DOI:10.32604/biocell.2022.018895

| BIOCELL DOI:10.32604/biocell.2022.018895 |  |
| Review |
Regulation mechanisms of endocrine disruptors on vasodilation and vasoconstriction: Insights from ex vivo models
1CICS-UBI, Health Sciences Research Centre, University of Beira Interior, Covilhã, 6200-506, Portugal
2FCS-UBI, Faculty of Health Sciences, University of Beira Interior, Covilhã, 6200-506, Portugal
*Address correspondence to: Elisa Cairrao, ecairrao@fcsaude.ubi.pt
Received: 23 August 2021; Accepted: 16 November 2021
Abstract: Cardiovascular diseases (CVD) are one of the leading causes of death worldwide. The knowledge and understanding of CVD are based on the study of vascular physiology and how the smooth muscle cells and tissues perform their different functions. Exposure to endocrine disruptors (EDCs), such as phytoestrogens, polycyclic aromatic hydrocarbons, flame retardants, plasticizers, pesticides, and cosmetics, is an integral and fundamental part of human exposure. Humans are exposed to EDCs by multiple pathways including air, food, water, and consumer products. However, this exposure can lead to several adverse effects on human health, including on the cardiovascular (CV) system. The negative impact that EDC toxicity has on human CV health is a serious problem that must not be overlooked. In this point of view, we proposed the use of the human umbilical artery as a human model to study the direct effects of EDCs on the vascular level. Several works where these cells were directly exposed to EDC’s were presented to highlight this well-established model as a great strategy to be used. In the future, we emphasize the need to continue to carry out different investigations using HUA to unveil and understand the vascular toxicity of EDCs and improve human CV health.
Keywords: Vascular tonus; Human umbilical artery; Human exposome
Cardiovascular diseases (CVD) are one of the leading causes of death worldwide (Mc Namara et al., 2019). The knowledge and understanding of CVD are based on the study of vascular physiology and how the smooth muscle cells and tissues perform their different functions.
Humans are daily exposed to endocrine disruptors (EDCs), “exogenous chemicals that interfere with hormone action” by different cellular and molecular mechanisms (see review (La Merrill et al., 2020)). Exposure to EDCs, such as phytoestrogens, polycyclic aromatic hydrocarbons, flame retardants, plasticizers, pesticides, and cosmetics is an integral and fundamental part of human exposure and can occur by multiple pathways including air, food, water, and consumer products. However, this exposure led to several adverse effects on human health, including on cancer, reproductive, metabolic, and neurobiology systems, and cardiovascular disorders (e.g., coronary artery disease, hypertension, atherosclerosis, or myocardial infarction) (Zlatnik, 2016; Gore et al., 2019; Papalou et al., 2019; Fu et al., 2020; Mariana and Cairrao, 2020; Mesquita et al., 2021). Concerns about endocrine exposure have increased as the modulation of EDCs on the actions of natural hormones is discovered to involve a range of additive, synergistic, or negative biological effects (Feron et al., 2002; Fowler et al., 2012; Ribeiro et al., 2017).
Currently, one of the main research challenges is to discover the mechanisms of action of EDCs (Satpathy, 2020) to improve human health. However, studying the toxicity of EDCs presents some challenges, namely the complex network through which EDCs can act. In this sense, La Merrill et al. (2020) have recently presented a suggestion to classify into 10 key characteristics EDCs according to their disruptive effects and respective hormonal actions: 1) EDCs can act by activating/agonism of hormone receptors; 2) EDCs can act by inactivating/antagonism of hormone receptors; 3) EDCs can act by altering the expression of hormone receptors; 4) EDCs can alter signaling transduction; 5) EDCs can induce epigenetic changes; 6) EDCs can change hormone synthesis; 7) EDCs can change hormone transport; 8) EDCs can change hormone distribution; 9) EDCs can alter metabolism; and 10) EDCs can cause modification in the fate of hormone-producing or reactive cells (La Merrill et al., 2020).
Furthermore, it is essential not to forget that the endocrine system is itself a complex, integrative system and involves a series of hormonal feedback processes, and therefore very difficult to understand (Couderq et al., 2020). The existence of non-monotonic responses by EDCs (in which their effects change, in an inverted U or U-shape) also makes the work of researchers very difficult, since it is necessary to be aware that a high dose of EDC is not always it is the most toxic, but it can be a lower dose—this event makes difficult to define the safe dose of a given compound (Diamanti-Kandarakis et al., 2009; Vandenberg et al., 2012; Couderq et al., 2020). Indeed, one of the greatest challenges in toxicology is the choice of concentrations for in vitro testing, an issue that has been discussed recently (Leist et al., 2017; Albrecht, 2020; Hengstler et al., 2020). Currently, the use of relatively high concentrations (20 to 200 times higher than in vivo blood concentrations (Cmax) is agreed upon, as these high concentrations lead to better accuracy results than lower concentrations. Overall, higher concentrations in the culture medium are required to observe cell damage compared to the Cmax that is known to cause adverse effects in vivo (in vitro–in vivo scaling factor). Thus, it is advised to use a concentration range close to and above the maximum concentrations observed in human plasma (Hengstler et al., 2020). Furthermore, most EDCs remain understudied, which constituted a major force of investigation but, highlight the need for more emergent investigations to discover the toxicological effects. Thus, evaluating EDCs as an integral part of the human exposome has been the current challenge of greatest interest.
In this point of view, we proposed the use of the human umbilical artery as a human model to study the direct effects of EDCs on the vascular level. Several works where these cells were directly exposed to EDC’s are presented to emphasize this well-established model as a great strategy to be used.
The Role of Smooth Muscle Cells
The main function of vascular smooth muscle is to regulate vasodilation and vasoconstriction of vessels. Thus, the vascular tone depends on the mechanisms that control the intracellular cytosolic Ca2+ concentration: vasoconstriction is due to the increase of Ca2+ levels, while vasodilation occur by decreasing them (Lorigo et al., 2018). The main mechanisms involved in the vasoconstriction are 1) a cell membrane depolarization and 2) an agonist stimulation. In the 1st mechanism, voltage-operated Ca2+ channels are activated causing a Ca2+ influx. In the 2nd mechanism, there is an activation of a G-protein, which induces Ca2+ release by intracellular reservoirs of the cell, such as the sarcoplasmic reticulum. On the other hand, the main mechanisms involved in vasorelaxation are the cyclic nucleotides and K+ channels activation. In this sense, the smooth muscle cells regulate the contractile properties of this highly specialized structure (Morgado et al., 2012; Manoury et al., 2020)—the human umbilical artery—through responses to a series of hormonal and hemodynamic stimuli (Owens et al., 2004), but also due to the expression of several contractile proteins, functional ion channels and signaling molecules (Owens et al., 1996; Kudryavtseva et al., 2013; Wang et al., 2015).
The functions of smooth muscle cells result from a multiplicity of phenotypes (contractile to the synthetic phenotypes range), with well-defined structural characteristics (Rensen et al., 2007). As smooth muscle cells are very plastic, in pathophysiological response, such as exposure to EDCs, they may alter their contractile state and signaling mechanisms (Owens et al., 2004; Gloria et al., 2018; Lorigo et al., 2018), including the cyclic nucleotides compartmentalization (Feiteiro et al., 2016). This phenotypic modulation, as it impairs vascular tone (comprise the vasoconstriction and vasorelaxation responses), is associated with vascular lesions (Huang et al., 2016) and may be an inductor of cardiovascular disorders, as atherosclerosis. As these underlying molecular mechanisms are not yet clear, the use of smooth muscle cells is crucial to elucidate them and thus, to understand the development of some vascular diseases. However, the obtaining of smooth muscle cells is limited by the difficulty of acquiring human tissue for isolation and cell culture.
In this sense, the human umbilical artery (HUA) is an excellent source of vascular smooth muscle cells (Cairrao et al., 2009) and could be a good model for studying the effects of endocrine disruptors on the vascular system (Gloria et al., 2018; Lorigo et al., 2018). Easily isolated from the tunica media of the vessels (Meyer et al., 1978; Rockelein and Schneider, 1992), human umbilical artery smooth muscle cells (HUASMC) play a critical role in vascular physiology and pathophysiology (Santos-Silva et al., 2008; Morgado et al., 2012; Lorigo et al., 2018; Lorigo et al., 2020) (Fig. 1).
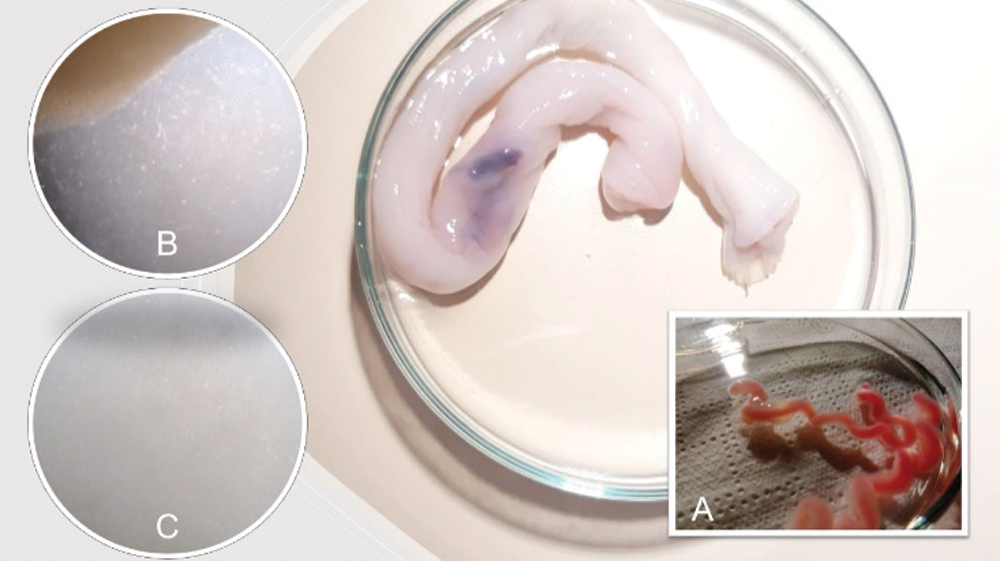
Figure 1: The human umbilical artery (HUA) can be easily obtained from the human umbilical cord (background) allowing the performance of human umbilical artery smooth muscle cells (HUASMC) cultures. (A) The HUA isolated. (B and C) HUASMC migrating from the smooth muscle layer and in a confluent state, respectively.
Why Study the Human Umbilical Artery?
Normal umbilical vascular reactivity is critical to maintaining the correct exchange of gases and nutrients between the fetus and the mother. As HUA does not have innervation (Santos-Silva et al., 2009; Provitera et al., 2019), unlike other arteries, its specific physiological regulatory control depends entirely on local mediators (such as serotonin, 5-HT, and histamine, His). On the other hand, also catecholamines (such as adrenaline, noradrenaline, and dopamine) play an important role in this regulation. However, it has been shown that the vascular response of HUA may differ from other vascular systems (Yoshikawa and Chiba, 1991), namely concerning the adrenergic system. This difference is mainly due to the portion of the cord that is collected because as shown by Kawano and Mori (1990), adrenergic nerve fibers are present only in HUA at the fetal end of the cord (Kawano and Mori, 1990). The main ex vivo methods used in HUA to study the vascular response are the organ bath and the human vessel perfusion system. Both systems allow to achievement of a physiological blood vessel environment. The main advantage of the perfusion system is that the vessel segments are maintained as 3D structures with an intact native endothelial lining (if isolated and mounted correctly), allowing quantification of nanoparticles accumulation and cellular response (Lysyy et al., 2020). Regarding the organ bath, the main advantage is allowing to isolate pharmacological responses, drug testing, and easy repeatable (Jespersen et al., 2015). Thus, we can conclude that HUA is a good model to evaluate the effects of EDCs on the local mediators and even to study adrenergic response in the portion proximal to the newborn.
Exposure to some EDCs can alter the hormone levels of sex steroids (Sathyanarayana et al., 2014; Johns et al., 2015). For example, it has been shown that perchlorate inhibits thyroid hormone synthesis (Wolff, 1998), while phthalates decrease the synthesis of testosterone (Parks, 2000; Mylchreest et al., 2002). On the other hand, the estradiol levels seem to increase by exposure to herbicide atrazine (Jin et al., 2013). In this sense, some studies have reported that HUA is more sensitive to the effects of estradiol than the human umbilical vein (Fausett et al., 1999) and that this artery allows the short-term and long-term effects of testosterone to be studied (Cairrao et al., 2008; Cairrao et al., 2010; Saldanha et al., 2013). Thus, the HUA can be considered an excellent tool/model for the study of genomic and non-genomic alterations that EDCs can induce at the vascular level, resulting from alterations in hormonal levels.
On the other hand, it is known that EDCs also impair thyroid hormone production (Ghassabian and Trasande, 2018; Vancamp et al., 2019; Street and Bernasconi, 2020), compromising CV homeostasis and consequently promoting or increasing the risk of developing CVD (Jain et al., 2013). The effects of EDCs on vascular contractility relating them to thyroid homeostasis is practically unexplored. However, a recent study showed that EDC octylmethoxycinnamate alters the contractility patterns of the HUA of pregnant women with hypothyroidism and competes with the natural hormone T3 for binding to the thyroid hormone receptor alpha active center. Although these computational simulations by docking molecular cannot clarify with certainty the absolute mode of action of an EDC, they are an asset in understanding them (Lorigo et al., 2021a), even supporting the different studies of contractility. Therefore, HUA may be also a good model for the vascular study of different pathologies, as is the case of thyroid pathologies, so closely related to the CV system.
Moreover, the HUA can also be used for the study of hypertensive disorders of pregnancy (HDP), such as pre-eclampsia or gestational hypertension (Naderi et al., 2017). Additionally, in HDP and specifically in pre-eclampsia, it would be interesting also use placenta or placental chorionic plaque arteries, using the ex vivo placental perfusion model as described by Hitzerd et al. (2019), as its pathophysiology is believed to be mainly originate in the placenta. On the other hand, the use of more peripheral placental arteries should also be considered as a model depending on the study to be performed. Overall, understanding the regulation of vascular reactivity and the remodeling of blood vessels in the umbilical cord is essential to understand the pathophysiology of HDP and to investigate the best therapeutic treatment strategies for HDP.
As mentioned before, HUA is mainly regulated by local mediators (such as serotonin, 5-HT, and histamine, His) (Santos-Silva et al., 2009; Provitera et al., 2019). Changes in 5-HT and His receptors increase HUA sensitivity and reactivity to these mediators, which causes an increase in vascular resistance (Bolte et al., 2001; Brew and Sullivan, 2006). Consequently, this may promote the development of gestational hypertension and pre-eclampsia (Bolte et al., 2001; Brew and Sullivan, 2006; Feinberg, 2006; Gupta et al., 2006; Lorigo et al., 2018). Different EDCs have already been shown to interfere with the HUA 5-HT and His receptors (Gloria et al., 2018), which highlights a possible role of these EDCs in promoting the development of HDP.
Furthermore, ion channels also play a key role in HDP (Kuo et al., 2011). The HUA was also used to demonstrate the role of EDCs tributyltin (Gloria et al., 2018), and octylmethoxycinnamate (Lorigo et al., 2019; Lorigo et al., 2021a; Lorigo et al., 2021b) in the blockade of L-type Ca2+ channels of HUA. Moreover, this effect in EDCs di-(2-ethylhexyl) phthalate and bisphenol A is also proved by patch-clamp in rat vascular smooth muscle cells (Feiteiro et al., 2018; Mariana et al., 2018). The EDC octylmethoxycinnamate also appears to have effects on K+ channel activation by cGMP-dependent protein kinase activation (Lorigo et al., 2021b), which can compromise vascular homeostasis and induce hypertension (Cox et al., 2001; Cox, 2005).
In summary, and according to the suggested for other authors (Kelley et al., 2019; Street and Bernasconi, 2020; Tang et al., 2020), the exposure to EDCs seems to be related to the promotion and development of some cardiovascular disorders, such as atherosclerosis, hypertension, or pre-eclampsia. Therefore, changes in HUA reactivity induced by EDCs exposure are an asset to understanding the risk of developing CVD, highlighting the clinical importance of this artery.
Cultures of HUASMC and Their Vascular Importance
In addition to being used in different studies of arterial contractility, the HUA is also an important model to perform isolation of HUASMC (as reviewed by Lorigo et al. (2018)). These cells can be directly exposed to different EDCs and used to perform cell contractility, gene expression, or cell cytotoxicity studies. As demonstrated by Cairrao et al. (2009) these cells express functional ion channels (Cairrao et al., 2009), enabling the successful realization of electrophysiological studies by Patch-Clamp, or cell contractility studies, by planar cell surface area. These cells can also be used for cell viability studies (Gloria et al., 2018), to assess the toxicity of an EDC to the CV system. Coherence between the in vitro results of HUASMC with those ex vivo (using HUA) and electrophysiological studies (Santos-Silva et al., 2008; Cairrao et al., 2009; Santos-Silva et al., 2009; Saldanha et al., 2013; Li et al., 2016; Mazza et al., 2016; Provitera et al., 2019) and the cell contractility studies (Lorigo et al., 2019; Lorigo et al., 2021a) was verified. Thus, we can conclude that HUASMC can be an excellent tool for the study of EDCs in vitro. The use of in silico methods also complements the assessment of disruptive effects on the vascular system, through the computational analysis of structure-activity relationships (Molecular Docking) (Lorigo et al., 2021a) (Fig. 2). Understanding how the normal physiological regulation of HUA is impaired because of exposure to EDCs is critical to understanding the development of CVD and targeting new possible treatments.
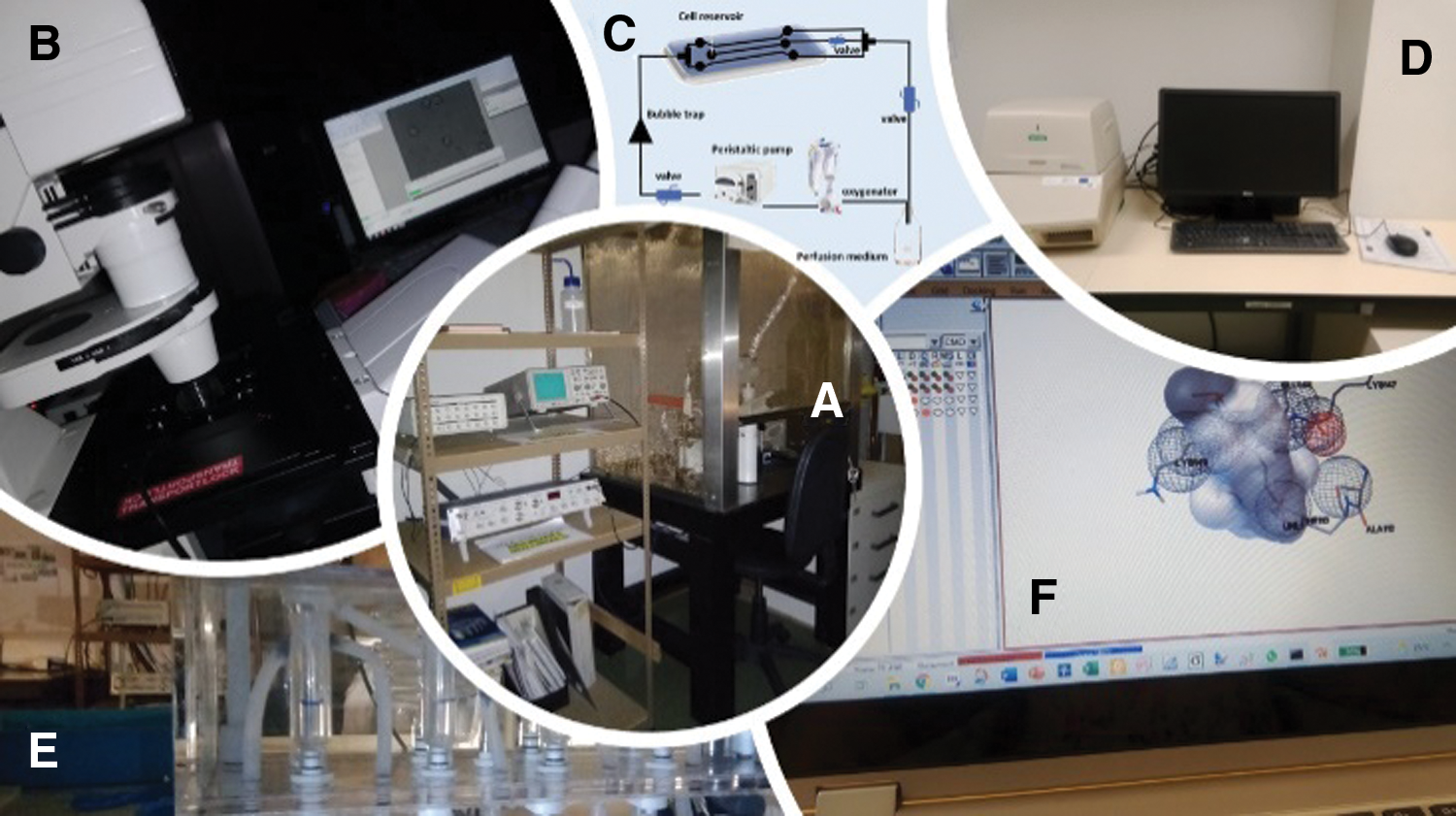
Figure 2: Different studies to evaluate the effects of EDCs on the human umbilical artery (HUA) and human umbilical artery smooth muscle cells (HUASMC). (A) Whole-cell patch-clamp experiments, (B) planar cell surface area technique, (C) vessel perfusion system (D) real-time polymerase chain reaction (PCR), (E) organ bath contractility, and (F) computational simulations by molecular docking.
In summary, the negative impact that EDC toxicity has on human cardiovascular health is a serious problem that must not be overlooked. Although several investigations are already starting to emerge in this field of research, there is still a long way to go. It should not be forgotten that exposure to EDCs is complex, often occurs through mixtures, there are non-monotonic responses, and there is also the possibility that it is influenced by various external factors (such as diet, genetics, or gender differences). Different studies on the vascular function using HUA will allow addressing this complexity: the studies can be performed in arteries from women whose births were to boys or girls, from women with different eating habits, carriers of various pathologies, whether genetic or not. However, the occurrence of pathologies, particularly CVD modulates the vascular response. This phenotypic modulation by smooth muscle cells, as it impairs vascular tone, is associated with vascular lesions, and can be studied. An example where vascular lesions have an important role to develop phenotypic modulation is atherosclerotic. In this pathological process, there is lipid accumulation (low-density lipoprotein levels increase) and mononuclear leukocyte infiltration in vascular tunica intima. Moreover, some conditions (e.g., hypertension, smoking, or diabetes) can be inductors of this disorder. In this sense, the human umbilical artery can be considered a good model to study endocrine disruption at the vascular level once allows for understanding the alterations in the regulation of contractile mechanisms controlled by smooth muscle cells. On the other hand, the possibility of non-monotonic responses induced by EDCs can be achieved by performing different techniques using the artery itself or its cells.
Therefore, we emphasize the need to continue to perform different investigations using HUA, to understand the mechanisms by which EDCs dysregulate the vascular response and lead to induce CVD in later life. In this way, it is possible to understand the vascular toxicity of EDCs and improve human cardiovascular health.
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Authors’ Contribution: The authors confirm contribution to the paper as follows: study conception and design: Elisa Cairrao; data collection, analysis, and interpretation of results: Margarida Lorigo and Elisa Cairrao; draft manuscript preparation: Margarida Lorigo. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This work was financed by the Foundation for Science and Technology (FCT), through funds from the State Budget, and by the European Regional Development Fund (ERDF), under the Portugal 2020 Program, through the Regional Operational Program of the Center (Centro2020), through the Project with the reference UIDB/00709/2020. M.L. acknowledges the Ph.D. fellowship from FCT (Reference: 2020.06616.BD).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Albrecht W (2020). Which concentrations are optimal for in vitro testing? EXCLI Journal 19: 1172–1173. [Google Scholar]
Bolte AC, van Geijn HP, Dekker GA (2001). Pathophysiology of preeclampsia and the role of serotonin. European Journal of Obstetrics & Gynecology and Reproductive Biology 95: 12–21. DOI 10.1016/S0301-2115(00)00367-5. [Google Scholar] [CrossRef]
Brew O, Sullivan MH (2006). The links between maternal histamine levels and complications of human pregnancy. Journal of Reproductive Immunology 72: 94–107. DOI 10.1016/j.jri.2006.04.002. [Google Scholar] [CrossRef]
Cairrao E, Alvarez E, Santos-Silva AJ, Verde I (2008). Potassium channels are involved in testosterone-induced vasorelaxation of human umbilical artery. Naunyn-Schmiedeberg’s Archives of Pharmacology 376: 375–383. DOI 10.1007/s00210-007-0213-3. [Google Scholar] [CrossRef]
Cairrao E, Santos-Silva AJ, Alvarez E, Correia I, Verde I (2009). Isolation and culture of human umbilical artery smooth muscle cells expressing functional calcium channels. Vitro Cellular & Developmental Biology 45: 175–184. DOI 10.1007/s11626-008-9161-6. [Google Scholar] [CrossRef]
Cairrao E, Santos-Silva AJ, Verde I (2010). PKG is involved in testosterone-induced vasorelaxation of human umbilical artery. European Journal of Pharmacology 640: 94–101. DOI 10.1016/j.ejphar.2010.04.025. [Google Scholar] [CrossRef]
Couderq S, Leemans M, Fini JB (2020). Testing for thyroid hormone disruptors, a review of non-mammalian in vivo models. Molecular and Cellular Endocrinology 508: 110779. DOI 10.1016/j.mce.2020.110779. [Google Scholar] [CrossRef]
Cox RH (2005). Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochemistry and Biophysics 42: 167–195. DOI 10.1385/CBB:42:2:167. [Google Scholar] [CrossRef]
Cox RH, Folander K, Swanson R (2001). Differential expression of voltage-gated K+ channel genes in arteries from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension 37: 1315–1322. DOI 10.1161/01.HYP.37.5.1315. [Google Scholar] [CrossRef]
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC (2009). Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocrine Reviews 30: 293–342. DOI 10.1210/er.2009-0002. [Google Scholar] [CrossRef]
Fausett MB, Belfort MA, Nanda R, Saade GR, Vedernikov Y (1999). The effects of sex steroids on human umbilical artery and vein. Journal of the Society for Gynecologic Investigation 6: 27–31. DOI 10.1016/S1071-5576(98)00040-9. [Google Scholar] [CrossRef]
Feinberg BB (2006). Preeclampsia: The death of Goliath. American Journal of Reproductive Immunology 55: 84–98. DOI 10.1111/j.1600-0897.2005.00362.x. [Google Scholar] [CrossRef]
Feiteiro J, Mariana M, Gloria S, Cairrao E (2018). Inhibition of L-type calcium channels by Bisphenol A in rat aorta smooth muscle. Journal of Toxicological Sciences 43: 579–586. DOI 10.2131/jts.43.579. [Google Scholar] [CrossRef]
Feiteiro J, Verde I, Cairrao E (2016). Cyclic guanosine monophosphate compartmentation in human vascular smooth muscle cells. Cellular Signalling 28: 109–116. DOI 10.1016/j.cellsig.2015.12.004. [Google Scholar] [CrossRef]
Feron VJ, Cassee FR, Groten JP, van Vliet PW, van Zorge JA (2002). International issues on human health effects of exposure to chemical mixtures. Environmental Health Perspectives 110: 893–899. DOI 10.1289/ehp.02110s6893. [Google Scholar] [CrossRef]
Fowler PA, Bellingham M, Sinclair KD, Evans NP, Pocar P et al. (2012). Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Molecular and Cellular Endocrinology 355: 231–239. DOI 10.1016/j.mce.2011.10.021. [Google Scholar] [CrossRef]
Fu X, Xu J, Zhang R, Yu J (2020). The association between environmental endocrine disruptors and cardiovascular diseases: A systematic review and meta-analysis. Environmental Research 187: 109464. DOI 10.1016/j.envres.2020.109464. [Google Scholar] [CrossRef]
Ghassabian A, Trasande L (2018). Disruption in thyroid signaling pathway: A mechanism for the effect of endocrine-disrupting chemicals on child neurodevelopment. Frontiers in Endocrinology 9: 204. DOI 10.3389/fendo.2018.00204. [Google Scholar] [CrossRef]
Gloria S, Marques J, Feiteiro J, Marcelino H, Verde I, Cairrao E (2018). Tributyltin role on the serotonin and histamine receptors in human umbilical artery. Toxicology in Vitro 50: 210–216. DOI 10.1016/j.tiv.2018.03.006. [Google Scholar] [CrossRef]
Gore AC, Krishnan K, Reilly MP (2019). Endocrine-disrupting chemicals: Effects on neuroendocrine systems and the neurobiology of social behavior. Hormones and Behavior 111: 7–22. DOI 10.1016/j.yhbeh.2018.11.006. [Google Scholar] [CrossRef]
Gupta S, Hanff LM, Visser W, Steegers EA, Saxena PR, Vulto AG, MaassenVanDenBrink A (2006). Functional reactivity of 5-HT receptors in human umbilical cord and maternal subcutaneous fat arteries after normotensive or pre-eclamptic pregnancy. Journal of Hypertension 24: 1345–1353. DOI 10.1097/01.hjh.0000234115.40648.88. [Google Scholar] [CrossRef]
Hengstler JG, Sjögren AK, Zink D, Hornberg JJ (2020). In vitro prediction of organ toxicity: The challenges of scaling and secondary mechanisms of toxicity. Archives of Toxicology 94: 353–356. DOI 10.1007/s00204-020-02669-7. [Google Scholar] [CrossRef]
Hitzerd E, Broekhuizen M, Neuman RI, Colafella KMM, Merkus D et al. (2019). Human placental vascular reactivity in health and disease: Implications for the treatment of pre-eclampsia. Current Pharmaceutical Design 25: 505–527. DOI 10.2174/1381612825666190405145228. [Google Scholar] [CrossRef]
Huang CH, Ciou JS, Chen ST, Kok VC, Chung Y et al. (2016). Identify potential drugs for cardiovascular diseases caused by stress-induced genes in vascular smooth muscle cells. PeerJ 4: e2478. DOI 10.7717/peerj.2478. [Google Scholar] [CrossRef]
Jain S, Murthy M, Ramteke K, Raparti G (2013). Thyroid: Disorders, disruptors and drugs. International Journal of Nutrition, Pharmacology, Neurological Diseases 3: 87. DOI 10.4103/2231-0738.112827. [Google Scholar] [CrossRef]
Jespersen B, Tykocki NR, Watts SW, Cobbett PJ (2015). Measurement of smooth muscle function in the isolated tissue bath-applications to pharmacology research. Journal of Visualized Experiments 95: 52324. DOI 10.3791/52324. [Google Scholar] [CrossRef]
Jin Y, Wang L, Fu Z (2013). Oral exposure to atrazine modulates hormone synthesis and the transcription of steroidogenic genes in male peripubertal mice. General and Comparative Endocrinology 184: 120–127. DOI 10.1016/j.ygcen.2013.01.010. [Google Scholar] [CrossRef]
Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-González LO et al. (2015). Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: A longitudinal analysis. Reproductive Biology and Endocrinology 13: 4. DOI 10.1186/1477-7827-13-4. [Google Scholar] [CrossRef]
Kawano M, Mori N (1990). Prostacyclin producing activity of human umbilical blood vessels in adrenergic innervated and non-innervated portions. Prostaglandins, Leukotrienes & Essential Fatty Acids 39: 239–245. DOI 10.1016/0952-3278(90)90079-Z. [Google Scholar] [CrossRef]
Kelley AS, Banker M, Goodrich JM, Dolinoy DC, Burant C, Domino SE, Smith YR, Song PXK, Padmanabhan V (2019). Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation. Scientific Reports 9: 5422. DOI 10.1038/s41598-019-41134-z. [Google Scholar] [CrossRef]
Kudryavtseva O, Aalkjaer C, Matchkov VV (2013). Vascular smooth muscle cell phenotype is defined by Ca2+-dependent transcription factors. FEBS Journal 280: 5488–5499. DOI 10.1111/febs.12414. [Google Scholar] [CrossRef]
Kuo IY, Wolfle SE, Hill CE (2011). T-type calcium channels and vascular function: The new kid on the block? Journal of Physiology 589: 783–795. DOI 10.1113/jphysiol.2010.199497. [Google Scholar] [CrossRef]
La Merrill MA, Vandenberg LN, Smith MT, Goodson W, Browne P et al. (2020). Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nature Reviews Endocrinology 16: 45–57. DOI 10.1038/s41574-019-0273-8. [Google Scholar] [CrossRef]
Leist M, Ghallab A, Graepel R, Marchan R, Hassan R et al. (2017). Adverse outcome pathways: Opportunities, limitations and open questions. Archives of Toxicology 91: 3477–3505. DOI 10.1007/s00204-017-2045-3. [Google Scholar] [CrossRef]
Li L, An L, Zhou X, Pan S, Meng X, Ren Y, Yang K, Guan Y (2016). Biological behaviour of human umbilical artery smooth muscle cell grown on nickel-free and nickel-containing stainless steel for stent implantation. Scientific Reports 6: 18762. DOI 10.1038/srep18762. [Google Scholar] [CrossRef]
Lorigo M, Mariana M, Feiteiro J, Cairrao E (2018). How is the human umbilical artery regulated? Journal of Obstetrics and Gynaecology Research 44: 1193–1201. DOI 10.1111/jog.13667. [Google Scholar] [CrossRef]
Lorigo M, Oliveira N, Cairrao E (2020). Clinical importance of the human umbilical artery potassium channels. Cells 9: 1956. DOI 10.3390/cells9091956. [Google Scholar] [CrossRef]
Lorigo M, Quintaneiro C, Breitenfeld L, Cairrao E (2021a). UV-B filter octylmethoxycinnamate alters the vascular contractility patterns in pregnant women with hypothyroidism. Biomedicines 9: 115. DOI 10.3390/biomedicines9020115. [Google Scholar] [CrossRef]
Lorigo M, Quintaneiro C, Lemos MC, Martinez-de-Oliveira J, Breitenfeld L, Cairrao E (2019). UV-B filter octylmethoxycinnamate induces vasorelaxation by Ca2+ channel inhibition and guanylyl cyclase activation in human umbilical arteries. International Journal of Molecular Sciences 20: 1376. DOI 10.3390/ijms20061376. [Google Scholar] [CrossRef]
Lorigo M, Quintaneiro C, Maia CJ, Breitenfeld L, Cairrao E (2021b). UV-B filter octylmethoxycinnamate impaired the main vasorelaxant mechanism of human umbilical artery. Chemosphere 277: 130302. DOI 10.1016/j.chemosphere.2021.130302. [Google Scholar] [CrossRef]
Lysyy T, Bracaglia LG, Qin L, Albert C, Pober JS, Tellides G, Saltzman WM, Tietjen GT (2020). Ex vivo isolated human vessel perfusion system for the design and assessment of nanomedicines targeted to the endothelium. Bioengineering & Translational Medicine 5: e10154. DOI 10.1002/btm2.10154. [Google Scholar] [CrossRef]
Manoury B, Idres S, Leblais V, Fischmeister R (2020). Ion channels as effectors of cyclic nucleotide pathways: Functional relevance for arterial tone regulation. Pharmacology & Therapeutics 209: 107499. DOI 10.1016/j.pharmthera.2020.107499. [Google Scholar] [CrossRef]
Mariana M, Cairrao E (2020). Phthalates implications in the cardiovascular system. Journal of Cardiovascular Development and Disease 7: 26. DOI 10.3390/jcdd7030026. [Google Scholar] [CrossRef]
Mariana M, Feiteiro J, Cairrao E (2018). Cardiovascular response of rat aorta to di-(2-ethylhexyl) phthalate (DEHP) exposure. Cardiovascular Toxicology 18: 356–364. DOI 10.1007/s12012-017-9439-6. [Google Scholar] [CrossRef]
Mazza G, Rossmanith E, Lang-Olip I, Pfeiffer D (2016). Marker profile for the evaluation of human umbilical artery smooth muscle cell quality obtained by different isolation and culture methods. Cytotechnology 68: 701–711. DOI 10.1007/s10616-014-9822-0. [Google Scholar] [CrossRef]
Mc Namara K, Alzubaidi H, Jackson JK (2019). Cardiovascular disease as a leading cause of death: How are pharmacists getting involved? Integrated Pharmacy Research & Practice 8: 1–11. DOI 10.2147/IPRP. [Google Scholar] [CrossRef]
Mesquita I, Lorigo M, Cairrao E (2021). Update about the disrupting-effects of phthalates on the human reproductive system. Molecular Reproduction and Development 88: 650–672. DOI 10.1002/mrd.23541. [Google Scholar] [CrossRef]
Meyer WW, Rumpelt HJ, Yao AC, Lind J (1978). Structure and closure mechanism of the human umbilical artery. European Journal of Pediatrics 128: 247–259. DOI 10.1007/BF00445610. [Google Scholar] [CrossRef]
Morgado M, Cairrao E, Santos-Silva AJ, Verde I (2012). Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cellular and Molecular Life Sciences 69: 247–266. DOI 10.1007/s00018-011-0815-2. [Google Scholar] [CrossRef]
Mylchreest E, Sar M, Wallace DG, Foster PM (2002). Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reproductive Toxicology 16: 19–28. DOI 10.1016/S0890-6238(01)00201-5. [Google Scholar] [CrossRef]
Naderi S, Tsai SA, Khandelwal A (2017). Hypertensive disorders of pregnancy. Current Atherosclerosis Reports 19: 15. DOI 10.1007/s11883-017-0648-z. [Google Scholar] [CrossRef]
Owens GK, Kumar MS, Wamhoff BR (2004). Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological Reviews 84: 767–801. DOI 10.1152/physrev.00041.2003. [Google Scholar] [CrossRef]
Owens GK, Vernon SM, Madsen CS (1996). Molecular regulation of smooth muscle cell differentiation. Journal of Hypertension 14: S55–64. [Google Scholar]
Papalou O, Kandaraki EA, Papadakis G, Diamanti-Kandarakis E (2019). Endocrine disrupting chemicals: An occult mediator of metabolic disease. Frontiers in Endocrinology 10: 112. DOI 10.3389/fendo.2019.00112. [Google Scholar] [CrossRef]
Parks LG (2000). The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicological Sciences 58: 339–349. DOI 10.1093/toxsci/58.2.339. [Google Scholar] [CrossRef]
Provitera L, Cavallaro G, Griggio A, Raffaeli G, Amodeo I et al. (2019). Cyclic nucleotide-dependent relaxation in human umbilical vessels. Journal of Physiology and Pharmacology 70: 619–630. [Google Scholar]
Rensen SS, Doevendans PA, van Eys GJ (2007). Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Netherlands Heart Journal 15: 100–108. DOI 10.1007/BF03085963. [Google Scholar] [CrossRef]
Ribeiro E, Ladeira C, Viegas S (2017). EDCs mixtures: A stealthy hazard for human health? Toxics 5: 5. DOI 10.3390/toxics5010005. [Google Scholar] [CrossRef]
Rockelein G, Schneider R (1992). Three-dimensional analysis of the tunica media of umbilical arteries. Scanning electron microscopy study. Zeitschrift fur Geburtshilfe und Perinatologie 196: 266–272. [Google Scholar]
Saldanha PA, Cairrao E, Maia CJ, Verde I (2013). Long- and short-term effects of androgens in human umbilical artery smooth muscle. Clinical and Experimental Pharmacology and Physiology 40: 181–189. DOI 10.1111/1440-1681.12047. [Google Scholar] [CrossRef]
Santos-Silva AJ, Cairrao E, Marques B, Verde I (2009). Regulation of human umbilical artery contractility by different serotonin and histamine receptors. Reproductive Sciences 16: 1175–1185. DOI 10.1177/1933719109343787. [Google Scholar] [CrossRef]
Santos-Silva AJ, Cairrao E, Morgado M, Alvarez E, Verde I (2008). PDE4 and PDE5 regulate cyclic nucleotides relaxing effects in human umbilical arteries. European Journal of Pharmacology 582: 102–109. DOI 10.1016/j.ejphar.2007.12.017. [Google Scholar] [CrossRef]
Sathyanarayana S, Barrett E, Butts S, Wang C, Swan SH (2014). Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction (Cambridge, England) 147: 401–409. DOI 10.1530/REP-13-0415. [Google Scholar] [CrossRef]
Satpathy R (2020). Application of molecular docking methods on endocrine disrupting chemicals: A review. Journal of Applied Biotechnology Reports 7: 74–80. [Google Scholar]
Street ME, Bernasconi S (2020). Endocrine-disrupting chemicals in human fetal growth. International Journal of Molecular Sciences 21: 1430. DOI 10.3390/ijms21041430. [Google Scholar] [CrossRef]
Tang ZR, Xu XL, Deng SL, Lian ZX, Yu K (2020). Oestrogenic endocrine disruptors in the placenta and the fetus. International Journal of Molecular Sciences 21: 1519. DOI 10.3390/ijms21041519. [Google Scholar] [CrossRef]
Vancamp P, Houbrechts AM, Darras VM (2019). Insights from zebrafish deficiency models to understand the impact of local thyroid hormone regulator action on early development. General and Comparative Endocrinology 279: 45–52. DOI 10.1016/j.ygcen.2018.09.011. [Google Scholar] [CrossRef]
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DRJr. et al. (2012). Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocrine Reviews 33: 378–455. DOI 10.1210/er.2011-1050. [Google Scholar] [CrossRef]
Wang G, Jacquet L, Karamariti E, Xu Q (2015). Origin and differentiation of vascular smooth muscle cells. Journal of Physiology 593: 3013–3030. DOI 10.1113/JP270033. [Google Scholar] [CrossRef]
Wolff J (1998). Perchlorate and the thyroid gland. Pharmacological Reviews 50: 89–105. [Google Scholar]
Yoshikawa F, Chiba S (1991). Pharmacological analysis of vasoconstrictor responses of isolated and perfused human umbilical arteries. Heart and Vessels 6: 197–202. DOI 10.1007/BF02125097. [Google Scholar] [CrossRef]
Zlatnik MG (2016). Endocrine-disrupting chemicals and reproductive health. Journal of Midwifery & Women’s Health 61: 442–455. DOI 10.1111/jmwh.12500. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |