DOI:10.32604/biocell.2022.016934

| BIOCELL DOI:10.32604/biocell.2022.016934 |  |
| Article |
IR780 loaded hollow MnO2 nanoparticles for dual-mode imaging and enhanced photodynamic therapy of oral squamous cell carcinoma
1Department of Oral and Maxillofacial Surgery, Stomatological Hospital Affiliated to Chongqing Medical University, Chongqing, 401147, China
2Chongqing Municipal Key Laboratory of Oral Biomedical Engineering of Higher Education, Chongqing Medical University, Chongqing, 401147, China
3Chongqing Key Laboratory of Ultrasound Molecular Imaging, Chongqing Medical University, Chongqing, 400010, China
*Address correspondence to: Wei Pan, 2018110849@stu.cqmu.edu.cn; Lihua Qiu, 500082@hospital.qmu.edu.cn
Received: 11 April 2021; Accepted: 07 June 2021
Abstract: Photodynamic therapy (PDT) has emerged as a novel therapeutic modality for cancer treatment, but its therapeutic efficacy is severely limited by the hypoxic tumor microenvironment (TME). Here we designed an innovative multifunctional nano-platform which consists of a hollow MnO2 shell and internal photosensitizer IR780. It is not only used for multimodal imaging of oral squamous cell carcinoma (OSCC), but also for adjustment hypoxic TME to enhance cancer treatment. Hollow MnO2 can promote decomposition of tumor endogenous H2O2 to relieve tumor hypoxia, thereby enhancing the effect of photodynamic therapy. Photosensitizer IR780 generates singlet oxygen under laser irradiation to kill tumor cells, playing photodynamic effect, can also act as the contrast agents for photoacoustic and fluorescence multiple imaging, providing potential imaging capability for cancer therapeutic guidance and monitoring. Our research results in this article show that HMnO2-IR780 nanocomposite exhibits good biocompatibility and nontoxicity, strong PA/FL imaging contrast, excellent oxygen production capacity and outstanding photodynamic therapy effect. This finding provides a new idea for multimodal imaging-guided nanotherapy for OSCC.
Keywords: Endogenous oxygen generation; Tumor hypoxia; Multimodal imaging; Photodynamic therapy; Oral squamous cell carcinoma
Oral squamous cell carcinoma (OSCC) is one of the most common malignant tumors in the oral and maxillofacial region, comprising 90% of all oral tumors (Li et al., 2020; Ren et al., 2017). It is reported that the global 5-year survival rate of patients with OSCC is less than 60%, due to local recurrence and cervical lymph node metastasis (Shi et al., 2018; Wang et al., 2019; Zhao et al., 2015). Surgery, post-operative adjuvant radiotherapy and chemotherapy are currently the primary clinical treatments for OSCC (Wang et al., 2020b; Xiong et al., 2019; Zhu et al., 2019). However, chemotherapeutic drugs are mostly hydrophobic medicine, with fast blood clearance and lack of targeting to the tumor site, resulting in poor therapeutic effect on OSCC (Zhao et al., 2015). Furthermore, drug resistance caused by long-term chemotherapy will also lead to bad therapeutic efficacy and further lead to treatment failure (Fan et al., 2020). Moreover, patients with OSCC are generally unable to receive ablation surgery because it will seriously damage oral structure and affect oral functions, such as speech, chewing and swallowing (Shi et al., 2018). In view of these facts, exploring more effective treatment strategies to treat OSCC at an early stage is essential to improve survival and life quality of OSCC patients.
Photodynamic therapy (PDT) has gradually gained attention as a recently developed therapy strategy for tumor treatments due to its outstanding merits, including noninvasiveness, high selectivity, and low systemic toxicity as compared to traditional chemotherapy and radiotherapy (Liu et al., 2018; Shao et al., 2020; Sun et al., 2019; Zhang et al., 2018). Due to most of the tumors of OSCC are relatively superficial, the tumor tissues are easy to receive laser irradiation, which is conducive to the application of photodynamic therapy (Li et al., 2020). Hence, it can be seen that PDT has the potential to become an effective alternative therapy for OSCC.
In the PDT process, oxygen is an indispensable substrate for causing cell death. However, the vast majority of tumors are in a hypoxic microenvironment and the oxygen consumption in the PDT process will further reduce the oxygen content of the tumor site, affecting the efficacy and prognosis of PDT, which greatly limits its application (He et al., 2018; Liu et al., 2018; Pan et al., 2019; Shao et al., 2020; Yang et al., 2019). Hence, scientists make attempts on advanced strategies to realize continuous and adequate oxygen supply in tumor tissues, to overcoming the limitations of tumor hypoxia, thereby improving the efficacy of PDT.
Thus far, various strategies have been proposed such as directly delivering oxygen into tumor sites by hemoglobin and perfluorocarbon, generating oxygen upon decomposition of chemicals (such as C3N4 and CaO2) carried by nanomaterials to the tumor site, providing endogenous oxygen by catalyzing Internal H2O2 to O2 (Wang et al., 2020c; Yang et al., 2020; Yang et al., 2018). Due to the high content of H2O2 in the tumor microenvironment (TME), among the above methods, the strategy of using nanomaterials as endogenous H2O2 catalysts to produce oxygen will show good tumor treatment efficiency (Wang et al., 2020c). In recent years, manganese dioxide (MnO2) as a unique oxygen-producing substrate, has attracted widespread attention due to its high reactivity and specificity to H2O2. MnO2NPs can function as nanozymes to generate O2 in situ by reacting with undesirable and abundantly available tumor metabolites (H2O2 and H+), meanwhile, could be decomposed to harmless water-soluble Mn2+ ions that can be rapidly excreted by hepatic and renal systems, avoiding accumulation of the metal oxide commonly found in other metal-based nanoparticle (NP) systems in the body, leading to the low long-term toxicity and good biosafety. In addition, Mn2+ ions can also significantly enhance the contrast of T1 magnetic resonance (MR) imaging, so it can be used as an effective magnetic resonance imaging (MRI) agent for tumor-specific imaging and diagnosis (Cao et al., 2020; Wang et al., 2020a; Yang et al., 2017; Zhu et al., 2020a; Zhu et al., 2020b).
IR780 iodide is a lipophilic dye with maximum absorption at 780 nm, which upon near infrared (NIR) light irradiation can effectively convert light to reactive oxygen species (ROS), thus being a potential agent for application in photodynamic therapy to kill cancer cells (Alves et al., 2018). Compared to indocyanine green (ICG), which had been reported as a widely used photosensitizer, IR780 exhibited better photostability in circulation, higher fluorescence intensity and higher 1O2 quantum yield (Liu et al., 2018; Zhang et al., 2018). Furthermore, it has been reported that IR780 have mitochondria active targeting activity, which could preferentially accumulate at intracellular mitochondria without additional chemical conjugation of target ligands. Mitochondrion, as an indispensable cellular organelle, plays key roles in cell aerobic respiration. Mitochondrial-targeted PDT agents can destroy the biological functions of organelles, not only can inhibit cell respiration in cancer cells, thereby reducing oxygen consumption, but can also cause tumor cell death. Thence, mitochondrial targeting is considered one of the effective strategies to improve the efficacy of photodynamic therapy (Yang et al., 2019; Yang et al., 2020; Zhang et al., 2018). More importantly, due to IR780 has excellent Photoacoustic (PA) imaging and NIR fluorescence imaging (FL) ability, research on nanosystems based on IR780 for cancer diagnosis and guiding tumor treatment, has become a hotspot. Constructing a nanoprobe based on IR780 can combine PA and FL imaging to form a multi-modal imaging platform, which can provide complementary advantages over single imaging methods, thereby helping to guide and monitor the photodynamic therapy process and results (Luo et al., 2020; Qiao et al., 2020; Sheng et al., 2018).
In this study, we loaded a photosensitizer IR780 into hollow mesoporous MnO2 (HMnO2) nanoshells, we for the first time designed and successfully synthesized HMnO2-IR780 nanoparticles to develop a simple, intelligent platform for TME-responsive generation of oxygen to overcome tumor hypoxia, so as to achieve enhanced photodynamic therapy under the guidance of PA imaging and FL imaging. In this system, silica nanoparticles were used as hard template which mesoporous MnO2 grew on the surface, and then the silica nanoparticles were removed by gentle etching to obtain hollow mesoporous MnO2 shells. Then Photosensitizers IR780 can be loaded into this hollow mesoporous HMnO2 nano-platform with high loading capacities (HMnO2-IR780). The HMnO2 shell not only promotes loading and transportation photosensitizers but also can effectively catalyze H2O2 into O2 which subsequently receives the energy transferred by IR780 from lasers to produce ROS for PDT. Owing to the superior photoluminescence and photothermal-conversion capability of IR780, this nanoparticle can also act as a PA and FL imaging contrast agent. We have verified the ability of HMnO2-IR780 to produce oxygen and ROS, as well as the PA and FL imaging effects in vitro. To evaluate the potential of HMnO2-IR780 NPs as NIR-triggered PDT treatment systems, in vitro cellular localization and phototoxicity effects of MnO2-based nanoplatform were also detected. As far as we know, it is the first time to combine hollow mesoporous HMnO2 structure with the photosensitizer IR780 for dual-mode imaging guided enhanced photodynamic therapy.
TritonX-100, tetraethyl orthosilicate (TEOS), (3-aminopropyl) triethoxysilane (APTES), cycloethane, n-hexanol, ammonia, anhydrous ethanol, and sodium carbonate (Na2CO3) were purchased from Ron Reagent Co., Ltd. (Shanghai, China) Potassium permanganate (KMnO4) were obtained from Sinopharm Chemical Reagent Co., Ltd. (China). IR780 iodide were purchased from Sigma-Aldrich. SOSG reagent was purchased from Invitrogen (Thermo Fisher Scientific).1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiI) and 2-(4-Amidinophenyl)-6-indolecarbamidinedihydrochloride (DAPI) were bought from Beyotime Technology. Calcein-AM/PI Double Staining Kit were obtained from Dojindo Molecular Technologies (China). Deionized water was obtained from a Millipore water purification system. All other reagents were of analytical grade and used as received without further purification.
The HMnO2 nanoparticles were synthesized following the previously reported method. First, Solid silica nanoparticles (sSiO2) were synthesized through inverse microemulsion method. Triton X-100 (53 mL), cyclohexane (225 mL) and n-Hexanol (54 mL) were added into a 500 mL flask under uniform stirring. Then, ammonia (7.5 mL) and H2O (10 mL) were immediately added and the mixed solution were stirred for 1 h. After that, TEOS (5 mL) and APTES (1 mL) were mixed and added to the above solution. The mixture was stirred for 24 h at the room temperature. sSiO2 nanoparticles were collected by centrifugation (12000 rpm, 10 min) and washed three times with ethanol and water. The obtained sSiO2 nanoparticles were dispersed in ultrapure water (100 mg, 1 mg/mL). Under ultrasonication, KMnO4 aqueous solution (10 mg/mL, 75 mL) were dropwise added into the above solution, and then the reaction was kept stirring for 6 h (Gao et al., 2019b). The as-prepared mesoporous MnO2-coated sSiO2 were dissolved in 2M Na2CO3 aqueous solution at 60°C for 12 h. The obtained hollow mesoporous MnO2 nanoshells (H-MnO2) were centrifuged and washed with water several times. The photosensitizer IR780 (ethanolic solution 5 mg/mL) was loaded into HMnO2 nanoparticles (50 mg), which was sonicated using an ultrasonic probe (Sonics & Materials Inc., USA) at a power of 100 W (5 s on and 5 s off) and for another 4 min at a power of 60 W (5 s on and 5 s off) in an ice bath. The HMnO2-IR780 powder were centrifuged and washed three times with ethanol and water. Finally, the prepared HMnO2-IR780 NPs were stored at 4°C for later use.
The morphological characteristics of HMnO2-IR780 NPs was observed by transmission electron microscope (TEM, JEM-2100F, Japan) and light microscope (Olympus CKX41; Canada). The sizes and zeta potentials of nanoparticles were determined by a Malvern particle size meter (Malvern, UK). In order to verify the loading of photosensitizer IR780, we also measured the UV-vis absorption spectrum of each component of HMnO2-IR780 NPs. The UV-vis absorption spectrum of HMnO2, IR780, HMnO2-IR780 was recorded respectively by UV-vis-NIR spectrophotometer (UV-3600, Shimadzu, Japan).
Evaluation of the generation of oxygen
Water, IR780, and HMnO2-IR780 (fixed the concentration of IR780 with 5 μg/mL) were added into 3% H2O2 solution (4mL), and then stood still for 3 min to observe the bubble formation of each sample (Zhu et al., 2020a). In order to further quantitatively verify the ability of HMnO2-IR780 nanoparticles as catalysts to trigger the decomposition of H2O2 to produce oxygen, a portable dissolved oxygen meter (YSI,550A, Japan) was used to monitor the dissolved oxygen in the solution. Similarly, water, IR780 and HMnO2-IR780 were added to the 3% H2O2 solution, and the oxygen concentration of each group was monitored and recorded in real time by YSI550A portable dissolved oxygen meter (Cao et al., 2020).
Detection of singlet oxygen generation
In order to further confirm that the HMnO2 nanostructures could enhance the PDT effect of IR780, the in vitro photodynamic properties of HMnO2-IR780 and IR780 were evaluated under the conditions of without 3% w/v H2O2 or 3% w/v H2O2 (Cao et al., 2020). Singlet oxygen fluorescent probe (SOSG) is a common commercial 1O2 detection kit, which was exerted to detect the production of singlet oxygen by HMnO2-IR780 and IR780 under laser irradiation. In this work, Free IR780 and HMnO2-IR780 with or without H2O2 added were incubated with SOSG (10 μM), and then irradiated under 808 nm light at 1 W/cm2 for 5 min. The generated Singlet Oxygen (SO) was measured by the recovered SOSG fluorescence under 494 nm excitation. The Spectra Max M2 multifunctional microplate reader was used to measure its fluorescence intensity (Zhang et al., 2018).
To examine the PA performance of HMnO2-IR780 NPs, an agarose gel phantom (3% agar w/v in deionized water) with a hole of 1 cm in diameter was prepared (Qiao et al., 2020). A Vevo LAZR Photoacoustic Imaging System (Visual Sonics Inc., Toronto, Canada) was used to obtain PA images. HMnO2-IR780 NPs suspension at the IR780 concentration of 2 mg/mL was used for PA imaging at different wavelengths scanning from 680 to 970 nm (interval = 5 nm) to detect the maximum absorbance (Zhang et al., 2018). Then, the PA signals of different IR780 concentrations of HMnO2-IR780 NPs (2,1, 0.5, 0.25, and 0.125 mg/mL) were measured at the excitation wavelength of 780 nm and the corresponding PA images were acquired. The quantified PA signal intensity of each image was then analyzed by Vevo LAZR software.
The fetal bovine serum (FBS) resuspension of HMnO2-IR780 NPs with different IR780 concentrations of (2,1, 0.5, 0.25 mg/mL) were prepared, and 100 μL of each concentration was placed in the hole of a 96-well plate. The Xenogen IVIS Spectrum imaging system (Perkin Elmer, USA) was used to acquire Fluorescence images (excitation wavelength 745 nm, emission wavelength 820 nm). Quantitative fluorescence and bio-luminescence imaging system was used for scanning imaging. The quantified fluorescence signal intensity of each image was then analyzed by the system’s own software.
A human tongue squamous cell carcinoma cell line (HN-6) was obtained from Dr. Chen (Chongqing Medical University), and the cells were cultured in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12, Gibco-BRL, USA) media with 10% fetal bovine serum (FBS, Australian origin, Gibco) and 1% penicillin-streptomycin and incubated under a 5% CO2 atmosphere at 37°C. The cells were sub-cultured when the cell growth reached approximately 80%.
For the in vitro cellular uptake test, HN-6 cells were seeded in laser confocal cell-culture dishes (1 × 105 cells per well) and cultured for 24 h. Then, the old media were replaced with new media without serum including HMnO2-IR780 NPs (stained with DiI, λ excitation/λ emission = 549 nm/565 nm) and incubated for various times (1, 2, and 4 h). After that, the cells were washed three times with phosphate buffer saline (PBS), fixed with 4% paraformaldehyde for 15 min, washed with PBS, and dyed with DAPI (λ excitation/λ emission = 364 nm/454 nm) for another 10 min (Odda et al., 2020). After being rinsed with PBS, the cells were observed by confocal laser scanning microscopy (CLSM) (Nikon A1, Japan).
In vitro photodynamic therapy against cancer cells
To test the photodynamic therapeutic effects of HMnO2-IR780 NPs, HN-6 cells were seeded in laser confocal cell-culture dishes (1 × 105 cells per well) for 24 h. Then these HN-6 cells received various treatments including: control, laser only, HMnO2-IR780 only, HMnO2-IR780+Laser, and IR780+Laser. The HMnO2-IR780 and IR780 were diluted according to the concentration of IR780 to 100 µg/mL. After 4 h coincubation, HN-6 cells were irradiated with an 808 nm laser at (1 W/cm2) for 5 min. Then, the living cells and dead cells were co-stained with CAM and PI solution for CLSM observation (Luo et al., 2020; Zhang et al., 2018).
All data were expressed as mean ± SD. A comparison of different groups was determined using one-way analysis of variance (ANOVA) and a significant difference was assumed at P ≤ 0.05.
Design, synthesis and characterization of HMnO2-IR780 NPs
HMnO2-IR780, a novel tumor microenvironment-responsive nano formulation, was designed for dual-modal imaging-guided photodynamic therapy with mitochondria-targeted property, oxygen self-sufficiency and enhanced PDT efficacy by NIR irradiation. These nanoparticles HMnO2-IR780 can accumulate into the tumors through the typical enhanced permeability and retention (EPR) effect, thereby exerting anti-tumor effects, improving efficacy and reducing systemic side effects (Gao et al., 2019a). After entering the tumor cells, the HMnO2 shell can react with tumor endogenous H2O2 to generate O2 in situ through the following reaction: MnO2 + H2O2 + 2H+ → Mn2+ + 2H2O + O2↑ (Pan et al., 2019; Wang et al., 2020a; Yang et al., 2017). The loaded IR780 not only function as the photosensitizers for enhanced PDT, but also act as PA and FL imaging contrast agents. In addition, IR780 can actively target cell mitochondria, which further improves the photocytotoxicity of tumor cells during PDT. Since mitochondria are the main organs of cellular respiration, they are highly sensitive to ROS.
The Fig. 1a shows the procedure for the synthesis of HMnO2-IR780NPs. Firstly, monodispersed solid silica nanoparticles (sSiO2) were synthesized as the hard template, by hydrolyzing the tetraethyl orthosilicate (TEOS). Then, by simply mixing sSiO2 with manganese permanganate (KMnO4), a uniform layer of mesoporous MnO2 was grown on the surface of freshly prepared silica nanoparticles. And KMnO4 can be reduced by unreacted organosilicon existing on those previously synthesized silica nanoparticles. The hollow mesoporous MnO2 (H-MnO2) nanoshells were acquired after incubating MnO2@SiO2 nanoparticles with a Na2CO3 solution to dissolve silica. At last, the loading of the photosensitizer IR780 in the hollow structure of HMnO2 was sonicated using an ultrasonic probe (Sonics & Materials Inc, USA). Using the above method, HMnO2-IR780 NPs for subsequent experiments were synthesized.
The solution of nanoparticles was dark brown when observed by naked eyes. The optical images of the HMnO2-IR780 NPs demonstrated that they were spherical, uniform in size, and well dispersed in pure water, which enabled them not to gather into a mass and adapt well to the external environment (Fig. 1b). Transmission electron microscope (TEM) images clearly showed the nanoparticles appeared round with numerous surface pores (Figs. 1c and 1d). The zeta potential absolute values of typically 30 mV suggested that the particles are very stable; 10–30 mV indicating relatively stable, and 0–10 mV represented rapid agglutination of particle instability. As measured using a Malvern particle size meter, the average zeta potential of the HMnO2-IR780 NPs was −38.9 ± 0.9 mV (Fig. 1e). Obviously, this shows that HMnO2-IR780 NPs have considerable stability, which is conducive to the later photodynamic function in vivo. The average size of NPs is crucial for its accumulation in tumors. Ultrasmall nanoparticles (<10 nm) are quickly filtered by the kidneys, resulting in a reduction in tumor site accumulation and retention, while microparticles (380–780 nm) cannot pass through the endothelium of tumor tissue blood vessels (Liu et al., 2018). As shown in Fig. 1f, the mean diameter of the HMnO2-IR780 NPs was 242.7 ± 22.2 nm, which was suitable size for achieving tumor accumulation by the EPR effect. UV–vis–NIR spectrum (Fig. 1g) showed that HMnO2-IR780 was featured with the characteristic absorption of IR780 at 780 nm, while pure HMnO2 exhibited no such a characteristic absorption peak, suggesting the successful encapsulation of IR780 into the nanoshell.
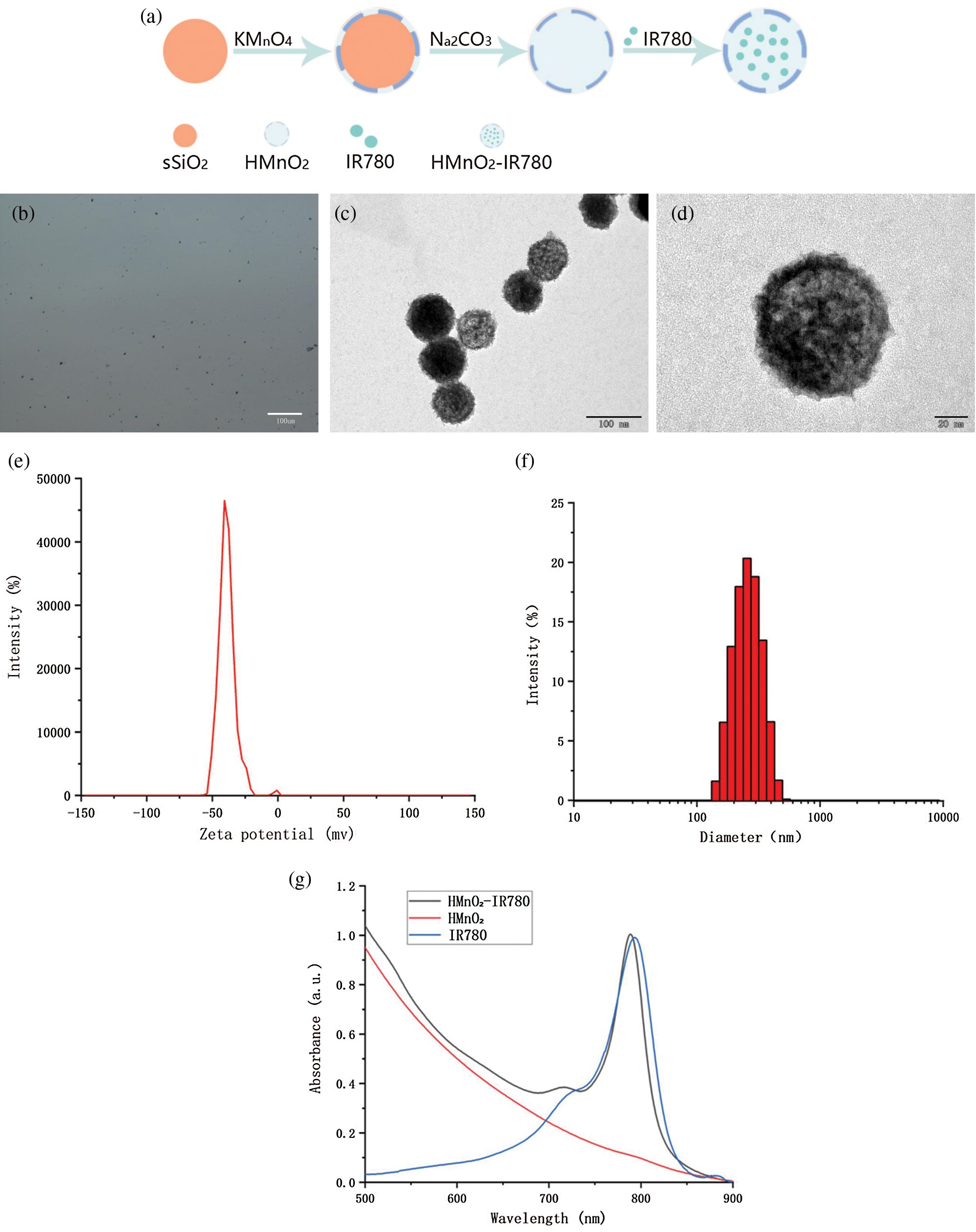
Figure 1: (a) Schematic illustration of synthetic procedure of HMnO2-IR780. (b) Optical image of HMnO2-IR780. (c) TEM image of HMnO2-IR780. (d) Enlarged TEM image (scale bar: 20 nm). (e) Zeta potential of HMnO2-IR780. (f) Size distribution of HMnO2-IR780. (g) Absorbance spectra of IR780, HMnO2, HMnO2-IR780 as recorded by UV–vis–NIR spectrophotometer.
Detection of O2 release and ROS
As found in many previous studies, the hypoxic TME is responsible for the limited PDT efficacy for treatment of solid tumors as oxygen is an important element in the process of PDT (Cai et al., 2019; Wang et al., 2018; Zhao et al., 2020c).
Therefore, after adding different concentrations of H-MnO2-IR780 to the 3% H2O2 solution, we tested the ability of H-MnO2 as a catalyst to trigger the decomposition of H2O2 by measuring the dissolved O2 using an oxygen probe, thereby indirectly verifying the H-MnO2 enhances the effect of photodynamic. As expected, as shown in Fig. 2A, after standing for 3 min, no obvious bubbles were seen in the IR780 group and the pure water group, while a large number of bubbles were seen in the HMnO2-IR780 group, which verified the catalase-like properties of MnO2. Fig. 2B more intuitively records the trend of the dissolved oxygen content of the three groups of samples over time. It is not difficult to see from the figure that ionized water or IR780 has no effect on H2O2, but HMnO2-IR780 all produce a large amount of O2 bubbles in a time-dependent manner.
In addition, in order to ensure that the O2 produced by HMnO2-IR780 catalyzed by H2O2 can be converted into 1O2 that can be used for PDT treatment, we use SOSG as a sensitive sensor through the electronic transition with 1O2 to detect the fluorescence intensity of SOSG-EP products. We tested the fluorescence intensity of ROS generated by the same concentration of HMnO2-IR780 and free IR780 under the condition of adding H2O2 and without H2O2, after receiving laser irradiation (808, 1 W/cm2, 5 min).
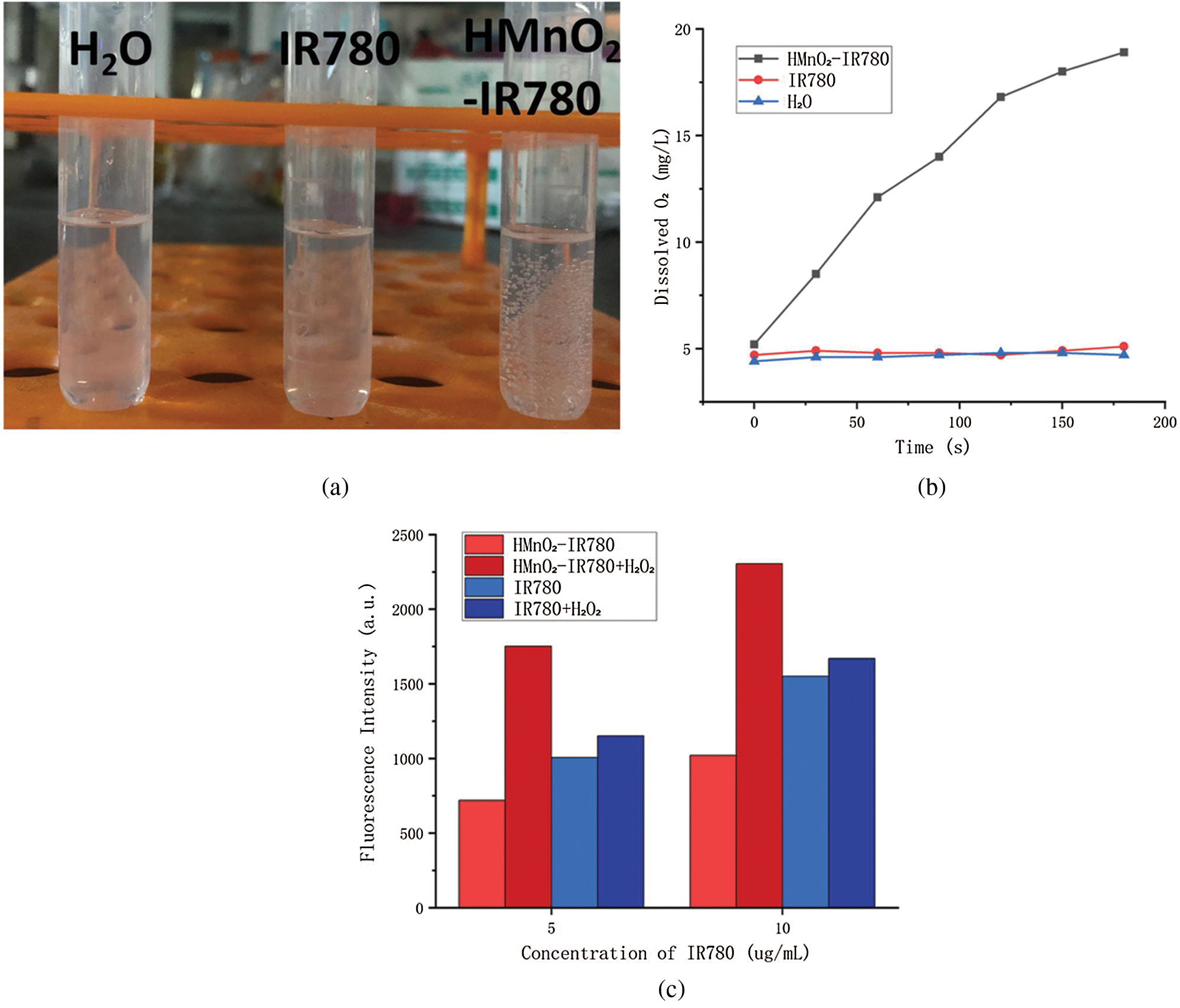
Figure 2: (a) The formation of O2 bubbles in different conditions. (b) Oxygen generation of H2O, IR780, HMnO2-IR780 measured using dissolved oxygen meter. (c) Singlet oxygen release reflected by SOSG fluorescence in different conditions.
As shown in the figure, the ROS level of HMnO2-IR780 is lower than that of free IR780 in the absence of H2O2. This may be due to the quenching effect of MnO2 on IR780 which weakens the photoactivation ability of IR780, so that the content of 1O2 is reduced. weakened. Under the condition of rich H2O2, HMnO2-IR780 group, however, due to the O2 produced by catalyzing H2O2 or protons, IR780 can convert O2 into 1O2 activated by 808 nm laser. As expected, no significant difference was observed in the amount of SO produced by free IR780 under light regardless of the addition of H2O2 (100 μM). Therefore, under the condition of low oxygen and high H2O2 content of TME, HMnO2-IR780 is expected to be a more effective PDT reagent.
Photoacoustic (PA) imaging is a rising and noninvasive imaging modality for the diagnosis of tumors, has higher sensitivity and better spatial resolution than CT imaging, holding great potential for clinical applications to visualize the accumulation of nanoprobe and to adjust the therapeutic time window and therapeutic response (Luo et al., 2020; Qiao et al., 2020; Sheng et al., 2018). IR780 iodide is a heptamethine cyanine dye, with a strong optical absorption and emission in the NIR region, is receiving an increasing attention in the area of cancer imaging. Taking into account the outstanding PA imaging capability of IR780, HMnO2-IR780 NPs were expected to be excellent contrast-enhanced agents for clinical application. Consequently, the imaging performance of the NPs was systematically evaluated in vitro. Firstly, we performed a full-spectrum scan to detect the imaging window of HMnO2-IR780NPs in PA. After scanned wavelengths from 680 nm to 950 nm at a spectrum interval of 5 nm, it has been seen that the wavelength at 780 nm was the optimal choice for HMnO2-IR780 NPs enhanced PA imaging. Therefore, the PA-imaging performance of HMnO2-IR780 NPs was evaluated at 780 nm. Fig. 3A showed the PA images of HMnO2-IR780 NPs at IR780 concentrations of 0.125, 0.25, 0.5, 1, and 2 mg/mL, which showed a concentration-dependent pattern that as the concentration of nanoparticles increased, the signal of photoacoustic imaging was also stronger. In addition, it is obvious that the signal intensity of photo-acoustic imaging was quite high when the concentration was 2 mg/mL, which verified that HMnO2-IR780 NPs have good photoacoustic imaging ability and revealed that HMnO2-IR780 NPs have the potential to become a photoacoustic contrast agent. And these results were further proofed by quantitative analysis of the corresponding signal intensity. As showed in Fig. 3B, quantitative analysis of the PA imaging signal values of different concentrations of HMnO2-IR780 NPs suggested that it is a linear growth relation.
Fluorescence imaging is considered to be one of the most promising imaging modalities for diagnostic applications in the clinic due to its able to detect the distribution of nanocontrast agents throughout the body (including tumors) in real time (Chen et al., 2019; Zhao et al., 2020b). The high NIR fluorescence intensity of IR780 in the 807-823 nm wavelength range enlightened us to further verify the performance of HMnO2-IR780 NPs as Fluorescence imaging agents. In addition, according to previous literature reports, the fluorescence intensity of IR780 in different solvents is also different, the fluorescence intensity is the highest in fetal bovine serum, and the lowest in PBS. Then, we tested the fluorescence intensity of HMnO2-IR780 NPs fetal bovine serum resuspension at different concentrations of IR780.Different concentrations of samples were added to 96-well plates. Xenogen IVIS Spectral Imaging system (Perkin Elmer, USA) was used to obtain fluorescence images and collect quantitative fluorescence signal intensity. As we can see in Fig. 4a, the fluorescence signal of the HMnO2-IR780 NPs became stronger as the NP concentration raised from 0.25 to 2 mg/mL, demonstrating that HMnO2-IR780 NP was an effective fluorescent imaging contrast agent.
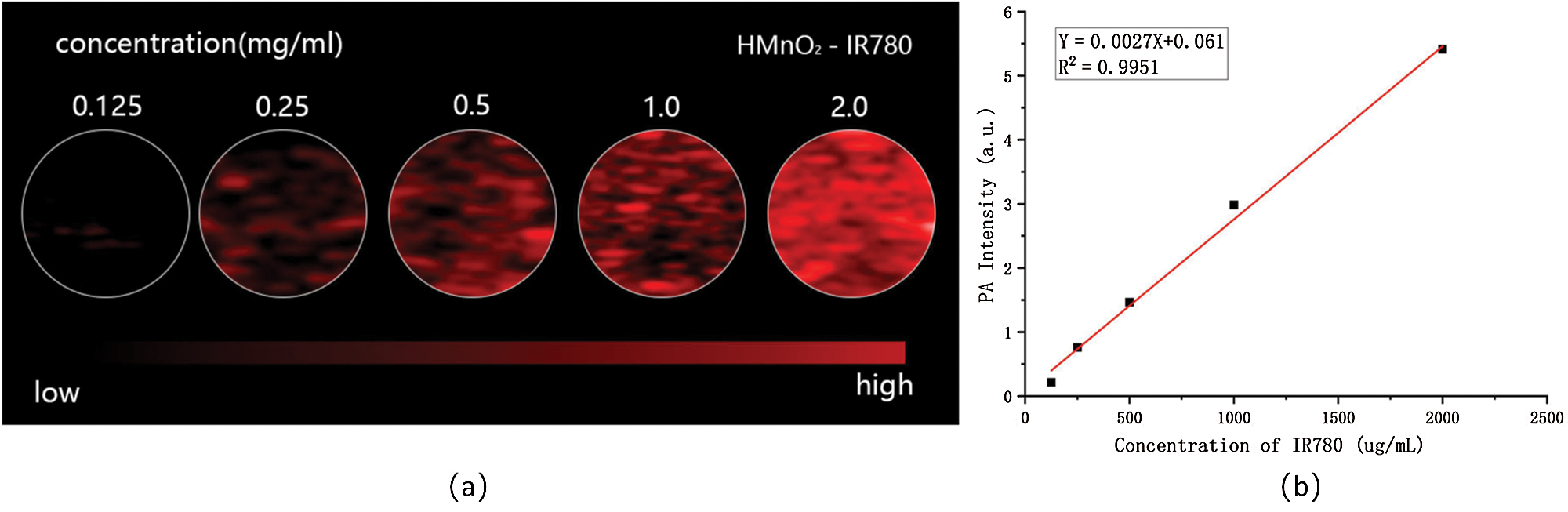
Figure 3: (a) In vitro PA contrast images of HMnO2-IR780 at different IR780 concentrations. (b) PA values of different concentrations of HMnO2-IR780 NPs.
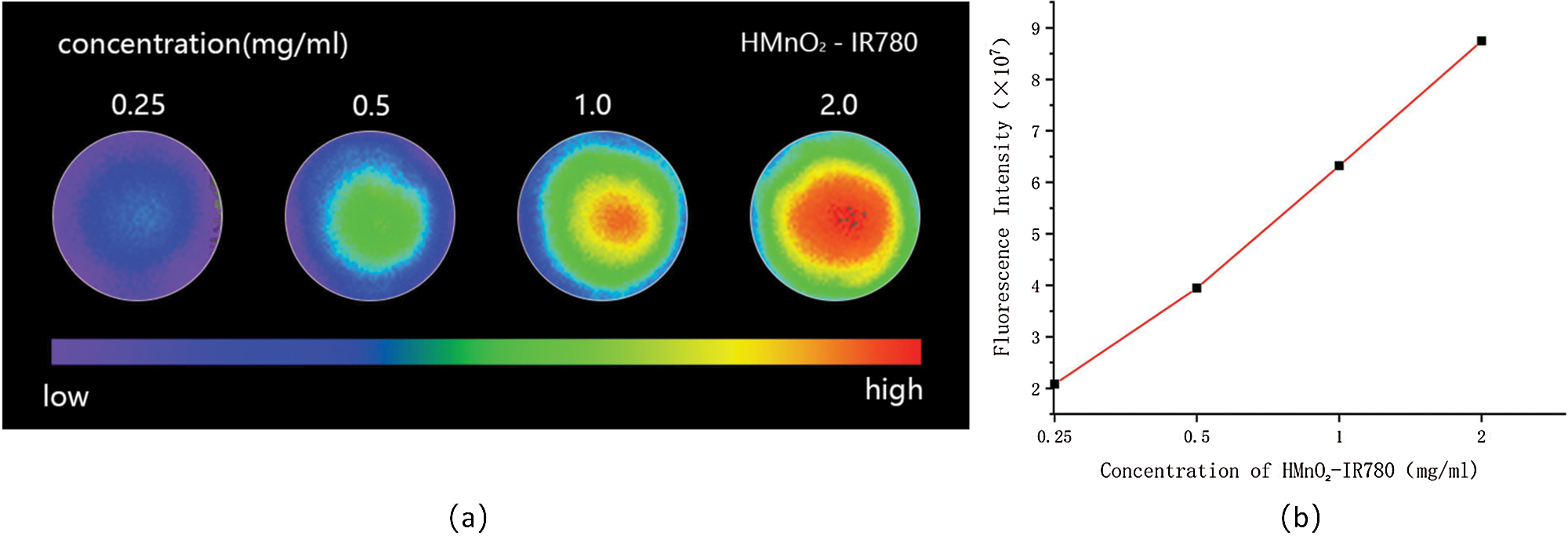
Figure 4: (a) In vitro NIR fluorescence images of HMnO2-IR780 at different IR780 concentrations. (b) Fluorescence intensities of various concentrations of HMnO2-IR780 NPs.
In summary, the above results show that HMnO2-IR780 NPs is expected to be effective in the treatment of tumors accumulate and act as agents for multimodal imaging. Although PA imaging is capable of displaying functional information of biological tis-sues, for example, it can be used to detect blood vessel distribution and oxygenation status in tumor areas, it remains inapplicable for deep tissue imaging. However, FL imaging is capable to visualize changes in chemical make-up of tissue in real time suiting for wide-field imaging. Consequently, the construction of IR780-based nanoplatform can combine PA imaging and FL imaging together to overcome the limitations of a single imaging mode and provide more accurate and valuable imaging information to adjust the treatment time window and follow-up treatment outcome monitoring.
The delivery efficiency of HMnO2-IR780 NPs depends on the cellular uptake of HMnO2-IR780 NPs. Therefore, the cellular uptake and accumulation of HMnO2-IR780 NPs in tumor tissues directly affect the treatment efficiency of PDT. The internalization of the HMnO2-based carriers in tumor cells was observed by confocal laser scanning microscopy. First, DiI-stained HMnO2-IR780 NPs was prepared. Then HN-6 cells were co-incubated with DiI-labeled samples for different times. The endocytosis of HMnO2-IR780 NPs was Intuitively observed by CLSM through the fluorescence of the DAPI (blue fluorescence) and DiI (red fluorescence). As shown in Fig. 5, the CLSM image indicated that the cellular uptake of HMnO2-IR780 NPs increased as the culture time increased from 1 h to 4 h. In addition, as it was predicted, after 4 h coculture, evident red fluorescence of HMnO2-IR780 (labeled with DiI) was observed in HN-6 cells. According to reports, IR780 has preferential accumulation properties for tumor cells. Cell endocytosis may play a major role in the absorption and accumulation of nano-carriers.
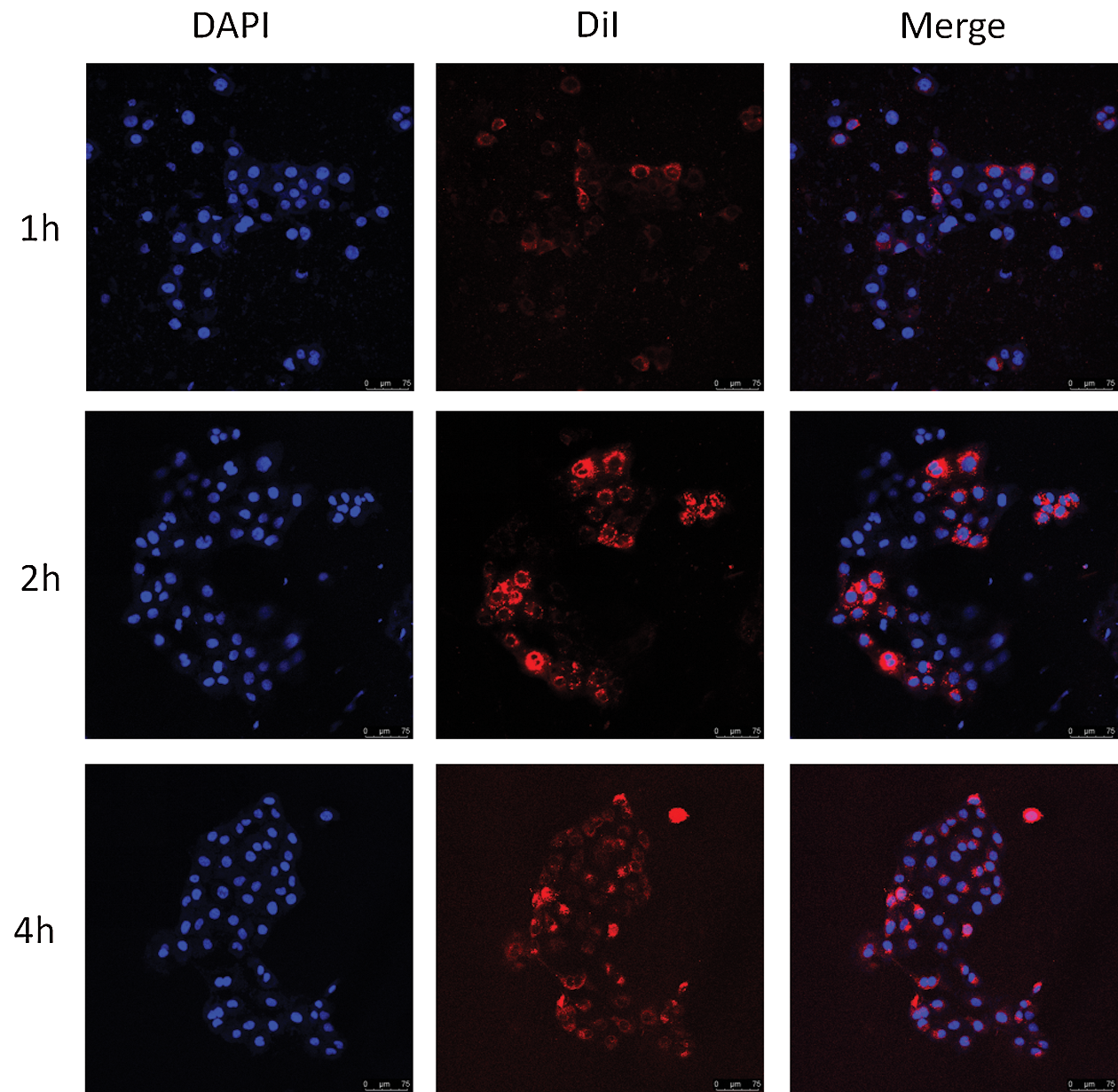
Figure 5: Confocal microscopy images (scale bar is 75 μm) of HN-6 cells co-cultured with DiI-labeled HMnO2-IR780 for different times (1, 2, and 4 h). From left column to right column were DAPI-labeled cell nucleus, DiI-labeled NPs and their merged images.
In vitro phototoxicity of HMnO2-IR780 NPs
The mechanism of photodynamic therapy is to activate photosensitizers with specific wavelength of laser light, releasing a large amount of reactive oxygen species (ROS), which can directly kill cancer cells by inducing oxidative damages to lipids, proteins and nucleic acids (Li et al., 2019; Xu et al., 2018; Zhao et al., 2020a). HMnO2-IR780 NPs have the ability to release drugs in response to TME, can up-regulate the level of ROS in tumor cells and destroy the redox cycle in tumor cells, thereby using tumor microenvironment to specifically inhibit tumor cells. This motivates us to further explore the effect and mechanism of enhanced PDT treatment at the cellular level. In order to further study the therapeutic effect of PDT, a collaborative identification test of calcein acetoxymethyl ester (calcein-AM) and propidium iodide (PI) was carried out, and the corresponding living cells and dead cells were examined by fluorescence under CLSM observation. HN-6 cells were grouped and received different treatments. From Fig. 6 we can intuitively see that green fluorescence of calcein AM and negligible red fluorescence for PI appeared in the control group, laser-only group and HMnO2-IR780 NPs only group, revealing that the 808-laser caused only minor damage and that HMnO2-IR780 NPs had excellent biocompatibility. It is worth noting that even at high concentrations as high as 100 µg/mL, without laser irradiation, HMnO2-IR780 nanoparticles still have no obvious dark toxicity. This show that HMnO2-IR780 nanoparticles are promising nanomedicines for clinical treatment, because good biocompatibility is one of the prerequisites for clinical application of nano-preparations. Moreover, both red fluorescence and green fluorescence were observed in the free IR780+Laser group, showing that photodynamic therapy mediated by photosensitizer IR780 had inhibitory effect on cancer cells to some extent.
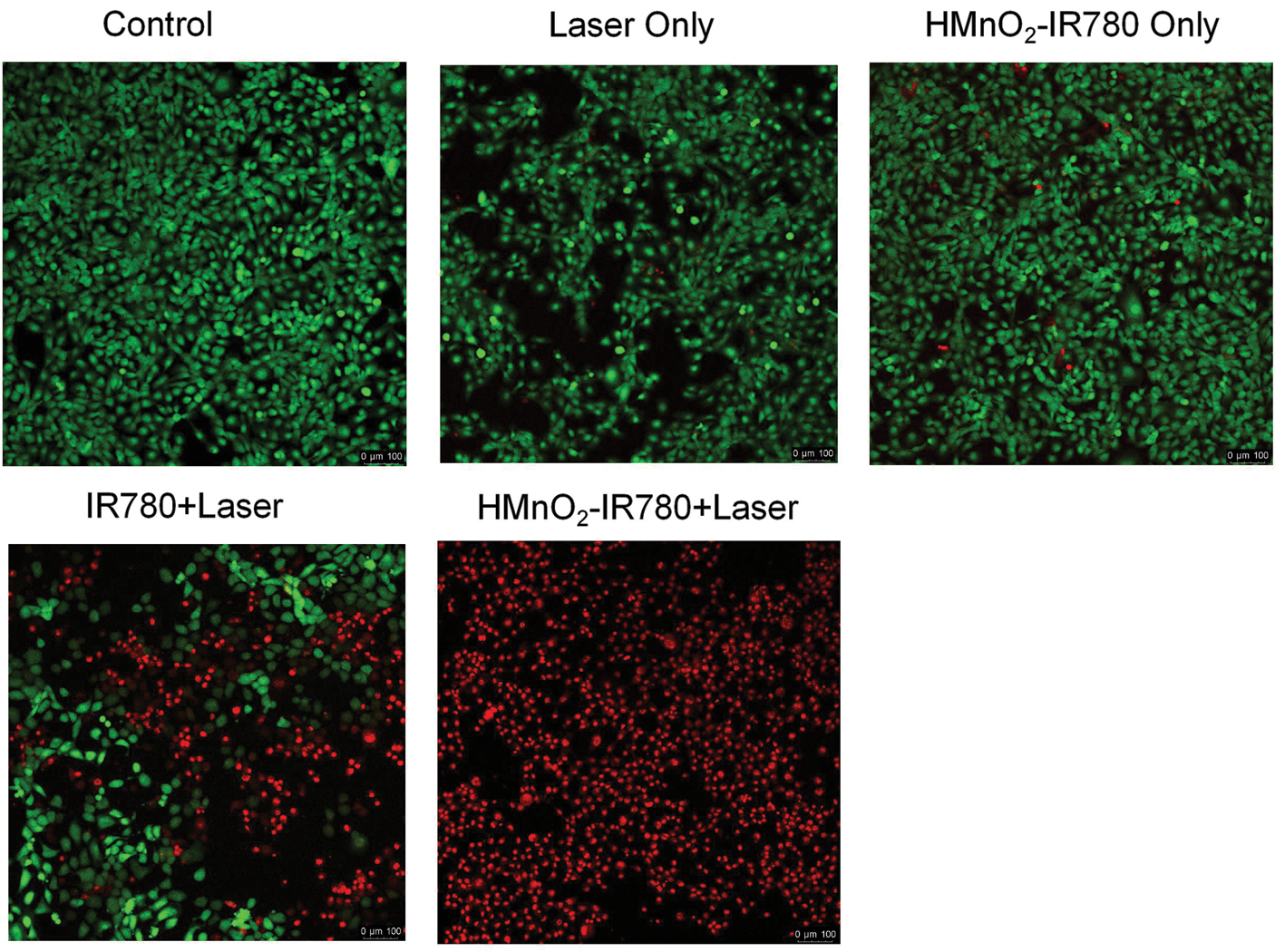
Figure 6: Confocal microscopy images (scale bar is 100 μm) of the HN-6 cells after coincubation with HMnO2-IR780 (100 µg/mL) or free IR780 (100 µg/mL) for 4 h followed by various treatments. stained with CAM (live cells are green) and PI (dead cells are red).
Notably, in the HMnO2-IR780+Laser group demonstrated potent photodynamic lethality of HMnO2-IR780 NPs under 808nm laser (1 W/cm2 5 min). In this group, almost all the cells were stained by PI (red) proving death. The fact that HMnO2-IR780 killed more cells than free IR780 may be due to the increase of IR780 uptake by IR780 encapsulated by HMnO2. In summary, these results reflected the high biocompatible and phototoxicity of HMnO2-IR780 and ensured HN-6 cells could be destroyed completely into death under laser irradiation.
In summary, we have successfully prepared a multifunctional nanoplatform consisting of a IR780-loaded hollow MnO2 for OSCC photoacoustic and fluorescent molecular imaging guidance/monitoring and oxygen-augmented photodynamic therapy. In vitro experiments confirmed that HMnO2-IR780 NPs have a high fluorescence intensity and strong PA signals, demonstrates the potential to become excellent PA/FL contrast agents. Furthermore, HMnO2-IR780 NPs not only improves the stability of IR780 and prolongs its blood circulation, but also decomposes H2O2 to produce oxygen, which enhances the effect of photodynamic therapy. In vitro cell experiments show that HMnO2-IR780 NPs can be well taken up by cells, and after laser irradiation HMnO2-IR780 NPs can effectively kill HN-6 cells through photodynamic therapy. In conclusion, this study demonstrated a new approach for self-sufficient oxygen-augmented PDT and multiple-imaging guidance/monitoring offering more inspiration to overcome tumor hypoxia and to improve the clinical outcome of OSCC treatment.
Acknowledgement: All synthesis and detection experiments were conducted within the Chongqing Key Laboratory of Ultrasound Molecular Imaging, Chongqing Medical University.
Availability of Data and Materials: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contributions: WP performed the majority of the experiments, including the preparation of nanoparticles, oxygen production experiments, imaging experiments and cell experiments, and wrote the manuscript. YH, MDH and FW analyzed and interpreted the oxygen production experimental data and revised the manuscript. LHQ designed and supervised the study. All authors read and approved the final manuscript.
Funding Statement: The present study was funded by the Chongqing Social Livelihood Science and Technology Innovation Project (Grant No. cstc2016shmszx00010), the Science and Technology Research Project of Chongqing Education Commission (Grant No. KJ1600231) and the Program for Innovation Team Building at Institutions of Higher Education in Chongqing (Grant No. CXTDG201602006).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Alves CG, Lima-Sousa R, de Melo-Diogo D, Louro RO, Correia IJ (2018). IR780 based nanomaterials for cancer imaging and photothermal, photodynamic and combinatorial therapies. International Journal of Pharmaceutics 542: 164–175. [Google Scholar]
Cai X, Xie Z, Ding B, Shao S, Liang S et al. (2019). Monodispersed copper (I)-based nano metal-organic framework as a biodegradable drug carrier with enhanced photodynamic therapy efficacy. Advanced Science 6: 1900848. [Google Scholar]
Cao W, Liu B, Xia F, Duan M, Hong Y et al. (2020). MnO2@Ce6-loaded mesenchymal stem cells as an oxygen-laden guided-missile for the enhanced photodynamic therapy on lung cancer. Nanoscale 12: 3090–3102. [Google Scholar]
Chen C, Sun J, Chen S, Liu Y, Zhu S et al. (2019). A multifunctional-targeted nanoagent for dual-mode image-guided therapeutic effects on ovarian cancer cells. International Journal of Nanomedicine 14: 753. [Google Scholar]
Fan L, Wang J, Xia C, Zhang Q, Pu Y et al. (2020). Glutathione-sensitive and folate-targeted nanoparticles loaded with paclitaxel to enhance oral squamous cell carcinoma therapy. Journal of Materials Chemistry B 8: 3113–3122. [Google Scholar]
Gao C, Lin Z, Wang D, Wu Z, Xie H, He Q (2019a). Red blood cell-mimicking micromotor for active photodynamic cancer therapy. ACS Applied Materials & Interfaces 11: 23392–23400. [Google Scholar]
Gao F, Tang Y, Liu WL, Zou MZ, Huang C et al. (2019b). Intra/extracellular lactic acid exhaustion for synergistic metabolic therapy and immunotherapy of tumors. Advanced Materials 31: 1904639. [Google Scholar]
He Z, Xiao Y, Zhang JR, Zhang P, Zhu JJ (2018). In situ formation of large pore silica-MnO2 nanocomposites with H+/H2O2 sensitivity for O2- elevated photodynamic therapy and potential MR imaging. Chemical Communications 54: 2962–2965. [Google Scholar]
Li M, Li L, Su K, Liu X, Zhang T et al. (2019). Highly effective and noninvasive near-infrared eradication of a Staphylococcus aureus biofilm on implants by a photoresponsive coating within 20 min. Advanced Science 6: 1900599. [Google Scholar]
Li Q, Zhou R, Xie Y, Li Y, Chen Y, Cai X (2020). Sulphur-doped carbon dots as a highly efficient nano-photodynamic agent against oral squamous cell carcinoma. Cell Proliferation 53: e12786. [Google Scholar]
Liu WL, Liu T, Zou MZ, Yu WY, Li CX et al. (2018). Aggressive man-made red blood cells for hypoxia-resistant photodynamic therapy. Advanced Materials 30: 1802006. [Google Scholar]
Luo Y, Qiao B, Zhang P, Yang C, Cao J et al. (2020). TME-activatable theranostic nanoplatform with ATP burning capability for tumor sensitization and synergistic therapy. Theranostics 10: 6987. [Google Scholar]
Odda AH, Li H, Kumar N, Ullah N, Khan MI et al. (2020). Polydopamine coated PB-MnO2 nanoparticles as an oxygen generator nanosystem for imaging-guided single-NIR-laser triggered synergistic photodynamic/photothermal therapy. Bioconjugate Chemistry 31: 1474–1485. [Google Scholar]
Pan W, Ge Y, Yu Z, Zhou P, Cui B et al. (2019). A cancer cell membrane-encapsulated MnO2 nanoreactor for combined photodynamic-starvation therapy. Chemical Communications 55: 5115–5118. [Google Scholar]
Qiao B, Luo Y, Cheng HB, Ren J, Cao J et al. (2020). Artificial nanotargeted cells with stable photothermal performance for multimodal imaging-guided tumor-specific therapy. ACS Nano 14: 12652–12667. [Google Scholar]
Ren S, Cheng X, Chen M, Liu C, Zhao P et al. (2017). Hypotoxic and rapidly metabolic PEG-PCL-C3-ICG nanoparticles for fluorescence-guided photothermal/photodynamic therapy against OSCC. ACS Applied Materials & Interfaces 9: 31509–31518. [Google Scholar]
Shao Y, Liu B, Di Z, Zhang G, Sun LD et al. (2020). Engineering of upconverted metal-organic frameworks for near-infrared light-triggered combinational photodynamic/chemo-/immunotherapy against hypoxic tumors. Journal of the American Chemical Society 142: 3939–3946. [Google Scholar]
Sheng D, Liu T, Deng L, Zhang L, Li X et al. (2018). Perfluorooctyl bromide & indocyanine green co-loaded nanoliposomes for enhanced multimodal imaging-guided phototherapy. Biomaterials 165: 1–13. [Google Scholar]
Shi S, Zhang L, Zhu M, Wan G, Li C et al. (2018). Reactive oxygen species-responsive nanoparticles based on PEGlated prodrug for targeted treatment of oral tongue squamous cell carcinoma by combining photodynamic therapy and chemotherapy. ACS Applied Materials & Interfaces 10: 29260–29272. [Google Scholar]
Sun Q, Bi H, Wang Z, Li C, Wang C et al. (2019). O2-generating metal-organic framework-based hydrophobic photosensitizer delivery system for enhanced photodynamic therapy. ACS Applied Materials & Interfaces 11: 36347–36358. [Google Scholar]
Wang C, Xiao Y, Zhu W, Chu J, Xu J et al. (2020a). Photosensitizer-modified MnO2 nanoparticles to enhance photodynamic treatment of abscesses and boost immune protection for treated mice. Small 16: 2000589. [Google Scholar]
Wang D, Shi R, Zhou J, Shi S, Wu H et al. (2018). Photo-enhanced singlet oxygen generation of prussian blue-based nanocatalyst for augmented photodynamic therapy. Iscience 9: 14–26. [Google Scholar]
Wang F, Wang Z, Pang L, Wan S, Qiu L (2020b). Preparation and in vitro study of stromal cell-derived factor 1-targeted Fe3O4/poly(lactic-co-glycolic acid)/perfluorohexane nanoparticles. Experimental and Therapeutic Medicine 20: 2003–2012. [Google Scholar]
Wang J, Sun J, Hu W, Wang Y, Chou T et al. (2020c). A porous Au@ Rh bimetallic core-shell nanostructure as an H2O2-driven oxygenerator to alleviate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Advanced Materials 32: 2001862. [Google Scholar]
Wang Y, Zhang W, Sun P, Cai Y, Xu W et al. (2019). A novel multimodal NIR-II nanoprobe for the detection of metastatic lymph nodes and targeting chemo-photothermal therapy in oral squamous cell carcinoma. Theranostics 9: 391. [Google Scholar]
Xiong J, Feng J, Qiu L, Gao Z, Li P et al. (2019). SDF-1-loaded PLGA nanoparticles for the targeted photoacoustic imaging and photothermal therapy of metastatic lymph nodes in tongue squamous cell carcinoma. International Journal of Pharmaceutics 554: 93–104. [Google Scholar]
Xu W, Qian J, Hou G, Wang Y, Wang J et al. (2018). PEGylated hydrazided gold nanorods for pH-triggered chemo/photodynamic/photothermal triple therapy of breast cancer. Acta Biomaterialia 82: 171–183. [Google Scholar]
Yang G, Xu L, Chao Y, Xu J, Sun X et al. (2017). Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nature Communications 8: 902. [Google Scholar]
Yang Z, Wang J, Ai S, Sun J, Mai X, Guan W (2019). Self-generating oxygen enhanced mitochondrion-targeted photodynamic therapy for tumor treatment with hypoxia scavenging. Theranostics 9: 6809. [Google Scholar]
Yang Z, Wang J, Liu S, Li X, Miao L et al. (2020). Defeating relapsed and refractory malignancies through a nano-enabled mitochondria-mediated respiratory inhibition and damage pathway. Biomaterials 229: 119580. [Google Scholar]
Yang ZL, Tian W, Wang Q, Zhao Y, Zhang YL et al. (2018). Oxygen-evolving mesoporous organosilica coated prussian blue nanoplatform for highly efficient photodynamic therapy of tumors. Advanced Science 5: 1700847. [Google Scholar]
Zhang L, Wang D, Yang K, Sheng D, Tan B et al. (2018). Mitochondria-targeted artificial Nano-RBCs for amplified synergistic cancer phototherapy by a single NIR irradiation. Advanced Science 5: 1800049. [Google Scholar]
Zhao H, Feng H, Liu D, Liu J, Ji N et al. (2015). Self-assembling monomeric nucleoside molecular nanoparticles loaded with 5-FU enhancing therapeutic efficacy against oral cancer. ACS Nano 9: 9638–9651. [Google Scholar]
Zhao LP, Zheng RR, Chen HQ, Liu LS, Zhao XY et al. (2020a). Self-delivery nanomedicine for O2 economized photodynamic tumor therapy. Nano Letters 20: 2062–2071. [Google Scholar]
Zhao XZ, Zhang W, Cao Y, Huang SS, Li YZ et al. (2020b). A cleverly designed novel lipid nanosystem: targeted retention, controlled visual drug release, and cascade amplification therapy for mammary carcinoma in vitro. International Journal of Nanomedicine 15: 3953. [Google Scholar]
Zhao Y, Wang J, Cai X, Ding P, Lv H, Pei R (2020c). Metal-organic frameworks with enhanced photodynamic therapy: Synthesis, erythrocyte membrane camouflage, and aptamer-targeted aggregation. ACS Applied Materials & Interfaces 12: 23697–23706. [Google Scholar]
Zhu D, Lyu M, Jiang W, Suo M, Huang Q, Li K (2020a). A biomimetic nanozyme/camptothecin hybrid system for synergistically enhanced radiotherapy. Journal of Materials Chemistry B 8: 5312–5319. [Google Scholar]
Zhu T, Shi L, Yu C, Dong Y, Qiu F et al. (2019). Ferroptosis promotes photodynamic therapy: Supramolecular photosensitizer-inducer nanodrug for enhanced cancer treatment. Theranostics 9: 3293. [Google Scholar]
Zhu X, Liu Y, Yuan G, Guo X, Cen J et al. (2020b). In situ fabrication of MS@MnO2 hybrid as nanozymes for enhancing ROS-mediated breast cancer therapy. Nanoscale 12: 22317–22329. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |