DOI:10.32604/biocell.2022.018594

| BIOCELL DOI:10.32604/biocell.2022.018594 |  |
| Viewpoint |
Mesenchymal stem cells-derived extracellular vesicles as ‘natural’ drug delivery system for tissue regeneration
Department of Nephrology, Rheumatology, Endocrinology and Metabolism, Okayama University Academic Field of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, 700-8558, Japan
*Address correspondence to: Kenji Tsuji, gmd422036@s.okayama-u.ac.jp
Received: 05 August 2021; Accepted: 22 September 2021
Abstract: Mesenchymal stem cells (MSCs) have abilities to mediate tissue protection through mechanisms of anti-apoptosis, anti-oxidative stress and anti-fibrosis as well as tissue regeneration through mechanisms of cell proliferation, differentiation and angiogenesis. These effects by MSCs are mediated by a variety of factors, including growth factors, cytokines and extracellular vesicles (EVs). Among these factors, EVs, containing proteins, mRNA and microRNAs (miRNA), may carry their contents into distant tissues with high stability. Therefore, the treatment with MSC-derived EVs may be promising as ‘natural’ drug delivery systems (DDS). Especially, the treatment of MSC-derived EVs with the manipulation of specific miRNAs expression has been reported to be beneficial under a variety of diseases and tissue injuries. The overexpression of specific miRNAs in the EVs might be through pre-loading method using the gene editing system by plasmid vector or post-loading method to load miRNA mimics into EVs by electroporation or calcium chloride-mediated transfection. Despite current several challenges for clinical use, it should open the next era of regenerative medicine for a variety of diseases. In this article, we highlight the therapeutic potential of MSC-derived EVs as ‘natural’ DDS and current challenges.
Keywords: Mesenchymal stem cells; Extracellular vesicles; Drug delivery system; microRNA; Regeneration
Mesenchymal stem cells (MSCs) are multipotent cells with the capacities to differentiate into mesodermal, endodermal and ectodermal lineages as well as the abilities of self-renewal and regeneration (Pittenger et al., 1999). MSCs have been shown to ameliorate multiple tissue injuries through the direct replacement into injured area (Tsuji et al., 2020b). In addition, MSCs have also been shown to induce regeneration indirectly through the tissue-protective secretome, including cytokines, chemokines, growth factors and extracellular vesicles (EVs) containing proteins, mRNAs and microRNAs (miRNAs) (Tsuji et al., 2020a). Recent evidence revealed that paracrine effect of MSCs is the dominant contributor of regenerative ability of MSCs, therefore the secretome from MSCs may provide a novel cell-free regenerative option in a variety of diseases. The mechanisms by which secretome mediates tissue protection and regeneration include suppression of pro-inflammatory response, modulation of immune system, inhibition of cell apoptosis and fibrosis as well as the stimulation of cell proliferation and tissue-intrinsic progenitor cells differentiation (Tsuji et al., 2020b). Although the therapy using secretome from MSCs is an attractive option for regenerative medicine, the quality control of the beneficial secretome is quite difficult (Allelein et al., 2021; Giebel, 2017) especially because beneficial effects may be mediated comprehensively with many beneficial factors.
Among the secretome from MSCs, EVs have been reported to be the main mediators to induce tissue regeneration (Park et al., 2019). EVs are small membrane vesicles released by a variety of cell types and can carry several factors into recipient cells and mediate cell-cell communications (Lee et al., 2012). EVs mediate high stability of their contents and carry contents into distant tissues, thus sometimes act as organ-to-organ communication. In this point, EVs are a kind of in vivo ‘natural’ drug delivery system (DDS). The DDS requires the delivery of their contents of appropriate amount toward appropriate position at appropriate timing with good biocompatibility. Various types of DDS such as adeno-associated virus vectors and nanoparticles, including liposomes, lipid nanoparticles, polymer micelles and polymeric nanoparticles have been reported and some of them are under trials (Deng et al., 2021; Prabhu et al., 2015). However, there are several concerns, including toxicity, immunogenicity, biocompatibility, ease of production, transfection efficiency, transfer to the target tissue and cellular uptake. Since EVs are the natural DDS derived from cells, thus free from the concerns of toxicity, immunogenicity and biocompatibility, it would be the promising DDS. Indeed, EVs treatment as DDS has been reported since 2010 (Alvarez-Erviti et al., 2011; Sun et al., 2010). While EVs secreted from MSCs may mediate beneficial effects, recent focus of the analysis is shifting to strengthen the effect by overexpressing specific factors.
Manipulation of MSC-derived EVs has emerged as a novel therapeutic option against several diseases and tissue injuries. Among the content of EVs, recent evidence using knockdown of the ribonuclease III Drosha gene, essential for the miRNAs production, implied that miRNAs are the main contributor of therapeutic effect, since knockdown of miRNAs abolished the therapeutic effect with MSC-secretome (Collino et al., 2015). miRNAs are the noncoding and single-stranded RNAs, which silence their targets genes through the binding to the 3’-UTR (Selbach et al., 2008). More than 200 mammalian miRNAs have been found currently and each miRNA may regulate several gene transcriptions. The prediction of the target gene of each miRNA is available in several public website, such as miRBase (Hsu et al., 2006). To explore specific miRNAs might be useful as disease biomarkers as well as novel therapeutic approaches. Indeed, some miRNAs are under clinical trials. For example, AntimiR of miR-122 (Miravirsen) to type C hepatitis (Ottosen et al., 2015), and MRX34 to regulate miR-34 against tumors are under clinical trials (Zhang et al., 2019b). Although the detail mechanisms as well as targeted genes of each miRNA are not fully uncovered, there are several miRNAs reported to mediate tissue beneficial effects, that may be divided into two aspects, tissue protection and regeneration. The protective mechanisms include anti-apoptosis, anti-oxidative stress and anti-fibrosis while the regenerative mechanisms include cell proliferation, differentiation and angiogenesis. miRNAs may regulate both aspects. For example, it is reported that let-7c may target TGFBR1, thus inhibit the progression of tissue fibrosis (Park et al., 2014) and miR-21 may regulate phosphatase and tensin homolog (PTEN) and protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling, thereby mediating anti-apoptosis effect (Song et al., 2018), both of which are the tissue protective effects. In addition, miR-26a-5p has been reported to target Toll-like receptor 4 (TLR4), thereby inactivating the NF-κB pathway and protecting against diabetic nephropathy (Duan et al., 2020). On the other hand, it is reported that miR-210 may promote angiogenesis through the activation of vascular endothelial growth factor (VEGF) pathway in ischemia/reperfusion-induced acute kidney injury (Zhang et al., 2019a), which is the regenerative aspect of the miRNA effects. Since MSCs may secrete these beneficial miRNAs-containing EVs, the overexpression of these beneficial miRNAs by way of miRNA mimic or gene editing systems might be potent to mediate therapeutic effects (Lv et al., 2020; Yang et al., 2020; Zhang et al., 2017). Important point is that the types of beneficial miRNAs are depending on the disease type. For example, recent analysis revealed that let-7 family therapy ameliorated diabetic kidney disease (Park et al., 2014) while overexpression of miR-21 protected against ischemia reperfusion-induced kidney injury via anti-apoptotic mechanisms (Song et al., 2018). We may need to choose the specific miRNAs depending on the patients’ diseases and conditions as the personalized medicine.
Despite these promising effects, there are still several challenges for the clinical use. First, we need to standardize the way to obtain EVs. Because of the quality fluctuation under the isolation of EVs, The International Society for Extracellular Vesicles (ISEV) proposed Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines in 2014 and updated in 2018 (Thery et al., 2018). It is required to optimize the way to isolate EVs and at least follow the guideline. Second, we need to explore how to transfer these EVs into particular target organs at high concentration. While MSCs have the ability of the homing by which MSCs may accumulate to the injured tissue in which MSCs may mediate therapeutic effect (Deak et al., 2010), EVs do not have the homing effect. It is reported, using fluorescent-labeled EVs, that injected EVs were localized mainly in liver, spleen, lung and kidney (Takahashi et al., 2013; Wiklander et al., 2015; Lai et al., 2014), suggesting that DDS approach using EVs is more likely to be advantageous in liver, lung and renal diseases. In addition to this natural localization of injected EVs, it is also important to elucidate how to enhance EVs delivery to specific organs. For example, the method of mounting specific molecules on the surface of exosomes to increase delivery toward specific tissue was reported (Alvarez-Erviti et al., 2011). Third, we need to clarify how to load and enclose the beneficial miRNAs in EVs. At present, there are two options, pre-loading method and post-loading method (Fig. 1). Pre-loading method is to use the gene editing system by plasmid vectors, in which modified cells strongly express miRNAs of interest and these miRNAs are enclosed in the secreted EVs. It is reported that the cargo of RNAs reflects the levels and types of cytoplasmic contents (Abels and Breakefield, 2016) and cells that overexpress specific miRNAs using plasmid vectors secrete EVs rich in that miRNAs (Kosaka et al., 2013) and mediated regeneration (Yang et al., 2020). Post-loading method is to load miRNA mimics into EVs for example by electroporation (Lv et al., 2020) or calcium chloride-mediated transfection (Zhang et al., 2017). On the other hand, for the pathogenic miRNAs, in which high expression may cause or worsen the tissue injury, the antisense of miRNAs may be useful to interfere the pathogenic miRNAs. Optimization of efficient loading of miRNAs is required.
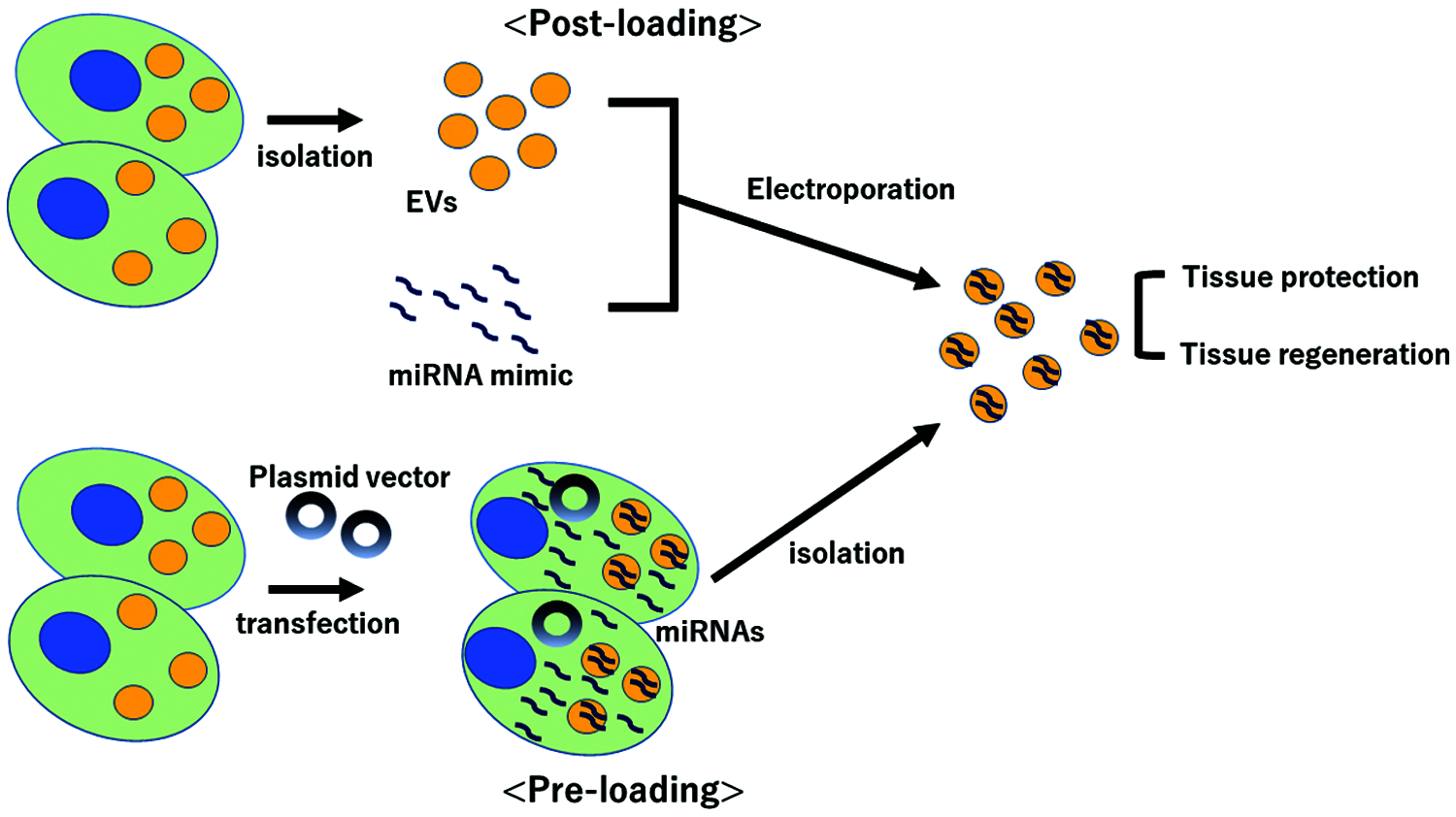
Figure 1: Scheme of two methods of loading miRNAs into extracellular vesicles. EVs; extracellular vesicles.
In summary, the EVs treatment as ‘natural’ DDS might be the promising and novel options for a variety of diseases. To establish these therapies, we need to deepen the understanding of the properties and roles of EVs. Especially for clinical applications, there are several challenges about how EVs should be isolated, what sources of EVs should be used, how to enclose the contents, and how to add target specificity. Despite current limitations, it should open the next era of regenerative medicine for a variety of diseases.
Authors’ Contribution: Study conception, design and data collection, KT and SK; manuscript preparation, KT, SK and JW.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abels ER, Breakefield XO (2016). Introduction to extracellular vesicles: Biogenesis, RNA cargo selection, content, release, and uptake. Cellular and Molecular Neurobiology 36: 301–312. DOI 10.1007/s10571-016-0366-z. [Google Scholar] [CrossRef]
Allelein S, Medina-Perez P, Lopes ALH, Rau S, Hause G, Kolsch A, Kuhlmeier D (2021). Potential and challenges of specifically isolating extracellular vesicles from heterogeneous populations. Scientific Reports 11: 11585. DOI 10.1038/s41598-021-91129-y. [Google Scholar] [CrossRef]
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology 29: 341–345. DOI 10.1038/nbt.1807. [Google Scholar] [CrossRef]
Collino F, Bruno S, Incarnato D, Dettori D, Neri F et al. (2015). AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying microRNAs. Journal of the American Society of Nephrology 26: 2349–2360. DOI 10.1681/ASN.2014070710. [Google Scholar] [CrossRef]
Deak E, Seifried E, Henschler R (2010). Homing pathways of mesenchymal stromal cells (MSCs) and their role in clinical applications. International Reviews of Immunology 29: 514–529. DOI 10.3109/08830185.2010.498931. [Google Scholar] [CrossRef]
Deng P, Halmai J, Waldo JJ, Fink KD (2021). Cell-based delivery approaches for DNA-binding domains into the central nervous system. Current Neuropharmacology. DOI 10.2174/1570159X19666210517144044. [Google Scholar] [CrossRef]
Duan Y, Luo Q, Wang Y, Ma Y, Chen F, Zhu X, Shi J (2020). Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. Journal of Biological Chemistry 295: 12868–12884. DOI 10.1074/jbc.RA120.012522. [Google Scholar] [CrossRef]
Giebel B (2017). On the function and heterogeneity of extracellular vesicles. Annals of Translational Medicine 5: 150. DOI 10.21037/atm.2017.02.14. [Google Scholar] [CrossRef]
Hsu PW, Huang HD, Hsu SD, Lin LZ, Tsou AP, Tseng CP, Stadler PF, Washietl S, Hofacker IL (2006). miRNAMap: Genomic maps of microRNA genes and their target genes in mammalian genomes. Nucleic Acids Research 34: D135–139. DOI 10.1093/nar/gkj135. [Google Scholar] [CrossRef]
Kosaka N, Takeshita F, Yoshioka Y, Hagiwara K, Katsuda T, Ono M, Ochiya T (2013). Exosomal tumor-suppressive microRNAs as novel cancer therapy: “Exocure” is another choice for cancer treatment. Advanced Drug Delivery Reviews 65: 376–382. DOI 10.1016/j.addr.2012.07.011. [Google Scholar] [CrossRef]
Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, Tannous BA, Breakefield XO (2014). Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8: 483–494. DOI 10.1021/nn404945r. [Google Scholar] [CrossRef]
Lee Y, El Andaloussi S, Wood MJ (2012). Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Human Molecular Genetics 21: R125–134. DOI 10.1093/hmg/dds317. [Google Scholar] [CrossRef]
Lv Q, Deng J, Chen Y, Wang Y, Liu B, Liu J (2020). Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Molecular Pharmaceutics 17: 1723–1733. DOI 10.1021/acs.molpharmaceut.0c00177. [Google Scholar] [CrossRef]
Ottosen S, Parsley TB, Yang L, Zeh K, van Doorn LJ, van der Veer E, Raney AK, Hodges MR, Patick AK (2015). In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrobial Agents and Chemotherapy 59: 599–608. DOI 10.1128/AAC.04220-14. [Google Scholar] [CrossRef]
Park JT, Kato M, Lanting L, Castro N, Nam BY, Wang M, Kang SW, Natarajan R (2014). Repression of let-7 by transforming growth factor-beta1-induced Lin28 upregulates collagen expression in glomerular mesangial cells under diabetic conditions. American Journal of Physiology-Renal Physiology 307: F1390–1403. DOI 10.1152/ajprenal.00458.2014. [Google Scholar] [CrossRef]
Park KS, Bandeira E, Shelke GV, Lasser C, Lotvall J (2019). Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Research & Therapy 10: 288. DOI 10.1186/s13287-019-1398-3. [Google Scholar] [CrossRef]
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147. DOI 10.1126/science.284.5411.143. [Google Scholar] [CrossRef]
Prabhu RH, Patravale VB, Joshi MD (2015). Polymeric nanoparticles for targeted treatment in oncology: Current insights. International Journal of Nanomedicine 10: 1001–1018. [Google Scholar]
Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N (2008). Widespread changes in protein synthesis induced by microRNAs. Nature 455: 58–63. DOI 10.1038/nature07228. [Google Scholar] [CrossRef]
Song N, Zhang T, Xu X, Lu Z, Yu X, Fang Y, Hu J, Jia P, Teng J, Ding X (2018). miR-21 protects against ischemia/reperfusion-induced acute kidney injury by preventing epithelial cell apoptosis and inhibiting dendritic cell maturation. Frontiers in Physiology 9: 790. DOI 10.3389/fphys.2018.00790. [Google Scholar] [CrossRef]
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010). A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Molecular Therapy 18: 1606–1614. DOI 10.1038/mt.2010.105. [Google Scholar] [CrossRef]
Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y (2013). Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. Journal of Biotechnology 165: 77–84. DOI 10.1016/j.jbiotec.2013.03.013. [Google Scholar] [CrossRef]
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles 7: 1535750. DOI 10.1080/20013078.2018.1535750. [Google Scholar] [CrossRef]
Tsuji K, Kitamura S, Sang Y, Fukushima K, Wada J (2020a). Adult kidney stem/progenitor cells contribute to regeneration through the secretion of trophic factors. Stem Cell Research 46: 101865. DOI 10.1016/j.scr.2020.101865. [Google Scholar] [CrossRef]
Tsuji K, Kitamura S, Wada J (2020b). Immunomodulatory and regenerative effects of mesenchymal stem cell-derived extracellular vesicles in renal diseases. International Journal of Molecular Sciences 21: 756. DOI 10.3390/ijms21030756. [Google Scholar] [CrossRef]
Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G et al. (2015). Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. Journal of Extracellular Vesicles 4: 26316. DOI 10.3402/jev.v4.26316. [Google Scholar] [CrossRef]
Yang C, Luo L, Bai X, Shen K, Liu K, Wang J, Hu D (2020). Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Archives of Biochemistry and Biophysics 681: 108259. DOI 10.1016/j.abb.2020.108259. [Google Scholar] [CrossRef]
Zhang C, Ma P, Zhao Z, Jiang N, Lian D, Huo P, Yang H (2019a). miRNAmRNA regulatory network analysis of mesenchymal stem cell treatment in cisplatininduced acute kidney injury identifies roles for miR210/Serpine1 and miR378/Fos in regulating inflammation. Molecular Medicine Reports 20: 1509–1522. [Google Scholar]
Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y (2017). Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. American Journal of Physiology-Lung Cellular and Molecular Physiology 312: L110–L121. DOI 10.1152/ajplung.00423.2016. [Google Scholar] [CrossRef]
Zhang L, Liao Y, Tang L (2019b). MicroRNA-34 family: A potential tumor suppressor and therapeutic candidate in cancer. Journal of Experimental & Clinical Cancer Research 38: 53. DOI 10.1186/s13046-019-1059-5. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |