DOI:10.32604/biocell.2022.018934

| BIOCELL DOI:10.32604/biocell.2022.018934 |  |
| Article |
Exogenous melatonin alleviated growth inhibition and oxidative stress induced by drought stress in apple rootstock
College of Horticulture, Henan Agricultural University, Zhengzhou, 450002, China
*Address correspondence to: Xianbo Zhang, xianbo@163.com; Tuanhui Bai, tuanhuibai88@163.com
Received: 27 August 2021; Accepted: 05 November 2021
Abstract: Drought stress is one of the major environmental obstacles that limit the production and development of apples (Malus domestica Borkh.). The role of melatonin is well known in the protection of plants under environmental stresses. In this study, we investigated the effect of melatonin on apple rootstock M. hupehensis Rehd under drought stress. The results showed that drought inhibited the growth of M. hupehensis and dramatically reduced root surface area, root volume, the number of tips and forks, and root diameter. Drought-induced growth inhibition was significantly decreased by adding melatonin. Net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), stomatal conductance (Gs), transpiration rate (Tr), were markedly reduced under drought stress. However, the application of melatonin could mitigate the damage to the photosynthetic apparatus and increase the quantum yield of PSII photochemistry. Additionally, generation of hydrogen peroxide (H2O2) and superoxide radicals (O2•–) sharply increased in apple leaves after 4 days under drought stress, and the accumulation of electrolyte leakage (EL) represented oxidative stress, while by applying melatonin under drought stress, the generation of O2•– and H2O2 were significantly reduced and protected the membrane from drought damages. These results suggest that the adverse effects of drought can be minimized by applying melatonin to apples.
Keywords: Apple; Chlorophyll fluorescence; Drought; Melatonin; Photosynthesis
Drought is one of the most important environmental stresses that affect plant growth and development worldwide (Chaves et al., 2003). Drought stress inhibits plant growth via many physiological processes, including chlorophyll biosynthesis, photosynthesis, energy consumption, and reactive oxygen species (ROS) metabolism (Barchet et al., 2014; Liang et al., 2019; Qiang et al., 2019). Photosynthesis is one of the physiological processes which is sensitive to environmental stresses (Johnson et al., 2015). It has been reported that drought inhibits photosynthesis through stomatal limitation and non-stomatal limitation (Barchet et al., 2014; Bai et al., 2019). Stomatal closure is one of the earliest responses of plants to drought stress, reducing transpiration and photosynthetic rate (Chaves et al., 2003). Notably, drought stress induced over-reduction of the electron transport chain, which in turn leads to photooxidation and excessive accumulation of ROS, such as superoxide radicals (O2•–) and hydrogen peroxide (H2O2) (Bano et al., 2012; Liu et al., 2012). These ROS are very reactive and damage the photosynthetic apparatus, reduce the photosynthetic efficiency, and ultimately result in cell death (Yin et al., 2005; Gupta et al., 2017; Gururani et al., 2015).
To overcome drought, plants have developed a variety of adaptation mechanisms, such as movement of leaves or chloroplasts, photorespiration, and ROS scavenging system (Yin et al., 2005; Liu et al., 2012; Basu et al., 2016). Drought resistance and adaptation in plants developed via morphological structure and physiological changes in leaves. Plants can induce stomatal closure, accumulation of compatible solutes, and biosynthesis of wax to avoid drought (Galmés et al., 2007). Additionally, the ROS scavenging system is also important for the physiological mechanisms which are supportive of drought resistance in plants. Enhanced ROS scavenging system alleviates oxidative damage and induces some molecular chaperones to protect the plant from damage (Bai et al., 2009; Basu et al., 2016). Drought stress was reported that stimulate the ROS scavenging system in apple leaves under mild to moderate stress, but that was lower under severe stress because of antioxidant enzyme damage (Liu et al., 2012). Furthermore, plants are regulated by the absorption of immobile nutrients via root systems under drought stress (Hanslin et al., 2019).
The application of biostimulators is one of the promising and practical strategies to enhance stress tolerance (Wei et al., 2015; Debnath et al., 2019). Melatonin is known as a biopromoter and plays important roles in regulating plant growth, development, and stress tolerance (Wang et al., 2012; Arnao and Hernández-Ruiz, 2014; Fan et al., 2018). In recent years, an increasing number of studies have reported the physiological functions of melatonin in plants to serve as the first line of defense against environmental stresses. Previous studies suggested that drought tolerance has increased by applying melatonin under environmental stress, such as drought, salinity, ultraviolet radiation, chilling, and heat (Posmyk et al., 2009; Galano et al., 2011; Li et al., 2012; Zhang et al., 2014; Wei et al., 2015). Stress alleviation effect of melatonin relies on various factors, including inhibiting chlorophyll degradation, improving the photosynthesis, enhancing the antioxidant system, and directly scavenging free radicals (Wang et al., 2012; Liu et al., 2015; Ke et al., 2018; Debnath et al., 2019; Asif et al., 2020).
Apple (Malus × domestica Borkh.) is one of the most economically important fruits worldwide, with the largest area of cultivation in China (Liu et al., 2012). Most apple trees are grown in arid or semi-arid regions, and drought is considered as the main environmental factor restricting the development and production of apples (Bai et al., 2019). The development of drought resistance is a priority goal in apples. Rootstocks play a vital role in productive apple orchards because of their contributions to water and nutrient uptake and abiotic stress resistance (Valverdi et al., 2019). M. hupehensis, which originates in China, is an excellent rootstock because of its resistance to abiotic stress. In addition, M. hupehensis is apomictic, resulting in uniform phenotypes and genotypes. So, it is widely used as rootstock for apple production (Huang et al., 2018). Evidence suggested melatonin is known as a biopromoter and plays important roles in regulating plant drought tolerance (Fleta-Soriano et al., 2017; Liang et al., 2019). Therefore, the objective of this work was to examine the influence of melatonin on the seedling growth, root architecture, photosynthesis, chlorophyll fluorescence of M. hupehensis exposed to drought.
Plant materials and experimental design
Seeds of M. hupehensis were laminated by sand at 4°C for 35 to 40 days (d). Then, one germinated seed was planted in one plastic pot (6 cm × 6 cm) filled with soil/organic substrate (1:5, v:v), the plastic pots were then placed in a greenhouse at 20–25/15–20°C (day/night), with a 16-hour photoperiod (photosynthetically active radiation, 180–200 μmol m−2 s−1), and the relative humidity was approximately 70%–80%. After growing for 70 d, the seedlings were assigned to a pretreatment group that received irrigation water containing 0.2 mM melatonin, whereas others continued to receive irrigation water containing 0 mM melatonin. After the 10-d pretreatment was completed, the seedlings were assigned to one of three treatments: (1) Control (CK), plants normally watered; (2) Drought treatment (D), plants exposed to progressive drought by withholding irrigation; and (3) Drought + melatonin treatment (DM), plants receiving melatonin pretreatment for 10 d and then exposed to drought stress. Each treatment contained three replications with 30 plants in each replicate, resulting in a total of 90 plants per treatment. At 0, 1, 2, 3, and 4 d after treatment, photosynthetic measurements were recorded, and leaves were sampled from plants of each treatment.
After the treatments, the seedlings were taken out, and the roots were then washed with water. The root system was scanned with a scanner (SNAPSCAN 310, Agfa, Beijing, China), and root length, root surface area, root volume, number of root tips, and branching angle of each root were measured by root image analysis software WinRHIZO (Canada, Regent Instruments).
Photosynthetic and chlorophyll fluorescence
Photosynthetic measurements were determined with a Li-Cor 6400 portable photosynthesis system (Li-6400XT, LICOR, Lincoln, Nebraska, USA). The net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr) were recorded from mature leaves between 09:00 AM and 11:00 AM. Chlorophyll fluorescence parameters in leaves were measured with a portable photosynthetic efficiency analyzer (DUAL-PAM-100, WALZ, Germany). Leaves were dark-adapted for 30 min in leaf clips before measurements. Measurements were taken between 10:00 AM and 02:00 PM from six leaves per treatment. Chlorophyll fluorescence parameters were calculated according to Genty et al. (1989). Fv/Fm = (Fm – F0)/Fm, qP = (Fm' – Fs)/(Fm' – F0'), NPQ = (Fm – Fm')/Fm'. That, Fv/Fm is the maximal potential quantum efficiency of photosystem II; Fm is the maximum fluorescence (in the dark-adapted leaves); Fo is the minimum chlorophyll fluorescence; the photochemical quenching value was evaluated using qP = (Fm' − Fs)/(Fm' − F0') formula representing the photochemical energy conversion of the PS II reaction centers when the primary acceptor QA has been oxidized; Fv is the variable fluorescence (dark) (Fm – F0); and NPQ is non-photochemical quenching.
Determination of chlorophyll content and electrolyte leakage
Chlorophyll was extracted from 0.1 g of leaf tissue in 80% (v/v) acetone under darkness at 25°C. After centrifugation for 15 min at 13000×g, values were measured at A663, A645, and A470 with a spectrophotometer (UV-9000S, Shanghai, China). Total chlorophyll (Chl t), chlorophyll a (Chl a), and chlorophyll b (Chl b) contents were calculated according to the equations (Porra et al., 1989). Electrolyte leakage (EL) was determined according to the method described by Dionisio-Sese and Tobita (1998).
Histochemical detections of H2O2 and O2•–
H2O2 and O2•– were detected by histochemical staining with 3,3'-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT), respectively, according to the method described by Rodríguez and Taleisnik (2012). For H2O2 measurement, leaves were stained in 1 mg/mL DAB solution and incubated for 12 h at room temperature in darkness, then photographed. For O2•– measurement, leaves were stained in 0.1 mg/mL NBT in 25 mM K-HEPES buffer (pH 7.9) for 1 h. Then, the samples were kept at 25°C for an additional 4 h. After 12 h of incubation, leaves were decolorized by immersing them in boiling 70% ethanol to detect the deep brown polymerization product. After cooling, photographs were taken after placing the leaves on the bench.
Data were statistically analyzed with IBM SPSS Statistics 17.0 (IBM China Company, Ltd., Beijing, China). Results were represented as the mean values ± standard deviation. The statistical significance between treatments was assessed by one-way analysis of variance at P < 0.05.
Exogenous melatonin improved the growth parameters
M. hupehensis responded to drought stress with morphological changes of the seedling showing leaf wilting due to dehydration (Fig. 1). However, melatonin-pretreated plants under drought stress exhibited a significant improvement compared to control plants. To evaluate the effect of melatonin on root traits under drought stress, we investigated the total root length, root diameter, root surface area, root volume, and the number of forks. Compared to control, root surface area, number of tips, forks, and root diameter significantly decreased under drought stress (Table 1). However, after treatment with irrigation water containing melatonin, the root surface area, root volume, and root diameter significantly increased, and were 42.71%, 133.33%, and 36.36% greater than those in the drought stress treatment, respectively.

Figure 1: Growth status of M. hupehensis under drought conditions for 4 d. CK: plants not pretreated with drought and melatonin; D: plants under drought stress; DM: melatonin-pretreated plants under drought stress.
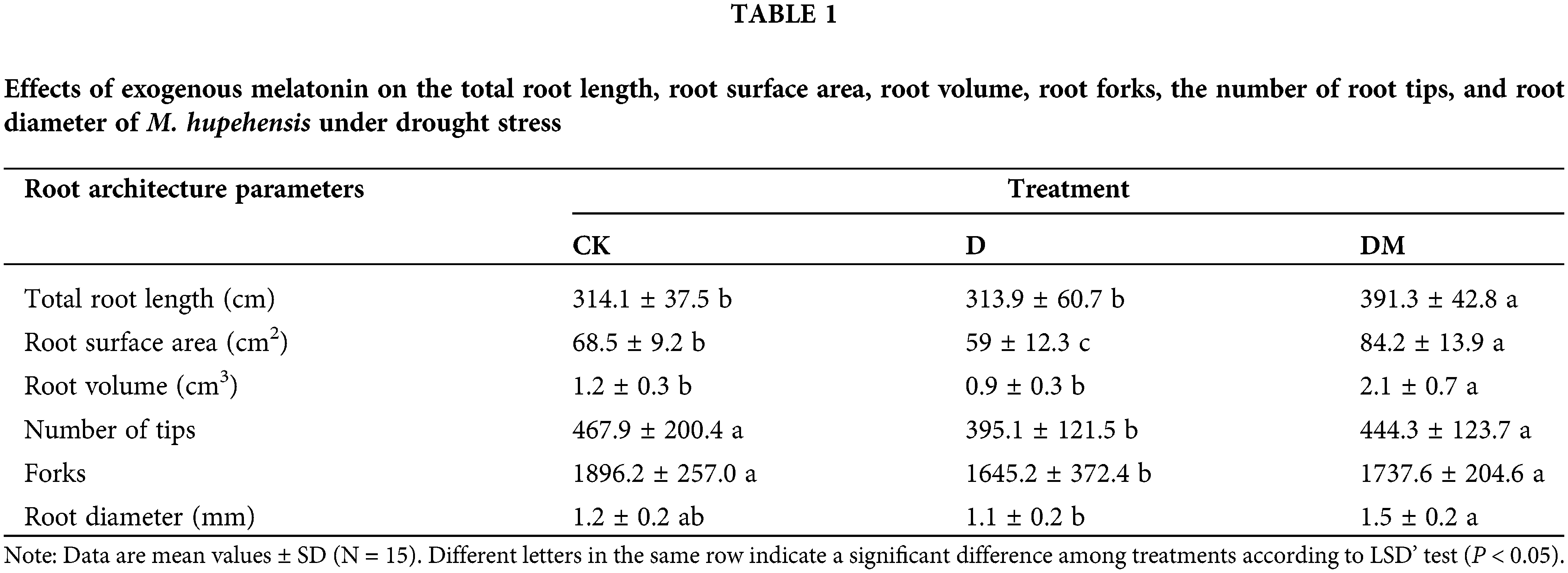
Additionally, the effects of exogenous melatonin on the root length proportion of M. hupehensis were detected under drought stress (Fig. 2). There was no obvious change in the proportion of short root lengths among control, drought stress, and melatonin-treated plants. However, drought reduced the proportion of medium and long root lengths, especially for the length of 3.0–3.5 cm. In contrast, melatonin significantly increased the proportion of medium and long root lengths.
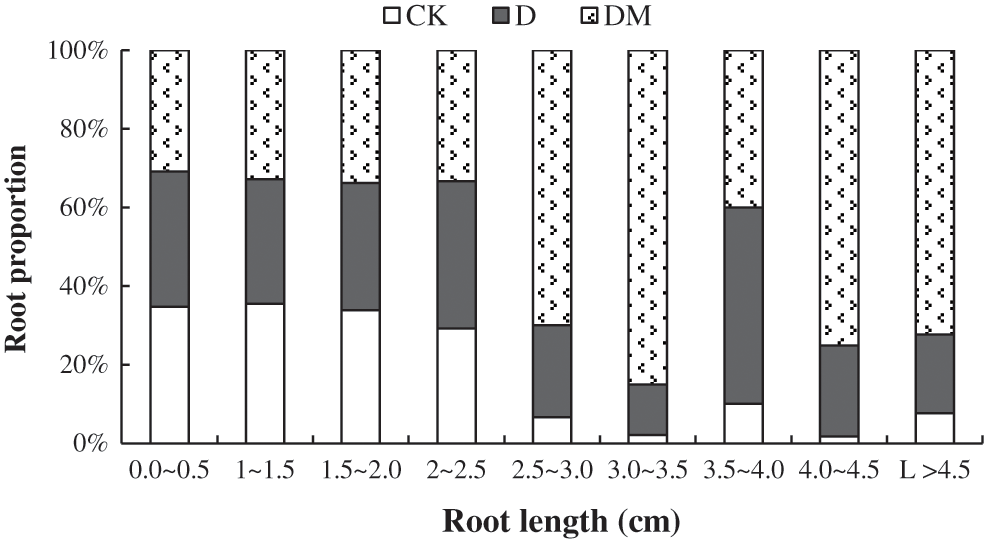
Figure 2: Root proportions of M. hupehensis under drought conditions with and without melatonin application. Data are mean values of 15 measurements ± standard deviation. CK: plants not pretreated with drought and melatonin; D: plants under drought stress; DM: melatonin-pretreated plants under drought stress.
Exogenous melatonin enhanced photosynthesis and fluorescence parameters
Drought stress caused a continuous decline in gas exchange parameters over time. With the extension of drought treatment time, all gas exchange parameters decreased sharply, except Ci, which exhibited a smooth decline (Fig. 3). However, the application of melatonin resulted in higher Gs, Ci, and Tr under drought stress. At the beginning of the second day, the Pn increased then decreased until the end of the second day. Additionally, the application of melatonin effectively improved Pn.
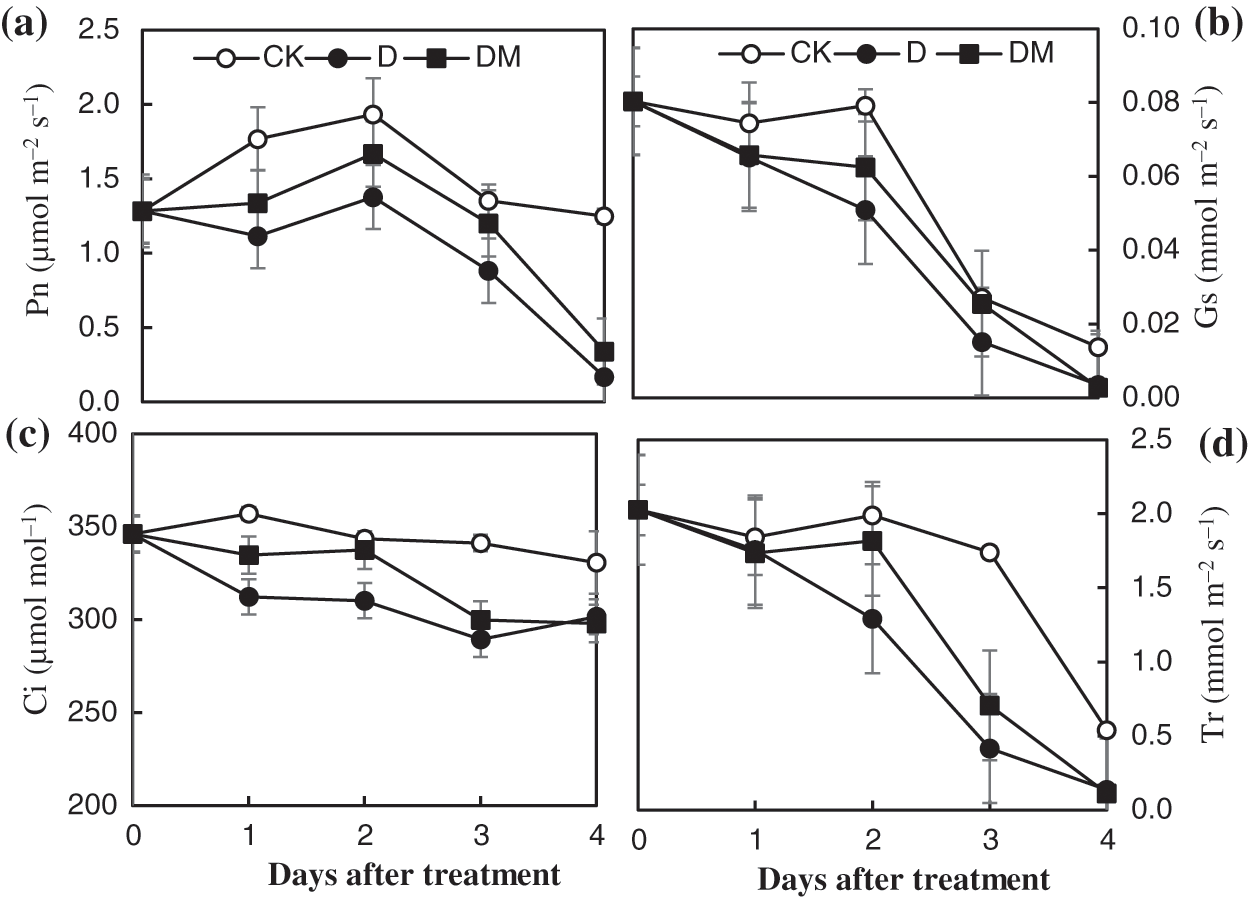
Figure 3: Effects of exogenous melatonin on the net photosynthetic rate (Pn), stomatal conductance (Gs), internal CO2 concentration (Ci), and transpiration (Tr) of M. hupehensis under drought stress. Each point represents the mean value from 6 replicates and the vertical bars indicate the standard deviations.
Chlorophyll fluorescence measurement technology was used to explore the effects of melatonin priming on the photosynthetic capacity of apple leaves under drought stress (Fig. 4). Fv/Fm and qP of apple leaves decreased sharply, over time, under drought treatment compared to control ones. However, drought stress remarkably increased the Fo value. In contrast, melatonin application improved the photochemical efficiency of PSII, which was expected to be sensitive to some environmental stresses, suggesting that melatonin priming could reduce damage to PSII of apple leaves under drought stress.
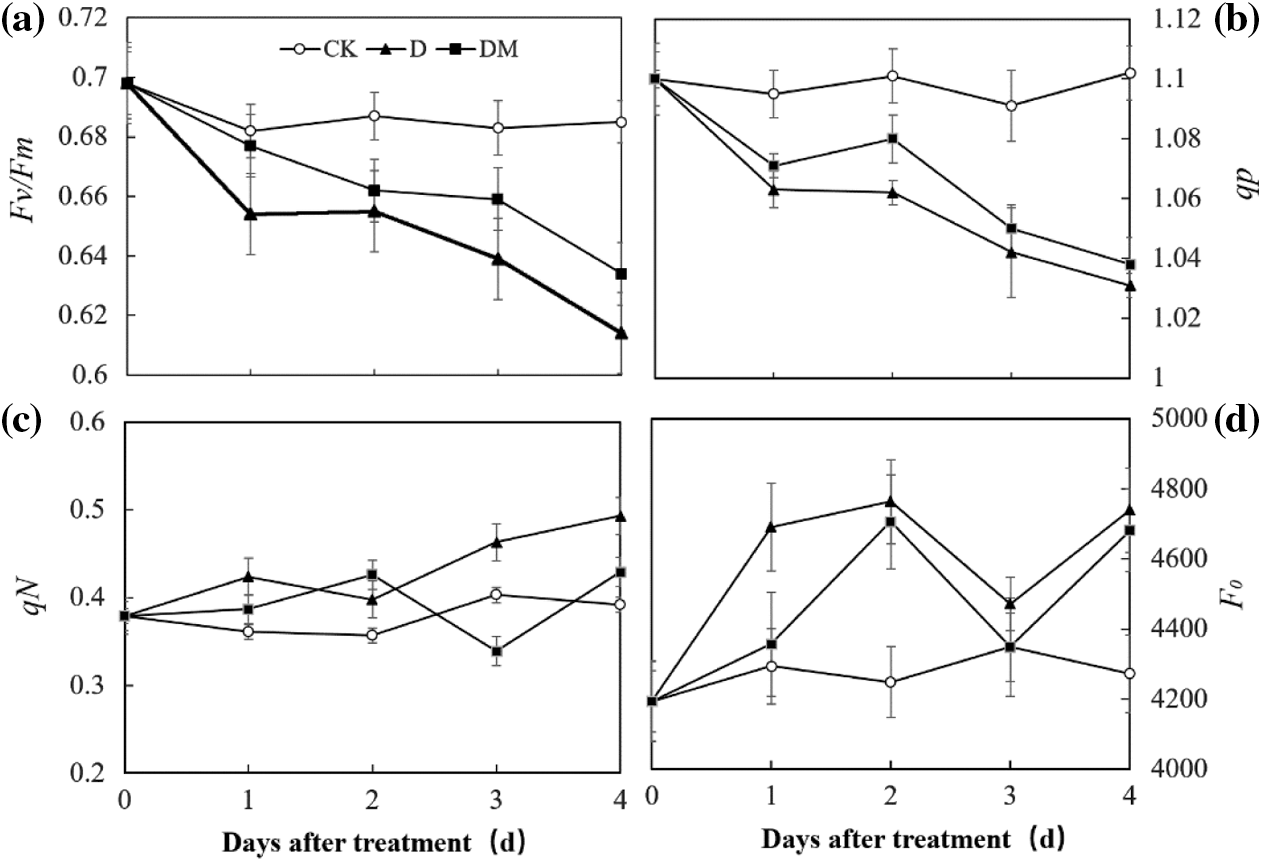
Figure 4: Effects of exogenous melatonin on Fv/Fm, qP, qN, and F0 of M. hupehensis under drought stress. Each point represents the mean value from 6 replicates and the vertical bars indicate the standard deviations.
Exogenous melatonin enhanced chlorophyll content
To illustrate the role of melatonin on chlorophyll content under drought stress, we detected the content of Chl a and Chl b. After the first 2 d, all chlorophyll a and b content and the ratio of Chl a/b smoothly declined (Fig. 5). Then, a sharp decrease was observed during the last days under drought stress. However, the reduction of Chl caused by drought stress was significantly alleviated by melatonin treatment.
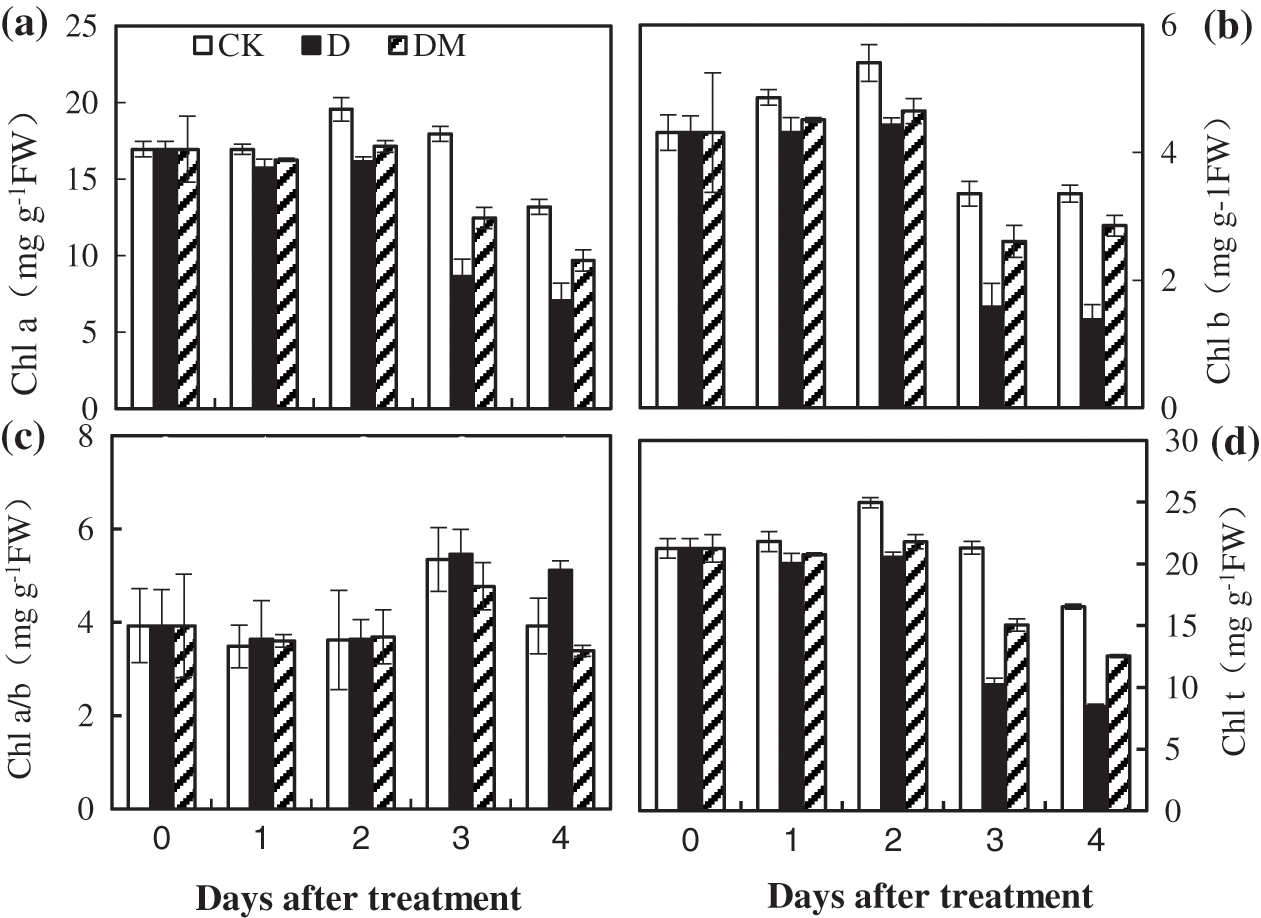
Figure 5: Effects of exogenous melatonin on Chl a, Chl b, Chl a/b, and the Chl t ratio of M. hupehensis under drought stress. Each point represents the mean value from 4 replicates and the vertical bars indicate the standard deviations.
Exogenous melatonin delayed membrane lipid peroxidation
The data showed that EL increased significantly with an increase in the duration of drought stress with maximum EL recorded at 4 d (Fig. 6). Compared to the control plants, the melatonin exhibited a significant effect on the drought response, which significantly decreased EL of the melatonin-treated plants.
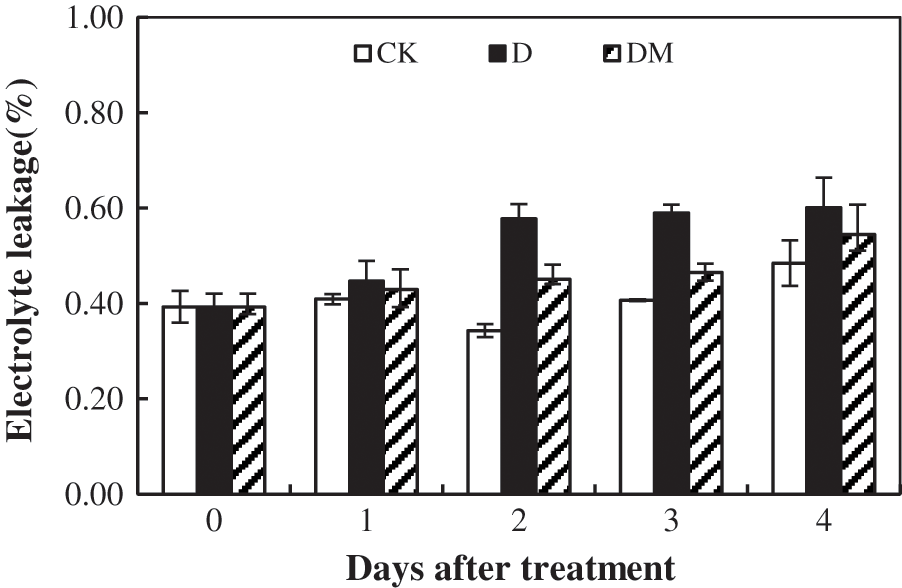
Figure 6: Effects of exogenous melatonin on electrolyte leakage of M. hupehensis under drought stress. Each point represents the mean value from 4 replicates and the vertical bars indicate the standard deviations.
Exogenous melatonin declined O2•– and H2O2 contents
As shown in Fig. 7, photographs of the stained leaves against a contrasting background were documented for oxidation conditions. H2O2 was visualized as a reddish-brown stain formed by the reaction of DAB with the endogenous H2O2. O2•– was detected as a dark blue stain of the formazan compound formed as a result of NBT reacting with the endogenous O2•–. When the time of drought treatment reached 2 d, the accumulation of O2•– and H2O2 was not significantly different from that of the melatonin treatment. However, H2O2 and O2•– were sharply increased in apple leaves under drought stress after 4 d compared to the control ones. Exogenous application of melatonin significantly reduced the generation of O2•– and H2O2, and the highest efficiency of O2•– and H2O2 removal was observed in the drought stress treatment after 4 d.
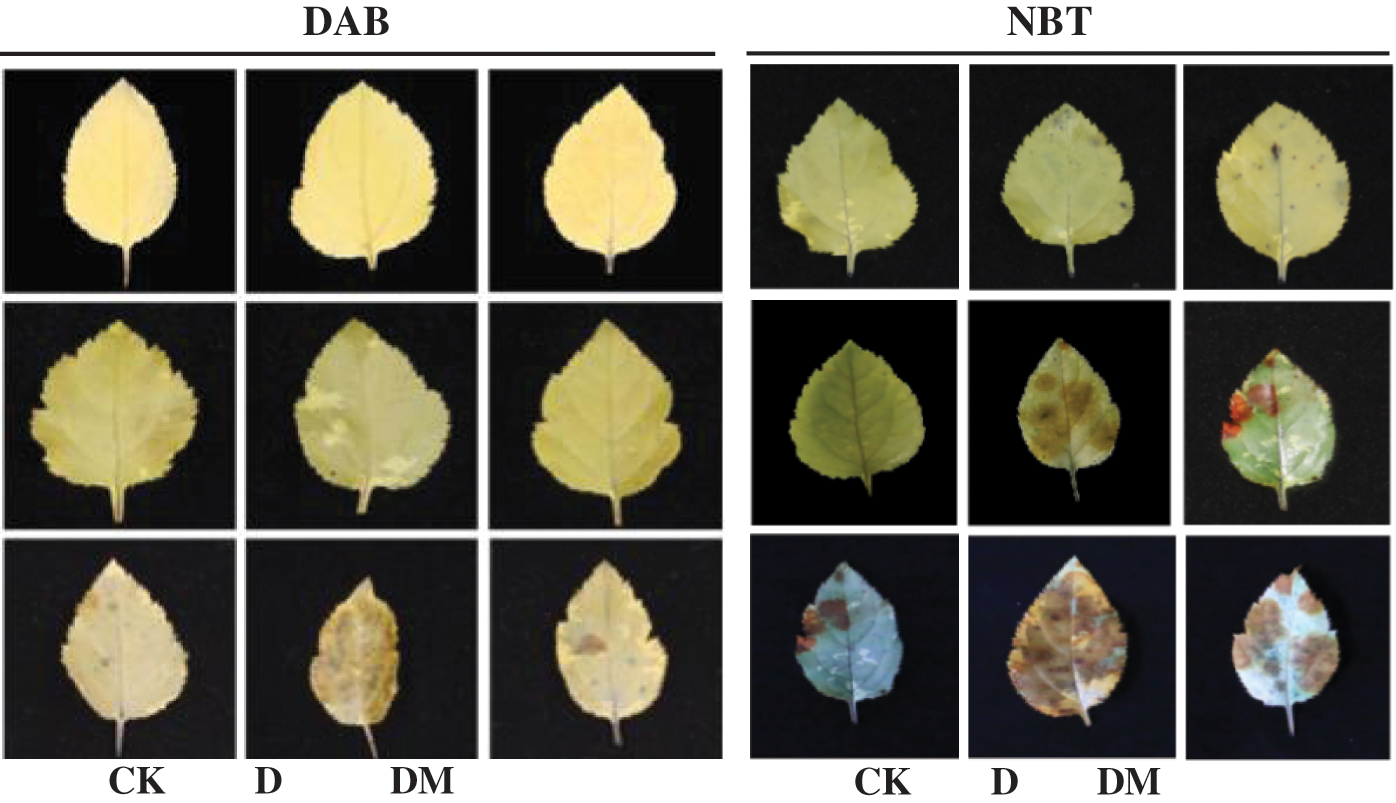
Figure 7: Effects of exogenous melatonin on H2O2 and O2•– accumulation of M. hupehensis under drought stress. H2O2 production in M. hupehensis leaves was detected using DAB after 4 d of drought stress. O2•– production in M. hupehensis leaves was detected using NBT after 4 d of drought stress.
Apple is known to be a large water consumer and drought stress is the major limitation for high quality and yield of apple (Atkinson et al., 1999; Liu et al., 2012). Identifying ways to produce more apples with less water consumption is essential for the high quality and yield of apples. Melatonin has been reported to play key roles in regulating environmental stresses tolerance (Galano et al., 2011; Zhang et al., 2014; Arnao and Hernández-Ruiz, 2015). In the present study, the protective effects of melatonin in drought response were investigated in M. hupehensis. The results showed that plants were wilting and that the leaves were seriously dehydrated, and almost all the measured root architecture parameters were significantly reduced under drought conditions. However, melatonin-treated plants under drought stress exhibited an effectively improved state. This agrees with the findings that melatonin-treated maize had a higher dry mass compared with the untreated plants under drought (Ye et al., 2016; Fleta-Soriano et al., 2017).
It has been reported that growth inhibition was related to photosynthesis (Galmés et al., 2007; Gururani et al., 2015; Fleta-Soriano et al., 2017). In the present study, drought stress caused a decline in all gas exchange parameters, especially for Pn on the last day of the treatment. Drought can result in photooxidation and reductions in photosynthetic efficiency (Skirycz and Inzé, 2010; Ma et al., 2015). The current results proved that exogenously applied melatonin could effectively ameliorate photosynthetic inhibition caused by drought. Additionally, exogenous application of melatonin significantly increased the proportion of medium and long root lengths. Roots play an effective role in soil water use by conveying it to the aerial parts of plants more effectively during drought (Palta and Turner, 2019). Previous research has also provided evidence for the potential role of melatonin in the rooting of plants (Li et al., 2012).
Stress-induced photosynthetic apparatus damage in the plant causes a decrease in photosynthesis, which is one of the main reasons for excessive accumulation of ROS (Bai et al., 2009; Liu et al., 2015; Gupta et al., 2017). Therefore, keeping the photosynthetic organ in good condition is important for the enhancement of plant tolerance to stress. Our study showed that drought significantly induced accumulation of H2O2 and O2•–, which resulted in higher EL. Similar results were obtained in maize (Liu et al., 2012). Also, Goharrizi et al. (2020a; 2020b; 2020c) showed that drought and osmotic stresses can increase the oxidative stress parameters, thereby elevating the content of EL. Oxidative stress caused by drought was removed via adding melatonin, which effectively protected apple plants from damage. To some extent, this could be attributed to the function of melatonin as a direct free radical scavenger (Galano et al., 2011; Arnao and Hernández-Ruiz, 2015). Substantial evidence supports that melatonin could play a vital role as an antioxidant molecule, especially under abiotic stress conditions (Li et al., 2012; Liu et al., 2015; Ye et al., 2016; Fleta-Soriano et al., 2017).
Chlorophyll plays an extremely critical role in plants, which absorb light energy and transport electrons to the reaction center during photosynthesis (Gururani et al., 2015). In plants under various environmental stresses, chlorophyll synthesis can be disrupted (Ahammed et al., 2018). Previous studies showed that exogenous melatonin helped in maintaining normal levels of chlorophyll in salt-stressed apples (Li et al., 2012). In our study, exogenous melatonin enhanced the level of chlorophyll under drought stress, suggesting that melatonin was linked to enhance the synthesis of chlorophyll. In addition, melatonin significantly decreased Chl a/Chl b at 4 d. This indicated that melatonin was able to inactive the inhibitory effect of drought stress on Chl b. The function of the photosynthetic apparatus was studied using chlorophyll fluorescence (Tang et al., 2016; Oikonomou et al., 2019). The current results showed that F0 increased under drought stress, implying that the PSII reaction center was damaged, which led to the decline of Fv/Fm. However, melatonin had a favorable effect and relieved drought-induced changes to F0 and Fv/Fm. These results suggested that 0.2 mM melatonin is an effective method to prevent damage to the PSII and to enhance the drought tolerance of apple rootstock.
Application of melatonin mitigated the growth inhibition, delayed membrane lipid peroxidation, and reduced oxidative damage in M. hupehensis under drought stress. Melatonin alleviated the adverse effects of drought stress on the growth of apple seedlings by enhancing the capacity of photosynthesis, improving chlorophyll fluorescence, and inhibiting the degradation of chlorophyll, as well as directly scavenging H2O2 and O2•– to reduce oxidative damage induced by drought stress. Thus, melatonin has been proved to improve drought stress tolerance in apple plants. Based upon the results of the current study, we present a model depicting melatonin-mediated drought tolerance of apple rootstock (Fig. 8).
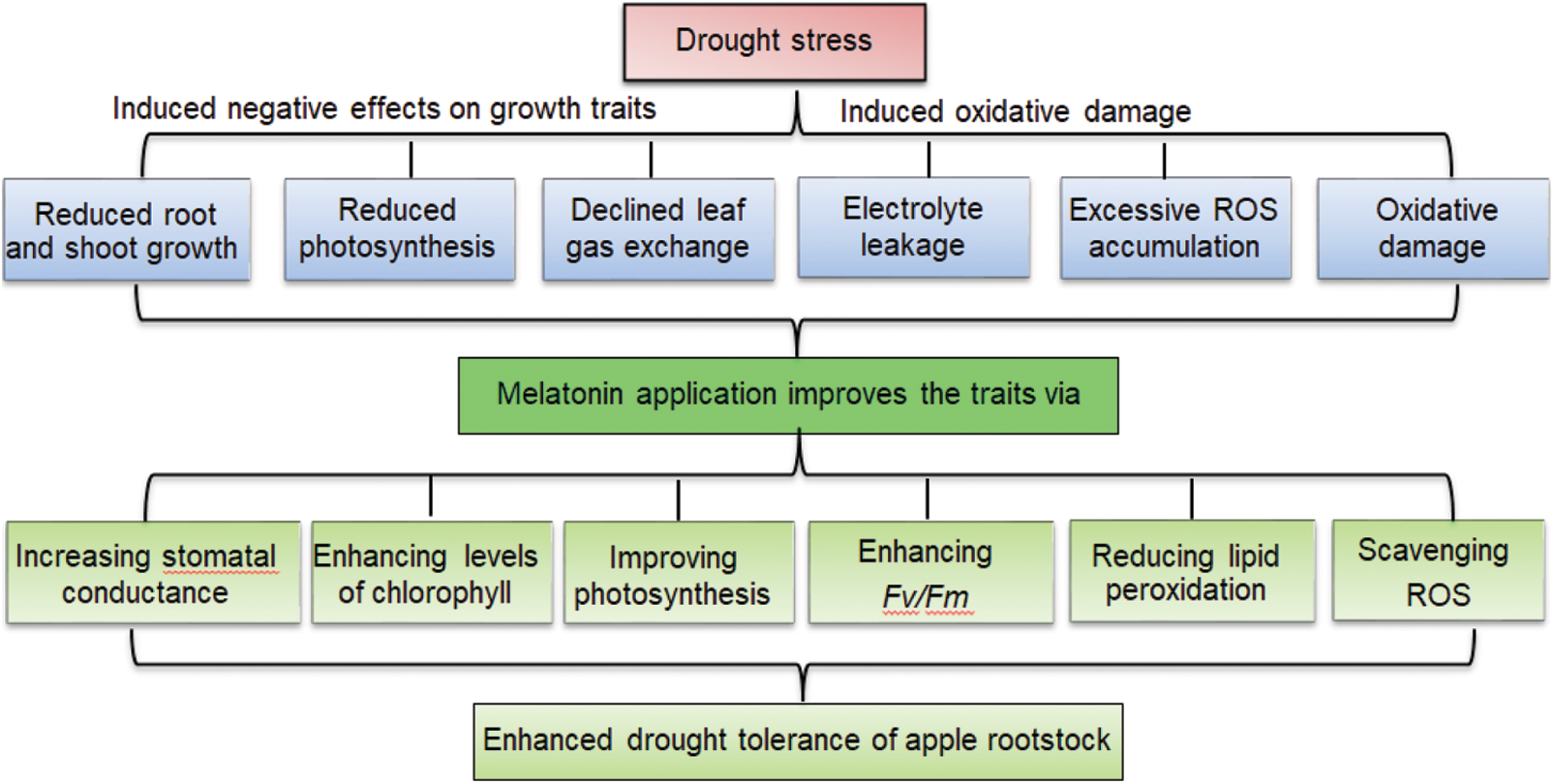
Figure 8: Proposed model depicting melatonin-mediated drought tolerance in apple rootstock. Drought caused oxidative damage and inhibited plant growth of apple rootstock seedlings. Melatonin alleviated the adverse effects of drought stress on the growth by enhancing capacity of photosynthesis, improving chlorophyll fluorescence, and inhibiting the degradation of chlorophyll, as well as directly scavenging H2O2 and O2•– to reduce oxidative damage.
Acknowledgement: The authors would like to thank Zhanying Li for providing technical assistance for photosynthesis system. The authors thank Anita K. Snyder for critical reading of the manuscript.
Author Contributions: TB and MW conceived and designed research; JG, CS, and ZW conducted experiments; XZ, SS, JJ, and MW reviewed and edited the manuscript. All the authors have read and agreed to the published version of the manuscript.
Funding Statement: This study was financially supported by the National Key Research and Development Projects (2019YFD1000100, 2018YFD1000301), the National Natural Science Foundation of China (31872058), Program of Young-Backbone Teacher of University in Henan Province (2018GGJS029) and the Special Fund for Henan Agriculture Research System (S2014-11-G02).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ahammed GJ, Xu W, Liu A, Chen S (2018). COMT1 silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II activity and electron transport efficiency in tomato. Frontiers in Plant Science 9: 998. [Google Scholar]
Asif M, Pervez A, Irshad U, Mehmood Q, Ahmad R (2020). Melatonin and plant growth-promoting rhizobacteria synergistically alleviate the cadmium and arsenic stresses and increase the growth of Spinacia oleracea L. Plant, Soil and Environment 66: 234–241. [Google Scholar]
Atkinson CJ, Policarpo M, Webster AD, Kuden AM (1999). Drought tolerance of apple rootstocks: Production and partitioning of dry matter. Plant and Soil 206: 223–235. [Google Scholar]
Arnao MB, Hernández-Ruiz J (2014). Melatonin: Plant growth regulator and/or biostimulator during stress? Frontiers in Plant Science 19: 789–797. [Google Scholar]
Arnao MB, Hernández-Ruiz J (2015). Functions of melatonin in plants: A review. Journal of Pineal Research 59: 133–150. [Google Scholar]
Bai T, Li C, Ma F, Shu H, Han M (2009). Exogenous salicylic acid alleviates growth inhibition and oxidative stress induced by hypoxia stress in Malus robusta Rehd. Journal of Plant Growth Regulation 28: 358–366. [Google Scholar]
Bai T, Li Z, Song C, Song S, Jiao J et al. (2019). Contrasting drought tolerance in two apple cultivars associated with difference in leaf morphology and anatomy. American Journal of Plant Sciences 10: 709–722. [Google Scholar]
Bano A, Ullah F, Nosheen A (2012). Role of abscisic acid and drought stress on the activities of antioxidant enzymes in wheat. Plant, Soil and Environment 58: 181–185. [Google Scholar]
Barchet GL, Dauwe R, Guy RD, Schroeder WR, Soolanayakanahally RY et al. (2014). Investigating the drought-stress response of hybrid poplar genotypes by metabolite profiling. Tree Physiology 34: 1203–1219. [Google Scholar]
Basu S, Ramegowda V, Kumar A, Pereira A (2016). Plant adaptation to drought stress. F1000Research 5: 1554. [Google Scholar]
Chaves MM, Maroco JP, Pereira JS (2003). Understanding plant responses to drought-from genes to the whole plant. Functional Plant Biology 30: 239–264. DOI 10.1071/FP02076. [Google Scholar] [CrossRef]
Debnath B, Islam W, Li M, Sun Y, Lu X et al. (2019). Melatonin mediates enhancement of stress tolerance in plants. International Journal of Molecular Sciences 20: 1040. DOI 10.3390/ijms20051040. [Google Scholar] [CrossRef]
Dionisio-Sese ML, Tobita S (1998). Antioxidant responses of rice seedlings to salinity stress. Plant Science 135: 1–9. DOI 10.1016/S0168-9452(98)00025-9. [Google Scholar] [CrossRef]
Fan J, Xie Y, Zhang Z, Chen L (2018). Melatonin: A multifunctional factor in plants. International Journal of Molecular Sciences 19: 1528. DOI 10.3390/ijms19051528. [Google Scholar] [CrossRef]
Fleta-Soriano E, Díaz L, Bonet E, Munné-Bosch S (2017). Melatonin may exert a protective role against drought stress in maize. Journal of Agronomy Crop Science 203: 286–294. DOI 10.1111/jac.12201. [Google Scholar] [CrossRef]
Galano A, Tan DX, Reiter RJ (2011). Melatonin as a naturally against oxidative stress: A physicochemical examination. Journal of Pineal Research 51: 1–16. DOI 10.1111/j.1600-079X.2011.00916.x. [Google Scholar] [CrossRef]
Galmés J, Medrano H, Flexas J (2007). Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytologist 175: 81–93. DOI 10.1111/j.1469-8137.2007.02087.x. [Google Scholar] [CrossRef]
Genty B, Briantais JM, Baker NR (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA General Subjects 990: 87–92. DOI 10.1016/S0304-4165(89)80016-9. [Google Scholar] [CrossRef]
Goharrizi KJ, Amirmahani F, Salehi F (2020). Assessment of changes in physiological and biochemical traits in four pistachio rootstocks under drought, salinity and drought+salinity stresses. Physiologia Plantarum 168: 973–989. DOI 10.1111/ppl.13042. [Google Scholar] [CrossRef]
Goharrizi KJ, Baghizadeh A, Kalantar M, Kalantar M, Fatehi F (2020). Combined effects of salinity and drought on physiological and biochemical characteristics of pistachio rootstocks. Scientia Horticulturae 261: 108970. DOI 10.1016/j.scienta.2019.108970. [Google Scholar] [CrossRef]
Goharrizi KJ, Moosavi SS, Amirmahani F, Salehi F, Nazari M (2020). Assessment of changes in growth traits, oxidative stress parameters, and enzymatic and non-enzymatic antioxidant defense mechanisms in Lepidium draba plant under osmotic stress induced by polyethylene glycol. Protoplasma 257: 459–473. [Google Scholar]
Gupta D, Pena LB, Romero-Puertas M, Hernández A, Inouhe M et al. (2017). NADPH oxidases differentially regulate ROS metabolism and nutrient uptake under cadmium toxicity. Plant, Cell & Environment 40: 509–526. [Google Scholar]
Gururani MA, Venkatesh J, Tran LSP (2015). Regulation of photosynthesis during abiotic stress-induced photoinhibition. Molecular Plant 8: 1304–1320. [Google Scholar]
Hanslin HM, Bischoff A, Hovstad KA (2019). Root growth plasticity to drought in seedlings of perennial grasses. Plant and Soil 440: 551–568. [Google Scholar]
Huang L, Li M, Shao Y, Sun T, Li C et al. (2018). Ammonium uptake increases in response to PEG-induced drought stress in Malus hupehensis Rehd. Environmental and Experimental Botany 151: 32–42. [Google Scholar]
Johnson GN, Lawson T, Murchie EH, Raines C (2015). Photosynthesis invariable environments. Journal of Experimental Botany 66: 2371–2372. [Google Scholar]
Ke Q, Ye J, Wang B, Ren J, Yin L et al. (2018) Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Frontiers in Plant Science 9: 914. [Google Scholar]
Liang D, Ni Z, Xia H, Xie Y, Lv X et al. (2019). Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Scientia Horticulturae 246: 34–43. [Google Scholar]
Li C, Wang P, Wei Z, Liang D, Liu C et al. (2012). The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. Journal of Pineal Research 53: 298–306. [Google Scholar]
Liu B, Li M, Cheng L, Liang D, Zou Y et al. (2012). Influence of rootstock on antioxidant system in leaves and roots of young apple trees in response to drought stress. Plant Growth Regulation 67: 247–256. [Google Scholar]
Liu N, Jin Z, Wang S, Gong B, Wen D et al. (2015). Sodic alkaline stress mitigation with exogenous melatonin involves reactive oxygen metabolism and ion homeostasis in tomato. Scientia Horticulturae 181: 18–25. [Google Scholar]
Ma P, Bai T, Ma F (2015). Effects of progressive drought on photosynthesis and partitioning of absorbed light in apple trees. Journal of Integrative Agriculture 14: 681–690. [Google Scholar]
Palta JA, Turner NC (2019) Crop root system traits cannot be seen as a silver bullet delivering drought resistance. Plant Soil 439: 31–43. [Google Scholar]
Qiang X, Ding J, Lin W, Li Q, Xu C et al. (2019). Alleviation of the detrimental effect of water deficit on wheat (Triticum aestivum L.) growth by an indole acetic acid-producing endophytic fungus. Plant and Soil 439: 373–391. [Google Scholar]
Oikonomou A, Ladikou EV, Chatziperou G, Margaritopoulou T, Landi M et al. (2019). Boron excess imbalances root/shoot allometry, photosynthetic and chlorophyll fluorescence parameters and sugar metabolism in apple plants. Agronomy 9: 731. [Google Scholar]
Porra RJ, Thompson WA, Kriedemann PE (1989). Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA Bioenergetics 975: 384–394. [Google Scholar]
Posmyk MM, Bałabusta M, Wieczorek M, Sliwinska E, Janas KM (2009). Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. Journal of Pineal Research 46: 214–223. [Google Scholar]
Rodríguez AA, Taleisnik EL (2012). Determination of reactive oxygen species in salt-stressed plant tissues. Methods in Molecular Biology 913: 225–236. [Google Scholar]
Skirycz A, Inzé D (2010). More from less: Plant growth under limited water. Current Opinion in Biotechnology 21: 197–203. [Google Scholar]
Tang G, Li X, Lin L, Gu Z, Zeng F (2016). The chlorophyll a fluorescence characteristic in different types of leaf senescence in Alhagi sparsifolia. Journal of Plant Growth Regulation 35: 952–964. [Google Scholar]
Valverdi NA, Cheng L, Kalcsits L (2019). Apple scion and rootstock contribute to nutrient uptake and partitioning under different belowground environments. Agronomy 9: 415. [Google Scholar]
Wang P, Yin L, Liang D, Li C, Ma F et al. (2012). Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. Journal of Pineal Research 53: 11–20. [Google Scholar]
Wei W, Li QT, Chu YN, Reiter RJ, Yu XM et al. (2015). Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. Journal of Experimental Botany 66: 695–707. [Google Scholar]
Ye J, Wang S, Deng X, Yin L, Xiong B et al. (2016). Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiologiae Plantarum 38: 48. [Google Scholar]
Yin C, Pen Y, Zang R, Zhu Y, Li C (2005). Adaptive responses of Populus kangdingens is to drought stress. Physiologia Plantarum 123: 445–451. [Google Scholar]
Zhang N, Sun Q, Zhang H, Cao Y, Weeda S et al. (2014). Roles of melatonin in abiotic stress resistance in plants. Journal of Experimental Botany 66: 647–656. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |