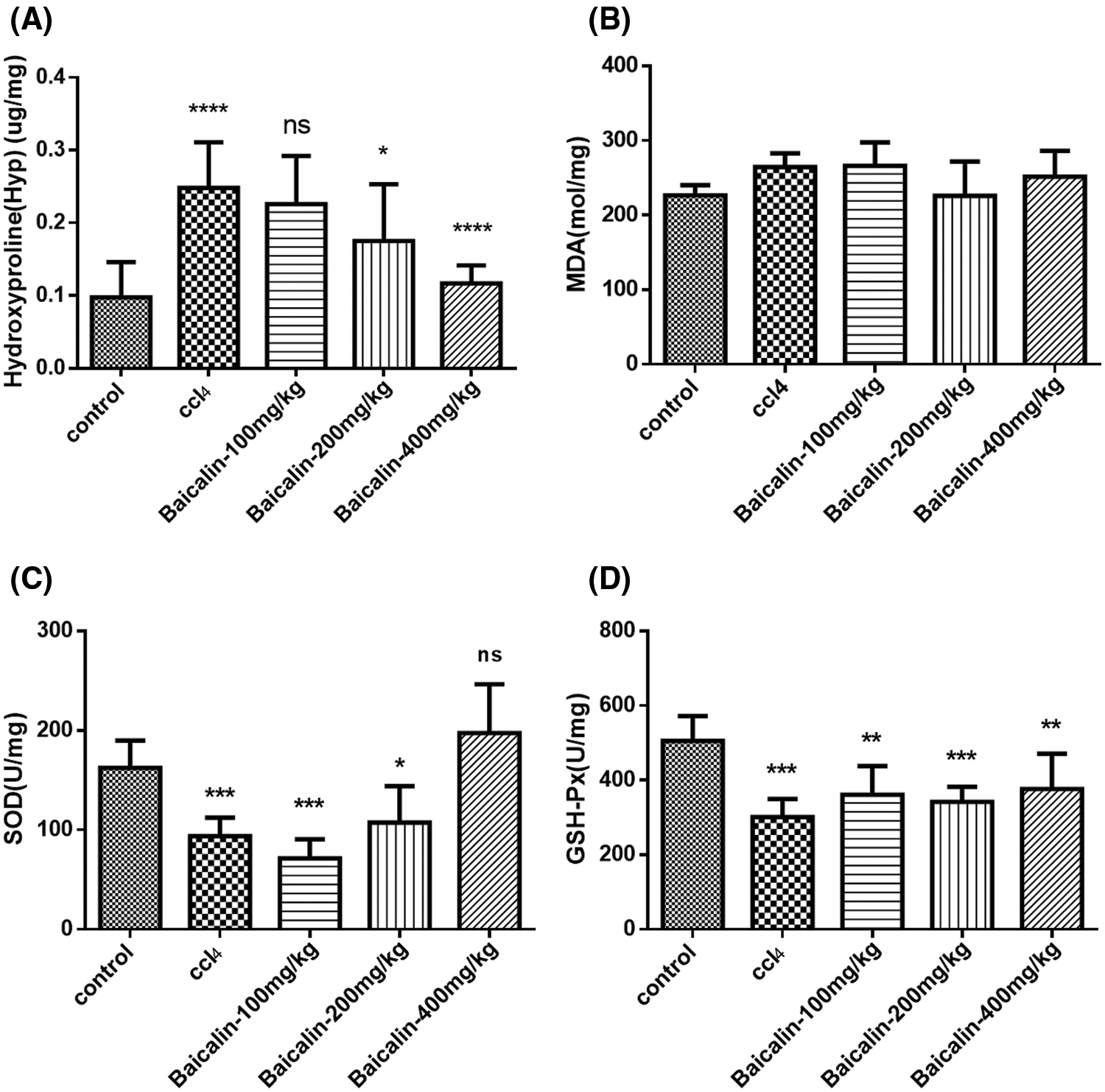
Figure 3: Hydroxyproline (HyP, A), malondialdehyde (MDA, B), superoxide dismutase (SOD, C), and glutathione peroxidase (GSH-Px, D) in experimental groups.
*, **, ***, and **** represent the significant difference from the control group with P < 0.5, P < 0.01, P < 0.001, and P < 0.0001, respectively.
The effect of protein expression level in the liver tissue
Liver fibrosis is often investigated by the accumulation of proteins in extracellular matrix, which occurs in the most types of chronic liver diseases (Chen et al., 2015; Li et al., 2019). To determine whether the protective effect of baicalin on CCl4-induced liver injury is mediated by cell apoptosis, the protein levels of AKT, phosphorylate (p-) AKT, ERK, and phosphorylate (p-) ERK in cell were detected. The results indicated that CCl4 injection enhanced the levels of AKT, p-AKT, p-JAK2, ERK, p-ERK and ROCK1, compared with the levels in control group (Figs. 4 and 5). However, as shown in Fig. 5, treating with a small dose of baicalin does not significantly affect the p-JAK2 and ERK level in CCl4-injuried rats. These levels were only suppressed significantly at higher doses of baicalin. On the other hand, a small dose of baicalin (100 mg/kg) can significantly suppress AKT, P-AKT, and ROCK1 level in the CCl4-injuried rats. It has been shown that high responsiveness of cell apoptosis is related to the increased expression of AKT and ROCK1 levels in liver cells (Liu et al., 2015b). Our findings indicate that baicalin treatment may suppress the AKT and ROCK1 signaling pathway in CCl4 induced liver injury, suggesting the potential capability of baicalin to suppress cell apoptosis.
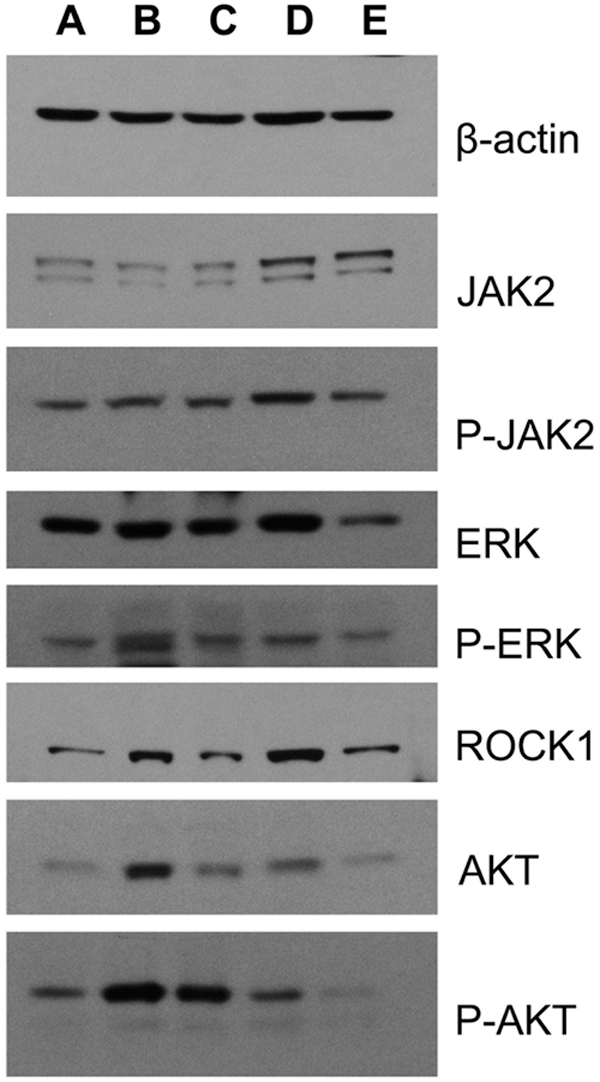
Figure 4: The effect of baicalin on β-actin, JAK2, p-ERK, ROCK1, AKT, and P-AKT in the rats’ livers.
(A) Control group, (B) CCl4 control group, (C) baicalin (100 mg/kg), (D) baicalin (200 mg/kg), (E) baicalin (400 mg/kg).
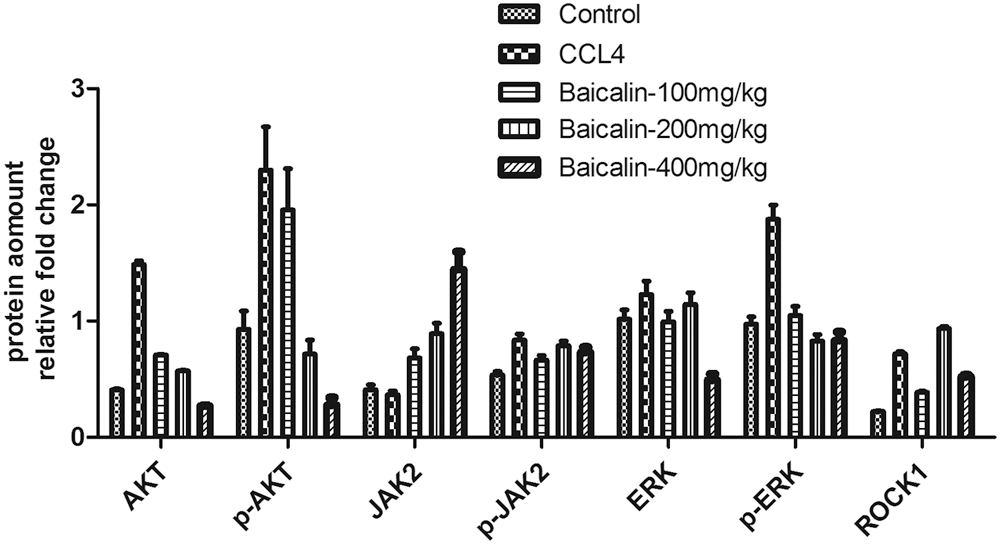
Figure 5: The effect of baicalin on AKT/ JAK2/ ERK and ROCK protein expression in the rats’ livers.
B-cell lymphoma-2 (Bcl-2) is considered to be an anti-apoptotic protein, and Bcl-2 associated X protein (Bax), mammalian target of rapamycin (mTOR), p53 and caspase-3 are known to be the biomarker of apoptosis (Cao et al., 2013; Guo et al., 2002). To better understand the role of baicalin on the cell apoptosis in liver injury, the western blot analysis was performed to detect the expression of apoptosis markers at mRNA and protein levels. As indicated in Figs. 6 and 7, the relative expression of Bax, Bcl-2, mTOR, p53, and cleaved caspase-3 was significantly enhanced after CCl4-treatment, compared with the control. However, this enhancement was markedly attenuated by treatment of baicalin. Several studies have shown that high level of Bax, Bcl-2, mTOR, and p53 associates with cell apoptosis (Gao et al., 2002, 2003; Liu et al., 2019). Thus, these results suggest that baicalin may attenuate hepatocyte apoptosis in liver injury in the dose dependence matter.
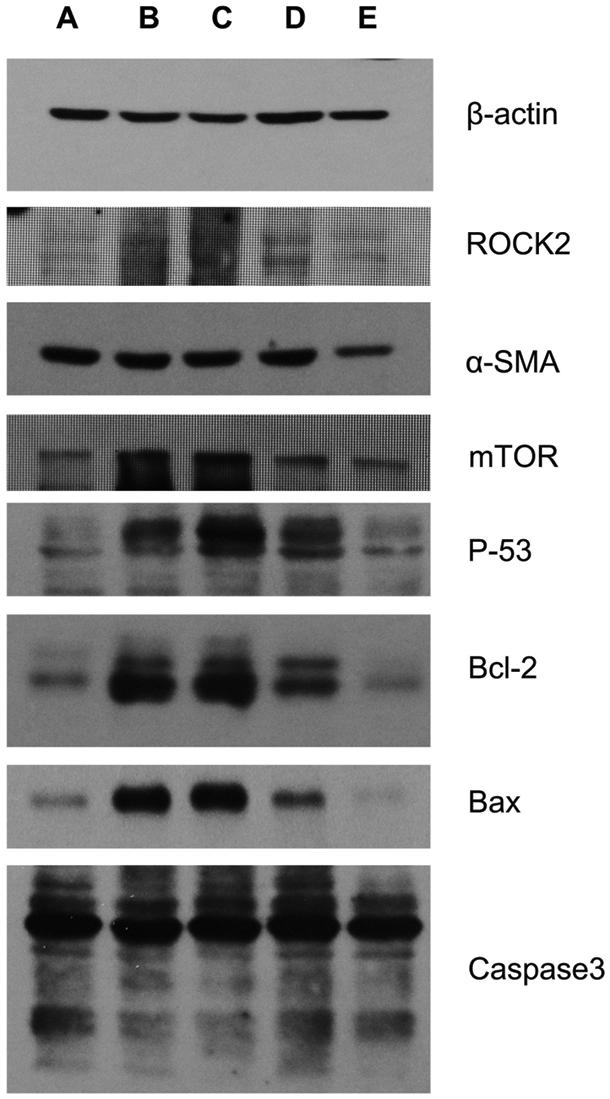
Figure 6: The effect of baicalin on β-actin, ROCK2, α-SMA, mTOR, p-53, Bcl-2, Bax in the rats’ livers mediated by cell apoptosis.
(A) Control group, (B) CCl4 control group, (C) baicalin (100 mg/kg), (D) baicalin (200 mg/kg), (E) baicalin (400 mg/kg).
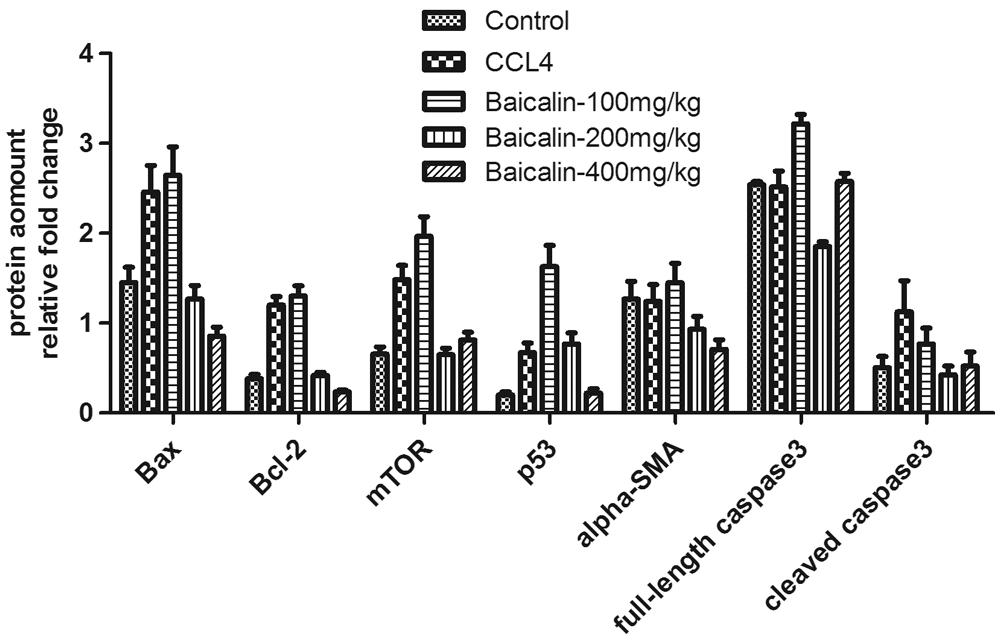
Figure 7: The effect of baicalin on the cell apoptosis in the rats’ livers.
Histological staining analysis
Histological characteristics of the liver in the control group showed a normal lobular architecture and structure (Fig. 8A). However, after treating with CCl4, the extensive hepatocellular damages were observed, as evidenced by the presence of hepatocellular degeneration and infiltration inflammatory cells (Fig. 8B). The treatment of baicalin in various dosages can attenuate the degree of injury changes (Figs. 8C–8E). These results confirmed that baicalin has the anti-fibrotic effects in CCl4–liver injury model, and the effect is dose dependent.
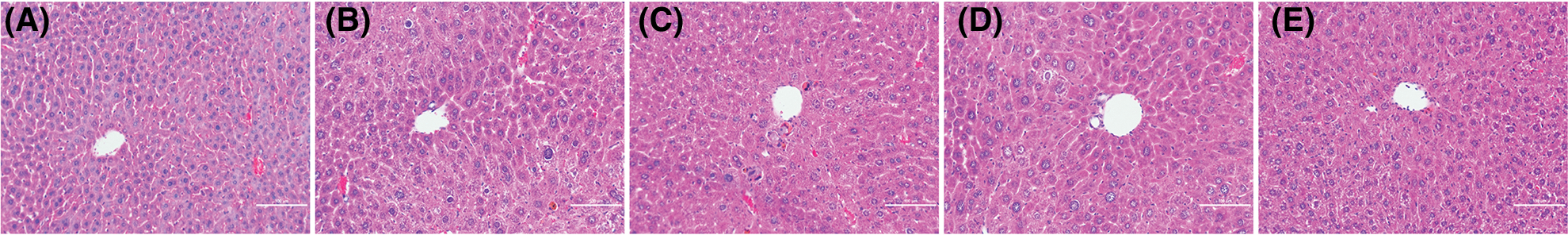
Figure 8: The representative hematoxylin-eosin staining (HE) histological photographs of (A) control group, (B) CCl4 control group, (C) CCl4 and low-dose baicalin group (100 mg/kg), (D) CCl4 and intermediate-dose baicalin group (200 mg/kg), (E) CCl4 and high-dose baicalin group (400 mg/kg).
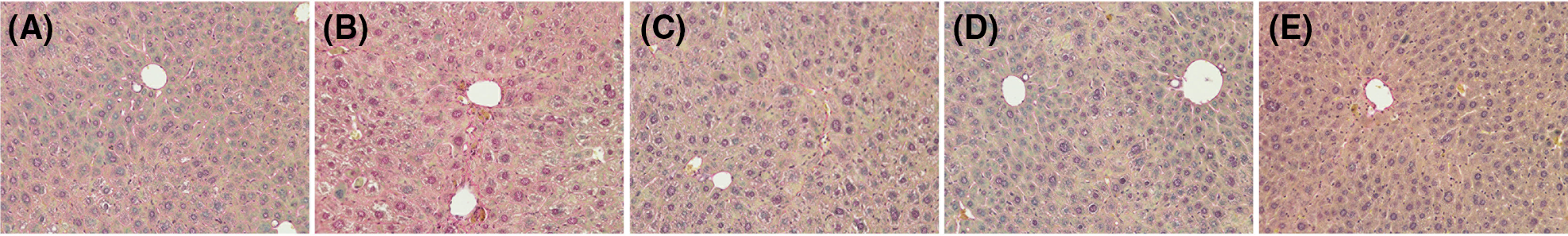
Figure 9: Representative Van Gieson’s (VG) Stain photographs x200 of (A) control group, (B) CCl4 control group, (C) CCl4 and low-dose baicalin group (100 mg/kg), (D) CCl4 and intermediate-dose baicalin group (200 mg/kg), (E) CCl4 and high-dose baicalin group (400 mg/kg).
The effect of baicalin on the changes of liver cells was presented in Fig. 9. The VG staining results indicated that CCl4 treated liver showed extensive changes in microstructures, including marked enlarged domains of portal inflammation, hepatic necrosis, and fibrotic septa (Fig. 9B). No abnormalities were observed in the control group (Fig. 9A). Baicalin treatment could significantly alleviate the degree of liver injury in the cellular level, and their cell morphologies are close to that of normal cells.
Herein, we evaluate the role of baicalin on liver fibrosis induced by CCl4 in rats. The treatment of CCl4 in rats has been commonly used as an ideal model system to study liver fibrosis (Kodavanti et al., 1989; Ritesh et al., 2015). In this work, liver fibrosis was induced successfully by subcutaneous 2% CCl4 injection for 6 weeks. The histological data has showed that the microstructure of liver lobules was destroyed and pseudolobules generated. In addition, the high hydroxyproline level in liver, as well as high HA, HPCIII, and AST in serum consistently confirmed the formation of liver fibrosis.
We showed that baicalin treatment appeared to be beneficial in this model of CCl4 injury by means of improving liver function, reducing liver index, and inhibiting the process of developed hepatic fibrosis. This protective function could associate with the increase of SOD and GSH-Px activities and the reduction of MDS level, thus suggesting antioxidant activity may be the possible mechanism of baicalin in liver fibrosis. Additionally, baicalin can suppress several biomarkers associated with cell apoptosis in CCl4 induced liver cells. It indicates that baicalin can alleviate liver cell injury by suppressing the cell apoptosis process.
The increased content of aminotransferase (AST) could be due to the leakage from the damaged hepatic cells and has been used as a marker for liver injury (Hu et al., 2010; Ozer et al., 2010). It was shown in Fig. 2D that the abnormally high AST level was noted in rats treated with CCl4. AST level is reduced by administration of baicalin. This finding provided strong evidence that the baicalin can improve the hepatic damages in rats without significant hepatotoxicity. The role of baicalin was further evaluated by the improvement in the histopathological analysis.
HPCIII and HA levels in serum are key markers of hepatic fibrogenesis. Hepatic HA level reflects the total amount of collagen, and it has been used to evaluate the extent of fibrosis (Alcock et al., 2019; Kim et al., 2009). Therefore, HPCIII and HA are important indexes to evaluate liver fibrosis. The result shown in Fig. 2 demonstrated that baicalin could significantly reduce HA and PCIII in serum, which suggested that baicalin could suppress deposition and accumulation of collagen in the liver.
The present study showed that baicalin from traditional medicine herb, Scutellaria baicalensis, has a rich bioactive profile and has preventive functions to liver fibrosis. The possible mechanism of the effects might be related to its antioxidant activities and suppression of cell apoptosis, decreasing the level of Hyp, SOD, GSH-Px, AKT/JAK2/ERK protein and inhibition of collagen synthesis. Baicalin could be a potential agent for liver fibrosis treatment, which targeting the apoptosis of hepatocytes and preventing cell oxidation. This research can provide alternative options for the development of pharmaceutical therapeutic compounds in medical practices.
Availability of Data and Materials: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: Meng-Ting Li; data collection: Kaifeng Zheng; analysis and interpretation of results: Yi-er Qiu; draft manuscript preparation: Mengting Li. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: The animal study was approved by the Institutional Animal Care and Use Committee of Shanghai Rat & Mouse Biotech Co., Ltd. (SHRM) (Approval No. SHRM-IACUC-045). It was carried out throughout the experiment following the international laboratory animal use and care guideline.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Alcock RD, Shaw GC, Tee N, Welvaert M, Burke LM (2019). Urinary hydroxyproline is only suitable as a biomarker for acute intake, up to 6 hr postingestion of collagen proteins in “free-living,” healthy, active males. International Journal of Sport Nutrition and Exercise Metabolism 29: 461–465. [Google Scholar]
Are VS, Vuppalanchi R, Vilar-Gomez E, Chalasani N (2020). Enhanced liver fibrosis score can be used to predict liver-related events in patients with nonalcoholic steatohepatitis and compensated cirrhosis. Clinical Gastroenterology and Hepatology 19: 1292–1293. [Google Scholar]
Cao J, Chen J, Wang J, Jia R, Xue W et al. (2013). Effects of fluoride on liver apoptosis and Bcl-2, Bax protein expression in freshwater teleost, Cyprinus carpio. Chemosphere 91: 1203–1212. [Google Scholar]
Cederbaum AI, Lu Y, Wu D (2009). Role of oxidative stress in alcohol-induced liver injury. Archives of Toxicology 83: 519–548. [Google Scholar]
Chen W, Han C, Zhang J, Song K, Wang Y et al. (2015). miR-150 deficiency protects against FAS-induced acute liver injury in mice through regulation of AKT. PLoS One 10: e0132734. [Google Scholar]
Dai LJ, Li HY, Guan LX, Ritchie G, Zhou JX (2009). The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Research 2: 16–25. [Google Scholar]
Dong Y, Xing Y, Sun J, Sun W, Xu Y, Quan C (2020). Baicalein alleviates liver oxidative stress and apoptosis induced by high-level glucose through the activation of the PERK/Nrf2 signaling pathway. Molecules 25: 599. [Google Scholar]
Friedman SL (2003). Liver fibrosis-from bench to bedside. Journal of Hepatology 38: S38–53. [Google Scholar]
Gao D, Sakurai K, Chen J, Ogiso T (1995). Protection by baicalein against ascorbic acid-induced lipid peroxidation of rat liver microsomes. Research Communications in Molecular Pathology and Pharmacology 90: 103–114. [Google Scholar]
Gao CJ, Sun WY, Christofidou-Solomidou M, Sawada M, Newman DK et al. (2003). PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood 102: 169–179. [Google Scholar]
Gao XS, Zhang Y, Arrazola P, Hino O, Kobayashi T et al. (2002). Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nature Cell Biology 4: 699–704. [Google Scholar]
Gebhardt R (2002). Oxidative stress, plant-derived antioxidants and liver fibrosis. Planta Medica 68: 289–296. [Google Scholar]
Guo XZ, Shao XD, Liu MP, Xu JH, Ren LN et al. (2002). Effect of bax, bcl-2 and bcl-xL on regulating apoptosis in tissues of normal liver and hepatocellular carcinoma. World Journal of Gastroenterology 8: 1059–1062. [Google Scholar]
Hu KQ, Schiff ER, Kowdley KV, Min AD, Shiffman ML et al. (2010). Histologic evidence of active liver injury in chronic hepatitis B patients with normal range or minimally elevated alanine aminotransferase levels. Journal of Clinical Gastroenterology 44: 510–516. [Google Scholar]
Huang HL, Wang YJ, Zhang QY, Liu B, Wang FY et al. (2012). Hepatoprotective effects of baicalein against CCl(4)-induced acute liver injury in mice. World Journal of Gastroenterology 18: 6605–6613. [Google Scholar]
Huang X, Mao W, Zhang T, Wang M, Wang X et al. (2018). Baicalin promotes apoptosis and inhibits proliferation and migration of hypoxia-induced pulmonary artery smooth muscle cells by up-regulating A2a receptor via the SDF-1/CXCR4 signaling pathway. BMC Complementary and Alternative Medicine 18: 330. [Google Scholar]
Huang X, Wang X, Lv Y, Xu L, Lin J et al. (2014). Protection effect of kallistatin on carbon tetrachloride-induced liver fibrosis in rats via antioxidative stress. PLoS One 9: e88498. [Google Scholar]
Katayama T, Yokoyama S, Mitomi T, Watanabe K (1997). Alterations in glutathione peroxidase activity following reperfusion injury to rat liver. Tokai Journal of Experimental and Clinical Medicine 22: 33–44. [Google Scholar]
Kim KY, Cho SE, Yu BS (2009). Intragastrically applicated CCl4-thiopental sodium enhanced lipid peroxidation and liver fibrosis (cirrhosis) in rat: malonedialdehyde as a parameter of lipid peroxidation correlated with hydroxyproline as a parameter of collagen synthesis (deposition). Toxicological Research 25: 71–78. [Google Scholar]
Kisseleva T, Brenner DA (2006). Hepatic stellate cells and the reversal of fibrosis. Journal of Gastroenterology and Hepatology 21: S84–S87. [Google Scholar]
Kodavanti PR, Joshi UM, Young RA, Meydrech EF, Mehendale HM (1989). Protection of hepatotoxic and lethal effects of CCl4 by partial hepatectomy. Toxicologic Pathology 17: 494–505. [Google Scholar]
Li S, Yi Z, Deng M, Scott MJ, Yang C et al. (2019). TSLP protects against liver I/R injury via activation of the PI3K/Akt pathway. JCI Insight 4: e129013. [Google Scholar]
Lin L, Zhou F, Shen S, Zhang T (2018). Fighting liver fibrosis with naturally occurring antioxidants. Planta Medica 84: 1318–1333. [Google Scholar]
Liu A, Huang L, Fan H, Fang H, Yang Y et al. (2015a). Baicalein pretreatment protects against liver ischemia/reperfusion injury via inhibition of NF-κB pathway in mice. International Immunopharmacology 24: 72–79. [Google Scholar]
Liu A, Wang W, Fang H, Yang Y, Jiang X et al. (2015b). Baicalein protects against polymicrobial sepsis-induced liver injury via inhibition of inflammation and apoptosis in mice. European Journal of Pharmacology 748: 45–53. [Google Scholar]
Liu Y, Jia L, Min D, Xu Y, Zhu J, Sun Z (2019). Baicalin inhibits proliferation and promotes apoptosis of vascular smooth muscle cells by regulating the MEG3/p53 pathway following treatment with oxLDL. International Journal of Molecular Medicine 43: 901–913. [Google Scholar]
Mccay PB, Lai EK, Poyer JL, Dubose CM, Janzen EG (1984). Oxygen- and carbon-centered free radical formation during carbon tetrachloride metabolism. Observation of lipid radicals in vivo and in vitro. Journal of Biological Chemistry 259: 2135–2143. [Google Scholar]
Ozer JS, Chetty R, Kenna G, Palandra J, Zhang Y et al. (2010). Enhancing the utility of alanine aminotransferase as a reference standard biomarker for drug-induced liver injury. Regulatory Toxicology and Pharmacology 56: 237–246. [Google Scholar]
Poli G (2000). Pathogenesis of liver fibrosis: Role of oxidative stress. Molecular Aspects of Medicine 21: 49–98. [Google Scholar]
Ritesh KR, Suganya A, Dileepkumar HV, Rajashekar Y, Shivanandappa T (2015). A single acute hepatotoxic dose of CCl4 causes oxidative stress in the rat brain. Toxicology Reports 2: 891–895. [Google Scholar]
Romani F, Vertemati M, Frangi M, Aseni P, Monti R et al. (1988). Effect of superoxide dismutase on liver ischemia-reperfusion injury in the rat: A biochemical monitoring. European Surgical Research 20: 335–340. [Google Scholar]
Sampey BP, Korourian S, Ronis MJ, Badger TM, Petersen DR (2003). Immunohistochemical characterization of hepatic malondialdehyde and 4-hydroxynonenal modified proteins during early stages of ethanol-induced liver injury. Alcoholism: Clinical and Experimental Research 27: 1015–1022. [Google Scholar]
Sastre J, Serviddio G, Pereda J, Minana JB, Arduini A et al. (2007). Mitochondrial function in liver disease. Frontiers in Bioscience 12: 1200–1209. [Google Scholar]
Sun WY, Wang L, Liu H, Li X, Wei W (2012). A standardized extract from Paeonia lactiflora and Astragalus membranaceus attenuates liver fibrosis induced by porcine serum in rats. International Journal of Molecular Medicine 29: 491–498. [Google Scholar]
Sun WY, Wei W, Wu L, Gui SY, Wang H (2007). Effects and mechanisms of extract from Paeonia lactiflora and Astragalus membranaceus on liver fibrosis induced by carbon tetrachloride in rats. Journal of Ethnopharmacology 112: 514–523. [Google Scholar]
Toledano MB, Mukherjee SK, Howell J, Westaby D, Khan SA et al. (2019). The emerging burden of liver disease in cystic fibrosis patients: A UK nationwide study. PLoS One 14: e0212779. [Google Scholar]
Wu R, Murali R, Kabe Y, French SW, Chiang YM et al. (2018). Baicalein targets GTPase-mediated autophagy to eliminate liver tumor-initiating stem cell-like cells resistant to mTORC1 inhibition. Hepatology 68: 1726–1740. [Google Scholar]
Yin H, Huang L, Ouyang T, Chen L (2018). Baicalein improves liver inflammation in diabetic db/db mice by regulating HMGB1/TLR4/NF-κB signaling pathway. International Immunopharmacology 55: 55–62. [Google Scholar]
Yu Z, Li Q, Wang Y, Li P (2020). A potent protective effect of baicalein on liver injury by regulating mitochondria-related apoptosis. Apoptosis 25: 412–425. [Google Scholar]
Zhou HC, Wang H, Shi K, Li JM, Zong Y, Du R (2018). Hepatoprotective effect of baicalein against acetaminophen-induced acute liver injury in mice. Molecules 24: 131. [Google Scholar]
Zimmermann A, Darstein F, Hoppe-Lotichius M, Toenges G, Lautem A et al. (2019). Cirrhosis risk score of the donor organ predicts early fibrosis progression after liver transplantation. Journal of Gastrointestinal and Liver Diseases 28: 53–61. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |