DOI:10.32604/biocell.2022.018559

| BIOCELL DOI:10.32604/biocell.2022.018559 |  |
| Article |
Hypoxia induced apoptosis of rat gastric mucosal cells by activating autophagy through HIF-1α/TERT/mTORC1 pathway
1Department of Digestive Medicine, Wuxi Huishan District People’s Hospital, Wuxi, China
2Department of Digestive Medicine, Qinghai Provincial People’s Hospital, Xining, China
*Address correspondence to: Yaping Wang, rose19820721@126.com
#These authors contributed equally to this work
Received: 02 August 2021; Accepted: 26 September 2021
Abstract: The pathogenesis of high altitude-related gastric mucosal injury remains poorly understood, this study aimed to investigate the role of autophagy in hypoxia-induced apoptosis of rat gastric mucosal cells. Rats were randomized into four groups which were maintained at an altitude of 400 m (P) or received no treatment (H), autophagy inducer rapamycin (H+AI) or autophagy inhibitor 3-MA (H+AB) at an altitude of 4,300 m for 1, 7, 14 and 21 days, respectively, and the morphology, ultrastructure, autophagy, and apoptosis of gastric mucosal tissues were examined. Gastric mucosal epithelial cells CC-R039 were cultured under conditions of normoxia, 2% O2 (hypoxia), or 2% O2+anti-mTORC1 for 0, 24, 48, and 72 h, respectively, and the autophagy and apoptosis were analyzed. CC-R039 cells were transfected with siHIF-1α, siTERT, or siRNA and the autophagy was examined. The results showed that the exposure to hypoxia increased the autophagy and apoptosis of gastric mucosal cells in rats, and apoptosis was aggravated by rapamycin treatment but alleviated by 3-MA treatment. Increased duration of hypoxia from 0 to 72 h could increase the autophagy and apoptosis but decrease the proliferation of gastric mucosal cells. Inhibition of mTORC1 with rapamycin led to further increase in apoptosis and even substantial cell death, and inhibition of HIF-1α and TERT increased mTORC1 expression and reduced autophagy. Moreover, the inhibition of HIF-1α reduced TERT expression. In conclusion, hypoxia could induce apoptosis of rat gastric mucosal cells by activating autophagy through HIF-1α/TERT/mTORC1 pathway.
Keywords: Hypoxia; Autophagy; Apoptosis; HIF-1α; TERT; mTORC1
Abbreviations
| 3-MA: | 3-methyladenine |
| HIF-1α: | Hypoxia-inducible factor 1α |
| LC3-II: | Microtubule-associated protein light chain 3-II |
| mTORC1: | Mammalian target of rapamycin complex 1 |
| TERT: | Telomerase reverse transcriptase |
The incidence of gastric mucosal injury is high even in the early period of hypoxia with symptoms of gastric mucosal erosion, ulcer, and some life-threatening conditions such as massive hemorrhage from the upper gastrointestinal tract (Netzer et al., 2013; Wu et al., 2007). Exposure to hypoxia at high altitude can increase the permeability of gastric mucosa and make individuals susceptible to bacterial translocation and systemic inflammatory response syndrome, and some individuals may develop potentially fatal multiple organ dysfunction syndrome (Khanna et al., 2019). Therefore, hypoxia-induced damage to gastric mucosal cells may cause maladaptation to high altitudes. However, the pathogenesis of high altitude-related gastric mucosal injury remains poorly understood.
Autophagy is a lysosome-dependent cellular catabolic process by which cytoplasmic components are isolated from the rest of the cell within double-membraned structures known as autophagosomes, which subsequently fuse with lysosomes for the degradation (Mostowy, 2013). Autophagy often occurs at a low level to maintain cellular homeostasis under physiological conditions but could accelerate in response to harmful stimuli such as oxygen deprivation (Zhang et al., 2020). However, it is important to note that hypoxia-induced autophagy differs substantially across cellular contexts, as it may promote cell survival under mild hypoxic conditions but apoptosis under severe hypoxic conditions (Mazure and Pouysségur, 2010). In this study, we aimed to investigate the role of autophagy in the apoptosis of gastric mucosal cells of rats exposed to high-altitude hypoxia and to explore the underlying molecular mechanism.
This study was approved by the Ethical Committee of Qinghai Provincial People’s Hospital (Approval No. 2017-16, approval date January 16, 2017). A total of 104 male Wistar rats (weight 180–200 g) were provided by the Experimental Animal Center of Medical College of Xi’an Jiaotong University (Xi’an, China) and were housed in a room with controlled temperature at 25 ± 1°C and a 12 h light/dark cycle.
The rats were randomized into four groups (N = 8) and then were maintained at an altitude of 400 m (P) or received no treatment (H), autophagy inducer rapamycin (H+AI) or autophagy inhibitor 3-MA (H+AB) at an altitude of 4300 m for 1, 7, 14, and 21 days, respectively. Rats in the H+AI and H+AB groups were intraperitoneally injected with rapamycin (1 mg/kg) and 3-MA (2 mg/kg) once a day for consecutive 21 days, respectively, while other rats were injected with an equal volume of PBS. The gastric mucosal tissues were collected from the greater curvature of the stomach after the rats were euthanized with sodium pentobarbital (dose > 50 mg/kg).
Gastric mucosal tissues were fixed with 4% paraformaldehyde, dehydrated in graded alcohols, cleared in xylene, and embedded in paraffin. Subsequently, paraffin-embedded tissues were sectioned and stained with hematoxylin for 10–30 min. After destaining, slides were re-stained with 0.5% eosin (alcoholic), and the morphology of gastric mucosal tissues was observed under the microscope.
Gastric mucosal tissues were fixed with 2.5% glutaraldehyde at 4°C for over 2 h, washed three times with phosphate buffer, fixed with 1% osmic acid for 2 h, washed again with phosphate buffer, dehydrated in graded alcohols, and embedded in epoxy resin (Epon 812). After polymerization, semi-thin sections were cut and stained with toluidine blue. Then, ultrathin sections of 50–70 nm were cut using an ultramicrotome and stained with lead citrate and uranyl acetate, and the ultrastructure and autophagy of gastric mucosal tissues were observed under the transmission electron microscope (Hitachi, Japan).
Rat gastric mucosal epithelial cells (CC-R039) were cultured in six-well plates until 95% confluence, and rapamycin, a specific inhibitor of mTORC1 (final concentration of 200 μM), was added. Cells were cultured in 2 mL of complete medium in six-well plates for about 24 h until 90% confluence. Then, cells were transfected with small interfering RNA (siRNA), including HIF-1α siRNA, TERT siRNA, and the negative control siRNA (all purchased from Invitrogen, USA), using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
CC-R039 cells were exposed to hypoxia for 0, 24, 48, and 72 h, respectively, and apoptosis was examined by using Annexin V-PE/7 AAD apoptosis kit (BD Biosciences, USA). About 1 × 106 suspended cells were collected and re-suspended in 200 μL of PBS. After that, 5 μL of Annexin V-PE and 5 μL of 7-AAD were added and incubated in the dark at 4°C for 30 min. Next, propidium iodide (PI) solution was added, and DNA content was determined using a flow cytometer to determine the percentage of cells at different cycles and cell apoptosis using NovoExpress software.
CC-R039 cells were washed with PBS, fixed with 4% paraformaldehyde, rinsed with 1 mL of 0.5% Triton X-100 at room temperature for 20 min, washed with PBS, and blocked with 1 mL of 5% BSA at 37°C for 1 h. After that, cells were incubated with primary antibodies for Beclin-1 and LC3 (Abcam, USA) overnight at 4°C, and then with FITC-labeled goat anti-rabbit secondary antibody at 37°C for 1 h. Then, cells were blocked with 300 μL of anti-fluorescence quenching sealing solution containing DAPI, and the localization and distribution of Beclin-1 and LC3 were observed under a fluorescence microscope (Leica, Germany).
The total proteins were extracted for protein quantification, and 20 μg proteins were loaded per well for electrophoresis on 8% polyacrylamide gel, transferred to membranes, blocked with 5% defatted milk at room temperature, and washed with PBST. Then, the membranes were incubated with primary antibodies (all from Abcam, USA) overnight at 4°C, washed three times with PBST, and then incubated with HRP-labeled secondary antibody at room temperature for 1 h, and visualized by using enhanced chemiluminescence (ECL) kit. The grey values were analyzed using TANON GIS software.
Data were expressed as mean ± standard deviation (SD) and analyzed by SPSS 19.0. Multiple-group differences were analyzed by single-factor analysis of variance, followed by post-hoc Bonferroni test for pairwise comparison. P < 0.05 was considered statistically significant.
Morphology of gastric mucosal tissues
HE-staining showed that rats exposed to hypoxia for 1 day (H1) and 7 days (H7) exhibited gastric gland rupture, inflammatory cell infiltration and bleeding, H14 group exhibited some hypertrophic cells, while the H21 group exhibited indistinct cellular boundaries, nuclear pyknosis and apoptosis. Inflammatory cell infiltration, gastric mucosal bleeding, gastric gland rupture, indistinct cellular boundaries, nuclear pyknosis and apoptosis were observed in rats treated with rapamycin but become increasingly severe in H+AI14 and H+AI21 groups. Gastric gland rupture occurred in all rats treated with 3-MA (H+AB), and inflammatory cell infiltration occurred only in H+AB7, H+AB14 and H+AB21 groups (Fig. 1A). Collectively, these results indicated that the exposure to hypoxia resulted in progressive deterioration of gastric mucosal injury, which can be aggravated by rapamycin but alleviated by 3-MA.
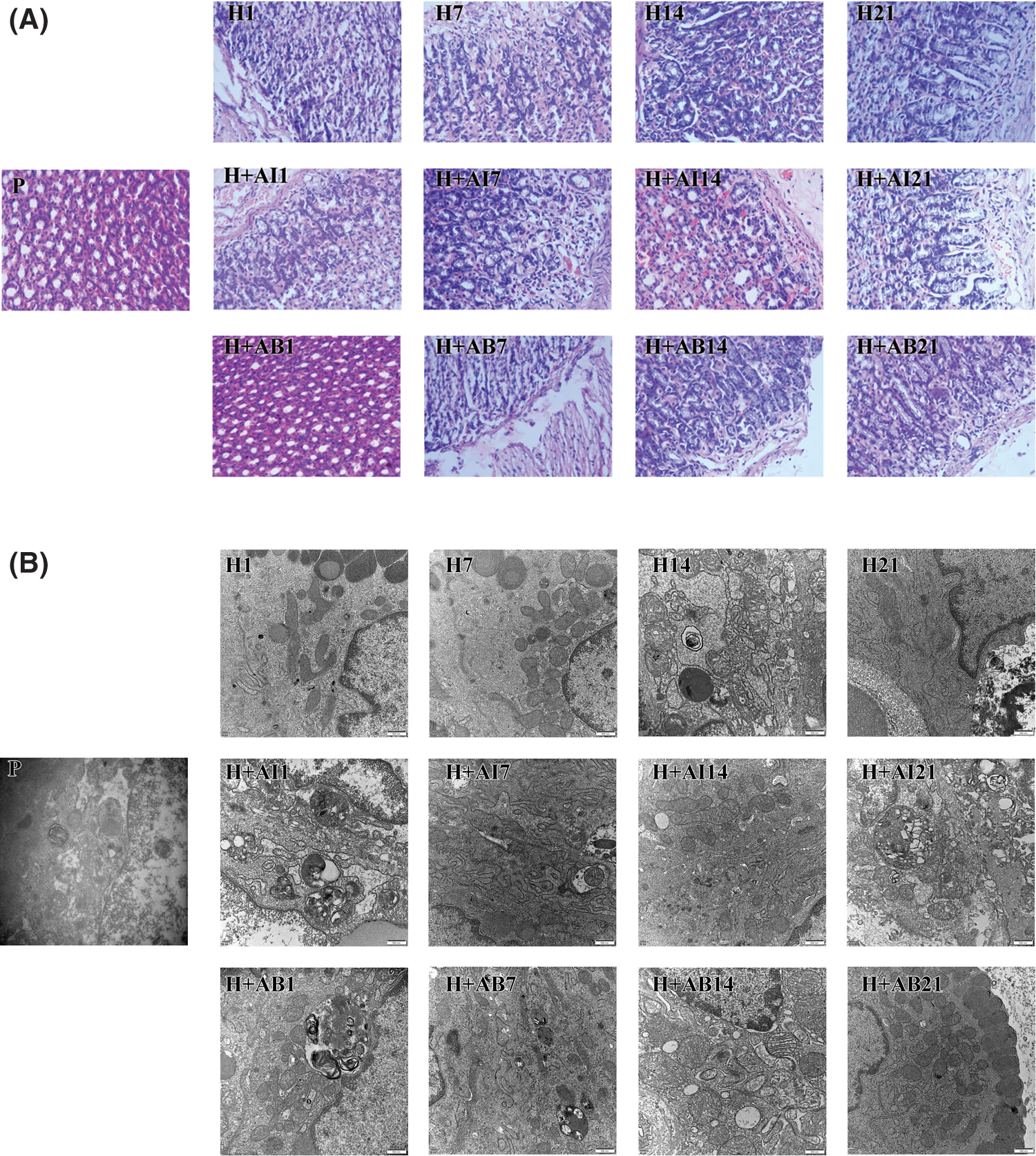
Figure 1: High-altitude hypoxia induced autophagy of rat gastric mucosal cells. (A) Morphology of gastric mucosal tissues. (B) Ultrastructure and autophagy of gastric mucosal cells. P, control; H, hypoxia; H+AI, hypoxia plus rapamycin treatment; H+AB, hypoxia plus 3-MA.
The autophagy of gastric mucosal cells
The changes in the ultrastructure and autophagy of gastric mucosal cells were observed under the electron microscope (Fig. 1B). We found the mitochondria in the cytoplasm but no autophagic structures in the H1 group. However, lipid droplets and autophagic structures were observed occasionally in the H7 group. In the H14 group, gastric mucous cells were arranged disorderly, accompanied by the aggregation and margination of the chromatin, mitochondrial swelling, and the formation of autophagic structures. In the H21 group, the intercellular space increased with the formation of autophagic structures in the cytoplasm. In rats treated with rapamycin, gastric mucous epithelial cells were arranged in a single layer with irregularly shaped nuclei and visible autophagic structures. In the H+AI14 and H+AI21 groups, most cells showed swelling and necrosis, but no autophagic or apoptotic structures were observed in the cytoplasm. In rats treated with 3-MA, gastric mucous epithelial cells were arranged disorderly, and the mitochondria were slightly swollen with the absence of autophagic structures. In the H+AB21 group, autophagic structures were occasionally observed with the absence of apoptotic cells.
The protein levels of Beclin-1, LC3-II, P53, Bcl-2, Caspase-3, and Caspase-9 in gastric mucosal tissues were analyzed by Western blot analysis (Figs. 2A and 2B). We found a time-dependent increase in Beclin-1 and LC3-II levels in rats exposed to hypoxia. Compared with the H group, Beclin-1 and LC3-II levels were upregulated by rapamycin treatment but downregulated by 3-MA treatment. The levels of P53, Caspase-3 and Caspase-9 increased, while the Bcl-2 level decreased with increased exposure to hypoxia. However, rapamycin treatment led to a significant increase in the levels of P53, Caspase-3 and Caspase-9 but a significant decrease in Bcl-2 level, while 3-MA treatment led to opposite effects. These data indicated that the exposure to high-altitude hypoxia resulted in progressive autophagy and deterioration of gastric mucosal injury, which can be aggravated by rapamycin but alleviated by 3-MA.
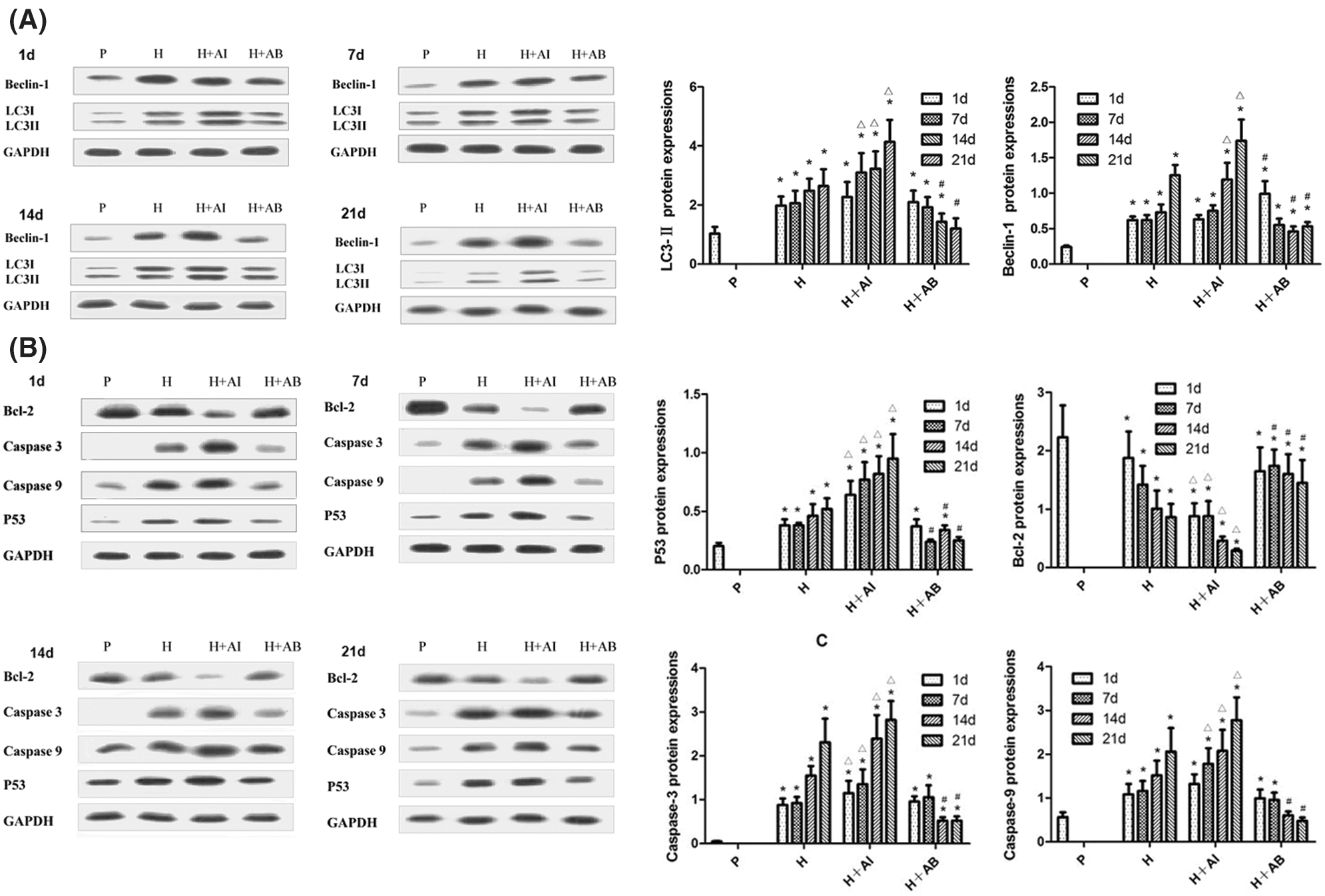
Figure 2: The levels of Beclin-1, LC3-II, P53, Bcl-2, Caspase-3, and Caspase-9 in gastric mucosal tissues. (A) Levels of Beclin-1 and LC3-II. (B) Levels of P53, Bcl-2, Caspase-3, and Caspase-9. Data were expressed as mean ± SD (N = 3). *P < 0.05 vs. P; ΔP < 0.05 vs. H at the same time; #P < 0.05 vs. H and H+AI at the same time.
Hypoxia induced apoptosis of CC-R039 cells
The apoptosis and cell cycle of CC-R039 cells cultured under hypoxia for 0, 24, 48, and 72 h were assayed by flow cytometry (Figs. 3A and 3B). The results showed that increased duration of hypoxia led to a significant increase in the apoptosis rate, and apoptosis rate was further increased by mTORC1 inhibitor rapamycin. Moreover, the percentage of cells in the G0/G1 phase increased and the percentage of cells in the S phase decreased with increased duration of hypoxia. Compared with cells exposed to hypoxia, treatment with rapamycin resulted in a further increase in the percentage of cells in the G0/G1 phase.
In addition, we detected the levels of Beclin-1, LC3-II, P53, Bcl-2, Caspases-3, and Caspases-9 in CC-R039 cells exposed to hypoxia. Western blot analysis showed that the levels of Beclin-1 and LC3II increased as the duration of hypoxia increased in both H and H+anti-mTORC1 groups, and the increase was enhanced in the latter group. Similarly, increasing the duration of hypoxia increased the levels of P53, Caspases-3 and Caspases-9, and reduced Bcl-2 levels in both H and H+anti-mTORC1 groups (Figs. 3C and 3D). These results suggested that hypoxia could induce apoptosis of gastric mucosal cells and inhibit the transition of the cell cycle from G0/G1 phase to S phase, and the inhibition of mTORC1 by rapamycin could further induce apoptosis and inhibit the proliferation of gastric mucosal cells.
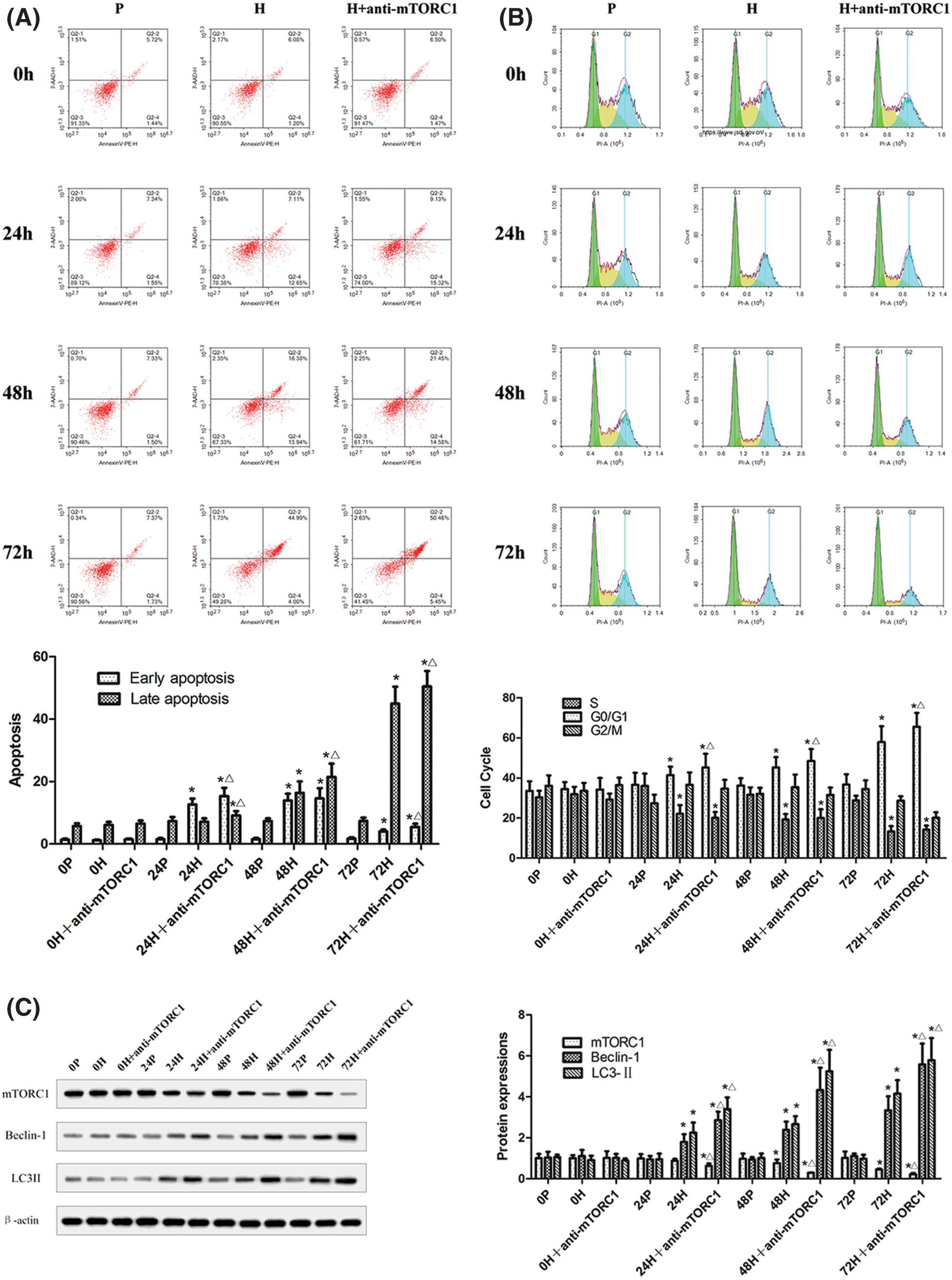
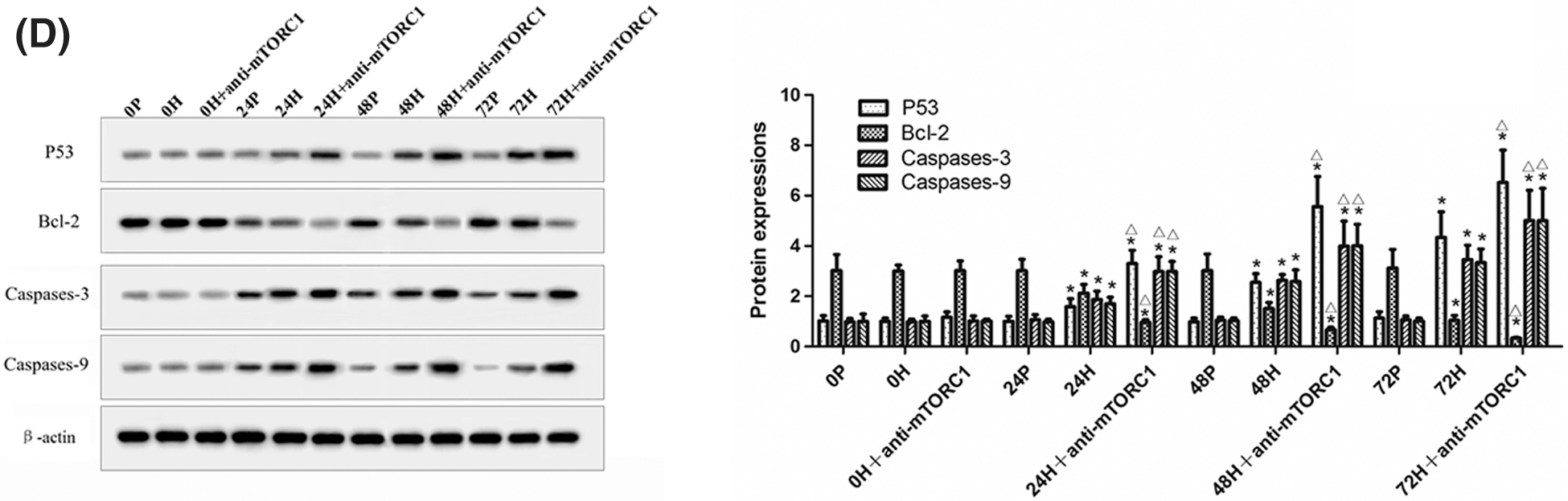
Figure 3: Autophagy and apoptosis of CC-R039 cells cultured under hypoxia. (A) Cell apoptosis rate. (B) Cell cycle distribution. (C) Levels of mTORC1, Beclin-1 and LC3-II. (D) Levels of P53, Bcl-2, Caspases-3, and Caspases-9. P: control; H: 2% O2; H + anti-mTORC1: 2% O2 + specific inhibitor of mTORC1. Data were expressed as mean ± SD (N = 3). *P < 0.05 vs. P and 0 h; ∆P < 0.05 vs. H at the same time.
HIF-1α knockdown inhibited the autophagy of CC-R039 cells
The localization and distribution of autophagy-specific proteins Beclin-1 and LC3 in CC-R039 cells were observed under a fluorescence microscope (Figs. 4A and 4B). The expression levels of Beclin-1 and LC3 increased as the duration of hypoxia increased. In addition, their expression levels were upregulated in CC-R039 cells exposed to hypoxia for 24, 48, and 72 h, but downregulated in CC-R039 cells transfected with si-HIF-1α.
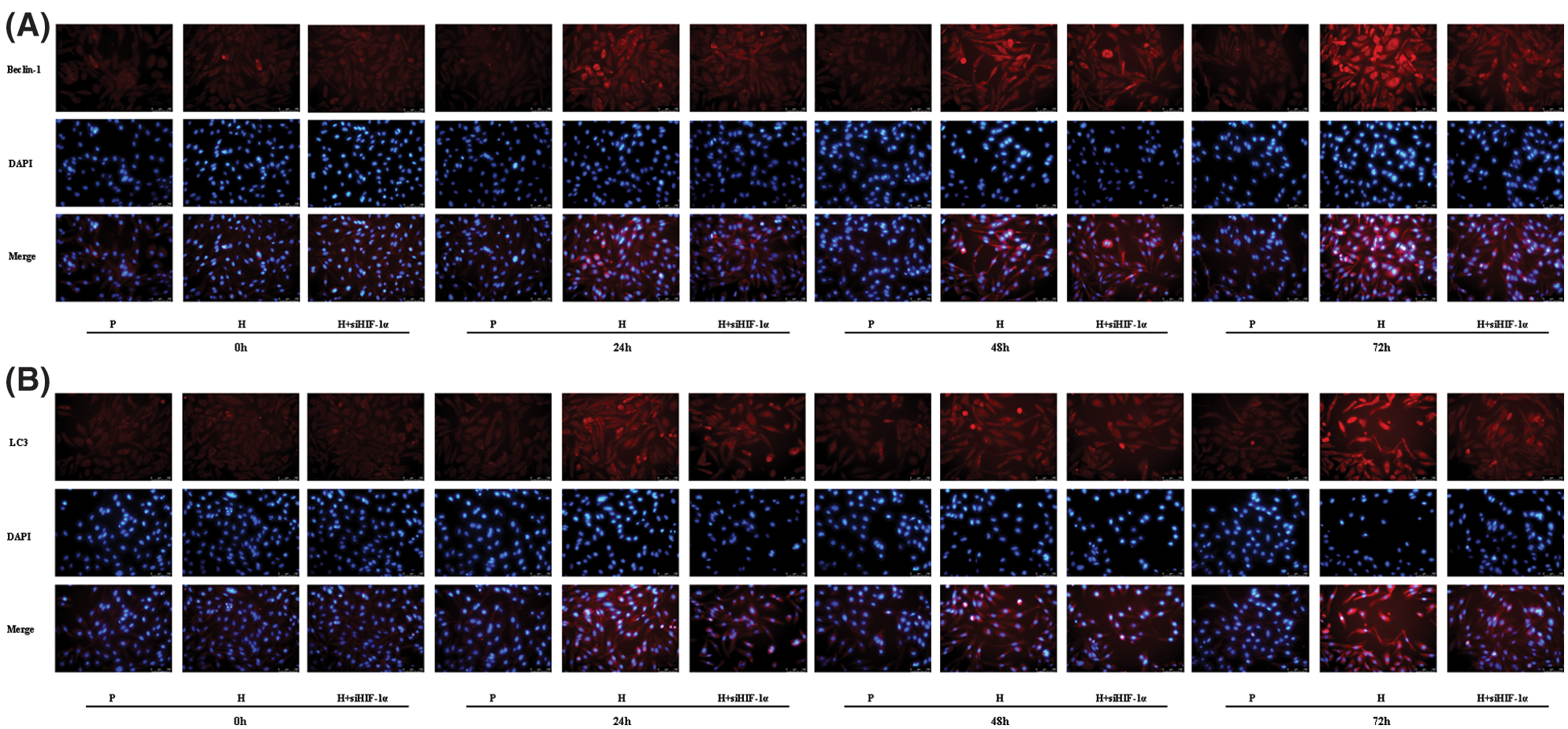
Figure 4: Autophagy of CC-R039 cells cultured under hypoxia after HIF-1α knockdown. (A) Localization and distribution of Beclin-1. (B) Localization and distribution of LC3. P: control; H: 2% O2; H + siHIF-1α: 2% O2 + small interfering RNA for siHIF-1α.
In CC-R039 cells exposed to hypoxia, as the duration of hypoxia increased the expression of mTORC1 decreased and the expression of Beclin-1 and LC3II increased. However, transfection with siHIF-1α activated mTORC1 and decreased Beclin-1 and LC3II expression. In addition, the expression levels of HIF-1α and TERT were upregulated in cells exposed to hypoxia but downregulated in cells transfected with siHIF-1α (Figs. 5A and 5B). These results indicated that knockdown of HIF-1α under hypoxic conditions could inhibit autophagy by activating mTORC1.
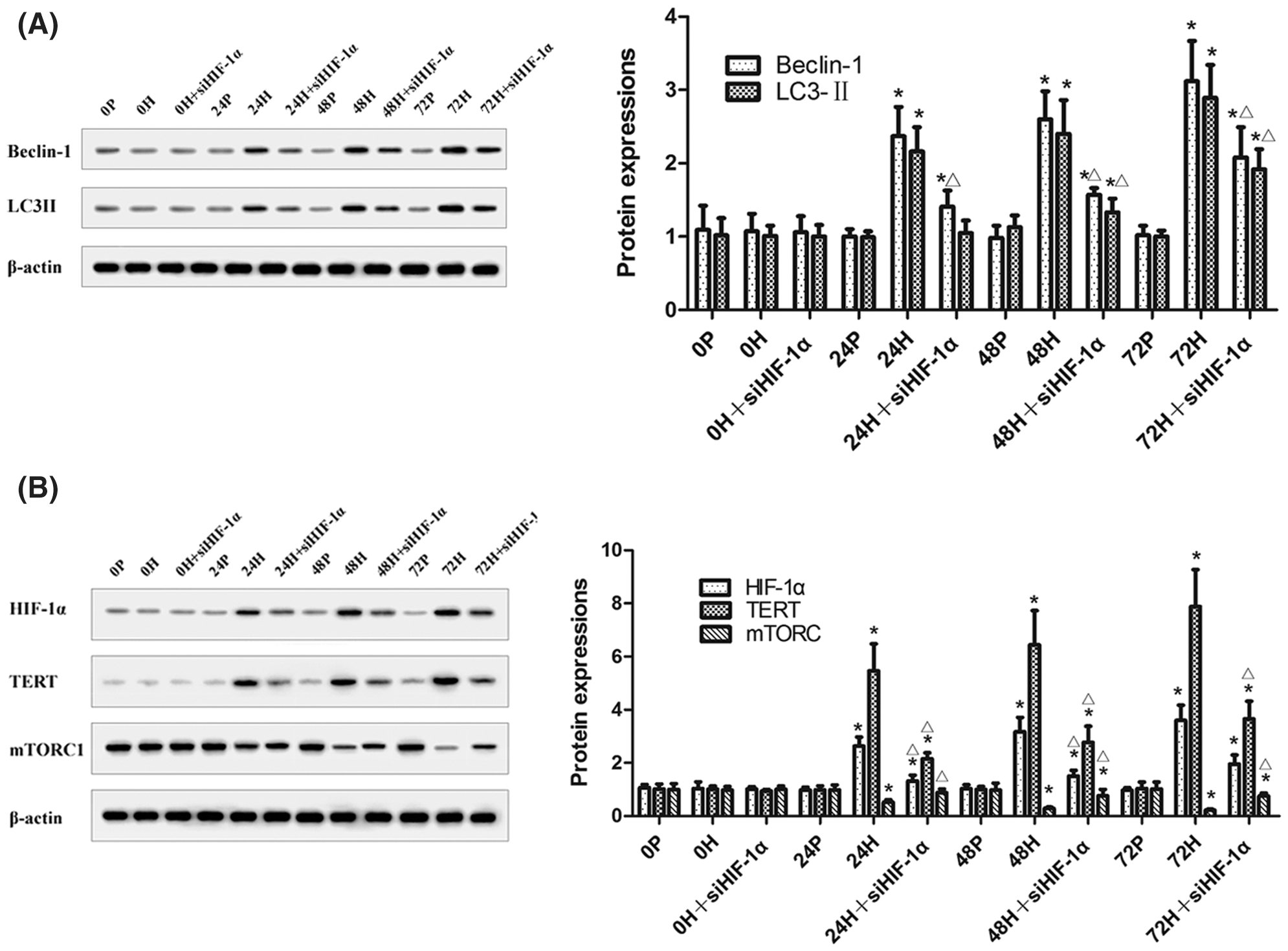
Figure 5: The levels of mTORC1, Beclin-1, LC3II, HIF-1α, and TERT in CC-R039 cells after HIF-1α knockdown. (A) Levels of Beclin-1 and LC3II. (B) Levels of mTORC1, HIF-1α, and TERT. Data were expressed as mean ± SD (N = 3). *P < 0.05 vs. P and 0 h; ΔP < 0.05 vs. H at the same time.
TERT knockdown inhibited the autophagy of CC-R039 cells
Because the upregulation of HIF-1α can induce TERT expression under hypoxic conditions, TERT may be a potential downstream target of HIF-1α. We found that the expression of Beclin-1 and LC3 in CC-R039 cells increased as the duration of hypoxia increased. Compared with the control group, the expression levels of Beclin-1 and LC3 were upregulated in cells exposed to hypoxia but downregulated in cells transfected with siTERT (Figs. 6A and 6B).
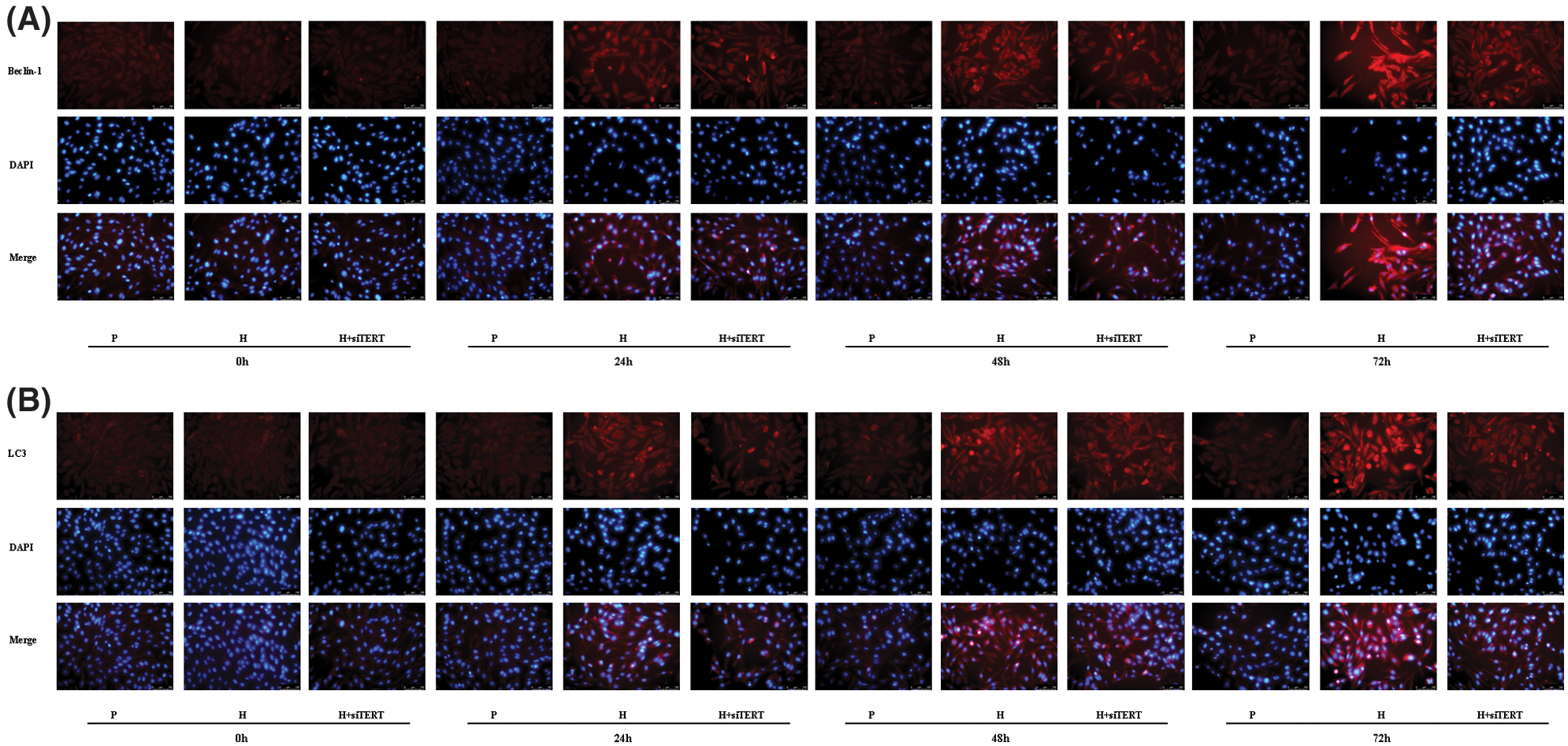
Figure 6: Autophagy of CC-R039 cells cultured under hypoxia after TERT knockdown. (A) Localization and distribution of Beclin-1. (B) Localization and distribution of LC3. P: control; H: 2% O2; H + siTERT: 2% O2 + small interfering RNA for TERT.
Western blot analysis showed that increased duration of hypoxia led to the downregulation of mTORC1 expression and the upregulation of Beclin-1 and LC3II expressions in CC-R039 cells. However, Beclin-1 and LC3II were downregulated while mTORC1 was upregulated in cells transfected with siTERT, indicating that knockdown of TERT could reduce autophagy by activating mTORC1 under hypoxic conditions. Increasing the duration of hypoxia also increased HIF-1α and TERT expression, but transfection with siTERT led to no significant change in HIF-1α expression (Figs. 7A and 7B). Collectively, these results suggested that knockdown of TERT under hypoxic conditions could inhibit autophagy by activating mTORC1, and TERT might be a downstream target of HIF-1α.
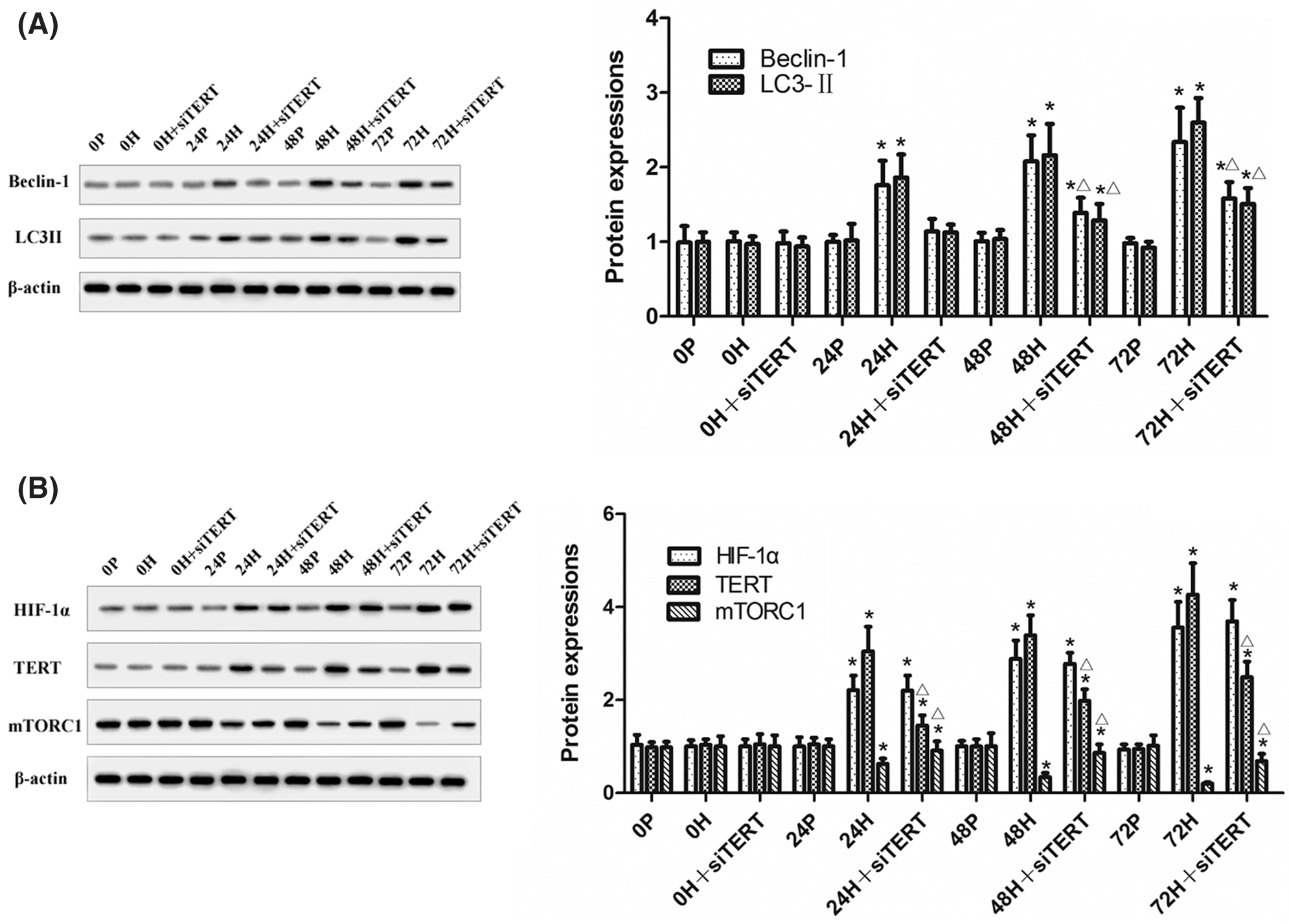
Figure 7: The expression of mTORC1, Beclin-1, LC3II, HIF-1α and TERT in CC-R039 cells after TERT knockdown. (A) Levels of Beclin-1 and LC3II. (B) Levels of mTORC1, HIF-1α, and TERT. Data were expressed as mean ± SD (N = 3). *P < 0.05 vs. P and 0 h; ΔP < 0.05 vs. H at the same time.
Hypoxia can induce oxidative stress and increase apoptosis of gastric mucosal cells, leading to various hypoxia-related gastric mucosal injuries (Liu et al., 2013). In this study, our results showed that increasing the duration of hypoxia could increase the apoptosis injury of gastric epithelial cells in vitro and in vivo.
Hypoxia-induced autophagy can cause cell apoptosis under conditions of severe hypoxia (Mazure and Pouysségur, 2010). Overactivation of autophagy causes the degradation of a large number of proteins and organelles (Mizushima et al., 2008). Cardiac cells may undergo apoptosis in response to exposure to hypoxia (Gong et al., 2020). Inhibiting the expression of autophagy-related proteins reduced ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy and renal failure (Chien et al., 2007). Hypoxia could also induce apoptosis of mouse spermatocyte GC-2 cells by activating autophagy (Zhou et al., 2018). The hyperactivation of autophagy is considered an important pathogenic mechanism for hypoxic-ischemic lung injury (Ye et al., 2020). Our results demonstrated that increasing the duration of hypoxia led to increased autophagy of gastric mucosal cells, and progressive aggravation of gastric mucosal apoptosis injury.
Apoptosis is a process of programmed cellular death regulated by specific genes. Autophagy often precedes apoptosis and cooperates with apoptosis to determine the fate of cells (Edinger and Thompson, 2004). Activation of autophagy may increase the apoptosis of microglial and myocardial cells in ischemia/reperfusion injury (Ouyang et al., 2016). Hypoxia can promote the apoptosis of cardiac microvascular endothelial cells by activating autophagy (Wang et al., 2016). Our study showed that as the duration of hypoxia increased, both autophagy and apoptosis of gastric mucosal cells increased and could be further aggravated by rapamycin treatment but alleviated by 3-MA treatment. Collectively, these data suggest that exposure to high-altitude hypoxia can induce the apoptosis of gastric mucosal cells by activating autophagy.
The signaling pathways involved in the regulation of autophagy remain to be elucidated. There is evidence that mTORC1 is associated with negative regulation of autophagy (Levine et al., 2011). mTORC1 is an evolutionarily conserved Ser/Thr kinase that is involved in gene transcription, protein translation, and autophagy. Upon hypoxia, autophagy is upregulated mainly by the mTORC1 signaling pathway, which is related to PI3K/Akt, HIF-1α, and ROS. HIF-1α is a specific transcription factor that plays a key role in cellular adaptation to hypoxia (Xiao et al., 2013). Hypoxia can induce autophagy through the activation of HIF-1α (Li et al., 2020). TERT is a ribonucleoprotein complex that synthesizes telomeric DNA responsible for maintaining telomere length at chromosome ends (Harley, 1994). Hypoxia can induce HIF-1α expression, which in turn can regulate TERT expression and improve telomerase activity (Nishi et al., 2004). A previous study reported that hypoxia induced HIF-1α and TERT expression (Millonig et al., 2008). It is known that mTORC1 is an interacting protein and downstream target gene of TERT, and TERT can induce basal and amino acid starvation-induced autophagy through mTORC1 (Ali et al., 2016). TERT deficiency inhibited autophagy and delayed renal recovery from ischemia-reperfusion injury in mice (Cheng et al., 2015). Inhibiting TERT can restrict autophagy-mediated nutrient supply to cancer cells (Roh et al., 2018). Under the hypoxic condition, HIF-1α could activate TERT promoter in papillary thyroid carcinoma to inhibit mTORC1 activity and activate autophagy (Song et al., 2021). Our previous study reported that mRNA and protein levels of TERT and HIF-1α were significantly higher in heart and lung tissues of rats exposed to hypoxia and increased with prolonged exposure (Wang et al., 2018). In this study, we found that knockdown of HIF-1α or TERT could activate mTORC1 and inhibit autophagy and apoptosis of gastric mucosal cells. However, knockdown of HIF-1α resulted in a significant decrease in TERT expression, while knockdown of TERT resulted in no significant change in HIF-1α expression. These results indicated that hypoxia could upregulate TERT expression by inducing HIF-1α expression in gastric epithelial cells, which in turn inhibits mTORC1 kinase activity and activates autophagy and apoptosis.
In conclusion, this is the first study to investigate the role of autophagy in hypoxia-induced gastric mucosal injury. Our results suggest that chronic exposure to severe hypoxia at high altitude induces autophagy and apoptosis of rat gastric mucosal cells through HIF-1α/TERT/mTORC1 signaling pathway. Inhibition of autophagy by interfering with HIF-1α/TERT/mTORC1 signaling pathway may be a novel therapy strategy for chronic and acute gastric mucosal injury induced by hypoxia at high altitude.
Availability of Data and Material: All data are available from the corresponding author on reasonable request.
Authors’ Contribution: YPW and XHX conceived and designed the experiments. YPW, XHX, XLL, ZZ and ZYZ performed the experiments. ZZ and XLL analyzed the data. All authors read and approved the manuscript.
Ethics Approval: This study was approved by the Ethical Committee of Qinghai Provincial People’s Hospital (Approval No. 2017-16; Approval Date: January 16, 2017).
Funding Statement: This study is supported by the National Natural Science Foundation of China (No. 81760334), Innovative and Entrepreneurial Doctor Program of Jiangsu Provincial, Top Talents Support Program for Young and Middle-Aged People of Wuxi Health Committee and The Scientific Research Project of Wuxi Health committee (Youth Project, No. Q201912). The funds have no role in the design of the study, the collection, analysis, and interpretation of data, and the composition of the manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ali M, Devkota S, Roh JI, Lee J, Lee HW (2016). Telomerase reverse transcriptase induces basal and amino acid starvation-induced autophagy through mTORC1. Biochemical and Biophysical Research Communications 478: 1198–1204. DOI 10.1016/j.bbrc.2016.08.094. [Google Scholar] [CrossRef]
Cheng H, Fan X, Lawson WE, Paueksakon P, Harris RC (2015). Telomerase deficiency delays renal recovery in mice after ischemia-reperfusion injury by impairing autophagy. Kidney International 88: 85–94. DOI 10.1038/ki.2015.69. [Google Scholar] [CrossRef]
Chien CT, Shyue SK, Lai MK (2007). Bcl-xL augmentation potentially reduces ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy. Transplantation 84: 1183–1190. DOI 10.1097/01.tp.0000287334.38933.e3. [Google Scholar] [CrossRef]
Edinger AL, Thompson CB (2004). Death by design: Apoptosis, necrosis and autophagy. Current Opinion in Cell Biology 16: 663–669. DOI 10.1016/j.ceb.2004.09.011. [Google Scholar] [CrossRef]
Gong X, Fan G, Liu H, Wang S, Huang R (2020). Human umbilical cord blood mesenchymal stem cells conditioned media inhibits hypoxia-induced apoptosis in H9c2 cells by activation of the survival protein Akt. BIOCELL 44: 329–336. DOI 10.32604/biocell.2020.09639. [Google Scholar] [CrossRef]
Harley C (1994). Telomerases: New approaches to anticancer chemotherapy: From basic concepts to therapeutic strategy. Pathologie et Biologie 42: 342–345. [Google Scholar]
Khanna K, Mishra KP, Chanda S, Eslavath MR, Ganju L et al. (2019). Effects of acute exposure to hypobaric hypoxia on mucosal barrier injury and the gastrointestinal immune axis in rats. High Altitude Medicine & Biology 20: 35–44. DOI 10.1089/ham.2018.0031. [Google Scholar] [CrossRef]
Levine B, Mizushima N, Virgin HW (2011). Autophagy in immunity and inflammation. Nature 469: 323–335. DOI 10.1038/nature09782. [Google Scholar] [CrossRef]
Li H, Liao C, Weng W, Zhong H, Zhou T (2020). Association of hypoxia-inducible factor-1α (HIF1α) 1772C/T gene polymorphism with susceptibility to renal cell carcinoma/prostate cancer. BIOCELL 44: 257–262. DOI 10.32604/biocell.2020.08826. [Google Scholar] [CrossRef]
Liu L, Liu Y, Cui J, Liu H, Liu YB et al. (2013). Oxidative stress induces gastric submucosal arteriolar dysfunction in the elderly. World Journal of Gastroenterology 19: 9439. DOI 10.3748/wjg.v19.i48.9439. [Google Scholar] [CrossRef]
Mazure NM, Pouysségur J (2010). Hypoxia-induced autophagy: Cell death or cell survival? Current Opinion in Cell Biology 22: 177–180. DOI 10.1016/j.ceb.2009.11.015. [Google Scholar] [CrossRef]
Millonig G, Hegedüsch S, Becker L, Seitz H-K, Schuppan D, Mueller S (2008). Hypoxia-inducible factor 1 alpha under rapid enzymatic hypoxia: Cells sense decrements of oxygen but not hypoxia per se. Free Radical Biology & Medicine 46: 182–191. DOI 10.1016/j.freeradbiomed.2008.09.043. [Google Scholar] [CrossRef]
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008). Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075. DOI 10.1038/nature06639. [Google Scholar] [CrossRef]
Mostowy S (2013). Autophagy and bacterial clearance: A not so clear picture. Cellular Microbiology 15: 395–402. DOI 10.1111/cmi.12063. [Google Scholar] [CrossRef]
Netzer N, Strohl K, Faulhaber M, Gatterer H, Burtscher M (2013). Hypoxia-related altitude illnesses. Journal of Travel Medicine 20: 247–255. DOI 10.1111/jtm.12017. [Google Scholar] [CrossRef]
Nishi H, Nakada T, Kyo S, Inoue M, Shay JW, Isaka K (2004). Hypoxia-inducible factor 1 mediates upregulation of telomerase (hTERT). Molecular and Cellular Biology 24: 6076–6083. DOI 10.1128/MCB.24.13.6076-6083.2004. [Google Scholar] [CrossRef]
Ouyang F, Huang H, Zhang M, Chen M, Huang H et al. (2016). HMGB1 induces apoptosis and EMT in association with increased autophagy following H/R injury in cardiomyocytes. International Journal of Molecular Medicine 37: 679–689. DOI 10.3892/ijmm.2016.2474. [Google Scholar] [CrossRef]
Roh JI, Kim Y, Oh J, Kim Y, Lee J et al. (2018). Hexokinase 2 is a molecular bridge linking telomerase and autophagy. PLoS One 13: e0193182. DOI 10.1371/journal.pone.0193182. [Google Scholar] [CrossRef]
Song H, Chen X, Jiao Q, Qiu Z, Shen C et al. (2021). HIF-1α-mediated telomerase reverse transcriptase activation inducing autophagy through mammalian target of rapamycin promotes papillary thyroid carcinoma progression during hypoxia stress. Thyroid 31: 233–246. DOI 10.1089/thy.2020.0023. [Google Scholar] [CrossRef]
Wang R, Yang Q, Wang X, Wang W, Li J et al. (2016). FoxO3α-mediated autophagy contributes to apoptosis in cardiac microvascular endothelial cells under hypoxia. Microvascular Research 104: 23–31. DOI 10.1016/j.mvr.2015.11.001. [Google Scholar] [CrossRef]
Wang Y, Zhao Z, Zhu Z, Li P, Li X et al. (2018). Telomere elongation protects heart and lung tissue cells from fatal damage in rats exposed to severe hypoxia. Journal of Physiological Anthropology 37: 5. DOI 10.1186/s40101-018-0165-y. [Google Scholar] [CrossRef]
Wu TY, Ding SQ, Liu JL, Jia JH, Dai RC et al. (2007). High-altitude gastrointestinal bleeding: An observation in Qinghai-Tibetan railroad construction workers on Mountain Tanggula. World Journal of Gastroenterology 13: 774. DOI 10.3748/wjg.v13.i5.774. [Google Scholar] [CrossRef]
Xiao H, Gu Z, Wang G, Zhao T (2013). The possible mechanisms underlying the impairment of HIF-1α pathway signaling in hyperglycemia and the beneficial effects of certain therapies. International Journal of Medical Sciences 10: 1412–1421. DOI 10.7150/ijms.5630. [Google Scholar] [CrossRef]
Ye C, Qi W, Dai S, Zou G, Liu W et al. (2020). microRNA-223 Promotes autophagy to aggravate lung ischemia-reperfusion injury by inhibiting the expression of transcription factor HIF2α. American Journal of Physiology-Lung Cellular and Molecular Physiology 319: L1–L10. [Google Scholar]
Zhang J, Huang Y, Liu W, Li L, Chen L (2020). Chaperone-mediated autophagy targeting chimeras (CMATAC) for the degradation of ERα in breast cancer. BIOCELL 44: 591–595. DOI 10.32604/biocell.2020.011642. [Google Scholar] [CrossRef]
Zhou J, Qian CY, Tong RQ, Wang B, Chen XL et al. (2018). Hypoxia induces apoptosis of mouse spermatocyte GC-2 cells through activation of autophagy. Cell Biology International 42: 1124–1131. DOI 10.1002/cbin.10971. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |