DOI:10.32604/biocell.2022.019086

| BIOCELL DOI:10.32604/biocell.2022.019086 |  |
| Viewpoint |
Exocytosis, endocytosis and recycling of secretory vesicles in neuroendocrine cells, and its regulation by cortical actin
1Centro Interdisciplinario de Neurociencia de Valparaíso, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso, 2360102, Chile
2Instituto de Fisiología, Biología Molecular y Neurociencias, CONICET. Departamento de Fisiología y Biología Molecular y Celular, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires, 1428, Argentina
*Address correspondence to: Fernando D. Marengo, fernando@fbmc.fcen.uba.ar; Ana M. Cárdenas, ana.cardenas@uv.cl
Received: 03 September 2021; Accepted: 08 December 2021
Abstract: The cortical actin network is a mesh of filaments distributed beneath the plasmalemma that dynamically reacts in response to stimuli. This dynamic network of cortical filaments, together with motor myosin partners, adjusts the plasmalemma tension, organizes membrane protein microdomains, remodels the cell surface and drives vesicle motion in order to fine-tune exocytosis, endocytosis and recycling of secretory vesicles. In this review, we discuss how these mechanisms work in secretory cells.
Keywords: Cortical actin; Neuroendocrine cell; Chromaffin cell; Exocytosis; Endocytosis
The cytoskeletal actin network is a highly dynamic mesh of filaments formed by globular actin monomers (G-actin) that assemble to form actin filaments (F-actin). Six actin isoforms have been described in mammals. Among them, the cytoplasmic isoforms β-actin and γ-actin are components of the cytoskeleton, with β-actin mostly expressed in stress fibers, contractile rings and cell-cell contacts, whereas γ-actin is mainly present in a dense branched meshwork at the cell cortex (cortical actin) and lamellipodia (Dugina et al., 2009). Nucleation, elongation, ramification, and depolymerization of these filaments are tightly orchestrated by a set of enzymes and auxiliary proteins such as the ARP2/3 complex, N-WASP, cortactin, Src kinases, scinderin, and cofilin, among others (Gasman et al., 2004; Olivares et al., 2014; González-Jamett et al., 2017; Carman and Dominguez, 2018). This armamentarium of proteins acts in concert to rearrange cortical actin filaments in response to stimuli (Rottner et al., 2017; Li et al., 2018), remodeling the cell surface, driving vesicles and other organelles to the plasmalemma as occurs during exocytosis (Papadopulos, 2017; Miklavc and Frick, 2020; Venkatesh et al., 2020), and/or removing patches of the plasmalemma and directing the formed vesicles to a given target membrane, as occurs during endocytosis (Houy et al., 2013; Hinze and Boucrot, 2018). Together with motor partners, such as myosin II, the cortical actin network further provides the membrane tension and drives forces for these processes to occur (Chugh and Paluch, 2018; Sonal et al., 2018; Svitkina, 2020). In this review, we will focus on the role of the cortical F-actin network on regulated exocytosis, compensatory endocytosis and vesicle recycling in secretory cells, with an emphasis on adrenal chromaffin cells.
How Does the Cortical Actin Control Exocytosis in Secretory Cells?
A selective set of proteins regulates Ca2+ and SNARE-dependent fusion of secretory granules with the plasmalemma to tightly control the release of neurohormones (Cárdenas and Marengo, 2016) and neurotransmitters (Jahn and Fasshauer, 2012). Among these proteins are the actin filaments (Marengo and Cárdenas, 2018). In this regard, increasing evidence during the last 40 years has demonstrated that cortical actin plays a pivotal role in different processes associated with the activity of secretory cells. In the early 1980s, it was noted that the actin filaments are densely localized in the periphery of neurosecretory chromaffin cells (i.e., cortical actin) (Lee and Trifaro, 1981), and interact with secretory granule membranes in a Ca2+-dependent manner (Fowler and Pollard, 1982). Thus, it was initially proposed that this cortical mesh of F-actin acts as a barrier that retains secretory vesicles away from the subplasmalemmal area, and that, upon a rise of cytosolic Ca2+ concentrations, this F-actin network disassembles, releasing the secretory vesicles to allow them to reach the cell periphery (Fowler and Pollard, 1982; Burgoyne and Cheek, 1987; Aunis and Bader, 1988). A similar mechanism was proposed later in pancreatic beta cells (Trexler and Taraska, 2017) and mast cells (Singh et al., 2013). According to this idea, treatment of chromaffin cells with cytochalasin D, a fungal metabolite that prevents the incorporation of new monomers into actin filaments by binding to the barbed ends (Cooper, 1987), or with latrunculin A, a toxin that sequesters actin monomers and promotes subunit dissociation from the F-actin ends (Fujiwara et al., 2018), increases the number of vesicles fusing with the plasmalemma (Berberian et al., 2009). These treatments facilitate a slow secretory component, as was visualized by electron microscopy and measured by single-cell amperometry (Gil et al., 2000). The disassembly of the F-actin network triggered by Ca2+ involves scinderin, an actin-binding protein that promotes disassembly of actin filaments upon cytosolic Ca2+ rises (Rodriguez Del Castillo et al., 1990), and MARCKS, a myristoylated alanine-rich C kinase substrate that in its non-phosphorylated state binds and cross-links actin filaments, but upon its PKC-induced phosphorylation such MARCKS´s properties are inhibited, allowing F-actin disassembly (Rosé et al., 2001). More recent studies show that MUNC18-1 also regulates the cortical F-actin mesh, as Munc18-1 knock-out (KO) chromaffin cells show a twice as dense F-actin network (Pons-Vizcarra et al., 2019). Curiously, the expression of a MUNC18-1 mutant (V263T) that recovers recruitment, docking and fusion of secretory vesicles in KO cells, does not rescue the F-actin phenotype (Pons-Vizcarra et al., 2019), suggesting that a dense cortical F-actin meshwork is not a rate-limiting barrier for the motion of the secretory vesicle towards the exocytotic sites.
As demonstrated by us, Ca2+ concentrations that trigger exocytosis not only promote disruption of a preexisting cortical actin network but also induce the formation of new actin filaments (Olivares et al., 2014; Figs. 1A and 1B), indicating that the cytoskeletal actin mesh is actively remodeled and adapted to facilitate different steps of the neurosecretory process (Fig. 1C). Among the proteins involved in de novo formation of actin filaments are the small GTPase Cdc42 and αII-spectrin, which recruit N-WASP, a promotor of Arp2/3-mediated actin nucleation, to the plasmalemma (Gasman et al., 2004; Houy et al., 2020). Src kinase and its substrate cortactin also contribute to the Ca2+-dependent formation of F-actin, as established by the enhanced incorporation of Alexa Fluor 488-G-actin into filaments (Olivares et al., 2014; González-Jamett et al., 2017). On the other hand, the β2a subunit of voltage-dependent Ca2+ channels reduces both the incorporation of G-actin monomers into actin filaments, and Ca2+ and Na+ currents in bovine chromaffin cells, indicating that the F-actin cortex also influences the traffic of vesicle carrying ion channels (Guerra et al., 2019).
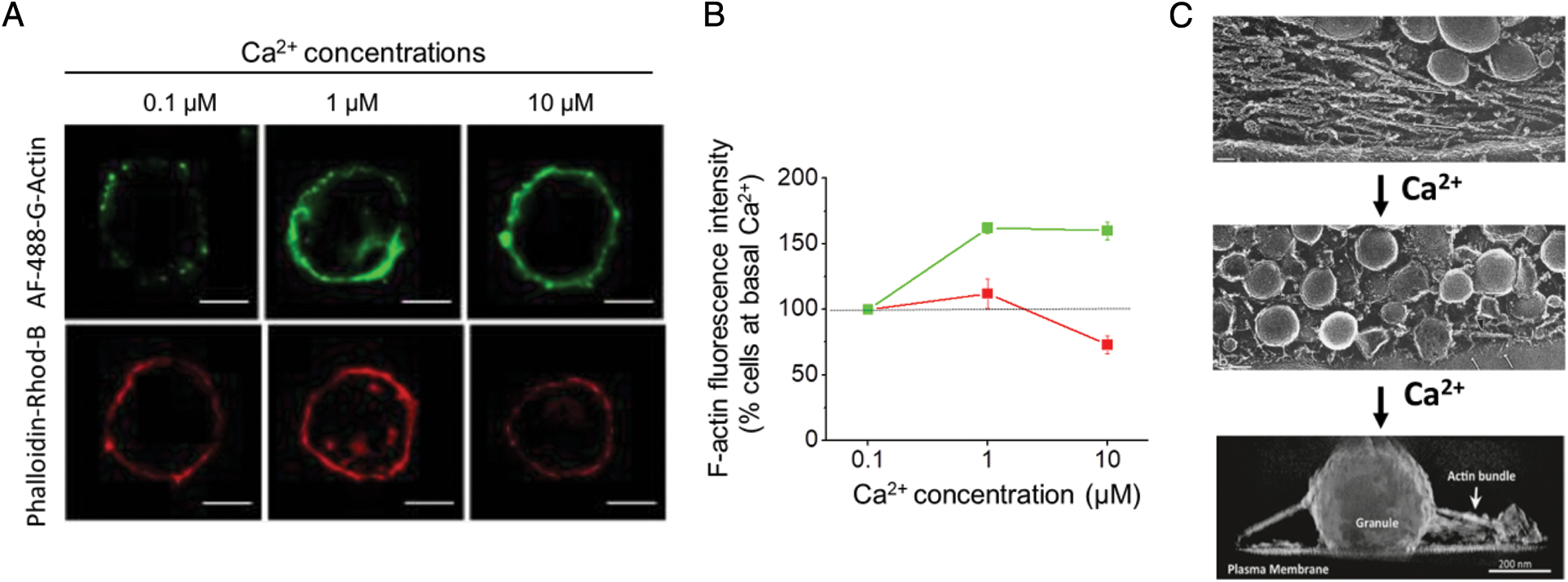
Figure 1: Ca2+ concentrations that induce exocytosis promote both disruption of the preexisting cortical actin network and formation of new actin filaments. A–B: As measured in permeabilized chromaffin cells, formation of new actin filaments (green) is observed at 1 and 10 μM free Ca2+ concentrations, whereas a significant disruption of the actin network (red) is manifested at 10 μM free Ca2+ concentrations. Figures reproduced from Olivares et al. (2014). C: Electron microscopy analyses show that in unstimulated chromaffin cells a dense cortical actin mesh separates the secretory vesicles from the plasmalemma (upper panel), whereas in stimulated cells, the secretory vesicles that reach the plasmalemma are still surrounded by actin filaments (middle panel), and their exocytosis is favored by actin bundles linking secretory vesicles (granule) to the plasmalemma (bottom panel). Upper and middle images adapted from Nakata and Hirokawa (1992); Copyright 1992, Society for Neuroscience, and bottom image adapted from Gabel et al. (2015).
The intensity of the stimulus, and therefore the magnitude of Ca2+ entry, determines the degrees of F-actin disassembly and assembly (Olivares et al., 2014) and their impact on the exocytotic release of transmitters (Doreian et al., 2008). The variable effects of F-actin disassembly/assembly on neurosecretion include increase or reduction of the number of exocytosis events, or the amount of transmitter released per event, as determined by single-cell amperometry (Doreian et al., 2008; Berberian et al., 2009; González-Jamett et al., 2013; Olivares et al., 2014; González-Jamett et al., 2017). Probably these effects are determined by a differential activation and/or recruitment of actin partners, such as those that promote actin assembly, like N-WASP (Gasman et al., 2004) and cortactin (González-Jamett et al., 2017), or F-actin disassembly, like scinderin (Rodriguez Del Castillo et al., 1990), among others. However, future investigations should be conducted to determine the submembrane Ca2+ levels that selectively recruit or activate proteins involved in F-actin assembly or disassembly, including protein kinase C, Src kinase, scinderin, among others.
The rearrangement of the cortical actin cytoskeleton is required for secretory vesicle mobility (Desnos et al., 2003; Neco et al., 2004; Wen et al., 2011). Biochemical and morphological analyses, together with live-cell imaging experiments, in PC12 cells, a cell line obtained from a pheochromocytoma of rat adrenal medulla, have shown that this mobility depends on myosin Va and VI, which bind to secretory vesicle and promote their motion towards the cell periphery (Rudolf et al., 2003; Tomatis et al., 2013).
The actin cytoskeleton also plays a role in the organization of the exocytotic site (Torregrosa-Hetland et al., 2011). As visualized by total internal reflection fluorescence microscopy (TIRFM) and Förster resonance energy transfer (FRET), SNARE proteins and voltage-dependent Ca2+ channels appear to be clustered together with F-actin structures (Torregrosa-Hetland et al., 2013). This actin function seems to involve annexin A2 (Gabel et al., 2015), an actin-binding protein that binds and remodels lipid membranes in a Ca2+-dependent way (Gabel and Chasserot-Golaz, 2016). In this regard, biochemical assays in chromaffin cells, together with immunogold electron microscopy, reveal that annexin A2 is recruited to the plasmalemma upon cell stimulation, where it bundles actin filaments that organize lipid platforms for docking and exocytosis of secretory vesicles (Gabel et al., 2015).
The cortical actin cytoskeleton further provides the membrane tension required for the late stage of the exocytotic process (Bretou et al., 2014; Shin et al., 2018; Wen et al., 2016). In this regard, measurements of exocytosis with single-cell amperometry shows that the disruption of the F-actin cortex, with cytochalasin D or latrunculin A or by interfering with the function of N-WASP or cortactin, delays the enlargement of the fusion pore (González-Jamett et al., 2013; Olivares et al., 2014; González-Jamett et al., 2017), a transient structure formed during exocytosis (Álvarez de Toledo et al., 2018). The membrane tension provided by cortical F-actin importantly depends on myosin II (Bretou et al., 2014). Indeed, inhibition of myosin II function also impairs the fusion pore expansion (Neco et al., 2008; Doreian et al., 2008; Berberian et al., 2009). Regarding the mechanism by which myosin II might control membrane tensions, optical trap experiments suggest that myosin-1a contributes to the adhesion between the plasmalemma and the F-actin cortex (Nambiar et al., 2009). Then, it remains to be proved that this mechanism is also valid for myosin II.
Although some of the findings aforementioned appear to be controversial, it is currently accepted that the cortical actin cytoskeleton is a dynamic structure that is constantly rearranged at specific submembrane regions in response to different stimulus intensity. This tightly controlled assembly and disassembly of actin filaments facilitates different stages of the secretory process, including secretory vesicle mobility, and its docking and fusion at exocytotic sites, wherein SNARE proteins and Ca2+ channels are organized together with actin structures. Despite different actin partners participating in these distinct events have been identified, the underlying mechanisms still remain ambiguous, in particular those that control membrane tension, and the signaling pathways activated by the stimulus that determine local assembly or disassembly of actin filaments. This should be resolved with the use of modern instruments with high temporal and spatial resolution.
How Does the Cortical Actin Drive Endocytosis and Vesicle Replenishment in Secretory Cells?
The cortical actin cytoskeleton plays a critical role in several forms of endocytosis by providing force for the invagination of endocytic pits (Kessels and Qualmann, 2021) and their later fission (Wu et al., 2016; Gormal et al., 2015). For example, using time-lapse imaging of Lifeact–GFP-transfected bovine chromaffin cells in combination with fluorescent 70 kDa dextran, Gormal and collaborators demonstrated that an acto-myosin II ring constricts the neck of nascent big vesicles or cisternae during bulk endocytosis, allowing the retrieval of large amounts of plasmalemma in response to sustained stimulation (Gormal et al., 2015). Actin polymerization can also provide the additional work against the membrane tension needed to complete membrane bending during clathrin-mediated endocytosis in cells (Boulant et al., 2012). In this regard, Huntingtin-interacting protein 1-related (HIP1R), a binding partner for clathrin light chains, is required for the productive interactions of clathrin coated vesicles with the actin cytoskeleton (Poupon et al., 2008). Additionally, using ‘flash-and-freeze’ electron microscopy, Watanabe and collaborators revealed that polymerized actin, possibly through the modulation of membrane tension, is needed for membrane invagination during the ultrafast endocytosis occurring in less than 0.1 s at sites flanking 100 nm the active zone in hippocampal pre-synapses (Watanabe et al., 2013). Moreover, Wu and collaborators, using measurements of membrane capacitance and fission pore conductance, imaging of vesicular protein endocytosis, and electron microscopy, also reported that cortical actin mediates overshoot (endocytosis that surpasses previous exocytosis), slow (time constant > 10 s) and rapid endocytosis (time constant ~1 s) in the calyx of Held, possibly by exerting mechanical forces required to bend membranes and thus to generate membrane pits (Wu et al., 2016). Finally, actin also has a fundamental participation in clathrin- and dynamin-independent endocytosis, a mechanism that is coordinated by the small GTPases Arf1 and Cdc42 and Bin/Amphiphysin/Rvs (BAR) domain proteins (Sathe et al., 2018). BAR domain proteins further provide a link between membrane remodeling and the actin cytoskeleton, coordinating the modulation of membrane curvature and actin assembly during cellular processes such as endocytosis and organelle trafficking (Carman and Dominguez, 2018).
We have recently found, by using membrane capacitance measurements in mouse chromaffin cells, that the fast dynamin-dependent endocytosis (time constant ~800 ms) which develops after the exocytosis triggered by action potential-type (ETAP) stimulus (Moya-Díaz et al., 2016, 2020) also depends on cortical F-actin (Montenegro et al., 2021). Particularly, depolymerization of actin filaments, induced by an increase of cytosolic Ca2+ or by pretreatment with cytochalasin D, significantly decelerates this endocytosis (Montenegro et al., 2021). This endocytic process is tightly associated with the replenishment of the group of vesicles secreted during ETAP. Inhibition of GTPase dynamin by different experimental approaches (cell dialysis with dynamin 829–842 blocking peptide or specific blocking antibodies against dynamin), modifications of cytosolic [Ca2+], as well as depolymerization of F-actin decelerate both processes in parallel (Moya-Díaz et al., 2016; Montenegro et al., 2021). We propose two alternative hypotheses (Fig. 2) to explain this tight relationship between fast dynamin-dependent endocytosis and rapid dynamin-dependent ETAP replenishment. One hypothesis is based on the idea that ETAP exocytosis is produced by a kiss-and-run type of mechanism. Through this process, the secretory vesicle is retrieved directly after the formation of a fusion pore, without collapse of the vesicle, and after the release of some portion of its content (Saheki and de Camilli, 2012). In such a case, vesicles would be rapidly retrieved and replenished locally, resulting in a rapid recovery of ETAP exocytosis. This hypothesis is supported by previous publications of the Corey Smith group showing that fast endocytosis, measured as membrane capacitance changes, and kiss-and-run, evaluated by small amperometric spikes and internalization of fluid phase fluorescent markers of small sizes, predominate in response to stimulation with action potentials applied alone or at low frequencies (Chan and Smith, 2001; Fulop et al., 2005). Later studies from the same group proposed that cortical F-actin plays a key role in stabilizing the kiss-and-run fusion event, whereas a stronger stimulation, as well cytochalasin D treatment, disrupts the actin cortex, driving full granule collapse (Doreian et al., 2008). The other possible hypothesis is that, after ETAP exocytosis, a fast endocytotic mechanism facilitates rapid vesicle replenishment by clearance of exocytotic materials from active zones, restoring the structure of the exocytotic sites (Hosoi et al., 2009). Following this hypothesis, if endocytosis is impaired by F-actin disruption (or by inhibition of dynamin) vesicle replenishment will be affected as well. In addition, the actomyosin network is also essential in the transport of vesicles to the exocytotic sites at the plasmalemma (Neco et al., 2003; Papadopulos et al., 2015). Therefore, in agreement with our experimental results, both hypotheses consider a pivotal role of cortical actin in the recycling of secretory vesicles occurring after ETAP. Future investigations directed to the fusion pore dynamics during ETAP should be conducted to discriminate between both hypotheses.
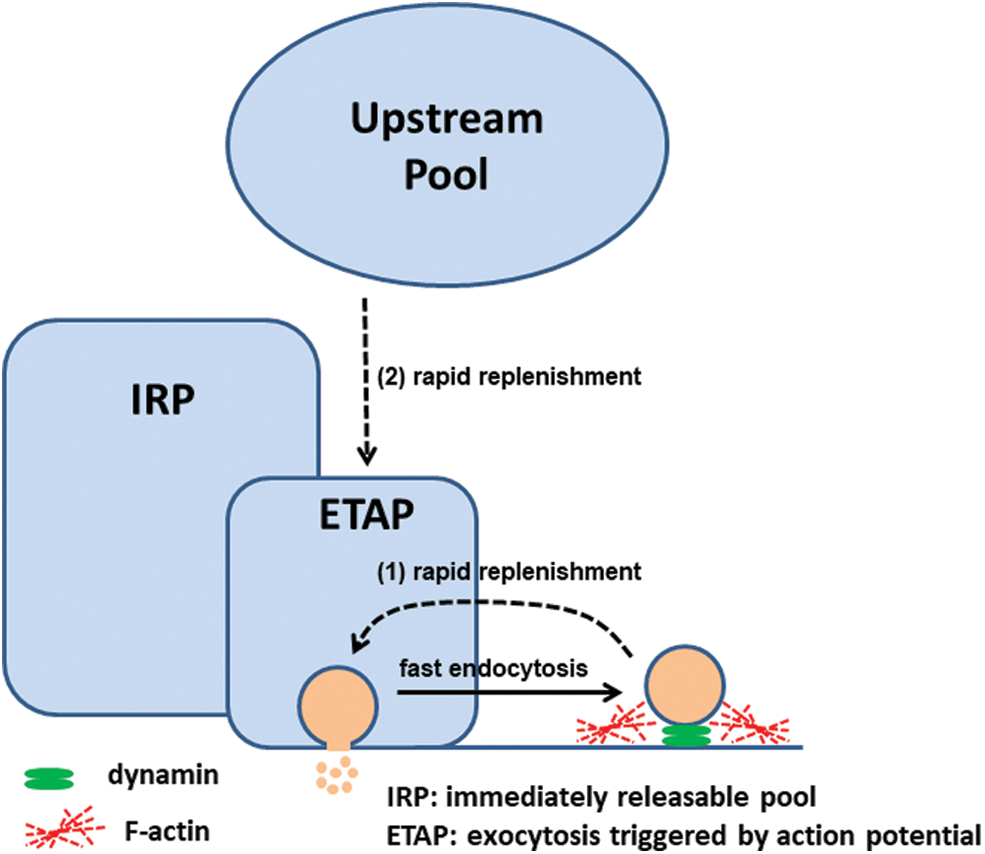
Figure 2: We propose two possible mechanisms for ETAP rapid replenishment: (1) local vesicle recovery through kiss and run, and/or (2) vesicle mobilization from upstream vesicular pools after clearance of exocytic materials by fast endocytosis. Both depend on a dynamin-dependent fast endocytosis and on an organized cortical F-actin. Low cytosolic [Ca2+] favors F-actin polymerization, and in consequence, accelerates fast endocytosis and rapid ETAP replenishment. Figure adapted from Moya-Díaz et al. (2016).
The localization of cortical filamentous actin in the sub-plasmalemmal region of secretory cells is strategic for the multiple roles that this protein has on diverse processes associated with secretion, as was summarized in this viewpoint. Cortical F-actin co-localizes with the secretory vesicles at different stages of the secretory process, even when secretory vesicles dock and fuse with the plasmalemma. Also, in this cortical area the compensatory membrane retrieval is produced for the reestablishment of plasmalemma surface and composition after vesicle fusion, and the transport of new endosomes to their respective membrane targets is initiated. Similar events have been observed in other systems, like in epithelial cells which undergo regulated secretion (Khandelwal et al., 2013, 2010). Some of the contributions of cortical actin to secretory vesicle exocytosis, endocytosis and recycling were already described in chromaffin cells, in pancreatic beta cells, in mast cells and in some neuronal models, as it was mentioned in this viewpoint. However, the strategic localization and properties of cortical actin open the door to many other regulatory functions associated with secretion. As aforementioned, how these many functions are distinctly and tightly regulated should be further investigated in the future.
Acknowledgement: Funders are thanked for supporting this work.
Authors’ Contribution: Study conception: Ana M Cárdenas, Fernando D Marengo; Draft manuscript preparation: Ana M Cárdenas, Luciana I Gallo, Fernando D Marengo. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: Not applicable.
Funding Statement: This work was supported by the Grants PICT 2764-2016, PICT 02849-2018 and PICT 02041-2019 from the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (Argentina), and ICN09_022 from ICM-ANID (Chile).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Álvarez de Toledo G, Montes MA, Montenegro P, Borges R (2018). Phases of the exocytotic fusion pore. FEBS Letters 592: 3532–3541. DOI 10.1002/1873-3468.13234. [Google Scholar] [CrossRef]
Aunis D, Bader MF (1988). The cytoskeleton as a barrier to exocytosis in secretory cells. Journal of Experimental Biology 139: 253–266. DOI 10.1242/jeb.139.1.253. [Google Scholar] [CrossRef]
Berberian K, Torres AJ, Fang Q, Kisler K, Lindau M (2009). F-actin and myosin II accelerate catecholamine release from chromaffin granules. Journal of Neuroscience 29: 863–870. DOI 10.1523/JNEUROSCI.2818-08.2009. [Google Scholar] [CrossRef]
Boulant S, Kural C, Zeeh JC, Ubelmann F, Kirchhausen T (2012). Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nature Cell Biology 13: 1124–1131. DOI 10.1038/ncb2307. [Google Scholar] [CrossRef]
Bretou M, Jouannot O, Fanget I, Pierobon P, Larochette N et al. (2014). Cdc42 controls the dilation of the exocytotic fusion pore by regulating membrane tension. Molecular Biology of the Cell 25: 3195–3209. DOI 10.1091/mbc.e14-07-1229. [Google Scholar] [CrossRef]
Burgoyne RD, Cheek TR (1987). Reorganization of peripheral actin filaments as a prelude to exocytosis. Bioscience Reports 7: 281–288. DOI 10.1007/BF01121449. [Google Scholar] [CrossRef]
Cárdenas AM, Marengo FD (2016). How the stimulus defines the dynamics of vesicle pool recruitment, fusion mode, and vesicle recycling in neuroendocrine cells. Journal of Neurochemistry 137: 867–879. DOI 10.1111/jnc.13565. [Google Scholar] [CrossRef]
Carman PJ, Dominguez R (2018). BAR domain proteins—A linkage between cellular membranes, signaling pathways, and the actin cytoskeleton. Biophysical Reviews 10: 1587–1604. DOI 10.1007/s12551-018-0467-7. [Google Scholar] [CrossRef]
Chan SA, Smith C (2001). Physiological stimuli evoke two forms of endocytosis in bovine chromaffin cells. Journal of Physiology 537: 871–885. DOI 10.1113/jphysiol.2001.012838. [Google Scholar] [CrossRef]
Chugh P, Paluch EK (2018). The actin cortex at a glance. Journal of Cell Science 131: jcs186254. DOI 10.1242/jcs.186254. [Google Scholar] [CrossRef]
Cooper JA (1987). Effects of cytochalasin and phalloidin on actin. Journal of Cell Biology 105: 1473–1478. DOI 10.1083/jcb.105.4.1473. [Google Scholar] [CrossRef]
Desnos C, Schonn JS, Huet S, Tran VS, El-Amraoui A et al. (2003). Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites. Journal of Cell Biology 163: 559–570. DOI 10.1083/jcb.200302157. [Google Scholar] [CrossRef]
Doreian BW, Fulop TG, Smith CB (2008). Myosin II activation and actin reorganization regulate the mode of quantal exocytosis in mouse adrenal chromaffin cells. Journal of Neuroscience 28: 4470–4478. DOI 10.1523/JNEUROSCI.0008-08.2008. [Google Scholar] [CrossRef]
Dugina V, Zwaenepoel I, Gabbiani G, Clément S, Chaponnier C (2009). Beta and gamma-cytoplasmic actins display distinct distribution and functional diversity. Journal of Cell Science 122: 2980–2988. DOI 10.1242/jcs.041970. [Google Scholar] [CrossRef]
Fowler VM, Pollard HB (1982). Chromaffin granule membrane-F-actin interactions are calcium sensitive. Nature 295: 336–339. DOI 10.1038/295336a0. [Google Scholar] [CrossRef]
Fujiwara I, Zweifel ME, Courtemanche N, Pollard TD (2018). Latrunculin A accelerates actin filament depolymerization in addition to sequestering actin monomers. Current Biology 28: 3183–3192.e2. DOI 10.1016/j.cub.2018.07.082. [Google Scholar] [CrossRef]
Fulop T, Radabaugh S, Smith C (2005). Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. Journal of Neuroscience 25: 7324–7332. DOI 10.1523/JNEUROSCI.2042-05.2005. [Google Scholar] [CrossRef]
Gabel M, Delavoie F, Demais V, Royer C, Bailly Y, Vitale N, Bader MF, Chasserot-Golaz S (2015). Annexin A2-dependent actin bundling promotes secretory granule docking to the plasma membrane and exocytosis. Journal of Cell Biology 210: 785–800. DOI 10.1083/jcb.201412030. [Google Scholar] [CrossRef]
Gabel M, Chasserot-Golaz S (2016). Annexin A2, an essential partner of the exocytotic process in chromaffin cells. Journal of Neurochemistry 137: 890–806. DOI 10.1111/jnc.13628. [Google Scholar] [CrossRef]
Gasman S, Chasserot-Golaz S, Malacombe M, Way M, Bader MF (2004). Regulated exocytosis in neuroendocrine cells: A role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Molecular Biology of the Cell 15: 520–531. DOI 10.1091/mbc.e03-06-0402. [Google Scholar] [CrossRef]
Gil A, Rueda J, Viniegra S, Gutiérrez LM (2000). The F-actin cytoskeleton modulates slow secretory components rather than readily releasable vesicle pools in bovine chromaffin cells. Neuroscience 98: 605–614. DOI 10.1016/S0306-4522(00)00132-9. [Google Scholar] [CrossRef]
González-Jamett AM, Momboisse F, Guerra MJ, Ory S, Báez-Matus X et al. (2013). Dynamin-2 regulates fusion pore expansion and quantal release through a mechanism that involves actin dynamics in neuroendocrine chromaffin cells. PLoS One 8: e70638. DOI 10.1371/journal.pone.0070638. [Google Scholar] [CrossRef]
González-Jamett AM, Guerra MJ, Olivares MJ, Haro-Acuña V, Baéz-Matus X, Vásquez-Navarrete J, Momboisse F, Martinez-Quiles N, Cárdenas AM (2017). The F-Actin binding protein cortactin regulates the dynamics of the exocytotic fusion pore through its SH3 domain. Frontiers in Cellular Neuroscience 11: 130. DOI 10.3389/fncel.2017.00130. [Google Scholar] [CrossRef]
Gormal RS, Nguyen TH, Martin S, Papadopulos A, Meunier FA (2015). An acto-myosin II constricting ring initiates the fission of activity-dependent bulk endosomes in neurosecretory cells. Journal of Neuroscience 35: 1380–1389. DOI 10.1523/JNEUROSCI.3228-14.2015. [Google Scholar] [CrossRef]
Guerra MJ, González-Jamett AM, Báez-Matus X, Navarro-Quezada N, Martínez AD, Neely A, Cárdenas AM (2019). The Ca2+ channel subunit Ca(V) beta2a-subunit down-regulates voltage-activated ion current densities by disrupting actin-dependent traffic in chromaffin cells. Journal of Neurochemistry 151: 703–715. DOI 10.1111/jnc.14851. [Google Scholar] [CrossRef]
Hinze C, Boucrot E (2018). Local actin polymerization during endocytic carrier formation. Biochemical Society Transactions 46: 565–576. DOI 10.1042/BST20170355. [Google Scholar] [CrossRef]
Hosoi N, Holt M, Sakaba T (2009). Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron 63: 216–229. DOI 10.1016/j.neuron.2009.06.010. [Google Scholar] [CrossRef]
Houy S, Croisé P, Gubar O, Chasserot-Golaz S, Tryoen-Tóth P, Bailly Y, Ory S, Bader MF, Gasman S (2013). Exocytosis and endocytosis in neuroendocrine cells: Inseparable membranes!. Frontiers in Endocrinology 4: 135. DOI 10.3389/fendo.2013.00135. [Google Scholar] [CrossRef]
Houy S, Nicolas G, Momboisse F, Malacombe M, Bader MF, Vitale N, Lecomte MC, Ory S, Gasman S (2020). αII-spectrin controls calcium-regulated exocytosis in neuroendocrine chromaffin cells through neuronal Wiskott–Aldrich Syndrome protein interaction. IUBMB Life 72: 544–552. DOI 10.1002/iub.2217. [Google Scholar] [CrossRef]
Jahn R, Fasshauer D (2012). Molecular machines governing exocytosis of synaptic vesicles. Nature 490: 201–207. DOI 10.1038/nature11320. [Google Scholar] [CrossRef]
Kessels MM, Qualmann B (2021). Interplay between membrane curvature and the actin cytoskeleton. Current Opinions in Cell Biology 68: 10–19. DOI 10.1016/j.ceb.2020.08.008. [Google Scholar] [CrossRef]
Khandelwal P, Ruiz WG, Apodaca G (2010). Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway. EMBO Journal 29: 1961–1975. DOI 10.1038/emboj.2010.91. [Google Scholar] [CrossRef]
Khandelwal P, Prakasam HS, Clayton DR, Ruiz WG, Gallo LI, van Roekel D, Lukianov S, Peränen J, Goldenring JR, Apodaca G (2013). A Rab11a-Rab8a-Myo5B network promotes stretch-regulated exocytosis in bladder umbrella cells. Molecular Biology of the Cell 24: 1007–1019. DOI 10.1091/mbc.e12-08-0568. [Google Scholar] [CrossRef]
Lee RW, Trifaro JM (1981). Characterization of anti-actin antibodies and their use in immunocytochemical studies on the localization of actin in adrenal chromaffin cells in culture. Neuroscience 6: 2087–2108. DOI 10.1016/0306-4522(81)90048-8. [Google Scholar] [CrossRef]
Li P, Bademosi AT, Luo J, Meunier FA (2018). Actin remodeling in regulated exocytosis: Toward a mesoscopic view. Trends in Cell Biology 28: 685–697. DOI 10.1016/j.tcb.2018.04.004. [Google Scholar] [CrossRef]
Marengo FD, Cárdenas AM (2018). How does the stimulus define exocytosis in adrenal chromaffin cells? Pflugers Archiv: European Journal of Physiology 470: 155–167. DOI 10.1007/s00424-017-2052-5. [Google Scholar] [CrossRef]
Miklavc P, Frick M (2020). Actin and myosin in non-neuronal exocytosis. Cells 9: 1455. DOI 10.3390/cells9061455. [Google Scholar] [CrossRef]
Moya-Díaz J, Alvarez YD, Montenegro M, Bayones L, Belingheri AV, Gonzalez-Jamett AM, Cardenas AM, Marengo FD (2016). Sustained exocytosis after action potential-like stimulation at low frequencies in mouse chromaffin cells depends on a dynamin-dependent fast endocytotic process. Frontiers in Cellular Neuroscience 10: 184. DOI 10.3389/fncel.2016.00184. [Google Scholar] [CrossRef]
Moya-Díaz J, Bayones L, Montenegro M, Cardenas AM, Koch H et al. (2020). Ca2+-independent and voltage-dependent exocytosis in mouse chromaffin cells. Acta Physiologica 228: e13417. DOI 10.1111/apha.13417. [Google Scholar] [CrossRef]
Montenegro M, Bayones L, Moya-Diaz J, Borassi C, Toscani MA, Gallo LI, Marengo FD (2021). Rapid vesicle replenishment after the immediately releasable pool exocytosis is tightly linked to fast endocytosis, and depends on basal calcium and cortical actin in chromaffin cells. Journal of Neurochemistry 157: 1069–1085. DOI 10.1111/jnc.15276. [Google Scholar] [CrossRef]
Nakata T, Hirokawa N (1992). Organization of cortical cytoskeleton of cultured chromaffin cells and involvement in secretion as revealed by quick-freeze, deep-etching, and double-label immunoelectron microscopy. Journal of Neuroscience 12: 2186–2197. DOI 10.1523/JNEUROSCI.12-06-02186.1992. [Google Scholar] [CrossRef]
Nambiar R, McConnell RE, Tyska MJ (2009). Control of cell membrane tension by myosin-I. Proceedings of the National Academy of Sciences 106: 11972–11977. DOI 10.1073/pnas.0901641106. [Google Scholar] [CrossRef]
Neco P, Fernández-Peruchena C, Navas S, Gutiérrez LM, de Toledo GA, Alés E (2008). Myosin II contributes to fusion pore expansion during exocytosis. Journal of Biological Chemistry 283: 10949–10957. DOI 10.1074/jbc.M709058200. [Google Scholar] [CrossRef]
Neco P, Giner D, Viniegra S, Borges R, Villarroel A, Gutiérrez LM (2004). New roles of myosin II during vesicle transport and fusion in chromaffin cells. Journal of Biological Chemistry 279: 27450–27457. DOI 10.1074/jbc.M311462200. [Google Scholar] [CrossRef]
Neco P, Giner D, Francés MM, Viniegra S, Gutiérrez LM (2003). Differential participation of actin- and tubulin-based vesicle transport systems during secretion in bovine chromaffin cells. European Journal of Neuroscience 18: 733–742. DOI 10.1046/j.1460-9568.2003.02801.x. [Google Scholar] [CrossRef]
Olivares MJ, González-Jamett AM, Guerra MJ, Baez-Matus X, Haro-Acuña V, Martínez-Quiles N, Cárdenas AM (2014). Src kinases regulate de novo actin polymerization during exocytosis in neuroendocrine chromaffin cells. PLoS One 9: e99001. DOI 10.1371/journal.pone.0099001. [Google Scholar] [CrossRef]
Papadopulos A, Gomez GA, Martin S, Jackson J, Gormal RS, Keating DJ, Yap AS, Meunier FA (2015). Activity-driven relaxation of the cortical actomyosin II network synchronizes Munc18-1-dependent neurosecretory vesicle docking. Nature Communications 6: 6297. DOI 10.1038/ncomms7297. [Google Scholar] [CrossRef]
Papadopulos A (2017). Membrane shaping by actin and myosin during regulated exocytosis. Molecular and Cellular Neurosciences 84: 93–99. DOI 10.1016/j.mcn.2017.05.006. [Google Scholar] [CrossRef]
Pons-Vizcarra M, Kurps J, Tawfik B, Sørensen JB, van Weering JRT, Verhage M (2019). MUNC18-1 regulates the submembrane F-actin network, independently of syntaxin1 targeting, via hydrophobicity in beta-sheet 10. Journal of Cell Science 132: jcs234674. DOI 10.1242/jcs.242552. [Google Scholar] [CrossRef]
Poupon V, Girard M, Legendre-Guillemin V, Thomas S, Bourbonniere L, Philie J, Bright NA, McPherson PS (2008). Clathrin light chains function in mannose phosphate receptor trafficking via regulation of actin assembly. Proceedings of the National Academy of Sciences USA 105: 168–173. DOI 10.1073/pnas.0707269105. [Google Scholar] [CrossRef]
Rodriguez Del Castillo A, Lemaire S, Tchakarov L, Jeyapragasan M, Doucet JP, Vitale ML, Trifaró JM (1990). Chromaffin cell scinderin, a novel calcium-dependent actin filament-severing protein. EMBO Journal 9: 43–52. DOI 10.1002/j.1460-2075.1990.tb08078.x. [Google Scholar] [CrossRef]
Rosé SD, Lejen T, Zhang L, Trifaró JM (2001). Chromaffin cell F-actin disassembly and potentiation of catecholamine release in response to protein kinase C activation by phorbol esters is mediated through myristoylated alanine-rich C kinase substrate phosphorylation. Journal of Biological Chemistry 276: 36757–36763. DOI 10.1074/jbc.M006518200. [Google Scholar] [CrossRef]
Rottner K, Faix J, Bogdan S, Linder S, Kerkhoff E (2017). Actin assembly mechanisms at a glance. Journal of Cell Science 130: 3427–3435. DOI 10.1242/jcs.206433. [Google Scholar] [CrossRef]
Rudolf R, Kögel T, Kuznetsov SA, Salm T, Schlicker O, Hellwig A, Hammer JA, Gerdes HH (2003). Myosin Va facilitates the distribution of secretory granules in the F-actin rich cortex of PC12 cells. Journal of Cell Science 116: 1339–1348. DOI 10.1242/jcs.00317. [Google Scholar] [CrossRef]
Saheki Y, de Camilli P (2012). Synaptic vesicle endocytosis. Cold Spring Harbor Perspectives in Biology 4: a005645. DOI 10.1101/cshperspect.a005645. [Google Scholar] [CrossRef]
Sathe M, Muthukrishnan G, Rae J, Disanza A, Thattai M, Scita G, Parton RG, Mayor S (2018). Small GTPases and BAR domain proteins regulate branched actin polymerisation for clathrin and dynamin-independent endocytosis. Nature Communications 9: 1835. DOI 10.1038/s41467-018-03955-w. [Google Scholar] [CrossRef]
Shin W, Ge L, Arpino G, Villarreal SA, Hamid E, Liu H, Zhao WD, Wen PJ, Chiang HC, Wu LG (2018). Visualization of membrane pore in live cells reveals a dynamic-pore theory governing fusion and endocytosis. Cell 173: 934–945.e12. DOI 10.1016/j.cell.2018.02.062. [Google Scholar] [CrossRef]
Singh RK, Mizuno K, Wasmeier C, Wavre-Shapton ST, Recchi C, Catz SD, Futter C, Tolmachova T, Hume AN, Seabra MC (2013). Distinct and opposing roles for Rab27a/Mlph/MyoVa and Rab27b/Munc13-4 in mast cell secretion. FEBS Journal 280: 892–903. [Google Scholar]
Sonal GKA, Vogel SK, Mücksch J, Blumhardt P, Schwille P (2018). Myosin-II activity generates a dynamic steady state with continuous actin turnover in a minimal actin cortex. Journal of Cell Science 132: jcs219899. DOI 10.1242/jcs.219899. [Google Scholar] [CrossRef]
Svitkina TM (2020). Actin cell cortex: Structure and molecular organization. Trends in Cell Biology 30: 556–565. DOI 10.1016/j.tcb.2020.03.005. [Google Scholar] [CrossRef]
Tomatis VM, Papadopulos A, Malintan NT, Martin S, Wallis T, Gormal RS, Kendrick-Jones J, Buss F, Meunier FA (2013). Myosin VI small insert isoform maintains exocytosis by tethering secretory granules to the cortical actin. Journal of Cell Biology 200: 301–320. DOI 10.1083/jcb.201204092. [Google Scholar] [CrossRef]
Torregrosa-Hetland CJ, Villanueva J, Giner D, Lopez-Font I, Nadal A et al. (2011). The F-actin cortical network is a major factor influencing the organization of the secretory machinery in chromaffin cells. Journal of Cell Science 124: 727–734. DOI 10.1242/jcs.078600. [Google Scholar] [CrossRef]
Torregrosa-Hetland CJ, Villanueva J, Garcia-Martínez V, Expósito-Romero G, Francés Mdel M, Gutiérrez LM (2013). Cortical F-actin affects the localization and dynamics of SNAP-25 membrane clusters in chromaffin cells. International Journal of Biochemistry & Cell Biology 45: 583–592. DOI 10.1016/j.biocel.2012.11.021. [Google Scholar] [CrossRef]
Trexler AJ, Taraska JW (2017). Regulation of insulin exocytosis by calcium-dependent protein kinase C in beta cells. Cell Calcium 67: 1–10. DOI 10.1016/j.ceca.2017.07.008. [Google Scholar] [CrossRef]
Venkatesh K, Mathew A, Koushika SP (2020). Role of actin in organelle trafficking in neurons. Cytoskeleton 77: 97–109. DOI 10.1002/cm.21580. [Google Scholar] [CrossRef]
Watanabe S, Rost BR, Camacho-Pérez M, Davis MW, Sohl-Kielczynski B, Rosenmund C, Jorgensen EM (2013). Ultrafast endocytosis at mouse hippocampal synapses. Nature 504: 242–247. DOI 10.1038/nature12809. [Google Scholar] [CrossRef]
Wen PJ, Osborne SL, Zanin M, Low PC, Wang HT et al. (2011). Phosphatidylinositol(4,5)bisphosphate coordinates actin-mediated mobilization and translocation of secretory vesicles to the plasma membrane of chromaffin cells. Nature Communications 2: 491. DOI 10.1038/ncomms1500. [Google Scholar] [CrossRef]
Wen PJ, Grenklo S, Arpino G, Tan X, Liao HS et al. (2016). Actin dynamics provides membrane tension to merge fusing vesicles into the plasma membrane. Nature Communications 7: 12604. DOI 10.1038/ncomms12604. [Google Scholar] [CrossRef]
Wu XS, Lee SH, Sheng J, Zhang Z, Zhao WD, Wang D, Jin Y, Charnay P, Ervasti JM, Wu LG (2016). Actin is crucial for all kinetically distinguishable forms of endocytosis at synapses. Neuron 92: 1020–1035. DOI 10.1016/j.neuron.2016.10.014. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |