DOI:10.32604/biocell.2022.018378

| BIOCELL DOI:10.32604/biocell.2022.018378 |  |
| Viewpoint |
MSCs-exosomes in regeneration medicine: Current evidence and future perspectives
1Jiangsu Key Laboratory of Laboratory Medicine, School of Medicine, Jiangsu University, Zhenjiang, 212013, China
2NHC Key Laboratory of Medical Embryogenesis and Developmental Molecular Biology & Shanghai Key Laboratory of Embryo and Reproduction Engineering, Shanghai, 200040, China
*Address correspondence to: Hui Qian, lstmmmlst@163.com
Received: 20 July 2021; Accepted: 09 October 2021
Abstract: Exosomes, especially from mesenchymal stem cells, have attracted extensive attention in regeneration medicine. Mesenchymal stem cells derived exosomes (MSCs-exosomes) have shown anti-inflammatory, anti-oxidant, anti-apoptosis and tissue regeneration effects in a variety of tissue injury repair models. MSCs-exosomes hold many excellent properties such as low immunogenicity, biocompatibility, and targeting capability. With the in-depth study on the generation and function of exosomes, MSCs-exosomes are considered to be the bright stars in the field of regenerative medicine. However, there are still many obstacles to overcome in terms of exosomes isolation, clinical trials and safety evaluation. In this article, what we should focus on about MSCs-exosomes in regeneration medicine will be discussed.
Keywords: Exosomes; Mesenchymal stem cells; Regeneration
Exosomes are nanosized vesicles with a diameter of 30–120 nm and can be secreted by most types of cells. These vesicles are widely distributed in various body fluids, such as blood, urine, milk, and saliva (Cho et al., 2021; Duca et al., 2021; Jiang et al., 2021; Wu et al., 2021c). Despite small size, exosomes play important roles in mediating intercellular communication, regulating immune response, and participating in the formation of microenvironment (Chen et al., 2021; Hou and Chen, 2021). Exosomes contain abundant cargos, such as protein, RNA, and lipid, which are considered to be the key molecules for exosomes to function. These cargos in exosomes and other types of extracellular vesicle can be transferred to the recipient cells and alter the function of the cells (Tkach and Théry, 2016).
In recent years, mesenchymal stem cells derived exosomes (MSCs-exosomes) exhibit treatment promise in multiple tissue repair models via various signaling pathways. Considering the abundant sources of exosomes, such as bone marrow, umbilical cord, adipose tissue, placenta, et al. (Burkova et al., 2021; Li et al., 2021; Pomatto et al., 2021; Tan et al., 2021), MSCs-exosomes are becoming the future star in the field of regeneration medicine. Here we provide a concise viewpoint at recent advances in MSCs-exosomes based regeneration medicine therapy, with a focus on treatment benefit and the main challenges in this field.
In past decades, MSCs-exosomes are applicated in a variety of tissue injury repair models. Zhang et al. (2015) showed that MSCs-exosomes mediated-Wnt4 activated the skin β-Catenin signal thus promoted the repair in cutaneous wound healing and restored the normal accessory structure of skin. In addition, MSCs-exosomes could attenuate unilateral ureteral obstruction induced rat renal fibrosis and cisplatin induced acute renal injury by regulating CK1δ/β-TRCP-mediated YAP degradation and enhancing autophagy (Jia et al., 2018; Ji et al., 2020). Jiang et al. (2018) found that MSCs-exosomes showed benefit in CCl4 induced liver injury through antioxidant effect. In intestinal diseases, research demonstrated that MSCs-exosomes carrying miR-326 inhibited neddylation to relieve inflammatory bowel disease in mice (Wang et al., 2020). In terms of myocardial injury repair, MSCs-exosomes could alleviate acute myocardial infarction, and also promote the repair of myocardial injury caused by viral myocarditis and myocardial ischemia-reperfusion (Gu et al., 2020; Li et al., 2020). Circular RNA 0001273 and AMPK/mTOR-mediated autophagy hold an important role in this process. In chronic wound including diabetic foot ulcers, adipose-derived MSCs-exosomes were able to enhance skin collagen production, angiogenesis and reduced inflammation in skin lesions (Zhao et al., 2021). What’s more, Tsai et al. (2021) demonstrated that MSCs-exosomes could rescue the hearing loss by promoting tissue remodeling and repair. Wu et al. (2021b) showed adipose-derived MSCs-exosomes could promote in vivo hair follicle regeneration. The therapeutic effects of MSCs-exosomes on multiple organs injury are shown in Fig. 1.
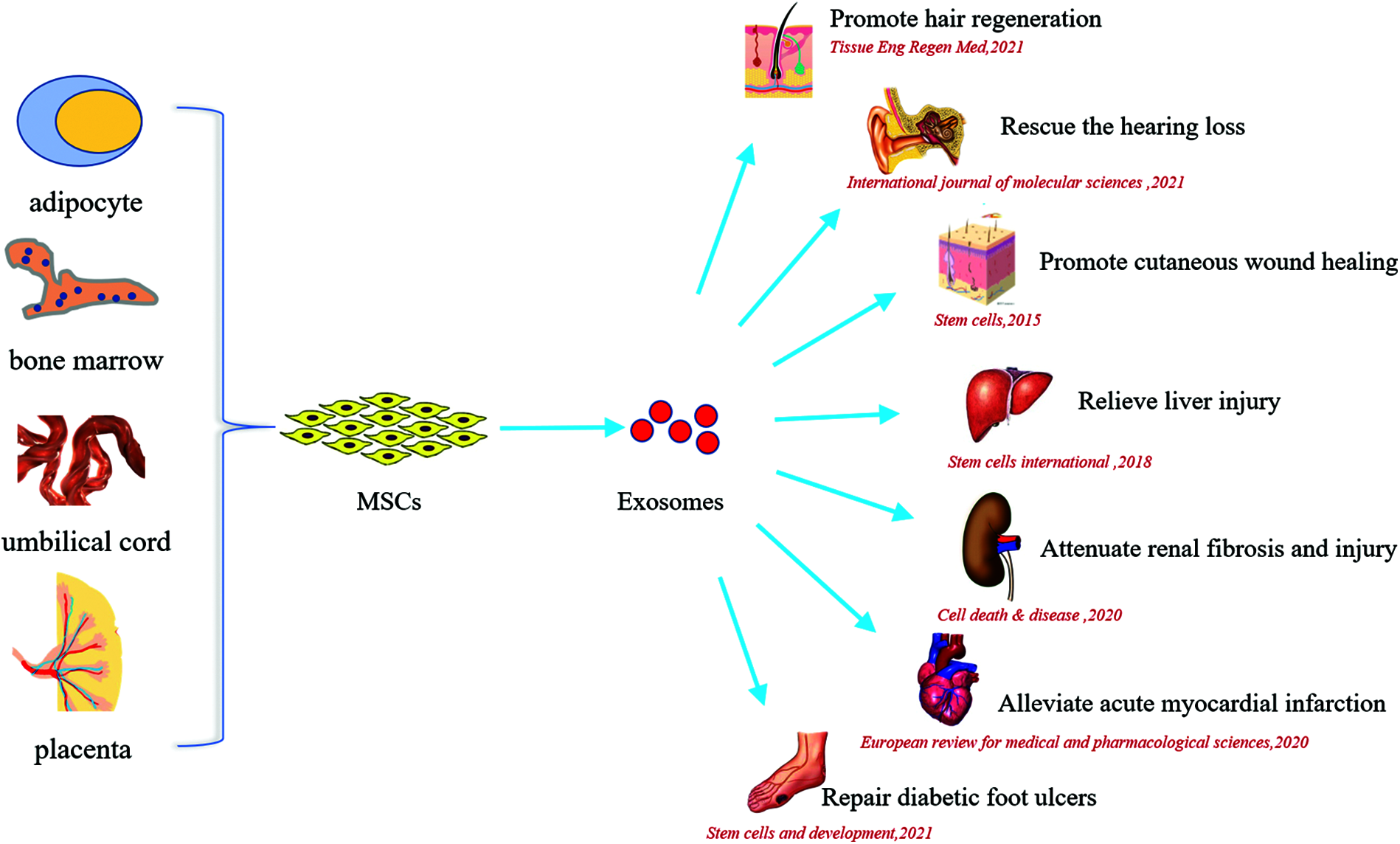
Figure 1: The beneficial effects of MSCs-exosomes on multiple organs injury.
To address these shortcomings of natural exosomes such as weak treatment effect, insufficient targeting, researchers have developed engineering strategies to enhance the functionality of MSCs-exosomes. Compared with exosomes of natural origin, genetic or non-genetic methods modified exosomes showed better therapeutic effects. For example, Akt-modified umbilical cord mesenchymal stem cells derived-exosomes were more effective in myocardial infarction by activating PDGF-D to promote angiogenesis (Ma et al., 2017). Yao et al. (2021) demonstrated that MSCs-exosomes overexpressing miR-29a-3p via miR-29a-3p-specific agonist could amplify the treatment effects of MSCs-exosomes on tendon healing. Besides, engineering exosomes via magnetic nanoparticles showed enhanced targeting ability to injury tissue under magnetic field, thus to promote wound healing and bone regeneration (Wu et al., 2021a). Moreover, the combination of MSCs-exosomes and new nanomaterials including new hydrogels, cardiac scaffolds and functional nanoparticles can provide great therapeutic effects. Zhang et al. (2021b) demonstrated that hyaluronic acid hydrogel encapsulated umbilical MSCs-exosomes could accelerate bone repair via enhancing angiogenesis, which potentially regulated through the miR-21/NOTCH1/DLL4 pathway.
These studies showed the benefit of MSCs-exosomes in multiple tissue injury models. Various exosomal cargos such as proteins, miRNAs, lncRNAs and lipids are considered as key components in the regeneration process (Zhao et al., 2020; Moghadasi et al., 2021). Put together, the employment of MSCs-exosomes in the treatment of damage disease models offers a novel therapeutical direction based on a new era of cell-free therapy. However, there are still many challenges in the application of exosomes in regeneration medicine.
The lack of clinical data is an obvious problem. Most of the experiments only stay at the level of animals and cannot truly reflect the effects of clinical application. The value of MSCs-exosomes in the field of tissue repair remains to be conclusively determined. Of course, this is also related to the complicated and strict approval of clinical trials. But it is undoubtedly correct to be cautious on issues involving clinical trials. At present, most of the industrialized products involving exosomes are concentrated in the aesthetic medical fields. Kwon et al. (2020) found adipose MSCs-exosomes had achieved a significantly greater improvement on acne scars than the control sides in a 12-week prospective, double-blind, randomized, split-face trial. A prospective nonrandomized open-label cohort study highlighted the capacity of bone marrow MSCs-exosomes to restore oxygenation, downregulate cytokine storm, and reconstitute immunity in severe COVID-19 (Sengupta et al., 2020). MSCs-exosomes are promising therapeutic candidate for improving patients’ clinical status with COVID-19. We look forward to more clinical trials on MSCs-exosomes to gain more understanding of the efficacy and safety of exosomes treatment.
The lack of large-scale and clinical-grade exosomes or extracellular vesicle isolation methods is another major obstacle. The ultracentrifugation method is currently considered the gold standard, but there are problems with time-consuming and high equipment cost (Witwer et al., 2013). The immunological separation method can directly separate exosomes from the supernatant and body fluids, but it is not suitable for large-volume samples (Oksvold et al., 2015). Commercial exosomes isolation kits provide efficient separation of exosomes from clinical samples, while avoiding exosomes being destroyed by long-term high-speed centrifugation. However, undesirable co-isolation of potential non-exosomal contents including polymer molecules limits its use for some quantitative examination (Taylor et al., 2011). Thus, new isolation methods need to be developed to improve the yield and bioactivity of exosomes. Recently, Kim et al. (2021) developed a tangential flow filtration (TFF) system-based method to significantly increase the yield and purity of exosomes compared to ultracentrifugation, and exosomes had higher bioactivity in promoting wound healing (Kim et al., 2021).
Moreover, the safety of MSCs-exosomes still needs more evidences. First, variations in effective dosing strategies are identified irrespective of the applied exosomes purification methods, which largely lead to the lack of stability between the current studies (Gupta et al., 2021). And it leads to the uncertainty of the potential harm of different quality of exosomes on the body. Secondly, most of researches are only conducted in a short period of time. Due to the lack of research on the in vivo effects of long-term use of exosomes, we emphasize the potential side effects of exosomes in a long range of time. Besides, some studies reported the favorable effects of MSCs-exosomes on tumor progression (Luo et al., 2020; Lyu et al., 2021). And vivo imaging has shown that exosomes are widely distributed in various organs of the body including liver, lung and kidney after intravenous administration. Therefore, we emphasize the safety evaluation of system administration of exosomes compared with local application.
In addition to the obstacles mentioned above, there are still the following challenges about the application of exosomes in regenerative medicine. In a number of studies, lack of comparison of the therapeutic effects of “MSCs” versus “MSCs-exosomes” is a limitation. The heterogeneity of mesenchymal stem cells is also an urgent problem to be solved. Zhang et al. (2021a) found two populations of umbilical cord mesenchymal stem cells through single-cell RNA sequencing based on differentially expressed genes. Besides, the difficulty of detecting exosomes in vivo makes it difficult to carry out dynamic analysis of exosomes at the tissue level (Fig. 2).
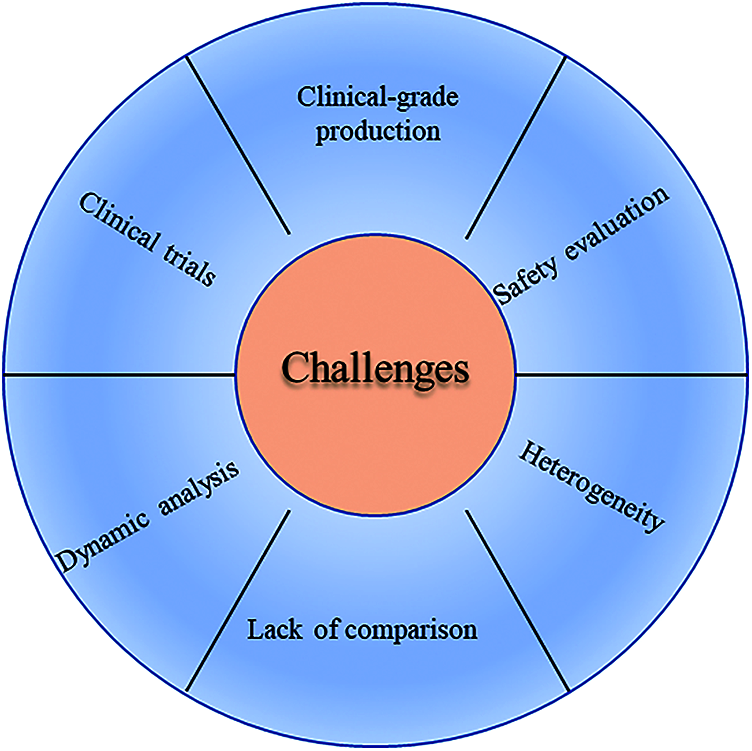
Figure 2: The main challenges of exosomes in the application of regeneration medicine.
To date, MSCs-exosomes therapy has been widely recognized and practiced in regenerative medicine based on multiple disease models. There is no doubt that MSCs-exosomes are bright stars in the field of regeneration medicine in the future. However, the complicated contents and signaling pathways involved in the regeneration process still remain unclear. Further research on the mechanisms of exosomes generation, isolation and function will help us to have a thorough understanding before further clinical trials. Exosomes are promising, but we still have a long way to go.
The relevant literatures and researches were searched in PubMed by using the keywords “mesenchymal stem cells”, “exosomes”, “injury repair” and “regenerative medicine”.
Acknowledgement: Yang Yang puts forward opinions for the idea of this paper.
Authors’ Contribution: Benshuai You conceived the work and wrote the first draft. Hui Qian critically reviewed the draft. All authors contributed to drafting the work, revised the final manuscript, and approved submission.
Funding Statement: This work was supported by the National Natural Science Foundation of China (81871496, 81971757), Zhenjiang Key Laboratory of High Technology Research on Exosomes Foundation and Transformation Application (ss2018003), Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD III).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Burkova EE, Sedykh SE, Nevinsky GA (2021). Human placenta exosomes: Biogenesis, isolation, composition, and prospects for use in diagnostics. International Journal of Molecular Sciences 22: 2158. DOI 10.3390/ijms22042158. [Google Scholar] [CrossRef]
Chen XD, Zhao J, Yang X, Zhou BW, Yan Z, Liu WF, Li C, Liu KX (2021). Gut-derived exosomes mediate memory impairment after intestinal ischemia/reperfusion via activating microglia. Molecular Neurobiology 58: 4828–4841. DOI 10.1007/s12035-021-02444-4. [Google Scholar] [CrossRef]
Cho NJ, Kim DY, Kwon SH, Ha TW, Kim HK, Lee MR, Chun SW, Park S, Lee EY, Gil HW (2021). Urinary exosomal microRNA profiling in type 2 diabetes patients taking dipeptidyl peptidase-4 inhibitor compared with sulfonylurea. Kidney Research and Clinical Practice 40: 383–391. DOI 10.23876/j.krcp.21.015. [Google Scholar] [CrossRef]
Duca RB, Massillo C, Dalton GN, Farré PL, Graña KD, Gardner K, de Siervi A (2021). MiR-19b-3p and miR-101-3p as potential biomarkers for prostate cancer diagnosis and prognosis. American Journal of Cancer Research 11: 2802–2820. [Google Scholar]
Gu X, Li Y, Chen K, Wang X, Wang Z et al. (2020). Exosomes derived from umbilical cord mesenchymal stem cells alleviate viral myocarditis through activating AMPK/mTOR-mediated autophagy flux pathway. Journal of Cellular and Molecular Medicine 24: 7515–7530. DOI 10.1111/jcmm.15378. [Google Scholar] [CrossRef]
Gupta D, Zickler AM, El Andaloussi S (2021). Dosing extracellular vesicles. Advanced Drug Delivery Reviews 178: 113961. DOI 10.1016/j.addr.2021.113961. [Google Scholar] [CrossRef]
Hou PP, Chen HZ (2021). Extracellular vesicles in the tumor immune microenvironment. Cancer Letters 516: 48–56. DOI 10.1016/j.canlet.2021.05.032. [Google Scholar] [CrossRef]
Ji C, Zhang J, Zhu Y, Shi H, Yin S et al. (2020). Exosomes derived from hucMSC attenuate renal fibrosis through CK1δ/β-TRCP-mediated YAP degradation. Cell Death & Disease 11: 327. DOI 10.1038/s41419-020-2510-4. [Google Scholar] [CrossRef]
Jia H, Liu W, Zhang B, Wang J, Wu P et al. (2018). HucMSC exosomes-delivered 14-3-3ζ enhanced autophagy via modulation of ATG16L in preventing cisplatin-induced acute kidney injury. American Journal of Translational Research 10: 101–113. [Google Scholar]
Jiang W, Tan Y, Cai M, Zhao T, Mao F, Zhang X, Xu W, Yan Z, Qian H, Yan Y (2018). Human umbilical cord msc-derived exosomes suppress the development of CCl(4)-induced liver injury through antioxidant effect. Stem Cells International 20: 6079642. [Google Scholar]
Jiang X, You L, Zhang Z, Cui X, Zhong H, Sun X, Ji C, Chi X (2021). Biological properties of milk-derived extracellular vesicles and their physiological functions in infant. Frontiers in Cell and Developmental Biology 9: 693534. DOI 10.3389/fcell.2021.693534. [Google Scholar] [CrossRef]
Kim JY, Rhim WK, Yoo YI, Kim DS, Ko KW, Heo Y, Park CG, Han DK (2021). Defined MSC exosome with high yield and purity to improve regenerative activity. Journal of Tissue Engineering 12: 20417314211008626. [Google Scholar]
Kwon HH, Yang SH, Lee J, Park BC, Park KY, Jung JY, Bae Y, Park GH (2020). Combination treatment with human adipose tissue stem cell-derived exosomes and fractional CO2 laser for acne scars: A 12-week prospective, double-blind, randomized, split-face study. Acta Dermato-Venereologica 100: adv00310. DOI 10.2340/00015555-3666. [Google Scholar] [CrossRef]
Li CX, Song J, Li X, Zhang T, Li ZM (2020). Circular RNA 0001273 in exosomes derived from human umbilical cord mesenchymal stem cells (UMSCs) in myocardial infarction. European Review for Medical and Pharmacological Sciences 24: 10086–10095. [Google Scholar]
Li Q, Jin Y, Ye X, Wang W, Deng G, Zhang X (2021). Bone marrow mesenchymal stem cell-derived exosomal microrna-133a restrains myocardial fibrosis and epithelial-mesenchymal transition in viral myocarditis rats through suppressing MAML1. Nanoscale Research Letters 16: 111. DOI 10.1186/s11671-021-03559-2. [Google Scholar] [CrossRef]
Luo T, Liu Q, Tan A, Duan L, Jia Y et al. (2020). Mesenchymal stem cell-secreted exosome promotes chemoresistance in breast cancer via enhancing miR-21-5p-mediated S100A6 expression. Molecular Therapy Oncolytics 19: 283–293. DOI 10.1016/j.omto.2020.10.008. [Google Scholar] [CrossRef]
Lyu T, Wang Y, Li D, Yang H, Qin B et al. (2021). Exosomes from BM-MSCs promote acute myeloid leukemia cell proliferation, invasion and chemoresistance via upregulation of S100A4. Experimental Hematology & Oncology 10: 24. DOI 10.1186/s40164-021-00220-7. [Google Scholar] [CrossRef]
Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, Qian H, Xu W, Zhu W (2017). Exosomes derived from akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Translational Medicine 6: 51–59. DOI 10.5966/sctm.2016-0038. [Google Scholar] [CrossRef]
Moghadasi S, Elveny M, Rahman HS, Suksatan W, Jalil AT et al. (2021). A paradigm shift in cell-free approach: The emerging role of MSCs-derived exosomes in regenerative medicine. Journal of Translational Medicine 19: 302. DOI 10.1186/s12967-021-02980-6. [Google Scholar] [CrossRef]
Oksvold MP, Neurauter A, Pedersen KW (2015). Magnetic bead-based isolation of exosomes. Methods in Molecular Biology 1218: 465–481. DOI 10.1007/978-1-4939-1538-5. [Google Scholar] [CrossRef]
Pomatto M, Gai C, Negro F, Cedrino M, Grange C et al. (2021). Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. International Journal of Molecular Sciences 22: 3851. DOI 10.3390/ijms22083851. [Google Scholar] [CrossRef]
Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N (2020). Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells and Development 29: 747–754. DOI 10.1089/scd.2020.0080. [Google Scholar] [CrossRef]
Tan KL, Chia WC, How CW, Tor YS, Show PL, Looi QHD, Foo JB (2021). Benchtop isolation and characterisation of small extracellular vesicles from human mesenchymal stem cells. Molecular Biotechnology 63: 780–791. DOI 10.1007/s12033-021-00339-2. [Google Scholar] [CrossRef]
Taylor DD, Zacharias W, Gercel-Taylor C (2011). Exosome isolation for proteomic analyses and RNA profiling. Methods in Molecular Biology 728: 235–246. DOI 10.1007/978-1-61779-068-3. [Google Scholar] [CrossRef]
Tkach M, Théry C (2016). Communication by extracellular vesicles: Where we are and where we need to go. Cell 164: 1226–1232. DOI 10.1016/j.cell.2016.01.043. [Google Scholar] [CrossRef]
Tsai SC, Yang KD, Chang KH, Lin FC, Chou RH et al. (2021). Umbilical cord mesenchymal stromal cell-derived exosomes rescue the loss of outer hair cells and repair cochlear damage in cisplatin-injected mice. International Journal of Molecular Sciences 22: 6664. DOI 10.3390/ijms22136664. [Google Scholar] [CrossRef]
Wang G, Yuan J, Cai X, Xu Z, Wang J et al. (2020). HucMSC-exosomes carrying miR-326 inhibit neddylation to relieve inflammatory bowel disease in mice. Clinical and Translational Medicine 10: e113. [Google Scholar]
Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C et al. (2013). Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. Journal of Extracellular Vesicles 2: 20360. DOI 10.3402/jev.v2i0.20360. [Google Scholar] [CrossRef]
Wu D, Chang X, Tian J, Kang L, Wu Y et al. (2021a). Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: Release of exosomal miR-1260a improves osteogenesis and angiogenesis. Journal of Nanobiotechnology 19: 209. DOI 10.1186/s12951-021-00958-6. [Google Scholar] [CrossRef]
Wu J, Yang Q, Wu S, Yuan R, Zhao X, Li Y, Wu W, Zhu N (2021b). Adipose-derived stem cell exosomes promoted hair regeneration. Tissue Engineering and Regenerative Medicine 18: 685–691. DOI 10.1007/s13770-021-00347-y. [Google Scholar] [CrossRef]
Wu M, Chen Z, Xie Q, Xiao B, Zhou G, Chen G, Bian Z (2021c). One-step quantification of salivary exosomes based on combined aptamer recognition and quantum dot signal amplification. Biosensors and Bioelectronics 171: 112733. DOI 10.1016/j.bios.2020.112733. [Google Scholar] [CrossRef]
Yao Z, Li J, Xiong H, Cui H, Ning J, Wang S, Ouyang X, Qian Y, Fan C (2021). MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. Journal of Nanobiotechnology 19: 169. DOI 10.1186/s12951-021-00906-4. [Google Scholar] [CrossRef]
Zhang B, Wang M, Gong A, Zhang X, Wu X et al. (2015). HucMSC-exosome Mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 33: 2158–2168. DOI 10.1002/stem.1771. [Google Scholar] [CrossRef]
Zhang S, Wang JY, Li B, Yin F, Liu H (2021a). Single-cell transcriptome analysis of uncultured human umbilical cord mesenchymal stem cells. Stem Cell Research & Therapy 12: 25. DOI 10.1186/s13287-020-02055-1. [Google Scholar] [CrossRef]
Zhang Y, Xie Y, Hao Z, Zhou P, Wang P, Fang S, Li L, Xu S, Xia Y (2021b). Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Applied Materials & Interfaces 13: 18472–18487. DOI 10.1021/acsami.0c22671. [Google Scholar] [CrossRef]
Zhao B, Zhang X, Zhang YL, Lu Y, Zhang W, Lu S, Fu Y, Zhou Y, Zhang J, Zhang J (2021). Human exosomes accelerate cutaneous wound healing by promoting collagen synthesis in a diabetic mice model. Stem Cells and Development 30: 922–933. DOI 10.1089/scd.2021.0100. [Google Scholar] [CrossRef]
Zhao G, Ge Y, Zhang C, Zhang L, Xu J, Qi L, Li W (2020). Progress of mesenchymal stem cell-derived exosomes in tissue repair. Current Pharmaceutical Design 26: 2022–2037. DOI 10.2174/1381612826666200420144805. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |