DOI:10.32604/biocell.2022.019448

| BIOCELL DOI:10.32604/biocell.2022.019448 |  |
| Review |
Mesenchymal stem cells, secretome and biomaterials in in-vivo animal models: Regenerative medicine application in cutaneous wound healing
1Department of Medical and Surgical Sciences, University of Foggia, Foggia, 71122, Italy
2Department of Clinical and Experimental Medicine, University of Foggia, Foggia, 71122, Italy
*Address correspondence to: Massimo Conese, massimo.conese@unifg.it
Received: 25 September 2021; Accepted: 13 December 2021
Abstract: The treatment of nonhealing and chronic cutaneous wounds still needs a clinical advancement to be effective. Both mesenchymal stem cells (MSCs), obtained from different sources, and their secretome derived thereof (especially exosomes) can activate signaling pathways related to promotion of cell migration, vascularization, collagen deposition, and inflammatory response demonstrating prohealing, angiogenetic and anti-scarring capacities. On the other hand, biodegradable biomimetic scaffolds can facilitate endogenous cell attachment and proliferation as well as extracellular matrix production. In this Review, we revise the complex composites made by biomimetic scaffolds, mainly hydrogels, and MSC-derived exosomes constructed for cutaneous wound healing. Studies demonstrate that there exists a synergistic action of scaffolds with encapsulated exosomes, displaying a sustained release profiles to facilitate long-lasting healing effects. It can be envisioned that dressings made by biomimetic hydrogels and MSC-derived exosomes will be clinically applied in the near future for the effective treatment of nonhealing and chronic wounds.
Keywords: Biomimetic scaffolds; Cutaneous wound healing; Exosomes; Hydrogels; Mesenchymal stem cells; Secretome
Wound healing is a complicated biological process that occurs in three distinct yet overlapping phases including inflammation, cell proliferation, and matrix remodeling, which need the support of nutrition and oxygen provided by blood vessels to cells participating in the healing process (Falanga, 2005; Arwert et al., 2012; Hu et al., 2014; Rodrigues et al., 2019). Fig. 1 provides the main events and cues determining intercellular signaling pathways and growth factors involved in the three phases of wound healing. In a wound, damage to the skin activates platelets and the formation of a clot. Platelets and epithelial cells at the margin of the lesion release a wide range of growth factors and chemo-attractants to recruit immune cells (neutrophils and macrophages) giving rise to the inflammatory phase. In the proliferative phase, macrophages acquire a M2 phenotype and secrete growth factors to develop the granulation tissue by the activation of fibroblasts and new vessels via transforming growth factor‑β (TGF-β) and platelet-derived growth factor (PDGF). Keratinocytes and activated fibroblasts can also stimulate angiogenesis. Proliferating and migrating keratinocytes are engaged in the re-epithelization and reconstitution of epidermal appendages. It is the presence of epidermal stem cells in different compartments of the skin such as inter-follicular compartments and epidermal appendages (sweat glands and hair follicles with their associated sebaceous glands) that allows skin self-repair capabilities (Mathes et al., 2014). In the remodeling phase, fibroblasts are stimulated by TGF-β3 to convert into myofibroblasts, which deposit extracellular matrix and determine wound contraction, reducing the surface area of the wound that must be re-epithelialized. Matrix remodelling is due to the secreton by myofibroblasts of MMPs and their respective inhibitors (tissue inhibitors of metalloproteinases, TIMPs). During time, the collagen III found in granulation tissue is gradually decreased and replaced with collagen I. In the last years, it has been elucidated that multiple signaling pathways are major players for regenerative wound healing, i.e., TGF-β, Notch, Hedgehog, and Wnt/β-catenin (Choi et al., 2022). While TGF-β1 functions as a fibrosis-stimulating factor, TGF-β3 regulates anti-scarring activity (Shah et al., 1995; Soo et al., 2003). The Notch pathway is involved in epidermal cell differentiation, maintenance of skin homeostasis and promotion of angiogenesis (Okuyama et al., 2008; Watt et al., 2008; Blanpain and Fuchs, 2009; Gridley, 2010; Shi et al., 2015). The Hedgehog pathway plays a role in skin morphogenesis and angiogenesis, and modulates dermal repair and wound vascularization during the healing process (Asai et al., 2006; Le et al., 2008). Finally, the Wnt/β-catenin pathway participates in multiple steps of the wound-healing process, including the formation of skin appendages by activation of stem cells residing within skin, the differentiation and migration of keratinocytes, the migration of fibroblasts and their transformation into myofibroblasts, and angiogenesis (Houschyar et al., 2015; Shi et al., 2015).
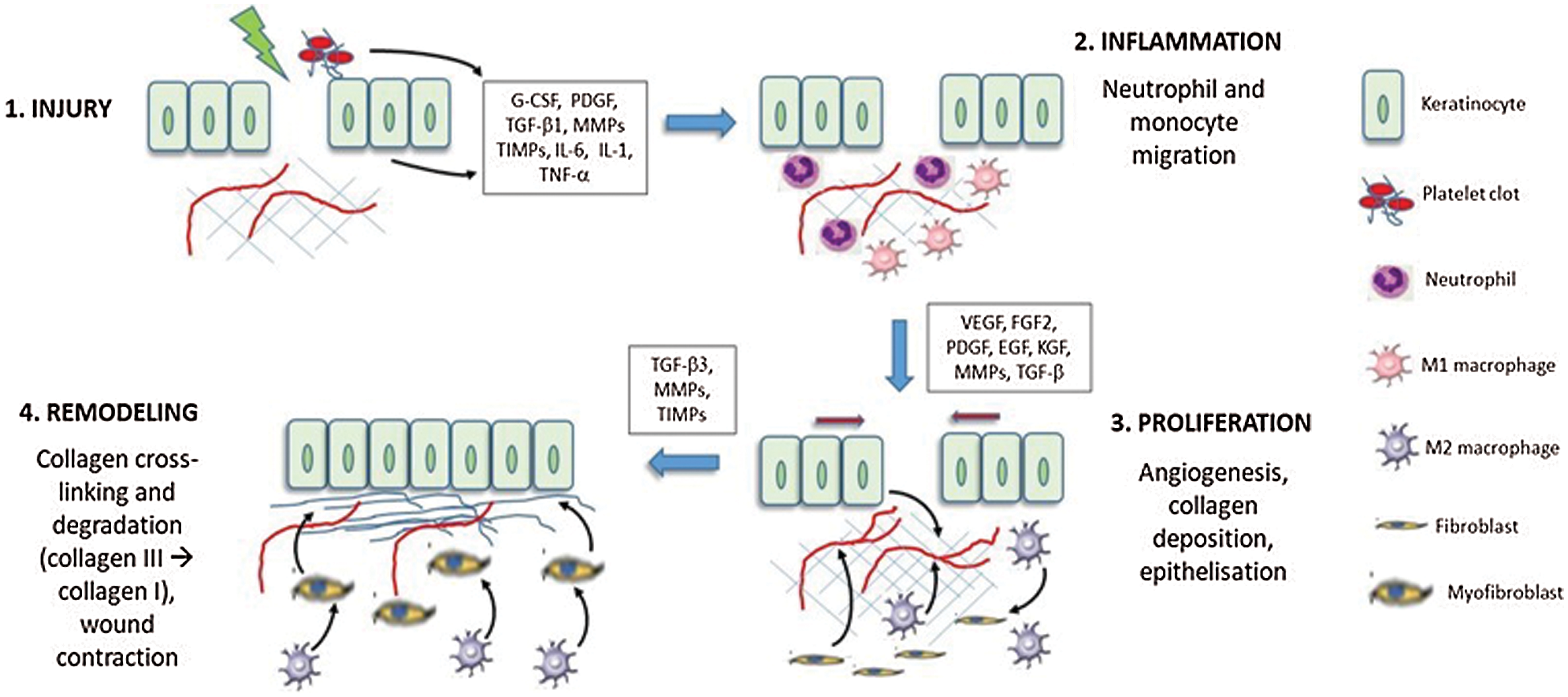
Figure 1: The three phases of skin wound healing after an injury. Cellular interplays are shown as black arrows. See text for details.
Impaired wound healing, characterized by insufficient angiogenesis and easy infection, is one of the most common complications of diabetes, leading to chronic and nonhealing ulcers, with a prevalence in Europe of 5.1%. Diabetic ulcers are recalcitrant to healing due to many cellular and molecular aberrations (Gary Sibbald and Woo, 2008). In diabetes mellitus, the persistence of hyperglycemia causes peripheral nerve injury and arterial disease. Sustained hyperglycemia and induced oxidative stress impair cell migration, alter nitric oxide production at the level of endothelial cells (Forstermann and Munzel, 2006), as well as determine insufficient angiogenesis to support the collagen synthesis necessary for mature granulation and subsequent re-epithelialization. In addition, high levels of blood glucose impair leukocyte function causing an insufficient immune response, inciting infections and difficulties in the healing of foot injuries and ulcers (Gary Sibbald and Woo, 2008). Vascular and peripheral neuritis complications and abnormal collagen lead to skin wounds that are refractory and which often ulcerate. Also trauma and burns can lead to scar formation and impaired wound healing (Cerqueira et al., 2016). In recent years, skin substitutes through the application of biomimetic scaffolds together with stem cells and bioactive substrates have provided an emerging therapeutic opportunity in the treatment of acute and chronic cutaneous wounds (Conese et al., 2020).
Mesenchymal stem cells (MSCs) are multipotent adult stem cells that can differentiate mainly into mesenchymal cell lineages, including adipocytes, osteoblast, chondrocytes, myoblasts, and endothelial cells, in different culture conditions and morphogens/growth factors. MSCs can be derived from diverse sources, such as bone marrow, adipose tissue, umbilical cord, fetal membranes, synovia, gingival tissue etc. (Lee et al., 2016). The main types investigated in wound healing are MSCs derived from bone marrow (BM), adipose tissue (AD) and umbilical cord (UC) (Riha et al., 2021; Sivaraj et al., 2021). Although there exist subtle variations in MSCs from different sources and in principle they can be used equally in wound healing, AD-derived MSCs (ADSCs) are the most easily accessible, can be isolated at higher yield and in large quantities with minimal patient morbidity, thus being the most favored cell type for wound repair and regeneration (Hassan et al., 2014; Bertozzi et al., 2017). A great number of animal studies have purported the notion that MSCs display positive healing actions, bringing their application to clinical trials (Huang et al., 2020). Especially with hard-to-heal wounds, MSC treatment results in enhanced angiogenesis, facilitated re-epithelialization, improved granulation, and accelerated wound closure. The underlying mechanisms of their therapeutic role is not completely understood, however MSCs actively respond to biological signals associated with inflammation, necrosis, and tissue injury (Prockop and Oh, 2012). MSCs can home to injured skin, operate direct differentiation into skin cells and are a reservoir of trophic factors that can be secreted and act paracrinally (Huang et al., 2020). Furthermore, in the harsh inflammatory milieu of non-healing wounds, MSCs can respond to inflammatory stimuli by becoming potently immunosuppressive (Zhang et al., 2015d; Cuenca et al., 2018; Yu et al., 2019), thus facilitating the transition from the inflammatory phase to the proliferative phase. In recent years, it has become increasingly clear that their engraftment in injury sites contribute little to their therapeutic effects. In the harsh environment of the wound, the contribution of MSC differentiation to diverse injury models have been limited, including poor postimplant cell survival, engraftment efficiency, and cell retention (Chen et al., 2012). Instead, it is increasingly appreciated that their secretome is the primary mechanism exerting multifaceted functions including immunomodulation, angiogenesis, anti-apoptosis, anti-scarring, chemoattraction and modulation of local stem and progenitor cells (Gnecchi et al., 2008; Singer and Caplan, 2011; Maxson et al., 2012; Khosrotehrani, 2013; Liang et al., 2014). The interest in MSCs and wound healing has been pointed out by their property of sensing the environment and creating an orchestrated network of molecules to promote the tissue repair/regeneration process. MSCs can secrete pro-angiogenic factors that can promote vascularisation in the wound area and formation of granulation tissue, among which vascular endothelial growth factor (VEGF), hepatocyte growth factors (HGF), PDGF, and basic fibroblast growth factor (bFGF) are of extreme importance (Chen et al., 2008; Yoon et al., 2010; An et al., 2015). MSCs can promote re-epithelization at the wound site via the secretion of epidermal growth factor (EGF) and keratinocyte growth factor (KGF) (Gnecchi et al., 2008). MSCs are anti-inflammatory, thanks to the secretion of indoleamine-2, 3-dioxygenase (IDO), prostaglandin E2 (PGE2) and tumor necrosis factor-α (TNF-α)-stimulated gene 6 (TSG-6), thereby modulating both innate and adaptive immune responses impeding scarring in favor of regeneration (Nemeth et al., 2009; Singer and Caplan, 2011; Ylostalo et al., 2012).
MSC secretome, represented roughly by conditioned medium (CM), is enriched in extracellular vesicles (EVs), membrane-surrounded structures released by cells that play important roles in the intercellular transmission of biological signals to regulate immunomodulatory and tissue repair processes. EVs released by MSCs are comprised of apoptotic bodies (1,000–5,000 nm), microparticles (or ectosomes, up to 1,000 nm), and exosomes (EXO, 30–150 nm) (Chen et al., 2017). Exosomes are considered the main contributor to stem cells efficacy (An et al., 2021). Indeed, exosomes display therapeutic effects on tissue injuries, which could be attributed to the transfer of membrane and cytosolic proteins, lipids and RNAs between cells (Raposo and Stoorvogel, 2013).
MSCs as well as their secretions (CM, extracellular vesicles, and EXO) have been shown to enhance wound healing and facilitate skin regeneration, as well as diabetic skin wound healing. MSC-conditioned medium has a potent healing effect on skin wounds (Chen et al., 2008; Yew et al., 2011; Shrestha et al., 2013; Li et al., 2017). The addition of EXO to the healing wound has been shown to promote proliferation and migration of related cells, enhance angiogenesis, re-epithelization, and regulating immune responses, highlighting exosomes as a promising approach to achieve a cell-free alternative to stem cell therapy (Zhang et al., 2015a; Zhang et al., 2015c; Cerqueira et al., 2016; Hu et al., 2016; Lee et al., 2016; Liang et al., 2016; Rani and Ritter, 2016; Phinney and Pittenger, 2017; Hu et al., 2018; Dalirfardouei et al., 2019; Ahangar et al., 2020; Manchon et al., 2021). Multiple studies have clarified that EXO can direct macrophage differentiation from pro-inflammatory M1 to anti-inflammatory M2 phenotype (He et al., 2019), induce fibroblast proliferation and migration for the first extracellular matrix (ECM) deposition (Zhang et al., 2015c; Ferreira et al., 2017; Choi et al., 2018), endothelial cell proliferation and migration to induce angiogenesis (Shabbir et al., 2015), keratinocyte proliferation and migration for re-epithelization (Ferreira et al., 2017), and to induce remodeling by ECM degradation and deposition by modulation of myofibroblasts with reduction of scar formation (Fang et al., 2016; Hu et al., 2016). Fig. 2 displays the main mechanisms by which MSCs and EXO determine healing at the wound injury site. The advantages of using EV-mediated cell-free therapies is of greater stability and storability, no risk of ectopic tissue formation and having a lower possibility of immune rejection as compared to MSC-based cell therapies (Merino-Gonzalez et al., 2016).
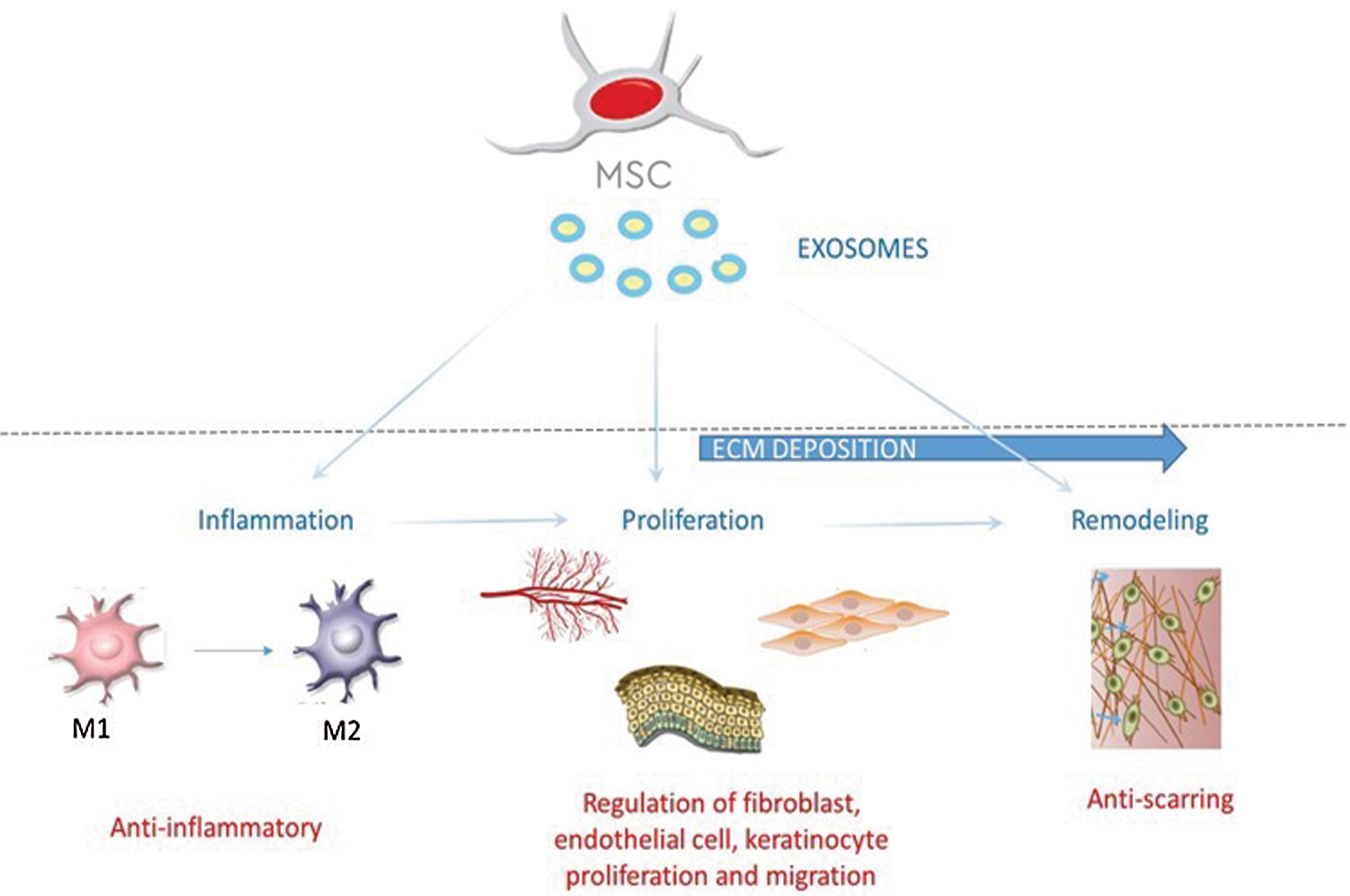
Figure 2: MSCs and exosomes derived thereof acting on the different stages of cutaneous wound healing, such as inflammation, proliferation and remodeling.
In order to improve survival of transplanted MSCs, a supportive microenvironment is pivotal to maximize cell viability (Kamoun et al., 2017). Biomaterial-based wound dressings have been thought to accelerate cell attachment and proliferation of various cell types and interact with the released growth factors enhancing their bioavailability (Tartarini and Mele, 2015). Four main approaches that have been envisioned include: (i) sheets of cells secreting ECM (Yu et al., 2018); (ii) pre-made porous scaffolds of synthetic, natural, and biodegradable biomaterial; (iii) decellularized ECM scaffolds, and (iv) cells entrapped in hydrogels (Chaudhari et al., 2016). To this end, many smart “skin substitutes”, made with varied combinations of synthetic and/or biologic substances, were used in order to perform many of skin’s functions and to treat deep dermal and full thickness injuries of various etiologies. These skin dressings were combined with MSCs to foster skin healing and include epidermal, dermal, and dermoepidermal (composite) skin substitutes, made by collagen and hyaluronic acid, i.e., the major components of the ECM (Hu et al., 2014). Other skin substitute are comprised of biocompatible and biodegradable synthetic polymers, such as polycaprolactone, polylactic acid, polyglycolic acid, poly(vinyl alcohol), poly(ethylene glycol), and polyurethanes, as well polysaccharides, such as chitosan and its derivatives (Moura et al., 2013). The current available commercial tissue-engineered products for wound healing comprise acellular products mainly made of collagen, hyaluronic acid, elastin or fibrin (Ho et al., 2017), among which Integra® (a bilayer made of bovine collagen and shark chondroitin sulfate with a silicone membrane, acting as a temporary barrier) was the first to be approved by U.S. Food and Drug Administration (FDA) to regenerate dermis (Savoji et al., 2018). These skin substitutes may tailor tissue-engineered products to the required patient groups, except Integra® that can be applied for a wide range of treatments including full-thickness burns, chronic ulcer and full-thickness nonthermal skin wound management, among others (Bello et al., 2001; Portincasa et al., 2018).
A wealth of preclinical studies has demonstrated that stem cell therapy combined with biomaterials improved wound healing capacity and regeneration to skin injury by accelerating healing time, by which the correction time was shortened from 7–28 days to 7–14 days with only MSCs and MSCs combined with biomaterials, respectively (Riha et al., 2021). These composites were made of nanfibrous scaffolds, gels and hydrogels, and were evaluated together with BM-MSCs, UC-MSCs, or ADSCs in mouse models representing burns, full-thickness excisional wounds, and nonhealing diabetic ulcers (Altman et al., 2009; Chung et al., 2016; Alapure et al., 2018; Xu et al., 2018; Tang et al., 2019; Chen et al., 2020; Lu et al., 2020). Although a tremendous progress has been made over the past few decades to develop skin substitutes for the management of acute and chronic wounds, most commercially used skin substitutes are manufactured from autologous adult cells (keratinocytes and fibroblast). Indeed, there are no existing commercial skin constructs available in the market that are constructed using both MSCs and biomaterials. The main challenges to be faced ahead include characterization, optimization, and delivery of treatment of stem cells composites (Savoji et al., 2018), and also unresolved drawbacks such as wound contraction and impaired vascularization should be further considered (Ho et al., 2017). Moreover, it is not well known if different types of wounds would be better healed with a specific type of MSCs; in other words, which of MSCs, BM-MSCs, ADSCs or UC-MSCs, would sense properly the wound microenvironment when combined with biomaterials in the patient’s setting.
The EXO incorporation into scaffolds as wound dressing for skin wound healing would make the MSCs and its secretome more realistic in clinical application, because of their direct contact with the injury site. Indeed, the common method of EXO administration is injection, which can affect their function due to the rapid clearance rate and relatively short half-life in vivo (Liu et al., 2017). On the other hand, diabetic wound repair and regeneration require a relatively long healing time. Herein, it is necessary to develop a novel biocompatible scaffold that can serve as a sustained release carrier for EXO to maintain their bioactivity at the diabetic wound area and further accelerate wound healing.
Among biomimetic composites, hydrogels, structurally similar to the natural ECM, have been considered promising biomaterials to deliver drugs/cells for wound treatments (Sharifzadeh and Hosseinkhani, 2017; Xi et al., 2018; Sivaraj et al., 2021). Hydrogels are physical or chemical cross-linked three-dimensional hydrophilic polymeric networks, which possess the capacity to absorb abundant amount of water ideally for hydrating and creating a supportive environment within the wound bed that accelerates angiogenesis and removal of cell debris and alleviating pain (Sivaraj et al., 2021). Hydrogels applied to wound healing should possess the following features: appropriate mechanical properties, good water retention, anti-infection capacity, injectable capacity, and excellent cell biocompatibility (Annabi et al., 2017). Moreover, they should be self-healing, meaning that maintain their structural stability during the wound healing (Taylor and In Het Panhuis, 2016; Li et al., 2018).
The works discussed in this Review were selected when they presented data on MSC secretome in combination with biomaterials for cutaneous wound healing in in vivo models. We have focused only on experimental works in animals without discussing clinical data and meta-analysis. Thus, we searched PubMed, MEDLINE, and Scopus using the keywords mesenchymal stem cell, conditioned medium, exosome, extracellular vesicle, and skin/cutaneous wound healing.
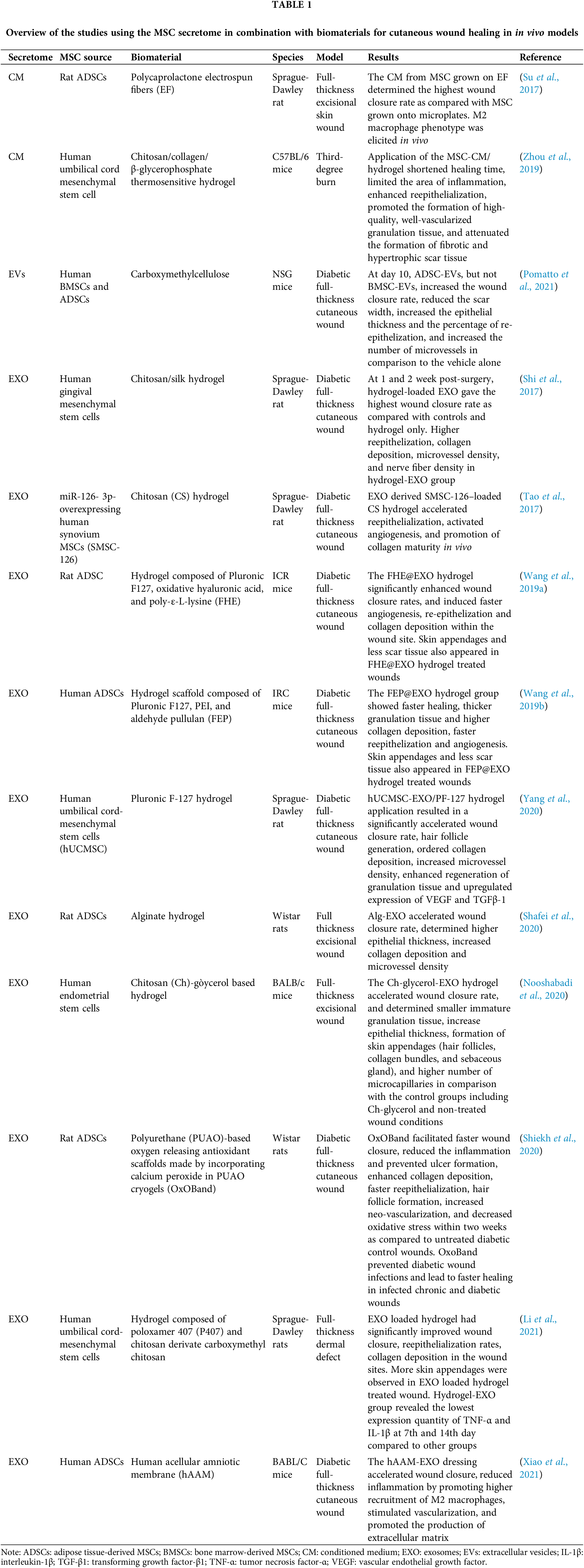
MSC-derived CM, EVs and exosomes were used in combination mostly with hydrogels, although also one study with electrospun fibers and one with decellularized amniotic membrane were found (Table 1). Diabetes chronic wounds/ulcers was the main medical problem that was considered in these studies. In general, MSC-CM/EVs/EXO scaffold application could shorten wound healing time, limit the inflammatory response, enhance re-epithelialization, promote the formation of high-quality, well vascularized granulation tissue, and attenuate the production of fibrotic or hypertrophic scar tissue, thereby improving wound healing rate and quality. In particular, therapeutic hydrogels addressed concerns such as desiccation (loss of moisture from the wound), bacterial infection, and prevention of debilitating scar formation. Notably, hydrogel-EXO treatments brought to the promotion of proper skin regeneration (growth of skin appendages, such as hair follicles, and other cutaneous glands) within the wound, indicating that epidermal stem cells were activated (Wang et al., 2019a; Wang et al., 2019b; Nooshabadi et al., 2020; Shiekh et al., 2020; Li et al., 2021). As fewer skin appendages could be found in diabetic wounds treated by pure exosomes, these results strongly indicate that the sustained release of exosomes may facilitate complete wound healing with abundant skin appendages and scarless tissue (Wang et al., 2019a).
EXO are rapidly cleared from the application site and survive in vivo for only a short time (Liu et al., 2017), and specifically EXO rapidly degrades at body temperature particularly in the chronic wound sites, which may decrease their therapeutic efficacy. On the other hand, chronic wound repair and regeneration, particularly in diabetic patients, require a long healing time (more than 3 months) during which many proteases are released by concurring cells (Zeng and Liu, 2021). The biomaterials used in the combination with MSC secretome are of paramount importance to stabilize and allow EXO biological activities. In the study by Yang et al. (2020), a hydrogel based on Pluronic 127 (PF-127), that provide a moist environment for wound healing and act as a barrier against harmful substances, was used with human UC (hUC)-MSC-EXO in the treatment of diabetic wounds, demonstrating that both hUC-MSC-EXO and hUC-MSC-EXO/PF-127 reduced the wound area in the first three days after treatment in vivo. Subsequently, hUC-MSC-EXO/PF-127 evoked a faster healing rate than the other treatments at 7, 10, and 14 days. A study with self-healing polypeptide-based hydrogel, made of PF-127, oxidative hyaluronic acid (OHA), and Poly-ε-L-lysine (EPL), showed a long-term exosomes release (up to 21 days) and an enhanced proliferation of human umbilical vein endothelial cells (HUVECs) than one-time treatment of exosomes, as well as a higher diabetic wound healing rate at 14 days as compared with the EXO-only group (Wang et al., 2019a). These results are compatible with the notion that, besides in the first phases of wound healing, the biological activity of hUC-MSC-EXO was prolonged by the protection of the PF-127 gel, and we can assume that these exosomes were continuously released, leading to increased, sustained, and rapid wound healing. In summary, from these studies we have learnt that developing a biocompatible scaffold that can maintain the function of EXO and sustained release would be critical for exosomes-based therapeutics for cutaneous wound healing. Moreover, it can be noticed a synergistic action of scaffolds with exosomes. In particular, the sustained release of bioactive factors in scaffold dressing could efficiently enhance the early angiogenesis in the diabetic wound and accelerate the healing. The main primeval action of EXO released by scaffolds is to increase cell proliferation and migration underlying the first stages during wound healing, i.e., re-epithelization and neoangiogenesis, giving rise to granulation tissue and subsequent matrix deposition and remodeling. It remains to understand the specific functional component of EXO and mechanism by which exosomes released by scaffolds operate and accelerate wound healing. Non-excluding mechanisms include the modulation of signaling pathways (Chen et al., 2017) and the delivery of anti-inflammatory and anti-scarring miRNAs (Golchin et al., 2018). Activation of AKT, ERK, and STAT3, and the induction of the expression of cell cycle genes and growth factors as well (including HGF, insulin-like growth factor (IGF)-1, nerve growth factor (NGF), and stromal cell-derived factor (SDF)-1) by MSC-derived EVs/EXO play a role in inhibiting stress-induced skin cell apoptosis, and in promoting their migration and proliferation (Shabbir et al., 2015; Kim et al., 2018; Ren et al., 2019). HUC-MSCs-EXO could promote wound healing in the rat model of skin deep second-degree burn injury through activation of Wnt/β-catenin to enhance proliferation and migration of skin cells and AKT signaling to reduce heat stress-induced apoptosis (Zhang et al., 2015a). The same group showed that the administration of hUC-MSCs-EXO in a deep second-degree burn injury skin model promoted wound healing and angiogenesis by delivering Wnt4 and activating Wnt/β-catenin signaling in endothelial cells (Zhang et al., 2015b).
MiR-181c in UC-MSC-EXO was demonstrated to reduce burn-induced excessive inflammation by downregulating the TLR4 signaling pathway (Li et al., 2016). In a rat deep second-degree burn injury model, hUC-MSC-derived EXO promoted activation of β-catenin and skin stem cell proliferation in the early stages of tissue repair and restricted excessive cell expansion by inhibiting Wnt signaling via transfer of the 14–3-3ζ protein, inducing cytoplasmic retention of the YAP protein (Zhang et al., 2016). hUC-MSC-Exo could promote wound healing and reduce scarring by delivering a group of specific microRNAs (miR-21, miR-23a, miR-125b, and miR-145) that were found to suppress myofibroblast formation by inhibiting excess α-smooth muscle actin and collagen deposition associated with activity of the TGF-β/SMAD2 signaling pathway (Fang et al., 2016).
As regarding the techniques used in the studies procuring biocomposites with MSC-EXO, electrospun biomaterials, which mimics ECM structure, have been shown to give rise to homogenous mixtures made of nanofibres with high tensile strength (Riha et al., 2021), however they are derived from a complicated process that produces ECM matrix structure with an unsatisfactory strain (Sadeghi-Avalshahr et al., 2017). Moreover, the elecrospinning process depends on many variables, and it is problematic to obtain 3D structures with the required pore size needed for biomedical application (Law et al., 2017; Keirouz et al., 2020). Decellularised scaffolds retain native ECM thus maintaining normal atomical features, as well as present less inflammatory and immune response with higher mechanical strength (Chaudhari et al., 2016). Although the human amniotic membrane (AM) presents many advantages, including anti-bacterial, anti-inflammatory, and non-immunogenic properties, promotes reduced pain and dehydration, and favors the reepithelialization process, disadvantages of AM include poor mechanical properties and a high biodegradability rate, which complicate its extensive use in clinic (Dussoyer et al., 2020). Due to the limited use of decellularized AM in the skin regeneration field combining composites and EXO, and unknown mechanisms of action, further studies are needed to comprehend its usefulness as compared with other decellularized sources and other composites, either made of natural or synthetic polymers.
Hydrogels, that were found the most used composites in the above outlined studies, however show on their own some limitation when used in conjunction with MSCs. First, MSCs pre-encapsulated within hydrogels may slowly alter hydrogel stability and mechanical properties due to secretion of proteases. Thereby, seeding premade hydrogels with MSCs should be operated at the point of care, implying that the un-seeded hydrogel must be easy to handle and encourage rapid cell seeding (Garg et al., 2014). The integration of MSC secretome, and significantly EXO, should overcome these limitations. Another critical issue is linked to already moist wounds, such as venous leg ulcers, as hydrogels may cause a high amount of output drainage and exudate from the site that further impedes healing by slowing down cell growth, degrading the tissue matrix structure, promoting inflammation or bacterial contamination (Murakami et al., 2010). Finally, these hydrogels are usually changed every 3 days, so production of these scaffolds must be simple, quick, and inexpensive to be commercially appealing for physicians (Sivaraj et al., 2021). All these issues will be the focus of future studies on hydrogels applied to the MSC secretome delivery, in particular EXO, to wound-healing settings in animal models first and in patients hereafter.
Although a direct comparison among CM, EVs and EXO has not been conducted in the setting of cutaneous wound repair in combination with biomaterials, it has been shown that MSC-EXO’s role is not static during the entire cutaneous tissue regeneration process and they exert distinct effects on skin cell proliferation at various cell densities (Zhang et al., 2016). However, further studies are deemed to definitively understand which MSC secretome would have better results in terms of application to different pathological skin wound healing processes.
Finally, from a logistic point of view, clinic application of MSC-derived exosomes in wound healing needs that the standard procedures for purification, storage, and administration of therapeutic exosomes with low cost ought to be developed (Hettich et al., 2020).
Nonhealing and chronic wounds (mainly diabetic) deserve more efficient treatment options that accelerate wound healing, favor neoangiogenesis, reduce scarring, and allow optimal epidermal reconstitution. Biomimetic materials and MSC-derived exosomes possess all these properties and therefore have great potential in achieving satisfactory healing in recalcitrant wounds. Due to their versatility, different fabrication techniques, and numerous biological properties, hydrogels represent a promising approach to advance the combination of EXO with tissue engineering scaffolds to the clinic.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: Massimo Conese; analysis of the literature: Massimo Conese, Aurelio Portincasa; draft manuscript preparation: Massimo Conese, Aurelio Portincasa. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ahangar P, Mills SJ, Cowin AJ (2020). Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. International Journal of Molecular Sciences 21: 7038. DOI 10.3390/ijms21197038. [Google Scholar] [CrossRef]
Alapure BV, Lu Y, He M, Chu CC, Peng H et al. (2018). Accelerate healing of severe burn wounds by mouse bone marrow mesenchymal stem cell-seeded biodegradable hydrogel scaffold synthesized from arginine-based poly(ester amide) and chitosan. Stem Cells and Development 27: 1605–1620. DOI 10.1089/scd.2018.0106. [Google Scholar] [CrossRef]
Altman AM, Yan Y, Matthias N, Bai X, Rios C et al. (2009). IFATS collection: Human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells 27: 250–258. DOI 10.1634/stemcells.2008-0178. [Google Scholar] [CrossRef]
An Y, Lin S, Tan X, Zhu S, Nie F et al. (2021). Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Proliferation 54: e12993. DOI 10.1111/cpr.12993. [Google Scholar] [CrossRef]
An Y, Wei W, Jing H, Ming L, Liu S et al. (2015). Bone marrow mesenchymal stem cell aggregate: An optimal cell therapy for full-layer cutaneous wound vascularization and regeneration. Scientific Reports 5: 17036. DOI 10.1038/srep17036. [Google Scholar] [CrossRef]
Annabi N, Rana D, Shirzaei Sani E, Portillo-Lara R, Gifford JL et al. (2017). Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 139: 229–243. DOI 10.1016/j.biomaterials.2017.05.011. [Google Scholar] [CrossRef]
Arwert EN, Hoste E, Watt FM (2012). Epithelial stem cells, wound healing and cancer. Nature Reviews Cancer 12: 170–180. DOI 10.1038/nrc3217. [Google Scholar] [CrossRef]
Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C et al. (2006). Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation 113: 2413–2424. DOI 10.1161/CIRCULATIONAHA.105.603167. [Google Scholar] [CrossRef]
Bello YM, Falabella AF, Eaglstein WH (2001). Tissue-engineered skin. Current status in wound healing. American Journal of Clinical Dermatology 2: 305–313. DOI 10.2165/00128071-200102050-00005. [Google Scholar] [CrossRef]
Bertozzi N, Simonacci F, Grieco MP, Grignaffini E, Raposio E (2017). The biological and clinical basis for the use of adipose-derived stem cells in the field of wound healing. Annals of Medicine and Surgery 20: 41–48. DOI 10.1016/j.amsu.2017.06.058. [Google Scholar] [CrossRef]
Blanpain C, Fuchs E (2009). Epidermal homeostasis: A balancing act of stem cells in the skin. Nature Reviews Molecular Cell Biology 10: 207–217. DOI 10.1038/nrm2636. [Google Scholar] [CrossRef]
Cerqueira MT, Pirraco RP, Marques AP (2016). Stem cells in skin wound healing: Are we there yet? Advances in Wound Care 5: 164–175. DOI 10.1089/wound.2014.0607. [Google Scholar] [CrossRef]
Chaudhari AA, Vig K, Baganizi DR, Sahu R, Dixit S et al. (2016). Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. International Journal of Molecular Sciences 17: 1974. DOI 10.3390/ijms17121974. [Google Scholar] [CrossRef]
Chen B, Li Q, Zhao B, Wang Y (2017). Stem cell-derived extracellular vesicles as a novel potential therapeutic tool for tissue repair. Stem Cells Translational Medicine 6: 1753–1758. DOI 10.1002/sctm.16-0477. [Google Scholar] [CrossRef]
Chen JS, Wong VW, Gurtner GC (2012). Therapeutic potential of bone marrow-derived mesenchymal stem cells for cutaneous wound healing. Frontiers in Immunology 3: 192. DOI 10.3389/fimmu.2012.00192. [Google Scholar] [CrossRef]
Chen L, Tredget EE, Wu PY, Wu Y (2008). Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3: e1886. DOI 10.1371/journal.pone.0001886. [Google Scholar] [CrossRef]
Chen S, Wang H, Su Y, John JV, McCarthy A et al. (2020). Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomaterialia 108: 153–167. DOI 10.1016/j.actbio.2020.03.035. [Google Scholar] [CrossRef]
Choi EW, Seo MK, Woo EY, Kim SH, Park EJ et al. (2018). Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Experimental Dermatology 27: 1170–1172. DOI 10.1111/exd.13451. [Google Scholar] [CrossRef]
Choi S, Yoon M, Choi KY (2022). Approaches for regenerative healing of cutaneous wound with an emphasis on strategies activating the Wnt/beta-catenin pathway. Advances in Wound Care 11: 70–86. DOI 10.1089/wound.2020.1284. [Google Scholar] [CrossRef]
Chung E, Rybalko VY, Hsieh PL, Leal SL, Samano MA et al. (2016). Fibrin-based stem cell containing scaffold improves the dynamics of burn wound healing. Wound Repair and Regeneration 24: 810–819. DOI 10.1111/wrr.12459. [Google Scholar] [CrossRef]
Conese M, Annacontini L, Carbone A, Beccia E, Cecchino LR et al. (2020). The role of adipose-derived stem cells, dermal regenerative templates, and platelet-rich plasma in tissue engineering-based treatments of chronic skin wounds. Stem Cells International 2020: 7056261. DOI 10.1155/2020/7056261. [Google Scholar] [CrossRef]
Cuenca J, Le-Gatt A, Castillo V, Belletti J, Diaz M et al. (2018). The reparative abilities of menstrual stem cells modulate the wound matrix signals and improve cutaneous regeneration. Frontiers in Physiology 9: 464. DOI 10.3389/fphys.2018.00464. [Google Scholar] [CrossRef]
Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E (2019). Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. Journal of Tissue Engineering and Regenerative Medicine 13: 555–568. DOI 10.1002/term.2799. [Google Scholar] [CrossRef]
Dussoyer M, Michopoulou A, Rousselle P (2020). Decellularized scaffolds for skin repair and regeneration. Applied Sciences 10: 3435. DOI 10.3390/app10103435. [Google Scholar] [CrossRef]
Falanga V (2005). Wound healing and its impairment in the diabetic foot. Lancet 366: 1736–1743. DOI 10.1016/S0140-6736(05)67700-8. [Google Scholar] [CrossRef]
Fang S, Xu C, Zhang Y, Xue C, Yang C et al. (2016). Umbilical cord-derived mesenchymal stem cell-derived exosomal micrornas suppress myofibroblast differentiation by inhibiting the transforming growth factor-beta/SMAD2 pathway during wound healing. Stem Cells Translational Medicine 5: 1425–1439. DOI 10.5966/sctm.2015-0367. [Google Scholar] [CrossRef]
Ferreira ADF, Cunha PDS, Carregal VM, da Silva PC, de Miranda MC et al. (2017). Extracellular vesicles from adipose-derived mesenchymal stem/stromal cells accelerate migration and activate AKT pathway in human keratinocytes and fibroblasts independently of miR-205 activity. Stem Cells International 2017: 9841035. DOI 10.1155/2017/9841035. [Google Scholar] [CrossRef]
Forstermann U, Munzel T (2006). Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 113: 1708–1714. DOI 10.1161/CIRCULATIONAHA.105.602532. [Google Scholar] [CrossRef]
Garg RK, Rennert RC, Duscher D, Sorkin M, Kosaraju R et al. (2014). Capillary force seeding of hydrogels for adipose-derived stem cell delivery in wounds. STEM CELLS Translational Medicine 3: 1079–1089. DOI 10.5966/sctm.2014-0007. [Google Scholar] [CrossRef]
Gary Sibbald R, Woo KY (2008). The biology of chronic foot ulcers in persons with diabetes. Diabetes/Metabolism Research and Reviews 24: S25–30. [Google Scholar]
Gnecchi M, Zhang Z, Ni A, Dzau VJ (2008). Paracrine mechanisms in adult stem cell signaling and therapy. Circulation Research 103: 1204–1219. DOI 10.1161/CIRCRESAHA.108.176826. [Google Scholar] [CrossRef]
Golchin A, Hosseinzadeh S, Ardeshirylajimi A (2018). The exosomes released from different cell types and their effects in wound healing. Journal of Cellular Biochemistry 119: 5043–5052. DOI 10.1002/jcb.26706. [Google Scholar] [CrossRef]
Gridley T (2010). Notch signaling in the vasculature. Current Topics in Developmental Biology 92: 277–309. DOI 10.1016/S0070-2153(10)92009-7. [Google Scholar] [CrossRef]
Hassan WU, Greiser U, Wang W (2014). Role of adipose-derived stem cells in wound healing. Wound Repair and Regeneration 22: 313–325. DOI 10.1111/wrr.12173. [Google Scholar] [CrossRef]
He X, Dong Z, Cao Y, Wang H, Liu S et al. (2019). MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells International 2019: 7132708. DOI 10.1155/2019/7132708. [Google Scholar] [CrossRef]
Hettich BF, Ben-Yehuda Greenwald M, Werner S, Leroux JC (2020). Exosomes for wound healing: Purification optimization and identification of bioactive components. Advanced Science 7: 2002596. DOI 10.1002/advs.202002596. [Google Scholar] [CrossRef]
Ho J, Walsh C, Yue D, Dardik A, Cheema U (2017). Current advancements and strategies in tissue engineering for wound healing: A comprehensive review. Advances in Wound Care 6: 191–209. DOI 10.1089/wound.2016.0723. [Google Scholar] [CrossRef]
Houschyar KS, Momeni A, Pyles MN, Maan ZN, Whittam AJ et al. (2015). Wnt signaling induces epithelial differentiation during cutaneous wound healing. Organogenesis 11: 95–104. DOI 10.1080/15476278.2015.1086052. [Google Scholar] [CrossRef]
Hu L, Wang J, Zhou X, Xiong Z, Zhao J et al. (2016). Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Scientific Reports 6: 32993. DOI 10.1038/srep32993. [Google Scholar] [CrossRef]
Hu MS, Borrelli MR, Lorenz HP, Longaker MT, Wan DC (2018). Mesenchymal stromal cells and cutaneous wound healing: A comprehensive review of the background, role, and therapeutic potential. Stem Cells International 2018: 6901983. DOI 10.1155/2018/6901983. [Google Scholar] [CrossRef]
Hu MS, Maan ZN, Wu JC, Rennert RC, Hong WX et al. (2014). Tissue engineering and regenerative repair in wound healing. Annals of Biomedical Engineering 42: 1494–1507. DOI 10.1007/s10439-014-1010-z. [Google Scholar] [CrossRef]
Huang YZ, Gou M, Da LC, Zhang WQ, Xie HQ (2020). Mesenchymal stem cells for chronic wound healing: Current status of preclinical and clinical studies. Tissue Engineering Part B: Reviews 26: 555–570. DOI 10.1089/ten.teb.2019.0351. [Google Scholar] [CrossRef]
Kamoun EA, Kenawy ES, Chen X (2017). A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. Journal of Advanced Research 8: 217–233. DOI 10.1016/j.jare.2017.01.005. [Google Scholar] [CrossRef]
Keirouz A, Chung M, Kwon J, Fortunato G, Radacsi N (2020). 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: A review. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology 12: e1626. DOI 10.1002/wnan.1626. [Google Scholar] [CrossRef]
Khosrotehrani K (2013). Mesenchymal stem cell therapy in skin: Why and what for? Experimental Dermatology 22: 307–310. DOI 10.1111/exd.12141. [Google Scholar] [CrossRef]
Kim S, Lee SK, Kim H, Kim TM (2018). Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. International Journal of Molecular Sciences 19: 3119. DOI 10.3390/ijms19103119. [Google Scholar] [CrossRef]
Law JX, Liau LL, Saim A, Yang Y, Idrus R (2017). Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Engineering and Regenerative Medicine 14: 699–718. DOI 10.1007/s13770-017-0075-9. [Google Scholar] [CrossRef]
Le H, Kleinerman R, Lerman OZ, Brown D, Galiano R et al. (2008). Hedgehog signaling is essential for normal wound healing. Wound Repair and Regeneration 16: 768–773. DOI 10.1111/j.1524-475X.2008.00430.x. [Google Scholar] [CrossRef]
Lee DE, Ayoub N, Agrawal DK (2016). Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Research & Therapy 7: 37. DOI 10.1186/s13287-016-0303-6. [Google Scholar] [CrossRef]
Li M, Luan F, Zhao Y, Hao H, Liu J et al. (2017). Mesenchymal stem cell-conditioned medium accelerates wound healing with fewer scars. International Wound Journal 14: 64–73. DOI 10.1111/iwj.12551. [Google Scholar] [CrossRef]
Li Q, Gong S, Yao W, Yang Z, Wang R et al. (2021). Exosome loaded genipin crosslinked hydrogel facilitates full thickness cutaneous wound healing in rat animal model. Drug Delivery 28: 884–893. DOI 10.1080/10717544.2021.1912210. [Google Scholar] [CrossRef]
Li X, Liu L, Yang J, Yu Y, Chai J et al. (2016). Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBioMedicine 8: 72–82. DOI 10.1016/j.ebiom.2016.04.030. [Google Scholar] [CrossRef]
Li Y, Wang X, Fu YN, Wei Y, Zhao L et al. (2018). Self-adapting hydrogel to improve the therapeutic effect in wound-healing. ACS Applied Materials & Interfaces 10: 26046–26055. DOI 10.1021/acsami.8b08874. [Google Scholar] [CrossRef]
Liang X, Ding Y, Zhang Y, Tse HF, Lian Q (2014). Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplantation 23: 1045–1059. DOI 10.3727/096368913X667709. [Google Scholar] [CrossRef]
Liang X, Zhang L, Wang S, Han Q, Zhao RC (2016). Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. Journal of Cell Science 129: 2182–2189. DOI 10.1242/jcs.170373. [Google Scholar] [CrossRef]
Liu X, Yang Y, Li Y, Niu X, Zhao B et al. (2017). Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 9: 4430–4438. DOI 10.1039/C7NR00352H. [Google Scholar] [CrossRef]
Lu TY, Yu KF, Kuo SH, Cheng NC, Chuang EY et al. (2020). Enzyme-crosslinked gelatin hydrogel with adipose-derived stem cell spheroid facilitating wound repair in the murine burn model. Polymers 12: 2997. DOI 10.3390/polym12122997. [Google Scholar] [CrossRef]
Manchon E, Hirt N, Bouaziz JD, Jabrane-Ferrat N, Al-Daccak R (2021). Stem cells-derived extracellular vesicles: Potential therapeutics for wound healing in chronic inflammatory skin diseases. International Journal of Molecular Sciences 22: 3130. DOI 10.3390/ijms22063130. [Google Scholar] [CrossRef]
Mathes SH, Ruffner H, Graf-Hausner U (2014). The use of skin models in drug development. Advanced Drug Delivery Reviews 69–70: 81–102. DOI 10.1016/j.addr.2013.12.006. [Google Scholar] [CrossRef]
Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA (2012). Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Translational Medicine 1: 142–149. DOI 10.5966/sctm.2011-0018. [Google Scholar] [CrossRef]
Merino-Gonzalez C, Zuniga FA, Escudero C, Ormazabal V, Reyes C et al. (2016). Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: Potencial clinical application. Frontiers in Physiology 7: 24. DOI 10.3389/fphys.2016.00024. [Google Scholar] [CrossRef]
Moura LI, Dias AM, Carvalho E, de Sousa HC (2013). Recent advances on the development of wound dressings for diabetic foot ulcer treatment--A review. Acta Biomaterialia 9: 7093–7114. DOI 10.1016/j.actbio.2013.03.033. [Google Scholar] [CrossRef]
Murakami K, Aoki H, Nakamura S, Nakamura S, Takikawa M et al. (2010). Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 31: 83–90. DOI 10.1016/j.biomaterials.2009.09.031. [Google Scholar] [CrossRef]
Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A et al. (2009). Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature Medicine 15: 42–49. [Google Scholar]
Nooshabadi VT, Khanmohamadi M, Valipour E, Mahdipour S, Salati A et al. (2020). Impact of exosome-loaded chitosan hydrogel in wound repair and layered dermal reconstitution in mice animal model. Journal of Biomedical Materials Research Part A 108: 2138–2149. DOI 10.1002/jbm.a.36959. [Google Scholar] [CrossRef]
Okuyama R, Tagami H, Aiba S (2008). Notch signaling: Its role in epidermal homeostasis and in the pathogenesis of skin diseases. Journal of Dermatological Science 49: 187–194. DOI 10.1016/j.jdermsci.2007.05.017. [Google Scholar] [CrossRef]
Phinney DG, Pittenger MF (2017). Concise review: MSC-Derived exosomes for cell-free therapy. Stem Cells 35: 851–858. DOI 10.1002/stem.2575. [Google Scholar] [CrossRef]
Pomatto M, Gai C, Negro F, Cedrino M, Grange C et al. (2021). Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. International Journal of Molecular Sciences 22: 3851. DOI 10.3390/ijms22083851. [Google Scholar] [CrossRef]
Portincasa A, Trecca EMC, Ciancio F, Annacontini L, Bufo P et al. (2018). The role of lipofilling in reconstructions with dermal regeneration template: Clinical and histological assessment. Journal of Biological Regulators and Homeostatic Agents 32: 171–176. [Google Scholar]
Prockop DJ, Oh JY (2012). Mesenchymal stem/stromal cells (MSCsRole as guardians of inflammation. Molecular Therapy 20: 14–20. DOI 10.1038/mt.2011.211. [Google Scholar] [CrossRef]
Rani S, Ritter T (2016). The exosome-A naturally secreted nanoparticle and its application to wound healing. Advanced Materials 28: 5542–5552. DOI 10.1002/adma.201504009. [Google Scholar] [CrossRef]
Raposo G, Stoorvogel W (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. Journal of Cell Biology 200: 373–383. DOI 10.1083/jcb.201211138. [Google Scholar] [CrossRef]
Ren S, Chen J, Duscher D, Liu Y, Guo G et al. (2019). Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Research & Therapy 10: 47. DOI 10.1186/s13287-019-1152-x. [Google Scholar] [CrossRef]
Riha SM, Maarof M, Fauzi MB (2021). Synergistic effect of biomaterial and stem cell for skin tissue engineering in cutaneous wound healing: A concise review. Polymers 13: 1546. DOI 10.3390/polym13101546. [Google Scholar] [CrossRef]
Rodrigues M, Kosaric N, Bonham CA, Gurtner GC (2019). Wound healing: A cellular perspective. Physiological Reviews 99: 665–706. DOI 10.1152/physrev.00067.2017. [Google Scholar] [CrossRef]
Sadeghi-Avalshahr A, Nokhasteh S, Molavi AM, Khorsand-Ghayeni M, Mahdavi-Shahri M (2017). Synthesis and characterization of collagen/PLGA biodegradable skin scaffold fibers. Regenerative Biomaterials 4: 309–314. DOI 10.1093/rb/rbx026. [Google Scholar] [CrossRef]
Savoji H, Godau B, Hassani MS, Akbari M (2018). Skin tissue substitutes and biomaterial risk assessment and testing. Frontiers in Bioengineering and Biotechnology 6: 86. DOI 10.3389/fbioe.2018.00086. [Google Scholar] [CrossRef]
Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, van Badiavas E (2015). Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells and Development 24: 1635–1647. DOI 10.1089/scd.2014.0316. [Google Scholar] [CrossRef]
Shafei S, Khanmohammadi M, Heidari R, Ghanbari H, Taghdiri Nooshabadi V, Farzamfar S, Akbariqomi M, Sanikhani NS, Absalan M, Tavoosidana G (2020). Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. Journal of Biomedical Materials Research Part A 108: 545–556. DOI 10.1002/jbm.a.36835. [Google Scholar] [CrossRef]
Shah M, Foreman DM, Ferguson MW (1995). Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. Journal of Cell Science 108: 985–1002. DOI 10.1242/jcs.108.3.985. [Google Scholar] [CrossRef]
Sharifzadeh G, Hosseinkhani H (2017). Biomolecule-responsive hydrogels in medicine. Advanced Healthcare Materials 6: 1700801. DOI 10.1002/adhm.201700801. [Google Scholar] [CrossRef]
Shi Q, Qian Z, Liu D, Sun J, Wang X et al. (2017). GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Frontiers in Physiology 8: 904. DOI 10.3389/fphys.2017.00904. [Google Scholar] [CrossRef]
Shi Y, Shu B, Yang R, Xu Y, Xing B et al. (2015). Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Research & Therapy 6: 120. DOI 10.1186/s13287-015-0103-4. [Google Scholar] [CrossRef]
Shiekh PA, Singh A, Kumar A (2020). Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials 249: 120020. DOI 10.1016/j.biomaterials.2020.120020. [Google Scholar] [CrossRef]
Shrestha C, Zhao L, Chen K, He H, Mo Z (2013). Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. International Journal of Endocrinology 2013: 592454. DOI 10.1155/2013/592454. [Google Scholar] [CrossRef]
Singer NG, Caplan AI (2011). Mesenchymal stem cells: Mechanisms of inflammation. Annual Review of Pathology: Mechanisms of Disease 6: 457–478. DOI 10.1146/annurev-pathol-011110-130230. [Google Scholar] [CrossRef]
Sivaraj D, Chen K, Chattopadhyay A, Henn D, Wu W et al. (2021). Hydrogel scaffolds to deliver cell therapies for wound healing. Frontiers in Bioengineering and Biotechnology 9: 660145. DOI 10.3389/fbioe.2021.660145. [Google Scholar] [CrossRef]
Soo C, Beanes SR, Hu FY, Zhang X, Dang C et al. (2003). Ontogenetic transition in fetal wound transforming growth factor-beta regulation correlates with collagen organization. American Journal of Pathology 163: 2459–2476. DOI 10.1016/S0002-9440(10)63601-2. [Google Scholar] [CrossRef]
Su N, Gao PL, Wang K, Wang JY, Zhong Y et al. (2017). Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials 141: 74–85. DOI 10.1016/j.biomaterials.2017.06.028. [Google Scholar] [CrossRef]
Tang KC, Yang KC, Lin CW, Chen YK, Lu TY et al. (2019). Human adipose-derived stem cell secreted extracellular matrix incorporated into electrospun poly(lactic-co-glycolic acid) nanofibrous dressing for enhancing wound healing. Polymers 11: 1609. DOI 10.3390/polym11101609. [Google Scholar] [CrossRef]
Tao SC, Guo SC, Li M, Ke QF, Guo YP et al. (2017). Chitosan wound dressings incorporating exosomes derived from MicroRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Translational Medicine 6: 736–747. DOI 10.5966/sctm.2016-0275. [Google Scholar] [CrossRef]
Tartarini D, Mele E (2015). Adult stem cell therapies for wound healing: Biomaterials and computational models. Frontiers in Bioengineering and Biotechnology 3: 206. [Google Scholar]
Taylor DL, In Het Panhuis M (2016). Self-healing hydrogels. Advanced Materials 28: 9060–9093. DOI 10.1002/adma.201601613. [Google Scholar] [CrossRef]
Wang C, Wang M, Xu T, Zhang X, Lin C et al. (2019a). Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 9: 65–76. DOI 10.7150/thno.29766. [Google Scholar] [CrossRef]
Wang M, Wang C, Chen M, Xi Y, Cheng W et al. (2019b). Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano 13: 10279–10293. DOI 10.1021/acsnano.9b03656. [Google Scholar] [CrossRef]
Watt FM, Estrach S, Ambler CA (2008). Epidermal Notch signalling: Differentiation, cancer and adhesion. Current Opinion in Cell Biology 20: 171–179. DOI 10.1016/j.ceb.2008.01.010. [Google Scholar] [CrossRef]
Xi Y, Ge J, Guo Y, Lei B, Ma PX (2018). Biomimetic elastomeric polypeptide-based nanofibrous matrix for overcoming multidrug-resistant bacteria and enhancing full-thickness wound healing/skin regeneration. ACS Nano 12: 10772–10784. DOI 10.1021/acsnano.8b01152. [Google Scholar] [CrossRef]
Xiao S, Xiao C, Miao Y, Wang J, Chen R et al. (2021). Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Research & Therapy 12: 255. DOI 10.1186/s13287-021-02333-6. [Google Scholar] [CrossRef]
Xu Q, AS, Gao Y, Guo L, Creagh-Flynn J et al. (2018). A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing. Acta Biomaterialia 75: 63–74. DOI 10.1016/j.actbio.2018.05.039. [Google Scholar] [CrossRef]
Yang J, Chen Z, Pan D, Li H, Shen J (2020). Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. International Journal of Nanomedicine 15: 5911–5926. [Google Scholar]
Yew TL, Hung YT, Li HY, Chen HW, Chen LL et al. (2011). Enhancement of wound healing by human multipotent stromal cell conditioned medium: The paracrine factors and p38 MAPK activation. Cell Transplantion 20: 693–706. DOI 10.3727/096368910X550198. [Google Scholar] [CrossRef]
Ylostalo JH, Bartosh TJ, Coble K, Prockop DJ (2012). Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 30: 2283–2296. [Google Scholar]
Yoon BS, Moon JH, Jun EK, Kim J, Maeng I et al. (2010). Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells and Development 19: 887–902. [Google Scholar]
Yu J, Wang MY, Tai HC, Cheng NC (2018). Cell sheet composed of adipose-derived stem cells demonstrates enhanced skin wound healing with reduced scar formation. Acta Biomaterialia 77: 191–200. [Google Scholar]
Yu Y, Yoo SM, Park HH, Baek SY, Kim YJ et al. (2019). Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis. Journal of Tissue Engineering and Regenerative Medicine 13: 1792–1804. [Google Scholar]
Zeng QL, Liu DW (2021). Mesenchymal stem cell-derived exosomes: An emerging therapeutic strategy for normal and chronic wound healing. World Journal of Clinical Cases 9: 6218–6233. [Google Scholar]
Zhang B, Shi Y, Gong A, Pan Z, Shi H et al. (2016). HucMSC exosome-delivered 14-3-3zeta orchestrates self-control of the Wnt response via modulation of YAP during cutaneous regeneration. Stem Cells 34: 2485–2500. DOI 10.1002/stem.2432. [Google Scholar] [CrossRef]
Zhang B, Wang M, Gong A, Zhang X, Wu X et al. (2015a). HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 33: 2158–2168. DOI 10.1002/stem.1771. [Google Scholar] [CrossRef]
Zhang B, Wu X, Zhang X, Sun Y, Yan Y et al. (2015b). Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta-catenin pathway. Stem Cells Translational Medicine 4: 513–522. DOI 10.5966/sctm.2014-0267. [Google Scholar] [CrossRef]
Zhang J, Guan J, Niu X, Hu G, Guo S et al. (2015c). Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. Journal of Translational Medicine 13: 49. DOI 10.1186/s12967-015-0417-0. [Google Scholar] [CrossRef]
Zhang J, La X, Fan L, Li P, Yu Y et al. (2015d). Immunosuppressive effects of mesenchymal stem cell transplantation in rat burn models. International Journal of Clinical and Experimental Pathology 8: 5129–5136. [Google Scholar]
Zhou P, Li X, Zhang B, Shi Q, Li D et al. (2019). A human umbilical cord mesenchymal stem cell-conditioned medium/chitosan/collagen/beta-glycerophosphate thermosensitive hydrogel promotes burn injury healing in mice. BioMed Research International 2019: 5768285. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |