DOI:10.32604/biocell.2022.018679

| BIOCELL DOI:10.32604/biocell.2022.018679 |  |
| Viewpoint |
Prognostic, diagnostic and therapeutic potential of endothelial progenitor cells for patients with ischaemic stroke: Hype or Hope
Academic Unit of Mental Health and Clinical Neuroscience, School of Medicine, Nottingham, NG5 1PB, UK
*Address correspondence to: Ulvi Bayraktutan, ulvi.bayraktutan@nottingham.ac.uk
Received: 10 August 2021; Accepted: 23 August 2021
Abstract: Ischaemic stroke is a debilitating disease with immense personal, societal and economic impact. Thrombolysis with recombinant tissue plasminogen activator remains the only approved pharmacotherapy for this disease. As each year less than 1% of eligible patients receive this therapy worldwide, efficacious new therapeutics are desperately needed. Emerging evidence suggest endothelial progenitor cells (EPCs), capable of repairing damaged vasculature, as one such therapeutics. However, questions regarding their optimal dose, delivery route and in vivo survivability remain largely unanswered. Outgrowth endothelial cells, generated in large numbers by ex vivo expansion of EPCs, enable effective assessment of these issues and may eventually serve as off-the-shelf therapeutics. Correlations between circulating EPC levels and stroke outcome imply that EPCs may also serve as clinical biomarkers for stroke. This viewpoint briefly evaluates the current evidence, pinpoints the gaps in the literature and proposes new directions for research.
Keywords: Ischaemic stroke; Endothelial Progenitor Cells; Stroke therapeutics; Clinical biomarkers; Regenerative medicine
Abbreviations
| BBB: | Blood-brain barrier |
| BMSC: | Bone marrow-derived mononuclear stem cell |
| EPC: | Endothelial progenitor cell |
| HLA: | Human leukocyte antigen |
| IS: | Ischaemic stroke |
| OEC: | Outgrowth endothelial cell |
| PDGF-BB: | Platelet-derived growth factor-BB |
| RACs: | Regeneration-associated cells |
| rtPA: | recombinant tissue plasminogen activator |
| TNF-α: | Tumour necrosis factor-α |
| VEGF: | Vascular endothelial growth factor |
Stroke annually affects about 13.5 million people worldwide and constitutes one of the leading causes of mortality and morbidity (Virani et al., 2021). Ischaemic stroke, stemming from the occlusion of an artery leading to the brain, represents the major subtype of stroke and accounts for approximately 85% of all strokes in the Western world (Hisham and Bayraktutan, 2013). At present, reperfusion therapy with recombinant tissue plasminogen activator (rtPA) and mechanical thrombectomy are the only approved treatment options for acute IS. However, due to short therapeutic windows associated with these treatment regimens, each year globally <1% of patients receive these therapies (Malhotra et al., 2019; Virani et al., 2021).
The pathophysiology of stroke is complex and involves many interrelated processes, including depletion of ATP, apoptosis, necrosis, excitotoxicity, inflammation, oxidative stress and an abrupt surge in intracellular calcium levels (Allen and Bayraktutan, 2008; Rakkar and Bayraktutan, 2016). Despite having tremendous success in preclinical studies, agents targeting these particular mechanisms have failed to produce similar benefits in clinical trials. The incessant failure of clinical trials with the so-called neuroprotectants prompted search for alternative therapeutics and placed cell-based therapeutic approaches targeting cerebrovascular integrity at the forefront of clinical investigation. Given that disruption of blood-brain barrier (BBB) and ensuing cerebral oedema constitute the main cause of death within the first week after an IS, adoption of a vascular restorative/reparative approach was somewhat inevitable (Hou and MacManus, 2002; Dankbaar et al., 2011). In this context, endothelial progenitor cells (EPCs), capable of angiogenesis, vasculogenesis, self-renewal and differentiation into mature endothelial cells, have attracted much of the attention (Asahara et al., 1999; Bayraktutan, 2017; Bayraktutan, 2019).
Overview of EPCs as Therapeutics
Phase I studies, performed with bone marrow-derived mononuclear stem cells (BMSCs) or CD34+ cells known to contain a population of progenitor cells, prove the safety and feasibility of intravenous and intra-arterial infusion of EPCs during the (sub)acute phase of IS (Fang et al., 2019; Savitz et al., 2011). Despite confirming these findings, a randomised, multicentre study using BMSCs yielded no benefit on stroke outcome (Prasad et al., 2014). Preclinical studies, investigating the degree of EPC incorporation into the damaged vasculature as a measure of therapeutic efficacy, also report inconsistent results in terms of functional outcome (Purhonen et al., 2008; Garbuzova-Davis et al., 2017; Hong et al., 2020). Indeed, while robust engraftment of intravenously administered human bone marrow EPCs within capillaries is associated with widespread repair of vasculature and near normal morphology of endothelial cells, astrocytes and pericytes in bilateral striatum and motor cortex of a rodent model of transient IS (Garbuzova-Davis et al., 2017), studies showing inability of circulating EPCs to engraft vasculature also exists (Hagensen et al., 2012). As ambiguities regarding the nature of EPCs somewhat account for these discrepancies, it is of utmost importance to standardise the definition of EPCs in order to accurately evaluate their therapeutic efficacy. As alluded above, CD34+ cells, isolated from peripheral blood by fluorescence activated cell sorting, are described in various studies as EPCs (Banerjee et al., 2014; Shyu et al., 2006). Although shown to promote revascularisation in ischaemic hearts and brains, it is unlikely that these haematopoietic stem cells can actually be true EPCs (Chen et al., 2014; Sobrino et al., 2011). Because, EPCs possess embryonic angioblast-like characteristics and are the precursors of mature endothelial cells, it is likely that only few circulating nonhaematopoietic cells (e.g., CD45- or CD14-), concomitantly expressing markers for stemness (e.g., CD34+ or CD117+), immaturity (e.g., CD133+) and endothelial cells (e.g., KDR+ or CD31+), may make up the true EPCs. It is of note that a recent study suggests ephrinB2 and bone morphogenetic protein 2 and 4 as important novel markers for identification and characterisation of EPC subpopulation in adult peripheral blood (Abdelgawad et al., 2021).
In addition to repairing vascular damage, EPCs can also stimulate the process of endogenous recovery by immunomodulation and secretion of various trophic factors, notably stromal-derived factor-1, angiopoietin-1, vascular endothelial growth factor (VEGF), basic fibroblast growth factor and platelet-derived growth factor-BB (Ohab et al., 2006; Rosell et al., 2013). Enhanced functional recovery coupled with decreases in infarct volume, angiogenesis and anti-inflammatory cytokine release provide indirect evidence for the beneficial effects of stem cells (and their secretome) in animal models of stroke (di Santo et al., 2009; Rosell et al., 2013). Our recent studies probing how EPCs, defined as CD45-CD34+CD133+KDR+ cells, may affect the integrity and function of an in vitro model of human BBB subjected to ischaemic injury or TNF-α, a key cytokine during the post-stroke inflammation, offers convincing direct evidence for their protective effects in that suppression of stress fibre formation, oxidative stress and apoptosis play a pivotal role (Abdulkadir et al., 2020; Alwjwaj et al., 2021). Crucially, these studies also indicate that much of the EPC-mediated beneficial effects are realised by their secretome and thus necessitate the scrutiny of EPC secretome as a potential therapeutic for IS. Microparticles, containing DNA, RNA and microRNAs, represent an important constituent of the secretome and deserve attention in future studies due to their seminal role in inducing vascular endothelial regeneration via promotion of tissue-resident endothelial cell proliferation and migration (Kadir et al., 2020). Concurrent manipulation of other key factors known to mediate mobilisation, homing and differentiation of EPCs, such as VEGF, stromal derived factor-1 and interleukin-10 also deserve attention in relevant future studies (Bayraktutan, 2019; Nagata et al., 2019).
Since EPCs repair the damaged vasculature and can also work as proangiogenic support cells, the number of circulating EPCs is widely considered as a reliable diagnostic and/or prognostic marker for IS. Indeed, observational studies scrutinising the correlation between EPC number and stroke outcome have correlated the increased cell numbers in acute and subacute phases of the disease to greater vascular repair, endothelial restoration and better clinical outcome (Paczkowska et al., 2009; Martí-Fàbregas et al., 2013). However, investigations reporting lower baseline levels of circulating EPCs in acute IS patients compared to healthy volunteers also exist (Tsai et al., 2014). In addition to EPC number, variations in the capacity of cultured EPCs to migrate, proliferate and form tubules and colonies are also regarded as important diagnostic and prognostic markers for IS. Even so, the current data on these EPC characteristics during different phases of stroke, particularly chronic phase, are rather scant and controversial. Besides, exclusion of healthy volunteers from most studies make it difficult to interpret the data generated (Chu et al., 2008; Martí-Fàbregas et al., 2013; Zhou et al., 2009). Considering the gap in the literature and bearing in mind the close association reported between EPC numbers and different subtypes of stroke (Tsai et al., 2014), a recent study has longitudinally assessed the diagnostic and prognostic value of EPCs in elderly patients with lacunar or cortical stroke during acute, subacute and chronic phases of the disease (Rakkar et al., 2020). By recruiting both elderly (≥65 years) and young (18–64 years) healthy volunteers, this study has also addressed the specific association between EPC characteristics (number and functional aspects) and chronological ageing, a prominent risk factor for IS. The data indicate that ageing process and other vascular risk factors, including hypertension and diabetes, adversely affect the release and function of EPCs in healthy volunteers and the count and function of EPCs are similar in lacunar and cortical stroke patients during different phases of the disease.
Cell culture, based on adhesion of cells to specific substrates, e.g., collagen, before culture in endothelial cell specific media is recognised as the best methodology to generate adequate numbers of homogeneous cells for therapeutic purposes. This procedure leads to generation of two functionally and morphologically distinct EPC subtypes: early EPCs (eEPCs) and outgrowth endothelial cells (OECs), also known as endothelial colony forming cells (Bayraktutan, 2019). The molecular profiling of early EPCs classifies them as haematopoietic cells (Medina et al., 2010a). OECs, on the other hand, constitute the functional subtype of EPCs and display strong proliferative, migratory and tubulogenic capacity in vitro. OECs also express endothelial and progenitor cell markers which differentiate them from haematopoietic cells and circulating mature endothelial cells, respectively (Abdulkadir et al., 2020). Attempts to expand OECs ex vivo have shown that after a certain number of passages, OECs go into senescence and display classical markers of senescence including enlarged cellular phenotype, S-β-galactosidase activity and DNA damage. Albeit associated with dysfunctionality and limited regenerative potential, OEC senescence is also associated with the reduced risk of tumourigenesis, indicating their safety in vivo (Medina et al., 2013). Ability of OECs to lodge and survive in nine different vascular beds for up to 7 months after injection without inducing any thrombosis or infarcts confirm their long-term efficacy and safety (Milbauer et al., 2009). Through modulation of key mechanisms involved in OEC senescence, such as interleukin-8 and growth differentiation function 15, it is possible to delay senescence in a controlled manner and/or improve vascular functionality (Medina et al., 2013; Ha et al., 2019).
Key features that make OECs potentially a very effective cell-based therapeutic include that OECs repair post-ischaemic vascular injuries by directly incorporating into host endothelium and inducing angiogenesis. Significant decreases in avascular areas in a murine model of retinal ischemia and improvements in cardiac function in a porcine model of acute myocardial infarction support this hypothesis (Medina et al., 2010b; Dubois et al., 2010). Other features include that OECs can be expanded from patients’ own blood for autologous therapy which markedly eliminate the risk of rejection. However, the time needed to cultivate the number of cells required for transplantation restricts autologous therapy to chronic phases of disease and necessitates consideration of an allogeneic approach for patients with acute and subacute stroke. Albeit conceptually fraught with immunological risk, a meta-analysis of large animals with ischaemic heart disease has shown that both autologous and allogeneic cell therapies are equally safe and efficacious (Jansen et al., 2015). Detection of a greater tissue repair in acute ischaemic stroke patients at one year of receiving intravenously injected allogeneic cells further confirm the safety of this therapeutic approach (Vaes et al., 2012) where suppression of both CD4 and CD8 T cells proliferation and activation by EPCs may be crucial (Naserian et al., 2020). It may be possible to further augment the immunosuppressive capacity of EPCs by priming them with TNF-α to specifically activate TNF/TNFR2 (TNF-α receptor 2) signalling pathway before administration (Barkestani et al., 2021). Creation of cell banks with detailed characterisation of HLA haplotypes matching population requirements and use of immunologically immature cord blood-OECs may also help address immune-(in)compatibility issue. By mediating the secretion of both interleukin-10, an anti-inflammatory cytokine and transforming growth factor-β1, (TGF-β1), cord blood-OECs restore a functional vascular network under ischaemic conditions in immunocompetent mice (Proust et al., 2020). TGF-β1 is a multifunctional protein that plays a crucial role in the formation of blood vessels, wound healing and inflammatory processes in the immune system (Fujio et al., 2016).
Administration of regeneration-associated cells (RACs) may be an alternative approach to potentiate the therapeutic efficacy of EPCs in ischaemic diseases. RACs, obtained by vasculogenic conditioning of peripheral mononuclear cells in the presence of human recombinant stem cell factor, thrombopoietin, Flt-3 ligand, VEGF and interleukin-6, can enhance EPC expansion and activate anti-inflammatory and angiogenic monocytes/macrophages and helper T lymphocytes (Masuda et al., 2012; Masuda et al., 2014). In accordance with these findings, administration of RACs to an animal model of human transient IS during the acute phase of ischaemic injury has successfully reduced infarct volume and promoted significant recovery of neural tissues through intensified angiogenic and anti-inflammatory effects (Nakayama et al., 2019).
Future Issues for Consideration
As summarised in Fig. 1, due to extremely low level of true EPCs in circulation, it is highly unlikely to sort sufficient number of cells from peripheral blood that can be used for therapeutic purposes. OECs, the functional subtype of EPCs generated by cell culture, appear to be a highly promising therapeutics with immense potential for post-ischaemic vascular repair, vasculogenesis, angiogenesis and possibly neurogenesis (Takizawa et al., 2016, Bayraktutan, 2019). Although limited replicative potential and the current immunological understanding, based on animal and clinical data, support the use of allogeneic therapy, various issues concerning the survivability, in vivo tracking, optimal dose and delivery route need clarification. Furthermore, questions regarding tolerance to repeat OEC dosing need to be addressed. Should cells from a different donor be used to avoid anamnestic reaction? Also, questions regarding the co-application of OECs with other stem cells or agents targeting oxidative stress, inflammation or increased intracellular Ca2+ levels need to be addressed. How would these applications affect the therapeutic impact of OECs? Similarly, questions regarding immunological reactions also need to be addressed. Should HLA-matching be performed before administration of OECs? Should immunological reaction alongside potential (serious) adverse effects be monitored after administration?
In conclusion, well-planned comprehensive studies, monitoring the levels and functional capacity of circulating EPCs and the immunologic profile of the recipients, are needed to unravel the true diagnostic or therapeutic value of EPCs/OECs for IS patients.
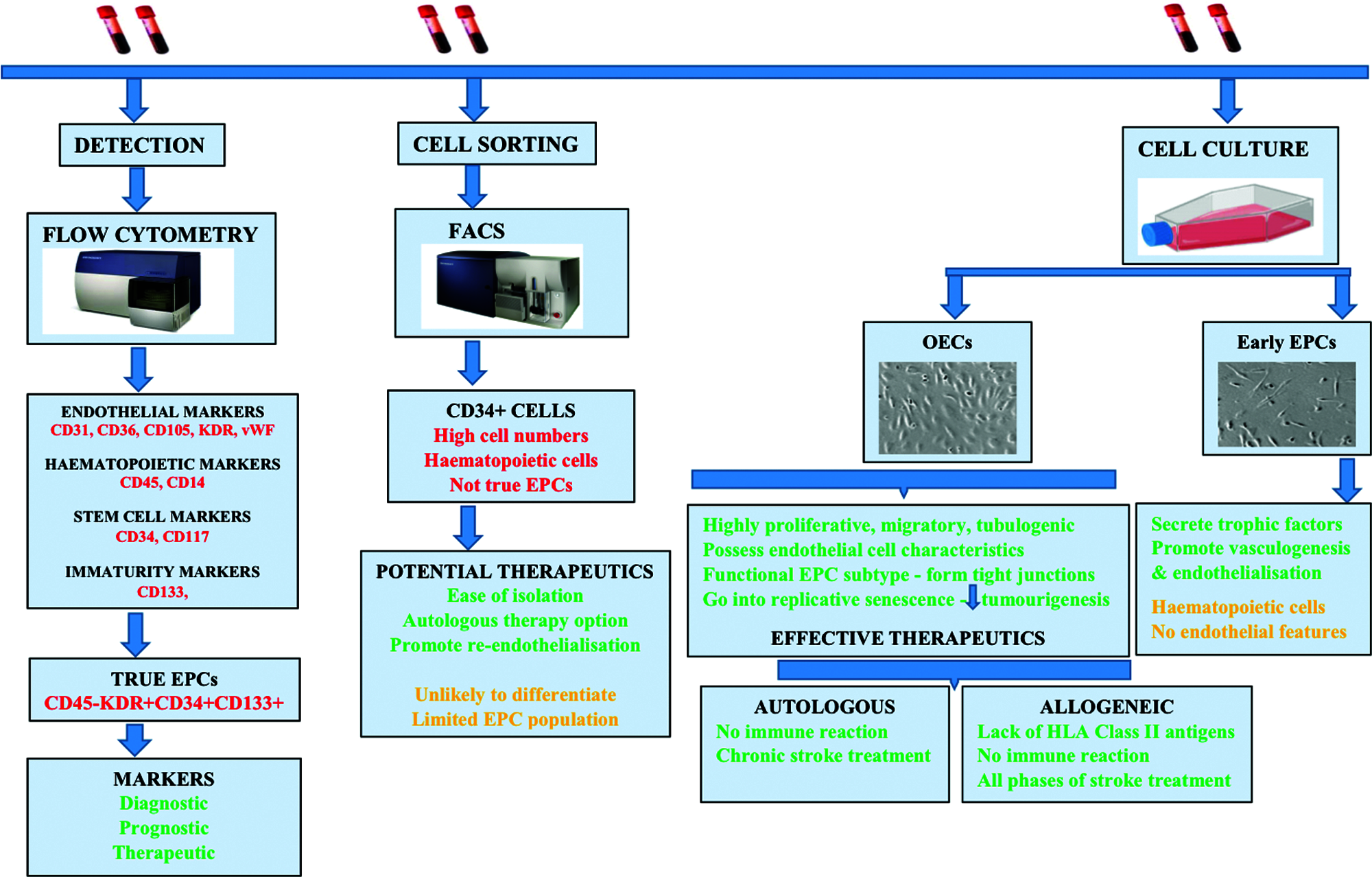
Figure 1: Summary of the main processes by which endothelial progenitor cells (EPCs) are obtained from peripheral blood and used for clinical purposes. EPCs can serve as diagnostic, prognostic or therapeutic biomarkers for ischaemic stroke. Alternatively, EPCs may be sorted by FACS or expanded by cell culture to obtain a large number of homogenous cells which successively yield CD34+ cells and outgrowth endothelial cells (OECs). These then can be used as efficacious therapeutics in autologous or allogeneic therapies.
Acknowledgement: Funders are thanked for support of this writing.
Availability of Data and Materials: No data are included in this viewpoint.
Author Contribution: The author confirms sole responsibility for the following: study conception and design, data collection, analysis and interpretation of results, and manuscript preparation.
Ethics Approval: No committees were required for this study.
Funding Statement: This work was supported by a grant to Dr. Ulvi Bayraktutan from the Dunhill Medical Trust (R459/0216). The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The contents are solely the responsibility of the author and do not necessarily represent the official views of the DMT.
Conflicts of Interest: The author declares that they have no conflicts of interest to report regarding the present study.
Abdelgawad ME, Desterke C, Uzan G, Naserian S (2021). Single-cell transcriptomic profiling and characterization of endothelial progenitor cells: New approach for finding novel markers. Stem Cell Research & Therapy 12: 1351. DOI 10.1186/s13287-021-02185-0. [Google Scholar] [CrossRef]
Abdulkadir RR, Alwjwaj M, Othman OA, Rakkar K, Bayraktutan U (2020). Outgrowth endothelial cells form a functional cerebral barrier and restore its integrity after damage. Neural Regeneration Research 15: 1071–1078. DOI 10.4103/1673-5374.269029. [Google Scholar] [CrossRef]
Allen CL, Bayraktutan U (2008). Risk factors for ischaemic stroke. International Journal of Stroke 3: 105–116. DOI 10.1111/j.1747-4949.2008.00187.x. [Google Scholar] [CrossRef]
Alwjwaj M, Kadir RRA, Bayraktutan U (2021). The secretome of endothelial progenitor cells: A potential therapeutic strategy for ischemic stroke. Neural Regeneration Research 16: 1483–1489. DOI 10.4103/1673-5374.303012. [Google Scholar] [CrossRef]
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C et al. (1999). Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation Research 85: 221–228. DOI 10.1161/01.RES.85.3.221. [Google Scholar] [CrossRef]
Banerjee S, Bentley P, Hamady M, Marley S, Davis J et al. (2014). Intra-arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Translational Medicine 3: 1322–1330. DOI 10.5966/sctm.2013-0178. [Google Scholar] [CrossRef]
Barkestani MN, Shamdani S, Bakshloo MA, Arouche N, Bambai B et al. (2021). TNFα priming through its interaction with TNFR2 enhances endothelial progenitor cell immunosuppressive effect: New hope for their widespread clinical application. Cell Communication and Signaling 19: 291. DOI 10.1186/s12964-020-00683-x. [Google Scholar] [CrossRef]
Bayraktutan U (2017). Endothelium, endothelial progenitor cells and stroke. Journal of Neurology and Clinical Neuroscince 1: 21–22. [Google Scholar]
Bayraktutan U (2019). Endothelial progenitor cells: Potential novel therapeutics for ischaemic stroke. Pharmacological Research 144: 181–191. DOI 10.1016/j.phrs.2019.04.017. [Google Scholar] [CrossRef]
Chen DR, Lin SZ, Fan JR, Lin CH, Lee W et al. (2014). Intracerebral Implantation of autologous peripheral blood stem cells in stroke patients: A randomized phase II study. Cell Transplantation 23: 1599–1612. DOI 10.3727/096368914X678562. [Google Scholar] [CrossRef]
Chu K, Jung KH, Lee ST, Park HK, Sinn DI et al. (2008). Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke 39: 1441–1447. DOI 10.1161/STROKEAHA.107.499236. [Google Scholar] [CrossRef]
Dankbaar JW, Hom J, Schneider T, Cheng SC, Bredno J et al. (2011). Dynamic perfusion-CT assessment of early changes in blood brain barrier permeability of acute ischaemic stroke patients. Journal of Neuroradiology 38: 161–166. DOI 10.1016/j.neurad.2010.08.001. [Google Scholar] [CrossRef]
di Santo S, Yang Z, von Ballmoos M Wyler, Voelzmann J, Diehm N et al. (2009). Novel cell-free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One 4: e5643. DOI 10.1371/journal.pone.0005643. [Google Scholar] [CrossRef]
Dubois C, Liu X, Claus P, Marsboom G, Pokreisz P et al. (2010). Differential effects of progenitor cell populations on left ventricular remodeling and myocardial neovascularization after myocardial infarction. Journal of the American College of Cardiology 55: 2232–2243. DOI 10.1016/j.jacc.2009.10.081. [Google Scholar] [CrossRef]
Fang J, Guo Y, Tan S, Li Z, Xie H et al. (2019). Autologous endothelial progenitor cells transplantation for acute ischemic stroke: A 4-year follow-up study. Stem Cells Translational Medicine 8: 14–21. DOI 10.1002/sctm.18-0012. [Google Scholar] [CrossRef]
Fujio K, Komai T, Inoue M, Morita K, Okamura T et al. (2016). Revisiting the regulatory roles of the TGF-β family of cytokines. Autoimmunity Reviews 15: 917–922. DOI 10.1016/j.autrev.2016.07.007. [Google Scholar] [CrossRef]
Garbuzova-Davis S, Haller E, Lin R, Borlongan CV (2017). Intravenously transplanted human bone marrow endothelial progenitor cells engraft within brain capillaries, preserve mitochondrial morphology, and display pinocytotic activity towards BBB repair in ischemic stroke rats. Stem Cells 35: 1246–1258. DOI 10.1002/stem.2578. [Google Scholar] [CrossRef]
Ha G, de Torres F, Arouche N, Benzoubir N, Ferratge S et al. (2019). GDF15 secreted by senescent endothelial cells improves vascular progenitor cell functions. PLoS One 14: e0216602. DOI 10.1371/journal.pone.0216602. [Google Scholar] [CrossRef]
Hagensen MK, Raarup MK, Mortensen MB, Thim T, Nyengaard JR et al. (2012). Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovascular Research 93: 223–231. DOI 10.1093/cvr/cvr278. [Google Scholar] [CrossRef]
Hisham NF, Bayraktutan U (2013). Epidemiology, pathophysiology, and treatment of hypertension in ischaemic stroke patients. Journal of Stroke and Cerebrovascular Diseases 22: e4–e14. DOI 10.1016/j.jstrokecerebrovasdis.2012.05.001. [Google Scholar] [CrossRef]
Hong Y, Yu Q, Kong Z, Wang M, Zhang R et al. (2020). Exogenous endothelial progenitor cells reached the deficient region of acute cerebral ischemia rats to improve functional recovery via Bcl-2. Cardiovascular Diagnosis and Therapy 10: 695–704. DOI 10.21037/cdt-20-329. [Google Scholar] [CrossRef]
Hou ST, MacManus JP (2002). Molecular mechanisms of cerebral ischemia induced neuronal death. International Review of Cytology 221: 93–148. DOI 10.1016/S0074-7696(02)21011-6. [Google Scholar] [CrossRef]
Jansen of Lorkeers SJ, Eding JEC, Vesterinen HM, van der Spoel TI, Sena ES et al. (2015). Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: Systematic review and meta-analysis of large animal studies. Circulation Research 116: 80–86. DOI 10.1161/CIRCRESAHA.116.304872. [Google Scholar] [CrossRef]
Kadir RRA, Alwjwaj M, Bayraktutan U (2020). MicroRNA: An emerging predictive, diagnostic, prognostic and therapeutic strategy in ischaemic stroke. Cellular and Molecular Neurobiology 15: 1071. DOI 10.1007/s10571-020-01028-5. [Google Scholar] [CrossRef]
Martí-Fàbregas J, Crespo J, Delgado-Mederos R, Martínez-Ramírez S, Peña E et al. (2013). Endothelial progenitor cells in acute ischemic stroke. Brain and Behavior 3: 649–655. DOI 10.1002/brb3.175. [Google Scholar] [CrossRef]
Masuda H, Iwasaki H, Kawamoto A, Akimaru H, Ishikawa M et al. (2012). Development of serum-free quality and quantity control culture of colony-forming endothelial progenitor cell for vasculogenesis. Stem Sanne Johanna Jansen of Lorkeers Cells Translational Medicine 1: 160–171. DOI 10.5966/sctm.2011-0023. [Google Scholar] [CrossRef]
Masuda H, Tanaka R, Fujimura S, Ishikawa M, Akimaru H et al. (2014). Vasculogenic conditioning of peripheral blood mononuclear cells promotes endothelial progenitor cell expansion and phenotype transition of anti-inflammatory macrophage and T lymphocyte to cells with regenerative potential. Journal of the American Heart Association 3: 362. DOI 10.1161/JAHA.113.000743. [Google Scholar] [CrossRef]
Medina RJ, O’Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA et al. (2010a). Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Medical Genomics 3: 496. DOI 10.1186/1755-8794-3-18. [Google Scholar] [CrossRef]
Medina RJ, O’Neill CL, Humphreys MW, Gardiner TA, Stitt AW (2010b). Outgrowth endothelial cells: Characterization and their potential for reversing ischemic retinopathy. Investigative Opthalmology and Visual Science 51: 5906–5913. DOI 10.1167/iovs.09-4951. [Google Scholar] [CrossRef]
Medina RJ, O’Neill CL, O’Doherty TM, Chambers SEJ, Guduric-Fuchs J et al. (2013). Ex vivo expansion of human outgrowth endothelial cells leads to IL-8-mediated replicative senescence and impaired vasoreparative function. Stem Cells 31: 1657–1668. DOI 10.1002/stem.1414. [Google Scholar] [CrossRef]
Milbauer LC, Enenstein JA, Roney M, Solovey A, Bodempudi V et al. (2009). Blood outgrowth endothelial cell migration and trapping in vivo: A window into gene therapy. Translational Research 153: 179–189. [Google Scholar]
Malhotra K, Ahmed N, Filippatou A, Katsanos AH, Goyal N et al. (2019). Association of elevated blood pressure levels with outcomes in acute ischemic stroke patients treated with intravenous thrombolysis: A systematic review and meta-analysis. Journal of Stroke 21: 78–90. [Google Scholar]
Nagata E, Masuda H, Nakayama T, Netsu S, Yuzawa H et al. (2019). Insufficient production of IL-10 from M2 macrophages impairs in vitro endothelial progenitor cell differentiation in patients with Moyamoya disease. Scientific Reports 9: 16752. [Google Scholar]
Nakayama T, Nagata E, Masuda H, Asahara T, Takizawa S (2019). Regeneration-associated cell transplantation contributes to tissue recovery in mice with acute ischemic stroke. PLoS One 14: e0210198. [Google Scholar]
Naserian S, Abdelgawad ME, Bakshloo1 MA, Ha G, Arouche N et al. (2020). The TNF/TNFR2 signaling pathway is a key regulatory factor in endothelial progenitor cell immunosuppressive effect. Cell Communication and Signaling 18: 94. [Google Scholar]
Ohab JJ, Fleming S, Blesch A, Carmichael ST (2006). A neurovascular niche for neurogenesis after stroke. Journal of Neuroscience 26: 13007–13016. DOI 10.1523/JNEUROSCI.4323-06.2006. [Google Scholar] [CrossRef]
Paczkowska E, Kucia M, Koziarska D, Halasa M, Safranow K et al. (2009). Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke 40: 1237–1244. DOI 10.1161/STROKEAHA.108.535062. [Google Scholar] [CrossRef]
Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S et al. (2014). Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke A multicentric, randomized trial. Stroke 45: 3618–3624. DOI 10.1161/STROKEAHA.114.007028. [Google Scholar] [CrossRef]
Proust R, Ponsen AC, Rouffia V, Schenowitz C, Montespan F et al. (2020). Cord blood-endothelial colony forming cells are immunotolerated and participate at post-ischemic angiogenesis in an original dorsal chamber immunocompetent mouse model. Stem Cell Research & Therapy 11: 677. DOI 10.1186/s13287-020-01687-7. [Google Scholar] [CrossRef]
Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I et al. (2008). Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proceedings of the National Academy of Sciences of the United States of America 105: 6620–6625. DOI 10.1073/pnas.0710516105. [Google Scholar] [CrossRef]
Rakkar K, Bayraktutan U (2016). Increases in intracellular calcium perturb blood-brain barrier via protein kinase C-alpha and apoptosis. Biochimica et Biophysica Acta (BBA)–Molecular Basis of Disease 1862: 56–71. [Google Scholar]
Rakkar K, Othman O, Sprigg N, Bath P, Bayraktutan U (2020). Endothelial progenitor cells, potential biomarkers for diagnosis and prognosis of ischemic stroke: Protocol for an observational case-control study. Neural Regeneration Research 15: 1300–1307. [Google Scholar]
Rosell A, Morancho A, Navarro-Sobrino M, Martınez-Saez E, Hernandez-Guillamon M et al. (2013). Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PLoS One 8: e73244. [Google Scholar]
Savitz SI, Misra V, Kasam M, Juneja H, Cox CS et al. (2011). Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Annals of Neurology 70: 59–69. [Google Scholar]
Shyu WC, Lin SZ, Chiang MF, Su CY, Li H (2006). Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. Journal of Neuroscience 26: 3444–3453. [Google Scholar]
Sobrino T, Arias S, Perez-Mato M, Agulla J, Brea D et al. (2011). CD34+ Progenitor Cells likely are involved in the good functional recovery after intracerebral hemorrhage in humans. Journal of Neuroscience Research 89: 979–985. DOI 10.1002/jnr.22627. [Google Scholar] [CrossRef]
Takizawa S, Nagata E, Nakayama T, Masuda H, Asahara T (2016). Recent progress in endothelial progenitor cell culture systems: Potential for stroke therapy. Neurologia Medico-Chirurciga (Tokyo) 56: 302–309. DOI 10.2176/nmc.ra.2016-0027. [Google Scholar] [CrossRef]
Tsai NW, Hung SH, Huang CR, Chang HW, Chang WN et al. (2014). The association between circulating endothelial progenitor cells and outcome in different subtypes of acute ischemic stroke. Clinica Chimica Acta 427: 6–10. DOI 10.1016/j.cca.2013.09.029. [Google Scholar] [CrossRef]
Vaes B, Van’t Hof W, Deans R, Pinxteren J (2012). Application of MultiStem(s) allogeneic cells for immunomodulatory therapy: Clinical progress and pre-clinical challenges in prophylaxis for graft versus host disease. Frontiers in Immunology 3: 345. DOI 10.3389/fimmu.2012.00345. [Google Scholar] [CrossRef]
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS et al. (2021). Heart disease and stroke statistics—2021 update, a report from the American Heart Association. Circulation 143: e254–e743. [Google Scholar]
Zhou WJ, Zhu DL, Yang GY, Zhang Y, Wang HY et al. (2009). Circulating endothelial progenitor cells in Chinese patients with acute stroke. Hypertension Research 32: 306–310. DOI 10.1038/hr.2009.16. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |